Abstract
Both myeloid cells, which contribute to innate immunity, and lymphoid cells, which dominate adaptive immunity, partake in defending against SARS-CoV-2. In response to the virus, the otherwise slow haematopoietic production supply chain quickly unleashes its preconfigured myeloid element, which largely resists a bullwhip-like effect. By contrast, the lymphoid element risks a bullwhip-like effect when it produces T cells and B cells that are specifically designed to clear the virus. As T-cell production is telomere-length dependent and telomeres shorten with age, older adults are at higher risk of a T-cell shortfall when contracting SARS-CoV-2 than are younger adults. A poorly calibrated adaptive immune response, stemming from a bullwhip-like effect, compounded by a T-cell deficit, might thus contribute to the propensity of people with inherently short T-cell telomeres to develop severe COVID-19. The immune systems of these individuals might also generate an inadequate T-cell response to anti-SARS-CoV-2 vaccination.
Introduction
Haematopoiesis in the red bone marrow generates and maintains the 25 trillion circulating red blood cells (RBCs) in adults.1 The production of these cells, which do not have nuclei, is tightly controlled. Haematopoiesis also produces nucleated white blood cells (WBCs) that belong to the myeloid lineage, which is central to innate immunity,2 and the lymphoid lineage, which is the core of adaptive immunity.3 Throughout the human life course, haematopoiesis strictly regulates the myeloid cells (known here as system 1) but has less influence on the lymphoid cells (known here as system 2). In fighting invading pathogens, system 1 is reflexive and largely general, whereas system 2 is plastic and specific. Both systems engage SARS-CoV-2, the virus that causes COVID-19.4, 5, 6
In an interview about decision making with The New Yorker's Maria Konnikova on April 6, 2020,7 88-year-old Nobel Prize winner Daniel Kahneman made a chillingly prescient prediction about COVID-19: “For old people like me the prospects are not good. I see very little reason for optimism. I mean at the best I would say for the older among us, it's pretty much a life sentence of incarceration at home, or a very long-term sentence. This is going to change the rest of our lives.”
In his book Thinking, Fast and Slow,8 Kahneman describes two systems of decision making, which he refers to as system 1 and system 2. System 1 of decision making is reflexive, whereas system 2 of decision making is analytical and often involves a series of steps. Immune cells do not make decisions, but portraying the myeloid element of innate immunity (ie, reflexive and general) as system 1 and the lymphoid element of the adaptive immunity (ie, plastic and specific) as system 2 might help to explain a puzzling person-to-person variation in the response to anti-SARS-CoV-2 vaccination and the susceptibility of older people to severe COVID-19.
Haematopoiesis
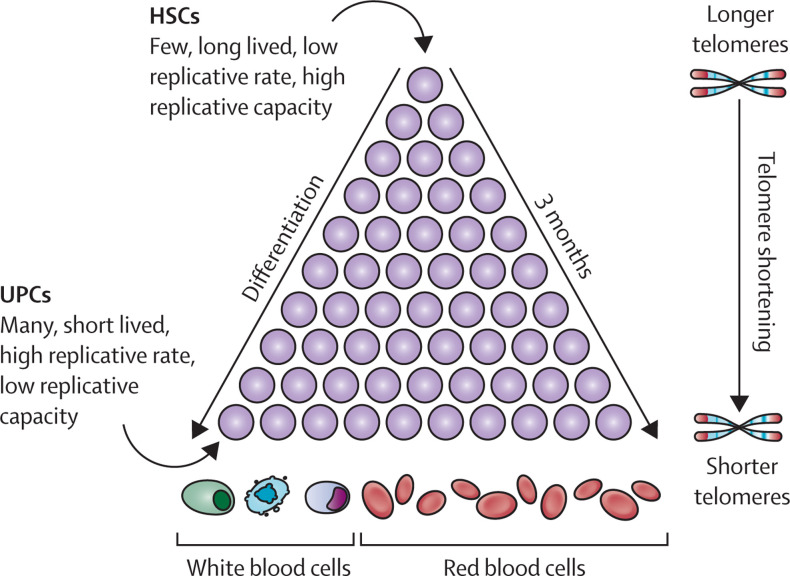
Roughly 400 long-lived haematopoietic stem cells at the top of the haematopoietic hierarchy replicate about once a year in an adult.9, 10, 11 Other work has estimated that the number of haematopoietic stem cells at the top of the hierarchy is 50 000–200 000,12 but it is unclear which number is correct. The pace of replication progressively quickens down the hierarchy, where the numbers of haematopoietic cells increase as they become differentiated (ie, mature) and take on functions of their specific lineages (figure 1 ). At the bottom of the hierarchy, short-lived unipotent haematopoietic progenitor cells replicate once or more per day, releasing into the circulation around 300 billion blood cells, most of which are RBCs. At least 30 sequential cell replications separate haematopoietic stem cells at the top of the hierarchy from circulating blood cells. Additionally, whereas haematopoietic stem cells have a high replicative capacity (ie, they can replicate numerous times), unipotent cells have a low replicative capacity and are continuously replaced by haematopoietic cells from higher strata of the hierarchy. Generally, 3 months elapse between the replication of haematopoietic stem cells and the release of their progenies into the circulation (figure 1).9, 10
Figure 1.
Haematopoiesis
HSCs are at the top of the hierarchy and UPCs are at the bottom of the hierarchy. The figure shows only eight of the more than 30 strata of cell replication. Telomeres are depicted as red caps on the chromosomal ends. HSCs=haematopoietic stem cells. UPCs=unipotent cells.
From the standpoint of homeostasis (ie, the maintenance of physiological stability), referred to as a steady state, this configuration makes little sense. In theory, fast haematopoietic cell replication up the hierarchy and propagation of replication waves downwards could, for instance, rapidly stabilise decreasing circulating RBCs due to bleeding and bring them back to a typical level. However, such a response might raise the risk of a process similar to the supply chain phenomenon known as the bullwhip effect.
The hierarchal dynamics of haematopoiesis attenuate a bullwhip-like effect
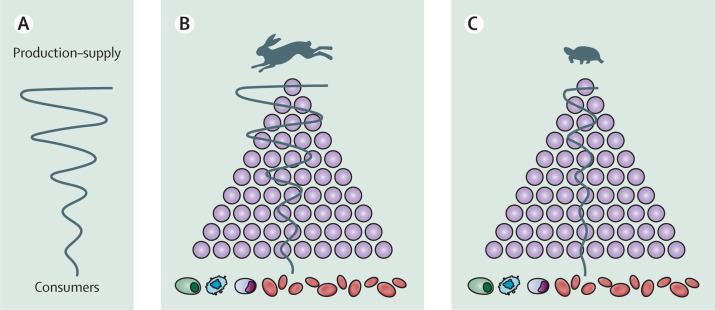
The bullwhip effect, in essence, arises when irregular variations in the consumer demand ripple across the production–supply chain, progressively increasing in magnitude as they move up the chain (figure 2A ).13, 14, 15, 16 This variance amplification interrupts the proper operation of the production–supply chain. Many models have been formulated to mitigate the bullwhip effect. In commerce, however, human decision making exerts a major influence on the effect,17 diminishing the likelihood of its abolition. By contrast, the cellular factors that define the dynamics of haematopoiesis have been created by evolution, which is free of human cognition.18
Figure 2.
Smoothing a bullwhip-like effect in haematopoiesis by slowing the production of blood cells
(A) Bullwhip effect in commerce. (B) Bullwhip-like effect in a setting of fast production–supply (hare) of blood cells. (C) Preferred system of slow production–supply (tortoise) that reduces the effect.
The Tortoise and the Hare fable, in which the fast-moving hare is beaten in a race by the slower-moving, but consistently persevering, tortoise competitor, has been invoked as a lesson for decreasing the bullwhip effect.13, 14 A slow but stable production–supply chain better withstands unanticipated factors that might disrupt its operation than does a fast production–supply chain. This analogy applies to haematopoiesis, for which the production–supply starts with haematopoietic stem cells and ends in circulating blood cells (figure 2B, C). As haematopoiesis occurs primarily in the red bone marrow of flat bones, such as the sternum, pelvis, shoulder, and skull, circulating blood cells originate not from a single production–supply chain but from multiple chains. Variance amplification in a fast proliferative response of haematopoietic cells up the hierarchy of these different chains might overcompensate for minor (within normal) fluctuations in the number of circulating blood cells. Therefore, when forced to react because of bleeding, for instance, this haematopoietic system relegates the immediate response to stabilise RBC count to haematopoietic cells lower down the hierarchy.19 Such reliance on haematopoietic cells down the hierarchy to dodge a bullwhip-like effect is also evident when the haematopoietic system responds to infection.
Decoupling adaptive immunity from innate immunity
WBCs that primarily belong to the myeloid lineage are a key component of innate immunity (ie, system 1). This immune defence of multicellular organisms2 responds promptly to an invading pathogen, although some viruses, including SARS-CoV-2, might manage to delay their detection.20 Except for monocytes, which traffic out of the blood to become macrophages with replicative capacity,21 cells of the myeloid lineage are terminally differentiated, and they do not replicate. Principally relying on cells down the haematopoietic hierarchy for their production and replenishment (ie, myelopoiesis), myeloid cells set off a largely preconfigured immune response to kill or contain invading pathogens.22, 23 However, these cells do not have the tactical plasticity of tailor-made mechanisms to clear specific pathogens.
Some pathogens cause mild infections, whereas others are life-threatening. The innate immune response is often sufficient to clear pathogens that cause mild infections. However, in engaging potentially life-threatening infections, such as SARS-CoV-2, this elemental system often only suppresses the pathogen, providing time to mobilise a more specific response. In fact, haematopoiesis seems to falter under severe SARS-CoV-2 assault, as shown by the spill of premature myeloid cells into the circulation from higher strata of the haematopoietic hierarchy (ie, emergency myelopoiesis).24, 25 Such a finding suggests that the supply chain of myeloid cells cannot accommodate the increased demand in patients with severe COVID-19. In the next stage of the response, the logistics of fighting the pathogen shift to an immune system that has evolved more recently than innate immunity (ie, adaptive immune response), which involves lymphoid cells (system 2).3
The adaptive immune response relies principally on two types of lymphocytes (ie, T cells and B cells), which are specifically designed to clear an offending pathogen. Both cell types are formed in the bone marrow but retain their replicative capacities after leaving it. The life histories of T cells and B cells and their biology are complex and beyond the scope of this Personal View.26, 27 Put simply, T cells and B cells continue to differentiate out of the bone marrow and are not constrained by the strict rules of haematopoiesis. These cells are generally stratified into two subcategories: naive T cells and B cells and the different types of pathogen-specific effector or memory (EM) cells that they generate through sequential replications in response to infection, a process that is referred to as clonal expansion.
In addition to T cells and B cells, natural killer cells are capable of clonal expansion.28, 29 Similarly to T and B cells, these cells are formed in the bone marrow and further differentiate outside of it. Although they are of lymphoid origin, natural killer cells are mobilised to kill virus-infected cells as part of the general innate immune response. Moreover, they appear to display adaptive-like features in chronic viral infection, conferring some specific immunity through clonal expansion.28 The adaptive immune response to acute infections, however, is principally driven by the clonal expansion of T cells and B cells.
Pathogens possess epitopes (ie, antigens) that a T cell recognises as non-self. When exposed to its cognate antigen, a naive T cell begins a series of replications that mark clonal expansion. While the innate immune response fends off the invading pathogen for several days, selected naive T cells generate an army of EM T cells through clonal expansion. Additionally, a subset of T cells, called follicular helper CD4+ T cells, interact with antigen-specific B cells, which are then stimulated to replicate and become EM B cells.30, 31 EM B cells secrete specific antibodies against the pathogen. Jointly, the custom-made EM T cells and antibody-secreting B cells provide a powerful and specific mechanism to clear an invading pathogen from the body.
By exposing T cells and B cells to antigens, vaccination provokes the adaptive immune response to generate EM cells and antibodies against a pathogen through mechanisms similar to a response to the real infection. What is so baffling about this response, however, is its wide interindividual variation after vaccination. For SARS-CoV-2 antigens, a hundred times or more variation across adults is observed after vaccination in virus-specific T-cell responses32, 33, 34, 35, 36, 37, 38, 39 and antibody titres.39, 40, 41, 42, 43, 44, 45
The dynamics of adaptive immunity risk a bullwhip-like effect
The myeloid component of the innate immune response (system 1) to acute infection mainly relies on circulating blood cells and unipotent cells down the production–supply chain of the haematopoietic hierarchy. Haematopoietic cells up the hierarchy hardly contribute to the process. By contrast, the adaptive immune response (system 2) to acute infection constructs an entire production–supply chain of EM cells specific for a given antigen in a few days. The chain depends on interactions within and between T cells and B cells and sequential waves of cell replication that take place not at the site where a pathogen enters the body (eg, the respiratory epithelium for SARS-CoV-2) but in regional lymph nodes.46 Clonal expansion at these lymph nodes is not only rapid but also exponential.
In his interview with Maria Konnikova about decision making and the expanding COVID-19 pandemic, Kahneman reflects: “This is an exponential event; that is, we see things doubling every two days, every three days, every four days, and people don’t, certainly, including myself, don’t seem to think straight about exponential growth.”7 Similar to the spreading pandemic, the exponential nature of clonal expansion means that the size of an expansion doubles with each cycle of replication (eg, 1 cell becomes 2 cells, then 22 cells, then 23 cells, etc). Such a process expeditiously produces an enormous number of EM cells. For instance, in 20 replicative cycles, one naive cell can produce a clone of about 1 million EM cells.
Rapidly expanding clones of T cells and B cells across regional lymph nodes, and the exponential nature of this process, provide an excellent setting for variance amplification up the EM cell production–supply chain. A bullwhip-like effect might thus contribute to a poorly calibrated adaptive immune response, as expressed after vaccination in the vast interindividual variation in EM T-cell counts and anti-SARS-CoV-2 antibodies. Notably, vaccination exposes different people to a fixed dose of SARS-CoV-2 antigens, whereas infection exposes people to different loads of the virus. The varying viral load would further exacerbate a bullwhip-like effect on the adaptive immune response.
The telomeric factor
Telomere length is another potential contributor to the interindividual variation in the adaptive immune response. Telomeres cap the ends of the chromosomes and serve to protect the genome from genomic instability and other hazards that might damage genes or alter their functions.47, 48, 49 In humans, telomere length has been linked to ageing-related diseases that largely fall under two major categories: cardiovascular disease and cancer.48, 50, 51, 52, 53 Throughout the human life course, as somatic cells replicate, their telomeres progressively shorten. Age-dependent shortening thus explains the shorter length of telomeres in somatic cells, including WBCs, of many older people compared with younger people, regardless of their health status.54, 55 Variation in telomere length across somatic cells of the individual reflects their different replicative histories,56 whereas the vast interindividual variation in telomere length at any age mainly relates to heredity.57, 58
The progressive shortening of telomeres ultimately triggers cell signals that stop replication. The outcome is a cellular state referred to as replicative senescence.59 An enzyme called telomerase,60 which is typically silent in most human somatic cells, increases its activity in T cells and B cells as they begin antigen-mediated proliferation. Telomerase can elongate telomeres, but its activation in T cells is insufficient to prevent telomere shortening,61, 62, 63 meaning that T-cell clonal expansion is dependent on their telomere length.
When short telomeres prevent further replication, the exponential feature of clonal expansion works in reverse (ie, 220 cells becomes 219 cells, then 218, then 217, etc), meaning that the clone size is smaller by 50% for each unachievable replicative cycle. This concept is even more perplexing than exponential growth. For instance, a telomere-length-mediated decline in the number of clonally expanding T cells from 20 replications to 15 replications would decrease the clone size from about 1 million EM T cells to approximately only 30 000 cells. As the T-cell response to vaccination or SARS-CoV-2 cross-recognises SARS-CoV-2 variants and is long-lasting,37, 38 short T-cell telomeres might result in a shorter-lasting response to SARS-CoV-2 variants.
T-cell clonal expansion and the age of onset
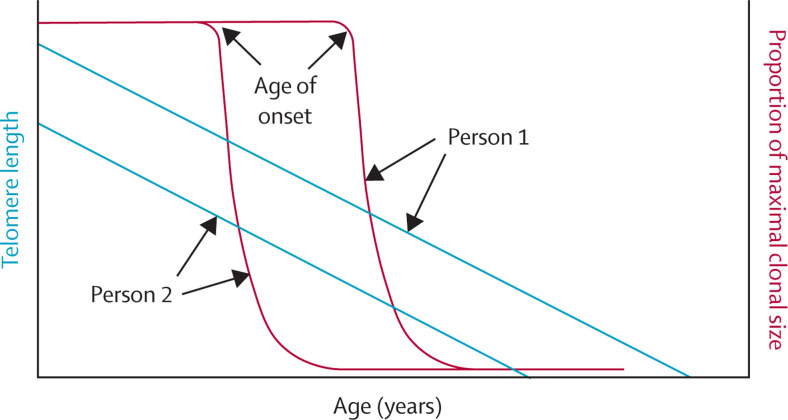
WBC telomeres shorten in a linear fashion (ie, at a slow pace of about 0·03 kilobases per year) during adult life.64, 65 These telomeres also show tracking and fixed ranking, such that people with comparatively short (or long) WBC telomeres in their twenties maintain their short (or long) telomeres throughout their adult lives.66 Until a particular age, referred to as the age of onset, 67 despite age-dependent telomere shortening, WBC telomeres are long enough so that T cells can achieve a maximal clone size of EM T cells through clonal expansion. After this age, WBC telomeres are too short to achieve the maximal clone size. The model used by Anderson and colleagues67 showed that, within 10 years after the age of onset, the T-cell clonal expansion decreases in an exponential fashion to less than 5% of its maximal clone size. This process happens while WBC telomeres continue to shorten at their slow pace (figure 3 ).
Figure 3.
The effect of age-dependent shortening of telomeres in white blood cells on T-cell clonal expansion in two adults
Person 1 has longer telomeres than person 2. Person 1 reaches age of onset at an older age than person 2. Both people start with maximal T-cell clone size.
Under steady-state conditions, circulating T cells in healthy adults show a low turnover, surviving for months if not years.68 Therefore, when these T cells die, their slow replacement exerts a minor demand on T-cell replication in most adults, regardless of the age of onset. By contrast, people who contract SARS-CoV-2 past the age of onset are at increased risk of severe COVID-19, because their T-cell production might not meet the increased T-cell demand for fighting the pathogen.67
T-cell lymphopenia and WBC telomere length are biomarkers of severe COVID-19
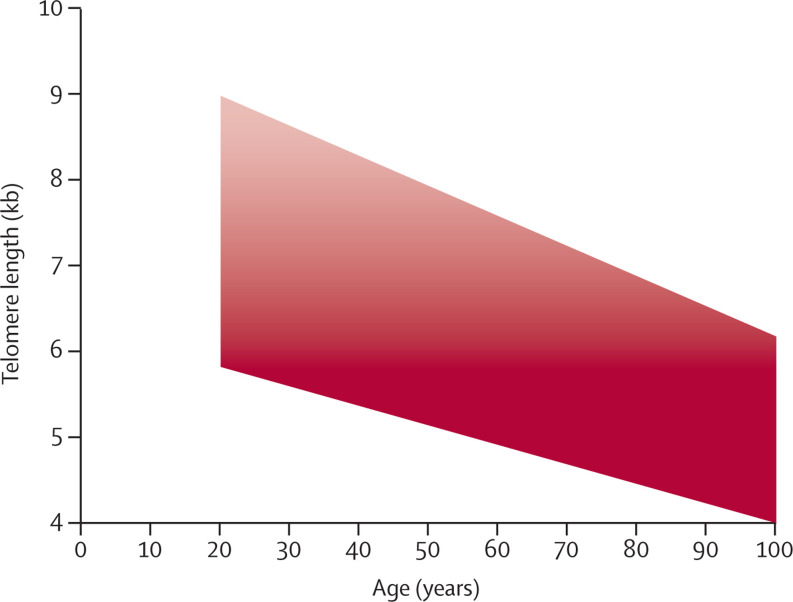
Patients who are admitted to hospital for COVID-19 often show a low number of blood lymphocytes (ie, lymphopenia), which typically stems from a decreasing number of T cells. COVID-19 lymphopenia is thus T-cell lymphopenia.4, 69, 70, 71, 72 This form of lymphopenia is more common in older people than in younger people,69, 70, 71, 72, 73 and it typically indicates severe disease. Much about the primary cause of COVID-19 lymphopenia is unknown. Notwithstanding underlying causes, offsetting the decline in T-cell number or recovering from COVID-19 T-cell lymphopenia requires prompt and massive telomere-length-dependent T-cell clonal expansion. Additionally, short telomeres might prevent sufficient T-cell expansion to attain the optimal number of EM T cells that are essential for SARS-CoV-2 clearance, even in patients showing a normal T-cell number. As feedback mechanisms link adaptive immunity to innate immunity, a poor telomere-length-mediated T-cell response to SARS-CoV-2 can unleash the innate response in the form of a cytokine storm that might cause severe lung injury.74 The correlation between telomere length in peripheral blood mononuclear cells and lymphocyte count in patients with COVID-19,75 and the propensity of adults with short WBC telomeres to have severe disease,76, 77, 78, 79, 80 support this overall scheme. Notably, as not only ageing but also heredity exert a profound effect on telomere length, WBC telomere length in some people in their forties is similar to that of many octogenarians (figure 4 ). These younger adults with inherently short WBC telomere length might also be at risk for severe COVID-19.
Figure 4.
Telomere length in white blood cells across the adult population and the risk of severe COVID-19 because of short telomeres
Colour intensity indicates risk of severe disease. White blood cell telomere length data are from Steenstrup and colleagues.55 Risk of telomere-length-dependent severe disease is based on a model by Anderson and colleagues.67
Conclusion
2·5 years into the COVID-19 pandemic, the severity of and the number of deaths due to COVID-19 have eased in the USA and Europe. However, as SARS-CoV-2 will continue evolving to escape immunity, COVID-19 is not going away anytime soon. Successive variants will probably be progressively more transmissible than the original SARS-CoV-2 strain. As the transmissibility plateau of these variants is unknown, SARS-CoV-2 will continue to be an unpredictable and formidable threat to older adults, such as Kahneman and me.
A major cause of mortality related to COVID-19 in older adults is probably the decreased capability of system 2 due to increased age. The adaptive immune response probably evolved because of the preference of haematopoiesis for stability. The tortoise model works well for the RBC and myeloid cell pipelines. However, the hurried construction of the adaptive immune response in the face of acute infections by lethal pathogens, such as SARS-CoV-2, risks variance amplification up the production–supply chain of EM cells. Learning more about the role of telomere-length dynamics in the T-cell response to SARS-CoV-2 is of particular interest since the telomere-length-dependent T-cell response is enduring and cross-recognises SARS-CoV-2 variants. When combined with a bullwhip-like effect, short telomeres probably predispose many older adults, and younger people with inherently short telomeres, to severe COVID-19. A comprehensive assessment of the age of onset based on measurements of WBC telomere length in the general population might help to identify individuals at high risk of telomere-length-dependent COVID-19 T-cell lymphopenia. These individuals might also generate a weak immune response to anti-SARS-CoV-2 vaccination.
Finally, a dialogue of behavioural economists with haematologists and evolutionary biologists could generate further insight into the bullwhip effect in both medicine and economy. Economists have developed many models for this effect, a few of which might improve understanding of haematopoiesis and adaptive immunity. In return, economists could learn how evolution has forged the supply chains of RBCs, myeloid cells, and EM T cells and B cells without the interference of Kahneman's two systems of human decision making, built on the work for which he was awarded the Nobel Memorial Prize in Economic Sciences. In its announcement of the award, the Royal Swedish Academy of Sciences credited Kahneman “for having integrated insights from psychological research into economic science, especially concerning human judgment and decision-making under uncertainty”.81
Declaration of interests
I declare no competing interests.
Acknowledgments
Acknowledgments
AA's telomere research is supported by the National Institutes of Health (grants R01 HL134840, U01AG066529, and 1R56AG073226) and a grant from the Norwegian Research Council (ES562296). I thank Konstantin Arbeev and Daniel Aviv for constructing the figures.
Contributors
AA is responsible for the conceptualisation and writing of the Personal View. He accepts responsibility for publication submission.
References
- 1.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riera Romo M, Pérez-Martínez D, Castillo Ferrer C. Innate immunity in vertebrates: an overview. Immunology. 2016;148:125–139. doi: 10.1111/imm.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm T, Swann JB. Origin and evolution of adaptive immunity. Annu Rev Anim Biosci. 2014;2:259–283. doi: 10.1146/annurev-animal-022513-114201. [DOI] [PubMed] [Google Scholar]
- 4.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKechnie JL, Blish CA. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe. 2020;27:863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickenden D. Why we underestimated COVID-19. April 6, 2020. https://www.newyorker.com/podcast/political-scene/why-we-underestimated-covid-19
- 8.Kahneman D. Thinking, fast and slow, 8th edn. Farrar, Straus and Giroux; New York: 2011. [Google Scholar]
- 9.Dingli D, Traulsen A, Pacheco JM. Compartmental architecture and dynamics of hematopoiesis. PLoS One. 2007;2:e345. doi: 10.1371/journal.pone.0000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner B, Beier F, Hummel S, et al. Reconstructing the in vivo dynamics of hematopoietic stem cells from telomere length distributions. eLife. 2015;4:e08687. doi: 10.7554/eLife.08687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidorov I, Kimura M, Yashin A, Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol. 2009;37:514–524. doi: 10.1016/j.exphem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee-Six H, Øbro NF, Shepherd MS, et al. Population dynamics of normal human blood inferred from somatic mutations. Nature. 2018;561:473–478. doi: 10.1038/s41586-018-0497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Disney SM. The bullwhip effect: progress, trends and directions. Eur J Oper Res. 2016;250:691–701. [Google Scholar]
- 14.Ohno T, Bodek N. Toyota production system: beyond large-scale production. CRC Press; Boca Raton, FL: 2019. [Google Scholar]
- 15.Forrester JW. Industrial dynamics. MIT Press; Cambridge, MA: 1961. [Google Scholar]
- 16.Bray RL, Mendelson H. Information transmission and the bullwhip effect: an empirical investigation. Manage Sci. 2012;58:860–875. [Google Scholar]
- 17.Yang Y, Lin J, Liu GL, Zhou L. The behavioural causes of bullwhip effect in supply chains: a systematic literature review. Int J Prod Econ. 2021;236:108120. [Google Scholar]
- 18.Hummert S, Bohl K, Basanta D, et al. Evolutionary game theory: cells as players. Mol Biosyst. 2014;10:3044–3065. doi: 10.1039/c3mb70602h. [DOI] [PubMed] [Google Scholar]
- 19.Mon Père NV, Lenaerts T, Pacheco JMDS, Dingli D. Multistage feedback-driven compartmental dynamics of hematopoiesis. iScience. 2021;24:102326. doi: 10.1016/j.isci.2021.102326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taefehshokr N, Taefehshokr S, Hemmat N, Heit B. COVID-19: perspectives on innate immune evasion. Front Immunol. 2020;11:580641. doi: 10.3389/fimmu.2020.580641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins SJ, Allen JE. The expanding world of tissue-resident macrophages. Eur J Immunol. 2021;51:1882–1896. doi: 10.1002/eji.202048881. [DOI] [PubMed] [Google Scholar]
- 22.Rivera A, Siracusa MC, Yap GS, Gause WC. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17:356–363. doi: 10.1038/ni.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilk AJ, Lee MJ, Wei B, et al. Multi-omic profiling reveals widespread dysregulation of innate immunity and hematopoiesis in COVID-19. J Exp Med. 2021;218:e20210582. doi: 10.1084/jem.20210582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzemaekers M, Cambier S, Blanter M, et al. Kinetics of peripheral blood neutrophils in severe coronavirus disease 2019. Clin Transl Immunol. 2021;10:e1271. doi: 10.1002/cti2.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Björkström NK, Strunz B, Ljunggren HG. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2022;22:112–123. doi: 10.1038/s41577-021-00558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams NM, Grassmann S, Sun JC. Clonal expansion of innate and adaptive lymphocytes. Nat Rev Immunol. 2020;20:694–707. doi: 10.1038/s41577-020-0307-4. [DOI] [PubMed] [Google Scholar]
- 30.Juno JA, Hill DL. T follicular helper cells and their impact on humoral responses during pathogen and vaccine challenge. Curr Opin Immunol. 2022;74:112–117. doi: 10.1016/j.coi.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Cui D, Tang Y, Jiang Q, et al. Follicular helper T cells in the immunopathogenesis of SARS-CoV-2 infection. Front Immunol. 2021;12:731100. doi: 10.3389/fimmu.2021.731100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 33.Painter MM, Mathew D, Goel RR, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54:2133–2142. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry H, Bruton R, Tut G, et al. Immunogenicity of single vaccination with BNT162b2 or ChAdOx1 nCoV-19 at 5–6 weeks post vaccine in participants aged 80 years or older: an exploratory analysis. Lancet Healthy Longev. 2021;2:e554–e560. doi: 10.1016/S2666-7568(21)00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angyal A, Longet S, Moore SC, et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 2022;3:e21–e31. doi: 10.1016/S2666-5247(21)00275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS-CoV-2 spike cross-recognize omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munro APS, Feng S, Janani L, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22:1131–1141. doi: 10.1016/S1473-3099(22)00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bates TA, Leier HC, Lyski ZL, et al. Age-dependent neutralization of SARS-CoV-2 and P.1 variant by vaccine immune serum samples. JAMA. 2021;326:868–869. doi: 10.1001/jama.2021.11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates TA, Leier HC, Lyski ZL, et al. Age-dependent neutralization of SARS-CoV-2 and P.1 variant by vaccine immune serum samples. JAMA. 2021;326:868–869. doi: 10.1001/jama.2021.11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 47.Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 48.Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160436. doi: 10.1098/rstb.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone RC, Horvath K, Kark JD, Susser E, Tishkoff SA, Aviv A. Telomere length and the cancer-atherosclerosis trade-off. PLoS Genet. 2016;12:e1006144. doi: 10.1371/journal.pgen.1006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNally EJ, Luncsford PJ, Armanios M. Long telomeres and cancer risk: the price of cellular immortality. J Clin Invest. 2019;129:3474–3481. doi: 10.1172/JCI120851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Codd V, Wang Q, Allara E, et al. Polygenic basis and biomedical consequences of telomere length variation. Nat Genet. 2021;53:1425–1433. doi: 10.1038/s41588-021-00944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8:e1002696. doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steenstrup T, Kark JD, Verhulst S, et al. Telomeres and the natural lifespan limit in humans. Aging (Albany NY) 2017;9:1130–1142. doi: 10.18632/aging.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 58.Hjelmborg JB, Dalgård C, Möller S, et al. The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52:297–302. doi: 10.1136/jmedgenet-2014-102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6:584–593. doi: 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blackburn EH, Collins K. Telomerase: an RNP enzyme synthesizes DNA. Cold Spring Harb Perspect Biol. 2011;3:a003558. doi: 10.1101/cshperspect.a003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodes RJ, Hathcock KS, Weng NP. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 62.Weng NP, Palmer LD, Levine BL, Lane HC, June CH, Hodes RJ. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 63.Patrick M, Weng NP. Expression and regulation of telomerase in human T cell differentiation, activation, aging and diseases. Cell Immunol. 2019;345:103989. doi: 10.1016/j.cellimm.2019.103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum--artifact or biology? Nucleic Acids Res. 2013;41:e131. doi: 10.1093/nar/gkt370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nettle D, Gadalla SM, Lai TP, Susser E, Bateson M, Aviv A. Measurement of telomere length for longitudinal analysis: implications of assay precision. Am J Epidemiol. 2021;190:1406–1413. doi: 10.1093/aje/kwab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benetos A, Kark JD, Susser E, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12:615–621. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson JJ, Susser E, Arbeev KG, et al. Telomere-length dependent T-cell clonal expansion: a model linking ageing to COVID-19 T-cell lymphopenia and mortality. EBioMedicine. 2022;78:103978. doi: 10.1016/j.ebiom.2022.103978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vrisekoop N, den Braber I, de Boer AB, et al. Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc Natl Acad Sci USA. 2008;105:6115–6120. doi: 10.1073/pnas.0709713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bartleson JM, Radenkovic D, Covarrubias AJ, Furman D, Winer DA, Verdin E. SARS-CoV-2, COVID-19 and the ageing immune system. Nat Aging. 2021;1:769–782. doi: 10.1038/s43587-021-00114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin S, An H, Zhou T, et al. Age cohorts stratified according to age-distributions of COVID-19 morbidity statistics identify uniquely age-dependent CD3+CD8+ T-cell lymphocytopenia in COVID-19 patients without comorbidities on admission. Aging (Albany NY) 2021;13:7713–7722. doi: 10.18632/aging.202691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aviv A. Short telomeres and severe COVID-19: the connection conundrum. eBioMedicine. 2021;70:103513. doi: 10.1016/j.ebiom.2021.103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benetos A, Lai TP, Toupance S, et al. The nexus between telomere length and lymphocyte count in seniors hospitalized with COVID-19. J Gerontol A Biol Sci Med Sci. 2021;76:e97–101. doi: 10.1093/gerona/glab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Q, Codd V, Raisi-Estabragh Z, et al. Shorter leukocyte telomere length is associated with adverse COVID-19 outcomes: a cohort study in UK Biobank. eBioMedicine. 2021;70:103485. doi: 10.1016/j.ebiom.2021.103485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Froidure A, Mahieu M, Hoton D, et al. Short telomeres increase the risk of severe COVID-19. Aging (Albany NY) 2020;12:19911–19922. doi: 10.18632/aging.104097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanchez-Vazquez R, Guío-Carrión A, Zapatero-Gaviria A, Martínez P, Blasco MA. Shorter telomere lengths in patients with severe COVID-19 disease. Aging (Albany NY) 2021;13:1–15. doi: 10.18632/aging.202463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGroder CF, Zhang D, Choudhury MA, et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021;76:1242–1245. doi: 10.1136/thoraxjnl-2021-217031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsilingiris D, Tentolouris A, Eleftheriadou I, Tentolouris N. Telomere length, epidemiology and pathogenesis of severe COVID-19. Eur J Clin Invest. 2020;50:e13376. doi: 10.1111/eci.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nobel Prize The Sveriges Riksbank Prize in Economic Sciences in Memory of Alfred Nobel 2002. https://www.nobelprize.org/prizes/economic-sciences/2002/summary