Abstract
Background:
Hypertension and diabetes are contraindications for living kidney donation in young candidates. However, little is known about long-term outcomes of women who had these pregnancy-related complications and subsequently become donors. In the general population, gestational hypertension (GHtn), preeclampsia/eclampsia, and gestational diabetes (GDM) are associated with long-term risks.
Methods:
Donors with the specified predonation complication were matched to contemporary control donors with pregnancies without the complication using nearest neighbor propensity score matching. Propensity scores were estimated using logistic regression with covariates for gravidity, blood pressure, glucose, BMI, age, and creatinine at donation, donation year, race, relationship to recipient, and family history of disease. Long-term incidence of hypertension, diabetes, cardiovascular disease (CVD), and reduced renal function (eGFR<30, eGFR<45 mL/min/1.73m2) were compared between groups using proportional hazards models.
Results:
Of 1862 donors with predonation pregnancies, 48 had preeclampsia/eclampsia, 49 had GHtn without preeclampsia, and 43 had GDM. Donors had a long interval between 1st pregnancy and donation (median 18.5 years, IQR: 10.6-27.5) and a long postdonation follow-up time (median 18.0, IQR: 9.2-27.7 years). GHtn was associated with development of hypertension (HR: 1.89, 95% CI: 1.26-2.83); GDM was associated with diabetes (HR: 3.04, 95% CI: 1.33-6.99). Pregnancy complications were not associated with eGFR<30 or eGFR<45 mL/min/1.73m2 .
Conclusions:
Our data suggests that even with a normal donor evaluation, women with predonation pregnancy-related complications have long-term risks. Donor candidates with a history of pregnancy-related complications should be counseled about these risks.
Introduction
In the last 2 decades, it has become clear that hypertensive disorders of pregnancy are associated with long-term maternal risk, including development of hypertension (HTN), cardiovascular disease (CVD), diabetes (DM), chronic kidney disease (CKD), and end stage kidney disease (ESKD).1-31 In addition, gestational diabetes (GDM) has been associated with subsequent development of type 2 DM and CVD.32-50 Importantly, these increased risks are often seen early postpartum, with HTN and/or type 2 DM often occurring within the first decade after delivery.4,6,29,41,47
By both historical and current criteria, women with early postpartum development of DM or HTN would not meet living donor (LD) acceptance criteria. However, a question remains about acceptance of LD candidates with a history of hypertensive disorders of pregnancy or GDM, and with a long interval of health between pregnancy and LD evaluation. These candidates might still have the long-term risks associated with their pregnancy-related complications, and the impact may be worse as a consequence of LD nephrectomy. Published guidelines vary or make no recommendations on whether to accept such candidates.51-54 Given that more than 60% of LDs are women and that many have had predonation pregnancies,55 an important knowledge gap exists in understanding if predonation pregnancy complications affect postdonation health.
To further inform donor candidate counselling and informed consent, we evaluated the impact of predonation pregnancy complications on risk of developing postdonation HTN, DM, CVD, and reduced renal function using 5 decades of information from the University of Minnesota living kidney donor program.
Materials and Methods
The Minnesota LD cohort and postdonation follow-up
Detailed descriptions of the University of Minnesota LD cohort have been published.56,57 LDs were screened to be healthy at the time of evaluation, including no evidence of proteinuria, HTN, or DM (using existing definitions at evaluation), and body mass index (BMI) ≤ 30 kg/m2 unless physical examination results warranted acceptance. Recently, non-Black candidates older than 55 who have well controlled HTN and no evidence of end-organ damage have been accepted as LDs.
Prior to 2003, LDs were surveyed intermittently. In 2003, all donors were contacted to be surveyed, and asked to consent to be surveyed every 3 years. Since 2003, consenting LDs are surveyed at 6, 12, and 24 months, and then every 3 years postdonation. As part of the survey, LDs are asked to provide information about their health including recent creatinine measurements and development of HTN requiring medication, DM requiring treatment (diet/oral hypoglycemic agent/ insulin use), and CVD (defined as coronary artery disease/angina; myocardial infarction, angioplasty, or stents; congestive heart disease/failure, and cerebrovascular accident or transient ischemic attack stroke). Donors are also asked to provide copies of their medical records or provide consent to contact their clinics for lab results, medical history, and physical examination notes. All data is maintained in a database approved by the University of Minnesota Institutional Review Board (HSC #0301M39762).
The most recently collected survey is crossvalidated with previous survey responses. Information about health problems is also obtained from medical charts, if provided. For this study we use the earliest date of reported problem as indicated on a survey, medical charts, or during follow-up. If the date of onset is not provided, the first date the condition was known to the survey team is used as date of onset.
Reduced renal function (eGFR<45 or eGFR<30 mL/min/1.73m2) was defined as having 2 measurements below the specified threshold, with the measurements separated by at least 28 days, and both measurements occurring at least 182 days after donation. The CKD-EPI creatinine equation (2009) was used to estimate GFR.58
Study cohort
Women with predonation pregnancy who underwent LD nephrectomy at our center between June 1963 and October 2020 and have subsequently provided postdonation information were included in this study. Methods for data collection on predonation pregnancies has varied across the eras. Chart review of all LD evaluations from 1963 to present has been conducted, and any available pregnancy information, including named diagnoses (eg, “toxemia”, “preeclampsia”, “hypertension during pregnancy”) has been abstracted. Post donation surveys were sent in the early 1990s asking about pregnancy numbers split by predonation and postdonation occurrence.59 Between 2003-2007 LDs known to be alive were sent questionnaires regarding pregnancy counts and outcomes. Since 2007, the pregnancy complication survey also has been given predonation.
Exposure definitions
Gestational hypertension and diabetes (GHtn and GDM, respectively) were defined as conditions requiring treatment only during the pregnancy. Preeclampsia is typically defined as HTN accompanied by new-onset proteinuria and edema. Eclampsia is typically defined as a more severe manifestation of preeclampsia characterized by other end-organ damage. For the present study, GHtn is defined as the condition occurring exclusive of preeclampsia or eclampsia. If no predonation pregnancy complications were noted in surveys or in chart review, it is assumed that none occurred.
Statistical Analysis
Continuous values were summarized using means and SD or medians (and quartiles) and compared between groups using ANOVA or Kruskal-Wallis rank sum tests, respectively. Categorical variables were summarized using proportions and compared using Fisher’s exact tests with simulated p-values.
LDs with the specified predonation complication (eclampsia/preeclampsia, GHtn, or GDM) were matched 1:10 to donors who had predonation pregnancies without the complication using nearest neighbor propensity score matching without replacement. Propensity scores were estimated using logistic regression with covariates for systolic and diastolic blood pressure (BP), glucose, BMI, age, gravidity and creatinine at the time of donation, smoking prior to donation, donation year, race (White versus non-White) relationship to recipient (biologically related versus not-biologically related), and family history of HTN, DM, kidney disease, and CVD (defined as family history of heart disease or transient ischemic attack/stroke). Matching balance was evaluated using standardized mean differences (SMDs).
Incidence of HTN, DM, CVD, eGFR<45 mL/min/1.73m2, and eGFR<30 mL/min/1.73m2 was estimated for each group (with versus without the given pregnancy complication) using Kaplan Meier estimators and compared using Cox proportional hazards models with robust standard errors. Each Cox proportional hazards model consisted of an indicator variable for the specific pregnancy complication under consideration. Individuals who are not recorded as developing HTN, DM, or CVD were censored at latest health survey or latest medical history and physical date. For models evaluating reduced renal function measures, “Time 0” was set to 182 days after donation and individuals who are not recorded as experiencing reduced renal function were censored at latest creatinine date. Individuals who died were censored at the date of last medical history/physical date or last creatinine date, respectively.
Matching and subsequent incidence analyses were conducted using data from LDs who had complete information for the matching variables and also using multiply imputed data sets. Multivariate imputation by chained equations using predictive mean matching for numeric data and logistic regression for binary data was used to impute variables used for matching. Auxiliary variables included in the imputation included ethnicity (Hispanic or non-Hispanic) and hemoglobin and cholesterol at donation. Cox proportional hazards results were pooled across the 30 imputations using Rubin's rules for computing the total variance.60 Survival probabilities provided in the text are averaged across imputations.
As a sensitivity analysis the matching procedure and analyses were restricted to LDs who had no recorded postdonation pregnancies. Statistical analyses were conducted in R version 3.6.0 (R Foundation for Statistical Computing; Vienna, Austria) using version 0.11.1 of the “tableone” package, version 1.2 of the “nephro” package, version 3.13.7 of the “mice” package, version 4.0.1 of the “MatchIt” package, and version 3.1-12 of the “survival” package.61-65 All p-values are 2-sided with statistical significance defined as p < 0.05.
Results
LD characteristics
Between 1963 and 2020, there were 2656 female LDs; of these, 1862 LDs had predonation pregnancies, pregnancy complication information, and postdonation follow-up information. The average (SD) age at donation was 42.4 (10.5) years; average age at first pregnancy, 23.3 (4.4) years. The median (IQR) time from first pregnancy to donation was 18.5 (10.6 to 27.5) years and the median postdonation follow-up time was 18.0 (9.2 to 27.7) years. The average number of predonation pregnancies was 3.0 (1.7); the majority (89%) did not have postdonation pregnancies. Most LDs were white (94.0%), non-Hispanic (88.7%) and were related to the recipient (72.7%). Average BMI at donation was 25.7 (4.5) kg/m2; creatinine, 0.81 (0.12) mg/dL; systolic BP, 118 (13) mm Hg; diastolic BP, 72 (10) mm Hg; and glucose, 92 (14) mg/dL. The majority (71.2%) did not have a history of smoking prior to donation. Of those with predonation pregnancies, 48 had preeclampsia or eclampsia; 43, GDM; and 49, GHtn (Table 1).
Table 1:
Donor characteristics by predonation pregnancy complications and average standardized mean differences (SMDs) across 30 imputations for characteristics after matching. Donors may be included in more than 1 complication category (see Figure 1). Factors included in the propensity score estimation for matching were systolic and diastolic blood pressure (BP), glucose, BMI, age, gravidity and creatinine at the time of donation, smoking prior to donation, donation year, race (White vs non-White) relationship to recipient (biologically related vs not-biologically related), and family history of HTN, DM, kidney disease, and CVD (defined as family history of heart disease or transient ischemic attack/stroke). PE/E = Pre-eclampsia; gDM = Gestational Diabetes; gHTN= Gestational Hypertension; Comp = Complications; DM=diabetes; HTN=hypertension; TIA= transient ischemic attack; BP=Blood Pressure.
| PE/E (N=48) |
gDM (N=43) |
gHTN (N=49) |
No Comp. (N=1746) |
Total (1862) |
SMDs after matching |
|||
|---|---|---|---|---|---|---|---|---|
| PE/E | gDM | gHTN | ||||||
| Years of follow-up post donation, median [IQR] | 17.50 [8.62, 32.07] | 15.25 [11.89, 22.72] | 24.03 [15.12, 33.75] | 18.04 [9.16, 27.56] | 18.05 [9.20, 27.71] | |||
| Age, years, at donation | 41.45 ± 8.98 | 43.12 ± 8.65 | 40.45 ± 9.56 | 42.47 ± 10.61 | 42.39 ± 10.53 | 0.034 | 0.038 | 0.031 |
| Race, n (%) | 0.031 | 0.001 | 0.027 | |||||
| American Indian or Alaska Native | 1 (2.1%) | 0 (0.0%) | 1 (2.0%) | 23 (1.3%) | 25 (1.3%) | |||
| Asian | 1 (2.1%) | 0 (0.0%) | 1 (2.0%) | 11 (0.6%) | 12 (0.6%) | |||
| Black or African American | 2 (4.2%) | 0 (0.0%) | 2 (4.1%) | 43 (2.5%) | 46 (2.5%) | |||
| Caucasian / White | 44 (91.7%) | 43 (100.0%) | 45 (91.8%) | 1640 (93.9%) | 1750 (94.0%) | |||
| Hawaiian Or Pacific Islander | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) | |||
| Multiracial | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 14 (0.8%) | 14 (0.8%) | |||
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 14 (0.8%) | 14 (0.8%) | |||
| Ethnicity, n (%) | ||||||||
| Hispanic/Latino | 1 (2.1%) | 1 (2.3%) | 1 (2.0%) | 49 (2.8%) | 52 (2.8%) | |||
| Non-Hispanic/Non-Latino | 46 (95.8%) | 42 (97.7%) | 46 (93.9%) | 1542 (88.3%) | 1652 (88.7%) | |||
| Unknown | 1 (2.1%) | 0 (0.0%) | 2 (4.1%) | 155 (8.9%) | 158 (8.5%) | |||
| Decade of donation, n (%) | 0.031 | 0.048 | 0.029 | |||||
| 1960 | 1 (2.1%) | 0 (0.0%) | 4 (8.2%) | 26 (1.5%) | 30 (1.6%) | |||
| 1970 | 5 (10.4%) | 2 (4.7%) | 8 (16.3%) | 184 (10.5%) | 196 (10.5%) | |||
| 1980 | 9 (18.8%) | 4 (9.3%) | 10 (20.4%) | 312 (17.9%) | 333 (17.9%) | |||
| 1990 | 10 (20.8%) | 15 (34.9%) | 11 (22.4%) | 433 (24.8%) | 464 (24.9%) | |||
| 2000 | 15 (31.2%) | 18 (41.9%) | 13 (26.5%) | 486 (27.8%) | 523 (28.1%) | |||
| After 2010 | 8 (16.7%) | 4 (9.3%) | 3 (6.1%) | 305 (17.5%) | 316 (17.0%) | |||
| Smoke prior to donation, n (%) | 0.026 | 0.031 | 0.019 | |||||
| No | 37 (77.1%) | 33 (76.7%) | 33 (67.3%) | 1241 (71.1%) | 1325 (71.2%) | |||
| Yes | 11 (22.9%) | 10 (23.3%) | 15 (30.6%) | 433 (24.8%) | 464 (24.9%) | |||
| Unknown | 0 (0.0%) | 0 (0.0%) | 1 (2.0%) | 72 (4.1%) | 73 (3.9%) | |||
| Relationship to recipient, n (%) | 0.039 | 0.031 | 0.023 | |||||
| Not-biologically related | 8 (16.7%) | 12 ( 27.9%) | 10 (20.4%) | 484 (27.7) | 508 (27.3) | |||
| Biologically related | 40 (83.3%) | 31 ( 72.1%) | 39 (79.6%) | 1262 (72.3) | 1354 (72.7) | |||
| Family history of DM, n (%) | 0.024 | 0.029 | 0.025 | |||||
| No | 22 (45.8%) | 16 (37.2%) | 24 (49.0%) | 825 (47.3%) | 878 (47.2%) | |||
| Yes | 26 (54.2%) | 24 (55.8%) | 25 (51.0%) | 808 (46.3%) | 868 (46.6%) | |||
| Unknown | 0 (0.0%) | 3 (7.0%) | 0 (0.0%) | 113 (6.5%) | 116 (6.2%) | |||
| Family history of HTN, n (%) | 0.022 | 0.03 | 0.023 | |||||
| No | 23 (47.9%) | 25 (58.1%) | 28 (57.1%) | 994 (56.9%) | 1056 (56.7%) | |||
| Yes | 23 (47.9%) | 14 (32.6%) | 19 (38.8%) | 568 (32.5%) | 616 (33.1%) | |||
| Unknown | 2 (4.2%) | 4 (9.3%) | 2 (4.1%) | 184 (10.5%) | 190 (10.2%) | |||
| Family history of CVD, n (%) | 0.027 | 0.031 | 0.026 | |||||
| No | 24 (50.0%) | 25 ( 58.1%) | 27 (55.1%) | 1010 (57.8%) | 1075 (57.7%) | |||
| Yes | 22 (45.8%) | 14 ( 32.6%) | 20 (40.8%) | 540 (30.9%) | 585 (31.4%) | |||
| Unknown | 2 (4.2%) | 4 (9.3%) | 2 (4.1%) | 196 (11.2%) | 202 (10.8%) | |||
| Family history of TIA/Stroke, n (%) | ||||||||
| No | 43 (89.6%) | 35 (81.4%) | 42 (85.7%) | 1407 (80.6%) | 1506 (80.9%) | |||
| Yes | 3 (6.2%) | 4 (9.3%) | 5 (10.2%) | 128 (7.3%) | 139 (7.5%) | |||
| Unknown | 2 (4.2%) | 4 (9.3%) | 2 (4.1%) | 211 (12.1%) | 217 (11.7%) | |||
| Family history of kidney disease, n (%) | 0.030 | 0.028 | 0.018 | |||||
| No | 13 (27.1%) | 12 (27.9%) | 15 (30.6%) | 530 (30.4%) | 563 (30.2%) | |||
| Yes | 34 (70.8%) | 29 (67.4%) | 34 (69.4%) | 1146 (65.6%) | 1226 (65.8%) | |||
| Unknown | 1 (2.1%) | 2 (4.7%) | 0 (0.0%) | 70 (4.0%) | 73 (3.9%) | |||
| Donation Creatinine, mg/dl | 0.83 ± 0.12 | 0.80 ± 0.11 | 0.80 ± 0.11 | 0.81 ± 0.12 | 0.81 ± 0.12 | 0.026 | 0.034 | 0.033 |
| Donation Cholesterol, mg/dl | 200.43 ± 38.02 | 208.37 ± 42.35 | 191.91 ± 25.50 | 192.07 ± 37.36 | 192.57 ± 37.47 | |||
| Donation BMI kg/m2 | 26.12 ± 4.76 | 27.15 ± 5.47 | 27.22 ± 6.00 | 25.63 ± 4.42 | 25.70 ± 4.50 | 0.036 | 0.022 | 0.053 |
| Donation Glucose mg/dL | 89.74 ± 11.20 | 93.88 ± 12.18 | 89.81 ± 11.51 | 92.34 ± 14.54 | 92.28 ± 14.37 | 0.021 | 0.028 | 0.038 |
| Donation Systolic BP mmHg | 121.06 ± 13.53 | 118.14 ± 11.76 | 121.02 ± 15.12 | 118.06 ± 13.02 | 118.20 ± 13.10 | 0.025 | 0.024 | 0.038 |
| Donation Diastolic BP mmHg | 73.79 ± 8.08 | 70.24 ± 9.01 | 75.73 ± 10.74 | 71.92 ± 9.74 | 72.00 ± 9.75 | 0.033 | 0.031 | 0.025 |
| Predonation pregnancies | 3.23 ± 1.94 | 3.42 ± 1.94 | 3.52 ± 2.10 | 2.94 ± 1.67 | 2.96 ± 1.67 | 0.026 | 0.023 | 0.024 |
| Known First Pregnancy Date, n (%) | ||||||||
| No | 10 (20.8%) | 8 (18.6%) | 16 (32.7%) | 499 (28.6%) | 526 (28.2%) | |||
| Yes | 38 (79.2%) | 35 (81.4%) | 33 (67.3%) | 1247 (71.4%) | 1336 (71.8%) | |||
| Age at first pregnancy | 24.60 ± 5.33 | 24.61 ± 5.03 | 23.90 ± 4.22 | 23.22 ± 4.35) | 23.29 ± 4.39 | |||
| Years from first preg. to don., median [IQR] | 14.56 [9.44, 19.73] | 19.48 [12.49, 26.35] | 17.49 [9.97, 24.36] | 18.66 [10.80, 27.68] | 18.54 [10.63, 27.46] | |||
| Postdonation pregnancies, n (%) | ||||||||
| No | 42 (87.5%) | 40 (93.0%) | 41 (83.7%) | 1560 (89.3%) | 1660 (89.2%) | |||
| Yes | 6 (12.5%) | 3 (7.0%) | 8 (16.3%) | 186 (10.7%) | 202 (10.8%) | |||
Comparison of Characteristics by Predonation Pregnancy Complication
The majority (84.8%) of LDs with predonation pre-eclampsia/eclampsia had the complication during the first pregnancy; 23.9% (N=11), in more than 1 pregnancy (Table 2). Two individuals had preeclampsia/eclampsia in both predonation and postdonation pregnancies. Less than half (45.2%) of LDs with predonation GDM had the complication during the first pregnancy; 16.7% (N=7), in more than 1 pregnancy. Two individuals had GDM in both predonation and postdonation pregnancies. Less than half (47.1%) of LDs with GHtn had the complication during the first pregnancy; 25.6% (N=11), in more than 1 pregnancy. Three LDs had both predonation and postdonation GHtn.
Table 2:
Pregnancy complication history by predonation pregnancy complication category. Donors may be included in more than 1 category. Six donors known to have predonation pregnancy complications but with uncertainty about during which pregnancy the complication occurred, were not included. PE/E = Pre-eclampsia; gDM = Gestational Diabetes; gHTN = Gestational Hypertension.
| PE/E (N=46) |
gDM (N=42) |
gHTN (N=43) |
|
|---|---|---|---|
| Known date of first specified complication, N (%) | |||
| No | 8 (17.4%) | 7 (16.7%) | 20 (46.5%) |
| Yes | 38 (82.6%) | 35 (83.3%) | 23 (53.5%) |
| Age at first complication | 25.25 ± 5.53 | 27.65 ± 6.16 | 26.03 ± 5.16 |
| Years from first complication to donation, median [IQR] | 12.88 [6.50, 19.73] | 14.41 [8.37, 26.17] | 12.97 [8.45, 21.31] |
| Complication during 1st pregnancy, N (%) | |||
| No | 7 (15.2%) | 23 (54.8%) | 18 (52.9%) |
| Yes | 39 (84.8%) | 19 (45.2%) | 16 (47.1%) |
| Number of pregnancies with the specified complication, N(%) | |||
| 1 | 35 (76.1%) | 35 (83.3%) | 32 (74.4%) |
| 2 | 8 (17.4%) | 3 (7.1%) | 6 (14.0%) |
| >2 | 3 (6.5%) | 4 (9.5%) | 5 (11.6%) |
| Number of pregnancies with the specified complication predonation, N(%) | |||
| 1 | 37 (80.4%) | 36 (85.7%) | 35 (81.4%) |
| 2 | 6 (13.0%) | 3 (7.1%) | 4 (9.3%) |
| >2 | 3 (6.5%) | 3 (7.1%) | 4 (9.3%) |
| Number of postdonation pregnancies with the specified complication, N (%) | |||
| 0 | 44 (95.7%) | 40 (95.2%) | 40 (93.0%) |
| 1 | 2 (4.3%) | 2 (4.8%) | 2 (4.7%) |
| 2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| >2 | 0 (0.0%) | 0 (0.0%) | 1 (2.3%) |
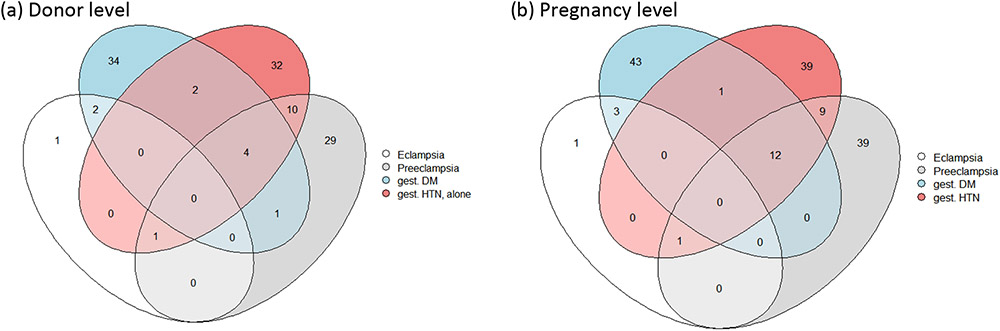
There were 20 LDs who experienced more than 1 type of complication prior to donation (Figure 1), with the majority (55%) experiencing both GHtn and pre-eclampsia/eclampsia, but in separate pregnancies. Characteristics were similar across the 5 exclusive groups defined by predonation pregnancy complications (pre-eclampsia/eclampsia alone, GDM alone, GHtn alone, >1 complication, and no complications) except for decade of donation, length of follow-up information, and BMI at donation (p-value≤0.02 Table 3).
Figure 1:
Overlap between pregnancy complications experienced (a) by the same donor across potentially multiple pregnancies, and (b) within the same pregnancy. If a donor was noted as experiencing both gestational hypertension and pre-eclampsia or eclampsia in a pregnancy they were classified as having pre-eclampsia or eclampsia in Figure 1a. Six individuals who were noted as having complications before donation, but with uncertain information about which pregnancies had the complication were excluded from Figure 1b.
Table 3:
Donor characteristics by exclusive predonation pregnancy complication categories. Donors are only included in 1 complication category. PE/E = Pre-eclampsia; gDM = Gestational Diabetes; gHTN = Gestational Hypertension; DM=diabetes; HTN=hypertension; TIA= transient ischemic attack; BP=Blood Pressure. P-value calculated using Kruskal-Wallis rank sum tests (for variables presented as median [IQR]), ANOVA (for variables presented as mean ± sd), or Fisher’s exact tests (for categorical variables).
| PE/E alone (N=30) |
gDM alone (N=34) |
gHTN alone (N=32) |
More than 1 complication (N=20) |
No complications (N=1746) |
p- value |
|
|---|---|---|---|---|---|---|
| Years of follow-up post donation, median [IQR] | 20.57 [10.08, 32.99] | 15.05 [11.84, 21.22] | 27.05 [20.81, 36.05] | 13.74 [6.50, 23.04] | 18.04 [9.16, 27.56] | 0.001 |
| Age, years, at donation | 40.07 ± 9.56 | 42.70 ± 8.79 | 39.50 ± 10.26 | 42.86 ± 7.69 | 42.47 ± 10.61 | 0.403 |
| Race, n (%) | 0.839 | |||||
| Black or African American | 1 (3.3%) | 0 (0.0%) | 1 (3.1%) | 1 (5.0%) | 43 (2.5%) | |
| Caucasian / White | 28 (93.3%) | 34 (100.0%) | 30 (93.8%) | 18 (90.0%) | 1640 (93.9%) | |
| Other | 1 (3.3%) | 0 (0.0%) | 1 (3.1%) | 1 (5.0%) | 49 (2.8%) | |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 14 (0.8%) | |
| Ethnicity, n (%) | 0.472 | |||||
| Hispanic/Latino | 1 (3.3%) | 1 (2.9%) | 1 (3.1%) | 0 (0.0%) | 49 (2.8%) | |
| Non-Hispanic/Non-Latino | 28 (93.3%) | 33 (97.1%) | 29 (90.6%) | 20 (100.0%) | 1542 (88.3%) | |
| Unknown | 1 (3.3%) | 0 (0.0%) | 2 (6.2%) | 0 (0.0%) | 155 (8.9%) | |
| Decade of donation, n (%) | 0.014 | |||||
| 1960 | 0 (0.0%) | 0 (0.0%) | 3 (9.4%) | 1 (5.0%) | 26 (1.5%) | |
| 1970 | 3 (10.0%) | 1 (2.9%) | 6 (18.8%) | 2 (10.0%) | 184 (10.5%) | |
| 1980 | 8 (26.7%) | 3 (8.8%) | 8 (25.0%) | 2 (10.0%) | 312 (17.9%) | |
| 1990 | 6 (20.0%) | 12 (35.3%) | 9 (28.1%) | 4 (20.0%) | 433 (24.8%) | |
| 2000 | 9 (30.0%) | 15 (44.1%) | 6 (18.8%) | 7 (35.0%) | 486 (27.8%) | |
| After 2010 | 4 (13.3%) | 3 (8.8%) | 0 (0.0%) | 4 (20.0%) | 305 (17.5%) | |
| Smoke prior to donation, n (%) | 0.915 | |||||
| No | 24 (80.0%) | 24 (70.6%) | 21 (65.6%) | 15 (75.0%) | 1241 (71.1%) | |
| Yes | 6 (20.0%) | 10 (29.4%) | 10 (31.2%) | 5 (25.0%) | 433 (24.8%) | |
| Unknown | 0 (0.0%) | 0 (0.0%) | 1 (3.1%) | 0 (0.0%) | 72 (4.1%) | |
| Family history of DM, n (%) | 0.616 | |||||
| No | 15 (50.0%) | 14 (41.2%) | 16 (50.0%) | 8 (40.0%) | 825 (47.3%) | |
| Yes | 15 (50.0%) | 17 (50.0%) | 16 (50.0%) | 12 (60.0%) | 808 (46.3%) | |
| Unknown | 0 (0.0%) | 3 (8.8%) | 0 (0.0%) | 0 (0.0%) | 113 (6.5%) | |
| Family history of HTN, n(%) | 0.276 | |||||
| No | 12 (40.0%) | 19 (55.9%) | 18 (56.2%) | 13 (65.0%) | 994 (56.9%) | |
| Yes | 17 (56.7%) | 12 (35.3%) | 13 (40.6%) | 6 (30.0%) | 568 (32.5%) | |
| Unknown | 1 (3.3%) | 3 (8.8%) | 1 (3.1%) | 1 (5.0%) | 184 (10.5%) | |
| Family history of heart disease | 0.261 | |||||
| No | 18 (60.0%) | 21 (61.8%) | 20 (62.5%) | 10 (50.0%) | 1078 (61.7%) | |
| Yes | 12 (40.0%) | 10 (29.4%) | 11 (34.4%) | 9 (45.0%) | 484 (27.7%) | |
| Unknown | 0 (0.0%) | 3 (8.8%) | 1 (3.1%) | 1 (5.0%) | 184 (10.5%) | |
| Family history of TIA/Stroke | 0.460 | |||||
| No | 27 (90.0%) | 27 (79.4%) | 27 (84.4%) | 18 (90.0%) | 1407 (80.6%) | |
| Yes | 2 (6.7%) | 4 (11.8%) | 4 (12.5%) | 1 (5.0%) | 128 (7.3%) | |
| Unknown | 1 (3.3%) | 3 (8.8%) | 1 (3.1%) | 1 (5.0%) | 211 (12.1%) | |
| Family history of kidney disease, n (%) | 0.981 | |||||
| No | 8 (26.7%) | 10 (29.4%) | 9 (28.1%) | 6 (30.0%) | 530 (30.4%) | |
| Yes | 21 (70.0%) | 22 (64.7%) | 23 (71.9%) | 14 (70.0%) | 1146 (65.6%) | |
| Unknown | 1 (3.3%) | 2 (5.9%) | 0 (0.0%) | 0 (0.0%) | 70 (4.0%) | |
| Donation Creatinine, mg/dl | 0.82 ± 0.14 | 0.79 ± 0.11 | 0.78 ± 0.11 | 0.84 ± 0.10 | 0.81 ± 0.12 | 0.439 |
| Donation Cholesterol, mg/dl | 205.00 ± 41.86 | 206.48 ± 45.70 | 190.92 ± 24.47 | 193.00 ± 27.74 | 192.07 ± 37.36 | 0.232 |
| Donation BMI, kg/m2 | 25.96 ± 4.93 | 27.03 ± 5.63 | 28.09 ± 6.52 | 26.01 ± 4.52 | 25.63 ± 4.42 | 0.014 |
| Donation Glucose, mg/dl | 88.93 ± 9.34 | 93.88 ± 10.74 | 91.07 ± 12.75 | 90.89 ± 13.35 | 92.34 ± 14.54 | 0.662 |
| Donation Systolic BP, mmHg | 121.33 ± 14.54 | 118.65 ± 12.45 | 121.22 ± 16.52 | 119.58 ± 12.22 | 118.06 ± 13.02 | 0.425 |
| Donation Diastolic BP, mmHg | 73.80 ± 8.34 | 70.15 ± 8.77 | 76.28 ± 12.43 | 73.05 ± 8.04 | 71.92 ± 9.74 | 0.067 |
| Predonation pregnancies | 2.87 ± 1.46 | 3.00 ± 1.33 | 3.42 ± 1.84 | 3.70 ± 2.39 | 2.94 ± 1.67 | 0.157 |
| Postdonation pregnancies, n (%) | ||||||
| No | 24 (80.0%) | 32 ( 94.1%) | 25 (78.1%) | 19 ( 95.0%) | 1560 (89.3%) | 0.105 |
| Yes | 6 (20.0%) | 2 ( 5.9%) | 7 (21.9%) | 1 ( 5.0%) | 186 (10.7%) |
Long term outcomes
Matching led to good balance with SMDs <0.1 for all factors included in the propensity score estimation (Table 1, Tables S1-S2, Figures S1-S2).
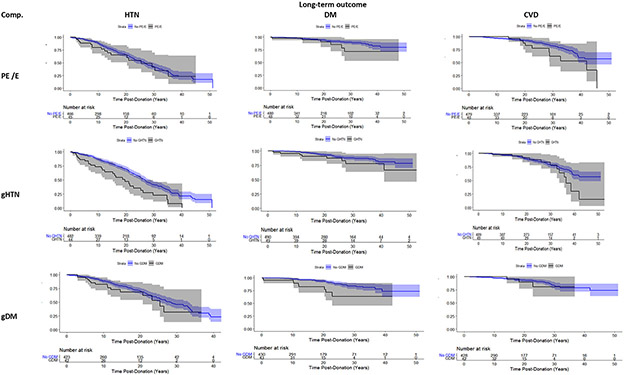
Compared to matched controls (10 LDs matched to every LD with the specific complication) those with predonation GHtn were estimated to have increased risk of developing postdonation HTN (HR: 1.89, 95% CI: 1.26 to 2.83; p-value ≤0.01; Table 4, Figure 2). There was not a strong association between GHtn and development of DM, CVD, or reduced renal function (Figure S3). The estimated probability of developing HTN within 20 years after donation was 53% for individuals with predonation GHtn compared to 32% for controls (Figure 2).
Table 4:
Proportional hazards regression results for time from donation to development of long-term outcomes based on predonation pregnancy complications. The outcomes of donors experiencing a given predonation pregnancy complication were compared to matched donors with predonation pregnancies without the given complication. Pooled cox proportional hazards results over 30 imputations are presented. Donors may be classified into more than 1 predonation pregnancy complication category.
| Pregnancy complication | N | HR (95% CI); p-value for outcome |
||||
|---|---|---|---|---|---|---|
| HTN | DM | CVD | eGFR<45 | eGFR<30 | ||
| Analysis including individuals with both pre and postdonation pregnancies | ||||||
| Gestational HTN | 49 | 1.89 (1.26, 2.83); ≤0.01 | 1.64 (0.75, 3.58); 0.21 | 1.54 (0.82, 2.88); 0.18 | 0.79 (0.30, 2.04); 0.61 | 1.02 (0.20, 5.24); 0.98 |
| Gestational DM | 43 | 1.26 (0.72, 2.20); 0.42 | 3.04 (1.33, 6.99); ≤0.01 | 0.96 (0.27, 3.40); 0.95 | 1.29 (0.47, 3.54); 0.61 | 1.72 (0.03, 92.96); 0.67 |
| Preeclampsia/ Eclampsia | 48 | 1.15 (0.70, 1.91); 0.57 | 1.51 (0.60, 3.79); 0.37 | 1.91 (0.94, 3.88); 0.07 | 1.25 (0.53, 2.93); 0.60 | 1.61 (0.25, 10.42); 0.58 |
| Analysis restricted to individuals with only predonation pregnancies | ||||||
| Gestational HTN | 41 | 1.75 (1.11, 2.78); 0.02 | 1.36 (0.55, 3.33); 0.50 | 1.43 (0.69, 2.99); 0.33 | 1.01 (0.38, 2.67); 0.99 | 1.35 (0.25, 7.45); 0.70 |
| Gestational DM | 40 | 1.37 (0.79, 2.40); 0.26 | 3.54 (1.52, 8.24); ≤0.01 | 1.02 (0.29, 3.57); 0.98 | 1.46 (0.53, 4.00); 0.45 | 2.06 (0.06, 70.10); 0.58 |
| Preeclampsia/ Eclampsia | 42 | 1.24 (0.73, 2.12); 0.42 | 1.32 (0.49, 3.58); 0.58 | 2.35 (1.12, 4.92); 0.02 | 1.24 (0.53, 2.89); 0.61 | 1.52 (0.26, 8.97); 0.62 |
Figure 2: Postdonation disease free probability by predonation pregnancy complications. Kaplan Meier Curves with confidence intervals from 1 imputation.
Outcomes of donors with a given predonation pregnancy complication were compared to matched donors without the given complication. Donors may be classified into more than 1 predonation pregnancy complication category.
Comp=Complication, PE/E=pre-eclampsia/eclampsia, gHTN= gestational hypertesion
Predonation GDM was associated with development of DM after donation (HR: 3.04, 95% CI: 1.33 to 6.99, p-value ≤ 0.01). There was not a strong association between GDM and development of HTN, CVD, or reduced renal function. Those with predonation GDM were estimated to have a 17% chance of developing DM within 20 years after donation compared to a 8% chance for controls.
Compared to matched controls, LDs with predonation preeclampsia/eclampsia were at increased risk of developing CVD (HR: 1.91, 95% CI: 0.94 to 3.88, p-value= 0.07), although the risk did not reach statistical significance. We did not identify an increased risk of developing HTN, DM, or reduced renal function. The estimated probability of developing CVD within 30 years after donation was 38% for those with predonation preeclampsia/eclampsia compared to approximately 17% for controls.
Results from the complete case analyses are presented in Table S3 and Figures S4-S5.
Sensitivity Analysis
In a restricted population of 1660 LDs with only predonation pregnancies reported there were 41 LDs with predonation GHtn, 40 with predonation GDM, and 42 with predonation preeclampsia/eclampsia. We continued to identify an association between GHtn and development of HTN postdonation (HR: 1.75, 95% CI: 1.11 to 2.78); and predonation GDM and increased risk of postdonation DM (HR: 3.54, 95% CI: 1.52 to 8.24, Table 4). The association between predonation preeclampsia/ eclampsia and risk of CVD was stronger in this subgroup than in the full population (HR: 2.35, 95% CI: 1.12 to 4.92).
Discussion
We are unaware of previous reports of the outcomes of LDs with a history of hypertensive disorders of pregnancy or GDM. General population studies have found GHtn, preeclampsia or GDM significantly increased long-term risk of HTN, DM, CVD, CKD and ESKD. The pathophysiology of the association between these pregnancy complications and long-term adverse outcomes is unclear. It may be that physiologic stress during pregnancy uncovers prepregnancy subclinical disorders.8 It may be that the pregnancy disorder disrupts metabolic pathways and is a cause of subsequent complications. Or it may be that the pregnancy complication and the long-term complications are due to a common underlying pathway.
GHtn among non-LDs has been reported to increase the risk of developing HTN by 2 to greater than 20-fold, depending on how long after pregnancy the risk is evaluated.2,3,5,7,11,19 Behrens et al reported that women with GHtn had a greater than 20 times increased risk of developing HTN within the 1st postpregnancy year, 6-times increased risk within 5 years, 4-times increased risk within 10 years and 2-times increased risk long-term.5 Engeland et al similarly reported a much higher relative risk of HTN early postpregnancy.3 Longer-term studies, 10 to 25 years after pregnancy, report a 2-3 fold increase in risk.5,7 In our study with a median (IQR) time from first pregnancy to donation of 18.5 (10.6 to 27.5) years and follow-up of 18.0 (9.2 to 27.7) years postdonation, LDs with GHtn during a predonation pregnancy had an almost 2-fold increased risk of developing HTN (HR: 1.89, 95% CI: 1.26 to 2.83; p-value≤0.01).
General population studies have also found that GHtn is associated with a 1.5-2-fold increased risk of developing CVD.7-19 We found a similar, although not statistically significant, risk in our cohort (HR: 1.54, 95% CI: 0.82 to 2.88, p-value= 0.18). In contrast to general population studies, we did not find an association between GHtn and increased risk of CKD (eGFR <45 mL/min/1.73m2or eGFR<30 mL/min/1.73m2) or DM.19,,28-33
Preeclampsia, in the general population is associated with a 2-3 fold increased risk for HTN, and a 1.5 to 2-fold increased risk of CVD (although a higher risk was reported by Tooher et al.11,16). In our LD cohort, we found a similar, although not statistically significant, risk for CVD (HR: 1.91, 95% CI: 0.94 to 3.88, p-value= 0.07), but no increased risk for HTN. Also, in contrast to general population studies, we did not find that preeclampsia was associated with an increased risk for CKD.
GDM, in the general population, is associated with development of type 2 DM and CVD,32-50 although much of the increased risk of developing DM is seen early after pregnancy. A review of 26 studies done between 1965 and 2001 reported that between 2.6% to up to 70% of women with GDM develop type 2 DM depending on length of follow-up time and testing rates.34 More recent studies report that between 20-30% of women with GDM develop type 2 DM.35-37 Overall, studies report a 3 to 5 fold increased risk. However, Noctor and Dunne warn that, “heterogeneity across studies with regard to diagnostic criteria, demographics, and duration of follow-up, limit direct comparison” across studies of GDM risk.38 In addition, the incidence varies considerably depending on maternal characteristics (eg, genetic factors, obesity).34-38 For women with GDM, the risk of CVD is in increased 1.5 to 2.5 fold.42-50 We found that LDs with a history of predonation GDM had a significantly increased risk of developing DM (HR: 3.04, 95% CI: 1.33 to 6.99, p-value≤ 0.01). In contrast to large general population studies, we did not observe an increased risk of CVD.
There are several possible reasons why we did not identify some of the same long-term risks seen in the general population. Firstly, our study was designed to answer a different question among a different population. Our study evaluates incidence of disease after kidney donation, not immediately after pregnancy. Both the “control” and “case” population in our study were healthy at time of donation, which typically occurred many years after pregnancy. As such, LDs with predonation pregnancy complications may be healthier than other populations studied. Of note, the overall rate of pregnancy complications in LDs was much lower than observed in the general population with only approximately 2% of predonation pregnancies complicated by hypertensive disorders, compared to 5-10% in the general population,4 and 1% complicated by GDM, compared to an estimated 14% in the general population.34 This is not surprising because the LD selection criteria excludes women who had many risk factors for pregnancy complications. In the general population, the greatest relative risk for developing postpartum HTN or DM is highest early after pregnancy and the likelihood of development of long-term complications after preeclampsia has been associated with the severity of the preeclampsia. It is likely that kidney LDs, who have not developed long-term complications a median of 12 years after pregnancy complications, represent a lower risk subgroup with less severe pregnancy-related complications than have been evaluated in general population studies.
Secondly, our study implemented different analytic methods and had fewer individuals with pregnancy complications than in other studies. We compared the incidence of outcomes for LDs with versus without predonation pregnancy complications, matched on similar lab values, age, gravidity, smoking history, year of donation, and family history characteristics. Given the extensive information collected during LD evaluation we can account for potentially more confounders than in other studies. In addition, matching, as compared to multivariate adjustment, avoids defining a functional form for the relationship between covariates and outcomes and may avoid model misspecification. While the analytic methods chosen for this study have several strengths, they do limit comparisons to other studies.
Of note, pregnancies among our LD cohort occurred across 8 decades and definitions for both pregnancy complications and development of conditions (HTN, DM, and CVD) have changed over time. Within that time-period there have been at least 12 different changes in GDM diagnoses, alone, with different groups recommending different definitions.38 In addition, diagnoses in our population were based on a mixture of chart review and self-report so retrospectively applying standardized definitions based on lab values (BP and glucose) and gestational age is not possible. While our study used contemporary controls, so the changing definitions should not affect study conclusions, comparing the results of our study to general population studies which occurred during different time periods or using different definitions is challenging.
Limited guidelines have been provided regarding acceptance of LD candidates with predonation pregnancy complications. The 2017 Kidney Disease Improving Global Outcomes (KDIGO) guideline recommends that female LD candidates should be asked about prior hypertensive disorders of pregnancy (recommendation 15.2), and that women with a prior hypertensive disorder of pregnancy may be acceptable for donation if their long-term postdonation risks are acceptable (recommendation 15.7).51 Other society or national guidelines do not comment.52-54
Regarding DM, the KDIGO guidelines recommend that: a) 2-hour glucose tolerance testing or HbA1c testing should be performed in LD candidates with elevated fasting blood glucose and a history of GDM, and results should be used to classify DM or pre-DM status (recommendation 11.7); b) The decision to approve LD candidates with pre-DM or type 2 DM should be individualized based on demographic and health profile in relation to the transplant program’s acceptable risk threshold (recommendation 11.9); and c) LD candidates with pre-DM or type 2 DM should be counseled that their condition may progress over time and may lead to end-organ complications (recommendation 11.10).51 In contrast, the Caring for Australians with Renal Impairment (CARI) guidelines consider a past history of GDM to be an absolute contraindication to donation.52 Other society or national guidelines do not comment.53,54
In our study approximately 70% of female LDs had a predonation pregnancy. Clearly, understanding the long-term risks associated with predonation pregnancy complications is important for both counseling LD candidates and for informed consent. While our study indicates that existing screening protocols may limit the risk of CKD, those with predonation pregnancy complications are still at increased risk for HTN, DM, and CVD. Our data does not address the risk of donation shortly after a pregnancy-related complication. Consideration should be given to having women with pregnancy complication wait before proceeding with donation because the relative risk of developing HTN or DM is highest in first postpartum years. All potential LDs considering future pregnancies should also be counseled that postdonation pregnancies, compared with predonation pregnancies, were associated with increased risk of GHtn and preeclampsia.66,67 And a study comparing postdonation pregnancies to pregnancies in matched healthy controls found that the LDs had an increased risk of developing GHtn and preeclampsia.68 Those with predonation pregnancy complications should be counseled that women who have GHtn or preeclampsia in greater than 1 pregnancy are at greater risk for long-term adverse outcomes.5,31-35
All LD candidates should be counseled that HTN and DM are potential causes of CKD, that DM and HTN are the 2 most common causes of ESKD,69 and that development of HTN and DM are also related to lifestyle. Donation should be linked to a long-term commitment to a healthy lifestyle.
There are limitations to our study. Firstly, our study population was 94% white so the study results may not be generalizable to other donor populations. Secondly, we have only done regular LD surveys since 2003 and we may not have accurate pregnancy complication information on all LDs. Underreporting of pregnancy complications is possible. Also, since our data is limited to a combination of self-report and medical records, some of the information about pregnancy complications and development of postdonation conditions could be inaccurate. Thirdly, as discussed above, predonation pregnancy complications were rare in our population, which limited our ability to fully assess all risks associated with these complications. We may be underpowered to identify some of the risks that have been reported in general population studies. Given the small number of LDs (20 LDs: 11 with pre-eclampsia/eclampsia and GHtn in separate pregnancies) who experienced more than 1 type of pregnancy complication, we are unable to assess the risks for these LDs as compared to LDs with a single complication.
Our data does not answer the question of whether kidney donation contributed to the long-term risks that we identified and thus does not suggest that pregnancy complications should be a contraindication to living donation. Encouragingly, in our population the long-term relative risks associated with pregnancy complications are not higher than those that have been reported in the general population. However, some women in the general population have several risk factors for pregnancy complications (eg, obesity, DM) that would preclude them from becoming kidney LDs. Large long-term studies are needed, such as a registry of pregnancy complications and outcomes for living donors, ideally with comparison to healthy selected controls, to: 1) confirm these risks in LDs and determine whether other long-term risks can be identified; and 2) study whether the risks are purely related to the prior pregnancies or whether donation is an additional risk factor.
Supplementary Material
Acknowledgments
We thank Stephanie Taylor for administrative assistance in preparation of the manuscript.
Financial Disclosure:
This work is supported by NIH NIDDKD 5R01DK125431-02.
Abbreviations:
- BMI
body mass index
- BP
blood pressure
- CARI
Caring for Australians with Renal Impairment
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- DM
diabetes
- ESKD
end stage kidney disease
- GDM
gestational diabetes
- GHtn
gestational hypertension
- HTN
hypertension
- KDIGO
Kidney Disease Improving Global Outcomes
- LD
living donor
- SMDs
standard mean differences
Footnotes
Disclaimer: The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation.
References
- 1.Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–1112. [DOI] [PubMed] [Google Scholar]
- 2.Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeland GM, Skurtveit S, Staff AC, et al. Pregnancy-related risk factors are associated with a significant burden of treated hypertension within 10 years of delivery: findings from a population-based Norwegian cohort. J Am Heart Assoc. 2018;7(10):e008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal A, Wenger NK. Hypertension during pregnancy. Curr Hypertens Rep. 2020;22(9):64. [DOI] [PubMed] [Google Scholar]
- 5.Behrens I, Basit S, Melbye M, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giorgione V, Ridder A, Kalafat E, et al. Incidence of postpartum hypertension within 2 years of a pregnancy complicated by pre-eclampsia: a systematic review and meta-analysis. BJOG. 2021;128(3):495–503. [DOI] [PubMed] [Google Scholar]
- 7.Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169(4):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varagic J, Desvigne-Nickens P, Gamble-George J, et al. Maternal morbidity and mortality: Are we getting to the "heart" of the matter? J Womens Health (Larchmt). 2021;30(2):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riise HKR, Sulo G, Tell GS, et al. Association between gestational hypertension and risk of cardiovascular disease among 617 589 Norwegian women. J Am Heart Assoc. 2018;7(10):e008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. 2018;7(17):e009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tooher J, Thornton C, Makris A, et al. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension. 2017;70(4):798–803. [DOI] [PubMed] [Google Scholar]
- 12.Wikström AK, Haglund B, Olovsson M, et al. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112(11):1486–1491. [DOI] [PubMed] [Google Scholar]
- 13.Veerbeek JH, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65(3):600–606. [DOI] [PubMed] [Google Scholar]
- 14.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003497. [DOI] [PubMed] [Google Scholar]
- 15.Dines V, Kattah A. Hypertensive disorders of pregnancy. Adv Chronic Kidney Dis. 2020;27(6):531–539. [DOI] [PubMed] [Google Scholar]
- 16.Tooher J, Chiu CL, Yeung K, et al. High blood pressure during pregnancy is associated with future cardiovascular disease: an observational cohort study. BMJ Open. 2013;3(7):e002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melchiorre K, Thilaganathan B, Giorgione V, et al. Hypertensive disorders of pregnancy and future cardiovascular health. Front Cardiovasc Med. 2020;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lykke JA, Langhoff-Roos J, Sibai BM, et al. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. [DOI] [PubMed] [Google Scholar]
- 20.Covella B, Vinturache AE, Cabiddu G, et al. A systematic review and meta-analysis indicates long-term risk of chronic and end-stage kidney disease after preeclampsia. Kidney Int. 2019;96(3):711–727. [DOI] [PubMed] [Google Scholar]
- 21.Ayansina D, Black C, Hall SJ, et al. Long term effects of gestational hypertension and pre-eclampsia on kidney function: Record linkage study. Pregnancy Hypertens. 2016;6(4):344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation. 2015;132(13):1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristensen JH, Basit S, Wohlfahrt J, et al. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019;365:l1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khashan AS, Evans M, Kublickas M, et al. Preeclampsia and risk of end stage kidney disease: a Swedish nationwide cohort study. PLoS Med. 2019;16(7):e1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersgaard AB, Acharya G, Mathiesen EB, et al. Recurrence and long-term maternal health risks of hypertensive disorders of pregnancy: a population-based study. Am J Obstet Gynecol. 2012;206(2):143.e1–e8. [DOI] [PubMed] [Google Scholar]
- 26.Vikse BE, Irgens LM, Leivestad T, et al. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359(8):800–809. [DOI] [PubMed] [Google Scholar]
- 27.Auger N, Fraser WD, Schnitzer M, et al. Recurrent pre-eclampsia and subsequent cardiovascular risk. Heart. 2017;103(3):235–243. [DOI] [PubMed] [Google Scholar]
- 28.Barrett PM, McCarthy FP, Kublickiene K, et al. Adverse pregnancy outcomes and long-term maternal kidney disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(2):e1920964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett PM, McCarthy FP, Evans M, et al. Hypertensive disorders of pregnancy and the risk of chronic kidney disease: a Swedish registry-based cohort study. PLoS Med. 2020;17(8):e1003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feig DS, Shah BR, Lipscombe LL, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med. 2013;10(4):e1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forest JC, Girouard J, Massé J, et al. Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet Gynecol. 2005;105(6):1373–1380. [DOI] [PubMed] [Google Scholar]
- 32.Callaway LK, Lawlor DA, O’Callaghan M, et al. Diabetes mellitus in the 21 years after a pregnancy that was complicated by hypertension: findings from a prospective cohort study. Am J Obstet Gynecol. 2007;197(5):492.e1–e7. [DOI] [PubMed] [Google Scholar]
- 33.Pouta A, Hartikainen AL, Sovio U, et al. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension. 2004;43(4):825–831. [DOI] [PubMed] [Google Scholar]
- 34.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. [DOI] [PubMed] [Google Scholar]
- 35.Rayanagoudar G, Hashi AA, Zamora J, et al. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. 2016;59(7):1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang C, Olsen SF, Hinkle SN, et al. ; Diabetes & Women’s Health Study Team. Diabetes & Women's Health (DWH) Study: an observational study of long-term health consequences of gestational diabetes, their determinants and underlying mechanisms in the USA and Denmark. BMJ Open. 2019;9(4):e025517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Rahman ML, Wu J, et al. Genetic factors and risk of type 2 diabetes among women with a history of gestational diabetes: findings from two independent populations. BMJ Open Diabetes Res Care. 2020;8(1):e000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noctor E, Dunne FP. Type 2 diabetes after gestational diabetes: the influence of changing diagnostic criteria. World J Diabetes. 2015;6(2):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am. 2019;48(3):479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8(11):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaiser K, Nielsen MF, Kallfa E, et al. Metabolic syndrome in women with previous gestational diabetes. Sci Rep. 2021;11(1):11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905–914. [DOI] [PubMed] [Google Scholar]
- 43.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Archambault C, Arel R, Filion KB. Gestational diabetes and risk of cardiovascular disease: a scoping review. Open Med. 2014;8(1):e1–e9. [PMC free article] [PubMed] [Google Scholar]
- 45.Daly B, Toulis KA, Thomas N, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study. PLoS Med. 2018;15(1):e1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowe WL Jr, Scholtens DM, Lowe LP, et al. ; HAPO Follow-up Study Cooperative Research Group. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goueslard K, Cottenet J, Mariet AS, et al. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol. 2016;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177(12):1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunderson EP, Chiang V, Pletcher MJ, et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc. 2014;3(2):e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177(12):1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lentine KL, Kasiske BL, Levey AS, et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101(8S Suppl 1):S1–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boudville N, Isbel N; CARI. The CARI guidelines. Donors at risk: impaired glucose tolerance. Nephrology (Carlton). 2010;15 Suppl 1:S133–S136. [DOI] [PubMed] [Google Scholar]
- 53.The Renal Association. Guidelines for living donor kidney transplantation. Available at https://bts.org.uk/wp-content/uploads/2018/07/FINAL_LDKT-guidelines_June-2018.pdf. Accessed July 2020. [DOI] [PubMed]
- 54.Abramowicz D, Cochat P, Claas FH, et al. European renal best practice guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant. 2015;30(11):1790–1797. [DOI] [PubMed] [Google Scholar]
- 55.Organ Procurement and Transplantation Network. National data. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed October 2021.
- 56.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360(5):459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibrahim HN, Foley RN, Reule SA, et al. Renal function profile in White kidney donors: the first 4 decades. J Am Soc Nephrol. 2016;27(9):2885–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wrenshall LE, McHugh L, Felton P, et al. Pregnancy after donor nephrectomy. Transplantation. 1996;62(12):1934–1936. [DOI] [PubMed] [Google Scholar]
- 60.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley and Sons; 1987:76. [Google Scholar]
- 61.Yoshida K tableone: Create 'Table 1' to Describe Baseline Characteristics. [Computer software]. R package version 0.11.1. 2020. Available at https://CRAN.R-project.org/package=tableone. [Google Scholar]
- 62.Pattaro C nephro: Utilities for Nephrology. [Computer software]. R package version 1.2. 2017. Available at https://CRAN.R-project.org/package=nephro. [Google Scholar]
- 63.van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. [Computer software]. J Stat Software. 2011;45(3):1–67. [Google Scholar]
- 64.Ho DE, Imai K, King G, et al. MatchIt: nonparametric preprocessing for parametric causal inference. [Computer software]. J Stat Software. 2011;42(8):1–28. [Google Scholar]
- 65.Therneau T A Package for Survival Analysis in R. [Computer software]. R package version 3.1–12. 2020. Available at https://CRAN.R-project.org/package=survival. [Google Scholar]
- 66.Ibrahim HN, Akkina SK, Leister E, et al. Pregnancy outcomes after kidney donation. Am J Transplant. 2009;9(4):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reisaeter AV, Røislien J, Henriksen T, et al. Pregnancy and birth after kidney donation: the Norwegian experience. Am J Transplant. 2009;9(4):820–824. [DOI] [PubMed] [Google Scholar]
- 68.Garg AX, McArthur E, Lentine KL; Donor Nephrectomy Outcomes Research (DONOR) Network. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372(15):1469–1470. [DOI] [PubMed] [Google Scholar]
- 69.Matas AJ, Hays RE, Ibrahim HN. A case-based analysis of whether living related donors listed for transplant share ESRD causes with their recipients. Clin J Am Soc Nephrol. 2017;12(4):663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.