Abstract
Lethal giant larvae homolog 2 (LLGL2) and solute carrier family 7 member 5 (SLC7A5) have been reported to be involved in resistance to endocrine therapy. This study aimed to assess the effects of LLGL2/SLC7A5 co-expression in predicting prognosis and response to tamoxifen therapy in ERα-positive breast cancer patients according to LLGL2/SLC7A5 mRNA and protein expression in long-term follow-up invasive breast cancer tissues. We identified that low LLGL2/SLC7A5 mRNA co-expression (LLGL2low/SLC7A5low) was associated with disease-free survival (DFS) compared with other combination groups in all breast cancer patients. In ERα-positive breast cancer patients, LLGL2low/SLC7A5low showed longer DFS and overall survival (OS) compared with LLGL2high/SLC7A5high and a positive trend of longer survival compared with the other combination groups. We also observed that LLGL2low/SLC7A5low showed longer survival compared with LLGL2high/SLC7A5high in ERα-positive breast cancer patients receiving adjuvant tamoxifen therapy. Multivariate analysis demonstrated that LLGL2low/SLC7A5low was an independent favorable prognostic factor of both DFS and OS, not only in all breast cancer patients, but also in ERα-positive breast cancer patients. High co-expression of LLGL2 and SLC7A5 protein showed a positive trend of shorter survival. Our study showed that co-expression of LLGL2 and SLC7A5 mRNA is a promising candidate biomarker in early breast cancer patients.
Subject terms: Breast cancer, Tumour biomarkers
Introduction
Breast cancer remains the leading cause of cancer-related deaths in women. Approximately 70% of all breast cancer cases express estrogen receptor α (ERα)1,2. In ERα-positive breast cancers, estradiol is a critical regulator of cell proliferation and survival. Estradiol directly regulates genes through binding to ERα or indirectly through activating plasma membrane-associated ERα. Treatment options for ERα-positive breast cancer patients include endocrine therapies that inhibit ERα signaling, either by antagonizing ligand binding to ERα, downregulating ERα, or suppressing estrogen production3.
Tamoxifen is one of the most common endocrine treatments for breast cancer. Tamoxifen treatment was demonstrated to reduce the risk of breast cancer recurrence and death in ERα-positive breast cancer patients4. Although endocrine therapy has dramatically improved survival in ERα-positive breast cancer patients, some tumors show de novo or acquired drug resistance to endocrine therapy5–7. Resistance to endocrine therapies including tamoxifen remains a major challenge in the treatment of ERα-positive breast cancer patients. Furthermore, there are few established biomarkers for tamoxifen treatment resistance that have been applied in current clinical practice.
Recently, Saito et al. reported that lethal giant larvae homolog 2 (LLGL2) functions as a promoter of tumor growth in ERα-positive breast cancer8. They observed that the intracellular concentration of leucine was decreased in MCF-7 ERα-positive breast cancer cells when LLGL2 expression was knocked down. They also demonstrated that the proliferation of MCF-7 cells was suppressed when LLGL2 expression was knocked down, and that excess leucine could rescue the proliferation of LLGL2-knockdown cells8.
LLGL2 is reported to be localized at cell junctions and membranes with a member of the solute carrier (SLC) family, SLC7A5, which is the primary leucine transporter in cells8,9. SLC7A5 is a sodium-independent amino acid transporter that imports leucine10. High SLC7A5 expression was reported to be associated with poor prognosis in various cancers including breast cancer11–15.
LLGL2 and SLC7A5 are involved in resistance to tamoxifen treatment8,12,16. LLGL2 was also reported to function with SLC7A5 at cell junctions and membranes in ERα-positive breast cancer cells, and LLGL2 interacts with SLC7A5 to promote cell proliferation8. Therefore, we hypothesized that both LLGL2 and SLC7A5 are required for the efficacy of tamoxifen treatment in ERα-positive breast cancer patients.
In this study, we assessed the effects of LLGL2/SLC7A5 co-expression in predicting prognosis and response to tamoxifen therapy in ERα-positive breast cancer patients with long-term follow up.
Results
LLGL2 mRNA expression and prognosis of breast cancer patients
We first investigated the association between LLGL2 mRNA expression level and prognosis of breast cancer patients with long-term follow-up. A total of 624 breast cancer tissue samples were subjected to LLGL2 mRNA expression analysis. The associations between LLGL2 mRNA expression and clinicopathological characteristics are shown in Supplementary Table S1. Low LLGL2 mRNA levels were positively associated with larger tumor size (P = 0.047) and lymph node negativity (P = 0.031). Low LLGL2 mRNA expression was positively associated with longer DFS in all breast cancer patients analyzed in this study (P = 0.023; Supplementary Fig. S1a). Furthermore, patients with tumors showing low LLGL2 mRNA expression showed a tendency towards longer OS (P = 0.072; Supplementary Fig. S1b).
LLGL2 was reported to be involved in prognosis only in ERα-positive breast cancer patients8. Therefore, we next investigated the association of LLGL2 mRNA expression with prognosis according to ERα status. In ERα-positive breast cancer patients, there was a positive correlation between low LLGL2 mRNA expression and longer DFS and OS (P = 0.0009 and P = 0.005, respectively; Supplementary Fig. S1c,d); however, in ERα-negative breast cancer patients, there was no association between LLGL2 mRNA expression and prognosis (Supplementary Fig. S1e,f). The clinicopathological characteristics of ERα-positive breast cancer patients are shown in Supplementary Table S2. Low LLGL2 mRNA expression level was positively associated with lymph node negativity (P = 0.007).
LLGL2 was reported to be involved in resistance to tamoxifen in ERα-positive breast cancer patients8. Therefore, we investigated the association of LLGL2 mRNA expression with prognosis in ERα-positive breast cancer patients receiving adjuvant tamoxifen therapy (n = 272). As shown in Supplementary Fig. S1g,h, positive associations were found between low LLGL2 mRNA expression and longer DFS and OS in ERα-positive breast cancer patients receiving adjuvant tamoxifen therapy (P = 0.016 and P = 0.018, respectively). Interestingly, no associations were identified between LLGL2 mRNA expression level and prognosis in ERα-positive breast cancer patients without adjuvant tamoxifen therapy (Supplementary Fig. S1i,j).
We next performed univariate and multivariate Cox regression analyses of clinicopathological factors associated with prognosis using stepwise linear regression in all breast cancer patients (Supplementary Table S3) and in ERα-positive breast cancer patients analyzed in this study (Supplementary Table S4). Although low LLGL2 mRNA expression was not an independent favorable prognostic factor in all breast cancer patients, we showed that low LLGL2 was an independent favorable prognostic factor for both DFS and OS in ERα-positive breast cancer patients, as well as nodal status (P = 0.012 and P = 0.011, respectively).
SLC7A5 mRNA expression and prognosis of breast cancer patients
Next, we investigated the association between SLC7A5 mRNA expression and the prognosis of breast cancer patients. The characteristics of ERα-positive breast cancer patients according to SLC7A5 mRNA expression are shown in Supplementary Table S5. Low SLC7A5 mRNA expression was positively associated with favorable prognosis in both DFS and OS in all breast cancer patients analyzed (P = 0.002 and P = 0.0005, respectively; Supplementary Fig. S2a,b). Low SLC7A5 mRNA expression was also positively associated with favorable prognosis in both DFS and OS in ERα-positive breast cancer patients (P = 0.004 and P = 0.004, respectively; Supplementary Fig. S2c,d), but no association was observed in ERα-negative breast cancer patients (Supplementary Fig. S2e,f). As shown in Supplementary Fig. S2g, positive associations were identified between low SLC7A5 mRNA expression and longer DFS in ERα-positive breast cancer patients receiving adjuvant tamoxifen therapy (P = 0.014).
Combination of LLGL2 and SLC7A5 mRNA expression and prognosis of breast cancer patients
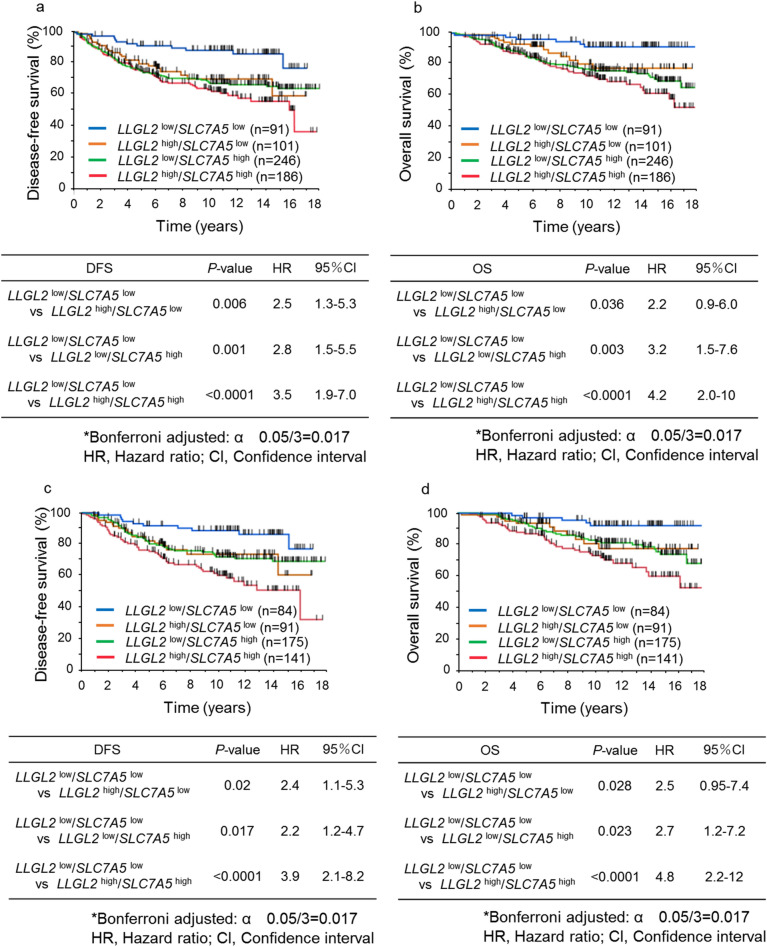
We then investigated the prognostic impact of the combination of LLGL2 and SLC7A5 mRNA expression. Supplementary Table S6 shows the characteristics of breast cancer patients classified by the combination of LLGL2 and SLC7A5 mRNA expression. Low LLGL2/SLC7A5 mRNA co-expression (LLGL2low/SLC7A5low) was positively associated with lower histological grade, lymph node negativity, and ERα positivity. As shown in Fig. 1a,b, LLGL2low/SLC7A5low was associated with longer survival compared with the other combination groups in all breast cancer patients analyzed in this study. The characteristics of ERα-positive breast cancer patients according to LLGL2/SLC7A5 mRNA co-expression are shown in Table 1. LLGL2low/SLC7A5low was associated with lower grade and lymph node negativity in ERα-positive breast cancer patients. As shown in Fig. 1c,d, LLGL2low/SLC7A5low showed longer survival compared with high LLGL2/SLC7A5 mRNA co-expression (LLGL2high/SLC7A5high) and a positive trend of longer survival compared with other combination groups in ERα-positive breast cancer patients. However, we did not observe the association between LLGL2/SLC7A5 mRNA expression and prognosis in ERα-negative breast cancer patients (Fig. 1g,h). Then, we investigated the association of prognosis with the combination of LLGL2 and SLC7A5 mRNA expression in ERα-positive breast cancer patients receiving adjuvant tamoxifen therapy. As shown in Fig. 1e,f, LLGL2low/SLC7A5low showed longer survival than LLGL2high/SLC7A5high and a positive trend of longer survival compared with other combination groups in ERα-positive breast cancer patients receiving adjuvant tamoxifen therapy. No significant difference was observed between these four combination groups in ERα-positive breast cancer patients who had not received adjuvant tamoxifen therapy (Supplementary Fig. S3a,b).
Figure 1.
Kaplan–Meier survival curves according to the combination of LLGL2 and SLC7A5 mRNA expression. Graphs show DFS and OS curves for all breast cancer patients (a,b), ERα-positive breast cancer patients (c,d), ERα-positive breast cancer patients receiving adjuvant tamoxifen therapy (e,f), and ERα-negative breast cancer patients (g,h).
Table 1.
Association between LLGL2 and SLC7A5 mRNA expression and clinicopathological characteristics in ERα-positive patients.
| LLGL2/SLC7A5 | P value | ||||
|---|---|---|---|---|---|
| Low/low | High/low | Low/high | High/high | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Patients | 84 | 91 | 175 | 141 | |
| Menopausal status | |||||
| Pre | 44 (52) | 43 (47) | 75 (43) | 54 (38) | 0.19 |
| Post | 40 (48) | 48 (53) | 100 (57) | 87 (62) | |
| Tumor size | |||||
| ≤ 2 cm | 29 (35) | 45 (50) | 71 (40) | 62 (44) | 0.22 |
| > 2 cm | 55 (65) | 46 (50) | 104 (60) | 79 (56) | |
| Unknown | 0 | 0 | 0 | 0 | |
| Nodal status | |||||
| Negative | 56 (66) | 50 (55) | 95 (54) | 57 (40) | 0.001 |
| Positive | 24 (29) | 37 (41) | 72 (41) | 76 (54) | |
| Unknown | 4 (5) | 4 (4) | 8 (5) | 8 (6) | |
| Grade | |||||
| 1 + 2 | 64 (76) | 64 (70) | 101 (58) | 86 (61) | 0.011 |
| 3 | 18 (22) | 25 (28) | 71 (40) | 50 (35) | |
| Unknown | 2 (2) | 2 (2) | 3 (2) | 5 (4) | |
| pStage TNM* | |||||
| I | 27 (32) | 30 (33) | 51 (29) | 36 (26) | 0.80 |
| II | 44 (52) | 43 (47) | 83 (47) | 71 (50) | |
| III | 9 (11) | 13 (14) | 33 (19) | 24 (17) | |
| Unknown | 4 (5) | 5 (5) | 8 (5) | 10 (7) | |
| Histology | |||||
| IDC | 73 (88) | 79 (85) | 160 (91) | 126 (89) | 0.35 |
| ILC | 4 (4) | 6 (7) | 5 (3) | 11 (8) | |
| Others | 6 (7) | 6 (7) | 10 (6) | 4 (3) | |
| Unknown | 1 (1) | 0 | 0 | 0 | |
| PgR status | |||||
| Positive | 76 (85) | 76 (90) | 155 (89) | 119 (84) | 0.39 |
| Negative | 8 (15) | 15 (10) | 20 (11) | 22 (16) | |
| Unknown | 0 | 0 | 0 | 0 | |
| HER2 status | |||||
| Positive | 6 (7) | 11 (12) | 15 (9) | 13 (9) | 0.73 |
| Negative | 75 (89) | 78 (86) | 152 (87) | 124 (88) | |
| Unknown | 3 (4) | 2 (2) | 8 (4) | 4 (3) | |
| Adjuvant therapy | |||||
| ET alone | 56 (67) | 47 (52) | 85 (49) | 63 (45) | |
| CT alone | 3 (4) | 1 (1) | 19 (10) | 6 (4) | |
| ET + CT | 23 (27) | 34 (37) | 54 (31) | 62 (44) | |
| None | 2 (2) | 8 (9) | 12 (7) | 9 (6) | |
| Unknown | 0 | 1 (1) | 5 (3) | 1 (1) | |
PgR progesterone receptor, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, HER2 human epidermal growth factor receptor 2, ET endocrine therapy, CT chemotherapy.
*AJCC stage.
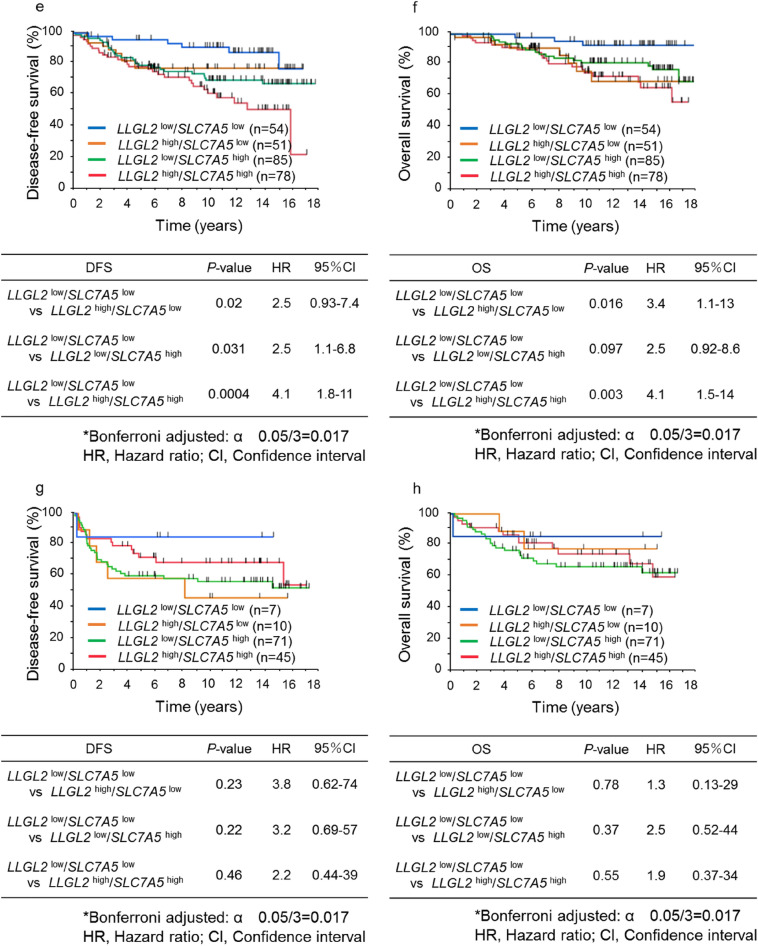
Next, we investigated the prognostic value of LLGL2low/SLC7A5low versus all other groups including LLGL2high/SLC7A5low, LLGL2low/SLC7A5high, and LLGL2high/SLC7A5high in patients who had received tamoxifen as an adjuvant therapy versus those who had not. As shown in Fig. 2, patients with tumors showing LLGL2low/SLC7A5low who had received tamoxifen as an adjuvant therapy (LLGL2low /SLC7A5low TAM+) showed not only significantly better DFS, but also significantly better OS compared with the patients of all other groups who had received tamoxifen as an adjuvant therapy (all other groups TAM+) (P = 0.006 and P = 0.02, respectively). However, there was no significant difference in DFS and OS between the patients with tumors showing LLGL2low/SLC7A5low who had not received tamoxifen as an adjuvant therapy (LLGL2low/SLC7A5low TAM−) compared with the patients of all other groups who had not received tamoxifen as an adjuvant therapy (all other groups TAM−).
Figure 2.
Kaplan–Meier survival curves according to LLGL2low/SLC7A5low versus all other groups with and without adjuvant tamoxifen therapy. Graphs show DFS and OS curves (a,b).
We performed univariate and multivariate Cox regression analyses of clinicopathological factors associated with prognosis using stepwise linear regression in each group of breast cancer patients. Multivariate analyses demonstrated that LLGL2low/SLC7A5low was an independent favorable prognostic factor for DFS as well as lymph node negativity and ERα positivity in all analyzed breast cancer patients (Supplementary Table S7). Then, we performed univariate and multivariate analyses in ERα-positive breast cancer patients, which identified LLGL2low/SLC7A5low as an independent favorable prognostic factor for both DFS and OS, as well as lymph node negativity (Table 2).
Table 2.
Univariate and multivariate analyses of factors associated with DFS and OS including LLGL2/SLC7A5 in ERα-positive patients.
| Variate | n (%) | Univariate (DFS) (DFS) (DFS) |
Univariate (OS) (OS) |
Multivariate (DFS) (DFS) |
Multivariate (OS) (OS) |
||
|---|---|---|---|---|---|---|---|
| P-value | P-value | P-value | HR (95% CI) | P-value | HR (95% CI) | ||
| Menopausal status | |||||||
| Pre | 206 (47) | ||||||
| Post | 237 (53) | 0.29 | 0.20 | ||||
| Tumor size | |||||||
| ≤ 2 cm | 184 (42) | ||||||
| > 2 cm | 259 (58) | 0.14 | 0.011 | 1 (Reference) | |||
| Nodal status | 0.045 | 1.72 (1.01, 2.94) | |||||
| Negative | 244 (55) | 1 (Reference) | 1 (Reference) | ||||
| Positive | 199 (45) | < 0.0001 | < 0.0001 | < 0.0001 | 2.90 (1.93, 4.35) | 0.0001 | 2.68 (1.62, 4.44) |
| Grade | |||||||
| 1 and 2 | 287 (65) | ||||||
| 3 | 156 (35) | 0.39 | 0.11 | ||||
| Histology | |||||||
| IDC | 402 (91) | ||||||
| ILC | 18 (4) | 0.59 | 0.37 | ||||
| Others | 23 (5) | 0.095 | 0.29 | ||||
| HER2 status | |||||||
| Negative | 402 (91) | 1 (Reference) | 1 (Reference) | ||||
| Positive | 41 (9) | 0.19 | 0.038 | 0.054 | 1.78 (0.99, 3.20) | 0.004 | 2.64 (1.37, 5.10) |
| LLGL2/SLC7A5 | |||||||
| Low/low | 76 (17) | 1 (Reference) | 1 (Reference) | ||||
| High/low | 84 (19) | 0.018 | 0.043 | 0.04 | 2.53 (1.04, 6.14) | 0.047 | 3.28 (1.02, 10.58) |
| Low/high | 157 (35) | 0.008 | 0.012 | 0.022 | 2.57 (1.15. 5.75) | 0.02 | 3.47 (1.22, 9.90) |
| High/high | 126 (28) | < 0.0001 | 0.0004 | 0.0008 | 3.97 (1.78, 8.85) | 0.001 | 5.58 (1.94, 16.02) |
IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, HER2 human epidermal growth factor receptor 2.
Protein expression of LLGL2 and SLC7A5 in breast cancer patients
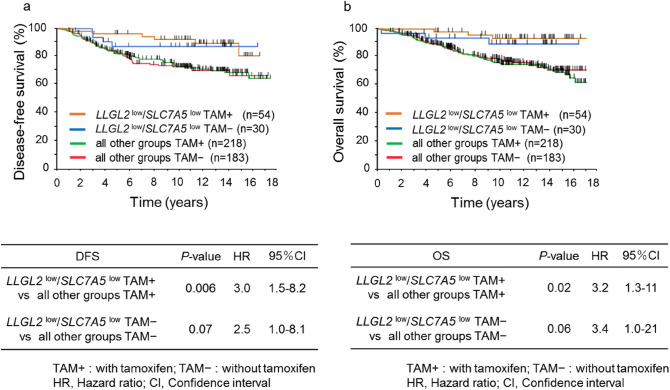
The expression levels of LLGL2 protein in breast cancer tissue samples were examined by Immnohistochemistry (IHC). LLGL2 protein expression was observed in the cytoplasm. Representative images of LLGL2 and SLC7A5 are shown in Fig. 3a,b. Immunostaining results were evaluated using the Aperio scanscopeCS2 and eSlide manager application, and H-scores were calculated by this digital pathological system. In this study, a total of 285 consecutive breast cancer tissue samples for which mRNA expression data were available were analyzed for LLGL2 protein expression.
Figure 3.
Representative images of LLGL2 protein expression in breast cancer tissues. H-score: 0, 90, and 270 from the left (a). Representative images of SLC7A5 protein expression in breast cancer tissues. 0 is negative and 1+ to 3+ is positive (b). Immunofluorescence staining for LLGL2 and SLC7A5. SLC7A5 (FITC, green, lt. upper), LLGL2 (TRITC, red, rt. upper), DAPI (blue, lt. lower), and merge (rt. lower) (c). Kaplan–Meier survival curves of DFS according to the combination of LLGL2 and SLC7A5 protein expression (d).
We also evaluated the expression levels of SLC7A5 protein in breast cancer tissue samples. The analysis was performed using the same tissue microarray as the protein expression analysis of LLGL2. SLC7A5 protein expression was observed in the cell membrane (Fig. 3a). Furthermore, SLC7A5 protein expression was observed in 10% of breast cancer tissues.
We then performed immunofluorescence to further clarify the localization of each protein. As shown in Fig. 3c, LLGL2 was expressed in the cytoplasm and SLC7A5 in the plasma membrane, indicating co-expression in the same tumor cells. Next, we investigated the association between prognosis and the combination of LLGL2 and SLC7A5 protein expression. The LLGL2high/SLC7A5pos group seemed to show the worst prognosis among the four groups, although there was no statistically significant difference between them (Fig. 3d). The median H-score was used as the cutoff value for LLGL2, and SLC7A5 was divided into two groups based on the presence or absence of staining.
Discussion
In this study, we evaluated the prognostic value of co-expression of LLGL2 and SLC7A5 in primary breast cancer patients with long-term follow-up. First, we showed that low LLGL2 or low SLC7A5 mRNA expression was an independent favorable prognostic factor in Erα-positive breast cancer patients. Second, we demonstrated that low LLGL2/SLC7A5 mRNA co-expression (LLGL2low/SLC7A5low) was also an independent favorable prognostic factor both in all breast cancer patients and in Erα-positive breast cancer patients. We also observed that LLGL2low/SLC7A5low showed longer survival compared with LLGL2high/SLC7A5high and a positive trend of longer survival compared with other combination groups in Erα-positive breast cancer patients receiving adjuvant tamoxifen therapy.
Saito et al. recently reported that LLGL2 promoted leucine uptake and conferred tumor growth and resistance to tamoxifen treatment by increasing the cell surface levels of SLC7A5 in Erα-positive breast cancer. They also reported that low LLGL2 mRNA expression was a favorable prognostic factor in Erα-positive breast cancer8. In this study, our data supported the report by Saito et al.8. However, in Erα-negative breast cancer, high expression of LLGL2 mRNA was a favorable prognostic trend. This result was also consistent with previous reports, suggesting that the function of LLGL2 differs depending on the ER status of the tumor.
In this study, we showed that low SLC7A5 mRNA expression was positively associated with favorable prognosis in Erα-positive breast cancer patients but not in Erα-negative breast cancer patients. Our data in this study supported the previous report by Ansari et al.11. Although SLC7A5 is a systemic L-amino acid transporter that carries branch-chain amino acids such as leucine and bulky amino acids such as glutamine, which are considered master regulators of the mTORC1 signaling pathway17,18, the mechanism by which SLC7A5 affects the prognosis of Erα-positive breast cancer patients is not yet fully understood. Recently, Ansari et al. reported that enhanced glutamine uptake by SLC family members including SLC7A5 affects the composition of immune cell infiltrates and might be involved in breast cancer progression19,20.
In this study, we demonstrated that LLGL2low/SLC7A5low was an independent favorable prognostic factor not only in all breast cancer patients, but also in Erα-positive breast cancer patients. In Erα-positive breast cancer patients receiving adjuvant tamoxifen therapy, we observed that LLGL2low/SLC7A5low showed longer survival compared with LLGL2high/SLC7A5high and a positive trend of longer survival compared with other combination groups. However, no significant difference was observed between these four combination groups in Erα-positive breast cancer patients who had not received adjuvant tamoxifen therapy, although it should be noted that the small sample size resulted in decreased power. Moreover, we showed that patients with tumors showing LLGL2low/SLC7A5low who had received tamoxifen as an adjuvant therapy were significantly associated with better prognosis compared with the patients of all other groups who had received tamoxifen as an adjuvant therapy. Saito et al. reported a novel mechanism by which LLGL2 interacts with its cargo, SLC7A5, in the cytoplasm and transports it to the membrane, increasing SLC7A5 levels on the cell surface in Erα-positive breast cancer. They also reported that knockdown of LLGL2 decreased cell surface levels of SLC7A5, and that knockdown of LLGL2 or SLC7A5 was sufficient to restore tamoxifen sensitivity to tamoxifen-resistant Erα-positive breast cancer cells under low leucine concentrations. Our data and that of other groups suggested that co-expression of LLGL2 and SLC7A5 is involved in tamoxifen resistance in Erα-positive breast cancer patients, and that the LLGL2–SLC7A5 axis may be an important determinant of the therapeutic effect of tamoxifen in ER-positive breast cancer.
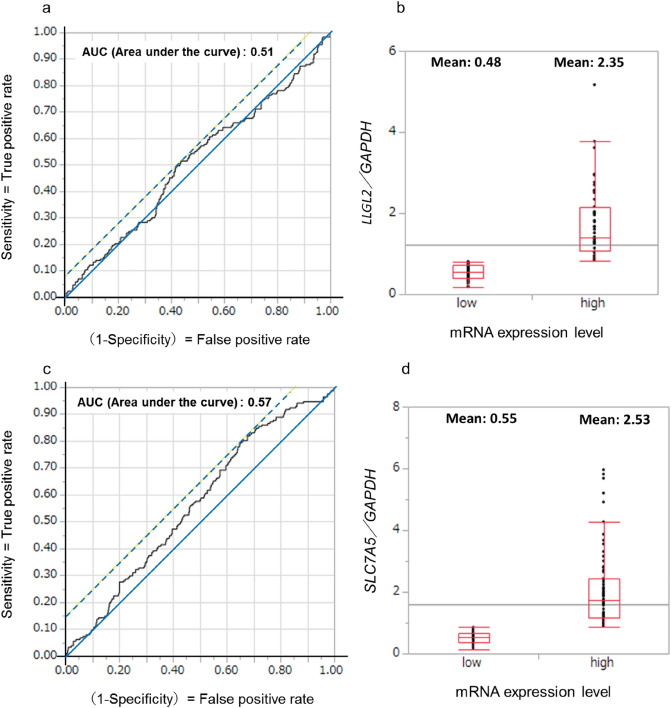
This study had some limitations. First, this was a retrospective analysis at a single institute using archived materials. For the mRNA analysis, we used samples from surgical specimens that were macro-dissected and cryopreserved immediately after resection. However, we did not confirm the amount of cancer tissue in the cryopreserved samples; therefore, the percentage of cancer cells was likely to vary. A total of 626 consecutive invasive breast cancer tissue samples collected between 1992 and 2008 from the archive of our institute were included in this study, and adjuvant therapies for breast cancer have progressed during this period. Therefore, we could not eliminate the effects of different adjuvant therapies in this study. Second, we determined the mRNA cutoff values of LLGL2 and SLC7A5 by Receiver Operating Characteristic (ROC) curve analysis (Fig. 4a–d). As a result of these analyses, we determined 0.51 and 0.57 as the cut-off levels of relative LLGL2 and SLC7A5 mRNA expression, respectively. Because AUC values of 0.51 and 0.57 do not indicate good discriminatory power, the cut-off value for both mRNAs should be re-evaluated using a different dataset in the future. Third, we did not identify a positive association between LLGL2/SLC7A5 protein expression and prognosis in this study. The positive rate of SLC7A5 was only 10% in this study. Because long-term follow-up tissues were used in this study, the positive rate of staining might have decreased as a result of tissue deterioration over time, but the prognosis of cases with staining was poor, which was consistent with the mRNA results of this study.
Figure 4.
ROC analysis to define the threshold for LLGL2 and quantitative differences in LLGL2 mRNA expression (a,b). ROC analysis to define the threshold for SLC7A5 and quantitative differences in SLC7A5 mRNA expression (c,d).
In summary, we showed that low LLGL2/SLC7A5 mRNA co-expression (LLGL2low/SLC7A5low) was an independent favorable prognostic factor in ERα-positive breast cancer patients, as well as low LLGL2 or low SLC7A5 mRNA expression. We also observed that LLGL2low/SLC7A5low showed longer survival compared with LLGL2high/SLC7A5high and a positive trend of longer survival compared with other combination groups in ERα-positive breast cancer patients receiving adjuvant tamoxifen therapy. Thus, our study showed that the co-expression of LLGL2 and SLC7A5 mRNA is a promising candidate biomarker and suggested that the LLGL2–SLC7A5 axis might be a therapeutic target in early breast cancer patients, especially in those receiving adjuvant tamoxifen therapy.
Methods
Patients and samples
A total of 626 consecutive invasive breast cancer tissue samples excluding Stage IV collected between 1992 and 2008 from the archive of the Department of Breast Surgery, Nagoya City University Hospital, Japan, were included in this study to measure LLGL2 and SLC7A5 mRNA expression. Furthermore, 415 consecutive invasive breast cancer tissues collected between 2000 and 2009 as tissue microarrays were included to evaluate LLGL2 and SLC7A5 protein expression. We performed a statistical analysis of protein expression in 285 of the 415 cases for which mRNA expression data were concurrently available. The tissues were fixed in 10% buffered formalin and embedded in paraffin to make a pathological diagnosis or snap-frozen in liquid nitrogen immediately after resection and stored at − 80 °C until RNA extraction. The histological grade was estimated according to the Bloom and Richardson method proposed by Elston and Ellis21. Disease-free survival (DFS) was defined as the interval from the date of primary surgery to the earliest occurrence of one of the following: locoregional recurrence, distant metastasis, or death from any cause. Overall survival (OS) was defined as the interval from the date of primary surgery to death from any cause. The median follow-up period was 10.1 years (range 0.2–215.1 months) and 9.7 years (range 0.8–215.1 months) for the mRNA and protein expression analyses, respectively. Written informed consent for comprehensive research use was obtained from all patients before surgery. This protocol was approved by the institutional review board of Nagoya City University Graduate School of Medical Sciences and conformed to the guidelines of the Declaration of Helsinki.
RNA extraction and quantitative real-time reverse transcription polymerase chain reaction (RT-PCR)
We used an miRNeasy Mini Kit (QIAGEN, Hilden, Germany) for total RNA extraction from breast cancer tissues and a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) for reverse transcription according to each manufacturer’s protocol22. mRNA expression levels of LLGL2, SLC7A5, and the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were measured using TaqMan Gene Expression assays (Thermo Fisher Scientific). Duplex quantitative RT-PCR assays were performed using the StepOnePlus real-time PCR system (Thermo Fisher Scientific). The reaction was analyzed using a FAM-labeled probe for LLGL2 or SLC7A5 (Thermo Fisher Scientific) and a VIC-labeled probe for GAPDH (Thermo Fisher Scientific) as a single assay for each sample. The compositions of the amplification reaction mixtures and reaction conditions were as previously reported22.
We performed a ROC curve analysis to determine the cut-off values of LLGL2 and SLC7A5 mRNA expression (Supplementary Figs. S4, S5). We used Youden’s index (sensitivity + specificity − 1), which corresponded to a point on the ROC curve with the highest vertical distance from the 45° diagonal line, and determined the cut-off values for LLGL2 and SLC7A5 mRNA expression to be 0.82 and 0.54, respectively.
IHC of ERα, progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2)
One 4-μm-thick section from each paraffin-embedded specimen was first stained with hematoxylin and eosin to ascertain whether an adequate number of invasive ductal carcinoma cells were present and that the quality of fixation was adequate for immunohistochemical analysis. Serial sections (4-μm-thick) were then prepared from suitable tissue blocks and float-mounted on adhesive-coated glass slides for ERα (Dako Envision FLEX-ER, EP1; Agilent Technologies, Santa Clara, CA, USA), PgR (Dako Envision FLEX-ER, PgR636; Agilent Technologies), and HER2 (HercepTest II; Agilent Technologies) staining. Staining of these hormone receptors was performed using the Autostainer Link 48 (Agilent Technologies). Immunostained specimens were scored after the entire section had been evaluated by light microscopy. ERα and PgR expression was evaluated by the percentage of cells with positive nuclear staining. A nuclear staining ratio of ≥ 1/100 was considered positive. Scoring of HER2 expression was based on the membrane staining pattern and was scored on a scale of 0–3 + . Tumors with scores of 0 or 1 were considered negative for HER2 overexpression, and those with a score of 3 were considered positive. Tumors with a score of 2+ were tested for gene amplification by fluorescence in situ hybridization (FISH) using the PathVysion assay (Vysis; Abbott Laboratories, Abbott Park, IL, USA) in accordance with the manufacturer’s protocol. A ratio of > 2.0 for HER2 (ERBB2) gene/chromosome 17 was considered positive. Tumors were considered HER2-positive if immunohistochemical staining was 3+ or positive by FISH.
IHC of LLGL2 and SLC7A5
For the immunohistochemical analysis of LLGL2 and SLC7A5, tissue microarrays on 2-mm-diameter slides were prepared after confirming whether an appropriate number of invasive ductal carcinoma cells was present and whether the fixation quality was suitable for immunohistochemical analysis. The primary antibodies for LLGL2 and SLC7A5 protein were rabbit monoclonal anti-LLGL2 antibody (1:300; Santa Cruz Biotechnology, CA, USA) and rabbit monoclonal anti-SLC7A5/LAT1 antibody (1:100; Abcam, Cambridge, UK). Immunostaining was performed using the Leica Bond-Max automated system and Leica Refine detection kits (Leica Biosystems).
Tissue microarray slides were scanned at × 20 magnification using an Aperio scanscopeCS2 (Leica Biosystems, San Diego, CA, USA). We evaluated at least 1000 tumor cells in each tissue core. The protein expression level of LLGL2 was evaluated according to H-score using the eSlide manager application, a digital pathological system (Leica Biosystems). H-score was calculated by classifying the immunostaining intensity into three categories (weak staining, 1; moderate, 2; and strong, 3) and adding the evaluation of each staining proportion. The threshold optical density for each staining intensity was defined as 210, 180, and 150, respectively. H-score was assigned using the following formula: [1 × (% cells with category 1) + 2 × (% cells with category 2) + 3 × (% cells with category 3)]23,24.
SLC7A5 protein expression was localized to the cell membrane, and therefore SLC7A5 immunostaining was evaluated in accordance with the same method employed by the HercepTest (Dako) for HER2 immunostaining, as described above. Scoring of SLC7A5 expression was based on the membrane staining pattern and scored on a scale of 0–3+25,26. For immunostaining evaluation, LLGL2 was divided into two groups (high/low) with the median H-score as the cutoff, and SLC7A5 was divided into two groups with 0 as negative and 1+ to 3+ as positive.
Immunofluorescence staining for LLGL2 and SLC7A5
The detailed methods for immunofluorescence staining employed in this study have been described previously27. Frozen sections were cut to 4-μm thickness and fixed in cold acetone and 10% buffered formalin. A mouse monoclonal anti-LLGL2 antibody (Abnova Corporation, Taipei, Taiwan) was used with biotin-conjugated anti-mouse IgG and TRITC-labeled streptavidin (Thermo Fisher Scientific) to visualize the endogenous proteins using an image analyzer (Keyence). A rabbit monoclonal anti-SLC7A5/LAT1 antibody (Abcam, Cambridge, UK) was used with biotin-conjugated anti-rabbit IgG and FITC-labeled streptavidin (Thermo Fisher Scientific).
Statistical analysis
The associations of LLGL2 and SLC7A5 mRNA expression with clinicopathological factors were assessed by χ2 and Fisher’s exact probability tests. Survival curves were analyzed using the Kaplan–Meier method and verified by the log-rank test. DFS was censored at the date of last follow-up if patients were still relapse-free and alive, and OS was censored at the time when patients were alive. Cox proportional hazards regression analysis was used for univariate and multivariate analyses of prognostic values using the stepwise variable selection method. The level of statistical significance was set at a P-value of less than 5%. Multiple survival curves were compared by the log-rank test with Bonferroni adjustment. In this study, there were three comparisons: LLGL2high/SLC7A5high vs LLGL2low/SLC7A5low; LLGL2high/SLC7A5low vs LLGL2low/SLC7A5low; and LLGL2low/SLC7A5high vs LLGL2low/SLC7A5low. Therefore, a two-sided P-value of 0.017 (0.05/3) was taken to indicate statistical significance. Missing data points were excluded from the analysis. Statistical calculations were performed with JMP12.2 software (SAS Institute, Inc., Cary, NC, USA).
Ethics declarations
This study was approved by the institutional review board of Nagoya City University Graduate School of Medical Sciences. All tissue samples were provided from a biobank that is maintained by the Department of Breast Surgery, Nagoya City University Graduate School of Medical Sciences and conformed to the guidelines of the Declaration of Helsinki. Written informed consent for comprehensive research use was obtained from all patients involved in the study.
Supplementary Information
Acknowledgements
T thank Mr. Hashimoto for the excellent statistical advice and analysis, Mrs. Makino for the excellent technical assistance, and H. Nikki March, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Abbreviations
- ERα
Estrogen receptor α
- LLGL2
Lethal giant larvae homolog 2
- SLC7A5
Solute carrier family 7 member 5
- DFS
Disease-free survival
- OS
Overall survival
- ROC
Receiver operating characteristic
- IHC
Immunohistochemistry
- PgR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- OD
Optical density
Author contributions
T.H. and T.T. contributed to the study conception and design. N.K., Y.W.-E., T.A., H.S., Y.K., M.T., A.K., K.O., and T.T. contributed to the acquisition of patient data. TH., Y.U., and S.N. performed the mRNA analyses and T.H. analyzed patient data. T.H., M.K., and H.K. evaluated the immunohistological analysis and S.T. supervised the pathological evaluation. S.O. performed the statistical analysis and S.M. supervised these analyses. T.H. drafted the manuscript. All authors read and approved the final manuscript. All authors have given consent for publication.
Funding
This work was supported, in part, by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) (KAKENHI Grant Number: 19K18065).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20225-4.
References
- 1.Rakha EA, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J. Clin. Oncol. 2007;25:4772–4778. doi: 10.1200/JCO.2007.12.2747. [DOI] [PubMed] [Google Scholar]
- 2.Dawson SJ, Rueda OM, Aparicio S, Caldas C. A new genome-driven integrated classification of breast cancer and its implications. EMBO J. 2013;32:617–628. doi: 10.1038/emboj.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez CG, et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 2011;13:R21. doi: 10.1186/bcr2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toy W, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arpino G, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: A southwest oncology group study. Clin. Cancer Res. 2004;10:5670–5676. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, et al. LLGL2 rescues nutrient stress by promoting leucine uptake in ER(+) breast cancer. Nature. 2019;569:275–279. doi: 10.1038/s41586-019-1126-2. [DOI] [PubMed] [Google Scholar]
- 9.Nawashiro H, et al. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int. J. Cancer. 2006;119:484–492. doi: 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- 10.Kanai Y, et al. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J. Biol. Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 11.El Ansari R, et al. The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res. 2018;20:21. doi: 10.1186/s13058-018-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfarsi LH, et al. Co-expression effect of SLC7A5/SLC3A2 to predict response to endocrine therapy in oestrogen-receptor-positive breast cancer. Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21041407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maimaiti M, et al. Expression of L-type amino acid transporter 1 as a molecular target for prognostic and therapeutic indicators in bladder carcinoma. Sci. Rep. 2020;10:1292. doi: 10.1038/s41598-020-58136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higuchi K, et al. Characterization of the expression of LAT1 as a prognostic indicator and a therapeutic target in renal cell carcinoma. Sci. Rep. 2019;9:16776. doi: 10.1038/s41598-019-53397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett JM, et al. Mammostrat as an immunohistochemical multigene assay for prediction of early relapse risk in the tamoxifen versus exemestane adjuvant multicenter trial pathology study. J. Clin. Oncol. 2012;30:4477–4484. doi: 10.1200/JCO.2012.42.8896. [DOI] [PubMed] [Google Scholar]
- 16.Mihaly Z, et al. A meta-analysis of gene expression-based biomarkers predicting outcome after tamoxifen treatment in breast cancer. Breast Cancer Res. Treat. 2013;140:219–232. doi: 10.1007/s10549-013-2622-y. [DOI] [PubMed] [Google Scholar]
- 17.Cha YJ, Kim ES, Koo JS. Amino acid transporters and glutamine metabolism in breast cancer. Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Holst J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015;5:1281–1294. [PMC free article] [PubMed] [Google Scholar]
- 19.El-Ansari R, et al. The combined expression of solute carriers is associated with a poor prognosis in highly proliferative ER+ breast cancer. Breast Cancer Res. Treat. 2019;175:27–38. doi: 10.1007/s10549-018-05111-w. [DOI] [PubMed] [Google Scholar]
- 20.Ansari RE, et al. Enhanced glutamine uptake influences composition of immune cell infiltrates in breast cancer. Br. J. Cancer. 2020;122:94–101. doi: 10.1038/s41416-019-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa S, et al. Low RAI2 expression is a marker of poor prognosis in breast cancer. Breast Cancer Res. Treat. 2021;187:81–93. doi: 10.1007/s10549-021-06176-w. [DOI] [PubMed] [Google Scholar]
- 23.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. J. Clin. Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John T, Liu G, Tsao MS. Overview of molecular testing in non-small-cell lung cancer: Mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S14–S23. doi: 10.1038/onc.2009.197. [DOI] [PubMed] [Google Scholar]
- 25.Hicks DG, Tubbs RR. Assessment of the HER2 status in breast cancer by fluorescence in situ hybridization: A technical review with interpretive guidelines. Hum. Pathol. 2005;36:250–261. doi: 10.1016/j.humpath.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Lebeau A, et al. Her-2/neu analysis in archival tissue samples of human breast cancer: Comparison of immunohistochemistry and fluorescence in situ hybridization. J. Clin. Oncol. 2001;19:354–363. doi: 10.1200/JCO.2001.19.2.354. [DOI] [PubMed] [Google Scholar]
- 27.Naiki-Ito A, Kato H, Asamoto M, Naiki T, Shirai T. Age-dependent carcinogenic susceptibility in rat liver is related to potential of gap junctional intercellular communication. Toxicol. Pathol. 2012;40:715–721. doi: 10.1177/0192623312441402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.