Abstract
Objective
To evaluate the association between initiation of fluoroquinolones and hospital admission or emergency department visit for suicidality.
Design
Population based cohort study.
Setting
IBM MarketScan database, USA.
Participants
2 756 268 adults (≥18 years) who initiated an oral fluoroquinolone (ciprofloxacin, levofloxacin, moxifloxacin, gemifloxacin, ofloxacin, gatifloxacin, norfloxacin, lomefloxacin, besifloxacin) or comparator antibiotic (January 2003 to September 2015) and had at least six months of continuous health plan enrollment and a diagnosis of pneumonia or urinary tract infection (UTI) three days or less before the drug initiation date. Comparator antibiotics were azithromycin in the pneumonia cohort and trimethoprim-sulfamethoxazole in the UTI cohort. Participants were matched 1:1 within each cohort on a propensity score, calculated from a multivariable logistic regression model that included 57 baseline covariates.
Main outcomes measure
Primary outcome was hospital admission or emergency department visit for suicidal ideation or self-harm within 60 days after treatment initiation. Cox proportional hazard models were used to estimate hazard ratios and 95% confidence intervals.
Results
The pneumonia cohort included 551 042 individuals, and the UTI cohort included 2 205 526 individuals. During the 60 day follow-up, 181 events were observed in the pneumonia cohort and 966 in the UTI cohort. The adjusted hazard ratios for fluoroquinolones were 1.01 (95% confidence interval 0.76 to 1.36) versus azithromycin in the pneumonia cohort and 1.03 (0.91 to 1.17) versus trimethoprim-sulfamethoxazole in the UTI cohort. Results were consistent across sensitivity analyses and subgroups of sex, age, or history of mental illnesses.
Conclusion
Initiation of fluoroquinolones was not associated with a substantially increased risk of admission to hospital or emergency department visits for suicidality compared with azithromycin or trimethoprim-sulfamethoxazole.
Introduction
Fluoroquinolones are a family of antibiotics that have been widely used as treatment for acute respiratory infections and uncomplicated urinary tract infections (UTIs).1 2 3 Partly owing to their desirable pharmacokinetic properties, excellent oral availability, and broad spectrum of antimicrobial activity, fluoroquinolones were the third most commonly prescribed antibiotic class in the United States in 2011.4 Despite their attractive profile as anti-infective agents, the toxic effects of fluoroquinolones on gastrointestinal, dermatological, cardiovascular, and nervous systems have been well established.1 5 6 However, the risks of serious associated adverse effects on mental health remain uncertain.
Although a range of mental health side effects from fluoroquinolone use has been reported,7 suicidal ideation is of concern. People attempting or completing suicide and suicidal ideation have been reported after initiation of fluoroquinolones.8 9 10 11 A review of US Food and Drug Administration reports of suicidal behaviors associated with all FDA approved fluoroquinolones suggested these events could occur even in people with no previous psychiatric illness.12 In 2016, after a review of the adverse event reports and case reports, the FDA revised the boxed warning for all oral and injectable fluoroquinolones because of the potential risks of serious side effects, including suicidal thoughts.7
Several neurobiological mechanisms have been proposed to explain the association between fluoroquinolones and central nervous system (CNS) events, which include suicidality.13 14 15 Given the similarity of the structure of fluoroquinolones to γ-aminobutyric acid (GABA) agonists, it is believed that fluoroquinolones may bind to brain GABA receptors, leading to neurotoxicity.6 13 14 15 It has also been suggested that fluoroquinolones may stimulate the excitatory N-methyl-d-aspartate (NMDA) and adenosine receptors, which on sufficient penetration of the CNS could manifest in observable CNS symptoms.13 14 Finally, fluoroquinolone induced reduction in serotonin levels, oxidative stress and lowered antioxidant levels, and alterations of various microRNAs may also contribute to neuropsychiatric adverse effects.13
Given the limitations of adverse event case reports and the broad use of fluoroquinolones, we evaluated the risk of hospital admission and emergency department visits for suicidal behaviors in a large cohort of people and quantified the magnitude of any increase in risk. We compared the rates of suicidality between fluoroquinolones and clinically appropriate comparators in patients with pneumonia or UTI; two of the most common indications for treatment with fluoroquinolones.
Methods
Data source and study cohort
The study data were derived from health insurance claims between 1 January 2003 and 30 September 2015, obtained from a national commercial US health insurance claims database (IBM MarketScan). The database contains person level data on healthcare use and expenditures for people enrolled in private insurance plans through a participating employer, health plan, or government organization. IBM MarketScan includes the MarketScan Commercial Claims and Encounters database, as well as the MarketScan Medicare Supplemental database that includes Medicare eligible retirees with employer sponsored Medicare Supplement plans. In addition to personal information and enrollment status, the database provides longitudinal records of reimbursed medical services. Information on inpatient and outpatient encounters includes diagnoses and procedures coded using ICD-9 (international classification of diseases, ninth revision) and CPT (Current Procedural Terminology) codes, as well as corresponding dates of service. For pharmacy claims, the database provides details such as national drug code, date of dispensing, quantity dispensed, and day’s supply for prescription drugs dispensed in the outpatient setting.
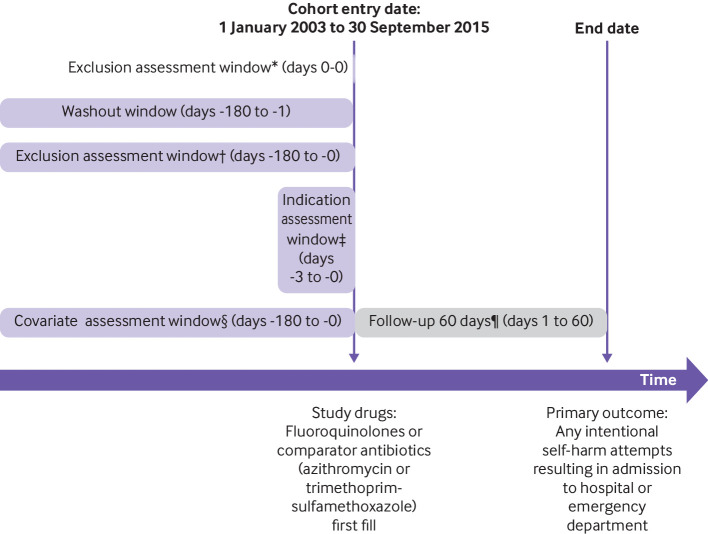
The study cohort included adults aged 18 years and older who initiated an oral fluoroquinolone or a comparator antibiotic between 1 January 2003 and 30 September 2015, and who had at least 180 days of continuous medical and prescription drug coverage enrollment before the drug initiation date (cohort entry date; fig 1). We defined initiation as the absence of dispensings for any fluoroquinolones or comparator antibiotics in the 180 days before the cohort entry date.
Fig 1.
Study design. *Age <18 years, antibiotics other than study drugs, nursing home or hospital stay. †History of intentional self-harm, any hospital admission >28 days, any diagnosis of HIV infection or cancer. ‡Diagnosis of pneumonia or urinary tract infection. §Demographic information, calendar year, other covariates (mental disorders, neurological disorders, other comorbidities, medication use, healthcare utilization). ¶Censoring criteria: occurrence of study outcome, death, nursing home or hospice admission, end of continuous health plan enrollment, end of study period (30 September 2015)
To control for confounding by indication and bias due to differential surveillance, we restricted the eligible study population to individuals with specific treatment indications and used an active comparator design. In particular, we restricted to those with a diagnosis of pneumonia or a UTI within three days before and including the cohort entry date. We chose azithromycin as the comparator antibiotic for the pneumonia cohort and combined trimethoprim and sulfamethoxazole for the UTI cohort.16 Supplementary table 1 provides definitions of pneumonia and UTI.
We excluded patients if they received antibiotics other than the study drugs (fluoroquinolones or a comparator) on the cohort entry date; had a diagnosis of attempted suicide, cancer, or HIV; had a prolonged hospital stay (length of stay >28 days) within the six months preceding the cohort entry date; or were in a hospital or nursing home on the cohort entry date. Supplementary table 2 presents definitions of exclusion criteria. For patients with multiple eligible cohort entries, we selected the first one.
Assessment of drug use
The fluoroquinolones of interest included ciprofloxacin, levofloxacin, moxifloxacin, gemifloxacin, ofloxacin, gatifloxacin, norfloxacin, lomefloxacin, and besifloxacin. We chose azithromycin and trimethoprim-sulfamethoxazole as the comparator drugs for the pneumonia and UTI cohorts, respectively, because they represented alternative treatment options for the infections of interest during the study period.17 18 Use of fluoroquinolones and comparator drugs was identified using pharmacy dispensing data.
Outcome and follow-up
The study outcome, suicidality, was defined as any hospital admission or emergency department visit with ICD-9 codes V62.84 (suicidal ideation) or E950.x-E958.x (suicide and self-inflicted injury) at any position.19 We also conducted a sensitivity analysis limiting the outcome definition to primary discharge diagnosis for hospital admissions and any emergency department visit (emergency department records do not differentiate between primary and other diagnoses).
Follow-up started on the day after the antibiotic was dispensed, and continued for a maximum of 60 days regardless of changes to treatment, until the occurrence of a study outcome, death, nursing home or hospice admission, end of continuous health plan enrollment, or end of the study period (30 September 2015), whichever came first (fig 1). Since the exact biological mechanism behind the association between fluoroquinolones and CNS symptoms is not established, but the usual course of fluoroquinolone treatment is short,20 we varied the duration of follow-up in sensitivity analyses.
Covariates
We assessed patient characteristics from 180 days before cohort entry and through the cohort entry date (day of antibiotic initiation). In addition to personal information (age, sex, region) and calendar year, conditions that may be associated with suicidality and the choice of study antibiotic were identified through ICD-9 codes and CPT-4 codes for comorbidities, and through pharmacy dispensings for drug use. These covariates included psychiatric disorders, such as anxiety disorder, depression, psychotic disorders, delirium, and substance misuse, and other comorbidities, such as neurological disorders, Parkinson’s disease, migraine, multiple sclerosis, diabetes mellitus, stroke, ischemic heart disease, skin disorders, renal dysfunction, and liver disease (see supplementary table 3 for full list). To ensure comparability between study groups, we measured indicators for healthcare utilization, including previous hospital admissions, office visits, and total number of unique drugs used. The combined comorbidity index and a validated, claims based frailty index were included as proxies for overall disease state and care intensity.21 22 Supplementary table 3 presents definitions for covariates.
Statistical analysis
To account for baseline differences between the study drug groups, we matched patients initiating fluoroquinolones with those initiating the comparator antibiotic on their predicted probability of drug use (propensity score) in a 1:1 ratio, using a nearest neighbor algorithm and a caliper of 0.05 on the propensity score scale. Propensity scores were estimated separately within each cohort using logistic regression and all predefined covariates (listed in supplementary tables 4 and 5). Standardized mean differences were used to examine the balance of covariates within the matched cohorts; a standardized difference greater than 0.1 was considered to show imbalance between groups.23 In each propensity score matched cohort, we estimated hazard ratios and corresponding 95% confidence intervals using Cox proportional hazards regression models.
To evaluate the robustness of the primary results we conducted several sensitivity analyses. First, we varied the follow-up period to 14 days, 30 days, and 90 days in sensitivity analyses. Additionally, we conducted a high dimensional propensity score analysis that included an additional 200 empirically identified proxies for confounders to further control for potential residual confounding,24 an analysis that included people with cancer, HIV, and a history of suicidality while adjusting for these risk factors, and an analysis using a more restrictive outcome definition.
Finally, we conducted several subgroup analyses. Analysis was stratified by age (age 18-24, 25-44, 45-64, ≥65 years), since the susceptibility for bacterial infections and suicidality may vary by age.25 26 The analysis was also stratified by sex (men and women) in light of potential sex differences in certain bacterial infections.27 Finally, the rate of hospital admission for self-harm within 60 days was assessed among subgroups of patients with or without a history of mental illness at baseline. All statistical analyses were performed on Aetion, platform version 4.20,28 with R, version 3.1.2.
Patient and public involvement
No patients were involved in setting the research question or study design nor in the interpretation of results, owing to training restrictions and budget constraints.
Results
Study population
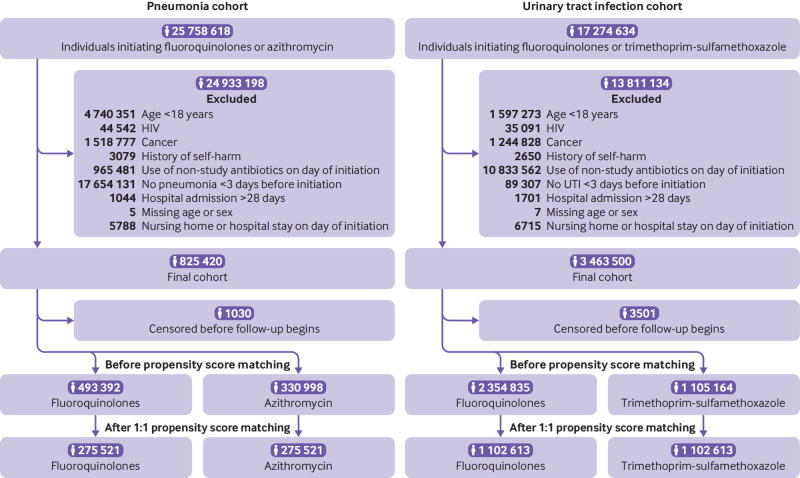
A total of 825 420 patients with pneumonia and 3 463 500 patients with UTI satisfied the inclusion criteria and were eligible for study inclusion (fig 2).
Fig 2.
Formation of study cohort
Before propensity score matching, fluoroquinolone initiators compared with azithromycin initiators in the pneumonia cohort were older (mean age 56.23 v 48.55 years), had a greater burden of comorbidities, and had a higher prevalence of characteristics suggestive of frailty (see supplementary table 4). Similarly, fluoroquinolone initiators compared with trimethoprim-sulfamethoxazole initiators in the UTI cohort were older (48.08 v 43.70 years), had a higher prevalence of characteristics suggestive of frailty (mean 0.13 (standard deviation 0.04) v 0.12 (0.03)), and were more likely to be men (15.4% v 8.1%) (see supplementary table 5).
After propensity score matching, the final study populations included 275 521 matched pairs in the pneumonia cohort and 1 102 613 matched pairs in the UTI cohort (fig 2). Both pneumonia and UTI cohorts were adequately balanced on all measured covariates across drug use and reference groups (table 1). Supplementary table 6 presents the median (interquartile range) duration of index prescription, as measured by days’ supply recorded by the pharmacy, and the distribution of individual fluoroquinolone agents. The most common fluoroquinolone used by the pneumonia cohort was levofloxacin (73.1% of patients using fluoroquinolones), whereas the most common fluoroquinolone used by the UTI cohort was ciprofloxacin (86.3% of patients using fluoroquinolones).
Table 1.
Selected baseline characteristics in propensity score matched pneumonia cohort and urinary tract infection cohort. Values are numbers (percentages) unless stated otherwise
| Characteristics | Pneumonia cohort | Standardized difference | Urinary tract infection cohort | Standardized difference | ||
|---|---|---|---|---|---|---|
| Fluoroquinolones* (n=275 521) | Azithromycin (n=275 521) | Fluoroquinolones* (n=1 102 613) | TMP-SMX (n=1 102 613) | |||
| Personal characteristics | ||||||
| Mean (SD) age (years) | 51.44 (16.93) | 51.69 (17.00) | 0.01 | 43.66 (17.41) | 43.76 (17.49) | 0.01 |
| Age categories (years): | ||||||
| 18-24 | 15 748 (5.7) | 14 228 (5.2) | −0.02 | 195 010 (17.7) | 189 810 (17.2) | −0.01 |
| 25-44 | 83 467 (30.3) | 82 206 (29.8) | −0.01 | 395 123 (35.8) | 396 915 (36.0) | 0.00 |
| 45-64 | 120 455 (43.7) | 123 594 (44.9) | 0.02 | 388 695 (35.3) | 392 357 (35.6) | 0.01 |
| ≥65 | 55 851 (20.3) | 55 493 (20.1) | 0.00 | 123 785 (11.2) | 123 531 (11.2) | 0.00 |
| Men | 133 903 (48.6) | 134 269 (48.7) | 0.00 | 92 621 (8.4) | 89 254 (8.1) | −0.01 |
| Psychiatric comorbidities | ||||||
| Anxiety disorder | 12 103 (4.4) | 11 872 (4.3) | 0.00 | 57 931 (5.3) | 58 236 (5.3) | 0.00 |
| Depression | 19 531 (7.1) | 19 034 (6.9) | −0.01 | 88 256 (8.0) | 88 806 (8.1) | 0.00 |
| Sleep disorder | 9703 (3.5) | 9676 (3.5) | 0.00 | 30 924 (2.8) | 30 920 (2.8) | 0.00 |
| Psychotic disorder | 1806 (0.7) | 1682 (0.6) | −0.01 | 5314 (0.5) | 5161 (0.5) | 0.00 |
| Mania | 2657 (1.0) | 2564 (0.9) | −0.01 | 11 079 (1.0) | 11 072 (1.0) | 0.00 |
| Delirium | 2096 (0.8) | 1736 (0.6) | −0.02 | 4554 (0.4) | 4344 (0.4) | 0.00 |
| Dementia | 4492 (1.6) | 4130 (1.5) | −0.01 | 10 892 (1.0) | 10 583 (1.0) | 0.00 |
| Substance misuse | 16 530 (6.0) | 16 186 (5.9) | 0.00 | 30 116 (2.7) | 30 050 (2.7) | 0.00 |
| Other mental disorder | 3028 (1.1) | 2977 (1.1) | 0.00 | 14 027 (1.3) | 14 066 (1.3) | 0.00 |
| Neurological comorbidities | ||||||
| Seizure or epilepsy | 2697 (1.0) | 2594 (0.9) | −0.01 | 8423 (0.8) | 8383 (0.8) | 0.00 |
| Neuropathy or neuropathic pain | 11 348 (4.1) | 11 104 (4.0) | −0.01 | 34 793 (3.2) | 34 891 (3.2) | 0.00 |
| Migraine | 5063 (1.8) | 4964 (1.8) | 0.00 | 30 016 (2.7) | 30 486 (2.8) | 0.01 |
| Parkinson’s disease | 906 (0.3) | 867 (0.3) | 0.00 | 2348 (0.2) | 2267 (0.2) | 0.00 |
| Other comorbidities | ||||||
| Congestive heart failure | 12 552 (4.6) | 11 453 (4.2) | −0.02 | 11 554 (1.0) | 10 921 (1.0) | 0.00 |
| Ischemic heart disease | 21 533 (7.8) | 20 463 (7.4) | −0.02 | 32 282 (2.9) | 31 478 (2.9) | 0.00 |
| Any stroke | 6332 (2.3) | 5916 (2.1) | −0.01 | 13 534 (1.2) | 13 104 (1.2) | 0.00 |
| Atrial fibrillation | 10 624 (3.9) | 10 182 (3.7) | −0.01 | 14 788 (1.3) | 14 423 (1.3) | 0.00 |
| Autoimmune disorder | 10 973 (4.0) | 10 927 (4.0) | 0.00 | 33 987 (3.1) | 34 204 (3.1) | 0.00 |
| Diabetes | 33 149 (12.0) | 32 909 (11.9) | 0.00 | 83 932 (7.6) | 84 031 (7.6) | 0.00 |
| Skin disorder | 37 778 (13.7) | 37 218 (13.5) | −0.01 | 147 370 (13.4) | 148 296 (13.4) | 0.00 |
| Renal dysfunction | 12 016 (4.4) | 11 079 (4.0) | −0.02 | 22 927 (2.1) | 21 465 (1.9) | −0.01 |
| Liver disease | 5631 (2.0) | 5343 (1.9) | −0.01 | 14 419 (1.3) | 14 253 (1.3) | 0.00 |
| Asthma or COPD | 81 428 (29.6) | 80 907 (29.4) | 0.00 | 139 285 (12.6) | 140 578 (12.7) | 0.00 |
| Mean (SD) combined comorbidity index | 0.38 (1.14) | 0.34 (1.13) | −0.04 | 0.10 (0.73) | 0.09 (0.73) | −0.01 |
| Mean (SD) frailty index | 0.14 (0.04) | 0.14 (0.04) | 0.00 | 0.12 (0.03) | 0.12 (0.03) | 0.00 |
| Drug use | ||||||
| Opioid | 80 621 (29.3) | 80 738 (29.3) | 0.00 | 243 198 (22.1) | 244 455 (22.2) | 0.00 |
| Lithium | 648 (0.2) | 641 (0.2) | 0.00 | 2649 (0.2) | 2666 (0.2) | 0.00 |
| Antipsychotic | 5603 (2.0) | 5432 (2.0) | 0.00 | 18 838 (1.7) | 18 792 (1.7) | 0.00 |
| Antidepressant | 55 432 (20.1) | 55 238 (20.0) | 0.00 | 228 466 (20.7) | 230 432 (20.9) | 0.00 |
| NSAID | 39 201 (14.2) | 39 355 (14.3) | 0.00 | 163 022 (14.8) | 164 539 (14.9) | 0.00 |
| Benzodiazepine | 30 314 (11.0) | 29 932 (10.9) | 0.00 | 116 039 (10.5) | 116 818 (10.6) | 0.00 |
| Migraine product | 4401 (1.6) | 4466 (1.6) | 0.00 | 28 652 (2.6) | 29 254 (2.7) | 0.01 |
| Antiparkinsonian agent | 3141 (1.1) | 3093 (1.1) | 0.00 | 8628 (0.8) | 8748 (0.8) | 0.00 |
| Dementia drug | 3526 (1.3) | 3396 (1.2) | −0.01 | 8418 (0.8) | 8332 (0.8) | 0.00 |
| Muscle relaxant | 19 897 (7.2) | 19 856 (7.2) | 0.00 | 84 960 (7.7) | 85 729 (7.8) | 0.00 |
| Anticonvulsant | 19 354 (7.0) | 19 004 (6.9) | 0.00 | 66 726 (6.1) | 66 997 (6.1) | 0.00 |
| CNS stimulant | 2396 (0.9) | 2390 (0.9) | 0.00 | 13 105 (1.2) | 13 272 (1.2) | 0.00 |
| Other anxiolytic or hypnotic | 19 113 (6.9) | 18 894 (6.9) | 0.00 | 73 809 (6.7) | 74 585 (6.8) | 0.00 |
| Health services utilization | ||||||
| Mean (SD) No of office visits | 4.11 (4.37) | 4.05 (4.18) | −0.01 | 3.83 (3.78) | 3.83 (3.80) | 0.00 |
| Mean (SD) No of unique psychotropics | 0.98 (1.49) | 0.97 (1.48) | −0.01 | 0.88 (1.42) | 0.88 (1.43) | 0.00 |
| Mean (SD) No of unique drugs | 6.22 (4.34) | 6.18 (4.37) | −0.01 | 5.09 (3.73) | 5.11 (3.76) | 0.01 |
| Mean (SD) No of hospital admissions | 0.17 (0.44) | 0.16 (0.44) | −0.02 | 0.07 (0.28) | 0.06 (0.29) | −0.04 |
| Mean (SD) No of emergency department visits | 0.56 (1.08) | 0.54 (0.98) | −0.02 | 0.33 (0.78) | 0.32 (0.83) | −0.01 |
TMP-SMX=trimethoprim and sulfamethoxazole; SD=standard deviation; COPD=chronic obstructive pulmonary disease; NSAID=non-steroidal anti-inflammatory drug; CNS=central nervous system.
Ciprofloxacin, levofloxacin, moxifloxacin, gemifloxacin, ofloxacin, gatifloxacin, norfloxacin, lomefloxacin, or besifloxacin.
Admissions for suicidality
Supplementary table 7 presents the median duration of follow-up and reasons for censoring. Most of the patients (92.3% in the pneumonia cohort and 93.0% in the UTI cohort) completed the 60 day follow-up. Overall, 91 (0.03%) fluoroquinolone initiators and 90 (0.03%) azithromycin initiators in the pneumonia cohort and 491 (0.04%) fluoroquinolone initiators and 475 (0.04%) trimethoprim-sulfamethoxazole initiators in the UTI cohort were admitted to hospital or an emergency department for suicidality during follow-up (see supplementary figure for cumulative incidence curves). The adjusted hazard ratio for use of fluoroquinolones and hospital admission for suicidality was 1.01 (95% confidence interval 0.76 to 1.36) in the pneumonia cohort and 1.03 (0.91 to 1.17) in the UTI cohort (table 2).
Table 2.
Association between fluoroquinolone initiation and hospital admission or emergency department visit for suicidality
| Analyses | Pneumonia cohort | Urinary tract infection cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Propensity score matching | High dimensional propensity score matching | Propensity score matching | High dimensional propensity score matching | ||||||
| Fluoroquinolones* (n=275 521) | Azithromycin (n=275 521) | Fluoroquinolones* (n=269 625) | Azithromycin (n=269 625) | Fluoroquinolones* (n=1 102 613) | TMP-SMX (n=1 102 613) | Fluoroquinolones* (n=1 098 770) | TMP-SMX (n=1 098 770) | ||
| No (%) of admissions | 91 (0.03) | 90 (0.03) | 86 (0.03) | 84 (0.03) | 491 (0.04) | 475 (0.04) | 492 (0.04) | 470 (0.04) | |
| Risk/1000 patients | 0.33 | 0.33 | 0.32 | 0.31 | 0.45 | 0.43 | 0.45 | 0.43 | |
| Hazard ratio (95% CI) | 1.01 (0.76 to 1.36) | Reference | 1.02 (0.76 to 1.38) | 1 (Reference) | 1.03 (0.91 to 1.17) | Reference | 1.05 (0.92 to 1.19) | 1 (Reference) | |
TMP-SMX=trimethoprim and sulfamethoxazole; CI=confidence interval.
Ciprofloxacin, levofloxacin, moxifloxacin, gemifloxacin, ofloxacin, gatifloxacin, norfloxacin, lomefloxacin, or besifloxacin.
The results were similar in the overall cohorts before propensity score matching. The crude hazard ratio for suicidality was 1.06 (95% confidence interval 0.84 to 1.35) in the pneumonia cohort and 0.98 (0.88 to 1.09) in the UTI cohort (see supplementary table 8).
Sensitivity and subgroup analyses
The high dimensional propensity score matched analysis (table 2) produced results consistent with those of the main analyses in both the pneumonia cohort (hazard ratio 1.02, 95% confidence interval 0.76 to 1.38) and the UTI cohort (1.05, 0.92 to 1.19). When the follow-up period was varied to 14 days, 30 days, or 90 days the results became qualitatively consistent with the primary findings (see supplementary tables 9-11), as did the analysis that included people with cancer, HIV, or a history of suicidality (see supplementary table 12), and the sensitivity analysis using a more restrictive outcome definition (see supplementary table 13).
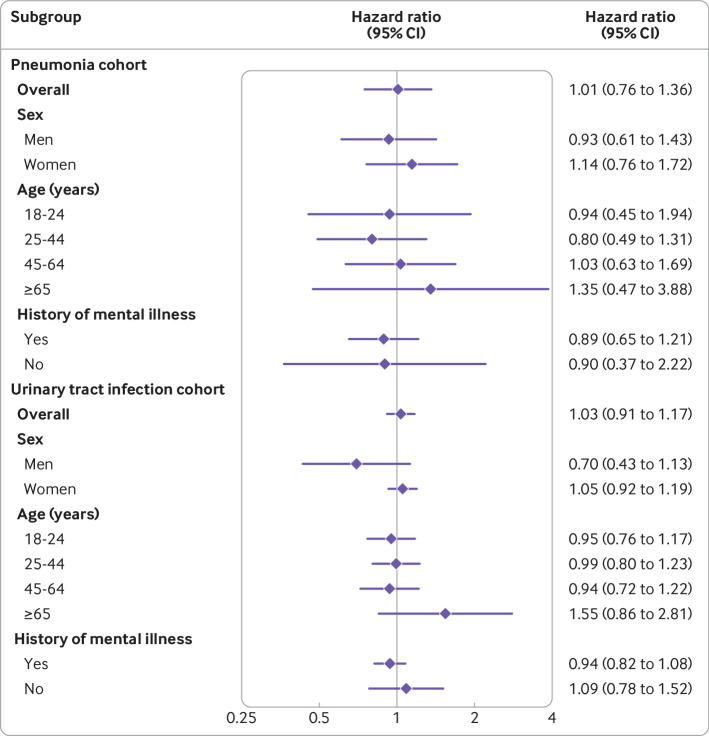
Results were similar in subgroup analyses (fig 3). In both cohorts, we found no evidence of effect modification by age, sex, or history of mental illness, although some subgroups were small (see supplementary table 14 for number of patients and events in each subgroup), which led to limited statistical precision and wide confidence intervals.
Fig 3.
Subgroup analyses of association between fluoroquinolone use and suicidality
Discussion
Principal findings
In this nationwide cohort study of more than a million US patients treated with fluoroquinolones for either pneumonia or UTI, we observed no increased risk of suicidality associated with short term use of fluoroquinolone compared with azithromycin or trimethoprim-sulfamethoxazole, as measured by hospital admissions and emergency department visits for suicidal ideation or self-harm. The lack of association persisted across several sensitivity and subgroups analyses. Moreover, the absolute risks of admission to hospital admission or an emergency department for suicidality were low in both cohorts (<0.1%) and consistent with the numbers reported in the general population.29
Comparison with other studies
Two previous studies have provided conflicting results.13 30 In a nested case-control study that included 348 people with attempted or completed suicide or suicidal ideation and 808 controls, Jick et al found that the use of quinolone antibiotics was not associated with an increased risk of suicidal behaviors.30 In contrast with this finding, the disproportionality analyses by Samyed et al using a global adverse drug reaction database, including 608 quinolone related suicidal behaviors, reported an increase in reporting of suicidal behaviors associated with quinolone use.13
Despite differences in data sources and design and analysis methods, our findings are consistent with those of Jick et al’s case-control study,30 which compared fluoroquinolone users with antibiotic non-users. The findings of our study, which used an active comparator design to tackle differences in individuals who did and did not use an antibiotic, bolster the findings of Jick et al’s study.16 Although Samyed et al’s analyses of suicidality associated with quinolone use also compared quinolone users with those receiving other antibiotics,13 the findings of this study should be interpreted in the context of the under-reporting and selective reporting of spontaneous adverse drug events.15 For example, factors such as experience of the reporter, local patterns of utilization, specificity of the adverse reaction, and knowledge about safety profile of the drug can affect reporting patterns of adverse drug reactions to pharmacovigilance systems, which can limit the interpretation of disproportionality studies.15 31
Strengths and limitations of this study
We used several approaches to ensure the validity of findings and to minimize bias that can affect non-randomized studies of treatments. Restricting the study cohorts to people with pneumonia or UTI, identifying comparable treatment alternatives within each indication, and implementing propensity score based adjustment for covariates increased our ability to control for confounding. An incident user, active comparator design enabled us to align time zero (start of follow-up) for the fluoroquinolone and comparator antibiotic groups and to avoid time related biases or selection bias due to depletion of individuals susceptible to the outcome.32 33 34 A large sample size allowed us to evaluate a rare outcome and to exclude a substantial increase in risk.
Our study does, however, have several limitations. First, our findings should be interpreted in the context of the limitations inherent to the nature of claims data, as such data are generated for administrative purposes and are generally limited to information relevant for billing. Administrative claims data lack information on certain patient characteristics that could affect the risk of suicidality, such as socioeconomic status and lifestyle factors, if they are different between the drug groups. To ensure robust control of confounding, we implemented multiple approaches, such as a clinically exchangeable comparator and adjustment for multiple variables measured in claims, many of which serve as proxies for unmeasured characteristics. Nevertheless, residual confounding cannot be completely excluded.
Second, as our outcome definition was based on hospital admissions and emergency department visits with billed ICD-9 codes, the observed absolute risks could underestimate the true incidence of suicidality in antibiotic users as our outcome definition only captured those who were admitted to hospital or obtained emergency care. Electronic healthcare records were not available to confirm outcomes, and outcomes among individuals with suicidal thoughts who were not admitted to hospital or did not seek emergency care, such as those who called their doctors, social workers, or hotlines, would have been missed in our study. Inasmuch as the degree of outcome misclassification is similar between the groups compared, relative risks remain unbiased.35 However, we cannot rule out that fluoroquinolone use might be associated with suicidal thoughts that do not lead to hospital admission. Future work, preferably using data that contain information on mental health services, including those not billed to medical insurance, may shed more light on this question. In addition to not capturing less extreme cases, our outcome definition would have also missed people who completed suicide and therefore did not attend a hospital. Outpatient deaths are not recorded in administrative claims data. Nevertheless, the percentages of individuals who died (in a hospital) or had their insurance terminated (which would happen after death) were almost identical between the antibiotic groups.
The results of our study should also be interpreted in the context of study inclusion criteria and study design choices. Our study included commercially insured individuals treated for pneumonia or UTI in an outpatient setting who are typically prescribed a treatment regimen that lasts less than two weeks; thus, our results may not be generalizable to patients who require long term use of fluoroquinolones, or require systemic fluoroquinolones. Although we did not find evidence of effect measure modification in subgroup analyses, it is possible that analysis was underpowered in these subgroups. Our results need to be confirmed in populations that may be underrepresented in our study cohort, such as older people who are eligible for Medicare in the US. We compared fluoroquinolones to therapeutically comparable antibiotics—namely, azithromycin and trimethoprim-sulfamethoxazole. If comparators are associated with suicidality, then our findings do not necessarily reflect an absence of risk associated with fluoroquinolones. To our knowledge, neither azithromycin nor trimethoprim-sulfamethoxazole are known to cause suicidal thoughts.
The study period was also limited to years before 2016 to avoid bias due to differential surveillance after the FDA labeling change in 2015. In the US, where transition to ICD-10 codes occurred in October 2015, only ICD-9 codes were in place during the study period. It is possible that suicidal coding changed with the introduction of the ICD-10 codes, and the rates we observed in our study are not generalizable to more recent years.
Next, although we had sufficient statistical power to exclude a substantial increase in risk associated with fluoroquinolone use in the overall study population, we cannot exclude a small increase in risk, particularly in individuals treated for pneumonia. Some of our subgroup analyses were also limited by the low number of events; thus, our study might have been underpowered to detect effect heterogeneity across subgroups. Finally, since individual fluoroquinolones differ in their pharmacokinetic properties, including brain tissue penetration, it is possible that some agents may be more likely to cause CNS adverse events, including suicidality. Most patients in our cohort were taking ciprofloxacin and levofloxacin, both of which are known to have poor to limited CNS penetration.36 Further work is needed to evaluate fluoroquinolones known to achieve good CNS penetration.
Conclusion
No substantially increased risk of hospital admission or emergency department visit for suicidality was observed among people treated for pneumonia or UTI with a fluoroquinolone, compared with those receiving azithromycin or combined trimethoprim and sulfamethoxazole. We cannot, however, exclude a small increase in risk, or an effect on suicidal thoughts that do not lead to hospital admission or emergency department visit.
What is already known on this topic
The US Food and Drug Administration’s revised boxed warning on fluoroquinolones in 2016 suggested an increased risk of suicidal thoughts associated with use of fluoroquinolones
No population-wide cohort study has investigated the association
What this study adds
In this population based study the short term use of outpatient oral fluoroquinolones for pneumonia or urinary tract infections was not associated with an increased risk of admission to hospital or an emergency department for suicidality compared with azithromycin or combined trimethoprim and sulfamethoxazole
The absolute risk of hospital admissions for suicidality was low
Web extra.
Extra material supplied by authors
Supplementary information: additional tables 1-14 and figure
Contributors: JW, JJG, and KB designed the study. JW and SKS processed the data. KB and JG directed the analyses, which were carried out by JW and SKS. All authors participated in the discussion and interpretation of the results. JW wrote the initial draft. All authors critically revised the manuscript for intellectual content, approved the final version, and meet the ICMJE criteria for authorship. JW is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded internally by the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital. KB was supported by a career development grant from the National Institute on Aging (K01AG068365). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital for the submitted work. JJG previously received salary support from investigator initiated grants from Eli Lilly and Novartis Pharmaceutical awarded to the Brigham and Women’s Hospital for projects not related to the study. JJG was previously a consultant for Optum, was previously employed by Exponent, and is currently an employee of Johnson & Johnson and reports stock options (Johnson & Johnson), all unrelated to the study.
The lead author (JW) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results of the study will be disseminated through social media postings and press releases explaining the result to news media and general public.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was approved by the Brigham and Women’s Hospital IRB under the umbrella IRB approval that included a waiver for informed consent owing to the use of deidentified data (IRB# 2011P002580). Signed data license agreements were in place.
Data availability statement
No patient level data are available. The study protocol was registered with the centralized research tracking of the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital (#2011P002580-201). Study protocol is available from the corresponding author upon request.
References
- 1. Cowling T, Farrah K. Fluoroquinolones for the Treatment of Other Respiratory Tract Infections: A Review of Clinical Effectiveness. Cost-Effectiveness, and Guidelines, 2019. [Google Scholar]
- 2. Daneman N, Chateau D, Dahl M, et al. Canadian Network for Observational Drug Effect Studies (CNODES) Investigators . Fluoroquinolone use for uncomplicated urinary tract infections in women: a retrospective cohort study. Clin Microbiol Infect 2020;26:613-8. 10.1016/j.cmi.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 3. Vardakas KZ, Siempos II, Grammatikos A, Athanassa Z, Korbila IP, Falagas ME. Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials. CMAJ 2008;179:1269-77. 10.1503/cmaj.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015;60:1308-16. 10.1093/cid/civ076. [DOI] [PubMed] [Google Scholar]
- 5. Stahlmann R, Lode HM. Risks associated with the therapeutic use of fluoroquinolones. Expert Opin Drug Saf 2013;12:497-505. 10.1517/14740338.2013.796362. [DOI] [PubMed] [Google Scholar]
- 6. Mathews B, Thalody AA, Miraj SS, Kunhikatta V, Rao M, Saravu K. Adverse Effects of Fluoroquinolones: A Retrospective Cohort Study in a South Indian Tertiary Healthcare Facility. Antibiotics (Basel) 2019;8:E104. 10.3390/antibiotics8030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics. Published 26 July 2016. Accessed 13 October 2021.
- 8. Ahmed AI, van der Heijden FM, van den Berkmortel H, Kramers K. A man who wanted to commit suicide by hanging himself: an adverse effect of ciprofloxacin. Gen Hosp Psychiatry 2011;33:82.e5-7. 10.1016/j.genhosppsych.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9. Behera C, Krishna K, Singh HR. Antitubercular drug-induced violent suicide of a hospitalised patient. BMJ Case Rep 2014;2014:bcr2013201469. 10.1136/bcr-2013-201469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. LaSalvia EA, Domek GJ, Gitlin DF. Fluoroquinolone-induced suicidal ideation. Gen Hosp Psychiatry 2010;32:108-10. 10.1016/j.genhosppsych.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 11. Labay-Kamara U, Manning S, McMahon T. Fluoroquinolone-induced suicidal ideation and suicidality. Psychosomatics 2012;53:97-8. 10.1016/j.psym.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 12. Kommalapati A, Wallam S, Tella SH, Qureshi ZP, Bennett CL. Fluoroquinolone-associated suicide. Eur J Intern Med 2018;55:e21-2. 10.1016/j.ejim.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 13. Samyde J, Petit P, Hillaire-Buys D, Faillie JL. Quinolone antibiotics and suicidal behavior: analysis of the World Health Organization’s adverse drug reactions database and discussion of potential mechanisms. Psychopharmacology (Berl) 2016;233:2503-11. 10.1007/s00213-016-4300-3. [DOI] [PubMed] [Google Scholar]
- 14. Kandasamy A, Srinath D. Levofloxacin-induced acute anxiety and insomnia. J Neurosci Rural Pract 2012;3:212-4. 10.4103/0976-3147.98256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scavone C, Mascolo A, Ruggiero R, et al. Quinolones-Induced Musculoskeletal, Neurological, and Psychiatric ADRs: A Pharmacovigilance Study Based on Data From the Italian Spontaneous Reporting System. Front Pharmacol 2020;11:428. 10.3389/fphar.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gopalakrishnan C, Bykov K, Fischer MA, Connolly JG, Gagne JJ, Fralick M. Association of Fluoroquinolones With the Risk of Aortic Aneurysm or Aortic Dissection. JAMA Intern Med 2020;180:1596-605. 10.1001/jamainternmed.2020.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta K, Hooton TM, Naber KG, et al. Infectious Diseases Society of America. European Society for Microbiology and Infectious Diseases . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52:e103-20. 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 18. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America. American Thoracic Society . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44(Suppl 2):S27-72. 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healthcare Cost and Utilization Project. Suicidal Ideation, Suicide Attempt, or Self-Inflicted Harm: Pediatric Emergency Department Visits, 2010-2014 and 2016. https://www.hcup-us.ahrq.gov/reports/ataglance/findingsataglance.jsp. Published 5 November 2019. Accessed 13 October 2021.
- 20. Wilson HL, Daveson K, Del Mar CB. Optimal antimicrobial duration for common bacterial infections. Aust Prescr 2019;42:5-9. 10.18773/austprescr.2019.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011;64:749-59. 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci 2018;73:980-7. 10.1093/gerona/glx229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512-22. 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kline KA, Bowdish DM. Infection in an aging population. Curr Opin Microbiol 2016;29:63-7. 10.1016/j.mib.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 26. Shah A. The relationship between suicide rates and age: an analysis of multinational data from the World Health Organization. Int Psychogeriatr 2007;19:1141-52. 10.1017/S1041610207005285. [DOI] [PubMed] [Google Scholar]
- 27. Vázquez-Martínez ER, García-Gómez E, Camacho-Arroyo I, González-Pedrajo B. Sexual dimorphism in bacterial infections. Biol Sex Differ 2018;9:27. 10.1186/s13293-018-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aetion. Aetion evidence platform. Software for real-world data analysis. 2020. Accessed May 24, 2022. http://aetion.com
- 29. Curtin SC, Warner M, Hedegaard H. Increase in Suicide in the United States, 1999-2014. NCHS Data Brief 2016;(241):1-8. [PubMed] [Google Scholar]
- 30. Jick SS, Vasilakis C, Martinez C, Jick H. A study of the relation of exposure to quinolones and suicidal behaviour. Br J Clin Pharmacol 1998;45:77-81. 10.1046/j.1365-2125.1998.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faillie JL. Case-non-case studies: Principle, methods, bias and interpretation. Therapie 2019;74:225-32. 10.1016/j.therap.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 32. Franklin JM, Schneeweiss S. When and How Can Real World Data Analyses Substitute for Randomized Controlled Trials? Clin Pharmacol Ther 2017;102:924-33. 10.1002/cpt.857. [DOI] [PubMed] [Google Scholar]
- 33. Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol 2016;183:758-64. 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suissa S, Dell’Aniello S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2020;29:1101-10. 10.1002/pds.5083. [DOI] [PubMed] [Google Scholar]
- 35. Funk MJ, Landi SN. Misclassification in administrative claims data: quantifying the impact on treatment effect estimates. Curr Epidemiol Rep 2014;1:175-85. 10.1007/s40471-014-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomé AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf 2011;34:465-88. 10.2165/11587280-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables 1-14 and figure
Data Availability Statement
No patient level data are available. The study protocol was registered with the centralized research tracking of the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital (#2011P002580-201). Study protocol is available from the corresponding author upon request.