Abstract
With the high prevalence of coronavirus disease-2019 (COVID-19), there has been increasing understanding of the pathologic changes associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This review summarizes the pathologic changes in the digestive system and liver associated with COVID-19, including the injuries induced by SARS-CoV2 infection of GI epithelial cells and the systemic immune responses. The common digestive manifestations associated with COVID-19 include anorexia, nausea, vomiting, and diarrhea; the clearance of the viruses in COVID-19 patients with digestive symptoms is usually delayed. COVID-19-associated gastrointestinal histopathology is characterized by mucosal damage and lymphocytic infiltration. The most common hepatic changes are steatosis, mild lobular and portal inflammation, congestion/sinusoidal dilatation, lobular necrosis, and cholestasis.
Keywords: COVID-19, Pathologic characteristics, SARS-CoV-2, Lymphocytic infiltration
Key points
-
•
The common digestive manifestations associated with coronavirus disease-2019 (COVID-19) include anorexia, nausea, vomiting, and diarrhea; the clearance of the viruses in COVID-19 patients with digestive symptoms is usually delayed.
-
•
COVID-19-associated gastrointestinal histopathology is characterized by mucosal damage and lymphocytic infiltration.
-
•
The most common hepatic changes are steatosis, mild lobular and portal inflammation, congestion/sinusoidal dilatation, lobular necrosis, and cholestasis.
Introduction
With the high prevalence of coronavirus disease-2019 (COVID-19), there has been increasing understanding of the pathologic changes associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus can infect multiple organs and cause multiorgan symptoms, causing a wide range of clinical manifestations,1 including respiratory, cardiovascular, gastrointestinal (GI), and neurologic symptoms (including loss of smell and taste),2 , 3 as well as skin manifestations4 (erythema and papules). A meta-analysis has shown that 17.6% of patients with COVID-19 have GI symptoms, and that viral RNA is detected in stool samples in 48.1% of patients.5 Neglecting GI symptoms may sometimes delay a timely diagnosis and may permit the unchecked fecal-oral transmission of the virus. Ulcerative lesions occur in the GI tract in some patients, but only a few studies have described the histopathology of these lesions.6 In addition, hepatic injury is a frequent complication of COVID-19 and is associated with the severity of the disease. Studies in patients with COVID-19 have shown the incidence of liver injury ranges from 14.8% to 62%, usually indicated by abnormal alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels accompanied by slightly elevated bilirubin levels.7, 8, 9, 10 In fatal cases, the incidence of liver injury may reach up to 58% to 78%.7 Pathologic findings in the GI tract and liver come mostly from autopsies or postmortem biopsies but may include pathologic examination of GI biopsies obtained premortem by GI endoscopy. This review summarizes the pathologic changes in the digestive system and liver associated with COVID-19, including the injuries induced by SARS-CoV2 infection of GI epithelial cells and the systemic immune responses.
Esophageal Pathology
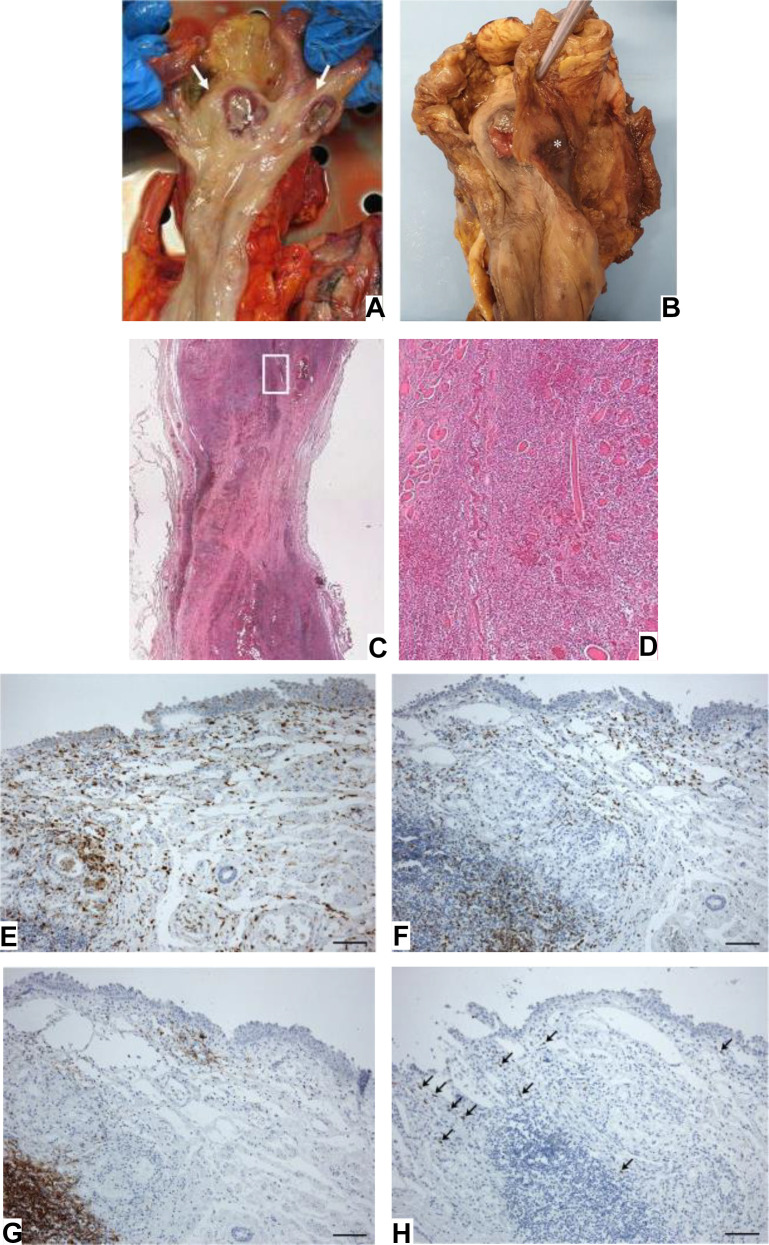
Although the clinical manifestations of COVID-19 are usually dominated by respiratory symptoms, some patients may lack symptoms and imaging features of COVID-19 pneumonia but only show GI symptoms.11 SARS-CoV-2 infection may lead to esophageal mucosal injury, with acute esophagus necrosis (AEN) occurring in critically ill patients.12 Two case reports have shown esophageal bleeding and multiple round herpetic-like erosions and ulcers by endoscopy in patients with GI symptoms, and SARS-CoV-2 RNA was detected in these esophageal lesions.12 , 13 At the autopsy, two necrotic ulcers were detected at the hypopharynx (Fig. 1 A, B). Histopathology showed full-thickness inflammatory cell infiltration with thinning of the pharyngeal wall at the level of the ulcer center (Fig. 1C, D).6 Meanwhile, in the presence of cells positive for SARS-CoV-2 spike protein subunit 1, histologic examination showed moderate lymphocytic infiltration in the esophageal mucosa (Fig. 1E–H),6 consistent with the histopathological features of viral esophagitis.
Fig. 1.
Macroscopic examination of fresh (A) and fixed (B) hypopharynx, with two necrotic ulcer (white arrows in A). (C) Histopathology of ulcer in hypopharynx. (D) Inflammatory infiltration of the muscle layer with necrosis and degeneration of the skeletal muscle fibers (E–H). Moderate lympho-monocytic infiltration in esophageal mucosa (E––anti-CD68; F––anti-CD3; G––anti-CD20; H––positive for SARS-CoV-2 spike subunit 1, black arrows).
(Porzionato A, Stocco E, Emmi A, et al. Hypopharyngeal Ulcers in COVID-19: Histopathological and Virological Analyses - A Case Report. Frontiers in immunology. 2021;12:676828. https://doi.org/10.3389/fimmu.2021.676828)
Gastric and Intestinal Pathology
The incidence of GI symptoms in patients with COVID-19 is shown in Table 1 .
Table 1.
Incidence of gastrointestinal symptoms in patients with coronavirus disease-2019
| Studies | Number of Patients, n | GI Symptoms, n (%) | Anorexia, n (%) | Nausea, n (%) | Vomiting, n (%) | Diarrhea, n (%) | Abdominal Pain, n (%) | Virus RNA in Stool (+),n (%) |
|---|---|---|---|---|---|---|---|---|
| Xiao et al,19 2020 | 73 | NA | NA | NA | NA | NA | NA | 39(53.4) |
| Nobel et al,17 2020 | 278 | 97(34.9) | NA | 63(64.9) | 56(57.7) | NA | NA | |
| Luo et al,68 2020 | 1141 | 183(16.0) | 180(98.4) | 134(73.2) | 119(65.0) | 68(37.2) | 45(24.6) | NA |
| Hunt et al,33 2021 | 206 | 48(23.3) | NA | NA | NA | 67(32.5) | NA | NA |
| Cheung et al,5 2020 | 59 | 15(25.4) | NA | NA | 1(6.7) | 13(86.7) | 7(46.7) | 9(60.0) |
| Pan et al,69 2020 | 204 | 103(50.5) | 81(78.6) | NA | 4(3.9) | 35(34.0) | 2(1.9) | NA |
| Jin et al,15 2019 | 651 | 74(11.4) | NA | 17(23.0) | 18(24.3) | 56(75.7) | NA | NA |
| Wang et al,70 2020 | 138 | NA | NA | 14(10.1) | 5(3.6) | 14(10.1) | 3(2.2) | NA |
| Ferm et al,71 2020 | 892 | 219(24.6) | 105(11.8) | 148(16.6) | 91(10.2) | 177(19.8) | 70(7.8) | NA |
Abbreviation: NA, not available.
The appearance of GI symptoms in patients with COVID-19 seems to indicate disease progression, as GI symptoms are more common in severe and critically ill patients, and are associated with an increased risk of adverse outcomes.14, 15, 16 Interestingly, other case-control studies had previously shown that the presence of GI symptoms was associated with longer illness duration, a trend toward lower ICU admissions, and lower mortality,17 and the presence of GI symptoms could predict reduced disease severity and mortality.18 The presence of SARS-CoV-2 RNA in feces is related to GI symptoms. Fecal shedding of viral RNA suggests prolonged GI infection.19 In addition, the virus may persist in the GI tract after it was cleared from the respiratory tract.19
A multicenter study showed that ulcers were the most common lesions observed in upper GI endoscopy in patients with COVID-19, with the lesions sometimes accompanied by active bleeding.20 Bhayana and colleagues21 retrospectively analyzed the abdominal imaging findings of 412 patients with COVID-19, and a variety of abnormalities were observed. Bowel-wall abnormalities were found on 13 computed tomography (CT) images (31%), which were associated with intensive care unit (ICU) admission. Pneumatosis or portal venous gas was observed in four abdominal CT images obtained in patients in the ICU. Unusual yellow discoloration of the bowel was observed in three cases and bowel infraction in two cases. Pathologic examinations revealed ischemic enteritis, with patchy necrosis and fibrin thrombi in arterioles. Amarapurkar and colleagues22 also reported a case of hemorrhagic enteritis associated with COVID-19. Histopathology revealed extensive transmural hemorrhages with many congested and dilated blood vessels, and fibrin thrombi were occasionally observed in capillaries.
The GI pathology of SARS-CoV-2 infection had been verified in autopsy and biopsy studies. Liu and colleagues23 observed alternating segmental dilatations and stenoses of the small bowel at the autopsy of a patient with COVID-19, associated with SARS-CoV-2 replication in GI mucosa.19 , 20 Another report described GI alterations in patients with COVID-19 as characterized by lymphoplasmacytic infiltration in the lamina propria of the GI tract.19 Coagulative necrosis, micro-hemorrhages, microthrombi, and vascular congestion had been found in the colonic mucosa, suggesting ischemia is one mechanism of injury. Such lesions have been found to be positive for COVID-19 by immunohistochemistry.20 Duodenitis may also occur in critically ill patients with COVID-19, with endoscopic manifestations of diffuse bleeding, mucosal edema, and severe inflammation with erosions. Intracytoplasmic and intranuclear inclusions consistent with a viral infection were identified in duodenal crypts.24
Angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine type 2 (TMPRSS2) receptors for SARS-CoV-2, are expressed in GI mucosa.25 , 26 Experimental studies have shown human gastric organoids are susceptible to SARS-CoV-2 infection.27 In addition, as both ACE2 and TMPRSS2 are expressed in the enteric nervous system, gut sensory-motor functions may be affected in susceptible patients with COVID-19.28
Pancreatic Pathology
SARS-CoV-2 receptors, including ACE2, TMPRSS2, NRP1,29 , 30 and TFRC,29 are expressed at very low levels in pancreatic β-cells; studies showed SARS-CoV-2 tropism for β cells in vitro.31 SARS-CoV-2 infection has been shown to suppress insulin secretion and injure β cells ex vivo, eventually causing pancreatic dysfunction,31 which leads to infection-related diabetes.32 Among patients hospitalized with COVID-19, the prevalence of acute pancreatitis is 0.27%. COVID-19-associated acute pancreatitis is more frequently associated with severe systemic disease and multi-organ complications.33
Liver Pathology
SARS-CoV-2 can cause hepatic injury via direct binding to ACE2 receptors in cholangiocytes and hepatocytes, antibody dependent enhancement of infection, systemic inflammatory response syndrome, inflammatory cytokine storms, ischemia/reperfusion injury, and adverse events due to drug therapy.9 , 34, 35, 36, 37
Findings in autopsies or postmortem biopsies of patients with coronavirus disease-2019
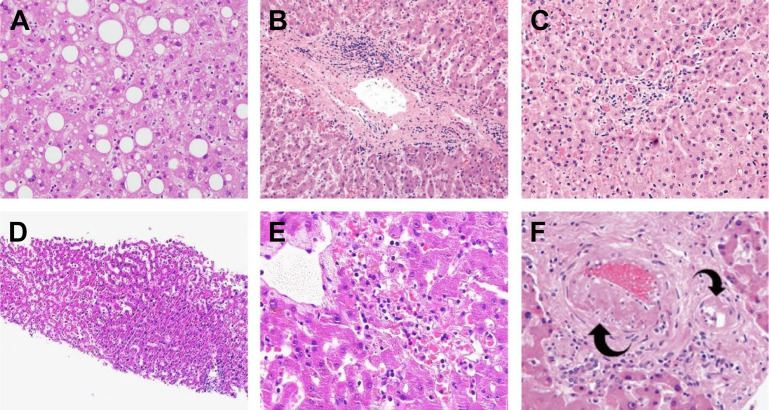
The main liver findings in patients with COVID-19 are shown in Table 2 and are illustrated in Fig. 2 A–E. The most common histopathological changes associated with SARS-CoV-2 are hepatic steatosis, mild lobular and portal inflammation, congestion/sinusoidal dilatation, lobular necrosis, and cholestasis.38 , 39
Table 2.
Summary of main hepatic findings in patients with coronavirus disease-2019
| Ref. | No. Cases | Specimen Type | Steatosis | Portal Inflammation | Lobular Inflammation | Congestion/Sinusoidal Dilation | Lobular Necrosis | Cholestasis | Hepatocyte Apoptosis | Vascular Pathology and/or Thrombosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Greuel et al,40 2021 | 6 | Autopsies | 2/6 (33.3%) | – | – | – | – | 1/6 (16.7%) | – | – |
| Xu et al,44 2020 | 1 | Postmortem biopsy | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | – | – | – | – | – |
| Tian et al,50 2020 | 4 | Postmortem biopsies | 1/4 (25%) | – | 1/4 (25%) | 3/4 (75%) | 1/4 (25%) | – | – | – |
| Wang et al,8 2020 | 2 | Postmortem biopsies | 2/2 (100%) | 2/2 (100%) | 1/2 (50%) | – | – | – | 2/2 (100%) | – |
| Sonzogni et al,45 2020 | 48 | Postmortem biopsies | 26/48 (54.2%) | 32/48 (66.7%) | 24/48 (50%) | – | 18/48 (37.5%) | – | – | 48/48 (100%) |
| Cai et al,9 2020 | 1 | Postmortem biopsy | 1/1 (100%) | – | 1/1 (100%) | – | – | – | – | – |
| McConnell et al,54 2021 | 43 | Postmortem biopsies | 20/43 (46.5%) | 10/43 (23.3%) | – | 42/43 (97.7%) | – | – | – | – |
| Beigmohammadi et al,47 2021 | 7 | Postmortem biopsies | 7/7 (100%) | 7/7 (100%) | – | 7/7 (100%) | 1/7 (14.3%) | 2/7 (28.6%) | – | – |
| Lagana et al,46 2020, | 40 | Autopsies | 30/40 (75%) | 20/40 (50%) | – | – | 20/40 (50%) | 15/40 (37.5%) | 10/40 (25%) | 6/40 (15%) |
| Yurdaisik et al,49 2021 | 7 | Postmortem biopsies | 4/7 (57.1%) | 2/7 (28.6%) | 5/7 (71.4%) | 1/7 (14.3%) | 6/7 (85.7%) | 2/7 (28.6%) | – | 1/7 (14.3%) |
| Ramos-Rincon et al,52 2022 | 5 | Postmortem biopsies | 1/2 (50%) | – | – | – | 1/5 (20%) | 1/5 (20%) | – | – |
| Barton et al,41 2020 | 2 | Autopsies | 1/2 (50%) | – | – | – | – | – | – | – |
| Zhao et al,43 2021 | 17 | Autopsies | 12/17 (70.6%) | 8/17 (47.1%) | 5/17 (29.4%) | – | 2/17 (11.8%) | – | – | – |
| Bradley et al,48 2020 | 14 | Autopsies | 9/14 (64.3%) | 4/14 (28.6%) | 1/14 (7.1%) | 11/14 (78.6%) | 4/14 (28.6%) | – | – | – |
| Wang XX et al,51 2021 | 1 | Postmortem biopsy | 1/1 (100%) | – | 1/1 (100%) | – | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | – |
| Chornenkyy et al,55 2021 | 8 | Autopsies | 4/8 (50%) | 7/8 (87.5%) | 6/8 (75%) | 6/8 (75%) | 4/8 (50%) | 1/8 (12.5%) | – | – |
| Falasca et al,58 2020 | 22 | Autopsies | 12/22 (54.5%) | – | 11/22 (50%) | 10/22 (45.5%) | – | – | – | – |
| Fassan et al,56 2021 | 25 | Autopsies | 9/25 (36%) | – | – | 21/24 (87.5%) | 2/25 (8%) | – | – | 3/25 (12%) |
| 3 | Liver biopsies | 2/3 (66.7%) | 2/3 (66.7%) | 1/3 (33.3%) | – | 1/3 (33.3%) | – | – | – | |
| Fraga et al,59 2020 | 1 | Liver biopsy | – | 1/1 (100%) | – | – | – | – | 1/1 (100%) | – |
| Fiel et al,57 2021 | 2 | Liver biopsies | – | 2/2 (100%) | 1/2 (50%) | – | 2/2 (100%) | – | 1/2 (50%) | – |
−, finding was not described or found.
Fig. 2.
Histology of liver changes in patients with COVID-19. (A) Steatosis. (B) Mild portal activity. (C) Mild lobular activity. (D) Mild sinusoidal dilatation with increased lymphocytic infiltration. (E) Focal centrilobular hepatic necrosis. (F) Portal arteriolar muscular hyperplasia (left arrow) and hyalinosis of a smaller branch of portal arteriole (right arrow).
([A] Zhao CL, Rapkiewicz A, Maghsoodi-Deerwester M, et al. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19). Human pathology. Mar 2021;109:59-68. https://doi.org/10.1016/j.humpath.2020.11.015 (Ref. 43); [B, C] Chornenkyy Y, Mejia-Bautista M, Brucal M, et al. Liver Pathology and SARS-CoV-2 Detection in Formalin-Fixed Tissue of Patients With COVID-19. American journal of clinical pathology. May 18 2021;155(6):802-814. https://doi.org/10.1093/ajcp/aqab009 (Ref. 55); [D, E] Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. Jun 2020;33(6):1007-1014. https://doi.org/10.1038/s41379-020-0536-x (Ref. 50); [F] Lagana SM, Kudose S, Iuga AC, et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. Nov 2020;33(11):2147-2155. https://doi.org/10.3389/fimmu.2021.676828)
In several autopsy studies, hepatic steatosis of variable severity was the main findings,40, 41, 42 which may be related to high BMI, as well as hypoxia and shock induced by COVID-19-related complications. It is well documented that shock and hypoxia can lead to lipid accumulation in hepatocytes and cause liver injury.43 Postmortem liver biopsy examination carried out by Xu and colleagues44 showed moderate microvesicular steatosis and mild lobular and portal activity, indicating the injury could have been caused by either SARS-CoV-2 infection or drug-induced liver injury. In another study of 48 postmortem liver biopsies performed on patients with COVID-19, the histologic assessment also revealed microvesicular and macrovesicular steatosis (54%), mild portal inflammation (66%), and lobular inflammation (50%).45 The same histologic findings were described in another study of 17 patients.43 In a study of 40 autopsies, Lagana and colleagues46 described gross findings of hepatic fibrosis in two patients. Histologically, macrovesicular steatosis was the most common finding, involving 30 patients (75%). Mild lobular necroinflammation and portal inflammation were present in 20 cases (50%) each.
In studies by Beigmohammadi and colleagues47 and Bradley and colleagues,48 congestion, steatosis and minimal-to-mild portal inflammation were the most common findings, whereas lobular inflammation was not prominent. Conversely, Yurdaisik and colleagues49 observed lobular inflammation in most cases. Tian and colleagues50 reported mild sinusoidal dilatation and focal macrovesicular steatosis in postmortem liver biopsies of four patients. There was mild lobular lymphocytic infiltration, which was insignificant in portal areas; the same findings were reported in another pathologic study.9 , 51
In addition, patchy hepatic necrosis has been described in postmortem liver biopsies and autopsies,43 , 48, 49, 50, 51, 52, 53 mainly in centrilobular areas (zone 3) and without evident inflammatory cellular infiltration. This pattern is consistent with acute ischemic injury. More severe changes such as confluent necrosis45 , 47 and coagulative necrosis51 were observed in rare cases.
Other histopathological changes frequently described in patients with COVID-19 include the proliferation of the intrahepatic bile ducts and the presence of intra-canalicular bile plugs, consistent with cholestasis.40 , 49 , 51, 52, 53 In fact, 38% of patients were shown to have lobular cholestasis among 40 autopsied cases, which were generally mild and focal.46 Four (10%) of these patients had ductular cholestasis.46 However, bile duct injury has not been observed.43
In postmortem wedge liver biopsies of 48 patients, Sonzogni and colleagues45 noted alterations of vascular structures, both acute (thrombosis of portal and sinusoidal vessels, luminal ectasia) and chronic (fibrous thickening of vascular wall or phlebosclerosis, and abnormalities of the portal intrahepatic vasculature). Lagana and colleagues46 reported similar changes, such as phlebosclerosis and sinusoidal microthrombi in six cases (15%).43 Portal arterioles were abnormal (Fig. 2F) in nine cases (22.5%), including arteriolar muscular hyperplasia, hyalinosis of the vessel wall, and fibrinoid necrosis with endothelial apoptosis. These findings strongly suggest marked derangement of the intrahepatic blood vessel network secondary to systemic changes induced by the viral infection.
Other uncommon histologic changes include histiocytic hyperplasia in the portal tract,43 platelet-fibrin microthrombi in the hepatic sinusoids, central vein, or portal vein, and rare megakaryocytes in sinusoids.43 Minor to massive hepatocytic apoptosis,8 , 51 and mild ballooning degeneration9 , 40 , 46 , 47 , 54 have been described as well. Presence of SARS- CoV-2 in hepatocytes has been confirmed by in situ hybridization or RT-PCR.45 , 46 , 50 , 52 , 55, 56, 57
Liver pathology of patients with coronavirus disease-2019 in controlled studies
To further delineate the role of pre-existing conditions, Falasca and colleagues58 showed in 22 COVID-19 autopsies (18 with comorbidities and 4 without comorbidities), that the incidence of macroscopic parenchyma congestion, histologic sinusoidal congestion, steatosis, and inflammatory infiltrate were similar between the two groups.58
In another postmortem study, patients with COVID-19 (n = 8) were compared with controls (n = 4). Minimal to focal mild portal tract chronic inflammation (P < 0.05) and mild focal lobular activity (P = 0.06) were more frequently observed in COVID patients.46
McConnell and colleagues54 compared postmortem liver biopsies between 43 patients with COVID-19 versus normal controls (n = 12). Dilated sinusoids with congestion (P < 0.01), lobular inflammation (P < 0.01), steatosis (P = 0.02), and sinusoidal erythrocyte aggregation (P < 0.01) were more frequently observed in patients with COVID-19.
Pathology of liver biopsies in living patients with coronavirus disease-2019
Although findings at autopsy are often “contaminated” by terminal iatrogenic changes, liver biopsies performed in patient's premortem likely present more specific pathologic findings. Such findings include mild portal inflammation, scattered hepatocyte apoptosis, ground-glass hepatocytes consistent with cytoplasmic accumulation of fibrinogen,59 activation of Kupffer cells, and steatosis.56 In another study of 2 patients without significant lung disease, acute hepatitis, prominent bile duct damage, foci of centrilobular necrosis, and endothelitis were identified, although some of these changes may be due to post-transplant changes in one of the patients.57
Liver pathology in patients with underlying chronic liver diseases
Overall, 2% to 11% of patients with COVID-19 had underlying chronic liver disease.37 Fatty liver disease or non-alcoholic steatohepatitis accounted for 42% of COVID-19 patients with preexisting liver diseases.60 Hepatic dysfunction was significantly higher in patients with preexisting liver disease, especially in patients with cirrhosis and this was associated with poor outcomes.60
In a study of 202 consecutive patients with COVID-19,61 patients with NAFLD had a higher risk of disease progression (P < 0.0001) and longer viral shedding (P < 0.0001) than those without NAFLD. Postmortem liver biopsies in one of these patients showed microvesicular steatosis with overactivation of T cells. However, other autopsy and biopsy studies only showed histologic findings consistent with shock liver62 or the preexisting liver disease.50
Liver pathology in patients after vaccination
Hepatitis has been observed in some individuals after vaccination, that share some histologic features with autoimmune liver disease63 , 64; some contain diffusely distributed highly activated T cells.65 Moreover, among the infiltrating T cells, there is an enrichment of T cells that are reactive to SARS-CoV-2, suggesting that the vaccine-induced cells can contribute to hepatic inflammation. In a cohort of 16 patients who presented with hepatic dysfunction after vaccination, 10 underwent liver biopsy. All showed portal inflammation (60% of which was graded as moderate or severe).66
In a case report of an 86-year-old man who died of acute renal and respiratory failure after receiving the first dose of the BNT162b2 mRNA COVID-19 vaccine, the autopsy showed stenosis and sinus dilatation in the liver.67
In summary, the most common histologic changes associated with SARS-CoV-2 in the liver are steatosis, mild lobular and portal hepatitis, congestion with sinusoidal dilatation, lobular necrosis, and cholestasis. Hepatocyte apoptosis, vascular pathology with or without thrombosis, histiocytic hyperplasia, and Kupffer cells hyperplasia may also occur.
Clinics care points
-
•
GI symtoms due to involvement by COVID-19 are non-specific. Diagnosis may be facilitated by exclusion of other etiology and positive COVID test.
-
•
Pathologically GI involvement is mainly characterized by lymphocytic infiltration of the mucosa.
-
•
In patients with hepatic involvement, non-specific portal and/or lobular lymphocytic infiltration, mild steatosis, and rarely, spotty necrosis may be pathologic findings.
References
- 1.Aiyegbusi O.L., Hughes S.E., Turner G., et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–442. doi: 10.1177/01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullol J., Alobid I., Marino-Sanchez F., et al. The Loss of Smell and Taste in the COVID-19 Outbreak: a Tale of Many Countries. Curr Allergy asthma Rep. 2020;20(10):61. doi: 10.1007/s11882-020-00961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glezer I., Bruni-Cardoso A., Schechtman D., et al. Viral infection and smell loss: The case of COVID-19. J Neurochem. 2021;157(4):930–943. doi: 10.1111/jnc.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daneshgaran G., Dubin D.P., Gould D.J. Cutaneous Manifestations of COVID-19: An Evidence-Based Review. Am J Clin Dermatol. 2020;21(5):627–639. doi: 10.1007/s40257-020-00558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung K.S., Hung I.F.N., Chan P.P.Y., et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porzionato A., Stocco E., Emmi A., et al. Hypopharyngeal Ulcers in COVID-19: Histopathological and Virological Analyses - A Case Report. Front Immunol. 2021;12:676828. doi: 10.3389/fimmu.2021.676828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L., Liu J., Lu M., et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Liu S., Liu H., et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Q., Huang D., Yu H., et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajifathalian K., Krisko T., Mehta A., et al. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020;159(3):1137–1140 e2. doi: 10.1053/j.gastro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L., Jiang X., Zhang Z., et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 12.Deliwala S.S., Gurvits G.E. Acute Esophageal Necrosis in a Patient With COVID-19. Am J Gastroenterol. 2021;116(10):1977. doi: 10.14309/ajg.0000000000001207. [DOI] [PubMed] [Google Scholar]
- 13.Rahim F., Kapliyil Subramanian S., Larson S. Case Report of Acute Esophageal Necrosis (Gurvits Syndrome) in Vaccinated, COVID-19-Infected Patient. Cureus. 2022;14(2):e22241. doi: 10.7759/cureus.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S.Y., Lian J.S., Hu J.H., et al. Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang, China. Infect Dis poverty. 2020;9(1):85. doi: 10.1186/s40249-020-00710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin X., Lian J.S., Hu J.H., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M.M., Chen L.N., Qian J.M. Gastrointestinal manifestations and possible mechanisms of COVID-19 in different periods. J Dig Dis. 2021;22(12):683–694. doi: 10.1111/1751-2980.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobel Y.R., Phipps M., Zucker J., et al. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology. 2020;159(1):373–375 e2. doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livanos A.E., Jha D., Cossarini F., et al. Intestinal Host Response to SARS-CoV-2 Infection and COVID-19 Outcomes in Patients With Gastrointestinal Symptoms. Gastroenterology. 2021;160(7):2435–2450 e34. doi: 10.1053/j.gastro.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao F., Tang M., Zheng X., et al. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833 e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanella G., Capurso G., Burti C., et al. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: an international multicentre study. BMJ open Gastroenterol. 2021;8(1) doi: 10.1136/bmjgast-2020-000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhayana R., Som A., Li M.D., et al. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology. 2020;297(1):E207–E215. doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amarapurkar A.D., Vichare P., Pandya N., et al. Haemorrhagic enteritis and COVID-19: causality or coincidence. J Clin Pathol. 2020;73(10):686. doi: 10.1136/jclinpath-2020-206743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q., Wang R.S., Qu G.Q., et al. Gross examination report of a COVID-19 death autopsy. Fa yi xue za zhi. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Neuberger M., Jungbluth A., Irlbeck M., et al. Duodenal tropism of SARS-CoV-2 and clinical findings in critically ill COVID-19 patients. Infection. 2022 doi: 10.1007/s15010-022-01769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C., Xiao S.Y. COVID-19 and inflammatory bowel disease: A pathophysiological assessment. Biomed Pharmacother. 2021;135:111233. doi: 10.1016/j.biopha.2021.111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trougakos I.P., Stamatelopoulos K., Terpos E., et al. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J Biomed Sci. 2021;28(1):9. doi: 10.1186/s12929-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giobbe G.G., Bonfante F., Jones B.C., et al. SARS-CoV-2 infection and replication in human gastric organoids. Nat Commun. 2021;12(1):6610. doi: 10.1038/s41467-021-26762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marasco G., Lenti M.V., Cremon C., et al. Implications of SARS-CoV-2 infection for neurogastroenterology. Neurogastroenterol Motil. 2021;33(3):e14104. doi: 10.1111/nmo.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantuti-Castelvetri L., Ojha R., Pedro L.D., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly J.L., Simonetti B., Klein K., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C.T., Lidsky P.V., Xiao Y., et al. SARS-CoV-2 infects human pancreatic beta cells and elicits beta cell impairment. Cell Metab. 2021;33(8):1565–1576 e5. doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller J.A., Gross R., Conzelmann C., et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 33.Hunt R.H., East J.E., Lanas A., et al. COVID-19 and Gastrointestinal Disease: Implications for the Gastroenterologist. Dig Dis. 2021;39(2):119–139. doi: 10.1159/000512152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamed D.Z., Ghoneim M.E., Abu-Risha S.E., et al. Gastrointestinal and hepatic diseases during the COVID-19 pandemic: Manifestations, mechanism and management. World J Of Gastroenterol. 2021;27(28):4504–4535. doi: 10.3748/wjg.v27.i28.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei H.Y., Ding Y.H., Nie K., et al. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed Pharmacother. 2021;133:111064. doi: 10.1016/j.biopha.2020.111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Xiao S.Y. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92(9):1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 37.Jothimani D., Venugopal R., Abedin M.F., et al. COVID-19 and the liver. J Hepatol. 2020;73(5):1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zghal M., Bouhamed M., Mellouli M., et al. Liver injury in COVID-19: pathological findings. Pan Afr Med J. 2022;41:56. doi: 10.11604/pamj.2022.41.56.31114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreira J.L.S., Barbosa S.M.B., Vieira J.G., et al. Liver histopathological changes and COVID-19: What does literature have to tell us? Dig Liver Dis. 2022;54(3):296–298. doi: 10.1016/j.dld.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greuel S., Ihlow J., Dragomir M.P., et al. COVID-19: Autopsy findings in six patients between 26 and 46 years of age. Int J Infect Dis : IJID. 2021;108:274–281. doi: 10.1016/j.ijid.2021.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barton L.M., Duval E.J., Stroberg E., et al. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikhaleva L.M., Cherniaev A.L., Samsonova M.V., et al. Pathological Features in 100 Deceased Patients With COVID-19 in Correlation With Clinical and Laboratory Data. Pathol Oncol Res : POR. 2021;27:1609900. doi: 10.3389/pore.2021.1609900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao C.L., Rapkiewicz A., Maghsoodi-Deerwester M., et al. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19) Hum Pathol. 2021;109:59–68. doi: 10.1016/j.humpath.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonzogni A., Previtali G., Seghezzi M., et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40(9):2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lagana S.M., Kudose S., Iuga A.C., et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33(11):2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beigmohammadi M.T., Jahanbin B., Safaei M., et al. Pathological Findings of Postmortem Biopsies From Lung, Heart, and Liver of 7 Deceased COVID-19 Patients. Int J Surg Pathol. 2021;29(2):135–145. doi: 10.1177/1066896920935195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley B.T., Maioli H., Johnston R., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yurdaisik I., Demiroz A.S., Oz A.B., et al. Postmortem Biopsies of the Lung, Heart, Liver, and Spleen of COVID-19 Patients. Cureus. 2021;13(12):e20734. doi: 10.7759/cureus.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian S., Xiong Y., Liu H., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X.X., Shao C., Huang X.J., et al. Histopathological features of multiorgan percutaneous tissue core biopsy in patients with COVID-19. J Clin Pathol. 2021;74(8):522–527. doi: 10.1136/jclinpath-2020-206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos-Rincon J.M., Alenda C., Garcia-Sevila R., et al. Histopathological and virological features of lung, heart and liver percutaneous tissue core biopsy in patients with COVID-19: A clinicopathological case series. Malays J Pathol. 2022;44(1):83–92. [PubMed] [Google Scholar]
- 53.Yao X.H., Li T.Y., He Z.C., et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies] Chin J Pathol. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 54.McConnell M.J., Kawaguchi N., Kondo R., et al. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J Hepatol. 2021;75(3):647–658. doi: 10.1016/j.jhep.2021.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chornenkyy Y., Mejia-Bautista M., Brucal M., et al. Liver Pathology and SARS-CoV-2 Detection in Formalin-Fixed Tissue of Patients With COVID-19. Am J Clin Pathol. 2021;155(6):802–814. doi: 10.1093/ajcp/aqab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fassan M., Mescoli C., Sbaraglia M., et al. Liver histopathology in COVID-19 patients: A mono-Institutional series of liver biopsies and autopsy specimens. Pathol Res Pract. 2021;221:153451. doi: 10.1016/j.prp.2021.153451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fiel M.I., El Jamal S.M., Paniz-Mondolfi A., et al. Findings of Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Cell Mol Gastroenterol Hepatol. 2021;11(3):763–770. doi: 10.1016/j.jcmgh.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falasca L., Nardacci R., Colombo D., et al. Postmortem Findings in Italian Patients With COVID-19: A Descriptive Full Autopsy Study of Cases With and Without Comorbidities. J Infect Dis. 2020;222(11):1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraga M., Moradpour D., Artru F., et al. Hepatocellular type II fibrinogen inclusions in a patient with severe COVID-19 and hepatitis. J Hepatol. 2020;73(4):967–970. doi: 10.1016/j.jhep.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh S., Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159(2):768–771 e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji D., Qin E., Xu J., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73(2):451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bril F., Al Diffalha S., Dean M., et al. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan C.K., Wong Y.J., Wang L.M., et al. Autoimmune hepatitis following COVID-19 vaccination: True causality or mere association? J Hepatol. 2021;75(5):1250–1252. doi: 10.1016/j.jhep.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boettler T., Csernalabics B., Salie H., et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022;77(3):653–659. doi: 10.1016/j.jhep.2022.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shroff H., Satapathy S.K., Crawford J.M., et al. Liver injury following SARS-CoV-2 vaccination: A multicenter case series. J Hepatol. 2022;76(1):211–214. doi: 10.1016/j.jhep.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansen T., Titze U., Kulamadayil-Heidenreich N.S.A., et al. First case of postmortem study in a patient vaccinated against SARS-CoV-2. Int J Infect Dis. 2021;107:172–175. doi: 10.1016/j.ijid.2021.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo S., Zhang X., Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18(7):1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan L., Mu M., Yang P., et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D., Hu B., Hu C., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferm S., Fisher C., Pakala T., et al. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY. Clin Gastroenterol Hepatol. 2020;18(10):2378–2379 e1. doi: 10.1016/j.cgh.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]