Abstract
Keystone virus (KEYV) is an under-studied orthobunyavirus that is transmitted via both horizontal and vertical cycles involving various mosquito species and vertebrate hosts. Historical evidence indicates that KEYV causes sub-clinical infections in humans, and some case studies draw links between this virus and encephalitis. Given KEYV’s potential to cause human infections, it is plausible that it causes an under-appreciated proportion of both generic viral infections and unidentified viral encephalitis cases. This review summarizes current knowledge of KEYV and its disease dynamics in order to better understand the virus’ medical and economic burden on human populations.
Keywords: Keystone virus, KEYV, Encephalitis, Meningitis, Vector-borne virus
Graphical Abstract
1. Introduction
Meningitis is inflammation of the meninges, which line the brain and spinal cord, and encephalitis is inflammation of the brain itself. Both are potentially life-threatening medical conditions, although those due to viral etiologies tend to be less severe. In the United States, it is estimated that they collectively account for over 104,000 hospitalizations and nearly 4300 deaths per year (adjusted for 2020 census population) (George et al., 2014; Holmquist et al., 2008; QuickFacts, 2021), and many survivors experience long-lasting sequelae, which can be serious. The causes of meningitis and encephalitis are diverse (e.g., bacterial, viral, fungal) and often poorly characterized during diagnosis: in the U.S., 34.9 and 17.2% of encephalitis and meningitis cases, respectively, are not given an etiological classification, and an additional 12.1% of encephalitis cases and 54.6% of meningitis cases are caused by undetermined viruses. Keystone virus (KEYV) is a poorly-studied pathogen that plausibly infects many people and is related to several viruses known to cause meningitis and/or encephalitis, making it a potentially important and underappreciated cause of these diseases.
KEYV (order Bunyavirales, genus Orthobunyavirus) was named after the region near Tampa, Florida where it was discovered (Bond et al., 1966). It is a mosquito-borne RNA virus with a single-stranded tripartite genome, and is presently the only member of the species Keystone orthobunyavirus recognized by the International Committee on Taxonomy of Viruses (Abudurexiti et al., 2019). KEYV infects some mammals, including humans, in the southern and eastern U.S. (Parkin et al., 1972).
KEYV belongs to a group of orthobunyaviruses that was traditionally referred to as the California encephalitis serogroup, California serogroup, or simply the California group (Alatoom and Payne, 2009). Although this nomenclature is no longer commonly used, for convenience we will use it for the purposes of referring to this collection of similar viruses. Viruses that were included in this group, such as California encephalitis virus (CEV), Jamestown Canyon virus (JCV), and La Crosse virus (LACV), are known to cause fever and rash, and less commonly, but of greater concern, meningitis and encephalitis (Alatoom and Payne, 2009) (see Table 1 for summary of virus acronyms).
Table 1.
Relevant viruses and their acronyms.
| Virus | Acronym |
|---|---|
| California encephalitis virus | CEV |
| Jamestown Canyon virus | JCV |
| Keystone virus | KEYV |
| La Crosse virus | LACV |
| Trivitattus virus | TVTV |
There is also some evidence that KEYV can cause encephalitis. Serology-based surveillance between 1963–1964 detected two cases of encephalitis in children, one severe, that might be linked to KEYV (Bond et al., 1966). As part of that surveillance effort, researchers at the Encephalitis Research Center of the Florida State Board of Health performed serologic tests for hemagluttination-inhibition antibodies to an antigen associated with California group viruses. In both cases, they observed a four-fold increase in antibody titers, suggesting infection with a California group virus. Furthermore, complement-fixation antibodies were observed in one case, providing additional support for this hypothesis.
While a specific California group virus was not identified for these encephalitis cases, they occurred during the same time period in which KEYV was first identified in Florida mosquitoes (Bond et al., 1966). It is plausible that the infections were instead caused by Trivitattus virus (TVTV), another California group virus, which was also detected in Florida during the same time. However, there are two lines of evidence that make KEYV a more likely causative pathogen than TVTV. First, KEYV appears to have been more common in Florida mosquito populations than TVTV during that general time period (Taylor et al., 1971). Second, an analysis of patient antibody crossreactivity characterized the virus as more related to CEV than TVTV (Quick et al., 1965). Subsequent serum-neutralization experiments with rabbits comparing the serologic responses to CEV, KEYV, and TVTV infections found that KEYV antibodies were more reactive to CEV than TVTV antigens (Jennings et al., 1968). Thus, while no etiologic agent was identified, KEYV is a plausible cause alongside other California group viruses.
Taken together, these facts are suggestive: (1) many meningitis and encephalitis cases do not receive an etiologic diagnosis; (2) viruses related to KEYV can cause meningitis and encephalitis; and (3) KEYV is both capable of infecting a substantial fraction of the population and is typically undiagnosed. Assessing the public health impact of KEYV in Florida involves numerous questions about basic science, disease outcomes, and costs. As many of these questions are currently unanswered—and some even unasked—the first step in answering them is to identify what they are. Broadly speaking, they can be grouped into three general questions:
What are the basic transmission dynamics of KEYV, and how, if at all, are they currently changing?
How many people are infected with KEYV each year in Florida?
What are the possible outcomes or consequences of KEYV infections in humans?
In the following sections, we review the current state of scientific knowledge with regard to each of these questions. Ultimately, answering these questions will allow us to better understand how to control transmission of KEYV and to evaluate how the pathogen should be prioritized relative to other public health concerns.
This review’s focus on the state of Florida was motivated by a combination of factors. Serological studies have suggested that a significant fraction of Floridians may have been exposed in their lifetimes, and ecological evidence suggests that transmission in Florida may be elevated compared to other regions of the southeastern U.S. The observation that KEYV was first discovered in Florida and a recent, confirmed KEYV infection in the state additionally motivate this review’s scope.
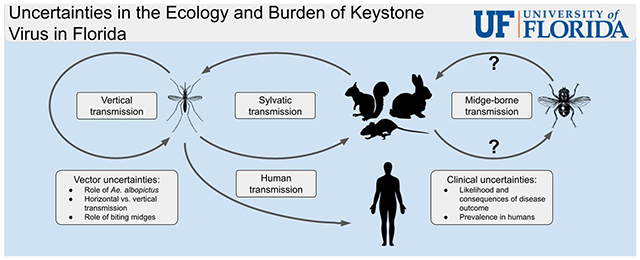
2. Transmission dynamics of KEYV
In order to understand the transmission dynamics of KEYV, we must first identify the virus’s mosquito vectors and mammalian hosts. We then need to understand the contributions of vertical versus horizontal transmission, e.g., how important are mosquito–mammal transmission cycles in sustaining sylvatic KEYV during periods of reduced mosquito populations? These questions provide the proper groundwork for understanding KEYV’s transmission dynamics, which allows for the exploration of potential interventions that disrupt these transmission cycles.
KEYV’s inclusion in the California group raises the possibility that its transmission dynamics share common features to those of other California group viruses, which also have been observed in mosquitoes and exhibit a mosquito–mammal host cycle. Because they are spread by mosquitoes, California group viruses fall into the broader category of arthropod-borne viruses, or arboviruses. In most cases, the mammals involved in transmission of California group viruses are small rodents or lagomorphs, although deer are the primary mammalian reservoir of JCV. Human-to-human transmission is not observed with better-understood California group viruses (e.g., LACV and JCV) (Transmission, 2019, 2018). Thus, because California group viruses share these basic transmission features, we expect similar features in the transmission dynamics of KEYV.
2.1. A note on mosquito taxonomy
The taxonomy of some relevant mosquito species has changed since KEYV was first described, and these revisions can make it difficult to interpret the relevant literature.
In 2000, the subgenus Ochlerotatus within the genus Aedes was elevated to be a genus in its own right. As a result of this reclassification, the mosquito species historically known as Ae. atlanticus now belongs to the genus Ochlerotatus (Reinert et al., 2000, 2004, 2008). Some sources identify the mosquito species historically known as Ae. albopictus as a member of this genus, others as a member of the subgenus Stegomyia within Aedes, and others still regard Stegomyia as a full genus in its own right (Reinert et al., 2004).
Because of historical usage, it is still very common to see these species, and other species that were formerly classified as Aedes—or whose classification in Aedes is currently a matter of debate—referred to as Aedes, even by those who accept the reclassifications. For this reason, in this paper, we will refer to these mosquitoes as Aedes species.
The mosquito species Ae. atlanticus and Ae. tormentor are extremely difficult to distinguish as adults, are closely related and biologically similar, and have similar ranges (Burkett-Cadena, 2013). They have historically been grouped together in mosquito surveys, sometimes described as Aedes atlanticus tormentor or atlanticus–tormentor (Bond et al., 1966). Other research may distinguish between the two, or may be conducted in areas where only one is present. For the purpose of this review, we will not distinguish between these cases; for the sake of brevity, we use Ae. atlanticusto encompass both species.
The mosquito species Ae. infirmatus is less similar to Ae. atlanticus and Ae. tormentor than they are to each other, but is still similar enough to be occasionally combined into one atlanticus–tormentor–infirmatus group when fine distinctions between the three are not important. In the case of KEYV, however, the distinction appears to be important, with Ae. infirmatus being an important factor in TVTV transmission cycles (Taylor et al., 1971). Therefore, we maintain this distinction.
2.2. KEYV hosts
KEYV was first isolated from mosquitoes found in 1962 in the Tampa Bay area during a St. Louis Encephalitis epidemic (Chamberlain et al., 1964; Taylor et al., 1971), and was first identified as a distinct strain in the California group in 1964 (Bond et al., 1966). It is primarily transmitted by the day-biting floodwater mosquito Ae. atlanticus. Ae. atlanticus has a narrow window of population activity during the summer months, tending to occupy shaded forests and nearby fields in the southeastern United States. As with other floodwater mosquitoes, population levels increase with heavy rainfall (Fig. 1) (LeDuc et al., 1975a; Roberts and Scanlon, 1975). Ae. infirmatus, another floodwater mosquito, appears to play a lesser, but perhaps still important, role in transmission (Taylor et al., 1971). The prevalence of KEYV in female Ae. atlanticus has consistently been found to be approximately 0.003 across several surveys conducted in various locations between 1969 and 1988 (Watts et al., 1988; Taylor et al., 1971; LeDuc et al., 1975a; Chamberlain et al., 1969). While human biting is known for Ae. atlanticus and Ae. albopictus, their contact patterns with humans do not appear to have been characterized.
Fig. 1.
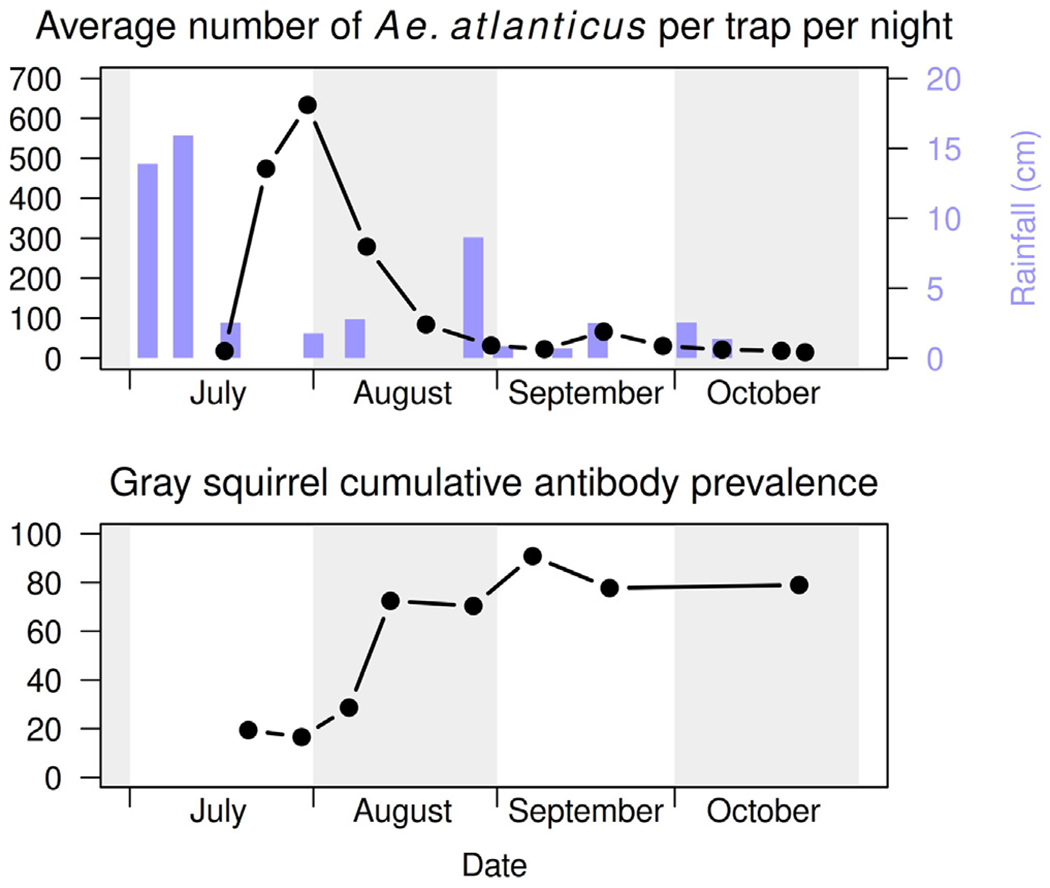
Ae. atlanticus abundance and rainfall (above). Cumulative KEYV antibody prevalence in gray squirrels (below). All data collected in the Pokomoke Cypress Swamp in 1975.
Source: Reproduction of Fig. 1 from Watts et al. (1988) using WebPlotDigitizer (Watts et al., 1988; Rohatgi, 2021).
Ae. albopictus, the Asian tiger mosquito, is an invasive species that has been spreading in the continental U.S. since its introduction in 1985 (Sprenger and Wuithiranyagool, 1986). It is more closely associated with humans than Ae. atlanticus, has a larger range, and is present for a greater portion of the year. Most of the work on prevalence of KEYV infection in different mosquito species was done before the arrival and spread of Ae. albopictus. This raises questions of whether it could play a role in increasing the enzootic range of KEYV and the frequency with which humans in that range are infected.
Multiple studies have since made it clear that Ae. albopictus can be infected with KEYV and can transmit KEYV vertically to offspring (Mitchell, 1991; Mitchell et al., 1996, 1992; Tesh, 1980). The role of Ae. albopictus in horizontal transmission is less clear, however. Grimstad et al. (1989) has been cited as providing evidence that Ae. albopictus is at most very poorly capable of transmitting KEYV to vertebrates, but similar experiments have also characterized Ae. atlanticus as transmitting KEYV poorly (Watts et al., 1988), despite the latter being established as a competent vector. These results suggest that certain experimental protocols may be inadequate for assessing vector competence.
As discussed further in Section 2.3, the transmission rate from horizontally-infected Ae. atlanticus is likely lower than the transmission rate from vertically-infected Ae. atlanticus. Further research is needed to quantify differences in the transmission potential between vertically- and horizontally-infected Ae. albopictus.
The greatest body of research on both vertebrate hosts and transmission dynamics of KEYV was done in the Pokomoke Cypress Swamp in Maryland, in the 1970s and 1980s (Watts et al., 1988). There, the primary vertebrate hosts of KEYV are rabbits and gray squirrels. In gray squirrels, cumulative measured antibody prevalence is approximately 40%–65% during a year and is documented to increase following the emergence of mosquito vectors (Watts et al., 1988, 1982). A smaller body of research conducted in Florida similarly supports a major role for rabbits and gray squirrels in KEYV transmission cycles (Jennings et al., 1970, 1968; Taylor et al., 1971). In addition, there is some evidence that cotton rats may also be important hosts in Florida (Jennings et al., 1970; Taylor et al., 1971); however, cotton rats are not found as far north as the Pokomoke Swamp and were therefore not studied (Watts et al., 1982). In all of these primary hosts, KEYV appears to produce a 1–5 day viremia (Watts et al., 1988; Jennings et al., 1968).
A number of other hosts including whitetail deer, horses, cattle, and dogs show a high seroprevalence of neutralizing antibodies when exposed to KEYV and do not appear to develop detectable viremia. Therefore, they are unlikely to play a significant role in KEYV transmission dynamics (Parkin, 1973; Watts et al., 1982, 1979), but they are still potentially useful sentinels for surveillance (Parkin, 1973).
Additionally, there is evidence that biting midges could potentially be a significantly underappreciated vector. Belonging to the genus Culicoides, these biting midges have been shown to transmit other members of the Orthobunyavirus genus such as the Simbu serogroup which contains significant viruses in veterinary public health (Sick et al., 2019). Further research is required to determine any possible role Culicoides midges may have in the transmission dynamics of KEYV and other California group viruses.
Another notable observation of the Pokomoke research was a strong relationship between the presence of adult Ae. atlanticus mosquitoes and infections in squirrels (Fig. 1) (Watts et al., 1988). In fact, few infections in squirrels were detected during periods of decreased mosquito populations (such as during periods of low rainfall). This highlights the significant impacts that climate and Ae. atlanticus population dynamics have on KEYV transmission. Numerous studies have estimated how climate change will impact vector-borne disease dynamics and the geographic ranges of mosquito vectors (Rocklöv and Dubrow, 2020; Githeko et al., 2000; Reiter, 2001). Specifically related to mosquito-borne encephalitic viruses, the World Health Organization estimates that increased temperatures may increase transmission rates for viruses such as St. Louis encephalitis as well as shift vector ranges toward higher latitudes (Githeko et al., 2000). The impact of climate change specifically on KEYV has not be studied; however, it is plausible that rising temperatures will have similar effects on KEYV transmission dynamics in Florida.
It is worth noting that transmission dynamics in the Pokomoke Cypress Swamp may be different from Florida, for a number of reasons. There are no cotton rats in the Pokomoke Swamp, meaning that any contribution these may make to Floridian KEYV transmission dynamics could not be observed there. Pokomoke is also near the northern range of Ae. atlanticus resulting in a shorter season for mosquito activity.
2.3. Routes of KEYV transmission
Since the initial discovery of KEYV, it has been believed to be transmitted horizontally in a two-host cycle: mosquito–vertebrate–mosquito (Taylor et al., 1971). This is indeed supported by experimental evidence. What was later discovered is that KEYV can also be transmitted vertically from an infected female mosquito to a fraction of her eggs (LeDuc et al., 1975b; Watts et al., 1988; LeDuc et al., 1975a). Studies have concluded that vertically infected mosquitoes retain the ability to infect vertebrate hosts and suggest that vertical transmission may be a major factor in the preservation of KEYV during the winter (LeDuc, 1978; Watts et al., 1988). There is evidence, from studies of CEV transmission dynamics, that vertically-infected mosquitoes are more capable of vertical transmission than horizontally infected mosquitoes, a phenomenon known as stabilized transmission (Turell et al., 1982). In transovarially-infected mosquito populations, vertical transmission rates exceeded 90%. These high rates suggest that stabilized vertical transmission may be a more significant factor in viral amplification than horizontal transmission. However, more research is required to determine if these transmission dynamics apply to KEYV. Somewhat more surprisingly, there is also evidence that suggests that vertically-infected mosquitoes may be more efficient horizontal transmitters as well. In fact, horizontally-infected Ae. atlanticus appear to transmit quite poorly, with one experiment finding only one definite transmission out of 82 mosquitoes (Watts et al., 1988). In contrast, the same study found that one of the two vertically-infected mosquitoes successfully transmitted KEYV to mice. This increased horizontal efficiency was also observed in the CEV study where mosquitoes from populations experiencing stabilized transmission infected all exposed mice (Turell et al., 1982).
There is circumstantial evidence that within-species horizontal transmission among mosquitoes (during copulation) (Thompson and Beaty, 1978) occurs. However, this evidence is for LACV and its vector (Ae. triseriatus). Further research is required to determine if venereal transmission occurs for KEYV in Ae. atlanticus populations. It is worth noting that there can be substantial variability in vector competence for different lineages of the same mosquito species (Chouin-Carneiro et al., 2016).
3. KEYV surveillance
In order to investigate the incidence of KEYV in Florida, we must first understand what is meant by a “relevant infection”. A definition of a relevant infection should allow us to characterize the probability that a given individual will experience a KEYV infection. Additionally, this definition should result in all relevant infections having a similar risk profile based on patient characteristics and not related to a history of KEYV exposure. Ideally, we want a definition that will encompass all (or almost all) exposures to KEYV that have non-negligible expected costs, have well-defined risks, and will be feasible to measure.
3.1. Measuring incidence
While both incidence and prevalence can provide useful insight into a KEYV’s transmission dynamics, in practice, this distinction is of little importance because the direct measurement of infections is highly unlikely at the present. In the vertebrates believed to be reservoir hosts, KEYV viremia lasts for less than one week and likely occurs only once in a lifetime (Watts et al., 1988, 1979). KEYV appears to rarely present with specific symptoms in humans, making it challenging to identify cases in the population. Thus, attempting to obtain a reliable estimate of the incidence or prevalence of acute KEYV infections directly would be highly cost-prohibitive.
However, there is an alternative approach that can be used to help estimate and comprehend the past incidence of KEYV: seroprevalence. This refers to the fraction of the population that has neutralizing antibodies to KEYV at titers that indicate a past infection by the virus. To assess KEYV seroprevalence, multiple serosurveys have been performed in the Tampa Bay area and have consistently found a human seroprevalence of approximately 20% (Parkin et al., 1972).
While using seroprevalence data to make inferences about past incidence allows us to avoid the challenges of measuring incidence directly, it introduces several problems of its own. First, it is not clear if all infections that carry a meaningful risk of severe negative health outcomes will result in seroconversion. Indeed, one would predict that immunosuppression (due to HIV, medications, genetic disorders, etc.) would both increase the risk of severe disease outcomes and decrease the likelihood of generating neutralizing antibodies. Patients who die as a result of infection will also not be measured by a serosurvey, although the expected bias introduced by this may be mitigated by the expected low fatality rate. Additionally, there is evidence from the Pokomoke studies that initially seropositive squirrels can revert to seronegativity (Watts et al., 1988); if this occurs among humans as well, then seroprevalence will underrepresent the cumulative fraction of the population with at least one past infection. The same is true if infection or re-infection is possible despite seropositivity. However, given evidence that reinfection appears to produce minimal or no viremia (at least in animal models) (Watts et al., 1988, 1979), it may not be relevant from a transmission dynamics perspective. If rapid containment by the immune system sufficiently minimizes the health risks of KEYV reinfection, secondary infection may also be irrelevant in determining the general burden of KEYV. On the other hand, cross-reactivity with neutralizing antibodies from other California group viruses could in principle lead to serosurveys overestimating the seroprevalence and thus the prevalence of past KEYV infection.
In addition to addressing the above concerns, in order to accurately estimate the incidence of relevant KEYV infections in Florida using serological data, it would also be necessary to perform a serosurvey for the entire state rather than solely for the Tampa Bay area. This statewide serosurvey is further complicated by the regular movement of people in and out of the state during the year (such as tourists and part-time residents). It would also be highly desirable to not only record the seroprevalence of the state’s population, but also to stratify the results by age. This would offer additional data that could help model patterns of seroconversion (taken as a proxy for infection) and, if applicable, seroreversion. However, care must be used in the interpretation of such data if infection rates are changing over time due to changing patterns of land use, or increased transmission due to the spread of Ae. albopictus or other invasive mosquito species.
An additional approach to better characterize KEYV incidence over time would be conducting a longitudinal study of people across the state of Florida. Compared to a serosurvey (a cross-sectional analysis), a longitudinal study would provide insight into the temporal dynamics of KEYV and KEYV-based immunity (e.g., the durability of KEYV immunity). Additionally, similar to a statewide serosurvey, a statewide longitudinal study would give insight into the spatial dynamics of KEYV in the state (i.e., where transmission is more likely) and would also help to better understand the frequency of seroreversion and the outcomes of reinfections in humans. These potential benefits of a longitudinal study may help to justify the higher costs of this kind of study compared to serosurveys.
3.2. Challenges in KEYV surveillance
While past serosurvey results are certainly suggestive of human KEYV infections, until recently there had been no confirmed human cases. In 2016, a 16-year-old male presented at a Northern Florida urgent care clinic with symptoms of low fever, mild fatigue, and a mild rash. These are common and non-specific symptoms in children and adolescents which often receive no diagnosis beyond a general viral etiology. In this case, however, an unusually extensive workup was performed due to concerns about possible Zika virus infection, as there was an outbreak of Zika virus disease in the Americas and Caribbean at that time. As a result, KEYV was detected in the patient’s urine (Lednicky et al., 2018). Combined with serosurvey data and what we know about other California group viruses, this recent case raises concerns regarding whether KEYV is a relatively common cause of childhood viral infections, and, if so, for what reasons these infections are severely underdiagnosed.
First of all, KEYV infections may generally be asymptomatic. Moreover, evidence from both presumptive reservoir hosts and the one confirmed human infection to date all point to infections that, when they do present with symptoms, present most frequently with mild and non-specific symptoms that are unlikely to provoke anywhere near the level of effort involved in the 2016 case. Viral fever and rash are often not investigated beyond excluding pathogens of major concern.
Furthermore, it may be difficult to detect KEYV infections even when looking for them. In the 2016 case, after screenings of the saliva and urine for a variety of viral RNAs using RT-PCR were negative, only a more intensive and broader screening procedure yielded a positive result for KEYV(Lednicky et al., 2018). In this second test, the patient’s saliva and urine were screened by amplifying any viral DNA or RNA present. This process was much more difficult and labor-intensive and would not be practical for routine diagnostic use.
Moreover, because this approach depended on finding a match for the sequencing results in the GenBank sequence database, it could not have identified KEYV infections prior to when the matching KEYV sequences were added to GenBank. The earliest (U12801.1) was added in 1995 (Keystone virus, 2019), with the two others (KT630293.1 and KT630290.1) added only in 2015 (Keystone virus strain, 2019a,b).
If KEYV infections are in fact a reasonably common and unrecognized childhood infection in Florida, then its public health significance has similarly been underestimated.
4. Possible outcomes or consequences of KEYV infections in humans
Having considered questions about the ecology and transmission dynamics of KEYV it remains to consider the potential consequences and costs associated with KEYV infections. It is important to recognize that the costs of disease are not exclusively financial: pain and suffering, both acute and chronic, may result as well, and should be considered. The same is true of disability, both as experienced on a short-term basis during infection and with regards to impairments resulting from long-term sequelae.
There are a number of economic and non-economic costs to consider for each acute disease state. Economic costs can be divided into short-term and long-term categories. The former includes hospitalization costs and the cost of missed work or school (for the patient and also for parents or other relatives if applicable). In the long-term, there may be economic costs associated with various sequelae. In addition to these monetary costs, non-economic costs may include the risk of death, impairment and pain caused by either the acute condition or long-term sequelae, and heightened long-term risk of death (if any). These costs must be evaluated in order to properly understand the potential burden of KEYV.
Several other California group viruses found in America are known to cause meningitis and/or encephalitis in humans including CEV, JCV, and LACV. The last of these is considered one of the most common and significant arboviral causes of encephalitis in the U.S. today (Harding et al., 2019; Rust et al., 1999). Furthermore, LACV is known to have age-structure regarding risks of infection and severe outcomes: approximately 90% of infections are estimated to occur in children under 16 years old; these children also have the highest risk of severe neuroinvasive disease caused by LACV (Harding et al., 2019; Symptoms, Diagnosis, & Treatment, 2018). While more research is required to explore any relationships between KEYV, meningitis, and encephalitis, the ability of other California group viruses to cause these disease states raises public health concerns for KEYV infections. Similarly, future research is required to investigate whether KEYV shares the age-structured risks observed for LACV. Once again, a longitudinal study would be beneficial towards quantifying the clinical and financial outcomes of KEYV infections of humans across age groups.
4.1. Fever and rash
As discussed in the previous section, it is likely that the most common acute symptoms caused by KEYV in humans are fever and rash. This is not necessarily innocuous, as these are still a potential source of numerous costs.
Costs may include suffering experienced by the infected individual and the patient’s family (especially in pediatric cases). While this will in general be far less severe than the cost of suffering in cases that develop meningitis or encephalitis, it is hardly negligible. There is also, in many cases, a cost from missed work for parents or adult patients, or from missed school for children.
There are also the costs of any medical services that may be needed for diagnosis or treatment. If antibiotics are prescribed, whether as a precaution or to placate the patient or parent, there is not only the monetary cost of the antibiotics themselves but also potentially health costs to the patient, often from the disruption of normal microflora.
4.2. Meningitis
Meningitis is an acute inflammation of the meninges, which are the three membranes that surround the brain and spinal cord. The classic symptoms of meningitis are headache, fever, stiff neck, photophobia, and altered mental status; however, symptoms, prognoses, and costs can vary substantially depending on the underlying etiology. These variations include the probability of hospitalization, the expected duration and cost of hospitalization, the probability of complications, the probability of death, and the risk of long-term sequelae (Ramers et al., 2000; Griffiths et al., 2018).
Over half of all meningitis cases in the modern U.S. are caused by viruses. These cases are typically less severe than bacterial or fungal meningitis. In fact, some sources refer to viral meningitis as “generally a benign and self-limited condition” (Khetsuriani et al., 2003) or “a self-limiting condition” (Balada-Llasat et al., 2019). Because it is relatively less severe, viral meningitis is believed to be heavily underdiagnosed.
Numerous genera of viruses can cause meningitis, but the most common viral causes in the U.S. are non-polio enteroviruses. There are some specific treatments for meningitis caused by certain viruses such as herpes simplex, varicella zoster, and cytomegalovirus, but for most viral cases, only supportive care is possible. Combined with the generally mild course of these viral meningitis cases, this results in many cases being diagnosed in outpatient settings without subsequent hospitalization or attempts to identify the specific agent beyond determining that it is not a herpesvirus. As a result, an estimated 84% of diagnosed viral meningitis cases do not receive a more specific diagnosis (Holmquist et al., 2008). Naturally, such cases will not be included in the statistics for the actual causative pathogen (such as KEYV) and will therefore contribute to underdetection.
Although most meningitis cases are preventatively referred to hospitals for care and diagnostic testing, viral cases often show high spontaneous recovery rates (Logan and MacMahon, 2008). Even if the physiological effects of the disease itself are limited, additional incurred costs may be associated with diagnostic practices and etiological uncertainty. And while hospitalization may be limited in most cases, with a median stay duration of 2 days (Hasbun et al., 2019), it is generally a major factor that increases overall costs of viral meningitis cases.
The duration and costs of hospitalization due to viral meningitis are typically the result of the necessary diagnostic and laboratory tests to determine the viral cause or to eliminate more serious pathogens (such as herpes simplex and rabies) as the cause. Cases of meningitis with arboviral causes were consistently more costly among adults and children compared to other viral causes (Balada-Llasat et al., 2019, 2018). These increased costs were derived from longer ICU stays, delays in lumbar puncture testing, and preventative antibiotic prescriptions. In addition to monetary costs, there are also non-economic costs to consider. Lumbar punctures represent a significant cause of these costs as this diagnostic procedure can be the cause of immediate pain and carries serious if unlikely risks of subsequent complications (Weir, 2000).
4.3. Encephalitis
Encephalitis is the inflammation of the brain itself and is generally associated with altered mental status and other neurological symptoms. In-hospital fatality rates average 5.6% in the U.S., but varies according to the underlying cause. For survivors, long-term sequelae, sometimes serious, are more common than with meningitis.
Of all cases of encephalitis in the U.S., approximately 38% of cases are diagnosed as viral encephalitis (George et al., 2014). Furthermore, approximately 32% of these viral cases receive no further diagnosis (i.e., identifying the specific viral agent). As with viral meningitis, this contributes to underdetection. Overall case fatality rates for viral encephalitis is 8.2%, but ranges from <1% for some pathogens—including LACV—to effectively 100% for some rare causes, such as rabies (George et al., 2014).
For context, herpes simplex encephalitis resides near the upper end of this spectrum. It is the most common cause of viral encephalitis in the U.S., and if untreated is typically fatal, with approximately 97% of survivors experiencing serious, long-term sequelae. Even with current treatment methods, prognoses remain poor with around 10% dying, and 75% of survivors suffering from severe, long-term sequelae (Bradshaw and Venkatesan, 2016). Because of this wide variability and the substantial overall risk of death, in contrast to viral meningitis, viral encephalitis cannot be generalized as a benign condition.
The cost of encephalitis includes many of the costs described for meningitis but often at a higher level. For example, both meningitis and encephalitis cases often receive empiric treatment with antibiotics; however, encephalitis cases—including those diagnosed as viral but without a specifically identified pathogen—often can receive empiric treatment with antivirals as well (Venkatesan and Geocadin, 2014). Empiric antiviral treatment is rarer in meningitis cases because the prognosis of untreated viral (typically herpetic) meningitis is much more promising compared to viral (herpetic) encephalitis, which when left untreated has fatality rates of up to 70% (Kennedy and Chaudhuri, 2002; Logan and MacMahon, 2008). These increased clinical concerns result in increased testing and treatment, and thus greater financial costs and risks of complications.
LACV is generally considered to be the California group virus that poses the largest public health threat in the U.S.; however, it appears to only cause encephalitis in a minority of infected individuals, with a ratio of encephalitis cases to infections that has been estimated at anywhere from 1 in 3 to 1 in 1500 (Rust et al., 1999). LACV encephalitis cases frequently exhibit a number of similar symptoms that have traditionally been regarded as highly specific for herpetic encephalitis (McJunkin et al., 2001). Thus, these LACV cases can receive similar preventative and diagnostic procedures as herpetic cases, ultimately increasing costs. As both LACV and KEYV are California group viruses, it is possible that KEYV infections can also develop these severe herpes-like symptoms.
The overall fatality rate for hospitalized viral encephalitis cases is just over 6% (George et al., 2014), compared to an overall fatality rate for hospitalized viral meningitis cases of 0.6% (Holmquist et al., 2008). This is partly due to the high fatality rate of herpetic encephalitis, but even if all (diagnosed) herpesviral encephalitis cases are excluded from consideration (including viruses such as varicella zoster virus and cytomegalovirus in addition to herpes simplex viruses 1 and 2), the fatality rate is still approximately 4.4%—over 7 times the fatality rate of all viral meningitis cases including those due to herpesviruses.
In addition to increased risk of death, LACV encephalitis has also been linked with an long-term neurological impairments. Specifically, children who suffered from LACV encephalitis saw declines in overall cognitive abilities as well as increased risk for attention deficit hyperactivity disorder (ADHD) development (McJunkin et al., 2001). Thus, because of its increased risks of both long-term sequelae and death, viral encephalitis represents a more costly disease state than meningitis both during and after hospitalization.
4.4. Meningitis/Encephalitis spectrum
Differentiating between meningitis and encephalitis can often be uncertain. Many of the same pathogens can cause each; this is particularly common for viruses. Sometimes, both conditions can be present simultaneously, a condition known as meningoencephalitis. Even when this is not the case, it can be difficult to distinguish between each condition as they share symptoms such as fever and altered mental status. Additionally, a viral infection of the meninges can, in some cases, produce inflammation of adjacent portions of the brain itself without the brain itself being infected (Ropper et al., 2014).
4.5. Reporting
Neither meningitis nor encephalitis as such is a reportable disease in Florida; however, bacterial and fungal meningitis are reportable, as are amoebic encephalitis and HSV encephalitis. Additionally, all “arboviral disease” is reportable whether specifically categorized by cause (including St. Louis encephalitis or California serogroup virus disease) or under the catch-all category “arboviral diseases not otherwise listed” (Reportable Diseases, 2016). Of course, such reporting cannot occur without a diagnosis more specific than “viral encephalitis or meningitis”. Thus, in order to include KEYV in these reporting practices, an efficient diagnostic procedure would be required.
5. Conclusions
Meningitis and encephalitis are serious public health burdens in the contemporary U.S., including in the state of Florida. Many cases of meningitis and encephalitis never have a specific etiology diagnosed, and there is reason to believe that a substantial fraction of these may be due to mosquito-transmitted viruses (arboviruses). KEYV is an arbovirus in the California group, which contains viruses known to produce infections in humans that are usually either asymptomatic or characterized by non-specific symptoms such as fever; however, some California group viruses can also cause meningitis or encephalitis with low but non-trivial frequencies. Serological evidence suggests that approximately 20% of Tampa Bay area residents have been infected with KEYV on one or more occasions, and there is evidence implicating KEYV in three cases: two encephalitis cases in the 1960s and one more recent, milder infection. Together, this evidence suggests that KEYV may be an underappreciated cause of viral meningitis and encephalitis cases in Florida and of the consequent costs to individuals and society. By exploring the frequency and costs associated with potential KEYV infections, further research would allow us to more accurately estimate the burden KEYV may cause in Florida. And while this review focuses specifically on Florida, the concerns and conclusions can be similarly applied to the entire potential range of KEYV in the southeastern region of the U.S.
There are many questions that need to be answered to properly assess the costs imposed by KEYV. From a public health perspective, the most important question is likely to be how many of the more than 68,000 hospitalizations that occur each year due to meningitis and encephalitis with an unidentified etiology or with an etiology that is only classified as “viral” are in fact due to KEYV (Holmquist et al., 2008; George et al., 2014).
If the techniques used by Lednicky et al. in the case discussed in Section 3.2 can be sufficiently refined for clinical use, there would then be a systematic way to address not only this question but also gain a better understanding of the role numerous other viruses play in these viral meningitis and encephalitis cases (Lednicky et al., 2018). More immediately, routine testing for additional clades of viruses known to contain species that can produce meningitis and encephalitis (such as the California group serogroup) could enhance our understanding of the relative importance of these viral clades, aid in surveillance, and provide more accurate prognoses. This could be done, for example, by performing (RT-)PCR with primers for sequences that are conserved within the clade of interest, with further refinement upon obtaining a positive result.
Additionally, serosurveys and longitudinal studies would provide valuable insight into the transmission and immune dynamics of KEYV in Florida. While both kinds of studies would help to quantify the burden of KEYV in the state, they differ in meaningful ways. A serosurvey provides a cross-sectional quantification of KEYV immunity (i.e., any kind of past exposure) and may be less costly. Alternatively, while longitudinal studies are more resource intensive, conducting one would provide more insight into the spatiotemporal dynamics of KEYV infections as well as their clinical and financial outcomes.
From the perspective of a disease ecologist or transmission modeler, the most pressing question is the extent to which each of the different routes of transmission contribute to maintaining endemic transmission of KEYV in Florida. The first step to addressing this question is to determine the how transmission route (i.e., vertical or horizontal) affects a female Ae. atlanticus mosquito’s ability to spread the virus vertically and horizontally. If results are informative, this might also be extended to similar studies on transmission probabilities for Ae. albopictus, and potentially for other mosquito species as well.
Given the uncertainties that exist about the public health burden of KEYV, it is understandable that funders may be reluctant to allocate limited resources investigating it further. However, this must be balanced against the human health impact that circumstantial evidence suggests that KEYV may have. Moreover, as discussed above, there is much research required that would greatly increase our understanding of the transmission dynamics and of the public health burden of KEYV which would also have widespread applications for other pathogens of public health significance.
Acknowledgments
This project was funded in part a by grant from the National Institutes of Health/National Institute of General Medical Sciences (U54 GM111274).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abudurexiti Abulikemu, Adkins Scott, Alioto Daniela, Alkhovsky Sergey V., Avšič-Županc Tatjana, Ballinger Matthew J., Bente Dennis A., Beer Martin, Bergeron Éric, Blair Carol D., et al. , 2019. Taxonomy of the order Bunyavirales: update 2019. Arch. Virol 164 (7), 1949–1965. 10.1007/s00705-019-04253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatoom Adnan, Payne Deborah, 2009. An overview of arboviruses and bunyaviruses. Lab. Med 40 (4), 237–240. 10.1309/LMPX9OEOAOPPBCJH. [DOI] [Google Scholar]
- Balada-Llasat Joan M., Rosenthal Ning, Hasbun Rodrigo, Zimmer Louise, Bozzette Samuel, Duff Steven, Chung Jessica, Ginocchio Christine C., 2019. Cost of managing meningitis and encephalitis among infants and children in the United States. Diagn. Microbiol. Infect. Dis 93 (4), 349–354. 10.1016/j.diagmicrobio.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Balada-Llasat Joan M., Rosenthal Ning, Hasbun Rodrigo, Zimmer Louise, Ginocchio Christine C., Duff Steven, Allison Jessica, Bozzette Samuel, 2018. Cost of managing meningitis and encephalitis among adult patients in the United States of America. Int. J. Infect. Dis 71, 117–121. 10.1016/j.ijid.2018.04.799. [DOI] [PubMed] [Google Scholar]
- Bond James O., Hammon William McD., Lewis Arthur L., Sather Gladys E., Taylor DJ, 1966. California group arboviruses in Florida and report of a new strain, Keystone virus. Public Health Rep. 81 (7), 607. 10.2307/4592788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw Michael J., Venkatesan Arun, 2016. Herpes simplex virus-1 encephalitis in adults: Pathophysiology, diagnosis, and management. Neurotherapeutics 13 (3), 493–508. 10.1007/s13311-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett-Cadena Nathan D., 2013. Mosquitoes of the Southeastern United States. University of Alabama Press, p. 79, URL: https://books.google.com/books?id=AafUAQAAQBAJ. [Google Scholar]
- Chamberlain RW, Sudia WD, Coleman PH, Beadle LD, 1964. Vector studies in the St. Louis encephalitis epidemic, Tampa Bay area, Florida, 1962. Amer. J. Trop. Med. Hyg 13, 456–461. 10.4269/ajtmh.1964.13.456. [DOI] [PubMed] [Google Scholar]
- Chamberlain RW, Sudia WD, Coleman PH, Johnston JG Jr., Work TH, 1969. Arbovirus isolations from mosquitoes collected in Waycross, Georgia, 1963, during an outbreak of equine encephalitis. Am. J. Epidemiol 89 (1), 82–88. 10.1093/oxfordjournals.aje.a120918. [DOI] [PubMed] [Google Scholar]
- Chouin-Carneiro Thais, Vega-Rua Anubis, Vazeille Marie, Yebakima André, Girod Romain, Goindin Daniella, Dupont-Rouzeyrol Myrielle, Lourenco-de Oliveira Ricardo, Failloux Anna-Bella, 2016. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl. Trop. Dis 10 (3), 10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George Benjamin P., Schneider Eric B., Venkatesan Arun, 2014. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000–2010. PLoS One 9 (9), e104169. 10.1371/journal.pone.0104169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko Andrew K., Lindsay Steve W., Confalonieri Ulisses E., Patz Jonathan A., 2000. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ 78 (9), URL: https://www.who.int/bulletin/archives/78(9)1136.pdf. [PMC free article] [PubMed] [Google Scholar]
- Griffiths Michael J., McGill Fiona, Solomon Tom, 2018. Management of acute meningitis. Clin. Med 18 (2), 164. 10.7861/clinmedicine.18-2-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstad PR, Kobayashi JF, Zhang MB, Craig GB Jr., 1989. Recently introduced Aedes albopictus in the United States: Potential vector of La Crosse virus (Bunyaviridae: California serogroup). J. Amer. Mosq. Control Assoc 5 (3), 422–427. [PubMed] [Google Scholar]
- Harding S, Greig J, Mascarenhas M, Young I, Waddell LA, 2019. La Crosse Virus: A scoping review of the global evidence. Epidemiol. Infect 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbun Rodrigo, Wootton Susan H., Rosenthal Ning, Balada-Llasat Joan Miquel, Chung Jessica, Duff Steve, Bozzette Samuel, Zimmer Louise, Ginocchio Christine C., 2019. Epidemiology of meningitis and encephalitis in infants and children in the United States, 2011–2014. Pediatr. Infect. Dis. J 38 (1), 37–41. 10.1097/INF.0000000000002081. [DOI] [PubMed] [Google Scholar]
- Holmquist Laurel, Russo C. Allison, Elixhauser Anne, 2008. Meningitis-related hospitalizations in the United States, 2006. In: HCUP Statistical Brief #57. Agency for Healthcare Research and Quality (US), Rockville (MD), URL: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb57.jsp. [PubMed] [Google Scholar]
- Jennings William L., Lewis Arthur L., Sather Gladys E., Hammon William McD., Bond James O., 1968. California-encephalitis-group viruses in Florida rabbits: Report of experimental and sentinel studies. Amer. J. Trop. Med. Hyg 17 (5), 781–787. 10.4269/ajtmh.1968.17.781. [DOI] [PubMed] [Google Scholar]
- Jennings William L., Lewis Arthur L., Sather Gladys E., Pierce LV, Bond James O., 1970. Tamiami virus in the Tampa Bay area. Amer. J. Trop. Med. Hyg 19 (3), 527–536. 10.4269/ajtmh.1970.19.527. [DOI] [PubMed] [Google Scholar]
- Kennedy PGE, Chaudhuri A, 2002. Herpes simplex encephalitis. J. Neurol. Neurosurg. Psychiatry 73 (3), 237–238. 10.1136/jnnp.73.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2019. Keystone virus C14031-33 S RNA segment, N and NSs protein genes, complete cds. National Center for Biotechnology Information, URL: https://www.ncbi.nlm.nih.gov/nuccore/U12801.1 (visited on 07/05/2019). [Google Scholar]
- 2019a. Keystone virus strain KEYV/Ochlerotatus atlanticus/USA/KEYVLK02/2005 segment S, complete sequence. National Center for Biotechnology Information, URL: https://www.ncbi.nlm.nih.gov/nuccore/KT630293.1 (visited on 07/05/2019). [Google Scholar]
- 2019b. Keystone virus strain KEYV/Ochlerotatus atlanticus/USA/KEYVLK01/2005 segment S, complete sequence. National Center for Biotechnology Information, URL: https://www.ncbi.nlm.nih.gov/nuccore/KT630290.1 (visited on 07/05/2019). [Google Scholar]
- Khetsuriani Nino, Quiroz Eva S., Holman Robert C., Anderson Larry J., 2003. Viral meningitis-associated hospitalizations in the United States, 1988–1999. Neuroepidemiology 22 (6), 345–352. 10.1159/000072924. [DOI] [PubMed] [Google Scholar]
- Lednicky John A., White Sarah K., Stephenson Caroline J., Cherabuddi Kartikeya, Loeb Julia C., Moussatche Nissin, Lednicky Andrew, Morris J. Glenn, 2018. Keystone virus isolated from a Florida teenager with rash and subjective fever: Another endemic arbovirus in the Southeastern United States? Clin. Infect. Dis 68 (1), 143–145. 10.1093/cid/ciy485. [DOI] [PubMed] [Google Scholar]
- LeDuc James W., 1978. Natural transmission of Keystone virus to sentinel rabbits on the Delmarva Peninsula. Amer. J. Trop. Med. Hyg 27 (5), 1041–1044. 10.4269/ajtmh.1978.27.1041. [DOI] [PubMed] [Google Scholar]
- LeDuc James W., Burger John F., Eldridge Bruce F., Russell Philip K., 1975a. Ecology of Keystone virus, a transovarially maintained arbovirus. Ann. New York Acad. Sci 266 (1), 144–151. 10.1111/j.1749-6632.1975.tb35095.x. [DOI] [PubMed] [Google Scholar]
- LeDuc James W., Suyemoto W, Eldridge Bruce F., Russell Philip K., Barr AR, 1975b. Ecology of California encephalitis viruses on the Del Mar Va peninsula: II. Demonstration of transovarial transmission. Amer. J. Trop. Med. Hyg 24 (1), 124–126. 10.4269/ajtmh.1975.24.124. [DOI] [PubMed] [Google Scholar]
- Logan Sarah A.E., MacMahon Eithne, 2008. Viral meningitis. BMJ 336, 36–40. 10.1136/bmj.39409.673657.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McJunkin James E., de los Reyes Emily C., Irazuzta Jose E., Caceres Manuel J., Khan Raheel R., Minnich Linda L., Fu Kai D., Lovett Gretchen D., Tsai Theodore, Thompson Ann, 2001. La Crosse encephalitis in children. N. Engl. J. Med 344 (11), 801–807. 10.1056/NEJM200103153441103. [DOI] [PubMed] [Google Scholar]
- Mitchell Carl J., 1991. Vector competence of North and South American strains of Aedes albopictus for certain arboviruses: A review. J. Amer. Mosq. Control Assoc 7 (3), 446–451. [PubMed] [Google Scholar]
- Mitchell CJ, Morris CD, Smith GC, Karabatsos N, Vanlandingham D, Cody E, 1996. Arboviruses associated with mosquitoes from nine Florida counties during 1993. J. Amer. Mosq. Control Assoc 12 (2), 255–262. [PubMed] [Google Scholar]
- Mitchell CJ, Niebylski ML, Smith GC, Karabatsos N, Martin D, Mutebi JP, Craig GB Jr., Mahler MJ, 1992. Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science 257 (5069), 526–527. 10.1126/science.1321985. [DOI] [PubMed] [Google Scholar]
- Parkin William E., 1973. The occurrence and effects of the local strains of the California encephalitis group of viruses in domestic mammals of Florida. Amer. J. Trop. Med. Hyg 22 (6), 788–795. 10.4269/ajtmh.1973.22.788. [DOI] [PubMed] [Google Scholar]
- Parkin William E., Hammon William McD., Sather Gladys E., 1972. Review of current epidemiological literature on viruses of the California arbovirus group. Amer. J. Trop. Med. Hyg 21 (6), 964–978. 10.4269/ajtmh.1972.21.964. [DOI] [PubMed] [Google Scholar]
- Quick Donald T., Smith Arthur G., Lewis Arthur L., Sather Gladys E., Hammon William McD., 1965. California encephalitis virus infection: A case report. Amer. J. Trop. Med. Hyg 14 (3), 456–459. 10.4269/ajtmh.1965.14.456. [DOI] [PubMed] [Google Scholar]
- 2021. QuickFacts: United States. United States Census Bureau, URL: https://www.census.gov/quickfacts/fact/table/US/PST045219 (visited on 08/25/2021). [Google Scholar]
- Ramers Christian, Billman Glenn, Hartin Michele, Ho Sandy, Sawyer Mark H., 2000. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. JAMA 283 (20), 2680–2685. 10.1001/jama.283.20.2680. [DOI] [PubMed] [Google Scholar]
- Reinert John F., Harbach Ralph E., Kitching Ian J., 2004. Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zool. J. Linn. Soc 142 (3), 289–368. 10.1111/j.1096-3642.2004.00144.x. [DOI] [Google Scholar]
- Reinert John F., Harbach Ralph E., Kitching Ian J., 2008. Phylogeny and classification of Ochlerotatus and allied taxa (Diptera: Culicidae: Aedini) based on morphological data from all life stages. Zool. J. Linn. Soc 153 (1), 29–114. 10.1111/j.1096-3642.2008.00382.x. [DOI] [Google Scholar]
- Reinert John F., et al. , 2000. New classification for the composite genus Aedes (Diptera: Culicidae: Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. J. Amer. Mosq. Control Assoc.-Mosq. News 16 (3), 175–188. [PubMed] [Google Scholar]
- Reiter Paul, 2001. Climate change and mosquito-borne disease. Environ. Health Perspect 109 (suppl 1), 141–161. 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2016. Reportable Diseases/Conditions in Florida: Practitioner List. Florida Department of Health, URL: http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/_documents/reportable-diseases-list-practitioners.pdf (visited on 07/05/2019). [Google Scholar]
- Roberts DR, Scanlon JE, 1975. The ecology and behavior of Aedes atlanticus D. & K. and other species with reference to Keystone virus in the Houston area, Texas. J. Med. Entomol 12 (5), 537–546. [DOI] [PubMed] [Google Scholar]
- Rocklöv Joacim, Dubrow Robert, 2020. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol 21 (5), 479–483. 10.1038/s41590-020-0648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi Ankit, 2021. Webplotdigitizer: Version 4.5. URL: https://automeris.io/WebPlotDigitizer.
- Ropper AH, Samuels MA, Klein J, 2014. Adams and Victor’s Principles of Neurology, Tenth Edition McGraw-Hill Education, URL: https://books.google.com/books?id=U3XhAgAAQBAJ. [Google Scholar]
- Rust Robert S., Thompson Wayne H., Matthews Charles G., Beaty Barry J., Chun Raymond W.M., 1999. La Crosse and other forms of California encephalitis. J. Child Neurol 14 (1), 1–14. 10.1177/088307389901400101. [DOI] [PubMed] [Google Scholar]
- Sick Franziska, Beer Martin, Kampen Helge, Wernike Kerstin, 2019. Culicoides biting midges—underestimated vectors for arboviruses of public health and veterinary importance. Viruses 11 (4), 10.3390/v11040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger D, Wuithiranyagool T, 1986. The discovery and distribution of Aedes albopictus in Harris County, Texas. J. Amer. Mosq. Control Assoc 2 (2), 217–219. [PubMed] [Google Scholar]
- 2018. Symptoms, Diagnosis, & Treatment | La Crosse encephalitis | CDC. Centers for Disease Control and Prevention, URL: https://www.cdc.gov/lac/symptoms/index.html (visited on 12/29/2020). [Google Scholar]
- Taylor DJ, Lewis Arthur L., Edman JD, Jennings William L., 1971. California group arboviruses in Florida: Host-vector relations. Amer. J. Trop. Med. Hyg 20 (1), 139–145. 10.4269/ajtmh.1971.20.139. [DOI] [PubMed] [Google Scholar]
- Tesh Robert B., 1980. Experimental studies on the transovarial transmission of Kunjin and San Angelo viruses in mosquitoes. Amer. J. Trop. Med. Hyg 29 (4), 657–666. 10.4269/ajtmh.1980.29.657. [DOI] [PubMed] [Google Scholar]
- Thompson Wayne H., Beaty Barry J., 1978. Venereal transmission of La Crosse virus from male to female Aedes trisenatus. Amer. J. Trop. Med. Hyg 27 (1), 187–196. 10.4269/ajtmh.1978.27.187. [DOI] [PubMed] [Google Scholar]
- 2018. Transmission | Jamestown Canyon virus | CDC. Centers for Disease Control and Prevention, URL: https://www.cdc.gov/jamestown-canyon/transmission/index.html (visited on 12/29/2020). [Google Scholar]
- 2019. Transmission | La Crosse encephalitis | CDC. Centers for Disease Control and Prevention, URL: https://www.cdc.gov/lac/tech/transmission.html (visited on 12/29/2020). [Google Scholar]
- Turell Michael J., Hardy James L., Reeves William C., 1982. Stabilized infection of California encephalitis virus in Aedes dorsalis, and its implications for viral maintenance in nature. Amer. J. Trop. Med. Hyg 31 (6), 1252–1259. 10.4269/ajtmh.1982.31.1252. [DOI] [PubMed] [Google Scholar]
- Venkatesan Arun, Geocadin Romergryko G., 2014. Diagnosis and management of acute encephalitis. Neurol. Clin. Pract 4 (3), 206–215. 10.1212/CPJ.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts Douglas M., Bailey Charles L., Roberts Nancy T., Tammariello Ralph F., Dalrymple Joel M., Clark Gary C., 1988. Maintenance and transmission of Keystone virus by Aedes atlanticus (Diptera: Culicidae) and the gray squirrel in the Pocomoke Cypress Swamp, Maryland. J. Med. Entomol 25 (6), 493–500. 10.1093/jmedent/25-6.493. [DOI] [PubMed] [Google Scholar]
- Watts Douglas M., LeDuc James W., Bailey Charles L., Dalrymple Joel M., Gargan Thomas P. II, 1982. Serologic evidence of Jamestown Canyon and Keystone virus infection in vertebrates of the DelMarVa peninsula. Amer. J. Trop. Med. Hyg 31 (6), 1245–1251. 10.4269/ajtmh.1982.31.1245. [DOI] [PubMed] [Google Scholar]
- Watts Douglas M., Tammariello Ralph F., Dalrymple Joel M., Eldridge Bruce F., Russell Philip K., Top Franklin H. Jr., 1979. Experimental infection of vertebrates of the Pocomoke Cypress Swamp, Maryland with Keystone and Jamestown Canyon viruses. Amer. J. Trop. Med. Hyg 28 (2), 344–350. 10.4269/ajtmh.1979.28.344. [DOI] [PubMed] [Google Scholar]
- Weir Evelyn C., 2000. The sharp end of the dural puncture. BMJ 320 (7227), 127. [PMC free article] [PubMed] [Google Scholar]


