Abstract
Research in adults suggests that higher peripheral inflammation is associated with increased threat-related amygdala activity and reduced cortico-amygdala connectivity. However, there is limited research in adolescents, which is striking given the major developmental changes that occur in cortico-amygdala circuitry during adolescence. In this study, we examine the association between peripheral inflammation and amygdala activity and connectivity to emotional faces in a community sample of adolescents. Participants included 88 adolescents 12 to 15 years old who provided a blood sample and underwent fMRI scanning while completing a face and shape matching task that included fearful, angry, and happy faces. Blood samples were assayed for interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α); IL-6 and CRP were combined into a composite due to their high correlation and TNF-α was analyzed separately. Results indicated that higher TNF-α, but not the composite of IL-6 and CRP, was associated with increased amygdala activity to threatening (fearful and angry) faces and to happy faces, relative to shape matching. Whole-brain analyses also identified associations between TNF-α and neural activity to angry and happy faces in regions outside of the amygdala. Psychophysiological interaction analysis indicated that higher TNF-α was associated with reduced bilateral amygdala connectivity to the left cuneus, right cuneus/calcarine fissure/precuneus, and left supramarginal gyrus/inferior parietal gyrus during angry and fearful faces > shapes and higher IL-6/CRP was associated with reduced bilateral amygdala connectivity to the right postcentral gyrus and right precuneus. Results suggest that peripheral inflammation is associated with increased amygdala activity to emotional face stimuli and reduced amygdala connectivity with occipital and parietal regions. These findings enhance our understanding of the association between peripheral inflammation and neural response to emotional faces, which could inform the development of interventions targeting inflammation for adolescents.
Keywords: Inflammation, fMRI, adolescence, amygdala, connectivity, threat, emotion
1. Introduction
Peripheral inflammatory mediators may cross the blood-brain barrier through several routes and influence brain function (Capuron & Miller, 2011). The neuro-immune network hypothesis proposes that bi-directional communication between peripheral inflammatory mediators and the brain, which becomes amplified under stress, underlies the development of a range of health problems (Nusslock & Miller, 2016). This hypothesis proposes that there is a bi-directional association between peripheral inflammation and the brain’s cortico-limbic network involved in threat processing, such that increased neural sensitivity to threat predicts increased peripheral inflammation, and increased peripheral inflammation in turn predicts increased neural sensitivity to threat. Increased neural sensitivity to threat could then increase risk for a range of behavioral and mental health problems associated with heightened negative affect and emotion regulation difficulties. The goal of the present paper is to test part of this hypothesis by examining cross-sectional associations between peripheral inflammatory markers and threat-related cortico-limbic function in adolescents with a focus on the amygdala, a key region within the cortico-limbic network.
Prior functional magnetic resonance imaging (fMRI) research conducted in adults has provided initial support for the neuro-immune network hypothesis. Specifically, several studies conducted in adults have shown that higher peripheral inflammation is associated with increased amygdala activity to threat, particularly social threat (Davies et al., 2021; Inagaki, Muscatell, Irwin, Cole, & Eisenberger, 2012; Muscatell et al., 2015; Swartz, Prather, & Hariri, 2017). For example, stimulation of inflammation through administration of endotoxin resulted in increased amygdala activity to fearful facial expressions (Inagaki et al., 2012). In another study, higher C-reactive protein (CRP) measured with dried blood spots was correlated with higher amygdala activity to fearful and angry facial expressions, which was significant for males but not females (Swartz et al., 2017). To our knowledge, only one study has examined the association between peripheral inflammation and amygdala activity in adolescents (Miller, White, Chen, & Nusslock, 2021), and found that higher peripheral inflammation (as measured by a composite of CRP, interleukin-6, interleukin-8, interleukin-10, and tumor necrosis factor-α) was associated with increased amygdala activity to angry faces. Moreover, this effect was moderated by socioeconomic status (SES) with more robust effects in adolescents with lower SES.
In addition to the association between inflammation and heightened amygdala reactivity, inflammation may also be associated with functional connectivity between the amygdala and a broader cortico-limbic network involved in threat processing and emotion regulation (Labrenz et al., 2016; Mehta et al., 2018; Muscatell et al., 2015). Most of this work on associations between inflammation and functional connectivity has examined resting-state connectivity, or intrinsic connectivity between brain regions when participants are “at rest,” and not performing an explicit task. For example, in a study of adults with depression, higher peripheral CRP was associated with decreased resting-state functional connectivity between the amygdala and ventromedial prefrontal cortex (Mehta et al., 2018). Further, we have found that higher peripheral tumor necrosis factor-α (TNF-α) was associated with increased resting-state functional connectivity between the amygdala and striatum, but not resting-state amygdala-cortical connectivity in adolescents (Swartz et al., 2021). Other research in adolescents has found that higher peripheral inflammation was associated with reduced resting-state functional connectivity in an emotion regulation network that included cortical regions, but not the amygdala (Nusslock et al., 2019). There is limited research examining the association between peripheral inflammation and task-based neural connectivity of the amygdala when viewing socially threatening facial expressions through psychophysiological interaction (PPI) analysis, which explicitly tests changes in functional connectivity during performance of a specific task condition (e.g., viewing threatening facial expressions). Moreover, there is a relative absence of research in adolescents, which is striking given that major developmental changes in threat-related amygdala activity and connectivity occur during adolescence (Gee et al., 2013; Swartz, Williamson, & Hariri, 2015; Tottenham & Gabard-Durnam, 2017) and that adolescence is a developmental stage when problems associated with emotion regulation often emerge (Thapar, Collishaw, Pine, & Thapar, 2012).
In this study we aimed to examine the association between peripheral inflammation and threat-related amygdala activity in a community sample of adolescents. We hypothesized that higher peripheral inflammation would be associated with increased threat-related amygdala activity. Our second goal was to examine the association between peripheral inflammation and amygdala connectivity during threat processing through the use of PPI analysis. We hypothesized that higher peripheral inflammation would be associated with reduced amygdala-prefrontal cortex connectivity when viewing socially threatening (i.e., angry and fearful) facial expressions. In addition, we explored socioeconomic status and gender as moderators of these effects, given prior findings (Miller et al., 2021; Swartz et al., 2017).
2. Methods and materials
2.1. Participants
Participants were from the Adolescent Health and Brain (AHB) Study, designed to examine associations between physical health, brain function, and mental health in adolescents. We have previously reported associations between peripheral inflammation and resting-state functional connectivity in adolescents from this sample (Swartz et al., 2021), and non-overlapping analyses using the face task from the study have been published in prior research (Swartz, Carranza, & Knodt, 2019). Please see Swartz et al. (2021) for full details regarding recruitment, inclusion, and exclusion criteria for the study. Briefly, adolescents 12-15 years old were recruited from the community. Exclusion criteria included select psychiatric diagnoses based on parent reports, chronic diseases or conditions that could affect cerebral blood flow, use of select medications, and any contraindications to MRI scanning. All procedures were approved by the University of California, Davis Institutional Review Board; parents provided informed consent and adolescents provided informed assent before beginning study procedures.
Data for the study were collected between August 2017 and March 2020. Of the 116 participants enrolled in the study, 7 did not provide a blood sample, 2 provided a blood sample that could not be used due to a labeling error, 4 did not attend the MRI scan, and 1 attended the MRI scan but did not complete the face processing task due to ending the scan early. Of the 102 participants who had both a blood sample and MRI scan, 13 were excluded due to not meeting the MRI quality control criteria (see quality control section below), resulting in a total of 89 participants with usable MRI data. In addition, one participant was removed from the current analyses due to reporting use of antibiotics for an infection, resulting in a sample of 88 available for analyses.
Participants were 12 to 15 years old (M = 14.07 years, SD = 1.07); 41 were female, 45 were male, and 2 identified as non-binary. There were 5 sets of siblings in the sample. Similar to our prior paper (Swartz et al., 2021), due to the small number of siblings in the sample, we did not statistically adjust for clustering within families. Participants’ race/ethnicity was self-reported as follows: 6 Asian, 4 Black or African American, 7 Hispanic/Latino, 2 Native Hawaiian or Pacific Islander, 31 White, 35 two or more races, 2 “other”, and 1 did not report. Participant characteristics are reported in Table 1 and bivariate correlations in Table 2.
Table 1.
Participant characteristics
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Age | 14.07 | 1.07 | 12.00 | 15.92 |
| BMI | 22.43 | 5.12 | 15.5 | 43.6 |
| Subjective SES | 6.06 | 1.23 | 4.00 | 9.00 |
| IL-6 (pg/mL) | 0.51 | 0.46 | 0.10 | 3.00 |
| TNF-α (pg/mL) | 2.36 | .65 | .94 | 4.35 |
| CRP (mg/L) | 1.57 | 3.83 | 0.10 | 23.90 |
| Inflammation composite (Z-score) | 0 | 1.80 | −2.92 | 5.04 |
| Task accuracy: All trials, % correct | 97.25 | 3.10 | 81 | 100 |
| Task accuracy: Faces, % correct | 98.61 | 2.79 | 89 | 100 |
| Task accuracy: Shapes, % correct | 95.89 | 4.46 | 74 | 100 |
| Task reaction time: All trials, ms | 1295.03 | 301.34 | 744.72 | 2165.61 |
| Task reaction time: Faces, ms | 1256.64 | 307.56 | 711.97 | 2145.64 |
| Task reaction time: Shapes, ms | 1333.43 | 311.90 | 777.48 | 2218.69 |
Note: SD=standard deviation; BMI=body mass index; SES=socioeconomic status; IL-6=interleukin-6; TNF-α=tumor necrosis factor-α; CRP=C-reactive protein; Inflammation composite=sum of log-transformed, Z-scored IL-6 and CRP.
Table 2.
Bivariate correlations
| Age | BMI | TNF-a | Inflammation composite | Subjective SES | Accuracy | RT | Amygdala Fearful + Angry | Amygdala Fearful | Amygdala Angry | Amygdala Happy | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | ||||||||||
| BMI | 0.28** | 1 | |||||||||
| TNF-a | −0.03 | 0.02 | 1 | ||||||||
| Inflammation composite | 0.23* | 0.65*** | 0.18 | 1 | |||||||
| Subjective SES | −0.12 | −0.18 | 0.06 | −0.17 | 1 | ||||||
| Accuracy | 0.17 | −0.15 | −0.12 | −0.19 | 0.11 | 1 | |||||
| RT | −0.04 | 0.16 | 0.03 | 0.02 | 0.001 | −0.36*** | 1 | ||||
| Amygdala Fearful + Angry | −0.03 | 0.02 | 0.33** | −0.04 | −0.16 | −0.13 | 0.06 | 1 | |||
| Amygdala Fearful | −0.10 | −0.04 | 0.26* | −0.06 | −0.15 | −0.22* | 0.14 | 0.78*** | 1 | ||
| Amygdala Angry | 0.05 | 0.07 | 0.26* | −0.01 | −0.10 | −0.01 | −0.02 | 0.83*** | 0.30** | 1 | |
| Amygdala Happy | −0.05 | −0.07 | 0.24* | −0.07 | −0.03 | 0.18 | −0.02 | 0.18 | 0.003 | 0.27* | 1 |
Note:
p<.05,
p<.01,
p<.001;
BMI = body mass index; TNF-α=tumor necrosis factor-α; Inflammation composite=sum of log-transformed, Z-scored IL-6 and CRP; SES=socioeconomic status; Accuracy=percent correct for all trials of matching task; RT=reaction time for all trials of matching task; amygdala variables indicate amygdala activity extracted for stated condition.
2.2. Procedure
Full details regarding the procedure are reported in Swartz et al. (2021). Briefly, the study was conducted in two visits. During the first visit, participants provided one non-fasting blood sample of 6 ml via antecubital venipuncture. Height and weight were measured to calculate body mass index (BMI), and the adolescent’s temperature was taken to confirm the absence of a fever. Visits generally occurred in the afternoon, but ranged from 8:44 am to 5:15 pm. Time of day of the blood sample was included as a covariate in analyses. During this visit, participants and their parents also completed self-report survey measures. During the second visit, participants completed an MRI scan. This visit generally took place within a week of the first visit (mean time between visits=3.98 days, SD=5.92; range=0 to 30 days). Participants completed three MRI tasks and a resting state scan; only results for the face matching task will be reported in this paper.
2.3. Inflammatory markers
Frozen serum samples were shipped to the UCLA Cousins Center for Psychoneuroimmunology to be assayed. A panel of six immune markers was assayed, including IL-6, IFN-g, IL-10, IL-8, TNF-α (using Multiplex assays by Meso Scale Discovery, Rockville, Maryland), and CRP (using a high-sensitivity ELISA assay by R&D Systems, Minneapolis, MN). Mean inter-assay CVs for IL-6, IFN-g, IL-10, IL-8, TNF-α, and CRP were 4.0%, 6.1%, 11.3%, 3.6%, 5.6%, and 1.2%, respectively. Mean intra-assay CVs were 1.0%, 1.2%, 12.5%, .5%, 4.9%, 1.0%, respectively. Samples were assayed in duplicate. Assays were conducted in three lots; we included two dummy-coded covariates to control for lot. Values below the lower limit of quantitation were coded as .10. All inflammatory marker variables were log-transformed and Z-scored. We used the same approach as in our prior study to examine inflammatory markers (Swartz et al., 2021). Specifically, we combined IL-6 and CRP into a composite variable due to their high correlation (by summing the log-transformed, Z-scored variables) and we examined TNF-α separately.
2.4. Face matching task
Participants performed a face matching task (Hariri et al., 2002), in which they viewed three faces and were asked to identify which of two faces matched a target face. Faces were presented in two blocks each of fearful faces, angry faces, and happy faces. Interleaved between face matching blocks were blocks of matching shapes. Face stimuli were taken from the NimStim Set of Facial Expressions (Tottenham et al., 2009). For face blocks, 6 trials were presented per block for 4 s each, with a variable interstimulus interval between 2 s and 6 s. For shape blocks, 6 trials were presented per block for 4 s each, with an interstimulus interval of 2 s. Accuracy and response times for all trials were recorded. Further details on the face matching task are provided in Swartz et al. (2019).
2.5. fMRI data acquisition, preprocessing, and quality control criteria
Full details regarding fMRI data acquisition, preprocessing, and quality control criteria are provided in the Supplement. Briefly, fMRI data were acquired on a 3T Siemens TIM Trio MRI system and a preprocessing pipeline including realignment, segmentation and spatial normalization, and smoothing was applied. Quality control criteria included <10% volumes censored for motion or signal intensity outliers, ≥ 90% signal coverage within the amygdala region of interest, and accuracy ≥75% on the matching task. A total of 8 participants were excluded for insufficient signal coverage in the amygdala and 5 were excluded for exceeding the motion and signal intensity outlier criterion, resulting in the total exclusion of 13 participants. Mean accuracy and reaction time for the face matching task are reported in Table 1.
2.6. Individual and group-level analyses and amygdala region of interest
Analyses were conducted with SPM12 software. For the individual-level model, boxcar regressors were entered for the conditions of angry faces, fearful faces, happy faces, and shapes. In addition, the regressors from artifact detection (described in Supplement) were included to control for outliers due to head motion. Next, individual contrast images for each condition were generated at the first level and then entered into second-level random effects models. We modeled the following contrasts: angry and fearful faces > shapes, fearful faces > shapes, angry faces > shapes, happy faces > shapes, and angry and fearful faces > happy faces.
In order to examine associations for our region of interest (ROI) analysis with the amygdala, we extracted mean contrast values from all voxels in the left and right amygdala anatomical ROIs from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) within the Wake Forest University (WFU) Pickatlas (Maldjian, Laurienti, Burdette, & Kraft, 2003). Contrast values were extracted and imported into SPSS v27 for further analyses.
2.7. Psychophysiological interaction (PPI) analysis
A PPI analysis was conducted using the generalized PPI (gPPI) toolbox (McLaren, Ries, Xu, & Johnson, 2012). For the gPPI, four psychological conditions were modeled (angry faces, fearful faces, happy faces, and shapes). The physiological regressor consisted of the amygdala time course, extracted from the bilateral amygdala ROI, created using the union of the left and right amygdala ROIs from the AAL atlas. The psychophysiological regressor consists of the interaction between psychological condition and the physiological regressor. Four psychophysiological regressors were modeled (amygdala time course x fearful faces, amygdala time course x angry faces, amygdala time course x happy faces, and amygdala time course x shapes). The main contrast of interest we examined for the gPPI was amygdala connectivity for angry and fearful faces > shapes. Because we hypothesized that inflammation would be associated with reduced amygdala-prefrontal cortex connectivity and prior research indicated altered amygdala connectivity with the ventral prefrontal cortex (Mehta et al., 2018), we created a ventral prefrontal cortex ROI (size: 4153 voxels), composed of the union of the following AAL regions: left and right superior frontal gyrus, orbital; middle frontal gyrus, orbital; inferior frontal gyrus, orbital; and superior frontal gyrus, medial orbital.
2.8. Self-report: Socioeconomic status
We used the MacArthur Scale of Subjective Social Status to operationalize SES in this paper (Adler, Espel, Castellazzo, & Ickovics, 2000; Singh-Manoux, Adler, & Marmot, 2003). For this question, adolescent participants were shown a figure of a ladder with rungs labeled from 1 to 10 with the statement: “Think of this ladder as representing where people stand in the United States. At the top of the ladder are the people who are the best off - those who have the most money, the most education and the most respected jobs. At the bottom are the people who are the worst off - who have the least money, least education, and the least respected jobs or no job. The higher up you are on this ladder, the closer you are to the people at the very top; the lower you are, the closer you are to the people at the very bottom.” They were then asked, “Where would you place yourself on this ladder compared to other people in the United States?” with response options ranging from 1 (bottom of the ladder) to 10 (top of the ladder). We used this subjective SES measure because research suggests that subjective SES can be a better predictor of health outcomes than more objective measures such as income (Adler et al., 2000; Singh-Manoux et al., 2003), and this measure can be assessed directly from adolescents themselves, who are not likely to be aware of their parents’ objective income levels. One participant was missing data because they skipped this item. Parents were also asked to report on their subjective SES using the same question. Parents’ and adolescents’ subjective SES reports were significantly correlated, r(83)=.43, p>.001. Because some parts of the subjective SES question (e.g., respected jobs) would be more relevant for parents than for adolescents, we also re-ran moderator analyses conducted in SPSS using the parent-reported subjective SES measure as a supplementary analysis. Two parents were missing data because they skipped this item.
2.9. Covariates
We included the following covariates in all analyses: gender (two dummy-coded covariates for male and non-binary groups, with female as the reference group); age; BMI (log-transformed); time of day for the blood draw; number of days between blood draw and MRI scan; lot for assays (dummy-coded); and medication use (0=no medications reported; 1=medications). The following medications were coded as 1 for this variable: antihistamines, oral contraceptives, nonsteroidal anti-inflammatory drugs such as ibuprofen, and bronchodilators such as albuterol. Twenty participants reported they were taking one or more of these medications. One participant was missing medication data due to technical difficulties with the Qualtrics questionnaire, so was coded as 0 for this variable.
2.10. 3dClustSim Analysis
For analyses involving whole-brain correction or the prefrontal cortex ROI, we implemented 3dClustSim in AFNI to determine corrected cluster thresholds. We used the same approach for all 3dClustSim analyses. First, we saved out residuals when estimating the second-level group analysis. We then used 3D to 4D file conversion in SPM to create one 4D residual file containing the residuals for all participants. This residual file was then entered into AFNI’s 3DFWHMx using AFNI version 20.3.02 to estimate the smoothness in the residuals of the data with the ‘acf’ option. Cluster size threshold was determined using 2-sided thresholding with the first-nearest neighbor clustering (NN1) option. For all analyses, we used an uncorrected voxelwise p-threshold of p<0.002 and cluster thresholds for each analysis required to achieve a whole-brain corrected threshold of p<0.05 are reported in Table 3.
Table 3.
Cluster size thresholds for whole-brain activity and gPPI analyses
| Analysis | Cluster threshold (voxels) |
|---|---|
| Association between TNF-α and activity to angry and fearful faces > shapes | 165 |
| Association between IL-6/CRP and activity to angry and fearful faces > shapes | 160 |
| Association between TNF-α and activity to angry faces > shapes | 164 |
| Association between IL-6/CRP and activity to angry faces > shapes | 151 |
| Association between TNF-α and activity to fearful faces > shapes | 154 |
| Association between IL-6/CRP and activity to fearful faces > shapes | 155 |
| Association between TNF-α and activity to happy faces > shapes | 151 |
| Association between IL-6/CRP and activity to happy faces > shapes | 151 |
| Association between TNF-α and activity to angry and fearful faces > happy faces | 170 |
| Association between IL-6/CRP and activity to angry and fearful faces > happy faces | 160 |
| Association between TNF-α and bilateral amygdala PPI for angry and fearful faces > shapes | 115 |
| Association between IL-6/CRP and bilateral amygdala PPI for angry and fearful faces > shapes | 142 |
| Association between TNF-α and bilateral amygdala PPI for angry faces > shapes | 129 |
| Association between IL-6/CRP and bilateral amygdala PPI for angry faces >shapes | 116 |
| Association between TNF-α and bilateral amygdala PPI for fearful faces > shapes | 137 |
| Association between IL-6/CRP and bilateral amygdala PPI for fearful faces > shapes | 127 |
| Association between TNF-α and bilateral amygdala PPI for angry and fearful faces > happy faces | 135 |
| Association between IL-6/CRP and bilateral amygdala PPI for angry and fearful faces > happy faces | 138 |
| Association between TNF-α and bilateral amygdala PPI for happy faces > shapes | 134 |
| Association between IL-6/CRP and bilateral amygdala PPI for happy faces > shapes | 126 |
| Association between TNF-α x SES and bilateral amygdala PPI for angry and fearful faces > shapes | 118 |
| Association between IL-6/CRP x SES and bilateral amygdala PPI for angry and fearful faces > shapes | 134 |
| Association between TNF-α x gender and bilateral amygdala PPI for angry and fearful faces > shapes | 118 |
| Association between IL-6/CRP x gender and bilateral amygdala PPI for angry and fearful faces > shapes | 140 |
Note: Cluster size threshold was determined using 2-sided thresholding with the first-nearest neighbor clustering (NN1) option. To examine whole-brain effects, we used an uncorrected voxelwise p-value of p<0.002
2.11. Statistical analyses
Our first goal was to examine associations between the peripheral inflammatory markers and amygdala activity to threatening faces during the face matching task. We first examined the main effect of task for each facial expression, to ensure that the task elicited significant amygdala activity as expected. Next, as described above, we used an anatomical ROI approach to extract amygdala activity for each face condition (angry and fearful > shapes, angry > shapes, fearful > shapes, happy > shapes, angry and fearful faces > happy faces), and import these into SPSS for analyses. The main contrast of interest for primary hypothesis testing was angry and fearful faces > shapes. To reduce the number of comparisons performed, we averaged left and right amygdala activity for each condition, resulting in a single measure of bilateral threat-related amygdala activity (angry and fearful faces > shapes) for the primary analyses. We conducted multiple regression analyses to examine associations between the IL-6/CRP composite and amygdala activity and between TNF-α and amygdala activity. All covariates described above were included in these regressions. In order to control for two comparisons (one regression for IL-6/CRP composite predicting amygdala activity and one regression for TNF-α predicting amygdala activity), we adopted a Bonferroni-corrected threshold of p<.025 for these analyses. As a sensitivity analysis to test whether effects were specific to any type of emotional face expression, we also conducted supplementary analyses examining regressions with bilateral amygdala activity for each condition separately (fearful faces>shapes, angry faces>shapes, and happy faces>shapes) and for the contrast of angry and fearful faces > happy faces. We did not correct for multiple comparisons for these supplementary sensitivity analyses.
As an exploratory analysis, we conducted whole-brain regressions to examine areas outside of the amygdala ROI in which activity was significantly associated with the inflammatory markers. To do so, we conducted separate regressions to regress each marker (TNF-α or IL-6/CRP composite) onto whole-brain activity, including all covariates described above. We conducted this exploratory whole-brain analysis for the following contrasts: angry and fearful faces > shapes, angry faces > shapes, fearful faces > shapes, happy faces > shapes, and angry and fearful faces > happy faces. We used 3dClustSim in AFNI, as described above, to determine corrected thresholds, which are reported in Table 3.
Our second goal was to examine the association between the inflammatory markers and threat-related amygdala connectivity using gPPI. We first examined the main effect of the PPI to test whether there was significant amygdala connectivity with the ventral prefrontal cortex for the contrast of angry and fearful faces > shapes, without including any covariates. We used 3dClustSim in AFNI to calculate the cluster size threshold needed to achieve a corrected statistical significance of p<.05 within the ventral prefrontal cortex ROI. Based on 3dClustSim, with an uncorrected voxelwise threshold of p<.002, a cluster size of 32 voxels was needed to obtain a corrected threshold of p<.05 within the ventral prefrontal cortex ROI. To test our hypothesis that the inflammatory markers would be associated with amygdala connectivity, we conducted separate multiple regressions in SPM12, regressing the inflammatory marker variable (IL-6/CRP composite or TNF-α), as well as all covariates described above, onto the gPPI for angry and fearful faces > shapes. We tested for associations between inflammation and amygdala-prefrontal cortex connectivity using the prefrontal cortex ROI described above and used 3dClustSim in AFNI to calculate cluster-size thresholds needed to achieve a corrected threshold within this ROI. Because we tested two inflammatory marker variables, we set the corrected threshold at p<0.025. 3dClustSim determined that with a voxelwise p-threshold of p<0.002 uncorrected, a cluster size of 33 voxels and 31 voxels was required to achieve a corrected threshold of p<0.025 within the prefrontal cortex ROI for the IL-6/CRP composite and for TNF-α, respectively.
As an exploratory analysis, we examined PPI effects for amygdala connectivity with the whole brain. As described above, we used 3dClustSim in AFNI to calculate cluster-size thresholds to achieve a whole-brain correction of p<0.05. Cluster extent thresholds are reported in Table 3. We did not correct for multiple comparisons for these exploratory whole-brain analyses. We examined whole-brain PPI effects for all contrasts.
We also planned to conduct exploratory analyses to examine whether effects were moderated by SES or gender. To test whether the association between inflammatory markers and amygdala activity was moderated by subjective SES, we added a regressor for SES and an interaction term for SES x inflammatory marker into the two main regression analyses (IL-6/CRP composite predicting threat-related amygdala activity and TNF-α predicting threat-related amygdala activity) using the PROCESS macro for SPSS (processmacro.org). We used a similar approach to examine gender as a moderator, looking at the dummy-coded variable of male vs. female as a moderator using the PROCESS macro for SPSS. We did not examine moderating effects for participants who identified as non-binary, given the small sample size of that group. For the PPI analyses, we created whole-brain multiple regressions in SPM 12 for the gPPI of angry and fearful faces > shapes, as described above. For moderation by subjective SES, we included regressors for SES and the interaction of SES x inflammatory marker in the regression. For the moderation by gender, we included the interaction of gender (male vs. female) x inflammatory marker in the regression. Similar to the methods described above for whole-brain gPPI, 3dClustSim was used to determine cluster sizes needed to achieve a whole-brain correction of p<.05, using an uncorrected voxelwise threshold of p<.002. Cluster size thresholds for these whole-brain multiple regression analyses are reported in Table 3. For all interaction analyses, continuous variables were mean-centered before calculating interaction terms. Because these moderator analyses were exploratory, we did not correct for multiple comparisons.
3. Results
3.1. Amygdala activity to faces
As expected, there was significant amygdala activity to all the face conditions vs. shapes. For angry and fearful faces > shapes, there was significant left, t(87)=11.47, p fwe-corr<0.001, k=136, [peak x,y,z]=[−20,−6,−18], and right amygdala activity, t(87)=11.46, p fwe-corr<0.001, k=202, [22, −6, −14]. For fearful faces > shapes, there was significant left, t(87)=8.54, p fwe-corr<0.001, k=117, [−20,−6,−18], and right amygdala activity, t(87)=9.80, p fwe-corr<0.001, k=188, [22, −6, −14]. For angry faces > shapes, there was significant left, t(87)=9.81, p fwe-corr<0.001, k=133, [−20, −6, −18], and right amygdala activity, t(87)=9.84, p fwe-corr<0.001, k=196, [22, −4, −18]. For happy faces > shapes, there was significant left, t(87)=7.97, p fwe-corr<0.001, k=97, [−20, −8, −16], and right amygdala activity, t(87)=9.49, p fwe-corr<0.001, k=19, [24, −8, −14]. There was also significant activity for the contrast of angry and fearful faces > happy faces for the left amygdala, t(87)=3.27, p fwe-corr=.023, k=15, [−28, 0, −22], and right amygdala, t(87)=3.53, p fwe-corr=.013, k=12, [30, −4, −20].
3.2. Associations between peripheral inflammatory markers and brain activity
There was a significant positive association between TNF-α and bilateral threat-related (angry and fearful faces > shapes) amygdala activity, B=0.07, SE=0.02, standardized Beta=0.47, p=0.002, change R2=0.12 (Figure 1). The IL-6/CRP composite was not significantly associated with bilateral threat-related amygdala activity (p=0.58). Supplementary sensitivity analyses indicated that TNF-α was significantly associated with amygdala activity to fearful faces, B=0.06, SE=0.03, standardized Beta=0.33, p=0.027, change R2=0.06, amygdala activity to angry faces, B=0.09, SE=0.03, Beta=0.42, p=0.004, change R2=0.10, and amygdala activity to happy faces, B=0.10, SE=0.02, Beta=0.61, p<0.001, change R2=0.20. The sensitivity analysis for the contrast of angry and fearful faces > happy faces was not significant, B=−0.03, SE=0.03, Beta=−0.15, p=.316, indicating that the strength of the association between TNF-α and amygdala activity to angry and fearful faces was not significantly different from the strength of the association between TNF-α and amygdala activity to happy faces. None of the sensitivity analyses conducted with the IL-6/CRP composite were significant.
Figure 1. Association between TNF-α and bilateral amygdala activity to angry and fearful faces > shapes.
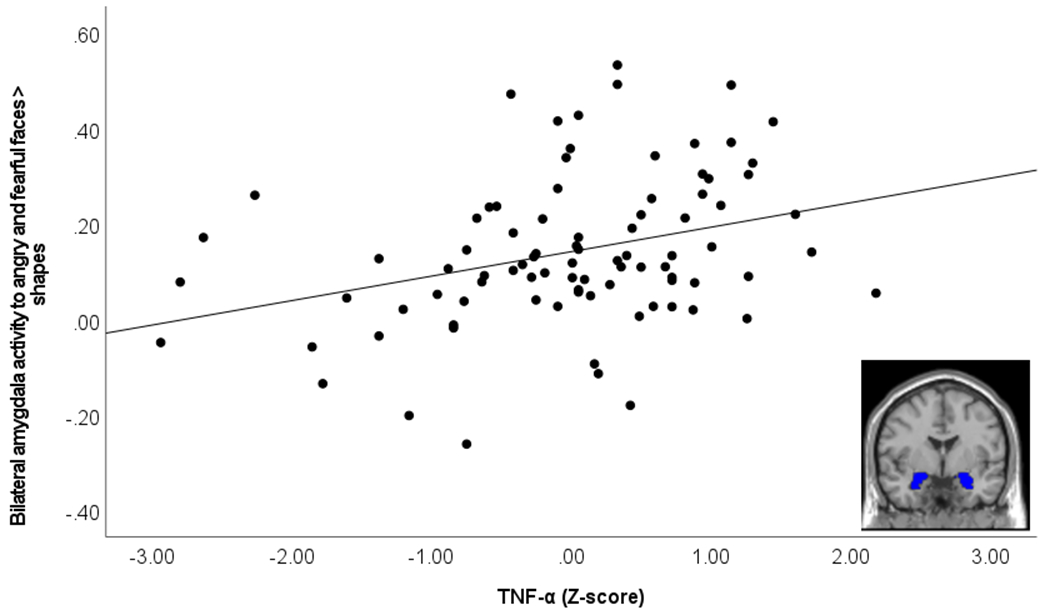
Scatterplot demonstrates association between TNF-α (log-transformed and Z-scored) and amygdala activity to angry and fearful faces > shapes. Image in bottom right of scatterplot demonstrates bilateral amygdala region of interest used to extract contrast values.
Results of the exploratory whole-brain activity analyses are reported in Table 4 and Figure 2. These results indicated that TNF-α was associated with increased left precentral gyrus activity to angry and fearful faces > shapes; increased activity in frontal and temporal regions to angry faces > shapes; and increased activity in occipital, parietal, frontal, subcortical, and temporal regions to happy faces > shapes. In addition, there was a negative association between TNF-α and activity in the cerebellum and occipital regions for the contrast of angry and fearful faces > happy faces, indicating there was a stronger positive association between TNF-α and activity to happy faces in these regions relative to angry and fearful faces.
Table 4.
Results from whole-brain activity analysis
| t(77)= | p-uncorrected | Cluster size | Peak coordinates (x, y, z) | Label of region | Direction of association | |
|---|---|---|---|---|---|---|
| Angry and fearful faces > Shapes | ||||||
| TNF-α | 3.74 | <.001 | 247 | (−48, 4, 36) | Left precentral gyrus | Positive |
| Angry faces > Shapes | ||||||
| TNF-α | 4.78 | <.001 | 1,714 | (−52, 18, 34) | Left inferior frontal gyrus, triangular part | Positive |
| 4.75 | <.001 | 187 | (−48, −42, −12) | Left inferior temporal gyrus | Positive | |
| 4.05 | <.001 | 414 | (18, −28, 72) | Right superior frontal gyrus, dorsolateral; right supplementary motor area | Positive | |
| 3.57 | <.001 | 273 | (−28, 34, 46) | Left superior frontal gyrus, dorsolateral; left superior frontal gyrus, medial | Positive | |
| Happy faces > Shapes | ||||||
| TNF-α | 5.64 | <.001 | 14,720 | (52, −66, −6) | White matter; left middle occipital gyrus; left inferior parietal gyrus; right superior occipital gyrus; left superior occipital gyrus | Positive |
| 5.14 | <.001 | 1,439 | (−50, 24, 26) | Left inferior frontal gyrus, triangular part | Positive | |
| 4.75 | <.001 | 301 | (44, −36, 12) | Right superior temporal gyrus | Positive | |
| 4.41 | <.001 | 396 | (24, −36, −6) | White matter; right putamen | Positive | |
| 4.13 | <.001 | 320 | (36, 18, 24) | Right inferior frontal gyrus, triangular part; white matter | Positive | |
| 3.84 | <.001 | 283 | (−32, −2, 62) | Left superior frontal gyrus, dorsolateral; left precentral gyrus | Positive | |
| Angry and fearful faces > happy faces | ||||||
| TNF-α | 4.66 | <.001 | 389 | (−8, −74, −18) | Left cerebellum | Negative |
| 4.42 | <.001 | 1,029 | (−8, −84, 10) | Left calcarine fissure; left middle occipital gyrus | Negative | |
| 4.41 | <.001 | 420 | (−26, 80, 34) | Left superior occipital gyrus; left cuneus; left precuneus | Negative | |
| 4.18 | <.001 | 188 | (22, −66, 18) | Right cuneus; right calcarine fissure | Negative | |
Note: Labels are based on the Automated Anatomical Labeling (AAL) atlas v3. Labels include regions in which 20% or more of the cluster fall within that label. For clusters in which no region was more than 20% of cluster, the region with the highest percentage is reported. For clusters in which white matter is the top region, the region below that is also reported. For the large cluster activated to happy faces > shapes, we report the top 5 regions in that cluster.
Figure 2. Associations between TNF-α and whole-brain activity to emotional face expressions.
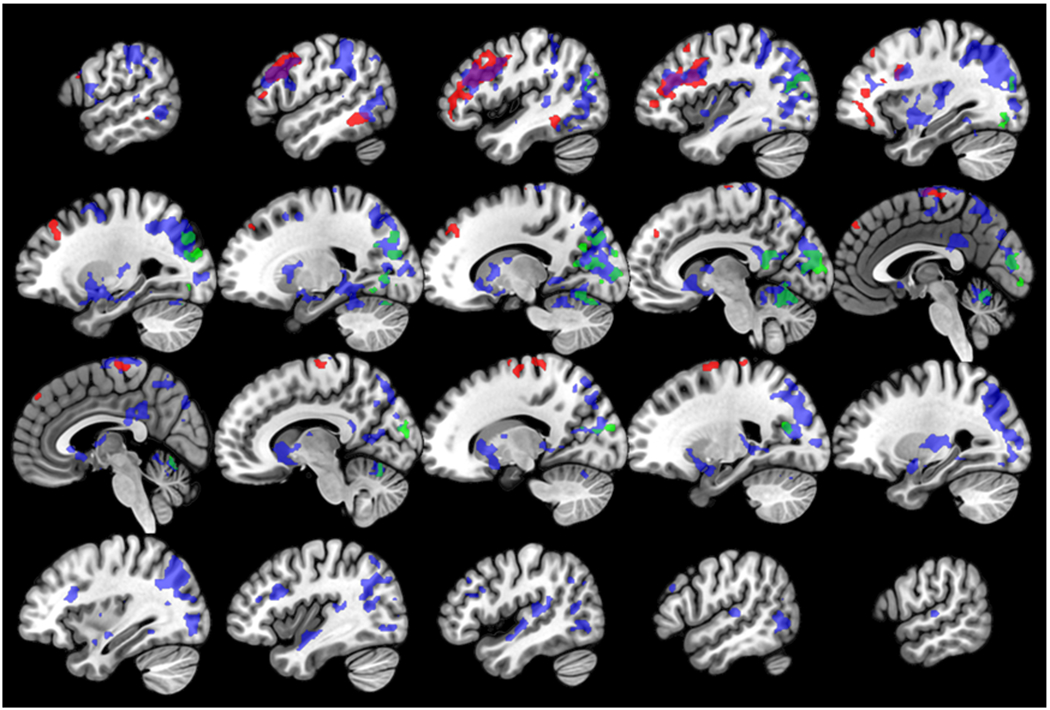
Figure demonstrates associations between TNF-α and activity for the contrasts of angry faces > shapes (red), happy faces > shapes (blue), and angry and fearful faces > happy faces (green). Purple regions demonstrate overlap between activity to the angry faces > shapes and happy faces > shapes contrasts, and teal regions demonstrate overlap between activity for the happy faces > shapes and angry and fearful faces > happy faces contrasts. Slices are shown in sagittal orientation, from X=−56 to X=58, in increments of 6.
3.3. Associations between peripheral inflammatory markers and amygdala connectivity
We first tested for a main effect of connectivity between the bilateral amygdala and ventral prefrontal cortex ROI for the contrast of angry and fearful faces > shapes. This analysis identified two clusters in the ventral prefrontal cortex ROI that demonstrated a significant negative PPI with the amygdala for this contrast. The first cluster was in the left superior frontal gyrus, orbital region, t(87)=4.98, p-uncorrected<0.001, cluster size = 38 voxels, peak MNI coordinates = (−24, 38, −14). The second cluster was in the right superior frontal gyrus, medial orbital region, t(87)=4.13, p-uncorrected<0.001, cluster size = 121 voxels, peak MNI coordinates = (8, 40, −4).
We next tested for associations with the inflammatory markers. There were no significant results for the association between TNF-α or IL-6/CRP and threat-related amygdala connectivity with the a priori ventral PFC ROI. Results of the exploratory whole-brain analyses are provided in Table 5. TNF-α was associated with reduced bilateral amygdala connectivity with the left cuneus, right cuneus, calcarine fissure, and precuneus, and left supramarginal gyrus and inferior parietal gyrus during angry and fearful faces > shapes (Table 5; Figure 3). IL-6/CRP was associated with reduced bilateral amygdala connectivity with the right postcentral gyrus and right precuneus during angry and fearful faces > shapes (Table 5; Figure 4) and reduced bilateral amygdala connectivity with left middle occipital gyrus and bilateral precuneus to angry faces > shapes (Table 5). There were no significant associations between the inflammatory marker variables and amygdala connectivity during happy faces > shapes.
Table 5.
Results from whole-brain gPPI analyses
| t(77)= | p-uncorrected | Cluster size | Peak coordinates (x, y, z) | Label of region | Direction of association | |
|---|---|---|---|---|---|---|
| Angry and fearful faces > Shapes | ||||||
| TNF-α | 3.96 | <.001 | 144 | (−6, −82, 26) | Left cuneus | Negative |
| 3.95 | <.001 | 147 | (14, −62, 20) | Right cuneus; right calcarine fissure; right precuneus | Negative | |
| 3.88 | <.001 | 119 | (−60, −50, 30) | Left inferior parietal gyrus; left supramarginal gyrus | Negative | |
| IL-6/CRP | 4.37 | <.001 | 154 | (42, −18, 32) | White matter; right postcentral gyrus | Negative |
| 4.18 | <.001 | 191 | (14, −52, 12) | Right precuneus | Negative | |
| Angry > Shapes | ||||||
| IL-6/CRP | 4.01 | <.001 | 172 | (−38, −74, 22) | Left middle occipital gyrus | Negative |
| 3.95 | <.001 | 292 | (12, −50, 12) | Right precuneus; left precuneus | Negative | |
Note: Labels are based on the Automated Anatomical Labeling (AAL) atlas v3. Labels include regions in which 20% or more of the cluster fall within that label.
Figure 3. Association between TNF-α and bilateral amygdala connectivity during angry and fearful faces > shapes.
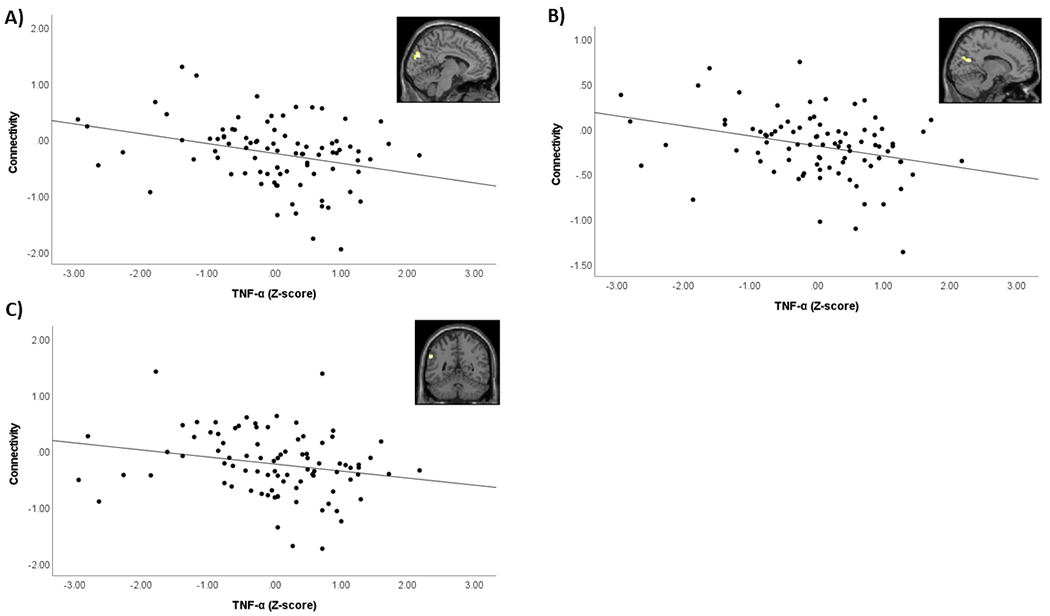
Scatterplots demonstrate association between TNF-α (log-transformed and Z-scored) and the PPI contrast value extracted from significant clusters. Images in top right of each scatterplot demonstrate location of significant cluster identified from whole-brain analysis. A) Association between TNF-α and bilateral amygdala connectivity with left cuneus. B) Association between TNF-α and bilateral amygdala connectivity with right cuneus/calcarine fissure/precuneus. C) Association between TNF-α and bilateral amygdala connectivity with left supramarginal gyrus/inferior parietal gyrus.
Figure 4. Association between IL-6/CRP composite and bilateral amygdala connectivity during angry and fearful faces > shapes.
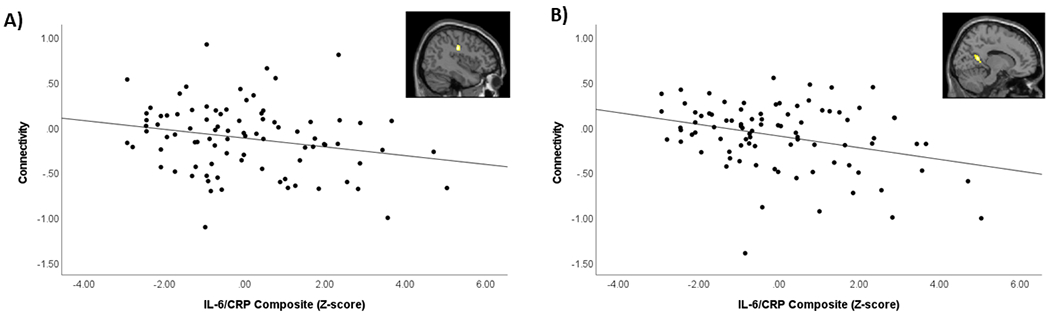
Scatterplots demonstrate association between IL-6/CRP composite (Z-score) and the PPI contrast value extracted from significant clusters. Images in top right of each scatterplot demonstrate location of significant cluster identified from whole-brain analysis. A) Association between IL-6/CRP composite and bilateral amygdala connectivity with right postcentral gyrus. B) Association between IL-6/CRP composite and bilateral amygdala connectivity with right precuneus.
3.4. Exploratory moderator analyses
We conducted exploratory analyses to examine whether associations between the inflammatory markers and amygdala activity were moderated by subjective SES or gender. Neither the interaction between TNF-α and subjective SES (p=0.66) nor the interaction between IL-6/CRP and subjective SES (p=0.21) were a significant predictor of bilateral threat-related amygdala activity. Supplementary analyses with parent-reported subjective SES were also not significant. Likewise, neither the interaction between TNF-α and gender (p=0.78) nor the interaction between IL-6/CRP and gender (p=0.08) were a significant predictor of bilateral threat-related amygdala activity. We also conducted whole-brain exploratory analyses to examine whether associations between the inflammatory markers and amygdala connectivity modeled with gPPI were moderated by subjective SES or gender. None were significant.
4. Discussion
The aims for this paper were to examine the association between peripheral inflammatory markers and threat-related amygdala activity and connectivity in adolescents. Our first hypothesis was that higher levels of peripheral inflammatory markers would be associated with increased threat-related amygdala activity. This hypothesis was supported for TNF-α, in that we found a significant positive association between TNF-α and bilateral threat-related amygdala activity. An unexpected finding from the supplementary sensitivity analyses was that not only was TNF-α associated with increased amygdala activity to threatening (angry and fearful) faces, but it was also associated with increased amygdala activity to happy faces. We did not find a significant association between the IL-6/CRP composite and amygdala activity to any of the facial expressions. Our second hypothesis was that higher levels of peripheral inflammatory markers would be associated with reduced threat-related connectivity between the amygdala and ventral prefrontal cortex. This hypothesis was not supported. However, the exploratory whole-brain analysis identified several regions outside of the ventral prefrontal cortex in which increased peripheral inflammation was associated with reduced amygdala connectivity when viewing threatening faces. Below, we discuss these findings in more detail.
Our finding that higher levels of peripheral TNF-α were associated with increased amygdala activity to threatening faces is generally consistent with prior research finding associations between increased peripheral inflammation and increased amygdala activity to social threat. However, differences across methodology make it difficult to directly compare the results to prior research. For example, one study conducted in adults used an endotoxin inflammatory challenge to examine the association between inflammation and amygdala activity (Inagaki et al., 2012), but specific inflammatory markers were not examined in this study. Another study conducted in adults found that CRP was associated with increased amygdala activity to threatening faces (Swartz et al., 2017), but TNF-α was not examined in that study and so it is unclear if similar effects would have been observed for TNF-α. In a study conducted in adolescents, higher peripheral inflammatory markers were associated with increased amygdala activity to threatening faces (Miller et al., 2021), but this study used an inflammatory marker composite that included TNF-α along with several other markers, and so it is unclear if this effect would be significant for TNF-α alone. Overall, studies are generally consistent in finding that higher peripheral inflammation is associated with increased threat-related amygdala activity, but more research is needed to examine whether effects are specific to certain inflammatory markers such as TNF-α. Research in animal models indicates that increased expression of TNF-α in the basolateral amygdala is associated with increased excitatory signaling and decreased inhibitory signaling (Chen et al., 2013), which could help explain the association between TNF-α and heightened amygdala activity.
An unexpected finding from this study was that higher TNF-α was associated with increased amygdala activity to happy facial expressions. This suggests that peripheral inflammation may increase amygdala sensitivity not just to threat, but to other types of salient, social stimuli such as positive facial expressions during adolescence. This is somewhat consistent with prior research in adults that has found that an inflammatory challenge with endotoxin increases neural sensitivity to both negative and positive social feedback (Muscatell et al., 2016). However, this is not consistent with prior research in adults that showed that an inflammatory challenge increased amygdala activity to social threat (fearful facial expressions) but not to non-threatening social images (happy facial expressions) (Inagaki et al., 2012) or with a study conducted in older adults from the Midlife in the United States study that found that higher peripheral inflammation was associated with reduced limbic activity when viewing positive images (Alvarez, Hackman, Miller, & Muscatell, 2020). Further research is needed to clarify how associations between peripheral inflammation and amygdala activity differ based on inflammatory marker, type of social stimuli, and developmental stage. If results of the current study are confirmed in future research with adolescents, this suggests that TNF-α may increase sensitivity to both negative and positive social stimuli in adolescents, and so its effect on later health-related outcomes may depend on the individual’s social context, with more negative health effects predicted in more stressful and negative social contexts. Contrary to the findings of Miller et al. (2021), we did not find that SES moderated the association between the inflammatory markers and amygdala activity. This could be due to the fact that we did not specifically target a low SES sample for recruitment, and likely did not have sufficient variation in this variable to observe strong moderating effects.
The exploratory whole-brain analyses identified additional regions in which activity was associated with TNF-α. Specifically, TNF-α was associated with increased activity in the left precentral gyrus to angry and fearful faces > shapes, increased activity to angry faces > shapes in frontal regions and temporal regions, and increased activity to happy faces > shapes in occipital, parietal, frontal, subcortical, and temporal regions. A negative association between TNF-α and the contrast of angry and fearful faces > happy faces indicated a stronger positive association between TNF-α and activity in occipital regions and the cerebellum to happy faces relative to angry and fearful faces. Although these results are exploratory and caution should be taken in interpreting whole-brain effects until confirmed in future analyses, there are a few interesting patterns to note. First, TNF-α was associated with increased activity to emotional faces across regions which, in other studies, have been involved in visual and face processing, sensorimotor processing, emotional processing, mentalizing, language processing, and executive functions such as attention regulation and working memory (Del Casale et al., 2017; du Boisgueheneuc et al., 2006; Eraldo et al., 1997; Fusar-Poli et al., 2009; Haber, 2016; Mukerji, Lincoln, Dodell-Feder, & Nelson, 2019; Rotshtein, Vuilleumier, Winston, Driver, & Dolan, 2007). Speculatively, this suggests TNF-α is associated with increased neural activity involved in a range of processes in response to emotional faces. Second, activity effects were detected for happy faces and for angry faces, whereas no whole-brain effects were identified for fearful faces. Some effects (especially in visual processing regions such as the occipital cortex) were significantly stronger for happy faces relative to angry and fearful faces. It is unclear what is driving the differences in effects for these different emotional face conditions. Differences could be driven by developmental differences in sensitivity to different emotional face expressions (Rutter et al., 2019), differences in emotion recognition accuracy for different emotional face expressions (Calvo & Beltran, 2013), differences in the approach-avoidance orientation of happy and angry faces compared to fearful faces with directed eye gaze (Adams Jr. & Kleck, 2003), or other factors. As discussed above, the finding that TNF-α is associated with increased neural activity to both positive and negative emotional stimuli suggests that it may be associated with increased sensitivity to emotional or salient stimuli in general, such that effects for later socio-emotional outcomes would be dependent on an individual’s social context. Further research is needed to confirm these whole-brain effects and to determine which aspects of the stimuli are driving differences in the effects to different emotional face expressions. It is also notable that the IL-6/CRP composite was not significantly associated with activity in the whole-brain analyses, and that effects for this marker were more evident for connectivity, as we discuss next.
Our second hypothesis that higher peripheral inflammation would be associated with reduced amygdala-prefrontal cortex connectivity was not supported. One possible explanation for why an association between inflammation and prefrontal cortex-amygdala connectivity is observed in adults (Mehta et al., 2018), but not adolescents, is that this circuitry undergoes major organizational changes across the period of adolescence, shifting from a pattern of positive to negative task-based connectivity (Gee et al., 2013). Thus, while this circuitry is going through a period of reorganization and flux, there may be weaker associations between peripheral inflammatory markers and prefrontal cortex-amygdala connectivity. Given the speculation that amygdala activity during childhood and early adolescence drives the development of connections with the prefrontal cortex (Tottenham & Gabard-Durnam, 2017), the finding here that TNF-α is associated with increased amygdala activity suggests that this could have later effects on the development of amygdala-prefrontal cortex connectivity that may become observable during late adolescence or adulthood. Further longitudinal research, as well as cross-sectional research that spans the periods of childhood through adulthood, is needed to examine these possibilities further.
Results of exploratory analyses did identify several patterns of amygdala connectivity with other parts of the brain outside of the ventral prefrontal cortex associated with the inflammatory markers. First, we found a significant negative association between TNF-α and bilateral amygdala connectivity with visual processing regions including the cuneus, calcarine sulcus, and precuneus during angry and fearful faces > shapes. We also found that the IL-6/CRP composite was associated with decreased bilateral amygdala connectivity with visual processing regions including the precuneus during angry and fearful faces>shapes and the left middle occipital gyrus and precuneus during angry faces > shapes. Second, we found a negative association between TNF-α and bilateral amygdala connectivity with the left supramarginal gyrus/inferior parietal gyrus. Finally, IL-6/CRP was associated with reduced bilateral amygdala connectivity with the postcentral gyrus. Interestingly, in our previous paper examining resting-state functional connectivity in this sample (Swartz et al., 2021), we did not observe any significant associations between IL-6/CRP and amygdala connectivity. The findings in the current study suggest that associations between IL-6/CRP and amygdala connectivity may only be observed during specific cognitive processes, in this case, processing threatening facial expressions. The regions identified in these connectivity analyses have previously been shown to be involved in visual processing, attention regulation in the context of emotional stimuli, emotion perception, and emotion regulation (Ferri, Schmidt, Hajcak, & Canli, 2016; Li et al., 2019; Loeffler et al., 2000; Sander et al., 2005; Yoon, Shim, Kim, & Lee, 2015). Speculatively, the decreased connectivity between the amygdala and these regions associated with higher TNF-α and IL-6/CRP may be associated with difficulties in functions that involve these processes. Because these whole-brain effects were not hypothesized, further research is needed to confirm whether these effects are replicated, and whether these connectivity patterns predict future socio-emotional difficulties. If results are replicated, further research using animal models and other mechanistic approaches will also be needed to determine the biological mechanisms through which peripheral TNF-α and IL-6/CRP have distinct associations with amygdala activity and connectivity.
Results should be interpreted with respect to several limitations. First, we were only able to examine peripheral markers of inflammation. Although peripheral inflammatory mediators have been shown to cross the blood-brain barrier through several routes (Capuron & Miller, 2011), the correspondence between peripheral and central inflammation in our sample of adolescent participants cannot be determined from this study. Second, this study is cross-sectional, so we cannot determine the direction of associations between peripheral inflammation and brain function. It could be that higher levels of peripheral inflammation cause increases in emotion-related amygdala activity and decreased connectivity, or that higher emotion-related amygdala activity and decreased connectivity cause higher levels of peripheral inflammation. Or it could be that both of these processes operate in a bi-directional manner, as hypothesized by the neuro-immune network hypothesis (Nusslock & Miller, 2016). Further research using inflammatory challenge paradigms and using longitudinal measures of inflammation and brain function will be necessary to examine the direction of effects underlying the cross-sectional associations observed here. Third, TNF-α was significantly associated with amygdala activity to both threatening (angry and fearful) and positively-valenced (happy) emotional faces, and there was no significant difference between these effects when we examined the contrast of angry and fearful faces > happy faces. Therefore, it is unclear which aspects of the stimuli are driving these effects (e.g., the social nature of the stimuli, the emotional nature, etc.). Further research is needed that manipulates different aspects of the emotional stimuli (e.g., social vs. nonsocial emotional stimuli, face morphs that vary in intensity, comparing activity to faces with directed vs. averted eye gaze, etc.) to tease apart these effects. Fourth, there was some variation in time of day of the blood sample and the number of days between collecting the blood sample and the fMRI scan across participants. There may also have been some variation introduced by participants who were taking medications. However, we controlled for all of these factors as covariates in analyses. Fifth, prior research in other samples suggests the within-subject test-retest reliability of amygdala activity to emotional faces is poor (Elliott et al., 2020; Plichta et al., 2012; Vetter et al., 2017), which could add noise to the estimates of amygdala activity and may help to explain the lack of significant effects in our moderator analyses. Sixth, BMI was highly correlated with the IL-6/CRP composite in this sample; therefore, controlling for BMI may have removed some effects of IL-6/CRP on activity and connectivity that were confounded with BMI. Finally, as discussed above, there were several limitations of the SES analysis that may explain why we did not detect a significant moderating effect. Moderating effects are likely to be strongest for families below the poverty line (Miller et al., 2021), but we did not target low-income families or families living below the poverty line for recruitment. Also, the subjective SES measure asked about facets of SES (e.g., respected job status) that would not be relevant to adolescents, and so a subjective SES measure worded towards asking adolescents about their parents’ occupational/educational status would be ideal in future research. Small sample size may have also contributed to the lack of significant moderating effects for SES and gender. Indeed, the interaction between IL-6/CRP and gender approached significance, suggesting that we may have observed a significant interaction with a larger sample size. Future research with larger sample sizes will be needed to better examine these potential moderating factors, and other factors that may moderate these effects such as pubertal status.
In summary, this study examined associations between peripheral inflammatory markers and amygdala activity and connectivity during emotional face matching. Results indicated that higher TNF-α is associated with increased amygdala activity to both threatening facial expressions as well as happy facial expressions, and increased activity in several other regions to angry and happy facial expressions. We also found that higher TNF-α and IL-6/CRP were associated with reduced amygdala connectivity with several occipital and parietal regions when matching threatening faces vs. shapes, which in prior research have been associated with emotion perception, attention regulation, and emotion regulation. These results lay the groundwork for translational work that could target inflammation, such as mind-body interventions (Bower et al., 2014; Bower & Irwin, 2016; Creswell et al., 2012; Morgan, Irwin, Chung, & Wang, 2014). Future translational work in adolescents can help to experimentally address whether targeting inflammation influences the function of the neural circuitry identified in this study, and whether this has effects on socio-emotional functioning during adolescence.
Supplementary Material
Acknowledgments
This work was supported by UC Davis, Prop. 63, the Mental Health Services Act and the Behavioral Health Center of Excellence at UC Davis (JRS) and the USDA National Institute of Food and Agriculture, Hatch project 1013485 (JRS). AFC was supported by the Eugene Cota Robles Fellowship from UC Davis. CEH was supported by the National Institute of Child Health and Human Development, grant R01 HD104185. This work was also supported in part by the Norman Cousins Center for Psychoneuroimmunology at UCLA (MRI). Part of this research was conducted at the UC Davis Clinical and Translational Science Center Clinical Research Center, which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. The authors would like to thank the participants of the study for their time.
Footnotes
Disclosures
No author has a conflict of interest to disclose.
References
- Adams RB Jr., & Kleck RE (2003). Perceived gaze direction and the processing of facial displays of emotion. Psychological Science, 14(6), 644–647. [DOI] [PubMed] [Google Scholar]
- Adler N, Espel E, Castellazzo G, & Ickovics J (2000). Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychol, 19(6), 586–592. [DOI] [PubMed] [Google Scholar]
- Alvarez GM, Hackman DA, Miller AB, & Muscatell KA (2020). Systemic inflammation is associated with differential neural reactivity and connectivity to affective images. Social Cognitive and Affective Neuroscience, 15(10), 1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CCC, Caballero C, Scherer E, West MR, Mrazek MD, Phillips DT, … Gabrieli JDE (2019). Mindfulness training reduces stress and amygdala reactivity to fearful faces in middle-school children. Behavioral Neuroscience, 133(6), 569–585. [DOI] [PubMed] [Google Scholar]
- Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, … Cole SW (2014). Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Pscyhoneuroendocrinology, 43, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, & Irwin MR (2016). Mind-body therapies and control of inflammatory biology: A descriptive review. Brain, Behavior, and Immunity, 51, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, & Beltran D (2013). Recognition advantage of happy faces: Tracing the neurocognitive processes. Neuropsychologia, 51(11), 2051–2061. [DOI] [PubMed] [Google Scholar]
- Capuron L, & Miller AH (2011). Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacology & Therapeutics, 130, 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Song Y, Yang J, Zhang Y, Zhao P, Zhu X, & Su H (2013). The contribution of TNF-a in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci Lett, 541, 275–280. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JMG, Ma J, … Cole SW (2012). Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain, Behavior, and Immunity, 26(7), 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KA, Cooper EA, Voon V, Tibble J, Cercignani M, & Harrison NA (2021). Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms. Molecular Psychiatry, 26, 5150–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Casale A, Kotzalidis GD, Rapinesi C, Janiri D, Aragona M, Puzella A, … Girardi P (2017). Neural functional correlates of empathic face processing. Neurosci Lett, 655, 68–75. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, … Dubois B (2006). Functions of the left superior frontal gyrus in humans; a lesion study. Brain, 129, 3315–3328. [DOI] [PubMed] [Google Scholar]
- Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S, … Hariri AR (2020). What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychological Science, 31(7), 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraldo P, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, … Fazio F (1997). Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. NeuroReport, 8(8), 2011–2016. [DOI] [PubMed] [Google Scholar]
- Ferri J, Schmidt J, Hajcak G, & Canli T (2016). Emotion regulation and amygdala-precuneus connectivity: Focusing on attentional deployment. Cognitive, Affective & Behavioral Neuroscience, 16, 991–1002. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, … Politi P (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 fucntional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34(6), 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys K, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci, 33(10), 4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe N, Khan I, Hayes J, Erlenbach E, & Damoiseaux JS (2019). Yoga effects on brain health: A systematic review of the current literature. Brain Plasticity, 5(1), 105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18(1), 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, … Weinberger DR (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science, 297(5580), 400–403. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, & Eisenberger NI (2012). Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage, 59(4), 3222–3226. doi: 10.1016/j.neuroimage.2011.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, … Naliboff BD (2011). Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. NeuroImage, 56(1), 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrenz F, Wrede K, Forsting M, Engler H, Schedlowski M, Elsenbruch S, & Benson S (2016). Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men. Brain Behav Immun, 54, 17–26. doi: 10.1016/j.bbi.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang M, Li K, Zou F, Wang Y, Wu X, & Zhang H (2019). The altered somatic brain network in state anxiety. Frontiers in Psychiatry, 10, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler LAK, Satterthwaite TD, Habel U, Schneider F, Radke S, & Derntl B (2000). Attention control and its emotion-specific association with cognitive emotion regulation in depression. Brain Imaging and Behavior, 13, 1766–1779. [DOI] [PubMed] [Google Scholar]
- Lutz J, Herwig U, Opialla S, Hittmeyer A, Jancke L, Rufer M, … Bruhl AB (2014). Mindfulness and emotion regulation--an fMRI study. Social Cognitive and Affective Neuroscience, 9(6), 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J, & Kraft R (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- McLaren D, Ries M, Xu G, & Johnson S (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, & Felger JC (2018). Inflammation negatively correlates with amygdala-ventromedial prefrontal connectivity in association with anxiety in patients with depression: Preliminary results. Brain, Behavior, and Immunity, 73, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, White SF, Chen E, & Nusslock R (2021). Association of inflammatory activity with larger neural responses to threat and reward among children living in poverty. American Journal of Psychiatry, 178, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N, Irwin MR, Chung M, & Wang C (2014). The effects of mind-body therapies on the immune system: meta-analysis. PloS One, 9(7), e100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji CE, Lincoln SH, Dodell-Feder D, & Nelson CA (2019). Neural correlates of theory-of-mind are associated with variation in children’s everyday social cognition. SCAN, 14(6), 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, & Bower JE (2015). Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun, 43, 46–53. doi: 10.1016/j.bbi.2014.06.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, … Eisenberger NI (2016). Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav Immun, 57, 21–29. doi: 10.1016/j.bbi.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Brody GH, Armstrong CC, Carroll AL, Sweet LH, Yu T, … Miller GE (2019). Higher peripheral inflammatory signaling associated with lower resting-state functional brain connectivity in emotion regulation and central executive networks. Biological Psychiatry, 86(2), 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry, 80(1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, … Meyer-Lindenberg A (2012). Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. NeuroImage, 60(3), 1746–1758. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Vuilleumier P, Winston J, Driver J, & Dolan R (2007). Distinct and convergent visual processing of high and low spatial frequency information in faces. Cereb Cortex, 17(11), 2713–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter LA, Dodell-Feder D, Vahia IV, Forester BP, Ressler KJ, Wilmer JB, & Germine L (2019). Emotion sensitivity across the lifespan: Mapping clinical risk periods to sensitivity to facial emotion intensity. Journal of Experimental Psychology: General, 148(11), 1993–2005. [DOI] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, & Vuilleumier P (2005). Emotion and attention interactions in social cognition: Brain regions involved in processing anger prosody. NeuroImage, 28(4), 848–858. [DOI] [PubMed] [Google Scholar]
- Sanger KL, & Dorjee D (2015). Mindfulness training for adolescents: A neurodevelopmental perspective on investigating modifications in attention and emotion regulation using event-related brain potentials. Cognitive, Affective & Behavioral Neuroscience, 15, 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, & Newberg AB (2015). Mind-body practices and the adolescent brain: Clinical neuroimaging studies. Adolesc Psychiatry, 5(2), 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Adler NE, & Marmot MG (2003). Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Social Science & Medicine, 56(6), 1321–1333. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Carranza AF, & Knodt AR (2019). Amygdala activity to angry and fearful faces relates to bullying and victimization in adolescents. Social Cognitive and Affective Neuroscience, 14(10), 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carranza AF, Tully LM, Knodt AR, Jiang J, Irwin MR, & Hostinar CE (2021). Associations between peripheral inflammation and resting state functional connectivity in adolescents. Brain, Behavior, and Immunity, 95, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Prather AA, & Hariri AR (2017). Threat-related amygdala activity is associated with peripheral CRP concentrations in men but not women. Psychoneuroendocrinology, 78, 93–96. doi: 10.1016/j.psyneuen.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, & Hariri AR (2015). Developmental change in amygdala reactivity during adolescence: Effects of family history of depression and stressful life events. Am J Psychiatry, 172(3), 276–283. doi: 10.1176/appi.ajp.2014.14020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, & Thapar AK (2012). Depression in adolescence. Lancet, 379(9820), 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Gabard-Durnam L (2017). The developing amygdala: a student of the world and a teacher of the cortex. Current Opinion in Psychology, 17, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res, 168(3), 242–249. doi: 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Vetter NC, Steding J, Jurk S, Ripke S, Mennigen E, & Smolka MN (2017). Reliability in adolescent fMRI within two years - a comparison of three tasks. Scientific Reports, 7, 2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Shim M, Kim HS, & Lee S-H (2015). Enhanced early posterior negativity to fearful faces in patients with anxiety disorder. Brain Topography, 29, 262–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


