Summary
Background
Real-world data is currently limited on the association between oral antiviral therapy and healthcare system burden in patients with mild-to-moderate COVID-19. This study aims to evaluate the clinical and cost effectiveness of Molnupiravir and Nirmatrelvir-ritonavir use in reducing mortality in this population.
Methods
This is a retrospective cohort study involving 54,355 COVID-19 patients during February 22–March 31,2022 in Hong Kong. Inverse probability of treatment weighting (IPTW) was used to adjust patient characteristics. Our exposure of interest was Molnupiravir/Nirmatrelvir-Ritonavir prescription, with all-cause mortality as the primary outcome. IPTW-adjusted multivariate regressions were used to estimate treatment impact on clinic re-attendance and unplanned admissions. Finally, attributed cost and incremental cost-effectiveness ratios (ICER) were estimated.
Findings
In the outpatient cohort (N = 33,217, 61.1%), 16.1% used Molnupiravir and 13.4% used Nirmatrelvir-Ritonavir, while in the inpatient cohort (N = 21,138, 38.9%), 3.8% used Molnupiravir and 1.3% used Nirmatrelvir-Ritonavir. IPTW-adjusted Cox model estimated that Molnupiravir (hazard ratio (HR)(95%CI)=0.31 (0.24-0.40), P< 0.0001) and Nirmatrelvir-Ritonavir (HR=0.10 (95%CI 0.05-0.21), P< 0.0001) were significantly associated with a reduced mortality hazard. In the outpatient cohort, both antiviral prescriptions were associated with reduced odds for unplanned hospital admissions (Molnupiravir: odds ratio (OR) =0.72 (0.52-0.98), P=0.039; Nirmatrelvir-Ritonavir: OR=0.37 (0.23-0.60), P<0.0001). Among hospitalised patients, both antiviral prescriptions were associated with significant reductions in the odds ratios for 28-days readmission (Molnupiravir: OR=0.71 (0.52-0.97), P=0.031; Nirmatrelvir-Ritonavir: OR=0.47 (0.24-0.93), P=0.030). ICERs for death averted for Molnupiravir stood at USD493,345.09 in outpatient settings and USD2,629.08 in inpatient settings. In outpatient settings, Nirmatrelvir-ritonavir cost USD331,105.27 to avert one death, but saved USD5,502.53 to avert one death in comparison with standard care.
Interpretation
In high-risk patients in Hong Kong with mild-to-moderate COVID-19, Molnupiravir and Nirmatrelvir-Ritonavir prescriptions were associated with reduced all-cause mortality and significant cost savings.
Funding
Centre for Health Systems & Policy Research is funded by The Tung's Foundation; and The Laboratory of Data Discovery for Health Limited(D24H) is funded the AIR@InnoHK platform administered by the Innovation and Technology Commission of Hong Kong. Funders did not have any role in study design, data collection, data analysis, interpretation and writing of this manuscript.
Keywords: Avoidable healthcare system cost, COVID-19, Oral antiviral
Research of context.
Evidence before this study
Oral antivirals, such as Molnupiravir and Nirmatrelvir-Ritonavir, are important treatment option caring for people with mild-to-moderate COVID-19. In randomized controlled trials, both antivirals demonstrated a significant reduction in risk of hospitalization or deaths. Recent real work data in Israel shows that Nirmatrelvir-Ritonavir is highly effective in reducing risk of severe COVID-19 and mortality, but currently no evidence reported the impact. Further investigation is needed to understand the association between oral antiviral therapy and healthcare system burden in this population.
Added value of this study
In this retrospective cohort study that used inverse probability of treatment weighting-adjusted analysis and included 54,355 patients with mild-to-moderate COVID-19, early oral antiviral (Molnupiravir or Nirmatrelvir-Ritonavir) use, compared with no oral antiviral use, was significantly associated with a lower risk of all-cause mortality (hazard ratios, 0.31 for Molnupiravir and 0.10 for Nirmatrelvir-ritonavir respectively).
Implications of all the available evidence
Among high-risk COVID-19 patients in mild condition, antiviral therapy was significantly associated with a lower risk of mortality.
Alt-text: Unlabelled box
Introduction
With increasing understanding of the SARS-CoV-2, there has been significant progress in therapeutic options available to COVID-19 patients,1 in outpatient facilities. Antiviral agents, such as Molnupiravir and Nirmatrelvir-ritonavir, are expected to reduce virus replication to prevent hospitalization, clinical deterioration and mortality. Molnupiravir, for instance, reduced hospitalization or death at 29 days by 30% in a randomized-controlled trial,2 while Nirmatrelvir-ritonavir, in a Phase III study, showed a 89% reduction in hospitalization or death.3 Based on this evidence, these two antiviral prescriptions have been recommended for patients with non-severe COVID-19 at highest risk of hospitalisation.4
The first cluster of COVID-19 patients attributable to the Omicron variant in Hong Kong were identified on 6 January, 2022.5 As of 30 April 2022, approximately 1.2 million cases and 9,095 deaths were reported in Hong Kong, although 82.6% and 43.1% of the population had been immunised with the 2nd and 3rd vaccine dose, respectively.6
Emerging evidence for the relative benefit of one oral antiviral agent strategy over another in high-risk COVID-19 patients remains limited. Having considered the socio-economic implications that public health interventions have incurred in different jurisdictions,7,8 oral antivirals are considered a potential protection against the potential of the health care system to be overwhelmed. However, the cost analysis of these agents is equally important to ensure that health care systems are financially sustainable. In order to address these issues, we analysed real-world data from the Hospital Authority, a publicly-funded health care system that covers 7.4 million population in Hong Kong, to evaluate the association of two antiviral prescriptions with all-cause mortality, and their economic implication among high-risk patients with mild to moderate COVID-19.
Methods
In line with World Health Organization guidance,9 COVID-19 can be confirmed through a positive nucleic acid amplification test (NAAT), or by a positive diagnostic rapid antigen test (RAT) with contact history. We used laboratory data and diagnostic codes (International Classification of Diseases, the Ninth Revision, Clinical Modification (ICD-9-CM): 519.8(8)) to identity COVID-19 patients admitted into hospitals through emergency departments (ED) (inpatient cohort) between 22 February and 31 March 2022. Our outpatient cohort were those patients who attended the Designated Clinics during that period. The Hospital Authority is the statutory body that operates all public hospitals and clinics in Hong Kong, including 23 time-limited designated clinics for outpatient COVID-19 treatments, and that was responsible for all COVID-19 related treatments during the study period. A unique identifier linked all health records, pharmaceutical data, and death cause. The outpatient cohort was followed-up until 15 April 2022; and the inpatient cohort was followed-up until 25 April 2022.
We defined the index date as the date of first designated clinic attendance (outpatient cohort), and the date of positive NAAT, or the date of admission of an episode coded for COVID-19 (inpatient cohort). We excluded patients from the inpatient cohort who visited designated clinics after discharge from hospital as a previous COVID-19 admission. This study was approved, and the requirement for obtaining patient informed consent was waived by the Hospital Authority Hong Kong West Cluster/The University of Hong Kong institutional review board (UW 20-112). The protocol for this study is attached in supplementary materials e-1.
Exposure
Our exposure of interest was Molnupiravir and Nirmatrelvir-ritonavir prescriptions among mild-to-moderate COVID-19 patients, with an increased risk of deterioration including old-age and chronic disease patients.10 We included patients who were aged ≥ 60 years or younger patients with at least one chronic disease. We excluded firstly those patients who received Remdesivir, Dexamethasone, Interferon-Beta 1b, and plasma infusion; secondly, considering the delayed access to antivirals during the early stage of roll out, patients who received Molnupiravir or Nirmatrelvir-ritonavir later than 7 days from the index date; and thirdly, those patients who received prescriptions before the index date.
The standard course of both antivirals was 5 days,10 followed by a second course with viable duration as needed. In Hong Kong, patients who had been treated with a full course of antiviral in outpatient clinics may be given another course in the ward after hospital admission, as determined by clinical judgment of ward physicians. e-Figure 1 outlines our selection of population.
Outcomes
The primary outcome was 28-day all-cause mortality (e-Table 1). The secondary outcomes include re-attendance to the designated clinic or subsequent hospital admission through ED within 28 days from index date in the outpatient cohort; and unplanned readmission through ED within 28 days in the inpatient cohort. The economic burden was evaluated through estimation of the total attributed healthcare cost reduction from both antivirals by operational cost. The incremental cost-effectiveness ratio (ICER) was estimated for each antiviral, compared with control subjects.
Covariates
For each patient, we obtained their demographics including age (<60, 60–69, 70–79, 80–89, and ≥90), sex, and their past health 2 years before the index date. We included the comorbidities that were predictive to one-year mortality (e-Table 2).11 The inpatient cohort was adjusted for care home residency. For the outpatient cohort, their ethnicities and socioeconomic status (denoted by social security status at index date) were adjusted in addition to their comorbidities.
Statistical analysis
Using univariate and multivariable analysis, we tested the hypothesis that both antivirals could impact on all-cause mortality, healthcare utilization outcomes, burden on the healthcare system, and its attributable cost. Descriptive statistics were presented as mean (SD), median (interquartile range, IQR) and percentage (%) as appropriate. We adopted inverse probability of treatment weighting-adjusted analysis (IPTW)12 to account for the observed differences in the baseline characteristics among groups. Covariate balance was evaluated by comparing the standardized mean differences (SMD) among groups.13,14 For each covariate, the chi-square test was used for each of the categorical and binary covariates. One-way analysis of variance (ANOVA) was used for each of continuous variables in order to determine inter-group differences. Sensitivity analyses were then conducted with IPTW adjustments using two groups (combined antivirals; and control subjects) instead of three.
For our primary outcome, IPTW-adjusted Kaplan-Meier curves were drawn and pairwise log-rank tests were used to compare the mortality among the three groups. An IPTW-adjusted Cox proportional hazard regression model was adopted in order to estimate survival time in hazard ratio (HR) with 95% confidence interval (95%CI), adjusted for age group, sex, care home residency, and comorbidities in the inpatient cohort. Results were stratified by the patients’ sex, age group, care home residency, and whether they had acute myocardial infarction, chronic obstructive pulmonary disease, peptic ulcer disease, mild liver disease, hemiplegia or paraplegia, moderate/severe liver disease, metastatic cancer, or AIDS/HIV at baseline. We also tested the proportional hazard assumption of the Cox model, to avoid biased effect estimate, with the global Schoenfeld Residual Test.
For our secondary outcomes, IPTW-adjusted binary logistic regression were used to estimate the effect of intervention on binary outcomes (28-day reattendance, 28-day hospital readmission, and ED visit). We have considered the following assumptions of binary logistic regressions, namely: (1) a binary outcome for the binary logistic regression; (2) absence of multicollinearity; and (3) no influential outliners. Models were checked for potential collinearity using standardized variance inflation factor (cut-off: >5), and influential outliners using Cook's distance (cutoff: >1) and standardized residuals (cutoff: >3). Significance level was set at 5% and all analyses were implemented using the R version 4.0.3 with RStudio 2022.07.0-548, and the packages “WeightIt (version 0.12.0)”14 for IPTW adjustment, “survminer (version 0.4.9)”15 and “survival (version 3.3.1)”16 for survival analysis; and the glm function in “stats (version 4.2.0)16,17” for logistic regressions. We then plotted the results with package “ggplot2 (version 3.3.6)”.18 We considered using zero-inflated negative binomial regressions in fitting the number of clinic reattendance and hospital admissions in earlier version of this analysis. However, we noticed that the models fit better in binary logistic regressions, and therefore transformed the count data into binary data for the main analysis.
For healthcare expenditure, we adopted the gross patient charge without subsidy, per clinic visit with a value of USD 151.6 (HKD1,190), ED visit USD 156.7 (HKD 1,230), and an acute hospital day USD 649.74 (HKD 5,100) – which included consultation, investigation, and treatment.19 We determined the attributable healthcare burden by multiplying the fraction of health utilization that could be attributable to the prescriptions. We also considered applying the mean hospital length of stay (LOS) in their first hospital re-admission after the index visit for the cost of inpatient admission. Cost saved from reduced mortality was estimated using the value of a statistical life method (VSL), which is an economic measure of a person's preference on death risk and wage in a given sample over a year.20 VSL could be estimated by maximizing a person's expected indirect utility by the following equation21:
where p is the probability of the person surviving the period, dw/dp is the negative rate of wealth with respect to the probability of the person survived (negative value of differentiation of w with respect to p), u(w) is the utility of wealth w if the person survives the period, v(w) is the utility of wealth w if they die, u'(w) is the first derivative of the function u(w), and v'(w) is the first derivative of the function v(w).
In this article, we referenced VSL in Hong Kong at USD 2.20 million per statistical life in 2016,22 adjusted for the inflation rate in 2021.23 The 95% CI for risk estimates were used to calculate the range of cost estimations. For cost-effectiveness, we considered the probability of survival for a COVID-19 patient admitted into hospital (inpatient cohort) or who attended the clinics (outpatient cohort), respectively, during the study observation period in this study. The patient could receive either antivirals, or standard care (control subjects). Costs were estimated by adding the costs of antivirals (Molnupiravir: USD141.4/day; Nirmatrelvir-ritonavir: USD105.8/day),24 clinic visit (for outpatient cohort), ED visit, and inpatient care during the period. All costs are given in US dollars (USD1 = HKD7.8).25 In an earlier version of this analysis, we considered adopting decision trees in ICER estimation based on secondary aggregate data,26,27; however, to better reflect cost-effectiveness in the local context, we used real-world treatment data for cost and effectiveness estimation instead based on the methods reported in a previous study.28
Role of the funding source
The funders did not have any role in study design, data collection, data analysis, interpretation and writing of this manuscript.
Results
Table 1 shows the unadjusted and IPTW-adjusted demographic information of COVID-19 patient in the outpatient and inpatient cohorts. During the study period, 33,217 COVID-19 patients attended designated outpatient clinics with 1,290,480 person-days follow-up (mean follow-up time: 38.85 days), and 29.5% were prescribed with either Molnupiravir (16.1%) or Nirmatrelvir-ritonavir (13.4%). More than half of these patients were female (53.1%). Close to half were under 69 years of age (48.4%). Over 90% of the outpatient patients were Chinese, and 16% were receiving public assistance. Diabetes without complications (8.0%), cerebrovascular disease (3.4%) and cancer (2.6%) were the most prevalent comorbidities. Also, a small proportion of deaths (n=73; 0.2%) was observed in the outpatient cohort.
Table 1.
Demographic information of COVID-19 patient admitted into public hospitals/ outpatient clinic between 22 February and 31 March 2022.
| Outpatient cohort |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
IPTW |
|||||||||||
| Overall | Control | Molnupiravir | Nirmatrelvir-ritonavir | p | SMD | Control | Molnupiravir | Nirmatrelvir-ritonavir | p | SMD | ||
| n | 33217 | 23430 | 5345 | 4442 | 23430 | 18637.1 | 14120.92 | |||||
| Follow-up time (days, mean (SD)) | 38.85 (8.95) | 41.34 (9.07) | 34.17 (5.10) | 31.32 (4.19) | 41.34 (9.07) | 36.58 (4.31) | 34.62 (4.68) | |||||
| Male (n(%)) | 15592 (46.9) | 11078 (47.3) | 2498 (46.7) | 2016 (45.4) | 0.064 | 0.025 | 11078.0 (47.3) | 8711.1 (46.7) | 6497.0 (46.0) | 0.62 | 0.017 | |
| Age group (n (%)) | <0.0001 | 0.531 | < 0.0001 | 0.148 | ||||||||
| <60 | 1722 (5.2) | 1526 (6.5) | 120 (2.2) | 76 (1.7) | 1526.0 (6.5) | 1022.8 (5.5) | 970.2 (6.9) | |||||
| 60–69 | 14339 (43.2) | 11531 (49.2) | 1126 (21.1) | 1682 (37.9) | 11531.0 (49.2) | 8169.8 (43.8) | 5527.6 (39.1) | |||||
| 70–79 | 10107 (30.4) | 6585 (28.1) | 1907 (35.7) | 1615 (36.4) | 6585.0 (28.1) | 5901.6 (31.7) | 4718.6 (33.4) | |||||
| 80–89 | 5396 (16.2) | 2987 (12.7) | 1562 (29.2) | 847 (19.1) | 2987.0 (12.7) | 2809.0 (15.1) | 2244.1 (15.9) | |||||
| >=90 | 1653 (5.0) | 801 (3.4) | 630 (11.8) | 222 (5.0) | 801.0 (3.4) | 733.9 (3.9) | 660.4 (4.7) | |||||
| Received public assistance (n (%)) | 5299 (16.0) | 2979 (12.7) | 1443 (27.0) | 877 (19.7) | <0.0001 | 0.242 | 2979.0 (12.7) | 2953.2 (15.8) | 2476.7 (17.5) | < 0.0001 | 0.090 | |
| Chinese ethnicities (n (%)) | 30755 (92.6) | 21622 (92.3) | 5083 (95.1) | 4050 (91.2) | <0.0001 | 0.104 | 21622.0 (92.3) | 17353.1 (93.1) | 13146.9 (93.1) | 0.24 | 0.021 | |
| Medical history with ICD-9 code (n (%)) | ||||||||||||
| Acute myocardial infarction | 229 (0.7) | 134 (0.6) | 83 (1.6) | 12 (0.3) | <0.0001 | 0.093 | 134.0 (0.6) | 127.9 (0.7) | 151.7 (1.1) | 0.22 | 0.037 | |
| Congestive heart failure | 540 (1.6) | 296 (1.3) | 210 (3.9) | 34 (0.8) | <0.0001 | 0.143 | 296.0 (1.3) | 296.8 (1.6) | 280.6 (2.0) | 0.17 | 0.038 | |
| Peripheral vascular disease | 59 (0.2) | 32 (0.1) | 23 (0.4) | 4 (0.1) | <0.0001 | 0.045 | 32.0 (0.1) | 28.1 (0.2) | 15.1 (0.1) | 0.80 | 0.008 | |
| Cerebrovascular disease | 1136 (3.4) | 757 (3.2) | 263 (4.9) | 116 (2.6) | <0.0001 | 0.081 | 757.0 (3.2) | 739.7 (4.0) | 532.9 (3.8) | 0.25 | 0.026 | |
| Dementia | 77 (0.2) | 36 (0.2) | 35 (0.7) | 6 (0.1) | <0.0001 | 0.056 | 36.0 (0.2) | 37.0 (0.2) | 25.2 (0.2) | 0.78 | 0.007 | |
| COPD | 730 (2.2) | 540 (2.3) | 131 (2.5) | 59 (1.3) | <0.0001 | 0.055 | 540.0 (2.3) | 479.4 (2.6) | 395.0 (2.8) | 0.54 | 0.021 | |
| Rheumatoid disease | 114 (0.3) | 95 (0.4) | 15 (0.3) | 4 (0.1) | 0.003 | 0.043 | 95.0 (0.4) | 78.4 (0.4) | 163.6 (1.2) | 0.09 | 0.057 | |
| Peptic ulcer disease | 301 (0.9) | 196 (0.8) | 60 (1.1) | 45 (1.0) | 0.099 | 0.019 | 196.0 (0.8) | 182.2 (1.0) | 113.6 (0.8) | 0.74 | 0.012 | |
| Mild liver disease | 530 (1.6) | 438 (1.9) | 61 (1.1) | 31 (0.7) | <0.0001 | 0.070 | 438.0 (1.9) | 275.2 (1.5) | 253.2 (1.8) | 0.55 | 0.02 | |
| Diabetes without complications | 2647 (8.0) | 1946 (8.3) | 432 (8.1) | 269 (6.1) | <0.0001 | 0.058 | 1946.0 (8.3) | 1649.0 (8.8) | 1060.4 (7.5) | 0.22 | 0.033 | |
| Diabetes with complications | 304 (0.9) | 200 (0.9) | 81 (1.5) | 23 (0.5) | <0.0001 | 0.067 | 200.0 (0.9) | 140.9 (0.8) | 152.9 (1.1) | 0.45 | 0.023 | |
| Hemiplegia or paraplegia | 47 (0.1) | 35 (0.1) | 5 (0.1) | 7 (0.2) | 00.59 | 0.012 | 35.0 (0.1) | 63.3 (0.3) | 17.6 (0.1) | 0.21 | 0.030 | |
| Renal disease | 613 (1.8) | 378 (1.6) | 192 (3.6) | 43 (1.0) | <0.0001 | 0.119 | 378.0 (1.6) | 417.0 (2.2) | 274.5 (1.9) | 0.33 | 0.030 | |
| Cancer | 878 (2.6) | 554 (2.4) | 205 (3.8) | 119 (2.7) | <0.0001 | 0.057 | 554.0 (2.4) | 557.6 (3.0) | 399.0 (2.8) | 0.15 | 0.026 | |
| Moderate/severe liver disease | 2 (0.0) | 2 (0.0) | 0 (0.0) | 0 (0.0) | 0.66 | 0.009 | 2.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.64 | 0.009 | |
| Metastatic cancer | 18 (0.1) | 18 (0.1) | 0 (0.0) | 0 (0.0) | 0.020 | 0.026 | 18.0 (0.1) | 0.0 (0.0) | 0.0 (0.0) | 0.10 | 0.026 | |
| AIDS/HIV | 11 (0.0) | 9 (0.0) | 2 (0.0) | 0 (0.0) | 0.43 | 0.019 | 9.0 (0.0) | 5.6 (0.0) | 0.0 (0.0) | 0.26 | 0.019 | |
| Died during observation (n (%)) | 73 (0.2) | 65 (0.2) | 8 (0.0) | 0 (0.0) | ||||||||
| Died with an ICD-10 diagnosis (n (%)) | 48 (0.1) | 42 (0.2) | 5 (0.1) | 1 (0.0) | ||||||||
| COVID-19 (n (% of all ICD-10 coded death)) | 29 (60.4) | 26 (61.9) | 2 (40.0) | 1 (100.0) | ||||||||
| Cancer (n (% of all ICD-10 coded death)) | 1 (2.1) | 1 (2.4) | 0 (0.0) | 0 (0.0) | ||||||||
| Heart (n (% of all ICD-10 coded death)) | 2 (4.2) | 1 (2.4) | 1 (20.0) | 0 (0.0) | ||||||||
| Stroke (n (% of all ICD-10 coded death)) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||||
| Nervous system (n (% of all ICD-10 coded death)) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||||
| Genitourinary (n (% of all ICD-10 coded death)) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||||
| Diabetes (n (% of all ICD-10 coded death)) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||||||
| Others (n (% of all ICD-10 coded death)) | 16 (33.3) | 14 (33.3) | 2 (40.0) | 0 (0.0) | ||||||||
| Inpatient cohort |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
IPTW |
|||||||||||
| Overall | Control | Molnupiravir | Nirmatrelvir-ritonavir | p | SMD | Control | Molnupiravir | Nirmatrelvir-ritonavir | p | SMD | ||
| n | 21138 | 20057 | 799 | 282 | ||||||||
| Follow-up time (day, mean (SD)) | 27.82 (14.38) | 27.87 (14.65) | 27.78 (8.17) | 24.34 (5.52) | 27.87 (14.65) | 28.77 (8.34) | 25.83 (5.19) | |||||
| Male (n (%)) | 11708 (55.4) | 11157 (55.6) | 404 (50.6) | 147 (52.1) | 0.01 | 0.068 | 11157.0 (55.6) | 11343.3 (56.1) | 10487.8 (55.3) | 0.93 | 0.010 | |
| Age group (n (%)) | <0 .0001 | 0.232 | 0.78 | 0.101 | ||||||||
| <60 | 588 (2.8) | 572 (2.9) | 14 (1.8) | 2 (0.7) | 572.0 (2.9) | 648.0 (3.2) | 366.1 (1.9) | |||||
| 60–69 | 3103 (14.7) | 2968 (14.8) | 101 (12.6) | 34 (12.1) | 2968.0 (14.8) | 3011.8 (14.9) | 2335.9 (12.3) | |||||
| 70–79 | 4842 (22.9) | 4600 (22.9) | 164 (20.5) | 78 (27.7) | 4600.0 (22.9) | 4994.5 (24.7) | 4266.6 (22.5) | |||||
| 80–89 | 7566 (35.8) | 7148 (35.6) | 299 (37.4) | 119 (42.2) | 7148.0 (35.6) | 7087.4 (35.0) | 7469.0 (39.4) | |||||
| >=90 | 5039 (23.8) | 4769 (23.8) | 221 (27.7) | 49 (17.4) | 4769.0 (23.8) | 4482.5 (22.2) | 4513.9 (23.8) | |||||
| Admitted from elderly home (n (%)) | 8002 (37.9) | 7731 (38.5) | 223 (27.9) | 48 (17.0) | <0 .0001 | 0.328 | 7731.0 (38.5) | 8012.8 (39.6) | 7679.8 (40.5) | 0.78 | 0.027 | |
| Medical history with ICD-9 code (n (%)) | ||||||||||||
| Acute myocardial infarction | 432 (2.0) | 418 (2.1) | 12 (1.5) | 2 (0.7) | 0.15 | 0.079 | 418.0 (2.1) | 408.2 (2.0) | 238.1 (1.3) | 0.63 | 0.043 | |
| Congestive heart failure | 1351 (6.4) | 1281 (6.4) | 58 (7.3) | 12 (4.3) | 0.21 | 0.086 | 1281.0 (6.4) | 128.0 (6.3) | 1277.1 (6.7) | 0.92 | 0.011 | |
| Peripheral vascular disease | 100 (0.5) | 98 (0.5) | 2 (0.3) | 0 (0.0) | 0.32 | 0.070 | 98.0 (0.5) | 93.0 (.5) | 0.0 (0.0) | 0.21 | 0.066 | |
| Cerebrovascular disease | 2107 (10.0) | 2016 (10.1) | 65 (8.1) | 26 (9.2) | 0.19 | 0.044 | 2016.0 (10.1) | 1917.8 (9.5) | 1798.7 (9.5) | 0.90 | 0.013 | |
| Dementia | 748 (3.5) | 719 (3.6) | 24 (3.0) | 5 (1.8) | 0.19 | 0.075 | 719.0 (3.6) | 750.4 (3.7) | 1168.9 (6.2) | 0.28 | 0.080 | |
| COPD | 1231 (5.8) | 1179 (5.9) | 39 (4.9) | 13 (4.6) | 0.34 | 0.038 | 1179.0 (5.9) | 1159.1 (5.7) | 1064.7 (5.6) | 0.96 | 0.007 | |
| Rheumatoid disease | 39 (0.2) | 38 (0.2) | 1 (0.1) | 0 (0.0) | 0.70 | 0.043 | 38.0 (0.2) | 33.6 (.2) | 0.0 (0.0) | 0.33 | 0.042 | |
| Peptic ulcer disease | 343 (1.6) | 315 (1.6) | 23 (2.9) | 5 (1.8) | 0.016 | 0.059 | 315.0 (1.6) | 309.1 (1.5) | 320.8 (1.7) | 0.92 | 0.009 | |
| Mild liver disease | 216 (1.0) | 201 (1.0) | 11 (1.4) | 4 (1.4) | 0.47 | 0.025 | 201.0 (1.0) | 204.6 (1.0) | 181.9 (1.0) | 0.98 | 0.003 | |
| Diabetes without complications | 2629 (12.4) | 2505 (12.5) | 105 (13.1) | 19 (6.7) | 0.010 | 0.144 | 2505.0 (12.5) | 2383.2 (11.8) | 2103.5 (11.1) | 0.77 | 0.029 | |
| Diabetes with complications | 459 (2.2) | 440 (2.2) | 17 (2.1) | 2 (0.7) | 0.24 | 0.083 | 440.0 (2.2) | 416.8 (2.1) | 429.6 (2.3) | 0.95 | 0.009 | |
| Hemiplegia or paraplegia | 132 (0.6) | 127 (0.6) | 5 (0.6) | 0 (0.0) | 0.41 | 0.075 | 127.0 (0.6) | 129.1 (.6) | 0.0 (0.0) | 0.039 | 0.076 | |
| Renal disease | 1401 (6.6) | 1352 (6.7) | 44 (5.5) | 5 (1.8) | 0.002 | 0.167 | 1352.0 (6.7) | 1475.6 (7.3) | 708.3 (3.7) | 0.22 | 0.104 | |
| Cancer | 793 (3.8) | 749 (3.7) | 28 (3.5) | 16 (5.7) | 0.22 | 0.069 | 749.0 (3.7) | 731.8 (3.6) | 554.2 (2.9) | 0.60 | 0.030 | |
| Moderate/severe liver disease | 1 (0.0) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0.97 | 0.007 | 1.0 (0.0) | 0.0 (.0) | 0.0 (0.0) | 0.88 | 0.007 | |
| Metastatic cancer | 2 (0.0) | 2 (0.0) | 0 (0.0) | 0 (0.0) | 0.95 | 0.009 | 2.0 (0.0) | 0.0 (.0) | 0.0 (0.0) | 0.82 | 0.009 | |
| AIDS/HIV | 10 (0.0) | 10 (0.0) | 0 (0.0) | 0 (0.0) | 0.76 | 0.021 | 10.0 (0.0) | .0 (.0) | 0.0 (0.0) | 0.61 | 0.021 | |
| Died during observation (n (%)) | 5291 (25.0) | 5211 (26.0) | 68 (8.5) | 12 (4.3) | <0 .0001 | 5211.0 (26.0) | 1795.8 (8.9) | 569.5 (3.0) | <0 .0001 | |||
| Died with an ICD-10 diagnosis (n (%)) | 3630 (17.2) | 3598 (17.9) | 26 (3.3) | 6 (2.1) | ||||||||
| COVID-19 (n (% of all ICD-10 coded death)) | 2108 (58.1) | 2094 (58.2) | 12 (46.2) | 2 (33.3) | ||||||||
| Cancer (n (% of all ICD-10 coded death)) | 60 (1.7) | 59 (1.6) | 1 (3.8) | 0 (0.0) | ||||||||
| Heart (n (% of all ICD-10 coded death)) | 108 (3.0) | 104 (2.9) | 2 (7.7) | 2 (33.3) | ||||||||
| Stroke (n (% of all ICD-10 coded death)) | 39 (1.1) | 39 (1.1) | 0 (0.0) | 0 (0.0) | ||||||||
| Nervous system (n (% of all ICD-10 coded death)) | 4 (0.1) | 4 (0.1) | 0 (0.0) | 0 (0.0) | ||||||||
| Genitourinary (n (% of all ICD-10 coded death)) | 57 (1.6) | 57 (1.6) | 0 (0.0) | 0 (0.0) | ||||||||
| Diabetes (n (% of all ICD-10 coded death)) | 7 (0.2) | 7 (0.2) | 0 (0.0) | 0 (0.0) | ||||||||
| Others (n (% of all ICD-10 coded death)) | 1247 (34.4) | 1234 (34.3) | 11 (42.3) | 2 (33.3) | ||||||||
aAIDS/HIV: Acquired immune deficiency syndrome/Human Immunodeficiency Virus; COPD: Chronic obstructive pulmonary disease; ICD-10: International Classification of Diseases; IPTW: Inverse Probability Treatment Weighting; SMD: Standardised mean difference; SD: Standard deviation.
bChi-square tests were performed for categorical/ binary variables, and 1-wat ANOVA were done for continuous variables.
There were 21,138 COVID-19 patients in the inpatient cohort, with a total follow-up of 588,059 person-days (mean follow-up time 27.82 days). Among these patients, 55.4% were male, almost 40% were from care homes, and 5.1% of these patients (N = 1,081) received either Molnupiravir (3.8%) or Nirmatrelvir-ritonavir (1.3%). Most patients received a single course (n=1058; 97.8%). Diabetes without complications (12.4%), cerebrovascular diseases (10.0%) and renal diseases (6.6%) were the most prevalent comorbidities. One-fourth (N = 5291) died, but less than one-fifth (17.2%; N = 3630) had their cause of death documented. The most frequent cause of death was COVID-19 (58.1%). The SMD of the baseline characteristics of patients in both cohorts showed that the three study groups (Molnupiravir, Nirmatrelvir-ritonavir and Control subjects) differed in terms of demographic, socioeconomic, and medical history before adjustments. Sensitivity analysis of IPTW adjustment using two groups (combined antivirals and Control subjects) is presented in e-Table 3 and e-Table 4.
Primary outcome: all-cause mortality
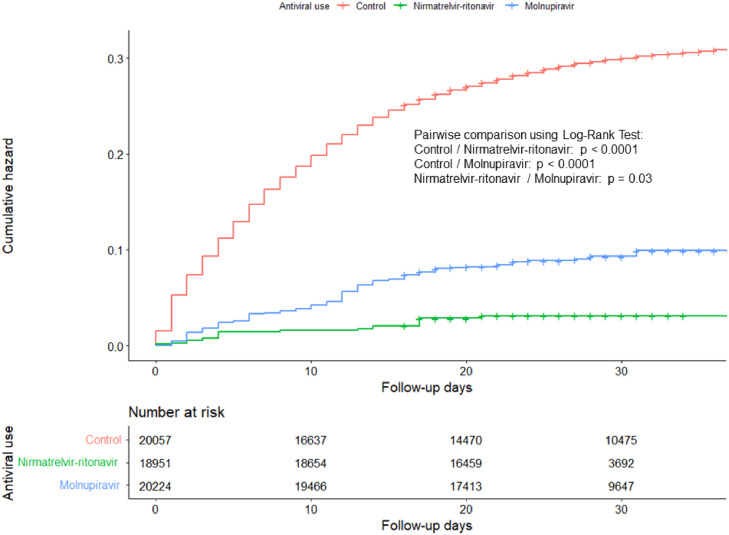
Figure 1 gives the mortality plot for IPTW-adjusted mortality in the inpatient cohort, while Table 2 illustrates the results for Cox regressions of survival estimation for COVID-19 patients being prescribed with either Molnupiravir or Nirmatrelvir-ritonavir in the inpatient setting. After IPTW adjustment, the overall mortality rate was 26.0%. Either prescribing Nirmatrelvir-ritonavir (hazard ratio (HR) = 0.10, 95%CI = 0.05 – 0.21, P <0.0001) or Molnupiravir (HR = 0.31, 95%CI = 0.24 – 0.40, P <0.0001) was significantly associated with reduced all-cause mortality in mild and moderate COVID-19 patients in hospital. There was no significant difference between the protective effect of both antivirals given the overlapping CIs of the hazard ratios. Sensitivity analysis showed an overall protective effect of prescribing either antiviral (HR = 0.24, 95%CI = 0.19 – 0.29, P <0.0001). For the outpatient cohort, a survival analysis was not performed due to the small number of deaths observed and there was not enough statistical power to conduct an IPTW-adjusted Cox regression. We observed no violation in the proportional hazard assumptions (Supplementary e-figures 2-5), and no influential outliner (Supplementary e-figures 6-7) of the Cox models we used.
Figure 1.
Mortality plot for hospitalized COVID-19 patients given Molnupiravir and Nirmatrelvir-ritonavir.
Table 2.
Cox regression of survival estimation for COVID-19 patient received antiviral medications.
| Unadjusted |
IPTW |
||||
|---|---|---|---|---|---|
| HR [95%CI] | P | HR [95%CI] | P | ||
| Antiviral medication | |||||
| Control (n=20057 in unadjusted, n= 17159.72 in IPTW) | Reference | Reference | |||
| Molnupiravir or Nirmatrelvir-ritonavir (n=1081 in unadjusted; n= 1081.0 in IPTW) | 0.28 (0.23, 0.35) | <0.0001 | 0.24 (0.19, 0.29) | <0.0001 | |
| Antiviral medication | |||||
| Control (n=20057, n=20057 in IPTW) | Reference | Reference | |||
| Nirmatrelvir-ritonavir (n=282 in unadjusted; n=172.25 in IPTW) | 0.18 (0.10, 0.31) | <0.0001 | 0.10 (0.05, 0.21) | <0.0001 | |
| Molnupiravir (n=799 in unadjusted; n=684 in IPTW) | 0.31 (0.25, 0.40) | <0.0001 | 0.31 (0.24, 0.40) | <0.0001 | |
aCI: Confidence intervals; HR: Hazard ratio; IPTW: Inverse Probability Treatment Weighting.
bAdjusted by age, sex, admitted from elderly homes, admitted through emergency department, the constituent comorbidities of the Charlson's Comorbidity Index including Acute myocardial infarction, Congestive heart failure, Peripheral vascular disease, Cerebrovascular disease, Dementia, COPD, Rheumatoid disease, Peptic ulcer disease, Mild liver disease, Diabetes without complications, Diabetes with complications, Hemiplegia or paraplegia, Renal disease, Cancer, Moderate/severe liver disease, Metastatic cancer, AIDS/HIV according to ICD-9 definition adopted by Quan et al, 2014.11
Secondary outcomes
Table 3 describes the regression model of predicting outpatient clinic re-attendance, and 28-days hospital admission through the ED among the outpatient cohort. Both antivirals were significantly associated with increased odds of 28-days re-attendance to the designated clinic after weighting (Molnupiravir: odds ratio (OR) = 1.80, 95%CI = 1.60 – 2.01, P < 0.0001; Nirmatrelvir-ritonavir: OR = 1.45, 95%CI = 1.11 – 1.91, P = 0.0069). A sensitivity analysis shows the same risk characteristics between them (OR = 1.65, 95%CI = 1.45 – 1.87, P =0.00033).
Table 3.
Binary logistic regression of unplanned readmission for COVID-19 patient received antiviral medications.
| Outpatient clinic |
Inpatient cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | IPTW | Unadjusted | IPTW | ||||||
| OR [95%CI] | P | OR [95%CI] | P | OR [95%CI] | P | OR [95%CI] | P | ||
| 28-days clinic re-attendance | |||||||||
| Antiviral medication | |||||||||
| Control | Reference | Reference | |||||||
| Molnupiravir or Nirmatrelvir-ritonavir | 1.21 (1.12, 1.30) | <0.0001 | 2.12 (1.84, 2.44) | <0.0001 | NA | NA | |||
| Antiviral medication | |||||||||
| Control | Reference | Reference | |||||||
| Nirmatrelvir-ritonavir | 0.94 (0.84, 1.05) | 0.273 | 1.45 (1.11, 1.91) | 0.0069 | NA | NA | |||
| Molnupiravir | 1.45 (1.32, 1.59) | <0.0001 | 1.80 (1.60, 2.01) | <0.0001 | |||||
| 28 days unplanned hospital readmission | |||||||||
| Antiviral medication | |||||||||
| Control | Reference | Reference | Reference | Reference | |||||
| Molnupiravir or Nirmatrelvir-ritonavir | 0.31 (0.25, 0.39) | <0.0001 | 0.12 (0.08, 0.18) | <0.0001 | 0.73 (0.56, 0.94) | 0.018 | 0.79 (0.61, 1.03) | 0.078 | |
| Antiviral medication | |||||||||
| Control | Reference | Reference | Reference | Reference | |||||
| Nirmatrelvir-ritonavir | 0.19 (0.12, 0.29) | <0.0001 | 0.37 (0.23, 0.60) | <0.0001 | 0.57 (0.31, 0.96) | 0.053 | 0.47 (0.24, 0.93) | 0.030 | |
| Molnupiravir | 0.37 (0.29, 0.48) | <0.0001 | 0.72 (0.52, 0.98) | 0.039 | 0.79 (0.58, 1.04) | 0.10 | 0.71 (0.52, 0.97) | 0.031 | |
aCI: Confidence intervals; IPTW: Inverse Probability Treatment Weighting; OR: Odds ratio.
bAdjusted by age, sex, admitted from elderly homes, admitted through emergency department, the constituent comorbidities of the Charlson's Comorbidity Index including Acute myocardial infarction, Congestive heart failure, Peripheral vascular disease, Cerebrovascular disease, Dementia, COPD, Rheumatoid disease, Peptic ulcer disease, Mild liver disease, Diabetes without complications, Diabetes with complications, Hemiplegia or paraplegia, Renal disease, Cancer, Moderate/severe liver disease, Metastatic cancer, AIDS/HIV according to ICD-9 definition adopted by Quan et al, 2014.11
Both antiviral prescriptions were significantly associated with lower odds in subsequent 28-day hospital admission in outpatient settings, and Nirmatrelvir-ritonavir had a significantly stronger effect (Molnupiravir: OR = 0.72, 95% CI = 0.52 – 0.98, P=0.039; Nirmatrelvir-ritonavir: OR = 0.37, 95% CI =0.23 – 0.60, P <0.0001). In the outpatient cohort, they significantly reduced the OR in subsequent hospitalization through the ED in the IPTW-adjusted sensitivity analysis (OR=0.30, 95%CI = 0.21 – 0.44, P <0.0001) by 70%.
The associations between both antivirals in the inpatient cohort and the risk of 28-day hospital readmission after IPTW adjustments were illustrated in Table 3. Both antivirals were significantly associated with a reduction in 28-day hospital readmission (Molnupiravir: OR = 0.71, 95%CI = 0.52 – 0.97, P=0.031; Nirmatrelvir-ritonavir: OR = 0.47, 95%CI = 0.24 – 0.93, P=0.030). However, a sensitivity analysis showed that, after adjustment for demographic characteristics and baseline comorbidities, they did not significantly reduce hospital readmission (p = 0.078). After assumption checking, we identified no influential outliners (supplementary materials e-Figure 8-13) nor multi-collinearity (supplementary materials e-Table 5-6) in the final models.
Cost analysis
Among 9,787 outpatients (USD 2,8080.7 per 1000 population) who received antivirals, USD 79,086.2 was saved including less clinic re-attendance, less unplanned admission and shorter hospital LOS.
In the outpatient setting, Molnupiravir cost an additional USD1356.70 per patient and reduced 0.275 percent of mortality for one patient compared with control subjects; and Nirmatrelvir-ritonavir cost an additional USD917.16 and reduced 0.277 percent (Table 4) for one patient compared with control subjects. The corresponding ICERs were USD493,345.09 and USD331,105.27 per death averted for Molnupiravir and Nirmatrelvir-ritonavir respectively. This was calculated by considering the cost incurred from designated clinical re-attendance, and subsequent hospital admissions through emergency department in this population. Given the 9.6% re-attendance rate among the patients without antiviral, there was an additional cost of USD 91,322.4 (USD 9331.0 per 1000 population) from the re-attendance with antiviral. This cost estimation was contributed to by an additional 45% of clinic re-attendance among 4442 patients with Nirmatrelvir-ritonavir and additional 80% of clinic re-attendance among 5345 patients with Molnupiravir. In 33,217 patients, the average operational cost of each clinic visit was USD 151.6 (HKD1190). Considering the subsequent hospital admissions, 1.89% patients who were not prescribed with antiviral were admitted to hospital through ED. We found that Nirmatrelvir-ritonavir and Molnupiravir in outpatient settings saved USD 12,720.4 (USD 1299.7 per 1000 population), which was contributed to by a reduction of 63% in the subsequent hospital admissions among 4442 patients with Nirmatrelvir-ritonavir and 28% among 5345 patients with Molnupiravir with an average operational cost of USD 156.7 (HKD 1230) per hospital emergency department visit. A total of USD 157,688.2 (USD 16,112.0 per 1000 population) was saved from the reduced LOS in subsequent hospital admissions. This cost estimation was contributed to by a reduction of 33% in total LOS among 4442 patients used Nirmatrelvir-ritonavir with a mean LOS of 9.39 days (SD = 6.93) of total hospital admissions and an average operational cost of USD 649.74 (HKD 5100) per acute bed-stay.
Table 4.
Cost-effectiveness analysis outcomes for outpatient and inpatient settings.
| Standard care (without any antiviral medication) | Molnupiravir | Nirmatrelvir-ritonavir | |
|---|---|---|---|
| Outpatient setting | |||
| Cost per person (USD) | 367.86 | 1724.56 | 1285.02 |
| Outpatient (designated clinic visit) b | 168.28 | 178.89 | 175.86 |
| Subsequent emergency room visit b | 50.14 | 37.61 | 18.80 |
| Antiviral medications | 0.00 | 1391.11 | 1044.88 |
| Subsequent inpatient healthcare costs b | 149.44 | 116.95 | 45.48 |
| Effectiveness: Probability of surviving during observation period | 99.723% | 99.998% | 100.000% |
| Incremental cost (USD) | - | 1356.70 | 917.16 |
| Incremental effectiveness | - | 0.275% | 0.277% |
| ICERa (USD per death averted) | - | 493,345.09 | 331,105.27 |
| Inpatient setting | |||
| Cost per person (USD) | 8306.35 | 8755.92 | 7040.77 |
| Inpatient healthcare costs b | 8290.68 | 8,440.12 | 6,828.77 |
| Antiviral medications | 0.00 | 306.40 | 205.94 |
| Subsequent emergency room visit b | 15.67 | 9.40 | 6.06 |
| Effectiveness: Probability of surviving during observation period | 74.00% | 91.10% | 97.00% |
| Incremental cost (USD) | - | 449.57 | −1265.58 |
| Incremental effectiveness | - | 17.10% | 23.00% |
| ICERa (USD per death averted) | - | 2629.08 | −5502.53 |
ICER: Incremental Cost-Effectiveness Ratio.
The cost includes costs occurred for doctor consultation, medical examinations, nursing and prescriptions.
In inpatient settings, both antivirals saved a total of USD 0.46 billion (USD 0.43 billion per 1000 populations) in 1081 patients, after considering the cost saved from reduced 28-days unplanned readmission and reduced all-cause mortality. Given 8.2% of 28-day unplanned re-admissions rate among the patients without the prescription of antiviral medication, we found that both antivirals saved a total of USD 4,897.8 (USD 4530.8 per 1000 population) in the inpatient cohort. The cost estimation was contributed to by a reduction of 53% unplanned readmissions among 282 patients prescribed with Nirmatrelvir-ritonavir and 29% among 799 patients with Molnupiravir with an average operational cost of USD 156.7 (HKD 1230) per ED visit. Nirmatrelvir-ritonavir was estimated to contribute a savings amount of USD 8041.29 (USD 28,514.7 per 1000 patients) in the inpatient cohort. Total LOS was reduced by 6% and the mean LOS was 8.92 days (SD = 6.72 days). On the other hand, patients with Molnupiravir contributed less savings by USD16,976.8 due to longer LOS (Mean LOS: 9.97 days, SD = 6.72 days). We found that the prescription of Nirmatrelvir-ritonavir and Molnupiravir saved USD 460.8 million (USD 426.2 million per 1000 population) in Hong Kong. This was calculated based on a 69% HR reduction for 799 patients who used Molnupiravir and 90% HR reduction for 282 patients who used Nirmatrelvir-ritonavir in this sample – given the all-cause in-hospital mortality rate in patients who were not prescribed of either antiviral was 26.0%, and the average VSL of USD 2.01 million for Hong Kong population in year 2021.
In summary, compared with control subjects in the inpatient cohort, Molnupiravir cost an additional USD449.57 per patient and reduced 17.1 percent point of mortality for one patient compared with control subjects, and had an ICER was USD2629.08 per death averted. Nirmatrelvir-ritonavir saved USD1265.58 and reduced mortality by 23.0 percentage points, indicating an ICER of USD5502.53 (Table 4).
Interpretation
In two cohorts of mild to moderate COVID-19 patients at high risk of complications, the use of oral antivirals was significantly associated with a lower risk of all-cause mortality. The association of antiviral agents with risk reduction of hospitalisation and mortality is emerging. Molnupiravir reduced hospitalisation and mortality by 30%, with a good safety profile.2 Nirmatrelvir-ritonavir was associated with 89.1% relative risk reduction in 28-day combined hospitalisation and mortality, without a significant difference in adverse events.3
During Hong Kong's fifth pandemic wave, numbers of COVID-19 cases soared and led to the world's highest 7-day rolling average of daily confirmed COVID-19 death rate (37.68 deaths per million population).29 The government in Hong Kong took a more liberal approach to the use of these antivirals both in the community and in hospitals.30 Risk factors for progression to severe COVID-19 in Hong Kong include age of 60 years or above, diabetes mellitus, Body Mass Index >=30kg/m2, immunocompromised state, underlying chronic illnesses and incomplete COVID-19 vaccination.10 During the study period, these antivirals were only available in Hospital Authority hospitals and clinics. Moreover, many patients sought medical consultation beyond the treatment window period because of the service limitations.
This is the first observational study to examine the treatment effect and economic analysis of both antivirals which were introduced to Hong Kong in early 2022 in response to the Omicron outbreak. It reflects the real-life application of these antiviral agents in a COVID-19 outbreak setting. Our study suggests the use of antivirals among hospitalised patients improved 28-day survival, in line with previous clinical trials that were conducted in non-hospitalised patient samples.2,3 Further analysis with adjustment reveals the significant superiority of Nirmatrelvir-ritonavir over Molnupiravir in terms of all-cause mortality, and the reduction of hospitalisation and related hospital LOS in the outpatient cohort, suggesting a preference for Nirmatrelvir-ritonavir unless other factors need consideration.
The 31,766 patients sampled from the clinics were of milder severity. Most of the patients were given antivirals due to age reasons. Compared to the non-users, the antiviral users had a higher proportion of senior age, while compared with Molnupiravir, Nirmatrelvir-ritonavir were given to younger patients and those with less comorbidities. This was due to a longer list of contraindications and more interactions with common long-term medications such as a statin. An interesting observation is that patients given antivirals had greater likelihood of reattending the clinic. While these patients were instructed to visit Emergency Departments if their condition deteriorated, reattendance is a possible marker of persistent symptoms, including Long COVID-19 syndrome, which warrants further analysis. However, it must be noted that each of the antivirals reduced ED visits after treatment. Moreover, the LOS was shorter if the patients were admitted after antiviral treatment. The effect on mortality among clinic patients could not be assessed because only a very small number of clinic patients died during the follow up period.
This study demonstrates the high value of these antivirals in population-wide infection control. The use of both antiviral medications saves cost with less subsequent hospitalisation, shorter length of stay, and fewer deaths (in terms of value of statistical life). In the inpatient cohort, the Nirmatrelvir-ritonavir saves inpatient healthcare expenditure while preventing death from COVID-19. This is more cost-effective than standard care, as it substantially reduced risk for all-cause mortality – as indicated by the primary outcomes and shorter length of stay. However, the ICERs for both antiviral medications were much larger in the outpatient setting. This suggests that the cost-effectiveness reduction in death from COVID-19, would be less when the medications were used among patients who were less likely to develop severe conditions and to die during the course of disease.
Primary care physicians in the clinic and ED are playing a critical role in controlling the impact of the infection on health systems. On one hand, COVID-19 patients are identified by them via screening and testing in designated fever areas, on the other hand, they provide appropriate management, including treatment and public health infection control advice, to COVID-19 patients with a wide spectrum of severity. The evidence based on this real-life data informs physicians in primary care setting of the efficacy of both antivirals against COVID-19, and its financial implication, so that more high-risk COVID-19 patients might benefit from community management, saving hospital beds for more severe patients.
Limitations
This is an analysis of real-life data from an administrative database in Hong Kong, from which symptomatology, SARS-CoV-2 vaccination status and vitals sign measurement were not available. Moreover, medical compliance data was not available, and therefore fidelity cannot be adjusted. Even though all COVID-19 patients were treated in the Hospital Authority, they could switch to private health facilities for follow up if they were negative in SARS-CoV-2 testing, leading to loss of follow up and immortal time bias. With the recent availability of these antivirals and the proximity to the outbreak, this study has short follow-up, meaning that late outcomes / effects, for example, prevention of Long-COVID syndrome or associated health service utilization, are not assessable during the study period. It is unclear how generalisable these findings are, as the treatment of COVID-19 is changing with the emergence of further evidence, and the patient spectrum in different countries and regions are highly variable. Given that the patients in this project were from Hong Kong, the adoption of these treatment regimens may only have limited applicability in different continents, where local cost-effectiveness evaluation, availability in market and healthcare financing models need to be further considered.
Conclusion
In Hong Kong, among outpatient and hospitalised patients with mild to moderate COVID-19 at high-risk of adverse complications, this population-based cohort study demonstrated that early initiation of 5-day Molnupiravir and Nirmatrelvir-ritonavir treatment within 5 days of symptom onset was associated with a lower risk of in-hospital death compared with control subjects. The use of these antivirals led to potential savings on health care cost, and thus decision- and policy-makers should be informed of these potential benefits.
Contributors
AKCW and CYC are the guarantors of the content of the manuscript, including the data and analysis. AKCW and CYC conceptualized, designed, implemented the study, performed the data analysis and drafted the manuscript. ELYW and THR conceptualized, designed and made critical revisions to the manuscript. AWLC, KW, SCLC, TTLL, LYKL, ETHY, OWKT, KWYC performed the data analysis. AWLC, KW, and JWKH made critical revisions to the manuscript. SL, CKT and TY contributed to acquisition of the data and data analysis. ELYW and THR contributed to conceptualization of the study and made critical revisions to the manuscript. All authors edited and approved the final version of the manuscript.
Data sharing statement
Individual participant data that underlie the results reported in this article, the study protocol, statistical analysis plan, and analytic code would be available upon request sent to the authors, and application to the Hospital Authority, after de-identification (text, tables, figures, and appendices).
Declaration of interests
No conflict of interest existed for AKCW, SCLC, TTLL, OWKT, KWYC, SL, CKT, TY, THR. CYC, AWLC, KW, ELYW of Centre for Health Systems & Policy Research are supported by funding from The Tung's Foundation. LYFL, ETFY, JWKH of The Laboratory of Data Discovery for Health Limited (D24H) are supported by funding from the AIR@InnoHK platform administered by the Innovation and Technology Commission of Hong Kong.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100602.
Contributor Information
Timothy Hudson Rainer, Email: thrainer@hku.hk.
Eliza Lai-Yi Wong, Email: lywong@cuhk.edu.hk.
Appendix. Supplementary materials
References
- 1.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 2.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Therapeutics and COVID-19: living guideline 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4. Accessed 14 July 2022. [PubMed]
- 5.Smith DJ, Hakim AJ, Leung GM, et al. COVID-19 mortality and vaccine coverage—hong kong special administrative region, China, January 6, 2022–March 21, 2022. Morbid Mortal Weekly Report. 2022;71(15):545. doi: 10.15585/mmwr.mm7115e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Health Protection Department of Health Hong Kong SAR. Statistics on 5th wave of COVID-19. 2022. https://www.coronavirus.gov.hk/eng/5th-wave-statistics.html. Accesseed 14 July 2022.
- 7.Richards F, Kodjamanova P, Chen X, et al. Economic burden of COVID-19: a systematic review. ClinEcon Outcomes Res. 2022;14:293. doi: 10.2147/CEOR.S338225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suthar S, Das S, Nagpure A, et al. Epidemiology and diagnosis, environmental resources quality and socio-economic perspectives for COVID-19 pandemic. J Environ Manage. 2021;280 doi: 10.1016/j.jenvman.2020.111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Public health surveillance for COVID-19: interim guidance 2022.www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/publications-and-technical-guidance/2022/public-health-surveillance-for-covid-19-interim-guidance,-14-february-2022. Accessed 14 February 2022.
- 10.Hospital Authority Task Force on Clinical Management on Infection. Interim recommendation on clinical management of adult cases with coronavirus disease 2019 (COVID-19). Hong Kong: Hospital Authority, 2022.
- 11.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 12.Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2022;15(1):14–20. doi: 10.1093/ckj/sfab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115(1):92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 14.Greifer N. WeightIt: weighting for covariate balance in observational studies. 2020. https://ngreifer.github.io/WeightIt/. Accessed 14 February 2022.
- 15.Kassambara A, Kosinski M, Biecek P, Fabian S. Package ‘survminer’. 2017. https://cran.r-project.org/web/packages/survminer/survminer.pdf. Accessed 14 February 2022.
- 16.Therneau T. A package for survival analysis in R. R package version 3.4-0. https://CRAN.R-project.org/package=survival. 2022. Accessed 14 February 2022.
- 17.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 18.Wickham H. ggplot2: elegant graphics for data analysis. Springer; USA, Houston: 2016. ISBN 978-3-319-24277-4. [Google Scholar]
- 19.Hospital Authority. Fees and charges. 2017. https://www.ha.org.hk/visitor/ha_visitor_index.asp?Content_ID=10045&Lang=ENG. Accessed 31 May 2022.
- 20.Viscusi WK, Aldy JE. The value of a statistical life: a critical review of market estimates throughout the world. J Risk Uncertainty. 2003;27(1):5–76. [Google Scholar]
- 21.Andersson H, Treich N. In: Handbook in Transport Economics. de Palma A, Lindsey C, Quinet E, Vickerman R, editors. Edward Elgar; Cheltenham, UK: 2011. The value of a statistical life; pp. 396–424. [Google Scholar]
- 22.Wong TW, Tam W, Wong A, Liu S. Environmental Protection Department Hong Kong SAR; Hong Kong: 2016. Developing an Instrument for Assessing the Health and Economic Impacts of Air Pollution in Hong Kong. Final Report. [Google Scholar]
- 23.Statistics & Census Department . Consumer Prices. 2022. The government of hong kong special administrative region. Accessed 27 May 2022. [Google Scholar]
- 24.Yeung K WM, Beinfeld M, Mohammed R, Wright A, Nhan E, Fluetsch N, Richardson M, Pearson SD. Final Evidence Report and Meeting Summary.: Institute for Clinical and Economic Review. 2022. Special assessment of outpatient treatments for COVID-19. [Google Scholar]
- 25.Gerlach S. Monetary operations by Hong Kong's currency board. J Asian Econ. 2005;15(6):1119–1135. [Google Scholar]
- 26.Congly SE, Varughese RA, Brown CE, Clement FM, Saxinger L. Treatment of moderate to severe respiratory COVID-19: a cost-utility analysis. Sci Rep. 2021;11(1):17787. doi: 10.1038/s41598-021-97259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheinson D, Dang J, Shah A, Meng Y, Elsea D, Kowal S. A cost-effectiveness framework for COVID-19 treatments for hospitalized patients in the United States. Adv Ther. 2021;38(4):1811–1831. doi: 10.1007/s12325-021-01654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black WC, Keeler EB, Soneji SS. Cost-effectiveness of CT screening in the national lung screening trial. N Engl J Med. 2015;372(4):388. doi: 10.1056/NEJMc1414726. [DOI] [PubMed] [Google Scholar]
- 29.Hannah Ritchie EM, Lucas Rodés-Guirao, Cameron Appel, et al.. Coronavirus pandemic (COVID-19). May 29, 2022. https://ourworldindata.org/covid-deaths. Accessed 30 May 30 2022.
- 30.The Government of the Hong Kong Special Administrative Region. Transcript of remarks of press conference on anti-epidemic measures. https://www.info.gov.hk/gia/general/202203/21/P2022032100446.htm. Accessed 30 May 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.