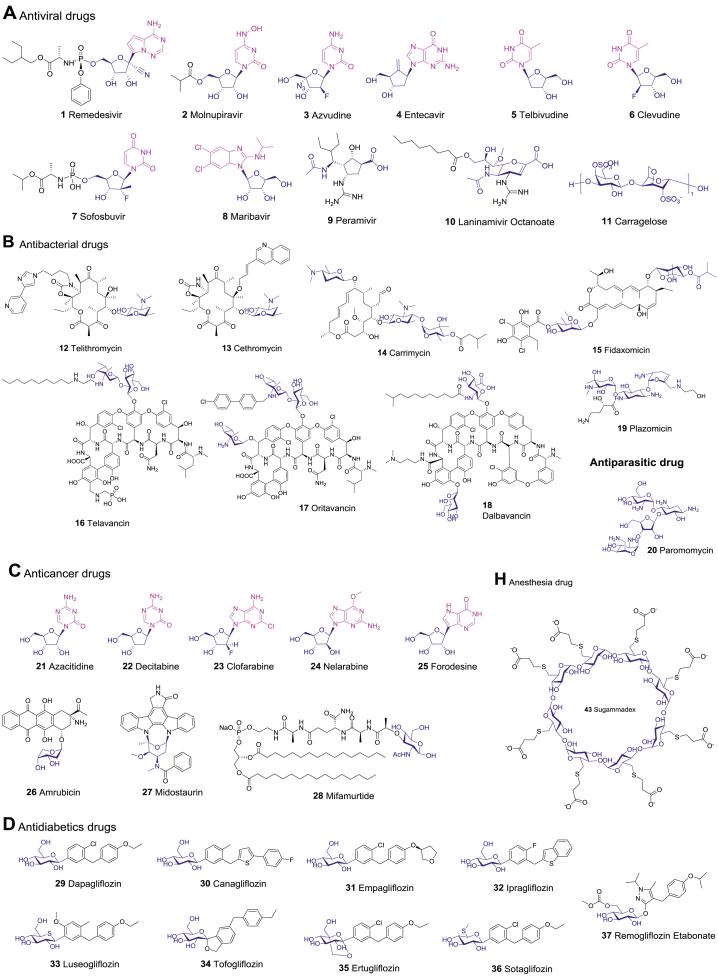
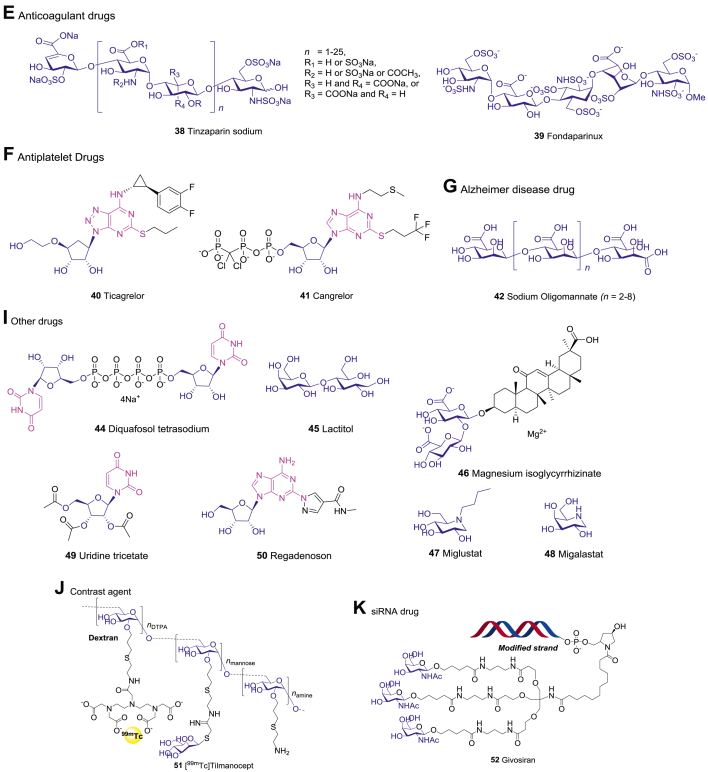
Abstract
Carbohydrates are fundamental molecules involved in nearly all aspects of lives, such as being involved in formating the genetic and energy materials, supporting the structure of organisms, constituting invasion and host defense systems, and forming antibiotics secondary metabolites. The naturally occurring carbohydrates and their derivatives have been extensively studied as therapeutic agents for the treatment of various diseases. During 2000 to 2021, totally 54 carbohydrate-based drugs which contain carbohydrate moities as the major structural units have been approved as drugs or diagnostic agents. Here we provide a comprehensive review on the chemical structures, activities, and clinical trial results of these carbohydrate-based drugs, which are categorized by their indications into antiviral drugs, antibacterial/antiparasitic drugs, anticancer drugs, antidiabetics drugs, cardiovascular drugs, nervous system drugs, and other agents.
Key words: Carbohydrate-based drug, Glycodrug, Glycoconjugate, Antibiotics, Antivirus drug, Anticancer drug
Graphical abstract
A systemic review of the total 54 carbohydrate-based drugs, which approved during 2000–2021, is comprehensively summarized according to their chemical structures, activities, and clinical trial results.
1. Introduction
Carbohydrates and carbohydrate-containing molecules are involved in almost every aspect of living organisms and perform various important biological functions1, 2, 3. Thus, the study of carbohydrate-based molecules has long been an important area of drug research4, 5, 6, 7. For examples, carbohydrate-based antibiotics, including streptomycin, neomycin, and gentamicin were discovered as anti-infectives in 1940s; adriamycin was isolated and developed as one of the most widely prescribed anticancer drugs; ganglioside GM1 was extracted and developed as an acute stroke drug; and the polysaccharide hyaluronic acid was investigated for arthritis treatment. In fact, the broad biological functions of carbohydrates lay the basis for the development of carbohydrate-based drugs.
d-Glucose is a energy source for most living organisms8, accordingly, the conjugates or derivatives of d-glucose can be used to treat metabolic disorders such as diabetes. Since d-ribose and -deoxyribose are the building blocks of RNA and DNA9, their derivatives are wiedely used to insert and interrupt the replication processes of pathological cells as well as viruses. Also prominent are the various glycans presenting on the surface of viruses, bacteria, and eukaryotic cells, which are responsible for recognition, communication, and invasion, therefore can be used as candidates for diagnosis and treatment of diseases10, 11, 12. In addition, microorganisms and plants secrete a large variety of carbohydrate-based secondary metabolites as defense or signaling moleculars13, which can be used as leads for drug development14.
Since 2000, great progresses have been achieved in the fields of carbohydrate chemistry15, 16, 17, 18, 19, 20, 21, glycobiology22,23, and chemical glycobiology24,25, bringing numerous opportunities for carbohydrate-based drug discovery26, 27, 28. A number of innovative carbohydrate-based drugs were designed, evaluated, and developed in the past twenty years29, 30, 31, 32, 33. For example, with a new mechanism of action, the sodium-glucose cotransporter type 2 (SGLT2) inhibitors bring great benefits to the type 2 diabetes mellitus (T2DM) patients, not only in blood glucose control, but also in kidney and heart functions34,35.
There have been excellent reviews and book chapters updating the research progresses on topics relevant to carbohydrate-based drugs, diagnostic agents, and vaccines during the past years36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55. Aiming to provide a comprehensive vision of the latest advances in the carbohydrate-based drugs and diagnostics, here we summarize the carbohydrate-based drugs and diagnostic agents approved during the period 2000–2021 around the world, including the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the National Medical Products Administration of China (NMPA), as well as in Japan, South Korea, and India, etc.
2. The approved carbohydrate-based drugs during 2000–2021
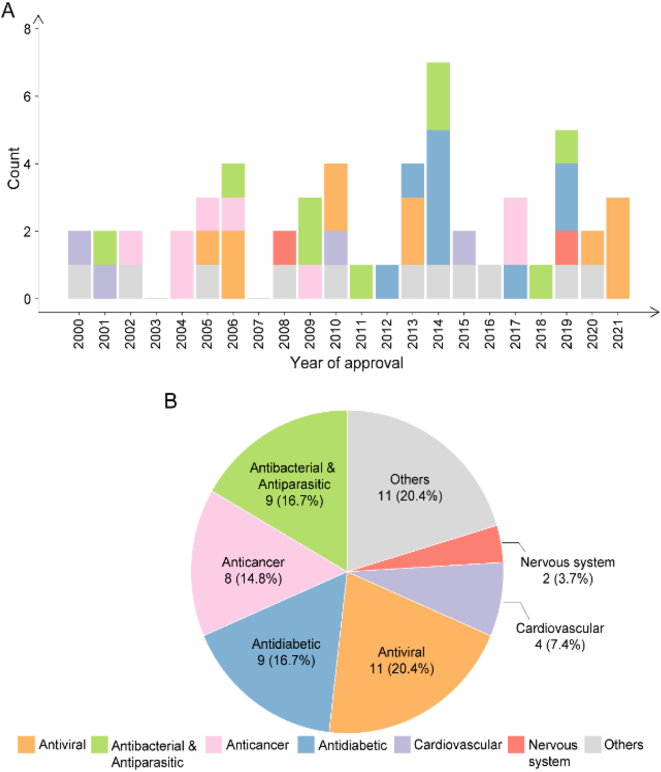
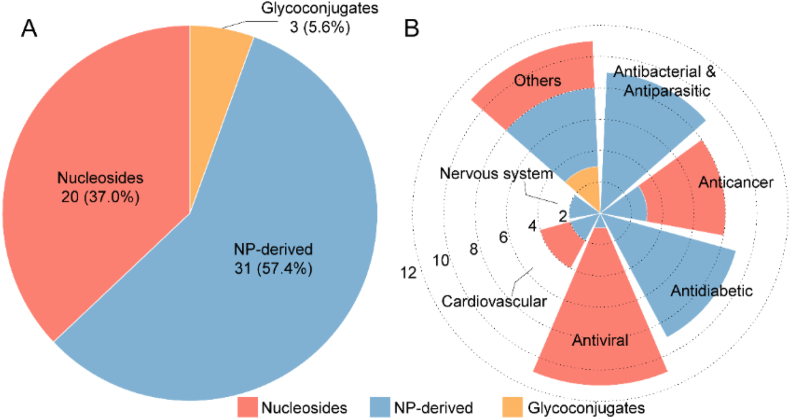
Over the past two decades, 54 carbohydrate-based new chemical entities (NCEs) have been launched worldwide (Table 1 and Fig. 1). Here we classify them into seven major categories based on their therapeutic indications, those include antiviral drugs, antibacterial/antiparasitic drugs, anticancer drugs, antidiabetic drugs, cardiovascular drugs, nervous system drugs, and other drugs. While the R&D of carbohydrate-based drugs is a continuous process, there have been a few explosive breakthroughs (Fig. 2A). Thus, the first SGLT2 inhibitor Dapagliflozin was launched in 2012 for T2DM treatment; then from 2012 to 2014, five analogues with the same mechanism, canagliflozin, empagliflozin, ipragliflozin, luseogliflozin, and tofogliflozin were successively approved, bringing more choices for T2DM patients. The top four categories, including antiviral drugs (10), antibacterial/antiparasitic drugs (9), anticancer drugs (8), and antidiabetic drugs (8), account for more than 60% of all CBNCEs (Fig. 2B). Prominently, all these CBNCEs are either natural products or derived from the natural carbohydrate scaffolds, among them 37% are nucleosides (Table 1 and Fig. 3).
Table 1.
The carbohydrate-based drugs launched during 2000–2021.
| No. | Generic name | Indications | Year | Country | Chemical category |
|---|---|---|---|---|---|
| 1 | Remedesivir | Antiviral | 2020 | USA | Nucleoside |
| 2 | Molnupiravir | Antiviral | 2021 | UK | Nucleoside |
| 3 | Azvudine | Antiviral | 2021 | China | Nucleoside |
| 4 | Entecavir | Antiviral | 2005 | USA | Nucleoside |
| 5 | Telbivudine | Antiviral | 2006 | USA | Nucleoside |
| 6 | Clevudine | Antiviral | 2006 | South Korea | Nucleoside |
| 7 | Sofosbuvir | Antiviral | 2013 | USA | Nucleoside |
| 8 | Maribavir | Antiviral | 2021 | USA | Nucleoside |
| 9 | Peramivir | Antiviral | 2010 | Japan | Nucleoside |
| 10 | Laninamivir octanoate | Antiviral | 2010 | Japan | Nucleoside |
| 11 | Carragelose | Antiviral | 2013 | Europe | NP-deriveda |
| 12 | Telithromycin | Antibacterial | 2001 | USA | NP-derived |
| 13 | Cethromycin | Antibacterial | 2009 | USA | NP-derived |
| 14 | Carrimycin | Antibacterial | 2019 | China | NP-derived |
| 15 | Fidaxomicin | Antibacterial | 2011 | USA | NP-derived |
| 16 | Telavancin | Antibacterial | 2009 | USA | NP-derived |
| 17 | Oritavancin | Antibacterial | 2014 | USA | NP-derived |
| 18 | Dalbavancin | Antibacterial | 2014 | USA | NP-derived |
| 19 | Plazomicin | Antibacterial | 2018 | USA | NP-derived |
| 20 | Paromomycin | Antiparasitic | 2006 | India | NP-derived |
| 21 | Azacitidine | Anticancer | 2004 | USA | Nucleoside |
| 22 | Decitabine | Anticancer | 2006 | USA | Nucleoside |
| 23 | Clofarabine | Anticancer | 2004 | USA | Nucleoside |
| 24 | Nelarabine | Anticancer | 2005 | USA | Nucleoside |
| 25 | Forodesine | Anticancer | 2017 | Japan | Nucleoside |
| 26 | Amrubicin | Anticancer | 2002 | Japan | NP-derived |
| 27 | Midostaurin | Anticancer | 2017 | USA | NP-derived |
| 28 | Mifamurtide | Anticancer | 2009 | Europe | NP-derived |
| 29 | Dapagliflozin | Antidiabetic | 2012 | Europe | NP-derived |
| 30 | Canagliflozin | Antidiabetic | 2013 | USA | NP-derived |
| 31 | Empagliflozin | Antidiabetic | 2014 | Europe | NP-derived |
| 32 | Ipragliflozin | Antidiabetic | 2014 | Japan | NP-derived |
| 33 | Luseogliflozin | Antidiabetic | 2014 | Japan | NP-derived |
| 34 | Tofogliflozin | Antidiabetic | 2014 | Japan | NP-derived |
| 35 | Ertugliflozin | Antidiabetic | 2017 | USA | NP-derived |
| 36 | Sotaglifozin | Antidiabetic | 2019 | Europe | NP-derived |
| 37 | Remogliflozin etabonate | Antidiabetic | 2019 | India | NP-derived |
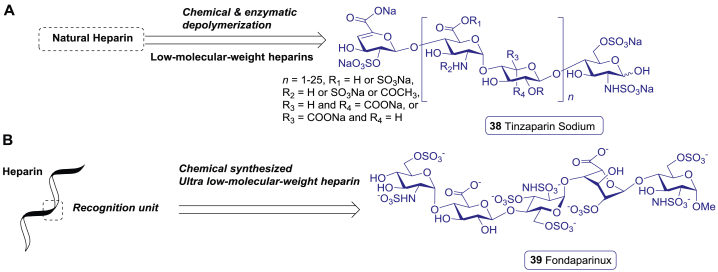
| 38 | Tinzaparin sodium | Cardiovascular/anticoagulant | 2000 | USA | NP-derived |
| 39 | Fondaparinux sodium | Cardiovascular/anticoagulant | 2001 | USA | NP-derived |
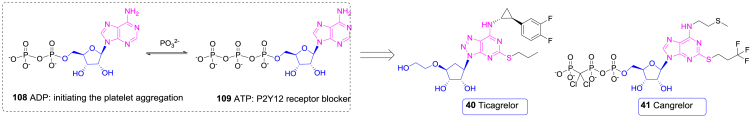
| 40 | Ticagrelor | Cardiovascular | 2010 | Europe | Nucleoside |
| 41 | Cangrelor | Cardiovascular | 2015 | USA | Nucleoside |
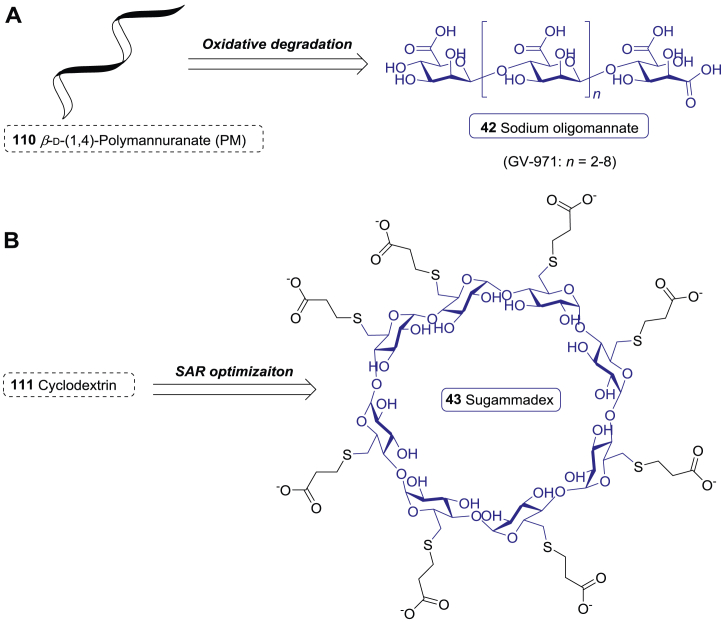
| 42 | Sodium oligomannate | Alzheimer disease | 2019 | China | NP-derived |
| 43 | Sugammadex | Anesthesia | 2008 | Europe | NP-derived |
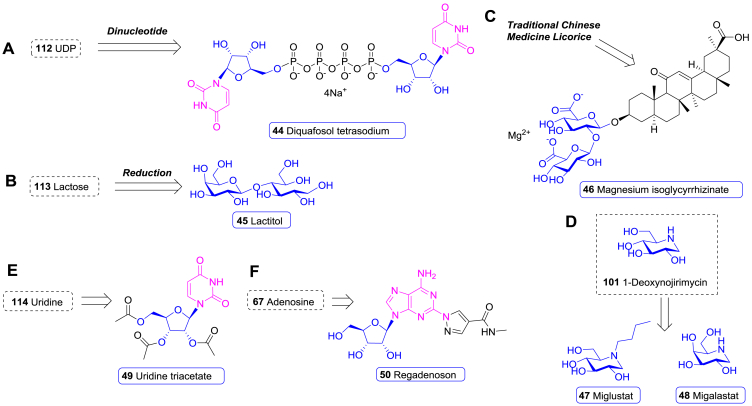
| 44 | Diquafosol tetrasodium | Dry eye disease | 2010 | Japan | Nucleoside |
| 45 | Lactitol | Chronic idiopathic constipation | 2020 | USA | NP-derived |
| 46 | Magnesium isoglycyrrhizinate | Anti-inflammatory | 2005 | China | NP-derived |
| 47 | Miglustat | Gaucher disease | 2002 | Europe | NP-derived |
| 48 | Migalastat | Fabry disease | 2016 | Europe | NP-derived |
| 49 | Uridine triacetate | Hereditary orotic aciduria | 2015 | USA | Nucleoside |
| 50 | Regadenoson | Myocardial perfusion imaging | 2008 | USA | Nucleoside |
| 51 | [99mTc]Tilmanocept | Contrast media | 2013 | USA | NP-derived |
| 52 | Givosiran | siRNA for AHP | 2019 | USA | Glycoconjugate |
| 53 | S. Pneumoniae vaccines | S. Pneumoniae prevention | 2000–2021 | USA, Europe, etc. | Glycoconjugate |
| 54 | Typhim Vi vaccine | Typhim prevention | 2014 | USA | Glycoconjugate |
NP-derived: derived from natural product.
Figure 1.
The chemical structures of carbohydrate-based drugs launched during 2000–2021.
Figure 2.
Statistics of carbohydrate-based drugs by launched year (A) and their medical indications (B).
Figure 3.
Statistics of the carbohydrate-based drugs according to chemical sources (A) and indications (B).
3. Carbohydrate-based antiviral drugs
Viral pathogens have been one of the great threats to public health throughout human history, accounting for more than 60 percent of the past pandemics, including the outbreaks of severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002–2003, Asian hghly pathogenic avian influenza (HPAI) A (H5N1) in 2009 and 2010, Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, Ebola virus in West Africa in 2014–2016, as well as the ongoing coronavirus disease 2019 (COVID-19)56, 57, 58. Besides, there is still no complete cure for acquired immune deficiency syndrome (AIDS) caused by human immunodeficiency virus (HIV) and hepatitis B disease caused by hepatitis B virus (HBV). Current treatments of HIV and HBV can only maintain low levels of the virus through lifelong antiviral therapy59,60.
The carbohydrate-based molecules show unique advantages in antiviral drug discovery. Nucleoside and nucleotide could selectively interrupt the replication of the viral RNA or DNA61. Eight new antiviral drugs have been developed based on this mechanism, including remedesivir (1), molnupiravir (2), azvudine (3), entecavir (4), telbivudine (5), clevudine (6), sofosbuvir (7), and maribavir (8). Some sugar moieties of glycoproteins on mammalian cell membrane can be hijacked by viruses and act as anchor receptors for host cell invasion. For example, the influenza A and B viruses infect host cells through binding to the α-2,3-linked sialic acid (N-acetyl-neuraminic acid, zanamivir)62, which is an important terminal sugar moiety of mammalian glycoproteins. For this reason, sialic acid analogues, including peramivir (9) and laninamivir octanoa (10), have been developed as neuraminidase inhibitors to block the invasion of influenza A and B. Moreover, since the natural carrageenans prevent viruses’ attachment to host cells and infection, the Iota-carrageenan carragelose (11) was approved as an OTC drug recently.
3.1. Drugs for COVID-19
Since late 2019, the world has been shaken by the ongoing COVID-19 pandemic. Though several inactivated, recombinant, and mRNA vaccines have been emergently authorized, the specific antiviral medicines are still in demand to stop the pandemic63. Rapid compound screenings have provided numerous potential antiviral agents for urgent clinical trials, such as chloroquine, hydroxychloroquine, favipiravir, lopinavir, ritonavir, and remdesivir (1)64, 65, 66, 67, 68, 69.
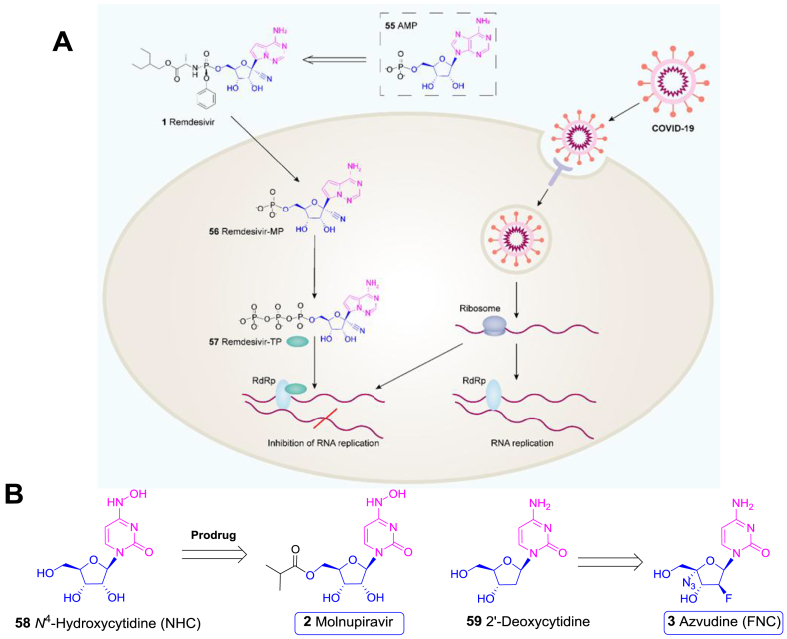
Remdesivir (1) is an AMP (55) nucleotide analogue (Fig. 4A), originally developed by Gilead and purposed for treatment of Ebola virus (IC50 = 100 nmol/L) and MERS-CoV (IC50 = 340 nmol/L). This compound, containing a necessary 1-β-cyano-ribose scaffold and a 1-α-C-nucleobase unit, is a phosphate prodrug, which can be readily hydrolysed into remdesivir-MP (56) and further converted to remdesivir-TP (57). The latter form can be incorporated into the nascent viral RNA to prevent viral replication70,71. However, it failed in a phase II anti-Ebola clinical trial conducted in West Africa in 201672.
Figure 4.
Carbohydrate-based drugs for COVID-19. (A) From AMP (55) to remdesivir (1) and its mode of action. RdRp, RNA-dependent RNA polymerase. (B) The potential antiviral nucleosides (2 and 3) for COVID-19 therapy and their parent compounds (58 and 59).
In vitro experiments revealed that remdesivir (1) could protect the Vero E6 cells against the infection of COVID-19 with an IC50 value of 770 nmol/L and IC90 value of 1760 nmol/L based on the qRT-PCR quantification of viral copy number in infected cells73, 74, 75. Several emergent large scale randomized, double-blind, placebo-controlled trials were conducted to evaluate the safety and efficacy of remdesivir (1) for severe COVID-19 patients76,77. The results showed that the patients receiving remdesivir (1) recovered 31% faster than those in the placebo group (11 vs. 15 days)69,70. A downtrend of mortality was also observed, although not statistically significant (8.0% vs. 11.6%). Therefore, remdesivir (1) was authorized by FDA for the treatment of hospitalized patients with severe COVID-19 under an Emergency Use Authorization (EUA)69,77,78.
Molnupiravir (2), also named EIDD-2801, is a prodrug of the cytidine nucleoside β-d-N-4-hydroxycytidine (NHC, 58) developed by Merck (Fig. 4B)79. This molecule was found effective in reducing nasopharyngeal COVID-19 virus and viral RNA, with good safety and tolerability80. Among 202 participants in a phase II clinical trial (NCT04405570), the viral load in the 800 mg molnupiravir (2) group (1.9%) was significantly inhibited compared with the placebo group (16.7%) after 3–5 days’ treatment. A more recent phase III clinical trial (MOVe-OUT) indicated that molnupiravir (2) reduced the risk of hospitalization or death by about 50% in non-hospitalized adults with mild to moderate COVID-1980. Accordingly, molnupiravir (2) was approved in UK, becoming the first oral antiviral drug for the treatment of COVID-19.
There are other antiviral nucleosides which have shown promise when repurposed to treat COVID-19. Azvudine (3), the 2′-deoxycytidine (59) anaologue developed by Genuine Biotech, is an oral effective 2′-deoxy-2′-β-fluoro-4′-azidocytidine (FNC) antiviral nucleoside (Fig. 4B)81. Azvudine (3) demonstrated highly potent replication inhibition against both HIV-1 and HIV-2 virus (EC50: 0.018–6.92 nmol/L). In phase II and III clinical trials, azvudine (3) displayed desirable pharmacokinetics, excellent efficacy, and safety for HIV treatment82, thus was approved as an anti-HIV drug in China in 2021. Azvudine (3) was found to be also effective in inhibiting COVID-19. A randomized, open-label, controlled clinical trial showed that Azvudine treatment significantly reduced the mean time of the first nucleic acid negative conversion (NANC) compared with standard antiviral treatment (2.6 vs. 5.6 days, P = 0.008)83. Such a convenient and inexpensive oral medicine could greatly benefit the treatment of mild to moderate COVID-1984.
3.2. Antiviral nucleosides and nucleotides
Nucleoside/nucleotides have been widely used to treat viral infections besides the COVID-1962. For example, idoxuridine (60), vidarabine (61), and ribavirin (62) were developed before 2000 (Supporting Information Fig. S1)85. In the past twenty years, four more nucleoside/nucleotide antiviral drugs, i.e., entecavir (4), telbivudine (5), clevudine (6), and sofosbuvir (7), have been marketed. All these four drugs are used for HBV treatment, with Sofosbuvir (7) originally used for hepatitis C virus (HCV).
Chronic HBV infection causes Hepatitis B and puts people at high risk of death from cirrhosis and liver cancer60. The FDA has approved a total of seven anti-hepatitis B drugs, including interferon α (IFNα), PEG-IFNα, lamivudine (63), entecavir (4), telbivudine (5), adefovir dipivoxil, and tenofovir disoproxil. However, none of them could eliminate HBV due to the occuurence of covalently closed circular DNA (cccDNA) of HBV86.
Entecavir (ETV, 4) is a novel carbocyclic nucleoside drug developed by Bristol-Myers Squibb (BMS). The cyclopentane pseudo sugar moiety mimicks 2′-deoxyguanosine (64, Fig. 5)87. The corresponding triphosphate (entecavir-TP) can compete with the natural nucleotide deoxyguanosine triphosphate (dGTP) to inhibit HBV DNA polymerase with a Ki value of 1.2 nmol/L. The inhibition of mammalian DNA polymerase α, β, or γ isoforms is relatively weak, with a Ki of 18–40 μmol/L88. In phase II and III clinical trials, entecavir (4) demonstrated superior advantages over lamivudine for all primary endpoints evaluated in both nucleoside-naive and lamivudine-resistant patients89. Since it was highly effective in both HBeAg-positive and HBeAg-negative nucleoside-naive patients, entecavir (4) was approved for the treatment of HBV in 2005.
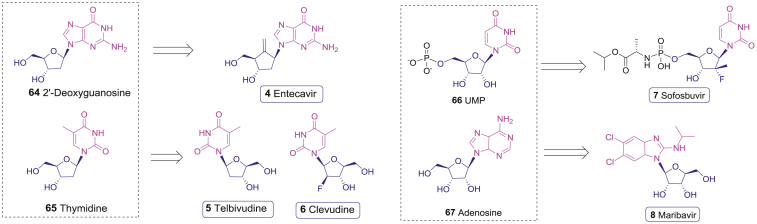
Figure 5.
The antivires drugs (4–8) are derived from natural nucleoside and nucleotide 64–67.
Telbivudine (5), a l-enantiomer of the natural d-thymidine (65), is developed by Idenix and Novartis (Fig. 5)90. The triphosphate form of telbivudine (telbivudine-TP) can compete with natural thymidine triphosphate to inhibit HBV DNA duplication with EC50 of 1.3 ± 1.6 μmol/L for the first strand (RNA-dependent) DNA synthesis and a preferential EC50 of 0.2 ± 0.2 μmol/L for the second strand (DNA-dependent) synthesis, wheras it does not inhibit mammalian DNA polymerases at concentrations up to 100 μmol/L91. In a 52-week phase III trial, the telbivudine (5) group showed greater reductions in serum HBV DNA compared with the lamivudine (63) group; in another one-year switching trial, the serum HBV DNA of telbivudine (5) group decreased more than that of the Adefovir group even at 24 weeks92,93. In addition, the adverse events of telbivudine (5) were mild and well tolerated by patients, thus it was approved by FDA in 2006 for the treatment of chronic HBV infection.
Clevudine (CLV, 6) is another l-enantiomeric analogue of natural d-thymidine (65) with an l-2-deoxy-2-fluoro-β-arabinofuranosyl moiety developed by Bukwang Pharma in South Korea (Fig. 5)94. The triphosphate form (clevudine-TP) inhibits the second strand (DNA-dependent) synthesis by HBV DNA polymerase with an EC50 of 16.3 ± 2.4 nmol/L and has the interaction with human DNA polymerases95. Clevudine (6) was tested in a 48-week follow-on phase III clinical trial in Korean for the treatment of HBV infection. After clevudine (6) treatment for 24 weeks, HBV DNA was still under the detectable line in 92% of HBeAg− patients and 59% of HBeAg+ patients96. Based on these meaningful results, clevudine (6) received the first approval in South Korea in 2006.
HCV is a bloodborne and hepatotropic RNA virus, which causes acute and chronic liver inflammation and leads to severe liver diseases such as cirrhosis, liver cancer, and chronic liver failure. According to WHO, an estimated 71 million people worldwide are chronically infected with HCV97. Until the recent discovery of sofosbuvir (7), HCV treatments require complicated long-term medications with limited efficacy and severe side effects98,99. Sofosbuvir (7), developed by Gilead, contains a β-d-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine-5′-monophosphate skeleton which mimics UMP (66, Fig. 5)100. After oral administration, sofosbuvir (7) is efficiently absorbed by liver cells and phosphorylated to its metabolite forms sofosbuvir-MP and sofosbuvir-TP. Sofosbuvir-TP inhibits the NS5B RNA-dependent RNA polymerase of HCV during the viral RNA replication with an EC50 of 92 nmol/L and an EC90 of 0.29 μmol/L, respectively101. Moreover, sofosbuvir (7) demonstrates low activity to human RNA polymerases and DNA polymerases, resulting in an overall safety profile. In a phase I clinic trial, sofosbuvir (7) showed favorable pharmacokinetic profile and was well tolerated at the tested doses102. Then a series of randomized, multicenter phase II and phase III clinical trials were carried out. The sustained virologic response rates at 12 weeks (SVR12) were 97%–99% in all administrated groups, including the combination of ledipasvir and sofosbuvir (7) for 12 weeks, the combination of ledipasvir, sofosbuvir (7), and ribavirin (62) for 12 weeks, the combination of ledipasvir and sofosbuvir (7) for 24 weeks, and the combination of ledipasvir, sofosbuvir (7), and ribavirin (62) for 24 weeks103. These data showed that the stubborn HCV disease could be cured by sofosbuvir (7) in combination with several synergetic drugs104. Therefore, sofosbuvir (7) was approved by FDA in December 2013 for the treatment of chronic HCV infection in all sub-genotypes, including those with liver cancer meeting Milan criteria and those with HIV-1 coinfections. Recently, WHO proposed a new global vision to eliminate hepatitis C infection by 2030, with the benefit of Sofosbuvir (7)97.
Maribavir (8), which has been developed in Takeda since 2000, is a dicholoro-benzimidazole l-riboside (Fig. 5). This substituted benzimidazole neucleoside mimics adenosine (67) and acts as an ATP competitive inhibitor with an IC50 of 3 nmol/L against the cytomegalovirus (CMV) UL97 kinase, which is involved in viral DNA assembly and capsids movement from virus to infected cells105. DNA hybridization assay showed that the IC50 of maribavir (8) against CMV viral replication was 0.12 ± 0.01 μmol/L107. An early preventive clinical trial revealed that maribavir (8) reduced the incidence of CMV infection after allogeneic stem-cell transplantation compared with placebo, without myelosuppression106. Accordingly, maribavir (8) was granted Orphan Drug Designation for the prevention of cytomegalovirus viremia and disease in high-risk populations by the FDA in 2007. Afterwards, more trials had focused on the efficacy of maribavir (8) in the treatment of drug-resistant or refractory CMV infection. According to the phase II/III trials of hematopoietic-cell or solid-organ transplants with CMV reactivation, 79% of patients in maribavir (8) arm had a response to the treatment, not inferior to valganciclovir (67%)107. Recently, FDA approved it as the first drug for treating adults and pediatric patients with post-transplant CMV infection/disease that does not respond to available antiviral treatment for CMV.
3.3. Neuraminidase inhibitors
Influenza A and B are the most common influenza virus types, causing seasonal influenza and a large number of deaths every year. While influenza A can infect humans and other animals, such as birds and pigs, influenza B appears to be found only in humans. Influenza A includes various subtypes based on different expression levels of hemagglutinin (H) and neuraminidase (N) on the viral surfaces108. The neuraminidase is a viral enzyme that recognizes the specific α-2,3-linked sialic acid moiety to invade host cells and cleaves the sialic acid moiety to release newly formed virus109. Targeting this specific enzyme, sialic acid analogues were developed to block influenza A and B with unprecedented success110.
Sialic acid (N-acetylneuraminic acid or Neu5Ac, 68) is a high-carbon sugar with a complex nine-carbon skeleton. Based on that the neuraminidase cleaves Neu5Ac with a six-membered planar oxonium transition state, zanamivir (69), with a 2,3-didehydro-2-deoxy-N-acetylneuraminic acid (Neu5Ac2en) moiety, is developed as a mimic of the transitions state. It efficiently inhibits the influenza viruses with IC50 of 0.95 and 2.7 nmol/L for influenza A and B, respectively111. Since the 4-guanidinium group increases the polarity of zanamivir (69) and affects the oral bioavailability, zanamivir is delivered by intranasal or dry powder inhalation. It was launched in 1999. In the same year, Roche launched a blockbuster neuraminidase inhibitor, oseltamivir (70). This drug contains a cyclohexene core instead of the 2,3-glycal of Neu5Ac (68), a 4-amine instead of the 4-guanidinium group, and a pentan-3′-O-ester instead of the 6-glycerol group112. Oseltamivir (70) inhibits influenza A-H3N2, A-H1N2, A-H1N1, and influenza B viruses with the IC50 values of 0.67, 0.9, 1.34, and 13 nmol/L, respectively113. Since the polarity has been greatly optimized by the structural modifications, both the oral bioavailability and therapeutic efficiency of oseltamivir (70) are improved compared to zanamivir (69).
Afterwards, peramivir (9) and laninamivir octanoate (10) were approved as new anti-influenza drugs around 2010. Peramivir (9) is a new neuraminidase inhibitor developed by BioCryst, with a cyclopentane core instead of the cyclohexene core of oseltamivir (70, Fig. 6A)114. The IC50 values of peramivir (9) in inhibiting influenza A-H1N1 virus, influenza A-H3N2 virus, and influenza B virus are 0.16, 0.13, and 0.99 nmol/L, respectively; and peramivir (9) also effectively prevents the release of influenza virus particles from infected cells115. Because the oral bioavailability of Peramivir (9) was low in a phase I clinical trial, further clinical trials used the intravenous administration. After more than ten years' complicated multicenter, open-label, uncontrolled clinical evaluations, and the 2009 H1N1 influenza pandemic emergency use authorization, peramivir (9) has proven to be effective in the treatment of human influenza A and B, including high-risk patients who have difficulties with oral drugs. It was firstly approved in Japan in 2010 for use in children's influenza treatment.
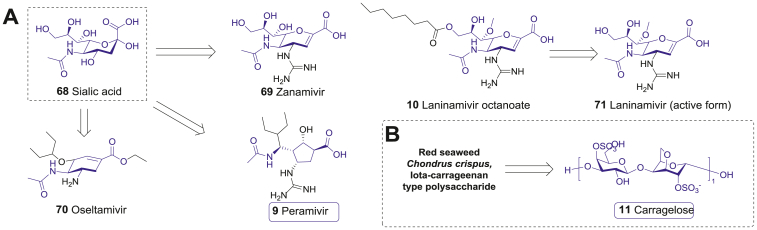
Figure 6.
The structure of other carbohydrate-based antiviral drugs. (A) From sialic acid (68) to the anti-influenza drugs launched before and after 2000–2021. (B) The red seaweed (Chondrus crispus) sourced carragelose (11).
Based on the success of zanamivir (69), a long-acting prodrug named laninamivir octanoate (10) was launched in Japan in 2010. Due to the long chain ester tail (Fig. 6A), the drug converts to its active form laninamivir (71) slowly in lungs, which allows a single inhaled administration to maintain an effective concentration for about 5 days116. Laninamivir octanoate (10) showed a significant clinical efficacy, which is comparable to oseltamivir (70) and zanamivir (69) for the treatment of the 2009 H1N1 pandemic influenza strain.
3.4. Antiviral polysaccharide
Carragelose (11), which developed by Marinomed, is a natural algae sourced linear Iota-carrageenan type polysaccharide drug from red seaweed (Chondrus crispus) (Fig. 6B). Generally, carrageenans are polygalactans (molecular weight higher than 100 kDa) with various sulfated galactose and 3,6-anhydrogalactose (3,6-AG) repeating units joined by alternative α-(1,3) and β-(1,4)-glycosidic linkages117. Among various sub-categories, iota-carrageenan demonstrates considerable and wide-spectrum antivirus activities. As demonstrated, the antivirus IC50 values of iota-carrageenan against influenza A H3N2 and H1N1 reach 0.04 and 0.20 μg/mL, respectively. Mechanism research reveals that Iota-carrageenan directly binds to virus and prevent its attachment to host cells, and thereby achieves effectiveness against several viruses117,118. Based on such bio-active characters, carragelose (11) was developed from iota-carrageenan polysaccharide.
Since carragelose (11) cannot penetrate nasal mucosa, it is safe in topically medical applications. A randomized, placebo-controlled, double-blind clinical trial enrolled 211 patients with common cold and showed that alleviation of symptoms was 2.1 days faster in the Iota-carrageenan nasal spray group than in placebo group (P = 0.037). Viral titers in nasal fluids also had a significantly decrease in iota-carrageenan group in the ITT population (P = 0.024) as well as in the per protocol population (P = 0.018)119. Consequently, carragelose (11) was approved as an over-the-counter (OTC) drug by EMA for cold treatment in 2013. It is worth noting that carragelose (11) inhibits COVID-19 virus with an IC50 of 2.6 μg/mL in vitro120. To date, several clinical studies (NCT04793984, NCT04681001 and NCT04590365) are on the way.
4. Carbohydrate-based antibacterial and antiparasitic drugs
Bacteria are closely associated with human health down the ages. There have been many bacteria incurred pandemics throughout history, including the bubonic plague caused by Yersinia pestis, tuberculosis caused by Tubercle bacilli, cholera caused by Vibrio cholera, and anthrax caused by Bacillus anthracis. Even today, bacterial infections remain severe threatens to human health and life121. Penicillin, discovered in 1928, became the first modern drug against bacteria, ushering in the “antibiotic era”. Thereafter, a large number of antibiotics, including many carbohydrate-conjugated compounds have been discovered for clinical use. Most of these antibiotics, produced by microbes over long periods of evolution, possess structures beyond chemists imagination.
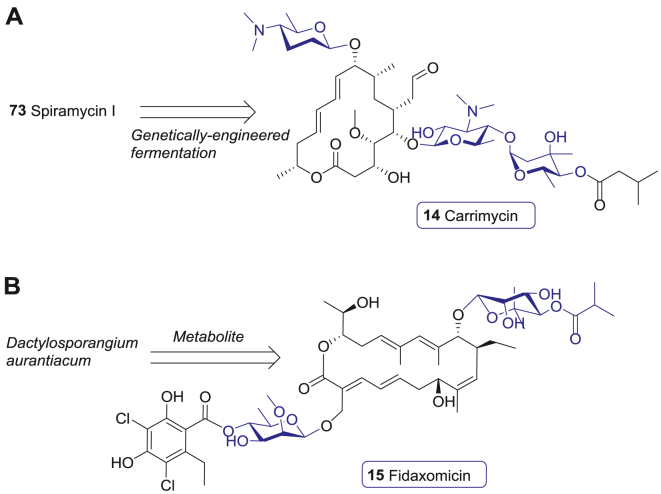
The protein synthesis machines are important targets of carbohydrate-based antibiotics. One important factor is that the 70S ribosome of bacteria is made up of a 30S small subunit and a 50S large subunit, which is significantly different from the 80S ribosome of eukaryotic cells122. Thus, carbohydrate-conjugated macrolide antibiotics and aminoglycoside antibiotics (AGs) can selectively disrupt the ribosomal functions required for the bacterial protein synthesis without affecting protein synthesis in eukaryotic cells123. Another unique structural feature of bacteria is their cell wall, which are mainly composed of peptidoglycans and glycolipids124. In order to produce these glycans, bacteria maintain sophisticated and distinctive biosynthesis systems, which are absent in eukaryotes125. This vital biosynthesis process of bacteria could be exploited by antibiotics to suppress bacterial infections125,126. However, with the widespread use of antibiotics, antimicrobial resistance (AMR) has become a global health threat121,127. Discovery of new antibiotic drugs is important to this global challenge127. During 2000 and 2021, nine new carbohydrate-based antibacterial drugs launched, including four glycomacrolide antibiotics telithromycin (12), cethromycin (13), carrimycin (14), and fidaxomicin (15), three glycopeptide antibiotics telavancin (16), oritavancin (17), and dalbavancin (18), and two aminoglycoside antibiotics plazomicin (19) and paromomycin (20).
4.1. Antibacterial drugs
Macrolide glycoside, consisting of macrocyclic lactones with one or more deoxysugar residues, are secondary metabolites of Streptomyces. They have broad spectrum antibacterial activities against aerobic Gram-positive and Gram-negative bacteria, some anaerobic bacteria, and atypical pathogens, and have been used to treat respiratory tract infections in patients allergic to penicillin124. All the macrolide antibiotics can interact with the nucleotides 2058–2062 in domain V of 23S rRNA, resulting in the premature release of peptidyl tRNA from the ribosomes, which inhibits protein synthesis and further kills bacteria128. Some glycomacrolide antibiotics can also block peptidyl-transferase activity and suppress bacterial ribosome assembly124,128.
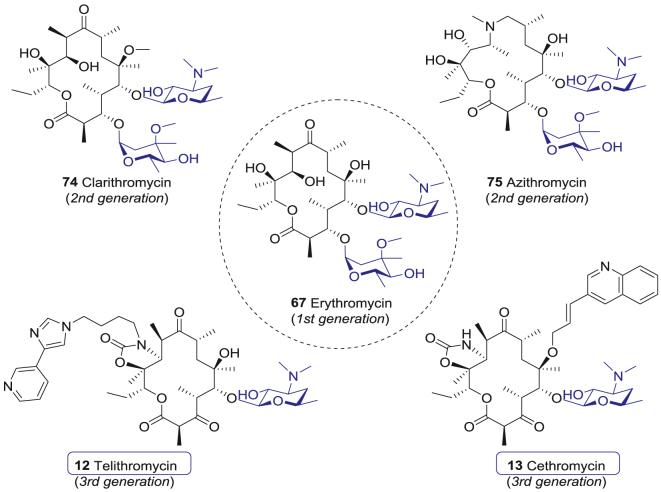
Hitherto, there have been three generations of macrolide antibiotics in clinical practices. The first-generation glycomacrolide antibiotics, including 14-membered-ring erythromycin (72) and 16-membered-ring spiramycin I (73, Fig. 7), are effective and well tolerated. However, their clinical efficacies are restricted by short half-life and poor oral bioavailability124. The second-generation macrolide antibiotics, such as clarithromycin (74) and azithromycin (75, Fig. 7), are modified at the 9-ketone, 6-hydroxy, or 12-hydroxy groups of the original macrocyclic lactones129,130. These modifications do not affect the antibacterial activity, but inhibit the isomerization of macrolides in acidic environments, thus improving their stability in gaster. Therefore, the second-generation macrolide antibiotics have more indications. For instance, clarithromycin (74) is also used to treat Helicobacter pylori infection and AIDS-related respiratory infections caused by Mycobacterium avium complex131,132.
Figure 7.
The representative first-, second- and third-generation macrolide glycoside antibiotics.
However, the application of the first- and second-generation macrolide antibiotics is gradually limited by antibiotic resistance, mainly mediated by erythromycin (72) resistance methylase (MLSB resistance phenotype, including constitutive and inducible MLSB resistance) and efflux of antibiotic from the bacteria133. Further optimization and structure–activity relationship (SAR) studies indicated that the 3-O-cladinose of erythromycin (72) was not an essential group, while the 5-O-desosamine dominated the antibacterial activity133. Thus, replacing the 3-O-cladinose with a ketone group improved the bacteriostatic sensitivity and resulted in the third-generation macrolide antibiotics, also known as ketolide antibiotics133. In 2001, FDA approved Aventis’ first third-generation ketolide antibiotic telithromycin (12), which was derived from erythromycin A. telithromycin (12) contains 3-ketone, 5-O-desosamine, 11,12-cyclocarbamate, and a butyl-imidazole-pyridine extension moiety attached to the lactone ring (Fig. 7)134. The alkylaryl extension enables the new drug to bind to a specific adenine (A752) in domain II of 23S subunit, which differs from the previous macrolide antibiotics and thus increases sensitivity against the erythromycin-resistant bacteria135. For erythromycin-sensitive Streptococcus pneumoniae, the 50% minimum inhibitory concentration (MIC50) and 90% minimum inhibitory concentration (MIC90) of telithromycin (12) are 0.016 and 0.03 μg/mL, respectively, which are about 10-fold lower than that of erythromycin (72)136. Besides, telithromycin (12) remains sensitive to these strains harboring inducible MLSB resistance137, 138, 139, as well as Gram-negative bacteria, and atypical bacteria. The MIC90 values of telithromycin (12) for Haemophilus influenza, Moraxella catarrhalis, Chlamydia pneumoniae, Mycoplasma pneumoniae and Legionella pneumophila are 4, 0.12, 0.125, 0.004, and 0.03 μg/mL, respectively140, 141, 142, 143.
Community-acquired pneumonia (CAP) is a common disease in outpatients. Annually, approximately 600,000 patients are hospitalized for CAP in the United States, resulting in $10.6 billion in health care expenditures144. The pooled analysis of several III/IV phase studies indicated that the clinical cure rate and bacteriologic eradication rate of oral telithromycin (12) treatment (800 mg/day) for 5 or 7–10 days reached 88.1% and 89.0%, respectively140,145. Thus, CAP became the first approved indication for telithromycin (12) in 2001146. After that, more clinical studies have proved that telithromycin (12) was also effective in treating tonsillopharyngitis, scrub typhus, and acute exacerbations of asthma147, 148, 149. However, it should be mentioned that side effects such as severe hepatotoxicity and visual impairment were reported and warned, limiting the use of terithromycin (12) for further indications146,150.
Abbott developed another third-generation ketolide antibiotic cethromycin (13), which also carries 3-ketone, 5-O-desosamine, and 11,12-cyclocarbamate moiety (Fig. 7). In cethromycin (13), the aryl-alkyl side chain is attached to the 6-hydroxyl group via an ether linkage. Cethromycin (13) binds to the domain II and V of 23S rRNA, sharing similar working mechanisms of telithromycin (12). The in vitro antibacterial activity against 1223 clinical isolated species showed that cethromycin (13) was effective in inhibiting S. pneumoniae and other Streptococci151. Cethromycin (13) inhibits macrolide-susceptible Streptococci and Staphylococci with the MIC90 ranging from 0.002 to 0.03 μg/mL, and inhibits macrolide-resistant S. pneumoniae and S. pyogenes with the MIC90 ranging from 0.015 to 0.12 μg/mL and from 0.12 to 0.5 μg/mL, respectively137. Cethromycin (13) is active for Gram-negative bacteria and atypical bacteria with the MIC90 values for H. influenzae, M. catarrhalis, C. pneumoniae, M. pneumoniae, and L. pneumophila being 4, 0.12, 0.016, 0.06, and ≤0.001 μg/mL, respectively151, 152, 153. It also inhibits methicillin-resistant Staphylococcus aureus (MRSA) with the MIC90 ≤ 0.002 μg/mL154. Further phase II/III studies for cethromycin (13) against CAP indicated that 10-day course treatment (150 mg/day) and 7-day course treatment (300 mg/day) achieved clinical cure rate of 83% and 84%, bacteriologic eradication rate of 83% and 85%, respectively155. Importantly, cethromycin (13) displayed outstanding antibacterial activity for B. anthracis, Y. pestis, and Francisella tularensis156, 157, 158. In 2009, FDA accelerated approval of cethromycin (13) as an orphan drug for prophylactic treatment of anthrax inhalation, tularemia, and plague.
Carrimycin (14) is a 16-membered macrolide glycoside antibiotic developed by Tonglian Pharmaceutical. It is produced from a genetically engineered bacteria strain of S. spiramyceticus (Fig. 8A). Compared with the 1st generation spiramycin I (73), carrimycin (14) contains an additional 4′-O-isovaleryl group at the terminal sugar residue, which makes it more lipophilic and more active. The in vitro activities of carrimycin (14) against Chlamydia trachomatis, C. pneumoniae, Ureaplasma urealyticum, and M. pneumoniae are similar to azithromycin (75) with MICs in the range of 0.03–0.5 μg/mL, while it is more potent than acetylspiramycin159. A Phase III clinical trial showed that the efficacy and safety of carrimycin (14) was superior to azithromycin (75)160. Thus, carrimycin (14) was approved by NMPA for pneumonia treatment in 2019. Of note, carrimycin (14) displays a broad-spectrum antiviral activity against human coronaviruses, including COVID-19, and is preferentially distributed in the lungs by oral administration161. As a result, several clinical trials are currently under way to investigate the efficacy of carrimycin (14) against COVID-19.
Figure 8.
Launched 16- and 18-membered macrolide glycoside antibiotic. (A) The macrolide glycoside antibiotic carrimycin (14) derived from spiramycin I (73). (B) Macrolide glycoside fidaxomicin (15).
Fidaxomicin (15), derived from the secondary metabolite of Dactylosporangium aurantiacum, is developed by Optimer as a novel member of macrolide glycoside antibiotics162. Structurally, fidaxomicin (15) is comprised of a 18-membered-ring with a 7-carbon sugar at 12-OH and a 4′-O-benzoyl-6′-deoxysugar at 21-OH (Fig. 8B). Unlike the macrolide antibiotic drugs mentioned above, fidaxomicin (15) shows a different mode of action by binding to the bacterial DNA template-RNA polymerase (RNAP) complex, which leads to the disruption of RNA transcription. However, the exact molecular mechanism of action still needs to be fully elucidated162,163.
Fidaxomicin (15) shows good inhibition activity against Clostridium difficile with the MIC90 values ranging from 0.008 to 0.25 μg/mL, but its activity against intestinal Gram-negative bacteria is relatively poor163,164. C. difficile infection (CDI) can cause severe infectious complications and death, especially in the elderly, and is the leading cause of healthcare-associated diarrhoea in the developed countries165. Previously, vancomycin (76) was the only agent approved by FDA for CDI treatment, while metronidazole was also used off-label as a treatment of mild-to-moderate CDI166. A multicenter, double-blind, randomized clinical trial, involving 629 patients with primary CDI or first recurrence, compared the safety and efficacy of fidaxomicin (15) and vancomycin (76). The results showed that the cure rate of fidaxomicin (15) was noninferior to that of vancomycin (76), and the recurrence rate was significantly lower than that of Vancomycin (76) with a similar adverse-event profile167. Thus, fidaxomicin (15) was endorsed by FDA for CDI treatment in 2011.
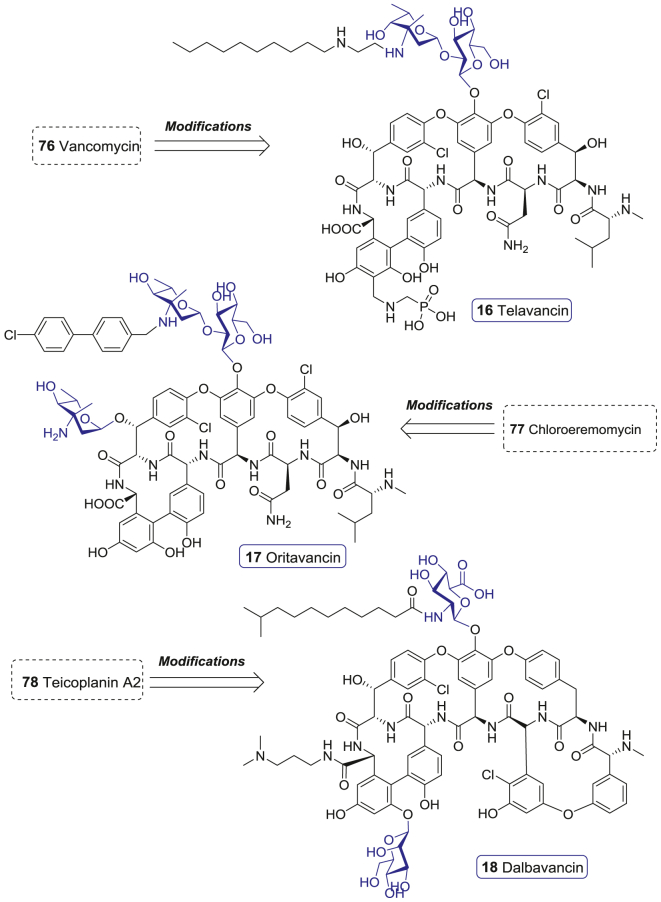
Glycopeptide antibiotcs (GPAs), including vancomycin (76), teicoplanins A2 (77), and chloroeremomycin (78), are secondary metabolites from Actinomycetes and Streptomyces. These molecules contain an intricate heptapeptide core modified by various glycosylation, acylation, chlorination, methylation, and/or sulfation modificaitons (Supporting Information Fig. S2)168. Sophisticated chemical structures endow GPAs with special antibiotic activity and mode of action. The heptapeptide core of GPAs binds to the C-terminal d-Ala-d-Ala of peptidoglycan precursors, which sequesters the substrate necessary for the enzyme-catalyzed bacterial cell wall cross-linking reaction and affects the trans-glycosylase catalyzed insertion of lipid intermediate II into the polysaccharide cell wall skeleton. Thus, GPAs hamper the bacterial cell wall construction to kill bacteria.
The emergence of drug-resistant Gram-positive bacteria, as represented by MRSA, motivated the development of GPAs. Among them, vancomycin (76) and teicoplanin A2 (77) are often described as the last defense, for their activity against a variety of Gram-positive bacteria. However, vancomycin-resistant bacteria has emerged, including vancomycin-resistant enterococcus (VRE) with the remodeling of d-Ala-d-Ala to d-Ala-d-Lac (this phenotype could be divided into VanA, VanB, VanC, VanD, VanE, and VanG types), vancomycin-intermediate S. aureus (VISA) induced by the thickening of cell wall, and vancomycin-resistant S. aureus (VRSA) resulted from an in vivo transfer of the vanA transposon from Enterococcus faecalis to MRSA168. Thus, there is an urgent need to develop new GPAs, and various chemical modifications on vancomycin (76) in the late 1990s paved ways169. Since 2000, three GPAs, also named lipoglycopeptides, for the common feature of the presence of lipid side chains, have been authorized by FDA.
Telavancin (16) is a semi-synthetic derivative of vancomycin (76) developed by Theravance. It has a lipophilic decylaminoethyl group on the vancosamine moiety and a hydrophilic aminomethyl group attached to the 4′-position of ring 7 (Fig. 9)170. Besides the shared action modes of GPAs, telavancin (16) also disrupts membrane barrier function via the interaction of the hydrophobic decylaminoethyl group with lipid II precursor171. As a result, though telavancin (16) has the same resistant mechanism of vancomycin (76), it demonstrates more potent activity against MRSA, methicillin-susceptible S. aureus (MSSA), methicillin-resistant Staphylococcus epidermidis (MRSE), methicillin-susceptible S. epidermidis (MSSE), VISA, and VanB-type VRE with the MIC90 values of 0.5, 0.5, 1.0, 1.0, 1.0, and 2.0 μg/mL172. A pooled analysis of two identically designed, randomized, double-blind, active control, phase III studies compared the efficacy of telavancin (16) and vancomycin (76) among 1867 patients with complicated skin and skin-structure infections (cSSSI) bred by suspected or confirmed Gram-positive bacteria. The clinical cure rates were 88.3% and 87.1% in telavancin (16) and vancomycin (76) treatment arms, respectively173. Among MRSA infected patients (n = 579), the achieved clinical cure rates were 88.3% and 87.1% in telavancin (16) and vancomycin (76) treatment arms, respectively173. Therefore, telavancin (16) was approved by FDA for the treatment of cSSSI in 2009.
Figure 9.
The lipopeptide glycoside antibiotics developed during 2000–2021.
Oritavancin (17) is a semisynthetic lipoglycopeptide drug developed by Eli Lilly from chloroeremomycin (78). It has excellent bactericidal activity against both glycopeptide-sensitive and glycopeptide-resistant Gram-positive bacteria174. The significant structural difference between oritavancin (17) and vancomycin (76) is that oritavancin (17) has an additional 4-epi-vancosamine in ring 6 and the replacement of vancosamine by 4-epi-vancosamine with a lipophilicity 4′-chlorobiphenylmethyl side chain (Fig. 9)168. Oritavancin (17) inhibits bacterial cell wall synthesis through impeding trans-glycosylation via binding to d-Ala-d-Ala/d-Ala-d-Lac and trans-peptidation via targeting a pentaglycine bridge. On the other hand, it also develops cell membrane anchoring and self-association into dimers, which results in perturbation of cell membrane integrity and ultrastructural changes in Gram-positive bacteria. Moreover, some researches indicated that oritavancin (17) might also inhibit the RNA synthesis of bacteria175. The antibacterial activity investigation showed that oritavancin (17) exhibited potent activity against MRSA, MSSA, MRSE, MSSE, VISA, VRSA, VanA-type, and VanB-type VRE with the MIC90 of 0.25, 0.12, 0.5, 0.5, 1.0, 0.5 (modal MIC), 0.25, and 0.03 μg/mL, respectively168. A randomized, double-blind clinical trial conducted for adults with acute bacterial skin and skin-structure infections (ABSSSI), revealed that the efficacy of oritavancin (17) was noninferior to vancomycin (76). The primary end point data of oritavancin (17) and vancomycin (76) were 82.3% and 78.9%, respectively176. These results impelled the approval of oritavancin (17) for ABSSSI by FDA in 2014.
Dalbavancin (18) is a semisynthetic lipoglycopeptide developed by Durata from the teicoplanin-like antibiotic A-40926, which was found from the actinomycete Nonomuria spp177. Dalbavancin (18) differes from teicoplanin A2 (77) in that it lacks the N-acetylglucosamine (GlcNAc) residue and a chlorine atom, but has an extra terminal methylamino group (Fig. 9). The lipophilic side chain of dalbavancin (18) enhances the binding affinity to the d-Ala-d-Ala site through dimer formation and membrane anchoring, leading to the destabilization of cell membranes168. Dalbavancin (18) exhibits potent antibacterial activity against MRSA, MSSA, MRSE, MSSE, VISA-type, and VanB-type VRE with the MIC90 of 0.06, 0.06, 0.06, 0.06, 2.0, and 0.03 μg/mL, but poor inhibition activity against VRSA and VanA VRE. According to three phase III studies carried out for comparing the efficacy of dalbavancin (18) to linezolid, cefazolin, and vancomycin (76) in cSSSI treatment, dalbavancin (18) displayed an activity non-inferior to the other three antibiotics168. Intention-to-treat (ITT) analysis revealed that dalbavancin (18) achieved a higher response rate than vancomycin (86% vs. 65.3%). A pooled analysis of DISCOVER 1 and DISCOVER 2 trials suggested that once-weekly intravenous dalbavancin (18) was not inferior to twice-daily intravenous vancomycin (76) followed by oral linezolid for ABSSSI treatment178. Accordingly, dalbavancin (18) was approved for treating ABSSSI by FDA in 2014.
4.2. Aminoglycoside antibiotics antiparasitic drugs
Aminoglycosides (AGs) are a class of broad-spectrum antibacterial antibiotics used mainly for the treatment of Gram-negative bacteria infections. AGs binds to the decoding A-site in helix 44 of 16S RNA, and converts bacterial ribosome 30S subunit into a special conformation that can bind to unpaired tRNA which resulting in protein mistranslation179. The clinical use of AGs has been limited by two considerations. In one aspect, the side effects of AGs, including neuromuscular block, ototoxicity, and nephrotoxicity are intolerable; in another aspect, the aminoglycoside-modifying enzymes (AMEs) induced resistance to extended-spectrum β-lactamase (ESBL)-producing enterobacteriaceae and carbapenem-resistant enterobacteriaceae (CRE). Thus, AGs are mainly used in treating severe Gram-negative bacteria infection during genetic disorders, Ménière's disease, and HIV treatments180, 181, 182.
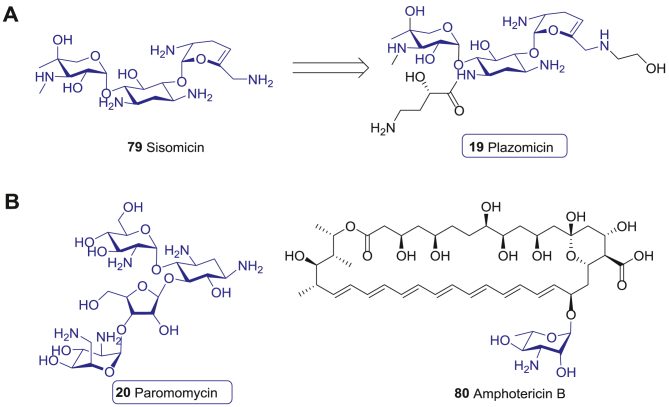
Plazomicin (19), a novel semisynthetic aminoglycoside derived from sisomicin (79), was approved recently (Fig. 10A)183. Compared with the traditional AGs, plazomicin (19) contains three key structural modifications, including 1-N amide substitution with 4-amino-2-hydroxybutanoic acid, dehydroxylation at the 3′- and 4′-positions, and 6′-N modificaiton of the hydroxyethyl group (Fig. 10A). These chemical modifications successfully provent the antibiotic from inactivation by such AMEs as O-nucleotidyltransferase ANT (reaction at 4′), O-phosphotransferase APH (reaction at 3′), and N-acetyltransferase AAC184. Therefore, plazomicin (19) possesses higher activity against CRE, ESBL-producing enterobacteriaceae, and AMEs mediated resistant bacteria compared to the traditional AGs184.
Figure 10.
Aminoglycoside antibiotics antiparasitic drugs. (A) Antibacterial aminoglycoside plazomicin (19) derived from sisomicin (79). (B) The antileishmanicidal drug paromomycin (20) and amphotericin B (80).
A multicenter, randomized, double-blind, phase II study in adults with complicated urinary tract infection (cUTI) indicated that in the groups receiving plazomicin (19) at 10 or 15 mg/kg, and levofloxacin at 750 mg, the microbiological eradication rates were 50.0%, 60.8%, and 58.6%, respectively, in modified ITT populations, and 85.7%, 88.6% and 81.0%, respectively, in the microbiologically evaluable population. In the modified ITT population, the clinically cured rates were 66.7%, 70.6%, and 65.5% in three groups, respectively185. Another phase III study in cUTI, which compared the efficacy and safety of plazomicin (19) to meropenem, suggested that plazomicin (19) was noninferior to meropenem with respect to the primary efficacy end points186. Given these advantages, FDA legalized the application of plazomicin (19) for the treatment of cUTI in 2018.
Paromomycin (20) is an old AG drug derived from the filtrates of Streptomyces krestomuceticus in the 1950s, and has a wide anti-bacterial spectra against most Gram-negative and many Gram-positive bacteria (Fig. 10B)187. Moreover, paromomycin (20) acts as an effective oral drug for treating the infections caused by intestinal protozoa, such as Entamoeba histolytica, Giardia lamblia, and Dientamoeba fragilis, leading to its FDA approval for amoebiasis treatment188. Paromomycin (20) is also found effective in the treatment of leishmaniasis, a fatal infectious disease that threatens 350 million people in 98 countries worldwide. Obligate intracellular protozoa of the genus Leishmania causes a range of diseases, broadly manifested as cutaneous (CL), mucosal (MCL), and visceral leishmaniasis (VL)189. The antileishmanicidal activity of paromomycin (20) may be through inhibition of parasite metabolism and mitochondrial respiration190. A phase III clinical trial conducted in India indicated that paromomycin (20) showed a reasonable safety profile and efficacy for Leishmania treatment, which was noninferior to amphotericin B (80, Fig. 10B). The final cure rates of paromomycin (20) and amphotericin B (80) were 94.6% and 98.8%, respectively191. Consequently, paromomycin (20) was legitimated in India in 2006.
5. Carbohydrate-based anticancer drugs
Cancer cells aberrantly express various glycans, which regulate different aspects of cancer progression, including proliferation, invasion, angiogenesis, and metastasis192, 193, 194, 195, 196, 197, 198. Based on the carbohydrates-related cancer hallmarks, various treatments for cancer have been developed over the past 20 years, including diagnosis, chemotherapy, radiotherapy, targeted therapy, and immunotherapy199−203.
Abnormally expressed glycans and glycoproteins are special markers of various cancers and provide valuable information for cancer diagnosis and prognosis195. Indeed, serum glycoproteins, including carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), CA19-9, and prostate-specific antigen (PSA), have been widely employed for early warning of colorectal, ovarian, pancreatic, and prostate cancers, respectively204, 205, 206. Cancer cells consume large amounts of glucose through aerobic glycolysis to support the biosynthetic requirements of uncontrolled proliferation, known as the Warburg effect198. Based on this phenomenon, positron emission tomography (PET) with in vivo 2-18F-2-deoxyglucose distribution monitor has become an important indicator of cancer diagnosis207.
Since 2000, there have eight carbohydrate-based anticancer drugs been approved for clinical cancer treatments, including five antineoplastic nucleosides or nucleotides azacitidine (21), decitabine (22), clofarabine (23), nelarabine (24), and forodesine (25), two carbohydrate conjugated chemotherapy drugs amrubicin (26) and midostaurin (27), and an immunomodulator drug mifamurtide (28).
5.1. Antineoplastic nucleosides and nucleotides
Although targeted therapy and immunotherapy have made breakthroughs, nucleosides and nucleotides mediated chemotherapy remains as the first-line therapy for various cancers199. Most antineoplastic nucleosides and nucleotides are pro-drugs that are transformed to active forms during metabolism199. The concentrative nucleoside transporters (CNT) and/or equilibrative nucleoside transporters (ENT) primarily mediate the diffusion of these pro-drugs into cells202,208. These pseudo nucleosides act as the substrates of DNA/RNA polymerases during DNA replication or RNA transcription202,208. These events result in stalled replication forks and chain termination, and trigger DNA damage response to arrest cell cycle progress and induce apoptosis199. Since DNA replication occurs more frequently in cancer cells than in normal cells, nucleoside and nucleotide therapies are selective for cancer cells202,208.
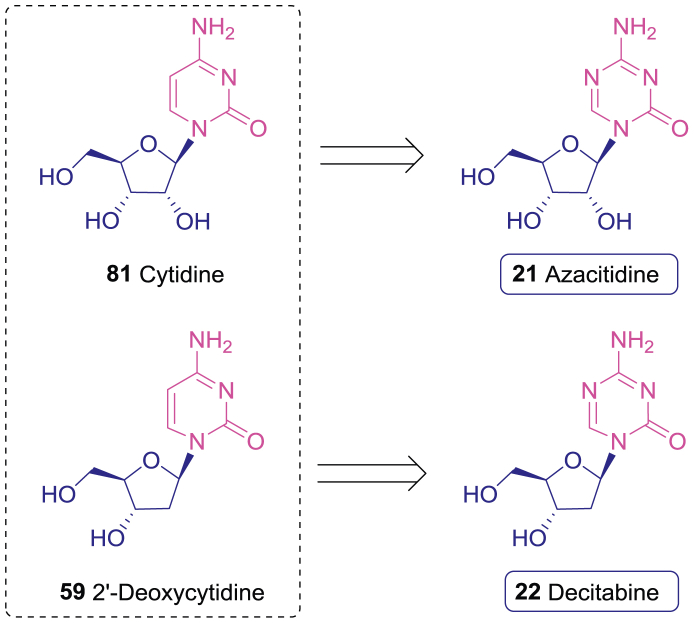
Azacitidine (21), developed by Pharmion, is a 5-N analogue of cytidine (81, Fig. 11), which can be converted to the 5′-O-triphosphate active form in cancer cells209. The antitumor activity of azacitidine (21) is mediated by multiple mechanisms, including inducing the cytotoxic effects by incorporating into DNA (10%–20%) and RNA (80%–90%), inhibiting proteins synthesis, and inducing apoptosis210. Decitabine (22), developed by MGI Pharma, is a 5-N analogue of 2′-deoxycytidine (59, Fig. 11). It can be converted to the 5′-O-triphosphate decitabine (82) active form and incorporated into DNA210.
Figure 11.
The anticancer nucleosides azacitidine (21) and decitabine (22) derived from cytidines (81) and 2′-deoxycytidine (59).
In addition to the cytotoxic activities, azocytidine (21) and decitabine (22) also affect epigenetic gene regulation in various cancer cells210. Specifically, the incorporation of these nuclesides into DNA leads to the inactivation of DNA methyl transferases (DNMTs) and subsequent hypomethylation of DNA, most likely restoring the expression of some tumor suppressor genes that are frequently silenced by aberrant DNA methylation in malignant tumors199. Active DNA replication and aberrant methylation could be frequently observed in myelodysplastic syndrome (MDS), a hematopoetic cell disease that could elicit cytopenias and acute myeloid leukemia (AML) progression211. Hence, azacitidine (21) and decitabine (22) are potential therapeutic agents for MDS. In a multicenter, randomized, open-label, phase III clinical trial, MDS patients (n = 191) were randomized to azacitidine (21) or supportive therapy group. The trial indicated that the response rates in azacitidine (21) and supportive therapy arms were 23% (7% complete response or CR, and 16% partial response or PR) and 0%, and the median time to leukemic transformation and death was 21 and 13 months, respectively211,212. Another phase III study carried out by International Working Group MDS criteria compared decitabine (22) group and supportive therapy group, showed that the response rate in supportive therapy group was significantly lower than that in the former group (0% vs. 17%), and the AML transfer rate in supportive therapy group was 1.68 folds higher than in the decitabine (22) group213, 214, 215. Accordingly, FDA approved azacitidine (21) and decitabine (22) for the treatment of MDS in 2004 and 2006, respectively. Recently, the clinical trials of azacitidine (21) and decitabine (22) for the treatment of more hematological malignancies as well as solid tumors, such as AML, lung cancer, colorectal cancer, and ovarian cancer, are under evaluations216, 217, 218, 219, 220, 221.
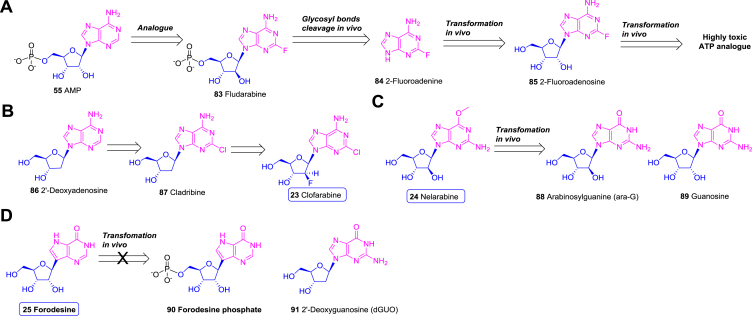
Fludarabine (83) and cladribine (87) are adenosine derived anticancer drugs developed in the 1980s; however, their clinical applications are hampered by in vivo cleavage of the glycosidic bonds222. Thus, 2-fluoroadenine (84) is produced by the cleavage of fludarabine (83). 2-Fluoroadenine (84) can be further transformed to 2-fluoroadenosine (85) triphosphate, which is highly toxic (Fig. 12A)222. Thus, structural modifications to enhance the stability of these nucleosides and decrease the toxicity are required.
Figure 12.
Carbohydrate-based antineoplastic nucleosides and nucleotides. (A) The metabolism and resultant toxicity of fludarabine (83). (B) The anticancer nucleoside clofarabine (23) derived from 2′-deoxyadenosine (86). (C) The in vivo metabolism of nelarabine (24). (D) The anticancer nucleoside forodesine (25) which increase the plasma 2′-deoxyguanosine (91).
Clofarabine (23), developed by Genzyme, is a 2′-deoxyadenosine (86) analogue with a C2′-fluorine substitution of cladribine (87), which significantly improves the stability of the glycosidic bond in acidic conditions (Fig. 12B)222,223. Clofarabine (23) is converted to the corresponding triphosphate active form, which was then incorporated into DNA by DNA polymerase224. The damaged DNA results in the release of cytochrome c from the mitochondria and induces cell apoptosis225. As expected, clofarabine (23) displays potent antitumor activity against various leukaemia and solid tumor cell lines with the IC50 values ranging from 28 to 290 nmol/L226.
Clofarabine (23) was further investigated in a series of clinical trials for hematological malignancies227, 228, 229. The clinical trial data from pediatric patients with acute lymphoblastic leukaemia (ALL) showed that 12% (6/49) patients achieved CR, 8% (4/49) achieved CR but without platelet recovery, and 10% (5/49) achieved PR. Combined with these data, clofarabine (23) was approved by FDA in 2004 for ALL treatment228,230.
Nelarabine (24), developed by GSK, is an purine arabinoside bearing a 2-amino-6-methoxy substitution in the adenine moiety (Fig. 12C)231. It acts as a prodrug, which can be demethoxylated to arabinosylguanine (ara-G, 88) in serum and cells231. Once in plasma, ara-G (88) acts as a guanine nucleoside (89) analogue and is phosphorylated by cellular kinases to form ara-G 5′-triphosphate199,232. The active ara-G 5′-triphosphate can be incorporated into DNA to result in cell death233,234. The cytotoxicity of nelarabine (24) towards human bone marrow progenitor cell lines is around micromolar concentrations in vitro235. Nelarabine (24) were evaluated for hematologic malignancy, especially T-cell relating diseases236, 237, 238. According to several phase II/III clinical trials on T-cell acute lymphoblastic leukemia (T-ALL)/lymphoblastic lymphoma (T-LBL), the CR rate and objective response rate (ORR) of nelarabine (24) were 26%–47% and 33%–60%, respectively239, 240, 241, 242. Base on these data, nelarabine (24) was approved by FDA in 2005 for the treatment of patients with recurrent or refractory T-cell lymphoblastic leukemia or lymphoma235.
Forodesine (25), developed by Mundi Pharma, is a C-glycoside analogue of purine nucleoside242,243. Being different from most antineoplastic nucleosides and nucleotides, forodesine (25) could not be phosphorylated as forodesine phosphate (90) and incorporated into DNA or RNA (Fig. 12D). Instead, forodesine (25) can increase plasma 2′-deoxyguanosine (dGuo, 91) via suppressing purine nucleoside phosphorylase (PNP) (Fig. 12D), whose deficiency facilitates a relatively selective depletion of T cells in humans242,243. The increased dGuo (91) is further converted to dGTP and leads to increased intercellular dGTP levels, resulting in the cell apoptosis243. In vitro assay indicated that under the treatment of forodesine (25), T-ALL cell lines were more sensitive to dGuo (91) than B-cell precursor-ALL (B-ALL) cell lines with the IC50 being 1.6 and 8.8 μmol/L, respectively244. A series of clinical studies were carried out to evaluate the efficacy of forodesine (25) in B-ALL and T-ALL, in which the CR rate was 16.7% (2/12) and 20.6% (7/34), respectively245,246. Afterwards, 37 patients with refractory cutaneous T-cell lymphoma (CTCL) were evaluated in a phase II study of oral forodesine (25), in which the ORR was 54% (7% CR, 46% PR)247. Therefore, forodesine (25) was approved for recurrent or refractory peripheral T-cell lymphoma treatment in 2017 in Japan.
5.2. Chemotherapy drugs
Anthracyclines, containing planar aromatic quinone rings decorated with a rare sugar moiety, constitute an important class of chemotherapy drugs248. Daunorubicin (92), epirubicin, and doxorubicin (93) are among the most prescribed drugs for the treatment of hematological malignancies and solid tumors (Supporting Information Fig. S3)248,249.
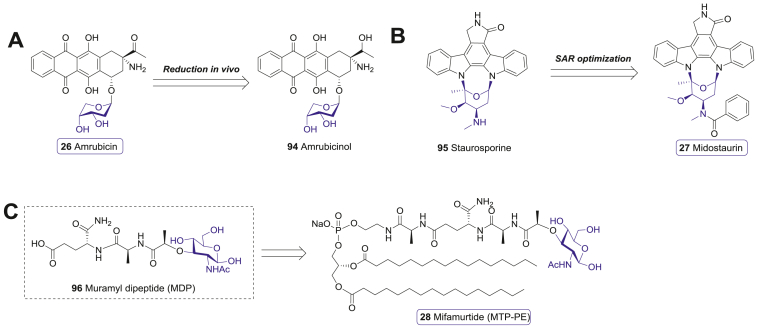
Amrubicin (26), developed by Sumitomo Pharma, is a third-generation synthetic anthracycline bearing 9-α-amino and 2-deoxypentose moieties (Fig. 13A)250. It can be converted to the more active metabolite amrubicinol (94), which inhibits the proliferation of various cancer cell lines, with IC50 values against lung cancer cells range from 0.16 to 0.64 μmol/L251, 252, 253. Both amrubicin (26) and amrubicinol (94) showed decreased DNA intercalation activity compared to the previous anthracyclines. Inhibition of topoisomerase II turns out to be their primary mechanism of action252. An important merit of amrubicin (26) is its low cardiotoxicity compared to doxorubicin (93)254. The safety and efficacy of amrubicin (26) as a chemotherapy agent have been studied extensively in clinical trials. A phase II study indicated that the response rates to amrubicin (26) in chemotherapy-naive patients with stage III or IV non-small cell lung cancer (NSCLC) and extensive-stage small cell lung cancer (SCLC) were 25% and 79%, respectively255,256. Accordingly, amrubicin (26) was approved in 2002 in Japan257.
Figure 13.
Other carbohydrate-based chemotherapy drugs. (A) The representative anthracycline anticancer drugs (92 and 93) and a active metabolit (94) of amrubicin (26). (B) The anticancer drug midostaurin (27) derived from staurosporine (95). (C) The immunomodulator anticancer drug mifamurtide (28) derived from MDP (96).
Staurosporine (95), a indolocarbazole glycoside isolated from Streptomyces staurosporus, is a pan-inhibitor of a series of serine/threonine protein kinases. However, the high toxicity of staurosporine (95) hinders its potential clinical application258. Thus, various modifications were explored to reduce the toxicity of staurosporine (95). Midostaurin (27), developed by Novartis, is a N-benzoate of staurosporine (95) (Fig. 13B)259, 260, 261, 262. It can inhibit a variety of kinases, including protein kinase C (PKC), protein kinase B (Akt), protein kinase A (PKA), and FMS-like tyrosine kinase 3 (FLT3) at nanomolar concentrations260. Midostaurin (27) selectively induced G1 arrest and apoptosis of AML cell lines with oncogenic FLT3 mutation (IC50 < 10 nmol/L) in vitro264. The multi-target ability of midostaurin (27) results in strong anti-proliferative activity against a variety of cancer cells258,260,263.
According to a phase II study, midostroin (27) achieved >50% (BR) reduction in peripheral blood or bone marrow blast-cells in more than half of the patients with mutated FLT3 AML and 42% of the patients with wild-type FLT3 AML, while no patients achieved CR265,266. Afterwards, a multi-institutional, multinational, randomized, double-blind, placebo-controlled phase III trial was carried out across 17 countries to evaluate the combinatory effects of midostaurin (27) with standard chemotherapy in AML patients with FLT3 mutant. In this trial, the addition of mitotolin (27) to standard chemotherapy in AML patients with FLT3-mutant showed significant clinical efficacy267. Thus, midostaurin (27) was approved by FDA for the treatment of FLT3-mutant AML in 2017. It is worth noting that midostaurin (27) is the first clinical agent approved for AML since 2000, as well as the first multi-kinase inhibitor for the FLT3-mutant subtype disease263. Another important progress for midostaurin (27) is its application for advanced systemic mastocytosis (SM). SM is a rare amyeloid neoplasm that results from the accumulation of abnormal mast cells in the bone marrow, liver, spleen, and skin268. 90% of the SM patients harbor a gain-of-function mutation (D816V) of KIT268. An open-label study of midostaurin (27) in 116 patients with SM demonstrated that the overall response rate of midostaurin (27) was 60%; the median overall survival (mOS) of midostaurin (27) reached 28.7 months, and the median progression-free survival (mPFS) was 14.1 months269. These data confirmed the efficacy of midostaurin (27) in SM treatment. Therefore, FDA also approved midostaurin (27) for treatment of SM in 2017.
5.3. Immunomodulator anticancer drug
Mifamurtide (MTP-PE, 28), developed by Ciba-Geigy AG, is a synthetic immunomodulator for cancer therapy270. It is derived from the covalent addition of alanine and dipalmitoyl phosphatidylethanolamine to muramyl dipeptide (MDP, 96), a common immune-stimulatory glycopeptide in the bacterial cell walls (Fig. 13C)270, 271, 272. These modifications make MTP-PE (28) possess the superior ability to activate human monocytes and macrophages, as well as a longer half-life in the plasma and lower toxicity273. Immunoassays indicated that MTP-PE (28) displayed enhanced stimulating activity for murine macrophages and human monocytes by 100-folds compared with MDP (96)274. Activation of these immune cells increased anti-tumor activities accordingly275. A phase III clinical trial in the patients with metastatic osteosarcoma showed that the addition of MTP-PE (28) to chemotherapy trended to improve 5-year event-free survival (EFS) and OS (42% vs. 26%, P = 0.23; 53% vs. 40%, P = 0.27)276. Therefore, MTP-PE (28) was approved for the treatment of non-metastatic osteosarcoma in European Union in 2009.
6. Carbohydrate-based antidiabetics
The incidence of diabetes mellitus (DM) is increasing rapidly. More than 90% of these cases are T2DM, and the remaining types include type 1 diabetes (T1DM) and hybrid forms of diabetes277,278. Effective control of blood glucose is the basis of treatment for all patients with diabetes. While T1DM patients need the lifelong insulin replacement therapy, for some T2DM patients, especially whose β-cells remain certain insulin secreting function, oral hypoglycemic agents (OADs) can be used. Since the blood glucose is a major driving factor of diabetes, glucose-based molecules have been extensively studied for the diabetes treatment. Two types of glucose-based OADs, namely the α-glucosidase inhibitors and SGLT2 inhibitors, have been on the market leading to significantly improved glycemic control in the majority of T2DM patients279, 280, 281, 282. The approved α-glucosidase inhibitors, including acarbose, voglibose, and miglitol, are a class of sugar mimics. They reversibly suppress the activity of α-glucosidase, block exogenous sugar uptakes from food digestion in small intestine, and are particularly suitable for the control of postprandial plasma glucose (PPG).
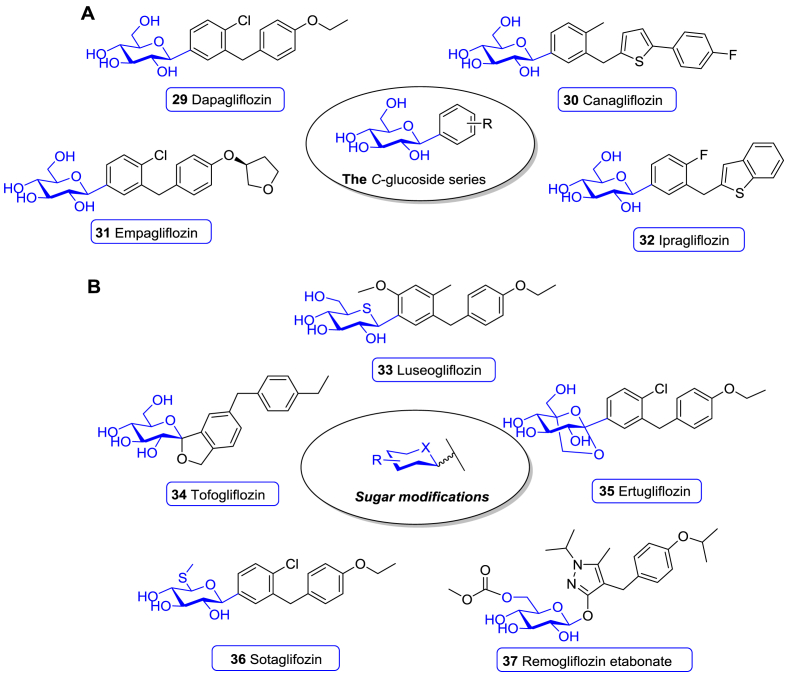
Based on the novel hypoglycemic concept, the SGLT2 inhibitors were discovered to have good hypoglycemic activity by enhancing urinary glucose excretion (UGE) and thereby decreasing the renal glucose reabsorption280. Since 2013, nine new SGLT1/2 inhibitors, including dapagliflozin (29), canagliflozin (30), empagliflozin (31), ipragliflozin (32), luseogliflozin (33), Tofogliflozin (34), ertugliflozin (35), sotaglifozin (36), and remogliflozin etabonate (37) have been approved worldwide.
6.1. α-Glucosidase and α-amylase inhibitors
Acarbose (97), a α-amylase inhibitor developed by Bayer in 1980s, is a pseudo-tetrasaccharide from the metabolites of Actinomycetes. Clinical data showed that acarbose (97) effectively reduced the fasting plasma glucose (FPG), PPG, postload insulin and glycosylated hemoglobin283, 284, 285. Voglibose (99), a potent α-glucosidase inhibitor developed by Taketa Pharma in 1990s, is a N-glycerol derivative of valiolamine (98, Supporting Information Fig. S4)286. Clinical trials indicated that voglibose (99) significantly reduced PPG, triglyceride, and increased the high-density lipoprotein cholesterol (HDL-C)286.
Nojirimycin (100) and 1-deoxynojirimycin (DNJ, 102), two natural iminosugars with the nitrogen atom substitution of the sugar ring oxygen, are inhibitors of α/β-glucosidase287. A series of N-substituted derivatives were developed as the second-generation α-glucosidase inhibitors288. Among them, miglitol (101) and emiglitate (103) were proved to be effective in controlling postsucrose glycaemia (Fig. S5); while miglitol (101) was approved in Germany for the treatment of T2DM in 1998289, 290, 291, 292, 293. Up to now, acarbose (97), voglibose (99), and miglitol (101) have been widely used in the treatment of T2DM. However, no new α-glucosidase inhibitors have been launched ever since.
6.2. SGLT1/2 inhibitors
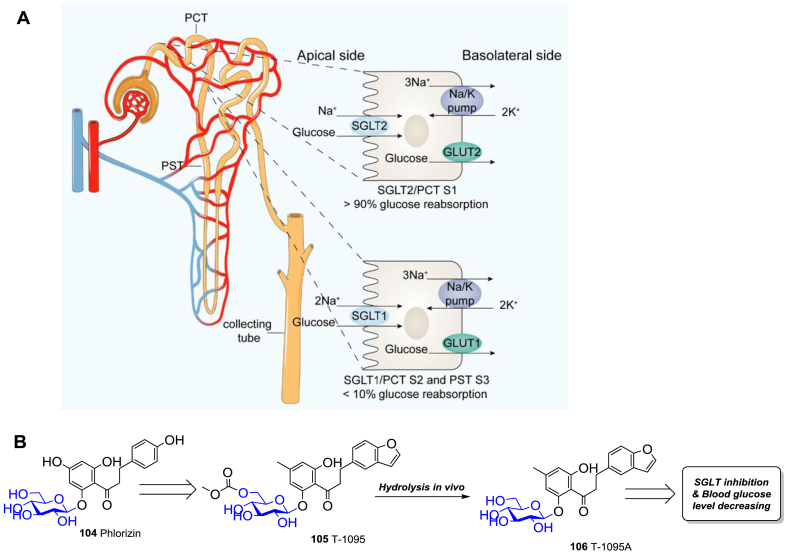
In the intestine and kidney, glucose is transported into the epithelial cells by sodium-glucose cotransporters (SGLTs) and glucose transporters (GLUTs)294. There are six members of the SGLT family (SGLT1−6), among which SGLT1 and SGLT2 have attracted the most attention295. SGLT1 is a low-capacity, high-affinity transporter, which primarily exists in the small intestine, responsible for the intestinal glucose and galactose absorption295. SGLT2 is a high-capacity, low-affinity transporter, which mainly expresses in the segment1 (S1) of the proximal convoluted tubule (PCT) in kidney, accounting for about 90% of the glucose reabsorption in kidney (Fig. 14A)296,297. The remaining reabsorption in kidney is via SGLT1 in the segment2 (S2) of PCT and segment3 (S3) of proximal straight tubule (PST)296,297. Significantly elevated SGLT2 and GLUT2 levels in PCT cells are found in T2DM patients, implying the increased capacity of renal glucose reuptake in T2DM patients298.
Figure 14.
The action mechanism of SGLT1/2 inhibitors. (A) The glucose reabsorption mechanism of SGLT1/2 in renal tubule. (B) The SGLT2 inhibitory natural glycoside phlorizin (104), an active derivative T-1095 (105), and its metabolite T-1095A (106).
Phlorizin (104, Fig. 14B), a naturally occurring glucoside of dihydrochalcone, was found to be able to increase urinary glucose of rats in the 1980s. Subsequent studies showed that the non-selective inhibition of SGLT1 and SGLT2 by phlorizin (104) was responsible for the glycosuria effect299, indicating a new approach to lower the glucose level. However, phlorizin (104) has a low bioavailability and is rapidly degraded by β-glucosidase in vivo. Modifications of phlorizin (104) are thus conducted to enhance the SGLT2 selectivity and to improve the stability and safety profile297. In order to improve the metabolic stability of the phenol O-glucoside, shielding of the 6-OH of the glucose moiety with an etabonic acid is a promising strategy. Thus T-1095 (105) turned out to be the first reported orally effective phlorizin analogue (Fig. 14B), whose active form T-1095A (106) can effectively reduce blood sugar and HbA1c, and improve hyperinsulinemia, hypertriglyceridemia, and microalbuminuria300. However, it demonstrated weak selectivity against SGLT1 and SGLT2, with the IC50 values being 0.20 and 0.05 μmol/L, respectively.300
Dapagliflozin (29), developed by AstraZeneca and BMS, is the first approved SGLT2 inhibitor301. SAR studies showed that the meta-substituted diarylmethanes had a stronger SGLT2 inhibitory activity than the other C-glucoside derivatives, leading to the final discovery of dapagliflozin (29, Fig. 15A)301. In vitro studies revealed that dapagliflozin (29) was highly selective for human SGLT2, with EC50 values for SGLT2 and SGLT1 being 1.12 and 1391 nmol/L, respectively (∼1200 fold)302. Meanwhile, dapagliflozin (29) displayed an extremely weak activity against GLUTs, showing 8%–9% inhibition in protein-free buffer at 20 μmol/L302. In a rat model, oral administration of dapagliflozin (29) effectively lowered FPG and improved the animals’ metabolic status302. A 24-week phase III trial with T2DM patients indicated that the mean HbA1c reduction in the placebo group and 10 mg dapagliflozin (29) group was 0.23% vs. 0.89% (P < 0.0001), mean FPG reduction was 4.1 mg/dL vs. 28.8 mg/dL (P < 0.0001), and mean body weight reduction was 2.2 kg vs. 3.2 kg, respectively303. The portion of patients who achieved glycemic control (HbA1c < 7%) at 24 weeks was 51% in the dapagliflozin (29) arm and 32% in the placebo arm303. Moreover, the addition of dapagliflozin (29) to nmol/letformin, pioglitazone, or insulin effectively improved disease control in patients with T2DM who had inadequate glycemic control with monotherapy304, 305, 306. Based on these data, dapagliflozin (29) was approved for T2DM therapy by EMA in 2012.
Figure 15.
Chemical structure of SGLT1/2 inhibitors. (A) C-Glucoside SGLT2 inhibitors (29–32) launched during 2000–2021. (B) SGLT1/2 inhibitors bearing modified glucose units (33–37) launched during 2000–2021.
Canagliflozin (30), developed by Mitsubishi Tanabe and marketed by Johnson & Johnson, is a C-glucoside derivative with a thiophene ring linked to a flourophenyl ring (Fig. 15A)307. The SAR studies revealed that the thiophene derivative markedly improved inhibitory potency against SGLT2307. Canagliflozin (30) showed good selectivity for SGLT2 and SGLT1 with IC50 of 2.2 and 910 nmol/L (410 times), respectively, and >10 μmol/L for GLUT1307,308. The phase I clinical trial indicated that this molecule effectively increased UEG and was well tolerated with no or rare hypoglycemia309. A phase III study showed that oral administration of 300 mg canagliflozin (30) strongly ameliorated the glycemic control. After 26 weeks of treatment, the least squares mean (LSM) changes of oral administration of 300 mg canagliflozin (30) and placebo in HbA1c were −1.03% vs. 0.14% (P < 0.001), in FPG were −34.2 vs. 9.0 mg/dL (P < 0.001), and in body weight were −3.4 vs. −0.5 kg (P < 0.001), respectively310. In addition, canagliflozin (30) significantly decreased the level of PPG, blood pressure, postprandial insulin, and increased HDL-C310,311. Canagliflozin (30), combined with other anti-diabetic drugs or add-on therapy, showed excellent therapeutic efficacy and enhanced glycemic control for patients who cannot achieve sufficient glycemic control with monotherapy311, 312, 313, 314. Consequently, canagliflozin (30) was approved for T2DM therapy by FDA in 2013314.
Empagliflozin (31), developed by Boehringer Ingelheim and Eli Lilly, is a analogue of dapagliflozin (Fig. 15A)315. The replacement of the ethoxy group in the distal phenyl unit in dapagliflozin (29) with 3-tetrahydrofuran greatly improves its selectivity against SGLT2. The IC50 values against SGLT2 and SGLT1/4/5/6 are 3.0 nmol/L, and 8.3, 11, 1.1 and 2.0 μmol/L, respectively315. A multi-center, randomized, placebo-controlled, phase III trial demonstrated that compared with placebo, oral administration of 25 mg empagliflozin (31) for 24 weeks caused a significant reduction of HbA1c (0.85%, P < 0.0001), FPG (36.2 mg/dL, P < 0.0001), and body weight (2.15 kg, P < 0.0001)316. Empagliflozin (31) elicited more portion of patients with HbA1c < 7.0% than that in the placebo arm at 24 weeks (43.6% vs. 12.0%, P < 0.0001)316. Empagliflozin (31) was also effective in reducing PPG, blood pressure, and postprandial insulin levels, and improved disease control in patients who cannot benefit from monotherapy317, 318, 319, 320, 321, 322. As a result, empagliflozin (31) was approved by the EMA and FDA in 2014 as the third SGLT2 inhibitor for clinical use.
Ipragliflozin (32), developed by Astellas, Kotobuki, and Merck Sharp & Dohme, is a p-fluorophenyl C-glucoside bearing a distal benzothiophene moiety (Fig. 15A)323. The in vitro assay indicated that ipragliflozin (32) demonstrated 254-fold selectivity for SGLT2 over SGLT1, with the IC50 values of 7.4 and 1876 nmol/L, respectively323,324. It showed no significant effects on human SGLT4 or SGLT5 isoforms (IC50 > 1.0 μmol/L) or GLUT at concentrations up to 3.0 μmol/L325. In a phase III study, patients in the ipragliflozin (32) arm had a reduction in mean HbA1c of 1.23% compared with placebo (P < 0.001), and in mean bodyweight of 1.47 kg326. 43.5% of the patients achieved HbA1c < 7.4% in the ipragliflozin (32) arm, while only 4.5% in the placebo arm326. More clinical trials in patients with T2DM, including monotherapy and combination therapy, showed that ipragliflozin (32) significantly reduced HbA1c, body weight, FPG, and blood pressure327. Based on these studies, ipragliflozin (32) received its first approval for the treatment of T2DM in 2014 by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan.
Luseogliflozin (33), developed by Novartis and Taisho, contains a similar phenyl aglycon as dapagliflozin (29) but a rare d-1-thioglucitol moiety in place of the glucose unit in dapagliflozin (Fig. 15B)328. It exhibited potent SGLT2 inhibition activity (IC50 = 2.26 nmol/L), with more than 1700-folds selectivity over SGLT1 (IC50 = 3.99 μmol/L) and 50,000-folds selectivity over GLUTs328, 329, 330. The randomized, double-blind, placebo-controlled, comparative phase III study with T2DM patients suggested that oral administration of 2.5 mg luseogliflozin (33) caused a significant decrease of HbA1c, FPG, and body weight compared to placebo, with the values of 0.75% (P < 0.001), 27.5 mg/dL (P < 0.001) and 1.77 kg (P < 0.001), respectively331. At the end of the trial, glycemic control with HbA1c < 7.0% was achieved by more patients in the luseogliflozin (33) arm than the placebo arm (24.1% vs. 3.8%)331. Further monotherapy or combination therapy trials further demonstrated that luseogliflozin (33) effectively ameliorated the glycemic control, including decreasing HbA1c, FPG, PPG, postprandial insulin and body weight330,331. Accordingly, luseogliflozin (33) was approved for the treatment of T2DM in 2014 by PMDA in Japan.
Tofogliflozin (34, Fig. 15B), developed by Chugai, Kowa, and Sanofi, is a O-spiroketal-C-aryl glucoside. This structure was identified through a structural database search according to the 3D pharmacophore model derived from reported inhibitors332. SAR studies showed that the para-substitution of small alkyl groups on the distal phenyl ring was conductive to increase both SGLT2 inhibitory potency and SGLT2 selectivity over SGLT1332. As observed, the para-ethyl group on the distal phenyl ring enhanced SGLT2 inhibition (IC50 = 2.9 nmol/L) with 2900 folds selectivity over SGLT1 (IC50 = 8.4 μmol/L)332. A multicenter, placebo-controlled, randomized, double-blind parallel-group, phase II/III study indicated that T2DM patients got significant benefits from tofogliflozin (34) arm (20 mg oral administration) compared to the placebo arm, with the mean reduction of HbA1c, FPG, 2-h PPG, and body weight being 0.99% (P < 0.001), 27.34 mg/dL (P < 0.001), 67.67 mg/dL (P < 0.001), and 2.50 kg (P < 0.001), respectively333. Tofogliflozin (34) also significantly improved blood pressure, HDL-C and triglyceride, and displayed well combination effects333,334. Accordingly, tofogliflozin (34) was authorized for T2DM treatment in 2014 by PMDA in Japan.
Ertugliflozin (35, Fig. 15B), developed by Merck Sharp & Dohme and Pfizer, is a C-aryl glucoside containing a dioxa-bicyclo[3.2.1]octane carbohydrate unit335. SAR studies revealed that the 4-Cl substitution on the central phenyl ring and the para-ethoxy group on the distal phenyl ring achieved a well-balanced profile for selective SGLT2 inhibition335. Ertugliflozin (35) showed an impressive SGLT2 inhibitory potency (IC50 = 0.92 nmol/L) and 2200-folds selectivity against SGLT1 (IC50 = 2.05 μmol/L)335. A set of phase III randomized double-blind trials indicated that oral administration of ertugliflozin (35) effectively improved glycaemic control and body weight336,337. At Week 26, 15 mg ertugliflozin (35) caused a reduction of LSM HbA1c (1.16%, P < 0.001), mean FPG (43.92 mg/dL, P < 0.001), mean 2-h PPG (67.32 mg/dL, P < 0.001), and mean body weight (2.16 kg, P < 0.001)336. The percentage of patients with HbA1c < 7.0% was 35.8% in the ertugliflozin (35) arm compared to 13.1% in the placebo group (P < 0.001)336. Moreover, ertugliflozin (35) improved blood pressure and displayed well combination effect with other anti-diabetic therapy, such as metformin338, 339, 340, 341, 342. These series of trials supported the FDA's approval of ertugliflozin (35) for the adult T2DM treatment in 2017343.
Sotaglifozin (36, Fig. 15B), which was developed by Lexicon Pharma and Sanofi, is a C-aryl glucoside with a special d-6-thioglucose moiety and the same aglycon with ertugliflozin (35). As reported, sotaglifozin (36) is the first dual inhibitor of SGLT1 and SGLT2 with the IC50 values of 36 and 1.8 nmol/L respectively, thus blunting and delaying absorption of glucose from the gastrointestinal tract and reabsorption of glucose by kidney344, 345. In the tandem clinical trial program, three double-blind placebo-controlled phase III clinical trials which enrolled more than 3000 T1DM patients showed that as an adjunct to optimized insulin therapy, sotaglifozin (36) demonstrated a significant reduction in HbA1c, FPG, weight as well as total daily insulin dose344,335. Compared with placebo group, more episodes of DKA and fewer episodes of severe hypoglycemia were observed in sotagliflozin group. Accordingly, sotaglifozin (36) achieved EMA's approval for treating T1DM patients with BMI ≥27 kg/m2 in 2019.
Remogliflozin etabonate (37, Fig. 15B), which was discovered by Kissei Pharmaceutical and marketed by Glenmark, is the only approved O-aryl glucoside SGLT2 inhibitor to date346. Following the stability improvent logic of T-1095 (105), an etabonic acid ester was introduced into the molecule. The etabonate quickly transformed into its active form remogliflozin in vivo346. Compared with T-1095 (105), remogliflozin demonstrated much potent and selective profile with the inhibition constant Ki values for SGLT2 and SGLT1 being 12.5 nmol/L and 4.52 μmol/L, respectively346. A double blind, double dummy phase III study conducted in India with T2DM patients indicated that 100 mg or 250 mg oral administration of remogliflozin etabonate (37) (twice daily) caused a significantly reduction of HbA1c by 0.72% and 0.77%, respectively, which was superior to dapagliflozin (29) (P < 0.001)347,348. Remogliflozin etabonate (37) also effectively decreased FPG and PPG, although noninferior to dapagliflozin (29)347,348. And no significant difference between these two drugs was observed when compared the percentage of patients who achieved glycemic control at 24 weeks347,348. Supported by these data, remogliflozin etabonate (37) was approved for T2DM therapy in 2019 in India348.
Insulin deficiency caused by pancreatic β-cell loss accounts for the major pathophysiological defect in T1DM. This determines the pivotal role of insulin for the treatment of T1DM349. As mentioned above, SGLT2 inhibitors can lower blood sugar level via an insulin-independent manner and improve β-cell function indirectly279,280,297,350,351. Hence, SGLT2 inhibitors were studied as potential adjunctive drugs for T1DM therapy. In a large long-term phase III study of dapagliflozin (29) for T1DM treatment, diabetic ketoacidosis (DKA) events occurred more frequently in the dapagliflozin (29) arms than that in the placebo arm, although the hypoglycemia events were comparable352,353. Similar results were observed from clinical trials for canagliflozin (30) and empagliflozin (31)354, 355, 356, 357. However, according to a related clinical trial for ipragliflozin (32) add-on therapy to insulin T1DM patients, at 24 weeks, oral administration of 50 mg ipragliflozin (32) acquired marked reductions in HbA1c (0.36%, P = 0.001), body weight (2.87 kg, P < 0.001), and insulin daily dose (7.35 IU, P < 0.001) compared to the placebo arm338. Of note, no DKA occurred in this study358. To date, only sotaglifozin has been recommended for T1DM, and the risk to benefit profile of other SGLT2 inhibitors deserves further studies359.
Besides the blood sugar control effects, SGLT2 inhibitors also have organ protective effects, especially the cardiorenal protective effects280,360, 361, 362, 363. Five large clinical trials have provided extensive clinical evidence of the cardiorenal benefits from SGLT2 inhibitors recently364, 365, 366, 367, 368, 369, 370, 371. Importantly, the cardiorenal benefits of SGLT2 inhibitors occurred rapidly after therapy initiation and maintained throughout the treatment, while the glycemic control took more time to show measurable effects360. Of note, cardiorenal benefits can also be achieved in patients without DM368. It was suggested that SGLT2 inhibitors induced aestivation-like hypometabolic patterns in kidney and heart, which economized their function and supported their longevity361. This explains, at least in part, the mechanisms behind the clinically observed cardiorenal benefits. Based on these results, SGLT2 inhibitors may find more applications in the future.
7. Carbohydrate-based cardiovascular drugs
Cardiovascular diseases (CVD) represent one of the most frequent causes of death globally, with cases of CVD nearly doubled from 271 million in 1990 to 523 million in 2019372. Thrombotic events are considered as the primary pathology underlying a board range of CVD. Physiologically, the process of thrombosis, also known as hemostasis or coagulation, plays an important role in maintaining the integrity of the circulatory system and normal cardiovascular functions373. Under pathologic conditions, thrombosis or thromboembolism blocks the natural blood flow, leading to the related organ dysfunction374, including ischemic stroke, peripheral artery disease, and ischemic heart disease (IHD) consisting of chronic coronary artery disease (CAD) and acute coronary syndrome (ACS)375, 376, 377, 378, 379, 380, 381. Therefore, antithrombotic therapy, including anticoagulant therapy, antiplatelet therapy, and thrombolytic therapy, is widely applied in these scenarios and regarded as the cornerstone of CVD therapy.
Carbohydrate-based drugs occupy an unshakable position in the field of antithrombotic therapy. The earliest natural anticoagulant heparin (107) was discovered from dog liver in 1916, and belongs to the glycosaminoglycan (GAG) polysaccharide family382. With a highly complex and heterogeneous polysaccharide structure, heparin (107) can bind a large variety of proteins and thus exhibit a wide range of biological functions. Importantly, a rare pentasaccharide unit of heparin binds strongly to antithrombin III to mediate the robust anticoagulant effects384. In addition, the purinergic receptor P2Y12, which is stimulated by ADP (108) but inhibited by ATP (109), plays an important role in platelet hemostasis387, 388, 389. Therefore, some adenine nucleotide analogs own antiplatelet effects389. From 2000 to 2021, there were two anticoagulation drugs tinzaparin sodium (38) and fondaparinux sodium (39), and two antiplatelet drugs, ticagrelor (40) and cangrelor (41), approved for CVD treatments.
7.1. Anticoagulation drugs
Heparin sulfate (107) is a negatively charged highly sulphated linear polysaccharide, consisting of 1,4-glycosidic bonds between d-glucosamine (GlcN) and d-glucuronic acid (GlcA) or l-iduronic acid (IdoA) units (Supporting Information Fig. S6)383, 384, 385, 386,390. In unfractionated heparin (UFH), a unique and uncommon pentasaccharide sequence, namely NS6SGlcNα→GlcAβ→NS3S6SGlcNα→2SIdoAα→NS6SGlcN, included in about one third of the chains, was proved to be the binding site for serine protease inhibitor AT-III391, 392, 393. This pentasaccharide motif can bind and change the conformation of AT-III, leading to inhibition of the serine proteases involved in the coagulation cascade385,393. In fact, UFH has been widely use as an anticoagulant drug in treating cardiopulmonary bypass, extracorporeal membrane oxygenation, hemodialysis, deep venous thrombosis (DVT), pulmonary embolism (PE), and other coagulation abnormalities384. However, the heparin materials are prepared from animal tissues, thus bringing a series of safety issues because of various contamination390.
The second generation heparin product is low-molecular-weight heparin (LMWH), being produced by chemical or enzymatic depolymerization of heparin384. Tinzaparin (Logiparin) sodium (38) developed by DuPont, is a representative LMWH drug with an average molecular weight of 5500–7500 Da. It is derived from the controlled enzymatic degradation of porcine heparin by the Flavobacterium heparinum heparinase (Fig. 16A)394,395. Tinzaparin binds to AT-III and induces its inhibitory function against multiple activated coagulation factors, particularly factor Xa, and the release of tissue factor pathway inhibitor (TFPI)394,395. Compared with heparin (107), Tinzaparin displayed a decreased binding affinity with platelets and plasma proteins396,397, but significantly prolonged the whole blood activated coagulation time (WBACT)398, 399, 400. In addition, the activated partial thromboplastin time (APTT) is only limitedly prolonged in the tinzaparinsodium (38) treatment, but is more pronounced than in enoxaparin sodium or bemiparin sodium treatments400, 401, 402, 403.
Figure 16.
Launched second and third generation heparin products. (A) The low-molecular-weight heparin tinzaparin sodium (38) derived from natural heparin. (B) The synthetic heparin pentasaccharide fondaparinux (39).
A series of clinical studies showed that tinzaparin sodium (38) was as effective and safe as enoxaparin sodium in the prophylaxis of DVT after total hip replacement (21.7% vs. 20.1%), and non-fatal PE were observed in both arms404. In patients after spinal cord injury, tinzaparin sodium (38) displayed a superior prophylaxis effect against thromboembolism to heparin (0% vs. 23.8%, P = 0.006)405. Further studies indicated that subcutaneous treatment of tinzaparin sodium (38) appeared to be as effective and safe as intravenous UFH in patients with acute PE and proximal DVT406,407. Therefore, tinzaparin sodium (38) was approved by FDA for the application in DVT in 2000.
The third generation heparin drug is the chemically synthesized ultra LMWH (ULMWH)384. Fondaparinux (39), developed by Sanofi and Organon, was the first launched ULMWH drug consisting of the mentioned active heparin pentasaccharide motif (Fig. 16B)408. Fondaparinux sodium (39) shows a high binding affinity to AT-III with the Kd value of 41–58 nmol/L409, 410, 411. This binding results in an irreversible conformational change of AT-III, triggering a robust inhibition of factor Xa and formation of thrombin and fibrin412. In vivo evaluation indicated that fondaparinux sodium (39) had no significant effect on APTT, PT, bleeding time, and plasma AT-III levels413,414. In addition, fondaparinux sodium (39) does not cause platelet aggregation and activation, but inhibits heparin-induced thrombocytopenia (HIT) antibody-induced platelet activation412.
One clinical study enrolled 1049 patients after elective major knee surgery showed that fondaparinux sodium achieved a superior prophylaxis effect against thrombotic events to enoxaparin sodium, including less venous thromboembolism (VTE) (12.5% vs. 27.8%, P < 0.001), DVT (12.5% vs. 27.1%, P < 0.001), and distal DVT (9.4% vs. 21.3%, P < 0.001)415. Another study involved 1711 patients after hip-fracture surgery revealed that fondaparinux sodium treatment caused a reduction of the incidence in VTE (8.3% vs. 19.1%, P < 0.001), DVT (7.9% vs. 18.8%, P < 0.001), proximal DVT (0.9% vs. 4.3%, P < 0.001), and distal DVT (6.7% vs. 15.0%, P < 0.001) compared with enoxaparin sodium416. Consequently, fondaparinux sodium (39) was approved by the FDA in 2001 for the prevention of DVT.
7.2. Antiplatelet drugs
Purinergic G-protein-coupled receptors (GPCR) P2Y1 and P2Y12 of platelet play fundamental roles in cardiovascular thrombosis387, 388, 389. When endothelium is injured, the activated platelets secrete prothrombotic signal molecule ADP (108), and initiate the platelet aggregation by activating P2Y1 receptor as well as the downstream processes, including granule release, platelet pro-inflammatory, and procoagulant activition387, 388, 389. Intriguingly, ATP (109) can act as an endogenous blocker against P2Y12 receptor389. Thus, it could be an effective way to mimic such process in blocking platelet function for IHD as well as stroke prevention389,417,418. Following this rationale, a series of reversible and competitive P2Y12 receptor antagonists were developed based on the scaffold of adenine nucleotide, among which ticagrelor (40) and cangrelor (41) have been approved (Fig. 17).
Figure 17.
The antiplatelet drugs ticagrelor (40) and cangrelor (41) derived from ADP (108) and ATP (109).
Ticagrelor (40), a pseudo adenine nucleotide developed by AstraZeneca, is a selective and reversible P2Y12 receptor antagonist419. Compared with AMP (55), the d-ribose was replaced with a cyclopentane moiety, and the 5′-O-phosphate was replaced by 2-hydroxyethoxy group, both of which improved the lability of the drug. The 5-propylthio side chain and the 7-phenylcyclopropylamino group on the purine base increased its binding affinity to P2Y12 receptor419. The in vitro assay indicated that the IC50 of ticagrelor (40) to inhibit platelets aggregate was 5 nmol/L420. It is worth noting that the metabolite AR-C124910XX in absence of the ethylene glycol moiety remained active421.
Clinical studies indicated that ticagrelor (40) had a more rapid onset action against platelet aggregation than clopidogrel422. Other two trials on patients with ACS revealed that ticagrelor (40) had a stronger and more stable antiplatelet effect compared with clopidogrel and prasugrel423,424. In addition to the antiplatelet effect, ticagrelor (40) also increased local endogenous adenosine by suppressing equilibrative nucleoside transporter-1 mediated adenosine uptake, leading to the sensation of dyspnea425,426. Data from the PLATO trial demonstrated that dyspnea occurred in the ticagrelor (40) group was frequently mild or moderate in intensity427. Taken these results together, ticagrelor (40) was approved for ACS treatment in Europe in 2010 and subsequently obtained FDA's approval in 2011.
Cangrelor (41), developed by Medicines Company, is an ATP analogue with reversible inhibition against P2Y12 receptor. Compared with ATP (109), the purine base was modified with 2-trifluoropropylthio and N-(2-(methylthio) ethyl) side chains, which enhanced its affinity with P2Y12 receptor (Fig. 17). Intriguingly, the β,γ-diphosphate of ATP (109) was reconnected by a dichloromethylene linkage, which increases cangrelor's resistance to ectonucleotidases mediated degradation428. The in vitro assays suggested the IC50 of cangrelor (41) against platelets aggregate was about 0.4 nmol/L428. Due to its rapid hydrolysis by nucleotide pyrophosphatases and metabolically unstable characters, cangrelor (41) was developed as an intravenously administered antiplatelet drug. Since its half-life was only 3–6 min, cangrelor (41) mediated a rapid offset of action389. These characteristics make it particularly useful when a rapid onset and offset antiplatelet effect is required.
A clinical trial was conducted in 11,145 patients receiving either urgent or elective treatments, and the primary efficacy endpoint was set as a composite of death, myocardial infarction (MI), ischemia-driven revascularization or stent thrombosis at 48 h after randomization429. These data showed that cangrelor (41) arm resulted in a significant reduction in the rate of primary efficacy endpoint compared with clopidogrel arm (4.7% vs. 5.9%, OR = 0.78, P = 0.005), with no significant increase in severe bleeding events (0.16% vs. 0.11%, OR = 1.50, P = 0.44)429. A frame-by-frame angiographic analysis revealed that cangrelor (41) caused a lower incidence of intraprocedural stent thrombosis (IPST) than clopidogrel (0.6% vs. 1.0%, OR = 0.65, P = 0.04)430. Accordingly, cangrelor (41) was approved by FDA in 2015 for reducing the risk of periprocedural MI, repeat coronary revascularization, and stent thrombosis in patients undergoing PCI431.
8. Carbohydrate-based nervous system drugs
In the past two decades, two carbohydrate-based drugs for the nervous system dieases have been approved, sodium oligomannate (42) for Alzheimer's disease (AD) and sugammadex (43) for reversing neuromuscular block (NMB).
8.1. Sodium oligomannate for Alzheimer's disease (AD)
AD, a severe neurodegenerative disease of the central nervous system, is a leading cause of dementia, affecting more than 40 million people around the world432,433. The neuropathology of AD is characterized by an extensive deposition of amyloid-β (Aβ) plaques in the neocortex and a hyper phosphorylated tau protein in neurofibrillary tangles located at limbic and cortical junction areas434. However, the pathogenesis of AD is complicated and has not been completely understood, which involves Aβ-tau synergy, oxidative stress, reactive glial, and microglial changes, while bacterial infections in brains as well as gut microbiota regulated central nervous system could also impact the course435,436. The present AD drugs could only relieve some clinical symptoms437,438. Despite enormous efforts have been made in the past two decades, including the exploration of various Aβ antibodies and tau centric therapy strategies, few success has been obtained in late-stage clinical trials439, 440, 441.
Sodium oligomannate (GV-971, 42), developed by Green Valley, is derived from marine brown algae β-d-(1,4)-polymannuranate (PM, 110) with 2–10 sugar units (Fig. 18A)436,443. Ethological experiments indicated that sodium oligomannate (42) had a significant ameliorative effect on the animal models of cognitive impairment436, with multiple targeting mechanisms. It was reported that sodium oligomannate (42) could penetrate the blood–brain barrier (BBB) in its original form via GLUT1. It can directly bind to Aβ, inhibit the formation of Aβ fibril, destabilize the existed fibrils into non-toxic monomers, and promote microglia-mediated Aβ phagocytosis436. Moreover, sodium oligomannate (42) has a specific impact on gut microbiota, suppressing accumulation of phenylalanine and isoleucine to reduce the Th1 cells’ infiltration to the brain. The cross talk with M1 microglia could be responsible for reducing AD-associated neuroinflammation and reversing the cognition impairment436.
Figure 18.
Carbohydrate-based nervous system drugs. (A) The AD drug sodium oligomannate (42) derived from brown algae β-d-(1,4)-polymannuranate (110). (B) Sugammadex (43) developed from cyclodextrin (111).
A recently reported 36-week randomized, double-blind, placebo-controlled phase III trial of mild-to-moderate AD participants (n = 818) showed remarkable drug-placebo differences at all measurement time points for the AD Assessment Scale-cognitive subscale 12-item (ADAScog12). The sodium oligomannate (42) oral administration group (900 mg/day) demonstrated noticeable efficacy in improving cognition, with sustained improvement in all observation periods of the trial (P < 0.0001), as well as great safety and tolerability444. Accordingly, sodium oligomannate (42) was approved by NMPA in 2019 for the treatment of mild to moderate AD443. Afterwards, an amplified phase III clinical trial named Green Memory was started in US for further evaluation of the safety, tolerability, and treatment efficacy of sodium oligomannate (42) in mild to moderate AD patients.
8.2. The selective reversal agent for nuromuscular block: Sugammadex
Neuromuscular blocking agents (NMBAs) are important drugs for general anesthesia. However, the residual neuromuscular block (NMB) effects after surgery are undesired and often cause side effects like hypoxia444. Acetylcholinesterase (AChE) inhibitors, for example neostigmine and pyridostigmine, are employed to reverse NMB through increasing the level of AChE445. However, increased AChE could stimulate nicotinic and muscarinic receptors, yielding a group of other side effects445.
Sugammadex (43), developed by Merck Sharp & Dohme, is a selective relaxant binding agent with novel and distinct mechanism for reversing NMB. It is a modified γ-cyclodextrin that consists of eight d-glucose units linked via β-1,4-glycosidic bonds (Fig. 18B)446. Due to the special cyclic structure and the hydroxyl group distribution, cyclodextrin (111) has a well-defined lipophilic cavity and a hydrophilic exterior447. Depending on the van der Waals and the hydrophobic interactions, cyclodextrin (111) could trap various drugs in the cavity and form a water-soluble guest-host complex446. Thus, it has been widely used as solubilizing agents in drug formulations447. It was found that the addition of negatively charged side chains at the 6-OH positions of the glucose residues in cyclodextrin could enhance its capture capacity towards the rigid rocuronium. Based on extensive SAR studies, sugammadex (43) was discovered as a unique drug for reversing NMB446.
The in vitro studies indicated that the EC50 of sugammadex's reversal activity against rocuronium mediated NMB was 1.2 ± 0.8 μmol/L446. Animal model studies showed that sugammadex (43) could effectively capture steroidal NMB agents in vivo, resulting in excretion of the rocuronium-sugammadex complex in urine445.
A number of clinical trials were conducted for sugammadex (43) to estimate its reversal effect of rocuronium or vecuronium induced NMB448. Its mediated recovery mean time from rocuronium induced NMB was shorter than that of neostigmine (1.5 vs. 18.6 min, P < 0.0001). Similar results were obtained in vecuronium induced NMB (2.7 vs. 17.9 min, P < 0.0001)449,450. A phase III trial enrolled surgical patients with American Society of Anesthesiologists (ASA) physical status I–IV suggested that sugammadex (43) treatment had a superior reversal effect against rocuronium induced NMB to neostigmine treatment, with the mean time to recovery to a train-of-four (TOF) ratio of 0.9 being 2.9 min versus 50.4 min (P < 0.0001)451. Consequently, sugammadex (43) was approved by EMA in 2008 as the first selective NMB reversal drug.
9. Other carbohydrate-based drugs
In addition to the above mentioned indications, carbohydrate-based drugs have been used in the treatment of dry eye disease, chronic idiopathic constipation (CIC), and liver injury. Besides, carbohydrate-based medicines have also found applications in orphan diseases, such as Gaucher's disease, hereditary lactobacteriuria (HOA), Fabry's disease, and acute hepatic porphyria (AHP), as well as diagnostic agents, including vasodilators and contrast agents. Besides, some other carbohydrate conjugate drugs, for example the carbohydrate-enhanced siRNA and carbohydrate-based vaccines are also discussed in this chapter. In 2000–2021, eleven carbohydrate-based molecules as well as glycoconjugate drugs are approved in the mentioned areas.
9.1. Drugs for other diseases and orphan diseases
Diquafosol tetrasodium (44), developed by Inspire, Allergan, and Santen, is a second-generation uridine nucleotide, containing a symmetrical structure of two UDP (112) units, namely Up4U, for the treatment of dry eye disease. This molecule belongs to the dinucleoside 5′,5′-polyphosphates (DNP) class, which is commonly present in tears, aqueous humour, and retina, and works for ocular functions (Fig. 19A)452,453. Diquafosol tetrasodium (44) is an agonist of P2Y2 purinergic receptor which is a G protein-coupled receptor, and can stimulate secretion of mucins from the conjunctiva into tears442, 443, 444, 445. SAR studies revealed that the length of the phosphate chains determined its selectivity against P2Y2, P2Y4, and P2Y6 receptors, in which four phosphates achieved favorable selectivity to P2Y2 (EC50 = 0.1 μmol/L)453, 454, 455. In animal experiments, diquafosol tetrasodium (44) significantly enhanced the corneal barrier function, including increase of tear fluid secretion and corneal epithelial resistance456, 457, 458.
Figure 19.
Carbohydrate-based drugs for other diseases. (A) Diquafosol tetrasodium (44) derived from UDP (112). (B) Lactitol (45) derived from lactose (113). (C) Magnesium isoglycyrrhizinate (46) prepared from licorice. (D) Miglustat (47) and migalastat (48) derived from 1-deoxynojirimycin (101). (E) Uridine triacetate (49) derived from uridine (114). (F) Regadenson (50) derived from adenosine (67).
A phase II trial showed that the diquafosol tetrasodium (44) treated arms (1% or 3% concentrations) resulted in a significant amelioration in fluorescein (FL) corneal staining scores compared with the placebo arm (1% concentration arm, P = 0.037; 3% concentration arm, P = 0.002), with a dose-dependent manner459,460. Rose bengal (RB) corneal and conjunctival staining scores revealed a significant improvement of diquafosol tetrasodium (44) compared with placebo (1% concentration arm, P = 0.007; 3% concentration arm, P = 0.004), and both diquafosol tetrasodium (44) groups achieved a favorable subjective dry eye symptom scores (P = 0.033)460. A phase III study indicated that no significant different in FL staining scores was observed between 3% diquafosol tetrasodium (44) and 0.1% sodium hyaluronate in the treatment of dry eye patients, whereas diquafosol tetrasodium (44) displayed a superior efficacy in improving RB staining scores461. According to these data, diquafosol tetrasodium (44) was approved for dry eye disease treatment in 2010 in Japan459,462.
Lactitol (45), the reduced form of disaccharide lactose (113, Fig. 19B), is a widely used sweetener in food industry. Since lactitol is hard to be absorbed in small intestine, oral intake of lactitol (45) displays almost no influence on blood sugar level and insulin excretion. In consequence, it is a suitable sweetener for diabetes mellitus patients463. Besides, as an osmotic laxative, lactitol (45) can enhance osmotic pressure in the intestinal lumen, increase the fecal volume and moisture content, and stimulate peristalsis464, 465, 466.
A large phase III study (NCT02819297) enrolled 1020 CIC patients, with the primary efficacy analysis being based on the first 12 weeks of the 6 months treatment period for 594 patients. As a result, lactitol (45) showed a greater response than the placebo group (25% vs. 13%, P < 0.05). Another phase III study (NCT02481947) provided a similar result, further confirming the efficacy and safety of lactitol (45). Therefore, lactitol (45) was approved by FDA for treating CIC in 2020467.
Magnesium isoglycyrrhizinate (MgIG, 46), developed by Chia Tai Tianqing Pharm for anti-inflammatory and hepatoprotective treatment, is a magnesium salt of 18α-glycyrrhizic acid (Fig. 19C)468,469. Glycyrrhizic acid, containing 18α and 18β glycyrrhizic acid stereoisomers, is a natural triterpene glycoside extracted from Licorice, which has been used to improve liver function in traditional Chinese medicine (TCM). Clinical evaluations demonstrated that the 18α stereoisomer possessed better liver protection efficacy and fewer side effects470, 471, 472, 473. Though the exact mechanism of action was not clear, MgIG (46) was approved for anti-inflammatory and hepatoprotective treatment by NMPA in 2005.
Orphan diseases, also known as rare diseases, are a group of disorders associated with the infrequent and unusual qualities, and 80 percent of the reported cases have a genetic basis474,475. Although hard to estimate, the burden of orphan diseases is tremendous, both in terms of loss of human life and economic burden474. Since 2000, an increasing number of orphan drugs have been approved, including three carbohydrate-based drugs, namely miglustat, uridine triacetate, and migalastat.
Miglustat (47, Fig. 19D), developed by Actelion, is a iminosugar for the treatment of Gaucher's disease (glycosphingolipid lysosomal storage disorder). Derived from the structure of deoxynojirimycin (102), SAR studies revealed that the presence of N-butyl residue led to the inhibition of glucosyltransferase inhibition476. Using ceramide as an acceptor, the Ki value of miglustat (47) against ceramide-specific glucosyltransferase, a pivotal enzyme for the glycosphingolipid biosynthesis, reached 7.4 μmol/L476. Data from in vitro Gaucher's disease model revealed that miglustat (47) effectively offset the cell storage of glucosylceramide (GlcCer)477. In a Sandhoff disease (another glycosphingolipid lysosomal storage disorder) mouse model, miglustat (47) caused a significant reduction of glycosphingolipids storage in brain and liver by 35%–86% (P < 0.05–0.001), delayed the onset time of symptoms (136 days vs. 40 days, P < 0.001), and prolonged life expectancy (170 days vs. 125 days, P < 0.001) compared with the untreated group478.
An open-labeled, phase II study that assigned 36 patients with type I Gaucher's disease indicated that in the patients clinically stable on enzyme replacement therapy, monotherapy with miglustat (47) could be an effective maintenance therapy and miglustat (47) plus imiglucerase could not achieve better benefits479. In addition, studies on patients with type III Gaucher's disease showed that miglustat (47) did not appear to achieve significant benefits on the neurological manifestations480. Though the incidence of diarrhea in patients who received miglustat (47) treatment was up to 80%, the severity of diarrhea was mild to moderate and often self-limiting481. For these reasons, miglustat (47) was approved as an orphan drug for treating type I Gaucher's disease by EMA and FDA in 2002 and 2003, respectively481.
Of note, several clinical trials also assessed the efficacy and safety of miglustat (47) for treating Niemann-Pick Disease Type C (NPC)482. A randomized controlled trial (RCT) about miglustat (47) in juveniles and adults with NPC indicated that miglustat (47) treatment for 12 months achieved an improved horizontal saccadic eye movement velocity (HSEM-α) compared with the standard care (excluding patients taking benzodiazepines, P = 0.028), as well several other clinically relevant symptoms, with a well safety profile483. A further study allowed these patients to continue treatment in a 12-month, non-controlled extension, and revealed that long-term miglustat (47) therapy could stabilize neurological symptoms caused by NPC, including its effect on HSEM-α, swallowing, ambulation, and cognition484. Accordingly, miglustat (47) was further authorized for NPC treatment by EMA in 2013482.
Migalastat (48), developed by Amicus Therapeutics, is a synthetic iminosugar for Fabry disease treatment (Fig. 19D)485. As 1-deoxygalactonojirimycin, migalastat (48) owns a galactose mimic structure and could selectively and reversibly bind to mutated α-galactosidase A, thereby to stabilize the enzyme in the endoplasmic reticulum and ensure its proper transportation to lysosome486. In the Fabry disease cell lines, migalastat (48) increased the level of 49 different missense mutant α-galactosidase A by 1.5–28 folds, with an EC50 ranging from 820 nmol/L to > 1 mmol/L486. Data from transgenic mice model suggested that oral administration of migalastat (48) resulted in a significant and dose-dependent increase of α-galactosidase A activity, which might account for globotriaosylceramid reduction in skin, heart, kidney, brain, and plasma487.
According to a phase III study, migalastat (48) caused a significant change in the mean globotriaosylceramid inclusions per kidney interstitial capillary (−0.25 vs. 0.07, P = 0.008), as well in the mean plasma globotriaosylsphingosine (−11.2 vs. 0.6, P = 0.003)488. In addition, for patients with suitable mutant α-galactosidase, migalastat (48) treatment was extended to 24 months and the data showed that migalastat (48) treatment caused a significant decrease of the left-ventricular-mass index from baseline488. Based on these results, migalastat (48) was approved for the treatment of Fabry disease by EMA and FDA in 2016 and 2018, respectively489.
Uridine triacetate (49), developed by Wellstar, is an acetylated prodrug of uridine for HOA treatment (Fig. 19E)490,491. It displayed 4- to 6-fold enhanced ability to enter the systemic circulation compared to uridine (114)491. As an orphan disease, a single arm phase III study suggested that all enrolled patients (n = 4) achieved stable predetermined principal hematologic parameters in 6 weeks treatment of Uridine Triacetate (NCT02110147). Moreover, uridine triacetate (49) could be well tolerated according to the trial data. Afterwards, uridine triacetate (49) was approved by FDA in 2015. Of note, it also obtained FDA's approval for another indication, the emergency treatment of adult and pediatric patients following a fluorouracil or capecitabine overdose, in the same year492.
9.2. Diagnostic agents
The coronary problem caused ischemic heart disease (IHD) is responsible for approximately 50% of CVD related deaths. Among clinical diagnosis of IHD, myocardial perfusion imaging (MPI) or stress MPI, is considered as a pivotal evaluation method372,493. Since the exercise stress MPI is only available for patients who could adequately perform exercise, the pharmacologic stress MPI serves more application. Adenosine (67), the nonselective inhibitor of A1, A2A, A2B, and A3 receptor, can vasodilate the coronary and peripheral arterial beds, enhance myocardial blood flow (MBF), and cause sympathoexcitation via A2A and A2B receptors activation, which results in pharmacologic stress MPI494. However, adenosine's activation activity against other receptors can yield undesirable side effects, and the short half-life necessitates a continuous intravenous infusion474. Hence, selective A2A receptor inhibitors are required.
Regadenoson (50) is a 2-[N-1-(4-N-methylcarboxamidopyrazolyl)] adenosine analogue developed by CV Therapeutics (Fig. 19F)495. The SAR studies revealed that the 4-substituted pyrazole derivative confered highly selective affinity with A2A receptor over A1, A2B, and A3 receptor495,496. The high A2A receptor binding affinity results in a long duration of action, while the low affinity leads to short actuation duration, and the latter is more suitable for stress MPI496. Therefore, the low-affinity agonist regadenoson (50) produces an equivalent response and becomes an ideal drug for pharmacologic stress MPI494,496.
A phase II study enrolled 36 patients suggested that regadenoson (50) stress MPI was well-tolerated and achieved 86% agreement with the results from adenosine stress MPI497. According to the results of phase III, the average agreement in adenosine-adenosine and adenosine-regadenoson were 0.62 ± 0.03 and 0.63 ± 0.02, respectively498. In addition, regadenoson (50) can be safely administered as a fixed unit bolus regardless of age, gender, or body mass index498. Afterwards, regadenoson (50) was approved as a pharmacological stress agent for myocardial perfusion imaging by FDA in 2008.
Lymph node metastasis is the hallmark of malignant tumors, which could be treated by lymph node dissection. However, how to trace the sentinel lymph node (SLN) in cancers, especially in melanoma and breast cancer, is considered as a fundamental issue for performing effective sentinel lymph node biopsy (SLNB) and lymph node dissection499, 500, 501. Technetium-99m (99mTc) is a radioisotope commonly used in nuclear medicine502. Based on its wide range of diagnostic uses, Navidea Biopharmaceuticals developed [99mTc]tilmanocept (Lymphoseek, 51; Supporting Information Fig. S7A), a CD206 mannose receptor targeted radio agent, for SLN detection496. [99mTc]tilmanocept (35.8 kDa) is a synthetic nanomolecule composed of a dextran backbone (9.5 kDa) attaching with multiple units, including diethylene triamine penta-acetic acid (DTPA) and mannose moieties. The DTPA group serves as the binding site for labeling the macromolecule with 99mTc503,504, and the mannose moieties in the macromolecule are responsible for binding to CD206, the mannose receptor with high level in tumor-associated macrophages (TAMs), conferring its tracing ability against SLN505. Animal model studies suggested that 99mTc-labeled-tilmanocept possessed several advantages of an ideal SLN imaging, which include the rapid clearance from the injection site, the rapid accumulation with prolonged retention in the SLN, as well a low uptake in distal lymph nodes503,506,507.
Clinical trials set the proportion of nodes detected by vital blue dye (VBD) and [99mTc]tilmanocept (51) as the primary endpoint, and assessed the lymphatic mapping performance of [99mTc]tilmanocept (51)508,509. Two phase III trials conducted in breast cancer showed that 207 of 209 lymph nodes detected by VBD were also detected by [99mTc]tilmanocept (51) with a concordance rate of 99.04% (P < 0.0001), and of 33 pathology-positive lymph nodes, [99mTc]tilmanocept (51) detected more than VBD did (93.9% vs. 75.8%, P < 0.05)508. In addition, 99mTc-labeled-tilmanocept detected at least 1 SLN in more patients than VBD (146 vs. 131, P < 0.0001)508. Another combined analysis of phase III trials in melanoma showed that [99mTc]tilmanocept (51) detected 232 of 235 lymph nodes detected by VBD (concordance rate = 99.04%, P < 0.001), and identified more pathology-positive lymph nodes than VBD (100% vs. 80%, P = 0.004) as well more patients with at least 1 SLN (150 vs. 138, P = 0.002)509. Moreover, no serious adverse events were observed in these trials508,509. Afterwards, [99mTc]tilmanocept (51) was approved as the first lymphatic mapping agent for patients with breast cancer or melanoma by FDA in 2013.
9.3. Carbohydrate-enhanced siRNA drug
Small interfering RNA (siRNA) is a general genetic therapy method for treating various diseases. However, the durability and therapeutic effect of siRNA were highly limited for its in vivo instable characters. To solve such problems, chemical modified nucleotides, enhanced stabilization chemistry (ESC) and delivery of molecularly targeted therapy technologies were developed for siRNA drug R & D. Among which, the chemical modified nucleotides efficiently increase the durability of the RNA strands, and the tri-GalNAc-conjugate technology enables subcutaneous dosing with increased potency, and therapeutic index, and demonstrates great potential for liver targeted siRNA drugs510.
Givosiran (52), an aminolevulinate synthase 1 (ALAS1)-directed small interfering RNA (siRNA) drug developed by Alnylam Pharma, is selected as an example for demonstrating the fast developed siRNA drug field recently. For structural detail, givosiran (52) consists of a 21-base sense strand and a 23-base antisense strand, which is modified with 16 nucleotides containing a 2′-F or 2′-OMe substituted nucleotides (Fig. S7B). Besides, six of its backbone linkages distributed at the ends of the strands are covalently conjugated to the tri-GalNAc moiety, which allows for high-uptake into the liver, the major site of ALAS1 synthesis, through subcutaneous administration511. Afterwards, the siRNA conjugate is efficiently and specifically delivered to hepatocytes511. In hepatocytes, this siRNA inhibits both the translation and expression of the ALAS1 protein, thus decreases systemic levels of neurotoxic precursors δ-aminolevulinic acid (ALA) and porphobilinogen (PBG), the main factors for causing acute porphyria attacks in AHP. A double-blind, placebo-controlled, phase Ⅲ clinical trial enrolled 94 AHP patients and found that the mean annualized attack rate was significantly decreased in Givosiran group than in placebo group (3.2 vs. 12.5, P < 0.01)512. Givosiran (52) resulted lower levels of urinary ALA and PBG, fewer days of hemin use, as well as better daily scores for pain than placebo. Hence, Givosiran received its first approval from FDA in 2019 for the treatment of adults with AHP.
9.4. Carbohydrate-based vaccines
Vaccination is a highly effective and cost-efficient interventions for infectious disease and even cancer preventions513. Since it was well recognized that some specific glycan units (e.g., capsular polysaccharide) are essential components for growth and virulence of pathogenic microorganism, carbohydrate-based vaccines have turn to be important vaccine categories that protected humans against diseases associated with severe bacterial pathogens, such as Haemophilus influenzae, S. pneumoniae, and Neisseria meningitides since the 1970s513.
In recent years, the developed conjugate vaccines that constructed by carrier proteins and antigen polysaccharides via covalent linkages induce long-lasting and stable immune responses for immune barrier establishment514. During 2000 and 2021, two categories of carbohydrate-based vaccines launched for preventing S. pneumoniae infection and typhoid515.
S. pneumoniae is an important pathogen that cause several diseases ranging from sinusitis and otitis media to life-threatening diseases such as pneumonia, bacteremia and meningitis516. In order to control pneumococcal disease, several pneumococcal conjugated vaccines (PCVs, 53) have been approved for S. pneumoniae infection prevention since 2000. PCV7 (including serotypes 1, 4, 6B, 9V, 14, 18C, 19F, and 23F) developed by Wyeth Pharma was introduced into children's immunization in USA in 2000. Afterwards, PCV13 (PCV7 plus serotypes 1, 3, 5, 6A, 7F, and 19A) developed by Pfizer was approved by FDA in 2010, and WHO comprehensively assessed the situation of serotype replacement since PCV7 introduction and decided to use PCV13 instead of PCV7 in the same year. Recently, PCV15 (PCV13 plus serotypes 22F and 33F) developed by Merck and PCV20 (PCV13 plus 8, 10A, 11A, 12F, 15B, 22F, and 33F) developed by Pfizer were approved by FDA in 2021. The promotion and application of these vaccines remarkably declined the disease burden of S. pneumoniae (Supporting Information Fig. S8)516.
Typhoid fever is an acute systemic infectious disease responsible for an estimated 12–20 million cases and over 150,000 casualties annually517. A typhoid Vi polysaccharide vaccine (Typhim Vi, 54) developed by Sanofi is composed of purified Vi polysaccharide from S. enterica subsp. enterica Typhi (S. Typhi; Fig. S8)7. A single vaccination dose of Typhim Vi (54) is sufficient to induce a 3-year cumulative protection from typhoid infection in 55% of the vaccinated population518. Accordingly, Typhim Vi (54) was approved by FDA in 2014 for preventing typhoid fever.
In addition to the carbohydrate enhanced pathogen-targeting vaccines, the tumor-associated carbohydrate antigens (TACAs), including glycoproteins (e.g., mucin O-antigens) and glycolipid antigens (e.g., gangliosides, globosides and Lewis determinants), based cancer vaccines have made great progress in the past decades for cancer prophylaxis, diagnosis and therapy514. Though there is still no TACA based cancer vaccine launched for some efficacy and safety problems, it has turn to be one of the most attractive fields for future carbohydrate drugs514.
10. Conclusions
Small molecular carbohydrates and carbohydrate-conjugated macromolecules are fundamental materials of life. Some of them are involved in energy metabolism; some take part in various signal transductions; and some others are weapons against viruses, bacteria, or parasites. Following the rationale of “learn from nature”, the carbohydrate-based drugs were developed with a variety of biological activities in the field of disease treatment and diagnosis. Since 2000, drug discovery and development processes are accelerating, along with the great progresses of basic chemistry and biology researches. The development of carbohydrate-based drugs has also made great achievements in the past 20 years, with 54 carbohydrate-based chemical entities approved worldwide for antiviral, antibacterial, antiparasitic, anticancer, antidiabetic, cardiovascular, neurological, and orphan diseases, siRNA, vaccines, as well as for diagnostic purposes. These drugs have brought enormous benefits to the world.
In many ways, there is huge scope for future sugar-based drugs. For example, the fourth generation heparin products, namely chemoenzymatically and bioengineered heparins have been proposed384. However, due to the huge difficulty in synthesis and purifications of the complex oligosaccharide, these works are still in progress. Besides, sugars play an important role in immunity, and most of the key molecules involved in innate and adaptive immune responses are glycoproteins519. The aberrant glycosylation or glyco-codes allows tumour to evade the perception of immune system and can also induce immunosuppressive signals200. The TACAs, including mucin-related TACAs, blood group Lewis-related TACAs, Globo class, and gangliosides, are uniquely or excessively expressed on the surface of tumor cells. They could be personalized cancer targets involved in tumor metastasis, development, and prognosis520. TACAs-based vaccines, including conjugated vaccines, nonconjugated vaccines based on self-assembly strategy, and vaccines based on modifications of TACAs, are expected to benefit in tumor treatments or recurrence prevention through immunity stimulation and activation520. Taken together, there is great potential for developing carbohydrate-based cancer diagnostics, vaccines, and treatments197,201, 202, 203. Continuous learning from nature, not only in the traditional glycochemistry and glycobiology areas, but also in broad glycosciences521,522, including glycomics, glycoproteomics, and glycogenomics, will inspire the creation of more innovative carbohydrate-based drugs, and bring more benefits to human health.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (82173662, 22031011, and 21621002), Natural Science Foundation of Shanghai (20ZR1410400, China), the Shanghai Municipal Science and Technology Major Project, the State Key Basic Research Program of the China (2018YFC0310905), the Extraordinary 2025 Elite Project of Fudan University, the Open Funding of Key Laboratory of Diagnosis and Treatment of Severe Hepato-pancreatic Diseases of Zhejiang Province (2018E10008, China), the Open Funding of State Key Laboratory of Bio-organic and Natural Products Chemistry.
Author contributions
Xin Cao and Biao Yu conceived the concept of the review. Xin Cao, Xiaojing Du, Heng Jiao, Quanlin An and Ruoxue Chen performed the literature review, organized and prepared the manuscript. Xin Cao, Biao Yu, Xiaojing Du, Pengfei Fang and Jing Wang revised the manuscript. All authors approved the submission of this manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.05.020.
Contributor Information
Xin Cao, Email: caox@fudan.edu.cn.
Biao Yu, Email: byu@sioc.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Finkelstein J. Glycochemistry & glycobiology. Nature. 2007;446:999. [Google Scholar]
- 2.Prestegard J.H., Liu J., Widmalm G. In: Essentials of glycobiology. 3rd ed. Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., et al., editors. Cold Spring Harbor Laboratory Press; New York: 2017. Oligosaccharides and polysaccharides. [Google Scholar]
- 3.Schjoldager K.T., Narimatsu Y., Joshi H.J., Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol. 2020;21:729–749. doi: 10.1038/s41580-020-00294-x. [DOI] [PubMed] [Google Scholar]
- 4.Kolb H.C., Ernst B. Recent progress in the glycodrug area. Pure Appl Chem. 1997;69:1879–1884. [Google Scholar]
- 5.Persidis A. The carbohydrate-based drug industry. Nat Biotechnol. 1997;15:479–480. doi: 10.1038/nbt0597-479. [DOI] [PubMed] [Google Scholar]
- 6.Simanek E.E., McGarvey G.J., Jablonowski J.A., Wong C.H. Selectin−carbohydrate interactions: from natural ligands to designed mimics. Chem Rev. 1998;98:833–862. doi: 10.1021/cr940226i. [DOI] [PubMed] [Google Scholar]
- 7.McAuliffe J.C., Hindsgaul O. Carbohydrate drugs–an ongoing challenge. Chem Ind. 1997;5:170–174. [Google Scholar]
- 8.Hui S., Ghergurovich J.M., Morscher R.J., Jang C., Teng X., Lu W., et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith D.F., Cummings R.D., Song X. History and future of shotgun glycomics. Biochem Soc Trans. 2019;47:1–11. doi: 10.1042/BST20170487. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M., Kizuka Y., Ohtsubo K., Gu J., Taniguchi N. Disease-associated glycans on cell surface proteins. Mol Aspect Med. 2016;51:56–70. doi: 10.1016/j.mam.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Nagae M., Yamaguchi Y. Sugar recognition and protein–protein interaction of mammalian lectins conferring diverse functions. Curr Opin Struct Biol. 2015;34:108–115. doi: 10.1016/j.sbi.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 12.del Carmen Fernández-Alonso M., Díaz D., Berbis M.Á., Marcelo F., Cañada J., Jiménez-Barbero J. Protein–carbohydrate interactions studied by NMR: from molecular recognition to drug design. Curr Protein Pept Sci. 2012;13:816–830. doi: 10.2174/138920312804871175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X.M., Liu H.W. Formation of unusual sugars: mechanistic studies and biosynthetic applications. Annu Rev Biochem. 2002;71:701–754. doi: 10.1146/annurev.biochem.71.110601.135339. [DOI] [PubMed] [Google Scholar]
- 14.Hobson C., Chan A.N., Wright G.D. The antibiotic resistome: a guide for the discovery of natural products as antimicrobial agents. Chem Rev. 2021;121:3464–3494. doi: 10.1021/acs.chemrev.0c01214. [DOI] [PubMed] [Google Scholar]
- 15.Stallforth P., Lepenies B., Adibekian A., Seeberger P.H. 2009 Claude S. Hudson award in carbohydrate chemistry. Carbohydrates: a frontier in medicinal chemistry. J Med Chem. 2009;52:5561–5577. doi: 10.1021/jm900819p. [DOI] [PubMed] [Google Scholar]
- 16.Crich D. En route to the transformation of glycoscience: a chemist's perspective on internal and external crossroads in glycochemistry. J Am Chem Soc. 2021;143:17–34. doi: 10.1021/jacs.0c11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galan M.C., Benito-Alifonso D., Watt G.M. Carbohydrate chemistry in drug discovery. Org Biomol Chem. 2011;9:3598–3610. doi: 10.1039/c0ob01017k. [DOI] [PubMed] [Google Scholar]
- 18.Shivatare S.S., Wong C.H. Synthetic carbohydrate chemistry and translational medicine. J Org Chem. 2020;85:15780–15800. doi: 10.1021/acs.joc.0c01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu B. Gold(I)-catalyzed glycosylation with glycosyl o-alkynylbenzoates as donors. Acc Chem Res. 2018;51:507–516. doi: 10.1021/acs.accounts.7b00573. [DOI] [PubMed] [Google Scholar]
- 20.Xavier N.M., Amélia P.R. Enantioselective synthesis in carbohydrate-based drug discovery: imino sugars, alkaloids and macrolide antibiotics. Curr Top Med Chem. 2014;14:1235–1243. doi: 10.2174/1568026614666140423103848. [DOI] [PubMed] [Google Scholar]
- 21.Lowary T.L. Twenty years of mycobacterial glycans: furanosides and beyond. Acc Chem Res. 2016;49:1379–1388. doi: 10.1021/acs.accounts.6b00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith B.A.H., Bertozzi C.R. The clinical impact of glycobiology: targeting selectins, siglecs and mammalian glycans. Nat Rev Drug Discov. 2021;20:217–243. doi: 10.1038/s41573-020-00093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reily C., Stewart T.J., Renfrow M.B., Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15:346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeberger P.H. Chemical glycobiology: why now? Nat Chem Biol. 2009;5:368–372. doi: 10.1038/nchembio0609-368. [DOI] [PubMed] [Google Scholar]
- 25.Boons G.J., Wu P. Chemical glycobiology. Glycobiology. 2016;26:788. doi: 10.1093/glycob/cww072. [DOI] [PubMed] [Google Scholar]
- 26.Becker B., Condie G.C., Le G.T., Meutermans W. Carbohydrate-based scaffolds in drug discovery. Mini Rev Med Chem. 2006;6:1299–1309. doi: 10.2174/138955706778993003. [DOI] [PubMed] [Google Scholar]
- 27.Meutermans W., Le G.T., Becker B. Carbohydrates as scaffolds in drug discovery. ChemMedChem. 2006;1:1164–1194. doi: 10.1002/cmdc.200600150. [DOI] [PubMed] [Google Scholar]
- 28.Reina J.J., Bernardi A. Carbohydrate mimics and lectins: a source of new drugs and therapeutic opportunities. Mini Rev Med Chem. 2012;12:1434–1442. doi: 10.2174/138955712803832690. [DOI] [PubMed] [Google Scholar]
- 29.Musser J.H., Fugedi P., Anderson M.B., Rao N., Peto C., Tyrrell D., et al. Carbohydrates as a source of molecular diversity for drug discovery. Drug News Perspect. 1996;9:133–141. [Google Scholar]
- 30.Sofia M.J. The generation of carbohydrate-based combinatorial libraries for drug discovery. Med Chem Res. 1998;8:362–378. [Google Scholar]
- 31.Islam T., von Itzstein M. Anti-influenza drug discovery: are we ready for the next pandemic?. Adv Carbohydr Chem Biochem. 2007;61:293–352. doi: 10.1016/S0065-2318(07)61006-3. [DOI] [PubMed] [Google Scholar]
- 32.Soengas R.G. Carbohydrate mimetics as potential drug candidates. Mini Rev Med Chem. 2012;12:1433. doi: 10.2174/138955712803832717. [DOI] [PubMed] [Google Scholar]
- 33.Lohof E., Planker E., Mang C., Burkhart F., Dechantsreiter M.A., Haubner R., et al. Carbohydrate derivatives for use in drug design: cyclic alpha(v)-selective RGD peptides. Angew Chem Int Ed Engl. 2000;39:2761–2764. doi: 10.1002/1521-3773(20000804)39:15<2761::aid-anie2761>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Khunti K. SGLT2 inhibitors in people with and without T2DM. Nat Rev Endocrinol. 2021;17:75–76. doi: 10.1038/s41574-020-00453-2. [DOI] [PubMed] [Google Scholar]
- 35.Brown E., Heerspink H.J.L., Cuthbertson D.J., Wilding J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262–276. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 36.Brennan M.B. Carbohydrate-based drug delivery. Chem Eng News. 1997;75:50–54. [Google Scholar]
- 37.McGeary R.P., Jablonkai I., Toth I. Carbohydrate-based templates for synthetic vaccines and drug delivery. Tetrahedron. 2001;57:8733–8742. [Google Scholar]
- 38.Gruner S.A., Locardi E., Lohof E., Kessler H. Carbohydrate-based mimetics in drug design: sugar amino acids and carbohydrate scaffolds. Chem Rev. 2002;102:491–514. doi: 10.1021/cr0004409. [DOI] [PubMed] [Google Scholar]
- 39.Badal S.C. vol. 862. American Chemical Society; Washington DC: 2003. Fermentation biotechnology. (ACS symposium series). [Google Scholar]
- 40.Wang P.G., Ichikawa Y. vol. 873. American Chemical Society; Washington DC: 2004. Synthesis of carbohydrates through biotechnology. (ACS symposium series). [Google Scholar]
- 41.Klyosov A.A., Witczak Z.J., Platt D. vol. 932. American Chemical Society; Washington DC: 2006. Carbohydrate drug design. (ACS symposium series). [Google Scholar]
- 42.Roy R. vol. 989. American Chemical Society; Washington DC: 2008. Carbohydrate-based vaccines. (ACS symposium series). [Google Scholar]
- 43.Klyosov A.A. vol. 1102. American Chemical Society; Washington DC: 2012. Glycobiology and drug design. (ACS symposium series). [Google Scholar]
- 44.Klyosov A.A., Traber P.G. vol. 1115. American Chemical Society; Washington DC: 2012. Galectins and disease implications for targeted therapeutics. (ACS symposium series). [Google Scholar]
- 45.Talisman I.J., Marzabadi C.H. Carbohydrate-based drugs in the treatment of epilepsy, depression and other affective disorders. Curr Top Med Chem. 2008;8:159–170. doi: 10.2174/156802608783378846. [DOI] [PubMed] [Google Scholar]
- 46.Ernst B., Magnani J.L. From carbohydrate leads to glycomimetic drugs. Nat Rev Drug Discov. 2009;8:661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiwari V.K., Mishra R.C., Sharma A., Tripathi R.P. Carbohydrate based potential chemotherapeutic agents: recent developments and their scope in future drug discovery. Mini Rev Med Chem. 2012;12:1497–1519. doi: 10.2174/138955712803832654. [DOI] [PubMed] [Google Scholar]
- 48.Seeberger P.H., Rademacher C. vol. 12. Springer; Berlin: 2014. Carbohydrates as drugs. (Topics in medicinal chemistry). [Google Scholar]
- 49.Fernández-Tejada A., Cañada F.J., Jiménez-Barbero J. Recent developments in synthetic carbohydrate-based diagnostics, vaccines, and therapeutics. Chem Eur J. 2015;21:10616–10628. doi: 10.1002/chem.201500831. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Wang F. Carbohydrate drugs: current status and development prospect. Drug Discov Ther. 2015;9:79–87. doi: 10.5582/ddt.2015.01028. [DOI] [PubMed] [Google Scholar]
- 51.Zhu D., Yu B. Synthesis of the diverse glycosides in traditional Chinese medicine. Chin J Chem. 2018;36:681–691. [Google Scholar]
- 52.Hossain F., Andreana P.R. Developments in carbohydrate-based cancer therapeutics. Pharmaceuticals. 2019;12:84. doi: 10.3390/ph12020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu J., Yang J., Seeberger P.H., Yin J. Glycoconjugates for glucose transporter-mediated cancer-specific targeting and treatment. Carbohydr Res. 2020;498:108195. doi: 10.1016/j.carres.2020.108195. [DOI] [PubMed] [Google Scholar]
- 54.Pan L., Cai C., Liu C., Liu D., Li G., Linhardt R.J., et al. Recent progress and advanced technology in carbohydrate-based drug development. Curr Opin Biotechnol. 2021;69:191–198. doi: 10.1016/j.copbio.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 55.Service R.F. Looking for a sugar rush. Science. 2012;338:321–323. doi: 10.1126/science.338.6105.321. [DOI] [PubMed] [Google Scholar]
- 56.Graham B.S., Sullivan N.J. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic. Nat Immunol. 2018;19:20–28. doi: 10.1038/s41590-017-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson P.T., de Roode J.C., Fenton A. Why infectious disease research needs community ecology. Science. 2015;349:1259504. doi: 10.1126/science.1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y. New virus, new challenge. Innovation. 2020;1:100005. doi: 10.1016/j.xinn.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margolis D.M., Archin N.M., Cohen M.S., Eron J.J., Ferrari G., Garcia J.V., et al. Curing HIV: seeking to target and clear persistent infection. Cell. 2020;181:189–206. doi: 10.1016/j.cell.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen M.H., Wong G., Gane E., Kao J.H., Dusheiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev. 2020;33 doi: 10.1128/CMR.00046-19. e00046−19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Clercq E. Strategies in the design of antiviral drugs. Nat Rev Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 62.Gamblin S.J., Skehel J.J. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem. 2010;285:28403–28409. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khashu M. COVID-19: scientific advisers must do more than just voice their concerns behind closed doors. BMJ. 2020;369:m2534. doi: 10.1136/bmj.m2534. [DOI] [PubMed] [Google Scholar]
- 64.Peng Q., Peng R., Yuan B., Wang M., Zhao J., Fu L., et al. Structural basis of SARS-CoV-2 polymerase inhibition by favipiravir. Innovation. 2021;2:100080. doi: 10.1016/j.xinn.2021.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature. 2020;578:347–348. doi: 10.1038/d41586-020-00444-3. [DOI] [PubMed] [Google Scholar]
- 66.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., et al. Effect of high vs. low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun X., Ni Y., Zhang M. Rheumotologitsts' view on the use of hydroxychloroquine to treat COVID-19. Emerg Microb Infect. 2020;9:830–832. doi: 10.1080/22221751.2020.1760145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wise J. COVID-19: remdesivir is recommended for authorisation by European Medicines Agency. BMJ. 2020;369:m2610. doi: 10.1136/bmj.m2610. [DOI] [PubMed] [Google Scholar]
- 70.Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., Zhang L., et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino]adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 71.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.PREVAIL II Writing Group. Multi-National PREVAIL II Study Team. Davey R.T., Jr., Dodd L., Proschan M.A., Neaton J., et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375:1448–1456. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trøseid M., Hites M., Barratt-Due A., Ader F., Yazdanpanah Y. Assessing the evidence on remdesivir. Lancet Infect Dis. 2021;21:1630–1631. doi: 10.1016/S1473-3099(21)00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ison M.G., Wolfe C., Boucher H.W. Emergency use authorization of remdesivir: the need for a transparent distribution process. JAMA. 2020;323:2365–2366. doi: 10.1001/jama.2020.8863. [DOI] [PubMed] [Google Scholar]
- 79.Wahl A., Gralinski L.E., Johnson C.E., Yao W., Kovarova M., Dinnon K.H., 3rd, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mahase E. COVID-19: molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ. 2021;375:n2422. doi: 10.1136/bmj.n2422. [DOI] [PubMed] [Google Scholar]
- 81.Sun L., Peng Y., Yu W., Zhang Y., Liang L., Song C., et al. Mechanistic insight into antiretroviral potency of 2′-deoxy-2′-β-fluoro-4′-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention. J Med Chem. 2020;63:8554–8566. doi: 10.1021/acs.jmedchem.0c00940. [DOI] [PubMed] [Google Scholar]
- 82.Yu B., Chang J.B. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct Targeted Ther. 2020;5:236. doi: 10.1038/s41392-020-00351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren Z., Luo H., Yu Z., Song J., Liang L., Wang L., et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci. 2020;7:2001435. doi: 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gentile I., Abenavoli L. COVID-19: perspectives on the potential novel global threat. Rev Recent Clin Trials. 2020;15:84–86. doi: 10.2174/1574887115999200228100745. [DOI] [PubMed] [Google Scholar]
- 85.De Clercq E. A 40-year journey in search of selective antiviral chemotherapy. Annu Rev Pharmacol Toxicol. 2011;51:1–24. doi: 10.1146/annurev-pharmtox-010510-100228. [DOI] [PubMed] [Google Scholar]
- 86.Zhao K., Liu A., Xia Y. Insights into hepatitis B virus DNA integration−55 years after virus discovery. Innovation. 2020;1:100034. doi: 10.1016/j.xinn.2020.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Billich A. Entecavir (Bristol-Myers Squibb) Curr Opin Invest Drugs. 2001;2:617–621. [PubMed] [Google Scholar]
- 88.Honkoop P., De Man R.A. Entecavir: a potent new antiviral drug for hepatitis B. Expet Opin Invest Drugs. 2003;12:683–688. doi: 10.1517/13543784.12.4.683. [DOI] [PubMed] [Google Scholar]
- 89.Dimou E., Papadimitropoulos V., Hadziyannis S.J. The role of entecavir in the treatment of chronic hepatitis B. Therapeut Clin Risk Manag. 2007;3:1077–1086. [PMC free article] [PubMed] [Google Scholar]
- 90.Hodge R.A. Telbivudine/torcitabine idenix/novartis. Curr Opin Invest Drugs. 2004;5:232–241. [PubMed] [Google Scholar]
- 91.Keam S.J. Telbivudine. Drugs. 2007;67:1917–1929. doi: 10.2165/00003495-200767130-00011. [DOI] [PubMed] [Google Scholar]
- 92.Chan H.L., Heathcote E.J., Marcellin P., Lai C.L., Cho M., Moon Y.M., et al. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med. 2007;147:745–754. doi: 10.7326/0003-4819-147-11-200712040-00183. [DOI] [PubMed] [Google Scholar]
- 93.Moon Y.M., Hwang S.G., Kim B.S., Rim K.S., Cho M., Kim D.J., et al. The efficacy and safety of telbivudine in Korean patients with chronic hepatitis B. Korean J Hepatol. 2007;13:503–512. doi: 10.3350/kjhep.2007.13.4.503. [DOI] [PubMed] [Google Scholar]
- 94.Peek S.F., Cote P.J., Jacob J.R., Toshkov I.A., Hornbuckle W.E., Baldwin B.H., et al. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, l-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax) Hepatology. 2001;33:254–266. doi: 10.1053/jhep.2001.20899. [DOI] [PubMed] [Google Scholar]
- 95.Korba B.E., Furman P.A., Otto M.J. Clevudine: a potent inhibitor of hepatitis B virus in vitro and in vivo. Expert Rev Anti Infect Ther. 2006;4:549–561. doi: 10.1586/14787210.4.4.549. [DOI] [PubMed] [Google Scholar]
- 96.Asselah T., Lada O., Moucari R., Marcellin P. Clevudine: a promising therapy for the treatment of chronic hepatitis B. Expet Opin Invest Drugs. 2008;17:1963–1974. doi: 10.1517/13543780802535760. [DOI] [PubMed] [Google Scholar]
- 97.Waheed Y., Siddiq M., Jamil Z., Najmi M.H. Hepatitis elimination by 2030: progress and challenges. World J Gastroenterol. 2018;24:4959–4961. doi: 10.3748/wjg.v24.i44.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarrazin C., Zeuzem S. Current guidelines for treatment of hepatitis C. The eradication of HCV as a goal. Pharmazie Unserer Zeit. 2011;40:52–59. doi: 10.1002/pauz.201100400. [DOI] [PubMed] [Google Scholar]
- 99.Cheng X., Ghany M. Key milestones in HCV discovery and therapeutics. Innovation. 2020;1:100067. doi: 10.1016/j.xinn.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Summers B.B., Beavers J.W., Klibanov O.M. Sofosbuvir, a novel nucleotide analogue inhibitor used for the treatment of hepatitis C virus. J Pharm Pharmacol. 2014;66:1653–1666. doi: 10.1111/jphp.12294. [DOI] [PubMed] [Google Scholar]
- 101.Murakami E., Tolstykh T., Bao H., Niu C., Steuer H.M., Bao D., et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J Biol Chem. 2010;285:34337–34347. doi: 10.1074/jbc.M110.161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gane E.J., Stedman C.A., Hyland R.H., Ding X., Svarovskaia E., Symonds W.T., et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 103.Lawitz E., Mangia A., Wyles D., Rodriguez-Torres M., Hassanein T., Gordon S.C., et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 104.Jin L., Örvell C., Myers R., Rota P.A., Nakayama T., Forcic D., et al. Genomic diversity of mumps virus and global distribution of the 12 genotypes. Rev Med Virol. 2015;25:85–101. doi: 10.1002/rmv.1819. [DOI] [PubMed] [Google Scholar]
- 105.Biron K.K., Harvey R.J., Chamberlain S.C., Good S.S., Smith A.A., 3rd, Davis M.G., et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46:2365–2372. doi: 10.1128/AAC.46.8.2365-2372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Winston D.J., Young J.A., Pullarkat V., Papanicolaou G.A., Vij R., Vance E., et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111:5403–5410. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maertens J., Cordonnier C., Jaksch P., Poiré X., Uknis M., Wu J., et al. Maribavir for preemptive treatment of cytomegalovirus reactivation. N Engl J Med. 2019;381:1136–1147. doi: 10.1056/NEJMoa1714656. [DOI] [PubMed] [Google Scholar]
- 108.Del Mar C., Collignon P. How can we prepare better for influenza epidemics?. BMJ. 2017;359:j5007. doi: 10.1136/bmj.j5007. [DOI] [PubMed] [Google Scholar]
- 109.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meindl P., Bodo G., Palese P., Schulman J., Tuppy H. Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1974;58:457–463. doi: 10.1016/0042-6822(74)90080-4. [DOI] [PubMed] [Google Scholar]
- 111.Baigent S.J., Bethell R.C., McCauley J.W. Genetic analysis reveals that both haemagglutinin and neuraminidase determine the sensitivity of naturally occurring avian influenza viruses to zanamivir in vitro. Virology. 1999;263:323–338. doi: 10.1006/viro.1999.9931. [DOI] [PubMed] [Google Scholar]
- 112.Service R.F. Researchers seek new weapon against the flu. Science. 1997;275:756–757. doi: 10.1126/science.275.5301.756. [DOI] [PubMed] [Google Scholar]
- 113.Gubareva L.V., Webster R.G., Hayden F.G. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother. 2001;45:3403–3408. doi: 10.1128/AAC.45.12.3403-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Babu Y.S., Chand P., Bantia S., Kotian P., Dehghani A., El-Kattan Y., et al. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 115.Alame M.M., Massaad E., Zaraket H. Peramivir: a novel intravenous neuraminidase inhibitor for treatment of acute influenza infections. Front Microbiol. 2016;7:450. doi: 10.3389/fmicb.2016.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamashita M. Laninamivir and its prodrug, CS-8958: long-acting neuraminidase inhibitors for the treatment of influenza. Antivir Chem Chemother. 2010;21:71–84. doi: 10.3851/IMP1688. [DOI] [PubMed] [Google Scholar]
- 117.van de Velde F., Antipova A.S., Rollema H.S., Burova T.V., Grinberg N.V., Pereira L., et al. The structure of kappa/iota-hybrid carrageenans II. Coil-helix transition as a function of chain composition. Carbohydr Res. 2005;340:1113–1129. doi: 10.1016/j.carres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 118.Leibbrandt A., Meier C., König-Schuster M., Weinmüllner R., Kalthoff D., Pflugfelder B., et al. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS One. 2010;5 doi: 10.1371/journal.pone.0014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ludwig M., Enzenhofer E., Schneider S., Rauch M., Bodenteich A., Neumann K., et al. Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respir Res. 2013;14:124. doi: 10.1186/1465-9921-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morokutti-Kurz M., Fröba M., Graf P., Große M., Grassauer A., Auth J., et al. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS One. 2021;16 doi: 10.1371/journal.pone.0237480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X., Bai H., Yang Y., Yoon J., Wang S., Zhang X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv Mater. 2019;31 doi: 10.1002/adma.201805092. [DOI] [PubMed] [Google Scholar]
- 122.Jomaa A., Stewart G., Martín-Benito J., Zielke R., Campbell T.L., Maddock J.R., et al. Understanding ribosome assembly: the structure of in vivo assembled immature 30S subunits revealed by cryo-electron microscopy. RNA. 2011;17:697–709. doi: 10.1261/rna.2509811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Katz L., Ashley G.W. Translation and protein synthesis: macrolides. Chem Rev. 2005;105:499–528. doi: 10.1021/cr030107f. [DOI] [PubMed] [Google Scholar]
- 124.Pasquina-Lemonche L., Burns J., Turner R.D., Kumar S., Tank R., Mullin N., et al. The architecture of the Gram-positive bacterial cell wall. Nature. 2020;582:294–297. doi: 10.1038/s41586-020-2236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Radkov A.D., Hsu Y.P., Booher G., Van Nieuwenhze M.S. Imaging bacterial cell wall biosynthesis. Annu Rev Biochem. 2018;87:991–1014. doi: 10.1146/annurev-biochem-062917-012921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Okano A., Isley N.A., Boger D.L. Total syntheses of vancomycin-related glycopeptide antibiotics and key analogues. Chem Rev. 2017;117:11952–11993. doi: 10.1021/acs.chemrev.6b00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Outterson K., Rex J.H., Jinks T., Jackson P., Hallinan J., Karp S., et al. Accelerating global innovation to address antibacterial resistance: introducing CARB-X. Nat Rev Drug Discov. 2016;15:589–590. doi: 10.1038/nrd.2016.155. [DOI] [PubMed] [Google Scholar]
- 128.Garza-Ramos G., Xiong L., Zhong P., Mankin A. Binding site of macrolide antibiotics on the ribosome: new resistance mutation identifies a specific interaction of ketolides with rRNA. J Bacteriol. 2001;183:6898–6907. doi: 10.1128/JB.183.23.6898-6907.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morimoto S., Takahashi Y., Watanabe Y., Omura S. Chemical modification of erythromycins. I. Synthesis and antibacterial activity of 6-O-methylerythromycins A. J Antibiot (Tokyo) 1984;37:187–189. doi: 10.7164/antibiotics.37.187. [DOI] [PubMed] [Google Scholar]
- 130.Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., et al. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against Gram-negative organisms. Antimicrob Agents Chemother. 1987;31:1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McColl K.E. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 132.Karakousis P.C., Moore R.D., Chaisson R.E. Mycobacterium avium complex in patients with HIV infection in the era of highly active antiretroviral therapy. Lancet Infect Dis. 2004;4:557–565. doi: 10.1016/S1473-3099(04)01130-2. [DOI] [PubMed] [Google Scholar]
- 133.Qiang G., Ma S.T. Modification on sugars of the macrolide antibiotics. Chin J Antibiot. 2013;38:12–21. [Google Scholar]
- 134.Denis A., Agouridas C., Auger J.M., Benedetti Y., Bonnefoy A., Bretin F., et al. Synthesis and antibacterial activity of HMR 3647 a new ketolide highly potent against erythromycin-resistant and susceptible pathogens. Bioorg Med Chem Lett. 1999;9:3075–3080. doi: 10.1016/s0960-894x(99)00534-x. [DOI] [PubMed] [Google Scholar]
- 135.Raja A., Lebbos J., Kirkpatrick P. Telithromycin. Nat Rev Drug Discov. 2004;3:733–734. doi: 10.1038/nrd1502. [DOI] [PubMed] [Google Scholar]
- 136.Pankuch G.A., Visalli M.A., Jacobs M.R., Appelbaum P.C. Susceptibilities of penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647), a new ketolide, compared with susceptibilities to 17 other agents. Antimicrob Agents Chemother. 1998;42:624–630. doi: 10.1128/aac.42.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shortridge V.D., Zhong P., Cao Z., Beyer J.M., Almer L.S., Ramer N.C., et al. Comparison of in vitro activities of ABT-773 and telithromycin against macrolide-susceptible and -resistant streptococci and staphylococci. Antimicrob Agents Chemother. 2002;46:783–786. doi: 10.1128/AAC.46.3.783-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jalava J., Kataja J., Seppälä H., Huovinen P. In vitro activities of the novel ketolide telithromycin (HMR 3647) against erythromycin-resistant Streptococcus species. Antimicrob Agents Chemother. 2001;45:789–793. doi: 10.1128/AAC.45.3.789-793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buxbaum A., Forsthuber S., Graninger W., Georgopoulos A. Comparative activity of telithromycin against typical community-acquired respiratory pathogens. J Antimicrob Chemother. 2003;52:371–374. doi: 10.1093/jac/dkg359. [DOI] [PubMed] [Google Scholar]
- 140.Low D.E., Felmingham D., Brown S.D., Rangaraju M., Nusrat R. Activity of telithromycin against key pathogens associated with community-acquired respiratory tract infections. J Infect. 2004;49:115–125. doi: 10.1016/j.jinf.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 141.Miyashita N., Fukano H., Niki Y., Matsushima T. In vitro activity of telithromycin, a new ketolide, against Chlamydia pneumoniae. J Antimicrob Chemother. 2001;48:403–405. doi: 10.1093/jac/48.3.403. [DOI] [PubMed] [Google Scholar]
- 142.Schülin T., Wennersten C.B., Ferraro M.J., Moellering R.C., Jr Eliopoulos G.M. Susceptibilities of Legionella spp. to newer antimicrobials in vitro. Antimicrob Agents Chemother. 1998;42:1520–1523. doi: 10.1128/aac.42.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bébéar C.M., Renaudin H., Aydin M.D., Chantot J.F., Bébéar C. In-vitro activity of ketolides against mycoplasmas. J Antimicrob Chemother. 1997;39:669–670. doi: 10.1093/jac/39.5.669. [DOI] [PubMed] [Google Scholar]
- 144.Lee J.S., Giesler D.L., Gellad W.F., Fine M.J. Antibiotic therapy for adults hospitalized with community-acquired pneumonia: a systematic review. JAMA. 2016;315:593–602. doi: 10.1001/jama.2016.0115. [DOI] [PubMed] [Google Scholar]
- 145.Fogarty C., de Wet R., Mandell L., Chang J., Rangaraju M., Nusrat R. Five-day telithromycin once daily is as effective as 10-day clarithromycin twice daily for the treatment of acute exacerbations of chronic bronchitis and is associated with reduced health-care resource utilization. Chest. 2005;128:1980–1988. doi: 10.1378/chest.128.4.1980. [DOI] [PubMed] [Google Scholar]
- 146.Shlaes D.M., Moellering R.C. Telithromycin and the FDA: implications for the future. Lancet Infect Dis. 2008;8:83–85. doi: 10.1016/S1473-3099(08)70002-1. [DOI] [PubMed] [Google Scholar]
- 147.Norrby S.R., Quinn J., Rangaraju M., Leroy B. Evaluation of 5-day therapy with telithromycin, a novel ketolide antibacterial, for the treatment of tonsillopharyngitis. Clin Microbiol Infect. 2004;10:615–623. doi: 10.1111/j.1469-0691.2004.00908.x. [DOI] [PubMed] [Google Scholar]
- 148.Kim D.M., Yu K.D., Lee J.H., Kim H.K., Lee S.H. Controlled trial of a 5-day course of telithromycin versus doxycycline for treatment of mild to moderate scrub typhus. Antimicrob Agents Chemother. 2007;51:2011–2015. doi: 10.1128/AAC.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnston S.L., Blasi F., Black P.N., Martin R.J., Farrell D.J., Nieman R.B. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 150.Clay K.D., Hanson J.S., Pope S.D., Rissmiller R.W., Purdum P.P., III, Banks P.M. Brief communication: severe hepatotoxicity of telithromycin: three case reports and literature review. Ann Intern Med. 2006;144:415–420. doi: 10.7326/0003-4819-144-6-200503210-00121. [DOI] [PubMed] [Google Scholar]
- 151.Barry A.L., Fuchs P.C., Brown S.D. In vitro activity of the ketolide ABT-773. Antimicrob Agents Chemother. 2001;45:2922–2924. doi: 10.1128/AAC.45.10.2922-2924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miyashita N., Fukano H., Yoshida K., Niki Y., Matsushima T. In vitro activity of cethromycin, a novel antibacterial ketolide, against Chlamydia pneumoniae. J Antimicrob Chemother. 2003;52:497–499. doi: 10.1093/jac/dkg371. [DOI] [PubMed] [Google Scholar]
- 153.Waites K.B., Crabb D.M., Duffy L.B. In vitro activities of ABT-773 and other antimicrobials against human mycoplasmas. Antimicrob Agents Chemother. 2003;47:39–42. doi: 10.1128/AAC.47.1.39-42.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luna V.A., Xu Z.Q., Eiznhamer D.A., Cannons A.C., Cattani J. Susceptibility of 170 isolates of the USA300 clone of MRSA to macrolides, clindamycin and the novel ketolide cethromycin. J Antimicrob Chemother. 2008;62:639–640. doi: 10.1093/jac/dkn227. [DOI] [PubMed] [Google Scholar]
- 155.Rafie S., MacDougall C., James C.L. Cethromycin: a promising new ketolide antibiotic for respiratory infections. Pharmacotherapy. 2010;30:290–303. doi: 10.1592/phco.30.3.290. [DOI] [PubMed] [Google Scholar]
- 156.Rosenzweig J.A., Brackman S.M., Kirtley M.L., Sha J., Erova T.E., Yeager L.A., et al. Cethromycin-mediated protection against the plague pathogen Yersinia pestis in a rat model of infection and comparison with levofloxacin. Antimicrob Agents Chemother. 2011;55:5034–5042. doi: 10.1128/AAC.00632-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frean J., Klugman K.P., Arntzen L., Bukofzer S. Susceptibility of Bacillus anthracis to eleven antimicrobial agents including novel fluoroquinolones and a ketolide. J Antimicrob Chemother. 2003;52:297–299. doi: 10.1093/jac/dkg364. [DOI] [PubMed] [Google Scholar]
- 158.Hammerschlag M.R., Sharma R. Use of cethromycin, a new ketolide, for treatment of community-acquired respiratory infections. Expet Opin Invest Drugs. 2008;17:387–400. doi: 10.1517/13543784.17.3.387. [DOI] [PubMed] [Google Scholar]
- 159.He W., Yang C., Zhao X., Wang Y. Antimicrobial activity of bitespiramycin, a new genetically engineered macrolide. Bioorg Med Chem Lett. 2017;27:4576–4577. doi: 10.1016/j.bmcl.2017.08.046. [DOI] [PubMed] [Google Scholar]
- 160.Liu J., Zhang Y., He W. Construction of a novel carrimycin-producing strain by using CRISPR-Cas9 and ribosome engineering techniques. Chin J Biotechnol. 2021;37:2116–2126. doi: 10.13345/j.cjb.200763. [DOI] [PubMed] [Google Scholar]
- 161.Yan H., Sun J., Wang K., Wang H., Wu S., Bao L., et al. Repurposing carrimycin as an antiviral agent against human coronaviruses, including the currently pandemic SARS-CoV-2. Acta Pharm Sin B. 2021;11:2850–2858. doi: 10.1016/j.apsb.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johnson A.P., Wilcox M.H. Fidaxomicin: a new option for the treatment of Clostridium difficile infection. J Antimicrob Chemother. 2012;67:2788–2792. doi: 10.1093/jac/dks302. [DOI] [PubMed] [Google Scholar]
- 163.Lin W., Das K., Degen D., Mazumder A., Duchi D., Wang D., et al. Structural basis of transcription inhibition by fidaxomicin (Lipiarmycin A3) Mol Cell. 2018;70:60–71. doi: 10.1016/j.molcel.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Finegold S.M., Molitoris D., Vaisanen M.L., Song Y., Liu C., Bolaños M. In vitro activities of OPT-80 and comparator drugs against intestinal bacteria. Antimicrob Agents Chemother. 2004;48:4898–4902. doi: 10.1128/AAC.48.12.4898-4902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rao K., Malani P.N. Diagnosis and treatment of Clostridioides (Clostridium) difficile infection in adults in 2020. JAMA. 2020;323:1403–1404. doi: 10.1001/jama.2019.3849. [DOI] [PubMed] [Google Scholar]
- 166.Cohen S.H., Gerding D.N., Johnson S., Kelly C.P., Loo V.G., McDonald L.C., et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 167.Louie T.J., Miller M.A., Mullane K.M., Weiss K., Lentnek A., Golan Y., et al. Fidaxomicin versus vancomycin for clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 168.Zhanel G.G., Calic D., Schweizer F., Zelenitsky S., Adam H., Lagacé-Wiens P.R., et al. New lipoglycopeptides: a comparative review of plazomicin, oritavancin and telavancin. Drugs. 2010;70:859–886. doi: 10.2165/11534440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 169.Ashford P.A., Bew S.P. Recent advances in the synthesis of new glycopeptide antibiotics. Chem Soc Rev. 2012;41:957–978. doi: 10.1039/c1cs15125h. [DOI] [PubMed] [Google Scholar]
- 170.Corey G.R., Stryjewski M.E., Weyenberg W., Yasothan U., Kirkpatrick P. Telavancin. Nat Rev Drug Discov. 2009;8:929–930. doi: 10.1038/nrd3051. [DOI] [PubMed] [Google Scholar]
- 171.Lunde C.S., Hartouni S.R., Janc J.W., Mammen M., Humphrey P.P., Benton B.M. Telavancin disrupts the functional integrity of the bacterial membrane through targeted interaction with the cell wall precursor lipid II. Antimicrob Agents Chemother. 2009;53:3375–3383. doi: 10.1128/AAC.01710-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Karlowsky J.A., Nichol K., Zhanel G.G. Telavancin: mechanisms of action, in vitro activity, and mechanisms of resistance. Clin Infect Dis. 2015;61(Suppl 2):S58−68. doi: 10.1093/cid/civ534. [DOI] [PubMed] [Google Scholar]
- 173.Stryjewski M.E., Graham D.R., Wilson S.E., O'Riordan W., Young D., Lentnek A., et al. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis. 2008;46:1683–1693. doi: 10.1086/587896. [DOI] [PubMed] [Google Scholar]
- 174.Allen N.E. From vancomycin to oritavancin: the discovery and development of a novel lipoglycopeptide antibiotic. Antinfect Agents Med Chem. 2010;9:23–47. [Google Scholar]
- 175.Belley A., Neesham-Grenon E., McKay G., Arhin F.F., Harris R., Beveridge T., et al. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother. 2009;53:918–925. doi: 10.1128/AAC.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Corey G.R., Kabler H., Mehra P., Gupta S., Overcash J.S., Porwal A., et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 177.Billeter M., Zervos M.J., Chen A.Y., Dalovisio J.R., Kurukularatne C. Dalbavancin: a novel once-weekly lipoglycopeptide antibiotic. Clin Infect Dis. 2008;46:577–583. doi: 10.1086/526772. [DOI] [PubMed] [Google Scholar]
- 178.Boucher H.W., Wilcox M., Talbot G.H., Puttagunta S., Das A.F., Dunne M.W. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370:2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 179.Trylska J., Kulik M. Interactions of aminoglycoside antibiotics with rRNA. Biochem Soc Trans. 2016;44:987–993. doi: 10.1042/BST20160087. [DOI] [PubMed] [Google Scholar]
- 180.Richardson R., Smart M., Tracey-White D., Webster A.R., Moosajee M. Mechanism and evidence of nonsense suppression therapy for genetic eye disorders. Exp Eye Res. 2017;155:24–37. doi: 10.1016/j.exer.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 181.Pullens B., van Benthem P.P. Intratympanic gentamicin for Ménière's disease or syndrome. Cochrane DB Syst Rev. 2011;3:CD008234. doi: 10.1002/14651858.CD008234.pub2. [DOI] [PubMed] [Google Scholar]
- 182.Schroeder R., Waldsich C., Wank H. Modulation of RNA function by aminoglycoside antibiotics. EMBO J. 2000;19:1–9. doi: 10.1093/emboj/19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Armstrong E.S., Miller G.H. Combating evolution with intelligent design: the neoglycoside ACHN-490. Curr Opin Microbiol. 2010;13:565–573. doi: 10.1016/j.mib.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 184.Eljaaly K., Alharbi A., Alshehri S., Ortwine J.K., Pogue J.M. Plazomicin: a novel aminoglycoside for the treatment of resistant Gram-negative bacterial infections. Drugs. 2019;79:243–269. doi: 10.1007/s40265-019-1054-3. [DOI] [PubMed] [Google Scholar]
- 185.Connolly L.E., Riddle V., Cebrik D., Armstrong E.S., Miller L.G. A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01989-17. e01989−17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wagenlehner F.M.E., Cloutier D.J., Komirenko A.S., Cebrik D.S., Krause K.M., Keepers T.R., et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729–740. doi: 10.1056/NEJMoa1801467. [DOI] [PubMed] [Google Scholar]
- 187.Tracy J.W., Wbester L.T.J. McGraw-Hill; New York: 2001. The pharmacological basis of therapeutics; pp. 1097–1120. [Google Scholar]
- 188.Moore T.A. 16th ed. McGraw-Hill; New York: 2005. Harrison's principles of internal medicine; pp. 1202–1214. [Google Scholar]
- 189.Gutiérrez V., Seabra A.B., Reguera R.M., Khandare J., Calderón M. New approaches from nanomedicine for treating leishmaniasis. Chem Soc Rev. 2016;45:152–168. doi: 10.1039/c5cs00674k. [DOI] [PubMed] [Google Scholar]
- 190.Davidson R.N., den Boer M., Ritmeijer K. Paromomycin. Trans R Soc Trop Med Hyg. 2009;103:653–660. doi: 10.1016/j.trstmh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 191.Sundar S., Jha T.K., Thakur C.P., Sinha P.K., Bhattacharya S.K. Injectable paromomycin for Visceral leishmaniasis in India. N Engl J Med. 2007;356:2571–2581. doi: 10.1056/NEJMoa066536. [DOI] [PubMed] [Google Scholar]
- 192.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 193.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 194.Girotti M.R., Salatino M., Dalotto-Moreno T., Rabinovich G.A. Sweetening the hallmarks of cancer: galectins as multifunctional mediators of tumor progression. J Exp Med. 2020;3 doi: 10.1084/jem.20182041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Costa A.F., Campos D., Reis C.A., Gomes C. Targeting glycosylation: a new road for cancer drug discovery. Trends Cancer. 2020;6:757–766. doi: 10.1016/j.trecan.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 196.Ferreira J.A., Magalhães A., Gomes J., Peixoto A., Gaiteiro C., Fernandes E., et al. Protein glycosylation in gastric and colorectal cancers: toward cancer detection and targeted therapeutics. Cancer Lett. 2017;387:32–45. doi: 10.1016/j.canlet.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 197.Fuster M.M., Esko J.D. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 198.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shelton J., Lu X., Hollenbaugh J.A., Cho J.H., Amblard F., Schinazi R.F. Metabolism, biochemical actions, and chemical synthesis of anticancer nucleosides, nucleotides, and base analogs. Chem Rev. 2016;116:14379–14455. doi: 10.1021/acs.chemrev.6b00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.RodrÍguez E., Schetters S.T.T., van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol. 2018;18:204–211. doi: 10.1038/nri.2018.3. [DOI] [PubMed] [Google Scholar]
- 201.Dube D.H., Bertozzi C.R. Glycans in cancer and inflammation-potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 202.Zhang J., Visser F., King K.M., Baldwin S.A., Young J.D., Cass C.E. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007;26:85–110. doi: 10.1007/s10555-007-9044-4. [DOI] [PubMed] [Google Scholar]
- 203.Astronomo R.D., Burton D.R. Carbohydrate vaccines: developing sweet solutions to sticky situations?. Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim S.S., Donahue T.R., Girgis M.D. Carcinoembryonic antigen for diagnosis of colorectal cancer recurrence. JAMA. 2018;320:298–299. doi: 10.1001/jama.2018.8424. [DOI] [PubMed] [Google Scholar]
- 205.Henderson J.T., Webber E.M., Sawaya G.F. Screening for ovarian cancer: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;319:595–606. doi: 10.1001/jama.2017.21421. [DOI] [PubMed] [Google Scholar]
- 206.Ray K. Pancreatic cancer: biomarkers for the early detection of PDAC. Nat Rev Gastroenterol Hepatol. 2017;14:504–505. doi: 10.1038/nrgastro.2017.111. [DOI] [PubMed] [Google Scholar]
- 207.Gambhir S.S. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 208.Matsuda A., Sasaki T. Antitumor activity of sugar-modified cytosine nucleosides. Cancer Sci. 2004;95:105–111. doi: 10.1111/j.1349-7006.2004.tb03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Qin T., Jelinek J., Si J., Shu J., Issa J.P. Mechanisms of resistance to 5-aza-2′-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–667. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Scott L.J. Azacitidine: a review in myelodysplastic syndromes and acute myeloid leukaemia. Drugs. 2016;76:889–900. doi: 10.1007/s40265-016-0585-0. [DOI] [PubMed] [Google Scholar]
- 211.Kornblith A.B., Herndon J.E., 2nd, Silverman L.R., Demakos E.P., Odchimar-Reissig R., Holland J.F., et al. Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized Phase III trial: a cancer and leukemia group B study. J Clin Oncol. 2002;20:2441–2452. doi: 10.1200/JCO.2002.04.044. [DOI] [PubMed] [Google Scholar]
- 212.Kaminskas E., Farrell A., Abraham S., Baird A., Hsieh L.S., Lee S.L., et al. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin Cancer Res. 2005;11:3604–3608. doi: 10.1158/1078-0432.CCR-04-2135. [DOI] [PubMed] [Google Scholar]
- 213.Saba H., Lübbert M., Wijermans P. Response rates of phase 2 and phase 3 trials of decitabine (DAC) in patients with myelodysplastic syndromes (MDS) Blood. 2005;106:2515. [Google Scholar]
- 214.Saba H., Rosenfeld C., Issa J.P., DiPersio J., Raza A., Klimek V., et al. First report of the phase III north American trial of decitabine in advanced myelodysplastic syndrome (MDS) Blood. 2004;104:67. [Google Scholar]
- 215.Wijermans P., Lübbert M., Verhoef G., Bosly A., Ravoet C., Andre M., et al. Low-dose 5-aza-2′-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol. 2000;18:956–962. doi: 10.1200/JCO.2000.18.5.956. [DOI] [PubMed] [Google Scholar]
- 216.Nie J., Wang C., Liu Y., Yang Q., Mei Q., Dong L., et al. Addition of low-dose decitabine to anti-PD-1 antibody camrelizumab in relapsed/refractory classical hodgkin lymphoma. J Clin Oncol. 2019;37:1479–1489. doi: 10.1200/JCO.18.02151. [DOI] [PubMed] [Google Scholar]
- 217.Welch J.S., Petti A.A., Miller C.A., Fronick C.C., O'Laughlin M., Fulton R.S., et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375:2023–2036. doi: 10.1056/NEJMoa1605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Licht J.D. DNA Methylation inhibitors in cancer therapy: the immunity dimension. Cell. 2015;162:938–939. doi: 10.1016/j.cell.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 219.Juergens R.A., Wrangle J., Vendetti F.P., Murphy S.C., Zhao M., Coleman B., et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011;1:598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Plimack E.R., Stewart D.J., Issa J.P. Combining epigenetic and cytotoxic therapy in the treatment of solid tumors. J Clin Oncol. 2007;25:4519–4521. doi: 10.1200/JCO.2007.12.6029. [DOI] [PubMed] [Google Scholar]
- 221.Nie J., Liu L., Li X., Han W. Decitabine, a new star in epigenetic therapy: the clinical application and biological mechanism in solid tumors. Cancer Lett. 2014;354:12–20. doi: 10.1016/j.canlet.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 222.Bonate P.L., Arthaud L., Cantrell W.R., Jr., Stephenson K., Secrist J.A., 3rd, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 223.Montgomery J., Shortnacy-Fowler A., Clayton S., Riordan J., Secrist J. Synthesis and biologic activity of 2′-fluoro-2-halo derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- 224.Xie K.C., Plunkett W. Deoxynucleotide pool depletion and sustained inhibition of ribonucleotide reductase and DNA synthesis after treatment of human lymphoblastoid cells with 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl) adenine. Cancer Res. 1996;56:3030–3037. [PubMed] [Google Scholar]
- 225.Genini D., Adachi S., Chao Q., Rose D.W., Carrera C.J., Cottam H.B., et al. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood. 2000;96:3537–3543. [PubMed] [Google Scholar]
- 226.Waud W.R., Schmid S.M., Montgomery J.A., Secrist J.A., 3rd Preclinical antitumor activity of 2-chloro-9-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)adenine (Cl-F-ara-A) Nucleos Nucleot Nucl. 2000;19:447–460. doi: 10.1080/15257770008033020. [DOI] [PubMed] [Google Scholar]
- 227.Jeha S., Gandhi V., Chan K.W., McDonald L., Ramirez I., Madden R., et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 228.Pui C.H., Jeha S., Kirkpatrick P. Clofarabine. Nat Rev Drug Discov. 2005;4:369–370. doi: 10.1038/nrd1724. [DOI] [PubMed] [Google Scholar]
- 229.Kantarjian H., Gandhi V., Cortes J., Verstovsek S., Du M., Garcia-Manero G., et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 230.Jeha S., Gaynon P.S., Razzouk B.I., Franklin J., Kadota R., Shen V., et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24:1917–1923. doi: 10.1200/JCO.2005.03.8554. [DOI] [PubMed] [Google Scholar]
- 231.Gravatt L.C., Chaffee S., Hebert M.E., Halperin E.C., Friedman H.S., Kurtzberg J. Efficacy and toxicity of 9-beta-d-arabinofuranosylguanine (araG) as an agent to purge malignant T cells from murine bone marrow: application to an in vivo T-leukemia model. Leukemia. 1993;7:1261–1267. [PubMed] [Google Scholar]
- 232.Rodriguez C.O., Jr., Mitchell B.S., Ayres M., Eriksson S., Gandhi V. Arabinosylguanine is phosphorylated by both cytoplasmic deoxycytidine kinase and mitochondrial deoxyguanosine kinase. Cancer Res. 2002;62:3100–3105. [PubMed] [Google Scholar]
- 233.Gandhi V., Keating M.J., Bate G., Kirkpatrick P. Nelarabine. Nat Rev Drug Discov. 2006;5:17–18. doi: 10.1038/nrd1933. [DOI] [PubMed] [Google Scholar]
- 234.Rodriguez C.O., Jr., Stellrecht C.M., Gandhi V. Mechanisms for T-cell selective cytotoxicity of arabinosylguanine. Blood. 2003;102:1842–1848. doi: 10.1182/blood-2003-01-0317. [DOI] [PubMed] [Google Scholar]
- 235.Cohen M.H., Johnson J.R., Massie T., Sridhara R., McGuinn W.D., Jr., Abraham S., et al. Approval summary: nelarabine for the treatment of T-cell lymphoblastic leukemia/lymphoma. Cancer Res Clin Cancer Res. 2006;12:5329–5335. doi: 10.1158/1078-0432.CCR-06-0606. [DOI] [PubMed] [Google Scholar]
- 236.Gandhi V., Plunkett W., Rodriguez C.O., Jr., Nowak B.J., Du M., Ayres M., et al. Compound GW506U78 in refractory hematologic malignancies: relationship between cellular pharmacokinetics and clinical response. J Clin Oncol. 1998;16:3607–3615. doi: 10.1200/JCO.1998.16.11.3607. [DOI] [PubMed] [Google Scholar]
- 237.Kisor D.F., Plunkett W., Kurtzberg J., Mitchell B., Hodge J.P., Ernst T., et al. Pharmacokinetics of nelarabine and 9-beta-d-arabinofuranosyl guanine in pediatric and adult patients during a Phase I study of nelarabine for the treatment of refractory hematologic malignancies. J Clin Oncol. 2000;18:995–1003. doi: 10.1200/JCO.2000.18.5.995. [DOI] [PubMed] [Google Scholar]
- 238.Kurtzberg J., Ernst T.J., Keating M.J., Gandhi V., Hodge J.P., Kisor D.F., et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–3403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 239.Berg S.L., Blaney S.M., Devidas M., Lampkin T.A., Murgo A., Bernstein M., et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children's Oncology Group. J Clin Oncol. 2005;23:3376–3382. doi: 10.1200/JCO.2005.03.426. [DOI] [PubMed] [Google Scholar]
- 240.DeAngelo D.J., Yu D., Johnson J.L., Coutre S.E., Stone R.M., Stopeck A.T., et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: cancer and leukemia group B study 19801. Blood. 2007;109:5136–5142. doi: 10.1182/blood-2006-11-056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Goekbuget N., Arnold R., Atta J., Brück P., Hermann S., Horst H., et al. Compound GW506U78 has high single-drug activity and good feasibility in heavily pretreated relapsed T-lymphoblastic leukemia (T-ALL) and T-lymphoblastic lymphoma (T-LBL) and offers the option for cure with stem cell transplantation (SCT) Blood. 2005;106:150. [Google Scholar]
- 242.Larson R.A. Three new drugs for acute lymphoblastic leukemia: nelarabine, clofarabine, and forodesine. Semin Oncol. 2007;34:S13−20. doi: 10.1053/j.seminoncol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 243.Gandhi V., Kilpatrick J.M., Plunkett W., Ayres M., Harman L., Du M., et al. Faderl S., et al. A proof-of-principle pharmacokinetic, pharmacodynamic, and clinical study with purine nucleoside phosphorylase inhibitor immucillin-H (BCX-1777, forodesine) Blood. 2005;106:4253–4260. doi: 10.1182/blood-2005-03-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Homminga I., Zwaan C.M., Manz C.Y., Parker C., Bantia S., Smits W.K., et al. In vitro efficacy of forodesine and nelarabine (ara-G) in pediatric leukemia. Blood. 2011;118:2184–2190. doi: 10.1182/blood-2011-02-337840. [DOI] [PubMed] [Google Scholar]
- 245.Ritchie E., Gore L., Roboz G., Feldman E., Ravandi F., Furman R. Phase II study of forodesine, a PNP inhibitor, in patients with relapsed or refractory B-lineage acute lymphoblastic leukemia. Blood. 2006;108:1881. [Google Scholar]
- 246.Furman R., Gore L., Ravandi F., Hoelzer D. Forodesine IV (Bcx-1777) is clinically active in relapsed/refractory T-cell leukemia: results of a phase II study (interim report) Blood. 2006;108:1851. [Google Scholar]
- 247.Duvic M., Forero-Torres A., Foss F., Olsen E.A., Kim Y. Oral forodesine (Bcx-1777) is clinically active in refractory cutaneous T-cell lymphoma: results of a phase I/II study. Blood. 2006;108:2467. [Google Scholar]
- 248.Kizek R., Adam V., Hrabeta J., Eckschlager T., Smutny S., Burda J.V., et al. Anthracyclines and ellipticines as DNA-damaging anticancer drugs: recent advances. Pharmacol Ther. 2012;133:26–39. doi: 10.1016/j.pharmthera.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 249.Vejpongsa P., Yeh E.T. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938–945. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 250.Morisada S., Yanagi Y., Noguchi T., Kashiwazaki Y., Fukui M. Antitumor activities of a novel 9-aminoanthracycline (SM-5887) against mouse experimental tumors and human tumor xenografts. Jpn J Cancer Res. 1989;80:69–76. doi: 10.1111/j.1349-7006.1989.tb02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ishizumi K., Ohashi N., Tanno N. Stereospecific total synthesis of 9-aminoanthracyclines: (+)-9-amino-9-deoxydaunomycin and related compounds. J Org Chem. 1987;52:4477–4485. [Google Scholar]
- 252.Hanada M., Mizuno S., Fukushima A., Saito Y., Noguchi T., Yamaoka T. A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complex. Jpn J Cancer Res. 1998;89:1229–1238. doi: 10.1111/j.1349-7006.1998.tb00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hatakeyama Y., Kobayashi K., Nagano T., Tamura D., Yamamoto M., Tachihara M., et al. Synergistic effects of pemetrexed and amrubicin in non-small cell lung cancer cell lines: potential for combination therapy. Cancer Lett. 2014;343:74–79. doi: 10.1016/j.canlet.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 254.Noda T., Watanabe T., Kohda A., Hosokawa S., Suzuki T. Chronic effects of a novel synthetic anthracycline derivative (SM-5887) on normal heart and doxorubicin-induced cardiomyopathy in beagle dogs. Invest N Drugs. 1998;16:121–128. doi: 10.1023/a:1006088907271. [DOI] [PubMed] [Google Scholar]
- 255.Sugiura T., Ariyoshi Y., Negoro S., Nakamura S., Ikegami H., Takada M., et al. Phase I/II study of amrubicin, a novel 9-aminoanthracycline, in patients with advanced non-small-cell lung cancer. Invest N Drugs. 2005;23:331–337. doi: 10.1007/s10637-005-1441-3. [DOI] [PubMed] [Google Scholar]
- 256.Yana T., Negoro S., Takada M., Yokota S., Takada Y., Sugiura T., et al. Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJTOG) study. Invest N Drugs. 2007;25:253–258. doi: 10.1007/s10637-006-9012-9. [DOI] [PubMed] [Google Scholar]
- 257.Ettinger D.S. Amrubicin for the treatment of small cell lung cancer: does effectiveness cross the Pacific?. J Thorac Oncol. 2007;2:160–165. doi: 10.1097/jto.0b013e31802f1cd9. [DOI] [PubMed] [Google Scholar]
- 258.Karaman M.W., Herrgard S., Treiber D.K., Gallant P., Atteridge C.E., Campbell B.T., et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 259.Fabbro D., Buchdunger E., Wood J., Mestan J., Hofmann F., Ferrari S., et al. Inhibitors of protein kinases: CGP 41251, a protein kinase inhibitor with potential as an anticancer agent. Pharmacol Ther. 1999;82:293–301. doi: 10.1016/s0163-7258(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 260.Bharate S.B., Sawant S.D., Singh P.P., Vishwakarma R.A. Kinase inhibitors of marine origin. Chem Rev. 2013;113:6761–6815. doi: 10.1021/cr300410v. [DOI] [PubMed] [Google Scholar]
- 261.Gani O.A., Engh R.A. Protein kinase inhibition of clinically important staurosporine analogues. Nat Prod Rep. 2010;27:489–498. doi: 10.1039/b923848b. [DOI] [PubMed] [Google Scholar]
- 262.Omura S., Iwai Y., Hirano A., Nakagawa A., Awaya J., Tsuchya H., et al. A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J Antibiot (Tokyo) 1977;30:275–282. doi: 10.7164/antibiotics.30.275. [DOI] [PubMed] [Google Scholar]
- 263.Rasko J.E.J., Hughes T.P. First approved kinase inhibitor for AML. Cell. 2017;171:981. doi: 10.1016/j.cell.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 264.Weisberg E., Boulton C., Kelly L.M., Manley P., Fabbro D., Meyer T., et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 265.Stone R.M., DeAngelo D.J., Klimek V., Galinsky I., Estey E., Nimer S.D., et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 266.Fischer T., Stone R.M., Deangelo D.J., Galinsky I., Estey E., Lanza C., et al. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Stone R.M., Mandrekar S.J., Sanford B.L., Laumann K., Geyer S., Bloomfield C.D., et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Reiter A., George T.I., Gotlib J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood. 2020;135:1365–1376. doi: 10.1182/blood.2019000932. [DOI] [PubMed] [Google Scholar]
- 269.Gotlib J., Kluin-Nelemans H.C., George T.I., Akin C., Sotlar K., Hermine O., et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530–2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 270.Schroit A.J., Fidler I.J. Effects of liposome structure and lipid composition on the activation of the tumoricidal properties of macrophages by liposomes containing muramyl dipeptide. Cancer Res. 1982;42:161–167. [PubMed] [Google Scholar]
- 271.Fogler W.E., Wade R., Brundish D.E., Fidler I.J. Distribution and fate of free and liposome-encapsulated [3H]nor-muramyl dipeptide and [3H]muramyl tripeptide phosphatidylethanolamine in mice. J Immunol. 1985;135:1372–1377. [PubMed] [Google Scholar]
- 272.Kansara M., Teng M.W., Smyth M.J., Thomas D.M. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–735. doi: 10.1038/nrc3838. [DOI] [PubMed] [Google Scholar]
- 273.Meyers P.A. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009;9:1035–1049. doi: 10.1586/era.09.69. [DOI] [PubMed] [Google Scholar]
- 274.Sone S., Mutsuura S., Ogawara M., Tsubura E. Potentiating effect of muramyl dipeptide and its lipophilic analog encapsulated in liposomes on tumor cell killing by human monocytes. J Immunol. 1984;132:2105–2110. [PubMed] [Google Scholar]
- 275.Kansara M., Leong H.S., Lin D.M., Popkiss S., Pang P., Garsed D.W., et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J Clin Invest. 2013;123:5351–5360. doi: 10.1172/JCI70559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chou A.J., Kleinerman E.S., Krailo M.D., Chen Z., Betcher D.L., Healey J.H., et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group. Cancer. 2009;115:5339–5348. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.International Diabetes Federation . 2019. IDF diabetes atlas.https://diabetesatlas.org/en/ 9th ed. Brussels, Belgium. Available from: [Google Scholar]
- 278.World Health Organization . World Health Organization; 2019. Classification of diabetes mellitus.https://apps.who.int/iris/handle/10665/325182 Availble from: [Google Scholar]
- 279.Bokor É., Kun S., Goyard D., Tóth M., Praly J.P., Vidal S., et al. C-Glycopyranosyl arenes and hetarenes: synthetic methods and bioactivity focused on antidiabetic potential. Chem Rev. 2017;117:1687–1764. doi: 10.1021/acs.chemrev.6b00475. [DOI] [PubMed] [Google Scholar]
- 280.Scheen A.J. Sodium-glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16:556–577. doi: 10.1038/s41574-020-0392-2. [DOI] [PubMed] [Google Scholar]
- 281.Mohan S., Eskandari R., Pinto B.M. Naturally occurring sulfonium-ion glucosidase inhibitors and their derivatives: a promising class of potential antidiabetic agents. Acc Chem Res. 2014;47:211–225. doi: 10.1021/ar400132g. [DOI] [PubMed] [Google Scholar]
- 282.Asano N. Sugar-mimicking glycosidase inhibitors: bioactivity and application. Cell Mol Life Sci. 2009;66:1479–1492. doi: 10.1007/s00018-008-8522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chen X., Fan Y., Zheng Y., Shen Y. Properties and production of valienamine and its related analogues. Chem Rev. 2003;103:1955–1977. doi: 10.1021/cr0102260. [DOI] [PubMed] [Google Scholar]
- 284.Ghani U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: finding needle in the haystack. Eur J Med Chem. 2015;103:133–162. doi: 10.1016/j.ejmech.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 285.van de Laar F.A., Lucassen P.L., Akkermans R.P., van de Lisdonk E.H., Rutten G.E., van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 286.Chen X., Zheng Y., Shen Y. Voglibose (Basen, AO-128), one of the most important alpha-glucosidase inhibitors. Curr Med Chem. 2006;13:109–116. doi: 10.2174/092986706789803035. [DOI] [PubMed] [Google Scholar]
- 287.Inouye S., Tsuruoka T., Nida T. The structure of nojirimycin, a piperidinose sugar antibiotic. J Antibiot (Tokyo) 1966;19:288–292. [PubMed] [Google Scholar]
- 288.Joubert P.H., Foukaridis G.N., Bopape M.L. Miglitol may have a blood glucose lowering effect unrelated to inhibition of alpha-glucosidase. Eur J Clin Pharmacol. 1987;31:723–724. doi: 10.1007/BF00541303. [DOI] [PubMed] [Google Scholar]
- 289.Taylor R.H., Barker H.M., Bowey E.A., Canfield J.E. Regulation of the absorption of dietary carbohydrate in man by two new glycosidase inhibitors. Gut. 1986;27:1471–1478. doi: 10.1136/gut.27.12.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Heinz G., Komjati M., Korn A., Waldhäusl W. Reduction of postprandial blood glucose by the alpha-glucosidase inhibitor miglitol (BAY m 1099) in type II diabetes. Eur J Clin Pharmacol. 1989;37:33–36. doi: 10.1007/BF00609420. [DOI] [PubMed] [Google Scholar]
- 291.Omar M.A., Seedat M.A., Hillebrand I. The clinical efficacy of a second-generation alpha-glucosidase inhibitor in non-insulin-dependent diabetic patients. S Afr Med J. 1987;71:422–423. [PubMed] [Google Scholar]
- 292.Schnack C., Prager R.J., Winkler J., Klauser R.M., Schneider B.G., Schernthaner G. Effects of 8-wk alpha-glucosidase inhibition on metabolic control, C-peptide secretion, hepatic glucose output, and peripheral insulin sensitivity in poorly controlled type II diabetic patients. Diabetes Care. 1989;12:537–543. doi: 10.2337/diacare.12.8.537. [DOI] [PubMed] [Google Scholar]
- 293.Scott L.J., Spencer C.M. Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs. 2000;59:521–549. doi: 10.2165/00003495-200059030-00012. [DOI] [PubMed] [Google Scholar]
- 294.Brown G.K. Glucose transporters: structure, function and consequences of deficiency. J Inherit Metab Dis. 2000;23:237–246. doi: 10.1023/a:1005632012591. [DOI] [PubMed] [Google Scholar]
- 295.Wright E.M., Loo D.D., Hirayama B.A. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 296.Wright E.M. Renal Na+-glucose cotransporters. Am J Physiol Ren Physiol. 2001;280:F10−18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- 297.Chao E.C., Henry R.R. SGLT2 inhibition-a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 298.Rahmoune H., Thompson P.W., Ward J.M., Smith C.D., Hong G., Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 299.Ehrenkranz J.R., Lewis N.G., Kahn C.R., Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–38. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 300.Oku A., Ueta K., Arakawa K., Ishihara T., Nawano M., Kuronuma Y., et al. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes. 1999;48:1794–1800. doi: 10.2337/diabetes.48.9.1794. [DOI] [PubMed] [Google Scholar]
- 301.Meng W., Ellsworth B.A., Nirschl A.A., McCann P.J., Patel M., Girotra R.N., et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2008;51:1145–1149. doi: 10.1021/jm701272q. [DOI] [PubMed] [Google Scholar]
- 302.Han S., Hagan D.L., Taylor J.R., Xin L., Meng W., Biller S.A., et al. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes. 2008;57:1723–1729. doi: 10.2337/db07-1472. [DOI] [PubMed] [Google Scholar]
- 303.Ferrannini E., Ramos S.J., Salsali A., Tang W., List J.F. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bailey C.J., Gross J.L., Pieters A., Bastien A., List J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 305.Rosenstock J., Vico M., Wei L., Salsali A., List J.F. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473–1478. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wilding J.P., Woo V., Soler N.G., Pahor A., Sugg J., Rohwedder K., et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 307.Nomura S., Sakamaki S., Hongu M., Kawanishi E., Koga Y., Sakamoto T., et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem. 2010;53:6355–6360. doi: 10.1021/jm100332n. [DOI] [PubMed] [Google Scholar]
- 308.Koga Y., Sakamaki S., Hongu M., Kawanishi E., Sakamoto T., Yamamoto Y., et al. C-Glucosides with heteroaryl thiophene as novel sodium-dependent glucose cotransporter 2 inhibitors. Bioorg Med Chem. 2013;21:5561–5572. doi: 10.1016/j.bmc.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 309.Sha S., Devineni D., Ghosh A., Polidori D., Chien S., Wexler D., et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metabol. 2011;13:669–672. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 310.Stenlöf K., Cefalu W.T., Kim K.A., Alba M., Usiskin K., Tong C., et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metabol. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Polidori D., Sha S., Mudaliar S., Ciaraldi T.P., Ghosh A., Vaccaro N., Farrell K., et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36:2154–2161. doi: 10.2337/dc12-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Cefalu W.T., Leiter L.A., Yoon K.H., Arias P., Niskanen L., Xie J., et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 313.Rosenstock J., Aggarwal N., Polidori D., Zhao Y., Arbit D., Usiskin K., et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–1238. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Elkinson S., Scott L.J. Canagliflozin: first global approval. Drugs. 2013;73:979–988. doi: 10.1007/s40265-013-0064-9. [DOI] [PubMed] [Google Scholar]
- 315.Grempler R., Thomas L., Eckhardt M., Himmelsbach F., Sauer A., Sharp D.E., et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metabol. 2012;14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 316.Roden M., Weng J., Eilbracht J., Delafont B., Kim G., Woerle H.J., et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 317.Ferrannini E., Berk A., Hantel S., Pinnetti S., Hach T., Woerle H.J., et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015–4021. doi: 10.2337/dc13-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nishimura R., Tanaka Y., Koiwai K., Inoue K., Hach T., Salsali A., et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11. doi: 10.1186/s12933-014-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tikkanen I., Narko K., Zeller C., Green A., Salsali A., Broedl U.C., et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 320.Häring H.U., Merker L., Seewaldt-Becker E., Weimer M., Meinicke T., Woerle H.J., et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–3404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rosenstock J., Jelaska A., Frappin G., Salsali A., Kim G., Woerle H.J., et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–1823. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 322.Ridderstråle M., Andersen K.R., Zeller C., Kim G., Woerle H.J., Broedl U.C. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 323.Imamura M., Nakanishi K., Suzuki T., Ikegai K., Shiraki R., Ogiyama T., et al. Discovery of ipragliflozin (ASP1941): a novel C-glucoside with benzothiophene structure as a potent and selective sodium glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes mellitus. Bioorg Med Chem. 2012;20:3263–3279. doi: 10.1016/j.bmc.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 324.Haider K., Pathak A., Rohilla A., Haider M.R., Ahmad K., Yar M.S. Synthetic strategy and SAR studies of C-glucoside heteroaryls as SGLT2 inhibitor: a review. Eur J Med Chem. 2019;184:111773. doi: 10.1016/j.ejmech.2019.111773. [DOI] [PubMed] [Google Scholar]
- 325.Kurosaki E., Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther. 2013;139:51–59. doi: 10.1016/j.pharmthera.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 326.Kashiwagi A., Takinami Y., Kazuta K., Yoshida S., Utsuno A., Nagase I. Ipragliflozin improved glycaemic control with additional benefits of reductions of body weight and blood pressure in Japanese patients with type 2 diabetes mellitus: BRIGHTEN study. Diabetologia. 2011;54:S68–S69. [Google Scholar]
- 327.Poole R.M., Dungo R.T. Ipragliflozin: first global approval. Drugs. 2014;74:611–617. doi: 10.1007/s40265-014-0204-x. [DOI] [PubMed] [Google Scholar]
- 328.Kakinuma H., Oi T., Hashimoto-Tsuchiya Y., Arai M., Kawakita Y., Fukasawa Y., et al. (1S)-1,5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-D-glucitol (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J Med Chem. 2010;53:3247–3261. doi: 10.1021/jm901893x. [DOI] [PubMed] [Google Scholar]
- 329.Yamamoto K., Uchida S., Kitano K., Fukuhara N., Okumura-Kitajima L., Gunji E., et al. TS-071 is a novel, potent and selective renal sodium-glucose cotransporter 2 (SGLT2) inhibitor with anti-hyperglycaemic activity. Br J Pharmacol. 2011;164:181–191. doi: 10.1111/j.1476-5381.2011.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Markham A., Elkinson S. Luseogliflozin: first global approval. Drugs. 2014;74:945–950. doi: 10.1007/s40265-014-0230-8. [DOI] [PubMed] [Google Scholar]
- 331.Seino Y., Sasaki T., Fukatsu A., Ubukata M., Sakai S., Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30:1245–1255. doi: 10.1185/03007995.2014.912983. [DOI] [PubMed] [Google Scholar]
- 332.Ohtake Y., Sato T., Kobayashi T., Nishimoto M., Taka N., Takano K., et al. Discovery of tofogliflozin, a novel C-arylglucoside with an O-spiroketal ring system, as a highly selective sodium glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2012;55:7828–7840. doi: 10.1021/jm300884k. [DOI] [PubMed] [Google Scholar]
- 333.Kaku K., Watada H., Iwamoto Y., Utsunomiya K., Terauchi Y., Tobe K., et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65. doi: 10.1186/1475-2840-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tanizawa Y., Kaku K., Araki E., Tobe K., Terauchi Y., Utsunomiya K., et al. Long-term safety and efficacy of tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open-label, randomized controlled trials. Expet Opin Pharmacother. 2014;15:749–766. doi: 10.1517/14656566.2014.887680. [DOI] [PubMed] [Google Scholar]
- 335.Mascitti V., Maurer T.S., Robinson R.P., Bian J., Boustany-Kari C.M., Brandt T., et al. Discovery of a clinical candidate from the structurally unique dioxa-bicyclo[3.2.1]octane class of sodium-dependent glucose cotransporter 2 inhibitors. J Med Chem. 2011;54:2952–2960. doi: 10.1021/jm200049r. [DOI] [PubMed] [Google Scholar]
- 336.Terra S.G., Focht K., Davies M., Frias J., Derosa G., Darekar A., et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metabol. 2017;19:721–728. doi: 10.1111/dom.12888. [DOI] [PubMed] [Google Scholar]
- 337.Chang P.Y., Heberer K., Lee J. Empagliflozin and HbA1c reduction in a national cohort. Diabetes. 2019;68(suppl_1):1208. [Google Scholar]
- 338.Rosenstock J., Frias J., Páll D., Charbonnel B., Pascu R., Saur D., et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET) Diabetes Obes Metabol. 2018;20:520–529. doi: 10.1111/dom.13103. [DOI] [PubMed] [Google Scholar]
- 339.Hollander P., Liu J., Hill J., Johnson J., Jiang Z.W., Golm G., et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: the VERTIS SU randomized study. Diabetes Ther. 2018;9:193–207. doi: 10.1007/s13300-017-0354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Dagogo-Jack S., Liu J., Eldor R., Amorin G., Johnson J., Hille D., et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metabol. 2018;20:530–540. doi: 10.1111/dom.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pratley R.E., Eldor R., Raji A., Golm G., Huyck S.B., Qiu Y., et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metabol. 2018;20:1111–1120. doi: 10.1111/dom.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Grunberger G., Camp S., Johnson J., Huyck S., Terra S.G., Mancuso J.P., et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. 2018;9:49–66. doi: 10.1007/s13300-017-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Markham A. Ertugliflozin: first global approval. Drugs. 2018;78:513–519. doi: 10.1007/s40265-018-0878-6. [DOI] [PubMed] [Google Scholar]
- 344.Markham A., Keam S.J. Sotagliflozin: first global approval. Drugs. 2019;79:1023–1029. doi: 10.1007/s40265-019-01146-5. [DOI] [PubMed] [Google Scholar]
- 345.Buse J.B., Garg S.K., Rosenstock J., Bailey T.S., Banks P., Bode B.W., et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 Study. Diabetes Care. 2018;41:1970–1980. doi: 10.2337/dc18-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Fujimori Y., Katsuno K., Nakashima I., Ishikawa-Takemura Y., Fujikura H., Isaji M. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Therapeut. 2008;327:268–276. doi: 10.1124/jpet.108.140210. [DOI] [PubMed] [Google Scholar]
- 347.Dharmalingam M., Aravind S.R., Thacker H., Paramesh S., Mohan B., Chawla M., et al. Efficacy and safety of remogliflozin etabonate, a new sodium glucose co-transporter-2 inhibitor, in patients with type 2 diabetes mellitus: a 24-week, randomized, double-blind, active-controlled trial. Drugs. 2020;80:587–600. doi: 10.1007/s40265-020-01285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Markham A. Remogliflozin etabonate: first global approval. Drugs. 2019;79:1157–1161. doi: 10.1007/s40265-019-01150-9. [DOI] [PubMed] [Google Scholar]
- 349.Eizirik D.L., Pasquali L., Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349–362. doi: 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- 350.Mather A., Pollock C. Renal glucose transporters: novel targets for hyperglycemia management. Nat Rev Nephrol. 2010;6:307–311. doi: 10.1038/nrneph.2010.38. [DOI] [PubMed] [Google Scholar]
- 351.Manoj A., Das S., Kunnath Ramachandran A., Alex A.T., Joseph A. SGLT2 inhibitors, an accomplished development in field of medicinal chemistry: an extensive review. Future Med Chem. 2020;12:1961–1990. doi: 10.4155/fmc-2020-0154. [DOI] [PubMed] [Google Scholar]
- 352.Dandona P., Mathieu C., Phillip M., Hansen L., Tschöpe D., Thorén F., et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care. 2018;41:2552–2559. doi: 10.2337/dc18-1087. [DOI] [PubMed] [Google Scholar]
- 353.Mathieu C., Dandona P., Gillard P., Senior P., Hasslacher C., Araki E., et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care. 2018;41:1938–1946. doi: 10.2337/dc18-0623. [DOI] [PubMed] [Google Scholar]
- 354.Rodbard H.W., Peters A.L., Slee A., Cao A., Traina S.B., Alba M. The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care. 2017;40:171–180. doi: 10.2337/dc16-1353. [DOI] [PubMed] [Google Scholar]
- 355.Peters A.L., Henry R.R., Thakkar P., Tong C., Alba M. Diabetic ketoacidosis with canagliflozin, a sodium-glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care. 2016;39:532–538. doi: 10.2337/dc15-1995. [DOI] [PubMed] [Google Scholar]
- 356.Henry R.R., Thakkar P., Tong C., Polidori D., Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38:2258–2265. doi: 10.2337/dc15-1730. [DOI] [PubMed] [Google Scholar]
- 357.Rosenstock J., Marquard J., Laffel L.M., Neubacher D., Kaspers S., Cherney D.Z., et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. 2018;41:2560–2569. doi: 10.2337/dc18-1749. [DOI] [PubMed] [Google Scholar]
- 358.Kaku K., Isaka H., Sakatani T., Toyoshima J. Efficacy and safety of ipragliflozin add-on therapy to insulin in Japanese patients with type 1 diabetes mellitus: a randomized, double-blind, phase 3 trial. Diabetes Obes Metabol. 2019;21:2284–2293. doi: 10.1111/dom.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Taylor S.I., Blau J.E., Rother K.I., Beitelshees A.L. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol. 2019;7:949–958. doi: 10.1016/S2213-8587(19)30154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Cowie M.R., Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 361.Marton A., Kaneko T., Kovalik J.P., Yasui A., Nishiyama A., Kitada K., et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat Rev Nephrol. 2021;17:65–77. doi: 10.1038/s41581-020-00350-x. [DOI] [PubMed] [Google Scholar]
- 362.Zelniker T.A., Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:422–434. doi: 10.1016/j.jacc.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 363.Lytvyn Y., Bjornstad P., Udell J.A., Lovshin J.A., Cherney D.Z.I. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 365.Guthrie R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. Postgrad Med. 2018;130:149–153. doi: 10.1080/00325481.2018.1423852. [DOI] [PubMed] [Google Scholar]
- 366.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 367.Perkovic V., Jardine M.J., Neal B., Bompoint S., Heerspink H.J.L., Charytan D.M., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 368.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Køber L., Kosiborod M.N., Martinez F.A., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 369.Cherney D.Z.I., Zinman B., Inzucchi S.E., Koitka-Weber A., Mattheus M., von Eynatten M., et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 370.Wanner C., Heerspink H.J.L., Zinman B., Inzucchi S.E., Koitka-Weber A., Mattheus M., et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol. 2018;29:2755–2769. doi: 10.1681/ASN.2018010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Mosenzon O., Wiviott S.D., Cahn A., Rozenberg A., Yanuv I., Goodrich E.L., et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 372.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Versteeg H.H., Heemskerk J.W., Levi M., Reitsma P.H. New fundamentals in hemostasis. Physiol Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 374.Furie B., Furie B.C. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 375.Franchini M., Mannucci P.M. Venous and arterial thrombosis: different sides of the same coin?. Eur J Intern Med. 2008;19:476–481. doi: 10.1016/j.ejim.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 376.Gallone G., Baldetti L., Pagnesi M., Latib A., Colombo A., Libby P., et al. Medical therapy for long-term prevention of atherothrombosis following an acute coronary syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2886–2903. doi: 10.1016/j.jacc.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 377.Campbell B.C.V., Khatri P. Stroke. Lancet. 2020;396:129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 378.Jackson S.P. Arterial thrombosis–insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 379.Silvis S.M., de Sousa D.A., Ferro J.M., Coutinho J.M. Cerebral venous thrombosis. Nat Rev Neurol. 2017;13:555–565. doi: 10.1038/nrneurol.2017.104. [DOI] [PubMed] [Google Scholar]
- 380.Wells P.S., Ihaddadene R., Reilly A., Forgie M.A. Diagnosis of venous thromboembolism: 20 years of progress. Ann Intern Med. 2018;168:131–140. doi: 10.7326/M17-0291. [DOI] [PubMed] [Google Scholar]
- 381.Jerjes-Sanchez C. Venous and arterial thrombosis: a continuous spectrum of the same disease?. Eur Heart J. 2005;26:3–4. doi: 10.1093/eurheartj/ehi041. [DOI] [PubMed] [Google Scholar]
- 382.Mclean J. The thromboplastic action of cephalin. Am J Physiol. 1916;41:250–257. [Google Scholar]
- 383.Bromfield S.M., Wilde E., Smith D.K. Heparin sensing and binding-taking supramolecular chemistry towards clinical applications. Chem Soc Rev. 2013;42:9184–9195. doi: 10.1039/c3cs60278h. [DOI] [PubMed] [Google Scholar]
- 384.Baytas S.N., Linhardt R.J. Advances in the preparation and synthesis of heparin and related products. Drug Discov Today. 2020;25:2095–2109. doi: 10.1016/j.drudis.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Lever R., Page C.P. Novel drug development opportunities for heparin. Nat Rev Drug Discov. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- 386.Tsai C.T., Zulueta M.M.L., Hung S.C. Synthetic heparin and heparan sulfate: probes in defining biological functions. Curr Opin Chem Biol. 2017;40:152–159. doi: 10.1016/j.cbpa.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 387.Dorsam R.T., Kunapuli S.P. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113:340–345. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Hollopeter G., Jantzen H.M., Vincent D., Li G., England L., Ramakrishnan V., et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 389.Baqi Y., Müller C.E. Antithrombotic P2Y(12) receptor antagonists: recent developments in drug discovery. Drug Discov Today. 2019;24:325–333. doi: 10.1016/j.drudis.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 390.Liu H., Zhang Z., Linhardt R.J. Lessons learned from the contamination of heparin. Nat Prod Rep. 2009;26:313–321. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Choay J., Petitou M., Lormeau J.C., Sinaÿ P., Casu B., Gatti G. Structure–activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983;116:492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- 392.Lindahl U., Bäckström G., Thunberg L., Leder I.G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci U S A. 1980;77:6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Linhardt R.J. 2003 Claude S. Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J Med Chem. 2003;46:2551–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 394.Friedel H.A., Balfour J.A. Tinzaparin. A review of its pharmacology and clinical potential in the prevention and treatment of thromboembolic disorders. Drugs. 1994;48:638–660. doi: 10.2165/00003495-199448040-00010. [DOI] [PubMed] [Google Scholar]
- 395.Hoy S.M., Scott L.J., Plosker G.L. Tinzaparin sodium: a review of its use in the prevention and treatment of deep vein thrombosis and pulmonary embolism, and in the prevention of clotting in the extracorporeal circuit during haemodialysis. Drugs. 2010;70:1319–1347. doi: 10.2165/11203710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 396.Sobel M., Adelman B. Characterization of platelet binding of heparins and other glycosaminoglycans. Thromb Res. 1988;50:815–826. doi: 10.1016/0049-3848(88)90341-6. [DOI] [PubMed] [Google Scholar]
- 397.Young E., Wells P., Holloway S., Weitz J., Hirsh J. Ex-vivo and in-vitro evidence that low molecular weight heparins exhibit less binding to plasma proteins than unfractionated heparin. Thromb Haemostasis. 1994;71:300–304. [PubMed] [Google Scholar]
- 398.Koutsikos D., Fourtounas C., Kapetanaki A., Dalamanga A., Tzanatos H., Agroyannis B., et al. A cross-over study of a new low molecular weight heparin (Logiparin) in hemodialysis. Int J Artif Organs. 1996;19:467–471. [PubMed] [Google Scholar]
- 399.Sabry A., Taha M., Nada M., Al Fawzan F., Alsaran K. Anticoagulation therapy during haemodialysis: a comparative study between two heparin regimens. Blood Coagul Fibrinolysis. 2009;20:57–62. doi: 10.1097/MBC.0b013e32831bec0f. [DOI] [PubMed] [Google Scholar]
- 400.Christidou F.N., Frangia T.K., Bamichas G.I., Gionanlis L.C., Natse T.A., Georgoulis I.E., et al. Comparison of two low-molecular weight heparins (LMWHs), tinzaparin and bemiparin, during hemodialysis. Int J Clin Pharm Ther. 2005;43:335–338. doi: 10.5414/cpp43335. [DOI] [PubMed] [Google Scholar]
- 401.Bara L., Planes A., Samama M.M. Occurrence of thrombosis and haemorrhage, relationship with anti-Xa, anti-IIa activities, and d-dimer plasma levels in patients receiving a low molecular weight heparin, enoxaparin or tinzaparin, to prevent deep vein thrombosis after hip surgery. Br J Haematol. 1999;104:230–240. doi: 10.1046/j.1365-2141.1999.01153.x. [DOI] [PubMed] [Google Scholar]
- 402.Fossler M.J., Barrett J.S., Hainer J.W., Riddle J.G., Ostergaard P., van der Elst E., et al. Pharmacodynamics of intravenous and subcutaneous tinzaparin and heparin in healthy volunteers. Am J Health Syst Pharm. 2001;58:1614–1621. doi: 10.1093/ajhp/58.17.1614. [DOI] [PubMed] [Google Scholar]
- 403.Mätzsch T., Bergqvist D., Hedner U., Ostergaard P. Effects of an enzymatically depolymerized heparin as compared with conventional heparin in healthy volunteers. Thromb Haemostasis. 1987;57:97–101. [PubMed] [Google Scholar]
- 404.Planès A., Samama M.M., Lensing A.W., Büller H.R., Barre J., Vochelle N., et al. Prevention of deep vein thrombosis after hip replacement–comparison between two low-molecular heparins, tinzaparin and enoxaparin. Thromb Haemostasis. 1999;81:22–25. [PubMed] [Google Scholar]
- 405.Green D., Lee M.Y., Lim A.C., Chmiel J.S., Vetter M., Pang T., et al. Prevention of thromboembolism after spinal cord injury using low-molecular-weight heparin. Ann Intern Med. 1990;113:571–574. doi: 10.7326/0003-4819-113-8-571. [DOI] [PubMed] [Google Scholar]
- 406.Simonneau G., Sors H., Charbonnier B., Page Y., Laaban J.P., Azarian R., et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. N Engl J Med. 1997;337:663–669. doi: 10.1056/NEJM199709043371002. [DOI] [PubMed] [Google Scholar]
- 407.Hull R.D., Raskob G.E., Pineo G.F., Green D., Trowbridge A.A., Elliott C.G., et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med. 1992;326:975–982. doi: 10.1056/NEJM199204093261502. [DOI] [PubMed] [Google Scholar]
- 408.Petitou M., Duchaussoy P., Herbert J.M., Duc G., El Hajji M., Branellec J.F., et al. The synthetic pentasaccharide fondaparinux: first in the class of antithrombotic agents that selectively inhibit coagulation factor Xa. Semin Thromb Hemost. 2002;28:393–402. doi: 10.1055/s-2002-34309. [DOI] [PubMed] [Google Scholar]
- 409.Lormeau J.C., Herault J.P., Gaich C., Barzu T., van Dinther T.G., Visser A., et al. Determination of the anti-factor Xa activity of the synthetic pentasaccharide SR 90107A/ORG 31540 and of two structural analogues. Thromb Res. 1997;85:67–75. doi: 10.1016/s0049-3848(96)00223-x. [DOI] [PubMed] [Google Scholar]
- 410.Petitou M., Duchaussoy P., Jaurand G., Gourvenec F., Lederman I., Strassel J.M., et al. Synthesis and pharmacological properties of a close analogue of an antithrombotic pentasaccharide (SR 90107A/ORG 31540) J Med Chem. 1997;40:1600–1607. doi: 10.1021/jm960726z. [DOI] [PubMed] [Google Scholar]
- 411.Herbert J.M., Hérault J.P., Bernat A., van Amsterdam R.G., Lormeau J.C., Petitou M., et al. Biochemical and pharmacological properties of SANORG 34006, a potent and long-acting synthetic pentasaccharide. Blood. 1998;91:4197–4205. [PubMed] [Google Scholar]
- 412.Keam S.J., Goa K.L. Fondaparinux sodium. Drugs. 2002;62:1673–1685. doi: 10.2165/00003495-200262110-00007. [DOI] [PubMed] [Google Scholar]
- 413.Boneu B., Necciari J., Cariou R., Sié P., Gabaig A.M., Kieffer G., et al. Pharmacokinetics and tolerance of the natural pentasaccharide (SR90107/Org31540) with high affinity to antithrombin III in man. Thromb Haemostasis. 1995;74:1468–1473. [PubMed] [Google Scholar]
- 414.Carrie D., Caranobe C., Saivin S., Houin G., Petitou M., Lormeau J.C., et al. Pharmacokinetic and antithrombotic properties of two pentasaccharides with high affinity to antithrombin III in the rabbit: comparison with CY216. Blood. 1994;84:2571–2577. [PubMed] [Google Scholar]
- 415.Bauer K.A., Eriksson B.I., Lassen M.R., Turpie A.G. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345:1305–1310. doi: 10.1056/NEJMoa011099. [DOI] [PubMed] [Google Scholar]
- 416.Eriksson B.I., Bauer K.A., Lassen M.R., Turpie A.G. Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001;345:1298–1304. doi: 10.1056/NEJMoa011100. [DOI] [PubMed] [Google Scholar]
- 417.Gao Y., Yu C., Pi S., Mao L., Hu B. The role of P2Y(12) receptor in ischemic stroke of atherosclerotic origin. Cell Mol Life Sci. 2019;76:341–354. doi: 10.1007/s00018-018-2937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Franchi F., Angiolillo D.J. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 419.Springthorpe B., Bailey A., Barton P., Birkinshaw T.N., Bonnert R.V., Brown R.C., et al. From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett. 2007;17:6013–6018. doi: 10.1016/j.bmcl.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 420.Husted S., van Giezen J.J. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27:259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Deeks E.D. Ticagrelor: a review of its use in the management of acute coronary syndromes. Drugs. 2011;71:909–933. doi: 10.2165/11206850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 422.Gurbel P.A., Bliden K.P., Butler K., Tantry U.S., Gesheff T., Wei C., et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 423.Storey R.F., Angiolillo D.J., Patil S.B., Desai B., Ecob R., Husted S., et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient outcomes) PLATELET substudy. J Am Coll Cardiol. 2010;56:1456–1462. doi: 10.1016/j.jacc.2010.03.100. [DOI] [PubMed] [Google Scholar]
- 424.Alexopoulos D., Galati A., Xanthopoulou I., Mavronasiou E., Kassimis G., Theodoropoulos K.C., et al. Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: a pharmacodynamic study. J Am Coll Cardiol. 2012;60:193–199. doi: 10.1016/j.jacc.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 425.Al-Salama Z.T., Keating G.M., Keam S.J. Ticagrelor: a review in long term secondary prevention of cardiovascular events. Drugs. 2017;77:2025–2036. doi: 10.1007/s40265-017-0844-8. [DOI] [PubMed] [Google Scholar]
- 426.Armstrong D., Summers C., Ewart L., Nylander S., Sidaway J.E., van Giezen J.J. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Therapeut. 2014;19:209–219. doi: 10.1177/1074248413511693. [DOI] [PubMed] [Google Scholar]
- 427.Storey R.F., Becker R.C., Harrington R.A., Husted S., James S.K., Cools F., et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011;32:2945–2953. doi: 10.1093/eurheartj/ehr231. [DOI] [PubMed] [Google Scholar]
- 428.Ingall A.H., Dixon J., Bailey A., Coombs M.E., Cox D., McInally J.I., et al. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 429.Bhatt D.L., Stone G.W., Mahaffey K.W., Gibson C.M., Steg P.G., Hamm C.W., et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 430.Généreux P., Stone G.W., Harrington R.A., Gibson C.M., Steg P.G., Brener S.J., et al. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: insights from the CHAMPION PHOENIX trial (clinical trial comparing cangrelor to clopidogrel standard of care therapy in subjects who require percutaneous coronary intervention) J Am Coll Cardiol. 2014;63:619–629. doi: 10.1016/j.jacc.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 431.Qamar A., Bhatt D.L. Current status of data on cangrelor. Pharmacol Ther. 2016;159:102–109. doi: 10.1016/j.pharmthera.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 432.Viceconti M., Hunter P. The virtual physiological human: ten years after. Annu Rev Biomed Eng. 2016;18:103–123. doi: 10.1146/annurev-bioeng-110915-114742. [DOI] [PubMed] [Google Scholar]
- 433.The Alzheimer’s Association 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16:391–460. [Google Scholar]
- 434.Busche M.A., Hyman B.T. Synergy between amyloid-β and tau in Alzheimer's disease. Nat Neurosci. 2020;23:1183–1193. doi: 10.1038/s41593-020-0687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Wang J., Gu B.J., Masters C.L., Wang Y.J. A systemic view of Alzheimer disease–insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol. 2017;13:612–623. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 436.Wang X., Sun G., Feng T., Zhang J., Huang X., Wang T., et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res. 2019;29:787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Dong Y., Li X., Cheng J., Hou L. Drug development for Alzheimer's disease: microglia induced neuroinflammation as a target?. Int J Mol Sci. 2019;20:558. doi: 10.3390/ijms20030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Cryan J.F., O'Riordan K.J., Sandhu K., Peterson V., Dinan T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 439.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimers Dement (NY) 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Kodamullil A.T., Zekri F., Sood M., Hengerer B., Canard L., McHale D., et al. Trial watch: tracing investment in drug development for Alzheimer disease. Nat Rev Drug Discov. 2017;16:819. doi: 10.1038/nrd.2017.169. [DOI] [PubMed] [Google Scholar]
- 441.Panza F., Lozupone M., Logroscino G., Imbimbo B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73–88. doi: 10.1038/s41582-018-0116-6. [DOI] [PubMed] [Google Scholar]
- 442.Syed Y.Y. Sodium oligomannate: first approval. Drugs. 2020;80:441–444. doi: 10.1007/s40265-020-01268-1. [DOI] [PubMed] [Google Scholar]
- 443.Xiao S.F. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer disease. Chin J Pharmacol Toxicol. 2019;6:403. doi: 10.1186/s13195-021-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Bowman W.C. Neuromuscular block. Br J Pharmacol. 2006;147(Suppl 1):S277−286. doi: 10.1038/sj.bjp.0706404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Kovac A.L. Sugammadex: the first selective binding reversal agent for neuromuscular block. J Clin Anesth. 2009;21:444–453. doi: 10.1016/j.jclinane.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 446.Adam J.M., Bennett D.J., Bom A., Clark J.K., Feilden H., Hutchinson E.J., et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure–activity relationships. J Med Chem. 2002;45:1806–1816. doi: 10.1021/jm011107f. [DOI] [PubMed] [Google Scholar]
- 447.Davis M.E., Brewster M.E. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 448.Keating G.M. Sugammadex: a review of neuromuscular blockade reversal. Drugs. 2016;76:1041–1052. doi: 10.1007/s40265-016-0604-1. [DOI] [PubMed] [Google Scholar]
- 449.Blobner M., Eriksson L.I., Scholz J., Motsch J., Della Rocca G., Prins M.E. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol. 2010;27:874–881. doi: 10.1097/EJA.0b013e32833d56b7. [DOI] [PubMed] [Google Scholar]
- 450.Khuenl-Brady K.S., Wattwil M., Vanacker B.F., Lora-Tamayo J.I., Rietbergen H., Alvarez-Gómez J.A. Sugammadex provides faster reversal of vecuronium-induced neuromuscular blockade compared with neostigmine: a multicenter, randomized, controlled trial. Anesth Analg. 2010;110:64–73. doi: 10.1213/ane.0b013e3181ac53c3. [DOI] [PubMed] [Google Scholar]
- 451.Jones R.K., Caldwell J.E., Brull S.J., Soto R.G. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008;109:816–824. doi: 10.1097/ALN.0b013e31818a3fee. [DOI] [PubMed] [Google Scholar]
- 452.Carracedo G., Crooke A., Guzman-Aranguez A., Pérez de Lara M.J., Martin-Gil A., Pintor J. The role of dinucleoside polyphosphates on the ocular surface and other eye structures. Prog Retin Eye Res. 2016;55:182–205. doi: 10.1016/j.preteyeres.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 453.Pendergast W., Yerxa B.R., Douglass J.G., 3rd, Shaver S.R., Dougherty R.W., Redick C.C., et al. Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5′-polyphosphates. Bioorg Med Chem Lett. 2001;11:157–160. doi: 10.1016/s0960-894x(00)00612-0. [DOI] [PubMed] [Google Scholar]
- 454.Cowlen M.S., Zhang V.Z., Warnock L., Moyer C.F., Peterson W.M., Yerxa B.R. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res. 2003;77:77–84. doi: 10.1016/s0014-4835(03)00068-x. [DOI] [PubMed] [Google Scholar]
- 455.Jumblatt J.E., Jumblatt M.M. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp Eye Res. 1998;67:341–346. doi: 10.1006/exer.1998.0520. [DOI] [PubMed] [Google Scholar]
- 456.Fujihara T., Murakami T., Fujita H., Nakamura M., Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001;42:96–100. [PubMed] [Google Scholar]
- 457.Li Y., Kuang K., Yerxa B., Wen Q., Rosskothen H., Fischbarg J. Rabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor agonists stimulate Cl– and fluid secretion. Am J Physiol Cell Physiol. 2001;281:C595−602. doi: 10.1152/ajpcell.2001.281.2.C595. [DOI] [PubMed] [Google Scholar]
- 458.Kojima T., Dogru M., Ibrahim O.M., Nagata T., Higa K., Shimizu T., et al. The effects of 3% diquafosol sodium application on the tear functions and ocular surface of the Cu,Zn-superoxide dismutase-1 (Sod1)-knockout mice. Mol Vis. 2014;20:929–938. [PMC free article] [PubMed] [Google Scholar]
- 459.Keating G.M. Diquafosol ophthalmic solution 3%: a review of its use in dry eye. Drugs. 2015;75:911–922. doi: 10.1007/s40265-015-0409-7. [DOI] [PubMed] [Google Scholar]
- 460.Matsumoto Y., Ohashi Y., Watanabe H., Tsubota K. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology. 2012;119:1954–1960. doi: 10.1016/j.ophtha.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 461.Takamura E., Tsubota K., Watanabe H., Ohashi Y. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012;96:1310–1315. doi: 10.1136/bjophthalmol-2011-301448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Watanabe H. Medical treatment for dry eye in Japan. Invest Ophthalmol Vis Sci. 2018;59:Des116–D120. doi: 10.1167/iovs.18-24130. [DOI] [PubMed] [Google Scholar]
- 463.Livesey G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev. 2003;16:163–191. doi: 10.1079/NRR200371. [DOI] [PubMed] [Google Scholar]
- 464.Miller L.E., Tennilä J., Ouwehand A.C. Efficacy and tolerance of lactitol supplementation for adult constipation: a systematic review and meta-analysis. Clin Exp Gastroenterol. 2014;7:241–248. doi: 10.2147/CEG.S58952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Prasad V.G., Abraham P. Management of chronic constipation in patients with diabetes mellitus. Indian J Gastroenterol. 2017;36:11–22. doi: 10.1007/s12664-016-0724-2. [DOI] [PubMed] [Google Scholar]
- 466.Mueller-Lissner S.A., Wald A. Constipation in adults. Clin Evid. 2010;2007:904–905. [PMC free article] [PubMed] [Google Scholar]
- 467.Food and Drug Administration . 2020. Advancing health through innovation: new drug therapy approvals.https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 Available from: [Google Scholar]
- 468.Wang L., Yang R., Yuan B., Liu Y., Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B. 2015;5:310–315. doi: 10.1016/j.apsb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Xie C., Li X., Wu J., Liang Z., Deng F., Xie W., et al. Anti-inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/arachidonic acid pathway. Inflammation. 2015;38:1639–1648. doi: 10.1007/s10753-015-0140-2. [DOI] [PubMed] [Google Scholar]
- 470.Cheng Y., Zhang J., Shang J., Zhang L. Prevention of free fatty acid-induced hepatic lipotoxicity in HepG2 cells by magnesium isoglycyrrhizinate in vitro. Pharmacology. 2009;84:183–190. doi: 10.1159/000235873. [DOI] [PubMed] [Google Scholar]
- 471.He Y., Zeng F., Liu Q., Ju W., Fu H., Hao H., et al. Protective effect of magnesium isoglycyrrhizinate on ethanol-induced testicular injuries in mice. J Biomed Res. 2010;24:153–160. doi: 10.1016/S1674-8301(10)60024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Mao Y.M., Zeng M.D., Chen Y., Chen C.W., Fu Q.C., Cai X., et al. Magnesium isoglycyrrhizinate in the treatment of chronic liver diseases: a randomized, double-blind, multi-doses, active drug controlled, multi-center study. Chin J Hepatol. 2009;17:847–851. [PubMed] [Google Scholar]
- 473.Song J.W., Xing R. Pharmacological effects and clinical applications of magnesium isoglycyrrhizinate. Chin J New Drugs Clin Remedies. 2012;31:578–582. [Google Scholar]
- 474.Spagnolo P., du Bois R.M., Cottin V. Rare lung disease and orphan drug development. Lancet Respir Med. 2013;1:479–487. doi: 10.1016/S2213-2600(13)70085-7. [DOI] [PubMed] [Google Scholar]
- 475.Tambuyzer E. Rare diseases, orphan drugs and their regulation: questions and misconceptions. Nat Rev Drug Discov. 2010;9:921–929. doi: 10.1038/nrd3275. [DOI] [PubMed] [Google Scholar]
- 476.Butters T.D., Dwek R.A., Platt F.M. Inhibition of glycosphingolipid biosynthesis: application to lysosomal storage disorders. Chem Rev. 2000;100:4683–4696. doi: 10.1021/cr990292q. [DOI] [PubMed] [Google Scholar]
- 477.Platt F.M., Neises G.R., Dwek R.A., Butters T.D. N-Butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J Biol Chem. 1994;269:8362–8365. [PubMed] [Google Scholar]
- 478.Jeyakumar M., Butters T.D., Cortina-Borja M., Hunnam V., Proia R.L., Perry V.H., et al. Delayed symptom onset and increased life expectancy in Sandhoff disease mice treated with N-butyldeoxynojirimycin. Proc Natl Acad Sci U S A. 1999;96:6388–6393. doi: 10.1073/pnas.96.11.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Elstein D., Dweck A., Attias D., Hadas-Halpern I., Zevin S., Altarescu G., et al. Oral maintenance clinical trial with miglustat for type I Gaucher disease: switch from or combination with intravenous enzyme replacement. Blood. 2007;110:2296–2301. doi: 10.1182/blood-2007-02-075960. [DOI] [PubMed] [Google Scholar]
- 480.Schiffmann R., Fitzgibbon E.J., Harris C., DeVile C., Davies E.H., Abel L., et al. Randomized, controlled trial of miglustat in Gaucher's disease type 3. Ann Neurol. 2008;64:514–522. doi: 10.1002/ana.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.McCormack P.L., Goa K.L. Miglustat. Drugs. 2003;63:2427–2434. doi: 10.2165/00003495-200363220-00006. [DOI] [PubMed] [Google Scholar]
- 482.Lyseng-Williamson K.A. Miglustat: a review of its use in Niemann-Pick disease type C. Drugs. 2014;74:61–74. doi: 10.1007/s40265-013-0164-6. [DOI] [PubMed] [Google Scholar]
- 483.Patterson M.C., Vecchio D., Prady H., Abel L., Wraith J.E. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6:765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 484.Wraith J.E., Vecchio D., Jacklin E., Abel L., Chadha-Boreham H., Luzy C., et al. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metabol. 2010;99:351–357. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 485.Karjalainen O.K., Passiniemi M., Koskinen A.M. Short and straightforward synthesis of (–)-1-deoxygalactonojirimycin. Org Lett. 2010;12:1145–1147. doi: 10.1021/ol100037c. [DOI] [PubMed] [Google Scholar]
- 486.Gaggl M., Sunder-Plassmann G. Fabry disease: a pharmacological chaperone on the horizon. Nat Rev Nephrol. 2016;12:653–654. doi: 10.1038/nrneph.2016.138. [DOI] [PubMed] [Google Scholar]
- 487.Khanna R., Soska R., Lun Y., Feng J., Frascella M., Young B., et al. The pharmacological chaperone 1-deoxygalactonojirimycin reduces tissue globotriaosylceramide levels in a mouse model of Fabry disease. Mol Ther. 2010;18:23–33. doi: 10.1038/mt.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Germain D.P., Hughes D.A., Nicholls K., Bichet D.G., Giugliani R., Wilcox W.R., et al. Treatment of Fabry's disease with the pharmacologic chaperone migalastat. N Engl J Med. 2016;375:545–555. doi: 10.1056/NEJMoa1510198. [DOI] [PubMed] [Google Scholar]
- 489.Markham A. Migalastat: first global approval. Drugs. 2016;76:1147–1152. doi: 10.1007/s40265-016-0607-y. [DOI] [PubMed] [Google Scholar]
- 490.Ramesh D., Vijayakumar B.G., Kannan T. Therapeutic potential of uracil and its derivatives in countering pathogenic and physiological disorders. Eur J Med Chem. 2020;207:112801. doi: 10.1016/j.ejmech.2020.112801. [DOI] [PubMed] [Google Scholar]
- 491.Cada D.J., Mbogu U., Bindler R.J., Baker D.E. Uridine triacetate. Hosp Pharm. 2016;51:484–488. doi: 10.1310/hpj5106-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Ison G., Beaver J.A., McGuinn W.D., Jr., Palmby T.R., Dinin J., Charlab R., et al. FDA approval: uridine triacetate for the treatment of patients following fluorouracil or capecitabine overdose or exhibiting early-onset severe toxicities following administration of these drugs. Clin Cancer Res. 2016;22:4545–4549. doi: 10.1158/1078-0432.CCR-16-0638. [DOI] [PubMed] [Google Scholar]
- 493.Bourque J.M., Beller G.A. Stress myocardial perfusion imaging for assessing prognosis: an update. JACC Cardiovasc Imag. 2011;4:1305–1319. doi: 10.1016/j.jcmg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 494.Al Jaroudi W., Iskandrian A.E. Regadenoson: a new myocardial stress agent. J Am Coll Cardiol. 2009;54:1123–1130. doi: 10.1016/j.jacc.2009.04.089. [DOI] [PubMed] [Google Scholar]
- 495.Palle V.P., Elzein E.O., Gothe S.A., Li Z., Gao Z., Meyer S., et al. Structure–affinity relationships of the affinity of 2-pyrazolyl adenosine analogues for the adenosine A2A receptor. Bioorg Med Chem Lett. 2002;12:2935–2939. doi: 10.1016/s0960-894x(02)00609-1. [DOI] [PubMed] [Google Scholar]
- 496.Gao Z., Li Z., Baker S.P., Lasley R.D., Meyer S., Elzein E., et al. Novel short-acting A2A adenosine receptor agonists for coronary vasodilation: inverse relationship between affinity and duration of action of A2A agonists. J Pharmacol Exp Therapeut. 2001;298:209–218. [PubMed] [Google Scholar]
- 497.Hendel R.C., Bateman T.M., Cerqueira M.D., Iskandrian A.E., Leppo J.A., Blackburn B., et al. Initial clinical experience with regadenoson, a novel selective A2A agonist for pharmacologic stress single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol. 2005;46:2069–2075. doi: 10.1016/j.jacc.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 498.Cerqueira M.D., Nguyen P., Staehr P., Underwood S.R., Iskandrian A.E. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging integrated ADVANCE-MPI trial results. JACC Cardiovasc Imag. 2008;1:307–316. doi: 10.1016/j.jcmg.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 499.Gershenwald J.E., Ross M.I. Sentinel-lymph-node biopsy for cutaneous melanoma. N Engl J Med. 2011;364:1738–1745. doi: 10.1056/NEJMct1002967. [DOI] [PubMed] [Google Scholar]
- 500.Wong S.L., Balch C.M., Hurley P., Agarwala S.S., Akhurst T.J., Cochran A., et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol. 2012;30:2912–2918. doi: 10.1200/JCO.2011.40.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Ahmed M., Purushotham A.D., Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014;15 doi: 10.1016/S1470-2045(13)70590-4. e351−62. [DOI] [PubMed] [Google Scholar]
- 502.Eckelman W.C., Jones A.G., Duatti A., Reba R.C. Progress using Tc-99m radiopharmaceuticals for measuring high capacity sites and low density sites. Drug Discov Today. 2013;18:984–991. doi: 10.1016/j.drudis.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 503.Vera D.R., Wallace A.M., Hoh C.K., Mattrey R.F. A synthetic macromolecule for sentinel node detection: 99mTc-DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–959. [PubMed] [Google Scholar]
- 504.Emerson D.K., Limmer K.K., Hall D.J., Han S.H., Eckelman W.C., Kane C.J., et al. A receptor-targeted fluorescent radiopharmaceutical for multireporter sentinel lymph node imaging. Radiology. 2012;265:186–193. doi: 10.1148/radiol.12120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Azad A.K., Rajaram M.V., Metz W.L., Cope F.O., Blue M.S., Vera D.R., et al. γ-Tilmanocept, a new radiopharmaceutical tracer for cancer sentinel lymph nodes, binds to the mannose receptor (CD206) J Immunol. 2015;195:2019–2029. doi: 10.4049/jimmunol.1402005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Méndez J., Wallace A.M., Hoh C.K., Vera D.R. Detection of gastric and colonic sentinel nodes through endoscopic administration of 99mTc-DTPA-mannosyl-dextran in pigs. J Nucl Med. 2003;44:1677–1681. [PubMed] [Google Scholar]
- 507.Ellner S.J., Méndez J., Vera D.R., Hoh C.K., Ashburn W.L., Wallace A.M. Sentinel lymph node mapping of the colon and stomach using lymphoseek in a pig model. Ann Surg Oncol. 2004;11:674–681. doi: 10.1245/ASO.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 508.Wallace A.M., Han L.K., Povoski S.P., Deck K., Schneebaum S., Hall N.C., et al. Comparative evaluation of [99mTc]tilmanocept for sentinel lymph node mapping in breast cancer patients: results of two phase 3 trials. Ann Surg Oncol. 2013;20:2590–2599. doi: 10.1245/s10434-013-2887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sondak V.K., King D.W., Zager J.S., Schneebaum S., Kim J., Leong S.P., et al. Combined analysis of phase III trials evaluating [99mTc]tilmanocept and vital blue dye for identification of sentinel lymph nodes in clinically node-negative cutaneous melanoma. Ann Surg Oncol. 2013;20:680–688. doi: 10.1245/s10434-012-2612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., et al. Therapeutic siRNA: state of the art. Signal Transduct Targeted Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Scott L.J. Givosiran: first approval. Drugs. 2020;80:335–339. doi: 10.1007/s40265-020-01269-0. [DOI] [PubMed] [Google Scholar]
- 512.Balwani M., Sardh E., Ventura P., Peiró P.A., Rees D.C., Stölzel U., et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382:2289–2301. doi: 10.1056/NEJMoa1913147. [DOI] [PubMed] [Google Scholar]
- 513.Khatun F., Toth I., Stephenson R.J. Immunology of carbohydrate-based vaccines. Adv Drug Deliv Rev. 2020;165−166:117–126. doi: 10.1016/j.addr.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 514.Sorieul C., Papi F., Carboni F., Pecetta S., Phogat S., Adamo R. Recent advances and future perspectives on carbohydrate-based cancer vaccines and therapeutics. Pharmacol Ther. 2022;235:108158. doi: 10.1016/j.pharmthera.2022.108158. [DOI] [PubMed] [Google Scholar]
- 515.Pan L., Cai C., Liu C., Liu D., Li G., Linhardt R.J., et al. Recent progress and advanced technology in carbohydrate-based drug development. Curr Opin Biotechnol. 2021;69:191–198. doi: 10.1016/j.copbio.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 516.Oliveira G.S., Oliveira M.L.S., Miyaji E.N., Rodrigues T.C. Pneumococcal vaccines: past findings, present work, and future strategies. Vaccines. 2021;9:1338. doi: 10.3390/vaccines9111338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Andrews J.R., Baker S., Marks F., Alsan M., Garrett D., Gellin B.G., et al. Typhoid conjugate vaccines: a new tool in the fight against antimicrobial resistance. Lancet Infect Dis. 2019;19 doi: 10.1016/S1473-3099(18)30350-5. [DOI] [PubMed] [Google Scholar]
- 518.Gayet R., Bioley G., Rochereau N., Paul S., Corthésy B. Vaccination against Salmonella infection: the mucosal way. Microbiol Mol Biol Rev. 2017;81:e00007–e17. doi: 10.1128/MMBR.00007-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Rudd P.M., Elliott T., Cresswell P., Wilson I.A., Dwek R.A. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 520.Wei M.M., Wang Y.S., Ye X.S. Carbohydrate-based vaccines for oncotherapy. Med Res Rev. 2018;38:1003–1026. doi: 10.1002/med.21493. [DOI] [PubMed] [Google Scholar]
- 521.Yamada I., Shiota M., Shinmachi D., Ono T., Tsuchiya S., Hosoda M., et al. The GlyCosmos portal: a unified and comprehensive web resource for the glycosciences. Nat Methods. 2020;17:649–650. doi: 10.1038/s41592-020-0879-8. [DOI] [PubMed] [Google Scholar]
- 522.West C.M., Malzl D., Hykollari A., Wilson I.B.H. Glycomics, glycoproteomics, and glycogenomics: an inter-taxa evolutionary perspective. Mol Cell Proteomics. 2021;20:100024. doi: 10.1074/mcp.R120.002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.