Abstract
Creatine is an organic compound which is utilized in biological activities, especially for adenosine triphosphate (ATP) production in the phosphocreatine system. This is a well-known biochemical reaction that is generally recognized as being mainly driven in specific parts of the body, such as the skeletal muscle and brain. However, our report shows a novel aspect of creatine utilization and ATP synthesis in innate immune cells. Creatine supplementation enhanced immune responses in neutrophils, such as cytokine production, reactive oxygen species (ROS) production, phagocytosis, and NETosis, which were characterized as antibacterial activities. This creatine-induced functional upregulation of neutrophils provided a protective effect in a murine bacterial sepsis model. The mortality rate in mice challenged with Escherichia coli K-12 was decreased by creatine supplementation compared with the control treatment. Corresponding to this decrease in mortality, we found that creatine supplementation decreased blood pro-inflammatory cytokine levels and bacterial colonization in organs. Creatine supplementation significantly increased the cellular ATP level in neutrophils compared with the control treatment. This ATP increase was due to the phosphocreatine system in the creatine-treated neutrophils. In addition, extracellular creatine was used in this ATP synthesis, as inhibition of creatine uptake abolished the increase in ATP in the creatine-treated neutrophils. Thus, creatine is an effective nutrient for modifying the immunological function of neutrophils, which contributes to enhancement of antibacterial immunity.
Keywords: creatine, neutrophils, adenosine triphosphate (ATP), antibacterial immunity, reactive oxygen species (ROS), neutrophil extracellular traps (NETs), sepsis
INTRODUCTION
Creatine is an organic compound produced from glycine and L-arginine through biosynthesis pathways in the kidney and liver [1]. In the initial step, glycine and L-arginine are converted to guanidinoacetate (GAA), which is accompanied by L-ornithine production, via the catalytic activity of L-arginine:glycine amidinotransferase (AGAT) in the kidney [2]. In the second step, this intermediate product is converted to creatine by guanidinoacetate N-methyltransferase (GAMT), which requires the participation of S-adenosylmethionine (SAM), in the liver [3]. Aside from endogenous synthesis, creatine can be taken in from several foods, such as fish and meat [4]. The majority of creatine is stored in skeletal muscle, and the brain and testes also hold relatively high levels of creatine as compared with other tissues and organs [5].
Although creatine is not an essential nutrient, excessive loss of creatine leads to critical situations in our health that are epitomized by creatine deficiency disorders (CDDs) [6]. Impaired creatine metabolism and transportation are major causes of CDDs [7]. Deficiencies of AGAT and GAMT lead to the impairment of creatine synthesis [8]. A deficiency or functional loss of the creatine transporter (CrT) critically downregulates the level and utilization of intracellular creatine [9]. The most famous symptoms of CDDs are neurological disorders, such as developmental delay, intellectual disability, and speech-language disorder [10, 11]. Therefore, creatine homeostasis is an indispensable factor for maintaining biological function and activity.
The most important use of creatine is for adenosine triphosphate (ATP) synthesis [12]. Creatine is used in the phosphocreatine system, which generates ATP from adenosine diphosphate (ADP) and phosphocreatine (PCr) via the catalytic activity of creatine kinase (CK) [13]. In the generation of PCr, CK catalyzes the reversible transfer of phosphate from ATP to creatine with its reversible catalytic activity [14]. Since creatine is able to conjugate with high-energy phosphate, most stored creatine molecules are bound with high-energy phosphate and form PCr [13]. Therefore, PCr functions as a carrier of phosphate used for rapid ATP generation in the phosphocreatine system [14, 15]. This is distinguishable from the oxidative phosphorylation-based ATP production in mitochondria and makes it possible to generate ATP in anaerobic manner [16].
The phosphocreatine system has been described as being mainly driven in specific organs and tissues, such as the skeletal muscle, heart, and brain, which have high energy demands [17, 18]. However, recent studies have revealed novel possibilities indicating that the immune system also requires creatine to enhance its activity. For instance, creatine supplementation promoted the activity of CD8+ T cells in anti-tumor immunity by increasing ATP production [19]. Innate immunity provides a strong front-line defense against various pathogenic invasions [20]. Neutrophils are especially important innate immune cells in this defensive mechanism [21]. In the acute phase of bacterial invasion, neutrophils are mobilized to the infected site and aggressively eliminate the bacteria by phagocytosis and producing antibacterial agents [22, 23]. Furthermore, they rapidly require sufficient sources of energy to exert these capabilities [24]. In this context, ATP is the primary energy source and is mainly provided through glycolysis rather than oxidative phosphorylation in mitochondria [25]. If rapid ATP production under inflammatory conditions involves another metabolic reaction, more neutrophil activity could be promoted than usual. Recent studies showed that various nutrients modify the activity of immune cells, such as T cells and dendritic cells (DCs) [26, 27]. However, the effects of nutrients on the activity of innate immune cells, especially for neutrophils, have not been described well. Thus, we investigated a nutrient that effectively modifies neutrophil activity and enhances the immune response.
In this report, we show that creatine supplementation enhanced the immunological activity of neutrophils and that this was based on increasing the cellular ATP level. This functional modification of neutrophils contributed to attenuation of the mortality rate, based on enhanced antibacterial immunity, in a murine bacterial sepsis model. Our finding indicates a novel aspect of the biological effect of creatine, especially for innate immunity.
MATERIALS AND METHODS
Reagents and antibodies
Creatine monohydrate, phorbol 12-myristate 13-acetate (PMA), lipopolysaccharide (LPS), β-guanidinopropionic acid (β-GPA), and BioTracker ATP-Red Live Cell Dye were purchased from Sigma Aldrich (St. Louis, MO, USA). CellROXTM Green, K-12 BioParticles (fluorescein labeled), SYTOXTM Green, 4’,6-diamidino-2-phenylindole (DAPI), anti-SLC6A8 polyclonal antibody (PA5-110393), HRP Goat Anti-Rabbit IgG (H+L), and TRIzol were purchased from Thermo Fisher Scientific (Waltham, MA, USA). CellTiter-Glo® 2.0 was purchased from Promega (Madison, WI, USA). Anti-CD45 (30-F11), anti-CD11b (M1/70), anti-Ly-6G (1A8), and 7-aminoactinomycin D (7-AAD) were purchased from BioLegend (San Diego, CA, USA).
Mice
C57BL/6 J mice were purchased from CLEA Japan (Tokyo, Japan) and the Jackson Laboratory (Bar Harbor, ME, USA). All mice were bred under specific pathogen-free (SPF) conditions with 12 hr day/night cycles and were allowed free access to food and water. Gender-matched 8- and 12-week-old mice were used for the experiments. Some mice received intraperitoneal (i.p.) injection of saline (200 µL) or creatine (200 µL of 50 mg/mL in saline) every 24 hr for 7 days. Both naive and treated mice were used for bone marrow (BM) neutrophil isolation. All experiments were approved by the animal care and use committees of Jichi Medical University (protocol no.: 20036-01, 20037-01), Central South University (protocol no.: 15-10-874), and Shibata Gakuen University (2021-019).
Bacterial sepsis model
Frozen Escherichia coli K-12 strain stock was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). The bacteria were thawed on ice, transferred to LB media (BD Biosciences, Franklin Lakes, NJ, USA), and then cultured at 37°C for 18–20 hr with shaking. Bacterial colony forming units (CFU) were determined in each culture. To establish a bacterial sepsis model, the mice that had been administrated saline or creatine (200 µL of 50 mg/mL in saline) for 7 days received an i.p. injection of live E. coli K-12 (100 µL of 1.0×109 CFU/mL suspension). Some mice received an oral administration of β-GPA (200 µL of 10 mg/mL in phosphate-buffered saline (PBS)) every 24 hr for 7 days prior to the K-12 challenge. The numbers of live and dead mice were counted every 12 hr up to 72 hr postinjection of K-12. At 24 hr, serum was collected from surviving mice, and the cytokine concentration was measured by enzyme-linked immunosorbent assay (ELISA). The frequency of NETosis in peripheral blood neutrophils was analyzed by flow cytometry. To measure K-12 colonization in organs of sepsis mice, K-12-challenged mice (a different group of mice from those used for live/dead monitoring) were sacrificed at 24 hr, and then whole spleens and livers were extracted from each mouse for the preparation of suspensions by homogenization in PBS. After centrifugation at 300 g for 5 min, supernatants were collected and used for determination of K-12 CFUs in the organs.
Flow cytometry
Flow cytometry analysis was performed by using a flow cytometer (LSR-II, BD Biosciences, Franklin Lakes, NJ, USA) with the fluorochrome-conjugated monoclonal antibodies and chemical probes described in the reagents and antibodies section. For surface marker staining, the cells were incubated with an FcR blocker (anti-CD16/32; 2.4G2) at 4°C for 10 min, and then the cells were incubated with antibodies at 4°C for 30 min. Finally, the samples were stained with 7-AAD and analyzed on the same day without fixation. The NETosis assay was performed by a previously reported method with minor modification [28, 29]. Briefly, peripheral blood was treated with 1× red blood cell (RBC) lysis buffer at room temperature (RT) for 10 min, washed and fixed with 1% paraformaldehyde (PFA) at RT for 10 min, and subjected to staining for surface markers as well as nuclear and DNA staining. All data were analyzed with BD FACSDiva (BD Biosciences, Franklin Lakes, NJ, USA) or FlowJo (BD Biosciences).
Isolation of BM neutrophils
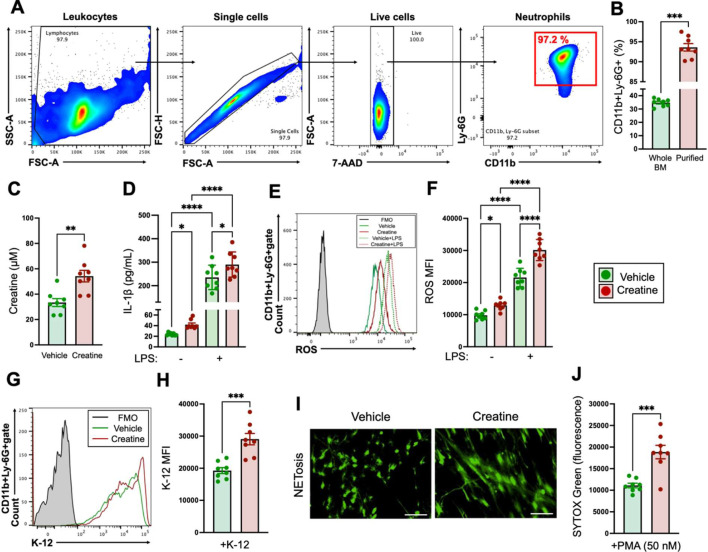
BM neutrophils were isolated from naive or treated (saline or creatine supplementation for 7 days) C57BL/6 J WT mice by following a protocol described in a previous report [28]. Briefly, tibias and femurs were extracted from the mice, and then BM cells were flushed out by using a 10 mL syringe with a 27G needle and cell culture medium (RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin). The cells were then washed, and RBCs were eliminated by treatment with 1×RBC lysis buffer at RT for 10 min. Subsequently, the cells were washed again with cell culture medium. The neutrophils were then enriched by using a Neutrophil Isolation Kit, mouse (Miltenyi Biotec, Bergisch Gladbach, North Rhine-Westphalia, Germany). All procedures were performed by following the product manual. Neutrophil purity was determined by flow cytometry. Samples with CD45+CD11b+Ly-6G+ >90% were used for experiments (Fig. 1A, 1B).
Fig. 1.
In vitro creatine treatment enhances the immunological activity of neutrophils.
A, B) Determination of the purity of neutrophils isolated from bone marrow (BM). A) Gating strategy of flow cytometry for determination of the purity of BM-isolated neutrophils. The purified neutrophils were determined to be the CD11b+Ly-6G+ population (red square) in 7-aminoactinomycin D (7-AAD-) gate (live cells). B) The percentages of CD11b+Ly-6G+ cells in whole BM and purified neutrophils. C) Intracellular creatine concentration in neutrophils. Neutrophils (1.0×107/mL) were treated with the vehicle control (PBS) or creatine (6.7 mM) at 37°C for 6 hr, and then the intracellular creatine concentration was measured by ELISA. D) IL-1β production in neutrophils. Neutrophils (1.0×107/mL) were treated with the vehicle control (PBS) or creatine (6.7 mM) and then further treated with the vehicle control (−, PBS) or lipopolysaccharide (LPS) (100 ng/mL) at 37°C overnight. The concentration of IL-1β in the cultured medium was measured by ELISA. E, F) reactive oxygen species (ROS) production in neutrophils. Neutrophils (1.0×107/mL) were treated with the vehicle control (PBS) or creatine (6.7 mM) and then further treated with the vehicle control (−, PBS) or LPS (100 ng/mL) at 37°C for 60 min. ROS production was analyzed by flow cytometry. E) Representative histogram image of ROS production in the flow cytometry analysis. F) Cumulative data for ROS MFIs. G, H) Phagocytosis activity of neutrophils. Neutrophils (1.0×106/mL) were treated with the vehicle control (PBS) or creatine (6.7 mM) in the presence of E. coli K-12 BioParticles (fluorescein labeled, 100 µg/mL). The cultures were incubated at 37°C for 120 min, and then the phagocytic activity against K-12 was analyzed by flow cytometry. G) Representative histogram image of phagocytosis in the flow cytometry analysis. H) Cumulative data for K-12 MFIs. I, J) NETosis of phorbol 12-myristate 13-acetate (PMA)-treated neutrophils. Neutrophils (5.0×105/mL) were treated with the vehicle control (PBS) or creatine (6.7 mM) in the presence of PMA (50 nM). The cultures were incubated at 37°C for 4 hr, the culture medium was collected, and the cell-free DNA (CFD) was then stained with SYTOXTM Green. The NETosis images were obtained with a fluorescence microscope. I) Representative image of NETosis. Bar=100 μM. J) Cumulative data for the fluorescence intensity of CFD. The cumulative data are shown as mean ± SEM values of eight samples in three independent experiments. Student’s t-test (B, C, H, J) or one-way ANOVA (D, F) was used to analyze data for significant differences. Asterisks indicate significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Cytokine production assay
Neutrophils (1.0×107/mL) were seeded in a 96-well flat-bottom plate with cell culture medium containing vehicle control (PBS) or LPS (100 ng/mL). Neutrophils isolated from naive mice were also treated with vehicle control (PBS) or creatine (6.7 mM). The culture was incubated at 37°C overnight, and the plate was immediately frozen at −80°C and stored until use. The cytokine concentration in the culture was measured by ELISA.
Reactive oxygen species (ROS) production assay
Neutrophils (1.0×107/mL) were seeded in a 96-well round-bottom plate with cell culture medium containing vehicle control (PBS) or LPS (100 ng/mL) and CellROXTM Green (1 μM). Neutrophils isolated from naive mice were also treated with vehicle control (PBS) or creatine (6.7 mM). The culture was incubated at 37°C for 60 min. The ROS signal (mean fluorescence intensity; MFI) was detected in the live neutrophil (7-AAD-CD11b+Ly-6G+) population by flow cytometry.
Phagocytosis assay
Neutrophils (1.0×107/mL) were seeded in a 96-well round-bottom plate with cell culture medium containing K-12 BioParticles (100 µg/mL). Neutrophils isolated from naive mice were also treated with vehicle control (PBS) or creatine (6.7 mM). The culture was incubated at 37°C for 2 hr, and then the phagocytosis activity was analyzed by flow cytometry. The incorporated K-12 signal mean fluorescence intensity (MFI) was detected in the neutrophil (CD11b+Ly-6G+) population.
NETosis assay
Neutrophils (5.0×105/mL) were seeded in a 96-well flat-bottom plate (poly-L-lysine coated) with cell culture medium supplemented with PMA (50 nM). Neutrophils isolated from naive mice were further treated with vehicle control (PBS) or creatine (6.7 mM). The culture was incubated at 37°C for 4 hr to induce NETosis. After incubation, the culture medium was harvested and stored at −80°C until use. The cells were stained with SYTOXTM Green (500 nM) at 37°C for 15 min. After being washed with PBS, they were treated with 1% PFA at 4°C for 10 min. NETosis was observed by fluorescence microscope. The cell-free DNA (CFD) in the culture medium was quantified by staining with SYTOXTM Green (500 nM) at 37°C for 15 min. Fluorescence was measured by microplate reader (Tecan, Männedorf, Zürich, Switzerland).
ELISA
Cytokine concentrations were measured by ELISA using a Ready-SET-Go!™ Kit (Thermo Fisher Scientific) for each target. All procedures were performed by following the product manuals.
Biochemical assay
Neutrophils (1.0×107/mL) isolated from naive mice were treated with vehicle control (PBS) or creatine (6.7 mM) at 37°C for 6 hr before assay. Creatine concentration was measured by using a Creatine Assay Kit (Abcam, Cambridge, UK). ATP level was measured by CellTiter-Glo® 2.0 (Promega). PCr levels were measured with a PCr ELISA Kit (MyBioSource, San Diego, CA, USA). CK activity was measured with a Creatine Kinase Activity Assay Kit (Thermo Fisher Scientific). All procedures were performed by following the product manuals. For blocking of mitochondrial ATP production, the neutrophils (1.0×107/mL) were pretreated with oligomycin A (1 µg/mL) at 37°C for 1 hr prior to creatine (6.7 mM) treatment at 37°C for 6 hr. For inhibition of creatine uptake, the neutrophils (1.0×107/mL) were pretreated with β-GPA (10 mM) at 37°C for 1 hr prior to creatine treatment (6.7 mM) at 37°C for 6 hr.
Real-time polymerase chain reaction (PCR)
Neutrophils (5.0×106/mL) were treated with vehicle (PBS) or creatine (6.7 mM) at 37°C for 6 hr, and then total RNA was isolated with TRIzol RNA Isolation Reagents (Thermo Fisher Scientific). cDNA was synthesized with 500 ng of total RNA using a PrimeScript RT Reagent Kit (TaKaRa, Tokyo, Japan). The expression level of CrT mRNA was quantified with a TB Green system (TaKaRa) and Thermal Cycler Dice (TaKaRa). GAPDH mRNA expression was used as an internal control. The following primers were used to specifically amplify the target genes: 5′-ACTGGGAGGTGACCTTGTGC-3′ (forward) and 5′-CGATCTTTCCTGTTGACTTG-3′ (reverse) for SLC6A8 and 5-TGTGTCCGTCGTGGATCTGA-3′, (forward) and 5′-TTGCTGTTGAAGTCGCAGGAG-3′ (reverse) for Gapdh. All procedures were performed by following the product manuals. The relative expression of the mRNA of interest was calculated using the 2ΔΔCT method.
Western blot
Neutrophils (1.0×107/mL) were treated with vehicle or creatine (6.7 mM) at 37°C for 6 hr, and then the cells were treated with 1× RIPA buffer. The protein concentration was determined by the bicinchoninic acid (BCA) method. The sample was diluted with distilled water (DW) and 5× sodium dodecyl sulfate (SDS) sample buffer (2% SDS; 62.5 mM Tris–HCl, pH 6.8; 10% glycerol; 0.01% bromophenol blue; 50 mM dithiothreitol (DTT) and boiled at 95°C for 5 min. The cell lysates (20 µg/well) were separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (10% gel), and proteins were transferred onto a PVDF membrane using a Trans-Blot Turbo™ Transfer System (Bio-Rad Laboratories, Hercules, CA, USA). The membrane was treated with tris-buffered saline, 0.05% Tween 20 (TBS-T) (20 mM Tris–HCl, pH 7.6; 150 mM NaCl; 0.1% Tween 20) containing 5% skim milk at room temperature for 1 hr and then further incubated with the primary antibody (anti-SAC6A8, 1:1,000) in TBST containing 1% skim milk at RT for 1 hr. After washing with TBST, the membrane was treated with the secondary antibody (HRP Goat Anti-Rabbit IgG (H+L), 1:5,000) in TBST containing 1% skim milk at RT for 30 min. After washing with TBST, the protein bands were visualized with an ECL Western Blotting Detection System (GE Healthcare Bioscience, Chicago, IL, USA), and the image was captured by a ChemiDoc XRS Imaging System (Bio-Rad, Hercules, CA, USA).
Statistical analyses
GraphPad Prism (GraphPad Software, La Jolla, CA, USA) was used for the statistical analysis. Student’s t-test and one-way analysis of variance (ANOVA) were used for comparisons between two groups and multiple groups, respectively. Values of p<0.05, p<0.01, p<0.001, and p<0.0001 were considered to be statistically significant.
RESULTS
Creatine enhanced the immune response of neutrophils
To investigate the contribution of creatine to neutrophil activity, we characterized the typical immunological response of neutrophils to creatine treatment. We used neutrophils isolated from BM for the experiments. BM cells were isolated from naive C57BL6/J mice, and then the neutrophils were purified from the whole BM cells by magnetic enrichment. The purity of the neutrophils was over 90% in an average of eight samples, which was confirmed by flow cytometry (Fig. 1A, 1B). We first measured the intracellular creatine concentration in the neutrophils. The neutrophils treated with creatine showed a significant increase in intracellular creatine concentration compared with the vehicle controls (Fig. 1C). To determine the suitable dose of creatine, the intracellular creatine concentrations of the neutrophils were measured at different doses of creatine during culture. When the dose reached 6.7 mM, the intracellular creatine concentration was significantly increased compared with the basal concentration as well as doses lower than 6.7 mM. In addition, the intracellular creatine concentration reached almost a plateau level at doses above 6.7 mM (Supplementary Fig. 1A). Therefore, we decided to use 6.7 mM of creatine in our in vitro experiments. IL-1β is a primary pro-inflammatory cytokine produced by neutrophils [30]. Creatine treatment significantly increased IL-1β production compared with the vehicle control even under the basal conditions, and the difference was clearly greater when the neutrophils were stimulated with LPS (Fig. 1D). ROS production, which is an antibacterial response, was also measured in the neutrophils [31]. Under the basal and LPS-stimulated conditions, ROS production was significantly increased in the creatine-treated neutrophils as compared with the vehicle controls (Fig. 1E, 1F). Neutrophils show aggressive phagocytosis against pathogenic bacteria [32]. In the phagocytosis assay using E. coli K-12 BioParticles, K-12 incorporation was significantly increased in the creatine-treated neutrophils as compared with the vehicle controls (Fig. 1G, 1H). Neutrophil extracellular traps (NETs) formation is another dynamic antibacterial response in neutrophils [28]. In the NETosis induced by PMA in this study, creatine treatment enhanced the formation of NET structures, which could be observed under fluorescence microscope (Fig. 1I). The magnitude of NETosis was quantified based on CFD release in the culture, and the creatine-treated cultures showed significantly high signals of CFD compared with the vehicle controls (Fig. 1J).
Taken together, creatine treatment enhanced the innate immune response of neutrophils.
Creatine supplementation modified the potential immunological activity of neutrophils and decreased the mortality rate of bacterial sepsis in the mice
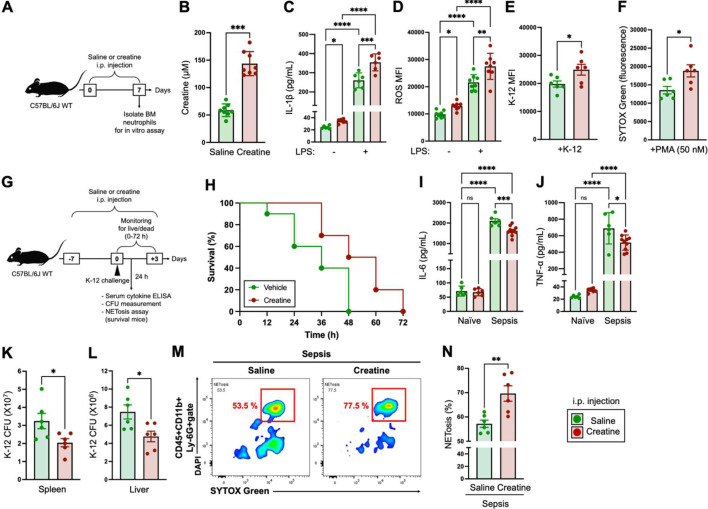
Next, we investigated the immunomodification of neutrophil function by creatine in the physiological environment. The C57BL/6 J mice received saline or creatine supplementation by i.p. injection every 24 hr for 7 days, and then neutrophils isolated from BM were used for a functional analysis (Fig. 2A).
Fig. 2.
In vivo creatine supplementation enhances the immunological activity of neutrophils and establishes an environment resistant to bacterial sepsis.
A) Design of in vivo creatine supplementation. The mice (n=8 in each group) received an i.p. injection of saline (200 µL) or creatine (200 µL of 50 mg/mL in saline) every 24 hr for 7 days. After completion of the administration procedures, neutrophils were isolated from bone marrow (BM) and used for subsequent in vitro experiments. B) Serum creatine concentrations of mice i.p. injected with saline or creatine at day 7. C) IL-1β production in neutrophils. Neutrophils (1.0×107/mL) were treated with the vehicle control (−, PBS) or lipopolysaccharide (LPS) (100 ng/mL) at 37°C overnight. The concentration of IL-1β in the culture medium was measured by ELISA. D) reactive oxygen species (ROS) production in neutrophils. Neutrophils (1.0×107/mL) were treated with the vehicle control (−, PBS) or LPS (100 ng/mL) at 37°C for 60 min. The ROS production was analyzed by flow cytometry. E) Phagocytosis activity in neutrophils. Neutrophils (1.0×107/mL) were incubated with E. coli K-12 BioParticles (100 µg/mL) at 37°C for 120 min. The phagocytosis activity against K-12 was analyzed by flow cytometry. F) NETosis of PMA-treated neutrophils. Neutrophils (5.0×105/mL) were treated with PMA (50 nM) at 37°C for 4 hr. The cell-free DNA (CFD) in the cultured medium was stained with SYTOXTM Green, and the fluorescence was analyzed with a microplate reader. G) Design of the bacterial sepsis model. The mice (n=10 in each group) received an i.p. injection of saline (200 µL) or creatine (200 µL of 50 mg/mL in saline) every 24 hr for 7 days (days −7 to 0). Live E. coli K-12 (100 µL of 1.0×109 CFU/mL of suspension in saline) was i.p. injected into the mice (at day 0). The numbers of live and dead mice in each group were monitored every 12 hr up to 72 hr (days 0 to +3). Plasma cytokine concentration, K-12 CFU in organs, and NETosis in peripheral blood circulating neutrophils were analyzed in the surviving mice at 24 hr post-challenge with K-12. H) Survival curve of bacterial sepsis mice. I, J) Serum cytokine levels in sepsis mice. The serum samples were collected from surviving mice at 24 hr post-challenge with K-12, and IL-6 (I) and TNF-α (J) concentrations were measured by ELISA. K–L) Bacteria colonization in organs of sepsis mice. The spleen and liver were extracted from surviving mice at 24 hr post-challenge with K-12 (a different group of mice from those used for live/dead monitoring). The K-12 CFUs in whole organs were measured in the spleen (K) and liver (L), respectively. M, N) NETosis activity of blood circulating neutrophils. Peripheral blood was collected from surviving mice at 24 hr post-challenge with K-12, and NETosis in neutrophils was analyzed by flow cytometry. The SYTOXTM Green+DAPI+ population in the CD45+CD11b+Ly-6G+ gate was determined to comprise NETosis neutrophils. L) Representative image of NETosis neutrophils in the flow cytometry analysis. M) Cumulative data for the percentage of NETosis neutrophils. The cumulative data are shown as mean ± SEM values of six or eight samples in two independent experiments. Student’s t-test (B, E, F, K, L, N) or one-way ANOVA (C, D, I, J) was used to analyze data for significant differences. Asterisks indicate significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. ns, not significant.
Intraperitoneal injection of creatine significantly increased the serum creatine concentration in the mice at day 7 compared with saline injection (Fig. 2B). The serum creatine concentration was dose-dependently increased; however, the level reached a plateau at injection with 50 mg/mL, which we used in the in vivo experiment (Supplementary Fig. 2).
In the stimulation assay, neutrophils isolated from creatine-supplemented mice showed a significantly larger amount of IL-1β production with LPS stimulation compared with that of the control mice. Interestingly, the basal IL-1β production was already slightly upregulated in the neutrophils that originated from creatine-supplemented mice (Fig. 2C). Both ROS production and K-12 phagocytosis were significantly enhanced in the neutrophils isolated from creatine-supplemented mice as compared with those of the control mice (Fig. 2D, 2E). Furthermore, NETosis was also enhanced in the neutrophils that originated from creatine-supplemented mice (Fig. 2F).
To investigate the contribution of creative supplementation to neutrophil-based antibacterial immunity, we established a murine bacterial sepsis model. C57BL/6 J mice first received saline or creatine administration for 7 days (Day −7 to 0). At day 0, live E. coli (K-12 strain) was i.p. injected into the mice. The mortality rate was monitored every 12 hr up to 72 hr after the K-12 challenge. The serum cytokine concentrations and NETosis of blood circulating neutrophils were measured in surviving mice at 24 hr. Furthermore, bacterial CFUs were assessed in the spleen and liver of the surviving mice (a different group of mice from those used for live/dead monitoring) at 24 hr (Fig. 2G).
In the saline-supplemented group of mice, 40% of the population was dead at 24 hr, and all of the mice were dead by 48 hr post-challenge with K-12. On the other hand, the creatine-supplemented mice showed high resistance to bacterial sepsis. At 24 hr and 48 hr, 100% and 50% of the creatine-supplemented mice were still alive, respectively. Ultimately, all of the mice in the creatine-supplemented group were dead by 72 hr; however, the surviving numbers of mice in this group were larger than those of the control group at all other time points (Fig. 2H).
Pro-inflammatory cytokines, such as interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α), are generally dramatically increased in bacteria sepsis [33, 34]. Abnormal levels of these cytokines are major causes of death in septic shock, as they induce cytokine storms and multiple organ dysfunction [35]. The plasma concentration of IL-6 was markedly increased in sepsis mice compared with naive mice. Creatine supplementation significantly decreased the plasma IL-6 level compared with that of the control mice (Fig. 2I). The TNF-α level was also slightly decreased in the creatine-supplemented mice compared with the control mice, although the difference was smaller than that for IL-6 (Fig. 2J). The creatine-supplemented mice showed significantly smaller K-12 CFUs compared with the control mice in both the spleen and liver at 24 hr of K-12 challenge (Fig. 2K, 2L). NETosis in blood circulating neutrophils was promoted in the creatine-supplemented mice at 24 hr postinjection of K-12 (Fig. 2M, 2N).
Taken together, creatine supplementation enhanced the potential immunological activity of neutrophils in the physiological environment. Furthermore, the modified immunological environment attenuated the mortality rate in bacterial sepsis by enhancing neutrophil activity.
Creatine supplementation increased the intracellular ATP level in neutrophils
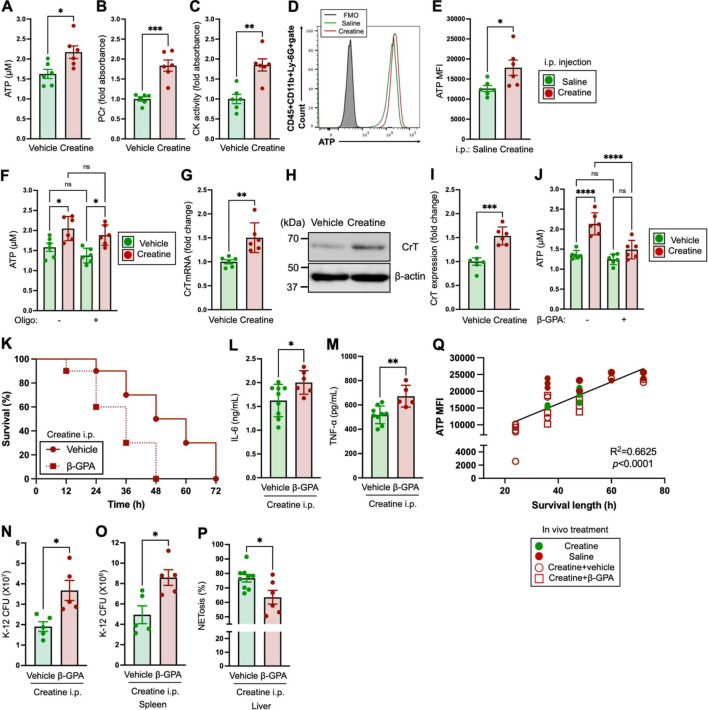
ATP is a universal energy in mammalian cells [36]. The cellular level increasing of uptake and the production of ATP are frequently correlated with cell activation [19, 37]. In fact, some reports have shown that increases in ATP promote the activity of immune cells [19, 38, 39]. Mammalian cells are capable of cytosolic ATP synthesis, which is distinct from oxidative phosphorylation-based ATP production in mitochondria [40]. The intracellular creatine concentration has an important role to this reaction, as high-energy phosphate-conjugated creatine (PCr) is utilized in the rapid production of ATP via the phosphocreatine system catalyzed by CK [41]. To investigate the effect of creatine supplementation on ATP production in neutrophils, we performed biochemical assays. We found a significant increase in the intracellular ATP concentration in creatine-treated neutrophils compared with the controls (Fig. 3A). The intracellular ATP concentration increased with the creatine dose; however, it reached a plateau at 6.7 mM of creatine, which was the dose we used in this study (Supplementary Fig. 1B).
Fig. 3.
Creatine supplementation increases cellular adenosine triphosphate (ATP), which is one of the major causes of the functional enhancement of neutrophils.
A–J) Biochemical assays in neutrophils. Neutrophils were isolated from naive mice for use in the assays (A–C, F–J). In vivo assays (D, E) were performed by using peripheral blood neutrophils collected from creatine-administered mice (following the administration schedule indicated in Fig. 2A). A–C) Neutrophils (1.0×107/mL) were treated with the vehicle control (PBS) or creatine (6.7 mM) at 37°C for 6 hr and then used for each assay. A) ATP concentration in neutrophils. B) phosphocreatine (PCr ) level of neutrophils. C) creatine kinase (CK) activity of neutrophils. D, E) ATP level in peripheral blood circulating neutrophils. The mice (n=6 in each group) received an i.p. injection of saline (200 µL) or creatine (200 µL of 50 mg/mL in saline) every 24 hr for 7 days. After completion of the administration procedures, peripheral blood was used for ATP assays by flow cytometry. D) Representative histogram image of ATP in the flow cytometry analysis. E) Cumulative data for ATP MFIs. F) ATP concentrations of neutrophils with blocking of oxidative phosphorylation-based ATP production. Neutrophils (1.0×106/mL) were treated with the vehicle control (PBS) or creatine (6.7 mM) in the presence or absence of oligomycin A (1 µg/mL) at 37°C for 6 hr, and then the intracellular ATP concentration was measured in the neutrophils. G–I) CrT expression in neutrophils. Neutrophils (1.0×107/mL) were treated with the vehicle control (PBS) or creatine (6.7 mM) at 37°C for 6 hr, and then total RNA or proteins were isolated for real-time PCR and western blotting (WB), respectively. G) Cumulative data for quantification of the CrT mRNA level. H) Total expression of CrT protein in WB. I) Cumulative data for CrT expression intensity in WB. J) ATP concentration in neutrophils with CrT blocking. Neutrophils (1.0×107/mL) were treated with the vehicle control (PBS) or β-PGA (10 mM) at 37°C for 1 hr followed by creatine (6.7 mM) treatment at 37°C for 6 hr, and then the intracellular ATP concentration was measured in the neutrophils. K–P) Comparison of mortality rate and antibacterial responses with or without the inhibition of ATP uptake in bacterial sepsis. The mice (n=10 in each group) received an i.p. injection of saline (200 µL) or creatine (200 µL of 50 mg/mL in saline) every 24 hr for 7 days (days −7 to 0). At the same time, some mice received oral administration of saline (200 µL) or β-GPA (200 µL of 100 mg/mL in saline). Live E. coli K-12 (100 µL of 1.0×109 CFU/mL of suspension in saline) was i.p. injected into the mice (at day 0). The numbers of live and dead mice in each group were monitored every 12 hr up to 72 hr (days 0 to +3 K). K) Survival curve of bacterial sepsis mice. L, M) Serum cytokine levels in sepsis mice. The serum samples were collected from surviving mice at 24 hr post-challenge with K-12, and IL-6 (L) and TNF-α (M) concentrations were measured by ELISA. N, O) Bacteria colonization in organs of sepsis mice. The spleen and liver were extracted from surviving mice at 24 hr post-challenge with K-12 (a different group of mice from those used for live/dead monitoring). The K-12 CFUs in whole organs were measured in the spleen (N) and liver (O), respectively. P) NETosis activity of blood circulating neutrophils. Peripheral blood was collected from surviving mice at 24 hr post-challenge with K-12, and NETosis in neutrophils was analyzed by flow cytometry. The SYTOXTM Green+DAPI+ population in the CD45+CD11b+Ly-6G+ gate was determined to comprise NETosis neutrophils. Q) Correlation between the neutrophil ATP levels and survival times in bacterial sepsis mice. Both the ATP MFIs in blood neutrophils and survival times in bacterial sepsis mice (four groups in total in this study) are summarized in a linear regression graph. The ATP levels were analyzed in peripheral blood neutrophils collected from surviving mice at 24 hr post-challenge with K-12. The cumulative data are shown as mean ± SEM values of six samples in two independent experiments. Student’s t-test (A–C, E, G, I, L–P) or one-way ANOVA (F, J) was used to analyze data for significant differences. Asterisks indicate significance: *p<0.05, **p<0.01, ****p<0.001. ns, not significant.
In conjunction with the ATP upregulation, the PCr levels increased in the creatine-treated neutrophils (Fig. 3B). In addition, CK activities were also significantly upregulated in the creatine-treated neutrophils compared with the controls (Fig. 3C).
In vivo creatine administration resulted in a significant increase in cellular ATP level in peripheral blood neutrophils compared with saline administration in the mice (Fig. 3D, 3E).
Treatment with oligomycin A, an inhibitor of complex V in mitochondrial electron transport chain (ETC), did not suppress the creatine-mediated ATP increase in neutrophils (Fig. 3F) [42]. This showed that this creatine-mediated ATP increase occurred via a pathway independent from ETC in mitochondria. Creatine is generally taken up by the creatine transporter, which is expressed on the cellular membrane [43]. Creatine treatment increased CrT mRNA expression in neutrophils (Fig. 3G). In addition, we confirmed creatine-mediated CrT protein upregulation in neutrophils by western blotting (WB; Fig. 3H). The expression intensity of CrT was significantly upregulated in neutrophils by creatine treatment compared with the vehicle control (Fig. 3I).
β-guanidinopropionic acid is a creatine mimetic substance which competitively inhibits creatine uptake by inhibiting the CrT on cells [44,45,46]. When neutrophils were treated with creatine in the presence of β-GPA, the cellular ATP concentration did not increase, and it was similar to that of the vehicle control (Fig. 3J). Interestingly, oral administration of β-GPA attenuated the creatine-mediated protective effect against bacterial sepsis in the mice. At 24 hr of K-12 challenge, 40% of the mice administered both creatine and β-GPA were dead, which was higher than the rate for the mice administered creatine only (Fig. 3K). Furthermore, the concentrations of serum IL-6 and TNF-α were both significantly higher in β-GPA-treated mice compared with those of the control mice (Fig. 3L, 3M). In addition, β-GPA-treated mice showed significantly larger bacterial CFUs in the spleen and liver compared with the control mice (Fig. 3N, 3O). The β-GPA treatment also downregulated the NETosis activity of neutrophils in the blood stream (Fig. 3P). Finally, we found a correlation between the cellular ATP levels in neutrophils and survival times (hours post-challenge with K-12) of the mice with bacterial sepsis. The mice with high ATP levels, which were mostly administered creatine but not treated with β-GPA, showed extended survival times compared with the mice without creatine administration or with administration of both creatine and β-GPA (Fig. 3Q).
Taken together, creatine supplementation increased the intracellular ATP level by driving the phosphocreatine system in neutrophils, and this was based on the uptake of extracellular creatine via CrT. In addition, the ATP increase was one of the major mechanisms in the functional modification of neutrophils that provided an environment resistant to bacterial sepsis.
DISCUSSION
In the present study, creatine supplementation effectively promoted the immunological activity of neutrophils by increasing ATP synthesis. Functional modification of neutrophils is generally a difficult approach, because neutrophils are biologically fragile and sensitive to the external environment. Hence, the finding of an effective approach to enhance neutrophil function is valuable from both the biological and immunological perspectives. In fact, we previously tried to enhance the activity of neutrophils with several nutrients and food-derived factors; however, all of our attempts to date have failed, except creatine. Eventually, we confirmed that creatine supplementation enhanced the antibacterial activity of neutrophils in both in vitro and in vivo environments (Fig. 1D–1J and Fig. 2B–2E). This phenomenon contributed to a reduction in the mortality late of bacterial sepsis in the mouse model (Fig. 2G). To the best of our knowledge, this is the first report of creatine-mediated functional modification in neutrophils.
We found that this creatine-induced functional upregulation is based on intracellular ATP increase in the neutrophils (Fig. 3A, 3D and 3E). In this biological mechanism, the phosphocreatine system might have primarily been involved in cytosolic ATP synthesis, as both the PCr level and CK activity were upregulated in the creatine-treated neutrophils (Fig. 3B, 3C). This is one of the possible mechanisms by which rapid ATP production was induced in the creatine-exposed neutrophils. This creatine-mediated ATP increase was observed in the neutrophils within 6 hr of creatine treatment (Fig. 3A). It was a relatively fast response, and this suggests that creatine was utilized for rapid ATP production in neutrophils by driving the phosphocreatine system in the cytosol. We also confirmed that the creatine-mediated ATP increase was an independent response from mitochondrial phosphorylation-based ATP production by treating neutrophils with oligomycin A, which selectively inhibits complex V in the ETC (Fig. 3F) [42]. A previous report showed that ATP supplementation enhanced the immunological activities of neutrophils [47, 48]. Neutrophils require ATP for their inflammatory responses, and previous studies reported that ATP treatment directly enhanced the activities of neutrophils, which were characterized by promoted cytokine production and chemotaxis [47, 48]. This evidence supports our findings. There were also interesting findings indicating that creatine administration promoted the potential activity and intracellular ATP level of neutrophils in the mice (Fig. 2B–2E and Fig. 3D, 3E). The neutrophils isolated from the creatine-supplemented mice showed significantly promoted immunological activities compared with the control mice, even at the basal level (Fig. 2B–2E). This means that creatine supplementation has sufficient potential to also modify our innate immunity. Previous reports indicated that the phosphocreatine system has a pathway via mitochondria and that mitochondrial CK has a role in this biosynthesis [49, 50]. This means that creatine might be utilized for ATP production via mitochondrial CK in neutrophils. We did not investigate this in the present study; however, we intend to perform another experiment to investigate it in the near future.
We found that CrT expression was upregulated in creatine-treated neutrophils at both the mRNA and protein levels (Fig. 3G–3I). The ATP increase in creatine-treated neutrophils was based on extracellular creatine uptake, which was proven by an experiment in which creatine transport was blocked with β-GPA. There was clear suppression of the increase in intracellular ATP in the neutrophils (Fig. 3J). We did not investigate the exact biological mechanism of CrT upregulation in creatine-treated neutrophils; however, we suspected that it was positive feedback to effectively utilize extracellular creatine depending on the circumstances. Since immune cells have no dynamic abilities to synthesize creatine, we hypothesize that neutrophils must sense the creatine abundance and promote uptake into the cytosol for maximum utilization of it. This is an interesting biological response of neutrophils in creatine utilization; therefore, we will work to describe this mechanism in detail.
Finally, we found that the inhibition of creatine uptake impaired the creatine-derived extension of survival time in bacterial sepsis mice (Fig. 3K). In addition, there was a strong correlation between the cellular ATP level in neutrophils and survival times of bacterial sepsis mice (Fig. 3L). These two findings were strong proof that the antibacterial response of neutrophils was enhanced by the uptake of creatine, which subsequently promoted ATP synthesis in the neutrophils. This finding is based on experiments in mice; however, we believe that this positive effect of creatine is conserved in the human immune system.
In this report, we introduced a positive aspect of creatine uptake in innate immunity; however, we must also consider the negative side effects on the immune response. A previous report indicated that creatine promoted the inflammatory response in the respiratory tract accompanied by increases in T helper 2-type cytokines and granulocyte accumulation in the inflamed region in a murine experimental asthma model [51]. Since some types of inflammatory diseases are also connected to overactivation of neutrophils, unsuitable creatine supplementation might have a potential risk of aggravating the inflammation [52, 53]. Similar to the characters of other nutrients, creatine supplementation must take into consideration the circumstances and background of the individual.
Creatine is easily supplemented orally in humans as a powder-based supplement. Therefore, it can be used for self-maintenance of the immune system on a daily basis. Our findings are still insufficient evidence for the use of creatine in a clinical setting; however, future studies have the potential to uncover further evidence indicating that creatine is highly effective for the treatment of several infectious diseases.
AUTHOR CONTRIBUTIONS
Conceptualization, S.S., D.Y.C., and A.O.; methodology, S.S.; experiments, S.S., D.Y.C., A.O., and Z.P.; data analysis, S.S., D.Y.C., A.O., X.L., and Z.P.; resources, S.S. and A.O.; discussion, S.S., D.Y.C., A.O., X.L., Z.P., N.M.T., and M.K.; writing manuscript, S.S., X.L., and M.K.; supervision, S.S.; project administration, S.S. and A.O.; funding acquisition, S.S. and A.O. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
This research was funded by the Japan Society for the Promotion of Science (Grant number 16H06814 to S.S., 21K15958 to S.S., and 21K20573 to A.O.), Mishima-Kaiun Memorial Fund (to S.S.), and private funds provided by the Shounai Midori Group (to S.S.).
REFERENCES
- 1.Kazak L, Cohen P. 2020. Creatine metabolism: energy homeostasis, immunity and cancer biology. Nat Rev Endocrinol 16: 421–436. [DOI] [PubMed] [Google Scholar]
- 2.Braissant O, Henry H. 2008. AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: a review. J Inherit Metab Dis 31: 230–239. [DOI] [PubMed] [Google Scholar]
- 3.Barsunova K, Vendelin M, Birkedal R. 2020. Marker enzyme activities in hindleg from creatine-deficient AGAT and GAMT KO mice—differences between models, muscles, and sexes. Sci Rep 10: 7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roschel H, Gualano B, Ostojic SM, Rawson ES. 2021. Creatine supplementation and brain health. Nutrients 13: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreider RB, Stout JR. 2021. Creatine in health and disease. Nutrients 13: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joncquel-Chevalier Curt M, Voicu PM, Fontaine M, Dessein AF, Porchet N, Mention-Mulliez K, Dobbelaere D, Soto-Ares G, Cheillan D, Vamecq J. 2015. Creatine biosynthesis and transport in health and disease. Biochimie 119: 146–165. [DOI] [PubMed] [Google Scholar]
- 7.Ostojic SM. 2019. Benefits and drawbacks of guanidinoacetic acid as a possible treatment to replenish cerebral creatine in AGAT deficiency. Nutr Neurosci 22: 302–305. [DOI] [PubMed] [Google Scholar]
- 8.Mercimek-Mahmutoglu S, Stoeckler-Ipsiroglu S, Adami A, Appleton R, Araújo HC, Duran M, Ensenauer R, Fernandez-Alvarez E, Garcia P, Grolik C, Item CB, Leuzzi V, Marquardt I, Mühl A, Saelke-Kellermann RA, Salomons GS, Schulze A, Surtees R, van der Knaap MS, Vasconcelos R, Verhoeven NM, Vilarinho L, Wilichowski E, Jakobs C. 2006. GAMT deficiency: features, treatment, and outcome in an inborn error of creatine synthesis. Neurology 67: 480–484. [DOI] [PubMed] [Google Scholar]
- 9.Skelton MR, Schaefer TL, Graham DL, Degrauw TJ, Clark JF, Williams MT, Vorhees CV. 2011. Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency. PLoS One 6: e16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braissant O, Henry H, Béard E, Uldry J. 2011. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 40: 1315–1324. [DOI] [PubMed] [Google Scholar]
- 11.Adriano E, Gulino M, Arkel M, Salis A, Damonte G, Liessi N, Millo E, Garbati P, Balestrino M. 2018. Di-acetyl creatine ethyl ester, a new creatine derivative for the possible treatment of creatine transporter deficiency. Neurosci Lett 665: 217–223. [DOI] [PubMed] [Google Scholar]
- 12.Adhihetty PJ, Beal MF. 2008. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromolecular Med 10: 275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahlin K, Harris RC. 2011. The creatine kinase reaction: a simple reaction with functional complexity. Amino Acids 40: 1363–1367. [DOI] [PubMed] [Google Scholar]
- 14.Bonilla DA, Kreider RB, Stout JR, Forero DA, Kerksick CM, Roberts MD, Rawson ES. 2021. Metabolic basis of creatine in health and disease: a bioinformatics-assisted review. Nutrients 13: 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papalazarou V, Zhang T, Paul NR, Juin A, Cantini M, Maddocks ODK, Salmeron-Sanchez M, Machesky LM. 2020. The creatine-phosphagen system is mechanoresponsive in pancreatic adenocarcinoma and fuels invasion and metastasis. Nat Metab 2: 62–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta MM, Weinberg SE, Chandel NS. 2017. Mitochondrial control of immunity: beyond ATP. Nat Rev Immunol 17: 608–620. [DOI] [PubMed] [Google Scholar]
- 17.Wallimann T, Tokarska-Schlattner M, Schlattner U. 2011. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40: 1271–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adriano E, Salis A, Damonte G, Millo E, Balestrino M. 2022. Effects of delivering guanidinoacetic acid or its prodrug to the neural tissue: possible relevance for creatine transporter deficiency. Brain Sci 12: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Biase S, Ma X, Wang X, Yu J, Wang YC, Smith DJ, Zhou Y, Li Z, Kim YJ, Clarke N, To A, Yang L. 2019. Creatine uptake regulates CD8 T cell antitumor immunity. J Exp Med 216: 2869–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CH, Liu H, Ge B. 2017. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol 14: 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castanheira FVS, Kubes P. 2019. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133: 2178–2185. [DOI] [PubMed] [Google Scholar]
- 22.Khan Z, Shen XZ, Bernstein EA, Giani JF, Eriguchi M, Zhao TV, Gonzalez-Villalobos RA, Fuchs S, Liu GY, Bernstein KE. 2017. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood 130: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao DY, Giani JF, Veiras LC, Bernstein EA, Okwan-Duodu D, Ahmed F, Bresee C, Tourtellotte WG, Karumanchi SA, Bernstein KE, Khan Z. 2021. An ACE inhibitor reduces bactericidal activity of human neutrophils in vitro and impairs mouse neutrophil activity in vivo. Sci Transl Med 13: eabj2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadiku P, Willson JA, Ryan EM, Sammut D, Coelho P, Watts ER, Grecian R, Young JM, Bewley M, Arienti S, Mirchandani AS, Sanchez Garcia MA, Morrison T, Zhang A, Reyes L, Griessler T, Jheeta P, Paterson GG, Graham CJ, Thomson JP, Baillie K, Thompson AAR, Morgan JM, Acosta-Sanchez A, Dardé VM, Duran J, Guinovart JJ, Rodriguez-Blanco G, Von Kriegsheim A, Meehan RR, Mazzone M, Dockrell DH, Ghesquiere B, Carmeliet P, Whyte MKB, Walmsley SR. 2021. Neutrophils fuel effective immune responses throughgluconeogenesis and glycogenesis. Cell Metab 33: 411–423.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohms M, Ferreira C, Busch H, Wohlers I, Guerra de Souza AC, Silvestre R, Laskay T. 2021. Enhanced glycolysis is required for antileishmanial functions of neutrophils upon infection with Leishmania donovani. Front Immunol 12: 632512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, Amodio V, Ceruti T, Sassi F, Arena S, Abrignani S, D’Incalci M, Zucchetti M, Di Nicolantonio F, Bardelli A. 2020. High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med 12: eaay8707. [DOI] [PubMed] [Google Scholar]
- 27.Barragan M, Good M, Kolls JK. 2015. Regulation of dendritic cell function by vitamin D. Nutrients 7: 8127–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito S, Cao DY, Victor AR, Peng Z, Wu HY, Okwan-Duodu D. 2021. RASAL3 is a putative RasGAP modulating inflammatory response by neutrophils. Front Immunol 12: 744300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zharkova O, Tay SH, Lee HY, Shubhita T, Ong WY, Lateef A, MacAry PA, Lim LHK, Connolly JE, Fairhurst AM. 2019. A flow cytometry-based assay for high-throughput detection and quantification of neutrophil extracellular traps in mixed cell populations. Cytometry A 95: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 31.Peng Z, Cao DY, Wu HY, Saito S. 2020. Immunization with a bacterial lipoprotein establishes an immuno-protective response with upregulation of effector CD4+ T cells and neutrophils against methicillin-resistant Staphylococcus aureus infection. Pathogens 9: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. 2021. The neutrophil. Immunity 54: 1377–1391. [DOI] [PubMed] [Google Scholar]
- 33.Carestia A, Mena HA, Olexen CM, Ortiz Wilczyñski JM, Negrotto S, Errasti AE, Gómez RM, Jenne CN, Carrera Silva EA, Schattner M. 2019. Platelets promote macrophage polarization toward pro-inflammatory phenotype and increase survival of septic mice. Cell Rep 28: 896–908.e5. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. 2008. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol 181: 5598–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar V. 2020. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int Immunopharmacol 89Pt B: 107087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A, Malinovska L, Saha S, Wang J, Alberti S, Krishnan Y, Hyman AA. 2017. ATP as a biological hydrotrope. Science 356: 753–756. [DOI] [PubMed] [Google Scholar]
- 37.Ring S, Enk AH, Mahnke K. 2010. ATP activates regulatory T cells in vivo during contact hypersensitivity reactions. J Immunol 184: 3408–3416. [DOI] [PubMed] [Google Scholar]
- 38.Zumerle S, Calì B, Munari F, Angioni R, Di Virgilio F, Molon B, Viola A. 2019. Intercellular calcium signaling induced by ATP potentiates macrophage phagocytosis. Cell Rep 27: 1–10.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. 2009. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J 23: 1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Aguilar A, Cuezva JM. 2018. A review of the inhibition of the mitochondrial ATP synthase by IF1 in vivo: reprogramming energy metabolism and inducing mitohormesis. Front Physiol 9: 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao TJ, Yan YB, Liu Y, Zhou HM. 2007. The generation of the oxidized form of creatine kinase is a negative regulation on muscle creatine kinase. J Biol Chem 282: 12022–12029. [DOI] [PubMed] [Google Scholar]
- 42.Cao DY, Spivia WR, Veiras LC, Khan Z, Peng Z, Jones AE, Bernstein EA, Saito S, Okwan-Duodu D, Parker SJ, Giani JF, Divakaruni AS, Van Eyk JE, Bernstein KE. 2020. ACE overexpression in myeloid cells increases oxidative metabolism and cellular ATP. J Biol Chem 295: 1369–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji L, Zhao X, Zhang B, Kang L, Song W, Zhao B, Xie W, Chen L, Hu X. 2019. Slc6a8-mediated creatine uptake and accumulation reprogram macrophage polarization via regulating cytokine responses. Immunity 51: 272–284.e7. [DOI] [PubMed] [Google Scholar]
- 44.Fitch CD, Shields RP, Payne WF, Dacus JM. 1968. Creatine metabolism in skeletal muscle. 3. Specificity of the creatine entry process. J Biol Chem 243: 2024–2027. [PubMed] [Google Scholar]
- 45.Fitch CD, Chevli R. 1980. Inhibition of creatine and phosphocreatine accumulation in skeletal muscle and heart. Metabolism 29: 686–690. [DOI] [PubMed] [Google Scholar]
- 46.Kurth I, Yamaguchi N, Andreu-Agullo C, Tian HS, Sridhar S, Takeda S, Gonsalves FC, Loo JM, Barlas A, Manova-Todorova K, Busby R, Bendell JC, Strauss J, Fakih M, McRee AJ, Hendifar AE, Rosen LS, Cercek A, Wasserman R, Szarek M, Spector SL, Raza S, Tavazoie MF, Tavazoie SF. 2021. Therapeutic targeting of SLC6A8 creatine transporter suppresses colon cancer progression and modulates human creatine levels. Sci Adv 7: eabi7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. 2016. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun 7: 10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Chen D. 2018. Purinergic regulation of neutrophil function. Front Immunol 9: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fritz-Wolf K, Schnyder T, Wallimann T, Kabsch W. 1996. Structure of mitochondrial creatine kinase. Nature 381: 341–345. [DOI] [PubMed] [Google Scholar]
- 50.Perry CG, Kane DA, Herbst EA, Mukai K, Lark DS, Wright DC, Heigenhauser GJ, Neufer PD, Spriet LL, Holloway GP. 2012. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J Physiol 590: 5475–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia M, Santos-Dias A, Bachi ALL, Oliveira-Junior MC, Andrade-Souza AS, Ferreira SC, Aquino-Junior JCJ, Almeida FM, Rigonato-Oliveira NC, Oliveira APL, Savio LEB, Coutinho-Silva R, Müller T, Idzko M, Siepmann T, Vieira RP. 2019. Creatine supplementation impairs airway inflammation in an experimental model of asthma involving P2 × 7 receptor. Eur J Immunol 49: 928–939. [DOI] [PubMed] [Google Scholar]
- 52.Wu X, Pippin J, Lefkowith JB. 1993. Platelets and neutrophils are critical to the enhanced glomerular arachidonate metabolism in acute nephrotoxic nephritis in rats. J Clin Invest 91: 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishi H, Furuhashi K, Cullere X, Saggu G, Miller MJ, Chen Y, Rosetti F, Hamilton SL, Yang L, Pittman SP, Liao J, Herter JM, Berry JC, DeAngelo DJ, Zhu C, Tsokos GC, Mayadas TN. 2017. Neutrophil FcγRIIA promotes IgG-mediated glomerular neutrophil capture via Abl/Src kinases. J Clin Invest 127: 3810–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.