Abstract
Long-lasting coronavirus disease 2019 (COVID-19) symptoms beyond 12 weeks, the so-called ‘long COVID’ have been increasingly reported worldwide. Long COVID can be manifested in various forms, and there is an increasing demand for proper assessment and management. However, it is challenging when trying to determine the best-practice standards of care based on the current evidence because there is no internationally agreed clinical definition or clear treatment pathway. Therefore, the present guidelines have been drafted to provide advice on diagnosis and management based on the latest updated available evidence and the consensus of expert opinion. So far, no standard test and drug treatment can be strongly recommended for patients with long COVID because of a lack of evidence. The present guidelines provide advice based on 12 key questions, including appropriate interventions for long COVID that can be used in clinical practice. Continuous careful observation and studies related to long COVID are needed for the long-term impact of COVID-19 and proper management for long COVID to be determined.
Keywords: SARS-CoV-2, Long COVID, Persistent symptoms, Post-COVID condition, Management
Background and purpose
By June 2022, more than 500 million people had been confirmed to have coronavirus disease 2019 (COVID-19) [1], and many of whom complained of several symptoms that persisted for a considerable time after the acute phase of COVID-19 (generally 4 weeks after diagnosis). These conditions are typically referred to as ‘long COVID’. Given the lack of objective diagnostic standards and the medical evidence for appropriate tests and treatment for various symptoms, treatment is prescribed based on individual clinicians’ empirical judgments. As such, the Korean Society of Infectious Diseases has developed these guidelines based on the medical judgment of the experts in the field and the latest evidence. Systematic literature review on diagnosis and treatment cannot be performed yet as medical research on long COVID is still lacking. Thus, these guidelines were developed as practical rapid guidelines based on expert consensus and the most recently published evidence rather than as evidence-based clinical practice guidelines.
Scope and subjects
These clinical guidelines cover the diagnostic tests and treatments for the persistent symptoms and signs developing during or after the acute COVID-19 phase. These guidelines do not cover the treatment of acute COVID-19. The target patients of these guidelines are pediatric and adult patients, including pregnant women and the elderly. The users of the guidelines are all general practitioners and specialists treating long COVID.
Composition of the committee developing the guidelines
A committee was formed to develop long COVID management guidelines in May 2022 by the Korean Society of Infectious Diseases. The committee comprised nine members, including infectious diseases, neurology, psychiatry, and pediatric infection experts.
Framing the key questions
The evidence for the epidemiology, diagnostic testing, interventions, and treatment of persistent symptoms/signs after COVID-19 in adults and children was collected and evaluated. In addition, other national and international guidelines were also reviewed to derive 12 key questions about long COVID.
Literature selection
A literature search was conducted on the treatment guidelines for long COVID published since 2020. Treatment guidelines were referenced from http://www.guideline.gov, http://www.nice.org.uk, https://www.nih.gov/, https://www.cdc.gov/, and https://www.who.int/ while a literature search was conducted in PubMed (www.pubmed.gov) database using the keywords ‘long COVID’, ‘chronic COVID syndrome’, ‘post-acute COVID-19 syndrome’, ‘post-acute COVID syndrome’, ‘post COVID syndrome’, ‘post COVID condition’, ‘post COVID’, ‘long term COVID’, and ‘post-acute sequelae of COVID-19’. In addition, for the diagnosis and treatment of the detailed symptoms/signs of long COVID, a literature search was conducted using keywords such as ‘dyspnea’, ‘cough’, ‘chest pain’, ‘chest discomfort’, ‘fatigue’, ‘arthralgia’, ‘myalgia’, ‘headache’, ‘brain fog’, ‘smell', ‘taste’, ‘disorder’, ‘dysfunction’, ‘neurocognitive’, ‘neurological’, ‘cognitive’, ‘depression’, ‘emotional’, ‘psychiatric’, ‘evaluation’, ‘assessment’, ‘treatment’, ‘therapy’, ‘SARS-CoV-2’, and ‘COVID-19’.
Level of recommendations and evidence
Due to the limitations in the literature at the clinically recommendable level, the levels of recommendation and evidence are not presented; instead, although lacking in clinical evidence, an expert consensus was presented, which can be recommended for use based on the expert consensus and clinical experience given the benefits and risk of the applicable treatment, level of evidence, value, preference, and resources. The disease definitions and key recommendations were determined through panel discussions and consensus using the Delphi method.
Limitations of the guidelines and future challenges
These guidelines were developed based on national and international guidelines and the latest publications available as a reference at the time of preparing these guidelines. Revisions to the guidelines will be considered in case new evidence becomes available or in case of significant changes to the treatment or diagnosis following the development of new drugs.
Definitions
As the definition of long COVID has not yet been clearly established, the definition may vary slightly by institution or guidelines. As the symptoms/signs of COVID-19 persist for <4 weeks from diagnosis, this phase is referred to as acute COVID-19. The World Health Organization (WHO) defines the post-COVID-19 condition as the symptoms and/or signs occurring during or after the acute COVID-19 phase that are not explained by other diagnoses and that persist for >2 months or even >12 weeks after diagnosis [2]. The guidelines from the National Institute for Health and Clinical Excellence (NICE) define ‘ongoing symptomatic COVID-19’ as the symptoms/signs occurring during or after the acute COVID-19 phase that improve within 4–12 weeks from diagnosis, and ‘post-COVID-19 syndrome’ is defined as symptoms/signs persisting after 12 weeks [3]. The guidelines from the European Society of Clinical Microbiology and Infectious Disease (ESCMID) use the definition based on the same specified period, while cases persisting after 12 weeks are referred to as ‘long COVID’ [4]. The National Institutes of Health (NIH) defines the ‘post-acute sequelae of SARS-CoV-2 infection’ as the symptoms/signs persisting for >4 weeks after the diagnosis of COVID-19 [5].
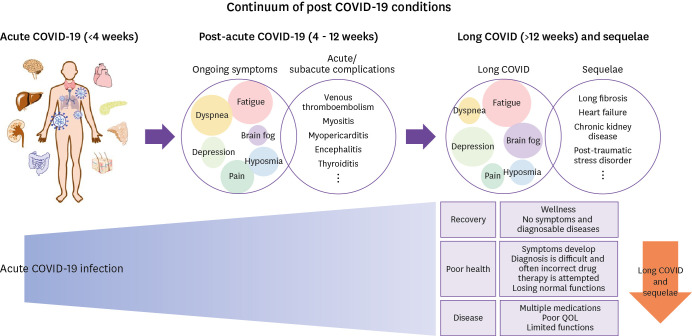
Although there is insufficient evidence for specific changes in pathophysiology at 12 weeks from COVID-19 onset, the consistent usage of the terminology is necessary to conduct research and clinical trials on the symptoms/signs persisting after acute COVID-19. In these guidelines, one or more symptoms/signs occurring during or after the acute phase that cannot be explained by other diseases are referred to as ‘post-acute COVID-19’ if the duration is 4 - 12 weeks from the diagnosis, while ‘long COVID’ is defined as the presence of symptoms/signs that persist after 12 weeks (Fig. 1). Several specific entities (venous thromboembolism, myocarditis, pericarditis, encephalitis, and thyroiditis) may occur as complications during the acute or post-acute phases of COVID-19. Hence, these are not categorized as long COVID.
Figure 1. Conceptual diagram of long COVID as a symptom/sign that persists post-COVID-19.
COVID-19, coronavirus disease 2019; QOL, quality of life.
Clinical characteristics of long COVID
1. Major symptoms and incidence
Long COVID may occur after recovery from acute COVID-19 symptoms, persist from acute phase symptoms, or may change or recur over time. According to the survey by the Centers for Disease Control and Prevention (CDC), related symptoms/signs persist after COVID-19 in 1 of 5 and 1 of 4 survivors aged 18 - 64 years and ≥ 65 years, respectively. About 200 symptoms were identified, including fatigue, dyspnea, depression/anxiety, cognitive decline, etc. [6]. The symptoms of long COVID that are likely to occur after acute COVID-19 infection are shown in Table 1.
Table 1. Overall and time-specific incidence of long COVID symptoms.
| Symptom | Meta-analysisa, % (95% CI) | Domestic (241 subjects) | ||
|---|---|---|---|---|
| >6 months % | >12 months % | |||
| Systemic | ||||
| Fever | 1.1 (0.2 - 4.7) | 1.2 | 0 | |
| Fatigue | 31.0 (23.9 - 39.0) | 25.3 | 16.2 | |
| Dizziness | 4.5 (2.5 - 7.9) | 14.9 | 10.4 | |
| Cardiopulmonary | ||||
| Cough | 8.2 (4.9 - 13.4) | 8.7 | 7.1 | |
| Sputum | 5.5 (3.2 - 9.2) | 8.7 | 7.1 | |
| Sore throat | 4.7 (2.4 - 8.9) | 12 | 7.1 | |
| Dyspnea | 25.1 (17.9 - 34.0) | 5.4 | 2.9 | |
| Chest pain/chest discomfort | 6.4 (3.2 - 12.4) | 8.3 | 4.6 | |
| Palpitation | 9.7 (6.0 - 15.3) | 2.5 | 2.5 | |
| Gastrointestinal | ||||
| Anorexia | 17.5 (4.1 - 51.0) | 5.4 | 2.9 | |
| Nausea/vomiting | 6.7 (1.6 - 23.6) | 6.2 | 0.8 | |
| Abdominal discomfort | 18.0 (11.5 - 26.1) | 8.7 | 5 | |
| Neurological | ||||
| Headache | 4.9 (2.3 - 10.1) | 12.4 | 4.6 | |
| Seizures/cramps | 1.3 (0.5 - 2.9) | 0.4 | 0 | |
| Taste disturbance | 13.5 (9.0 - 19.9) | 6.6 | 3.3 | |
| Smell disturbance | 15.2 (10.8 - 21.0) | 8.7 | 6.2 | |
| Tingling/paresthesia | 9.1 (2.2 - 30.9) | 11.2 | 10 | |
| Neurocognitive | ||||
| Concentration impairment | 26.0 (21.0 - 31.7) | 25.3 | 22.4 | |
| Memory impairment | 17.9 (5.3 - 46.3) | 25.7 | 19.9 | |
| Other cognitive impairment | 17.8 (0.1 - 98.2) | 25.3 | 21.2 | |
| Psychological | ||||
| Depression | 8.1 (4.1 - 15.1) | 24.9 | 17.8 | |
| Anxiety | 18.7 (9.0 - 35.3) | 24.1 | 16.2 | |
| Sleep disorder (insomnia) | 18.2 (9.6 - 31.6) | 21.2 | 13.3 | |
| Post-traumatic stress disorder | 9.1 (3.7 - 21.0) | 7.9 | 5 | |
| Musculoskeletal | ||||
| Muscle pain/myalgia | 11.3 (6.2 - 19.8) | 6.2 | 1.7 | |
| Joint pain/arthralgia | 9.4 (5.7 - 15.0) | 11.2 | 6.6 | |
| Other | ||||
| Hair loss | 14.3 (5.3 - 33.2) | 17 | 14.9 | |
| Skin rash | 2.8 (1.0 - 8.2) | 10.8 | 6.6 | |
aMeta-analysis was conducted on a total of 10,951 patients with confirmed or clinically suspected COVID-19 in 12 countries, 12 weeks or more from the onset of symptoms.
COVID, coronavirus disease; CI, 95% confidence interval.
Symptoms/signs that persist after COVID-19 usually improve over time [4]. Although the time to recovery varies individually, most people show improvement by week 12 [7]. According to the UK Office for National Statistics, 1 in 5 patients can have symptoms lasting >5 weeks, with 1 in 10 having symptoms for >12 weeks [8]. A decrease in quality of life has been confirmed in 57% of patients with long COVID [9], with 66% of patients with persisting symptoms/signs after COVID-19 showing organ damage and 25% showing multiple organ damage [10]. The results of a comparative study between influenza-infected and COVID-19 patient groups using data from the National Health Insurance Review and Assessment Service reported significantly higher incidence rates of dementia, heart failure, mood disorder, and alopecia among patients with COVID-19 patients after the acute phase of infection [4].
The most common symptoms of long COVID lasting up to 6 months were fatigue, post-exercise malaise, and cognitive dysfunction [11]. According to the online survey conducted among the domestic patient population in the Daegu region, 66% of patients complained of various clinical symptoms 6 months after acute COVID-19, including fatigue, concentration difficulties, amnesia, cognitive dysfunction, anxiety, depression, alopecia, dizziness, headaches, brain fog, anosmia, ageusia, and dyspnea [12]. Brain fog refers to a state in which thoughts and expressions are not clearly expressed due to a persistent feeling of mental fogginess. A survey study conducted 1 year after COVID-19 diagnosis in the same patient population, reported that 127 of 241 patients (53%) showed persistent long COVID symptoms, with the incidence decreasing in the order of concentration difficulty, cognitive dysfunction, amnesia, depression, fatigue, and anxiety. Specifically, neurological and psychiatric symptoms were confirmed to be long-lasting, and the duration of each symptom of long COVID showed a decreasing tendency over time [13]. A prospective survey conducted at a tertiary hospital in Daegu observed that various clinical symptoms persisted 1 year after COVID-19 diagnosis, including amnesia (24%), insomnia (15%), fatigue (14%), and anxiety (13%) [14]. The overall and period-specific incidence of the symptoms of long COVID are shown in Table 1 [12,13,15]. Post-COVID-19 symptoms/signs may persist regardless of the severity of the acute phase. The incidence of long COVID was higher in patients who received inpatient treatment than in patients who had not been hospitalized [4].
2. Risk factors for long COVID
The risk factors associated with long COVID include demographic factors (sex, age, etc.), obesity, underlying disease, hospitalization, severe illness, β2-agonist use, and non-vaccinated status [4,13,16,17,18,19,20]. However, depending on the study, the relevance of these risk factors varies for each symptom of long COVID. Two consistent risk factors for long COVID-19 are sex and the severity of the acute phase of COVID-19. Women are twice as likely to develop long COVID-19 as men [4]. The results of a domestic study confirmed that women are at high risk of neuropsychiatric long COVID-19 [12]. Severe COVID-19 shows a robust association with fatigue [4]. Some studies reported a higher risk of developing long COVID-19 in younger age groups, with no racial differences [21].
Key questions
Key question 1) When should long COVID be suspected?
Recommendation
• Patients with symptoms that persist beyond 12 weeks after the diagnosis of COVID-19 should be evaluated for the possibility of long COVID.
• The possibility that the patient’s symptoms are caused by other underlying diseases, complications of COVID-19 (e.g., thromboembolism, myocarditis, encephalitis, etc.), or other diseases with the same symptoms (e.g., allergic rhinitis, asthma, adrenal insufficiency, tumor, etc.) must first be excluded, and tests for the suspected diseases should be performed.
• Long COVID is diagnosed after excluding underlying diseases, COVID-19 complications, and other diseases that can also show the same symptoms and when the symptoms persist even after 12 weeks from COVID-19 diagnosis.
Long COVID is diagnosed by exclusion. The diagnostic approach begins by excluding complications from COVID-19 and other diseases unrelated to COVID-19.
1) Assessment for COVID-19 Complications
(1) Cardiopulmonary sequelae
COVID-19 may result in cardiopulmonary sequelae due to lung or heart damage during the acute phase in severely ill patients who require hospitalization, which may lead to persistent symptoms (fatigue, dyspnea, chest pain, cough, etc.) and abnormal laboratory test findings. Several studies reported a significant proportion of patients with abnormal findings in respiratory function (54%) and chest computed tomography study (40 - 94%) at 1 month after infection [22,23,24]. Lung fibrosis was observed in 26% of patients (50% of patients admitted to the intensive care unit [ICU]) at 3 months [25]. In addition, pleural thickening associated with acute COVID-19 occurs in 27% of cases and pleural effusions in 5 - 6% of cases [26,27]. Thus, it is necessary to determine whether the symptoms persist due to the sequelae associated with the lungs. Myocarditis in patients with COVID-19 typically occurs within the first 2 weeks, but there are reports of cases occurring several weeks after the infection has been resolved [28]. In addition, pericardial effusion has been reported in 5% of patients with COVID-19 patients, mostly in those with myocarditis. Cardiac tamponade has also been reported in 1% of hospitalized patients [26]. Therefore, the possibility that the patient’s persistent symptoms are due to these cardiac and pulmonary complications should be considered.
(2) COVID-19-associated coagulopathy
Thrombosis may occur in some patients with COVID-19. The incidence of thrombosis in discharged patients from acute COVID-19 is typically 0.5 - 2.5% [29,30]. COVID-19-related thromboembolism appears in various forms as venous thrombosis (deep vein thrombosis and pulmonary embolism) and arterial thrombosis (stroke and myocardial infarction). Up to one-third of critical COVID-19 patients admitted to the ICU are confirmed to have thromboembolism [31]. Thromboembolism as a complication associated with COVID-19 may manifest itself in various forms; thus, the possibility of thromboembolism developing as a complication of COVID-19 in patients complaining of long-term, persistent symptoms must be considered.
(3) Endocrine complications
COVID-19 can cause various endocrine and metabolic diseases, showing persistent abnormalities even after recovery from COVID-19 [32]. Among these, adrenal insufficiency may occur due to the direct effect of the COVID-19 virus on the adrenal glands or an iatrogenic cause from glucocorticoid therapy for COVID-19 pneumonia [33,34]. Diabetes, newly diagnosed without apparent risk factors, is also reported as a disease related to COVID-19 [35]. Vitamin D deficiency, hypocalcemia, and vertebral fractures may also occur as endocrine sequelae of COVID-19 [36,37]. In addition, thyroid disease may develop as a complication of COVID-19 [38,39]. Therefore, appropriate evaluations according to the existing practice guidelines for these diseases are needed in cases with suspected hypothalamic-pituitary-adrenal axis suppression, bone metabolism abnormality, or thyroid function.
(4) Neurological complications
Complications and sequelae of the central nervous system after acute COVID-19 have been reported, and the risk of these complications increases especially in severe COVID-19 [40,41]. Therefore, appropriate neurological evaluation is required in cases with neurological abnormalities after acute COVID-19.
(5) Post-COVID-19 immune-mediated manifestations
Immune-mediated diseases may develop in patients with COVID-19; although rare, it may be associated with autoimmunity. Arthritis, myositis, pancreatitis, perniosis, neurological diseases (encephalitis, Guillain-Barré syndrome, myelitis), kidney diseases (tubulopathies, glomerulonephritis), hematologic diseases (idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia), endocrine diseases (thyroiditis, thyrotoxicosis), and systemic autoimmune diseases (lupus, vasculitis, sarcoidosis, etc.) have been reported [28]. The possibility that persistent symptoms after acute COVID-19 may be primarily due to immune-related diseases should be considered.
2. Assessment of diseases unrelated to COVID-19
In addition to the complications of COVID-19, it is also necessary to assess the aggravation of the underlying disease that may cause the symptoms or other diseases unrelated to COVID-19. Appropriate laboratory or radiologic assessments are necessary by considering that various symptoms corresponding to various respiratory, cardiovascular, hematologic, endocrine, allergic, and neuropsychiatric diseases may appear alone or in combination (Table 2) [7,42].
Table 2. Diseases that may develop after COVID-19.
| Classification | Systemic diseases |
|---|---|
| Circulatory system | Myocarditis, pericarditis, microvascular angina, cardiac arrhythmias (atrial flutter, atrial fibrillation), dysautonomia (postural orthostatic tachycardia syndrome) |
| Respiratory system | Interstitial lung disease, pulmonary emboli |
| Gastrointestinal system | Hepatitis, abnormal liver enzymes, pancreatitis |
| Endocrine system | New-onset diabetes (diabetic ketoacidosis, etc.), thyroiditis (subacute thyroiditis, Graves’ disease, Hashimoto thyroiditis, etc.), adrenal insufficiency |
| Neurological system | Cerebral venous thrombosis, myelopathy, neuropathy, neurocognitive disorders, dysphonia, encephalitis, Guillain-Barré syndrome |
| Musculoskeletal system | Arthritis, myositis |
| Other | Renal impairment (tubulopathies, glomerulonephritis) |
| Autoimmune diseases (systemic lupus erythematosus, vasculitis, sarcoidosis) | |
| Mast cell activation syndrome | |
| New-onset allergies/anaphylaxis | |
| Perniosis |
Key question 2) What tests are needed for long COVID symptoms?
Recommendation
• There is no specific test for the diagnosis of long COVID.
• For diagnosis by exclusion, relevant blood tests should be performed for diseases that can explain the symptoms and signs of which the patient complained.
1. Dyspnea
• The pulmonary function test is a simple and non-invasive test. Therefore, regardless of symptoms, pulmonary function tests including diffusion capacity for carbon monoxide (DLCO) can be considered 3 months after the diagnosis of COVID-19 in patients with severe or critical COVID-19, persistent dyspnea after acute COVID-19, or underlying lung disease.
• When respiratory symptoms persist for 3 months, chest X-ray can be considered to exclude other diseases and detect early lung fibrosis. Chest computed tomography (CT) can be considered if there are abnormalities on chest X-rays or if symptoms persist even without chest X-ray abnormalities.
2. Cough
• If cough persists for >3 months, chest X-ray and chest computed tomography can be considered to check for pulmonary parenchymal fibrosis or bronchial inflammation.
3. Chest pain
• Transthoracic echocardiography can be considered to evaluate pericarditis/myocarditis or heart failure in patients with symptoms of pericardial or myocardial injury (chest pain, palpitations, dyspnea) >12 weeks from the acute stage.
• Functional tests such as the 6-minute walking test (6MWT) or 15 – 30-second sit-to-stand test to evaluate cardiopulmonary function can be considered when initiating patient evaluation or rehabilitation.
• Evidence is insufficient to recommend or advise against cardiac imaging (echocardiography, computed tomography) or functional studies (6MWT or sit-to-stand test) in patients with chronic chest pain after 12 weeks that did not exist during acute COVID-19.
4. Fatigue
• No specific test method exists to assess fatigue symptoms lasting >12 weeks after COVID-19.
• The degree of fatigue can be measured using a fatigue severity scale.
• A detailed medical history is needed to differentiate underlying diseases that can explain the symptoms of fatigue, the complications of COVID-19, and other diseases unrelated to COVID-19.
• If an organic cause that can explain fatigue is not identified, the possibility of COVID-19-related chronic fatigue syndrome should be considered.
5. Joint pain and muscle pain
• Evidence is insufficient to recommend or advise against specific laboratory tests (creatine kinase, lactate dehydrogenase, C-reactive protein, rheumatologic factor, anti-nuclear antibody) in patients with arthralgia or myalgia lasting >12 weeks after COVID-19.
6. Headache
• Assessing long COVID-related headaches should include history-taking, neurological examination, and vital sign measurements.
• If it is necessary to exclude an organic cause, brain imaging examinations should be considered, with a referral to a neurologist for further assessment and treatment.
7. Cognitive symptoms
• There is insufficient evidence to recommend brain imaging tests for cognitive symptoms related to long COVID. However, brain imaging tests can be considered to exclude other causes or for research purposes. In addition, a neuropsychological examination may be considered in cases with decreased occupational and social functioning due to cognitive symptoms.
8. Psychological/mental symptoms
• Attending physicians should be aware of the psychological sequelae of COVID-19 and can conduct psychosocial assessments and, if necessary, refer to a psychiatrist for further assessment and treatment.
• Patients with serious psychiatric symptoms or risk of self-harm or suicide should be immediately referred to a psychiatrist.
• There is insufficient evidence to recommend brain imaging tests for psychiatric symptoms. However, these tests can be performed to rule out other organic causes or for research purposes.
• When patients with pre-existing mental illness complain of symptoms related to other organs corresponding to long COVID (e.g., respiratory symptoms), they should not be discriminated against in diagnosis and treatment.
1) Blood tests required for diagnosis by exclusion
No studies have evaluated the usefulness of routine blood tests in patients with long COVID-19. A recent study reported that a small proportion (approximately 1 - 5%) of patients had abnormal findings in laboratory tests at follow-up assessment 12 months after the diagnosis of COVID-19 [43]. Nevertheless, blood tests according to symptoms should be performed to exclude other diseases. Care should be taken in interpreting whether the results indicate persistent abnormal findings after COVID-19. As recommended by several guidelines, the following blood tests may be considered depending on the symptoms [4,44,45]; C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), complete blood count (CBC), and liver function test (LFT). Myocardial enzyme tests (troponin, creatine kinase-myoglobin binding [CK-MB]) and brain natriuretic peptide tests (B-type natriuretic peptide, BNP) can be considered in patients with heart-related problems. If clinically suspected, thyroid function tests (TFTs) are performed to rule out thyroiditis. Arterial blood gas analysis is performed in patients with reduced oxygen saturation. D-dimer testing is not recommended in patients without respiratory symptoms. Patients at high risk for diabetes or with impaired fasting blood glucose should undergo measurement of the fasting blood glucose and glycosylated hemoglobin level.
2) Dyspnea
Diagnostic tests may be required if dyspnea persists for >4 - 12 weeks after acute COVID-19. Some guidelines recommend using the modified Medical Research Council [mMRC] dyspnea scale to evaluate the severity of dyspnea; however, there is currently no standard for assessing the severity of persistent dyspnea after acute COVID-19. Therefore, further research is needed [44]. Respiratory function abnormalities are reported at various rates in patients recovering from COVID-19, which vary depending on the definition of respiratory function abnormality, follow-up period, severity at the time of acute COVID-19, use of mechanical ventilation, and previous respiratory function [46].
The most common pulmonary function test abnormality is abnormal diffusion capacity for carbon monoxide (DLCO). The restrictive pattern is the most common result of pulmonary function tests including forced vital capacity (FVC) and total lung capacity (TLC). Up to 80% of patients with critical COVID-19 treated in ICU had DLCO impairment (<80% of predicted) at discharge, and 50 - 70% had DLCO impairment at 3 - 6 months after discharge. At 3 months, impaired DLCO was associated with a high chest CT total severity score at hospitalization (evaluated by percentage of involvement in each lobe and overall lung) and acute respiratory distress syndrome (ARDS) [46]. At 6 months, DLCO impairement was observed in 29% of patients with severe COVID-19 and in 58% with critical COVID-19 [47]. A study performing 1 year follow-up after severe-critical COVID-19 reported DLCO of <80% in 23 - 54% of patients. TLC of <80% of predicted was observed in 39% of critically ill patients at 6 months and in 29% at 12 months [43]. Limited data on pulmonary function tests are available in patients with mild-to-moderate COVID-19. Several studies included some patients with mild-to-moderate disease as a control group for severe COVID-19 patients, in which 10 - 20% of these patients showed abnormalities in pulmonary function tests including DLCO [4,25,47].
Two observational studies examined the results of chest X-rays 6 - 8 weeks after acute COVID-19. The follow-up chest X-ray findings correlated with the severity of acute COVID-19 but not with persistent respiratory symptoms and the degree of symptom recovery [48,49]. Among 110 patients hospitalized for COVID-19 and followed up for 8 - 12 weeks after discharge, 14% showed abnormalities in chest X-rays. In addition, reticular opacities were observed in 53% of patients with chest X-ray abnormalities, while peripheral atelectasis was observed in 33% of these patients [50].
In patients with severe or critical COVID-19, ground-glass opacities (GGOs), consolidation, or fibrotic changes were observed in 60 - 75% of patients with chest CT performed 3 months after diagnosis [51]. A systematic literature review of studies on chest CT findings 3 - 6 months after COVID-19 diagnosis reported abnormal findings in 59% of patients regardless of severity, most commonly GGOs (39%), followed by fibrosis and reticular opacities (30%) [52]. However, whether these abnormal findings predict future lung damage remains unknown. One study that performed chest magnetic resonance imaging (MRI) in 53 patients 2 – 3 months after recovery from acute COVID-19 observed lung parenchymal abnormalities in 60% of cases. However, these were not associated with clinically persistent symptoms [53].
If dyspnea persists for >4 - 12 weeks after acute COVID-19, a chest radiology examination can be considered to exclude other diseases, even though chest X-ray and chest CT findings poorly correlate with symptoms and can not predict future lung damage.
3) Cough
A cough that persists after acute COVID-19 is usually associated with chronic fatigue and dyspnea. Since lung fibrosis increases the sensitivity of the cough reflex, it is important to differentiate a persistent cough from other lung diseases and to check for fibrosis of the lung parenchyma or damage to the bronchus. Lung parenchymal fibrosis and bronchial damage can be detected by chest CT. It is also necessary to check for gastroesophageal reflux disease, a common cause of chronic cough, or for the use of angiotensin-converting enzyme (ACE) inhibitor [54].
4) Chest pain
Reports on pericarditis, myocarditis, heart failure, and cardiac arrhythmia after COVID-19 have been published, although causality is not always evident. One study showed that among patients with chest pain, palpitation, dyspnea, or edema 2 months after COVID-19, 28% (14/51) had severe cardiovascular disease [55]. However, this rate may be overestimated due to selection bias of patients with severe symptoms. Another study reported that 25% (35/150) of patients with mild to moderate COVID-19 showed abnormalities in transthoracic echocardiography performed 3 months after COVID-19 diagnosis, mostly reduced ejection fraction (EF), elevated pulmonary artery pressure, diastolic dysfunction, and thickened pericardium [56]. In transthoracic echocardiography studies performed 30 - 100 days after COVID-19, a considerable number of asymptomatic COVID-19 patients showed left ventricular strain, diastolic dysfunction, and pulmonary hypertension [57,58]. Considering the frequency of these cardiac complications, it is appropriate to perform follow-up transthoracic echocardiography within 2 – 3 months in patients with confirmed myocarditis, pericarditis, or heart failure in acute COVID-19. Transthoracic echocardiography can also be considered in patients who have experienced chest pain, palpitations, or dyspnea from the acute stage but have not undergone diagnostic tests for cardiac complications.
Several studies of cardiac MRI have shown abnormalities in 19 - 71% of patients approximately 1 - 4 months after the diagnosis of COVID-19 [53,59,60]. These findings usually did not correlate with symptoms and were resolved 6 months after the diagnosis of COVID-19 [61]. Therefore, the need for cardiac MRI should be determined according to the individual patient's clinical situation and symptoms.
To assess the frailty and physical activity after COVID-19, physicians can consider checking the distance and oxygen saturation before and after 6 MWT, sit-to-stand test (counting the number of sitting and standing for 15 - 30 seconds), and short physical performance test battery (includes balance assessment in a standing position, walking speed for 4 minutes, and standing up from a char with five repetitions) [4]. Most studies assessing patients with long COVID using these methods showed that higher disease severity during the acute stage was associated with a more severe dysfunction [25,47]. Therefore, when considering rehabilitation in long COVID patients with dyspnea and chest pain, the assessment of cardiac function can be considered first.
A Korean study reported the presence of chest pain after COVID-19 in 13% of patients after 6 months and 7% of patients after 12 months [13]. A meta-analysis reported a 6% rate (95% confidence interval [CI]: 3.2 - 12.4) at 3 - 6 months after the COVID-19 diagnosis [15], showing a decreasing trend over time. Therefore, if chest pain develops 12 weeks after the diagnosis of COVID-19, the possibility that chest pain is associated with a disease other than long COVID should be considered, and cardiac imaging studies for diagnosing/excluding other cardiac diseases can be performed, if necessary. However, there are currently no known specific cardiac image findings for long COVID. Therefore, there is insufficient evidence to recommend or against cardiac imaging for the diagnosis of long COVID, except to diagnose or exclude other cardiac diseases.
5) Fatigue
Fatigue is one of the common non-respiratory symptoms of patients with COVID-19, typically occurring in about 41% of patients [45]. This symptom occurs in 35 - 45% of patients at 4 weeks [62,63], 30 - 77% of patients at 8 weeks [64,65,66], and 16 - 55% of patients at 12 weeks after infection [22,67]. The fatigue in patients with COVID-19 has characteristics similar to those of the Middle East Respiratory Syndrome (MERS) or Severe Acute Respiratory Syndrome (SARS), and the chronic fatigue syndrome described after community-acquired pneumonia [68,69]. Fatigue is a difficult symptom to define, is generally non-specific, has subjective characteristics, and is difficult to assess objectively. To evaluate the degree of fatigue in patients complaining of fatigue, physicians can apply a fatigue severity scale (FSS) (Table 3). The FSS consists of a total of nine items, in which the level of fatigue during the past week is evaluated on a scale of 1 to 7. The scores for each item are summed, and the average value divided by nine is calculated as the final FSS score. The higher the scores, the higher the degree of fatigue. This evaluation method shows a sensitivity of 84% and a specificity of 86% based on a cutoff of 3.22 points [70].
Table 3. Fatigue severity scale.
| Statement | Strongly disagree Strongly agree | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | My motivation is lower when I am fatigued | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 2 | Exercise brings on my fatigue | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 3 | I easily get fatigued | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 4 | Fatigue interferes with my physical functioning | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 5 | Fatigue causes frequent problems for me | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 6 | My fatigue prevents sustained physical functioning | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 7 | Fatigue interferes with carrying out certain duties and responsibilities | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | Fatigue is among my three most disabling symptoms | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 9 | Fatigue interferes with work, family, or social life | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
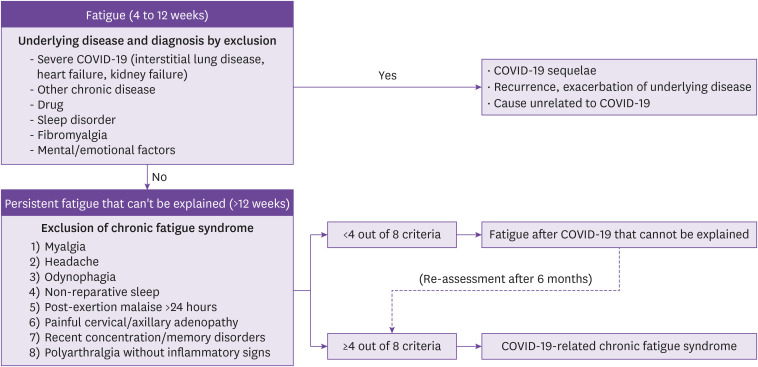
To date, no association has been reported between the symptoms of long-term fatigue associated with COVID-19 and the severity of COVID-19 or laboratory test values related to inflammation. However, excessive fatigue symptoms have been reported in female patients previously diagnosed with depression or anxiety [71]. If fatigue persists as a major symptom >12 weeks after COVID-19, the date of onset, the presence of symptoms or signs accompanying fatigue, socio-psychological/emotional factors or drugs that may cause fatigue, sleep disorders, and exposure to toxins are first investigated. In addition, it is essential to confirm the specific medical history of other current symptoms that coexist with fatigue, such as the presence of an underlying disease that may be related to the current fatigue or whether there are sequelae from severe COVID-19. In addition to the above, CBC with differential counts, electrolyte tests (sodium, potassium, chloride, bicarbonate, calcium, and phosphate), LFT, renal profile, ESR, CRP, ferritin, d-dimer, TFT, muscle enzyme, and plasma cortisol levels are evaluated. At the same time, measurements of vital signs, oxygen saturation, electrocardiogram, chest X-ray, spirometry, and nutritional assessment are also recommended. If no organic abnormalities are revealed in these evaluations, it is recommended to evaluate whether the criteria for chronic fatigue syndrome are met (Table 4).
Table 4. Evaluation criteria related to the diagnosis of chronic fatigue syndrome.
| Chronic Fatigue Syndrome Evaluation Items |
|---|
| • Myalgia |
| • Headache |
| • Odynophagia |
| • Non-reparative sleep |
| • Post-exertion malaise >24 hours |
| • Painful cervical/axillary adenopathy |
| • Recent concentration/memory disorders |
| • Polyarthralgia without inflammatory signs |
The presence of four or more of the evaluation criteria in Table 4 can be diagnosed as chronic fatigue syndrome associated with COVID-19 [45]. If the criteria are not met, it is categorized as unexplained post-COVID-19 fatigue, and each evaluation item of the criteria, psychological factors, and related physical conditions are re-evaluated. After applying a physical activity program such as rehabilitation support, the evaluation criteria for chronic fatigue syndrome are re-evaluated after 6 months (Fig. 2).
Figure 2. Diagnostic approach for chronic fatigue syndrome related to COVID-19.
COVID-19, coronavirus disease 2019.
6) Joint pain and muscle pain
A study in Korea performed 6 and 12 months after the diagnosis of COVID-19 reported that arthralgia occurred in 11% of patients after 6 months and 7% after 12 months, while myalgia occurred in 6% and 12% after 6 and 12 months, respectively [13], similar to the results of other studies reporting arthralgia in 10 - 48% of patients at 4 - 12 weeks, and 9% at 3 - 6 months (95% CI: 5.7 - 15), while myalgia was reported in 1 - 32% at 4 - 12 weeks and 11% of patients at 3 - 6 months (95% CI: 6.2 – 19.8) [9,15]. Several case reports described rhabdomyolysis in COVID-19 patients [72,73,74,75], and most cases occurred in the acute stage (within 4 weeks of symptom onset). In the case of severe COVID-19, there have been reports of rhabdomyolysis in the sub-acute stage (4 - 12 weeks from symptom onset), but there is no case of rhabdomyolysis occurring more than 12 weeks after symptom onset. Rheumatologic serology tests (anti-cardiolipin antibody, anti-nuclear antibody, and rheumatoid factor) did not differ significantly between patients with persistent symptoms and the control group in a study of 189 patients with symptoms persisting 6 weeks after the onset of COVID-19 [76]. However, in this study, a few patients suffered myalgia or arthralgia (11 and 6 patients, respectively); therefore, it can not be concluded that rheumatologic serology tests are not useful in patients with long COVID. Although several studies have reported arthralgia and myalgia as the symptoms of long COVID, no study has performed both muscle biopsy (histopathology) and skeletal muscle imaging. Most studies assumed that muscle weakness was related to cytokine storms. The pathophysiologic mechanism causing myalgia in long COVID is unknown [77].
7) Headache
Headache is one of the common symptoms of patients with COVID-19 (14 - 60% of cases) and may persist for several weeks after acute COVID-19 [78]. A meta-analysis reported that 15% of patients (95% CI: 4.5 - 25.8) complained of headaches even 3 months after COVID-19 [79]. Long COVID-related headache can be a worsening of the existing headache or a new onset headache. A common feature is the near-daily persistence of headache that occurrs at the time of COVID-19 diagnosis or with a slight time lag. Immune or inflammatory responses activated during infection cause headache or may aggravate existing migraines [80]. SARS-CoV-2 virus does not directly cause long COVID-related headache; thus, it is reasonable to regard it as a symptom that can persist long after viral infection [78]. Previous reports have described new daily persistent headache lasting >3 months after infection with Epstein-Barr virus and even after the 1890 Russian flu pandemic [81,82]. The similarities between new daily persistent headache after these infections and long COVID-related headache require further research.
The most important action in patients with a headache is to rule out secondary headaches with organic causes. First, the headache pattern should be identified through medical history taking, and focal neurologic deficit should be assessed through neurological examinations. Brain imaging tests (MRI or CT) should be considered, and a referral should be made to a neurologist for professional evaluation and treatment for headache suspected to be due to organic causes, such as headache with fever, vomiting, or weight loss; headache in cancer patients or immunosuppressed patients; headache accompanied by neurological abnormalities; headache with papilledema; severe thunderclap headache peaking within 1 minute; headache with new onset after 50 years of age; headache that worsens over time; and headache that occurs in situations similar to the Valsalva maneuver or that worsens when standing up. If organic causes are excluded, the treatment used for primary headache can be applied [45].
8) Neurocognitive symptom
A meta-analysis that examined the prevalence of neurological symptoms 3 months after COVID-19 reported a 22% (95% CI: 7.3 - 36.4) prevalence for decreased concentration, a 32% (95% CI: 10.3 - 54.0) prevalence of brain fog, and a 28% (95% CI: 21.5 - 35.4) prevalence of memory impairment [79]. A domestic study that examined the residual symptoms in patients at 6 and 12 months after COVID-19 reported the prevalence rates of neurological symptoms, including 22% for concentration difficulties, 20% for amnesia, and 21% for cognitive impairment [13].
In patients showing cognitive symptoms related to long COVID, brain imaging and neuropsychological tests can be considered when necessary to differentiate these symptoms from other causes or if the symptoms are severe enough to lead to decreased occupational or social function [4,83]. Referral should be made to a neurologist for professional evaluation and treatment [3,69]. Because the neuropsychological tests usually performed in clinical practice were developed for elderly patients with dementia, these assessments are often unsuitable for young patients, as in the case of those with long COVID who complain of concentration difficulties rather than a severe cognitive impairment that limits daily life. Therefore, a sensitive cognitive test tool suitable for this population should be developed and validated [3].
Recent studies have reported the brain imaging findings of patients with COVID-19. In a cohort of the UK brain bank, a comparison of MRI findings between 401 patients who had recovered from COVID-19 and 384 control subjects showed reduced gray matter thickness of the orbitofrontal cortex and parahippocampal gyrus in COVID-19 patients. Moreover, in areas functionally connected to the primary olfactory cortex, the markers related to tissue damage were more changed, and the overall brain size was decreased in COVID-19 patients [84]. In addition, in a study of brain positron emission tomography and computerized tomography (PET-CT) in 35 patients with long COVID-19, the lower the metabolism of the brainstem and cerebellum, the greater the number of complaints of symptoms [85].
There are several reports of cognitive tests after COVID-19. Analysis of the neuropsychological examination findings and health records of 740 patients who recovered from COVID-19 with no previous dementia showed decreased processing speed (in 18% of patients), decreased executive functioning (in 16%), and functional decline in tasks including phonemic fluency (in 15%), category fluency (in 20%), memory encoding (transformation of short-term memory into long-term memory) (in 24%), and memory recall (in 23%). Patients who had severe COVID-19 were more likely to develop cognitive sequelae [86]. Another study enrolled 1,438 patients >60 years of age 1 year after COVID-19 to assess for changes in cognitive function; the incidence of cognitive impairment was 12%, and the cognitive test scores were significantly lower in the COVID-19 group than in the control group. Patients with severe COVID-19 had a lower score for cognitive function and a higher risk of progressive cognitive impairment than those with mild COVID-19 or the control group [41]. Although studies based on neuropsychological and brain imaging tests for long COVID-related cognitive symptoms have been recently reported, further research is needed regarding the long-term prognosis.
9) Psychological/psychiatric symptom
Assessment of mental health in patients with COVID-19 is important [87], whether the cause is the direct effect of the virus or is a reaction to the experience of stressful events. This is because mental health can affect the quality of life of patients as well as the occurrence of other symptoms of long COVID [45]. Psychosocial assessment includes screening for depression, anxiety, post-traumatic stress disorder, psychotic symptoms, risk of harming self or others, mourning for sudden death, and life stress related to COVID-19 (e.g., debt, job loss or difficulty finding a job, interpersonal problems) [88]. In particular, patients with severe psychiatric symptoms or risks of harming themselves or others should be promptly referred for a psychiatric assessment to avoid delays in proper interventions [3,88]. The earlier help is received, the more effective the intervention. Patients without social support may experience anxiety and mental health deterioration [3].
Anxiety, depression, and post-traumatic stress disorder are reported in 16 - 47% of patients 2 - 3 months after hospitalization and discharge among COVID-19 survivors [89,90]. During admission in COVID-19 isolation wards, patients may experience concerns about their own health or that of others, physical and social isolation, potential risk of death, the risk of infecting others, leaving family behind, threats to livelihood, helplessness, boredom, and loneliness. Such stress may trigger new psychiatric symptoms or exacerbate preexisting mental health conditions [91]. Preexisting neuropsychiatric disorders or substance use disorders may worsen the course of COVID-19 or increase the risk of long-term sequelae [83,92]. Patients admitted to COVID-19 isolation wards have reduced physical activity, which is particularly problematic for the elderly [45]. Decreased physical function can increase mental health problems such as anxiety and depression. In addition, post-COVID-19 acute stress, hospitalization environmental factors, invasive medical procedures (e.g., mechanical ventilation), and multiple medications may increase the risk of sleep disruption [93]. The experience of ICU admission in COVID-19 survivors may increase long-lasting functional limitations, post-traumatic stress disorder, and depression [94]. A recent study reported incidence rates of anxiety and psychotic disorders of 17% and 1.2%, respectively, with particularly high rates in patients who had been admitted to the ICU [40]. When treating patients with psychological or psychiatric symptoms, it is important to consider social factors such as whether they are in situations of poverty, discrimination, or social exclusion [45]. Social connectedness, social support, and other collective or community-based measures can be beneficial for patient mental health and well-being.
The comparison of PET-CT between 35 patients with COVID-19 and a control group at mean 96 days after diagnosis showed hypometabolisms in specific brain regions of patients with COVID-19. These findings were associated with hyposmia, anosmia, memory or cognitive decline, pain, and insomnia [95]. In a prospective study of 58 patients with moderate-to-severe COVID-19, brain MRIs performed 2–3 months after diagnosis showed abnormalities in the thalamus and sagittal stratum in 32 COVID-19 patients compared with the control group [53].
Key question 3) Should thromboprophylaxis be used in patients with long COVID?
Recommendation
• The routine administration of anticoagulants or antiplatelet agents to prevent thrombosis in patients with long COVID is not recommended. The decision to prevent thrombosis in patients with long COVID is based on a general assessment of thrombotic risk and bleeding risk. If it is determined that thromboprophylaxis is necessary, refer a patient to a specialist in the relevant field.
The NIH guidelines for COVID-19 treatment recommend maintaining anticoagulants or antithrombotic drugs in patients who have been prescribed these drugs for an underlying disease. Routine screening for thrombosis is not recommended in patients without symptoms or signs suggestive of thrombosis. In addition, the administration of anticoagulants or antithrombotic drugs to prevent thrombosis is not recommended in patients with COVID-19 who do not require hospitalization. For patients requiring hospitalization due to COVID-19, the use of low molecular weight heparin (LMWH) or unfractionated heparin (UFH) is recommended for non-pregnant adults. However, the routine administration of drugs is not recommended for thrombosis prevention after discharge [96]. A multicenter randomized controlled clinical study in Brazil reported a significantly lower occurrence of thrombosis in the group of patients with COVID-19 receiving rivaroxaban (10 mg/day) up to 35 days after discharge who were at risk for thrombosis, compared with the control group [97]. However, it is difficult for the results of this study to support long-term thromboprophylaxis in patients with long COVID-19 due to the short duration of drug administration. The NIH guidelines also do not recommend the continued routine administration of drugs to prevent thrombosis in patients with COVID-19 after discharge, except for clinical research purposes. It is recommended to determine whether to prevent thrombosis by considering the risk of thrombosis and bleeding risk regardless of the presence of long COVID-19 [96]. As such, there is no evidence to recommend the routine prevention of thrombosis in patients with long COVID-19. Therefore, it would be reasonable to decide whether to prevent thrombosis in patients with long COVID by considering the general risk of thrombosis and the risk of bleeding according to the underlying diseases and current conditions.
Key question 4) Is general rehabilitation/respiratory rehabilitation necessary for long COVID?
Recommendation
• If long COVID-19 persists in patients with severe COVID-19 treated in ICU or patients >65 years of age, appropriate and specific respiratory rehabilitation can be considered in consultation with a rehabilitation specialist.
A systematic literature review and meta-analysis evaluating the effects of respiratory rehabilitation in interstitial lung diseases, including COVID-19 reported that respiratory rehabilitation improved patient walking distance and quality of life, as well as dyspnea and lung function [98]. The results of a randomized controlled clinical trial including 72 patients >65 years of age showed that 10 minutes of daily respiratory rehabilitation (respiratory muscle training, cough exercise, diaphragmatic training, stretching exercise, and home exercise) administered for 6 weeks at 6 months after COVID-19 diagnosis improved several indicators of pulmonary function tests (forced expiratory volume in 1 second, FVC, and DLCO), 6MWT, quality of life, anxiety, depression, and activities of daily living [99]. A prospective cohort study that applied a cardiopulmonary rehabilitation program for 2 - 4 weeks from the 10th day after admission in patients with severe COVID-19 who were mostly hospitalized in the ICU observed improvements in 6MWT and mood [100]. A prospective cohort study including 33 patients with COVID-19 who had been hospitalized in ICU and were discharged, reported improved 6MWT and quality of life after performing various therapeutic exercises for 30 minutes daily [101]. In a recent study of 106 patients with long COVID, 44 performed virtual physical therapy, 25 home physical therapy, 17 independent exercise program, and 20 did not perform therapy. The virtual and home physical therapy groups met the clinically meaningful differences for improvements in lower limb strength and cardiopulmonary endurance [102]. In Korea, the National Rehabilitation Center of the Ministry of Health and Welfare has distributed an informational rehabilitation guide for people released from quarantine for COVID-19. The guide contains information on the management of breathing, daily life activities, physical activity and exercise, cognition (concentration and memory), swallowing, and voice [103].
Key question 5) How should persistent respiratory symptoms be treated?
Recommendation
• Evidence is insufficient to recommend or against specific medical treatments (corticosteroids, antihistamines, ipratropium bromide, aminophylline, or codeine) in patients with respiratory symptoms (dyspnea and cough) in long COVID.
A small prospective study on the use of prednisolone (0.5 mg/kg/day) for 3 weeks in 30 patients hospitalized for COVID-19 and diagnosed with organizing pneumonia 6 weeks after discharge reported improvement of symptoms and DLCO, FVC, and imaging tests in all patients [104]. However, another study reported spontaneous improvement of symptoms within 12 weeks in a patient group with similar characteristics [105]. Therefore, the benefit of corticosteroid treatment is unclear. In addition, the results of a small prospective observational study in which a combination of antihistamines (H1 antagonist + H2 antagonist for at least 4 weeks) was administered to 49 patients at least 3 months after COVID-19 showed improvement in clinical symptoms in 72% of patients [106]. However, the efficacy of steroids and antihistamines in long COVID has not been confirmed by well-designed randomized controlled clinical trials. In addition, no studies have reported the efficacy of ipratropium bromide, aminophylline, or codeine in treating long COVID. Therefore, there is insufficient evidence to recommend or against specific drug treatments for patients with persistent respiratory symptoms. According to the clinical situation of individual patients, the risks and benefits of each drug can be considered for use in symptomatic treatment.
Key question 6) How should smell and taste disturbances be managed?
Recommendation
• There is insufficient evidence to recommend specific treatments for smell/taste disorders caused by COVID-19. These disorders usually resolve slowly within weeks to months. Olfactory training, which is helpful in the treatment of postinfectious olfactory dysfunction that is often of respiratory viral origin and is a safe and easily available treatment, can also be considered in patients with olfactory dysfunction due to COVID-19.
In the case of complaints of taste abnormalities after the acute phase of COVID-19, the possibility of taste disorders due to dental diseases such as gingivitis or oral diseases such as oral thrush should first be excluded. In addition, it is necessary to check whether a new drug that may cause taste disturbance as a side effect (ACE inhibitor, angiotensin receptor blocker, calcium channel blocker, macrolide, etc.) has been started. Most of the symptoms of taste abnormalities are caused by smell abnormalities; hence, it is necessary to first check whether there is an olfactory disorder [107]. Taste disorders caused by the aftereffects of COVID-19 usually improve over time. There are no research data on the treatment of these taste disorders.
In the case of persistent complaints of olfactory impairment due to long COVID, nasal or sinus diseases such as rhinosinusitis, nasal polyp, and allergic rhinitis should be excluded. Olfactory dysfunction usually improves over time but may persist for several months in some patients. Olfactory dysfunction that improves relatively quickly, is understood to occur due to the closure of the olfactory system by local inflammation. In contrast, long-lasting olfactory dysfunction is explained by damage to the olfactory epithelium or nerve damage to the olfactory system [108]. Research on the treatment of olfactory dysfunction in COVID-19 patients is still lacking. In the meantime, randomized controlled clinical studies on the treatment of postinfectious olfactory dysfunction caused by respiratory viruses can be used as a reference. The Clinical Olfactory Working Group by Addison et al. presented recommendations based on a systematic literature review focusing on randomized controlled clinical studies on the treatment of olfactory disorders after respiratory infections [109]. They recommended olfactory training and quitting smoking to help improve olfactory disorders. In addition, the need for additional research was cited, as high-quality studies on intranasal or systemic steroids, zinc, α-lipoic acid, vitamin A, theophylline, minocycline, or caroverine treatment are lacking [109]. A randomized controlled clinical study of 100 patients with COVID-19 observed no significant difference between olfactory training and treatment with topical corticosteroid nasal spray (mometasone furoate) versus olfactory training alone [110].
As the olfactory nerve is regenerable, it is possible to restore olfactory function through repeated olfactory training. In general, the improvement of olfactory disorders after infection can be expected by repeating the olfactory training to sniff at least four different olfactory stimulants through the nose for 10 seconds each, twice daily, morning and evening [111,112]. Traditionally, the four odorants are used in olfactory training; namely, clove, eucalyptus, rose, and lemon but the same effect can be expected for odorants more familiar to Koreans [113]. Patients can also perform olfactory training at home using scented essential oils or commercially available smell training kits as odorants. Olfactory training has the advantages of low cost and few systemic side effects, so can be readily used to treat olfactory disorders that persist after COVID-19.
Key question 7) How should fatigue be managed?
Recommendation
• There is insufficient evidence to recommend or limit specific treatments for fatigue in patients with long COVID-19.
Various medications, alternative medicine, cognitive behavioral therapy, and exercise therapy for chronic fatigue syndrome associated with long COVID have been considered. Although some studies have suggested the benefit of rintatolimod, counseling therapy, and step-by-step exercise therapy [114,115], these studies have limitations in using different fatigue interventions and evaluation methods for the outcome. High-quality evidence-based studies are lacking regarding the effectiveness of treatments and interventions for fatigue in patients with long COVID-19.
Key question 8) How should headache and cognitive symptoms be treated?
Recommendation
• In the case of long COVID-related headache, the same symptomatic treatment used for primary headache can be considered after organic causes have been excluded. In particular, if the migraine pattern persists and interferes with daily life, migraine prophylaxis can be considered.
• For cognitive impairment associated with long COVID, evidence is insufficient to recommend or limit specific treatment.
Few studies have provided evidence of the appropriate treatment of long COVID-related headache. For patients with preexisting primary headache and COVID-19 as an exacerbating factor, symptomatic and preventive treatment may be considered according to the patient's characteristics and headache features [116]. If the diagnostic criteria for new daily persistent headache or chronic headache due to systemic infection are met, and if the clinical phenotype is similar to migraines, then prophylactic treatment for migraines can be considered. In the case of long COVID-related headache, there is a high risk of the development of medication-overuse headache due to the daily nature of the headache. This medication overuse headace may develop from using triptans or narcotic analgesics for >10 days a month and simple analgesics for >15 days a month for 3 months. Patients should be informed about the risk, and appropriate preventive treatment for such headache can be considered [116]. When a headache attack occurs, non-steroidal anti-inflammatory drugs (NSAIDs) and triptans can be used to treat acute attacks. NSAIDs can be safely used for mild headache attacks, despite early reports that questioned their safety in patients with COVID-19 owing to their potential role in the overexpression of ACE2 [117]. Triptans may be considered for moderate to severe headache attacks. Table 5 shows the most commonly used headache treatments [118].
Table 5. Drugs used for the treatment of headache.
| Category | Drug | Mechanism | Daily dose range | Side effects |
|---|---|---|---|---|
| Prophylactic treatment | Propranolol | Beta-blocker | 20 - 160 mg | Fatigue, dizziness, depression, vivid dreams |
| Flunarizine | Calcium channel blocker | 5 - 10 mg | Weight gain, sleepiness, dry mouth, dizziness, hypotension, depression, Parkinsonism | |
| Amitriptyline | Tricyclic antidepressant | 2.5 - 50 mg | Weight gain, constipation, asthenia, dizziness, drowsiness, fatigue, blurred vision, dry mouth | |
| Topiramate | Epilepsy medication | 12.5 - 150 mg | Paresthesia, weight loss, memory impairment | |
| Acute-phase treatment | Frovatriptan | Triptan | 2.5 - 5 mg | Triptan sensation (numbness, throbbing, strange feeling, warmth, burning, cold, tightness in body including neck and chest), dizziness, drowsiness, fatigue, asthenia, headache, nausea |
| Naratriptan | Triptan | 1 - 2 mg | Triptan sensation, dizziness, drowsiness, fatigue, asthenia, headache, nausea | |
| Zolmitriptan | Triptan | 2.5 - 7.5 mg | Triptan sensation, dizziness, drowsiness, fatigue, asthenia, headache, nausea |
When headache is associated with stress from mood disorder, sleep disorder, family, work, and pandemic-related socioeconomic difficulties, flunarizine and beta-blockers may exacerbate depressive symptoms. As topiramate can cause cognitive dysfunction, caution should be exercised if long COVID symptoms accompany cognitive or memory impairment. Along with drug treatment, lifestyle modifications such as maintaining a regular lifestyle, exercising, and avoiding long-term fasting should be performed simultaneously [116]. In particular, since sleep disorders that are common after COVID-19 are associated with headache, treating them may help with headache control [47].
Specific evidence for treating and managing cognitive impairment related to long COVID is still lacking. However, the brain fog seen in long COVID is not severe enough to limit daily life, so it is sometimes compared to chemo brain/chemo fog in that it shows mild abnormalities in cognitive tests or the abnormal findings are not clear [119]. Chemobrain/chemo fog is a condition in which survivors who have received chemotherapy complain of cognitive impairment, such as concentration difficulties and amnesia, without any abnormal findings in cognitive tests. According to the chemobrain/chemo fog-related recommendations suggested by the Mayo Clinic, regular exercise, tracking what affects cognitive dysfunction, and developing stress relief and coping strategies should be considered. In addition, it is necessary to actively manage fatigue, depression, anxiety, and sleep problems, which are associated with long COVID-related cognitive symptoms [120]. While drug treatments such as methylphenidate, modafinil, donepezil, and memantine have been proposed [120], further research is needed. Moreover, luteolin, a natural flavonoid, has been proposed as a potential treatment for long COVID-related brain fog symptoms by inhibiting the activation of mast cells and microglia; however, additional research is needed [121].
Key question 9) How should psychological/psychiatric symptoms be treated?
Recommendation
• While some studies have reported that selective serotonin reuptake inhibitors (SSRIs) are effective in major depressive disorder caused by long COVID, there is insufficient evidence to recommend or limit specific treatment.
• If patients with long COVID require psychiatric and drug treatment, referrals for psychiatric treatment are recommended.
When SSRIs were administered to 60 patients in Italy reporting major depressive episodes after COVID-19, 92% of patients reported >50% decrease in Hamilton Depression Rating Scale scores after 4 weeks [122]. Clomipramine, a tricyclic antidepressant with an anti-inflammatory action that penetrates the central nervous system, has been proposed as a potential treatment for central nervous system sequelae after COVID-19. However, no randomized controlled clinical trial has been performed [123]. Other antidepressants with increased tolerance, such as SSRIs, may also have anti-inflammatory properties. Clinical research and clinical trials are needed regarding these candidate therapeutics. Fluvoxamine, a type of SSRI, has been proposed as a novel treatment for long COVID [124,125,126]. The use of fluvoxamine in the early stages of acute COVID-19 was effective in preventing the worsening of clinical symptoms [127]. Therefore, there is no evidence to recommend routine administration of antidepressants in long COVID patients without mood disorders. If antidepressant administration is necessary, it would be desirable to seek treatment from a psychiatrist.
Research results have also suggested that COVID-19-related alteration in tryptophan absorption and metabolism could be the underlying pathophysiology of long-COVID symptoms [128,129]. By interfering with the long-term control of L-tryptophan absorption and favoring the kynurenine pathway in metabolism, the increased levels of kynurenine might cause symptoms such as depression, insomnia, fatigue, and muscle weakness, similar to L-tryptophan deficiency. However, it is not yet known whether L-tryptophan administration is an effective treatment for long COVID-19 [130].
Key question 10) Is steroid administration helpful for patients with long COVID?
Recommendation
• There is insufficient evidence to recommend or limit steroid administration in patients with long COVID. The decision to administer steroids should be made considering their benefits and side effects.
Several studies have hypothesized that cognitive impairment, headache, and olfactory/taste impairment of long COVID are caused by persistent neuroinflammation [131]. Another hypothesis is that the inflammatory response induced by the coronavirus causes microvascular disorders and pulmonary fibrosis, resulting in cardiovascular and respiratory symptoms [131]. A cross-sectional study in Spain of 121 patients with mild COVID-19 reported that even if the combination of neutrophil count, CRP, and fibrinogen was in the normal range, patients with relatively high levels of these inflammatory markers were more likely to experience long COVID [132]. Despite these hypotheses, it is not clear whether steroid administration lowers the risk of developing long COVID and improves function or symptoms in patients with long COVID. A randomized controlled clinical trial conducted in Egypt observed no significant difference in smell score between the patient group receiving corticosteroid nasal spray therapy for 3 weeks and the control group [110]. An observational study in the UK reported significant improvement in pulmonary function when steroids were administered for 3 weeks to 35 patients with COVID-19 with interstitial lung lesions that persisted beyond 4 weeks. However, there are limitations in interpreting these results, as this was a single-arm study [104]. In addition, several reports suggest that the administration of steroids to treat acute COVID-19 is associated with side effects such as mucormycosis and avascular necrosis [133,134]. Therefore, when considering steroid administration to improve the function or symptoms of patients with long COVID, the benefits and risks should be individually weighed and decided. In addition, caution should be exercised because there are cases in which COVID-19 aggravates existing adrenal insufficiency or that new onset of adrenal insufficiency after COVID-19 is sometimes mistaken for long COVID.
Key question 11) What special cautions are needed for the diagnosis and treatment of long COVID in children and adolescents?
Recommendation
• In children and adolescents, the diagnosis of long COVID should be carefully made by sufficiently excluding other acute/chronic viral infections and somatic symptoms under various stress.
• There is no evidence yet to recommend or limit specific treatment for long COVID in children and adolescents. However, symptomatic treatment according to general recommendations should be administered first and, if symptoms persist for a long time or if the severity is high, the patients should be referred to a pediatric specialist in each field for additional evaluation and management.
In children and adolescents, non-specific respiratory, gastrointestinal, and/or systemic symptoms easily occur due to frequent viral infections. Therefore, it is not easy to define symptoms that develop or persist after acute COVID-19 as having been caused by COVID-19. In addition, in adolescents, it is difficult to diagnose long COVID since various somatic and neuropsychiatric symptoms can easily develop due to mental/physical stress. Furthermore, studies for signs/symptoms of long COVID symptoms in children and adolescents are lacking. Therefore, the WHO recommends using different criteria for defining long COVID in children and adolescents. However, no specific criteria have been officially suggested. Nevertheless, the incidence of long COVID in children and adolescents is relatively low when the general definition for adults is applied.
Recent studies reported incidence rates of symptoms lasting up to 4 weeks after COVID-19 diagnosis; 15 - 32% in small prospective observational studies conducted in children <18 years of age [135,136], and up to 50% in a large retrospective study of only adolescents [137]. The common symptoms in these studies were cough, shortness of breath, fatigue, headache, febrile sensation, insomnia, asthenia, constipation, and abdominal pain. In the CLoCk study conducted in the UK of 3,065 adolescents (11 - 17 years of age) diagnosed between January and March 2021, fatigue (39%), headache (23%), and dyspnea (23%) were the common symptoms present 3 months after COVID-19 diagnosis; however, fatigue (24%) and headache (14%) were also relatively common among 3,739 control adolescents tested negative for COVID-19 [138]. In particular, as fatigue and headache were complaints in 30% and 20% of 11 - 15-year-olds, respectively, even before the pandemic, there is considerable difficulty in considering these to be symptoms of long COVID [139].
In a case-control study of 28,270 adolescents aged 15 – 18 years in Denmark, the common symptoms lasting >2 months after COVID-19 were fatigue (18%), loss of appetite (10%), and headache (9%); however, respiratory difficulty (odds ratio [OR]: 2.7), cough (OR: 1.6), and sore throat (OR: 1.6) are significantly more common in COVID-19 adolescents compared with the control group [137]. In another study comparing 37,522 patients and 78,037 controls aged 0 - 17 years, preschool children with COVID-19 had significantly more symptoms (lasting >4 weeks) of fatigue, loss of smell/taste, and muscle weakness, and school-age children had more symptoms of loss of smell/taste, fatigue, respiratory symptoms, dizziness, muscle weakness, and chest pain compared with the control group [140]. In existing studies, children and adolescents show diverse symptoms with various frequencies after acute COVID-19, and many types of symptoms are subjective or non-specific. Therefore, careful consideration and evaluation to exclude other causes are needed to diagnose long COVID.
Long COVID develops more common in adolescents compared with young infants/children, especially in those with low pre-existing physical or mental health. Recent studies of children with COVID-19 showed no significant association between the incidence of long COVID and the severity of the acute phase or gender, unlike in the results in adults [135,136]. However, a study that included only adolescents showed that long COVID was more common in girls [137]. A UK study of non-hospitalized adolescents reported that long COVID symptoms involving two or more organs were more likely to be observed in girls compared with boys, in patients aged 15 - 17 years than in those aged 11 - 4 years, and in those with poorer previous physical and mental health before COVID-19 [138]. Therefore, in adolescents who currently have physical or mental comorbidities, the possibility of long COVID should be considered.
Most of the symptoms persisting after acute COVID-19 in children and adolescents are improved within 3 months [135,136,140]; thus, they might be resolved spontaneously without meeting the general definition for long COVID. If persistent symptoms beyond 12 weeks after COVID-19 are present, symptomatic treatments recommended in cases of usual viral infection or functional disorder can be considered after sufficiently excluding other causes. There is no evidence to recommend or limit specific drugs. Excessive exercise, physical/mental activity, and stress may contribute to the exacerbation or recurrence of long COVID [6]; therefore, careful attention and management are required regarding these factors. Because meaningful long COVID in adolescents tends to occur as a symptom group involving three or more organs [136,138], a coordinated multidisciplinary approach is required when interventions such as drug therapy are needed.
Key question 12) Does COVID-19 vaccination affect the development of long COVID?
Recommendation
• There is no evidence that COVID-19 vaccination increases the incidence of long COVID after acute COVID-19. Therefore, there is no need to avoid vaccination because of this concern.
Vaccination against COVID-19 reportedly reduces the risk of developing long COVID-19 after infection by approximately 15%. In a study conducted in Israel, patients who had received two doses of COVID-19 vaccination before COVID-19 diagnosis were less likely to develop long COVID symptoms than those who had not been vaccinated [141]. In addition, in a UK study comparing vaccinated and unvaccinated patients with similar sociodemographic characteristics, the incidence of long COVID was significantly lower in the vaccinated group (9.5% vs. 14.6%) [20]. However, there remains a lack of definitive evidence on the effect of COVID-19 vaccination on the incidence of long COVID. Therefore, further research is needed.
Footnotes
Funding: None.
Conflict of Interest: No conflict of interest.
- Conceptualization: YJK, SEK, KWY, SHL, YPC.
- Data curation: YJK, TK, EL, JWS, YHJ, YPC.
- Investigation: YJK, SEK, TK, KWY, SHL, EL, JWS, YHJ, YPC.
- Methodology: YPC.
- Project administration: YPC.
- Supervision: YPC.
- Validation: YJK, YPC.
- Visualization: YJK, YPC.
- Writing - original draft: YJK, SEK, TK, KWY, SHL, EL, JWS, YHJ, YPC.
- Writing - review & editing: YJK, SEK, TK, KWY, SHL, EL, JWS, YHJ, YPC.
SUPPLEMENTARY MATERIAL
References
- 1.World Health Organization (WHO) WHO coronavirus (COVID-19) dashboard. [Accessed 15 June 2022]. Available at: https://covid19.who.int/
- 2.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence (NICE) COVID-19 rapid guideline: managing the long-term effects of COVID-19. [Accessed 15 June 2022]. Available at: https://www.nice.org.uk/guidance/ng188. [PubMed]
- 4.Yelin D, Moschopoulos CD, Margalit I, Gkrania-Klotsas E, Landi F, Stahl JP, Yahav D. ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect. 2022;28:955–972. doi: 10.1016/j.cmi.2022.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institutes of Health (NIH) NIH launches new initiative to study “Long COVID”. [Accessed 13 June 2022]. Available at: https://covid19.nih.gov/covid-19-topics/long-covid.
- 6.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, Redfield S, Austin JP, Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorna R, MacDermott N, Rayner C, O’Hara M, Evans S, Agyen L, Nutland W, Rogers N, Hastie C. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455–457. doi: 10.1016/S0140-6736(20)32705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayoubkhani D. Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 1 April 2021. [Accessed 13 June 2022]. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021.
- 9.Jennings G, Monaghan A, Xue F, Mockler D, Romero-Ortuño R. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. 2021;10:5913. doi: 10.3390/jcm10245913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacobucci G. Long covid: damage to multiple organs presents in young, low risk patients. BMJ. 2020;371:m4470. [Google Scholar]
- 11.Ziauddeen N, Gurdasani D, O’Hara ME, Hastie C, Roderick P, Yao G, Alwan NA. Characteristics and impact of long Covid: findings from an online survey. PLoS One. 2022;17:e0264331. doi: 10.1371/journal.pone.0264331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y, Kim SW, Chang HH, Kwon KT, Bae S, Hwang S. Significance and associated factors of long-term sequelae in patients after acute COVID-19 infection in Korea. Infect Chemother. 2021;53:463–476. doi: 10.3947/ic.2021.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y, Bitna-Ha, Kim SW, Chang HH, Kwon KT, Bae S, Hwang S. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect Dis. 2022;22:93. doi: 10.1186/s12879-022-07062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Kim SW, Chang HH, Kwon KT, Hwang S, Bae S. One year follow-up of COVID-19 related symptoms and patient quality of life: a prospective cohort study. Yonsei Med J. 2022;63:499–510. doi: 10.3349/ymj.2022.63.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, O’Hara M, Suett J, Dahmash D, Bugaeva P, Rigby I, Munblit D, Harriss E, Burls A, Foote C, Scott J, Carson G, Olliaro P, Sigfrid L, Stavropoulou C. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6:e005427. doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75:e14357. doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 18.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu HY. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4:e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund LC, Hallas J, Nielsen H, Koch A, Mogensen SH, Brun NC, Christiansen CF, Thomsen RW, Pottegård A. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021;21:1373–1382. doi: 10.1016/S1473-3099(21)00211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayoubkhani D, Bosworth ML, King S, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, Khunti K, Alwan NA, Walker AS. Risk of long Covid in people infected with SARS-CoV-2 after two doses of a COVID-19 vaccine: community-based, matched cohort study. medRxiv. 2022:2022.02.23.22271388. doi: 10.1093/ofid/ofac464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18:e1003773. doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, Jia JL, Li LM, Mao HL, Zhou XM, Luo H, Gao YF, Xu AG. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frija-Masson J, Debray MP, Gilbert M, Lescure FX, Travert F, Borie R, Khalil A, Crestani B, d’Ortho MP, Bancal C. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30\xe2\x80\x85days post-infection. Eur Respir J. 2020;56:2001754. doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, Lei C, Chen R, Zhong N, Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H, van Hees HWH, van Helvoort H, van den Boogaard M, van der Hoeven H, Reijers MH, Prokop M, Vercoulen J, van den Heuvel M. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2021;73:e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17:701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J, Zhong Z, Li H, Ji P, Pang J, Li B, Zhang J. CT imaging features of 4121 patients with COVID-19: a meta-analysis. J Med Virol. 2020;92:891–902. doi: 10.1002/jmv.25910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos-Casals M, Brito-Zerón P, Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. 2021;17:315–332. doi: 10.1038/s41584-021-00608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patell R, Bogue T, Koshy A, Bindal P, Merrill M, Aird WC, Bauer KA, Zwicker JI. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts LN, Whyte MB, Georgiou L, Giron G, Czuprynska J, Rea C, Vadher B, Patel RK, Gee E, Arya R. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136:1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Liu Y, Cai X, Zhang W, Li Y, Fu C. Prevalence of venous thromboembolism in critically ill patients with coronavirus disease 2019: a meta-analysis. Front Med (Lausanne) 2021;8:603558. doi: 10.3389/fmed.2021.603558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puig-Domingo M, Marazuela M, Yildiz BO, Giustina A. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine. 2021;72:301–316. doi: 10.1007/s12020-021-02734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isidori AM, Arnaldi G, Boscaro M, Falorni A, Giordano C, Giordano R, Pivonello R, Pofi R, Hasenmajer V, Venneri MA, Sbardella E, Simeoli C, Scaroni C, Lenzi A. COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J Endocrinol Invest. 2020;43:1141–1147. doi: 10.1007/s40618-020-01266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carosi G, Morelli V, Del Sindaco G, Serban AL, Cremaschi A, Frigerio S, Rodari G, Profka E, Indirli R, Mungari R, Resi V, Orsi E, Ferrante E, Dolci A, Giavoli C, Arosio M, Mantovani G. Adrenal insufficiency at the time of COVID-19: a retrospective study in patients referring to a tertiary center. J Clin Endocrinol Metab. 2021;106:e1354–e1361. doi: 10.1210/clinem/dgaa793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, Mingrone G, Boehm B, Cooper ME, Chai Z, Del Prato S, Ji L, Hopkins D, Herman WH, Khunti K, Mbanya JC, Renard E. New-onset diabetes in Covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.di Filippo L, Formenti AM, Doga M, Frara S, Rovere-Querini P, Bosi E, Carlucci M, Giustina A. Hypocalcemia is a distinctive biochemical feature of hospitalized COVID-19 patients. Endocrine. 2021;71:9–13. doi: 10.1007/s12020-020-02541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchings N, Babalyan V, Baghdasaryan S, Qefoyan M, Sargsyants N, Aghajanova E, Martirosyan A, Harutyunyan R, Lesnyak O, Formenti AM, Giustina A, Bilezikian JP. Patients hospitalized with COVID-19 have low levels of 25-hydroxyvitamin D. Endocrine. 2021;71:267–269. doi: 10.1007/s12020-020-02597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, Ferrante E, Orsi E, Resi V, Longari V, Cuzzocrea M, Bandera A, Lazzaroni E, Dolci A, Ceriotti F, Re TE, Gori A, Arosio M, Salvi M. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8:739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105:dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu YH, Chen Y, Wang QH, Wang LR, Jiang L, Yang Y, Chen X, Li Y, Cen Y, Xu C, Zhu J, Li W, Wang YR, Zhang LL, Liu J, Xu ZQ, Wang YJ. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022;79:509–517. doi: 10.1001/jamaneurol.2022.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cicco S, Vacca A, Cittadini A, Marra AM. Long-term follow-up may be useful in coronavirus disease 2019 survivors to rrevent chronic complications. Infect Chemother. 2020;52:407–409. doi: 10.3947/ic.2020.52.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, Zhang X, Qu Y, Fan Y, Li X, Li C, Yu T, Xia J, Wei M, Chen L, Li Y, Xiao F, Liu D, Wang J, Wang X, Cao B. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivan M, Taylor S. NICE guideline on long covid. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 45.Sisó-Almirall A, Brito-Zerón P, Conangla Ferrín L, Kostov B, Moragas Moreno A, Mestres J, Sellarès J, Galindo G, Morera R, Basora J, Trilla A, Ramos-Casals M On Behalf Of The CAMFiC Long Covid-Study Group. Long Covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Public Health. 2021;18:4350. doi: 10.3390/ijerph18084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin W, Chen S, Zhang Y, Dong F, Zhang Z, Hu B, Zhu Z, Li F, Wang X, Wang Y, Zhen K, Wang J, Wan Y, Li H, Elalamy I, Li C, Zhai Z, Wang C. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J. 2021;58:2003677. doi: 10.1183/13993003.03677-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallia P, Meghji J, Wong B, Kumar K, Pilkington V, Chhabra S, Russell B, Chen J, Srikanthan K, Park M, Owles H, Liew F, Alcada J, Martin L, Coleman M, Elkin S, Ross C, Agrawal S, Gardiner T, Bell A, White A, Hampson D, Vithlani G, Manalan K, Bramer S, Martin Segura A, Kucheria A, Ratnakumar P, Sheeka A, Anandan L, Copley S, Russell G, Bloom CI, Kon OM. Symptomatic, biochemical and radiographic recovery in patients with COVID-19. BMJ Open Respir Res. 2021;8:e000908. doi: 10.1136/bmjresp-2021-000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Cruz RF, Waller MD, Perrin F, Periselneris J, Norton S, Smith LJ, Patrick T, Walder D, Heitmann A, Lee K, Madula R, McNulty W, Macedo P, Lyall R, Warwick G, Galloway JB, Birring SS, Patel A, Patel I, Jolley CJ. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021;7:00655–02020. doi: 10.1183/23120541.00655-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, Attwood M, Noel A, Gunning S, Hatrick J, Hamilton S, Elvers KT, Hyams C, Bibby A, Moran E, Adamali HI, Dodd JW, Maskell NA, Barratt SL. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Froidure A, Mahsouli A, Liistro G, De Greef J, Belkhir L, Gérard L, Bertrand A, Koenig S, Pothen L, Yildiz H, Mwenge B, Aboubakar F, Gohy S, Pilette C, Reychler G, Coche E, Yombi JC, Ghaye B. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir Med. 2021;181:106383. doi: 10.1016/j.rmed.2021.106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Ramirez DC, Normand K, Zhaoyun Y, Torres-Castro R. Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines. 2021;9:900. doi: 10.3390/biomedicines9080900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, Okell T, Sheerin F, Xie C, Mahmod M, Mózes FE, Lewandowski AJ, Ohuma EO, Holdsworth D, Lamlum H, Woodman MJ, Krasopoulos C, Mills R, McConnell FAK, Wang C, Arthofer C, Lange FJ, Andersson J, Jenkinson M, Antoniades C, Channon KM, Shanmuganathan M, Ferreira VM, Piechnik SK, Klenerman P, Brightling C, Talbot NP, Petousi N, Rahman NM, Ho LP, Saunders K, Geddes JR, Harrison PJ, Pattinson K, Rowland MJ, Angus BJ, Gleeson F, Pavlides M, Koychev I, Miller KL, Mackay C, Jezzard P, Smith SM, Neubauer S. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song WJ, Hui CKM, Hull JH, Birring SS, McGarvey L, Mazzone SB, Chung KF. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9:533–544. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewek J, Jatczak-Pawlik I, Maciejewski M, Jankowski P, Banach M. COVID-19 and cardiovascular complications - preliminary results of the LATE-COVID study. Arch Med Sci. 2021;17:818–822. doi: 10.5114/aoms/134211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tudoran C, Tudoran M, Pop GN, Giurgi-Oncu C, Cut TG, Lazureanu VE, Oancea C, Parv F, Ciocarlie T, Bende F. Associations between the severity of the post-acute COVID-19 syndrome and echocardiographic abnormalities in previously healthy outpatients following infection with SARS-CoV-2. Biology (Basel) 2021;10:469. doi: 10.3390/biology10060469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayama H, Ide S, Moroi M, Kitami Y, Bekki N, Kubota S, Uemura Y, Hara H, Kutsuna S, Ohmagari N, Hiroi Y. Elevated high-sensitivity troponin is associated with subclinical cardiac dysfunction in patients recovered from coronavirus disease 2019. Glob Health Med. 2021;3:95–101. doi: 10.35772/ghm.2021.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, Kurz K, Koppelstätter S, Haschka D, Petzer V, Boehm A, Aichner M, Tymoszuk P, Lener D, Theurl M, Lorsbach-Köhler A, Tancevski A, Schapfl A, Schaber M, Hilbe R, Nairz M, Puchner B, Hüttenberger D, Tschurtschenthaler C, Aßhoff M, Peer A, Hartig F, Bellmann R, Joannidis M, Gollmann-Tepeköylü C, Holfeld J, Feuchtner G, Egger A, Hoermann G, Schroll A, Fritsche G, Wildner S, Bellmann-Weiler R, Kirchmair R, Helbok R, Prosch H, Rieder D, Trajanoski Z, Kronenberg F, Wöll E, Weiss G, Widmann G, Löffler-Ragg J, Tancevski I. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57:2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Małek ŁA, Marczak M, Miłosz-Wieczorek B, Konopka M, Braksator W, Drygas W, Krzywański J. Cardiac involvement in consecutive elite athletes recovered from Covid-19: a magnetic resonance study. J Magn Reson Imaging. 2021;53:1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan C, Zhang Z, Luo L, Wu W, Jia T, Lu L, Liu WV, Qin Y, Hu F, Ding X, Qin P, Qian L, Chen J, Li S. Cardiac T1 and T2 mapping showed myocardial involvement in recovered COVID-19 patients initially considered devoid of cardiac damage. J Magn Reson Imaging. 2021;54:421–428. doi: 10.1002/jmri.27534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joy G, Artico J, Kurdi H, Seraphim A, Lau C, Thornton GD, Oliveira MF, Adam RD, Aziminia N, Menacho K, Chacko L, Brown JT, Patel RK, Shiwani H, Bhuva A, Augusto JB, Andiapen M, McKnight A, Noursadeghi M, Pierce I, Evain T, Captur G, Davies RH, Greenwood JP, Fontana M, Kellman P, Schelbert EB, Treibel TA, Manisty C, Moon JC COVIDsortium investigators. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc Imaging. 2021;14:2155–2166. doi: 10.1016/j.jcmg.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS, Smithline HA, Gong MN, Aboodi MS, Exline MC, Henning DJ, Wilson JG, Khan A, Qadir N, Brown SM, Peltan ID, Rice TW, Hager DN, Ginde AA, Stubblefield WB, Patel MM, Self WH, Feldstein LR IVY Network Investigators; CDC COVID-19 Response Team; IVY Network Investigators. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daher A, Balfanz P, Cornelissen C, Müller A, Bergs I, Marx N, Müller-Wieland D, Hartmann B, Dreher M, Müller T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosales-Castillo A, García de Los Ríos C, Mediavilla García JD. Persistent symptoms after acute COVID-19 infection: importance of follow-up. Med Clin (Engl Ed) 2021;156:35–36. doi: 10.1016/j.medcle.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carfì A, Bernabei R, Landi F Gemelli Against COVID-19 Post-Acute Care Study Group. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, Heightman M, Hillman TE, Jacob J, Jarvis HC, Lipman MCI, Naidu SB, Nair A, Porter JC, Tomlinson GS, Hurst JR ARC Study Group. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, Doucet L, Berkani S, Oliosi E, Mallart E, Corre F, Zarrouk V, Moyer JD, Galy A, Honsel V, Fantin B, Nguyen Y. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rimmer A. Covid-19: impact of long term symptoms will be profound, warns BMA. BMJ. 2020;370:m3218. doi: 10.1136/bmj.m3218. [DOI] [PubMed] [Google Scholar]
- 69.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 70.Chung KI, Song CH. Clinical usefulness of fatigue severity scale for patients with fatigue, and anxiety or depression. Korean J Psychosom Med. 2001;9:164–173. [Google Scholar]
- 71.Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, O’Connor L, Leavy D, O’Brien K, Dowds J, Sugrue JA, Hopkins D, Martin-Loeches I, Ni Cheallaigh C, Nadarajan P, McLaughlin AM, Bourke NM, Bergin C, O’Farrelly C, Bannan C, Conlon N. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alrubaye R, Choudhury H. Severe Rhabdomyolysis in a 35-year-old woman with COVID-19 due to SARS-CoV-2 infection: a case report. Am J Case Rep. 2020;21:e926733. doi: 10.12659/AJCR.926733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan KH, Farouji I, Abu Hanoud A, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19) Am J Emerg Med. 2020;38:1548.e1–1548.e3. doi: 10.1016/j.ajem.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Husain R, Corcuera-Solano I, Dayan E, Jacobi AH, Huang M. Rhabdomyolysis as a manifestation of a severe case of COVID-19: a case report. Radiol Case Rep. 2020;15:1633–1637. doi: 10.1016/j.radcr.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khosla SG, Nylen ES, Khosla R. Rhabdomyolysis in patients hospitalized with COVID-19 infection: five case series. J Investig Med High Impact Case Rep. 2020;8:2324709620984603. doi: 10.1177/2324709620984603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, Raza H, Okeke O, Dewar RL, Higgins BP, Tolstenko K, Kwan RW, Gittens KR, Seamon CA, McCormack G, Shaw JS, Okpali GM, Law M, Trihemasava K, Kennedy BD, Shi V, Justement JS, Buckner CM, Blazkova J, Moir S, Chun TW, Lane HC. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med. 2022;175:969–979. doi: 10.7326/M21-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dos Santos PK, Sigoli E, Bragança LJG, Cornachione AS. The musculoskeletal involvement after mild to moderate COVID-19 infection. Front Physiol. 2022;13:813924. doi: 10.3389/fphys.2022.813924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martelletti P, Bentivegna E, Spuntarelli V, Luciani M. Long-COVID headache. SN Compr Clin Med. 2021;3:1704–1706. doi: 10.1007/s42399-021-00964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, Suen J, Robba C, Fraser J, Cho SM. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bolay H, Gül A, Baykan B. COVID-19 is a real headache! Headache. 2020;60:1415–1421. doi: 10.1111/head.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diaz-Mitoma F, Vanast WJ, Tyrrell DL. Increased frequency of Epstein-Barr virus excretion in patients with new daily persistent headaches. Lancet. 1987;1:411–415. doi: 10.1016/s0140-6736(87)90119-x. [DOI] [PubMed] [Google Scholar]
- 82.Rozen TD. Daily persistent headache after a viral illness during a worldwide pandemic may not be a new occurrence: Lessons from the 1890 Russian/Asiatic flu. Cephalalgia. 2020;40:1406–1409. doi: 10.1177/0333102420965132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nurek M, Rayner C, Freyer A, Taylor S, Järte L, MacDermott N, Delaney BC Delphi panellists. Recommendations for the recognition, diagnosis, and management of long COVID: a Delphi study. Br J Gen Pract. 2021;71:e815–e825. doi: 10.3399/BJGP.2021.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JLR, Griffanti L, Duff E, Jbabdi S, Taschler B, Keating P, Winkler AM, Collins R, Matthews PM, Allen N, Miller KL, Nichols TE, Smith SM. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, Guis S, Barthelemy F, Habert P, Ceccaldi M, Million M, Raoult D, Cammilleri S, Eldin C. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:2823–2833. doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Becker JH, Lin JJ, Doernberg M, Stone K, Navis A, Festa JR, Wisnivesky JP. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw Open. 2021;4:e2130645. doi: 10.1001/jamanetworkopen.2021.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.British Thoracic Society. British Thoracic Society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia. [Accessed 5 June 2022]. Available at: https://www.brit-thoracic.org.uk/covid-19/covid-19-information-for-the-respiratory-community/
- 88.NHS England. National commissioning guidance for post COVID services. [Accessed 15 June 2022]. Available at: https://allcatsrgrey.org.uk/wp/download/commissioning/C1248-national-guidance-post-covid-syndrome-assessment-clinics-v2.pdf.
- 89.Bonazza F, Borghi L, di San Marco EC, Piscopo K, Bai F, Monforte AD, Vegni E. Psychological outcomes after hospitalization for COVID-19: data from a multidisciplinary follow-up screening program for recovered patients. Res Psychother. 2021;23:491. doi: 10.4081/ripppo.2020.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong F, Liu HL, Dai N, Yang M, Liu JP. A living systematic review of the psychological problems in people suffering from COVID-19. J Affect Disord. 2021;292:172–188. doi: 10.1016/j.jad.2021.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.World Health Organization (WHO) Living guidance for clinical management of COVID-19. [Accessed 10 June 2022]. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2.
- 92.Li L, Li F, Fortunati F, Krystal JH. Association of a prior psychiatric diagnosis with mortality among hospitalized patients with coronavirus disease 2019 (COVID-19) infection. JAMA Netw Open. 2020;3:e2023282. doi: 10.1001/jamanetworkopen.2020.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bourne RS, Mills GH. Sleep disruption in critically ill patients--pharmacological considerations. Anaesthesia. 2004;59:374–384. doi: 10.1111/j.1365-2044.2004.03664.x. [DOI] [PubMed] [Google Scholar]
- 94.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6:60. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guedj E, Campion JY, Horowitz T, Barthelemy F, Cammilleri S, Ceccaldi M. The impact of COVID-19 lockdown on brain metabolism. Hum Brain Mapp. 2022;43:593–597. doi: 10.1002/hbm.25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.National Institutes of Health (NIH) COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. [Accessed 10 June 2022]. Available at: https://www.covid19treatmentguidelines.nih.gov/
- 97.Ramacciotti E, Barile Agati L, Calderaro D, Aguiar VCR, Spyropoulos AC, de Oliveira CCC, Lins Dos Santos J, Volpiani GG, Sobreira ML, Joviliano EE, Bohatch Júnior MS, da Fonseca BAL, Ribeiro MS, Dusilek C, Itinose K, Sanches SMV, de Almeida Araujo Ramos K, de Moraes NF, Tierno PFGMM, de Oliveira ALML, Tachibana A, Chate RC, Santos MVB, de Menezes Cavalcante BB, Moreira RCR, Chang C, Tafur A, Fareed J, Lopes RD MICHELLE investigators. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;399:50–59. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reina-Gutiérrez S, Torres-Costoso A, Martínez-Vizcaíno V, Núñez de Arenas-Arroyo S, Fernández-Rodríguez R, Pozuelo-Carrascosa DP. Effectiveness of pulmonary rehabilitation in interstitial lung disease, including coronavirus diseases: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2021;102:1989–97.e3. doi: 10.1016/j.apmr.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract. 2020;39:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hermann M, Pekacka-Egli AM, Witassek F, Baumgaertner R, Schoendorf S, Spielmanns M. Feasibility and efficacy of cardiopulmonary rehabilitation after COVID-19. Am J Phys Med Rehabil. 2020;99:865–869. doi: 10.1097/PHM.0000000000001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Udina C, Ars J, Morandi A, Vilaró J, Cáceres C, Inzitari M. Rehabilitation in adult post-COVID-19 patients in post-acute care with therapeutic exercise. J Frailty Aging. 2021;10:297–300. doi: 10.14283/jfa.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hameed F, Palatulan E, Jaywant A, Said R, Lau C, Sood V, Layton A, Gellhorn A. Outcomes of a COVID-19 recovery program for patients hospitalized with SARS-CoV-2 infection in New York City: a prospective cohort study. PM R. 2021;13:609–617. doi: 10.1002/pmrj.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.National Rehabilitation Center. Rehabilitation guide for patients with COVID-19 related illness. [Accessed 20 June 2022]. Available at: http://www.nrc.go.kr/portal/board/boardView.do?no=18152&menu_cd=09_01_00_01&board_id=NRC_NOTICE_BOARD&bn=qnaView&fno=40&pageIndex=1.
- 104.Myall KJ, Mukherjee B, Castanheira AM, Lam JL, Benedetti G, Mak SM, Preston R, Thillai M, Dewar A, Molyneaux PL, West AG. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Denneny EK, Garthwaite HS, Heightman MJ, Porter JC. A role for steroids in COVID-19-associated pneumonitis at six-week follow-up? Ann Am Thorac Soc. 2021;18:1082–1083. doi: 10.1513/AnnalsATS.202101-048LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70:61–67. doi: 10.1136/jim-2021-002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malaty J, Malaty IA. Smell and taste disorders in primary care. Am Fam Physician. 2013;88:852–859. [PubMed] [Google Scholar]
- 108.Xydakis MS, Albers MW, Holbrook EH, Lyon DM, Shih RY, Frasnelli JA, Pagenstecher A, Kupke A, Enquist LW, Perlman S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021;20:753–761. doi: 10.1016/S1474-4422(21)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Addison AB, Wong B, Ahmed T, Macchi A, Konstantinidis I, Huart C, Frasnelli J, Fjaeldstad AW, Ramakrishnan VR, Rombaux P, Whitcroft KL, Holbrook EH, Poletti SC, Hsieh JW, Landis BN, Boardman J, Welge-Lüssen A, Maru D, Hummel T, Philpott CM. Clinical Olfactory Working Group consensus statement on the treatment of postinfectious olfactory dysfunction. J Allergy Clin Immunol. 2021;147:1704–1719. doi: 10.1016/j.jaci.2020.12.641. [DOI] [PubMed] [Google Scholar]
- 110.Abdelalim AA, Mohamady AA, Elsayed RA, Elawady MA, Ghallab AF. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am J Otolaryngol. 2021;42:102884. doi: 10.1016/j.amjoto.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Damm M, Pikart LK, Reimann H, Burkert S, Göktas Ö, Haxel B, Frey S, Charalampakis I, Beule A, Renner B, Hummel T, Hüttenbrink KB. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014;124:826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]
- 112.Hura N, Xie DX, Choby GW, Schlosser RJ, Orlov CP, Seal SM, Rowan NR. Treatment of post-viral olfactory dysfunction: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2020;10:1065–1086. doi: 10.1002/alr.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim DY, Ha JH, Lee JH, Kim HJ, Park DY. Comparing the effectiveness of olfactory training, according as type and preference of odorant. J Rhinol. 2019;26:92–98. [Google Scholar]
- 114.Smith ME, Haney E, McDonagh M, Pappas M, Daeges M, Wasson N, Fu R, Nelson HD. Treatment of myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review for a National Institutes of Health pathways to prevention workshop. Ann Intern Med. 2015;162:841–850. doi: 10.7326/M15-0114. [DOI] [PubMed] [Google Scholar]
- 115.Fowler-Davis S, Platts K, Thelwell M, Woodward A, Harrop D. A mixed-methods systematic review of post-viral fatigue interventions: are there lessons for long Covid? PLoS One. 2021;16:e0259533. doi: 10.1371/journal.pone.0259533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Membrilla JA, Caronna E, Trigo-López J, González-Martínez A, Layos-Romero A, Pozo-Rosich P, Guerrero-Peral Á, Gago-Veiga AB, Andrés-López A, de Terán JD. Persistent headache after COVID-19: Pathophysioloy, clinic and treatment. Neurology Perspectives. 2021;1:S31–S36. doi: 10.1016/j.neurop.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MaassenVanDenBrink A, de Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21:38. doi: 10.1186/s10194-020-01106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim BS, Chung JM, Chung PW, Park KY, Ahn JY, Moon HS, Park HK, Bae DW, Seo JG, Sohn JH, Song TJ. Clinical practice guideline of pharmacologic treatment for migraine prevention in adults 2021: The committee of clinical practice guideline of the Korean Headache Society. Korean J Headache. 2021;21:56–66. [Google Scholar]
- 119.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 120.Mayo Clinic. Chemo brain. [Accessed 11 July 2022]. Available at: https://www.mayoclinic.org/diseases-conditions/chemo-brain/diagnosis-treatment/drc-20351065.
- 121.Theoharides TC, Cholevas C, Polyzoidis K, Politis A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors. 2021;47:232–241. doi: 10.1002/biof.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mazza MG, Zanardi R, Palladini M, Rovere-Querini P, Benedetti F. Rapid response to selective serotonin reuptake inhibitors in post-COVID depression. Eur Neuropsychopharmacol. 2022;54:1–6. doi: 10.1016/j.euroneuro.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nobile B, Durand M, Olié E, Guillaume S, Molès JP, Haffen E, Courtet P. The anti-inflammatory effect of the tricyclic antidepressant clomipramine and its high penetration in the brain might be useful to prevent the psychiatric consequences of SARS-CoV-2 infection. Front Pharmacol. 2021;12:615695. doi: 10.3389/fphar.2021.615695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khani E, Entezari-Maleki T. Fluvoxamine and long COVID-19; a new role for sigma-1 receptor (S1R) agonists. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01545-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seo H, Kim H, Bae S, Park S, Chung H, Sung HS, Jung J, Kim MJ, Kim SH, Lee SO, Choi SH, Kim YS, Son KY, Chong YP. Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial. Infect Chemother. 2022;54:102–113. doi: 10.3947/ic.2021.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sookaromdee P, Wiwanitkit V. Correspondence on fluvoxamine treatment of patients with symptomatic COVID-19. Infect Chemother. 2022;54:369. doi: 10.3947/ic.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hashimoto Y, Suzuki T, Hashimoto K. Old drug fluvoxamine, new hope for COVID-19. Eur Arch Psychiatry Clin Neurosci. 2022;272:161–163. doi: 10.1007/s00406-021-01326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lionetto L, Ulivieri M, Capi M, De Bernardini D, Fazio F, Petrucca A, Pomes LM, De Luca O, Gentile G, Casolla B, Curto M, Salerno G, Schillizzi S, Torre MS, Santino I, Rocco M, Marchetti P, Aceti A, Ricci A, Bonfini R, Nicoletti F, Simmaco M, Borro M. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166042. doi: 10.1016/j.bbadis.2020.166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, Hudson KE, Zimring JC, Hansen KC, Hod EA, Spitalnik SL, D’Alessandro A. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5:e140327. doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eroğlu İ, Eroğlu BÇ, Güven GS. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19) Nutrition. 2021;90:111308. doi: 10.1016/j.nut.2021.111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, Primus-de Jong C, Cleemput I, Van den Heede K. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. 2022;54:1473–1487. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maamar M, Artime A, Pariente E, Fierro P, Ruiz Y, Gutiérrez S, Tobalina M, Díaz-Salazar S, Ramos C, Olmos JM, Hernández JL. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: a cross-sectional study. Curr Med Res Opin. 2022;38:901–909. doi: 10.1080/03007995.2022.2042991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Agarwala SR, Vijayvargiya M, Pandey P. Avascular necrosis as a part of ‘long COVID-19’. BMJ Case Rep. 2021;14:e242101. doi: 10.1136/bcr-2021-242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bhanuprasad K, Manesh A, Devasagayam E, Varghese L, Cherian LM, Kurien R, Karthik R, Deodhar D, Vanjare H, Peter J, Michael JS, Thomas M, Samuel P, Varghese GM. Risk factors associated with the mucormycosis epidemic during the COVID-19 pandemic. Int J Infect Dis. 2021;111:267–270. doi: 10.1016/j.ijid.2021.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bossley CJ, Kavaliunaite E, Harman K, Cook J, Ruiz G, Gupta A. Post-acute COVID-19 outcomes in children requiring hospitalisation. Sci Rep. 2022;12:8208. doi: 10.1038/s41598-022-12415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Buonsenso D, Munblit D, Pazukhina E, Ricchiuto A, Sinatti D, Zona M, De Matteis A, D’Ilario F, Gentili C, Lanni R, Rongai T, Del Balzo P, Fonte MT, Valente M, Zampino G, De Rose C, Sigfrid L, Valentini P FIMP-Roma. FIMP-Roma. Post-COVID condition in adults and children living in the same household in Italy: a prospective cohort study using the ISARIC global follow-up protocol. Front Pediatr. 2022;10:834875. doi: 10.3389/fped.2022.834875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kikkenborg Berg S, Dam Nielsen S, Nygaard U, Bundgaard H, Palm P, Rotvig C, Vinggaard Christensen A. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. 2022;6:240–248. doi: 10.1016/S2352-4642(22)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stephenson T, Pinto Pereira SM, Shafran R, de Stavola BL, Rojas N, McOwat K, Simmons R, Zavala M, O’Mahoney L, Chalder T, Crawley E, Ford TJ, Harnden A, Heyman I, Swann O, Whittaker E CLoCk Consortium. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. 2022;6:230–239. doi: 10.1016/S2352-4642(22)00022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rimes KA, Goodman R, Hotopf M, Wessely S, Meltzer H, Chalder T. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: a prospective community study. Pediatrics. 2007;119:e603–e609. doi: 10.1542/peds.2006-2231. [DOI] [PubMed] [Google Scholar]
- 140.Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur J Pediatr. 2022;181:1597–1607. doi: 10.1007/s00431-021-04345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuodi P, Gorelik Y, Zayyad H, Wertheim O, Wiegler KB, Abu Jabal K, Dror AA, Nazzal S, Glikman D, Edelstein M. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020-21, Israel. NPJ Vaccines. 2022;7:101. doi: 10.1038/s41541-022-00526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.