SUMMARY
γδ T cells are generally considered innate-like lymphocytes, however, an ‘‘adaptive-like’’ γδ compartment has now emerged. To understand transcriptional regulation of adaptive γδ T cell immunobiology, we combined single-cell transcriptomics, T cell receptor (TCR)-clonotype assignment, ATAC-seq, and immunophenotyping. We show that adult Vδ1+ T cells segregate into TCF7+LEF1+Granzyme Bneg (Tnaive) or T-bet+Eomes+ BLIMP-1+Granzyme B+ (Teffector) transcriptional subtypes, with clonotypically expanded TCRs detected exclusively in Teffector cells. Transcriptional reprogramming mirrors changes within CD8+ αβ T cells following antigen-specific maturation and involves chromatin remodeling, enhancing cytokine production and cytotoxicity. Consistent with this, in vitro TCR engagement induces comparable BLIMP-1, Eomes, and T-bet expression in naive Vδ1+ and CD8+ T cells. Finally, both human cytomegalovirus and Plasmodium falciparum infection in vivo drive adaptive Vδ1 T cell differentiation from Tnaive to Teffector transcriptional status, alongside clonotypic expansion. Contrastingly, semi-invariant Vγ9+Vδ2+ T cells exhibit a distinct ‘‘innate-effector’’ transcriptional program established by early childhood. In summary, adaptive-like γδ subsets undergo a pathogen-driven differentiation process analogous to conventional CD8+ T cells.
Graphical Abstract
In brief
Using single-cell transcriptomics, TCR repertoire analysis, ATAC-seq, and immunophenotyping, McMurray et al. show naive Vδ1+ T cells can undergo transcriptional reprogramming to an effector state extremely similar to CD8 TEMRA cells. Infections, including CMV and malaria, drive both clonotypic Vδ1+ T cell expansion and differentiation to this highly conserved effector program.
INTRODUCTION
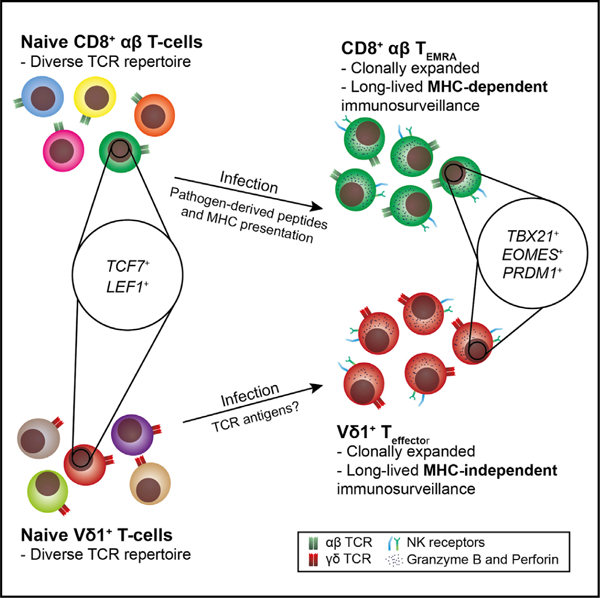
γδ T cells have been retained alongside αβ T cells and B cells for ~450 million years, with each compartment expressing distinct but related somatically recombined antigen receptors (Hayday, 2000; Vantourout and Hayday, 2013). Functioning in both anti-microbial and anti-tumor immunity (Silva-Santos et al., 2019; Vantourout and Hayday, 2013), γδ T cells display a distinct tissue distribution, can be enriched at epithelial surfaces, and employ unique recognition modes to sense target cells dysregulated by infection or transformation (Willcox and Willcox, 2019; Willcox et al., 2020). This poorly understood process involves a combination of γδ T cell receptor (TCR)-intrinsic but non-major histocompatibility complex (MHC)-restricted interactions, and TCR-extrinsic receptor/ligand interactions (Willcox and Willcox, 2019). The differentiation status of responding γδ T cells likely critically affects the outcome of such recognition processes.
How established lymphocyte differentiation paradigms apply to human γδ T cell function is unclear. While γδ T cells are often assumed to be ‘‘innate-like,’’ or ‘‘bridging innate and adaptive immunity,’’ increasing evidence suggests that parallel innate-like and adaptive paradigms operate in the human γδ T cell compartment, and apply to discrete γδ T cell subsets. Vγ9+Vδ2+ T cells prevalent in adult peripheral blood (PB) seem to adopt an innate-like immunobiology (Davey et al., 2018b; Willcox et al., 2018). They display a semi-invariant TCR repertoire from birth (Davey et al., 2018b), respond polyclonally to host and microbially derived phosphoantigens (Davey et al., 2018b), and display polyclonal recognition of butyrophilin superfamily (BTN)-2A1 via germline-encoded regions of the Vγ9+ TCR chain (Karunakaran et al., 2020; Rigau et al., 2020). Similarly, human intestinal γδ T cells include an intraepithelial lymphocyte (IEL) population polyclonally reactive to butyrophilin-like 3.8 heterodimer via germline-encoded regions of the Vγ4+ TCR chain (Melandri et al., 2018; Willcox et al., 2019), likely reflecting a distinct innate-like biology.
In contrast, PB Vδ1+ T cells adopt a more adaptive-like immunobiology (Davey et al., 2017; Ravens et al., 2017), as they display highly focused clonal expansion, including after viral (Farnault et al., 2013; Fujishima et al., 2007; Ravens et al., 2017) or parasitic (Rutishauser et al., 2020; von Borstel et al., 2021) infection, concomitant with apparent differentiation of select clones from a TCR-diverse Tnaive pool to generate long-lived, TCR-focused Teffector populations (Davey et al., 2018a; Davey et al., 2017; von Borstel et al., 2021). Also, Vδ1+ Tnaive and Teffector subsets bear immunophenotypic similarities to conventional adaptive CD8+ Tnaive and TEMRA populations, respectively (Davey et al., 2018a). Recently we defined a subset of Vγ9negVδ2+ T cells that shares critical adaptive-like hallmarks with PB Vδ1+ T cells (Davey et al., 2018b) and is linked to anti-viral (Davey et al., 2018b; Kaminski et al., 2020) and autoimmune (Bank et al., 1995; Hohlfeld et al., 1991; Pluschke et al., 1992) responses. The relevance of these observations likely extends to solid tissues. Hepatic γδ T cells included heavily expanded Vδ1+ and Vγ9negVδ2+ Teffector clonotypes, with some Vδ1+ clonotypes shared with PB, and Vδ1+ Teffector prevalence linked to viral exposure (Davey et al., 2018b; Hunter et al., 2018). Moreover, adaptive-like Vγ4negVδ1+ T cells were recently shown to outcompete endogenous γδ T cell populations during intestinal autoimmunity (Mayassi et al., 2019). In summary, a seemingly adaptive-like γδ immunobiology appears to drive potent T cell responses in several contexts, including viral and parasitic infections, and in autoimmunity.
While conventional αβ T cell differentiation is relatively well understood, the transcriptional basis of adaptive-like γδ T cell differentiation has not been addressed. CD8+ T cells transition from a TCR-diverse naive status through a series of differentiation states, via altered expression of a set of critical transcription factors (Fu et al., 2017; Henning et al., 2018; Kaech and Cui, 2012; Lazarevic et al., 2013; Vieira Braga et al., 2015). Initial antigen recognition and TCR signaling, culminating in clonal expansion, is considered critical in initiating this adaptive transition. Whether this model applies to adaptive-like γδ T cell populations is unclear. To date, transcriptional analyses of γδ T cells have stressed features of ‘‘innateness’’ shared between innate-like γδ subsets and unconventional αβ T cells, notably mucosal-associated innate T cells (MAITs) and invariant natural killer T cells (iNKTs) (Gutierrez-Arcelus et al., 2019; Koay et al., 2019; Pizzolato et al., 2019), but have not delineated differentiation processes fundamental to ‘‘adaptive-like’’ γδ T cell responses. To address this, we analyzed the transcriptional paradigms underpinning human γδ T cell function in both adaptive-like ( Vδ1+, Vγ9negVδ2+) and innate-like ( Vγ9+Vδ2+) subsets. We reveal striking similarities in transcriptional regulatory mechanisms between adaptive-like γδ T cell and CD8+ αβ T cell populations, including a tight link between clonotypic expansion and differentiation, and suggest they adopt a fundamentally different transcriptional control paradigm to innate-like Vγ9+Vδ2+ T cells.
RESULTS
Human PB Vδ1+ T cells exhibit two transcriptional profiles
To understand adaptive-like γδ T cell differentiation, we performed single-cell transcriptomic analyses of human Vδ1+ T cells. Vδ1+ T cells include distinct phenotypically naive (CD27hi) and effector-like (CD27lo/neg) sub-compartments (Davey et al., 2017, 2018a). A total of 447 single Vδ1+ T cells were sorted from 3 individuals with cell surface staining for CD27 and CD45RA; roughly equal numbers of CD27hi and CD27lo/neg cells were sorted (Figures S1A and S1B), with acceptable quality control statistics (transcripts per cell, genes per cell, fraction of mitochondrial reads per cell) (Figure S1C). Principal-component dimension reduction analysis and subsequent unsupervised clustering of Vδ1+ T cell transcriptomes identified two clusters of cells (Figures 1A–1C).
Figure 1. Clustering of single-cell transcriptomes reveals two distinct states of Vδ1+ cells reminiscent of effector and naive phenotypes.
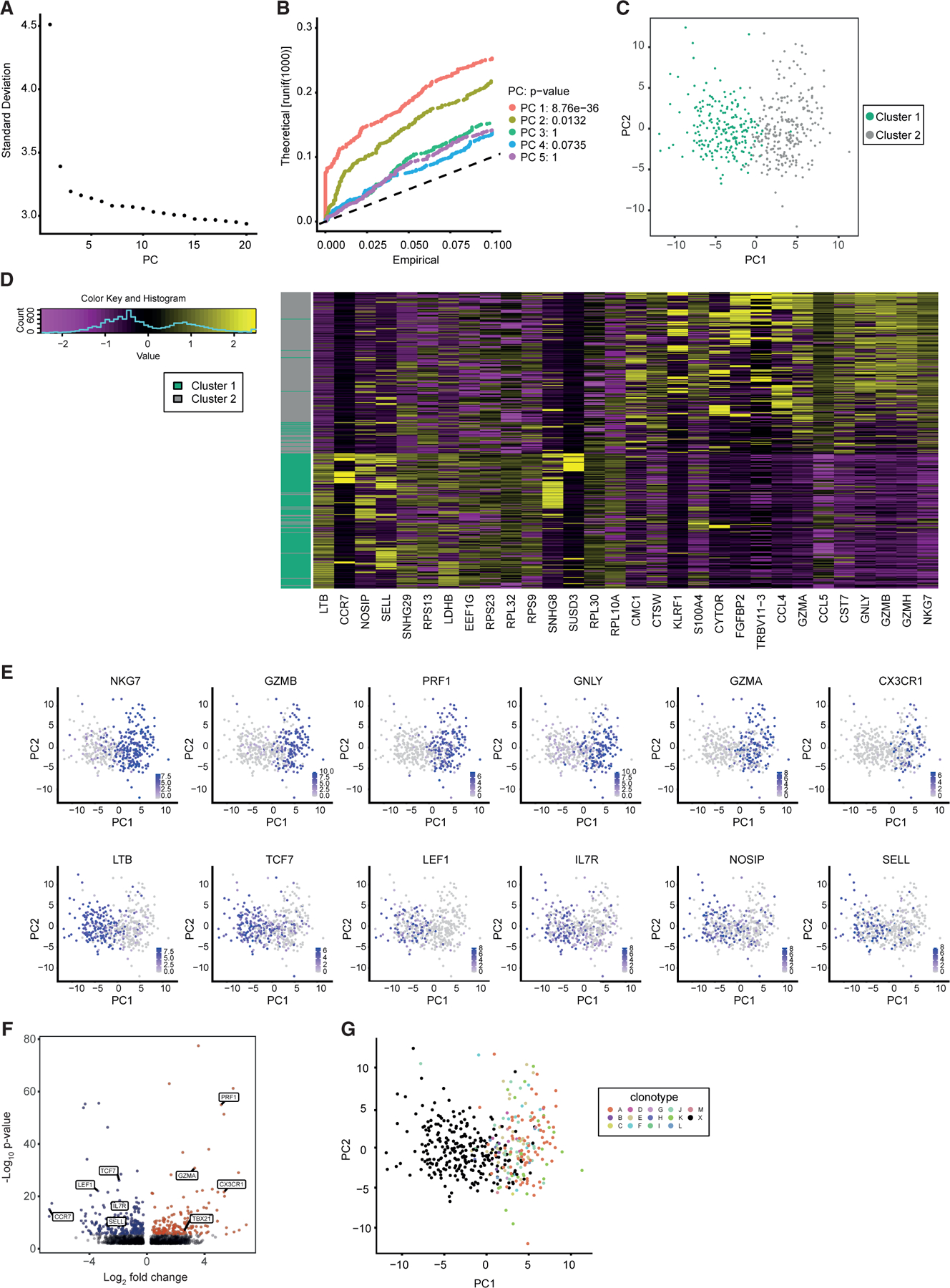
(A and B) Principal-component analysis was performed on single-cell RNA sequencing data from 447 FACS-sorted Vδ1+ cells (CD27hi versus CD27lo/neg) from three donors. (A) An elbow plot was constructed using the 20 dimensions. An ‘‘elbow’’ is observed at PC2. (B) A JackStraw plot was used to investigate the top 5 principal components (PCs). PC1 and PC2 were significant and used for visualization.
(C) Shared nearest neighbor clustering was used to find clusters in the data. Two clusters were found and colored.
(D) A heatmap was created using the top 30 genes contributing to the differences between cluster 1 and 2 (PC1). An even number of positive and negative factors are shown. These highlight genes previously shown to be associated with effector or naive status.
(E) Expression of genes previously shown to be associated with either effector (top) or naive (bottom) status were projected onto the PCA plot. Purple indicates higher expression.
(F) Differential gene expression between cluster 1 (blue) and cluster 2 (red) cells revealed 368 differentially expressed genes. Only those that had a LogFC greater than 0.25 and an adjusted p value of less than 0.05 were colored (Bonferroni corrected). Notable genes previously associated with effector or naive status are labeled.
(G) TCR clonality analysis. Thirteen expanded clonotypes were identified by the analysis (clones A–M), and projected onto the PCA taken from (B). ‘‘X’’ represents cells with a unique TCR sequence. Cells bearing expanded clonotypes fall within CD27lo/neg sorted cells and almost exclusively within the ‘‘Teffector’’ cluster (cluster 2).
Differential gene expression comparisons between cluster 1 and cluster 2 Vδ1+ T cells revealed 368 differentially expressed genes (Table S1). Cluster 1 Vδ1+ T cells exhibited enhanced expression of SELL, IL7R, and CCR7 and low expression of effector-related genes, including CX3CR1, GZMA, and PRF1 (Figures 1D–1F), whereas cluster 2 Vδ1+ T cells had an inverse expression pattern. This indicated close concordance with cell surface Vδ1+ Tnaive and Tefffector (cluster 1 and cluster 2, respectively) phenotypes we previously defined and noted were similar to CD8+ Tnaive and TEMRA cells, respectively (Davey et al., 2017, 2018a). Indexing of individual transcriptomes indicated a 91.1% concordance with flow phenotype (Figure S1D), whereby cluster 1 Vδ1+ T cells were predominantly CD27hi and cluster 2 Vδ1+ T cells CD27lo, confirming Vδ1+ Tnaive and Teffector status, respectively (Figures S1E–S1H). While cluster 1 and 2 Vδ1+ T cell transcriptomes (referred to as Vδ1+ Tnaive and Teffector programs) differentially expressed several key transcription factors, including TCF7, LEF1, and TBX21 (Figure 1F), the challenges of detecting low expression genes using single-cell transcriptomics suggested that this was an incomplete list.
We next probed the link between Vδ1+ clonotypic expansion and possession of a Tnaive/Teffector program. We extended the TraCeR algorithm (Stubbington et al., 2016) to reconstruct human γ and δ TCR sequences from standard single-cell transcriptomes. This leveraged approaches previously applied to αβ TCR sequence extraction (Stubbington et al., 2016), although the γ and δ complementarity determining region 3 length parameters were informed by bulk TCR sequencing on human γδ T cells (Davey et al., 2017, 2018b; Hunter et al., 2018). We reconstructed both γ and δ TCR sequences from ~70% of cells, a similar success rate to αβ T cells (Stubbington et al., 2016). Whereas γδ TCR clonotypes assigned to the Tnaive cellular cluster were overwhelmingly distinct ‘‘singlets’’ (95.6%), expanded clonotypes (those observed in more than one cell) were restricted almost entirely (95.3%) to Vδ1+ cells bearing a Teffector transcriptional program (cluster 2) (Figure 1G). Compelling statistical significance (p < 2.2e–16, Pearson’s chi-square test, Yates continuity correction) was evident in three associations: cluster status (1 or 2) with sorted phenotype (CD27hi or CD27lo/neg); clonality status (singlet or expanded clonotype) with cluster status (1 or 2); between clonality status (singlet or expanded clonotype) and sort phenotype (CD27hi or CD27lo/neg) (Figures S1E–S1H). Finally, TCR sequence analysis enabled us to probe transcriptional differences between distinct expanded clonotypes. While few interclonal differences were observed, two observations validated the approach. Firstly, interclonal discrepancies in Vγ chain expression were apparent and, secondly, expression of XIST, an X-linked transcript involved in X chromosome inactivation in females, was restricted to the female donor’s major clone (Figures S1I and S1J; Table S2).
These results suggested that distinct naive and effector transcriptional programs underpin Vδ1+ T cell differentiation, that these two states can be accurately delineated by CD27 status, and established that effector status was intrinsically linked to clonotypic expansion at a single-cell level.
Analogous transcriptional reprogramming in CD8+ and Vδ1+ T cells
To gain a deeper knowledge of the Vδ1+ Tnaive and Teffector transcriptional programs and how they compare with CD8+ differentiation states with a similar cell surface phenotype, we performed bulk RNA-seq on Vδ1+ Tnaive (CD27hi) and Teffector (CD27lo) populations and both CD8+ Tnaive and TEMRA populations, sorted from human PB mononuclear cells from healthy donors or buffy packs (see STAR Methods). Using all filtered genes, multidimensional scaling revealed strong similarity between CD8+ TEMRA and Vδ1+ Teffector populations (Figure 2A), both of which were clearly separated from CD8+ Tnaive and Vδ1+ Tnaive populations in dimension 1. While Vδ1+ Tnaive and CD8+ Tnaive cells were broadly similar transcriptionally, they exhibited some differences, particularly in dimension 2; however, this made a minor contribution to the observed variance (7%). In contrast, Vδ1+ Tnaive and CD8+ Tnaive cells were more concordant in dimension 1, which made a larger contribution (37%).
Figure 2. Bulk RNA sequencing on sorted Vδ1 populations reveals transcriptomic similarity between Vδ1+ and CD8+ populations.
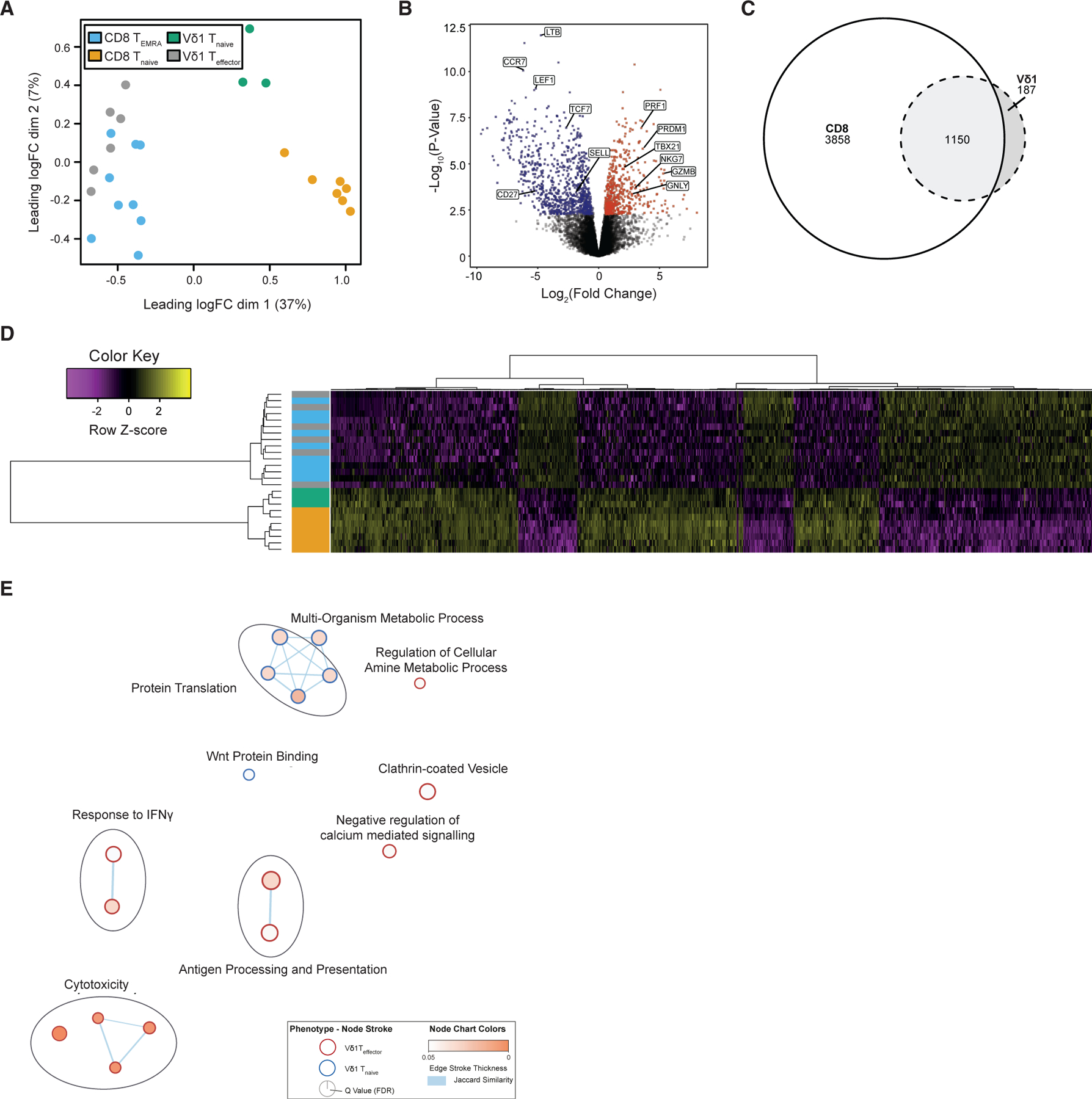
(A) Multidimensional scaling plot using 12,627 filtered genes revealed close transcriptomic similarity between bulk sorted Vδ1+ Teffector and CD8+ TEMRA.
(B) Differential gene expression between Vδ1+ Teffector (red) and Vδ1+ Tnaive (blue) revealed 1,337 differentially expressed transcripts. Among these were genes related to effector or naive status.
(C) Differential gene expression between Vδ1+ and CD8+ populations revealed a core of 1,150 differentially expressed transcripts shared between comparisons.
(D) Hierarchically clustered heatmap of the core 1,150 transcripts differentially expressed in both Vδ1+ and CD8+ comparisons highlighted similarity between Vδ1+ Teffector and CD8+ TEMRA cells and between Vδ1+ Tnaive and CD8+ Tnaive cells. Colors for the cell populations are taken from (A). Genes are plotted row-wise and scaled to represent Z scores across samples.
(E) Gene set enrichment analysis using the GO and KEGG databases revealed enrichment of 11 pathways associated with effector function in Vδ1+ Teffector cells. In contrast, 6 pathways were associated with Vδ1+ Tnaive status. Genes differentially expressed between Vδ1+ populations were used as input. Cytoscape was used to produce the enrichment map, where node size indicates the gene set size, edge thickness represents Jaccard similarity scores between gene sets, and node color is based on FDR value. Only gene sets with an FDR below 0.05 are shown.
These data are in accordance with, but extend, our previous phenotypic data on adaptive-like Vδ1+ T cells (Davey et al., 2017, 2018a). Differential gene expression analysis between Vδ1+ Tnaive/Teffector populations revealed 1,337 differentially expressed transcripts (Figure 2B), substantially more than revealed by single-cell analysis.
We next compared genes differentially expressed in both Vδ1+ and CD8+ subpopulations. Interestingly, 86% of all genes differentially expressed between Vδ1+ Tnaive/Teffector populations were also differentially expressed between CD8+ Tnaive/TEMRA (Figure 2C). Within this conserved set of 1,150 differentially expressed genes were those canonically related with effector (GNLY, GZMA/B/H, and PRF1) and naive (CCR7, LEF1) status (Figure 2B). Genes downregulated in Vδ1+ Teffector cells contributed more strongly than upregulated genes to this core gene list (Figure S2A). Heatmap analysis, using hierarchical clustering, on this 1,150-gene list segregated Vδ1+ Teffector cells with CD8+ TEMRA cells, and the Vδ1+ Tnaive subset alongside CD8+ Tnaive cells, highlighting clear concordance of gene expression within each subgroup (Figure 2D). Interestingly, Vδ1+ Teffector populations were not clustered together as a group, but instead clustered among CD8+ TEMRA populations, emphasizing close transcriptional concordance (Figure 2D). In contrast, Tnaive subsets clustered separately by compartment ( Vδ1+ and CD8+ T cells) (Figure 2D), consistent with greater transcriptional differences between naive Vδ1+ and CD8+ T cells (Figure 2A).
Gene set enrichment analysis highlighted 11 pathways significantly upregulated in Vδ1+ Teffector versus Vδ1+ Tnaive subsets (Figures 2E; Table S3). Chief among these were effector/cytotoxicity, antigen processing/presentation, and IFN-γ-responsive genes, mirroring changes in CD8+ TEMRA versus Tnaive compartments (Figures S2B; Table S3). Also, analysis of bespoke gene sets linked to cytotoxic molecules (Figure S3A), cytokine and chemokine receptors (Figure S3B), adhesion molecules (Figure S3C), and TCR/co-stimulatory molecules (Figure S3D) underlined close concordance between Vδ1+ Teffector and CD8+ TEMRA cells. Conversely, a minority of processes were upregulated on Vδ1+ Tnaive cells relative to Vδ1+ Teffector cells, including protein translation and metabolism (Figure 2E); these were also upregulated on CD8+ Tnaive cells versus CD8+ TEMRA cells (Figure S2B). Of note, several activating co-stimulatory molecules (e.g., CD28, CD27, and JAML) displayed higher expression on both Vδ1+ Tnaive and CD8+ Tnaive cells relative to Vδ1+ Teffector and CD8+ TEMRA subsets, whereas several inhibitory co-signaling molecules (e.g., PDCD1, TIGIT, and LAG3) were increased on Teffector/EMRA cells (Figure S3D). Furthermore, numerous NKRs, including KLRK1 (NKG2D), NCR1 (NKp46), and KLRF1 (NKp80), were upregulated in Vδ1+ Teffector and CD8+ TEMRA cells relative to Tnaive subsets (Figure S3E). Vδ1+ and CD8+ Teffector subset concordance was close, with differential gene expression analysis revealing just 20 transcripts (Table S1). Of these, seven were TCR chain-specific genes, and two, CD8A and CD8B, known to be expressed differentially in CD8+ αβ versus γδ T cells, leaving 11 genes. Several of these (CD5, THEMIS, and SIT1) have established roles regulating TCR signaling thresholds in response to peptide MHC (Fu et al., 2009; Simeoni et al., 2005; Voisinne et al., 2018), and were preferentially expressed in CD8+ T cells. ITGAD was upregulated in Vδ1+ Teffector cells, consistent with previous findings (Siegers et al., 2017). Notably, the Vδ1+ Tnaive compartment displayed marginally higher transcription of several NKR genes than CD8+ Tnaive cells, with some specific NKRs (NKG2A/C/E (KLRC1/3), and FCRL3) displaying particularly high relative expression in the Vδ1+ Tnaive compartment (Figure S3E).
A shared set of TCR-linked transcription factors underpin Vδ1+ and CD8+ T cell differentiation
Transcriptional analyses also indicated differential expression of a core set of transcription factors delineated CD8+/Vδ1+ Tnaive versus CD8+ TEMRA/Vδ1+ Teffector subsets. TCF7, LEF1, and Myc were enriched in Tnaive subsets, whereas TBX21 (T-bet), and PRDM1 (BLIMP-1) were enriched in Vδ1+ Teffector cells and CD8+ TEMRA cells (Figures 1E and 2B). Overall, 122 transcription factors were significantly differentially expressed between Vδ1+ Tnaive and Teffector subsets (Table S4). Moreover, hierarchical clustering based on these transcription factors separated Vδ1+ Tnaive with CD8+ Tnaive cells as distinct clusters, whereas Vδ1+ Teffector cell clustering was interspersed with that of CD8+ TEMRA cell populations (Figure S3F). Flow cytometry confirmed differential transcription factor expression at a protein level, establishing equivalently high TCF7 and IL-7Rα expression in Vδ1+ and CD8+ Tnaive cells and absence in Teffector cells, whereas T-bet, Eomes, and BLIMP-1 were strikingly and equivalently upregulated in Vδ1+ Teffector and CD8+ TEMRA cells relative to Tnaive subsets (Figures 3A and 3B). Also, HOBIT expression was higher in Vδ1+ Teffector and CD8+ TEMRA cells than Tnaive cells (Figures 3A and 3B). T-bet and Eomes expression correlated with lower CD27 expression (Figures 3C and 3D). These changes correlated with established differentiation markers, as confirmed by assessment of IL7Rα (Tnaive) and CX3CR1 (Davey et al., 2017) (Teffector/TEMRA) expression (Figures 3A and 3B). Of note, despite transcriptional differences in CXCR3 between Vδ1+ Tnaive and CD8+ Tnaive subsets, both expressed CXCR3 protein, whereas CXCR3 expression was lower on Vδ1+ Teffector and CD8+ TEMRA cells (Figures S4A–S4C).
Figure 3. Transcription factors key to CD8+ differentiation demonstrate analogous and TCR-dependent expression in Vδ1+ subsets.
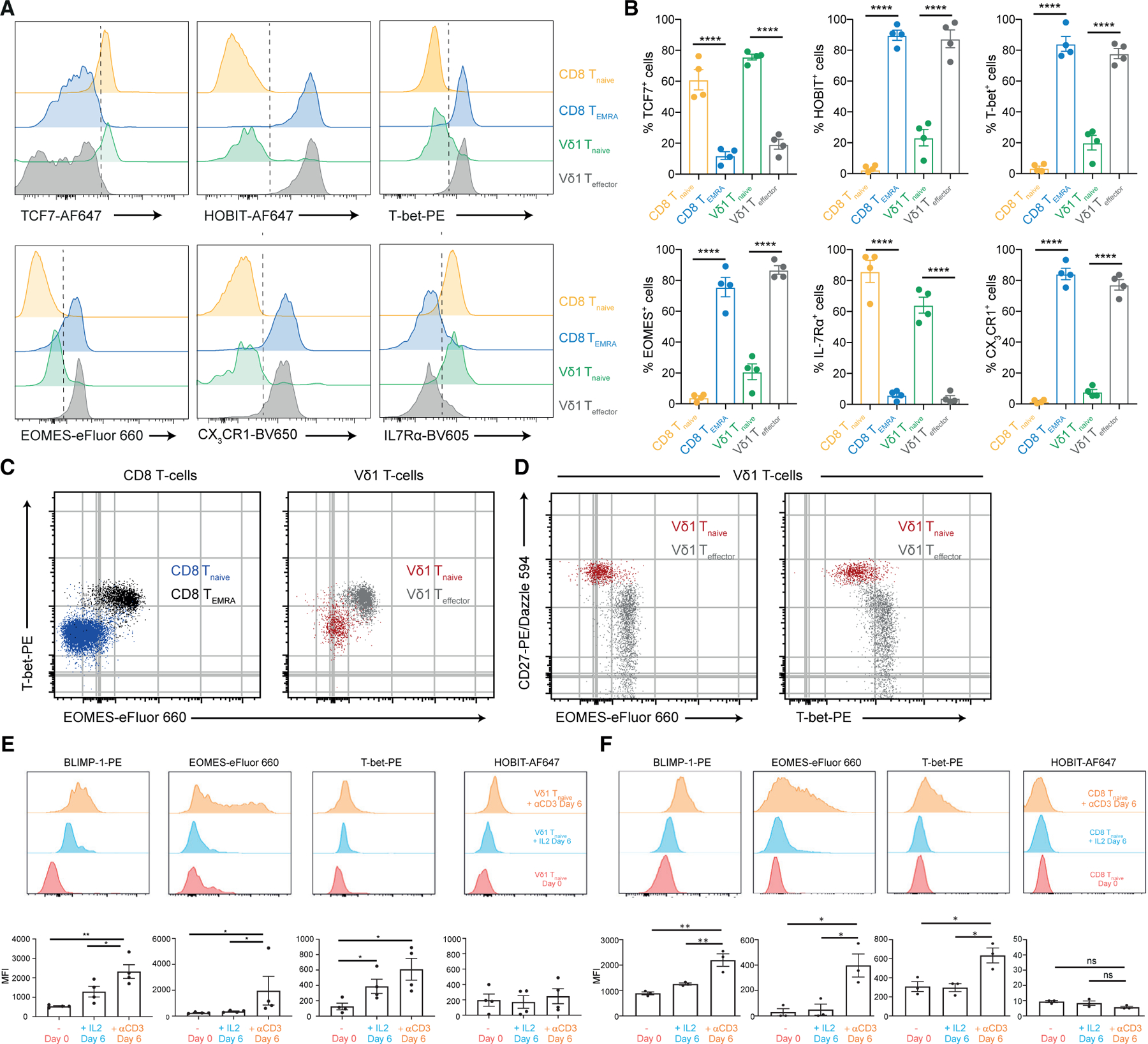
(A) Flow cytometry staining of transcription factors and homing receptors key to effector/naive status highlight similarities between Vδ1+ Teffector and CD8+ TEMRA, and Vδ1+ Tnaive and CD8+ Tnaive populations. Representative of four donors.
(B) Percentage of each naive and effector marker in each total cell population (CD8+ Tnaive, CD8+ TEMRA, Vδ1+ Tnaive, Vδ1+ Teffector). Graphs show mean ± SEM from four donors.
(C and D) Overlaid flow cytometry dot plots of the transcription factors Eomes and T-bet in total Vδ1+ and CD8+ populations (C) and the overlap of each transcription factor with CD27 (D). Representative of four donors. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons (****p < 0.0001).
(E and F) TCR stimulation of enriched Tnaive cells leads to expression of Teffector transcription factors. Flow cytometric analysis of BLIMP-1, Eomes, T-bet, and HOBIT in Vδ1+ Tnaive (E) and CD8+ Tnaive (F) on day 0 (unstimulated) and day 6 of stimulation with either plate-bound anti-CD3 antibodies or IL-2. The top panel shows representative histograms for flow cytometric analysis, whereas the lower panel shows bar charts representing data from four donors. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons (*p < 0.05, **p < 0.01). Only significant comparisons are shown.
To address how Vδ1+ Tnaive to Teffector transition affected chromatin structure, we carried out assay for transposase-accessible chromatin using sequencing (ATAC-seq) on purified Vδ1+ and CD8+ subpopulations (Figure S4D; Table S5). For a defined subset of loci, clear and analogous differences in chromatin accessibility were observed between CD8+/Vδ1+ Tnaive populations and CD8+ TEMRA/Vδ1+ Teffector subsets (Figure S4D). These included CD27, which was more active in Tnaive Vδ1+/CD8+ T cells than in TEMRA/Vδ1+ Teffector subsets. Conversely, PRDM1 (BLIMP-1) and EOMES and CX3CR1 all showed an inverse pattern of accessibility. In contrast, numerous loci displayed unaltered chromatin accessibility between CD8+/Vδ1+ Tnaive populations and CD8+ TEMRA/Vδ1+ Teffector subsets, including CD3E, B2M, and RPL13A (Figure S4E). These results confirm that Vδ1+ Tnaive and Teffector subsets represent two distinct differentiation states.
In CD8+ T cells, downregulation of TCF7/LEF1 and upregulation of Eomes, T-bet, and BLIMP-1 are associated with exposure of Tnaive subsets to antigen-induced TCR signaling (Fu et al., 2017; Kaech and Cui, 2012; Lazarevic et al., 2013; Willinger et al., 2006). Consistent with this also operating in Vδ1+ T cells, Vδ1+ clonal expansion correlates with a transition from Tnaive to Teffector status. To assess whether TCR signaling also induced similar changes in adaptive γδ T cells, we stimulated purified Vδ1+ T cells with plate-bound CD3 antibodies and assessed changes in transcription factor expression and phenotype in the Vδ1+ CD27hi (i.e., Tnaive) subset (Figure 3E). Exposure of Vδ1+ T cells to CD3 stimulation induced BLIMP-1, Eomes, and T-bet expression in CD27hi Vδ1+ T cells. TCR-induced expression of BLIMP-1 was previously shown for CD4+ T cells (Martins et al., 2006); however, here we show this extends to Vδ1+ and also CD8+ T cells (Figure 3F). While IL-2 (Figures 3E and 3F) and anti-CD28 (Figures S4F and S4G) appeared to amplify transcription factor expression, TCR signaling induced by anti-CD3 had the most profound effect on BLIMP-1, Eomes, and T-bet upregulation. This suggested that TCR signaling in Vδ1+ Tnaive cells can initiate transition from Tnaive to Teffector status, mirroring CD8+ T cell differentiation.
The Vδ1+ Teffector program permits rapid cytokine production kinetics
Production of inflammatory cytokines and enhanced cytotoxicity are hallmarks of γδ T cell responses. While cytotoxic pathways were upregulated at both the transcriptional and protein level in resting Vδ1+ Teffector cells, cytokines were not differentially transcribed in Vδ1+ Teffector relative to Tnaive cells. Yet, ATAC-seq indicated substantially greater chromatin accessibility at the IFNγ locus in CD8+ TEMRA than CD8+ Tnaive cells. A similar pattern was observed in Vδ1+ subsets, albeit with a lower fold difference due to greater accessibility in Vδ1+ Tnaive than CD8+ Tnaive cells. This indicated the potential for enhanced/expedited IFN-γ production in Teffector/TEMRA subsets (Figure S4H). While similarly low ATAC-seq reads mapped to the TNF-α locus in Tnaive and Teffector/TEMRA subsets, our analyses were performed on resting cells, and further signals may be needed to affect chromatin accessibility at this locus. Also, differential transcription factor expression in the Tnaive versus Teffector/TEMRA states may influence cytokine production, as suggested for TCF7-mediated repression of IFN-γ expression in CD4 T cells (Maier et al., 2011). To test if their distinct chromatin and transcriptional profiles affected cytokine production, we stimulated both Vδ1+ Tnaive and Teffector subsets and assessed production of IL-2, IL-17A, GM-CSF, IFN-γ, TNF-α, and CCL5. Vδ1+ Teffector cells displayed dramatically amplified and expedited production of IFN-γ, TNF-α, and CCL5 relative to Vδ1+ Tnaive cells (but not IL-2, IL-17A, or GM-CSF) (Figures 4A, 4B, and S4I) in a manner highly analogous to CD8+ TEMRA versus Tnaive subsets. This suggests that Vδ1+ Tnaive to Teffector transition, as for CD8+ T cells, results in transcriptional reprogramming and chromatin remodeling, enabling increased cytotoxicity and cytokine production, allowing amplified and expedited effector responses upon subsequent stimulation.
Figure 4. Vδ1+ Teffector and CD8+ TEMRA populations display similar cytokine production profiles.
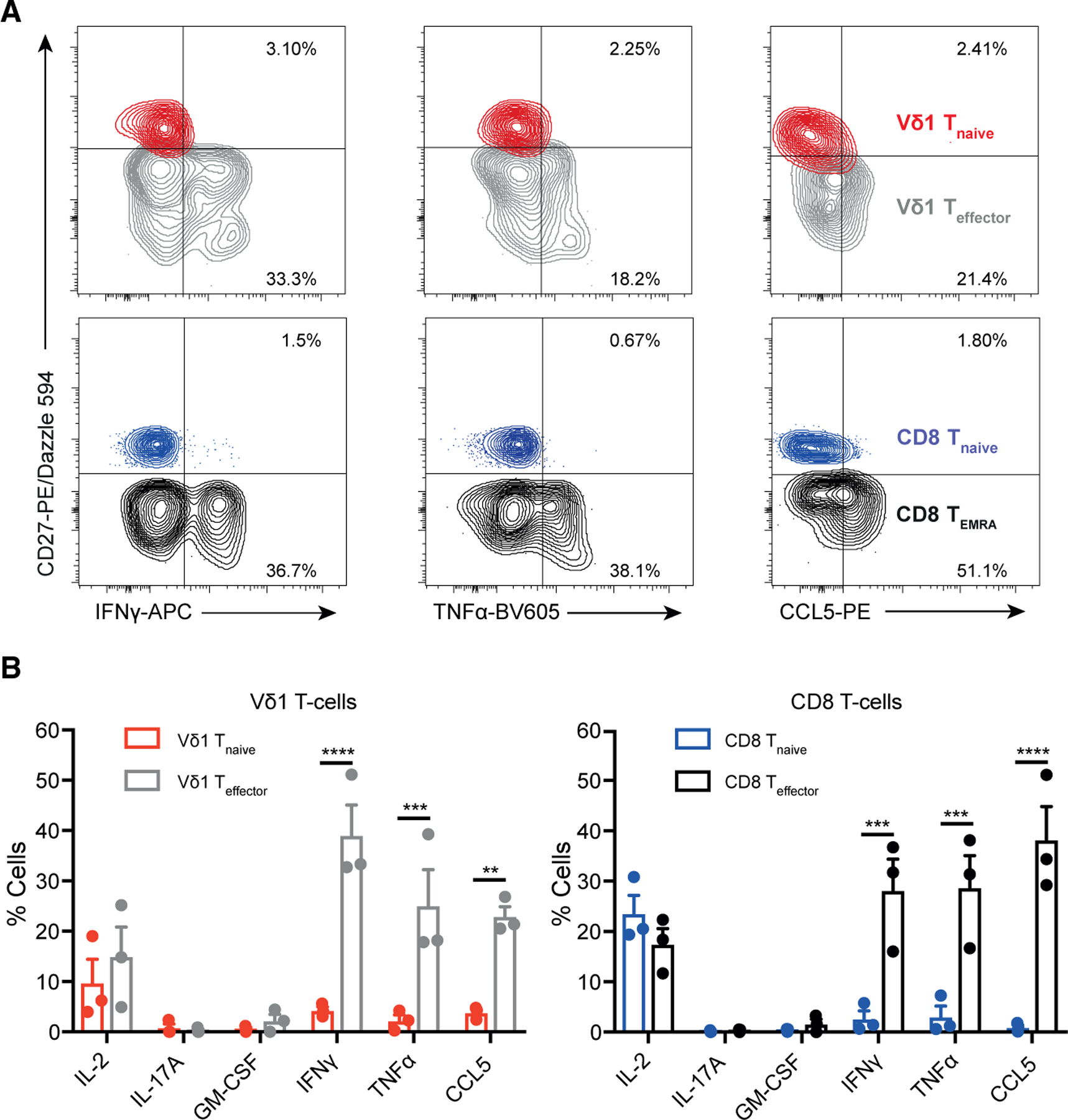
(A) Cytokine production by Vδ1+ Teffector and CD8+ TEMRA and Tnaive populations after 4 h of PMA/ionomycin stimulation. Overlaid contour flow cytometry plots of gated populations of Vδ1+ (top) and CD8+ (bottom).
(B) Graphs highlight mean ± SEM and represent three donors. Data were analyzed by two-way ANOVA with Sidak’s multiple comparisons (*p < 0.05, **p < 0.01, ****p < 0.0001). Only significant comparisons are shown.
Vγ9+Vδ2+ T cells display a distinct innate-like effector transcriptome from early life
RNA-seq data from Vγ9+Vδ2+ T cells sorted from healthy adults indicated an effector rather than naive transcriptional program. Multidimensional scaling (Figure 5A) positioned Vγ9+Vδ2+ cells closer to Vδ1+/CD8+ Teffector than Tnaive populations. Unsupervised hierarchical clustering based on the 1,150 genes differentially expressed between Tnaive and Teffector subsets also clustered Vγ9+ Vδ2+ cells with effector Vδ1+/CD8+ subsets (Figures 5B and S5A). Similar clustering approaches highlighted Vγ9+Vδ2+ T cell alignment with Vδ1+ Teffector/CD8+ TEMRA populations based on bespoke lists of cytotoxicity markers and NK receptors (Figures 5C and S5B). Moreover, Vγ9+Vδ2+ T cells were at least as potent producers of IFN-γ, TNF-α, and CCL5 upon anti-CD3 stimulation as Vδ1+ Teffector cells (Figures 4B and 5D).
Figure 5. Transcriptomic analysis of Vδ2+ cells reveals an effector-like program.
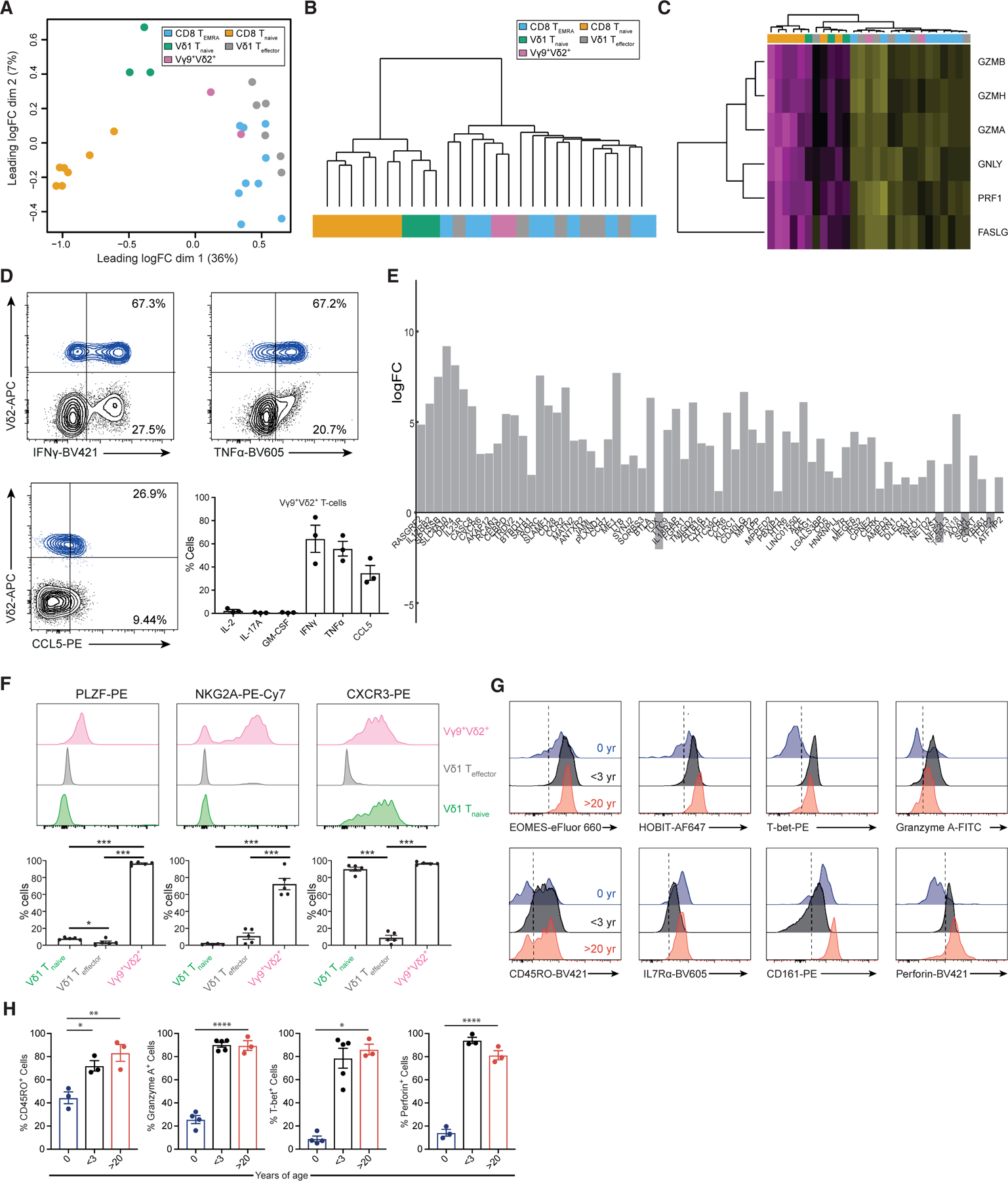
(A) Multidimensional scaling plot on 12,627 filtered genes placed Vγ9+Vδ2+ T cells closer to Vδ1+ Teffector and CD8+ TEMRA than to Tnaive cells, highlighting increased transcriptional similarity of Vγ9+Vδ2 to effector populations.
(B) Using the previously defined effector-naive gene list based on shared differentially expressed transcripts (1,150-gene list) between Vδ1+ and CD8+ populations, Vγ9+Vδ2+ cells were hierarchically clustered with the other effector populations. The dendrogram shown places Vδ2+ cells on the effector ‘‘arm,’’ suggesting more similar expression of these genes. Full heatmap in Figure S4A.
(C) Vγ9+Vδ2+ cells segregated with the effector compartment based on a bespoke gene set of cytotoxic markers and were hierarchically clustered. Colors for the cell populations are taken from (A). Genes are plotted row-wise and scaled to represent Z scores across samples.
(D) Cytokine production by Vγ9+Vδ2+ cells in total gated CD3+ T cells after 4 h PMA/ionomycin treatment. Flow plots are representative of three donors. Graphs depict mean ± SEM of an extended set of cytokine protein expression by intracellular flow cytometry.
(E) Differential gene expression between Vγ9+Vδ2+ and Vδ1+ Teffector cells revealed close transcriptomic similarity, with 69 genes significantly differentially expressed (p < 0.05). Transcripts were ordered according to p value, with the most significant being RASGRF2, IL12RB2, and SPTSSB. A positive log fold change indicates an enrichment in Vγ9+Vδ2+ cells.
(F) Vγ9+Vδ2+ T cells exhibit a unique innate-like effector program. Prevalence of γδ T cell subsets expressing PLZF, NKG2A, and CXCR3 was determined using flow cytometry. The top panel shows representative histograms for flow cytometric analysis; the lower panel shows bar charts representing data from five donors. Data were compared with one-way ANOVA with Tukey’s multiple comparisons (*p < 0.05, **p < 0.01). Only significant comparisons are shown.
(G) Comparison of Vγ9+Vδ2+ T cell phenotype in cord blood, from unrelated children under 3 years of age, and from adult donors over 20 years of age.
(H) Quantitation of MFI levels from (G), three samples of cord blood, five samples from children under the age of 3 years, and from three adult donors. Data were analyzed by two-way ANOVA with Sidak’s multiple comparisons (*p < 0.05, **p < 0.01). Only significant comparisons are shown.
Despite such similarities, several genes were enriched in Vγ9+Vδ2+ relative to Vδ1+ Teffector cells (Figure 5E; Table S1), including transcription factors (CEBPδ, SATB1, and RORγt), NKRs (KLRK1 [NKG2A]), co-stimulatory molecules (JAML), cytokine receptors (IL23R), sphingolipid metabolism genes (SPTSSB and KDSR), and an alternative splicing gene (HNRNPLL), which is responsible for generation of the CD45RO isoform (Oberdoerffer et al., 2008) expressed by Vδ2+ T cells (Parker et al., 1990), but not generally by Vδ1+ Teffector or CD8+ TEMRA cells. Vγ9+ Vδ2+ T cells also had a distinct overall profile of chemokine/cytokine receptors (Figure S5C) and transcription factors (Figure S5D) compared with Vδ1+ Teffector/CD8+ TEMRA subsets. While they shared expression of TBX21, EOMES, ZEB2, and PRDM1 with these effectors, like Tnaive subsets they were enriched for Myc, and also for both ZBTB16 (PLZF, also expressed in iNKT and MAIT cells) and CEBPD (C/EBPδ), which is also expressed by MAIT cells and supports CCR6 expression (Lee et al., 2018) on Vγ9+Vδ2+ T cells (Figure S5C). Higher transcription of RORγt and IL-23R in Vγ9+Vδ2+ than in Vδ1+ Teffector cells is consistent with the suggestion that adult Vγ9+Vδ2+ T cells may retain some IL-17-producing cells (Tan et al., 2020). Also, flow cytometry confirmed that, in adults, a high frequency of Vγ9+Vδ2+ T cells expressed PLZF, NKG2A, and CXCR3 (Figure 5F). Therefore, in adults, almost all Vγ9+Vδ2+ T cells adopt an ‘‘innate-like effector’’ program distinct from adaptive subsets.
To assess if Vγ9+Vδ2+ T cells were effector-like from birth, we analyzed cord blood samples, a setting where Vδ1+ T cells subsets are phenotypically highly naive and have an unfocused TCR repertoire. Cord blood Vγ9+Vδ2+ T cells displayed high levels of Eomes, HOBIT, CD161, and IL-7Rα. However, relative to adult Vγ9+Vδ2+ T cells, they had lower T-bet, HOBIT, granzyme A, and perforin expression, indicating an immature effector phenotype (Figures 5G and 5H). However, Vγ9+Vδ2+ T cells in young healthy children (≤3 years) displayed a transition to a T-bet+ HOBIT+ phenotype, increased levels of granzyme A and perforin, and a dominant CD45RO+ phenotype (Figures 5G and 5H). These results suggest Vγ9+Vδ2+ T cells are a dedicated effector subset at birth but transition to a more mature innate-effector program similar to that present in adults, in early life.
Adaptive γδ T cell subsets undergo transcriptional reprogramming in vivo
To assess transition from Tnaive to Teffector status in adaptivelike γδ T cells in vivo, we examined the transcriptional phenotype of such cells before and after pathogen challenge in two separate human in vivo infection models; firstly CMV infection in patients undergoing kidney transplantation, and secondly repeated controlled human malaria challenge (CHMI) in malaria-naive individuals. Changes in Vδ1+ T cells in patients who became CMV seropositive after transplantation were consistent with an infection-induced increase in the proportion of CD27lo/neg T-bet+ Eomes+ granzyme B+ Teffector cells (Figure 6A). We also examined changes in the adaptive-like Vγ9negVδ2+ T cell compartment. This was previously shown to undergo clonotypic expansion with concomitant phenotypic differentiation from a naive TCR-unfocused CD27hi repertoire to a CD27lo/neg phenotype after CMV infection (Davey et al., 2018b; Kaminski et al., 2020). Here, we demonstrate that while CMV-seronegative donor Vγ9negVδ2 T cells lacked T-bet, HOBIT and BLIMP-1, these markers were markedly upregulated upon CMV infection and seroconversion post-transplantation, alongside transition from CD27hi to CD27lo/neg status, suggesting transition to the Teffector transcriptional program (Figure 6B); in contrast, Vγ9+Vδ2+ T cells were strongly granzyme A/B+, T-bet+ and Eomes+ before and after CMV infection and seroconversion (Figure S6A). Secondly, CHMI with Plasmodium falciparum drives clonal expansion of Vδ1+ T cells (Rutishauser et al., 2020; von Borstel et al., 2021), providing an opportunity to assess acquisition of transcription factors in this context. At baseline, in a malaria-naive individual, 76%–78% of Vδ1+ T cells expressed a CD27+ Tnaive phenotype (Figure 6C). Upon sequential CHMIs over 378 days, Vδ1+ T cells transitioned from a Tnaive to Teffector subtype, as indicated by loss of CD27 and acquisition of T-bet, HOBIT, and Eomes—mirroring that seen after CMV seroconversion; HOBIT expression was most pronounced after a fourth infectious challenge (Figure 6C). Finally, in healthy children (12 months to 14 years) Vδ1+ Teffector cell levels correlated with the percentage of CD8+ Teffector cells (TCM + TEM + TEMRA) as measured by cell surface phenotype (CD27lo/neg Vδ1+ Teffector) or perforin expression (Figures 6D, 6E, and S6B–S6E). As expected, CMV-seropositive children had higher frequencies of Vδ1+ and CD8+ Teffector cells, but some CMV-seronegative children also had effector Vδ1+ and CD8+ T cells, suggesting Vδ1+ and CD8+ T cells can act as part of a coordinated adaptive immune response, which is dependent on antigen exposure. In contrast, Vγ9+Vδ2+ T cells in healthy children were perforin positive regardless of Vδ1+ or CD8+ Teffector cell levels or CMV status (Figures S6F and S6G).
Figure 6. Adaptive differentiation of human γδ T cells in vivo.
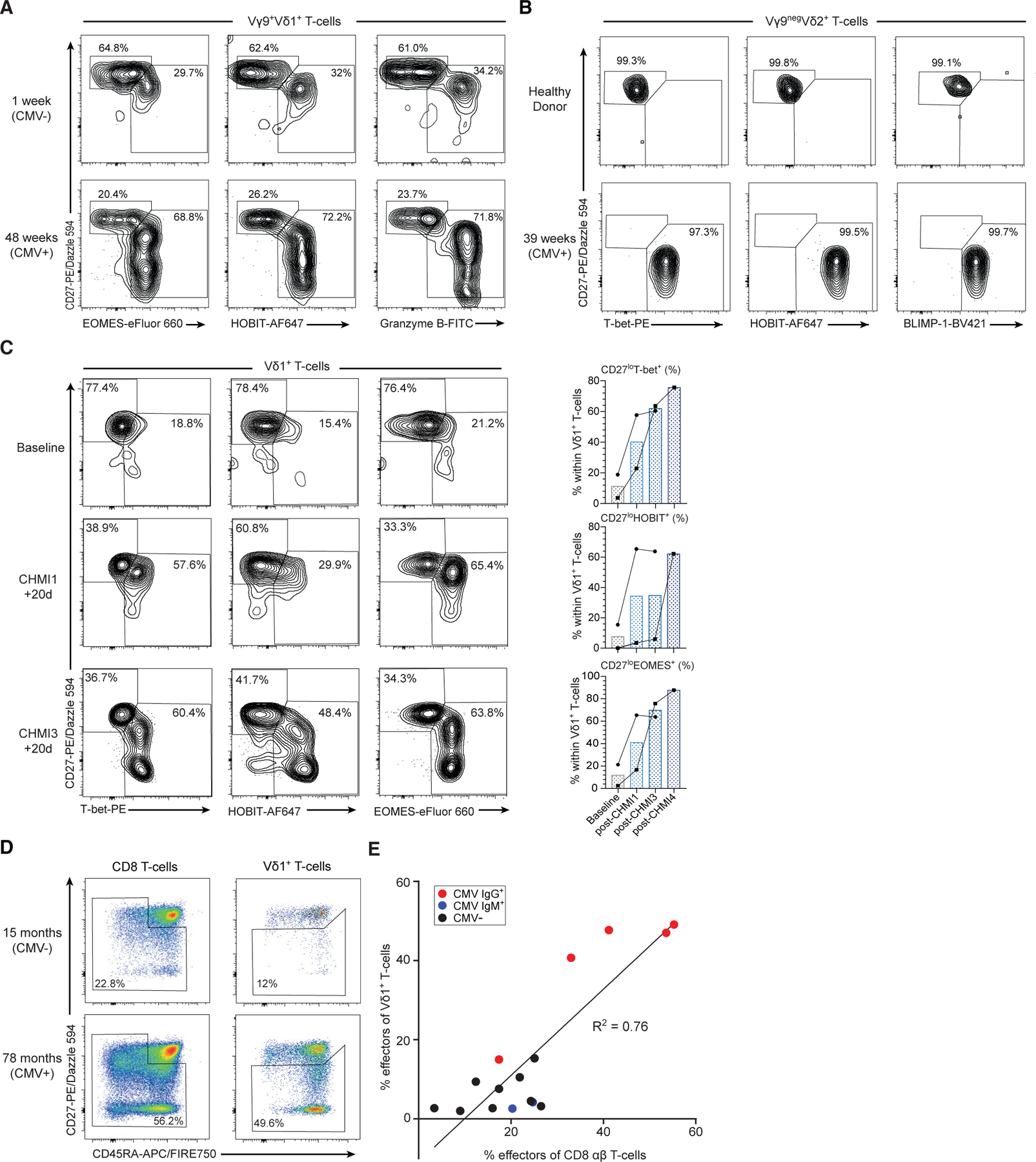
(A) Transition of Vγ9+Vδ1+ T cells to a Teffector transcriptional program following CMV infection after kidney transplantation involving increased Eomes+, HOBIT+, and granzyme B+ Teffector cells.
(B) Vγ9negVδ2+ T cells transition from a predominantly CD27hi, T-betneg, HOBITneg, BLIMP-1neg phenotype to a CD27lo/neg, T-bet+, HOBIT+, BLIMP-1+ phenotype 39 weeks post-CMV infection.
(C) Transition of Vδ1+ T cells from a Tnaive to a Teffector transcriptional program following consecutive controlled human malaria challenge (CHMI) in two malarianaive individuals. (Left panel) Flow cytometry plots from one individual showing baseline (malaria naive), CHMI challenge 1 + 20 days after first infection and CHMI challenge 3 + 20 days and measure expression of T-bet, HOBIT, and Eomes versus CD27. (Right panel) Frequencies of T-bet+, HOBIT+, and Eomes+ Vδ1+ T cells from the same individual and second individual undergoing CHMI.
(D) Assessment of Vδ1+ Teffector (CD27lo/neg) and CD8+ effector (TCM + TEM + TEMRA) cells in 16 healthy children (aged 12 months to 12 years).
(E) Percentage of CD27lo/neg Vδ1+ cells correlates with percentage of CD8+ effector αβ T cells (R2 = 0.76, Spearman coefficient = 0.73, p = 0.0017).
DISCUSSION
Evidence is emerging that an adaptive immune paradigm underpins the function of human γδ T cell subsets, including Vδ1+ (Davey et al., 2017, 2018a; Ravens et al., 2017; von Borstel et al., 2021) and Vγ9negVδ2+ T cells (Davey et al., 2018b, 2018c), both in PB and at least some solid tissues (Davey et al., 2018b; Hunter et al., 2018). Such adaptive biology likely impacts both anti-viral (Davey et al., 2018b; Dechanet et al., 1999; Ravens et al., 2017) and anti-parasite (Rutishauser et al., 2020; von Borstel et al., 2021) immunity and autoimmune responses (Hohlfeld et al., 1991; Mayassi et al., 2019; Pluschke et al., 1992). In contrast, Vγ9+Vδ2+ lymphocytes predominant in PB (Davey et al., 2018b; Willcox et al., 2018), and likely intestinal Vg4+ T cells (Mayassi et al., 2019; Melandri et al., 2018), appear to be more innate-like. Although some functional and clono/phenotypic changes have been observed during adaptive γδ T cell differentiation, its transcriptional and epigenetic basis (factors affecting chromatin accessibility), and how this compares with conventional αβ T cell differentiation and innate-like γδ T cell subsets, has remained unclear. Here, we used a multi-pronged approach to address this question.
Single-cell methods showed that, in adult PB, most Vδ1+ lymphocytes in the majority of healthy donors occupy one of two transcriptional states. These align closely with phenotypically distinct Vδ1+ Tnaive and Teffector subtypes defined previously, which differentially express markers, such as CD27, IL7Rα, CX3CR1, perforin, and granzyme A/B (Davey et al., 2017, 2018a), and exhibit differential chromatin accessibility. Our data reveal close transcriptional parallels with conventional αβ CD8+ Tnaive and CD8+ TEMRA subsets, to which the Vδ1+ Tnaive and Teffector subsets respectively exhibit phenotypic similarity (Davey et al., 2017, 2018a). There is a strong overlap in the transcription factors governing these naive/effector states in the Vδ1+ and CD8+ compartments. Naive Vδ1+ and CD8+ T cells are discriminated by TCF7/LEF1 expression, previously highlighted as crucial for CD8+ identity (Xing et al., 2016). The Vδ1+ Teffector and CD8+ TEMRA state is distinguished by upregulation of Eomes, T-bet, and BLIMP-1, all critical to T cell effector program maintenance (Fu et al., 2017; Henning et al., 2018; Kaech and Cui, 2012; Lazarevic et al., 2013; Vieira Braga et al., 2015). Vδ1+ Teffector and CD8+ TEMRA transcriptomes were especially closely matched, with a majority of genes increased in Vδ1+ Teffector cells versus Tnaive cells also increased in CD8+ TEMRA versus Tnaive cells, reflecting upregulation of pathways such as cytotoxicity and IFN-γ production. Co-expression of HOBIT alongside T-bet/Eomes, as observed in Vδ1+ Teffector cells, is a feature of long-lived CD8+ T cells contributing to immunity to persistent viruses that periodically reactivate (Vieira Braga et al., 2015) and Eomes expression in CD8+ T cells favors long-term persistence and effector memory function (Kaech and Cui, 2012).
Conserved regulation by such critical transcription factors and involving similar downstream effector pathways strongly suggests that Vδ1+ T cells undergo ‘‘transcriptional reprogramming’’ upon differentiation from Tnaive to Teffector status, analogous to CD8+ T cells. This transition likely applies to a second adaptive-like subset, Vγ9negVδ2+ T cells (Davey et al., 2018b), and may be a general feature of human adaptive γδ T cell differentiation. This common differentiation program suggests that PB Vδ1+ Teffector subsets may respond to intracellular pathogens, such as persistent viruses, using effector functions analogous to CD8+ T cells. Our results suggest that, in children, adaptive γδ subsets may often operate in parallel with adaptive CD8+ arms. However, such adaptive γδ T cell populations likely complement conventional αβ T cell responses by providing an alternative, MHC-unrestricted immune surveillance arm that may be particularly significant in certain recurrent or chronic pathogen infections, and conceivably tumor immunosurveillance. Consistent with this, genes involved in regulating thresholds for TCR/peptide MHC recognition signaling thresholds were among the rare genes differentially expressed between the Vδ1+ Teffector and CD8+ TEMRA subsets, with CD5, THEMIS, and SET1 all enriched in CD8 TEMRA cells.
Despite similarities between Vδ1+ T cell and CD8+ T cell differentiation, we note some differences. Firstly, Vδ1+ Tnaive and CD8+ Tnaive subsets clearly exhibit greater differences than is evident between Vδ1+ and CD8+ Teffector/TEMRA subsets, which are exceedingly concordant. These include an enhanced level of some NKR transcripts in Vδ1+ Tnaive cells relative to CD8+ Tnaive cells. Understanding the transcriptional, epigenetic, and functional significance of this is a key future aim, and may clarify signals that trigger Vδ1+ Tnaive to Teffector cell differentiation. Secondly, Vδ1+ lymphocytes clustered into only two subpopulations, whereas CD8+ T cells adopt several phenotypically distinct memory states. This may denote a fundamental difference in differentiation, or may alternatively reflect our study’s limitations.
A striking feature of several transcription factors differentially expressed between Vδ1+ Tnaive and Teffector subsets is their strong regulation by TCR triggering in CD8+ T cells. TCF7/LEF1 is lost from CD8+ Tnaive cells upon TCR-dependent antigenic stimulation and priming (Willinger et al., 2006), and T-bet and Eomes are upregulated upon TCR signaling in CD8+ T cells, with amplification via cytokine receptor signaling (Kaech and Cui, 2012). Several observations suggest that adaptive γδ T cell differentiation is similarly driven or initiated by TCR triggering. Firstly, BLIMP-1, T-bet, and Eomes upregulation in Tnaive Vδ1+ cells was dependent on CD3 stimulation, consistent with γδ TCR ligation triggering upregulation of transcription factors linked to the Teffector state. These findings were mirrored in CD8+ αβ T cells, and HOBIT was upregulated on neither Vδ1+ nor CD8+ Tnaive populations, consistent with its discordant regulation relative to BLIMP-1 after antigen stimulation (Kragten et al., 2018). Given the long duration of this assay, such CD3-driven upregulation of Teffector transcription factors may result from either direct signaling downstream of the TCR, or indirect effects, such as TCR-triggered cytokine production, or a combination of both. Secondly, the Tnaive to Teffector transition is tightly linked to clonotypic expansion: consistent with single-cell data presented here, both Vδ1+ and Vγ9negVδ2+ Tnaive cells are clonotypically diverse and unfocused, whereas clonally expanded TCRs are only detected in Teffector subsets (Davey et al., 2017, 2018b). Finally, in CMV and malaria infection models, pathogen exposure elicited Vδ1+ (and for CMV also Vγ9negVδ2+) clonal expansions (Davey et al., 2018b, 2018c; Ravens et al., 2017; Rutishauser et al., 2020; von Borstel et al., 2021), and drove phenotypic differentiation (Davey et al., 2018b, 2018c; Rutishauser et al., 2020; von Borstel et al., 2021). Collectively, these observations suggest that, as for conventional αβ T cells, cognate γδ TCR antigen recognition plays a critical role in triggering adaptive γδ T cell differentiation. The fact that Teffector populations display upregulated and expedited cytotoxicity/cytokine secretion relative to Tnaive cells, retain CD3/TCR sensitivity, and exhibit long-term persistence in vivo, suggests ongoing potential for γδ TCR ligand recognition during secondary/tertiary responses.
Previous studies on transcriptional regulation of T cell differentiation have focused largely on conventional αβ T cells. This study outlines comprehensively the transcriptional basis for adaptive γδ T cell biology. The results are in accordance with, but extend substantially, our previous work on Vδ1+ Tnaive and Teffector cells (Davey et al., 2017, 2018a). These studies established phenotypic and clonotypic changes consistent with some kind of adaptive differentiation, but did not define underlying transcriptional paradigms and their alignment with conventional αβ T cell differentiation. Many previous studies have assessed Vδ1+ T cells en masse, an example being Gutierrez-Arcelus et al. (2019), which positioned Vδ1+ T cells on an ‘‘innateness gradient.’’ In light of our results, caution should be exerted in such interpretations, as factors such as age and infectious status heavily influence the balance between Tnaive and Teffector Vδ1+ populations and are major confounding factors, limiting the utility and validity of such a gradient; the innateness scale proposed is also likely to overlap largely with effector program signatures. Also, expression of markers, such as NKG2D, on Vδ1+ T cells is sometimes interpreted as denoting an innate-like biology (Wu et al., 2019), whereas NKG2D is upregulated on both Vδ1+ Teffector and CD8+ TEMRA cells, and may alternatively reflect the presence of adaptive Vδ1+ Teffector populations.
Our results confirm that, relative to adaptive subsets, Vγ9+Vδ2+ T cells adopt a fundamentally different and innate-like immunobiology. Unlike Vδ1+ T cells, the adult Vγ9+Vδ2+ T cell subset lacked a distinct Tnaive subpopulation devoid of effector markers, and universally expressed T-bet and Eomes. The transcriptional profile of Vγ9+Vδ2+ T cells unmistakably denoted a type of Teffector status based on expression of granzyme A/B, perforin, and T-bet/Eomes. However, elements of this profile were notably different from both Vδ1+ and CD8+ Teffector cells, including expression of PLZF, a transcription factor linked with innate-like T cells (Fergusson et al., 2014; Kovalovsky et al., 2008; Kreslavsky et al., 2009; Savage et al., 2008), NKG2A and GZMK, both expressed by NK cells (Bratke et al., 2005; Brooks et al., 1997), and distinct cytokine and chemokine receptor expression patterns. A caveat is that we assessed Vγ9+Vδ2+ T cells en masse, whereas some other studies (Ryan et al., 2016; Wragg et al., 2020) have defined phenotypically distinct subsets present in multiple individuals. The central memory phenotype of Vγ9+Vδ2+ T cells present in most donors is consistent with profiles observed by Ryan et al. (2016); we observe NKG2A expression on most Vγ9+Vδ2+ cells in all donors, consistent with Wragg et al.’s findings (2020). Also, we observe markers linked with innate-like T cells (e.g., NKG2A, PLZF, C/EBPd), consistent with previous work (Gutierrez-Arcelus et al., 2019), which indicated similarities between Vγ9+Vδ2+, MAIT, and iNKT transcriptional profiles. While we did not directly compare NK transcriptional profiles, Pizzolato et al. (2019) detected a subpopulation of γδ T cells clustering with NK cells and this may align with the Vγ9+Vδ2+ subset. In contrast to Vδ1+ T cells, our results suggest a predisposition to effector status from birth and maturation early in life, since Vγ9+Vδ2+ T cell expression of Eomes and HOBIT was evident in cord blood, and universal by <3 years, consistent with recent findings (Papadopoulou et al., 2020). Such an innate-like paradigm is likely facilitated by the semi-invariant Vγ9+Vδ2+ TCR repertoire present from birth, featuring prevalence of public Vγ9+ clonotypes (Davey et al., 2018b; Willcox et al., 2018). While our results support post-natal maturation of Vγ9+Vδ2+ T cells (Ryan et al., 2016; Wragg et al., 2020), they also suggest that, from early life onward, changes reflect modification of an innate-effector transcriptional profile/phenotype, rather than ongoing potential for the more radical transcriptional reprogramming that applies to adaptive γδ T cell subsets.
In summary, we show that certain γδ T cell subsets can, throughout life and in response to specific immunological stimuli, undergo adaptive ‘‘transcriptional reprogramming’’ akin to changes in CD8+ T cells upon antigen-induced differentiation. This paradigm applies to Vδ1+ T cells present in PB and diverse tissues (Davey et al., 2017, 2018a; Hunter et al., 2018; Ravens et al., 2017) and the less prevalent Vγ9negVδ2+ subset (Davey et al., 2018b, 2018c). We suggest that adaptive differentiation drives inflammatory/cytotoxic γδ T cell responses to specific immune challenges in diverse biological scenarios. These likely include beneficial responses to pathogen infection (Davey et al., 2018a, 2018c), including CMV (Davey et al., 2018b; Ravens et al., 2017), EBV (Farnault et al., 2013; Fujishima et al., 2007), and malaria (Rutishauser et al., 2020; von Borstel et al., 2021). They likely complement conventional adaptive αβ T cell immunity, by permitting pathogen-specific responses to microenvironmental niches with compromised MHC expression, either resulting from viral immune evasion (for CMV and EBV), infection of cells devoid of MHC (e.g., red blood cells in the case of P. falciparum), or conceivably class I MHC loss during tumor development and immune evasion. However, adaptive γδ responses may sometimes promote immunopathology, as in autoimmune myositis driven by clonotypically focused Vγ9negVδ2+ T cells apparently Teffector in phenotype (Hohlfeld et al., 1991; Pluschke et al., 1992). A recent study reported that, in coeliac disease, the intestinal IEL population is enriched with inflammatory, T-bet+, clonotypically focused Vδ1+ cells that may be antigen driven, aligning with the adaptive γδ T cell differentiation paradigm outlined here (Mayassi et al., 2019). Future studies will no doubt extend the relevance of this adaptive paradigm to different settings. Understanding the transcriptional regulation of γδ T cell differentiation may also facilitate development of γδ T cell immunotherapy approaches in infectious disease and cancer.
Limitations of the study
We highlight three study limitations. Firstly, we focus on a modest number of individuals and on a limited number of γδ T cells in PB; a wider set of Vδ1+ T cell differentiation states therefore likely exist. Future studies will no doubt elaborate on our findings by exploring larger cohorts and/or cell numbers, and assessing γδ T cells in diverse scenarios, including active infection, and different tissues. Consistent with this, we recently defined a tissue-resident-like hepatic Vδ1+ subset phenotypically and functionally distinct from PB subsets (Hunter et al., 2018). Secondly, we used CD8+ TEMRA cells as a conventional effector T cell comparator; however, more marked differences between Vδ1+ Teffector cells and other CD8+ T cell subtypes may exist; we did not directly compare transcriptional profiles of CD4+ T cell populations, and alignment of Vδ1+ to CD4+ T cell differentiation warrants further study. Finally, despite strong similarities between Vδ1+ and CD8+ T cell subsets, our results do not exclude the possibility that, aside from MHC-unrestricted target cell recognition, adaptive γδ T cell populations may exhibit substantial functional differences to conventional counterparts, such as in their dynamics or relative importance of TCR-independent regulatory axes (e.g., mediated via NKRs).
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Benjamin Willcox (b.willcox@bham.ac.uk).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Raw data utilised in this study has been uploaded to the SRA database, under the accession code PRJNA562324 and the project title ‘‘Epigentic and transcriptional profiling of human gamma delta T cells’’.
This paper does not report any original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Ethical approval and samples
Peripheral blood samples were obtained from healthy donors or from buffy packs. All donors provided written informed consent for sample collection and subsequent analysis; project approval for this aspect of the study was granted by the NRES Committee West Midlands ethical board (REC reference 14/WM/1254) or for buffy packs by the Australian Red Cross (ARC) Lifeblood ethics committee and Monash University Human Research Ethics Committee (Reference, 19,488 and 14,487). Samples from patients undergoing renal transplantation were obtained at the Academic Medical Centre, Amsterdam; the medical ethics committee of the Academic Medical Center, Amsterdam, approved this arm of the study and all subjects provided written informed consent in accordance with the Declaration of Helsinki. Umbilical cord blood units were obtained from the Anthony Nolan Cell Therapy Centre Nottingham (ANCTC) under generic tissue bank ethics held by ANCTC and extended to the researchers under a material transfer agreement (MTA). Paediatric healthy volunteer samples were obtained as part of the TRICICL study (Tracking the Immune changes in children with cancer) with IRAS number 233593 and REC reference 17/WM/0453; health research authority and ethics approval was obtained from South Birmingham research ethics committee. Peripheral blood samples from two subjects participating in the controlled human malaria infection (CHMI) study were also included. Heparinized venous blood was collected at baseline (before infection), and twenty days (d) after the first (CHMI1), third (CHMI3), and for one subject, fourth P. falciparum infection (CHMI4). Subjects were infected by bites of mosquitos carrying P. falciparum (strain NF54) and were treated with anti-malarial drugs (Malarone and CoArtem as a secondary treatment) based upon the detection of two unquestionable parasites by blood smear. Both subjects gave written informed consent and the study was approved by the medical ethics committee of the University of Maryland, Baltimore (ClinicalTrials.gov, NCT03014258).
METHOD DETAILS
T-cell isolation, culture, activation
PBMC were isolated from heparinised venous blood or buffy packs by using lymphoprep© (Stem Cell Technologies) density gradients. Resulting PBMC were frozen in liquid nitrogen and thawed to be used for subsequent experiments. Cells were sorted on an ARIA III Fusion Cell Sorter (BD Bioscience) at the University of Birmingham or FlowCore, Monash University. PBMC and purified T-cells were cultured in RPMI-1640 medium (Invitrogen) supplemented with 2 mM L-glutamine, 1% sodium pyruvate, 50 μg/mL penicillin/streptomycin (Invitrogen) and 10% fetal calf serum (Sigma). In total, Vδ1+ Teffector (CD27lo/neg) populations were sorted from 6 healthy donors, and Vδ1+ Tnaïve (CD27hi) from 3, CD8+ Tnaïve from 7, and CD8+ TEMRA populations were obtained from 9, with comparator Vδ2+ populations obtained from 2 individuals (Davey et al., 2017).
Antibodies and flow cytometry
For phenotypic analysis and cell sorting, freshly isolated, frozen PBMC or cultured cells were labelled with Zombie Aqua viability dye (Biolegend), and Vδ1+ and Vγ9+Vδ2+ populations were identified with anti-CD3 (UCHT1; Biolegend), TCR ab (IP26; ThermoFisher), TCR Vδ1 (REA173; Miltenyi), TCR Vδ2 (123R3; Miltenyi), TCR Vγ9 (IMMU360; Beckman Coulter), CD27 (M-T271; Biolegend) and CD45RA (HI100; Biolegend) and various combinations of CXCR3 (GO25H7), CX3CR1 (2A9–1), CD127 (A019D5), CD45RO (UCHL1), CD161 (HP-3G10), all Biolegend; or NKG2A (Z199; Beckman Coulter). Populations were gated as outlined previously (Davey et al., 2017). For intracellular staining, cells were fixed in IC Fixation buffer (eBioscience) after surface antibody staining and finally stained in Permeabilisation Buffer (eBioscience) with antibodies directed against Granzyme A (CB9), Granzyme B (GB11), Perforin (B-D48), TCF7 (7F11A19), all Biolegend; EOMES (WD1928, Invitrogen); Blimp-1 (6D3) and Hobit (Sanquin-Hobit/1), both BD Biosciences; and T-bet (eBio4B10) and PLZF (21F7), both eBioscience. Cells were acquired on a Fortessa (BD Biosciences) and data analysed using FlowJo V10.1 (TreeStar & BD Biosciences). For detection of intracellular cytokines, cells were stimulated with 10 ng/mL PMA and 1 μg/mL ionomycin (both Sigma) for 1 h before adding 5 μg/mL brefeldin A (ThermoFisher) and 2 μM monensin (BD Biosciences) to the cultures for 4 h prior to harvesting. Surface-stained cells were labeled using the Fix & Perm kit (ThermoFisher) and monoclonal antibodies against IFNγ (4S.B3), TNFα (mAb11), CCL5 (VL1), IL-2 (MQ1–17H12), IL-17A (BL168), GM-CSF (BVD2–21C11), all Biolegend.
Single cell transcriptomics analysis
Single CD3+ TCR-αβ neg Vδ1+ T-cells (CD27hi or CD27lo/neg) from three donors were sorted directly into the wells of 96 well PCR plates containing 2 μL of 0.2% Triton X-100 (Sigma) with 2 U/μL recombinant RNase inhibitor (Takara Bio) and frozen at −80°C. cDNA was generated using the SmartScribe Reverse Transcriptase (Takara Bio Cat. 639,538), preamplified using the KAPA HiFi Hot Start Ready Mix, and sequencing libraries generated with the Nextera XT kit, following the protocol of Picelli et al. (2014).
Gene expression was quantified using Salmon (Patro et al., 2017), exporting transcripts per million (TPM) (version 0.6.0). Downstream analysis was completed using the Seurat package (v 2.3.4) (Butler et al., 2018) in R (v 3.5.3) (Team, 2000). Ensembl genes were mapped to HUGO gene symbols using the biomaRt package for R(Durinck et al., 2009). Upon conversion, 1,483 genes were not annotated and were discarded. Where symbols were not available, ensembl IDs were used. Genes were subsequently re-scaled to sum to 1 million and summated for those Ensembl genes which resolved to the same gene name. Three cells were deemed outliers by PCA analysis, and genes were filtered according to the Seurat package user guide. Data were separated into two clusters using the FindClusters() function under default conditions other than a resolution of 0.5. Data were displayed using PCA dimension reduction. To determine the number of significant principal components, both an Elbow plot and a JackStraw plot (100 replications) were constructed. An ‘elbow’ was observed at PC2, and PC1 and PC2 were deemed significant by JackStraw analysis. PC1 was the most significant principal component and was, therefore, further investigated between the clusters. Differential gene expression utilised a bimodal test within Seurat, an FDR cut-off of 0.05 (Bonferroni corrected), and a Log(Fold Change) threshold of 0.25. Interclone differences were calculated by a one-versus-many approach. The largest expanded clones for each donor (A, E, and K) were taken and individually compared to the others i.e. A versus E and K.
Reconstruction of γδ TCR sequences from single cell RNAseq
γδ TCR sequences were reconstructed from single cell transcriptomes, using TraCeR as previously described (Stubbington et al., 2016). TCRγ and TCRδ reference files were constructed from IMGT (Lefranc et al., 2009) reference sequences using the ‘build’ command of TraCeR. Given previous observations of long junctional regions for TCRδ (Davey et al., 2017; Hunter et al., 2018), the ‘max_junc_len’ parameter was increased to 100.
Bulk RNAseq analysis
Naive (CD27hi) and effector (CD27lo/neg) Vδ1+ T-cells or CD8+ naive (CD27hi CD45RAhi) and TEMRA (CD27–CD45RAhi) cells were sorted into RNAlater (Sigma Aldrich). Total RNA was isolated using the RNAmicro kit (Qiagen) according to the manufacturer’s instructions and RNA sequencing was performed by Genomics Birmingham (Birmingham, UK) or the Medical Genomics facility (MHTP, Clayton, Australia). FASTQ files were aligned to hg38 using HISAT2 (version 2.1.0) (Kim et al., 2015) under default conditions for paired-end alignment (average alignment of 90.90%). Reads were indexed using SAMtools (version 1.4) (Li et al., 2009) and counts were generated from the bam files with the Rsubread package (version 1.32.2) (Liao et al., 2013) in R (version 3.5.1) using the inbuilt annotation for the hg38 genome assembly. Batch effects were removed from the count data using the CountClust package for R (Dey et al., 2017; Taddy, 2012). Outliers were determined using PCA and the arrayQualityMetrics R package (Kauffmann et al., 2009) in accordance with outlier removal peformed by Guinney et al. (Guinney et al., 2015). Downstream analyses utilised the edgeR package (version 3.24.2) (Robinson et al., 2010) (McCarthy et al., 2012). As suggested in the edgeR user guide, reads were filtered based on counts per million (CPM). Using a threshold of 6–7 reads in the sample with the smallest library, the CPM scaling factor was calculated. Genes with a CPM above 3.04 in at least two samples (smallest cell population) were kept for further analysis as suggested. This filtering step removed 15,768 lowly expressed transcripts, including 5,640 that were 0 in all 27 samples. Filtered reads were passed to the plotMDS() function, using all available reads for multidimensional scaling. Utilisation of voom transformation allowed the use of the limma (version 3.38.3) package for differential gene expression analysis (Law et al., 2014; Ritchie et al., 2015). Genes were adjusted using the Benjamin-Hochberg method, as is the default and were considered significant if p < 0.05. Geneset enrichment analysis utilised the GO and KEGG database of terms, using the camera method (limma package). Genesets with less than 10 or more than 300 genes were discarded. The output of geneset enrichment analysis were visualised using Cytoscape (Shannon et al., 2003) (version 3.7.1) and the RCy3 package (version 2.2.6) (Ono et al., 2015). A similarity threshold of 0.25 was based on the Jaccard coefficient, and only genesets passing the 0.001 q value threshold were plotted. The graphic output used a force-directed layout. Heatmaps were constructed utilising the gplots R package (Warnes et al., 2020), using Euclidean distance and Ward linkage.
ATACseq analysis
Vδ1+ T-cells, CD8+ T-cells and Vγ9+Vδ2+ T-cells (20,000 of each population) were sorted into MACS buffer and cells were pelleted by centrifugation at 400 3 g. ATACseq libraries were generated using the Nextera DNA Library Prep Kit (Illumina Cat. FC-121–1030) in the presence of 0.01% digitonin (Promega Cat. G9441) and amplified using NEBNext High-Fidelity 2× PCR Master Mix (New England Labs Cat #M0541) for 9–13 cycles (Buenrostro et al., 2015). Amplified libraries were purified using Ampure beads (Beckman Coulter) and resuspended in 0.1× TE before quantification on Tapestation (Agilent), pooling and sequencing using the NextSeq 500/550 High Output kit v2.5 (Illumina) at Genomics Birmingham (Birmingham, UK). Paired end FASTQ files were aligned to the GRCh38 genome assembly using Bowtie2 (Langmead and Salzberg, 2012) (version 2.3.5.1), and sorted and indexed using SAMtools (version 1.9). Average number of fragments was 34,056,451, with 89.09% alignment to the reference genome. Samples had a relatively low alignment to the mitochondrial genome (4.21%) Duplicate sequences due to PCR were removed using Picard (Broad Institute, 2019) (version 2.21.1) and peaks were called using MACS2 (Zhang et al., 2008) (version 2.2.7.1) using settings of –shift 100 and –extsize 200. An average of 55,236 peaks were detected. HOMER (Heinz et al., 2010) (version 4.11) was used to find consensus peaks amongst the samples, using a distance argument of 100. 21,208 consensus peaks were found of which counts were subsequently normalised to 1e7 per sample and annotated using HOMER with a –size 400. Raw bam files were opened in IGV Genomics Viewer (version 2.8.12), and group autoscaled for visualisation.
CD3/CD28/IL-2 stimulation assays
For assessing TCR-mediated regulation of transcription factor expression, enriched Vδ1+ and CD8+ T-cells were used. Individuals with a substantial proportion of Tnaive Vδ1+ T-cells were prioritised for these experiments. Vδ1+ T-cells were negatively enriched from PBMC through depletion of αβ T-cells, B-cells, Vδ2+ T-cells, and monocytes by using a cocktail of APC-conjugated anti-αβ TCR (clone IP26, Thermo Scientific), CD19 antibody (clone HIB19, TONBO biosciences), Vδ2 TCR antibody (clone 123R3, Miltenyi Biotec), and CD14 antibody (clone 61D3, TONBO bioscience). CD8+ T-cells were negatively enriched from PBMC through the depletion of CD4+ T-cells, B-cells, Vδ2+ T-cells, Vδ1+ T-cells and monocytes by using a cocktail of APC-conjugated anti-CD4 (RPA-T4, Cy7-conjugated, BD Pharminogen), anti-CD19 antibody (clone HIB19, TONBO biosciences), anti-Vδ2 TCR antibody (clone 123R3, Miltenyi Biotec), anti-Vδ1 TCR antibody (clone REA173, Miltenyi Biotec), and anti-CD14 antibody (clone 61D3, TONBO bioscience). The APC-labelled cells in both settings were eliminated using EasySep Human APC Selection Kit (Stemcell Technologies). Enriched T-cells were then incubated at 37°C in the presence of plate-bound anti-CD3 antibodies (OKT-3, Biolegend) or 100 U/mL IL-2 (Peprotech) for 6 days. The expression level of relevant transcription factors in the Vδ1+ Tnaïve (CD27hi) and CD8+ Tnaïve subsets were measured on day 0 (unstimulated) and on day 6 of culture using flow cytometry. Data were analysed using FlowJo (version 10) software.
QUANTIFICATION AND STATISTICAL ANALYSIS
Tabulated data were analysed in Graphpad PRISM 7 (Graphpad Software Inc). Each dataset was assessed for normality using Shapiro-Wilko normality tests. Differences between groups were analyzed using one-way ANOVA with Holm-sidak’s or Tukey’s post-tests for normally distributed data; two-way ANOVA was used when comparing groups with independent variables. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. Correlation was assessed with Spearman correlation, and R2 values are reported.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Anti-human CD3, clone UCHT1 | Biolegend | Cat# 300460 |
| TCR αβ, clone IP26 | ThermoFisher | Cat#535531 |
| TCR Vδ1, clone REA173 | Miltenyi | Cat#130–118-362 |
| TCR Vδ2, clone 123R3 | Miltenyi | Cat#130–095-796 |
| TCR Vγ9, clone IMMU360 | Beckman Coulter | Cat#A63663 |
| CD27, clone M-T271 | Biolegend | Cat#356422 |
| CD45RA, clone HI100 | Biolegend | Cat# 304151 |
| CXCR3, clone GO25H7 | Biolegend | Cat#353705 |
| CX3CR1, clone 2A9–1 | Biolegend | Cat#341626 |
| CD127, clone A019D5 | Biolegend | Cat#351334 |
| CD45RO, clone UCHL1 | Biolegend | Cat# 304237 |
| CD161, clone HP-3G10 | Biolegend | Cat# 339903 |
| NKG2A, clone Z199 | Beckman Coulter | Cat#A60797 |
| Granzyme A, clone CB9 | Biolegend | Cat#507204 |
| Granzyme B, clone GB11 | Biolegend | Cat#515403 |
| Perforin, clone B-D48 | Biolegend | Cat#353305 |
| TCF-7, clone 7F11A19 | Biolegend | Cat#655203 |
| EOMES, clone WD1928 | ThermoFisher | Cat #50–4877-42 |
| Blimp-1, clone 6D3 | BD Biosciences | Cat#565276 |
| Hobit, clone Sanquin-Hobit/1 | BD Biosciences | Cat#566250 |
| T-bet, clone eBio4B10 | ThermoFisher | Cat#12–5825-82 |
| PLZF, clone 21F7 | ThermoFisher | Cat#12–9320-82 |
| IFNγ, clone 4S.B3 | Biolegend | Cat#502511 |
| TNFα, clone mAb11 | Biolegend | Cat#502935 |
| CCL5, clone VL1 | Biolegend | Cat#515503 |
| IL-2, clone MQ1–17H12 | Biolegend | Cat#500347 |
| IL-17A, clone BL168 | Biolegend | Cat#512315 |
| GM-CSF, clone B VD2–21C11 | Biolegend | Cat#502305 |
| CD19, clone HIB19 | TONBO biosciences | Cat#20–0199-T100 |
| CD14, clone 61D3 | TONBO biosciences | Cat#20–0149-T100 |
| CD3, clone OKT-3 | Biolegend | Cat#317326 |
|
| ||
| Biological samples | ||
|
| ||
| Healthy donor peripheral blood mononuclear cells | This study | |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Triton X-100 | Sigma | Cat#X100 |
| recombinant RNase inhibitor | Takara Bio | Cat#2313A |
| RNAlater | Sigma | Cat #R0901 |
| digitonin | Promega | Cat# G9441 |
| Recombinant IL-2 | Peprotech | Cat# 200–02 |
| RPMI-1640 | ThermoFisher | Cat#21875034 |
| L-Glutamine | ThermoFisher | Cat#25030081 |
| Sodium pyruvate | ThermoFisher | Cat#11360070 |
| penicillin/streptomycin | ThermoFisher | Cat#15070063 |
| Fetal Calf serum | Sigma | Cat#F7524 |
| Permeabilisation Buffer | ThermoFisher | Cat#00–8333-56 |
| PMA | Sigma | Cat#P1585 |
| Ionomycin | Sigma | Cat#I0634 |
| Brefeldin A | ThermoFisher | Cat#00–4506-51 |
| Monensin | BD Biosciences | Cat#554724 |
|
| ||
| Critical commercial assays | ||
|
| ||
| SmartScribe Reverse Transcriptase | Takara Bio | Cat#639538 |
| KAPA HiFi Hot Start Ready Mix | Fisher Scientific | Cat#50–196-5217 |
| Nextera XT kit | Illumina | Cat#CFC-131–1024 |
| RNeasy Plus Micro kit | Qiagen | Cat#74034 |
| Nextera DNA Library Prep Kit | Illumina | Cat#FC-121–1030 |
| NextSeq 500/550 High Output kit v2.5 | Illumina | Cat#20024907 |
| EasySep Human APC Selection Kit | Stemcell Technologies | Cat#17661 |
|
| ||
| Deposited data | ||
|
| ||
| Epigenetic and transcriptional profiling of human gamma delta T cells | This study | SRA database accession code PRJNA562324 |
| Human genome build (GRCh38/hg38) | Genome Reference Consortium | https://www.ncbi.nlm.nih.gov/grc/human |
|
| ||
| Software and algorithms | ||
|
| ||
| FlowJo version 10 | FlowJo LLC | https://www.flowjo.com/ |
| GraphPad Prism version 8.0.2 | GraphPad Software LLC | https://www.graphpad.com |
| R version 3.5.1/3.5.3 | R Project | https://www.r-project.org/ |
| Salmon version 0.6.0 | Patro et al. (2017) | https://salmon.readthedocs.io/en/latest/salmon.html |
| TraCeR | Stubbington et al. (2016) | https://doi.org/10.1038/nmeth.3800 |
| HISAT2 version 2.1.0 | Daehwankim lab | https://daehwankimlab.github.io/hisat2/ |
| SAMTools version 1.4 & version 1.9 | Li et al. (2009) | http://www.htslib.org/ |
| Cytoscape version 3.7.1 | Shannon et al., (2003) | https://cytoscape.org/ |
| Bowtie2 version 2.3.5.1 | Langmead and Salzberg (2012) | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| Picard version 2.21.1 | GATK | https://sourceforge.net/projects/picard/ |
| MACS2 version 2.2.7.1 | Zhang et al. (2008) | https://pypi.org/project/MACS2/ |
| HOMER version 4.11 | University of California San Diego | http://homer.ucsd.edu/homer/ |
| IGV Genomics Viewer version 2.8.12 | Broad Institute (2019) | https://software.broadinstitute.org/software/igv/ |
| Seurat version 2.3.4 (R package) | Butler et al. (2018) | https://satijalab.org/seurat/ |
| edgeR version 3.24.2 (R package) | Robinson et al. (2010) McCarthy et al. (2012) | https://bioconductor.org/packages/release/bioc/html/edgeR.html |
Highlights.
Vδ1+ T cells undergo transcriptional reprogramming from Tnaive to Teffector status
Vδ1+Teffector cells are highly transcriptionally similar to CD8+ TEMRA cells
CD3 ligation of Vδ1+Tnaive cells induces Teffector-linked transcription factors
Infections drive transition from a Vδ1+Tnaive to Teffector transcriptional profile
ACKNOWLEDGMENTS
We thank all donors and patients who participated in the study, AMC biobank staff for provision of renal transplant patient samples, and the Anthony Nolan Cell Therapy Centre for cord blood samples. We thank Dr. Matthew McKenzie and the University of Birmingham CMDS Cell Sorting Facility for γδ T cell isolation, the University of Birmingham Protein Expression Facility for use of facilities, FlowCore (Monash University) for cell sorting assistance, and the Medical Genomics facility (MHTP) for their services. We thank US CHMI study volunteers for their contribution and commitment to malaria research; Dr. Gregory Deye, of the National Institutes of Allergy and Infectious Diseases at the National Institutes of Health, for service as program medical officer of the repetitive challenge study at the University of Maryland, Baltimore (UMB); and Faith Pa’ahana-Brown, RN, Lisa Chrisley, RN, Alyson Kwon, Brenda Dorsey, Ana Raquel Da Costa, Jeffrey Crum, Kathleen Strauss, and Biraj Shrestha for their roles in the repetitive challenge study at UMB. We thank Sanaria, Inc. for providing mosquitoes for human malaria infections. The work was supported by Wellcome Trust Investigator award funding, supporting C.R.W., M.S.D., F.M., T.E.T. and M.S. (099266/Z/12/Z and 221725/Z/20/Z to B.E.W.). J.L.McM. was supported by a CRUK non-clinical studentship; F.A.V.B. by Open Targets (https://www.opentargets.org/); J.R. is supported by an Australian Research Council (ARC) Laureate Fellowship; K.W. and K.E.L. are supported by a National Institutes of Health (NIH), Division of Allergy and Infectious Diseases (NIAID) U01 (AI-110852), distributed by the Henry M. Jackson Foundation (no. 1701447C); and K.E.L. is further supported by additional funding from the NIAID (U01-HD092308, R01-AE141900, and AI110820–06), The Geneva Foundation (V-12VAXHRFS-03), the Medical Technology Enterprise Consortium (MTEC-17–01), and Pfizer (C4591001, site 1002). M.S.D. is supported by an ARC Discovery Early Career Researcher Award (DE200100292), Rebecca L. Cooper Medical Research Foundation Project Grant (PG2020668), and ARC Discovery Project (DP210103327). The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.110858.
DECLARATION OF INTERESTS
M.J.T.S. has been employed by 10× Genomics since April 2018; this employment had no bearing on this work. The other authors declare no competing financial interests.
REFERENCES
- Bank I, Tanay A, Migdal A, Book M, and Livneh A (1995). Vγ9-Vδ2+ γδ T cells from a patient with felty syndrome that exhibit aberrant response to triggering of the CD3 molecule can regulate immunoglobulin secretion by B cells. Clin. Immunol 74, 162–169. [DOI] [PubMed] [Google Scholar]
- Bratke K, Kuepper M, Bade B, Virchow JC Jr., and Luttmann W (2005). Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur. J. Immunol 35, 2608–2616. [DOI] [PubMed] [Google Scholar]
- Broad Institute (2019). ‘‘Picard Toolkit’’. In GitHub Repository http://broadinstitute.github.io/picard/ (Broad Institute; ). [Google Scholar]
- Brooks AG, Posch PE, Scorzelli CJ, Borrego F, and Coligan JE (1997). NKG2A complexed with CD94 defines a novel inhibitory natural killer cell receptor. J. Exp. Med 185, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Chang HY, and Greenleaf WJ (2015). ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol 21, 21.29.1–21.29.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, and Satija R (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol 36, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MS, Willcox CR, Baker AT, Hunter S, and Willcox BE (2018a). Recasting human Vδ1 lymphocytes in an adaptive role. Trends Immunol 39, 446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MS, Willcox CR, Hunter S, Kasatskaya SA, Remmerswaal EBM, Salim M, Mohammed F, Bemelman FJ, Chudakov DM, Oo YH, and Willcox BE (2018b). The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9− subsets. Nat. Commun 9, 1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MS, Willcox CR, Hunter S, Oo YH, and Willcox BE (2018c). Vδ2+ T cells-two subsets for the price of one. Front. Immunol 9, 2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, Hunter S, Salim M, Mohammed F, Price DA, et al. (2017). Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun 8, 14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet MM, Kourilsky P, et al. (1999). Implication of gammadelta T cells in the human immune response to cytomegalovirus. J. Clin. Invest 103, 1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey KK, Hsiao CJ, and Stephens M (2017). Visualizing the structure of RNA-seq expression data using grade of membership models. PLoS Genet 13, e1006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S, Spellman PT, Birney E, and Huber W (2009). Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc 4, 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnault L, Gertner-Dardenne J, Gondois-Rey F, Michel G, Chambost H, Hirsch I, and Olive D (2013). Clinical evidence implicating gamma-delta T cells in EBV control following cord blood transplantation. Bone Marrow Transplant 48, 1478–1479. [DOI] [PubMed] [Google Scholar]
- Fergusson JR, Smith KE, Fleming VM, Rajoriya N, Newell EW, Simmons R, Marchi E, Bjorkander S, Kang YH, Swadling L, et al. (2014). CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep 9, 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Vallee S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C, Salek M, Fallen PR, Hoerter JAH, Munshi A, et al. (2009). Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat. Immunol 10, 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SH, Yeh LT, Chu CC, Yen BLJ, and Sytwu HK (2017). New insights into Blimp-1 in T lymphocytes: a divergent regulator of cell destiny and effector function. J. Biomed. Sci 24, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima N, Hirokawa M, Fujishima M, Yamashita J, Saitoh H, Ichikawa Y, Horiuchi T, Kawabata Y, and Sawada KI (2007). Skewed T cell receptor repertoire of Vdelta1(+) gammadelta T lymphocytes after human allogeneic haematopoietic stem cell transplantation and the potential role for Epstein-Barr virus-infected B cells in clonal restriction. Clin. Exp. Immunol 149, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. (2015). The consensus molecular subtypes of colorectal cancer. Nat. Med 21, 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Arcelus M, Teslovich N, Mola AR, Polidoro RB, Nathan A, Kim H, Hannes S, Slowikowski K, Watts GFM, Korsunsky I, et al. (2019). Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat. Commun 10, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday AC (2000). [gamma] [delta] cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol 18, 975–1026. [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, and Glass CK (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning AN, Roychoudhuri R, and Restifo NP (2018). Epigenetic control of CD8(+) T cell differentiation. Nat. Rev. Immunol 18, 340–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R, Engel AG Ii, K., and Harper MC (1991). Polymyositis mediated by T lymphocytes that express the gamma/delta receptor. N. Engl. J. Med 324, 877–881. [DOI] [PubMed] [Google Scholar]
- Hunter S, Willcox CR, Davey MS, Kasatskaya SA, Jeffery HC, Chudakov DM, Oo YH, and Willcox BE (2018). Human liver infiltrating γδ T cells are composed of clonally expanded circulating and tissue-resident populations. J. Hepatol 69, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, and Cui W (2012). Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol 12, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski H, Menard C, El Hayani B, Adjibabi AN, Marseres G, Courant M, Zouine A, Pitard V, Garrigue I, Burrel S, et al. (2020). Characterization of a unique gammadelta T cell subset as a specific marker of CMV infection severity. J. Infect. Dis 223, 655–666. [DOI] [PubMed] [Google Scholar]
- Karunakaran MM, Willcox CR, Salim M, Paletta D, Fichtner AS, Noll A, Starick L, Nohren A, Begley CR, Berwick KA, et al. (2020). Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity 52, 487–498.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann A, Gentleman R, and Huber W (2009). arrayQualityMetrics–a bioconductor package for quality assessment of microarray data. Bioinformatics 25, 415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, and Salzberg SL (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay HF, Su S, Amann-Zalcenstein D, Daley SR, Comerford I, Miosge L, Whyte CE, Konstantinov IE, d’Udekem Y, Baldwin T, et al. (2019). A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci. Immunol 4, eaay6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. (2008). The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol 9, 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragten NAM, Behr FM, Vieira Braga FA, Remmerswaal EBM, Wesselink TH, Oja AE, Hombrink P, Kallies A, van Lier RAW, Stark R, and van Gisbergen KPJM (2018). Blimp-1 induces and Hobit maintains the cytotoxic mediator granzyme B in CD8 T cells. Eur. J. Immunol 48, 1644–1662. [DOI] [PubMed] [Google Scholar]
- Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, and von Boehmer H (2009). TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc. Natl. Acad. Sci. U S A 106, 12453–12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, and Smyth GK (2014). voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15, R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Glimcher LH, and Lord GM (2013). T-bet: a bridge between innate and adaptive immunity. Nat. Rev. Immunol 13, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Zhang HH, Singh SP, Koo L, Kabat J, Tsang H, Singh TP, and Farber JM (2018). C/EBPδ drives interactions between human MAIT cells and endothelial cells that are important for extravasation. eLife 7, e32532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, et al. (2009). IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res 37, D1006–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, and Durbin R; 1000 Genome Project Data Processing Subgroup (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W (2013). The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res 41, e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier E, Hebenstreit D, Posselt G, Hammerl P, Duschl A, and Horejs-Hoeck J (2011). Inhibition of suppressive T cell factor 1 (TCF-1) isoforms in naive CD4+ T cells is mediated by IL-4/STAT6 signaling. J. Biol. Chem 286, 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, and Calame K (2006). Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat. Immunol 7, 457–465. [DOI] [PubMed] [Google Scholar]
- Mayassi T, Ladell K, Gudjonson H, McLaren JE, Shaw DG, Tran MT, Rokicka JJ, Lawrence I, Grenier JC, van Unen V, et al. (2019). Chronic inflammation permanently reshapes tissue-resident immunity in celiac disease. Cell 176, 967–981.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, and Smyth GK (2012). Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40, 4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O, Polyakova O, Roberts NA, Wesch D, Kabelitz D, et al. (2018). The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol 19, 1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerffer S, Moita LF, Neems D, Freitas RP, Hacohen N, and Rao A (2008). Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein, hnRNPLL. Science 321, 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Muetze T, Kolishovski G, Shannon P, and Demchak B (2015). CyREST: turbocharging cytoscape access for external tools via a RESTful API. F1000Research 4, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou M, Dimova T, Shey M, Briel L, Veldtsman H, Khomba N, Africa H, Steyn M, Hanekom WA, Scriba TJ, et al. (2020). Fetal public Vγ9Vδ2 T cells expand and gain potent cytotoxic functions early after birth. Proc. Natl. Acad. Sci. U S A 117, 18638–18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, and Brenner MB (1990). Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J. Exp. Med 171, 1597–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, and Kingsford C (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, BJörklund AK, Winberg G, Sagasser S, and Sandberg R (2014). Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc 9, 171–181. [DOI] [PubMed] [Google Scholar]
- Pizzolato G, Kaminski H, Tosolini M, Franchini DM, Pont F, Martins F, Valle C, Labourdette D, Cadot S, Quillet-Mary A, et al. (2019). Single-cell RNA sequencing unveils the shared and the distinct cytotoxic hallmarks of human TCRVδ1 and TCRVδ2 γδ T lymphocytes. Proc. Natl. Acad. Sci. U S A 116, 11906–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G, Ruegg D, Hohlfeld R, and Engel AG (1992). Autoaggressive myocytotoxic T lymphocytes expressing an unusual gamma/delta T cell receptor. J. Exp. Med 176, 1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdorfer L, Reinhardt A, Ravens I, Beck M, Geffers R, et al. (2017). Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol 18, 393–401. [DOI] [PubMed] [Google Scholar]
- Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, McWilliam HEG, Hudson C, Tutuka C, Wheatley AK, et al. (2020). Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367, eaay5516. [DOI] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK (2015). Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser T, Lepore M, Di Blasi D, Dangy JP, Abdulla S, Jongo S, Ramadhani K, Sim BKL, Hoffman SL, Tanner M, et al. (2020). Activation of TCR Vδ1+ and Vδ1−Vδ2− γδ T cells upon controlled infection with Plasmodium falciparum in Tanzanian volunteers. J. Immunol 204, 180–191. [DOI] [PubMed] [Google Scholar]
- Ryan PL, Sumaria N, Holland CJ, Bradford CM, Izotova N, Grandjean CL, Jawad AS, Bergmeier LA, and Pennington DJ (2016). Heterogeneous yet stable Vδ2(+) T-cell profiles define distinct cytotoxic effector potentials in healthy human individuals. Proc. Natl. Acad. Sci. U S A 113, 14378–14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, and Bendelac A (2008). The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 29, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, and Ideker T (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegers GM, Barreira CR, Postovit LM, and Dekaban GA (2017). CD11d β2 integrin expression on human NK, B, and γδ T cells. J. Leukoc. Biol 101, 1029–1035. [DOI] [PubMed] [Google Scholar]
- Silva-Santos B, Mensurado S, and Coffelt SB (2019). γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 19, 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeoni L, Posevitz V, Kolsch U, Meinert I, Bruyns E, Pfeffer K, Reinhold D, and Schraven B (2005). The transmembrane adapter protein SIT regulates thymic development and peripheral T-cell functions. Mol. Cell. Biol 25, 7557–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbington MJT, Lonnberg T, Proserpio V, Clare S, Speak AO, Dougan G, and Teichmann SA (2016). T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods 13, 329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddy M (2012). On estimation and selection for topic models. Proceedings of the Fifteenth International Conference on Artificial Intelligence and Statistics In Proceedings of Machine Learning Research, PMLR, Neil DL and Mark G, eds., pp. 1184–1193. [Google Scholar]
- Tan L, Fichtner AS, Bubke A, Odak I, Schultze-Florey C, Koenecke C, Förster R, Jarek M, von Kaisenberg C, Borchers A, et al. (2020). A fetal wave of human type-3 γδ T cells with restricted TCR diversity persists into adulthood. Preprint at bioRxiv 10.1101/2020.08.14.248146. [DOI] [PubMed] [Google Scholar]
- Team, R.C. (2000). R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing) https://www.R-project.org/. [Google Scholar]
- Vantourout P, and Hayday A (2013). Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol 13, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Braga FA, Hertoghs KML, Kragten NAM, Doody GM, Barnes NA, Remmerswaal EBM, Hsiao CC, Moerland PD, Wouters D, Derks IAM, et al. (2015). Blimp-1 homolog Hobit identifies effector-type lymphocytes in humans. Eur. J. Immunol 45, 2945–2958. [DOI] [PubMed] [Google Scholar]
- Voisinne G, Gonzalez de Peredo A, and Roncagalli R (2018). CD5, an undercover regulator of TCR signaling. Front. Immunol 9, 2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Borstel A, Chevour P, Arsovski D, Krol JMM, Howson LJ, Berry AA, Day CL, Ogongo P, Ernst JD, Nomicos EYH, et al. (2021). Repeated Plasmodium falciparum infection in humans drives the clonal expansion of an adaptive γδ T cell repertoire. Sci. Transl. Med 13, eabe7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, et al. (2020). Gplots: Various R Programming Tools for Plotting Data. R package version 3.1.1 [Google Scholar]
- Willcox BE, and Willcox CR (2019). γδ TCR ligands: the quest to solve a 500-million-year-old mystery. Nat. Immunol 20, 121–128. [DOI] [PubMed] [Google Scholar]
- Willcox CR, Davey MS, and Willcox BE (2018). Development and selection of the human Vγ9Vδ2+ T-cell repertoire. Front. Immunol 9, 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox CR, Mohammed F, and Willcox BE (2020). The distinct MHC-unrestricted immunobiology of innate-like and adaptive-like human gammadelta T cell subsets-Nature’s CAR-T cells. Immunol. Rev 298, 25–46. [DOI] [PubMed] [Google Scholar]
- Willcox CR, Vantourout P, Salim M, Zlatareva I, Melandri D, Zanardo L, George R, Kjaer S, Jeeves M, Mohammed F, et al. (2019). Butyrophilin-like 3 directly binds a human Vγ4+ T cell receptor using a modality distinct from clonally-restricted antigen. Immunity 51, 813–825.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, and Callan MFC (2006). Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J. Immunol 176, 1439–1446. [DOI] [PubMed] [Google Scholar]
- Wragg KM, Tan HX, Kristensen AB, Nguyen-Robertson CV, Kelleher AD, Parsons MS, Wheatley AK, Berzins SP, Pellicci DG, Kent SJ, and Juno JA (2020). High CD26 and low CD94 expression identifies an IL-23 responsive Vδ2+ T cell subset with a MAIT cell-like transcriptional profile. Cell Rep 31, 107773. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kyle-Cezar F, Woolf RT, Naceur-Lombardelli C, Owen J, Biswas D, Lorenc A, Vantourout P, Gazinska P, Grigoriadis A, et al. (2019). An innate-like Vδ1+ γδ T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci. Transl. Med 11, eaax9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S, Li F, Zeng Z, Zhao Y, Yu S, Shan Q, Li Y, Phillips FC, Maina PK, Qi HH, et al. (2016). Tcf1 and Lef1 transcription factors establish CD8(+) T cell identity through intrinsic HDAC activity. Nat. Immunol 17, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, and Liu XS (2008). Model-based analysis of ChIP-seq (MACS). Genome Biol 9, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data utilised in this study has been uploaded to the SRA database, under the accession code PRJNA562324 and the project title ‘‘Epigentic and transcriptional profiling of human gamma delta T cells’’.
This paper does not report any original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


