Abstract
The clinical severity of coronavirus disease 2019 (COVID-19) is largely determined by host factors. Recent advances point to cellular senescence, an ageing-related switch in cellular state, as a critical regulator of SARS-CoV-2-evoked hyperinflammation. SARS-CoV-2, like other viruses, can induce senescence and exacerbates the senescence-associated secretory phenotype (SASP), which is comprised largely of pro-inflammatory, extracellular matrix-degrading, complement-activating and pro-coagulatory factors secreted by senescent cells. These effects are enhanced in elderly individuals who have an increased proportion of pre-existing senescent cells in their tissues. SASP factors can contribute to a ‘cytokine storm’, tissue-destructive immune cell infiltration, endothelialitis (endotheliitis), fibrosis and microthrombosis. SASP-driven spreading of cellular senescence uncouples tissue injury from direct SARS-CoV-2-inflicted cellular damage in a paracrine fashion and can further amplify the SASP by increasing the burden of senescent cells. Preclinical and early clinical studies indicate that targeted elimination of senescent cells may offer a novel therapeutic opportunity to attenuate clinical deterioration in COVID-19 and improve resilience following infection with SARS-CoV-2 or other pathogens.
Subject terms: Cell biology, Immunology
Many viruses, including SARS-CoV-2, can induce cellular senescence and exacerbate the senescence-associated secretory phenotype, leading to detrimental hyperinflammatory responses. Here, Schmitt and colleagues discuss the role of cellular senescence in COVID-19 as well as progress in the development of therapeutic approaches to eliminate senescent cells.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a continuing major threat to global health and the economy. Infections with SARS-CoV-2 and its variants of concern (VOC) evoke a broad spectrum of clinical manifestations, ranging from asymptomatic infections to disease associated with profound tissue damage, organ failure and death1–3. The excessive release of pro-inflammatory cytokines, a condition termed ‘cytokine storm’ (see Box 1 with glossary terms for further explanation), and massive immune stimulation, resulting in macrophage activation syndrome (MAS) (Box 1), aberrant neutrophil activation or T cell hyperactivation, are characteristic features of severe COVID-19 (refs.4–8). Extensive efforts have been undertaken to reduce viral spread and avoid severe disease pathology, including the administration of antiviral agents such as Molnupiravir (EIDD-2801) or Paxlovid (PF-07321332)9,10 and, importantly, vaccines, most of which induce immune responses targeted at the SARS-CoV-2 spike protein11–13. Another strategy is the mitigation of destructive hyperinflammation by using steroids such as dexamethasone, which has significantly reduced mortality rates, or treatment with other anti-inflammatory agents, for which clinical results have been mixed14. The recently reported promising clinical effects observed with the IL-1α/IL-1β blocker anakinra and the anti-IL-6 receptor antibodies tocilizumab and sarilumab confirm the critical role of overactive cytokine networks in COVID-19 (ref.15).
Although vaccines and previous infections largely protect against severe clinical courses of COVID-19 — particularly in the current phase of the pandemic, which is dominated by sublineages of the SARS-CoV-2 Omicron variant — there is still a significant unmet medical need for therapeutics to treat severe courses of disease in unvaccinated or immunosuppressed individuals as well as in elderly patients and patients with chronic disease conditions. Moreover, given the high global incidence of SARS-CoV-2 infections, no one can predict whether and when a distinct, much more virulent VOC might emerge.
Despite an advanced understanding of the molecular mechanisms underlying angiotensin-converting enzyme 2 (ACE2) receptor-mediated viral entry and viral propagation in susceptible host cells16 and new insights into innate and adaptive immune responses to SARS-CoV-2 infection17,18, less is known about cause–consequence relationships between the primary virus infection and subsequent pivotal pathogenetic events that can collectively lead to organ failure. SARS-CoV-2-related lung disease is characterized by extensive activation of both M1-like and M2-like macrophages (Box 1), infiltration by a heterogeneous spectrum of neutrophils, neutrophil extracellular trap (NET) formation, complement activation, and characteristic patterns of SARS-CoV-2-specific T cell immunity composed of predominantly IFNγ-releasing T helper 1 (TH1) cells with a central memory phenotype and perforin-expressing CD8+ T cells with an effector phenotype17,19–22. This can lead to immunopathology, resulting in alveolar and capillary damage, including endothelialitis (also referred to as endotheliitis) and endothelial cytolysis, fibrosis and widespread microthrombosis23–26. Interestingly, the most destructive phase of immune activation often occurs when the viral mRNA is no longer detectable.
Disease manifestations of SARS-CoV-2 infection might, at least partially, be seen as a programmed cellular stress response to the initiating viral insult of the upper airway respiratory cells. Recently, it was found that SARS-CoV-2 can evoke a form of cellular senescence, virus-induced senescence (VIS) (Box 1), through multiple mechanisms27–29 (Fig. 1).
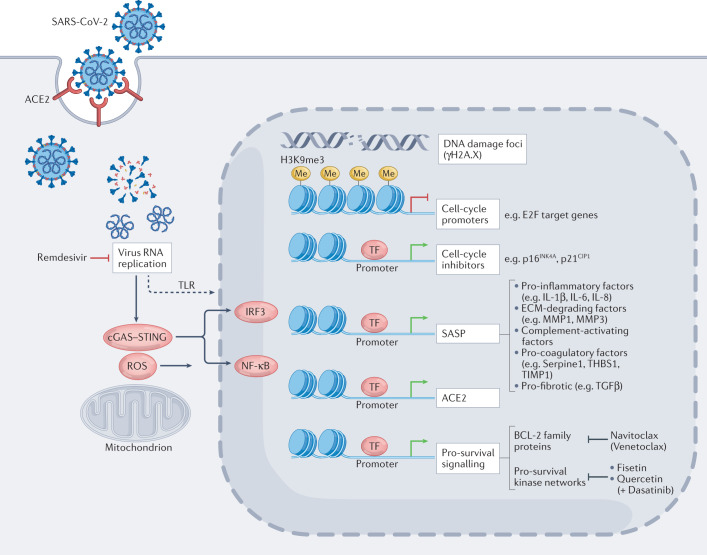
Fig. 1. Senescence-modulated host cell responses upon SARS-CoV-2 infection.
SARS-CoV-2 cellular entry induces senescence-typical transcriptional responses (see also Boxes 2 and 3), in both non-senescent or pre-existing senescent cells via viral replication stress, reactive oxygen species (ROS) production and cellular stress sensors such as toll-like receptor 3 (TLR3) and cGAS–STING signalling. Virus-induced senescence is characterized by senescence-associated, H3K9me3-positive heterochromatin foci (repressing, among others, S-phase-promoting E2F target genes), marks of DNA damage (that is, γH2A.X foci), the induction of cell-cycle inhibitors such as p16INK4a and p21CIP1, and pro-inflammatory, extracellular matrix-degrading, complement-activating, pro-coagulatory and pro-fibrotic senescence-associated secretory phenotype (SASP) factors (predominantly via IRF3, NF-κB and C/EBPβ transcription factors) that exacerbate tissue-destructive host immunity and potentiate the burden of senescent cells through paracrine and endocrine spread of senescence27–29,44 and may include elevated angiotensin-converting enzyme 2 (ACE2) expression27. Notably, pro-survival mechanisms selected for in stressed senescent cells, among them induction of anti-apoptotic BCL-2 family members and survival signalling-enhancing kinase networks involving, for example, SRC family kinases, are attractive targets for the selective pharmacological elimination of these cells; such agents are termed ‘senolytics’. ECM, extracellular matrix; MMP, matrix metalloproteinases; TF, transcription factors.
Cellular senescence is a stress-inducible cellular state switch that includes terminal cell-cycle arrest and the acquisition of a senescence-associated secretory phenotype (SASP). The SASP is predominantly characterized by the secretion of pro-inflammatory cytokines and extracellular matrix-modifying factors and plays important roles in embryonic development, ageing, oncogene-evoked and cancer therapy-evoked insults, as well as tissue injury30–33 (see Box 2 for details). In this Perspective, we propose that a propensity to senescence-governed immune escalation, which can be related to ageing or chronic disease, can also be acutely triggered by SARS-CoV-2 infection and lead to severe disease. This view of SARS-CoV-2 infection may allow new insights into the triggers and effectors involved in COVID-19 and into the dynamics of the inflammatory process seen in this disease. Moreover, we propose that the selective removal of senescent cells by candidate agents, termed senolytics, or of VIS cells as a collateral result of SARS-CoV-2 spike (S) protein-based vaccination, could prevent or mitigate severe COVID-19.
Box 1 Glossary terms used in this article.
Acute respiratory distress syndrome. Is a life-threatening response to lung-damaging insults, leading to respiratory failure characterized by rapid onset of widespread inflammation and fluid leakage into the alveoli, thereby severely impairing alveolar gas exchange.
Classic senescence response. Is a full-featured senescence response composed of a terminal cell-cycle arrest, signs of DNA damage and enhanced DNA damage response signalling, elevated MAPK pathway activity, global genomic reorganization, and chromatin remodelling (such as ‘senescence-associated heterochromatin foci’), expansion of the lysosomal compartment and a pronounced senescence-associated secretory phenotype (SASP).
Cytokine storm. This is the secretion of vast amounts of largely pro-inflammatory cytokines, also known as cytokine release syndrome, that occurs in various hyperinflammatory disease contexts; it serves as an indicator and mediator of immune hyperactivation and subsequent immune-mediated tissue damage.
Ki67 expression. Is widely used in routine pathological investigation as a nuclear proliferation marker as its expression is strongly associated with cell division.
Lipofuscin. Also considered ‘age pigment’, is brownish-yellowish, electron-dense material that progressively accumulates in the lysosomes of postmitotic cells and hence plays some role as a senescence surrogate marker.
M1-like and M2-like macrophages. ‘M1’ and ‘M2’ are classifications historically used to define macrophages activated in vitro as pro-inflammatory (when ‘classically’ activated with IFNγ and lipopolysaccharides) or anti-inflammatory (when ‘alternatively’ activated with IL-4 or IL-10), respectively. However, in vivo macrophages are highly specialized, transcriptomically dynamic and extremely heterogeneous with regards to their phenotypes and functions, which are continuously shaped by their tissue microenvironment. Therefore, the M1 or M2 classification is too simplistic to explain the true nature of in vivo macrophages, although these terms are still often used to indicate whether the macrophages in question are more pro-inflammatory or anti-inflammatory.
Macrophage activation syndrome. Is an uncontrolled, mechanistically not fully elucidated, often life-threatening activation and proliferation of macrophages with a marked increase in circulating cytokines (see ‘cytokine storm’) in response to virus infections or other pro-inflammatory triggers.
Paracrine senescence. Is a form of secondary, full-featured senescence evoked by adjacent, primary senescent cells owing to their secretion of SASP factors with pro-senescent potential (for example, plasminogen activator inhibitor 1 (PAI1)).
Pyroptosis. Is a highly inflammatory mode of regulated cell death, which plays a role in the removal of intracellular pathogens such as viruses. Initiated by a large supramolecular complex termed the inflammasome, a specific subset of caspases triggers pro-inflammatory cytokine activation and eventual cell pore formation.
scATACseq. Single-cell assay for transposase-accessible chromatin-sequencing is a sequencing method used to assess genome-wide chromatin accessibility (for transcription factor binding, for instance) by probing open chromatin with a hyperactive transposase that inserts sequencing adapters selectively into open regions of the genome.
Virus-induced senescence. A stress-induced state switch of host cells in response to virus entry and propagation as the sensed cellular insult, comprised of a lasting cell-cycle arrest and an associated secretory response (termed SASP).
Box 2 The biology of cellular senescence.
Senescence30–33 was originally observed in cells that have exhausted their proliferative potential owing to the critical erosion of their telomeres190,191. This process occurs during ageing and disease as well as at critical developmental steps in embryogenesis192,193. Importantly, it is also an essential component of wound healing194, indicating its tissue-protective and tissue-restoring role if it occurs at an adequate level and at the right time. Cellular insults, especially DNA damage but also other severe functional impairments, during tissue injury halt proliferation, which ensures that only intact cells contribute to the parenchymal cell pool. Driven by the cGAS–STING pathway and NF-κB and C/EBPβ signalling in response to DNA damage, senescent cells typically activate a vast, largely pro-inflammatory secretome that constitutes the senescence-associated secretory phenotype (SASP), which operates as a central component of wound healing but also causes tissue pathology74,194. Regardless of the initiating stress, ‘full-featured’ senescence locks the cells into an essentially terminal growth arrest, typically accompanied by the release of predominantly NF-κB-driven pro-inflammatory cytokines (for example, IL-1α/IL-β, IL-6 and IL-8) and chemokines (such as CCL2 or CXCL10), extracellular matrix-remodelling peptides (among them matrix metalloproteases (MMPs), serpins and thrombospondins), reactive metabolites, bradykinins and prostanoids, and secreted non-coding, potentially tissue-destructive nucleotides (such as microRNAs or mitochondrial DNA)71,72,74,75,195. SASP-mediated paracrine (secondary) senescence ensures that critically damaged but not primarily senescent cells also stop dividing — normally a tissue-safeguard principle that may also lead to paracrine senescence of non-damaged parenchymal cells in pathological settings with an excessive production of SASP factors63,71,72. SASP factors, such as MMP1 and MMP3, digest extracellular matrix barriers and attract immune cells to clear up cellular debris and, if present, microbial pathogens28,194,196. Once exogenous stresses are resolved, functional tissue cellularity might be replenished by senescence-reprogrammed cells177,197,198, which acquired stem cell-like capacity and may occasionally re-enter the cell cycle50,177,199. Cellular senescence also occurs in other pathological stress scenarios, such as profound metabolic deregulation200–202, the activation of oncogenes or cancer therapy-conferred cell damage, which function as a barrier to tumour development or as an alternative effector mechanism to treatment-induced apoptosis46,47,51,203–205. Hence, senescence plays a critical role in tissue homeostasis, bearing the risk that chronically senescent cells, owing to their ongoing secretion of pro-inflammatory factors and their reprogrammed nature, account for detrimental biological outputs33, implying that their selective removal can be beneficial112,138.
The identification of senescent cells and their discrimination from other types of cell-cycle arrest, such as quiescence, are confounded by the phenotypic heterogeneity of the senescent cell state, thereby necessitating the analysis of multiple markers.
Virus-evoked cellular stress
VIS appears to be a universal stress response in host cells and can be induced by many different virus species. These include single-stranded and double-stranded DNA and RNA viruses belonging to the Retroviridae, Polyomaviridae, Paramyxoviridae, Parvoviridae, Rhabdoviridae and Coronaviridae families, and has been explicitly shown for lentiviruses, adeno-associated viruses, vesicular stomatitis viruses and, notably, SARS-CoV-2 (refs.28,29,34–38). The molecular mechanisms by which different viruses evoke cellular senescence appear to vary as both replication-competent and assembly-defective viruses that cannot produce intact virions can induce a senescence response, although this requires higher viral titres for replication-incompetent viruses. For example, viruses such as the Merkel cell polyoma virus, HIV and SIV affect genome integrity, which can induce a senescence response. Respiratory syncytial or measles viruses enforce cell fusions, which act as cellular stress that can lead to VIS34–36,39. This is also observed in SARS-CoV-2 infection, where the Delta variant was shown to be particularly fusogenic40.
Although senescence reportedly leads to enhanced expression of entry receptors for different viruses, with the underlying mechanisms and the spectrum of affected entry receptors yet to be elucidated27,41,42, it can also represses virus propagation, which is thought to be due to the enhanced release of interferons as part of the SASP43. Moreover, at least in certain settings, antiviral therapies may induce cellular senescence37. However, some agents that interfere with viral replication can also inhibit VIS responses. For example, this was shown for the reverse transcriptase inhibitor zidovudine in the context of retroviral infection and for the remdesivir derivative GS-441425, a viral RNA polymerase inhibitor, in the context of SARS-CoV-2 infection28 (Fig. 1). Importantly, VIS reflects a ‘full-featured’ senescent state switch of host cells, accompanied by a pronounced SASP that is detectable by a typical set of molecular markers (Box 2).
The direct induction of senescence through infection with SARS-CoV-2 was demonstrated in vitro with a SARS-CoV-2-susceptible kidney cell line (Vero) as detected by an increase in lipofuscin (Box 1), the secretion of SASP factors, including IL-1β, IL-6 and IL-8, and the DNA damage mark γH2A.X, as well as a loss of Ki67 expression44 (Box 1). It is not entirely clear whether virus propagation stress or specific viral molecular components induce VIS. Interestingly, isolated recombinant S protein has been shown to increase the SASP in senescent ACE2-expressing cells27,29, similar to other pathogen-associated molecular patterns such as LPS45. However, whether the internalization of the S protein alone would suffice to induce senescence in non-senescent cells remains to be determined. Moreover, an elevated expression of the ACE2 receptor, which could contribute to enhanced susceptibility to SARS-CoV-2 in the elderly, became detectable in primary human lung epithelial cells in vitro in response to the SASP from pre-adipocytes or endothelial cells that had become senescent owing to ageing27. Senescent cells also express elevated levels of Toll-like receptor 3 (TLR3), which can detect SARS-CoV-2 viral RNA in endosomes. It is therefore conceivable that SARS-CoV-2 infection of senescent cells may amplify their pro-inflammatory SASP, potentially inducing a positive feedback loop in a VIS-dependent manner29 (Fig. 1).
The programmed VIS response is morphologically and transcriptionally largely indistinguishable from other types of senescence, in particular from oncogene-induced senescence28,46,47. It is also similar to the senescence induced by the retrovirus-related endogenous retrotransposon LINE-1 (refs.48,49). Importantly, in vitro experiments showed that cells that lack functional p53 or overexpress the H3K9me3 demethylase JMJD2c46,50 — which therefore cannot enter senescence in response to pro-senescent oncogenes such as Ras-G12V or Braf-V600E46,51 — also failed to enter VIS and lacked a SASP upon infection with SARS-CoV-2 (ref.28). This demonstrates that SARS-CoV-2-related cytokine production by infected cells is dependent on an intact capacity of these cells to enter cellular senescence. Additional experiments in SARS-CoV-2-susceptible but genetically senescence-compromised animal models are needed to confirm this observation in vivo.
Cells that undergo VIS show signs of DNA damage such as γH2A.X foci. Such foci were also detected in epithelial and endothelial cells from the lungs of patients with COVID-19 (ref.52), and are presumably caused by an increase in reactive oxygen species (ROS) produced by mitochondria. In turn, lowering cellular ROS levels with N-acetyl-cysteine in in vitro VIS models profoundly reduced DNA damage foci and senescence-associated β-galactosidase (SA-β-gal) reactivity (Box 3). Of note, mitochondrial outer membrane shedding has been reported in response to infection-induced stress. It has also been discussed as a potential mechanism for DNA damage induction in SARS-CoV-2-infected cells, where the viral protein Orf9b is thought to inhibit the translocase of the mitochondrial outer membrane complex53,54. Collectively, these observations indicate that ROS levels appear to play a crucial role in triggering VIS in the context of SARS-CoV-2 infection.
Senescence-associated DNA damage is sensed via the cGAS–STING pathway, which activates the transcription factors interferon regulatory factor 3 (IRF3) and NF-κB, thereby inducing an interferon and SASP response55–58 (Fig. 1). Although cGAS–STING inhibitors have limited impact on primary VIS, they can reduce the SASP and its subsequent non-cell-autonomous effects28,55. Lasting cGAS–STING activation in response to SARS-CoV-2 infection was recently confirmed to drive type I interferon responses and the secretion of other pro-inflammatory cytokines, such as IL-1α, IL-6 and tumour necrosis factor (TNF), by CD163+ macrophages and damaged lung endothelial cells in patients with severe COVID-19 (ref.59). Notably, in vitro experiments showed that cells that were engineered to be unable to undergo senescence failed to activate the cGAS–STING pathway in response to viral infection28. These observations indicate that viral entry causes high levels of cytokine secretion in host cells via the induction of VIS.
Box 3 Markers of senescence.
Compared to their normal counterparts, senescent cells are enlarged, have a more granule-rich cytoplasm and appear to exhibit vanishing cell borders under light microscopy. Their typically expanded lysosomal compartment, reflecting enhanced autophagocytotic activity owing to senescence-associated secretory phenotype-related proteotoxic stress206,207, forms the basis for the senescence-associated β-galactosidase (SA-β-gal) assay. This assay detects senescence-enhanced lysosomal galactosidase activity at a suboptimal, acidic pH, which is a condition that achieves best discrimination from non-senescent cells208. Beyond the ‘gold-standard’ SA-β-gal assay in its classic blue-stained end point form in fixed cells or as a fluorescence-based variation applicable to viable cells, there is no single senescence-defining, highly sensitive and specific marker of the senescent condition; hence, numerous markers are typically applied to collectively label a cell status as being senescent209,210. Among the most robustly established and widely applied markers in this regard are the cell-cycle inhibitors p16INK4a and p21CIP1, signs of DNA damage such as histone variant H2A.X phosphorylated at its serine 139 (γH2A.X), hypophosphorylated (that is, G1 phase-reminiscent) retinoblastoma (Rb) protein, activated MAPK signalling (such as phospho-ERK1/ERK2), and the transcriptionally repressive lysine 9-trimethylated histone H3 (H3K9me3) mark33. DAPI (4,6-diamidino-2-phenylindole)-dense senescence-associated heterochromatin foci indicate the profound epigenomic reorganization observed in senescent cells211–218. The Rb/E2F-mediated formation of H3K9me3-containing senescence-associated heterochromatin foci accounts for the stable senescent G1-phase arrest via the firm transcriptional repression of S-phase-promoting E2F target gene promoters, thereby turning off genes, such as CDK2 or CCND2, that are flanked by decreased expression of the proliferation marker Ki-67 (refs.47,50,211).
Senescence in patients with COVID-19
If VIS is of relevance as a cellular response to SARS-CoV-2 infection, then signs of cellular senescence should be detectable in ACE2-expressing ciliated epithelial cells of the upper airway mucosa — the site of primary virus–host encounter and viral replication — in patients with COVID-19. In general, the definitive demonstration of cellular senescence in vivo is a long-standing and challenging goal in the field because no single marker exists that defines senescence30,32 (Box 3). In addition, cryopreserved clinical samples from patients with COVID-19 cannot be analysed without virus-inactivating fixation, thereby precluding their use for enzymatic assays such as SA-β-gal staining in situ. Nevertheless, when comparing samples of nasopharyngeal mucosa from patients with COVID-19 to pre-pandemic biopsy samples from individuals without a manifest respiratory tract infection, a panel of senescence markers was shown to robustly discriminate these two groups, with significantly higher reactivity in samples from patients with COVID-19 (refs.28,29,44,52). Specifically, senescence markers, such as p16INK4a, p21CIP1, H3K9me3 and lipofuscin (detected by the GL13-SenTraGorTM reagent in fixed tissues60), as well as IL-8 (a component of the SASP) were markedly elevated in samples from the upper respiratory mucosa from patients with COVID-19 (ref.28) (Fig. 2). Similar analyses of lung specimens from patients with COVID-19, consistent with multiple independent patient datasets29,44,52, also showed much stronger signs of senescence compared to SARS-CoV-2 negative controls28. Notably, SARS-CoV-2 mRNA was detectable by in situ hybridization in many but not all VIS-positive samples, suggesting that VIS may persist beyond the initial infection28,61,62 and may be further aggravated by SASP-mediated paracrine spreading of senescence28,63 (paracrine senescence; Box 1). The results are also consistent with the observed gradient of SARS-CoV-2 infectivity, where proximal pulmonary epithelial cells from patients with COVID-19 show higher levels of infection compared to cells from the distal respiratory tract64. Moreover, these findings might explain, at least in part, the limited clinical efficacy of convalescent plasma in preventing severe COVID-19 given that the severe course of disease is thought to be caused by immune-mediated tissue damage independent of persistent virus65.
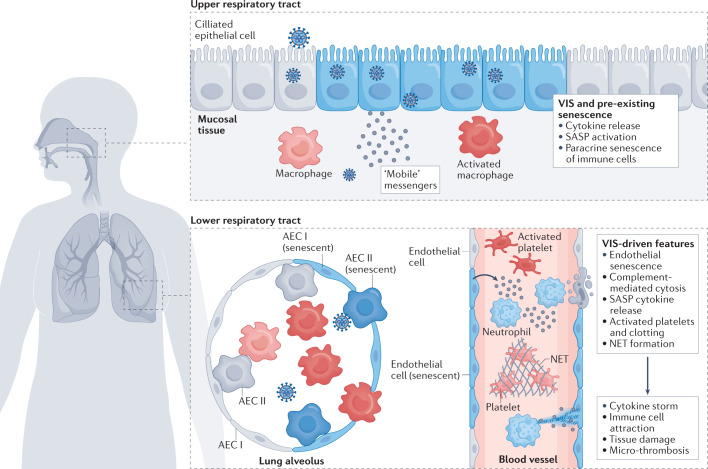
Fig. 2. A mechanistic working model of senescence-driven severe COVID-19.
The infection of susceptible respiratory mucosa cells in the upper airways with SARS-CoV-2 evokes virus-induced senescence (VIS). Like pre-existing, ageing-associated and chronic disease-associated senescent cells, they release large amounts of largely pro-inflammatory cytokines and other senescence-associated secretory phenotype (SASP) factors (see Fig. 1). Macrophages that are attracted to senescent cells via cytokines and chemokines (such as macrophage colony-stimulating factor (M-CSF) or CCL2 (ref.28)) can acquire a senescence-like condition via paracrine SASP action. These macrophages amplify the production of SASP factors and serve as ‘mobile messengers’ that provide a broad spectrum of SASP factors to more distal airways. Together with the direct viral infection of cells in the lower respiratory tract and an enhanced burden of senescent cells due to paracrine, SASP-mediated induction of secondary senescence, macrophages and other SASP-producing cells collectively promote lung pathology by contributing to local tissue damage. This is partly due to SASP-mediated cytotoxicity and partly via direct macrophage phagocytosis of infected or otherwise altered cells. Moreover, SASP factors attract other immune cells and excessive activation of these cells can result in direct or indirect cytotoxicity at lung epithelial and endothelial cells. Specifically, VIS-driven features of severe, tissue-destructive coronavirus disease 2019 (COVID-19) include endothelial cell senescence, complement-mediated cytolysis of endothelial cells, neutrophil extracellular trap (NET) formation, platelet activation and microthrombosis and, presumably, SASP-enhanced T cell-mediated cytotoxicity towards alveolar epithelial cells (AECs) I and II. Pre-existing senescent, virus-induced senescent and secondary SASP-induced senescent cells are depicted in blue. See main text for details.
Bulk and single-cell transcriptomic analyses further support the concept that SARS-CoV-2-triggered senescence drives pathology in COVID-19. Ciliated epithelial cells of the upper and lower airway mucosa of patients with COVID-19 displayed elevated levels of transcription of p16INK4a and various SASP factors28,66. Moreover, lungs from patients who died from severe COVID-19 showed higher levels of p16INK4a-positive cells compared to lungs from individuals who died from other causes29. The levels of SASP-typical cytokines and extracellular matrix-active proteases as well as SASP-related pro-coagulant and complement-activating factors (such as IL-1α, IL-6, CC-chemokine ligand 2 (CCL2, also known as MCP-1), CXC-chemokine ligand 10 (CXCL10), matrix metalloproteinase 9 (MMP9), plasminogen activator inhibitor 1 (PAI1, encoded by SERPINE1) and metalloproteinase inhibitor 1 (TIMP1)) were also strongly elevated in the serum of patients with COVID-19 compared to healthy controls28,52,67–70. Importantly, WHO-graded clinical severity of COVID-19 correlated positively with increasing concentrations of the pro-inflammatory SASP cytokine IL-6, the coagulation-related SASP representative SERPINA3 and D-Dimer-based clotting activity28. In particular, patients with COVID-19 who had detectable microthrombosis in their lungs presented with more pronounced levels of SASP-typical factors28, giving rise to the hypothesis that more extensive senescence underlies more profound COVID-19 pathobiology.
Macrophages in senescence spreading
Importantly, the senescent state can spread to non-senescent and, potentially, to non-infected cells as the increase in SASP evoked by viral infection can lead to the induction of paracrine senescence63,71,72. This observation was further supported by single-cell analyses that detected senescence marker profiles in cells that were not susceptible to primary SARS-CoV-2 infection owing to the lack of ACE2 expression28. We suspect that senescent cells acutely reinforce systemic inflammatory responses to pathogens and facilitate the spread of senescence, a hypothesis termed the ‘SASP amplifier/rheostat model’27,28. This model might also explain the increased risk of a cytokine storm and adverse outcomes after SARS-CoV-2 infection in the elderly or in individuals with chronic conditions associated with an increased burden of senescent cells such as obesity, diabetes or previous chemotherapy.
A central aspect of severe COVID-19 is the MAS8,73. Consistent with the recruitment of macrophages to VIS cells within the upper airway mucosa, the macrophage chemoattractant CCL2 and other macrophage-recruiting chemokines were identified as prominent components of the SASP of VIS cells28,74,75. Recruited macrophages may clear some of the senescent cells by phagocytic engulfment76. Importantly, in vitro and in vivo experiments have shown that resident and non-resident macrophages in the vicinity of SASP-producing virus-infected epithelial cells can undergo paracrine secondary senescence or acquire a senescence-like condition28,63,66,77,78. They are characterized by positive SA-β-gal staining and elevated p16INK4a expression, and present with a SASP, thus further amplifying the senescent secretory profile28. The state switch induced by the encounter with VIS cells reprogrammes the macrophages into CD86+CD14+CD163+ pro-inflammatory, M1-like cells79. Given their mobility, these macrophages may then respond to chemokines secreted by cells in the lower airway that were damaged by SARS-CoV-2 infection66. Accordingly, lungs from patients with severe COVID-19 exhibited much higher levels of infiltration by CD86+ macrophages and much stronger SASP-reminiscent cytokine expression compared to lungs from patients who died without notable signs of a respiratory infection and who were not infected with SARS-CoV-2 (refs.28,61). The finding that SARS-CoV-2 triggers a profibrotic response in CD14+CD163+ macrophages, which promotes manifest pulmonary fibrosis and acute respiratory distress syndrome (Box 1), complements these observations and further underscores the previously established link between cellular senescence and fibrosis in the lung via pro-fibrogenic SASP components52,80,81. Moreover, using a combination of single-cell RNA sequencing, mass cytometry and single-cell assay for transposase-accessible chromatin sequencing (scATAC-seq; Box 1) of blood samples from young and elderly individuals as well as patients with COVID-19, it was found that coronavirus susceptibility genes, among them CD147, CD26 and ANPEP (encoding aminopeptidase N; see ref.82 for details), are upregulated with age. Additionally, SARS-CoV-2 infection was shown to induce polarization of peripheral blood immune cell subsets that is typical for ageing and includes the expression of genes associated with inflammation and senescence82. Specifically, the authors reported T cell polarization from naive and memory cell populations to effector, cytotoxic, exhausted and regulatory T cell populations, and found increased levels of inflammatory monocytes in the blood of elderly patients with COVID-19. Collectively, the data indicate that activated monocytes and decreased T cell activity characterize hyperinflammation in severe COVID-19.
Notably, although activation status, pro-inflammatory M1-like polarization and mobility of macrophages make it compelling to view these as senescence-primed messengers that further spread senescence in a paracrine manner in the lungs or systemically28, it is still debated whether the macrophages enter a classic senescence response (Box 1) as opposed to a senescence-like condition characterized by the secretion of factors typical for the SASP77,78. Moreover, it was recently shown that monocytes and macrophages not only act as cytokine-releasing sensors of SARS-CoV-2-infected cells but that a small fraction of macrophages is susceptible to Fcγ receptor-mediated SARS-CoV-2 uptake and subsequent termination of virus replication via inflammasome activation and IL-1β-induced pyroptosis (Box 1). These features partially overlap with the senescence-like and SASP-reminiscent condition described here43,83. More detailed profiling, dynamic tracing and functional analyses are required to delineate the roles of senescence marker-positive tissue-resident versus senescence-marker positive infiltrating macrophages and circulating monocytes as well as the poorly understood functional consequences of paracrine senescence on distinct resident or mobile T cell subsets84,85. Notably, age-related immunosenescence, a term often used to describe immune dysfunction in the elderly, seems to enhance COVID-19 severity via T cell-mediated cytotoxicity directed at virus-infected or collaterally damaged host tissues86,87. However, the precise functional consequences of the induction of senescent T cell subsets, which is an emerging field of research, specifically in COVID-19, are much less clear, with limited data on the presence, quantity and function of T cells that display markers of immune senescence such as CD57 or other cellular features associated with ‘classic cellular senescence’88.
Overall, the escalating production of SASP-related factors in patients with severe COVID-19 appears to be mediated by the direct induction of primary senescence in virus-susceptible cells by SARS-CoV-2 infection, the paracrine senescence effects on adjacent cells and the systemic impact of the SASP even on distant cells. The disease-promoting effects of senescence-primed macrophages in different organs (see below) is likely to be further aggravated by the age-related burden of pre-existing senescent cells (Fig. 2).
Senescence-induced COVID-19 pathology
Despite the strong correlation between the extent of senescence (including VIS) and COVID-19 severity, a causal relationship between these processes is yet to be demonstrated. Based on the concept that the SASP links virus-evoked and pre-existing cellular senescence to macrophage priming and to specific pathognomonic features, such as endothelial cell senescence and complement-mediated cytolysis, NET formation, platelet activation and microthrombosis, in the damaged lung and other organ systems of patients with COVID-19, functional in vitro assays have been developed that can reproduce the aforementioned cellular responses to SARS-CoV-2 infection. For example, it was demonstrated that exposure to the supernatant of VIS cells induced macrophage activation that was marked by an M1-like polarization profile79,89, CD86 upregulation and signs of secondary macrophage senescence as detected by SA-β-gal staining and senescence-typical gene expression. By contrast, the culture supernatant of equally virus-infected but senescence-incapable cells failed to evoke these cellular effects28.
Another hallmark of COVID-19 is widespread thrombo-occlusive microangiopathy, which comprises lymphocytic endothelialitis with endothelial damage, platelet activation and capillary microclots. Neutrophils contribute to thrombosis by ejecting DNA, leading to intravascular net structures that activate platelets23,90,91. All of these individual components were recapitulated in vitro and mechanistically dissected as senescence inflicted28. Specifically, conditioned medium from senescent cells that is rich in SASP-associated factors evoked paracrine senescence in endothelial cells or triggered their complement-mediated cytolysis, promoted NET formation by neutrophils, activated platelets, and accelerated clotting. These effects represent highly disease-relevant components of COVID-19-related immunothrombosis, which were detected to a much lesser extent if the supernatant from virus-infected but senescence-resistant cells was tested28 (Fig. 2). Of note, complement activation has recently been shown to aggravate tissue injury in patients with COVID-19 by inducing excessive T cell cytotoxicity via immune complex-induced and T cell receptor-independent degranulation, thereby potentially linking VIS and the SASP to uncontrolled T cell-mediated tissue damage92. Overall, these in vitro observations suggest an essential, secretome-dependent role of VIS and senescence in general in the pathobiological manifestation of the infection27–29.
Senolytic strategies
VIS cells, pre-existing senescent cells in the elderly and in those with underlying conditions, and SASP-induced secondary senescent cells appear to play a key role in the development of severe COVID-19. If cellular senescence is indeed a causative component of severe COVID-19, then the early elimination of senescent cells would be expected to attenuate disease severity. This would confirm that senescent cells are a pivotal trigger of rapidly uncontrolled SASP-governed hyperinflammation in patients with severe COVID-19, thus expanding current therapeutic strategies. In addition to VIS, the burden of pre-existing senescent cells that is associated with older age or with chronic diseases, even in younger individuals, could also represent a key target to reduce complications and mortality in patients with COVID-19 (ref.93).
Targeting senescent cells is increasingly viewed as a novel opportunity for delaying, preventing, alleviating or treating a wide spectrum of human diseases, among them age-related disorders, cancer and, now, viral infections with profound hyperinflammatory tissue damage, with early clinical trials currently running in many of these indications94–97. A fundamental property and dependency of senescent cells is their protection from apoptotic death despite the stresses they encounter98. Hence, a characteristic shared across senescent cells, especially those with a profound SASP, is their enhanced insensitivity to pro-apoptotic signals, which is mediated via upregulation of anti-apoptotic BCL-2 family members or kinase networks that promote pro-survival signalling — collectively referred to as senescent cell anti-apoptotic pathways (SCAPs)98,99.
The SCAPs vary between different types of senescent cells (for example, between senescent human endothelial cells versus fat cell progenitors). In addition to BCL-2 family members, SCAPs are mediated by SRC family tyrosine kinases as well as pro-survival pathways related to serpin, heat shock protein and p21CIP1 (refs.99–104). Indeed, the expression of BCL-xL and BCL-w and the activity of SRC, AKT and p38 kinases were found to be elevated in retrovirus-infected fibroblast models of VIS, in SARS-CoV-2-infected hamsters and in patients with COVID-19 (ref.28). This suggests therapeutic opportunities in COVID-19 for senolytic agents that interfere with SCAPs, thereby inducing apoptosis in VIS cells and in pre-existing senescent and secondary senescent cells. Drugs that have been shown to be effective in killing senescent cells in various clinical and preclinical models are of particular interest: these include the investigational BCL-xL and BCL-w inhibitor navitoclax (ABT-263)100,101, BCL-xL-degrading proteolysis-targeting chimeric proteins with reduced platelet toxicity105–108, and the kinase-inhibiting flavonoids fisetin and quercetin99,103,104, the latter typically employed in combination with the SRC kinase inhibitor dasatinib99,100,109–114.
In vitro findings with senolytics in VIS
Navitoclax, fisetin, and a combination of dasatinib and quercetin demonstrated significant cytotoxic activity against VIS cells (including human nasal epithelial cells exposed to SARS-CoV-2) in vitro, leaving uninfected or virus-infected but genetically senescence-incapable cells virtually unaffected28. Importantly, the SARS-CoV-2 Alpha and Beta variants elicited a significantly stronger pro-inflammatory SASP upon infection compared to the ancestral SARS-CoV-2 strain, and these VIS cells exhibited excellent susceptibility to the aforementioned senolytics in vitro, comparable to cells infected with the ancestral virus28. Additional investigations are needed to determine the relevance of senescence-related and SASP-related pathophysiology induced by Delta and Omicron variants given their distinctly different degrees of clinical severity115. Of note, senescent cells have enhanced activity of the apolipoprotein B mRNA-editing catalytic polypeptide-like (APOBEC) enzymes. Given that APOBEC enforces viral mutagenesis, it is also conceivable that host cell senescence actually promotes the generation of new SARS-CoV-2 variants44,116.
A machine learning-based assessment of 45 FDA-approved drugs for their potential to be repurposed as antiviral agents in COVID-19 failed to predict a significant direct anti-viral activity of navitoclax and dasatinib, further underscoring that the mode of action of these agents in COVID-19 might be indirect, for example, through the selective elimination of senescent cells117. However, given the complexity of virus entry and replication, additional direct anti-viral activity cannot be excluded for any of the candidate senolytics tested and was reportedly observed upon quercetin pre-treatment118. Thus, to what extent the cytotoxicity exerted by anti-viral compounds is due to their direct interference with viral propagation or rather relates to VIS as a state-specific vulnerability of the host cell needs to be dissected in appropriate model systems, including the use of in vitro tests using cells that are genetically incapable of undergoing senescence. Given the broad deregulation of prominent pathways such as NF-κB, MAPK, ROS, JAK–STAT, p53–p21CIP1 and Rb–p16INK4a in senescent cells in general and in VIS cells in particular, there is an encouragingly large and growing list of additional compounds that may indeed have senolytic potential in COVID-19 (refs.113,119–121).
In vivo testing of senolytics in COVID-19
Importantly, animal models of SARS-CoV-2 infection, such as the Syrian golden hamster (these animals typically have a mild course of disease) and the Roborovski dwarf hamster, as well as mice transgenic for human ACE2 (these have a more severe, typically fatal course of disease), implicated VIS as a feature of both mild and severe COVID-19, especially as a central driver in the latter, and highlighted the clinical potential of senolytics as a novel interventional therapeutic strategy28,122–127. Navitoclax, fisetin, or a combination of dasatinib and quercetin were found to quantitatively eliminate senescent cells in situ and to improve at least some of the central histopathological findings of SARS-CoV-2 infection in the lung in all the animal models mentioned28. Particularly pronounced effects were observed in the Roborovski dwarf hamster model, where treatment with a combination of dasatinib and quercetin resulted in significantly attenuated disease features with respect to the overall pneumonia score, bronchial epithelial hyperplasia and alveolar damage28. Strikingly, the broadly elevated levels of SASP-typical cytokines and interleukins — among them granulocyte colony-stimulating factor (G-CSF), IFNγ, IL-1α, IL-7, IL-17A, TNF and vascular endothelial growth factor (VEGF) — that were detected (despite the challenge to do this at the protein level in hamsters) in the serum of infected animals, were reduced by all agents tested to levels that were virtually indistinguishable from those in uninfected animals28. In animal models of fatal COVID-19, senolytic regimens based on flavonoids, such as the dasatinib and quercetin combination or fisetin, enhanced survival rates28. Targeting of the cGAS–STING axis, which induces type I interferons and other SASP factors, with the small-molecule STING inhibitor H-151 had a SASP-suppressing, senomorphic effect28,121,128,129 and led to reduced lung pathology and prolonged survival in a human ACE2-transgenic mouse model of COVID-19 (ref.59). These observations provide further evidence for the pathogenic connection between senescence, SASP and severe COVID-19. Similarly, the inhibition of pro-inflammatory JAK signalling in the same model was shown to attenuate pathogenesis by reducing the infiltration of monocytes to the lung130–132. Age-related elevations of eicosanoid levels, especially prostaglandin D2 (PGD2), were detected in mouse models of SARS-CoV infection133. The levels of PGD2 and related prostaglandins were also found to be increased in older individuals and in individuals infected with SARS-CoV-2 (ref.134). These prostaglandins are known to promote senescence and the SASP135–137. In turn, genetic or pharmacological blockade of eicosanoid signalling was shown to protect aged mice from severe COVID-19 (ref.134), unveiling another mechanism by which inflammation, senescence, ageing and severe COVID-19 appear to be linked.
Therapeutic targeting of pre-existing senescent cells was also addressed in a model of coronavirus infection in aged mice. A cohort of aged INK-ATTAC transgenic mice, in which cells with high INK4a promoter activity, which is indicative of senescence, can be forced to undergo apoptosis138,139, exhibited increased resilience to pathogens, including the mouse hepatitis virus (MHV, a mouse β-coronavirus), when p16INK4a-high senescent cells were subjected to ATTAC-dependent depletion27. Of note, the therapeutic benefit was evident not only if the senescent cell burden was reduced prior to infection but also following infection, consistent with an increased burden of senescent cells that persists following pathogen encounter.
The central role of senescent cells and the potential of senolytics as therapeutics were further investigated in aged specific-pathogen-free mice that were exposed to pet-store mouse pathogens, including MHV, termed a ‘normal microbial experience’ (NME)140–142. Nearly all of the aged mice exposed to NME died within 2 weeks, whereas there was no mortality observed in younger mice27. Within 7 days following NME exposure, the expression of senescence markers (such as p16INK4a and p21CIP1) and SASP factors (such as IL-6, CCL2 and TNF) in liver, lung and kidney significantly increased in old mice compared to young mice27. Treatment before and/or following NME exposure with the senolytic fisetin or the combination of dasatinib and quercetin reduced mortality. This is consistent with the adverse effects of an increased senescent cell burden with age and the lethal spread of senescence and inflammation following pathogen exposure, possibly through both VIS and the exacerbated tissue-destructive SASP caused by pathogen-associated molecular patterns27. Notably, the mitigating activity observed when these agents were applied prior to NME exposure reflects a reduction of the pre-existing load of senescent cells, and not a direct antimicrobial mechanism, as the key mode of action.
Further corroborating and extending these preclinical findings to the human condition, two randomized clinical trials (NCT04578158 and NCT04861298) that, in addition to standard care, used a special formulation of quercetin with enhanced oral bioavailability as an early intervention in patients with mild COVID-19 symptoms, met their clinical end points143–145. Specifically, a collective analysis of both trials demonstrated a significantly better outcome in terms of avoiding hospitalization, need for oxygen, referral to the intensive care unit and death28. Of note, a recent clinical pilot study exploring a lecithin-based formulation of quercetin in combination with dasatinib as a senolytic treatment for idiopathic pulmonary fibrosis, an age-related and senescence-associated disease also occurring as a fibrotic complication in the course of COVID-19 (refs.52,80,81,146), showed encouraging signals of clinical improvement as evaluated by walk distance, speed and coordination tests, demonstrating the feasibility of this approach147.
The clinical trials underscored that quercetin exerts meaningful senolytic activity when tested as a single agent in individuals with SARS-CoV-2 infection and appeared to have relevant clinical potential to prevent severe COVID-19. Several other randomized clinical trials are currently investigating the use of fisetin in patients with COVID-19 (NCT04476953, NCT04537299 and NCT04771611). These trials examine whether fisetin decreases SARS-CoV-2 morbidity and mortality in PCR-positive older individuals (as well as in younger patients with pre-existing cellular senescence-associated comorbidities, including diabetes, obesity, cardiovascular disorders or chronic lung diseases)148. If successful, larger randomized studies with optimized dosing schedules will be needed to establish senolytics as preventive or early interventional treatments for patients with severe COVID-19 (ref.149).
VIS and vaccination
The majority of COVID-19 vaccines are designed to evoke humoral and cellular immune responses against the SARS-CoV-2 spike protein — responses that may not prevent infection but robustly protect against severe COVID-19, at least for certain periods of time11,150–157. Increasing numbers of breakthrough infections in vaccinated individuals, including those with detectable antibody responses, are observed, albeit with increasing evidence that disease severity is less pronounced in vaccinated compared to unvaccinated patients with COVID-19 (ref.158). Moreover, asymptomatic infections are likely to be underreported in vaccinated individuals because they tend to be less frequently monitored when local regulations do not require testing. It is becoming increasingly clear that community protection owing to reduced viral loads in infected but fully vaccinated individuals is diminishing over time159, thereby facilitating further outbreaks and the rapid expansion of new VOCs160–162. The fact that viral entry and replication occur despite successful vaccination is interesting as VIS would be the assumed consequence and could trigger a cytokine storm potentially leading to severe COVID-19. The fact that this is typically not the case might be either owing to reduced viral replication and a limited viral load that does not elicit a significant senescence response and/or may be a consequence of vaccine-evoked T cell responses.
Unlike neutralizing antibodies that inhibit virus entry but require high levels to fully protect, SARS-CoV-2-specific T cells cannot block primary host cell infection. However, pre-existing SARS-CoV-2-specific cytotoxic T cells can selectively eliminate infected cells — similar to the removal of VIS cells by early senolytic intervention. Hence, such intrinsic ‘T cell immunity-based senolysis’ might interrupt a potentially deleterious cytokine cascade upon SARS-CoV-2 infection in vaccinated individuals, possibly even in those with a compromised humoral but intact cellular, and hence discordant, immune response. Such discordance is frequently detected in patients with rheumatological diseases or haematological cancers, particularly after anti-CD20 antibody treatment163–166. Consistent with this view, convalescent plasma or recombinant therapeutic S protein-targeted antibodies achieved modest or mixed results in preventing severe COVID-19 (however, the emergence of SARS-CoV-2 VOC with mutations in the spike protein that may allow the evasion of neutralizing antibodies may further complicate the interpretation of these results) (refs.65,162,167).
In essence, the early elimination of spike peptide-presenting VIS cells either by exogenous pharmacological intervention using prototypic senolytics or by endogenous immune clearance, especially by vaccination-primed spike peptide-specific T cells or a virus-specific T cell response elicited by previous SARS-CoV-2 infection, targets a critical pathogenic inflexion point for COVID-19. Both approaches might be complementary and synergistic as they eliminate, in a partly overlapping fashion, virus-infected and hence virus-propagating cells as well as VIS and pre-existent and secondary senescent cells, all of which are a source of the SASP that can contribute to a SARS-CoV-2-triggered cytokine storm.
Conclusions and outlook
Rapidly expanding evidence implicates cellular senescence as a host cell response to viral infection in general and to SARS-CoV-2 in particular, similar to other cellular insults such as telomere dysfunction, oncogene activation or chemotherapy27–29. Although alternative mechanisms independent from VIS may contribute to the hyperinflammation observed in severe COVID-19, senescence operates as a central pathogenic principle in severe COVID-19 and may also be a general feature of other viral infections that induce hyperinflammatory responses. This could potentially also be of relevance in future epidemics or pandemics with viruses that induce VIS28. Accordingly, targeting VIS and senescent cells in general is a testable objective for attenuating the potentially severe course of disease following SARS-CoV-2 infection. However, a key role for senescence as a pathogenic driver and potential therapeutic target in other non-SARS-CoV-2 infection-related pathologies, such as other types of viral pneumonia, infectious and non-infectious acute respiratory distress syndrome, as well as non-pulmonary manifestations in viral infections, needs to be determined. A large variety of viruses have been shown to induce senescence in vitro and in vivo27,28,38, whereas no VIS phenotype was observed upon infection with the mild seasonal influenza A (H1N1) virus in Syrian hamsters62, implying that not all virus infections might be susceptible to senolytic strategies. Although preclinical and first clinical data are encouraging27,28,62,143–145, additional clinical studies are required to optimize regimens and dosing schedules. Appropriate timing of such intervention might be important. The preclinical data discussed here support further investigation of senolytic intervention with respect to their anti-inflammatory and pneumonia-attenuating efficacy and to minimize toxicities. Notably, the toxicities of agents with senolytic potential that are approved or currently in clinical trials, such as the tyrosine kinase inhibitor dasatinib or the BCL-2 family inhibitors navitoclax and venetoclax, profoundly differ between the dose/scheduling regimens as used in haemato-oncological indications compared to the regimens tested in preclinical and clinical investigations of COVID-19. Flavonoids, such as fisetin or quercetin, are freely available ‘over-the-counter’ agents and have favourable safety profiles168. Specifically, in the two COVID-19 trials referred to above, quercetin was generally well tolerated with no apparent toxicity and no particular side effects were reported by the patients143–145. Moreover, strategies that combine a senolytic agent with a viral replication blocker, such as Molnupiravir or Paxlovid, or possibly with TLR3 antagonists to attenuate viral induction of senescence appear attractive29 as mechanistically distinct approaches to reduce the prevalence of VIS cells may complement each other. However, more definitive preclinical data from SARS-CoV-2-infected animal models are needed to demonstrate that such approaches truly lead to disease attenuation via SASP reduction and not to extended viral persistence owing to impaired virus clearance.
The underlying reasons for the well-established higher risk in the elderly or in individuals with chronic disease to experience severe COVID-19 or to succumb to SARS-CoV-2 infection are multifactorial84,93. As recently shown in mice, the accumulation of (pre-)senescent cells during ageing primes for particularly SASP-intense VIS manifestation upon SARS-CoV-2 infection27, even if the senescent status of host cells might suppress further virus propagation as currently debated41,43. COVID-19-related VIS may also aggravate age-related pathologies via enhanced SASP-mediated inflammation29,169, as demonstrated for cardiovascular diseases and diabetes in preclinical models170, and might further deteriorate immune competence by inducing senescence in immune cells171,172. Further analyses into the mechanisms underlying immune cell senescence are urgently needed as is research into general similarities and differences between immune cell senescence and other types of cellular senescence, specifically in the context of immune cell senescence as a paracrine effect of VIS.
Damaged mucosal cells are subject to turnover in their respective tissues, foremost being the respiratory epithelia in the upper and lower airway tract during regeneration after acute COVID-19. More specifically, senescent cells are, at least in part, cleared by cells of both the innate and adaptive immune systems76,173–175 if such clearing function is not compromised, which remains to be studied in greater detail in the context of COVID-19 (ref.176). Persistent senescent cells are known to exert detrimental long-term effects on organ function and can promote tumorigenesis33,96,112,177,178. So far, no evidence has been reported for SARS-CoV-2-induced senescent cells in this regard and no direct pro-tumorigenic role has been attributed to SARS-CoV-2. However, given that a chronic SASP can deregulate immune functions, it remains to be investigated to what extent non-cleared VIS cells may contribute to the long-term disabilities collectively referred to as ‘long COVID’, which include fatigue, dyspnoea and cognitive dysfunction93,179–181. If the burden of senescent cells is chronically enhanced in patients with long COVID, then senolytic interventions might help to reduce the associated clinical symptoms.
Encouragingly, the senolytic agent navitoclax was shown to significantly reduce extended signs of cellular senescence and SASP in SARS-CoV-2-infected mice that survived beyond 2 weeks after infection at a time when the virus was no longer detectable62. Additionally, clearing VIS could further reduce the viral load in patients who exhibit viral persistence for months following infection182. Lastly, beyond a potential link between SARS-CoV-2 infection, immunosenescence, autoinflammatory disease and autoimmunity following COVID-19 (refs.86,183,184), there is increasing evidence for viral infections as triggers of autoimmune diseases in general. For example, type 1 diabetes mellitus was linked to enterovirus infections185 and multiple sclerosis to Epstein–Barr virus (EBV) infections186. This may imply that VIS operates as a pivotal amplifier of the underlying chronic and destructive autoinflammation in these conditions. EBV infection has long been known to induce the expression of central signalling mediators of the senescence response such as p16INK4a or p21CIP1. These proteins act as a primary host defence against virus propagation, and EBV-positive cells losing expression of these mediators subsequently get selected for in manifest chronic infection187,188. Therefore, research focusing on VIS and SASP in these contexts is urgently needed as it may not only help to elucidate the mechanism of autoaggressive pathogeneses but also to open new therapeutic avenues.
With new insights into COVID-19 pathogenesis and the potential for novel treatment opportunities, a central point has yet to be addressed: what factors, beyond age, determine the strength of the SASP response in individuals newly infected with SARS-CoV-2? Are predictors of the individual susceptibility to VIS or their senescent cell burden also indicators for a more severe clinical course upon SARS-CoV-2 infection? Are the induction of senescence, the extent of the SASP and symptomatic disease linked to the quantitative load of infectious virions as some preclinical data suggest33,189? In addition to senolytics, can we empower endogenous mechanisms that help to clear VIS cells more thoroughly during the initial phase of infection by stimulating NK cell or CD8+ T cell functions, for example? Will we be able to collect the relevant patient-individual data at the outset of the disease to inform personalized COVID-19 intervention strategies, including potential senolytic administration for those at need? And, is it possible that individuals who are at risk for severe COVID-19 may benefit from prophylactic, perhaps repetitive, exposure to senolytics in the context of very high incidence rates and a clinically challenging VOC? Finally, can these approaches be used to improve the effectiveness and duration of the immune response to COVID-19 vaccines in the elderly?
Acknowledgements
The authors apologize to all colleagues whose valuable insights and contributions to the discussed topic may have been missed or not explicitly mentioned owing to space restrictions. The authors thank numerous funding sources that enabled research at the intersection of senescence and COVID-19 in their laboratories, members of their research teams as well as their collaboration partners for helpful scientific exchange of conceptual ideas, controversies in the field, and clinical implications, as well as all the patients and their families, whose participation in clinical investigations and trials enhance our knowledge of pathogenetic mechanisms and therapeutic interventions.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Immunology thanks Henrike Maass who co-reviewed with Luca Cicin-Sain, Jesus Gill and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Competing interests
C.A.S. received travel support, honoraria and consulting fees from Abbvie, AstraZeneca, Bayer, Bristol-Myers Squibb/Celgene, Gilead/Kite, Janssen-Cilag, MSD, Novartis, Octapharma, Pierre Fabre, Roche, Sanofi, Takeda, and TissUse. Patents on senolytic drugs and their uses are held by Mayo Clinic (J.L.K. and T.T.) and the University of Minnesota (P.D.R. and L.J.N.). S.L. declares no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Clemens A. Schmitt, Email: clemens.schmitt@charite.de
Soyoung Lee, Email: soyoung.lee@jku.at.
References
- 1.Oberfeld B, et al. SnapShot: COVID-19. Cell. 2020;181:954–954.e1. doi: 10.1016/j.cell.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su Y, et al. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020;183:1479–1495.e20. doi: 10.1016/j.cell.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021 doi: 10.1038/s41579-021-00630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter MJ, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 5.Turnquist C, Ryan BM, Horikawa I, Harris BT, Harris CC. Cytokine storms in cancer and COVID-19. Cancer Cell. 2020;38:598–601. doi: 10.1016/j.ccell.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalfaoglu B, Almeida-Santos J, Tye CA, Satou Y, Ono M. T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis. Front. Immunol. 2020;11:589380. doi: 10.3389/fimmu.2020.589380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D, Ramanan AV, Bridgewood C. Immune cartography of macrophage activation syndrome in the COVID-19 era. Nat. Rev. Rheumatol. 2021;17:145–157. doi: 10.1038/s41584-020-00571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderbeke L, et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat. Commun. 2021;12:4117. doi: 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 10.Wahl A, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falsey AR, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JAC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriazopoulou E, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat. Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dan JM, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodda LB, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rydyznski Moderbacher C, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reusch N, et al. Neutrophils in COVID-19. Front. Immunol. 2021;12:652470. doi: 10.3389/fimmu.2021.652470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy RK, et al. Macrophage activation syndrome and COVID 19: impact of MAPK driven immune-epigenetic programming by SARS-Cov-2. Front. Immunol. 2021;12:763313. doi: 10.3389/fimmu.2021.763313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toor SM, Saleh R, Sasidharan Nair V, Taha RZ, Elkord E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology. 2021;162:30–43. doi: 10.1111/imm.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bert N, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 25.Ren X, et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184:1895–1913.e19. doi: 10.1016/j.cell.2021.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camell CD, et al. Senolytics reduce coronavirus-related mortality in old mice. Science. 2021 doi: 10.1126/science.abe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, et al. Virus-induced senescence is driver and therapeutic target in COVID-19. Nature. 2021 doi: 10.1038/s41586-021-03995-1. [DOI] [PubMed] [Google Scholar]
- 29.Tripathi U, et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging. 2021;13:21838–21854. doi: 10.18632/aging.203560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herranz N, Gil J. Mechanisms and functions of cellular senescence. J. Clin. Invest. 2018;128:1238–1246. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorgoulis V, et al. Cellular senescence: defining a path forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Schmitt CA. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019;21:94–101. doi: 10.1038/s41556-018-0249-2. [DOI] [PubMed] [Google Scholar]
- 34.Chuprin A, et al. Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev. 2013;27:2356–2366. doi: 10.1101/gad.227512.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, et al. Merkel cell polyomavirus large T antigen disrupts host genomic integrity and inhibits cellular proliferation. J. Virol. 2013;87:9173–9188. doi: 10.1128/JVI.01216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez I, et al. Induction of DNA double-strand breaks and cellular senescence by human respiratory syncytial virus. Virulence. 2016;7:427–442. doi: 10.1080/21505594.2016.1144001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohli J, Veenstra I, Demaria M. The struggle of a good friend getting old: cellular senescence in viral responses and therapy. EMBO Rep. 2021;22:e52243. doi: 10.15252/embr.202052243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seoane R, Vidal S, Bouzaher YH, El Motiam A, Rivas C. The interaction of viruses with the cellular senescence response. Biology. 2020 doi: 10.3390/biology9120455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorwood J, et al. SIV infection and the HIV proteins Tat and Nef induce senescence in adipose tissue and human adipose stem cells, resulting in adipocyte dysfunction. Cells. 2020 doi: 10.3390/cells9040854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora P, et al. B.1.617.2 enters and fuses lung cells with increased efficiency and evades antibodies induced by infection and vaccination. Cell Rep. 2021 doi: 10.1016/j.celrep.2021.109825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh TH, et al. Senescence in monocytes facilitates dengue virus infection by increasing infectivity. Front. Cell Infect. Microbiol. 2020;10:375. doi: 10.3389/fcimb.2020.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maremanda KP, Sundar IK, Li D, Rahman I. Age-dependent assessment of genes involved in cellular senescence, telomere, and mitochondrial pathways in human lung tissue of smokers, COPD, and IPF: associations with SARS-CoV-2 COVID-19 ACE2-TMPRSS2-Furin-DPP4 axis. Front. Pharmacol. 2020;11:584637. doi: 10.3389/fphar.2020.584637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baz-Martinez M, et al. Cell senescence is an antiviral defense mechanism. Sci. Rep. 2016;6:37007. doi: 10.1038/srep37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evangelou K, et al. Pulmonary infection by SARS-CoV-2 induces senescence accompanied by an inflammatory phenotype in severe COVID-19: possible implications for viral mutagenesis. Eur. Respir. J. 2022 doi: 10.1183/13993003.02951-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 46.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 47.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 48.De Cecco M, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019;566:73–78. doi: 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorbunova V, et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature. 2021;596:43–53. doi: 10.1038/s41586-021-03542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, et al. Targeting the senescence-overriding cooperative activity of structurally unrelated H3K9 demethylases in melanoma. Cancer Cell. 2018;33:322–336. doi: 10.1016/j.ccell.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 52.D’Agnillo F, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci. Transl. Med. 2021 doi: 10.1126/scitranslmed.abj7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mårtensson CU, et al. Mitochondrial protein translocation-associated degradation. Nature. 2019;569:679–683. doi: 10.1038/s41586-019-1227-y. [DOI] [PubMed] [Google Scholar]
- 54.Li X, et al. Mitochondria shed their outer membrane in response to infection-induced stress. Science. 2022;375:eabi4343. doi: 10.1126/science.abi4343. [DOI] [PubMed] [Google Scholar]
- 55.Dou Z, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017 doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gluck S, et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 2017;19:1061–1070. doi: 10.1038/ncb3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller KN, et al. Cytoplasmic DNA: sources, sensing, and role in aging and disease. Cell. 2021;184:5506–5526. doi: 10.1016/j.cell.2021.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021;21:548–569. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Domizio J, et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022 doi: 10.1038/s41586-022-04421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barbouti A, et al. In situ evidence of cellular senescence in thymic epithelial cells (TECs) during human thymic involution. Mech. Ageing Dev. 2019;177:88–90. doi: 10.1016/j.mad.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Desai N, et al. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. Nat. Commun. 2020;11:6319. doi: 10.1038/s41467-020-20139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuji S, et al. SARS-CoV-2 infection triggers paracrine senescence and leads to a sustained senescence-associated inflammatory response. Nat. Aging. 2022 doi: 10.1038/s43587-022-00170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Acosta JC, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou YJ, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Estcourt LJ, et al. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2021 doi: 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chua RL, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 67.Messner CB, et al. Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection. Cell Syst. 2020;11:11–24.e4. doi: 10.1016/j.cels.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demichev V, et al. A time-resolved proteomic and prognostic map of COVID-19. Cell Syst. 2021 doi: 10.1016/j.cels.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sacco K, et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat. Med. 2022;28:1050–1062. doi: 10.1038/s41591-022-01724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.COvid-19 Multi-omics Blood ATlas (COMBAT) Consortium A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell. 2022;185:916–938. doi: 10.1016/j.cell.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Acosta J, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 72.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 73.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jing H, et al. Opposing roles of NF-kB in anti-cancer treatment outcome unveiled by cross-species investigations. Genes Dev. 2011 doi: 10.1101/gad.17620611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall BM, et al. p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging. 2017;9:1867–1884. doi: 10.18632/aging.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Behmoaras J, Gil J. Similarities and interplay between senescent cells and macrophages. J. Cell Biol. 2021 doi: 10.1083/jcb.202010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roussel M, et al. Mass cytometry deep phenotyping of human mononuclear phagocytes and myeloid-derived suppressor cells from human blood and bone marrow. J. Leukoc. Biol. 2017;102:437–447. doi: 10.1189/jlb.5MA1116-457R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schafer MJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wendisch D, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021 doi: 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng Y, et al. A human circulating immune cell landscape in aging and COVID-19. Protein Cell. 2020;11:740–770. doi: 10.1007/s13238-020-00762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Junqueira C, et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022 doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartleson JM, et al. SARS-CoV-2, COVID-19 and the ageing immune system. Nat. Aging. 2021;1:769–782. doi: 10.1038/s43587-021-00114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martínez-Zamudio RI, et al. Senescence-associated β-galactosidase reveals the abundance of senescent CD8+ T cells in aging humans. Aging Cell. 2021;20:e13344. doi: 10.1111/acel.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Omarjee L, et al. Immunometabolism at the cornerstone of inflammaging, immunosenescence, and autoimmunity in COVID-19. Aging. 2020;12:26263–26278. doi: 10.18632/aging.202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang W, Thomas R, Oh J, Su DM. Thymic aging may be associated with COVID-19 pathophysiology in the elderly. Cells. 2021 doi: 10.3390/cells10030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Franzin R, Stasi A, Castellano G, Gesualdo L. Methods for characterization of senescent circulating and tumor-infiltrating T-cells: an overview from multicolor flow cytometry to single-cell RNA sequencing. Methods Mol. Biol. 2021;2325:79–95. doi: 10.1007/978-1-0716-1507-2_6. [DOI] [PubMed] [Google Scholar]
- 89.Li X, et al. The flavonoid quercetin ameliorates liver inflammation and fibrosis by regulating hepatic macrophages activation and polarization in mice. Front. Pharmacol. 2018;9:72. doi: 10.3389/fphar.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laridan E, Martinod K, De Meyer SF. Neutrophil extracellular traps in arterial and venous thrombosis. Semin. Thromb. Hemost. 2019;45:86–93. doi: 10.1055/s-0038-1677040. [DOI] [PubMed] [Google Scholar]
- 91.Radermecker C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020 doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Georg P, et al. Complement activation induces excessive T cell cytotoxicity in severe COVID-19. Cell. 2021 doi: 10.1016/j.cell.2021.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wissler Gerdes EO, et al. Role of senescence in the chronic health consequences of COVID-19. Transl. Res. 2021 doi: 10.1016/j.trsl.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hickson LJ, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J. Intern. Med. 2020;288:518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gasek NS, Kuchel GA, Kirkland JL, Xu M. Strategies for targeting senescent cells in human disease. Nat. Aging. 2021;1:870–879. doi: 10.1038/s43587-021-00121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wissler Gerdes EO, Misra A, Netto JME, Tchkonia T, Kirkland JL. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 2021;200:111591. doi: 10.1016/j.mad.2021.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Y, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu Y, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fuhrmann-Stroissnigg H, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017;8:422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu Y, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging. 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yousefzadeh MJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khan S, et al. A selective BCL-X(L) PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 2019;25:1938–1947. doi: 10.1038/s41591-019-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He Y, et al. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat. Commun. 2020;11:1996. doi: 10.1038/s41467-020-15838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pal P, et al. Discovery of a Novel BCL-X(L) PROTAC degrader with enhanced BCL-2 inhibition. J. Med. Chem. 2021;64:14230–14246. doi: 10.1021/acs.jmedchem.1c00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lv D, et al. Development of a BCL-xL and BCL-2 dual degrader with improved anti-leukemic activity. Nat. Commun. 2021;12:6896. doi: 10.1038/s41467-021-27210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walaszczyk A, et al. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell. 2019;18:e12945. doi: 10.1111/acel.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.González-Gualda E, et al. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell. 2020;19:e13142. doi: 10.1111/acel.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma AK, et al. The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Front. Cell Dev. Biol. 2020;8:354. doi: 10.3389/fcell.2020.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu M, et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Robbins PD, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu. Rev. Pharmacol. Toxicol. 2021;61:779–803. doi: 10.1146/annurev-pharmtox-050120-105018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tchkonia T, Palmer AK, Kirkland JL. New horizons: novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J. Clin. Endocrinol. Metab. 2021;106:e1481–e1487. doi: 10.1210/clinem/dgaa728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lubin JH, et al. Structural models of SARS-CoV-2 Omicron variant in complex with ACE2 receptor or antibodies suggest altered binding interfaces. bioRxiv. 2021 doi: 10.1101/2021.12.12.472313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karakasiliotis I, Lagopati N, Evangelou K, Gorgoulis VG. Cellular senescence as a source of SARS-CoV-2 quasispecies. FEBS J. 2021 doi: 10.1111/febs.16230. [DOI] [PubMed] [Google Scholar]
- 117.Verma AK, Aggarwal R. Repurposing potential of FDA-approved and investigational drugs for COVID-19 targeting SARS-CoV-2 spike and main protease and validation by machine learning algorithm. Chem. Biol. Drug Des. 2021;97:836–853. doi: 10.1111/cbdd.13812. [DOI] [PubMed] [Google Scholar]
- 118.Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front. Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohiuddin M, Kasahara K. The emerging role of cellular senescence in complications of COVID-19. Cancer Treat. Res. Commun. 2021;28:100399. doi: 10.1016/j.ctarc.2021.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu Q, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021;3:1706–1726. doi: 10.1038/s42255-021-00491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ, Robbins PD. Recent advances in the discovery of senolytics. Mech. Ageing Dev. 2021;200:111587. doi: 10.1016/j.mad.2021.111587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bao L, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 123.Kreye J, et al. A therapeutic non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 Hamster model. Cell. 2020;183:1058–1069.e19. doi: 10.1016/j.cell.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osterrieder N, et al. Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters. Viruses. 2020 doi: 10.3390/v12070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trimpert J, et al. The Roborovski dwarf hamster is a highly susceptible model for a rapid and fatal course of SARS-CoV-2 infection. Cell Rep. 2020;33:108488. doi: 10.1016/j.celrep.2020.108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Winkler ES, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020;21:1327–1335. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.White KM, et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science. 2021;371:926–931. doi: 10.1126/science.abf4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hao X, Wang C, Zhang R. Chromatin basis of the senescence-associated secretory phenotype. Trends Cell Biol. 2022 doi: 10.1016/j.tcb.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hubackova S, Krejcikova K, Bartek J, Hodny Z. IL1- and TGFbeta-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘bystander senescence’. Aging. 2012;4:932–951. doi: 10.18632/aging.100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu M, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. Usa. 2015;112:E6301–E6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dobosh B, et al. Baricitinib attenuates the proinflammatory phase of COVID-19 driven by lung-infiltrating monocytes. Cell Rep. 2022 doi: 10.1016/j.celrep.2022.110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wong L-YR, et al. Eicosanoid signalling blockade protects middle-aged mice from severe COVID-19. Nature. 2022;605:146–151. doi: 10.1038/s41586-022-04630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang HH, Kim C, Jung B, Kim KS, Kim JR. Involvement of IGF binding protein 5 in prostaglandin E(2)-induced cellular senescence in human fibroblasts. Biogerontology. 2011;12:239–252. doi: 10.1007/s10522-010-9318-z. [DOI] [PubMed] [Google Scholar]
- 136.Gonçalves S, et al. COX2 regulates senescence secretome composition and senescence surveillance through PGE(2) Cell Rep. 2021;34:108860. doi: 10.1016/j.celrep.2021.108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wiley CD, et al. Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Cell Metab. 2021;33:1124–1136.e5. doi: 10.1016/j.cmet.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu M, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. 2015;4:e12997. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beura LK, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huggins MA, Jameson SC, Hamilton SE. Embracing microbial exposure in mouse research. J. Leukoc. Biol. 2019;105:73–79. doi: 10.1002/JLB.4RI0718-273R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huggins MA, et al. Microbial exposure enhances immunity to pathogens recognized by TLR2 but increases susceptibility to cytokine storm through TLR4 sensitization. Cell Rep. 2019;28:1729–1743.e5. doi: 10.1016/j.celrep.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Di Pierro F, et al. Quercetin phytosome® as a potential candidate for managing COVID-19. Minerva Gastroenterol. 2020 doi: 10.23736/s1121-421x.20.02771-3. [DOI] [PubMed] [Google Scholar]
- 144.Di Pierro F, et al. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int. J. Gen. Med. 2021;14:2359–2366. doi: 10.2147/IJGM.S318720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Di Pierro F, et al. Potential clinical benefits of Quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial. Int. J. Gen. Med. 2021;14:2807–2816. doi: 10.2147/IJGM.S318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schneider JL, et al. The aging lung: physiology, disease, and immunity. Cell. 2021;184:1990–2019. doi: 10.1016/j.cell.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Justice JN, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Verdoorn BP, et al. Fisetin for COVID-19 in skilled nursing facilities: senolytic trials in the COVID era. J. Am. Geriatr. Soc. 2021 doi: 10.1111/jgs.17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chaib S, Tchkonia T, Kirkland JL. Obesity, senescence, and senolytics. Handb. Exp. Pharmacol. 2021 doi: 10.1007/164_2021_555. [DOI] [PubMed] [Google Scholar]
- 150.Corbett KS, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jackson LA, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mulligan MJ, et al. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020 doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 153.Sahin U, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 154.Oberhardt V, et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597:268–273. doi: 10.1038/s41586-021-03841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sahin U, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 156.Wang Z, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kissler SM, et al. Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated persons. N. Engl. J. Med. 2021;385:2489–2491. doi: 10.1056/NEJMc2102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Butt AA, et al. Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination. Int. J. Infect. Dis. 2021;110:353–358. doi: 10.1016/j.ijid.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Levine-Tiefenbrun M, et al. Viral loads of delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021 doi: 10.1038/s41591-021-01575-4. [DOI] [PubMed] [Google Scholar]
- 160.Schmidt F, et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Andrews N, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hoffmann M, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456.e11. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Apostolidis SA, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021 doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ehmsen S, et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mairhofer M, et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;39:1171–1172. doi: 10.1016/j.ccell.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liebers N, et al. Humoral and cellular responses after COVID-19 vaccination in anti-CD20-treated lymphoma patients. Blood. 2022;139:142–147. doi: 10.1182/blood.2021013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cohen MS, et al. Effect of Bamlanivimab vs Placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 2021;326:46–55. doi: 10.1001/jama.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kashyap D, et al. Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules. 2019 doi: 10.3390/biom9050174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bektas A, Schurman SH, Franceschi C, Ferrucci L. A public health perspective of aging: do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short- and long-term inflammaging? Immun. Ageing. 2020;17:23. doi: 10.1186/s12979-020-00196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma Y, et al. SARS-CoV-2 infection aggravates chronic comorbidities of cardiovascular diseases and diabetes in mice. Anim. Model. Exp. Med. 2021;4:2–15. doi: 10.1002/ame2.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.De Biasi S, et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020;11:3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cox LS, et al. Tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults. Lancet Healthy Longev. 2020;1:e55–e57. doi: 10.1016/S2666-7568(20)30011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reimann M, et al. Adaptive T-cell immunity controls senescence-prone MyD88- or CARD11-mutant B-cell lymphomas. Blood. 2021;137:2785–2799. doi: 10.1182/blood.2020005244. [DOI] [PubMed] [Google Scholar]
- 174.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 175.Arora S, et al. Invariant natural killer T cells coordinate removal of senescent cells. Med. 2021;2:938–950. doi: 10.1016/j.medj.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Knoll R, Schultze JL, Schulte-Schrepping J. Monocytes and macrophages in COVID-19. Front. Immunol. 2021;12:720109. doi: 10.3389/fimmu.2021.720109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Milanovic M, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100. doi: 10.1038/nature25167. [DOI] [PubMed] [Google Scholar]
- 178.Wyld L, et al. Senescence and cancer: a review of clinical implications of senescence and senotherapies. Cancers. 2020 doi: 10.3390/cancers12082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marshall M. The four most urgent questions about long COVID. Nature. 2021;594:168–170. doi: 10.1038/d41586-021-01511-z. [DOI] [PubMed] [Google Scholar]
- 180.Nalbandian A, et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sfera A, et al. Endothelial senescence and chronic fatigue syndrome, a COVID-19 based hypothesis. Front. Cell Neurosci. 2021;15:673217. doi: 10.3389/fncel.2021.673217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Avanzato VA, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912.e9. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020;16:413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kaklamanos A, et al. COVID-19 immunobiology: lessons learned, new questions arise. Front. Immunol. 2021;12:719023. doi: 10.3389/fimmu.2021.719023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus–why the β cells? Nat. Rev. Endocrinol. 2016;12:263–273. doi: 10.1038/nrendo.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bjornevik K, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022 doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 187.Yang X, He Z, Xin B, Cao L. LMP1 of Epstein-Barr virus suppresses cellular senescence associated with the inhibition of p16INK4a expression. Oncogene. 2000;19:2002–2013. doi: 10.1038/sj.onc.1203515. [DOI] [PubMed] [Google Scholar]
- 188.Chen YL, et al. The Epstein-Barr virus replication and transcription activator, Rta/BRLF1, induces cellular senescence in epithelial cells. Cell Cycle. 2009;8:58–65. doi: 10.4161/cc.8.1.7411. [DOI] [PubMed] [Google Scholar]
- 189.Dabisch PA, et al. Seroconversion and fever are dose-dependent in a nonhuman primate model of inhalational COVID-19. PLoS Pathog. 2021;17:e1009865. doi: 10.1371/journal.ppat.1009865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hayflick L, Moorhead PS. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 191.Yu GL, Bradley JD, Attardi LD, Blackburn EH. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990;344:126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- 192.Munoz-Espin D, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 193.Storer M, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 194.Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Iske J, et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 2020;11:4289. doi: 10.1038/s41467-020-18039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Prata L, Ovsyannikova IG, Tchkonia T, Kirkland JL. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin. Immunol. 2018;40:101275. doi: 10.1016/j.smim.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mosteiro L, et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science. 2016 doi: 10.1126/science.aaf4445. [DOI] [PubMed] [Google Scholar]
- 198.Ritschka B, et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017;31:172–183. doi: 10.1101/gad.290635.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Beausejour CM, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Minamino T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 201.Aird KM, et al. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 2013;3:1252–1265. doi: 10.1016/j.celrep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Palmer AK, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18:e12950. doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schmitt CA, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/S0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 204.Collado M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 205.Prasanna PG, et al. Therapy-induced senescence: opportunities to improve anticancer therapy. J. Natl Cancer Inst. 2021;113:1285–1298. doi: 10.1093/jnci/djab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Young AR, Narita M. Spatio-temporal association between mTOR and autophagy during cellular senescence. Autophagy. 2011;7:1387–1388. doi: 10.4161/auto.7.11.17348. [DOI] [PubMed] [Google Scholar]
- 207.Dorr JR, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 208.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat. Rev. Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 210.Fan DNY, Schmitt CA. Genotoxic stress-induced senescence. Methods Mol. Biol. 2019;1896:93–105. doi: 10.1007/978-1-4939-8931-7_10. [DOI] [PubMed] [Google Scholar]
- 211.Narita M, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/S0092-8674(03)00401-X. [DOI] [PubMed] [Google Scholar]
- 212.Zhang R, et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 213.Chandra T, et al. Global reorganization of the nuclear landscape in senescent cells. Cell Rep. 2015;10:471–483. doi: 10.1016/j.celrep.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.De Cecco M, et al. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell. 2013;12:247–256. doi: 10.1111/acel.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sadaie M, et al. Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev. 2013;27:1800–1808. doi: 10.1101/gad.217281.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shah PP, et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27:1787–1799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tasdemir N, et al. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 2016;6:612–629. doi: 10.1158/2159-8290.CD-16-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Martinez-Zamudio RI, et al. AP-1 imprints a reversible transcriptional programme of senescent cells. Nat. Cell Biol. 2020;22:842–855. doi: 10.1038/s41556-020-0529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]