Abstract
Objective
To assess whether maternal ultra-processed food intake during peripregnancy and during the child rearing period is associated with offspring risk of overweight or obesity during childhood and adolescence.
Design
Population based prospective cohort study.
Setting
The Nurses’ Health Study II (NHSII) and the Growing Up Today Study (GUTS I and II) in the United States.
Participants
19 958 mother-child (45% boys, aged 7-17 years at study enrollment) pairs with a median follow-up of 4 years (interquartile range 2-5 years) until age 18 or the onset of overweight or obesity, including a subsample of 2925 mother-child pairs with information on peripregnancy diet.
Main outcome measures
Multivariable adjusted, log binomial models with generalized estimating equations and an exchangeable correlation structure were used to account for correlations between siblings and to estimate the relative risk of offspring overweight or obesity defined by the International Obesity Task Force.
Results
2471 (12.4%) offspring developed overweight or obesity in the full analytic cohort. After adjusting for established maternal risk factors and offspring’s ultra-processed food intake, physical activity, and sedentary time, maternal consumption of ultra-processed foods during the child rearing period was associated with overweight or obesity in offspring, with a 26% higher risk in the group with the highest maternal ultra-processed food consumption (group 5) versus the lowest consumption group (group 1; relative risk 1.26, 95% confidence interval 1.08 to 1.47, P for trend<0.001). In the subsample with information on peripregnancy diet, while rates were higher, peripregnancy ultra-processed food intake was not significantly associated with an increased risk of offspring overweight or obesity (n=845 (28.9%); group 5 v group 1: relative risk 1.17, 95% confidence interval 0.89 to 1.53, P fortrend=0.07). These associations were not modified by age, sex, birth weight, and gestational age of offspring or maternal body weight.
Conclusions
Maternal consumption of ultra-processed food during the child rearing period was associated with an increased risk of overweight or obesity in offspring, independent of maternal and offspring lifestyle risk factors. Further study is needed to confirm these findings and to understand the underlying biological mechanisms and environmental determinants. These data support the importance of refining dietary recommendations and the development of programs to improve nutrition for women of reproductive age to promote offspring health.
Introduction
Childhood obesity is rising at alarming rates in the United States.1 2 According to the National Center for Health Statistics and National Health and Nutrition Examination Surveys, the prevalence of overweight, obesity, and severe obesity among children and young people aged 2-19 years have increased from 10.2%, 5.2%, and 1.0% in 1971-74 to 16.1%, 19.3%, and 6.1% in 2017-18, respectively.1 Childhood obesity increases the risk of major chronic diseases such as cardiovascular disease,3 diabetes, and cancers,4 and premature death.5 One of the potential contributors to the obesity epidemic among children and young people is the unhealthy Western style diet characterized by increased consumption of ultra-processed foods, which constitutes more than half of all energy intake among young people and adults in the US.6 7
Ultra-processed foods are extremely palatable, energy dense, convenient, and shelf stable products made from refined and inexpensive ingredients using a series of industrial processes.8 Ultra-processed foods contain various types of additives, including stabilizers, artificial flavors, and artificial colors, and contain little, if any, whole food ingredients.8 9 Further, ultra-processed foods generally have higher sugar, sodium, and saturated fat content compared with less processed foods.8 9 Consistent evidence has linked ultra-processed food intake to excess body fat, overweight, and obesity in adults10 11 12 and children.13 14 Because the development of obesity can be attributed to the combined influence of genetic susceptibility and environmental factors,15 maternal diet might influence offspring’s predisposition to obesity and diet choice.16 17 18 While Strohmaier and colleagues and Chen and colleagues have linked a healthy pregnancy diet to a reduced risk of obesity in children,17 18 Dhana and colleagues showed that a healthier maternal lifestyle during offspring’s childhood and adolescence was associated with lower obesity risk in offspring.16 However, the specific impact of maternal ultra-processed food consumption during these two critical periods on offspring’s body weight remains unknown.
We used cohorts of mother-child pairs to test our hypothesis that maternal intake of ultra-processed food during offspring childhood and adolescence (that is, child rearing period) was positively associated with the risk of incident overweight or obesity in offspring at age 7-18 years. We then analyzed the association between consumption of ultra-processed food during peripregnancy and offspring risk of overweight or obesity. Understanding these associations might help advance dietary recommendations and inspire actionable policies to improve maternal and offspring health.
Methods
Study population
We included longitudinal data from mothers and their offspring who participated in the Nurses’ Health Study II (NHS II) and the Growing Up Today Study (GUTS I and II), respectively. The NHS II enrolled 116 429 female registered nurses aged 25-42 years when established in 1989, with questionnaires mailed biennially to gain information on relevant medical history and risk factors.19 From 1991, a validated semiquantitative food frequency questionnaire was also mailed every four years.20 The GUTS I cohort was established in 1996 when 16 882 children (aged 8-15 years) of NHS II participants completed the initial questionnaire on health and lifestyle and were followed up every year between 1997 and 2001, and biennially thereafter. In 2004, 10 918 children (aged 7-17 years) of NHS II participants joined the extended GUTS II cohort and were followed up in 2006, 2008, and 2011, and biennially thereafter.
The study was approved by the Committees on the Use of Human Subjects in Research at the Harvard T.H. Chan School of Public Health and the Brigham and Women’s Hospital. Voluntarily returning the self-administered questionnaire was considered informed consent in both cohorts.
Participants
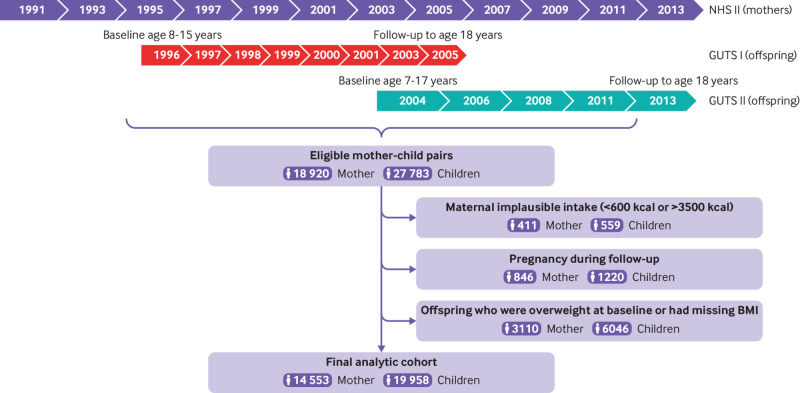
A total of 18 920 mothers and 27 783 children were matched between NHS II and two GUTS cohorts and were eligible for our study (fig 1). We excluded mother-child pairs with implausible maternal energy intake (<600 kcal/day or >3500 kcal/day, 411 mothers to 559 children) and pregnancy during follow-up (846 mothers, 1220 children). Additionally, we excluded mother-child pairs when children had missing baseline height and weight, or when they were overweight or had obesity at baseline (3110 mothers, 6046 children). The final analytic cohort included 19 958 children born to 14 553 mothers. To assess maternal consumption of ultra-processed food during peripregnancy (one year dietary assessment period that covered at least part of pregnancy), we restricted the analytic cohort to mother-child pairs when the mother had a singleton pregnancy because a multiple pregnancy might require more nutrients and could have an increased risk of complications, and encompassed by the NHS II 1991 or 1995 dietary assessment period. This left a subsample of 2790 mothers and 2925 offspring from GUTS II.
Fig 1.
Study design. Each number in the process chart indicates the year questionnaire was administered. BMI=body mass index, FFQ=food frequency questionnaire; GUTS=Growing Up Today Study; NHS=Nurses’ Health Study
Assessment of ultra-processed food consumption
We classified food items according to established NOVA food criteria based on the nature, purpose, and extent of food processing: unprocessed or minimally processed foods, processed culinary ingredients, processed foods, and ultra-processed foods.8 21 Food products that have undergone freezing, roasting, grinding, pasteurization, non-alcoholic fermentation, or vacuum packaging are considered to be unprocessed or minimally processed foods, including milk, bananas, and broccoli. In addition to these processes, processed culinary ingredients like olive oil and butter have undergone refining, centrifuging, or extracting, and processed foods like beer and peanut butter have undergone preservation methods such as bottling and canning. Ultra-processed foods include products like bacon, cola, energy bars, and ice cream that have undergone intensive industrial processing, such as extrusion, hydrogenation, and prefrying.22 After listing all food items on our food frequency questionnaires, three researchers independently assigned foods to the four groups based on the NOVA definitions and the example foods provided by Monteiro and colleagues.22 When all researchers did not reach consensus about assignment of a food item, the item underwent further review by three senior nutritional epidemiologists who finalized their categorization using information from research dietitians, cohort specific documents, and supermarket scans. More detailed procedures of food classification in NHS II and GUTS have been previously reported.23 24 We totaled the amount of each food item in each NOVA group to estimate the total consumption in servings per day, which is consistent with previous studies.25 26
Assessment of offspring overweight status
We calculated body mass index for GUTS participants at each follow-up using self-reported weight and height and following standardized directions. Participants were instructed to measure their weight in pounds without shoes or heavy clothing and to measure their height from their feet to the top of their head while standing up straight against a wall with feet flat on the floor and without wearing shoes or hats. Self-reported weight and height have been shown to be highly correlated with measured weight and height among US adolescents.27 28 29 We used age and sex specific body mass index cutoff points to define normal weight and overweight according to the International Obesity Task Force for participants aged 18 years or younger.30 Development of incident overweight or obesity was the primary outcome.
We also considered birth weight, which was reported by mothers in 2009, and somatotype (pictorial body diagram) at age 5, which was reported by offspring in the baseline GUTS II questionnaire in 2004 as a secondary outcome. The long term maternal recall of offspring birth weight has been shown to be reproducible and accurate (Pearson’s correlation coefficient 0.94).31 Somatotypes were selected by offspring from eight pictograms that most accurately represented their body shape, ranging from most lean (1) to most obese (8). We classified offspring into two groups by the median distribution of reported body shape.
Covariates
We considered maternal risk factors, including race (white or others), body mass index, total energy intake (categorized into five equal groups), chronic diseases (yes or no: cardiovascular disease, diabetes, or cancer), smoking (never, past, or current), parity (1, 2, 3, ≥4), gestational age (≤37, 37-39, 40-42, ≥43) and pregnancy complications (gestational diabetes, pre-eclampsia, pregnancy induced hypertension, cesarean delivery) as covariates. Maternal age (in years) at delivery was calculated based on birth dates of mothers and children. Annual household income (<$50 000 (£43 100; €49 730), $50 000-99 999, ≥$100 000, missing), which was estimated in 2001, and educational attainment of their partner (high school or less, college degree, graduate degree, missing), which was queried in 1999, were used as indicators of socioeconomic status. We assessed overall diet quality using the 2010 Alternative Healthy Eating Index (five equal groups)32 and physical activity over the past year (three equal groups) using a validated questionnaire.33 Offspring level covariates include age (years), sex (boy or girl), ultra-processed food intake (five equal groups), physical activity (three equal groups), and sedentary time (three equal groups), which were calculated based on reported hours per week spent on physical activity and sedentary activity (eg, using the computer, watching TV, reading or doing homework, surfing the internet) during the preceding year.
Statistical analysis
We followed offspring until the onset of overweight or obesity, loss to follow-up, or the age of 18 years (after which maternal diet might be expected to have little influence on their health; 2005 in GUTS I or 2013 in GUTS II), whichever occurred first. We estimated relative risks and 95% confidence intervals of overweight or obesity in offspring across cohort specific groups of maternal ultra-processed food consumption using a multivariable log binomial model with generalized estimating equations and exchangeable correlation structure, accounting for correlations between siblings born to the same mother. In the case of model convergence, relative risk was approximated by using a Poisson model with robust variance estimators.34 Linear trend was tested using standardized maternal ultra-processed food consumption as a continuous variable.
We adjusted for established risk factors for offspring obesity, including maternal age,35 total energy intake16 and diet quality (Alternative Healthy Eating Index 2010), physical activity, smoking, and offspring sex. We also adjusted for maternal race, overweight status, personal history of chronic disease, household income, living status (with partner or not), and partner’s education as indicators for socioeconomic status, which has been shown to be strongly correlated with childhood obesity.36 Additionally, to assess the role of offspring lifestyle factors, we further adjusted for offspring’s consumption of ultra-processed foods, physical activity, and sedentary time. To capture long term lifestyle factors for mothers and children over the child rearing period, total energy intake, Alternative Healthy Eating Index 2010 score, ultra-processed food consumption, maternal body mass index, physical activity, and sedentary time were cumulatively averaged from the baseline until censoring. For categorical covariates (smoking, personal history of chronic disease), we used the most recent information before censoring. Missing continuous variables were imputed with medians and a missing indicator was introduced where a categorical covariate had missing values. Missing data were rare (<0.1%): for example, maternal body mass index (n=14, 0.1%), offspring physical activity (n=6, 0.03%), and sedentary time (n=12, 0.06%); however, household income (n=2903, 20%) and partner’s education (n=1152, 8%) data were more frequently missing.
In the subsample analysis for peripregnancy consumption of ultra-processed foods, we adjusted for established prepregnancy risk factors for offspring health, including maternal age at pregnancy,35 total energy intake,37 diet quality, prepregnancy body mass index, prepregnancy physical activity,38 prepregnancy smoking status, parity,39 and gestational age at delivery; additionally, we adjusted for offspring’s lifestyle risk factors, including sex, birth weight, ultra-processed food intake, physical activity, and sedentary time. We adjusted for race, and as indicators for socioeconomic status, household income and partner’s education. Further, in a separate model, we mutually adjusted for maternal consumption of ultra-processed food during prepregnancy and during child rearing to assess whether maternal consumption of ultra-processed food during these two periods was independently associated with offspring overweight or obesity. We then assessed the association of the change in maternal consumption of ultra-processed food between these two periods with risk of offspring overweight or obesity.
In secondary analyses, we further categorized all ultra-processed foods into nine subgroups: ultra-processed breads and breakfast foods; sauces, cheeses, spreads and gravies; beverages; packaged sweets and desserts; dairy based desserts; frozen and ready-to-eat meals; packaged savory snacks; meats and meat substitute products; others (eg, liquor, non-dairy creamers); these categories are consistent with previous studies.24 We estimated the relative risk and 95% confidence interval of offspring overweight or obesity for each one standard deviation increase in intake of each mutually adjusted ultra-processed food subgroup. Moreover, we evaluated potential effect modification by offspring age, sex, birth weight, and maternal factors, including gestational age, parity, and maternal body mass index (prepregnancy and concurrent), pregnancy complications, and gestational weight gain using stratified analysis and Cochran’s Q test.
We conducted several sensitivity analyses to test the robustness of our findings. We considered incident offspring obesity (not just overweight), body mass index, birth weight, and offspring somatotype at age 5 as secondary outcomes. We estimated mean differences and 95% confidence intervals in offspring body mass index and birth weight across categories of maternal ultra-processed food consumption using a linear mixed model. Then, to assess the impact of missing values on our results, we used a multiple imputation approach (SAS PROC MI procedure, Markov Chain Monte Carlo method) to estimate missing body mass index values among offspring during follow-up, in line with previous work.16 We included age, sex, physical activity, sedentary time, diet quality, total energy intake, unprocessed or minimally processed food intake, processed culinary ingredient intake, processed food intake, ultra-processed food intake, and reported body mass index at baseline and during follow-up in the model to generate five imputed datasets. The validity of this method was found to be high: 97.4% of offspring were correctly classified by obesity status using imputed body mass index.16 We used PROC MIANALYZE to calculate the composite relative risks and 95% confidence intervals for offspring incident overweight or obesity associated with maternal ultra-processed food consumption. We also conducted a sensitivity analysis excluding participants with missing covariates.
Patient and public involvement
No patients were specifically involved in defining the research hypothesis or the outcome measures, nor were they involved in the design and implementation of the study. Participants, however, have provided feedback about our questionnaires throughout follow-up, which have been incorporated when feasible. We understand the tremendous value of patient and public involvement to research and have incorporated the suggestions from an internal review panel to improve this work.
Results
Participant characteristics
The consumption of ultra-processed foods among 14 553 mothers in our cohort slightly decreased from 1991 (mean±standard deviation 6.71±3.0 servings/day) to 2015 (5.81±3.1 servings/day; supplementary fig 1). While the consumption of some types of ultra-processed foods like ultra-processed bread and breakfast foods, beverages, and packaged sweets and desserts decreased, the consumption of dairy based desserts, packaged savory snacks, and other ultra-processed foods (eg, liquor, non-dairy creamers) increased. Across five groups of maternal ultra-processed food consumption during the child rearing period, maternal age at delivery (30.0±4.0 years), maternal body mass index before pregnancy (22.1 ±3.3), and baseline age of offspring (12.2±1.9 years) were similar (table 1). As maternal ultra-processed food consumption increased, maternal intake of carbohydrates, trans fatty acids, and sodium increased, while maternal intake of protein and overall diet quality assessed by the Alternative Healthy Eating Index 2010 decreased. Similarly, as maternal ultra-processed food consumption increased, the consumption of ultra-processed foods among 19 958 offspring also increased (Spearman’s correlation coefficient 0.21; P<0.001), whereas offspring’s overall diet quality decreased. Similar trends for these characteristics by maternal ultra-processed food consumption during peripregnancy were observed among a subsample of 2790 mothers and 2925 children (supplementary table 1).
Table 1.
Baseline characteristics of maternal (NHS II) and offspring (GUTS) participants according to maternal consumption of ultra-processed foods during child rearing period
| Characteristics | Maternal ultra-processed food consumption | ||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| Maternal characteristics | |||||
| No of participants | 2931 | 2899 | 2878 | 2878 | 2967 |
| Ultra-processed foods (servings/day)* | 3.4 (0.8) | 5.2 (0.5) | 6.6 (0.5) | 8.4 (0.7) | 12.1 (2.4) |
| Ultra-processed breads and breakfast foods | 0.9 (0.6) | 1.3 (0.7) | 1.7 (1) | 2.1 (1.2) | 2.8 (1.6) |
| Sauces, cheeses, spreads, and gravies | 0.9 (0.5) | 1.3 (0.6) | 1.6 (0.8) | 2 (1) | 2.6 (1.4) |
| Beverages | 0.4 (0.5) | 0.8 (0.8) | 1.1 (1) | 1.5 (1.3) | 2.3 (1.8) |
| Packaged sweets and desserts | 0.4 (0.3) | 0.7 (0.5) | 0.8 (0.6) | 1.1 (0.9) | 1.6 (1.4) |
| Dairy based desserts | 0.2 (0.2) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.4) | 0.4 (0.4) |
| Frozen and ready-to-eat meals | 0.2 (0.2) | 0.3 (0.2) | 0.4 (0.2) | 0.4 (0.3) | 0.5 (0.3) |
| Packaged savory snacks | 0.1 (0.2) | 0.2 (0.2) | 0.2 (0.2) | 0.2 (0.3) | 0.3 (0.5) |
| Meats and meat substitute products | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.2) | 0.2 (0.2) | 0.2 (0.3) |
| Others | 0.1 (0.3) | 0.2 (0.5) | 0.4 (0.8) | 0.6 (1.1) | 1.3 (1.9) |
| Unprocessed or minimally processed foods (servings/day) | 13.1 (4.9) | 13.6 (4.8) | 14.3 (5) | 14.9 (5.2) | 15.3 (5.1) |
| Processed culinary ingredients (servings/day) | 1 (1.3) | 1 (1.3) | 1.1 (1.3) | 1.2 (1.5) | 1.5 (1.7) |
| Processed foods (servings/day) | 1.4 (0.9) | 1.7 (0.9) | 1.9 (1) | 2 (1) | 2.3 (1.1) |
| Age at delivery (years) | 30.5 (4) | 30.1 (4.1) | 30 (4) | 29.8 (3.9) | 29.7 (3.9) |
| Age at study baseline (years) | 42.9 (4.4) | 42.4 (4.4) | 42.2 (4.3) | 42.1 (4.3) | 42 (4.2) |
| White race, n (%) | 2725 (93.0) | 2786 (96.1) | 2781 (96.6) | 2797 (97.2) | 2886 (97.3) |
| Prepregnancy body mass index | 21.8 (3.1) | 21.8 (3.1) | 22 (3.2) | 22.2 (3.3) | 22.7 (3.8) |
| Average body mass index during follow-up | 24.1 (4.4) | 24.2 (4.5) | 24.6 (4.7) | 24.9 (5) | 26 (5.8) |
| Chronic disease†, n (%) | 136 (4.6) | 127 (4.4) | 161 (5.6) | 142 (4.9) | 154 (5.2) |
| Physical activity (METs-h/week) | 20.9 (26.5) | 20.6 (26.9) | 20.3 (24.1) | 19.9 (23.5) | 19.9 (27.2) |
| AHEI-2010 diet score | 54.3 (11.5) | 52.1 (11.3) | 50.3 (11.4) | 49 (11.5) | 46.6 (11.3) |
| Total energy intake (kcal/day) | 1409 (385) | 1667 (386) | 1864 (434) | 2053 (474) | 2364 (541) |
| Carbohydrate intake (g/day) | 229.9 (40.8) | 230.7 (35.4) | 231.8 (35) | 232.5 (35.5) | 231.6 (33.9) |
| Protein intake (g/day) | 85.6 (15.6) | 84.5 (14.1) | 83.2 (13.4) | 82.5 (13.5) | 80.7 (13.1) |
| Total fat intake (g/day) | 60.7 (14.2) | 60.6 (12.4) | 60.7 (12.5) | 60.5 (12.5) | 61.4 (12.2) |
| Trans fatty acid intake (g/day) | 2.8 (1.3) | 3 (1.1) | 3.1 (1.2) | 3.2 (1.2) | 3.5 (1.3) |
| Sodium intake (mg/day) | 1922 (478) | 2021 (384) | 2072 (398) | 2137 (403) | 2256 (465) |
| Smoking status, n (%) | |||||
| Current | 193 (6.6) | 173 (6.0) | 170 (5.9) | 168 (5.8) | 221 (7.4) |
| Past | 693 (23.6) | 742 (25.6) | 646 (22.4) | 666 (23.1) | 702 (23.7) |
| Never | 2045 (69.8) | 1984 (68.4) | 2062 (71.6) | 2044 (71.0) | 2044 (68.9) |
| Living with a spouse or partner, n (%) | 2598 (88.6) | 2626 (90.6) | 2644 (91.9) | 2622 (91.1) | 2683 (90.4) |
| Household income, n (%) | |||||
| <$50 000 | 304 (10.4) | 263 (9.1) | 280 (9.7) | 293 (10.2) | 310 (10.4) |
| $50 000 to $99 999 | 995 (33.9) | 1079 (37.2) | 1069 (37.1) | 1069 (37.1) | 1185 (39.9) |
| ≥$100 000 | 1009 (34.4) | 995 (34.3) | 960 (33.4) | 931 (32.3) | 908 (30.6) |
| Missing | 623 (21.3) | 562 (19.4) | 569 (19.8) | 585 (20.3) | 564 (19.0) |
| Education attainment of spouse or partner, n (%) | |||||
| High school or less | 414 (14.1) | 410 (14.1) | 433 (15.0) | 439 (15.3) | 502 (16.9) |
| College degree | 1365 (46.6) | 1319 (45.5) | 1327 (46.1) | 1331 (46.2) | 1382 (46.6) |
| Graduate degree | 901 (30.7) | 923 (31.8) | 910 (31.6) | 889 (30.9) | 856 (28.9) |
| Missing data | 251 (8.6) | 247 (8.5) | 208 (7.2) | 219 (7.6) | 227 (7.7) |
| Offspring characteristics | |||||
| No of participants | 3978 | 4011 | 3985 | 3991 | 3993 |
| Body mass index | 18.2 (2.2) | 18.2 (2.3) | 18.2 (2.3) | 18.3 (2.3) | 18.4 (2.3) |
| Age (years) | 12.2 (1.9) | 12.1 (1.9) | 12.1 (1.9) | 12.2 (1.9) | 12.2 (1.9) |
| Male sex, n (%) | 1799 (45.2) | 1740 (43.4) | 1848 (46.4) | 1784 (44.7) | 1765 (44.2) |
| Ultra-processed foods (servings/day) | 6.3 (3.2) | 6.8 (3.2) | 7.1 (3.3) | 7.4 (3.4) | 8 (3.7) |
| AHEI-10 score | 36.8 (7.5) | 35.7 (7.2) | 35.3 (7.5) | 34.8 (7.4) | 33.9 (7.3) |
| Total energy intake (kcal/day) | 2026 (675) | 2107 (664) | 2173 (697) | 2220 (701) | 2289 (732) |
| Physical activity (h/week) | 16.2 (10.9) | 16.4 (10.9) | 16.3 (10.4) | 16.5 (10.8) | 16.7 (11) |
| Sedentary time (h/week) | 46.3 (23.3) | 45.8 (22.7) | 46.6 (22.9) | 46.2 (23.1) | 47.4 (23.1) |
Values are shown in mean (standard deviation) unless indicated otherwise. $1.00=£0.86, €0.99.
AHEI=Alternative Healthy Eating Index. METs-h/week=metabolic equivalent task hours/week.
The interquintile range of ultra-processed foods (servings/day) for each group (1-5) was 2.9-4.0, 4.8-5.6, 6.3-7.1, 7.9-8.9, and 10.4-13.1, respectively.
Includes diabetes, hypertension, cardiovascular disease, and cancer.
Risk of overweight or obesity in offspring
Over a median follow-up of 4 years (interquartile range 2-5 years), 2472 (12%) offspring developed overweight or obesity in the full analytic cohort. Maternal ultra-processed food consumption during the child rearing period was associated with an increased risk of incident overweight or obesity in offspring. We observed a 26% higher risk of overweight or obesity in the group with the highest maternal ultra-processed food consumption (group 5: 12.1±2.4 servings/day) compared with the group with the lowest consumption (group 1: 3.4±0.8 servings/day; relative risk 1.26, 95% confidence interval 1.08 to 1.47, P for trend<0.001; table 2, supplementary table 2), after controlling for established risk factors (including maternal body mass index, physical activity, smoking, and socioeconomic factors, and offspring’s ultra-processed food consumption, physical activity, and sedentary time).
Table 2.
Association between maternal consumption of ultra-processed foods during child rearing period and offspring body weight measures
| Measure | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | P for trend† |
|---|---|---|---|---|---|---|
| Overweight or obesity | ||||||
| No (%) | 458 (11.5) | 438 (11.0) | 501 (12.6) | 480 (12.0) | 594 (14.9) | — |
| Relative risk (95% CI)* | 1 (reference) | 1.00 (0.87 to 1.14) | 1.11 (0.97 to 1.27) | 1.07 (0.92 to 1.23) | 1.26 (1.08 to 1.47) | <0.001 |
| Obesity | ||||||
| No (%) | 164 (3.4) | 181 (3.8) | 171 (3.6) | 210 (4.4) | 272 (5.7) | — |
| Relative risk (95% CI)* | 1 (reference) | 1.1 (0.89 to 1.37) | 1.02 (0.81 to 1.28) | 1.14 (0.9 to 1.44) | 1.35 (1.06 to 1.72) | <0.001 |
| Body mass index percentile | ||||||
| Mean (SD) | 53.6 (27.9) | 54.5 (28.1) | 55.4 (28.0) | 56.3 (28.1) | 58.5 (28.0) | — |
| Mean difference (95% CI)a | 1 (reference) | 0.87 (0.32 to 1.42) | 1.35 (0.7 to 1.99) | 1.59 (0.88 to 2.29) | 2.11 (1.31 to 2.91) | <0.001 |
Ultra-processed food intake categorized into five equal groups.
Relative risk and 95% confidence interval (CI) for overweight or obesity and obesity were estimated by generalized estimating equation and mean difference (95% CI) for BMI was estimated by mixed linear model. All models were adjusted for maternal risk factors (baseline age, race, total energy intake, 2010 Alternative Healthy Eating Index, body mass index, physical activity, smoking, personal history of chronic disease, living status, household income, and spouse’s education) and offspring’s sex, ultra-processed food intake, physical activity, and sedentary time.
Linear trend was tested using standardized maternal ultra-processed food consumption as a continuous variable.
Similarly, maternal ultra-processed food consumption during the child rearing period was associated with an increased risk of childhood obesity and increased body mass index (table 2, supplementary table 2). In sensitivity analysis, we assessed offspring overweight or obesity with multiple imputation of missing body mass index data and found that the positive association between maternal ultra-processed food intake during the child rearing period and risk of childhood overweight or obesity was not materially altered (group 5 v group 1, relative risk 1.26, 95% confidence interval 1.15 to 1.37, P for trend=0.01; supplementary table 3). The analysis that excluded participants with missing covariates showed similar results (1.24, 1.04 to 1.48, P for trend=0.001; supplementary table 4).
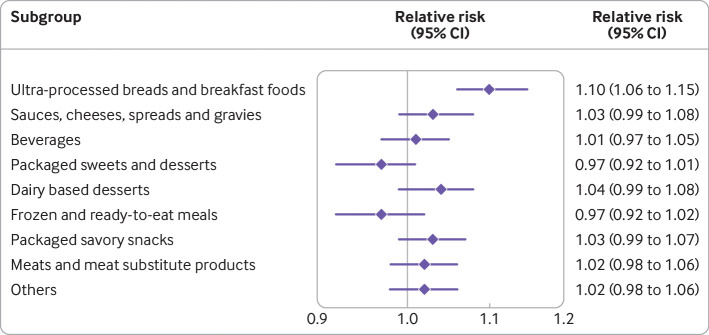
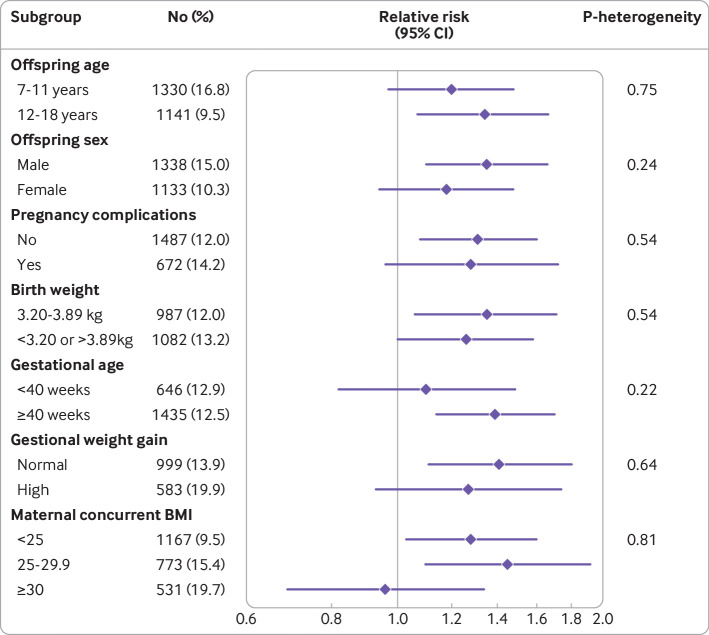
When assessing subtypes of ultra-processed foods, ultra-processed breads and breakfast foods were independently associated with childhood risk of overweight or obesity (relative risk per one standard deviation increase 1.10, 95% confidence interval 1.06 to 1.15; fig 2). In a stratified analysis (fig 3), we found positive associations between maternal ultra-processed food consumption during child rearing and risk of overweight or obesity among boys, older children, children with normal birth weight, children born over term, and children born to mothers without pregnancy complications, excess gestational weight gain, or obesity. However, tests of heterogeneity were not statistically significant, suggesting the association of maternal ultra-processed food intake and offspring adiposity did not substantially differ by offspring age, sex, pregnancy complications, birth weight, gestational age, gestational weight gain, or maternal body mass index.
Fig 2.
Association between maternal consumption of individual types of ultra-processed foods during child rearing period and risk of overweight or obesity in offspring. Relative risks and 95% confidence intervals were estimated for each one standard deviation increase in ultra-processed food intake using generalized estimating equation adjusted for maternal risk factors (baseline age, race, smoking, physical activity, total energy intake, Alternative Healthy Eating Index 2010, body mass index, personal history of chronic disease, living status, household income, spouse’s education), and offspring’s risk factors (sex, consumption of ultra-processed foods, physical activity, sedentary time). Individual types of ultra-processed foods were mutually adjusted
Fig 3.
Association between maternal consumption of ultra-processed foods during child rearing period and risk of overweight or obesity in offspring by risk factors. Relative risks and 95% confidence intervals for group with highest consumption of ultra-processed food (group 5) compared with group with lowest consumption (group 1) estimated using generalized estimating equation adjusted for maternal risk factors (baseline age, race, smoking, physical activity, total energy intake, 2010 Alternative Healthy Eating Index, body mass index (BMI), personal history of chronic disease, living status, household income, spouse’s education), and offspring’s risk factors (sex, consumption of ultra-processed foods, physical activity, sedentary time). Information for gestational weight gain is only available in GUTS I. P for heterogeneity was calculated using Cochran’s Q test
Peripregnancy ultra-processed food consumption in subsample
A total of 845 (28.9%) offspring with overweight or obesity were reported in the subsample. Peripregnancy consumption of ultra-processed foods was not significantly associated with an increased risk of overweight or obesity in offspring when comparing the group with the highest ultra-processed food intake (group 5: 11.7±2.1 servings/day) with the group with the lowest intake (group 1: 3.3 ±0.7 servings/day; relative risk 1.17, 95% confidence interval 0.89 to 1.53, P for trend=0.07; supplementary table 5). The associations of peripregnancy ultra-processed food consumption and offspring obesity, body mass index, birth weight, and body somatotype at age 5 were null (supplementary table 6). In a sensitivity analysis with multiple imputation of offspring body mass index, the association between peripregnancy ultra-processed food intake and offspring overweight or obesity was slightly attenuated (group 5 v group 1, relative risk 1.12, 95% confidence interval 0.83 to 1.51, P for trend=0.26; supplementary table 7). Among the nine subgroups of ultra-processed foods (supplementary fig 2), sugar sweetened beverages (relative risk per one standard deviation increase 1.08, 95% confidence interval 1.01 to 1.16) and dairy based desserts (1.08, 1.01 to 1.15) were more strongly associated with the risk of overweight or obesity in offspring. The associations between peripregnancy ultra-processed food consumption and offspring overweight or obesity were not modified by offspring’s age, sex, birth weight, gestational age, parity, or maternal prepregnancy body mass index according to tests for heterogeneity in a stratified analysis (supplementary fig 3).
Comparing maternal ultra-processed food consumption during peripregnancy and child rearing period in subsample
Maternal consumption of ultra-processed foods changed little from peripregnancy to child rearing period (Spearman’s correlation coefficient 0.46, P<0.001, mean±standard deviation −0.1±3.1 servings/day; supplementary tables 1 and 8), which had a null association with the risk of overweight or obesity in offspring. With additional adjustment for peripregnancy consumption of ultra-processed foods in the fully adjusted model (supplementary table 9), maternal ultra-processed food consumption during child rearing remains positively associated with childhood overweight or obesity (relative risk per one standard deviation increase 1.15, 95% confidence interval 1.01 to 1.32, P=0.03).
Discussion
In this large cohort study of mothers and children with long term follow-up, we found that maternal consumption of ultra-processed foods during the child rearing period was associated with an increased risk of developing overweight or obesity in offspring during childhood and adolescence, independent of offspring’s intake of ultra-processed foods, physical activity, and sedentary time. Offspring of mothers who were in the highest consumption group of ultra-processed food had a 26% increased risk of incident overweight or obesity compared with mothers in the lowest consumption group. These associations were similar among participants with different risk profiles, including maternal body weight, history of pregnancy complications, gestational weight gain, offspring sex, birth weight, and gestational age. In a subsample of mother-child pairs, the positive association between maternal ultra-processed food consumption during child rearing and offspring overweight or obesity remained even after adjusting for peripregnancy ultra-processed food consumption, suggesting that maternal ultra-processed food consumption during child rearing might have a stronger association with offspring overweight or obesity than peripregnancy ultra-processed food consumption.
Strengths and limitations of study
Our study has several strengths. We used data from several large ongoing prospective cohorts with standardized questionnaires covering a wide range of socioeconomic, lifestyle, and other health risk factors. The long term follow-up from preconception among mothers and through childhood and adolescence of offspring ensured that maternal risk factors were assessed prospectively, before incident overweight or obesity in offspring. Additionally, detailed dietary assessments using validated food frequency questionnaires allowed us to distinguish ultra-processed foods from other foods and estimate maternal ultra-processed food intake in detail.
Our study also has limitations. Although we have adjusted for various potential risk factors in our models, we cannot rule out the possibility of residual confounding due to the observational nature of our study. Nevertheless, a randomized controlled trial of ultra-processed food is infeasible and unethical given what is already known about ultra-processed food intake and chronic disease risk.10 11 12 Additionally, self-reported diet and weight measures might be subject to misreporting. However, body weight reported by NHS II participants has been validated in a large random subsample with measured versus reported weight40 and similar validation studies of US children suggested good accuracy with a tendency to underreport among those with obesity,29 41 which might have attenuated our results.
Similar to all transgenerational studies, some offspring participants were lost to follow-up, which resulted in a few of our analyses being underpowered, particularly those related to peripregnancy intake. However, our sensitivity analysis with multiple imputation of missing offspring body mass index data produced consistent associations, and we do not anticipate loss to follow-up to be related to our primary exposure (differential misclassification), which would have attenuated our results. A previous study has shown that loss to follow-up in our offspring cohort (16.5%) was unrelated to maternal lifestyle, and obesity classified using imputed body mass index had excellent specificity (99.3%) and moderate sensitivity (61.3%).16
Mothers in our cohort were predominantly white, had similar familial and personal educational attainments, and were of comparable socioeconomic backgrounds, which could restrict study generalizability but increase internal validity. Although we lack more detailed information on maternal educational attainments, we adjusted for partner’s education and household income in this study of US based nurses. Furthermore, our food frequency questionnaires were not specifically designed for pregnancy intake or administered specifically during pregnancy. Instead, we used a subsample design in which mothers who had completed questionnaires that encompassed their pregnancy period to assess peripregnancy maternal ultra-processed food intake. We used the same food frequency questionnaire during peripregnancy and child rearing to ensure consistency, and no major differences were observed in maternal ultra-processed food intake between these two periods. This finding is in line with a previous study in the United Kingdom showing relative consistency in dietary intake during prepregnancy and pregnancy.42
We used data collected from offspring from age 7 years onwards and were therefore unable to assess the risk of overweight in early childhood, although our results showed that maternal ultra-processed food intake was not associated with birth weight and body shape at age 5. Finally, we did not collect specific information on whether offspring lived with their mothers at the time of a given assessment. However, consistent with previous studies,16 we only followed offspring until age 18 years, a common age at which offspring leave their maternal home.
Comparison with other studies
Several studies have investigated the impact of ultra-processed food consumption on maternal and child health.43 44 45 For example, Silva and colleagues linked ultra-processed food consumption to increased gestational weight gain and glucose levels in pregnant women with gestational diabetes,44 and a prospective birth cohort study showed that the trajectories of body mass index and waist circumference from 7 to 24 years of age were greater among British children who had higher ultra-processed food consumption.45 According to a recent systematic review,43 only one cohort study tested the association between maternal ultra-processed food consumption and offspring body composition.46 Among 45 US women, Rohatgi and colleagues found that ultra-processed food intake during pregnancy, which was assessed by a one month food frequency questionnaire, was associated with increased thigh skinfold, subscapular skinfold, and total body adiposity in the neonate.46 Our study enrolled a larger population using a more detailed and validated dietary inventory with longer follow-up.46 This design filled the research gap of large longitudinal investigations examining the association between maternal ultra-processed food intake and offspring body weight into adolescence and early adulthood.
Most previous transgenerational studies have focused on the relation between overall maternal diet quality with offspring adiposity and suggested that adherence to a healthier dietary pattern during pregnancy might be associated with lower risk of offspring overweight or obesity.17 18 However, these dietary patterns are often unable to determine the level of industrial modifications among foods of the same food group (eg, brown rice v whole wheat bread and plain yogurt v sweetened yogurt). In contrast, our study using the NOVA classification system to distinguish ultra-processed foods from other foods, which provides robust epidemiological evidence for the role of maternal ultra-processed food consumption in the development of childhood obesity. Additionally, by showing that the relation between maternal ultra-processed food consumption and offspring adiposity is not fully explained by the overall maternal diet quality, our results offer further lines of inquiry on the specific biological interactions between ultra-processed food, diet quality, and adiposity. Moreover, our findings might offer support for more actionable and concrete dietary guidance to reduce ultra-processed food intake for risk mitigation of overweight or obesity compared with broader recommendations to consume a less Western diet.
Potential mechanisms
Although the underlying pathways of our findings have not yet been fully elucidated and remain beyond the scope of this investigation, maternal diet during child rearing is likely to shape offspring’s diet and lifestyle choices, which subsequently exert a profound impact on their risk of overweight or obesity.47 48 Randomized controlled trials have previously shown that parent-only interventions are similarly effective compared with parent-child interventions on child weight loss.49 50 The positive correlation between maternal and offspring consumption of ultra-processed foods in our cohort supports these hypotheses. Our results showed that the association between maternal ultra-processed food intake during the child rearing period and offspring risk of overweight or obesity was independent of offspring’s lifestyle risk factors. This finding indicates that there might be other pathways through which maternal ultra-processed food intake might influence childhood overweight risk; for example, long term in utero imprinting and the presence of uncharacterized gene by environment factors.51 52 53 Further research is needed to investigate these pathways.
There are a few potential mechanisms by which peripregnancy ultra-processed food intake could affect offspring adiposity, including epigenetic modification of offspring’s susceptibility to obesity. Animal and human studies have shown that maternal undernutrition and poor diet quality could lead to persistent epigenetic change in genes involved in the regulation of growth, energy balance, and insulin resistance in offspring.51 52 53 Other biological mechanisms might involve the proinflammatory additives in ultra-processed foods, including sodium,54 emulsifiers,55 56 57 sugar,58 and artificial sweeteners.59 Chronic maternal inflammation, possibly mediated through ultra-processed food intake, has been linked to increased offspring adiposity in mice and humans.60 61 62 For example, using an experimental model for human gut microbial communities, Chassaing and colleagues showed that synthetic emulsifiers polysorbate 80 and carboxymethylcellulose increased the proinflammatory potential of human gut bacteria.55 Emulsifiers and sweeteners are common ingredients found in store bought beverages and dairy based desserts such as ice cream and frozen yogurt, both of which were associated with childhood overweight or obesity in our peripregnancy analysis. However, larger studies with dietary assessment specifically targeting the pregnancy period are needed to confirm our findings.
Conclusion and public health implications
We found that maternal consumption of ultra-processed foods was associated with an increased risk of incident overweight or obesity in offspring, independent of various maternal and offspring factors. Our study highlights the potential benefits of limiting ultra-processed food consumption among mothers and women of reproductive age to reduce the risk of overweight in their children. However, we should not overlook social determinants of health that could impede women from reducing ultra-processed food intake. These might include a lack of adequate time to prepare unprocessed food, the additional costs of a more healthy diet (including limited shelf life that might result in greater waste), the possibility that mothers are not solely responsible for household foods, and limited access to healthy food options due to geographical location.63 Additionally, many women might already experience shame for weight related health behaviors during pregnancy and child rearing,64 and we caution against using these data to further stigmatize their food choices.
Addressing these financial and social structural barriers to making healthy food choices is critical for developing achievable and responsible dietary guidelines for women of child bearing age. Further studies are warranted to investigate specific biological mechanisms and socioeconomic determinants underlying the observed associations between maternal ultra-processed food intake and offspring overweigh and obesity.
What is already known on this topic
Ultra-processed foods are commonly found in contemporary Western style diets and are associated with weight gain in adults
It is unclear whether a transgenerational association exists between maternal consumption of ultra-processed foods and offspring body weight
What this study adds
Maternal consumption of ultra-processed foods during the child rearing period was associated with an increased risk of overweight or obesity in offspring during childhood and adolescence
The findings suggest that mothers might benefit from limiting intake of ultra-processed foods to prevent offspring overweight
Dietary recommendations should be refined and financial and social barriers removed to improve nutrition for women of child bearing age and to reduce childhood obesity
Web extra.
Extra material supplied by authors
Web appendix: Supplemental online content
Contributors: YW, LHN, and ATC conceived and designed the study. YW conducted statistical analysis, interpreted the findings, and drafted the manuscript for intellectual content. LHN and ATC interpreted the results, critically revised the manuscript, provided intellectual content, and supervised the study. JEC, CH, MS, LHN, and ATC obtained funding. All authors acknowledge full responsibility for the analyses and interpretation of data. All authors have provided intellectual content and revised the manuscript. LHN and ATC have equal contributions and are the guarantors. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by the National Institutes of Health (U01 CA176726 to the Nurses’ Health Study II, U01 HL145386 to the Growing Up Today Study, Loan Repayment Program and K23 DK125838 to LHN, R00 CA215314 to MS, R01 CA202704 to CH and ATC, and R35 CA253185 to ATC), American Gastroenterological Association (Research Scholars Award to LHN), the Crohn’s and Colitis Foundation (Research Fellowship Award and Career Development Award to LHN and Senior Investigator Award to ATC), American Cancer Society (Mentored Research Scholar Grant in Applied and Clinical Research to MS, Clinical Research Professorship to ATC), and Massachusetts General Hospital (Stuart and Suzanne Steele Research Scholar Award to ATC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The study sponsors and funders played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. All authors are independent from funders and have full access to all data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from National Institutes of Health, American Gastroenterological Association, the Crohn’s and Colitis Foundation, American Cancer Society, and Massachusetts General Hospital for the submitted work; ATC serves as a consultant for Pfizer, Boehringer Ingelheim, Bayer Pharma AG outside the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results will be disseminated to all study participants via an annual newsletter accessible by the general public (https://nurseshealthstudy.org/participants/newsletters), and through lay and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was approved by the Committees on the Use of Human Subjects in Research at the Harvard T.H. Chan School of Public Health and the Brigham and Women’s Hospital (IRB approval number: 2001P001128). Voluntarily returning the self-administered questionnaire was considered informed consent in both cohorts.
Data availability statement
Study data can be made available upon request to the corresponding author under usual cohort procedures (https://nurseshealthstudy.org/researchers). Cohort consent specifically precludes deposition of this data into public repositories as NHS II and GUTS participants agreed to participate only if data were made available to cohort investigators and external researchers after vetting.
References
- 1.Fryar CD CM, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. NCHS Health E-Stats 2020.
- 2. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics 2018;141:e20173459. 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sommer A, Twig G. The impact of childhood and adolescent obesity on cardiovascular risk in adulthood: a systematic review. Curr Diab Rep 2018;18:91. 10.1007/s11892-018-1062-9 [DOI] [PubMed] [Google Scholar]
- 4. Weihe P, Spielmann J, Kielstein H, Henning-Klusmann J, Weihrauch-Blüher S. Childhood obesity and cancer risk in adulthood. Curr Obes Rep 2020;9:204-12. 10.1007/s13679-020-00387-w [DOI] [PubMed] [Google Scholar]
- 5. Abdullah A, Wolfe R, Stoelwinder JU, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol 2011;40:985-96. 10.1093/ije/dyr018 [DOI] [PubMed] [Google Scholar]
- 6. Wang L, Martínez Steele E, Du M, et al. Trends in consumption of ultraprocessed foods among US youths aged 2-19 years, 1999-2018. JAMA 2021;326:519-30. 10.1001/jama.2021.10238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Juul F, Parekh N, Martinez-Steele E, Monteiro CA, Chang VW. Ultra-processed food consumption among US adults from 2001 to 2018. Am J Clin Nutr 2022;115:211-21. 10.1093/ajcn/nqab305 [DOI] [PubMed] [Google Scholar]
- 8. Monteiro CA, Cannon G, Moubarac J-C, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr 2018;21:5-17. 10.1017/S1368980017000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poti JM, Mendez MA, Ng SW, Popkin BM. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr 2015;101:1251-62. 10.3945/ajcn.114.100925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nardocci M, Leclerc B-S, Louzada M-L, Monteiro CA, Batal M, Moubarac JC. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health 2019;110:4-14. 10.17269/s41997-018-0130-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Askari M, Heshmati J, Shahinfar H, Tripathi N, Daneshzad E. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes (Lond) 2020;44:2080-91. 10.1038/s41366-020-00650-z [DOI] [PubMed] [Google Scholar]
- 12. Machado PP, Steele EM, Levy RB, et al. Ultra-processed food consumption and obesity in the Australian adult population. Nutr Diabetes 2020;10:39. 10.1038/s41387-020-00141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa CDS, Assunção MCF, Loret de Mola C, et al. Role of ultra-processed food in fat mass index between 6 and 11 years of age: a cohort study. Int J Epidemiol 2021;50:256-65. 10.1093/ije/dyaa141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costa CS, Del-Ponte B, Assunção MCF, Santos IS. Consumption of ultra-processed foods and body fat during childhood and adolescence: a systematic review. Public Health Nutr 2018;21:148-59. 10.1017/S1368980017001331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 1997;27:325-51. 10.1023/A:1025635913927 [DOI] [PubMed] [Google Scholar]
- 16. Dhana K, Haines J, Liu G, et al. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: results from two prospective cohort studies of mother-child pairs in the United States. BMJ 2018;362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strohmaier S, Bogl LH, Eliassen AH, et al. Maternal healthful dietary patterns during peripregnancy and long-term overweight risk in their offspring. Eur J Epidemiol 2020;35:283-93. 10.1007/s10654-020-00621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen L-W, Aubert AM, Shivappa N, et al. Maternal dietary quality, inflammatory potential and childhood adiposity: an individual participant data pooled analysis of seven European cohorts in the ALPHABET consortium. BMC Med 2021;19:33. 10.1186/s12916-021-01908-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078-83. 10.1001/jama.1997.03550130052036 [DOI] [PubMed] [Google Scholar]
- 20. Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570-84. 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monteiro CA, Cannon G, Lawrence M, et al. Ultra-processed foods, diet quality, and health using the NOVA classification system. FAO, 2019. [Google Scholar]
- 22. Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr 2019;22:936-41. 10.1017/S1368980018003762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khandpur N, Rossato S, Drouin-Chartier J-P, et al. Categorising ultra-processed foods in large-scale cohort studies: evidence from the Nurses’ Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. J Nutr Sci 2021;10:e77. 10.1017/jns.2021.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lo C-H, Khandpur N, Rossato SL, et al. Ultra-processed foods and risk of crohn’s disease and ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol 2022;20:e1323-37. 10.1016/j.cgh.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I, et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. bmj 2019;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong G-C, Gu H-T, Peng Y, et al. Association of ultra-processed food consumption with cardiovascular mortality in the US population: long-term results from a large prospective multicenter study. Int J Behav Nutr Phys Act 2021;18:21. 10.1186/s12966-021-01081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Field AE, Aneja P, Rosner B. The validity of self-reported weight change among adolescents and young adults. Obesity (Silver Spring) 2007;15:2357-64. 10.1038/oby.2007.279 [DOI] [PubMed] [Google Scholar]
- 28. Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics 2000;106:52-8. 10.1542/peds.106.1.52 [DOI] [PubMed] [Google Scholar]
- 29. Himes JH, Faricy A. Validity and reliability of self-reported stature and weight of US adolescents. Am J Hum Biol 2001;13:255-60. [DOI] [PubMed] [Google Scholar]
- 30. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240-3. 10.1136/bmj.320.7244.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology 1999;10:774-7. 10.1097/00001648-199911000-00022 [DOI] [PubMed] [Google Scholar]
- 32. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009-18. 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991-9. 10.1093/ije/23.5.991 [DOI] [PubMed] [Google Scholar]
- 34. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199-200. 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 35. Barclay K, Myrskylä M. Maternal age and offspring health and health behaviours in late adolescence in Sweden. SSM Popul Health 2016;2:68-76. 10.1016/j.ssmph.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Lim H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psychiatry 2012;24:176-88. 10.3109/09540261.2012.688195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brion M-JA, Ness AR, Rogers I, et al. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: exploring parental comparisons and prenatal effects. Am J Clin Nutr 2010;91:748-56. 10.3945/ajcn.2009.28623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhana K, Zong G, Yuan C, et al. Lifestyle of women before pregnancy and the risk of offspring obesity during childhood through early adulthood. Int J Obes (Lond) 2018;42:1275-84. 10.1038/s41366-018-0052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab 2010;95:5365-9. 10.1210/jc.2010-0697 [DOI] [PubMed] [Google Scholar]
- 40. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466-73. 10.1097/00001648-199011000-00009 [DOI] [PubMed] [Google Scholar]
- 41. Strauss RS. Comparison of measured and self-reported weight and height in a cross-sectional sample of young adolescents. Int J Obes Relat Metab Disord 1999;23:904-8. 10.1038/sj.ijo.0800971 [DOI] [PubMed] [Google Scholar]
- 42. Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s dietary patterns change little from before to during pregnancy. J Nutr 2009;139:1956-63. 10.3945/jn.109.109579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Oliveira PG, de Sousa JM, Assunção DGF, et al. Impacts of consumption of ultra-processed foods on the maternal-child health: a systematic review. Front Nutr 2022;9:821657. 10.3389/fnut.2022.821657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silva CFM, Saunders C, Peres W, et al. Effect of ultra-processed foods consumption on glycemic control and gestational weight gain in pregnant with pregestational diabetes mellitus using carbohydrate counting. PeerJ 2021;9:e10514. 10.7717/peerj.10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang K, Khandpur N, Neri D, et al. Association between childhood consumption of ultraprocessed food and adiposity trajectories in the Avon longitudinal study of parents and children birth cohort. JAMA Pediatr 2021;175:e211573. 10.1001/jamapediatrics.2021.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rohatgi KW, Tinius RA, Cade WT, Steele EM, Cahill AG, Parra DC. Relationships between consumption of ultra-processed foods, gestational weight gain and neonatal outcomes in a sample of US pregnant women. PeerJ 2017;5:e4091. 10.7717/peerj.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stang J, Loth KA. Parenting style and child feeding practices: potential mitigating factors in the etiology of childhood obesity. J Am Diet Assoc 2011;111:1301-5. 10.1016/j.jada.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 48. Acharya K, Feese M, Franklin F, Kabagambe EK. Body mass index and dietary intake among Head Start children and caregivers. J Am Diet Assoc 2011;111:1314-21. 10.1016/j.jada.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boutelle KN, Rhee KE, Liang J, et al. Effect of attendance of the child on body weight, energy intake, and physical activity in childhood obesity treatment: a randomized clinical trial. JAMA Pediatr 2017;171:622-8. 10.1001/jamapediatrics.2017.0651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ewald H, Kirby J, Rees K, Robertson W. Parent-only interventions in the treatment of childhood obesity: a systematic review of randomized controlled trials. J Public Health (Oxf) 2014;36:476-89. 10.1093/pubmed/fdt108 [DOI] [PubMed] [Google Scholar]
- 51. Begum G, Davies A, Stevens A, et al. Maternal undernutrition programs tissue-specific epigenetic changes in the glucocorticoid receptor in adult offspring. Endocrinology 2013;154:4560-9. 10.1210/en.2013-1693 [DOI] [PubMed] [Google Scholar]
- 52. Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 2008;105:17046-9. 10.1073/pnas.0806560105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gonzalez-Nahm S, Mendez M, Robinson W, et al. Low maternal adherence to a Mediterranean diet is associated with increase in methylation at the MEG3-IG differentially methylated region in female infants. Environ Epigenet 2017;3:dvx007. 10.1093/eep/dvx007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Monteleone I, Marafini I, Dinallo V, et al. Sodium chloride–enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J Crohns Colitis 2017;11:237-45. 10.1093/ecco-jcc/jjw139 [DOI] [PubMed] [Google Scholar]
- 55. Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017;66:1414-27. 10.1136/gutjnl-2016-313099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shang Q, Sun W, Shan X, et al. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol Lett 2017;279:87-95. 10.1016/j.toxlet.2017.07.904 [DOI] [PubMed] [Google Scholar]
- 57. Viennois E, Merlin D, Gewirtz AT, Chassaing B. Dietary emulsifier–induced low-grade inflammation promotes colon carcinogenesis. Cancer Res 2017;77:27-40. 10.1158/0008-5472.CAN-16-1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. DiNicolantonio JJ, Mehta V, Onkaramurthy N, O’Keefe JH. Fructose-induced inflammation and increased cortisol: A new mechanism for how sugar induces visceral adiposity. Prog Cardiovasc Dis 2018;61:3-9. 10.1016/j.pcad.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 59. Laudisi F, Di Fusco D, Dinallo V, et al. The food additive maltodextrin promotes endoplasmic reticulum stress–driven mucus depletion and exacerbates intestinal inflammation. Cell Mol Gastroenterol Hepatol 2019;7:457-73. 10.1016/j.jcmgh.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parisi F, Milazzo R, Savasi VM, Cetin I. Maternal low-grade chronic inflammation and intrauterine programming of health and disease. Int J Mol Sci 2021;22:1732. 10.3390/ijms22041732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dudele A, Hougaard KS, Kjølby M, et al. Chronic maternal inflammation or high-fat-feeding programs offspring obesity in a sex-dependent manner. Int J Obes (Lond) 2017;41:1420-6. 10.1038/ijo.2017.136 [DOI] [PubMed] [Google Scholar]
- 62. Gaillard R, Rifas-Shiman SL, Perng W, Oken E, Gillman MW. Maternal inflammation during pregnancy and childhood adiposity. Obesity (Silver Spring) 2016;24:1320-7. 10.1002/oby.21484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seguin R, Connor L, Nelson M, et al. Understanding barriers and facilitators to healthy eating and active living in rural communities. J Nutr Metab 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Skorinko JL, Incollingo Rodriguez AC, Doyle JK. Overlapping stigmas of pregnancy, motherhood, and weight: Policy implications for employment and higher education. Policy Insights Behav Brain Sci 2020;7:123-31 10.1177/2372732220943233. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplemental online content
Data Availability Statement
Study data can be made available upon request to the corresponding author under usual cohort procedures (https://nurseshealthstudy.org/researchers). Cohort consent specifically precludes deposition of this data into public repositories as NHS II and GUTS participants agreed to participate only if data were made available to cohort investigators and external researchers after vetting.