Abstract
Background
Post-COVID-19 condition is frequently comprised of persistent cognitive sequela, including deficits in attention and executive functions (EFs), which can act as a barrier for regaining pre-illness functional levels. Goal Management Training (GMT) is a cognitive rehabilitation (CR) intervention for improving attention and EFs that has received empirical support in studies of other patient groups. The present study aims to determine the efficacy of GMT for improving everyday attention and EFs in adults who experience persistent cognitive deficits after COVID-19.
Methods
This study protocol describes an open-label randomized controlled trial comparing the efficacy of GMT to a wait list control condition (WL), for improving persistent (> 2 months) cognitive sequela in post-COVID-19 condition. The study aims to recruit 240 participants aged 18 to 65 years with a history of SARS-CoV-2 infection and perceived attentional and EF difficulties in daily life. Participants will be block randomized (computer-algorithm) to either group-based GMT (n = 120) or WL (n = 120). GMT will be internet-delivered to groups of six participants in six two-hour sessions delivered once a week. The primary outcome will be the Metacognition Index of the Behavior Rating Inventory of Executive Function – Adult Version, a self-report measure assessing everyday EF difficulties, specifically metacognition, at six months post-treatment. Secondary outcomes include performance-based neurocognitive measures, and tertiary outcomes include rating scales of cognition, emotional health, quality of life, and fatigue.
Conclusion
Study findings could contribute to providing an evidence-based treatment option for symptoms that are frequent and debilitating following a prevalent condition.
Trial registration number
Keywords: COVID-19, Cognition, Cognitive rehabilitation, Executive functions, Randomized controlled trial
1. Introduction
According to the WHO clinical case definition, post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually three months from the onset of COVID-19, with symptoms (e.g., fatigue, shortness of breath, and cognitive dysfunction) that last for at least two months and cannot be explained by an alternative diagnosis [1]. Cognitive deficits are among the most frequent symptom of post-COVID-19 condition [2], identified in more than one fifth of individuals with confirmed diagnosis [3]. These deficits include perceived difficulties with attention, memory, and executive functions (EFs), that may compromise everyday functioning, quality of life, and mental health [1,3,4]. Consistent with self-reported cognitive symptoms, a heterogenous pattern of mild deficits has been identified using performance-based neurocognitive measures, with the most pronounced impairment in processing speed, attention, and EFs [[5], [6], [7], [8]]. Such deficits can occur even after mild illness and in individuals who were otherwise asymptomatic, and persist over the course of several months [5,[9], [10], [11]].
Due to the recent emergence of the disease, long-term trajectories of cognitive deficits lack detailing, and knowledge on outcomes beyond 12 months is currently limited. Initial findings suggest that even if many patients experience improvement of cognitive functioning the first 12 months following disease [11], a substantial proportion remain impaired one year following COVID-19, and relatedly that time since illness exerts a relativity small influence on the magnitude of the cognitive deficits [3,7,12,13].
While the etiology of cognitive dysfunction in post-COVID-19 condition is unknown, numerous factors including direct viral damage, hypoxia, microvascular injury, persistent immune hyperactivation, as well as neuropsychiatric comorbidities, have been proposed [5,14]. As noted above, the association with illness severity during the acute stage is not clear. While a selection of studies have found cognitive deficits to increase with more a severe disease course [6,12], others have noted that these are only weakly associated with many proxies of illness severity [3,4,15,16]. The available evidence concerning the direct impact from COVID-19 on long-term mental health is mixed [17], but a selection of studies have identified increased risk of neuropsychiatric sequela following disease [18,19]. In this context, it should still be noted that there is an overall increased prevalence of conditions with known detrimental effect on cognition, such as depression, anxiety, and sleep disorders, in the general population following the COVID-19 pandemic [19,20]. Concerning neurocognitive outcomes specifically, prior research suggest that persistent cognitive difficulties following COVID-19 are associated with elevated levels of psychological distress [21] and concurrently that cognitive deficits are more prevalent in those with pre-existing mental disorders [8].
Given the above, there is a urgent need to develop systematic approaches for the management of persistent cognitive- and neuropsychiatric sequela following COVID-19 [3,8,[22], [23], [24]]. Interventions to improve everyday cognitive functioning, such as cognitive rehabilitation (CR), could prove useful in this context. Importantly, such interventions have additionally displayed durable secondary effects on emotional health in other patient populations [25]. In a recent proof-of-concept study, computerized cognitive remediation therapy displayed pro-cognitive effects, while concurrently improving quality of life, in patients with persistent cognitive deficits following COVID-19 [26]. However, there are currently no reports on the efficacy of CR interventions in post-COVID-19 condition.
Goal Management Training (GMT) is a CR intervention that relies on metacognitive strategies to reengage executive attention processes, in addition to teaching problem-solving techniques, specifically aiming to improve everyday attention and EF abilities [27]. As such, GMT targets some of the cognitive domains identified to be most impaired in post-COVID-19 condition [5,6,11,28]. Being among the most well tested CR interventions, GMT has received empirical support in samples with neurological- and neuropsychiatric disorders, including reports of improvement on performance-based neurocognitive measures and laboratory analogs of real-life tasks, as well as improved self-reported cognitive functioning and emotional health [[29], [30], [31], [32]].
The main objective of the described randomized controlled trial (RCT) is to examine the efficacy of GMT as an internet-delivered group-based CR intervention for adults with post-COVID-19 condition experiencing persistent cognitive deficits, when compared to a wait list control condition (WL). Furthermore, we are interested in the potential transfer effects of GMT to aspects of emotional health, quality of life, and fatigue. The study hypotheses are: 1) GMT will result in greater improvement in self-reported daily-life EF, compared to WL (primary hypothesis), 2) GMT will result in greater improvement on performance-based neurocognitive measures of EF and attention, compared to WL (secondary hypothesis), and 3) GMT will result in greater improvement on rating scales of emotional health, quality of life, and fatigue, compared to WL (tertiary hypothesis).
2. Materials and methods
2.1. Study design
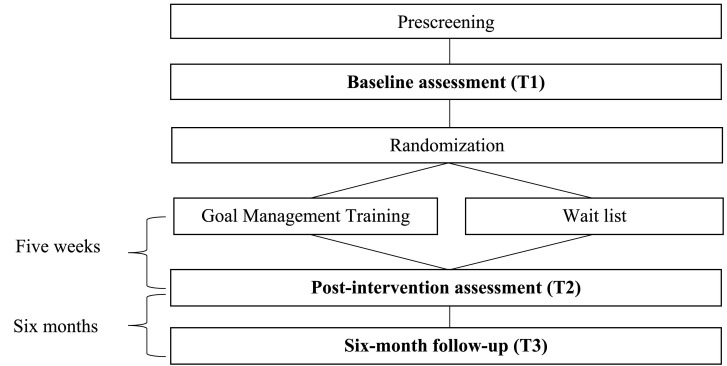
The proposed study is a parallel RCT, comparing GMT to WL, using a repeated-measures design across three time-points, including the baseline (T1), post-intervention (T2), and a six-month follow-up (T3). All participants will be assessed for eligibility and complete the baseline assessment (T1), prior to randomization. The individual follow-up assessments (T2 and T3) must be completed within a timeframe of four weeks following treatment completion, or the six-month post-intervention mark, to be included in the final analyses. Aiming to give weight to any durability of treatment effects, efficacy is evaluated based on outcomes at the six-month follow-up (See Fig. 1 ).
Fig. 1.
Study flowchart.
2.2. Study setting
The trial is single-center and conducted at Lovisenberg Diaconal Hospital, Oslo, Norway. However, all assessments, and the intervention, will be internet-delivered or completed off-site using secure digital platforms.
2.3. Intervention
GMT is a standardized and manual-based CR intervention, consisting of PowerPoint slides and participant workbooks available in Norwegian translation [32], teaching compensatory strategies for wide appliance [27]. The present version of GMT includes six group sessions (Table 1 ). The strategies taught are aimed at promoting goal-directed behavior in daily life through improving EF control, with a specific focus on periodically stopping ongoing behavior (“stop-and-think”), monitoring performance, and applying a step-by-step approach to problem-solving [27].
Table 1.
Description of the GMT sessions.
| Session | Description and objectives |
|---|---|
|
Introduction to goal hierarchies. Monitoring absentmindedness/Practice presentmindedness |
|
Identifying condition for, and consequences of, absentmindedness |
|
How automatic behavior may lead to errors |
|
Make a habit of stopping ongoing behavior, and bring attention to the present |
|
Check the content of working memory |
|
State goals to facilitate goal maintenance |
In the present study, GMT will be internet-delivered to groups of six participants as weekly two-hour sessions, spanning five weeks, and led by a clinical psychologist trained in GMT. To match the expected clinical needs of patients with post-COVID-19 condition, the length of the original GMT protocol (nine sessions) developed for patients with acquired brain injury is reduced [33], and minor adaptations are done to make the content more relevant to the target population. Homework assignments will include practicing strategies in daily life, logging of activities, and exercises in mindfulness. To be considered a completer of GMT treatment, participants need to attend a minimum of four sessions. Participants not attending the weekly group-session will be contacted by study staff, and offered a short individual summary of the content, prior to the subsequent session.
2.4. Sampling and eligibility criteria
The study aims to include 240 participants, primarily recruiting from the Norwegian Corona Cohort (clinical.trials.gov identifier: NTC04320732) [34]. The recruitment period is estimated to run from January 2023 to June 2023.
2.4.1. Inclusion criteria
Inclusion criteria are a history of laboratory- or home-test confirmed, SARS-CoV-2 infection (> 3 months since infection), age between 18 and 65 years, and perceived cognitive difficulties (attention, memory, EF) affecting everyday functioning that have lasted for at least two months and that cannot be explained by an alternative diagnosis [1]. The presence of cognitive difficulties will be determined by yes/no screening questions: 1. Are you having any difficulties with concentration, memory, or decision-making that have lasted for more than two months after COVID-19? 2. Do these cognitive difficulties affect your daily life activities?
2.4.2. Exclusion criteria
Exclusion criteria are ongoing alcohol- or substance abuse, premorbid insult and/or comorbid neurological disease (e.g., acquired brain injury, epilepsy, dementias, or multiple sclerosis), severe neurocognitive problems interfering with the capacity to participate (defined as scoring <10 points on the shortened version of the Montreal Cognitive Assessment), sensory disorders biasing cognitive assessment, schizophrenia spectrum disorders or bipolar disorder with mood congruent psychotic features, lack of proficiency in Norwegian, and being previously enrolled in a GMT trial. All eligibility evaluations are intended completed by the same rater, a clinical psychologist, in conference with a senior researcher.
2.5. Recruitment procedure
Those enrolled in the Norwegian Corona Cohort will be contacted and informed about the possibility to participate in the present study, receiving written information. Prior to enrollment, all participants will give their electronically signed written informed consent (identification through social security number and BankID, used by all public services in Norway). Subsequently, eligibility will be evaluated through a semi-structured telephone screening interview administered by a health care professional, lasting approximately 15 min. Eligible individuals will proceed to a baseline assessment (T1), completed in two parts. First, participants will complete an off-site web-based battery of performance-based neurocognitive tests and rating scales, before subsequently attending a neuropsychological assessment conducted under videoconferencing with a clinical psychologist.
2.6. Randomization
Following completion of the baseline assessment, participants will be randomized to either GMT or WL in a 1:1 ratio using unstratified block randomization with a block-size of six. The allocation sequence will be computer generated by a person not otherwise involved in the study and stored inaccessible the to study staff. To promote retention and interest in the study, a brief individual report from the assessments will be provided upon request after study completion, but participants will not be otherwise compensated for their participation.
2.7. Data collection, baseline variables, and outcome measures
The data will be stored using the Service for Sensitive Data facilities, owned by the University of Oslo, and all web-based assessments use a secure digital platform. Collection of data is estimated to begin January 2023 and completed by June 2024. Study staff involved in the assessment of outcomes will be blind to treatment allocation. If complete adherence to the protocol is not possible, any effort to collect as much data as feasible will be made.
2.7.1. Baseline variables
Participant characteristics and sociodemographic data will be collected through the telephone screening interview, or at the baseline assessment. Clinical characteristics, and information associated with COVID-19, will be collected at the baseline assessment, including various disease severity parameters (e.g., symptoms, symptom duration, hospitalization, duration in intensive care unit (ICU)), vaccination status, which virus variant the participant was infected with (if available, if not using date of infection as proxy). Information on any concurrent treatment that participants receive which is likely to influence any symptoms associated with post-COVID-19 condition (e.g., prescription medicines) will be collected throughout the study.
The Mini International Neuropsychiatric Interview [35] is used to assess a selection of current- or previous Diagnostic and Statistical Manual, Fourth edition, mental disorders. Rating scales applied only at baseline includes the Resilience Scale for Adults [36], Insomnia Severity Index [37], Brief-COPE [38], and Duke-UNC Functional Social Support Questionnaire [39].
Some performance-based neurocognitive measures are included at baseline only. A shortened version of the Montreal Cognitive Assessment [miniMoCA; [40]] is used to assess several cognitive domains. Attention span/working memory, processing speed, and long-term memory is assessed using the Digit Span subtest from the Wechsler Adult Intelligence Scale – Fourth edition [41], the Symbol Digit Modalities Test [42], and the California Verbal Learning Test – Second edition – Short form [43], respectively. The Vocabulary subtest from the Wechsler Abbreviated Scale of Intelligence [44] is used to measure verbal reasoning, and the matrix reasoning item bank [45] is used to measure non-verbal reasoning.
2.7.2. Primary outcome measure
The Metacognition Index (MI) from the Behavior Rating Inventory of Executive Function – Adult Version [BRIEF-A; 46] will be applied as the primary outcome measure of the present study. The BRIEF-A is a self-report questionnaire consisting of 75 items rated on a three-point scale assessing everyday difficulties with EF. The MI comprises 40 items and includes the subscales Initiate, Working Memory, Plan/Organize, Task Monitor, and Organization of Materials. As such, the MI assess everyday problems with activities involving metacognition, more specifically the ability to initiate activity and generate problem-solving ideas, to sustain working memory, to plan and organize problem-solving approaches, to monitor success and failure in problem-solving, and to organize one's materials and environment. The MI has excellent psychometric properties, with a Cronbach's alpha of 0.94 and a one-month test–retest reliability of 0.93 in the normative sample [46].
2.7.3. Secondary outcome measures
Secondary outcomes include a selection of tests from the Cambridge Neuropsychological Test Automated Battery [CANTAB; [47]], a performance-based neurocognitive test battery, used to assess attention and EFs. To assess the three main dimensions of EFs according to Miyake et al. [48], namely inhibition, working memory, and shifting, the Stop Signal Task (inhibition), Spatial Working Memory (working memory), and Intra-Extra Dimensional Set Shift (shifting) is used. In addition, Rapid Visual Information Processing from CANTAB is used as a measure of sustained attention.
2.7.4. Tertiary outcome measures
The following self-report measures are included as tertiary outcomes. The Hospital Anxiety and Depression Scale [49] is used to measure symptoms of anxiety and depression. Self-efficacy will be assessed using the Generalized Self-Efficacy Scale [50], while fatigue is measured by the Fatigue Severity Scale [51]. The Perceived Deficits Questionnaire [52], Everyday Memory Questionnaire [53], and BRIEF-A Behavior Regulation Index, are applied as measures of daily-life cognitive difficulties. Quality of life will be measures using the RAND 36-Item Health Survey [54] and EQ-5D [55] (See Table 2 ).
Table 2.
List of measures employed at each time point.
| Measure |
Mode |
Time point |
|||
|---|---|---|---|---|---|
| Prescreening | T1 | T2 | T3 | ||
| Informed consent | Digital | X | |||
| Eligibility screening | Telephone | X | |||
| Sociodemographic data | Telephone | X | |||
| Clinical characteristics | Videoconference/Digital | X | |||
| MiniMoCA | Videoconference | X | |||
| WAIS-IV: Digit span | Videoconference | X | |||
| SDMT | Videoconference | X | |||
| CVLT-II Short form | Videoconference | X | |||
| WASI: Vocabulary | Videoconference | X | |||
| MaRs-IB | Videoconference | X | |||
| MINI | Videoconference | X | |||
| RSA | Digital | X | |||
| ISI | Digital | X | |||
| Brief-COPE | Digital | X | |||
| FSSQ | Digital | X | |||
| BRIEF-A | Digital | X | X | X | |
| CANTAB – SST | Digital | X | X | X | |
| CANTAB - SWM | Digital | X | X | X | |
| CANTAB – IED | Digital | X | X | ||
| CANTAB - RVP | Digital | X | X | X | |
| HADS | Digital | X | X | X | |
| PDQ | Digital | X | X | X | |
| EMQ | Digital | X | X | X | |
| GSE | Digital | X | X | X | |
| FSS | Digital | X | X | X | |
| EQ-5D | Digital | X | X | ||
| RAND-36 | Digital | X | X | ||
Note. BRIEF-A = Behavior Rating Inventory of Executive Function – Adult Version; CANTAB = Cambridge Neuropsychological Test Automated Battery; CVLT-II = California Verbal Learning Test – Second edition; EQ-5D = EuroQol – 5 Dimension; EMQ = Everyday Memory Questionnaire; FSS = Fatigue Severity Scale; FSSQ = Duke-UNC Functional Social Support Questionnaire; GSE = Generalized Self-Efficacy Scale; HADS = The Hospital Anxiety and Depression Scale; IED = Intra-Extra Dimensional Set Shift; ISI = Insomnia Severity Index; MaRs-IB = The Matrix Reasoning Item Bank; MINI = The Mini International Neuropsychiatric Interview; MiniMoCA = Shortened Montreal Cognitive Assessment; PDQ = Perceived Deficits Questionnaire; RAND-36 = RAND 36-Item Health Survey; RSA = Resilience Scale for Adults; RVP = Rapid Visual Information Processing; SDMT = Symbol Digit Modalities Test; SST = Stop Signal Task; SWM = Spatial Working Memory; WAIS-IV = Wechsler Adult Intelligence Scale – Fourth edition; WASI = Wechsler Abbreviated Intelligence Scale.
2.8. Statistical analyses
Results from all primary-, secondary-, and tertiary outcomes will be presented separately by treatment allocation using an intention-to-treat (ITT) approach. First, baseline differences between groups will be investigated using chi-square tests or t-tests (or its non-parametrical equivalent), depending on the nature of the variables. Secondly, mixed model analyses will be carried out to assess both within- and between differences regarding the primary outcome measure, with BRIEF-A MI as dependent variable, and Group (treatment allocation) and Time (T1, T2, T3) as factors. Group, Time, and Group-Time interactions will be included as fixed group differences, and the restricted maximum likelihood method (REML) selected for estimation. Analysis of the primary outcome will be done with raw scores, due to lack of Norwegian norms for the BRIEF-A. For more details, please see the statistical analysis plan (Supplementary data)
2.9. Sample size justification and power calculations
The lack of previous studies on GMT in post-COVID-19 condition represents a challenge in estimating the required sample size for documenting moderate effects on our primary outcome measure. However, due to the somewhat comparable EF profile between post-COVID-19 condition and depression [56], we anticipated a change of seven points in BRIEF-A MI raw score in the intervention group and a change of at most two points in the control group, based on our previous research in a depression sample [31]. We further assumed a common standard deviation of nine points. To adjust for multiple testing, we lowered the predefined significant level to 1%, and used a power of 90% (β = 0.1). Based on the above, we would need 99 individuals in each group, but to allow for a dropout rate of about 20%, we aim to include 120 participants in each group, totaling 240.
2.10. Ethical considerations and quality control
Study initiation awaits approval from the Regional Committees for Medical and Health Research Ethics, South-Eastern Norway. The trial is registered at clinical.trials.gov (identifier: NCT05494424), and conducted and reported according to CONSORT guidelines [57]. Participants will be informed that there are no restraints concerning receiving other treatments during the study. Any substantial deviation from the study protocol will be described in publications reporting trial outcomes.
To improve quality control, all personnel involved in data collection and delivery of treatment will be given training by a senior researcher with extensive knowledge in neuropsychology and GMT. Furthermore, participants' compliance with the intervention will be assessed through the number of sessions attended, and by monitoring participants completion of the between-session assignments. Participants satisfaction with treatment and partaking in the study will be assessed and evaluated following study completion using a brief custom-made questionnaire (Likert scale).
3. Discussion
The current RCT will provide important data on the efficacy of CR for adults who experience persistent cognitive deficits after COVID-19, and findings may contribute to the development of an evidence-based treatment for this group of patients. Post-COVID-19 condition is prevalent, also among those in working age, and many experience persistent cognitive deficits with debilitating effect on functioning in the absence of treatment [3]. This suggests that it is critical that effective CR options are made available to these patients. GMT could offer an adequate approach in this context, targeting the everyday cognitive difficulties that are typically associated with the condition [4,23]. Furthermore, GMT might prove useful for managing persistent or new mood symptoms associated with post-COVID-19 condition [8,19,31]. Preliminary evidence further suggests that EF deficits in particular impair quality of life in post-COVID-19 condition [21], and also that targeting cognitive processes, including EFs, hold the potential to improve quality of life in this group of patients [26]. The probable multifactorial underlying mechanisms for the cognitive deficits in post-COVID-19 condition could be taken as an argument for applying a strategy approach, previously displaying positive effects on EF across various etiologies [25]. The expected outcome of the trial is that GMT will be more effective in improving EF when compared with WL, also concerning long-term effects. Ultimately, intervention effects following GMT may contribute to successful participation in work and social life. Should internet-delivered group-based GMT prove to display durable effects, it would represent a viable non-pharmacological treatment option that is easily disseminated.
Participants are included in the present study based solely on perceived cognitive difficulties, using two yes/no questions. In our opinion this represents a valid approach, as post-COVID-19 condition is currently predominantly defined by subjective difficulties, with no additional specific clinical characteristics, nor a distinct neurocognitive profile or testing criteria cut-off. Still, the existing literature concerning the correspondence between self-reported cognitive difficulties and neurocognitive performance in COVID-19 is mixed [4,23,58,59]. In one study by García-Sánchez and colleagues [23] in patients with subjective cognitive complaints following COVID-19 (n = 63), all displayed objective cognitive deficits in at least one cognitive domain. Indeed, in a recent meta-analysis, the prevalence rate of cognitive deficits was higher in studies including performance-based neurocognitive measures [3], indicating that the present study could fail to identify some of those who are not aware of their cognitive deficits. Importantly, previous research in other patient populations have shown that even those not performing below a specific normative cut-off on performance-based neurocognitive measures might improve their everyday cognitive functioning following interventions targeting cognition [60]. As numerous conditions are associated with perceived cognitive difficulties, some of which have increased in prevalence following the COVID-19 pandemic, the use of such liberal inclusion criteria is likely to result in a heterogenous sample. At the same time, this approach might increase the representability of patients with post-COVID-19 condition that are likely to seek pro-cognitive treatment. In line with the above, there are no inclusion criteria related to illness severity or other clinical characteristics, besides the duration of cognitive deficits (> 2 months), as these seem to inconsistently relate to cognitive functioning in the long-term [3,11].
The primary outcome of the study is a self-report measure of cognitive functioning in daily life. This is done primarily to give priority to functional outcomes, as self-reported cognitive functioning is considered to measure typical everyday performance [61], but also because the main aim of GMT is to improve everyday cognitive functioning. On the other hand, a performance-based neurocognitive measure of cognition is not applied as primary outcome, based on challenges with ecological validity associated with such measures [62]. However, applying a subjective measure as the primary outcome makes the study more susceptible to biases and confounding sources associated with self-report (e.g., demand characteristics, extreme responding, social desirability bias), and opposes recommendations in other patient populations for trials aiming to improve cognitive functioning [63].
The strength of the proposed study includes a rigorous RCT design, large sample size, multimodal cognitive assessment, and the use of a well-validated and theory-driven CR protocol. The most important limitation is the lack of an active control condition for comparison. Furthermore, the sampling method inflates the risk of bias, as the group of patients suspecting that COVID-19 has negatively influenced their cognitive functioning, might also display increased symptom awareness. We will not be able to control the effects of other treatments that participants receive during the study, including treatments with expected pro-cognitive effects. The extensive time invested by the participants could become an obstacle for participation, and additionally hinder achievement of the proposed sample size. Publishing a study protocol increases the transparency of the study, but at the cost of making hypotheses available to the participants.
The use of a WL control condition requires ethical consideration, as such designs has previously been associated with symptom worsening. Therefore, participants in WL will be offered the active treatment (GMT) following their six-month follow-up. Even if implementing an internet-delivered intervention could increase availability of treatment, extending the geographic reach, the requirement of digital competence might also act as a barrier to certain groups. More generally, the rapidly changing situation related to COVID-19 represents a challenge for study planning. For example, at the time of writing we are not aware of reports concerning the impact of vaccination on the prevalence and magnitude of cognitive sequela, or the cognitive outcome associated with different viral variants.
4. Conclusion
Results from this RCT will provide critical information on the efficacy of CR for adults who experience persistent cognitive deficits after COVID-19. If shown to be effective, integrating a group-based internet-delivered CR intervention in the public health services may be a cost-effective way to provide an important treatment option to this population. Given the relatively high rate of cognitive deficits after COVID-19, the public health relevance of this study is considerable. Further research utilizing a clinical trial with more biological and functional measures is warranted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by the South-Eastern Norway Regional Health Authority. The funding sources were not otherwise involved in the research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2022.106955.
Appendix A. Supplementary data
Statistical Analysis Plan
Data availability
No data was used for the research described in the article.
References
- 1.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V., Group WHOCCDW A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Premraj L., Kannapadi N.V., Briggs J., Seal S.M., Battaglini D., Fanning J., et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceban F., Ling S., Lui L.M.W., Lee Y., Gill H., Teopiz K.M., et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bungenberg J., Humkamp K., Hohenfeld C., Rust M.I., Ermis U., Dreher M., et al. Long COVID-19: objectifying most self-reported neurological symptoms. Ann. Clin. Transl. Neurol. 2022 doi: 10.1002/acn3.51496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker J.H., Lin J.J., Doernberg M., Stone K., Navis A., Festa J.R., et al. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw. Open. 2021;4:e2130645. doi: 10.1001/jamanetworkopen.2021.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henneghan A.M., Lewis K.A., Gill E., Kesler S.R. Cognitive impairment in non-critical, mild-to-moderate COVID-19 survivors. Front. Psychol. 2022;365 doi: 10.3389/fpsyg.2022.770459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan K., Miller A.K., Reiter K., Bonner-Jackson A. Neurocognitive profiles in patients with persisting cognitive symptoms associated with COVID-19. Arch. Clin. Neuropsychol. 2022 doi: 10.1093/arclin/acac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apple A.C., Oddi A., Peluso M.J., Asken B.M., Henrich T.J., Kelly J.D., et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann. Clin. Transl. Neurol. 2022 doi: 10.1002/acn3.51498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amalakanti S., Arepalli K.V.R., Jillella J.P. Cognitive assessment in asymptomatic COVID-19 subjects. Virusdisease. 2021;32:146–149. doi: 10.1007/s13337-021-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci R., Dini M., Rosci C., Capozza A., Groppo E., Reitano M.R., et al. One-year cognitive follow-up of COVID-19 hospitalized patients. Eur. J. Neurol. 2022 doi: 10.1111/ene.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampshire A., Chatfield D.A., Jolly A., Trender W., Hellyer P.J., Del Giovane M., et al. Multivariate profile and acute-phase correlates of cognitive deficits in a COVID-19 hospitalised cohort. EClinicalMedicine. 2022;47 doi: 10.1016/j.eclinm.2022.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fjelltveit E.B., Blomberg B., Kuwelker K., Zhou F., Onyango T.B., Brokstad K.A., et al. Symptom burden and immune dynamics 6 to 18 months following mild SARS-CoV-2 infection -a case-control study. Clin. Infect. Dis. 2022 doi: 10.1093/cid/ciac655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanderlind W.M., Rabinovitz B.B., Miao I.Y., Oberlin L.E., Bueno-Castellano C., Fridman C., et al. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Curr. Opin. Psychiatry. 2021;34:420. doi: 10.1097/YCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46:2235–2240. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourmistrova N.W., Solomon T., Braude P., Strawbridge R., Carter B. Long-term effects of COVID-19 on mental health: a systematic review. J. Affect. Disord. 2022;299:118–125. doi: 10.1016/j.jad.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnúsdóttir I., Lovik A., Unnarsdóttir A.B., McCartney D., Ask H., Kõiv K., et al. Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: an observational study. Lancet Public Health. 2022 doi: 10.1016/S2468-2667(22)00042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santomauro D.F., Herrera A.M.M., Shadid J., Zheng P., Ashbaugh C., Pigott D.M., et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., et al. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramírez-Moreno J.M., Muñoz-Sanz A., Vaz-Leal F.J. Cognitive function and neuropsychiatric disorders after COVID-19: a long term social and clinical problem? BioMed. 2022;2:50–59. doi: 10.3390/biomed2010005. [DOI] [Google Scholar]
- 23.García-Sánchez C., Calabria M., Grunden N., Pons C., Arroyo J.A., Gómez-Anson B., et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. 2022;12 doi: 10.1002/brb3.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolin S., Chakales A., Verduzco-Gutierrez M. Rehabilitation strategies for cognitive and neuropsychiatric manifestations of COVID-19. Curr. Phys. Med. Rehabil. Rep. 2022:1–6. doi: 10.1007/s40141-022-00352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamenova V., Levine B. Effectiveness of goal management training® in improving executive functions: a meta-analysis. Neuropsychol. Rehabil. 2019:1–31. doi: 10.1080/09602011.2018.1438294. [DOI] [PubMed] [Google Scholar]
- 26.Palladini M., Bravi B., Colombo F., Caselani E., Di Pasquasio C., D’Orsi G., et al. Cognitive remediation therapy for post-acute persistent cognitive deficits in COVID-19 survivors: a proof-of-concept study. Neuropsychol. Rehabil. 2022:1–18. doi: 10.1080/09602011.2022.2075016. [DOI] [PubMed] [Google Scholar]
- 27.Levine B., Robertson I.H., Clare L., Carter G., Hong J., Wilson B.A., et al. Rehabilitation of executive functioning: an experimental–clinical validation of goal management training. J. Int. Neuropsychol. Soc. 2000;6:299–312. doi: 10.1017/s1355617700633052. [DOI] [PubMed] [Google Scholar]
- 28.Favieri F., Forte G., Agostini F., Giovannoli J., Di Pace E., Langher V., et al. The cognitive consequences of the COVID-19 pandemic on members of the general population in Italy: a preliminary study on executive inhibition. J. Clin. Med. 2021;11:170. doi: 10.3390/jcm11010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tornås S., Løvstad M., Solbakk A.-K., Schanke A.-K., Stubberud J. Goal management training combined with external cuing as a means to improve emotional regulation, psychological functioning, and quality of life in patients with acquired brain injury: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2016;97:1841–1852.e3. doi: 10.1016/j.apmr.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Jensen D.A., Halmøy A., Stubberud J., Haavik J., Lundervold A.J., Sørensen L. An exploratory investigation of goal management training in adults with ADHD: improvements in inhibition and everyday functioning. Front. Psychol. 2021;4007 doi: 10.3389/fpsyg.2021.659480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagen B.I., Lau B., Joormann J., Småstuen M.C., Landrø N.I., Stubberud J. Goal management training as a cognitive remediation intervention in depression: a randomized controlled trial. J. Affect. Disord. 2020;275:268–277. doi: 10.1016/j.jad.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Stubberud J., Langenbahn D., Levine B., Stanghelle J., Schanke A.-K. Goal management training of executive functions in patients with spina bifida: a randomized controlled trial. J. Int. Neuropsychol. Soc. 2013;19:672–685. doi: 10.1017/S1355617713000209. [DOI] [PubMed] [Google Scholar]
- 33.Boyd J.E., O’Connor C., Protopopescu A., Jetly R., Rhind S.G., Lanius R.A., et al. An open-label feasibility trial examining the effectiveness of a cognitive training program, goal management training, in individuals with posttraumatic stress disorder. Chronic Stress. 2019;3 doi: 10.1177/2470547019841599. 2470547019841599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Søraas A., Bø R., Kalleberg K.T., Støer N.C., Ellingjord-Dale M., Landrø N.I. Self-reported memory problems 8 months after COVID-19 infection. JAMA Netw. Open. 2021;4:e2118717. doi: 10.1001/jamanetworkopen.2021.18717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., et al. The Mini-international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 36.Friborg O., Hjemdal O., Rosenvinge J.H., Martinussen M. A new rating scale for adult resilience: what are the central protective resources behind healthy adjustment? Int. J. Methods Psychiatr. Res. 2003;12:65–76. doi: 10.1002/mpr.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastien C.H., Vallières A., Morin C.M. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 38.Carver C.S. You want to measure coping but your protocol’too long: consider the brief cope. Int. J. Behav. Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 39.Broadhead W.E., Gehlbach S.H., De Gruy F.V., Kaplan B.H. The Duke-UNC Functional Social Support Questionnaire: Measurement of social support in family medicine patients. Med. Care. 1988:709–723. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 41.Wechsler D. San Antonio; Texas Psychol Corp: 2014. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV) [Google Scholar]
- 42.Smith A. Western psychological services Los Angeles; 2007. Symbol digit modalities test. [Google Scholar]
- 43.Delis D.C., Kramer J.H., Kaplan E., Ober B.A. Psychological Corporation; 2000. CVLT-II: California verbal learning test: adult version. [Google Scholar]
- 44.Wechsler D. San Antonio; TX Psychol Corp: 1999. Manual for the Wechsler Abbreviated Intelligence Scale (WASI) [Google Scholar]
- 45.Chierchia G., Fuhrmann D., Knoll L.J., Pi-Sunyer B.P., Sakhardande A.L., Blakemore S.-J. The matrix reasoning item bank (MaRs-IB): novel, open-access abstract reasoning items for adolescents and adults. R. Soc. Open Sci. 2019;6 doi: 10.1098/rsos.190232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth R.M., Isquith P.K., Gioia G. Psychological Assessment Resources; Lutz, FL: 2005. Behavioral Rating Inventory of Executive Function-Adult Version. [Google Scholar]
- 47.CANTAB CC . 2016. Cognitive Assessment Software. Cambridge Cogn Cambridge, UK. [Google Scholar]
- 48.Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 49.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 50.Schwarzer R., Jerusalem M. Generalized self-efficacy scale. Meas Heal Psychol. A User’s Portfolio Causal Control Beliefs. 1995;1:35–37. [Google Scholar]
- 51.Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan M.J., Edgley K., Dehoux E. A survey of multiple sclerosis: I. Perceived cognitive problems and compensatory strategy use. Can. J. Rehabil. 1990;4:99–105. [Google Scholar]
- 53.Royle J., Lincoln N.B. The everyday memory questionnaire–revised: development of a 13-item scale. Disabil. Rehabil. 2008;30:114–121. doi: 10.1080/09638280701223876. [DOI] [PubMed] [Google Scholar]
- 54.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med. Care. 1992:473–483. [PubMed] [Google Scholar]
- 55.The EuroQol Group EuroQol-a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 56.Poletti S., Palladini M., Mazza M.G., De Lorenzo R., Irene B., Sara B., et al. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2021 doi: 10.1007/s00406-021-01346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vannorsdall T.D., Brigham E., Fawzy A., Raju S., Gorgone A., Pletnikova A., et al. Cognitive dysfunction, psychiatric distress, and functional decline after COVID-19. J. Acad. Consult. Psychiatry. 2022;63:133–143. doi: 10.1016/j.jaclp.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delgado-Alonso C., Valles-Salgado M., Delgado-Álvarez A., Yus M., Gómez-Ruiz N., Jorquera M., et al. Cognitive dysfunction associated with COVID-19: a comprehensive neuropsychological study. J. Psychiatr. Res. 2022;150:40–46. doi: 10.1016/j.jpsychires.2022.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strawbridge R., Tsapekos D., Hodsoll J., Mantingh T., Yalin N., McCrone P., et al. Cognitive remediation therapy for patients with bipolar disorder: a randomised proof-of-concept trial. Bipolar Disord. 2021;23:196–208. doi: 10.1111/bdi.12968. [DOI] [PubMed] [Google Scholar]
- 61.Toplak M.E., West R.F., Stanovich K.E. Practitioner review: do performance-based measures and ratings of executive function assess the same construct? J. Child Psychol. Psychiatry Allied Discip. 2013;54:131–143. doi: 10.1111/jcpp.12001. [DOI] [PubMed] [Google Scholar]
- 62.Chaytor N., Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: a review of the literature on everyday cognitive skills. Neuropsychol. Rev. 2003;13:181–197. doi: 10.1023/b:nerv.0000009483.91468.fb. [DOI] [PubMed] [Google Scholar]
- 63.Miskowiak K., Burdick K.E., Martinez-Aran A., Bonnin C.M., Bowie C.R., Carvalho A.F., et al. Methodological recommendations for cognition trials in bipolar disorder by the International Society for Bipolar Disorders targeting cognition task force. Bipolar Disord. 2017;19:614–626. doi: 10.1111/bdi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Analysis Plan
Data Availability Statement
No data was used for the research described in the article.