Abstract
Background:
It has been suggested that individuals predisposed to or recovered from anorexia nervosa experience a hyperserotonergic state associated with anxiety that might be mitigated by restricted food intake, because diminished levels of the tryptophan precursor lower the central availability of serotonin (5-HT). At the neural level, the salience network is a system of functionally connected brain regions; it has been closely associated with 5-HT functioning and mental disorders (including anorexia nervosa). The aim of the present study was to investigate the effect on the salience network of a temporary dietary manipulation of 5-HT synthesis in patients with anorexia nervosa.
Methods:
In this double-blind crossover study, we obtained data on resting-state functional connectivity from 22 weight-recovered female patients with a history of anorexia nervosa, and 22 age-matched female healthy controls. The study procedure included acute tryptophan depletion (a dietary intervention that lowers the central 5-HT synthesis rate) and a sham condition.
Results:
We identified an interaction of group and experimental condition in resting-state functional connectivity between the salience network and the orbitofrontal cortex extending to the frontal pole (F1,42 = 12.52; pFWE = 0.026). Further analysis revealed increased resting-state functional connectivity during acute tryptophan depletion in patients recovered from anorexia nervosa, resembling that of healthy controls during the sham condition (T42 = −0.66; p = 0.51).
Limitations:
The effect of acute tryptophan depletion on the central availability of 5-HT can be judged only indirectly using plasma ratios of tryptophan to large neutral amino acids. Moreover, the definition of anorexia nervosa recovery varies widely across studies, limiting comparability.
Conclusion:
Taken together, our findings support the notion of 5-HT dysregulation in anorexia nervosa and indicate that reduced 5-HT synthesis and availability during acute tryptophan depletion (and possibly with food restriction) may balance hyperserotonergic functioning and the associated resting-state functional connectivity of the salience network.
Introduction
Mood regulation, anxiety and the modulation of appetite are functionally associated with the brain serotonin (5-HT) system,1–3 which is hypothesized to play an important role in the etiology of anorexia nervosa.4 Anorexia nervosa is characterized by food restriction (despite severe undernutrition),5 depressive symptoms and difficulties in emotion regulation,6 as well as high anxiety and harm avoidance.
Several studies have suggested that people experience reduced serotonergic tone in an acute undernourished state, as measured by parameters such as a decreased level of the 5-HT metabolite 5-hydroxyindolacetic acid in cerebrospinal fluid,7,8 blunted plasma prolactin response to 5-HT agonists9,10 and reduced platelet imipramine11 and paroxetine12,13 binding. As well, reduced 5-HT2A receptor density in the periphery14 and lower receptor binding capacity in the central nervous system15 have been reported. However, patients recovered from anorexia nervosa may be in a hyperserotonergic state after weight recovery,16 as indicated by decreased blood platelet MAO-B activity,17 increased 5-HT content,18 altered central 5-HT1A and 5-HT2A receptor binding capacity19,20 and elevated 5-hydroxyindolacetic acid in the cerebrospinal fluid.16
Based on these findings, it has been hypothesized that people with anorexia nervosa are in a hyperserotonergic state both premorbidly and after recovery. Because increased 5-HT availability appears to be associated with increased negative affect (such as anxiety21), people at risk for anorexia nervosa may experience some form of reinforcement (e.g., reduced anxiety) when they lower their intake of the essential amino acid tryptophan (the precursor of central 5-HT synthesis) via food restriction. Such dieting may eventually spin out of control (i.e., become habitual) and lead to a hyposerotonergic state during the acute phase of the disorder.22 However, although reduced tryptophan levels during acute undernutrition is an accepted and well-replicated finding, some studies have found no difference in tryptophan ratios between healthy controls and weight-restored patients directly after intensive treatment.23,24
An experimental procedure that has been used widely to study the role of 5-HT in cognitive function25,26 and mental disorders27 is acute tryptophan depletion. This method allows researchers to mimic the reduction in dietary plasma tryptophan described above. Participants ingest a beverage loaded with large neutral amino acids but lacking tryptophan. This approach has 2 effects: first, the beverage’s large neutral amino acid load competes with tryptophan for transport across the blood–brain barrier and reduces the availability of tryptophan in the brain, reducing 5-HT synthesis; second, protein synthesis in the liver is stimulated, depleting plasma tryptophan levels.28–30
Over the years, a large body of literature has accumulated, showing the effects of acute tryptophan depletion on cognitive function using functional MRI.25,31–33 Acute tryptophan depletion modulates neural responses in brain areas associated with affective or cognitive function, such as the amygdala during emotion processing31 and prefrontal areas during executive functioning.32 More recently, a growing number of studies has used acute tryptophan depletion and resting-state functional connectivity to study serotonergic effects on neural function.34–36 This method focuses on task-independent intrinsic neural networks — so-called resting state networks. Such networks are defined as temporally correlated, low-frequency, blood-oxygen-level-dependent signal fluctuations of distinct and widespread brain regions while the participant is at rest.
Several methods have been developed to analyze restingstate functional connectivity data.37 Seed-based analysis, which we used in the current study, is the most widely applied hypothesis-driven approach, with straightforward interpretability and high reliability.38 This approach involves the selection of specific brain regions (seed regions) from which time-series data are extracted to calculate whole-brain, voxel-wise connectivity maps of covariance with the seed region.39,40
One functional network of particular interest for understanding the underlying 5-HT-related neural alterations in anorexia nervosa is the salience network.41 Core brain regions of the network — including the dorsal anterior cingulate cortex, thalamus and insular cortex — are among the serotonergic projections of the nucleus raphe magnus.42 The salience network plays an important role in detecting, filtering and integrating biologically relevant “salient” stimuli, including emotional information, homeostatic regulation and reward value.43,44 Because deficits in salience detection have cascading consequences for higher-order cognitive function and dysfunction, the salience network has been associated with multiple mental disorders.45
In anorexia nervosa, altered functioning of the salience network has been reported repeatedly in studies that have applied both task-based and resting-state functional MRI.46 In particular, the insular cortex (a central node of the salience network) has shown altered resting-state functional connectivity in people with anorexia nervosa.47–49 However, the specific direction of the reported effect has been heterogeneous, a finding that might be explained by the different statistical approaches applied. Interestingly, we have shown that in patients with anorexia nervosa, epigenetic variation of the 5-HT transporter gene is associated with the resting-state functional connectivity of the salience network and with eating disorder psychopathology.47
Surprisingly, despite the theoretical considerations and empirical findings that suggest an important role for 5-HT in anorexia nervosa (as outlined above and in a growing number of resting-state functional connectivity studies50) no known studies have investigated how acute tryptophan depletion affects resting-state functional connectivity. In the present double-blind crossover study, we used seed-based analyses to investigate the effects of acute tryptophan depletion on the resting-state functional connectivity of the salience network; we compared a sample of patients recovered from anorexia nervosa with age-matched healthy controls.
We predicted that if people predisposed to or recovered from anorexia nervosa were truly in a hyperserotonergic state,22 then temporary dietary manipulation of 5-HT synthesis via acute tryptophan depletion would “balance” restingstate functional connectivity in the salience network in that group. This hypothesis was undirected, because previous studies have not provided a sufficient basis for predicting whether acute tryptophan depletion would result in increased or decreased resting-state functional connectivity in the salience network. To interrogate this hypothesis, we tested for group differences in resting-state functional connectivity in the salience network in acute tryptophan depletion and in a sham condition (i.e., interaction of group and experimental condition).
Methods
Sample
For the present study, we recruited a total of 49 female participants (to see our justification for including only female participants, see Appendix 1, available at www.jpn.ca/lookup/doi/10.1503/jpn.210161/tab-related-content). After pair-wise age-matching,51 we included data from 44 female volunteers: 22 patients recovered from anorexia nervosa (18 of the restrictive subtype at the time of acute illness, and 4 of the binge–purge subtype) and 22 healthy controls.
To be considered recovered, participants had to have previously met the DSM-5 diagnostic criteria5 for anorexia nervosa; be maintaining a body mass index greater than 18.5 kg/m2 (or above the 10th percentile if younger than 18 years); be menstruating; and not have engaged in substantial restrictive eating behaviour (or bingeing–purging) for at least 6 months before study participation. To be included in the healthy controls group, participants had to have a body mass index between 18.5 and 24.9 kg/m2, be eumenorrheic and have no history of psychiatric illness.
We recruited participants in both groups via advertisements among high school and university students, or they had participated in previous related studies. We used several additional exclusion criteria for each group (Appendix 1): psychotropic medication within 4 weeks before the study; binge eating or a diagnosis of bulimia nervosa; substance abuse; or neurologic or medical conditions. All participants were of European descent. The Institutional Review Board of the Technische Universität Dresden approved the study, and all participants (or their legal guardians) provided written informed consent.
Instruments
For all participants, we ascertained the presence or absence of a current eating disorder by administering the expert version of the Structured Interview for Anorexic and Bulimic Syndromes (SIAB-EX).52 We also used a structured clinical interview (Mini-DIPS)53 to assess participants for other active psychiatric disorders. To complement the information obtained from the clinical interviews, we assessed eating-disorder-specific psychopathology using the Eating Disorder Inventory (EDI-2)54 and depressive symptoms using the Beck Depression Inventory II (BDI-II).55
We assessed the motivational behavioural inhibition system (which corresponds to motivation to avoid aversive outcomes) using the BIS subscale of the Behavioural Inhibition System/Behavioural Activation System (BIS/BAS) Scale.56 We estimated IQ using the Wechsler Adult Intelligence Scale.57 We computed body mass index standard deviation scores (BMI-SDS)58,59 to provide an age-corrected index of weight-to-height ratio.
We managed study data using the Research Electronic Data Capture (REDCap)60 web interface.
Procedure
The acute tryptophan depletion procedure entailed a double-blind, randomized crossover design; each participant underwent both the depletion and sham conditions within 7 to 14 days to avoid potential carryover effects (mean ± standard deviation [SD] 9.72 ± 3.32 days). For the day before the study, participants received a list of meal instructions and were asked to restrict their diet to low-protein food. At 8.30 am, immediately before intake of the beverage for the depletion or sham condition, participants were weighed to ensure a weight-adapted dosing regimen (for a more detailed description of the acute tryptophan depletion mixture, see Appendix 1).61 After they drank the depletion or sham condition beverage, participants were given a tryptophan-free breakfast. The MRI session took place 4 hours after intake of the depletion or sham condition beverage. Participants were continuously supervised by trained staff.
Biochemical measures
Using a venous catheter (Braunüle) and EDTA tubes, we took blood samples before intake of the depletion or sham condition beverage and approximately 5 hours later (immediately after the MRI session). For more details on the biochemical measures and analyses, see Appendix 1.
To quantify the depletion effect for each participant, we calculated the ratio of plasma tryptophan to large neutral amino acids (valine, methionine, isoleucine, leucine, phenylalanine, tyrosine) using the values from the blood samples taken immediately after the MRI session. Because of complications during blood sampling, this value was missing for 7 participants.
MRI data acquisition and preprocessing
We acquired MRI data using a 3 T Siemens Trio scanner. We acquired T1-weighted structural brain scans using a magnetization-prepared rapid acquisition gradient-echo sequence (parameters described in Appendix 1). We acquired an 8-minute resting state functional MRI scan using a gradient-echo T2*-weighted echo-planar imaging sequence using standard parameters (Appendix 1). During the functional MRI, participants were instructed to lie still with their eyes closed but without falling asleep. Functional and structural images were processed using SPM8 (www.fil.ion.ucl.ac.uk/spm/) in the Nipype framework62 following standard procedures (Appendix 1).
We created a sample-specific template using structural images from all participants. The slice-time-corrected functional data were realigned and registered to their mean, and the realigned files were coregistered to the participant’s structural brain image. The echo-planar imaging volumes were then normalized to Montreal Neurological Institute space using the sample-specific template and corresponding flow field. We evaluated the quality of the functional MRI data by manual inspection and using artifact detection tools (Appendix 1).
Next, we used the CONN toolbox63 implemented in Matlab to apply the denoising procedure, including temporal filtering (0.01–0.08 Hz); regression of 5 CompCorr nuisance components from white matter and cerebrospinal fluid; and 24 motion parameters. We applied scrubbing to ignore time points with a frame-wise displacement of greater than 0.9 mm and global signal z value of greater than 5. Subsequent inspection revealed no group differences for the number of invalid scans (mean ± SD: healthy controls 2.41 ± 4.52, patients recovered from anorexia nervosa 3.14 ± 3.81; T42 = 0.58; p = 0.57) or mean motion (T42 = 0.15; p = 0.303).
Seed-based analysis
The following 7 seed regions, which encompass the salience network, were chosen using the network regions of interest provided by CONN (based on an independent component analysis of 497 healthy participants): the anterior cingulate cortex, left and right anterior insula, left and right rostral prefrontal cortex, and left and right supramarginal gyrus. We computed individual and condition-specific seed-based connectivity maps as Fisher-transformed bivariate correlation coefficients between the blood-oxygen-level-dependent time series for each seed region and all individual voxel time series in the brain. We subjected the seed-based connectivity maps to a second-level repeated-measures analysis of variance, using experimental condition as a within-subject factor and group as a between-subject factor. We first explored the main effects of group and condition individually. Then, to address the main research question about a potential group difference in the effect of acute tryptophan depletion versus the sham condition, we calculated the interaction of group and experimental condition. To guard against type I errors, results had to fall below a family-wise error (FWE)–corrected cluster-wise threshold of p < 0.05.
To aid in the interpretation of significant whole-brain results, we extracted connectivity values averaged across all voxels from significant clusters using CONN for each participant and condition and submitted them to a repeated-measures group × condition analysis of variance using SPSS statistical software version 27.0 (SPSS Inc.).
Additional exploratory analyses investigated the associations between the extracted connectivity values for the depletion and sham conditions and relevant clinical variables in patients recovered from anorexia nervosa, including EDI-2 total score, BMI-SDS and the BIS subscale of the BIS/BAS Scale using Pearson r.
Results
Participants and biochemical measures
As summarized in Table 1, we found no significant group differences in age, BMI-SDS or BDI-II score, but patients recovered from anorexia nervosa showed some residual symptoms of an eating disorder.
Table 1.
Demographic and clinical characteristics
| Characteristic | Group | Statistics | ||
|---|---|---|---|---|
|
|
|
|||
| Patients recovered from anorexia nervosa | Healthy controls | t | p value | |
| Age, yr* | 23.02 ± 2.83 | 23.05 ± 2.53 | 0.03 | 0.98 |
| Body mass index standard deviation score | −0.43 ± 0.62 | −0.46 ± 0.66 | −1.65 | 0.87 |
| Body mass index, kg/m2 | 21.03 ± 1.48 | 21.09 ± 2.14 | 0.10 | 0.92 |
| Eating Disorder Inventory 2 total score | 20.23 ± 5.77 | 15.59 ± 2.13 | −3.53 | 0.001 |
| Beck Depression Inventory II score | 6.18 ± 8.19 | 2.45 ± 3.40 | 1.97 | 0.06 |
| Behavioural Inhibition System subscore† | 21.95 ± 4.13 | 20.83 ± 2.64 | 1.04 | 0.30 |
| Recovery, mo‡ | 57.81 ± 34.42 | NA | NA | NA |
NA = not applicable.
Age range 17.5–27.8 yr (patients recovered from anorexia nervosa) and 17.8–26.4 yr (healthy controls).
From the Behavioural Inhibition System/Behavioural Activation System (BIS/BAS) Scale.
Recovery range = 17–140 mo.
Demonstrating successful reduction in tryptophan levels in the depletion condition, participants’ ratios of large neutral amino acids to tryptophan were significantly lower compared to the sham condition (t35 = −15.96, p < 0.001).
Resting-state functional connectivity analysis
Analyses revealed a main effect of group, indicating increased resting-state functional connectivity between seeds of the salience network and prefrontal regions such as the inferior and medial frontal gyrus (commonly associated with higher cognitive control function) in patients recovered from anorexia nervosa compared to healthy controls.
A main effect of condition was also significant, indicating elevated resting-state functional connectivity between seeds of the salience network and the thalamus (a region known to receive projections of the 5-HT producing raphe nuclei) during the depletion condition compared to the sham condition (Appendix 1).
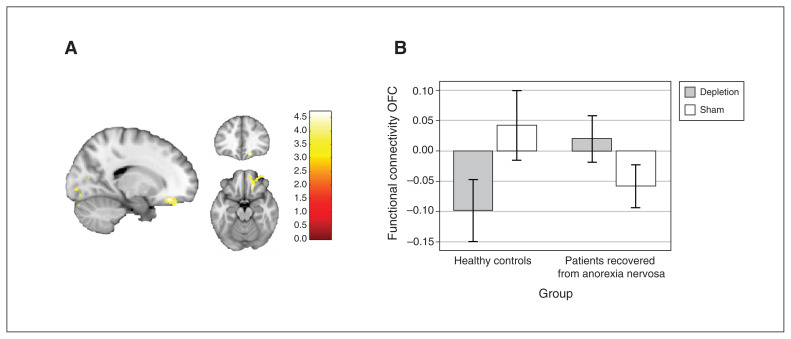
For the critical interaction of group and experimental condition, we found a significant group difference in the effect of acute tryptophan depletion compared to the sham condition in terms of resting-state functional connectivity between the right supramarginal gyrus and the right orbitofrontal cortex extending to the frontal pole (F1,42 = 12.52; pFWE = 0.026; k = 169; x, y, z = 18, 34, −16; Figure 1A). The cluster withstood Bonferroni correction for 7 correlated comparisons (7 seed regions; r = 0.86; Dubey/Armitage–Parmar method64).
Figure 1.
Group differences in functional connectivity between the right supramarginal gyrus and the right orbitofrontal cortex in the depletion versus sham conditions. (A) Cluster of the group × condition interaction at the orbitofrontal cortex, spreading to the frontal pole; the cluster withstood Bonferroni correction for 7 seed regions (number tested and correlated; r = 0.86). (B) Connectivity values between the seed and the orbitofrontal cortex, by group and condition.
Further analysis of resting-state functional connectivity values indicated that the interaction of group and experimental condition (F1,42 = 32.6; p < 0.001) was driven in part by the fact that patients recovered from anorexia nervosa showed elevated connectivity during the depletion versus the sham condition (t42 = −3.61; p = 0.002), and by a similar pattern of resting-state functional connectivity in patients recovered from anorexia nervosa during the depletion condition compared to healthy controls in the sham condition (t42 = −0.66; p = 0.512; Figure 1B).
In healthy controls, resting-state functional connectivity was reduced during the depletion condition; during the sham condition, functional connectivity was similar to that of patients recovered from anorexia nervosa in the depletion condition.
Results for all post hoc t tests can be found in Appendix 1. No other seed region revealed a significant interaction of group and experimental condition.
Exploratory correlation analyses showed no significant relationships between orbitofrontal cortex connectivity during the acute tryptophan depletion or sham conditions for any of the investigated clinical measures in patients recovered from anorexia nervosa.
Discussion
The aim of the present study was to investigate the influence of experimental reduction of central 5-HT synthesis (via acute tryptophan depletion) on resting-state functional connectivity in the salience network in patients recovered from anorexia nervosa. We found a significant group × condition interaction for resting-state functional connectivity between a seed in the right supramarginal gyrus and a region of the right orbitofrontal cortex. Patients recovered from anorexia nervosa showed increased resting-state functional connectivity in the depletion condition compared to the sham condition. The opposite pattern was true for healthy controls: restingstate functional connectivity was reduced in the depletion condition and increased in the sham condition (similar that of patients recovered from anorexia nervosa in the depletion condition).
In light of the 5-HT hypothesis of anorexia nervosa, which posits that patients restrict food intake to mitigate a hyperserotonergic state,22 it may be that acute tryptophan depletion “normalizes” 5-HT functioning and associated functional connectivity of the salience network in people with a history of anorexia nervosa (and possibly in those predisposed to it). However, although connectivity between the right supramarginal gyrus and the orbitofrontal cortex seemed to be affected by acute tryptophan depletion, no influence was evident for other parts of the salience network.
Mounting evidence suggests the salience network may have particular significance for psychiatric disorders,43 including anorexia nervosa.46,65 Its crucial role lies in mediating the switch between large-scale networks involved in externally oriented attention (e.g., the central executive network) and internally oriented self-related mental processes (e.g., the default mode network). Disturbance in this mapping can lead to altered cognitive and affective function.45 Our findings suggest that 5-HT depletion in patients recovered from anorexia nervosa may balance the mapping between the salience network and midline default mode network regions, as observed in the present study with the orbitofrontal cortex. This finding is in line with previous studies showing that the acute tryptophan depletion challenge affects activation of the default mode network mainly in the orbitofrontal cortex,35,66 a brain region innervated by dense serotonergic projections.67
The orbitofrontal cortex receives projections from several modality-specific brain regions, including the insula, the visual cortex and the somatosensory cortex, which includes the supramarginal gyrus, a seed region of the current study. These strong connections to brain regions involved with physical stimuli is critical for decoding the affective value of reward (primary reinforcers) and punishment via the senses, such as taste, touch and face expression. By representing the reward value of stimuli, the orbitofrontal cortex is also involved in representation of emotions, because emotions can be considered as states evoked by the value of rewards or aversive stimuli.68
In anorexia nervosa, reduced functional connectivity with the salience network (as we found in patients recovered from anorexia nervosa during the sham condition) may be related to aberrant emotional processing.69 Importantly, alterations associated with the somatosensory cortex (particularly the supramarginal gyrus) may be implicated in social–emotional dysfunction, leading to problems with interpersonal relationships70 and reduced empathy,71 aspects well recognized in a subgroup of patients with anorexia nervosa.72–74 However, given the widely discussed role of the insular cortex in anorexia nervosa,48,49,75 it is surprising that we did not find an effect of acute tryptophan depletion on insular connectivity. Reasons for this might include the modest sample size of the present study.
Our finding — of a potential relative normalization of functional connectivity between the salience network and the orbitofrontal cortex during acute tryptophan depletion in patients recovered from anorexia nervosa — lends further support to the hypothesis that a hyperserotonergic state might be a predisposing factor for anorexia nervosa. Initially, this hypothesis was put forward by Kaye and colleagues,22 who reported an anxiolytic effect of acute tryptophan depletion in anorexia nervosa. These findings may explain the rather poor therapeutic effect of selective serotonin reuptake inhibitors in the treatment of anorexia nervosa:76 the pharmacological increase of central 5-HT availability may actually worsen the pre-existing hyperserotonergic state. This hypothesis dovetails with reports from patients of dysphoric mood with increasing food intake during and after treatment, whereas food restriction was associated with positive effects on mood77 and affect lability.78 Nevertheless, refeeding of undernourished patients with anorexia nervosa constitutes an important part of a successful treatment protocol, despite possible initial aggravation of symptoms.79
Limitations
Interpretation of the present study rests on the following limitations. First, future studies with larger sample sizes are needed to replicate and confirm these results. Second, although acute tryptophan depletion has been used widely and validated for adults and adolescents (for a review, see Stewart and colleagues80), it has weaknesses; the effect of acute tryptophan depletion on central 5-HT availability can be judged only indirectly by measurement of the ratio of plasma tryptophan to large neutral amino acids. Still, the effectiveness of acute tryptophan depletion was confirmed by a positron emission tomography study measuring the actual influx of tryptophan into the brain.81 Third, the definition of recovery from anorexia nervosa varies widely across studies, limiting comparability. In our sample, one of the inclusion criteria for patients recovered from anorexia nervosa was the resumption of menses, and participants’ duration of recovery exceeded the recommended time period of 12 months.82 Fourth, future studies could benefit from longer scan lengths to improve the reliability of the estimates of resting-state functional connectivity. Finally, this study included only female participants of European descent, limiting generalizability.
Conclusion
The present study adds further preliminary evidence to the 5-HT hypothesis in anorexia nervosa, indicating that tryptophan depletion “normalizes” the functional connectivity of the salience network — a network closely associated with the processing of “salient” stimuli such as (social–)emotional information and reward value. If these findings are replicated, such knowledge may spur future research into treatment approaches that lower or modulate 5-HT levels, such as 5-HT-antagonists during refeeding.
Supplementary Material
Acknowledgements
The authors thank the Centre for Information Services and High Performance Computing (ZIH) at Technische Universität Dresden for generous allocations of computer time. This work was kindly supported by the DFG, SFB 940 “Volition and Cognitive Control: Mechanisms, Modulators and Dysfunctions,“ TP C03. The authors thank the numerous medical students and other interns for their assistance with participant recruitment and data collection all participants for their time and cooperation.
Footnotes
Competing interests: V. Roessner has received payment for consulting and writing activities from Lilly, Novartis and Shire Pharmaceuticals/Takeda; lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals/Takeda and Medice Pharma; and support for research from Shire/Takeda and Novartis. V. Roessner has carried out clinical trials in cooperation with Novartis, Shire Pharmaceuticals/Takeda and Otsuka. F.D. Zepf received honoraria from Takeda Pharmaceuticals and Medice Pharmaceuticals; payments from several local courts for expertise in the field of child and adolescent psychiatry, psychosomatics and psychotherapy; and is the current president of the International Society for Tryptophan Research. No other competing interests were declared.
Contributors: V. Roessner, F.D. Zepf and S. Ehrlich designed the study. F. Ritschel, D. Geisler, J.A. King and I. Lesch acquired the data, which I. Boehm and J. Hennig analyzed. I. Boehm and S. Ehrlich wrote the article, which J. Hennig, F. Ritschel, D. Geisler, J.A. King, I. Lesch, V. Roessner and F.D. Zepf reviewed. All authors approved the final version to be published, agree to be accountable for all aspects of the work and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (EH 367/5-1, EH 367/7-1 and SFB 940).
References
- 1.Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Halford JCG, Blundell JE. Separate systems for serotonin and leptin in appetite control. Ann Med 2000;32:222–32. [DOI] [PubMed] [Google Scholar]
- 3.Lam DD, Garfield AS, Marston OJ, et al. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav 2010;97:84–91. [DOI] [PubMed] [Google Scholar]
- 4.Kaye WH, Wierenga C, Bailer U, et al. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci 2013;36:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth edition. Arlington (VA): American Psychiatric Association Publishing; 2013. [Google Scholar]
- 6.Haynos AF, Fruzzetti AE. Anorexia nervosa as a disorder of emotion dysregulation: evidence and treatment implications. Clin Psychol Sci Pract 2011;18:183–202. [Google Scholar]
- 7.Demitrack MA, Heyes MP, Altemus M, et al. Cerebrospinal fluid levels of kynurenine pathway metabolites in patients with eating disorders: relation to clinical and biochemical variable. Biol Psychiatry 1995;37:512–20. [DOI] [PubMed] [Google Scholar]
- 8.Kaye WH, Ebert MH, Raleigh M, et al. Abnormalities in CNS monoamine metabolism in anorexia nervosa. Arch Gen Psychiatry 1984;41:350–5. [DOI] [PubMed] [Google Scholar]
- 9.Brewerton TD, Jimerson DC. Studies of serotonin function in anorexia nervosa. Psychiatry Res 1996;62:31–42. [DOI] [PubMed] [Google Scholar]
- 10.Monteleone P, Brambilla F, Bortolotti F, et al. Prolactin response to d-fenfluramine is blunted in people with anorexia nervosa. Br J Psychiatry 1998;172:439–42. [DOI] [PubMed] [Google Scholar]
- 11.Weizman R, Carmi M, Tyano S, et al. High affinity [3H] imipramine binding and serotonin uptake to platelets of adolescent females suffering from anorexia nervosa. Life Sci 1986;38:1235–42. [DOI] [PubMed] [Google Scholar]
- 12.Bruce KR, Steiger H, Ng Ying Kin NMK, et al. Reduced platelet [3H] paroxetine binding in anorexia nervosa: relationship to eating symptoms and personality pathology. Psychiatry Res 2006;142:225–32. [DOI] [PubMed] [Google Scholar]
- 13.Ramacciotti CE, Coli E, Paoli R, et al. Serotonergic activity measured by platelet [3H]paroxetine binding in patients with eating disorders. Psychiatry Res 2003;118:33–8. [DOI] [PubMed] [Google Scholar]
- 14.Sigurdh J, Allard P, Spigset O, et al. Platelet serotonin transporter and 5-HT 2A receptor binding in adolescents with eating disorders. Int J Neurosci 2013;123:333–8. [DOI] [PubMed] [Google Scholar]
- 15.Audenaert K, Van Laere K, Dumont F, et al. Decreased 5-HT2a receptor binding in patients with anorexia nervosa. J Nucl Med 2003;44:163–9. [PubMed] [Google Scholar]
- 16.Kaye WH, Gwirtsman HE, George DT, et al. Altered serotonin activity in anorexia nervosa after long-term weight restoration: does elevated cerebrospinal fluid 5-hydroxyindoleacetic acid level correlate with rigid and obsessive behavior? Arch Gen Psychiatry 1991;48:556–62. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich S, Franke L, Schott R, et al. Platelet monoamine oxidase activity in underweight and weight-recovered females with anorexia nervosa. Pharmacopsychiatry 2008;41:226–31. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich S, Franke L, Scherag S, et al. The 5-HTTLPR polymorphism, platelet serotonin transporter activity and platelet serotonin content in underweight and weight-recovered females with anorexia nervosa. Eur Arch Psychiatry Clin Neurosci 2010;260:483–90. [DOI] [PubMed] [Google Scholar]
- 19.Bailer UF, Frank G, Henry S, et al. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [carbonyl11C]WAY-100635. Arch Gen Psychiatry 2005;62:1032–41. [DOI] [PubMed] [Google Scholar]
- 20.Frank GK, Kaye W, Meltzer CC, et al. Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biol Psychiatry 2002;52:896–906. [DOI] [PubMed] [Google Scholar]
- 21.Charney DS, Woods SW, Krystal JH, et al. Serotonin function and human anxiety disorders. Ann N Y Acad Sci 1990;600:558–72. [DOI] [PubMed] [Google Scholar]
- 22.Kaye WH, Barbarich NC, Putnam K, et al. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Disord 2003;33:257–67. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier C, Hassler C, Mattar L, et al. Symptoms of depression and anxiety in anorexia nervosa: links with plasma tryptophan and serotonin metabolism. Psychoneuroendocrinology 2014;39:170–8. [DOI] [PubMed] [Google Scholar]
- 24.Attia E, Wolk S, Cooper T, et al. Plasma tryptophan during weight restoration in patients with anorexia nervosa. Biol Psychiatry 2005;57:674–8. [DOI] [PubMed] [Google Scholar]
- 25.Aquili L. The role of tryptophan and tyrosine in executive function and reward processing. Int J Tryptophan Res 2020;13:1178646920964825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendelsohn D, Riedel WJ, Sambeth A. Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev 2009;33:926–52. [DOI] [PubMed] [Google Scholar]
- 27.Bell CJ, Hood SD, Nutt DJ. Acute tryptophan depletion. Part II: clinical effects and implications. Aust N Z J Psychiatry 2005;39:565–74. [DOI] [PubMed] [Google Scholar]
- 28.Biskup CS, Sánchez CL, Arrant A, et al. Effects of acute tryptophan depletion on brain serotonin function and concentrations of dopamine and norepinephrine in C57BL/6J and BALB/cJ mice. PLoS ONE 2012;7:e35916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dingerkus VLS, Gaber TJ, Helmbold K, et al. Acute tryptophan depletion in accordance with body weight: influx of amino acids across the blood–brain barrier. J Neural Transm (Vienna) 2012;119:1037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood SD, Bell CJ, Nutt DJ. Acute tryptophan depletion. Part I: rationale and methodology. Aust N Z J Psychiatry 2005;39:558–64. [DOI] [PubMed] [Google Scholar]
- 31.Evers EAT, Sambeth A, Ramaekers JG, et al. The effects of acute tryptophan depletion on brain activation during cognition and emotional processing in healthy volunteers. Curr Pharm Des 2010;16:1998–2011. [DOI] [PubMed] [Google Scholar]
- 32.Fusar-Poli P, Allen P, McGuire P, et al. Neuroimaging and electrophysiological studies of the effects of acute tryptophan depletion: a systematic review of the literature. Psychopharmacology (Berl) 2006;188:131–43. [DOI] [PubMed] [Google Scholar]
- 33.Raab K, Kirsch P, Mier D. Understanding the impact of 5-HTTLPR, antidepressants, and acute tryptophan depletion on brain activation during facial emotion processing: a review of the imaging literature. Neurosci Biobehav Rev 2016;71:176–97. [DOI] [PubMed] [Google Scholar]
- 34.Bär K-J, Köhler S, de la Cruz F, et al. Functional consequences of acute tryptophan depletion on raphe nuclei connectivity and network organization in healthy women. Neuroimage 2020;207:116362. [DOI] [PubMed] [Google Scholar]
- 35.Helmbold K, Zvyagintsev M, Dahmen B, et al. Serotonergic modulation of resting state default mode network connectivity in healthy women. Amino Acids 2016;48:1109–20. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Huettel SA, Mullette-Gillman OA, et al. Exploring common changes after acute mental stress and acute tryptophan depletion: resting-state fMRI studies. J Psychiatr Res 2019;113:172–80. [DOI] [PubMed] [Google Scholar]
- 37.Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol 2013;34:1866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shehzad Z, Kelly AMC, Reiss PT, et al. The resting brain: unconstrained yet reliable. Cereb Cortex 2009;19:2209–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–41. [DOI] [PubMed] [Google Scholar]
- 40.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 2008;21:424–30. [DOI] [PubMed] [Google Scholar]
- 41.Palma-Gudiel H, Fañanás L. An integrative review of methylation at the serotonin transporter gene and its dialogue with environmental risk factors, psychopathology and 5-HTTLPR. Neurosci Biobehav Rev 2017;72:190–209. [DOI] [PubMed] [Google Scholar]
- 42.Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev 2006;30:203–14. [DOI] [PubMed] [Google Scholar]
- 43.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010;214:655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011;15:483–506. [DOI] [PubMed] [Google Scholar]
- 46.Peters SK, Dunlop K, Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front Syst Neurosci 2016;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boehm I, Walton E, Alexander N, et al. Peripheral serotonin transporter DNA methylation is linked to increased salience network connectivity in females with anorexia nervosa. J Psychiatry Neurosci 2020;45:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehrlich S, Lord A, Geisler D, et al. Reduced functional connectivity in the thalamo-insular subnetwork in patients with acute anorexia nervosa. Hum Brain Mapp 2015;36:1772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geisler D, Borchardt V, Lord AR, et al. Abnormal functional global and local brain connectivity in female patients with anorexia nervosa. J Psychiatry Neurosci 2016;41:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaudio S, Wiemerslage L, Brooks SJ, et al. A systematic review of resting state functional-MRI studies in anorexia nervosa: evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neurosci Biobehav Rev 2016;71:578–89. [DOI] [PubMed] [Google Scholar]
- 51.Munkres J. Algorithms for the assignment and transportation problems. J Soc Ind Appl Math 1957;5:32–8. [Google Scholar]
- 52.Fichter MM, Quadflieg N. Strukturiertes Inventar für anorektische und bulimische Essstörungen nach DSM-IV und ICD-10 (SIAB). Göttingen, Germany: Hogrefe; 1999. [Google Scholar]
- 53.Margraf J. Mini-DIPS: diagnostisches Kurz-Interview bei psychischen Störungen; Handbuch. Berlin Heidelberg: Springer-Verlag; 2013. [Google Scholar]
- 54.Paul T, Thiel A. Eating disorder inventory 2: EDI-2, Deutsche version. Manual. Boston (MA): Hogrefe; 2005. [Google Scholar]
- 55.Hautzinger M, Keller F, Kühner C. Beck Depressions-Inventar Revision. FBDI-II. Frankfurt am Main: Pearson; 2009. [Google Scholar]
- 56.Strobel A, Beauducel A, Debener S, et al. Eine deutschsprachige Version des BIS/BAS-Fragebogens von Carver und White. Z Differ Diagn Psychol 2001;22:216–27. [Google Scholar]
- 57.Petermann F, Petermann M. HAWIK-IV. Bern: Huber; 2010. [Google Scholar]
- 58.Kromeyer-Hauschild K, Wabitsch M, Kunze D, et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 2001;149:807–18. [Google Scholar]
- 59.Hemmelmann C, Brose S, Vens M, et al. Percentiles of body mass index of 18–80-year-old German adults based on data from the Second National Nutrition Survey. Dtsch Med Wochenschr 1946;2010:848–52. [DOI] [PubMed] [Google Scholar]
- 60.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) — a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moja EA, Stoff DM, Gessa GL, et al. Decrease in plasma tryptophan after tryptophan-free amino acid mixtures in man. Life Sci 1988;42:1551–6. [DOI] [PubMed] [Google Scholar]
- 62.Gorgolewski K, Burns CD, Madison C, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform 2011;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–41. [DOI] [PubMed] [Google Scholar]
- 64.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 1997;16:2529–42. [DOI] [PubMed] [Google Scholar]
- 65.McFadden KL, Tregellas JR, Shott ME, et al. Reduced salience and default mode network activity in women with anorexia nervosa. J Psychiatry Neurosci 2014;39:178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kunisato Y, Okamoto Y, Okada G, et al. Modulation of default-mode network activity by acute tryptophan depletion is associated with mood change: a resting state functional magnetic resonance imaging study. Neurosci Res 2011;69:129–34. [DOI] [PubMed] [Google Scholar]
- 67.Roberts AC. The importance of serotonin for orbitofrontal function. Biol Psychiatry 2011;69:1185–91. [DOI] [PubMed] [Google Scholar]
- 68.Rolls ET. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia 2019;128:14–43. [DOI] [PubMed] [Google Scholar]
- 69.Oldershaw A, Startup H, Lavender T. Anorexia nervosa and a lost emotional self: a psychological formulation of the development, maintenance, and treatment of anorexia nervosa. Front Psychol 2019;10:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosen ML, Sheridan MA, Sambrook KA, et al. Salience network response to changes in emotional expressions of others is heightened during early adolescence: relevance for social functioning. Dev Sci 2018;21:e12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kogler L, Müller VI, Werminghausen E, et al. Do I feel or do I know? Neuroimaging meta-analyses on the multiple facets of empathy. Cortex 2020;129:341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doba K, Nandrino J-L. Cognitive and emotional empathy in anorexia nervosa: the role of attachment insecurity, intrapersonal, and interpersonal emotional competences. J Nerv Ment Dis 2020;208:312–8. [DOI] [PubMed] [Google Scholar]
- 73.Harrison A, Tchanturia K, Treasure J. Attentional bias, emotion recognition, and emotion regulation in anorexia: state or trait? Biol Psychiatry 2010;68:755–61. [DOI] [PubMed] [Google Scholar]
- 74.Russell TA, Schmidt U, Doherty L, et al. Aspects of social cognition in anorexia nervosa: affective and cognitive theory of mind. Psychiatry Res 2009;168:181–5. [DOI] [PubMed] [Google Scholar]
- 75.Nunn K, Frampton I, Gordon I, et al. The fault is not in her parents but in her insula — a neurobiological hypothesis of anorexia nervosa. Eur Eat Disord Rev 2008;16:355–60. [DOI] [PubMed] [Google Scholar]
- 76.Frank GK. Pharmacotherapeutic strategies for the treatment of anorexia nervosa — too much for one drug? Expert Opin Pharmacother 2020;21:1045–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fitzsimmons-Craft EE, Accurso EC, Ciao AC, et al. Restrictive eating in anorexia nervosa: examining maintenance and consequences in the natural environment. Int J Eat Disord 2015;48:923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Selby EA, Cornelius T, Fehling KB, et al. A perfect storm: examining the synergistic effects of negative and positive emotional instability on promoting weight loss activities in anorexia nervosa. Front Psychol 2015;6:1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ehrlich S, Tam FI. Editorial: refeeding in anorexia nervosa: quo vadis? J Am Acad Child Adolesc Psychiatry 2021;60:566–7. [DOI] [PubMed] [Google Scholar]
- 80.Stewart RM, Wong JWY, Mahfouda S, et al. Acute tryptophan depletion moja-de: a method to study central nervous serotonin function in children and adolescents. Front Psychiatry 2020;10:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishizawa S, Benkelfat C, Young SN, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A 1997;94:5308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wade TD, Lock J. Developing consensus on the definition of remission and recovery for research. Int J Eat Disord 2020;53:1204–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.