Abstract
Background:
The management and repair of knee cartilage lesions currently represents a challenge for the orthopaedic surgeon. Identificable causes are the characteristics of the involved tissues themselves and the presence of poor vascularization, which is responsible for overall reduced repair capacity.
Materials and Methods:
For our article, we have evaluated the research systems of PubMed and google scholar considering the scientific articles of the last twenty years concerning our topic.
Results:
The literature reports three types of cartilage lesions’ treatment modalities: chondroprotection, chondroreparation and chondroregeneration. The preference for one or the other therapeutic option depends on the pattern of the lesion and the clinical conditions of the patient.
Conclusions:
Each treatment technique is distinguished by the quality of the restorative tissue that is generated. In particular, the chondroregeneration represents the last frontier of regenerative medicine, as it aims at the complete restoration of natural cartilage. However, the most recent literature documents good results only in the short and medium terms. In recent years, the optimization of chondroregeneration outcomes is based on the modification of the scaffolds and the search for new chondrocyte sources, in order to guarantee satisfactory long-term results. (www.actabiomedica.it)
Keywords: cartilaginous injuries, regenerative medicine, staminal cells, cartilaginous repair
Introduction
The cartilage of the knee is a highly specialized hyaline connective tissue, consisting of a cellular component (chondrocytes) and an extracellular component formed for 90% of water and 10% from proteoglycans, glycosaminoglycans, collagen (mainly type 2) and non-collagen proteins; these substances are organized in 4 layers, whose characteristics are based on different cell density, different composition of extracellular matrix and different orientation of the collagen fibers; these morphological characteristics carry an intrinsic limit, in relation to a reduced vascularization (1). These conditions are obviously in contrast with the tissue repair and therefore with an appropriate treatment of the lesion; cartilage lesions are debilitating for the patients’ daily life activities, manifesting themselves with swelling, functional analgesic limitation, local pain / tenderness. Failure or delayed treatment of the cartilage lesion can lead to arthritic degeneration with consequent various degrees of joint deformation and degeneration (2). The current knowledge on the management of cartilage lesions is provide with a clinical-instrumental classification; once the clinical-diagnostic process is completed, the cartilage lesion should be treated accordingly. The choice of the type of treatment depends mainly on three factors: pattern of injury, clinical conditions and the patients’ daily needs. The treatment options differ substantially concerning the quality of the tissue needing to be restored. The treatment methods can be conservative or surgical (3). The literature documents three type of surgical treatments: chondroprotection, chondroreparation and chondroregeneration. Chondrorigeneration is the most innovative technique, as it is meant to determine the restoration of natural cartilage. It is based on the use of cells supported by a scaffold (three-dimensional support formed by synthetic and biological substances). However, the literature documents satisfactory results only in the short and medium term. Now days the goal of the research is to increasingly improve the outcomes of chondroregeneration through the optimization of scaffolds and the use of stem cells, which represent the key to cartilage repair (4).
Types of Cartilaginous Injuries Treatment
Chondroprotection, Chondroreparation, Chondroregeneration
The treatment of knee cartilage lesions involves the evaluation of three factors: analysis of the lesion pattern, clinical condition of the patient, age and / or the patient’s daily needs. In the international literature, three main methods of treatment are described: chondroprotection, chondroreparation and chondroregeneration. Chondroprotection does not require direct treatment on the tissue under examination. It represents a set of therapeutic aids with the aim of reducing symptoms as much as possible, avoiding the progression of damage, and therefore preventing premature osteoarthritis. As part of this treatment, a conservative or surgical approach can be adopted. The first involves walking with assisted loading, abstention from physical activity, use of painkillers (in the acute phase), oral chondroprotectors, intra-articular knee joint injections with hyaluronic acid (HA), corticosteroids and PRP (Platelet Rich Plasma) (5). Concerning the utilization of intra-articular steroids, the most accepted guidelines recommend the use low-doses in order to relieve pain. In fact, it has been shown that an excessive use of intra-articular steroids could be toxic to the chondrocytes themselves (6). Hyaluronic acid is a natural long-chain polymer with repeated disaccharide units that provides lubrication, hence an improvement in the absorption of forces on the joint. Reported benefits of additional HA injections include pain relief, improved function and reduced stiffness, although the possible mechanisms of action for HA have not been fully elucidated (7,8).
PRP is also used for pain relief and potential tissue repair. However, there are no studies that acknowledge its real efficacy due to a lack of reliable high level of clinical evidence (9).
However, in the context of chondroprotection, there are also surgical options that reduce the stress at the joint knee, thus preventing damage progression and development of early osteoarthritis. Common options involve ligament reconstruction and / or meniscal repairs for joint stabilization, or osteotomies to correct the axes. (10). The indications for surgical treatment for cartilage lesions are symptomatic grade III and IV lesions with dimensions> 2 cm2 in patients aged 16 to 55 and active in everyday life (11). Contraindications of surgical treatment for cartilage injuries are distinguished in absolute and relative. The first are: diffuse degenerative arthritis, systemic diseases, infectious processes in progress, BMI> 30, smoking and alcoholism, full thickness cartilage lesion, neoplasms and instability. The second are axial deviations, ligament and meniscal alterations (12). Chondroreparation consists in stimulating the subchondral bone through various methods; abrasion, drilling and microfractures/nanofractures. This method stimulates tissue bleeding, thus favoring the recruitment of mesenchymal cells, responsible for tissue repair.
This involves the development of fibrocartilage, a tissue characterized mainly by collagen type I, which determines rigidity, poor resistance to forces, therefore biomechanically unfavorable. The indications for chondroreparation include: cartilage lesions up to 2 cm2 in size. Contraindications include age> 50 years, infection, tumors, avascular necrosis. Most common and relevant complications include iatrogenic fractures and the development of exuberant callus. However, microfractures have significant advantages: relatively simple, feasible and safe execution; minimally invasive; one-step execution technique; low costs (13,14). The chondral abrasion is an evolution of the open Magnusson debridement arthroplasty which was popularized by Lanny Johnson; it consists in the in the arthroscopic execution of a debridement of the damaged cartilage surface up to guaranteeing the leakage of blood which will allow tissue healing with the deposition of fibrocartilaginous tissue (15). The chondral drilling is a minimally invasive procedure to repair damaged cartilage in the knee joint; it consists in a small hole are drilled into the bone at the base of the damaged area to stimulate the growth of healthy cartilage (16). The microfractures technique, described by Steadman in the 1980s, represents the first-line treatment for chondral injuries; it consists in the execution of microfractures (after careful cleaning of the lesion site) through the use of specific angled tip awls; these are introduced at the injury site until fat droplets are observed from the medullary cavity, which detects the correct depth (approximately 2 to 4 mm) stimulation of tissue healing; the nanofractures provide a more precise subchondral stimulation, as it involves the use of special instruments capable of performing a deeper and thinner micro-perforation of the cartilage tissue than the standard microfracture technique; this innovative technique involves the use of a cannulated awl with a 15° angled tip to facilitate access to the lesion site; subchondral bone is then perforated with a special one-millimeter-thick needle which is advanced through the cannulated chisel to a depth of nine millimeters (17,18) (Figures 1,2).
Figure 1:
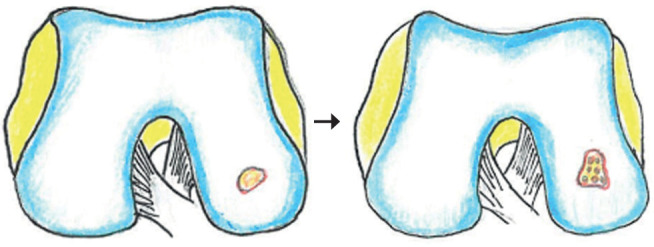
condylar defect treated with technique of microfracture
Figure 2:
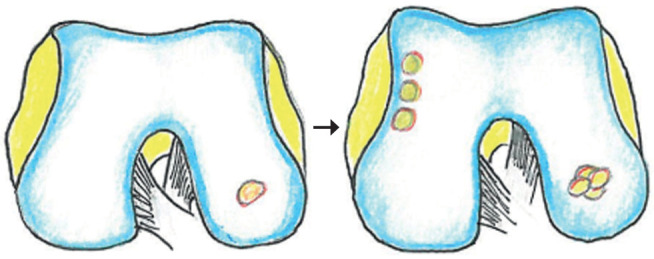
chondral defect treated with osteochondral autograft transfer
Chondroregeneration represents the last frontier in the treatment of cartilage lesions of the knee. It aims to restore articular cartilage through the application of chondral and / or osteochondral tissue (autograft or allograft) at the level of the lesion zone. The chondroregenerative techniques are: ACI (Autologous Chondrocyte Implantation), OATS (Osteochondral autograft transfer) or Mosaicoplasty and the Cellular matrix induced microfractures (19) (Figures 3,4).
Figure 3:
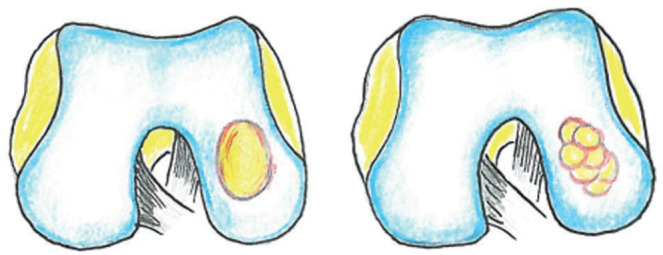
chondral defect treated with osteochondral allograft transplant
Figure 4:
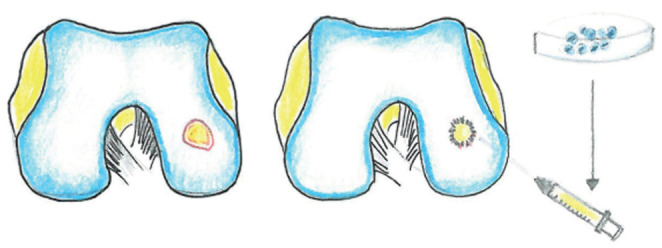
chondral defect treated autologous chondrocyte implantation
The OATS (Osteochondral Autograft Transfer System) technique is used for lesions ranging in size from 3 to 4 cm2; it consists in the removal of one or more osteochondrocytic “cylinders” or plugs (single or multiple plugs technique) from articular areas with low load, which are subsequently transferred to the damaged cartilage area according to a “mosaic” arrangement. This technique is reproducible using osteochondrocytic grafts from cadaver (allograft), whose main advantage is saving patients’ own tissues. Main significant complications documented in the literature include iatrogenic infections and fractures (20).
The ACI (Autologous Chondrocyte Implantation) technique consists in a two steps procedure: first, the removal of chondrocytes from patients’ knee through surgery, which will be cultured in vitro; chondrocytes will subsequently be implanted at the level of the injured cartilage area through a second procedure.
This regenerative method has undergone a recent technical evolution, involving three tissue generations that are distinguished by the “trophic support” of the implanted cells. The first generation involves the application of a periosteal membrane taken from the tibia; the second generation involves the use of a collagen patch (I-III), while the third generation, known as the MACI (matrix-induced ACI) technique, consists in the nutrition of chondrocytes through three-dimensional structures (scaffolds). They are provided by synthetic and / or biological components, which will ensure the trophic environment for cell growth and the consequent repair of the lesion. Indications for the ACI technique are: Outerbridge grade III or IV symptomatic chondral or osteochondral lesions> 2 cm2, age 18 to 55 years (daily active patients). Contraindications are: lesions with loss of more than 50% of cartilage thickness (narrowing of the joint space), smoking, systemic diseases, BMI> 30, age> 55 years (relative contraindication), collagen allergy. The advantage of this technique is that it tends to restore as much as possible the hyaline-type cartilage tissue compared to the microfracture technique. On the other hand, disadvantages include prolonged tissue maturation period, late return to sport, high costs, and poor long-term results (21). However, the literature describes a regenerative technique that does not involve the removal of chondrocytes. This is the AMIM (Acellular matrix induced microfractures) technique. It consists in the direct application of the scaffold (e.g. Maioregen® Finceramica) at the level of the lesion, and in some cases in association with microfractures, according to the extent of the damage. The advantage of this repair technique is that it allows the formation of the newly formed cartilage tissue without cell removal, with gains in economic terms and surgical timing (22).
The Future of Cartilage Repair: Cellular Sources and New Scaffolds
-
- Mesenchymal stem cells (MSCs)
These are cells with high proliferative activity from different tissue sources, such as bone marrow, adipose tissue, synovial membrane, umbilical cord, etc. To date, they represent the most used cells in regenerative medicine thanks to their wide potential for differentiation / proliferation, anti- inflammatory and immunomodulation properties. In the context of cartilage repair, chondrocyte differentiation and proliferations are variable about mesenchymal cells. The review conducted by. Medvedeva EV et al. states that synovial mesenchymal cells demonstrate good differentiation potential into joint chondrocytes. Despite this, they are not free of limits; in fact, it seems that such cells can cause mixed differentiated cells or tissues, which are poorly functional, because they weigh accordingly to the outcome of the cartilage treatment (23-24-25).
-
- Embryonic stem cells (ESC)
These cells are characterized by an unlimited potential for proliferation, a condition that allows us to give life to various types of cell lines.
The limit of these cells is the high regenerative capacity. Studies suggest that a number of growth factors are required in order to generate the chondrocyte line, including BMP4, TGF-beta, and bFGF. This method involves numerous steps, which makes the cell highly unstable, therefore potentially promoter of neoplasms. Disadvantages associated with using ESCs for cartilage regeneration include ethical concerns about the destruction of a human embryo, host immune rejection, poor survival of human ESCs following cell mass disintegration, and the risk of teratoma formation (26).
-
- Induced pluripotent stem cells (iPSC)
These cells acquire the proliferative and differentiative properties thanks to conversion mechanisms in the laboratory by means of the assistance of a number of transcription factors, which are: Yamanaka factors (TF 4 (ott4) the binder octomom, sex determining region Y (SRY) -box 2 (Sox2), cMyc and factor 4 similar to Krüppel (Klf4)). The cells most used in this conversion are fibroblasts, keratinocytes, mesenchymal cells, adipose stem cells, melanocytes and neurons. Strategies and procedures for the generation of chondrocytes from human iPSCs (hiPSCs) are currently under development. The phases of this process are variable in the various laboratories. However, the limit of these cells is similar to embryonic stem cells; in fact, the conversion itself involves various biochemical steps responsible for genetic instability, potential development of tumors or immune responses on the part of the recipient. Some scientific works have tried to analyze and resolve the problem of the abnormal immune response through an evaluation of the genome of the cells obtained in order to find immunological compatibility (27).
-
- Nasal chondrocytes
The regenerative potential of these cells was demonstrated in the study by Wenliang Chen et all. They demonstrated their regenerative potential for the development of cartilage tissue. These cells, when cultured in vitro, expressed chondrogenic markers (Col2A1, ACAN SOX9) (28).
-
- Scaffolds
They guarantee the mechanical properties (biocompatibility and wear resistance) and the nutritional environment for the cartilage tissue. The most common scaffolds are based on poly-glycolic acid or poly-lactic acid and are constructed like tissues, with good porosity and a surface to which cells can easily adhere to. To date, such “supports” are produced using various techniques, including freeze drying, molding, electrospinning, 3D bio- printing and stereolithography. Each scaffold has mechanical characteristics (breaking strength, manageability, three-dimensionality and visco- elasticity), chemical characteristics (hydrophilicity) and biological characteristics (chondro-conduction, biocompatibility and chondro- induction) (29). The last frontier in the realization of scaffolds is 3D bio- printing, based on computer-aided design (CAD), a condition that allows us to customize the construct based on the shape of the single defect. This innovation is documented by Zak L. et all who highlights a good short-term clinical-radiographic result in the treatment of large focal chondral lesions (30).
Now days the scaffolds are formed of synthetic material and biological material, both having advantages and disadvantages. As for synthetic products, the use of hydrogel-based bio-inks is in vogue, which according to studies represents a good compromise in ensuring the right environment for cell growth and mechanical support, as its water (~ 80 % by weight) is similar to that of articular cartilage. The polymers used in hydrogels are often found in nature. Among these, alginate, agarose and silk fibroin favor a low rate of biodegradation and compatibility with chondrocytes. However, they tend to reduce the chondrocyte growth due to their adhesiveness.
With regard to natural substances (such as collagen and hyaluronic acid, considered intrinsic components of joint cartilage), it can be said that they support cell attachment and stimulate the formation of the ECM, but show poor mechanical stability and are subject to intense biodegradation. In contrast, synthetic polymers are superior to natural ones in terms of biodegradation and biomechanical stability, but often demonstrate poor biocompatibility and modifications to provide specific biological functions (31).
Discussions and Conclusions
In this article, we have analyzed the most relevant treatments for knee cartilage repair, each of which stands out for its clinical indications and for their restorative quality and outcomes. Consequently, the closer one gets to restoring natural cartilage, the better the outcomes of regenerative medicine will be. To date, the protocols documented in the literature include both conservative and surgical treatment methods. In the context of co-repair, the goal is the reduction of symptoms but above all the prevention of premature osteoarthritis. In other words, we could consider chondroprotection as the first therapeutic step. There are many scientific papers on the results of various co-repair techniques; as regards the chondral abrasion, in the review by Johnson LL et al. it is believed that this method is effective in resolving pain symptoms, but does not prevent the development of osteoarthritis; this stems from the fact that articular fibrocartilage does not exhibit the same biomechanical characteristics as natural cartilage and to conclude by believing that the future use of growth factors could be the key to healing chondral lesions with mature cartilage tissue (32). Regarding chondral drilling, the systemic review by Liang Gao et al. about the cartilage repair following subchondral drilling on animal models, where 12 publications were evaluated from which the degree of cartilage repair was assessed by comparing the technique of chondral abrasion with cartilage drilling; the data from this systematic review indicate that subchondral perforation produces an improvement in structural repair of the articular cartilage short- term versus spontaneous repair in multiple small and large animal models (33). In the randomized controlled study of Solheim et al. 40 patients aged from 18 to 50 years with symptomatic cartilage lesions at the level of the trochlea or femoral condyles with dimensions ranging from 2 to 6 cm2 were evaluated, of which 20 treated with microfractures and 20 treated with mosaic plastic; symptoms were evaluated at 12 months, 5, 10 and 15 years after surger, noting that the mean Lysholm score was significantly higher in the mosaicplasty group than the microfracture group at 12 months, median 5 years, median 10 years, and minimum 15 years; the authors conclude that at short, medium, and long term (minimum 15 years), mosaicplasty results in a better, clinically relevant outcome than microfracture in articular cartilage defects (2-5 cm2) of the distal femur of the knee in patients aged 18 to 50 years (34).
In the pilot study of Enea et al. nine patients with focal lesions of the condylar articular cartilage treated with arthroscopic microfractures (MFX) and collagen membrane dipped in autologous bone marrow concentrate (BMC) from the iliac crest are evaluated. Patients were retrospectively assessed using various outcome assessment tools and MRI scans. at 12 months all repairs appeared near normal, histological analysis showed hyaline cartilage repair in one lesion, fibrocartilage repair in two lesions, and a mixture of both in one lesion (35). Comparing the microfracture technique with the nanofractures technique, the latter reduces trabecular compaction, allowing deeper access to the subchondral bone than conventional micro fractures, improving the filling of the lesion and developing a greater amount of hyaline cartilage, as stated by Peñalver JM et al. which describe a particular technique for the treatment of grade III-IV chondral lesions; it concerns a modification of the NAMIC procedure using drills for mosaicplasty to prepare the surface on which to subsequently prepare the microholes, subsequently a collagen membrane is applied arthroscopically, preventing the loss of regenerating cells and growth factors in the joint space; the authors conclude that nanofractures reduces trabecular compaction and allows deeper access to subchondral bone than conventional microfracture, improving lesion filling and production of higher hyaline cartilage (36).
Chondroregeneration, aiming at the complete restoration of hyaline cartilage, would represent the most performing technique. Considering the most recent scientific articles, there are differences in terms of outcomes regarding the various techniques. Hangody L. et all. talks about the ten-year results of autologous osteochondral mosaicplasty in 831 patients were analyzed through clinical scores, imaging techniques, arthroscopy, histological examination of biopsy specimens, and cartilage stiffness measurements were used to evaluate clinical outcomes and quality of transplanted cartilage; in according to these surveys, good to excellent results were obtained in 92% of treated patients with femoral condylar implants, 87% of those treated with tibial resurfacing, 79% of those treated with patellar and / or trochlear mosaicplasty, and 94% of those treated with talus procedures. Long-term disturbances of the donor site, evaluated with the use of the Bandi score, they showed that patients had 3% morbidity after mosaic plastics, sixty-nine out of eighty-three arthroscopically evaluated patients followed arthroscopically showed good tissue healing; they conclude that the autologous osteochondral mosaic plastic appears to be an alternative for small to medium sized focal condo treatment (37).
D’ Ambrosi R. et all. this study evaluates the clinical and radiological efficacy of three-dimensional acellular scaffolds (MaioRegen) in osteochondral defects of the knee. In a total of 471 patients (mean age 34.07 ± 5.28 years), 500 lesions (202 (40.4%) medial femoral condyles, 107
(21.4%) lateral femoral condyles, 28 (5, 6%) tibial plateaus, 46 (9.2%), 74 trochleas (14.8%) patellae and 43 (8.6%) unspecified femoral condyles). In almost all studies, significant clinical improvement was reported with further improvement up to 5 years after surgery, a total of 59 complications were reported of which 52 (11.1%) had minor complications and 7 (1, 48%) major complications with a total of 16 (3.39%) failures; conclude that the treatment of osteochondral defects of the knee with the osteochondral substitute MaioRegen reporting satisfactory and reliable results that are promising at medium-term follow-up with a low rate of complications and failures (38).
The study by Bentley R. et all. represents the first long-term randomized study comparing autologous chondrocyte implantation and mosaicplasty in 100 patients with a minimum follow-up of ten years. The mean age of the patients at the time of surgery was 31.3 years (16 to 49); the mean duration of symptoms before surgery was 7.2 years (9 months to 20 years). The mean size of lesions analyzed for the ACI group was 440.9 mm2 (100 to 1050), while for mosaicplasty it was 399.6 mm2 (100 to 2000). Patients had an average of 1.5 operations before (0 to 4) the joint cartilage defect. Assessments were performed through the modified Cincinnati knee score and the Stanmore-Bentley functional assessment system. The number of patients whose repair failed at ten years was ten of 58 (17%) in the ACI group and 23 of 42 (55%) in the plastic mosaic group (p <0.001). The authors conclude that the functional outcome of those patients with a surviving graft was significantly better in patients undergoing ACI than in mosaic plastic (p = 0.02) (39). The same can be said for the ACI technique, based on the transplantation of chondrocytes, that provides advantages, but at the same time the limits of this technique. In fact, the literature shows discordant results; in the systemic review by Kraeutler MJ et al. The systemic review compares the medium- and long-term (5 years) clinical outcomes of MFx versus ACI for focal chondral knee defects. The authors evaluated treatment failure rates, MRIs, and patient-reported outcome scores (Lysholm, Knee Injury and Osteoarthritis Outcome Score [KOOS] and Tegner scores); Five studies (3 level I studies, 2 level II studies) were identified that met the inclusion criteria, including a total of 210 patients (211 lesions) undergoing MFx and 189 patients (189 lesions) undergoing ACI. The mean follow-up across all studies was 7.0 years. Four studies used first generation periosteum-based ACI (P-ACI) and 1 study used third generation matrix-associated ACI (M-ACI). Treatment failure occurred in 18.5% of patients undergoing ACI and 17.1% of patients undergoing MFx (P = .70). The authors found that Lysholm and KOOS scores improved for both groups in the studies, with no significant differences in improvement between groups. They conclude that both techniques analyzed can give good results better clinicians in medium- and long-term follow-up with no significant differences between groups (40). On the contrary Na Y et all. state that the more innovative ACI versions (ACI III gen. or MACI) give more satisfactory results in the medium term than microfractures (41). After careful consideration of the results analyzed in this review, we can state that the poor long-term results (particularly about chondroregeneration) are attributable to the reduced effectiveness of the technique itself. Consequently, we hypothesize that the key to achieve the best results could be the creation of an increasingly favorable environment for chondrocyte growth, together with the design of scaffolds ever closer to the characteristics of a natural EMC, in order to obtain cell lines able to give life to mature and performing chondrocytes when appropriately stimulated. However, although biotechnology represents the basis of regenerative medicine, we believe that other factors of equal importance should be currently considered, such as the prevention of cartilage lesions, the optimization of the surgical technique and the clinical- diagnostic setting of the patient (42-43-44). We advocate the need of high level of evidence works that could provide definitive results on the outcomes and complications, with regards to the different aforementioned techniques for the treatment of knee cartilage lesions. It seems that the choice among the studied technique is often and currently left to the surgeons’ experience and institution availability.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Contributorship Statement:
GRT, FM, PC, VP, PA, GR and AC planned the study. GRT, FM, PC, VP, PA, GR and AC made the literature search, selected and fully reviewed the articles. GRT collected the data. GRT, FM, PC, VP, PA, GR and AC reviewed and discussed the results and findings and made discussion and conclusions. GRT and VP wrote the paper, which was reviewed by FM, PC, PA, GR and AC. GRT submitted the article.
References
- Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth P, Cucchiarini M, Kohn D, Madry H. Alterations of the subchondral bone in osteochondral repair-translational data and clinical evidence. European Cells & Materials. 2013 Jun;25:299–316. doi: 10.22203/ecm.v025a21. discussion 314-6. DOI: 10.22203/ecm.v025a21. PMID: 23813020. [DOI] [PubMed] [Google Scholar]
- Murray IR, Benke MT, Mandelbaum BR. Management of knee articular cartilage injuries in athletes: chondroprotection, chondrofacilitation, and resurfacing. Knee Surg Sports Traumatol Arthrosc. 2016 May;24(5):1617–26. doi: 10.1007/s00167-015-3509-8. [DOI] [PubMed] [Google Scholar]
- Dall’Oca C, Cengarle M, Costanzo A, Giannini N, Vacchiano A, Magnan B. Current concepts in treatment of early knee osteoarthritis and osteochondral lesions; the role of biological augmentations. Acta Biomed. 2017;88(4S):5–10. doi: 10.23750/abm.v88i4-S.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg MC, Altman RD, April K.T, et al. American College of Rheumatology American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- Wernecke C, Braun HJ, Dragoo JL. The Effect of Intra-articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop J Sports Med 3. 2015:2325967115581163. doi: 10.1177/2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari M, Bannuru RR, Babins EM, et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Ther Adv Musculoskelet Dis. 2017 Sep;9(9):231–246. doi: 10.1177/1759720X17729641. Epub 2017 Sep 12. Erratum in: Ther Adv Musculoskelet Dis. 2017 Nov;9(11):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.E, Maurer S.G, Di Cesare P.E. Viscosupplementation for osteoarthritis. Am. J. Orthop. 2000;29:80–88. discussion 88–89. [PubMed] [Google Scholar]
- Laver L, Marom N, Dnyanesh L, Mei-Dan O, Espregueira-Mendes J, Gobbi A. PRP for Degenerative Cartilage Disease: A Systematic Review of Clinical Studies. Cartilage. 2017;8(4):341–364. doi: 10.1177/1947603516670709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozmeriç A, Alemdaroğlu KB, Aydoğan NH. Treatment for cartilage injuries of the knee with a new treatment algorithm. World J Orthop. 2014 Nov 18;5(5):677–84. doi: 10.5312/wjo.v5.i5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedeva EV, Grebenik EA, Gornostaeva SN, et al. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int J Mol Sci. 2017;19(8) doi: 10.3390/ijms19082366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Oca C, Cengarle M, Costanzo A, Giannini N, Vacchiano A, Magnan B. Acta Biomed. Current concepts in treatment of early knee osteoarthritis and osteochondral lesions. the role of biological augmentations. 2017;88(4S):5–10. doi: 10.23750/abm.v88i4-S.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozmeriç A, Alemdaroğlu KB, Aydoğan NH. Treatment for cartilage injuries of the knee with a new treatment algorithm. World J Orthop. 2014 Nov 18;5(5):677–84. doi: 10.5312/wjo.v5.i5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DL, Schenck RC Jr, Wascher DC, Treme G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health. 2016;8(2):153–60. doi: 10.1177/1941738115611350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. J, Steadman J. R. Bone Marrow Stimulation Techniques: Microfractures, Drilling, and Abrasion. In: Articular Cartilage Lesions. New York, NY: Springer; 2004. [Google Scholar]
- Gao Liang, et al. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Disease models & mechanisms. 19 Jun. 2018;11:6. doi: 10.1242/dmm.034280. dmm034280. doi: 10.1242/dmm.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benthien JP, Behrens P. Reviewing subchondral cartilage surgery: considerations for standardized and outcome predictable cartilage remodeling: a technical note. Int Orthop. 2013;37(11):2139–2145. doi: 10.1007/s00264-013-2025-z. doi: 10.1007/s00264-013-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedde P, Cudoni S, Giachetti G, et al. Subchondral bone remodeling: comparing nanofracture with microfracture. An ovine in vivo study. Joints. 2016 Aug 18;4(2):87–93. doi: 10.11138/jts/2016.4.2.087. doi: 10.11138/jts/2016.4.2.087. PMID: 27602348; PMCID: PMC4993551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahla J, Stone J, Mandelbaum BR. How to Manage Cartilage Injuries? Arthroscopy. 2019 Oct;35(10):2771–2773. doi: 10.1016/j.arthro.2019.08.021. doi: 10.1016/j.arthro.2019.08.021. PMID: 31604490. [DOI] [PubMed] [Google Scholar]
- Eirik Solheim. Osteochondral Autograft Transplant (Mosaicplasty) for Knee Articular Cartilage Defects. JBJS Essent Surg Tech. 2019 Oct-Dec;9(4):e34.1–2. doi: 10.2106/JBJS.ST.18.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecca L, Davies, Nicola J. Kuiper. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering (Basel) 2019 Mar;6(1):22. doi: 10.3390/bioengineering6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linghui Dai, Zhenming He, Yanfang Jiang, et al. One-step strategy for cartilage repair using acellular bone matrix scaffold based in situ tissue engineering technique in a preclinical minipig model. Am J Transl Res. 2019;11(10):6650–6659. [PMC free article] [PubMed] [Google Scholar]
- Medvedeva EV, Grebenik EA, Gornostaeva SN, et al. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Mol Sci. 2018 Aug 11;19(8) doi: 10.3390/ijms19082366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhou X, Guan J, Wu M, Zhou J. Co-implantation of bone marrow mesenchymal stem cells and chondrocytes increase the viability of chondrocytes in rat osteo-chondral defects. Oncol Lett. 2018 May;15(5):7021–7027. doi: 10.3892/ol.2018.8195. Jul 3;6(3):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulak J, Szade K, Szade A, Nowak W, Józkowicz A. Adult stem cells: hopes and hypes of regenerative medicine. Acta Biochim Pol. 2015;62(3):329–37. doi: 10.18388/abp.2015_1023. doi: 10.18388/abp.2015_1023. Epub 2015 Jul 22. PMID: 26200199. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Jaenisch R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell. 2016 May 5;18(5):573–86. doi: 10.1016/j.stem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Li C, Peng M, Xie B, Zhang L, Tang X. Autologous nasal chondrocytes delivered by injectable hydrogel for in vivo articular cartilage regeneration. Cell Tissue Bank. 2018 Mar;19(1):35–46. doi: 10.1007/s10561-017-9649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu N, Dong T, Meng A, Meng Z, Zhu B, Lin Y. Research Progress of the Types and Preparation Techniques of Scaffold Materials in Cartilage Tissue Engineering. Curr Stem Cell Res Ther. 2018;13(7):583–590. doi: 10.2174/1574888X12666170718152611. [DOI] [PubMed] [Google Scholar]
- Zak L, Albrecht C, Wondrasch B, et al. Results 2 Years After Matrix-Associated Autologous Chondrocyte Transplantation Using the Novocart 3D Scaffold: An Analysis of28 Clinical and Radiological Data. Am J Sports Med. Jul 2014;42(7):1618–27. doi: 10.1177/0363546514532337. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kennedy P, Bonazza N, Yu Y, Dhawan A, Ozbolat I. Three-Dimensional Bioprinting of Articular Cartilage: A Systematic Review. Cartilage. 2018 Oct 29:1947603518809410. doi: 10.1177/1947603518809410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonson LL. Arthroscopic abrasion arthroplasty: a review. Clin Orthop. 2001;(391 Suppl):S306–S317. [PubMed] [Google Scholar]
- Gao L, Goebel LKH, Orth P, Cucchiarini M, Madry H. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis Model Mech. 2018 Jun 19;11(6) doi: 10.1242/dmm.034280. dmm034280. doi: 10.1242/dmm.034280. PMID: 29728409; PMCID: PMC6031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized Study of Long-term (15-17 Years) Outcome After Microfracture Versus Mosaicplasty in Knee Articular Cartilage Defects. Am J Sports Med. 2018 Mar;46(4):826–831. doi: 10.1177/0363546517745281. [DOI] [PubMed] [Google Scholar]
- Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot studies. Knee. 2015 Jan;22(1):30–5. doi: 10.1016/j.knee.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Peñalver JM, Villalba J, Yela-Verdú CP, Sánchez J, Balaguer-Castro M. All-Arthroscopic Nanofractured Autologous Matrix-Induced Chondrogenesis (A-NAMIC) Technique for the Treatment of Focal Chondral Lesions of the Knee. Arthrosc Tech. 2020;9(6):e755–e759. doi: 10.1016/j.eats.2020.02.007. Published 2020 May 14. doi: 10.1016/j.eats.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of the weight-bearing joints: ten years of experimental and clinic experience. J Bone Joint Surg Am. 2003;85-A(suppl 2):25–32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- D’Ambrosi R, Valli F, De Luca P, Ursino N, Usuelli FG. MaioRegen Osteochondral Substitute for the Treatment of Knee Defects: A Systematic Review of the Literature. J Clin Med. 2019 Jun 1;8(6):783. doi: 10.3390/jcm8060783. doi: 10.3390/jcm8060783. PMID: 31159439; PMCID: PMC6617307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012 Apr;94(4):504–9. doi: 10.1302/0301-620X.94B4.27495. doi: 10.1302/0301-620X.94B4.27495. PMID: 22434467. [DOI] [PubMed] [Google Scholar]
- Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture Versus Autologous Chondrocyte Implantation for Articular Cartilage Lesions in the Knee: A Systematic Review of 5-Year Outcomes. Am J Sports Med. 2018 Mar;46(4):995–999. doi: 10.1177/0363546517701912. [DOI] [PubMed] [Google Scholar]
- Na Y, Shi Y, Liu W, et al. Is implantation of autologous chondrocytes superior to microfracture for articular-cartilage defects of the knee? A systematic review of 5-year follow-up data. Int J Surg. 2019 Aug;68:56–62. doi: 10.1016/j.ijsu.2019.06.007. [DOI] [PubMed] [Google Scholar]
- Placella G, Bartoli M, Peruzzi M, Speziali A, Pace V, Cerulli G. Return to sport activity after anterior cruciate ligament reconstruction in skeletally immature athletes with manual drilling original all inside reconstruction at 8 years follow-up. Acta orthopaedica et traumatologica turcica. 2016;50(6):635–638. doi: 10.1016/j.aott.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinolfi P, Bartoli M, Placella G, et al. Acute patellofemoral instability in children and adolescents. Joints. 2016 Jun 13;4(1):47–51. doi: 10.11138/jts/2016.4.1.047. doi: 10.11138/jts/2016.4.1.047. PMID: 27386447; PMCID: PMC4914373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placella G, Pace V, Foster P. Reconstruction of the medial patellofemoral ligament reconstruction for patients with recurrent patellar dislocation: review of surgical techniques and tips to achieve successful reconstruction. Ann Transl Med. 2016 Dec;4(24):540. doi: 10.21037/atm.2016.11.60. doi: 10.21037/atm.2016.11.60. PMID: 28149901; PMCID: PMC5233527. [DOI] [PMC free article] [PubMed] [Google Scholar]


