Abstract
Background
Toxoplasmosis is a zoonotic disease. It is due to an obligate intracellular protozoan called Toxoplasma gondii (T. gondii). Felids are considered definitive hosts, and humans take part as intermediate hosts. At least one-third of the world’s population is seropositive to the parasite. In addition, to the known modes of transmission, the infection can be transmitted through blood transfusions. The aim of this study is to assess the immune status of blood donors about this disease and estimate the potential risk by blood components.
Methodology
A single cross-sectional study was conducted based on the search for T. gondii antibodies (IgG and IgM) in blood donors. This research was performed using a latex particle agglutination assay confirmed by an enzyme-linked immunosorbent assay (ELISA).
Results
In all, 103 blood donors were involved in this study. The sex ratio of male/ female was 0.75. The recorded rate of exposure to toxoplasmosis in blood donors was 47.7% (95% CI: 35.1-54.3). Significant differences were observed between the prevalence and those of other African countries in West, East, and Central Africa, but not with those of Algerian pregnant women and neighboring North African countries. There was no association between T. gondii seropositivity and the following factors: sex, age, and blood group ABO or Rhesus. Antitoxoplasma IgG was detectable in all positive donors, while IgM was undetectable. All seropositive donors had an IgG titer ≥9 IU/ml. The potential risk of T. gondii transmission ranges from 1 per 100,000 to 17 per 100,000 blood donations.
Conclusion
The seroprevalence of T. gondii infection was comparable to those found in Algerian pregnant women and neighboring North African countries. However, the seroprevalence rate was lower than recorded in other African countries. There is even a risk of transmission of toxoplasmosis through blood transfusions. There is a need to enhance blood safety measures for pregnant, immunocompromised, and multi-transfused people. As the immune status of blood donors may vary by region, there is a need to extend the national studies to the entire country. This study provides the first data on the seroprevalence of T. gondii infection among Algerian blood donors and the risk of its transmission by transfusion of blood components.
Keywords: blood transfusion, risk of transfusion transmission, blood component, seroprevalence, blood donors, toxoplasma gondii, toxoplasmosis
Introduction
Toxoplasmosis is a zoonotic disease. It occurs due to an obligate intracellular protozoan called Toxoplasma gondii (T. gondii). It is known to be one of humans' most common parasitic infections. Felids are definitive hosts, and humans take part as intermediate hosts. There are two forms of T. gondii in humans: Tachyzoite is the active proliferating form seen in the initial, more acute stage of the infection, and Bradyzoite is the slow dividing form. Bradyzoite was developed from tachyzoite. In tissues, bradyzoites form cysts due to the host immune response [1]. Tissue cysts are found in the brain and muscles. In the environment, there is a sporozoite form enclosed in an oocyst. Infection is acquired by ingesting uncooked or contaminated meat, food, or water and by getting in contact with cat or dog feces in the soil. It can also be passed from mother to fetus if a woman is infected during or just before pregnancy [2]. In addition to these known modes of transmission, it has been shown that the infection can be transmitted through blood transfusion or organ transplant from an infected donor [3]. The disease in immunocompetent humans is asymptomatic or with very few clinical manifestations. Still, the parasite may "hide," stay inactive for years, and reactivate when the immune system is compromised. The disease is a significant public health concern. It can have severe or fatal consequences for immunocompromised patients, transplant recipients, and fetuses.
Toxoplasmosis has been reported in almost every part of the world. About a third of the human population is seropositive to the parasite [3]. It is now well established from various studies that the frequency varies from region to region according to geographical areas, dietary habits, and transmission routes. In the case of Algeria, it has been recorded that the prevalence in pregnant women was 47.8% (95% CI: 44.8-51.0) [4]. So far, there is no data on the disease in blood donors in Algeria. Furthermore, there is no recommendation to screen blood donors for T. gondii. So, in Algerian blood banks, screening tests for infectious diseases do not contain T. gondii.
Moreover, this disease is not notifiable in Algeria. To our knowledge, seroprevalence in blood donors provides essential data on the prevalence of infection in the general population. Thus, this study aims to assess the immune status of blood donors and estimate the risk of toxoplasmosis transmission through blood components.
Materials and methods
Selection of subjects and study design
We conducted a single cross-sectional study from October 2018 to December 2018. We carried it out at the blood transfusion department of Sidi Bel Abbès city. We invited regular blood donors who live in Sidi Bel Abbès city to participate and give their consent. The criteria for inclusion and non-inclusion were those of blood donors as recommended by the National guidelines of blood donor selection. We included in this study any Algerian adult male and female able to donate after the physical examination and routine questionnaire. The ethics committee of the University Hospital of Sidi Bel Abbès approved the study. The purpose and procedures of the study were explained to donors. Written informed consent was obtained from all of them. The population consists of about 606 regular donors. The sample size was obtained by using a sample size calculator (https://www.calculator.net/sample-size-calculator.html). For these purposes, we considered a 95% CI, a 5% error, and a 6% prevalence for regular blood donors. Therefore, the minimum sample size needed for this survey was 76 donors.
Donor sampling and processing
About 5 mL of venous blood was collected from each blood donor. The serum was separated from the whole blood by centrifugation at 3500 x g for 15 min. The labeled tubes of serums were frozen and stored at -30∘C until analysis.
Assay
All samples were screened for T. gondii antibodies by latex particle agglutination using Pastorex™ Toxo, Biorad, France. All positive results were tested by enzyme-linked immunosorbent assay (ELISA). We used both ELISA kits. Platelia™ Toxo IgM Biorad kit was used to qualitatively detect IgM antibodies. Platelia™ Toxo IgG Biorad kit for the quantitative determination of IgG antibodies. The test results for IgG were interpreted as recommended by the manufacturer. Titer of IgG were <6 I.U./ml, negative; 6-9 I.U./ml, equivocal; and >9 I.U./ml, positive. All tests were performed following the instructions of the manufacturer.
Risk assessment
To estimate the risk of contamination of a blood donation by T. gondii, we used the mathematical model of the French Institute for Public Health Surveillance. It was estimated by taking the incidence in Algerian pregnant women. The risk is equal to the probability of taking a blood donor during parasitemia multiplied by the incidence of infection. The probability of taking such donors ranges from one to 21 days out of 365 days, with an incidence of 0.3 % [5].
Data analysis
The Kolmogorov-Smirnov test of normality was used to analyze the values. Continuous variables are expressed as the mean and SD or the median and range in the case of not normally distributed data. Paired and unpaired data were compared using the Wilcoxon and U-Whitney tests. A t-test was used to compare the means between groups. Besides, qualitative variables are reported as numbers or percentages with 95% CIs. Chi-square or Fisher's test was used to check the association of seropositivity with transfusion data. The ORs and their 95% CIs were calculated. Data were analyzed using IBM SPSS Statistics software. For all statistical tests, p<0.05 was considered statistically significant.
Results
A total of 103 blood donors were enrolled in this study. The sex ratio of male/female was 0.75. Baseline data for all patients, men and women, are shown in Table 1, where we can observe a similar distribution between genders.
Table 1. Baseline data of 103 qualified blood donors.
Chi-square test and *Mann-Whitney U test. Data are expressed as median value (minimum-maximum).
NS: Nonsignificant difference.
| All | Male | Female | P-value | |
| Number of subjects | 103 | 44 | 59 | |
| Age (years) | 31 (19-61) | 32.5 (20-49) | 31 (19-61) | NS* |
| Blood Group ABO | NS | |||
| O | 44 | 18 | 26 | |
| A | 30 | 14 | 16 | |
| B | 22 | 9 | 13 | |
| AB | 7 | 3 | 4 | |
| RH Positive/RH Negative | 84/19 | 33/11 | 51/8 | NS |
| Anti-Toxoplasma gondii antibodies | NS | |||
| IgG Negative/IgG positive | 57/46 | 24/20 | 33/26 | |
| IgM Negative/IgM positive | 103/0 | 44/0 | 59/0 |
The prevalence of exposure to toxoplasmosis in blood donors was 47.7%. (95% CI: 35.1-54.3). The comparison of the prevalence of the current study and other reports is summarized in Table 2. It is apparent from this table that the prevalence found in this study was not significantly different from those found among pregnant women in Algeria and neighboring North African countries. However, significant differences were observed between the prevalence and those of other African countries in West, East, and Central Africa, with 0.05 > p>0.01 and p<0.001.
Table 2. Seroprevalence of toxoplasmosis from reports of neighboring countries.
ELISA: Enzyme-linked immunosorbent assay; EIA: Enzyme immuno assay; IFA: Immuno fluorescence assay; NS: Nonsignificant difference.
| Author | Country | Population | Number | Method | Prevalence % (95% CI) | P-value | Reference |
| Belkacemi M and Heddi B (2022) | Algeria | Blood donors | 103 | ELISA | 44.7 (35.1-54.3) | Current Study | |
| Messerer L et al. (2014) | Algeria | Pregnant women | 1028 | EIA | 47.80 (44.80-51.00) | NS | [5] |
| Lachkhem A et al. (2020) | Tunisia | Blood donors | 800 | ELISA-IFA | 44.40 (40.93-47.82) | NS | [6] |
| Laboudi M (2014) | Morocco | Pregnant women | 1169 | ELISA | 47.00 (46.97-47.03) | NS | [7] |
| Mousa DA et al. (2011) | Libya | Pregnant women | 143 | ELISA | 44.80 (44.72-44.88) | NS | [8] |
| Elsheikha HM et al. (2004) | Egypt | Blood donors | 260 | ELISA | 59.60 (95.54-59.66) | NS | [9] |
| Siransy L et al. (2016) | Ivory Coast | Blood donors | 106 | ELISA | 67.92 (67.83-68.01) | = 0.0210 | [10] |
| Abamecha F and Awel H (2016) | Ethiopia | Pregnant women | 232 | ELISA | 85.30 (85.35-85.25) | <0.0001 | [11] |
| Doudou Y et al. (2014) | Congo | Pregnant women | 781 | ELISA | 80.30 (77.50-83.10) | <0.0001 | [12] |
As Table 3 shows, there is no association between T. gondii seropositivity and the following factors: sex, age, and blood group ABO or Rhesus.
Table 3. Factors related to blood donors associated with toxoplasmosis.
NS: Nonsignficant difference.
| Variable | Anti-Toxoplasma | T. gondii Antibodies | P-value |
| Positive | Negative | ||
| Age (years) | 31.5 (19-58) | 31 (19-61) | NS |
| Sex | NS | ||
| Male | 20 | 24 | |
| Female | 26 | 33 | |
| Blood Group | NS | ||
| O | 21 | 23 | |
| A | 12 | 18 | |
| B | 10 | 12 | |
| AB | 3 | 4 | |
| RH Blood Group | NS | ||
| RH Positive | 38 | 46 | |
| RH Negative | 8 | 11 | |
| Total | 46 | 57 |
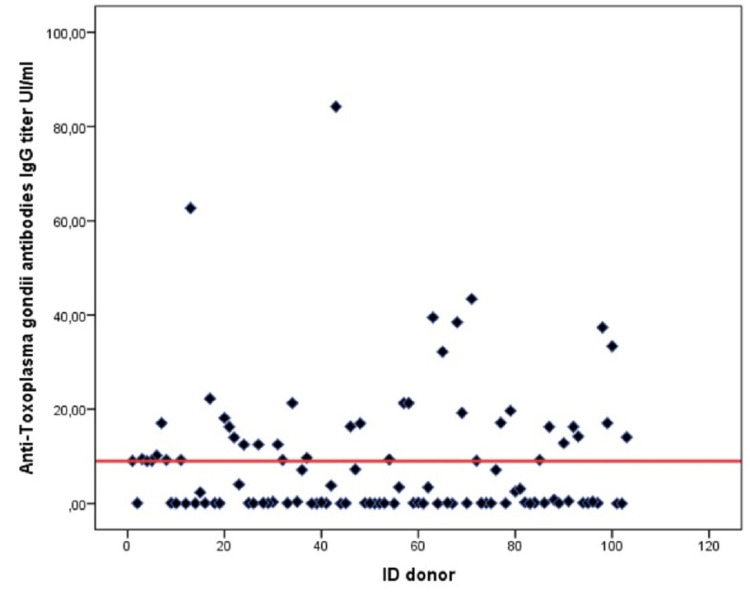
Antitoxoplasma IgG was detectable in all positive donors, while IgM was undetectable. From the graph below, shown in Figure 1, we can see that all seropositive donors had an IgG titer ranging from 9 to 84 IU/ml.
Figure 1. Distribution of anti-Toxoplasma gondii antibodies IgG titer among blood donors.
Table 4 presents a quantitative assessment of the risk of contamination of a blood donation by T. gondii in an endemic situation. A closer look at this table shows that the risk of transfusion transmission of toxoplasmosis ranges from 1 per 100,000 to 17 per 100,000 for donations.
Table 4. The quantitative estimation of the risk of contamination of a blood donation by Toxoplasma gondii.
| Items | Low hypothesis | High hypothesis |
| Number of days | 365 | 365 |
| Span of parasitemia (days) | 1 | 21 |
| Probability to take a blood donor in parasitemia stage | 1/365 | 21/365 |
| Incidence of infection % | 0.3 | 0.3 |
| Risk of infected donation for 100,000 blood donations | 1 | 17 |
Discussion
This study permitted us to estimate the seroprevalence of toxoplasmosis in blood donors. The prevalence rate obtained in this study is in line with that found in Algerian pregnant women [5]. Moreover, this prevalence was comparable to those described in neighboring countries in North Africa due to the same socio-cultural behaviors [6-9]. However, the prevalence was lower than recorded in other African countries of West, East and Central Africa [10-12]. This discrepancy in prevalence between these countries may be ascribed to differences in economic development levels, hygienic conditions, and feeding habits, such as eating raw meat in Ethiopia [13]. Another reason for this difference may be linked to the local climatic conditions. It is well known that T. gondii is widely spread, mainly in warm and humid areas [14]. It has been shown that at temperate to tropical temperatures, oocysts remain infectious for up to one year [2].
It is notable that there was no link between sex and the seropositivity rate. Thus, both genders are at equal risk of exposure to toxoplasmosis. This observation is in accord with the available data [9,15]. Furthermore, we found no association between age and the presence of T. gondii antibodies. However, some studies describe an increase in seroprevalence with age due to the increased risk of being exposed to infection sources [16,17]. Therefore, our result may be explained by the fact that most of our blood donors are young. Moreover, no association was found between the ABO or RhD blood group and the presence or absence of T. gondii antibodies. These findings support evidence that these blood groups are not a crucial factor in high or reduced risk of infection [18,19].
Consistent with the literature, this study showed that blood donors had the infection in the past, which may be occurred at a younger age [19-21]. It is well known that a hallmark of toxoplasmosis is that the parasite persists for months to years or even a lifetime in tissues. Chronic disease underlies recrudescent ocular toxoplasmosis in immunocompetent individuals. It has been associated with several psychiatric disorders and behavioral changes [22]. This finding implies that the blood donors are exposed to retinochoroiditis, encephalitis, or epilepsy later in life. In some cases, it has been observed that latent toxoplasmosis can reactivate and become acute in blood [23]. Thus, the active disease may lead to the possibility of a hematogenous transmission. It has been shown that T. gondii was isolated from the blood of infected donors up to four years after infection [24]. Hence, the presence of the parasite in the bloodstream can be a source of infection for patients receiving a blood transfusion. It has been demonstrated that latent toxoplasmosis is correlated with various disease burdens. Thus, we think it is a significant health issue in our area [25].
The key finding to emerge from the analysis is that there is a risk of transfusion-transmissible toxoplasmosis in our region. So, the danger of transmission of T. gondii infection by receiving a blood transfusion from apparently healthy asymptomatic persons is of great societal concern. However, this risk has been neglected. This fact may explain that screening for T. gondii infection is not currently performed in blood banks. Moreover, It is well known that parasites can survive in leukocytes for up to seven weeks between 2 °C and 4 °C [26]. This ability has been observed to be a factor that increases the risk of transmission through blood transfusion [27]. As far we know, not all blood products are routinely leukoreduced in our blood bank. Thus, it can pose a threat to immunosuppressed patients who are at higher risk of exposure to transfusion-transmitted infections. It has been reported that a history of blood transfusion is a risk factor for toxoplasmosis [28]. Thus, the multi-transfused patient has a risk of infection with T. gondii. Therefore, there is a need to increase security measures for patients at higher risk of infection with T. gondii. Thus, multi-transfused, immunocompromised people and pregnant women should receive T. gondii-free blood. The approach could be keeping a stock of blood anti-T. gondii negative, or provision of leukoreduced (filtered) blood components, or even better, using the pathogen inactivation method [29,30].
Conclusions
T. gondii infection obtained in this study was comparable to that of Algerian pregnant women and neighboring North African countries. However, this rate was lower than that recorded in other West, East, and Central African countries. Coming back to the issue highlighted at the beginning of this paper, it is now possible to state that an old infection was found in the blood donors of our region. It is well known that chronic toxoplasmosis plays a role in blood transmission as latent toxoplasmosis. It can be reactive in some circumstances and be acute in blood. In light of our results, we concluded that there is a risk of toxoplasmosis transmission through blood transfusion. To reduce the morbidity from a blood transfusion, there is a need to strengthen transfusion security measures for persons immunocompromised, multi-transfused, and pregnant women. This research appears to be the first to provide data on the seroprevalence of T. gondii infection among blood donors in Algeria and the risk of transmission by blood components. This report would help develop prevention strategies for transfusion-transmitted T. gondii infection in our country. There is a need to expand national studies, as the immune status of blood donors may vary by region due to the climatic differences in the country.
Acknowledgments
We would like to thank all the staff of the Blood Transfusion Department of Sidi Bel Abbès city.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Ethics Committee of Sidi Bel Abbès University Hospital issued approval CE/CHUSBA/05/ 2018
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Epidemiology of and diagnostic strategies for toxoplasmosis. Robert-Gangneux F, Dardé ML. Clin Microbiol Rev. 2012;25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neglected parasitic infections in the United States: toxoplasmosis. Jones JL, Parise ME, Fiore AE. Am J Trop Med Hyg. 2014;90:794–799. doi: 10.4269/ajtmh.13-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toxoplasma gondii: from animals to humans. Tenter AM, Heckeroth AR, Weiss LM. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toxoplasmosis: stages of the protozoan life cycle and risk assessment in humans and animals for an enhanced awareness and an improved socio-economic status. S Al-Malki E. Saudi J Biol Sci. 2021;28:962–969. doi: 10.1016/j.sjbs.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.[Seroprevalence of toxoplasmosis in pregnant women in Annaba, Algeria] Messerer L, Bouzbid S, Gourbdji E, Mansouri R, Bachi F. Rev Epidemiol Sante Publique. 2014;62:160–165. doi: 10.1016/j.respe.2013.11.072. [DOI] [PubMed] [Google Scholar]
- 6.Seroprevalence of Toxoplasma gondii among healthy blood donors in two locations in Tunisia and associated risk factors. Lachkhem A, Lahmar I, Galal L, et al. Parasite. 2020;27:51. doi: 10.1051/parasite/2020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Review of toxoplasmosis in Morocco: seroprevalence and risk factors for toxoplasma infection among pregnant women and HIV- infected patients. Laboudi M. Pan Afr Med J. 2017;27:269. doi: 10.11604/pamj.2017.27.269.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toxoplasma gondii infection in pregnant women with previous adverse pregnancy outcome. Mousa DA, Mohammad MA, Toboli AB. https://medicaljournal-ias.org/jvi.aspx?pdir=ias&plng=eng&un=IAS-84803 Med J Islamic World Acad Sci. 2011;19:95–102. [Google Scholar]
- 9.Seroprevalence of and risk factors for Toxoplasma gondii antibodies among asymptomatic blood donors in Egypt. Elsheikha HM, Azab MS, Abousamra NK, Rahbar MH, Elghannam DM, Raafat D. Parasitol Res. 2009;104:1471–1476. doi: 10.1007/s00436-009-1350-z. [DOI] [PubMed] [Google Scholar]
- 10.Immunity status of blood donors regarding Toxoplasma gondii infection in a low-income district of Abidjan, Côte d'Ivoire, West Africa. Siransy L, Dasse SR, Dou Gonat SP, et al. J Immunol Res. 2016;2016:6830895. doi: 10.1155/2016/6830895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seroprevalence and risk factors of Toxoplasma gondii infection in pregnant women following antenatal care at Mizan Aman General Hospital, Bench Maji Zone (BMZ), Ethiopia. Abamecha F, Awel H. BMC Infect Dis. 2016;16:460. doi: 10.1186/s12879-016-1806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toxoplasmosis among pregnant women: high seroprevalence and risk factors in Kinshasa, Democratic Republic of Congo. Doudou Y, Renaud P, Coralie L, et al. Asian Pac J Trop Biomed. 2014;4:69–74. doi: 10.1016/S2221-1691(14)60211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seroprevalence and potential risk factors of T. gondii infection in pregnant women attending antenatal care at Bonga Hospital, Southwestern Ethiopia. Negero J, Yohannes M, Woldemichael K, Tegegne D. Int J Infect Dis. 2017;57:44–49. doi: 10.1016/j.ijid.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Changing climate-changing pathogens: Toxoplasma gondii in North-Western Europe. Meerburg BG, Kijlstra A. Parasitol Res. 2009;105:17–24. doi: 10.1007/s00436-009-1447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epidemiology of toxoplasmosis in SERBIA: a cross-sectional study on blood donors. Stopić M, Štajner T, Marković-Denić L, et al. Microorganisms. 2022;10 doi: 10.3390/microorganisms10030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prevalence of antibodies to Toxoplasma gondii among blood donors in Abha, Asir Region, south-western Saudi Arabia. Al-Amari OM. https://pubmed.ncbi.nlm.nih.gov/7775896/ J Egypt Public Health Assoc. 1994;69:77–88. [PubMed] [Google Scholar]
- 17.Seroprevalence and associated risk factors for Toxoplasma gondii infection in healthy blood donors: a cross-sectional study in Sonora, Mexico. Alvarado-Esquivel C, Rascón-Careaga A, Hernández-Tinoco J, et al. Biomed Res Int. 2016;2016:9597276. doi: 10.1155/2016/9597276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basic researchLack of association between ABO histo-blood groups, secretor and non-secretor phenotypes, and anti-Toxoplasma gondii antibodies among pregnant women from the northwestern region of of São Paulo State, Brazil. Brandao de Mattos CC, Cintra JR, Ferreira AI, et al. https://www.termedia.pl/Basic-research-Lack-of-association-between-ABO-histo-blood-groups-secretor-and-non-secretor-phenotypes-and-anti-Toxoplasma-gondii-antibodies-among-pregnant-women-from-the-northwestern-region-of-S-227-,19,11195,0,1.html Archives of Medical Science. 2008;4:254–258. [Google Scholar]
- 19.Toxoplasma gondii seroprevalence among blood donors in Zahedan, Southeastern Iran. Modrek MJ, Mousavi M, Saravani R. Int J Infect. 2014;1:0. [Google Scholar]
- 20.Prevalence of IgG and IgM anti-Toxoplasma gondii antibodies in blood donors at Urmia Blood Transfusion Organization, Iran. Tappeh KH, Musavi J, Safa MB, Galavani H, Alizadeh H. Turkiye Parazitol Derg. 2017;41:1–4. doi: 10.5152/tpd.2017.5066. [DOI] [PubMed] [Google Scholar]
- 21.Seroprevalence of Toxoplasma gondii infection in blood donors in Makkah Al Mukarramah. Mohamed K, Zamzami H, Deqnah N, et al. https://scialert.net/abstract/?doi=aje.2019.25.31 Asian J Epidemiol. 2019;12:25–31. [Google Scholar]
- 22.Long-term impact of Toxoplasma gondii infection on human monocytes. Ehmen HG, Lüder CG. http://10.3389/fcimb.2019.00235. Front Cell Infect Microbiol. 2019;9:235. doi: 10.3389/fcimb.2019.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Silveira C, Vallochi AL, Rodrigues da Silva U, et al. Br J Ophthalmol. 2011;95:396–400. doi: 10.1136/bjo.2008.148205. [DOI] [PubMed] [Google Scholar]
- 24.Provision of a panel of anti-toxoplasma-negative blood donors. McDonald CP, Barbara JA, Contreras M, Brown S. Vox Sang. 1989;57:55–58. doi: 10.1111/j.1423-0410.1989.tb04984.x. [DOI] [PubMed] [Google Scholar]
- 25.Toxoplasmosis--a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. Flegr J, Prandota J, Sovičková M, Israili ZH. PLoS One. 2014;9:0. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seroprevalence and molecular diagnosis of Toxoplasma gondii infection among blood donors in southern Iran. Sarkari B, Shafiei R, Zare M, Sohrabpour S, Kasraian L. J Infect Dev Ctries. 2014;8:543–547. doi: 10.3855/jidc.3831. [DOI] [PubMed] [Google Scholar]
- 27.[Quantitative estimate of the risk of blood donation contamination by infectious agents] Pillonel J, Brouard C, Laperche S, Barin F, Bernillon P, de Valk H. Transfus Clin Biol. 2009;16:138–145. doi: 10.1016/j.tracli.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Association between Toxoplasma gondii infection and history of blood transfusion: a case-control seroprevalence study. Alvarado-Esquivel C, Sánchez-Anguiano LF, Hernández-Tinoco J, et al. J Int Med Res. 2018;46:1626–1633. doi: 10.1177/0300060518757928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seroprevalence of Toxoplasma gondii antibodies in North Indian blood donors: implications for transfusion transmissible toxoplasmosis. Elhence P, Agarwal P, Prasad KN, Chaudhary RK. Transfus Apher Sci. 2010;43:37–40. doi: 10.1016/j.transci.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Pathogen inactivation of cellular blood products-an additional safety layer in transfusion medicine. Seltsam A. Front Med (Lausanne) 2017;4:219. doi: 10.3389/fmed.2017.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]