Abstract
We analyzed 72 children’s textile products marketed as stain-resistant from US and Canadian stores, particularly school uniforms, to assess if clothing represents a significant route of exposure to per- and polyfluoroalkyl substances (PFAS). Products were first screened for total fluorine (total F) using particle-induced γ-ray emission (PIGE) spectroscopy (n = 72), followed by targeted analysis of 49 neutral and ionic PFAS (n = 57). PFAS were detected in all products from both markets, with the most abundant compound being 6:2 fluorotelomer alcohol (6:2 FTOH). Total targeted PFAS concentrations for all products collected from both countries ranged from 0.250 to 153 000 ng/g with a median of 117 ng/g (0.0281–38 100 μg/m2, median: 24.0 μg/m2). Total targeted PFAS levels in school uniforms were significantly higher than in other items such as bibs, hats, stroller covers, and swimsuits, but comparable to outdoor wear. Higher total targeted PFAS concentrations were found in school uniforms made of 100% cotton than synthetic blends. Perfluoroalkyl acids (PFAAs) precursors were abundant in school uniforms based on the results of hydrolysis and total oxidizable precursor assay. The estimated median potential children’s exposure to PFAS via dermal exposure through school uniforms was 1.03 ng/kg bw/day. Substance flow analysis estimated that ∼3 tonnes/year (ranging from 0.05 to 33 tonnes/year) of PFAS are used in US children’s uniforms, mostly of polymeric PFAS but with ∼0.1 tonne/year of mobile, nonpolymeric PFAS.
Keywords: school uniforms; children’s products; PFAS; fluorotelomer alcohols (FTOHs); fluorotelomer methacrylates (FTMAcs); PFAS dermal exposure; PFAS substance flow analysis, PFAS hydrolysis; total oxidizable precursor (TOP) assay
Short abstract
High per- and polyfluoroalkyl substance (PFAS) levels in school uniforms could be an important source of PFAS exposure for children.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are anthropogenic chemicals that have been used in industrial and consumer products such as fire-fighting foams, food packaging materials, and textiles for decades.1−3 Numerous studies have reported the presence of PFAS in environmental and biological matrices,4−7 with consumer products being one source. Recently, Glüge et al.8 summarized the uses of over 1400 PFAS, and their function in each application or specific type of products (where available). The extensive list included consumer products like textiles, cookware, personal care products, and sports articles.8
PFAS-treated goods such as clothing may be an important source of direct human exposure, especially for children, as well as a source of PFAS to the environment (and hence indirect human exposure). The Danish Environmental Protection Agency9 in 2015 tested children’s textile products and assessed children’s health risk associated with PFAS released from these products. The study identified that items most often containing PFAS were weather-proof items including snowsuits, skiwear, and matching items such as mittens, hoods, and hats, where PFAS are added to confer water resistance.9 The report concluded that the major routes of exposure for children wearing these clothes are dermal contact (mainly of ionic PFAS), air inhalation and dust ingestion (mainly of neutral/volatile PFAS), and direct oral exposure due to frequent hand-to-mouth behaviors.9−11 Because the report focused mostly on outdoor wear items, the authors estimated that dermal exposure would be limited to hands touching the outer body of the clothing. Several other studies, primarily focused on adult products available in the European market, have focused on PFAS in outdoor wear clothing.12−20 Several studies have examined children’s clothing, with most focused on outdoor wear items (ND–10791 μg/m2);12,15,19−23 five studies have measured PFAS concentrations in clothing that comes into direct contact with the skin, including infant apparel (ND–203 ng/g) like bibs (ND–16 ng/g)17,21,22 and uniforms (ND–976 ng/g).24−26 PFAS have also been analyzed in daycare carpets (32.2–8500 ng/g), dust (8.1–6470 ng/g) and nap-mats (1.6–600 ng/g),27,28 and children’s car seats (ND–268 ng/g).29 In the European Union, there are limits only for perfluorooctanoic acid (PFOA) (<25 ng/g) and perfluorooctyl sulfonate (PFOS) (<1 μg/m2) in textiles; PFOS and PFOA concentration in some of the aforementioned products exceeded these limits.30,31 PFAS are not regulated in textiles products in Canada. In these applications, PFAS are used to impart stain resistance, making them particularly useful for school uniforms.
The previous studies that measured PFAS in textile items focused on the following eight groups of PFAS: perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkyl sulfonic acids (PFSAs), perfluoroalkyl sulfon-amides and -amidoethanols (FASAs and FASEs), and fluorotelomer carboxylic acids (FTCAs), sulfonic acids (FTSAs), alcohols (FTOHs), and acrylates and methacrylates (FTAcs/FTMAcs), for a total of about 20–60 individual compounds.12,15,20−22 However, it is challenging to measure the unknown PFAS that may be used in consumer products due to a lack of information related to production and use, and a lack of analytical standards.21,29,32 These PFAS include side-chain fluorinated polymers, which are used extensively for antiwetting and antistain surface protection in, notably, the textile industry,3 with highly uncertain estimates of the release over time of nonpolymeric PFAS.33−37 The total oxidizable precursor (TOP) assay was developed38 to address the issue of unknown PFAS that are precursors to perfluoroalkyl acids (PFAAs). This assay has been used to analyze car seats fabric,29 textiles,20,21,39 water,38,40 and biota,41 finding concentrations of unknown PFAA precursors of up to 97 mol %.42 Although the assay does not represent releases under environmental conditions, it allows for an “upper bound limit” for exposure assessment and also, for the ultimate release of mobile PFAS to the environment.39 In addition to the total oxidizable precursor (TOP) assay, hydrolysis was recently suggested as a simple and feasible method to detect additional unknown PFAS, e.g., overall presence of side-chain fluorinated polymers, in textiles.43 For example, Nikiforov reported that the FTOH content increased by up to 500–1300 times after hydrolysis of textile samples.43 The presence of large amounts of PFAS that usually go undetected with targeted analyses carries the potential for release during use, including releases to wastewater during laundering,9,14,17,22,44,45 and the end-of-life stage and hence for exposure.
Children’s exposure to PFAS is of particular concern. Due to their lower body weight and sensitive developmental period, children’s exposure may result in a greater body burden and higher health risks compared to adults.4,10 Prenatal and/or postnatal exposure to several well-studied PFAS, especially perfluorooctanoic acid (PFOA), was associated with overweight and obesity, neurodevelopmental and behavioral problems, dyslipidemia, immunity including vaccine response and asthma, renal function, and age at menarche in children.10,46−50 A recent update from the United States (US) Center for Disease Control and Prevention reported geometric mean concentrations of 3.38 and 4.15 μg/L of perfluorooctanesulfonic acid (PFOS), and 2.00 and 1.89 μg/L of PFOA, in serum samples collected in 2013–2014 in the United States for children ages 3–5 and 6–11 years, respectively.51 These geometric means were similar to those reported for the general population (4.99 and 1.94 μg/L for PFOS and PFOA, respectively), and suggest that children might be exposed to higher levels of PFAS, but sources and pathways are not well understood.
To test the hypothesis that children’s products, in particular products marketed as stain-resistant or waterproof, contain high levels of PFAS and as such, be a source of exposure to children and the ecosystem at large, we purchased children’s clothing items in the US and Canada (n = 72), ranging from school uniforms and outdoor wear, to infant products like bibs. Following our previous work on PFAS in cosmetics,52 children’s clothing items were first screened for total fluorine using particle-included γ-ray emission spectroscopy (PIGE); (n = 72), and then a subset (n = 57) was selected for targeted PFAS analysis using mass spectrometry. A further subset of products (n = 5) was subjected to both the TOP assay and hydrolysis to assess unknown PFAA precursors in textiles. Data obtained from PIGE and targeted analyses were used for calculating dermal exposure from clothing and for a simplified substance flow analysis to model the mass use, pathways, and final sinks of PFAS in children’s clothing items.
Materials and Methods
Samples
Thirty-four children’s products were purchased online in the US in March 2021 and 38 were purchased from the Canadian market in November 2020 and February 2021, for a total of 72 products. Products were selected if they were labeled waterproof/water-resistant/durable water-repellent, stain-proof/stain-resistant/easy care stain release, windproof, or wrinkle resistant. Information regarding products, e.g., fabric composition, country of manufacture, brand, product type and functionality were obtained from the label and from online descriptions. Products selected included school uniforms, weather-resistant outdoor wear such as rainsuits, snowsuits, snowshoes, and mittens, and miscellaneous children’s products such as bibs, hats, stroller covers, swim wear, sweatshirts, and baby shoes. Layers were sampled from products comprised of different parts or composite fibers (e.g., mittens have a waterproof exterior and a fleece interior layer), yielding a total of 134 fabric subsamples (n = 51 from the US products and n = 83 from the Canadian products). Results for different subsamples from the same product were averaged for all subsequent analyses, and the concentrations after averaging decreased but not by much. Subsamples were cut (2 × 2 cm) from products using scissors precleaned with dichloromethane and methanol, placed in resealable Ziploc bags, and shipped to the University of Notre Dame for PIGE analysis and an additional set of samples was sent to Indiana University for targeted PFAS analyses. Detailed information on individual products and subsamples is listed in Table S1, and a summary of the sampling scheme is presented in Figure S1.
PIGE Sample Preparation and Analysis
The PIGE technique can measure the surface concentrations of total fluorine (both inorganic and organic); the contribution of inorganic fluoride is considered negligible in textiles.53 All samples (n = 134) were measured directly via PIGE without any additional treatment or preparation.53 Total fluorine analysis and quantification via PIGE was based on a previously published approach that utilized a thin (180 μm) fluorine-free qualitative filter paper spiked with known concentrations of inorganic fluoride.35,52 This method was modified to account for the thickness of samples, especially for thicknesses larger than the expected penetration depth of the proton beam (200 μm). In the modified approach, four fabric samples purchased from Fabric Wholesale Direct and papers of varying composition and thickness (Tables S2 and S3) were spiked with known concentrations of inorganic fluoride, and a calibration curve of the total F concentration vs PIGE response was generated in units of both μg F/cm2. Data were fitted using linear equations with forced y-intercepts. All curves had R2 > 0.99, and deviation for replicates (n = 7) was below 10% for all four fabrics. The limit of detection for each material was estimated using the LINEST function to measure standard error and slope which were plotted against measured thickness to determine relationships between material thickness and response. To quantify the amount of fluorine present in samples, each sample’s thickness was measured, and this value was inputted into the fit equation to relate sample thickness to a predicted slope which was used to convert total fluorine signals into concentrations. A similar approach was used to determine thickness-dependent limits of detection for total fluorine concentrations.
Sample Preparation
Procedures for liquid chromatography with tandem mass spectrometry (LC-MS/MS)- and gas chromatography mass spectrometry (GC-MS)-based targeted analysis of a subset of samples (n = 74) followed the previously reported methods.20,54 In brief, a 4 cm2 textile sample was further cut into 2 × 2 mm pieces for better extraction efficiency, then weighed into a 15 mL polypropylene tube, spiked with 20 ng each of the surrogate standards and extracted twice with 3 mL of 4:1 hexane/isopropyl alcohol, followed by 3 mL of 1:1 methanol/acetonitrile. For each extraction step, the sample was sonicated for 30 min and then centrifuged at 3000g for 5 min. The supernatants were combined, reduced in volume to ∼5 mL, and cleaned-up with ∼100 mg Envi-Carb activated carbon by vortexing for 1 min and centrifuging at 3000g for 5 min. The resulting sample was concentrated to 500 μL under nitrogen, filtered using a centrifugal filter, transferred into a 1 mL polypropylene vial, and spiked with 50 ng each of the internal standards (1 mg/L in MeOH/IPA (80:20)) for a final sample volume of 1 mL. (See Table S4 for details on standards.) Concentrations obtained from mass spectrometry measurements (ng/g) were converted to μg/m2 using the sample surface area (2 × 2 cm) and weight (mg) to compare results from this study with those from the literature.
To investigate the presence of PFAA precursors, five samples were selected for the TOP assay, following the protocol developed by Houtz and Sedlak38 with slight modifications. Briefly, the extracts of the original sample, not spiked with surrogate standards, were completely dried under a gentle nitrogen flow to avoid oxidant consumption by the solvent. A fixed volume (15 mL) of a freshly prepared oxidation solution (60 mM K2S2O8, 125 mM NaOH in water) was added to the sample tube and incubated for reaction at 85–90 °C for 6 h. The sample was then cooled to room temperature, adjusted to pH = 4.0 using formic acid, spiked with surrogate standards, and cleaned up on an Oasis WAX cartridge following a protocol described in previous studies.55−57
Additional samples from the same five products analyzed with the TOP assay were subjected to hydrolysis using the approach developed by Nikiforov with minor modifications.43 In brief, 0.5 mL of a 1 M NaOH solution in methanol/water (90:10) was added to a 15 mL glass vial containing ∼30 mg small-pieces of textile samples, and spiked with 20 ng each of the surrogate standards. The vial was vortexed for 1 min and placed in an oven at 60 °C for 16 h. After the vial was cooled to room temperature, the solution was transferred to a new clean vial, and 0.6 mL of a 1:1 mixture of methyl tert-butyl ether/n-hexane and 2 mL of LC-MS grade water was added. Samples were shaken for 30 min, and the top aqueous layer was removed with a glass pipette. Anhydrous Na2SO4 was added to remove the water in the sample until the organic layer became clear. Finally, the extracts were transferred into a 1 mL PP vial and analyzed using GC-MS.
LC-MS/MS and GC-MS Analysis
For the targeted analysis of 74 samples from the original extraction procedure plus the five extracts from the TOP assay, 49 PFAS with 15 surrogates and 7 internal standards were measured (see Table S4). FASAs and ionic PFAS, namely PFCAs, PFSAs, FTCAs, FTSAs, and fluorotelomer phosphate esters (PAPs), were analyzed by ultrahigh-performance liquid chromatography interfaced with a triple quadrupole MS in the negative ionization mode (Agilent 1290 Infinity II UPLC–6470 ESI—QQQ-MS). A gas chromatograph mass spectrometer operated in the positive chemical ionization mode (Agilent 7890 GC – 5977B PCI-MS) was used to measure neutral PFAS (i.e., FASEs, FTOHs, FTAcs, and FTMAcs). For extracts of the five samples from the hydrolysis assay, only neutral PFAS were measured. Detailed instrumental parameters were provided elsewhere,39 and are also summarized in Tables S5–S11.
Quality Assurance and Quality Control
Field blanks consisting of Kimwipes were shipped with the samples and processed using the same procedures as actual samples. Five lab duplicates and two field duplicates were also measured (Table S1). For the LC-MS and GC-MS analyses, a procedural blank and a matrix spike consisting of 20 ng for each of targeted PFAS were processed along with every batch of 7 samples to evaluate background contamination from laboratory operations (see Table S12) and the performance of our methods. Samples were quantitated using the internal standards except for hydrolysis samples where the surrogate standards were used for quantitation following the method from Nikiforov.43 Recoveries of surrogate standards were generally in the range of 60–140% (Table S13). The results for field blanks and procedural blanks were similar to one another, so they were averaged for blank analysis. Results were blank corrected by subtracting the corresponding average blank on a mass basis. If the mass of a detected compound was below the method detection limit (MDL), it was considered a nondetect, otherwise the value present in blanks was subtracted. The MDLs (ng/g) were defined as 3 times the standard deviation of the blank level or the amount of chemical generating a signal-to-noise ratio of 5 if the compound was not detected in procedural blanks divided by the average sample weight (Table S12). The results of five lab duplicates and two field duplicates showed a relative standard deviation generally lower than 20% both for total targeted PFAS and for individual target compounds (Table S14). Concentrations from duplicates were averaged for each product for reporting purposes.
A 7-point calibration curve was prepared over a concentration range of 0.25–25 ng/mL. The regression coefficients of calibration curves were all >0.99. To test the impact of the TOP assay on PFCAs and PFSAs (C3–C9), we spiked 5 ng each of our analytes into empty tubes and treated them in the same manner as samples. The recoveries of PFAAs in matrix spikes after TOP were in the range of 64.3–144% (Table S12), which meant that there was no significant effect on PFAAs (C3–C9) during TOP.29 Detailed QA/QC procedures for the PIGE analysis are provided elsewhere.52,53
Data Analysis
Since not all our data fit the normal distribution, a nonparametric Kruskal–Wallis ANOVA test was used for comparison of group means. Plotting and statistical analyses, including calculation of medians and Kruskal–Wallis ANOVA, were performed using OriginPro 2021 (OriginLab Corp.). Cells containing values below MDLs were replaced with 1/2 MDL only for individual PFAS (not for totals) and only for median calculations. At least one ionic PFAS was detected in each product before 1/2 MDL replacement. The statistical significance was set at p < 0.05 for all analyses.
Substance Flow Analysis
Substance Flow Analysis (SFA) is a useful tool in modeling the sources, life stages, and final sinks of chemical substances.58,59 A substance flow analysis was conducted to quantify the annual flow of PFAS in the purchased children’s school uniform. Full details are provided in the Supporting Information. The analysis considered the annual flow of both polymeric and nonpolymeric PFAS for a given year between 2019 and 2021 for children and adolescents 5–19 years of age (Flow 1 in Figure S3). This flow provides a numerical estimate of the input of PFAS to the entire downstream network of flows and sinks, which were described qualitatively due to uncertainties and data gaps.
The flow of PFAS in children’s school uniforms purchased (Flow 1) was calculated as
| 1 |
where Mschool uniform is the mass of children’s school uniforms purchased in a given year; Cfunctional is the PFAS concentration in school uniforms that were intentionally treated with PFAS; values for Cfunctional for polymeric PFAS were taken from literature recommendations of polymeric PFAS deposition to fabrics (0.1, 0.3, and 0.45% for low, middle, and high values, respectively),60−62 whereas values for nonpolymeric PFAS were taken from the results after the TOP assay presented here (0.00352, 0.011, and 0.013%; Table S16). CnoPFAS was assumed to be zero for polymeric and nonpolymeric PFAS in untreated school uniforms,%functional is the percentage of school uniforms that were intentionally treated with PFAS (1.3, 5.84, and 16.2%) taken from estimates of school uniforms marketed as stain-resistant but corrected for those marketed as such that were found to have total F < 0.1% (Table S19), and %noPFAS is the percentage of school uniforms that were not treated with PFAS.
We compiled four SFAs, one for each of the US and Canada, for polymeric PFAS (notably side-chain fluorinated polymers) and for nonpolymeric PFAS (i.e., sum of FASAs, FASEs, PFCAs, PFSAs, n:2 FTCAs, n:2 FTSAs, n:2 FTOHs, n:2 FTAcs, and/or n:2 FTMAcs). Low, middle, and high estimates were calculated for each parameter in eq 1 to account for uncertainties (a Monte Carlo analysis was not conducted due to uncertain distributions of input parameters). Estimates of the mass of school uniforms marketed as “stain-resistant” (%functional) were taken from websites of major retailers of school uniforms (Table S15). The total estimated number of such uniforms was corrected for the percentage likely to contain PFAS using total fluorine data presented here (Table S1), since some uniforms could either achieve stain-repellency using other textile surface treatments60 or were mislabeled. A detailed description of individual estimates used in the equations and data sources are provided in the Supporting Information.
Dermal Absorption
Since school uniforms are worn for extended periods of time and adhere to children’s bodies, we estimated the daily intake via dermal absorption (EDIderm, ng/kg bw/day) using the following equation
| 2 |
where C is the total extractable concentration of PFAS in school uniforms based on the original solvent extraction (before the TOP assay or hydrolysis) (ng/m2), SA is the total body surface area (1.08 m2 for 6–11 years children),40Fcontact is the fraction of the skin contact area (unitless; 0.824),63Fmig is the daily migration rate of nonpolymeric PFAS from textile to skin (days–1; 0.001),64Fpen is the fraction of PFAS penetrated into the skin (unitless; 0.5),64,65T is the contact duration (days; 0.42 corresponding to a wearing time of 10 h in a day), N is the mean daily number of events (days–1, 1), BW is the body weight (kg; 31.8 kg for 6–11 years children).63 Values for Fmig and Fpen are estimates for reasonable maximum exposure (RME) scenario since measurements are unavailable.21,22,64 The number of events in a day was set to 1 since uniforms are worn continuously, as opposed to outdoor wear that is often removed several times in a day. The dermal absorption calculated by eq 2 is a RME scenario as 50% extractable PFAS are assumed to penetrate the skin. As such, these values are likely overestimates of the true dermal absorption capacity of PFAS since they account for volatile FTOHs, and nonuniform PFAS concentration in textile.66 It is likely that the PFAS concentration in school uniforms will decrease over time due to laundering since some PFAS is likely washed out and then released to surface waters via wastewater treatment plant (WWTP) or septic field discharges; however, mass loss is anticipated to be minimal relative to the total amount added.44
Results and Discussion
Screening of Children’s Products
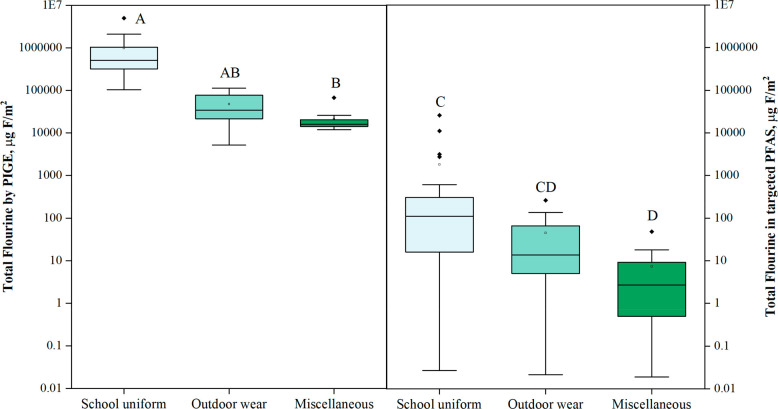
Using PIGE, fluorine was detected in 65% of the tested products (n = 72) with a range of ND–5 020 000 μg F/m2, and a median of 67200 μg F/m2; see Table S1 for individual concentrations. PIGE analyses showed that the highest levels of total fluorine were measured in school uniforms, followed by weather-resistant outdoor wear and then miscellaneous products (p < 0.001, see Figure 1). The same concentration trend was also observed for total targeted PFAS measured with targeted mass spectrometry analysis in a subset of the products (n = 57; Figure 1).
Figure 1.
Box and whisker plots of concentrations of total fluorine (left; μg F/m2; n = 134) by PIGE (excluding nondetects) and total fluorine calculated from total targeted PFAS (right, μg F/m2; n = 57) in the three categories of tested products. Shown are the medians (black lines inside the box), the 25th to 75th percentiles (box), the 10th and 90th percentiles (whiskers), the outliers (circles), and the Kruskal–Wallis ANOVA results (letters at the top of each box). Compounds sharing the same letter do not have statistically different concentrations (p > 0.05).
Total targeted PFAS concentrations in school uniforms ranged from 0.283 to 153 000 ng/g with a median of 728 ng/g (on surface-based units, these concentrations correspond to a range of 0.041–38100 μg/m2 and a median of 178 μg/m2) (Table 1). These levels were similar to those measured in outdoor wear with a median of 111 ng/g (a range of 0.032–376 μg/m2 and a median of 19.8 μg/m2), but significantly higher than those reported in the miscellaneous category, for which the median was 35.5 ng/g (a range of 0.028–71.5 μg/m2 and a median of 3.9 μg/m2; p = 0.0014).
Table 1. Detection Frequencies (DF, %), Median and Range of Concentration (ng/g) and Contributions (%) of the Most Abundant PFAS in Children’S Products Samplesa.
| School
uniform (n = 25) |
Outdoor wear (n = 16) |
Miscellaneous (n = 16) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | Median | Range | % of Σ PFAS | DF | Median | Range | % of Σ PFAS | DF | Median | Range | % of Σ PFAS | |
| PFCAs | ||||||||||||
| PFPrA | 4.00 | 0.651b | ND–0.651 | 0.000 | 37.5 | 1.84 | ND–45.9 | 0.618 | 18.8 | 1.68 | ND–2.24 | 0.373 |
| PFBA | 20.0 | 2.61 | ND–22.6 | 0.012 | 18.8 | 4.09 | ND–6.05 | 0.150 | 6.25 | 3.87 | ND–3.87 | 0.311 |
| PFPeA | 4.00 | 0.0441 | ND–1.68 | 0.001 | 6.25 | 0.0719 | ND–5.77 | 0.464 | ||||
| PFHxA | 84.0 | 1.60 | ND–6.47 | 0.018 | 81.3 | 0.502 | ND–3.38 | 0.153 | 62.5 | 0.213 | ND–6.42 | 0.767 |
| PFHpA | 72.0 | 0.0706 | ND–1.13 | 0.001 | 87.5 | 0.263 | ND–0.635 | 0.045 | 75.0 | 0.0845 | ND–0.719 | 0.191 |
| PFOA | 20.0 | 0.161 | ND–0.986 | 0.001 | 75.0 | 0.802 | ND–4.32 | 0.237 | 56.3 | 0.431 | ND–3.53 | 0.969 |
| PFNA | 16.0 | 0.0806 | ND–0.293 | 0.000 | 56.3 | 0.168 | ND–1.25 | 0.043 | 37.5 | 0.104 | ND–0.532 | 0.131 |
| PFDA | 24.0 | 0.0514 | ND–0.397 | 0.001 | 87.5 | 0.277 | ND–1.79 | 0.072 | 43.8 | 0.0988 | ND–0.967 | 0.203 |
| PFUdA | 24.0 | 0.0345 | ND–0.090 | 0.000 | 43.8 | 0.134 | ND–0.298 | 0.010 | 37.5 | 0.119 | ND–0.862 | 0.105 |
| PFDoA | 12.0 | 0.0443 | ND–0.213 | 0.000 | 50.0 | 0.106 | ND–0.495 | 0.022 | 43.8 | 0.0882 | ND–0.596 | 0.139 |
| PFTrDA | 20.0 | 0.0428 | ND–0.274 | 0.000 | 31.3 | 0.0676 | ND–0.865 | 0.015 | 37.5 | 0.0834 | ND–2.76 | 0.452 |
| PFTeDA | 4.00 | 0.0342 | ND–0.101 | 0.000 | 43.8 | 0.0806 | ND–0.512 | 0.016 | 31.3 | 0.0686 | ND–0.632 | 0.081 |
| PFSAs | ||||||||||||
| PFBS | 24.0 | 0.0109 | ND–6.47 | 0.002 | 37.5 | 0.0134 | ND–2.44 | 0.028 | 6.25 | 0.0108 | ND–0.021 | 0.002 |
| PFHxS | 20.0 | 0.0643 | ND–0.258 | 0.000 | 50.0 | 0.0915 | ND–1.33 | 0.025 | 43.8 | 0.0865 | ND–0.260 | 0.064 |
| PFOS | 24.0 | 0.0247 | ND–0.225 | 0.000 | 75.0 | 0.0719 | ND–0.308 | 0.015 | 43.8 | 0.0521 | ND–0.338 | 0.088 |
| PFNS | 12.0 | 0.0098 | ND–0.080 | 0.000 | 50.0 | 0.0233 | ND–0.298 | 0.006 | 43.8 | 0.0239 | ND–0.244 | 0.059 |
| PFDS | 20.0 | 0.0392 | ND–0.733 | 0.001 | ||||||||
| 8Cl-PFOS | 12.0 | 0.0247 | ND–0.078 | 0.000 | 12.5 | 0.0364 | ND–0.325 | 0.005 | ||||
| FTSs | ||||||||||||
| 4:2 FTS | 8.00 | 0.0162 | ND–0.038 | 0.000 | 6.25 | 0.0226 | ND–0.028 | 0.000 | ||||
| 6:2 FTS | 20.0 | 0.505 | ND–2.93 | 0.003 | 6.25 | 0.746 | ND—-1.00 | 0.011 | ||||
| 8:2 FTS | 8.00 | 0.0148 | ND–0.049 | 0.000 | 50.0 | 0.0355 | ND–1.27 | 0.023 | 25.0 | 0.0221 | ND–0.120 | 0.029 |
| PAPs | ||||||||||||
| 6–2_PAP | 20.0 | 0.242 | ND–0.576 | 0.001 | 25.0 | 0.335 | ND–1.78 | 0.035 | 18.8 | 0.278 | ND–2.12 | 0.401 |
| 8–2_PAP | 12.5 | 0.506 | ND–2.79 | 0.300 | ||||||||
| 6–2_diPAP | 4.00 | 0.0506 | ND–1.12 | 0.000 | 12.5 | 0.128 | ND–11.8 | 0.130 | ||||
| Neutral PFAS | ||||||||||||
| FBSA | 4.00 | 0.000978 | ND–0.284 | 0.000 | 6.25 | 0.0145 | ND–0.033 | 0.000 | ||||
| FHxSA | 12.5 | 0.0145 | ND–0.025 | 0.000 | ||||||||
| FOSA | 25.0 | 0.0411 | ND–0.247 | 0.004 | 6.25 | 0.0357 | ND–0.035 | 0.003 | ||||
| MeFOSA | 8.00 | 0.0147 | ND–0.215 | 0.000 | 6.25 | 0.0218 | ND–0.083 | 0.001 | 12.5 | 0.0240 | ND–0.314 | 0.045 |
| EtFOSA | 4.00 | 0.0196 | ND–0.186 | 0.000 | 6.25 | 0.0291 | ND–0.023 | 0.000 | 18.8 | 0.0348 | ND–0.493 | 0.046 |
| 4:2 FTOH | 4.00 | 1.81 | ND–5.25 | 0.002 | ||||||||
| 6:2 FTOH | 76.0 | 445 | ND–153000 | 97.8 | 68.8 | 30.4 | ND–931 | 30.4 | 37.5 | 6.74 | ND–510 | 65.0 |
| 8:2 FTOH | 20.0 | 9.50 | ND–202 | 0.09 | 56.3 | 16.8 | ND–2070 | 25.8 | 43.8 | 13.8 | ND–50.0 | 12.3 |
| 10:2 FTOH | 8.00 | 1.08 | ND–90.6 | 0.04 | 62.5 | 3.79 | ND–1630 | 19.4 | 50.0 | 2.16 | ND–19.5 | 5.64 |
| 6:2 FTAcr | 28.0 | 0.160 | ND–39.6 | 0.04 | 12.5 | 0.213 | ND–20.7 | 0.38 | ||||
| 8:2 FTAcr | 12.0 | 0.192 | ND–41.4 | 0.02 | 25.0 | 0.375 | ND–5.28 | 0.13 | 6.25 | 0.312 | ND–25.5 | 2.05 |
| 10:2 FTAcr | 8.00 | 0.383 | ND–26.7 | 0.01 | 50.0 | 0.644 | ND–4.71 | 0.18 | 6.25 | 0.392 | ND–18.8 | 1.51 |
| 6:2 FTMAcr | 76.0 | 132 | ND–1020 | 1.95 | 56.3 | 0.844 | ND–748 | 22.0 | 18.8 | 0.270 | ND–58.3 | 8.23 |
| 8:2 FTMAcr | 6.25 | 0.226 | ND–0.255 | 0.003 | ||||||||
| Σ Ionic PFAS | 100 | 2.30 | 0.10–34.6 | 0.04 | 100 | 7.49 | 0.214–48.8 | 1.50 | 100 | 2.63 | 0.021–9.40 | 4.41 |
| Σ PAPs | 24.0 | 1.27 | ND–1.12 | 0.00 | 37.5 | 1.624 | ND–11.8 | 0.167 | 18.8 | 1.36 | ND–4.30 | 0.706 |
| Σ FASAs | 16.0 | 0.0934 | ND–0.284 | 0.00 | 50.0 | 0.131 | ND–0.247 | 0.006 | 31.3 | 0.135 | ND–0.807 | 0.093 |
| Σ FTAc | 36.0 | 0.741 | ND–68.1 | 0.07 | 62.5 | 2.84 | ND–20.7 | 0.690 | 6.25 | 0.937 | ND–44.3 | 3.56 |
| Σ FTOHs | 76.0 | 456 | ND–153000 | 97.9 | 87.5 | 102 | ND–4140 | 75.6 | 68.8 | 42.5 | ND–510 | 83.0 |
| Σ FTMAc | 84.0 | 134 | ND–1020 | 2.02 | 87.5 | 6.46 | ND–762 | 22.7 | 25.0 | 1.71 | ND–53.3 | 11.8 |
| Σ Neutral PFAS | 92.0 | 740 | ND–153000 | 100 | 93.8 | 111 | ND–4180 | 98.3 | 68.8 | 49.9 | ND–543 | 94.9 |
| Σ PFAS | 100 | 728 | 0.283–153000 | 100 | 100 | 111 | 0.296–4190 | 100 | 100 | 35.5 | 0.250–547 | 100 |
PFAS that were not detected in any sample were not included here. The data in this table are the targeted PFAS analysis from original extraction before TOP assay and hydrolysis. Non-detect (ND) values were replaced by 1/2 MDLs for median calculations.
For compounds with DF < 50%, the median actually corresponds to 1/2MDL.
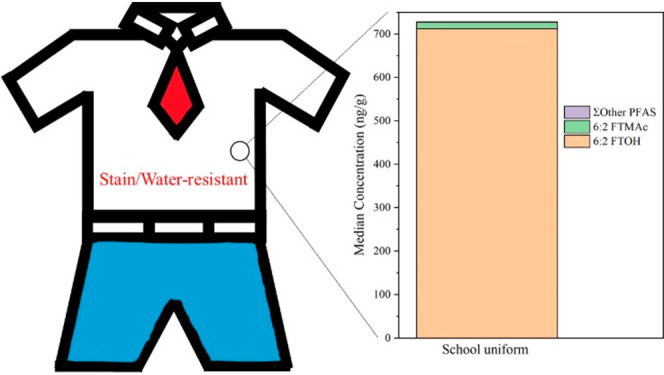
Total targeted PFAS concentrations in all three products’ categories were dominated by FTOHs and FTMAcs, although their contributions were different within the three categories (see Figure 2 and Table 1). School uniforms were almost entirely dominated by 6:2 FTOH, which represented 98% of the total targeted PFAS concentration. For outdoor wear and miscellaneous items, the compositions were more diverse with lower contributions from 6:2 FTOH (31% and 65%, respectively) and higher contributions from 6:2 FTMAc (22% and 7.9%, respectively) and 8:2 FTOH (26% and 12%, respectively). The abundance of FTMAc and FTOH suggests that these products were treated with side-chain fluorinated polymers. Ionic PFAS, including PFCAs, PFSAs, or FTSAs, were detected in all these products, but contributed less than 5% of the total targeted PFAS. The dominance of 6:2 FTOH is consistent with the industry’s transition to 6:2 fluorotelomer-based side-chain fluorinated polymers.16,19,67 In previous studies, textile products purchased before 2011/2012 were dominated by long-chain (C > 8) fluorotelomer alcohols, mainly 8:2 FTOH (C8),12,13,15,68 while in products purchased after 2012, 6:2 FTOH (C6) was detected more frequently and at higher concentrations than 8:2 FTOH.12,14,16,19,20,22 The significance of these results is that 6:2 FTOH measured here can be released from clothing via volatilization, with implications for inhalation exposure and dermal transfer (discussed below), and also it can be released to the environment during laundering (to WWTP effluent and biosolids during washing, and to the air via volatilization and fiber loss during drying) (see Figure S3).44,69
Figure 2.
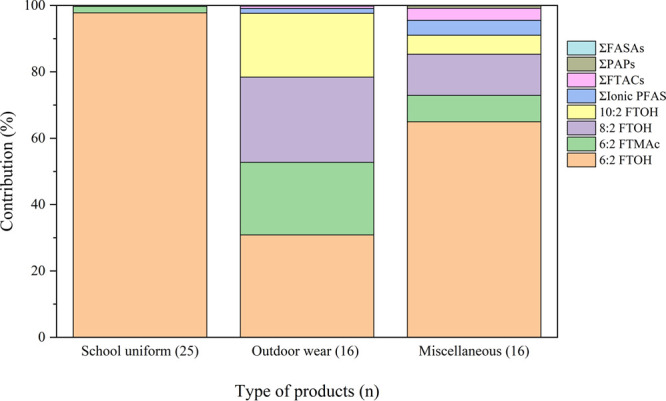
Average compounds’ contribution (%) to total targeted PFAS for the three types of products tested (n = 57).
Overall, the median total targeted PFAS concentration in the tested products (n = 57 resulting from 74 subsamples) from targeted analysis was 117 ng/g or 24.0 μg/m2 (Table S18), comparable to values presented in earlier publications for textiles (see Table S18).12−17,19−23,18−22 The median concentrations of ionic PFAS (2.99 ng/g or 0.67 μg/m2) (Table S18) were comparable to the levels reported in earlier studies for children’s clothing that did not include uniforms from North America,16,20,21 but lower than the levels from two previous studies that tested ionic PFAS (PFCAs and PFSAs) in US-marketed children’s uniforms, noting differences in sample analysis and target compounds measured.24,25 The median concentration of 6:2 FTOH (355 ng/g or 55.3 μg/m2) (Table S18) was higher than the previously reported levels.16,19,22,23 Only one study detected 6:2 FTMAc in outdoor clothes (mostly adult) at a median concentration of 10 μg/m2,14 similar to the median of 28.2 μg/m2 reported here.
Although a systematic comparison of products from the US and Canada was beyond the scope of this study, no significant differences were detected between comparable products in each of the three categories purchased in each country (p = 0.13). This similarity suggests that the US and Canadian markets can be considered as one entity, which has been observed for other products like cosmetics.52 Below we further discuss our findings regarding school uniforms.
The Role of Fabric Type
The total median targeted PFAS concentration in school uniforms labeled as 100% cotton (19 100 ng/g or 4620 μg/m2) and cotton Spandex (1240 ng/g or 291 μg/m2) were similar, but higher than in uniforms made of cotton/polyester (1.7 ng/g or 0.3 μg/m2) (p = 0.014; Figure S4). It should be noted that the items labeled as cotton/Spandex contained 97–98% of cotton and the items labeled as cotton/polyester contained about 50% cotton (see Table S1 for composition details). Cotton likely requires additional PFAS treatment to achieve the desired water-repellent or stain-resistant qualities than cotton blends or synthetic fabrics due to cotton’s greater hydrophilicity.70 Berger et al.15 also found that cotton materials contained a higher amount of PFAS than other fabric types. Zhu. et al. and Zheng and Salamova observed an opposite trend in fabric, but these studies did not select clothing items based on labeling of being “water-resistant” or “stain-resistant”, and thus nontreated textile samples may have been included and thus cause bias in the data interpretation.21,22
What Is Missing? The Quest for Unknown PFAS
The median concentrations from PIGE and MS analyses (97 200 and 15.9 μg F/m2, respectively) were significantly correlated (r2 = 0.55, p < 0.05; Figure S5), but the PIGE results were at least 2 orders of magnitude higher, similarly to prior studies (Figure 1).20,52 This confirms, once more, the presence of numerous unknown or unquantifiable PFAS in common consumer products, due to a lack of analytical methods and standards, as well as unextractable compounds such as fluorinated polymers. These undetected compounds likely included, for example, PFAA precursors such as unextractable fluorotelomer-based side-chain fluorinated polymers, which are widely used in textile finishing processes, and their degradation products.
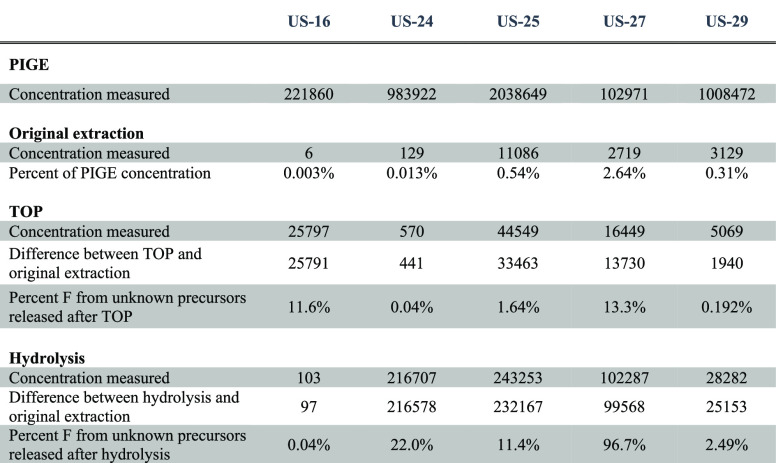
To attempt to quantify the contributions of unknown PFAS, we applied the TOP assay and hydrolysis treatment, in combination with targeted analysis of 49 PFAS, to five school uniform samples that were selected to be representative of fabric type, brands, and PFAS concentration range (low, medium, and high). We note that our results are likely underestimates of the total concentration and full range of oxidizable precursors for three possible reasons: first, we applied the TOP analysis to extracts and not the fabrics themselves; second, there could be other untargeted oxidation intermediates and terminal products generated by the TOP assay that cannot be detected/quantitated; third, precursors have not been completely oxidized.
TOP Assay
Overall, after the TOP assay, the mean concentration of total targeted PFAS increased from 21600 ng/g to 126,000 ng/g. In particular, concentrations of PFPrA, PFBA, PFPeA, PFHxA, and PFHpA increased by at least 1000 times, while the concentrations of 6:2 FTOH, 6:2 FTAc, and 6:2 FTMAc decreased by 70 times or were < MDL (see Figure 3 and Tables S16 and S17). This is consistent with results from previous studies20,21,29 that showed similar increases in PFCA concentrations, indicating the presence of extractable, nonvolatile, and oxidizable precursors as a significant source of human exposure to and environmental releases of PFAS.
Figure 3.
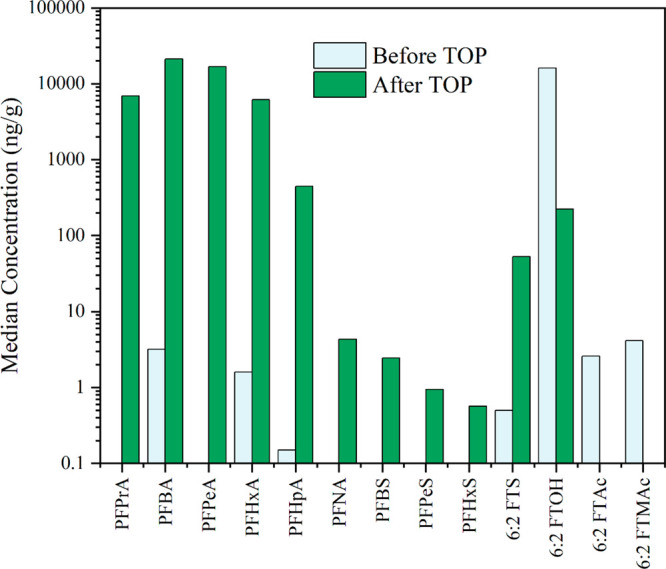
Median concentrations (ng/g) of detected PFAS in selected school uniform samples (n = 5) before and after TOP assay on original extracts.
In one uniform sample (US-16), we observed an increase of PFBS and PFBA after the TOP assay, which was surprising since PFSAs generally have lower yields with this treatment. Based on the results from Liagkouridis et al.,39 we speculate that this specific sample was also treated with a perfluorobutanesulfonamido ethanol-based side-chain fluorinated polymer. Interestingly, in at least two samples, 6:2 and 10:2 FTOHs were detected after the TOP assay, even though they were not present in the original analysis. These results indicates that school uniforms contained unknown precursors that can be converted to FTOHs, which in turn can be converted into PFCAs, noting that the TOP assay does not deliver 100% conversion (see mass balance in Table 2).
Table 2. Mass Balance of Total Fluorine for Five School Uniform Samples (Total F Concentration, μg F/m2).
Hydrolysis
The hydrolysis treatment can free the chemically bound FTOHs and other volatile PFAS from impurities or other precursors, e.g., side-chain fluorinated polymers in textiles.29 Summary statistics and full data of pre- and post-hydrolysis for five samples are presented in Tables S16 and S17, and the median concentrations are plotted in Figure 4. Consistent with the results of Nikiforov,43 after hydrolysis, the concentrations of 6:2 FTOH increased on average 42 times, that of 10:2 FTOH by 7 times, and those of 4:2 FTOH, 8:2 FTOH, N-MeFOSE, and 8:2 FTMAc by smaller amounts. 8:2 FTMAc was detected in only two samples with a median of 0.93 ng/g. Overall, these findings suggest that school uniforms contain even more unknown precursors (e.g., precursors that can release N-MeFOSE) than the TOP assay revealed; the PFAS freed up by hydrolysis likely came from side-chain fluorinated polymers. The hydrolysis treatment and TOP assay applied here provide a reasonable “upper-bound” estimate, but not a maximum, since both the TOP assay and hydrolysis do not provide a 100% conversion of numerous precursors that are of concern in school uniforms (Table 2).71
Figure 4.
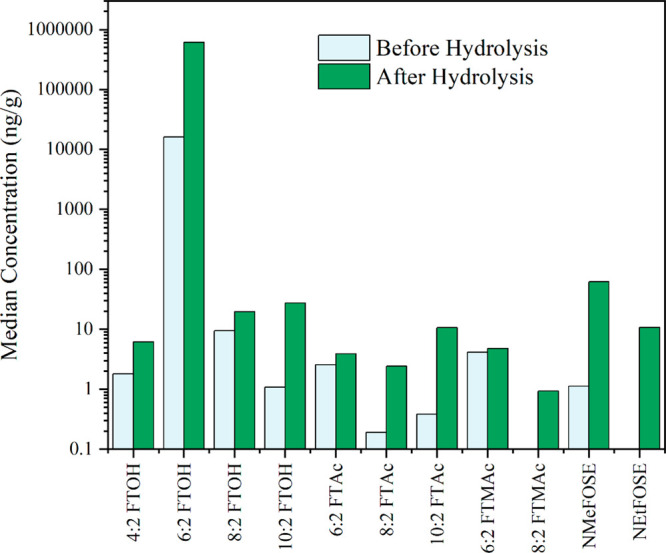
Median concentrations (ng/g) of detected PFAS in selected school uniform samples (n = 5) before and after hydrolysis.
In the mass balance calculations, unknown precursors from the TOP assay accounted for 0.04–13.3% of the total fluorine determined by PIGE, similarly to what was reported by Robel et al.20 Compared to the TOP assay, unknown precursors from hydrolysis accounted for 2.49–96.7% of the total fluorine determined by PIGE for all samples except US-16, indicating that hydrolysis could be an additional useful tool other than the TOP assay to assess the amount of precursors in textiles. The sample US-16, with the smallest increase from the targeted analysis, was likely treated with perfluorobutanesulfonamido ethanol-based side-chain fluorinated polymers, which cannot be hydrolyzed, nor readily extracted by the TOP assay.
There is still a large gap between concentrations of fluorine from PIGE and from the targeted analysis after additional treatments (either TOP assay or hydrolysis) in all samples, with the exception of US-27. This persistent gap suggests that there are other fluorinated polymers that cannot be completely converted to PFAA by the TOP assay or hydrolysis, or that untargeted intermediates are generated during these two processes. It could also be that some volatile FTOHs, especially the shorter ones, could be lost during the TOP assay due to heat and mixing. For US-27, total fluorine analyzed by MS after hydrolysis accounted for 99% of the total fluorine determined by PIGE.
Substance Flow Analysis
We estimated that, in a given year between 2019 and 2021, a midrange value for the mass of children’s (aged 5–19 years) school uniforms purchased per year was approximately 16 200 and 1600 tonnes/year for the US and Canada, respectively (Table S19). Based on this estimate and assumptions explained in the Supporting Information, we estimated that 2.8 (0.05–33 for the low and high estimates, respectively) and 0.3 (0.005–3.3) tonnes of polymeric PFAS, and 0.1 (0.0014–0.92) and 0.01 (1.4 × 10–4–0.092) tonnes of nonpolymeric PFAS, were used in school uniforms purchased annually in the US and Canada, respectively (Table 3). Thus, the usage of polymeric PFAS surpassed that of nonpolymeric PFAS by about 30 times.
Table 3. Estimations for the Mass of PFAS in Children’s School Uniforms (tonnes/year) Purchased Annually in US and Canada in a Year Representative of 2019–2021, Expressed in tonnes PFAS/year.
| Estimates |
||||
|---|---|---|---|---|
| Country | Description | Low | Middle | High |
| US | polymeric | 0.05 | 2.8 | 33 |
| nonpolymeric | 0.0014 | 0.099 | 0.92 | |
| Canada | polymeric | 0.005 | 0.28 | 3.3 |
| nonpolymeric | 1.4 × 10–04 | 0.01 | 0.092 | |
To put these values in perspective, global consumption of all fluoropolymers was 297 000 tonnes, with the US market72 taking up to 22%, which equates to roughly 65 000 tonnes per year (with the consideration that fluoropolymer production volumes are much higher than many other PFAS production volumes). Given the myriad list of PFAS applications, our estimate of US polymeric PFAS use in school uniforms constitutes much less than 0.1% of total fluoropolymer use in the US.73
Although we were unable to quantify the fate of PFAS in school uniforms due to a lack of data (for example PFAS releases due to laundering, at end-of-life of the uniforms; Tables S21 and S22), it is clear that a fraction of these PFAS flows will enter the environment throughout the life cycle of a school uniform and will contribute to human and environmental exposures. For example, PFAS enter the environment upon synthesis and use in textile manufacturing.74,75 During use, PFAS are released into water, soil, and air, through clothes washing (PFAS are released to the receiving waters from wastewater treatment plant effluent and to agricultural soils from biosolids application) and drying (release to indoor and outdoor air).9,17,76 PFAS can also be released via fiber loss, while processing, wearing, washing, and drying of treated garments.77−80 In particular, side-chain fluorinated polymers could be hydrolyzed to the related precursors and terminal PFAAs during anaerobic and aerobic digestion treatments (which are similar to hydrolysis and the TOP assay) in WWTP and in the environment over time.36,37 Laundering PFAS-containing school uniforms together with non-PFAS-containing clothes could also contaminate the latter, analogously to suggested transfer of other textile additives.81 To our knowledge, there are no data on the increases or decreases in PFAS release over the lifetime of treated garments.
Minimal information is available on the disposition of school uniforms at their end-of-life; however this represents opportunities for further release of PFAS to the environment (e.g., after first use, school uniforms may be sold or handed down together with other clothing). Approximately 25% of used clothing is exported or sold abroad, notably to some low-income countries where most end up as unmanaged waste dumped into the environment.82−84 Waste textiles comprise 1 to 5% of municipal solid waste,85 of which most is landfilled. The landfill results in PFAS release to leachates, from which they are poorly removed and thus can enter the environment.86,87 Textile recycling is generally insignificant relative to disposal in municipal solid waste. However, ∼20 and 30% of recyclable textiles can be used to manufacture new products, or be used in other applications such as industrial polishing and wiping clothes, respectively,83,84 during which PFAS will be released.
This analysis and the associated uncertainties point to the lack of knowledge of the fate of PFAS from their use in school uniforms. Such calculations require product-based inventories of PFAS uses, and adequate information on where PFAS are used and which PFAS are used for each individual application. Until that information becomes available, quantification of environmental releases of PFAS from children’s uniforms (and other consumer products) will remain uncertain.
Implications for Exposure
Surprisingly, school uniforms tested here contained PFAS levels similar to those measured in outdoor wear, where a high amount of PFAS is known to be used in order to impart stain- and water-repellency.14,16,19 However, the use of PFAS in school uniforms offers greater potential for direct children’s exposure in comparison to uses in outdoor wear. Uniforms are worn directly on the skin and for extended periods of time (i.e., 8–10 h per day). Further, this exposure route could pertain to about a quarter of US and Canadian school-aged children, as estimates of children wearing uniforms show for 2018.88 The calculated EDI values ranged from 0.0002 to 222 ng/kg bw/day with a median of 1.03 ng/kg bw/day (see Table S23 for complete data). This estimate may be both an under- and overestimate of the true value given all the uncertainties in the values used in eq 2. Furthermore, we did not include exposure due to mouthing clothing.
Acceptable doses for dermal absorption have not been published, and reference doses or acceptable daily intake levels are available only for a few individual PFAS. For example, the US EPA has established an oral noncancer reference dose of 20 ng/kg bw/day each for PFOA and PFOS,89 and has proposed a draft chronic reference dose of 300 ng/kg bw/day for PFBS.90 The Agency for Toxic Substances and Disease Registry (ATSDR) uses minimal risk levels of 3, 2, 20, and 3 ng/kg bw/day for PFOA, PFOS, PFHxS, and PFNA, respectively.91 The tolerable daily intake of the sum of four PFAS (PFOA, FPNA, PFHxS, and PFOS) suggested by the European Food Safety Authority (EFSA) is 0.63 ng/kg bw/day for children.92 No reference doses exist for neutral PFAS, which are the most abundant PFAS detected in children’s uniforms, although an FDA study indicated that 6:2 FTOH is more toxic than PFHxA.71
All these reference doses and daily intake values refer to individual compounds with exposure from all routes, and they also apply to adults. Conversely, our estimates consider 49 PFAS with exposure via dermal absorption for children. All these caveats and limitations prevent a sound comparison between the EDI estimated in this study with the few EDIs available. Nevertheless, the following aspects are a cause for concern: the high concentration of PFAS in school uniforms, the potential for dermal absorption from garments treated with PFAS, the possibility of metabolic transformation from FTOHs into PFAAs.93 Further concern is warranted due to the lack of information on EDIs and more importantly on toxicity.
Limitations and Implications
We recognize that this study has several limitations. First, the sample size of different fabric and clothing types was limited. Further studies with a more systematic approach targeting different fabric and clothing types are required to confirm that cotton is generally treated with higher amounts of PFAS compared with other fibers.70 Laundering experiments could be useful to evaluate the release of PFAS upon washing, and the impact of these releases for intermediate and ultimate sinks of PFAS added to children’s products.17,45 Finally, specific experiments on the efficiency of dermal absorption of PFAS from children’s clothing need to evaluate the extent of transfer from the fabric to skin and the magnitude of dermal absorption, to ultimately weigh the role of skin absorption from clothes as a source of PFAS exposure for children. The potential of PFAS ingestion from mouthing of clothing should also be considered for younger children (i.e., toddlers).
This study has supported the hypothesis that many children’s products that are marketed as “stain-resistant” or “waterproof” do indeed contain PFAS. Further, we provide support for the hypothesis that PFAS in school uniforms with high levels of PFAS could be a source of exposure to these harmful chemicals for millions of children each day via inhalation, ingestion and possibly also via dermal absorption, as well an important source of PFAS release to the environment during laundering and at the end-of-life stages. The need for children’s products to be stain-resistant should be re-evaluated and if deemed necessary by consumers, non-PFAS safer alternatives should be used.
Acknowledgments
Funding was provided by Environment and Climate Change Canada, Great Lakes Protection Initiative (GCXE21P039). Z.W. gratefully acknowledges financial support by the European Union under the Horizon 2020 Research and Innovation Programme (grant agreement number 101036756).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c02111.
Calibration information for PIGE; SFA calculation and source of parameters; sample details; instrument parameters for LC-MS/MS and GC-MS; targeted PFAS analytes; blanks and matrix spikes; surrogate recoveries; lab/field duplicate; data for pre- and post-TOP assay and hydrolysis; summary of previously published data on PFAS in textile; data for EDIs; summary of sampling scheme; substance flow analysis flowchart; mean total targeted PFAS concentration in different fabric types for school uniform; correlation between total fluorine (μg F/m2) by PIGE and total targeted PFAS (μg/m2) by MS (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Buck R. C.; Franklin J.; Berger U.; Conder J. M.; Cousins I. T.; de Voogt P.; Jensen A. A.; Kannan K.; Mabury S. A.; van Leeuwen S. P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7 (4), 513–41. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Cooperation and Development) . Toward a new comprehensive global database of per-and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per-and polyfluoroalkyl substances (PFASs); 2018, https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en (accessed October 10, 2021).
- Holmquist H.; Schellenberger S.; van der Veen I.; Peters G. M.; Leonards P. E.; Cousins I. T. Properties, performance and associated hazards of state-of-the-art durable water repellent (DWR) chemistry for textile finishing. Environ. Int. 2016, 91, 251–64. 10.1016/j.envint.2016.02.035. [DOI] [PubMed] [Google Scholar]
- Sunderland E. M.; Hu X. C.; Dassuncao C.; Tokranov A. K.; Wagner C. C.; Allen J. G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29 (2), 131–147. 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S. F.; Yoshikane M.; Onoda Y.; Nishihama Y.; Iwai-Shimada M.; Takagi M.; Kobayashi Y.; Isobe T. Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. Trends Analyt. Chem. 2019, 121, 115410. 10.1016/j.trac.2019.02.011. [DOI] [Google Scholar]
- Yao Y.; Zhu H.; Li B.; Hu H.; Zhang T.; Yamazaki E.; Taniyasu S.; Yamashita N.; Sun H. Distribution and primary source analysis of per- and poly-fluoroalkyl substances with different chain lengths in surface and groundwater in two cities, North China. Ecotoxicol. Environ. Saf. 2014, 108, 318–328. 10.1016/j.ecoenv.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Al Amin M.; Sobhani Z.; Liu Y.; Dharmaraja R.; Chadalavada S.; Naidu R.; Chalker J. M.; Fang C. Recent advances in the analysis of per- and polyfluoroalkyl substances (PFAS)—A review. Environ. Technol. Innov. 2020, 19, 100879. 10.1016/j.eti.2020.100879. [DOI] [Google Scholar]
- Gluge J.; Scheringer M.; Cousins I. T.; DeWitt J. C.; Goldenman G.; Herzke D.; Lohmann R.; Ng C. A.; Trier X.; Wang Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process Impacts 2020, 22 (12), 2345–2373. 10.1039/D0EM00291G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish EPA (Danish Environmental Protection Agency) . Polyfluoroalkyl substances (PFASs) in textiles for children-Survey of chemical substances in consumer products No. 136, 2015; Danish Environmental Protection Agency: Copenhagen, Denmark, 2015, https://www2.mst.dk/Udgiv/publications/2015/04/978-87-93352-12-4.pdf (accessed October 20, 2021).
- Rappazzo K. M.; Coffman E.; Hines E. P. Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int. J. Environ. Res. Public Health 2017, 14 (7), 691. 10.3390/ijerph14070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnarsdottir O.; Abdallah M. A.; Harrad S. Dermal uptake: An important pathway of human exposure to perfluoroalkyl substances?. Environ. Pollut. 2022, 307, 119478. 10.1016/j.envpol.2022.119478. [DOI] [PubMed] [Google Scholar]
- Gremmel C.; Fromel T.; Knepper T. P. Systematic determination of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in outdoor jackets. Chemosphere 2016, 160, 173–80. 10.1016/j.chemosphere.2016.06.043. [DOI] [PubMed] [Google Scholar]
- Kotthoff M.; Muller J.; Jurling H.; Schlummer M.; Fiedler D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. and Pollut. Res. 2015, 22 (19), 14546–59. 10.1007/s11356-015-4202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I.; Hanning A. C.; Stare A.; Leonards P. E. G.; de Boer J.; Weiss J. M. The effect of weathering on per- and polyfluoroalkyl substances (PFASs) from durable water repellent (DWR) clothing. Chemosphere 2020, 249, 126100. 10.1016/j.chemosphere.2020.126100. [DOI] [PubMed] [Google Scholar]
- Berger U.; Herzke D. Per- and polyfluorinated alkyl substances (PFAS) extracted from textile samples. Organohalogen Compd. 2006, 68, 2023–2026. [Google Scholar]
- Santen M.; Brigden K.; Cobbing M.. Leaving Traces-The hidden hazardous chemicals in outdoor gear Greenpeace product test 2016; 2016, https://www.greenpeace.org/static/planet4-international-stateless/2016/01/d9343da2-leaving-traces.pdf (accessed October 10, 2021).
- CEC (Commission for Environmental Cooperation) . Furthering the Understanding of the Migration of Chemicals from Consumer Products - A Study of Per- and Polyfluoroalkyl Substances (PFASs) in Clothing, Apparel, and Children’s Items; Commission for Environmental Cooperation, Montreal, Canada, 2017. (accessed January 11, 2021).
- Peaslee G. F.; Wilkinson J. T.; McGuinness S. R.; Tighe M.; Caterisano N.; Lee S.; Gonzales A.; Roddy M.; Mills S.; Mitchell K. Another Pathway for Firefighter Exposure to Per- and Polyfluoroalkyl Substances: Firefighter Textiles. Environ. Sci. Technol. Lett. 2020, 7 (8), 594–599. 10.1021/acs.estlett.0c00410. [DOI] [Google Scholar]
- Santen M.; Kallee U.. Chemistry for any weather-Greenpeace tests outdoor clothes for perfluorianted toxins; 2012, https://wayback.archive-it.org/9650/20200429191052/http://p3-raw.greenpeace.org/romania/Global/romania/detox/Chemistry%20for%20any%20weather.pdf (accessed October 10, 2021).
- Robel A. E.; Marshall K.; Dickinson M.; Lunderberg D.; Butt C.; Peaslee G.; Stapleton H. M.; Field J. A. Closing the Mass Balance on Fluorine on Papers and Textiles. Environ. Sci. Technol. 2017, 51 (16), 9022–9032. 10.1021/acs.est.7b02080. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Kannan K. Total oxidizable precursor assay in the determination of perfluoroalkyl acids in textiles collected from the United States. Environ. Pollut. 2020, 265 (Pt B), 114940. 10.1016/j.envpol.2020.114940. [DOI] [PubMed] [Google Scholar]
- Zheng G.; Salamova A. Are Melamine and Its Derivatives the Alternatives for Per- and Polyfluoroalkyl Substance (PFAS) Fabric Treatments in Infant Clothes?. Environ. Sci. Technol. 2020, 54 (16), 10207–10216. 10.1021/acs.est.0c03035. [DOI] [PubMed] [Google Scholar]
- Borg D.; Ivarsson J. Analysis of PFASs and TOF in Products. Nordisk Ministerråd 2017, 47. [Google Scholar]
- Liu X.; Guo Z.; Krebs K. A.; Pope R. H.; Roache N. F. Concentrations and trends of perfluorinated chemicals in potential indoor sources from 2007 through 2011 in the US. Chemosphere 2014, 98, 51–7. 10.1016/j.chemosphere.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Guo Z.; Liu X.; Krebs K. A.; Roache N. F.. Perfluorocarboxylic acid content in 116 articles of commerce; US Environmental Protection Agency: Washington, DC, 2009, https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NRMRL&dirEntryId=206124 (accessed June 15, 2021).
- Rodgers K. M.; Swartz C. H.; Occhialini J.; Bassignani P.; McCurdy M.; Schaider L. A. How Well Do Product Labels Indicate the Presence of PFAS in Consumer Items Used by Children and Adolescents?. Environ. Sci. Technol. 2022, 56 (10), 6294–6304. 10.1021/acs.est.1c05175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Romanak K.; Bruton T.; Blum A.; Venier M. Per- and polyfluoroalkyl substances in paired dust and carpets from childcare centers. Chemosphere 2020, 251, 126771. 10.1016/j.chemosphere.2020.126771. [DOI] [PubMed] [Google Scholar]
- Zheng G.; Boor B. E.; Schreder E.; Salamova A. Indoor exposure to per- and polyfluoroalkyl substances (PFAS) in the childcare environment. Environ. Pollut. 2020, 258, 113714. 10.1016/j.envpol.2019.113714. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Miller G. Z.; Gearhart J.; Peaslee G.; Venier M. Side-chain fluorotelomer-based polymers in children car seats. Environ. Pollut. 2021, 268 (Pt B), 115477. 10.1016/j.envpol.2020.115477. [DOI] [PubMed] [Google Scholar]
- EU (European Union) . Directive 2006/122/ECOF the European parliament and of the council of 12 December 2006. In Official Journal of the European Union, 2006; pp L 372/32–L 372/34. [Google Scholar]
- EU (European Union) . COMMISSION REGULATION (EU) 2017/1000 of 13 June 2017 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards perfluorooctanoic acid (PFOA), its salts and PFOA-related substances. In Official Journal of the European Union, 2017; pp L 150/14–L 150/18. [Google Scholar]
- Wang Z.; DeWitt J. C.; Higgins C. P.; Cousins I. T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?. Environ. Sci. Technol. 2017, 51 (5), 2508–2518. 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Washington J. W.; Ellington J. J.; Jenkins T. M.; Evans J. J.; Yoo H.; Hafner S. C. Degradability of an Acrylate-Linked, Fluorotelomer Polymer in Soil. Environ. Sci. Technol. 2009, 43 (17), 6617–6623. 10.1021/es9002668. [DOI] [PubMed] [Google Scholar]
- Rankin K.; Lee H.; Tseng P. J.; Mabury S. A. Investigating the biodegradability of a fluorotelomer-based acrylate polymer in a soil-plant microcosm by indirect and direct analysis. Environ. Sci. Technol. 2014, 48 (21), 12783–90. 10.1021/es502986w. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Cousins I. T.; Scheringer M.; Buck R. C.; Hungerbuhler K. Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environ. Int. 2014, 70, 62–75. 10.1016/j.envint.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Washington J. W.; Jenkins T. M. Abiotic Hydrolysis of Fluorotelomer-Based Polymers as a Source of Perfluorocarboxylates at the Global Scale. Environ. Sci. Technol. 2015, 49 (24), 14129–35. 10.1021/acs.est.5b03686. [DOI] [PubMed] [Google Scholar]
- Washington J. W.; Jenkins T. M.; Rankin K.; Naile J. E. Decades-scale degradation of commercial, side-chain, fluorotelomer-based polymers in soils and water. Environ. Sci. Technol. 2015, 49 (2), 915–23. 10.1021/es504347u. [DOI] [PubMed] [Google Scholar]
- Houtz E. F.; Sedlak D. L. Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environ. Sci. Technol. 2012, 46 (17), 9342–9. 10.1021/es302274g. [DOI] [PubMed] [Google Scholar]
- Liagkouridis I.; Awad R.; Schellenberger S.; Plassmann M. M.; Cousins I. T.; Benskin J. P. Combined Use of Total Fluorine and Oxidative Fingerprinting for Quantitative Determination of Side-Chain Fluorinated Polymers in Textiles. Environ. Sci. Technol. Lett. 2022, 9 (1), 30. 10.1021/acs.estlett.1c00822. [DOI] [Google Scholar]
- Zhang C.; Hopkins Z. R.; McCord J.; Strynar M. J.; Knappe D. R. U. Fate of Per- and Polyfluoroalkyl Ether Acids in the Total Oxidizable Precursor Assay and Implications for the Analysis of Impacted Water. Environ. Sci. Technol. Lett. 2019, 6 (11), 662–668. 10.1021/acs.estlett.9b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gockener B.; Fliedner A.; Rudel H.; Fettig I.; Koschorreck J. Exploring unknown per- and polyfluoroalkyl substances in the German environment - The total oxidizable precursor assay as helpful tool in research and regulation. Sci. Total Environ. 2021, 782, 146825. 10.1016/j.scitotenv.2021.146825. [DOI] [PubMed] [Google Scholar]
- Wang B.; Yao Y.; Wang Y.; Chen H.; Sun H. Per- and Polyfluoroalkyl Substances in Outdoor and Indoor Dust from Mainland China: Contributions of Unknown Precursors and Implications for Human Exposure. Environ. Sci. Technol. 2021, 56 (10), 6036. 10.1021/acs.est.0c08242. [DOI] [PubMed] [Google Scholar]
- Nikiforov V. A. Hydrolysis of FTOH precursors, a simple method to account for some of the unknown PFAS. Chemosphere 2021, 276, 130044. 10.1016/j.chemosphere.2021.130044. [DOI] [PubMed] [Google Scholar]
- Schellenberger S.; Liagkouridis I.; Awad R.; Khan S.; Plassmann M.; Peters G.; Benskin J. P.; Cousins I. T. An Outdoor Aging Study to Investigate the Release of Per- And Polyfluoroalkyl Substances (PFAS) from Functional Textiles. Environ. Sci. Technol. 2022, 56 (6), 3471–3479. 10.1021/acs.est.1c06812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I.; Schellenberger S.; Hanning A. C.; Stare A.; de Boer J.; Weiss J. M.; Leonards P. E. G. Fate of Per- and Polyfluoroalkyl Substances from Durable Water-Repellent Clothing during Use. Environ. Sci. Technol. 2022, 56 (9), 5886–5897. 10.1021/acs.est.1c07876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. S.; Commodore S.; Ferguson P. L.; Neelon B.; Pearce J. L.; Baumer A.; Newman R. B.; Grobman W.; Tita A.; Roberts J.; Skupski D.; Palomares K.; Nageotte M.; Kannan K.; Zhang C.; Wapner R.; Vena J. E.; Hunt K. J. Association between gestational PFAS exposure and Children’s adiposity in a diverse population. Environ. Res. 2022, 203, 111820. 10.1016/j.envres.2021.111820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. M.; Chen A.; Romano M. E.; Calafat A. M.; Webster G. M.; Yolton K.; Lanphear B. P. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 2016, 24 (1), 231–7. 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger S. D.; Yao P.; Vaughn M. G.; Qian Z. PFAS exposure and overweight/obesity among children in a nationally representative sample. Chemosphere 2021, 268, 128852. 10.1016/j.chemosphere.2020.128852. [DOI] [PubMed] [Google Scholar]
- Harris M. H.; Oken E.; Rifas-Shiman S. L.; Calafat A. M.; Bellinger D. C.; Webster T. F.; White R. F.; Sagiv S. K. Prenatal and childhood exposure to per- and polyfluoroalkyl substances (PFAS) and child executive function and behavioral problems. Environ. Res. 2021, 202, 111621. 10.1016/j.envres.2021.111621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogheim T. S.; Weyde K. V. F.; Aase H.; Engel S. M.; Suren P.; Oie M. G.; Biele G.; Reichborn-Kjennerud T.; Brantsaeter A. L.; Haug L. S.; Sabaredzovic A.; Auyeung B.; Villanger G. D. Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environ. Res. 2021, 202, 111692. 10.1016/j.envres.2021.111692. [DOI] [PubMed] [Google Scholar]
- US CDC (U.S. Centers for Disease Control and Prevention) . Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2021; U.S. Centers for Disease Control and Prevention: Atlanta, GA, 2021, https://stacks.cdc.gov/view/cdc/105344 (accessed October 10, 2021).
- Whitehead H. D.; Venier M.; Wu Y.; Eastman E.; Urbanik S.; Diamond M. L.; Shalin A.; Schwartz-Narbonne H.; Bruton T. A.; Blum A.; Wang Z.; Green M.; Tighe M.; Wilkinson J. T.; McGuinness S.; Peaslee G. F. Fluorinated Compounds in North American Cosmetics. Environ. Sci. Technol. Lett. 2021, 8 (7), 538–544. 10.1021/acs.estlett.1c00240. [DOI] [Google Scholar]
- Ritter E. E.; Dickinson M. E.; Harron J. P.; Lunderberg D. M.; DeYoung P. A.; Robel A. E.; Field J. A.; Peaslee G. F. PIGE as a screening tool for Per- and polyfluorinated substances in papers and textiles. Nucl. Instrum. Methods Phys. Res. B 2017, 407, 47–54. 10.1016/j.nimb.2017.05.052. [DOI] [Google Scholar]
- Winkens K.; Giovanoulis G.; Koponen J.; Vestergren R.; Berger U.; Karvonen A. M.; Pekkanen J.; Kiviranta H.; Cousins I. T. Perfluoroalkyl acids and their precursors in floor dust of children’s bedrooms - Implications for indoor exposure. Environ. Int. 2018, 119, 493–502. 10.1016/j.envint.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Taniyasu S.; Kannan K.; So M. K.; Gulkowska A.; Sinclair E.; Okazawa T.; Yamashita N. Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J. Chromatogr. A 2005, 1093 (1–2), 89–97. 10.1016/j.chroma.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Pickard H. M.; Criscitiello A. S.; Spencer C.; Sharp M. J.; Muir D. C. G.; De Silva A. O.; Young C. J. Continuous non-marine inputs of per- and polyfluoroalkyl substances to the High Arctic: a multi-decadal temporal record. Atmospheric Chemistry and Physics 2018, 18 (7), 5045–5058. 10.5194/acp-18-5045-2018. [DOI] [Google Scholar]
- Wu Y.; Simon K. L.; Best D. A.; Bowerman W.; Venier M. Novel and legacy per- and polyfluoroalkyl substances in bald eagle eggs from the Great Lakes region. Environ. Pollut. 2020, 260, 113811. 10.1016/j.envpol.2019.113811. [DOI] [PubMed] [Google Scholar]
- Van der Voet E.Substance flow analysis methodology. In A handbook of industrial ecology, Ayres R. U., Ayres L. W., Eds.; Edward Elgar Publishing: Cheltenham, UK, 2002; pp 91–101. [Google Scholar]
- Huang C.-L.; Vause J.; Ma H.-W.; Yu C.-P. Using material/substance flow analysis to support sustainable development assessment: A literature review and outlook. Resour. Conserv. Recycl. 2012, 68, 104–116. 10.1016/j.resconrec.2012.08.012. [DOI] [Google Scholar]
- Roy Choudhury A. K.7 - Repellent finishes. In Principles of Textile Finishing; Roy Choudhury A. K., Ed.; Woodhead Publishing: 2017; pp 149–194. 10.1016/B978-0-08-100646-7.00007-2. [DOI] [Google Scholar]
- Audenaert F.; Lens H.; Rolly D.; Vander Elst P. Fluorochemical Textile Repellents—Synthesis and Applications: A 3M Perspective. Journal of The Textile Institute 1999, 90 (3), 76–94. 10.1080/00405009908659480. [DOI] [Google Scholar]
- Sayed U.; Dabhi P.. 6 - Finishing of textiles with fluorocarbons. In Waterproof and Water Repellent Textiles and Clothing; Williams J., Ed.; Woodhead Publishing: 2014; pp 139–152. 10.1016/B978-0-08-101212-3.00006-X. [DOI] [Google Scholar]
- US EPA (U.S. Environmental Protection Agency) . Exposure Factors Handbook; Washington, D.C., 2011, https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 (accessed June 23, 2021).
- für Risikobewertung B.Introduction to the problems surrounding garment textiles, Updated BfR Opinion; 041/2012; 2012, https://www.bfr.bund.de/cm/349/introduction-to-the-problems-surrounding-garment-textiles.pdf (accessed June 23, 2021).
- Washburn S. T.; Bingman T. S.; Braithwaite S. K.; Buck R. C.; Buxton L. W.; Clewell H. J.; Haroun L. A.; Kester J. E.; Rickard R. W.; Shipp A. M. Exposure Assessment and Risk Characterization for Perfluorooctanoate in Selected Consumer Articles. Environ. Sci. Technol. 2005, 39 (11), 3904–3910. 10.1021/es048353b. [DOI] [PubMed] [Google Scholar]
- Tokranov A. K.; Nishizawa N.; Amadei C. A.; Zenobio J. E.; Pickard H. M.; Allen J. G.; Vecitis C. D.; Sunderland E. M. How Do We Measure Poly- and Perfluoroalkyl Substances (PFASs) at the Surface of Consumer Products?. Environ. Sci. Technol. Lett. 2019, 6 (1), 38–43. 10.1021/acs.estlett.8b00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer A.; Neugebauer F.; Neuhaus T.; Selke S. Emission of perfluoroalkyl substances (PFASs) from textiles. Organohalogen Compd. 2014, 76, 1211–1213. [Google Scholar]
- Schulze P.-E.; Norin H.. Fluorinated pollutants in all-weather clothing; The Swedish Society for Nature Conservation: Friends of the Earth Norway, 2006, https://www.fluoridealert.org/wp-content/pesticides/2006/clothing.foe.norway.feb.2006.pdf (accessed June 23, 2021).
- Morales-McDevitt M. E.; Becanova J.; Blum A.; Bruton T. A.; Vojta S.; Woodward M.; Lohmann R. The Air That We Breathe: Neutral and Volatile PFAS in Indoor Air. Environ. Sci. Technol. Lett. 2021, 8 (10), 897–902. 10.1021/acs.estlett.1c00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Zhang X.-q. The textile fluoro-surfactants on the POP_S list and its novel substitute. Textile Auxiliaries 2009, 26 (2), 1–6. [Google Scholar]
- Rice P. A.; Aungst J.; Cooper J.; Bandele O.; Kabadi S. V. Comparative analysis of the toxicological databases for 6:2 fluorotelomer alcohol (6:2 FTOH) and perfluorohexanoic acid (PFHxA). Food Chem. Toxicol. 2020, 138, 111210. 10.1016/j.fct.2020.111210. [DOI] [PubMed] [Google Scholar]
- Alpizar F.; Backhaus T.; Decker N.; Eilks I.; Escobar-Pemberthy N.; Fantke P.; Geiser K.; Ivanova M.; Jolliet O.; Kim H.-S.. UN Environment Global Chemicals Outlook II-From Legacies to Innovative Solutions: Implementing the 2030 Agenda for Sustainable Development; 9280737457; United Nations Environment Programme: 2019, http://hdl.handle.net/20.500.11822/28113 (accessed June 23, 2021).
- US EPA (U.S. Environmental Protection Agency) . Long-chain perfluorinated chemicals (PFCs) action plan; 2009, https://www.epa.gov/sites/default/files/2016-01/documents/pfcs_action_plan1230_09.pdf (accessed June 23, 2021).
- OECD (Organisation for Economic Cooperation and Development) . PFCs: outcome of the 2009 survey survey on the production, use and release of PFOS, PFAS, PFOA PFCA, their related substances products/mixtures containing these substances; 2011. (accessed October 10, 2021). [Google Scholar]
- Lohmann R.; Cousins I. T.; DeWitt J. C.; Gluge J.; Goldenman G.; Herzke D.; Lindstrom A. B.; Miller M. F.; Ng C. A.; Patton S.; Scheringer M.; Trier X.; Wang Z. Are Fluoropolymers Really of Low Concern for Human and Environmental Health and Separate from Other PFAS?. Environ. Sci. Technol. 2020, 54 (20), 12820–12828. 10.1021/acs.est.0c03244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper T.; Frömel T.; Gremmel C.; Van Driezum I.; Weil H.; Vestergren R.; Cousins I.. Understanding the exposure pathways of per-and polyfluoralkyl substances (PFASs) via use of PFASs-containing products-risk estimation for man and environment; Umweltbundesamt: 2014, https://www.umweltbundesamt.de/en/publikationen/understanding-the-exposure-pathways-of-per (accessed October 11, 2021).
- Athey S. N.; Adams J. K.; Erdle L. M.; Jantunen L. M.; Helm P. A.; Finkelstein S. A.; Diamond M. L. The Widespread Environmental Footprint of Indigo Denim Microfibers from Blue Jeans. Environ. Sci. Technol. Lett. 2020, 7 (11), 840–847. 10.1021/acs.estlett.0c00498. [DOI] [Google Scholar]
- Carney Almroth B. M.; Astrom L.; Roslund S.; Petersson H.; Johansson M.; Persson N. K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. and Pollut. Res. 2018, 25 (2), 1191–1199. 10.1007/s11356-017-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp K. J.; Miller R. Z. Electric clothes dryers: An underestimated source of microfiber pollution. PLoS One 2020, 15 (10), e0239165 10.1371/journal.pone.0239165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirc U.; Vidmar M.; Mozer A.; Krzan A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. and Pollut. Res. 2016, 23 (21), 22206–22211. 10.1007/s11356-016-7703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Zhang Y.; Liu Y.; Gong X.; Zhang T.; Sun H. Widespread Occurrence of Bisphenol A in Daily Clothes and Its High Exposure Risk in Humans. Environ. Sci. Technol. 2019, 53 (12), 7095–7102. 10.1021/acs.est.9b02090. [DOI] [PubMed] [Google Scholar]
- Besser L.Dead White Man’s Clothes. https://www.abc.net.au/news/2021-08-12/fast-fashion-turning-parts-ghana-into-toxic-landfill/100358702 (accessed January 11, 2022).
- Jay P.Here’s where your donated clothing really ends up. https://www.cbc.ca/news/canada/ottawa/donated-clothing-where-it-ends-up-1.4662023 (accessed June 28, 2021). [Google Scholar]
- SMART (Secondary Material and Recycled Textiles Association) . How are textiles recycled? https://www.smartasn.org/resources/frequently-asked-questions/ (accessed June 25, 2021).
- Burns & McDonnell Engineering Company . 2013 Statewide Waste Characterization; Minnesota Pollution Control Agency: 2013. (accessed June 22, 2022).
- Thorpe B.Scoping Per-and Polyfluoroalkyl Substances Releases from the Recycling of Paper and Textiles and their Implications for the Great Lakes-St Lawrence River Ecosystem: Identifying Opportunities to Address Toxicity of Products in a Circular Economy; Canadian Environmental Law Association: 2019, https://cela.ca/wp-content/uploads/2019/10/Report-PFAS-Sept-2019.pdf (accessed June 23, 2021). [Google Scholar]
- Choi Y. J.; Kim Lazcano R.; Yousefi P.; Trim H.; Lee L. S. Perfluoroalkyl Acid Characterization in U.S. Municipal Organic Solid Waste Composts. Environ. Sci. Technol. Lett. 2019, 6 (6), 372–377. 10.1021/acs.estlett.9b00280. [DOI] [Google Scholar]
- Coresight Research . Back-to-School: Share of Children Wearing School Uniforms in the United States from 2008 to 2018. https://www.statista.com/statistics/896612/share-of-children-wearing-school-uniforms-us/ (accessed October 05, 2021).
- US EPA (U.S. Environmental Protection Agency) . Technical Fact Sheet - Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). https://19january2021snapshot.epa.gov/sites/static/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf (accessed November 5, 2021).
- US EPA (U.S. Environmental Protection Agency) . Fact Sheet: Human Health Toxicity Assessment for GenX Chemicals. https://www.epa.gov/system/files/documents/2021-10/genx-final-tox-assessment-general_factsheet-2021.pdf (accessed November 05, 2021).
- Agency for Toxic Substances and Disease Registry . Toxicological Profile for Perfluoroalkyls. https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed November 05, 2021). [PubMed]
- Efsa Panel on Contaminants in the Food Chain (EFSA CONTAM Panel).Schrenk D.; Bignami M.; Bodin L.; Chipman J. K.; Del Mazo J.; Grasl-Kraupp B.; Hogstrand C.; Hoogenboom L. R.; Leblanc J. C.; Nebbia C. S.; Nielsen E.; Ntzani E.; Petersen A.; Sand S.; Vleminckx C.; Wallace H.; Barregard L.; Ceccatelli S.; Cravedi J. P.; Halldorsson T. I.; Haug L. S.; Johansson N.; Knutsen H. K.; Rose M.; Roudot A. C.; Van Loveren H.; Vollmer G.; Mackay K.; Riolo F.; Schwerdtle T. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18 (9), e06223 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson H.; Karrman A.; Rotander A.; van Bavel B.; Lindstrom G.; Westberg H. Biotransformation of fluorotelomer compound to perfluorocarboxylates in humans. Environ. Int. 2013, 51, 8–12. 10.1016/j.envint.2012.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.