Abstract
Background
GSK3640254 (GSK’254) is a next-generation human immunodeficiency virus type 1 (HIV-1) maturation inhibitor with pharmacokinetics (PK) supporting once-daily therapy.
Methods
This phase IIa double-blind (sponsor-unblinded), randomized, placebo-controlled, adaptive study evaluated antiviral effect, safety, tolerability, and PK of once-daily GSK’254 monotherapy administered with food (moderate-fat meal) in HIV-1–positive, treatment-naive adults. In part 1, participants received GSK’254 10 or 200 mg for 10 days. In part 2, participants received GSK’254 40, 80, or 140 mg for 7 days, modified from 10 days by a protocol amendment to decrease potential for resistance-associated mutations (RAMs). The primary endpoint was maximum change from baseline in HIV-1 RNA.
Results
Maximum changes in HIV-1 RNA of −0.4, −1.2, −1.0, −1.5, and −2.0 log10 occurred with GSK’254 10, 40, 80, 140, and 200 mg, respectively. Regardless of dosing duration, doses ≥40 mg resulted in ≥1-log10 declines in HIV-1 RNA. Plasma PK was generally dose proportional to 140 mg but non-proportional between 140 and 200 mg. Four participants in the 200-mg group developed RAMs on day 11 in part 1, 1 with phenotypic resistance. No RAMs occurred in part 2. Adverse events (AEs) were reported by 22 (65%) participants; headache was the most common (n = 4). Two non–drug-related serious AEs occurred. All AEs were of mild-to-moderate intensity, except for 2 grade 3 non–drug-related AEs in 1 participant.
Conclusions
This monotherapy study established a dose–antiviral response relationship for GSK’254. No safety or tolerability concerns were noted. These results supported dose selection for the ongoing phase IIb study (ClinicalTrials.gov: NCT04493216).
Clinical Trials Registration
Keywords: HIV infection, HIV-1 RNA, pharmacodynamics, tolerability, treatment-naive
This proof-of-concept monotherapy study of treatment-naive adults confirmed the antiviral activity of the next-generation HIV-1 maturation inhibitor GSK3640254 and established a dose–antiviral response relationship. No major safety or tolerability findings were observed, supporting the ongoing phase IIb study.
Combination antiretroviral therapy (ART) regimens targeting the reverse transcriptase, protease, or integrase proteins are highly effective at achieving virologic suppression and reducing morbidity and mortality in people living with human immunodeficiency virus type 1 (HIV-1) [1]. However, drug resistance and toxicities can occur with multidrug HIV-1 regimens, potentially resulting in treatment failure [1, 2]. Therefore, needs exist for therapeutic agents with novel mechanisms of action for people living with HIV-1 who have had previous virologic failure.
Maturation inhibitors are a new class of ART agents in clinical development [3]. Maturation is among the last steps of the HIV-1 life cycle in which the viral protease facilitates cleavage of HIV-1 structural protein (Gag) precursors into mature Gag proteins. This cleavage initiates a structural rearrangement in the immature viral particles that have been released, resulting in the formation of mature, infectious virions. Maturation inhibitors prevent HIV-1 maturation by blocking the protease-mediated cleavage of capsid-spacer protein 1 from the Gag polyprotein through binding the Gag substrate, which results in the release of immature, noninfectious particles [3]. Thus, maturation inhibitors are distinct from protease inhibitors, which specifically inhibit HIV-1 protease enzymatic activity. Findings from in vitro analyses and phase I/II clinical studies demonstrate that maturation inhibitors inhibit replication of HIV-1 isolates [2, 4–8]. Thus, maturation inhibitors represent a promising new ART class for HIV-1 treatment.
GSK3640254 (GSK’254) is a next-generation maturation inhibitor that has demonstrated inhibition across all HIV-1 subtypes and efficacy against a broad range of polymorphisms [9]. In multiple phase I clinical trials in healthy participants, GSK’254 has been well tolerated under short-term administration and demonstrated a pharmacokinetic (PK) profile that supports once-daily dosing [9–11]. No drug-drug interactions were observed when GSK’254 was coadministered with dolutegravir or tenofovir alafenamide/emtricitabine [10, 11]. Based on these safety and PK profiles, a range of GSK’254 doses were selected for evaluation in a phase IIa proof-of-concept study [9]. Here we report the antiviral effect, tolerability, and PK of GSK’254 in treatment-naive adults with HIV-1.
METHODS
Study Design
This was a phase IIa, global, double-blind (sponsor-unblinded), randomized, placebo-controlled, adaptive study of GSK’254 in treatment-naive adults with HIV-1 (ClinicalTrials.gov: NCT03784079). Part 1 consisted of a treatment period from days 1 to 10, a postdosing follow-up period from days 11 to 17, and final follow-up between days 18 and 24. Participants in part 1 received GSK’254 10 or 200 mg or placebo once daily for 10 days and could start combination ART after the final follow-up visit. A planned, sponsor-unblinded, interim analysis was performed after part 1 to allow for the selection of doses to be studied in part 2. Immediately after this analysis, treatment-emergent resistance was seen at day 11 in the 200-mg group (described in the Results), and the sponsor temporarily halted the study to conduct resistance analyses. As a result, part 2 was modified to decrease the likelihood of treatment-emergent resistance by decreasing the duration of GSK’254 monotherapy from 10 to 7 days and requiring participants to start combination ART immediately after completing monotherapy. Part 2 consisted of a treatment period from days 1 to 7, postdosing follow-up on day 8, and final follow-up between days 10 and 12. Participants in part 2 were administered GSK’254 40, 80, or 140 mg or placebo once daily for 7 days and began combination ART on day 8. All participants consumed a moderate-calorie, moderate-fat meal (ie, ≥400 calories with ≥120 calories from fat) 30 minutes before GSK’254 or placebo dosing. Doses were defined to adequately describe the dose– and exposure–antiviral response relationship of GSK’254 in HIV-1–positive, treatment-naive adults. Doses were selected to provide approximately 30%, 50%, 75%, 90%, and 95% of the median maximum anticipated effect on HIV-1 RNA for a maturation inhibitor in a 10-day study. Although, as noted, after assessments in part 1, monotherapy duration was reduced to 7 days for part 2.
The target trough concentration for GSK’254 was established using a value of 3× the protein-binding adjusted 90% effective concentration (3× PBA EC90) for 1 of the least sensitive variants, a triple-mutant polymorph (R361K/V362I/L363M), from a library of 35 Gag/Pr genotyped viruses [12]. This virus exhibited half maximal effective concentration (EC50) and 3× PBA EC90 values of 3 and 150 nM (110 ng/mL), respectively. Leading into this proof-of-concept study, the 3× PBA EC90 value of 110 ng/mL represented the anticipated minimal effective trough concentration of GSK’254 for a population of individuals with HIV-1.
This study was conducted at 27 sites in 6 countries in accordance with Declaration of Helsinki principles. The study protocol was reviewed and approved by national, regional, or investigational center ethics committees or institutional review boards in each country. All participants provided written informed consent and could withdraw from the study at any time.
Participants
Eligible participants were men and women aged 18 to 65 years with documented HIV-1 infection, body weight ≥50 kg (men) or ≥ 45 kg (women), and body mass index between 18.5 and 31.0 kg/m2. Participants were ART naive with plasma HIV-1 RNA ≥5000 copies/mL and CD4 + T-cell count ≥350 cells/mm3 at screening but were otherwise healthy, as determined by the investigator based on medical history, laboratory assessments, and electrocardiographic findings. Exclusion criteria are summarized in the Supplementary Materials.
Assessments
Primary endpoints were maximum change from baseline and change from baseline to day 11 (part 1) and day 8 (part 2) in plasma HIV-1 RNA. Secondary endpoints included area under the concentration-time curve (AUC) from time 0 to 24 hours (AUC0-24), maximum observed concentration (Cmax), and time to Cmax (tmax) after single-dose GSK’254 administration on day 1; AUC from time 0 to the end of the dosing interval (AUC0-τ), Cmax, tmax, and concentration at the end of the dosing interval (Cτ) after repeat-dose GSK’254 administration; PK parameter accumulation ratios; relationship between GSK’254 dose and PK parameters; relationship between maximum change from baseline in HIV-1 RNA and Cτ; and safety and tolerability parameters. Exploratory endpoints were treatment-emergent resistance-associated mutations (RAMs) and determination of steady-state GSK’254 concentrations.
Sample collection times for plasma HIV-1 RNA, resistance analyses, and PK assessments are summarized in the Supplementary Materials. GSK’254 concentrations were analyzed by PPD (Middletown, Wisconsin, USA) via liquid chromatography with tandem mass spectrometry, as previously described [10]. Plasma assay range for GSK’254 was 3.00 to 1000 ng/mL. Inter-assay coefficient of variation values for quality control samples ranged from 2.32% to 6.41%. Safety and tolerability were assessed by adverse events (AEs), clinical laboratory assessments, electrocardiograms, and vital signs.
Data Analyses
No formal hypotheses were tested. Sample size was based on feasibility to provide adequate precision for estimations. The Bayesian posterior probability testing based on the GSK’254 200-mg dose in part 1 determined a 99.79% probability of achieving a 1.3-log10 maximum mean decline in HIV-1 RNA and met the protocol-defined criteria to proceed to part 2. Based on these results, part 2 proceeded with GSK’254 doses of 40, 80, and 140 mg once daily that were estimated to result in maximum effects of 37%, 66%, and 84%, respectively.
Plasma HIV-1 RNA maximum change from baseline was analyzed using a mixed-effects linear model by treatment, baseline log10 HIV-1 RNA, and treatment × baseline log10 HIV-1 RNA as fixed effects and participant as a random effect. Pharmacokinetic parameters were calculated by standard noncompartmental analysis with Phoenix WinNonlin software (version 6.4; Certara, St Louis, Missouri, USA). A linear mixed model with day as a fixed effect and participant as a random effect evaluated the achievement of steady state using the Helmert transformation approach [13]. Pharmacokinetic parameters were assessed using a fixed-effects power model fitted by restricted maximum likelihood to evaluate GSK’254 dose proportionality. The relationship between maximum change from baseline in log10 plasma HIV-1 RNA and GSK’254 dose or Cτ was assessed using maximum effect (Emax) models combining doses from parts 1 and 2; models were selected based on Akaike information criterion (AIC) values. Safety data were descriptively summarized.
RESULTS
Study Population and Baseline Characteristics
Of the 106 individuals screened, 34 were randomized to receive either GSK’254 (6 participants/dose) or placebo (2 participants each in parts 1 and 2); all participants completed the study. Mean age of the overall study population was 31.8 years, and most participants were men (94%; Table 1). Primary reasons for study exclusion were not meeting inclusion or meeting exclusion criteria (n = 58) and physician decision (n = 13).
Table 1.
Baseline Demographics
| Parameter | GSK’254 10 mg (n = 6) | GSK’254 40 mg (n = 6) | GSK’254 80 mg (n = 6) | GSK’254 140 mg (n = 6) | GSK’254 200 mg (n = 6) | Placebo (n = 4) |
Total (n = 34) |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), ya | 32.7 (8.3) | 27.7 (6.9) | 32.8 (6.2) | 33.2 (8.2) | 29.3 (3.9) | 36.5 (9.3) | 31.8 (7.2) |
| Sex, no. (%) | |||||||
| Female | 0 | 1 (17) | 0 | 1 (17) | 0 | 0 | 2 (6) |
| Male | 6 (100) | 5 (83) | 6 (100) | 5 (83) | 6 (100) | 4 (100) | 32 (94) |
| Body mass index, mean (SD), kg/m2 | 25.3 (3.7) | 23.9 (4.3) | 24.8 (3.7) | 23.4 (1.6) | 22.6 (2.2) | 23.0 (1.3) | 23.9 (3.0) |
| Height, mean (SD), cm | 177.8 (5.7) | 172.8 (7.3) | 176.2 (12.3) | 173.6 (8.6) | 179.4 (6.0) | 174.0 (6.3) | 175.7 (7.9) |
| Weight, mean (SD), kg | 80.3 (14.9) | 70.7 (8.4) | 77.4 (16.7) | 70.9 (9.5) | 72.9 (9.8) | 69.8 (4.8) | 73.9 (11.5) |
| Ethnicity, no. (%) | |||||||
| Hispanic/Latino | 6 (100) | 2 (33) | 2 (33) | 1 (17) | 1 (17) | 2 (50) | 14 (41) |
| Not Hispanic/Latino | 0 | 4 (67) | 4 (67) | 5 (83) | 5 (83) | 2 (50) | 20 (59) |
| Race, no. (%) | |||||||
| American Indian/Alaska Native | 2 (33) | 0 | 0 | 0 | 0 | 1 (25) | 3 (9) |
| Asian/Southeast Asian heritage | 1 (17) | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Black/African American | 0 | 1 (17) | 2 (33) | 1 (17) | 0 | 0 | 4 (12) |
| White/Caucasian/European heritage | 2 (33) | 5 (83) | 4 (67) | 5 (83) | 5 (83) | 3 (75) | 24 (71) |
| Multiple | 1 (17) | 0 | 0 | 0 | 1 (17) | 0 | 2 (6) |
Abbreviations: GSK’254, GSK3640254; SD, standard deviation.
Age was imputed when full date of birth was not provided.
Pharmacodynamics
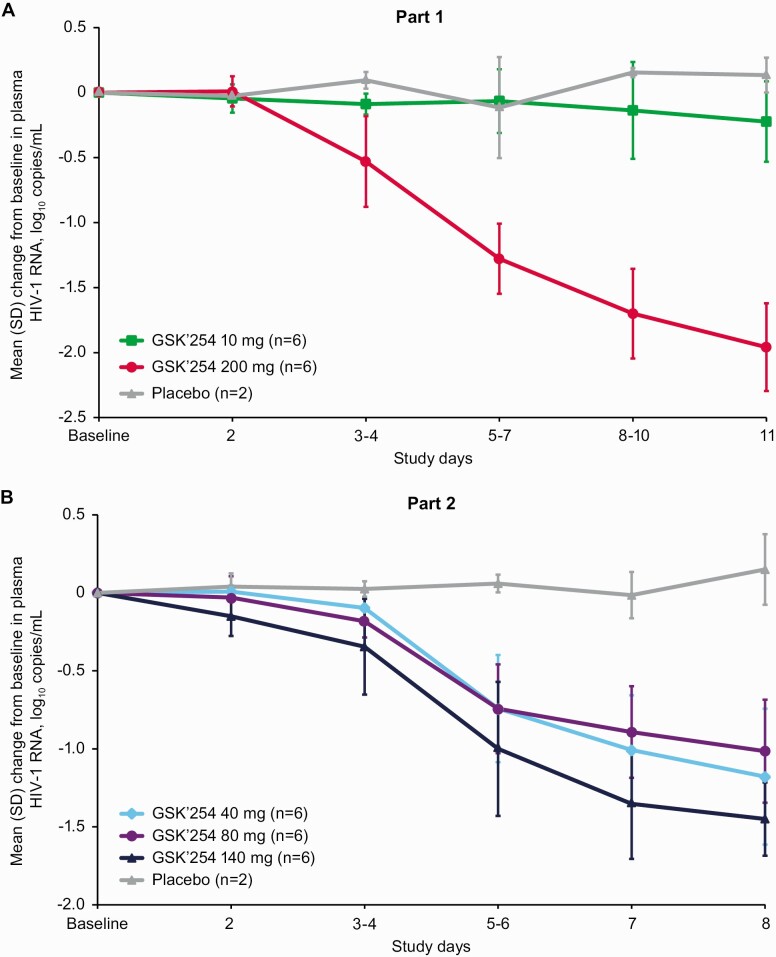
Plasma HIV-1 RNA mean change from baseline to days 11 and 8 in parts 1 and 2, respectively, and to nadir in parts 1 and 2 is summarized in Table 2. The largest decreases in HIV-1 RNA occurred in the 200- and 140-mg GSK’254 groups, with maximum mean declines of 2.0 and 1.5 log10, respectively. Median time to nadir in HIV-1 RNA was 11.5 days in part 1 and 8.0 days in part 2, consistent with the dosing intervals of 10 and 7 days in parts 1 and 2, respectively.
Table 2.
Mean Change From Baseline to Primary Endpoint and Nadir in Plasma HIV-1 RNA
| Parameter | Part 1 | Part 2a | |||||
|---|---|---|---|---|---|---|---|
| (Primary endpoint = Day 11) | (Primary endpoint = Day 8) | ||||||
| GSK’254 10 mg (n = 6) | GSK’254 200 mg (n = 6) | Placebo (n = 2) | GSK’254 40 mg (n = 6) | GSK’254 80 mg (n = 6) | GSK’254 140 mg (n = 6) | Placebo (n = 2) | |
| Plasma HIV-1 RNA (log10 copies/mL), mean (SD) | |||||||
| Baseline | 4.19 (0.31) | 4.82 (0.48) | 4.25 (0.42) | 4.67 (0.23) | 4.43 (0.51) | 4.53 (0.58) | 4.75 (1.78) |
| Primary endpoint | −0.22 (0.31) | −1.96 (0.34) | 0.14 (0.13) | −1.18 (0.44) | −1.02 (0.33) | −1.45 (0.24) | 0.15 (0.23) |
| Nadir | −0.36 (0.25) | −2.01 (0.33) | −0.21 (0.26) | −1.18 (0.44) | −1.02 (0.33) | −1.49 (0.27) | −0.03 (0.13) |
| Modeled plasma HIV-1 RNA rate of change (log10 copies/mL per day), mean (90% CI) | |||||||
| Primary endpointb | −0.02 (−.04 to .01) | −0.22 (−.24 to −.20) | 0.02 (−.02 to .06) | −0.21 (−.23 to −.19) | −0.17 (−.19 to −.15) | −0.25 (−.27 to −.22) | 0.01 (−.03 to .05) |
| Modeled plasma HIV-1 RNA maximum change (log10 copies/mL), mean (90% CI) | |||||||
| Nadirc | −0.34 (−.67 to −.00) | −1.93 (−2.24 to −1.62) | −0.07 (−.58 to .45) | −1.05 (−1.29 to −.82) | −0.94 (−1.16 to −.72) | −1.49 (−1.70 to − 1.28) | −0.02 (−.39 to .36) |
Abbreviations: CI, confidence interval; GSK’254, GSK3640254; HIV-1, human immunodeficiency virus type 1; SD, standard deviation.
Assessments on days 10–12 are excluded from nadir calculation because of administration of combination antiretroviral therapy from day 8 in part 2.
Change from baseline model estimates were generated using a mixed-effects model with baseline, treatment day, treatment, and treatment × treatment day as main effects and participant as a random effect.
Nadir model estimates are least squares means generated using a mixed-effects model with baseline, treatment, and treatment × baseline as main effects.
In part 1, log10 HIV-1 RNA decreased in the GSK’254 200-mg group from day 2 to day 11 (Figure 1A). A >1.5-log10 decline from baseline in HIV-1 RNA was observed at day 11 in all participants from the 200-mg group. Values for log10 HIV-1 RNA remained close to baseline levels for both the 10-mg and placebo groups. In part 2, log10 HIV-1 RNA steadily decreased from days 3 and 4 through day 8 in all GSK’254 groups (Figure 1B). A> 1.5-log10 decline in HIV-1 RNA from baseline was observed at Day 8 in 3 participants from the 140-mg group and 1 participant from the 40-mg group.
Figure 1.
Mean (SD) change from baseline in log10 plasma HIV-1 RNA by treatment in (A) part 1 and (B) part 2. Abbreviations: GSK’254, GSK3640254; HIV-1, human immunodeficiency virus type 1; SD, standard deviation.
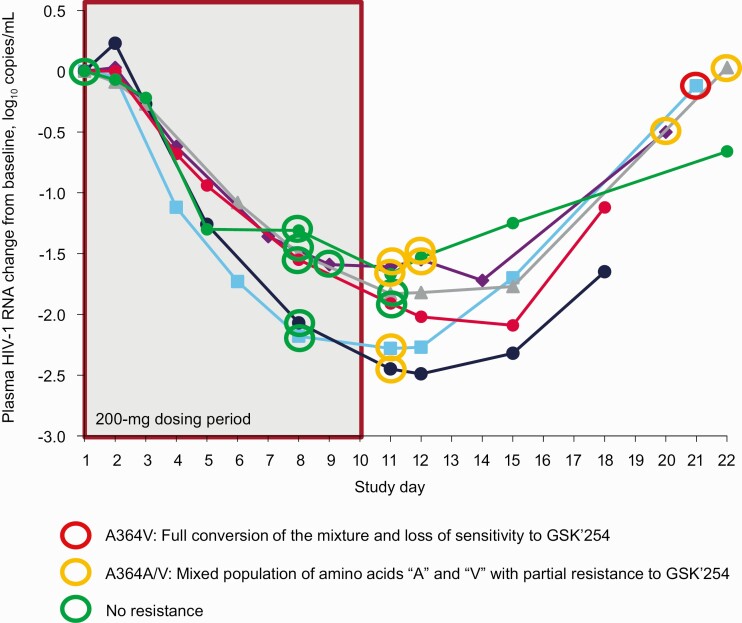
Of the 12 participants who received GSK’254 monotherapy for 10 days in part 1, 4 in the 200-mg group developed the RAM A364A/V at day 11 (Figure 2). One of 4 participants with full conversion to the A364V mutation developed phenotypic resistance with a fold change in half maximal inhibitory concentration of 132. However, no participant had treatment-emergent RAMs at days 8, 9, or 10. No emergent RAMs were observed in part 2 when GSK’254 monotherapy was administered for 7 days.
Figure 2.
Change from baseline in log10 plasma HIV-1 RNA and HIV-1 Gag genotyping results from day 8 to end of study in the 200-mg group. Abbreviations: GSK’254, GSK3640254; HIV-1, human immunodeficiency virus type 1.
Pharmacokinetics
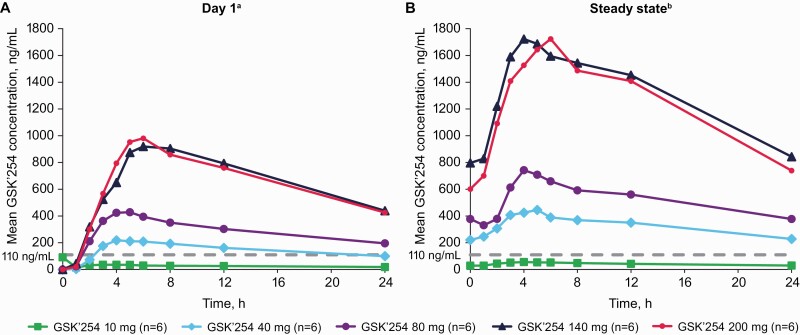
Across all doses, and similar to the PK observed in healthy participants, GSK’254 reached maximum plasma concentrations with a median tmax of 2.93 to 5.53 hours on Day 1 (after the first dose) and 4.02 to 5.48 hours at steady state (Table 3; Figure 3) [9]. Plasma Cmax and AUC0-24 or AUC0-τ values were generally dose proportional on day 1 and at steady state for doses up to 140 mg; a plateau in these parameters was observed between the 140- and 200-mg doses, indicating nonproportionality. The power model approach was applied to evaluate GSK’254 dose proportionality. After single-dose administration on day 1, the estimated slopes (90% confidence interval [CI]) for AUC0-24, Cmax, and concentration at 24 hours were 1.02 (90% CI: .88–1.16), 0.96 (90% CI: .77–1.15), and 1.06 (90% CI: .92–1.20), respectively. Estimated slopes (90% CI) for AUC0-τ, Cmax, and Cτ were 1.18 (90% CI: 1.07–1.28), 1.20 (90% CI: 1.11–1.30), and 1.14 (90% CI: 1.02–1.26), respectively, after repeat administration at steady state.
Table 3.
Plasma GSK’254 PK Parameters After Single- and Repeat-Dose Administration
| Geometric mean, (% CVb)a | GSK’254 10 mg (n = 6) |
GSK’254 40 mg (n = 6) |
GSK’254 80 mg (n = 6) |
GSK’254 140 mg (n = 6) |
GSK’254 200 mg (n = 6)b |
|---|---|---|---|---|---|
| Day 1 | |||||
| AUC0-24, µg∗h/mL | 0.70 (13.5) | 3.25 (31.7) | 6.12 (38.8) | 14.0 (36.6) | 12.40 (91.3) |
| 95% CI | .59–.82 | 2.35–4.50 | 4.13–9.07 | 9.67–20.40 | 5.48–28.00 |
| Range | 0.59–0.81 | 2.41–5.60 | 3.80–11.20 | 10.15–23.82 | 2.69–23.20 |
| Cmax, µg/mL | 0.06 (177.4) | 0.23 (30.5) | 0.43 (33.6) | 0.92 (41.5) | 0.94 (82.3) |
| 95% CI | .02–.21 | .17–.32 | .31–.61 | .60–1.40 | .44–2.00 |
| Range | 0.02–0.55 | 0.17–0.40 | 0.29–0.75 | 0.58–1.80 | 0.23–1.53 |
| tmax, median (range), h | 2.93 (0.00–5.00) | 4.42 (3.97–8.00) | 4.08 (2.95–6.17) | 5.51 (3.00–6.25) | 5.53 (3.92–8.05) |
| Day 8, 9, or 10 (part 1) or day 7 (part 2) | |||||
| AUC0-τ, µg∗h/mL | 0.91 (44.7) | 7.46 (26.8) | 11.80 (26.7) | 29.30 (27.9) | 27.90 (18.4) |
| 95% CI | .58–1.42 | 5.66–9.84 | 8.98–15.60 | 22.00–39.00 | 23.10–33.80 |
| Range | 0.41–1.33 | 5.76–11.47 | 8.96–16.68 | 17.76–37.70 | 22.27–34.79 |
| Cmax, µg/mL | 0.06 (41.3) | 0.47 (20.6) | 0.75 (23.7) | 1.86 (26.0) | 1.86 (19.5) |
| 95% CI | .04–.08 | .38–.58 | .59–.96 | 1.42–2.43 | 1.51–2.27 |
| Range | 0.03–0.08 | 0.35–0.61 | 0.60–1.13 | 1.17–2.52 | 1.43–2.45 |
| tmax, median (range), h | 4.02 (1.87–5.00) | 4.06 (2.00–8.00) | 4.58 (4.00–5.18) | 4.08 (2.92–5.20) | 5.48 (3.00–6.20) |
| Cτ, µg/mL | 0.03 (47.0) | 0.22 (30.1) | 0.36 (31.1) | 0.80 (34.1) | 0.70 (29.6) |
| 95% CI | .02–.04 | .16–.30 | .26–.50 | .56–1.13 | .52–.95 |
| Range | 0.01–0.04 | 0.16–0.35 | 0.25–0.51 | 0.49–1.19 | 0.46–0.91 |
| CL/F, mL/h | 11 011 (44.7) | 5360 (26.8) | 6765 (26.7) | 4779 (27.9) | 7159 (18.4) |
| 95% CI | 7033–17 238 | 4064–7070 | 5139–8905 | 3587–6367 | 5914–8667 |
| Range | 7519–24 568 | 3487–6941 | 4795–8933 | 3713–7881 | 5749–8981 |
| Accumulation ratio | |||||
| AUC0-τ | 1.54 (24.5) | 2.29 (11.2) | 1.93 (18.6) | 2.09 (37.5) | 2.25 (72.1) |
| 95% CI | 1.14–2.07 | 2.04–2.58 | 1.59–2.34 | 1.38–2.96 | 1.14–4.44 |
| Range | 1.09–1.95 | 2.05–2.69 | 1.49–2.59 | 1.31–2.73 | 1.50–8.27 |
| Cmax | 0.93 (171.7) | 2.03 (24.4) | 1.73 (17.6) | 2.02 (37.5) | 1.98 (69.4) |
| 95% CI | .27–3.18 | 1.57–2.61 | 1.47–2.07 | 1.38–2.96 | 1.03–3.82 |
| Range | 0.09–1.86 | 1.52–2.91 | 1.45–2.29 | 1.17–3.25 | 1.34–6.93 |
| Cτ | 1.48 (22.2) | 2.30 (6.4) | 1.94 (20.8) | 1.90 (16.0) | 1.98 (61.2) |
| 95% CI | 1.17–1.86 | 2.15–2.46 | 1.56–2.41 | 1.61–2.24 | 1.10–3.58 |
| Range | 1.09–2.07 | 2.08–2.46 | 1.48–2.55 | 1.44–2.26 | 1.35–6.09 |
Abbreviations: AUC0-24, area under the concentration-time curve from 0 to 24 hours; AUC0-τ, area under the concentration-time curve from time 0 to the end of the dosing interval; CI, confidence interval; CL/F, apparent oral clearance; Cmax, maximum observed concentration; Cτ, concentration at the end of the dosing interval; CVb, between-participant coefficient of variation; GSK’254, GSK3640254; HIV-1, human immunodeficiency virus type 1; PK, pharmacokinetic; tmax, time to Cmax.
Except where noted for tmax.
One participant was excluded from PK analysis on day 1 due to vomiting postdose ≤1 × tmax.
Figure 3.
Plasma GSK’254 PK parameters after (A) single-dose administration on day 1 and (B) repeat-dose administration at steady state. Dashed line indicates the clinical efficacy target value for which ≥95% of participants in a phase IIb study are projected to exceed target trough concentrations (110 ng/mL). Abbreviations: GSK’254, GSK3640254; PK, pharmacokinetics. aOne participant in the 10-mg group had a predose concentration that was inconsistent with the expected PK profile. One participant in the 200-mg group was excluded from PK analysis due to vomiting postdose ≤1 × time to maximum observed concentration. bSteady state was measured on day 8, 9, or 10 in part 1 and day 7 in part 2.
Although the HIV-1 RNA declines in the ≤80-mg groups were among the lowest in the study, mean GSK’254 concentrations in the ≥40-mg groups were above the target trough concentration (110 ng/mL). Except for Cmax in the 10-mg group, accumulation ratios were >1 for all parameters evaluated, ranging from 1.54 to 2.29 for AUC0-τ, 0.93 to 2.03 for Cmax, and 1.48 to 2.30 for Cτ, consistent with the observed terminal phase half-life in other studies (~24 hours) [9]. Steady-state GSK’254 concentrations were reached by days 5–7 in all dose groups.
Dose– and Exposure–Antiviral Response Relationships
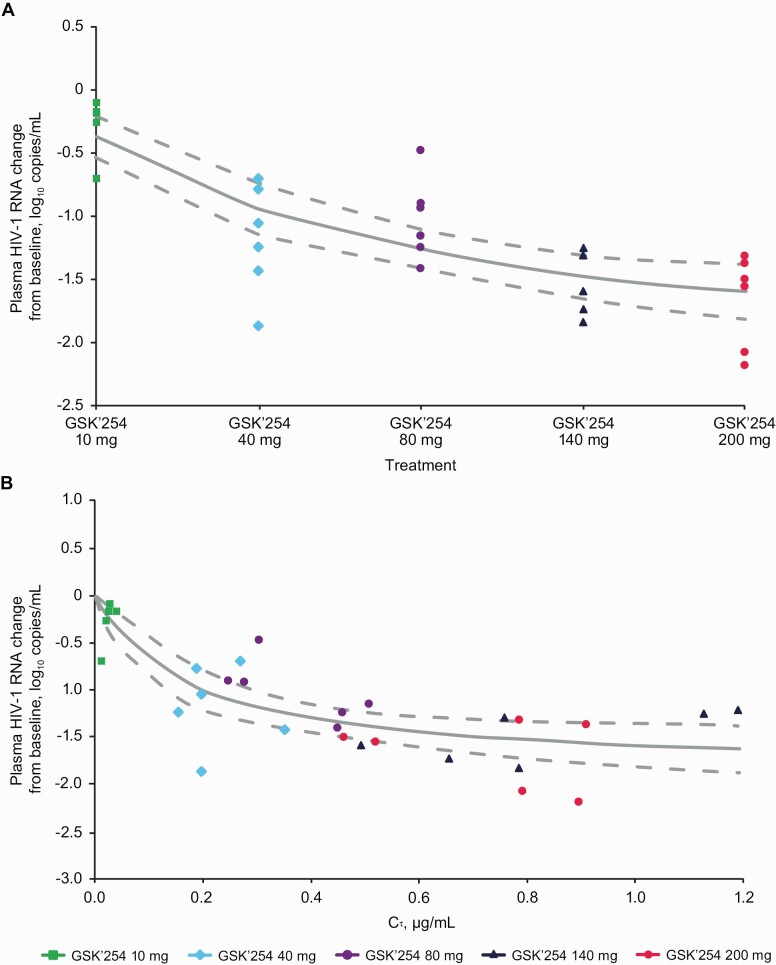
The relationship between dose and maximum change in HIV-1 RNA from baseline to day 8, noting that the maximum antiviral effect was not observed in 8 days of monotherapy with this late-stage HIV-1 life cycle inhibitor, was fitted to an Emax nonlinear model (Figure 4A). Multiple models were assessed based on AIC values, and the Emax nonlinear model had the lowest AIC value and was considered the best model. Estimated values (95% CI) from the dose-response model were − 1.93 log10 HIV-1 RNA (−2.43 to − 1.44) for Emax, 42.67 mg GSK’254 (7.56–77.77) for half maximal effective dose (ED50), and 0.12 (0.06–0.18) for variance.
Figure 4.
Maximum change from baseline to day 8 in log10 plasma HIV-1 RNA vs (A) GSK’254 dose and (B) Cτ fitted to an Emax nonlinear model. Solid and dashed lines represent the fitted value from the Emax model and 95% confidence interval, respectively. Abbreviations: Cτ, concentration at the end of the dosing interval; Emax, maximum effect; GSK’254, GSK3640254; HIV-1, human immunodeficiency virus type 1.
The relationship between GSK’254 Cτ and maximum change in HIV-1 RNA from baseline to Day 8 was fitted to an Emax nonlinear model (Figure 4B). Estimated values (95% CI) were −1.86 log10 HIV-1 RNA (−2.33 to −1.40), 0.17 µg/mL (.02–.32), and 0.12 (.06–.19) for Emax, EC50, and variance, respectively.
Safety
Overall, 22 (65%) participants reported AEs during the study, all of whom received GSK’254 (Table 4). The most commonly reported AEs were headache (n = 4) and oropharyngeal pain (n = 3). Gastrointestinal AEs occurred in 7 (21%) participants, with diarrhea (n = 3), abdominal pain (n = 2), and vomiting (n = 2) reported in >1 participant. Two participants each reported nasopharyngitis and lymphadenopathy. Other AEs were reported by 1 participant each. Fourteen drug-related AEs were reported by 9 (26%) participants, with diarrhea, abdominal pain, and vomiting being the most common. Two participants reported serious AEs (SAEs) of congestive cardiomyopathy and anal abscess, both of which were considered unrelated to the study drug; both SAEs required hospitalization and resolved. All AEs and SAEs were mild to moderate in intensity, except for in 1 participant who developed congestive cardiomyopathy and myocarditis, both of which were grade 3 and considered unrelated to the study drug. No AEs led to treatment discontinuation, and no deaths were reported during the study.
Table 4.
Summary of AEs
| Preferred Term, n (%) | GSK’254 10 mg (n = 6) | GSK’254 40 mg (n = 6) | GSK’254 80 mg (n = 6) | GSK’254 140 mg (n = 6) | GSK’254 200 mg (n = 6) | Placebo (n = 4) | Total (N = 34) |
|---|---|---|---|---|---|---|---|
| Total AEsa | |||||||
| Any event | 3 (50) | 5 (83) | 4 (67) | 5 (83) | 5 (83) | 0 | 22 (65) |
| Headache | 0 | 1 (17) | 0 | 1 (17) | 2 (33) | 0 | 4 (12) |
| Diarrhea | 1 (17) | 1 (17) | 0 | 0 | 1 (17) | 0 | 3 (9) |
| Oropharyngeal pain | 0 | 0 | 0 | 1 (17) | 2 (33) | 0 | 3 (9) |
| Abdominal pain | 0 | 0 | 2 (33) | 0 | 0 | 0 | 2 (6) |
| Nasopharyngitis | 0 | 0 | 0 | 0 | 2 (33) | 0 | 2 (6) |
| Lymphadenopathy | 1 (17) | 0 | 0 | 0 | 1 (17) | 0 | 2 (6) |
| Vomiting | 1 (17) | 0 | 0 | 0 | 1 (17) | 0 | 2 (6) |
| Drug-related AEs | |||||||
| Any event | 2 (33) | 2 (33) | 2 (33) | 1 (17) | 2 (33) | 0 | 9 (26) |
| Diarrhea | 1 (17) | 1 (17) | 0 | 0 | 1 (17) | 0 | 3 (9) |
| Abdominal pain | 0 | 0 | 2 (33) | 0 | 0 | 0 | 2 (6) |
| Vomiting | 1 (17) | 0 | 0 | 0 | 1 (17) | 0 | 2 (6) |
| Chromaturia | 1 (17) | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Epistaxis | 0 | 1 (17) | 0 | 0 | 0 | 0 | 1 (3) |
| Fatigue | 0 | 1 (17) | 0 | 0 | 0 | 0 | 1 (3) |
| Headache | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (3) |
| Maculopapular rash | 0 | 0 | 0 | 1 (17) | 0 | 0 | 1 (3) |
| Nausea | 1 (17) | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Pruritus | 0 | 0 | 0 | 1 (17) | 0 | 0 | 1 (3) |
| Serious AEsb | |||||||
| Anal abscess | 0 | 0 | 0 | 1 (17) | 0 | 0 | 1 (3) |
| Congestive cardiomyopathy | 1 (17) | 0 | 0 | 0 | 0 | 0 | 1 (3) |
Abbreviations: AE, adverse event; GSK’254, GSK3640254.
AEs reported in >1 participant.
Serious AEs were considered not related to the study drug and resolved.
Eleven participants reported 14 abnormal laboratory evaluations of potential clinical importance, including low neutrophil counts (n = 6), low leukocyte counts (n = 3), and elevated leukocyte counts or chloride levels (n = 2 each). Increases in pulse rate and blood pressure of potential clinical significance were each reported in 4 participants. No clinically significant trends in laboratory values, electrocardiograms, or vital signs across treatment groups in parts 1 or 2 were observed.
DISCUSSION
This proof-of-concept study evaluated the antiviral efficacy, PK, and safety of the next-generation maturation inhibitor GSK’254 in treatment-naive adults with HIV-1 and established a dose– and exposure–antiviral response relationship. Monotherapy with each GSK’254 dose resulted in declines from baseline in HIV-1 RNA, with the greatest mean decreases of 2.0 and 1.5 log10 observed in the 200- and 140-mg groups, respectively. Moreover, the 200-mg dose showed the greatest short-term decline in HIV-1 RNA after monotherapy with any developmental maturation inhibitor in phase IIa studies and approximated that of integrase inhibitor monotherapy [4, 5, 8, 14–18]. The 10-mg dose demonstrated a 0.36-log10 maximum mean decline from baseline in HIV-1 RNA, which was lower than the previous maturation inhibitor GSK3532795 at similarly low doses [5]. The maximum decline in HIV-1 RNA for each GSK’254 dose was reached after the treatment period was completed by a median of 11.5 and 8.0 days in parts 1 and 2, respectively, as a function of the duration of GSK’254 monotherapy. Thus, it is possible that the declines in HIV-1 RNA with GSK’254 doses in part 2 may have been greater with a longer dosing period before the introduction of combination ART. Plasma GSK’254 PK parameters were generally dose proportional after single- and repeat-dose administration between the 10- and 140-mg doses but non-proportional between the 140- and 200-mg doses. In a previous study of healthy participants, non-proportionality of GSK’254 was observed but at doses higher than those used in the present study [9]. Thus, the nonproportionality observed may be a result of the small participant numbers and PK variability. Overall, GSK’254 parameters were generally consistent with those observed in healthy participants [9]. Exposures of GSK’254 increased 1.5- to 2.3-fold from day 1 to steady state across all doses, in line with previous estimates of terminal phase half-life (~24 hours) [9].
Administration of GSK’254 in participants with HIV-1 did not result in any clinically significant tolerability findings. The most common AE was headache, similar to observations in healthy participants [9]. Two SAEs were reported, both of which were considered unrelated to the study drug. No AEs led to treatment discontinuation, and no deaths were reported. Overall, results from this study support further clinical development of GSK’254.
Maximum change in HIV-1 RNA from baseline to day 8 was fitted to Emax nonlinear models for GSK’254 dose and Cτ to evaluate dose– and exposure–antiviral response relationships. The GSK’254 dose estimated to achieve 50% of the maximum decline in HIV-1 RNA from the model was 42.7 mg. Although the 95% CI for this estimate is wide (7.6–77.8), the values are near or within the dose ranges included in this study. In addition, PK parameter concentrations estimated to attain maximum HIV-1 RNA declines were also within the range of PK results observed in this study. For example, the EC50 estimate for Cτ was 0.17 µg/mL, slightly decreased compared with the PK parameters observed at steady state in the GSK’254 40-mg group. Except for the lowest GSK’254 dose of 10 mg, all dose groups achieved trough concentrations above the pre-determined target of 110 ng/mL. The EC50 value derived from the PK/pharmacodynamic model of these data (~170 ng/mL; ~43 mg once daily) is similar to the target value. However, doses selected for phase IIb evaluation (100–200 mg once daily) were conservatively chosen to optimize efficacy (ie, to achieve population trough concentrations well above this value).
Treatment emergence of the RAM A364A/V occurred on day 11 in 4 participants who received monotherapy with GSK’254 200 mg for 10 days in part 1. One of 4 participants with full conversion to A364V demonstrated phenotypic resistance to GSK’254. After shortening the duration of GSK’254 monotherapy in part 2 to 7 days, no emergent RAMs were observed. A364V is a primary maturation inhibitor RAM [18]. Of 37 participants with available data who received monotherapy with the structurally similar maturation inhibitor GSK3532795 in a phase IIa trial, 18 developed treatment-emergent RAMs at Day 10, and 6 developed A364A/V. Of those 6 participants, 5 demonstrated phenotypic resistance to GSK3532795 at day 10 [19]. Combined, GSK’254 showed a more potent antiviral profile relative to GSK3532795 [12]. Of note, like all antiretroviral agents, GSK’254 will always be part of an antiretroviral regimen. An ongoing phase IIb study is evaluating GSK’254 in combination with 2 nucleoside reverse transcriptase inhibitors, which will provide data on the potential for treatment-emergent resistance over longer-term administration; this will inform the clinical development plan relative to the resistance barrier of GSK’254.
This study had some limitations. Sample sizes were small, and only 2 (6%) participants were women, potentially limiting generalizability. Although dose proportionality was evaluated, this study was not powered for that determination. Differences were observed in dosing duration between parts 1 and 2 (10 vs 7 days), making direct comparisons between GSK’254 doses in part 1 and those in part 2 challenging.
This proof-of-concept monotherapy study established the potency of GSK’254 as an antiviral (2-log10 maximum change) and a GSK’254 dose– and exposure–antiviral response relationship. Across all doses evaluated and regardless of dosing duration, GSK’254 140- and 200-mg doses achieved similar exposures and demonstrated the greatest declines in HIV-1 RNA. No major tolerability findings were observed with any GSK’254 dose in this treatment-naive population with HIV-1. Overall, results from this study support the clinical development of GSK’254 and informed dose selection for the ongoing phase IIb study evaluating GSK’254 in combination with 2 nucleoside reverse transcriptase inhibitors (ClinicalTrials.gov identifier: NCT04493216).
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Geraldine Ferron-Brady, Anu Shilpa Krishnatry, Varsha Vasi, and Heather Sevinsky for their scientific and clinical contributions. Editorial assistance was provided under the direction of the authors by Megan Schmidt, PhD, and Sherri Damlo, ELS, MedThink SciCom, and was funded by ViiV Healthcare.
Financial support. This work was supported by ViiV Healthcare.
Contributor Information
Christoph D Spinner, Technical University of Munich, School of Medicine, University Hospital Rechts der Isar, Department of Internal Medicine II, Munich, Germany.
Franco Felizarta, Office of Franco Felizarta, MD, Bakersfield, California, USA.
Giuliano Rizzardini, Infectious Diseases, ASST Fatebenefratelli Ospedale Sacco, Milan, Italy; School of Clinical Medicine, Faculty of Health Science, University of the Witwatersrand, Johannesburg, South Africa.
Patrick Philibert, Infectious Disease, Hôpital Européen de Marseille, Marseille, France.
Essack Mitha, Newtown Clinical Research, Johannesburg, South Africa.
Pere Domingo, Infectious Diseases Unit, Hospital Santa Creu i Sant Pau, Barcelona, Spain.
Christoph J Stephan, Infectious Diseases Unit, Universitätsklinikum Frankfurt, Frankfurt, Germany.
Michelle DeGrosky, ViiV Healthcare, Branford, Connecticut, USA.
Veronica Bainbridge, GlaxoSmithKline, Stockley Park, United Kingdom.
Joyce Zhan, GlaxoSmithKline, Collegeville, Pennsylvania, USA.
Teodora Pene Dumitrescu, GlaxoSmithKline, Collegeville, Pennsylvania, USA.
Jerry L Jeffrey, ViiV Healthcare, Research Triangle Park, North Carolina, USAand.
Jianfeng Xu, GlaxoSmithKline, Upper Providence, Pennsylvania, USA.
Fiona Halliday, GlaxoSmithKline, Stockley Park, United Kingdom.
Jianjun Gan, GlaxoSmithKline, Collegeville, Pennsylvania, USA.
Mark Johnson, ViiV Healthcare, Research Triangle Park, North Carolina, USAand.
Martin Gartland, ViiV Healthcare, Research Triangle Park, North Carolina, USAand.
Samit R Joshi, ViiV Healthcare, Branford, Connecticut, USA.
Max Lataillade, ViiV Healthcare, Branford, Connecticut, USA.
References
- 1. Arts EJ, Hazuda DJ.. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med 2012; 2:a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morales-Ramirez J, Bogner JR, Molina J-M, et al. Safety, efficacy, and dose response of the maturation inhibitor GSK3532795 (formerly known as BMS-955176) plus tenofovir/emtricitabine once daily in treatment-naive HIV-1-infected adults: week 24 primary analysis from a randomized phase IIb trial. PLoS One 2018; 13:e0205368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Lu W, Li F.. Pharmacological intervention of HIV-1 maturation. Acta Pharm Sin B 2015; 5:493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeJesus E, Harward S, Jewell RC, et al. A phase IIa study evaluating safety, pharmacokinetics, and antiviral activity of GSK2838232, a novel, second-generation maturation inhibitor, in participants with human immunodeficiency virus type 1 infection. Clin Infect Dis 2020; 71:1255–62. [DOI] [PubMed] [Google Scholar]
- 5. Hwang C, Schürmann D, Sobotha C, et al. Antiviral activity, safety, and exposure-response relationships of GSK3532795, a second-generation human immunodeficiency virus type 1 maturation inhibitor, administered as monotherapy or in combination with atazanavir with or without ritonavir in a phase 2a randomized, dose-ranging, controlled trial (AI468002). Clin Infect Dis 2017; 65:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li F, Goila-Gaur R, Salzwedel K, et al. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc Natl Acad Sci U S A 2003; 100:13555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nowicka-Sans B, Protack T, Lin Z, et al. Identification and characterization of BMS-955176, a second-generation HIV-1 maturation inhibitor with improved potency, antiviral spectrum, and Gag polymorphic coverage. Antimicrob Agents Chemother 2016; 60:3956–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith PF, Ogundele A, Forrest A, et al. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-o-(3’,3’-dimethylsuccinyl) betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob Agents Chemother 2007; 51:3574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joshi SR, Fernando D, Igwe S, et al. Phase I evaluation of the safety, tolerability, and pharmacokinetics of GSK3640254, a next-generation HIV-1 maturation inhibitor. Pharmacol Res Perspect 2020; 8:e00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pene Dumitrescu T, Joshi SR, Xu J, et al. A phase I evaluation of the pharmacokinetics and tolerability of the HIV-1 maturation inhibitor GSK3640254 and tenofovir alafenamide/emtricitabine in healthy participants. Antimicrob Agents Chemother 2021; 65:e02173–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pene Dumitrescu T, Joshi SR, Xu J, et al. Phase I evaluation of pharmacokinetics and tolerability of the HIV-1 maturation inhibitor GSK3640254 and dolutegravir in healthy adults. Br J Clin Pharmacol 2021; 87:3501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dicker I, Jeffrey JL, Protack T, et al. GSK3640254 is a novel HIV-1 maturation inhibitor with an optimized virology profile. Antimicrob Agents Chemother 2022; 66:e0187621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maganti L, Panebianco DL, Maes AL.. Evaluation of methods for estimating time to steady state with examples from phase 1 studies. AAPS J 2008; 10:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeJesus E, Berger D, Markowitz M, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr 2006; 43:1–5. [DOI] [PubMed] [Google Scholar]
- 15. Markowitz M, Morales-Ramirez JO, Nguyen B-Y, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr 2006; 43:509–15. [DOI] [PubMed] [Google Scholar]
- 16. Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 2011; 25:1737–45. [DOI] [PubMed] [Google Scholar]
- 17. Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials 2013; 14:192–203. [DOI] [PubMed] [Google Scholar]
- 18. Gallant JE, Thompson M, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics of bictegravir as 10-day monotherapy in HIV-1-infected adults. J Acquir Immune Defic Syndr 2017; 75:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dicker I, Zhang S, Ray N, et al. Resistance profile of the HIV-1 maturation inhibitor GSK3532795 in vitro and in a clinical study. PLoS One 2019; 14:e0224076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.