Abstract
Purpose of the review:
Commercial wearable biosensors are commonly used among athletes and highly active individuals, although their value in sports cardiology is not well established. In this review, we discuss the evidence for the current applications of wearables and provide our outlook for promising future directions of this emerging field.
Recent findings:
The integration of routine assessment of physiological parameters, activity data, and features such as electrocardiogram recording has generated excitement over a role for wearables to help diagnose and monitor cardiovascular disease. Presently, however, there are significant challenges limiting their routine clinical use. While studies suggest that wearable-derived data may help guide training, evidence for the use of wearables in guiding exercise regimens for individuals with cardiovascular disease is lacking. Further, there is a paucity of data to demonstrate its efficacy in detecting exercise-related arrhythmias or conditions associated with sudden cardiac death. Further technological developments may lead to a greater potential for wearables to aid in sports cardiology practice.
Summary:
The ability to collect vast amounts of physiological information can help athletes personalize training regimens. However, interpretation of these data and separating the signal from the noise are paramount, especially when used in a clinical setting. While there are currently no standardized approaches for the use of wearable-derived data in sports cardiology, we outline three domains in which they could guide the care of athletes in the future: (1) optimizing athletic performance (2) guiding exercise in athletes with known cardiovascular disease, and (3) screening for cardiovascular disease.
Keywords: Wearables, sports cardiology, athlete, cardiovascular performance, recovery
Introduction
Advances in digital technologies have led to the rapid uptake of commercial wearable biosensors (hereafter called “wearables”) among consumers in the general public, including athletes. At present, 20% of United States residents currently own a smart wearable device, with the market expected to exceed 929 million connected devices this year.1 Wearables are relatively low-cost technology that have a wide variety of potential uses, including monitoring for arrhythmia and hemodynamic changes, tracking physical activity and calorie expenditure, as well as providing information about general health and well-being. Furthermore, these devices can be accessed through smartphone technologies to provide almost instantaneous feedback about personal health metrics in real time to the user.
Many athletes and highly active individuals already use wearables (e.g., Polar chest straps (Polar Electro, Finland), Garmin (Garmin, USA) and Apple (Apple Inc, Cupertino, CA) watches) to monitor personal health metrics and quantify fitness levels and performance. With their rise in popularity, wearables have garnered the attention of sports cardiologists for their potential clinical utility. From detecting exercise-related arrhythmias and identifying subclinical cardiac pathology to providing information about training and recovery, the possible roles for wearables in cardiology practice appear broad at first glance, with the goal of reducing the risk of exercise-related adverse cardiac events and improving cardiovascular performance. However, these hopes have been tempered by a number of factors, particularly data accuracy and lack of actionable information. As sensor technologies mature and their use among the general and athlete population increases,2 it will be important for physicians to not only recognize their utility and benefits, but even more critically adjudicate such technologies to evaluate their potential and limitations. For instance, false positive alerts for suspected arrhythmias may cause unwarranted anxiety in asymptomatic individuals, generate unnecessary and costly tests, and, at worst, lead to inappropriate disqualification from sports participation. Furthermore, at present, there is no clear benefit of biosensors in relation to health care costs or utilization,3 and professional societies have raised concerns about the routine use of wearables for electrophysiologic monitoring in the community.4
In this review, we provide an overview of commercially available and commonly used wearables and discuss the evidence for their utility (or current lack thereof) among regular exercisers and athletes. Additionally, we discuss our outlook for promising future directions of this emerging field.
Types and Capabilities of Wearables
Popular wearables include chest straps, watches, wristbands, and rings5–7 (Figure 1). In the past decade, wearables have progressed from being primarily step-counters and fitness-trackers, to recording both physiological and activity-related data with enhanced connectivity.
Figure 1.
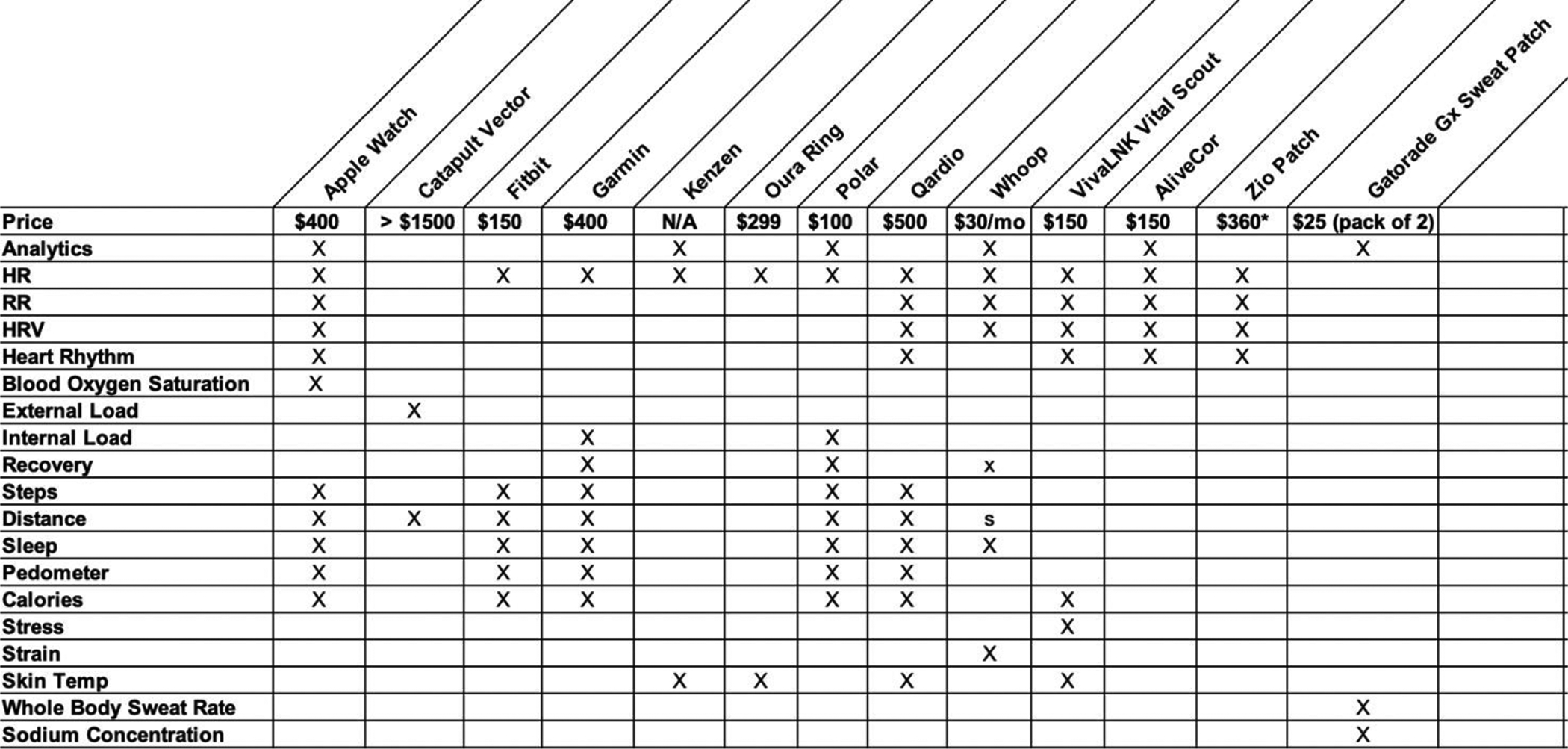
Commercially available wearable sensors for physiological and biochemical monitoring
Physiological Sensors
Physiological measurements that are monitored by currently available wearables include but are not limited to heart rate (HR), heart rate variability (HRV), respiratory rate (RR), blood oxygen saturation (SpO2), and skin temperature. More recent devices have the ability to estimate VO2max, a measure of aerobic capacity.
Measurement of Heart Rate During Exercise
In order to measure HR and SpO2, most wearables use photoplethysmography (PPG) technology, which is an optical volumetric assessment of blood volume changes in the microvasculature.8 This technology assesses small volume changes by illuminating the capillary bed with a small light source (such as an LED, e.g. 365 nm wavelength). The intensity of light transmitted through or reflected from the bed is then measured by a photodiode.9 Over a wide range of HR at rest, there are no detectable differences between a traditional vital signs monitor used in clinical settings and many commercial wearables.10 Furthermore, commercial wearables correlate fairly well with standard electrocardiographic (ECG) monitoring during aerobic exercise.11 Among both healthy adult volunteers and patients enrolled in cardiac rehabilitation, chest strap monitors (Polar H7) have the best agreement with ECG (r = 0.99), whereas optically based wrist-worn HR monitors vary across models with generally lower accuracy (r = 0.52–0.92).11,12 Importantly, the performance of wrist-worn HR monitors varies with exercise modality, and overall, they perform poorly during elliptical trainer activity with use of arm levers (all r <0.80).11 In comparison of commonly used wrist-worn wearables, the Apple Watch Series III shows the best agreement with ECG monitoring on the treadmill at various speeds (r = 0.96), while other tested devices (Fitbit Iconic (Fitbit, USA), Garmin Vivosmart HR, Tom Tom Spark 3 (TomTom, Netherlands)) have similarly lesser agreement (r = 0.89).13 It is important to also consider that skin color, moisture, and tattoos may affect the accuracy of wrist-worn monitors.14 In summary, given the variable performance across optically based wrist-worn monitors, data from these devices should be interpreted with caution, and chest strap monitors should be considered if more accurate ambulatory HR measurements during exercise are required for clinical or performance purposes.
More recently, smartwatches have introduced direct ECG electrode recording for heart rate and rhythm monitoring. Some smartwatches, such as the Apple Watch and the Fitbit Sense, can record a lead I-like rhythm strip by incorporating a negative and positive electrode on the side and back of the watch, respectively. Therefore, in order to record a single-lead ECG using the Apple Watch, the user simply places a finger of the contralateral hand on the digital crown. In addition, certain manoeuvres can be performed to obtain leads II and III (by moving the watch to the ankle and placing the right or left hand on the digital crown for lead II and II, respectively), and the precordial leads can also be acquired through manipulation of the device.15 This capability therefore offers individuals continuous PPG heart rhythm monitoring with single-lead ECG monitoring as needed.
Activity-related Sensors
In addition to physiological metrics, wearables can also precisely capture activity-related parameters, such as walking, cycling, running, and other activities. This capability is facilitated through the use of biaxial or triaxial accelerometers to measure movement and changes in motion based on principles of inertia. Accelerometers are often used in conjunction with global positioning systems to assess precise positioning in space during movement, barometers to detect changes in altitude/elevation, and gyroscopes to measure angular motion.16
Respiratory Rate Sensors
PPG exhibits respiratory modulations that are used by wearables to develop algorithms to estimate respiratory rate.17,18 These modulations include baseline wander, amplitude modulation, and frequency modulation, and are well summarized here.19 In short, changes in tissue blood volume detected by PPG occur due to i) reduced stroke volume during inspiration (due to reduced intrathoracic pressure) ii) arterial vasoconstriction during inspiration iii) increased HR in inspiration (baroreflex-mediated response to decreased stroke volume). These modulations differ across demographics; for example, frequency modulation-based respiratory signals are of lower quality in elderly individuals.20 A plethora of PPG-derived RR algorithms are reported in the literature, and many wearables use these algorithms to provide estimates of RR.19
VO2max Estimation
Data collected from HR and activity-related sensors can be used to derive surrogate measurements of other physiological data such as exergy expenditure21–23 and VO2max. Several formulae have been used to predict VO2max ranging from simple to complex. For example, the formula “VO2max = 15.3 × (HRmax/HRrest)” estimates VO2max in well-trained men with a standard error of estimate of 0.21 l/min or 2.7 ml/min/kg.24 By contrast, complex mathematical modeling and machine learning techniques, namely artificial neural networks (ANN), use more variables such as demographic information (age, sex, height, weight), HR, and activity-related data to estimate VO2max with similar precision.25
Implementation of Wearables in Sports Cardiology
Wearables to Screen for Cardiovascular Disease in Athletes
In sports cardiology, wearables may play an important role in risk stratification and prevention of sudden cardiac death (SCD) in athletes. Intense exercise may trigger ventricular arrhythmias in any individual with an underlying cardiac defect associated with SCD. While the true incidence of SCD in athletes remains uncertain and is influenced by age, sex and sport, it is reported to be as high as 1:15,000 among male adolescent soccer players.26 Although an ambitious goal, in the future, it is conceivable that wearables may hold the potential to facilitate detection of otherwise undiagnosed exercise-related arrhythmias in athletes with underlying cardiovascular disease. More ambitious still, through algorithm development and refinement, future wearables may provide automated health event prediction, identifying at-risk athletes whom would otherwise go undetected with traditional screening measures. Ultimately, these advances could lead to early recognition of conditions associated with SCD in athletes, with the intention to subsequently modify SCD risk in these individuals.
An important cause of SCD in young athletes is Long QT syndrome. Limited data suggest that using ECG equivalents of lead I, II, and V6, ECG tracings from the Apple Watch Series 4 allow for adequate QT interval measurements in 85% of patients in sinus rhythm, with the major limitation being ECG tracing quality and T-wave amplitude.27 Although the feasibility of remote detection of Long QT syndrome using wearables is yet to be proven, it is plausible that these devices may provide an opportunity to screen large numbers of young individuals for this condition in the future.
While it is not currently possible, nor feasible, to use wearables to accurately screen for other conditions associated with SCD in athletes, a growing body of evidence suggest that wearables may be used to identify arrhythmias, namely atrial fibrillation. In the Apple Heart Study, which virtually enrolled 419,297 participants within 8 months, a PPG-based algorithm detected an irregular pulse in 0.52% of participants.28 Of note, only 0.16% of those aged between 22 to 40 years received a notification compared to 3.1% of individuals 65 years and older. Of all study participants who had irregular pulse notifications during simultaneous use of an ECG patch, the positive predictive value for the irregular pulse notification was 84%. In the Huawei Heart Study that used smart devices to monitor the pulse rhythm, 424 out of 187,912 participants received a notification for suspected atrial fibrillation. Consistent with the findings from the Apple Heart Study, of the 62% of notified participants that pursued medical evaluation, the PPV of the Huawei Heart Study algorithm was 87%.29 Given that atrial fibrillation is more prevalent in male masters endurance athletes than the general population, the PPV in theory may in fact be higher in this specific athlete population.30
Wearables to Guide Exercise Training Regimens
Heart Rate Zone-Guided Training:
Wearables provide an opportunity for individuals to use HR as a tool to guide training intensity. This concept may be useful in creating training regimens for athletes as well as developing personalized exercise prescriptions for patients with cardiac disease. For example, popular endurance training regimens involve long periods of aerobic exercise at an intensity whereby lactate production does not exceed clearance (commonly referred to as Zone 2) coupled with high-intensity interval training (HIIT), which involves repeated, short bouts of high intensity efforts. For many individuals, specific training intensities can be most accurately ascertained by performing a cardiopulmonary exercise test. Once these intensities are determined, the correlated heart rate at the given intensity can be used as a proxy to guide future training sessions. For athletes without access to a cardiopulmonary exercise test, both heart rate zone- and HIIT training can be estimated, with specific zones determined as a percentage of predicted maximal heart rate for an individual.
In addition, providing safe exercise recommendations for athletes with coronary artery disease or conditions associated with SCD is an important, challenging, and evolving area of sports cardiology. While the American Heart Association and the American College of Cardiology recommendations for many of these conditions include restricting athletes to low intensity (i.e., Class IA) competitive sports,31 recent updates recommend a more liberal approach, especially when the relationship between exercise and the progression of disease/risk of SCD remains unclear.32 It follows that wearables, through the potential to provide real-time objective feedback to patients during exercise, may help ensure adherence to certain training intensities. Importantly, a wearable-based strategy for athletes with known cardiovascular disease demands demonstration of the accuracy of the device and of the clinical safety and utility in patients in a clinical trial. Perhaps the growing interest of home-based cardiac rehabilitation for patients with underlying cardiovascular disease may result in demonstrating the accuracy, safety, and efficacy of wearables in this population.33
As mentioned earlier, the accuracy of wrist-worn HR monitors varies considerably, especially during exercises that involve significant arm movement. Thus, when counseling athletes on HR-guided training regimens, or providing heart rate guided exercise prescriptions for individuals with underlying cardiac conditions, it is important to understand the accuracy limitations of wearables, with the most accurate guidance likely to result from the use of a chest strap monitor.
Heart Rate Variability-guided Training:
In addition to HR metrics, wearable devices present an opportunity to provide objective data related to internal workload and recovery. While external workload is defined as the output exerted by an athlete (generally quantified based on biomechanical movements quantified through acceleration and velocity), internal workload represents the physiological response to the external load. Internal workload is commonly measured using wellness questionnaires aimed at gauging the athlete’s response to prior workouts as well as recent levels of stress, recovery, and/or sleep.34,35 Internal workload also comprises of subjective scaled assessments during exercise (e.g rating of perceived exertion (RPE) using the Borg’s or Foster’s Scale). RPE and the duration of the training session helps to guide sports scientists to quantify the internal workload of the athlete during a particular workout. These metrics can then be used by training and coaching staff to determine whether there is sufficient recovery between training sessions (Table 1). In competitive sports, it is well established that recovery is important to prevent injury and training plateaus. Overreaching, and possibly overtraining may develop when there is a lack of balance between training load/stress and recovery (Figure 2).36
Table 1.
Subjective and Objective Methods used to Quantify Internal Workload. RPE: Rating of Perceived Exertion (RPE); Training Impulse Response (TRIMP); Workload (WL); D = duration of training session in minutes; HRex is average heart rate of the exercise; session, HRrest is resting heart rate; HRmax is maximal heart rate.
| Method | Description | Equation: Internal WL = | Wearables Exists to Measure? |
|---|---|---|---|
| Subjective Measures | |||
| Borg’s RPE Scale | Scale from 6–20 with increases in workout intensity correlating with increased score | Session RPE * Duration (minutes) | No |
| Foster’s RPE Scale | Scale from 1–10 with increases in workout intensity correlating with increased score | Session RPE * Duration (minutes) | No |
| Objective Measures | |||
| Banister TRIMP | Length of the session (in minutes) multiplied by an intensity factor defined for both men and women | D * (A * ΔHR * exp(B * ΔHR)) A = 0.64 (men); 0.86 (women) B = 1.92 (men); 1.67 (women) ΔHR = (HRex – HRrest)/(HRmax – HRrest) |
Yes |
| Morton’s TRIMP | Similar to Banister’s TRIMP model, except gives greater weight to high-intensity training | D * (A * ΔHR * 2.718exp(B * ΔHR)) A = 0.64 (men); 0.86 (women) B = 1.92 (men); 1.67 (women) ΔHR = (HRex – HRrest)/(HRmax – HRrest) |
Yes |
| Edwards TRIMP | Summation of the HR zone score method. Product of the cumulated training duration (in minutes) for 5 heart rate zones multiplied by a coefficient relative to each zone Zone 1: 50%−60% HRmax Zone 2: 60%−70% HRmax Zone 3: 70%−80% HRmax Zone 4: 80%−90% HRmax Zone 5: 90%−100% HRmax |
duration in zone 1 * 1 + duration in zone 2 * 2 + duration in zone 3 * 3 + duration in zone 4 * 4 + duration in zone 5 * 5 |
Yes |
| Lucia TRIMP | Individually determined lactate thresholds and the onset of blood lactate [La] accumulation. The duration spent in each of three heart rate zones (zone 1: below the ventilator threshold; zone 2: between the ventilator threshold and the respiratory compensation point and zone 3: above the respiratory compensation point) is multiplied by a coefficient (k) relative to each zone (k = 1 for zone 1, k = 2 for zone 2, and k = 3 for zone 3) and the adjusted scores are then summated | duration in zone 1 * 1 + duration in zone 2 * 2 + duration in zone 3 * 3 |
Yes |
| Stagno TRIMP | Modified version of the Banister’s TRIMP to quantify training load based on the direct measurement of the athletes’ [La] profile. | D * ΔHR * 0.1225 * exp (3.9434 × ΔHR) | Yes |
| LacTRIMP | Training load is calculated through [La] concentration. Three intensity zones were adopted (zone 1: [La] ≤ 2, zone 2: 2 > [La] < 4, zone 3: [La] ≥ 4). A relative coefficient was attributed to each zone (k = 1, for zone 1; k = 2, for zone 2; and k = 3, for zone 3). The LacTRIMP was calculated by the sum of the multiplications of the times spent in the different zones by the coefficient relative to each zone |
duration in zone 1 * 1 + duration in zone 2 * 2 + duration in zone 3 * 3 + duration in zone 4 * 4 |
No |
Figure 2.
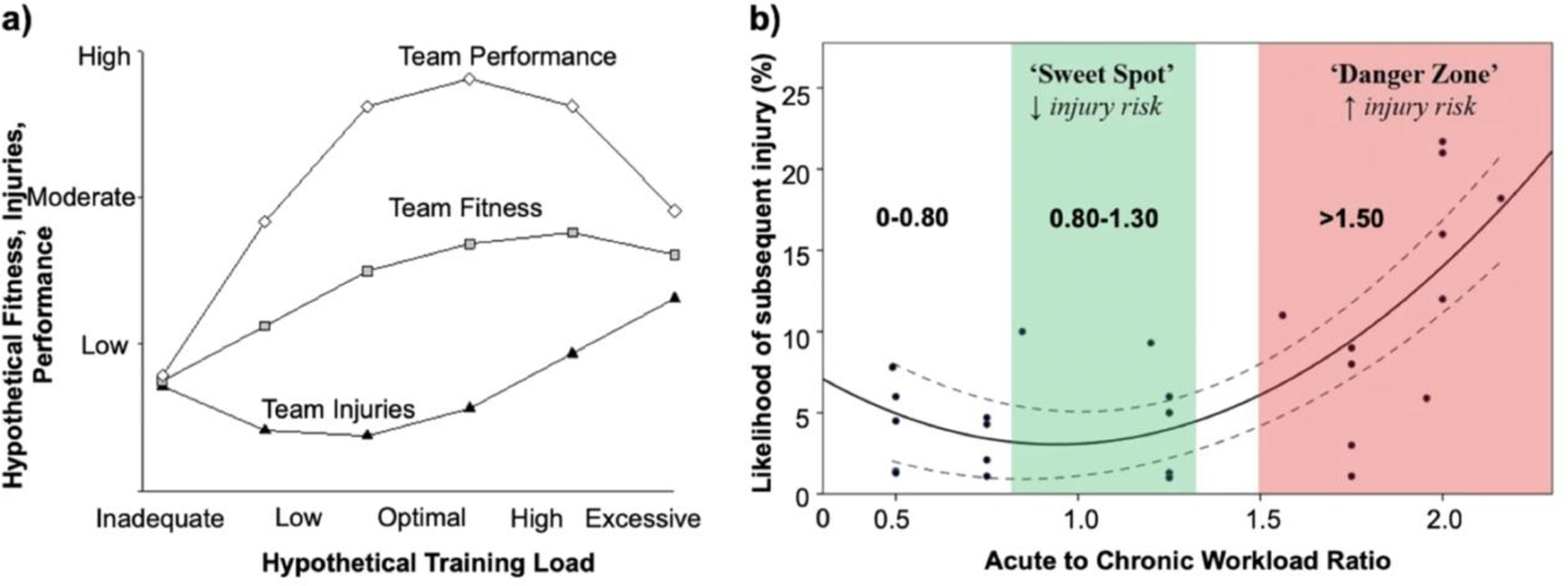
Relationship between workload, team fitness, and team performance. (a) Hypothetical relationship between training loads, fitness, injuries, and performance. Inadequate and excessive training loads could result in increased injuries, reduced fitness, and poor team performance. (b) Interpreting and applying training load data to assess the likelihood of subsequent injury. Modified and used with permission from Gabbett et al.35
Due the subjective nature of wellness questionnaires and RPE, objective data from wearables may be used to complement these assessments in order to more accurately determine the internal workload profile of athletes. Specifically, variations in skin temperature and RR from baseline circadian patterns, as well as the measurement of HRV in particular are increasingly being used as an objective marker of internal workload and recovery by wearable devices. HRV represents changes in patterns of the autonomic nervous system and may function as a holistic assessment of training, sleep, nutrition, psychological and emotional stress. Kiviniemi et al studied whether the use of daily HRV measurements as part of an endurance training strategy could result in a better training response compared to a pre-defined training program in healthy, moderately fit male endurance athletes.37 Using a Polar S810i heart rate monitor, HRV was calculated each morning using Polar software. When HRV decreased, training intensity for that day was decreased compared to the previous day. When HRV increased or remained the same, training intensity increased. A maximum of two consecutive high-intensity exercise days and resting days were allowed, and rest was prescribed after 9 consecutive days of training regardless of HRV that day. Despite similar training frequencies (6 times per week) and fewer high-intensity workouts (3/week vs 4/week in the pre-defined training program), athletes who followed an HRV-guided training protocol had significantly greater changes in their maximal running velocity achieved on maximal exercise testing, and a greater (but not statistically significant) increase in VO2peak. The same group performed a similar analysis on both men and women.38 While the findings were consistent with previous observations in men, women in the HRV-guided group had similar improvements in cardiovascular performance with a lower training load. These findings are supported by subsequent studies showing greater improvement in athletic performance using HRV guided training regimens.39,40 It is important to note that there are several limitations pertaining to the accuracy of wearables to measure HRV. Some studies assessing the effectiveness of HRV guided training use a 7-day rolling average of root mean square of successive differences (RMSSD) between normal heartbeats because it is thought that this is more sensitive to detect changes in the training status compared with single-day measurements.41 Taken together, despite the limitations in obtaining accurate HRV data and the challenges in interpreting its significance, it is plausible wearable-derived HRV can help guide training regimens to provide marginal gains in performance.
Collaborate to Compete: Integrating Basic Science and Sports Cardiology
Further advances in material science, manufacturing, and data analytics will generate more data for the user and physician and at a lower cost, making wearables a ubiquitous item, much like the personal computer of the past and smartphones of today.42 All devices in the market today leverage silicon-based process technology. Silicon chips are now able to be made very thin (~50 um) and can be stacked in three dimensions to enable complex sensing, signal processing, data storage and communication capabilities in a small unit volume.43,44 Silicon technology can also be integrated in epidermal sensors. Recently, nanomaterial-based flexible sensors have garnered increased attention due to their interaction with the human body. These materials can be attached onto clothing45 or applied directly on skin for real-time monitoring of various physical, chemical, biological, and environmental signals,46–49 thus making them extremely promising for sports-related applications.50 Such developments can enable the measurement of biochemical markers, such as sodium, potassium, lactate, cortisol and other physiological markers related to exercise. Gatorade (in collaboration with Epicore Biosystems) recently commercialized the Gx Sweat Patch, an epifluidic calorimetric system that wicks eccrine sweat and measures whole body sweat rate, hydration status, and sodium loss. Whether the real-time assessment of these analytes provide clinically meaningful data that can contribute to the assessment and monitoring of sports cardiology patients remains to be determined.
Conclusion
Athletes are continually searching for new approaches to efficiently maximize performance while reducing injury burden. An ever-increasing ability to collect physiological information can help with this mission through personalizing training regimens and making athletes aware of the importance of recovery. For sports cardiologists and athletes alike, it will be of paramount importance to understand how best to interpret and use the plethora of information and separate the signal from the noise. As technologies mature, and exercise recommendations for athletes with cardiac conditions become less restrictive, wearables may be an important tool to both monitor the safety of exercise in athletes with cardiac conditions as well as detect, and even predict, de novo disease. While there currently are no standardized approaches or guidelines to implementing the use of wearable-derived data in sports cardiology practice, we envision three domains in which they could guide care of athletes: (1) screening for cardiovascular disease, (2) improving cardiovascular performance, and (3) guiding exercise in athletes with known cardiovascular disease (Figure 3).
Figure 3.
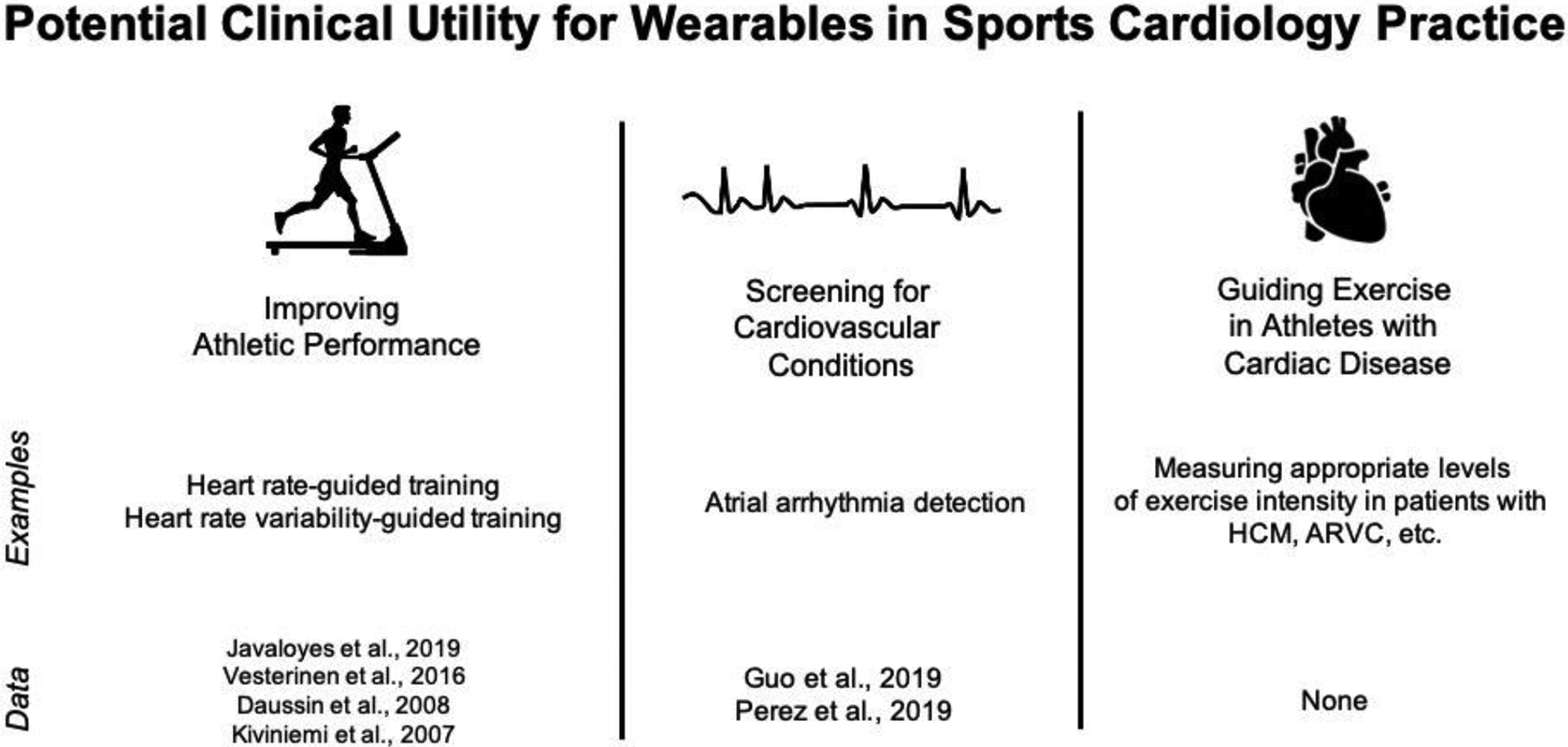
Potential clinical utility for wearables in sports cardiology practice.
Funding:
J.J.H. is supported by a career development grant from the NIH (1K08HL151961-01).
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest relative to the material discussed in this work.
References
- 1.Liu S Number of Connected Wearable Devices Worldwide from 2016 to 2022. 2019. 2019.
- 2.Samydurai K Technology: A key to patient satisfaction. Manag Heal Care Exec 2016. [Google Scholar]
- 3.Bloss CS, Wineinger NE, Peters M, Boeldt DL, Ariniello L, Kim JY, Sheard J, Komatireddy R, Barrett P, Topol EJ. A prospective randomized trial examining health care utilization in individuals using multiple smartphone-enabled biosensors. PeerJ 2016;4:e1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen JC, Lin Y-J, de Oliveira Figueiredo MJ, Sepehri Shamloo A, Alfie A, Boveda S, Dagres N, Toro D Di, Eckhardt LL, Ellenbogen K, Hardy C, Ikeda T, Jaswal A, Kaufman E, Krahn A, Kusano K, Kutyifa V, Lim HS, Lip GYH, Nava-Townsend S, Pak H-N, Diez GR, Sauer W, Saxena A, Svendsen JH, Vanegas D, Vaseghi M, Wilde A, Bunch TJ, Buxton AE, Calvimontes G, Chao T-F, Eckardt L, Estner H, Gillis AM, Isa R, Kautzner J, Maury P, Moss JD, Nam G-B, Olshansky B, Pava Molano LF, Pimentel M, Prabhu M, Tzou WS, Sommer P, Swampillai J, Vidal A, Deneke T, Hindricks G, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome. Hear Rhythm 2020;17:e269–e316. [DOI] [PubMed] [Google Scholar]
- 5.Väliaho E-S, Kuoppa P, Lipponen JA, Martikainen TJ, Jäntti H, Rissanen TT, Kolk I, Castrén M, Halonen J, Tarvainen MP. Wrist band photoplethysmography in detection of individual pulses in atrial fibrillation and algorithm-based detection of atrial fibrillation. EP Eur 2019;21:1031–1038. [DOI] [PubMed] [Google Scholar]
- 6.Brasier N, Raichle CJ, Dörr M, Becke A, Nohturfft V, Weber S, Bulacher F, Salomon L, Noah T, Birkemeyer R. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Ep Eur 2019;21:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbrugge FH, Proesmans T, Vijgen J, Mullens W, Rivero-Ayerza M, Van Herendael H, Vandervoort P, Nuyens D. Atrial fibrillation screening with photoplethysmography through a smartphone camera. EP Eur 2019;21:1167–1175. [DOI] [PubMed] [Google Scholar]
- 8.Kamiŝalić A, Fister I, Turkanović M, Karakatiĉ S. Sensors and functionalities of non-invasive wrist-wearable devices: A review. Sensors (Switzerland) 2018;18. Available at: https://pubmed.ncbi.nlm.nih.gov/29799504/. Accessed March 30, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shelley KH. Photoplethysmography: Beyond the calculation of arterial oxygen saturation and heart rate. Anesth Analg 2007;105. Available at: https://pubmed.ncbi.nlm.nih.gov/18048895/. Accessed April 3, 2021. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Dunn J, Salins D, Zhou G, Zhou W, Schüssler-Fiorenza Rose SM, Perelman D, Colbert E, Runge R, Rego S, Sonecha R, Datta S, McLaughlin T, Snyder MP. Digital Health: Tracking Physiomes and Activity Using Wearable Biosensors Reveals Useful Health-Related Information. PLoS Biol 2017;15:e2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillinov S, Etiwy M, Wang R, Blackburn G, Phelan D, Gillinov AM, Houghtaling P, Javadikasgari H, Desai MY. Variable accuracy of wearable heart rate monitors during aerobic exercise. Med Sci Sport Exerc 2017;49:1697–1703. [DOI] [PubMed] [Google Scholar]
- 12.Etiwy M, Akhrass Z, Gillinov L, Alashi A, Wang R, Blackburn G, Gillinov SM, Phelan D, Marc Gillinov A, Houghtaling PL, Javadikasgari H, Desai MY. Accuracy of wearable heart rate monitors in cardiac rehabilitation. Cardiovasc Diagn Ther 2019;9:262–271. Available at: https://pubmed.ncbi.nlm.nih.gov/31275816/. Accessed March 30, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasadyn SR, Soudan M, Gillinov M, Houghtaling P, Phelan D, Gillinov N, Bittel B, Desai MY. Accuracy of commercially available heart rate monitors in athletes: A prospective study. Cardiovasc Diagn Ther 2019;9:379–385. Available at: https://pubmed.ncbi.nlm.nih.gov/31555543/. Accessed March 30, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagher L, Shi H, Zhao Y, Marrouche NF. Wearables in cardiology: Here to stay. Hear Rhythm 2020;17:889–895. Available at: https://pubmed.ncbi.nlm.nih.gov/32354455/. Accessed March 30, 2021. [DOI] [PubMed] [Google Scholar]
- 15.Gil MÁC. Standard and precordial leads obtained with an apple watch. Ann Intern Med 2020;172:436–437. Available at: https://pubmed.ncbi.nlm.nih.gov/31766051/. Accessed March 30, 2021. [DOI] [PubMed] [Google Scholar]
- 16.Yang C-C, Hsu Y-L. A Review of Accelerometry-Based Wearable Motion Detectors for Physical Activity Monitoring. Sensors 2010;10:7772–7788. Available at: www.mdpi.com/journal/sensors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlen W, Raman S, Ansermino JM, Dumont GA. Multiparameter respiratory rate estimation from the photoplethysmogram. IEEE Trans Biomed Eng 2013;60:1946–1953. Available at: https://pubmed.ncbi.nlm.nih.gov/23399950/. Accessed April 3, 2021. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel MAF, Johnson AEW, Charlton PH, Birrenkott D, Watkinson PJ, Tarassenko L, Clifton DA. Toward a robust estimation of respiratory rate from pulse oximeters. IEEE Trans Biomed Eng 2017;64:1914–1923. Available at: https://pubmed.ncbi.nlm.nih.gov/27875128/. Accessed April 3, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlton PH, Birrenkott DA, Bonnici T, Pimentel MAF, Johnson AEW, Alastruey J, Tarassenko L, Watkinson PJ, Beale R, Clifton DA, Birrenkott DA, Pimentel MAF, Tarassenko L, Clifton DA, Johnson AEW, Alastruey J, Watkinson PJ. Breathing Rate Estimation From the Electrocardiogram and Photoplethysmogram: A Review. IEEE Rev Biomed Eng 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlton PH, Bonnici T, Tarassenko L, Alastruey J, Clifton DA, Beale R, Watkinson PJ. Extraction of respiratory signals from the electrocardiogram and photoplethysmogram: Technical and physiological determinants. Physiol Meas 2017;38:669–690. Available at: https://pubmed.ncbi.nlm.nih.gov/28296645/. Accessed April 3, 2021. [DOI] [PubMed] [Google Scholar]
- 21.Crouter SE, Clowers KG, Bassett DR. A novel method for using accelerometer data to predict energy expenditure. J Appl Physiol 2006;100:1324–1331. Available at: https://pubmed.ncbi.nlm.nih.gov/16322367/. Accessed April 3, 2021. [DOI] [PubMed] [Google Scholar]
- 22.Lin CW, Yang YTC, Wang JS, Yang YC. A wearable sensor module with a neural-network-based activity classification algorithm for daily energy expenditure estimation. IEEE Trans Inf Technol Biomed 2012;16:991–998. Available at: https://pubmed.ncbi.nlm.nih.gov/22875251/. Accessed April 3, 2021. [DOI] [PubMed] [Google Scholar]
- 23.Rothney MP, Neumann M, Béziat A, Chen KY. An artificial neural network model of energy expenditure using nonintegrated acceleration signals. J Appl Physiol 2007;103:1419–1427. Available at: https://pubmed.ncbi.nlm.nih.gov/17641221/. Accessed April 3, 2021. [DOI] [PubMed] [Google Scholar]
- 24.Uth N, Henrik AE, Ae S, Overgaard K, Pedersen PK. Estimation of _ V O 2max from the ratio between HR max and HR rest-the Heart Rate Ratio Method.
- 25.Abut Mehmet Fatih Akay F, Fatih Akay M. Machine learning and statistical methods for the prediction of maximal oxygen uptake: recent advances. Med Devices Evid Res 2015;8:369–379. Available at: 10.2147/MDER.S57281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra A, Dhutia H, Finocchiaro G, Gati S, Beasley I, Clift P, Cowie C, Kenny A, Mayet J, Oxborough D, Patel K, Pieles G, Rakhit D, Ramsdale D, Shapiro L, Somauroo J, Stuart G, Varnava A, Walsh J, Yousef Z, Tome M, Papadakis M, Sharma S. Outcomes of Cardiac Screening in Adolescent Soccer Players. N Engl J Med 2018;379:524–534. [DOI] [PubMed] [Google Scholar]
- 27.Strik M, Caillol T, Daniel Ramirez F, Abu-Alrub S, Marchand H, Welte N, Ritter P, Haïssaguerre M, Ploux S, Bordachar P. Validating QT-Interval Measurement Using the Apple Watch ECG to Enable Remote Monitoring During the COVID-19 Pandemic Circulation https://www.ahajournals.org/journal/circ. Circulation 2020;142:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 30.Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol 2009;11:1156–1159. [DOI] [PubMed] [Google Scholar]
- 31.Maron BJ, Zipes DP, Kovacs RJ. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Preamble, Principles, and General Considerations: A Scientific Statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2343–2349. [DOI] [PubMed] [Google Scholar]
- 32.Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV., Semsarian C, Sorajja P. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020;142:533–557. Available at: https://pubmed.ncbi.nlm.nih.gov/33215938/. Accessed April 7, 2021. [DOI] [PubMed] [Google Scholar]
- 33.Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. Home-based versus centre-based cardiac rehabilitation. Cochrane database Syst Rev 2017;6:CD007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seshadri DR, Thom ML, Harlow ER, Gabbett TJ, Geletka BJ, Hsu JJ, Drummond CK, Phelan DM, Voos JE. Wearable Technology and Analytics as a Complementary Toolkit to Optimize Workload and to Reduce Injury Burden. Front Sport Act Living 2021;2. Available at: https://pubmed.ncbi.nlm.nih.gov/33554111/. Accessed March 30, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabbett TJ. The training—injury prevention paradox: should athletes be training smarter and harder? Br J Sport Med 2016;50:273–280. Available at: https://bjsm.bmj.com/content/50/5/273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halson SL, Jeukendrup AE. Does overtraining exist? An analysis of overreaching and overtraining research. Sport Med 2004;34:967–981. Available at: https://pubmed.ncbi.nlm.nih.gov/15571428/. Accessed March 30, 2021. [DOI] [PubMed] [Google Scholar]
- 37.Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP. Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol 2007;101:743–751. Available at: https://pubmed.ncbi.nlm.nih.gov/17849143/. Accessed March 30, 2021. [DOI] [PubMed] [Google Scholar]
- 38.Kiviniemi AM, Hautala AJ, Kinnunen H, Nissilä J, Virtanen P, Karjalainen J, Tulppo MP. Daily exercise prescription on the basis of hr variability among men and women. Med Sci Sports Exerc 2010;42:1355–1363. Available at: https://pubmed.ncbi.nlm.nih.gov/20575165/. Accessed March 30, 2021. [DOI] [PubMed] [Google Scholar]
- 39.Vesterinen V, Nummela A, Heikura I, Laine T, Hynynen E, Botella J, Häkkinen K. Individual Endurance Training Prescription with Heart Rate Variability. Med Sci Sports Exerc 2016;48:1347–1354. Available at: https://pubmed.ncbi.nlm.nih.gov/26909534/. Accessed March 30, 2021. [DOI] [PubMed] [Google Scholar]
- 40.Javaloyes A, Sarabia JM, Lamberts RP, Moya-Ramon M. Training prescription guided by heart-rate variability in cycling. Int J Sports Physiol Perform 2019;14:23–32. Available at: https://pubmed.ncbi.nlm.nih.gov/29809080/. Accessed March 30, 2021. [DOI] [PubMed] [Google Scholar]
- 41.Plews DJ, Laursen PB, Kilding AE, Buchheit M. Heart-rate variability and training-intensity distribution in Elite rowers. Int J Sports Physiol Perform 2014;9:1026–1032. Available at: https://pubmed.ncbi.nlm.nih.gov/24700160/. Accessed March 30, 2021. [DOI] [PubMed] [Google Scholar]
- 42.Fischer A, Forsberg F, Lapisa M, … S B -M&, 2015. undefined. Integrating mems and ics. nature.com. Available at: https://www.nature.com/articles/micronano20155. Accessed March 31, 2021. [Google Scholar]
- 43.Hsieh AC, Hwang T, Chang MT, Tsai MH, Tseng CM, Li HC. TSV redundancy: Architecture and design issues in 3D IC. In: Proceedings -Design, Automation and Test in Europe, DATE.; 2010:166–171. [Google Scholar]
- 44.Wu B, Kumar A, Ramaswami S. 3D IC Stacking Technology. 1 edition. Place of publication not identified: McGraw-Hill Education; 2011. Available at: https://www.amazon.com/3D-IC-Stacking-Technology-Banqiu/dp/007174195X. [Google Scholar]
- 45.Farahbakhsh N, Venditti RA, Jur JS. Mechanical and thermal investigation of thermoplastic nanocomposite films fabricated using micro- and nano-sized fillers from recycled cotton T-shirts. Cellulose 2014;21:2743–2755. Available at: https://link.springer.com/article/10.1007/s10570-014-0285-4. [Google Scholar]
- 46.Xu R, Lee JW, Pan T, Ma S, Wang J, Han JH, Ma Y, Rogers JA, Huang Y. Designing Thin, Ultrastretchable Electronics with Stacked Circuits and Elastomeric Encapsulation Materials. Adv Funct Mater 2017;27:1604545. Available at: http://doi.wiley.com/10.1002/adfm.201604545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu S, Zhang Y, Jia L, Mathewson KE, Jang K-I, Kim J, Fu H, Huang X, Chava P, Wang R, Bhole S, Wang L, Na YJ, Guan Y, Flavin M, Han Z, Huang Y, Rogers JA. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 2014;344:70–74. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24700852. [DOI] [PubMed] [Google Scholar]
- 48.Araki H, Kim J, Zhang S, Banks A, Crawford KE, Sheng X, Gutruf P, Shi Y, Pielak RM, Rogers JA. Materials and Device Designs for an Epidermal UV Colorimetric Dosimeter with Near Field Communication Capabilities. Adv Funct Mater 2017;27:1604465. Available at: http://doi.wiley.com/10.1002/adfm.201604465. [Google Scholar]
- 49.Webb RC, Pielak RM, Bastien P, Ayers J, Niittynen J, Kurniawan J, Manco M, Lin A, Cho NH, Malyrchuk V, Balooch G, Rogers JA. Thermal Transport Characteristics of Human Skin Measured In Vivo Using Ultrathin Conformal Arrays of Thermal Sensors and Actuators. Ugaz VM, ed. PLoS One 2015;10:e0118131. Available at: http://dx.plos.org/10.1371/journal.pone.0118131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yildiz O, Stano K, Faraji S, Stone C, Willis C, Zhang X, Jur JS, Bradford PD. High performance carbon nanotube – polymer nanofiber hybrid fabrics. Nanoscale 2015;7:16744–16754. Available at: http://pubs.rsc.org/en/content/articlelanding/2015/nr/c5nr02732b. [DOI] [PubMed] [Google Scholar]


