Abstract
We aimed to systematically review the prevalence of potentially inappropriate prescribing (PIP) in older adults in Central and Eastern Europe (CEE) in all care settings. We searched Embase and MEDLINE (up to June 2019) and checked the reference lists of the included studies and relevant reviews. Eligible studies used validated explicit or implicit tools to assess the PIP prevalence in older adults in CEE. All study designs were considered, except case‒control studies and case series. We assessed the risk of bias using the Joanna Briggs Institute Prevalence Critical Appraisal Tool and the certainty of evidence using the GRADE approach. Meta-analysis was inappropriate due to heterogeneity in the outcome measurements. Therefore, we used the synthesis without meta-analysis approach—summarizing effect estimates method. This review included twenty-seven studies with 139,693 participants. Most studies were cross-sectional and conducted in high-income countries. The data synthesis across 26 studies revealed the PIP prevalence: the median was 34.6%, the interquartile range was 25.9–63.2%, and the range was 6.5–95.8%. The certainty of this evidence was very low due to the risk of bias, imprecision, and inconsistency. These findings show that PIP is a prevalent issue in the CEE region. Further well-designed studies conducted across countries are needed to strengthen the existing evidence and increase the generalizability of findings.
Subject terms: Geriatrics, Public health
Introduction
Potentially inappropriate prescribing (PIP) in older people is associated with increased morbidity, lower quality of life, increased use of health care services, and increased health care costs1–3. PIP, therefore, poses a clinical, humanistic and economic problem for older adults, their carers and health care systems. Furthermore, it is a prevalent global health issue in all settings of care that is likely to grow as the world population ages4. Although PIP is considered a highly prevalent problem worldwide, its prevalence varies widely due to differences in country contexts, health care settings, populations and measurement tools5. Additional information about the magnitude of the problem from relevant systematic reviews is presented in the Discussion section, illustrating that PIP is a global issue of major concern.
PIP encompasses the prescribing of potentially inappropriate medications (PIMs) and potential prescribing omissions (PPOs)6. PIM use refers to the prescribing of ineffective medications or medicines with higher risks than benefits (especially when safer therapeutic alternatives exist) and the prescribing of medications without a clinical indication or at the wrong dose, frequency or duration of treatment7. A PPO involves the omission of a clinically indicated medication6. The appropriateness of prescribing can be assessed using criterion-based (explicit) or judgment-based (implicit) tools8. Explicit tools are easily applied, reliable and reproducible but do not consider individual patient characteristics. On the other hand, implicit tools are time-consuming to use and have low reliability and reproducibility as they depend on clinician judgment but are person-specific and consider patient preferences9. The Beers criteria7,10–14 and Screening Tool of Older Person's Prescriptions (STOPP)15,16, which are explicit tools, and the Medication Appropriateness Index (MAI)17, which is an implicit tool, are among the most commonly used criteria to quantify prescribing appropriateness18. Furthermore, the Beers7,10–14 and STOPP15,16 criteria served as a basis for the development of most other validated tools19.
The clinical and economic consequences of PIP can be more devastating for older adults residing in regions and countries with fewer financial resources and worse health status, which contributes to deepening health inequalities globally. One such region is Central and Eastern Europe (CEE), which encompasses the following countries: Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Czechia, Estonia, Hungary, Latvia, Lithuania, Montenegro, North Macedonia, Poland, Romania, Serbia, Slovakia, Slovenia, and the territory of Kosovo. In all countries in CEE, the healthy life expectancy (HALE) at age 60 (years) is lower than those in other European Union (EU) countries and other more developed countries, such as Australia, New Zealand, Canada, the Republic of Korea, Singapore and Japan (range 14.9–17.8 versus 18.2–20.4 years, respectively), except for the United States of America (16.4 years)20. Additionally, all countries in CEE have a lower standard of living, expressed as gross domestic product (GDP) per capita, purchasing power parity (PPP) (current international $), than the EU average, the Organisation for Economic Co-operation and Development (OECD) average and the high-income economies' average (48436.3, 48482.1 and 54602.9013, respectively)21. According to the World Bank country classification, all non-EU countries in CEE and Bulgaria are upper-middle-income economies. In contrast, the rest of the EU countries in CEE are high-income economies22.
PIP has been extensively explored over the past three decades, and a number of systematic reviews have been published on this topic. However, only a few systematic reviews have investigated the prevalence of PIP. Additionally, some of these systematic reviews have focused only on single countries (two systematic reviews by Bhagavathula et al.23,24), specific measurement tools (the systematic reviews by Hill-Taylor et al., Opondo et al., Praxedes et al., Thomas et al., and Storms et al.25–29), or specific sources of data (the systematic review by Guaraldo et al.30). Only three systematic reviews had similar inclusion and exclusion criteria but focused only on specific settings: community (Tommelein et al.31), primary care (Liew et al.32) and long-term care (LTC) (Morin et al.33). Furthermore, these reviews included only a few studies conducted in countries in CEE. Morin et al.33 included no studies from CEE, Liew et al.32 included only one study from CEE, and Tommelein et al.31 included five studies from CEE. Thus, whether the findings from these reviews, which focused on wealthier countries, can be generalized to the CEE region that encompasses former communist states with less developed medication safety programs is uncertain because of differences in country contexts, the availability of resources, and health systems.
We believe that carrying out an up-to-date comprehensive systematic review across a range of settings can inform policy-makers about the issue of PIP in the CEE region and subsequently reduce global health disparities and accelerate the development of medication safety measures in this region. Therefore, we aimed to systematically review the PIP prevalence in older adults in all care settings in countries in CEE.
Methods
We conducted the review according to the registered protocol (PROSPERO: CRD42020152713; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=152713)34 and reported according to the Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline35, and the Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidance36–38 (see Supplementary Tables S1–S4). At all stages of the review process, we contacted the study authors via email to obtain or confirm relevant information. We did not use automation tools in our review.
Search strategy and selection criteria
We searched Embase (Embase.com; 14 June 2019) and MEDLINE (Ovid; 16 June 2019). Search strategies were adapted from two Cochrane systematic reviews39,40 and tailored to each database and specific interface (full search strategies are provided in Supplementary Tables S5 and S6). No filters or limits were used. Additionally, two authors independently checked the reference lists of the included studies and the reviews on similar topics. Duplicate records were removed using EndNote 20 and manually. We conducted a 'top-up' search in August 2022, and we listed the potentially eligible studies that were not incorporated into the review in the 'Studies awaiting classification' table.
We included studies that used validated explicit or implicit tools to measure the PIP prevalence in older adults aged 60 years and over (the United Nations standard)41 in all care settings in countries in CEE. We excluded studies focused on a single disease or condition, terminally ill patients and specific medications/classes of medications (because their results are not applicable to the older population as a whole). If a study reported that some participants were younger than 60 years, we attempted to contact the authors to obtain separate data for older adults (post hoc decision; see Differences between the protocol and review in Supplementary Table S7). All study designs were eligible except for case‒control studies and case series. Regarding interventional studies, participants could not be selected based on the presence of PIMs/PPOs, and only the PIP prevalence before the intervention was considered. Only primary studies published as full papers in peer-reviewed journals were included. We did not apply any language or date restrictions.
Data collection and analysis
Two review authors independently screened the titles and abstracts to exclude clearly ineligible studies. The same two authors then independently screened the full texts of the remaining potentially relevant studies. All disagreements were resolved by discussion without the need for a third reviewer. The reviewers were not blinded to the names of the authors, their institutions or the journal of publication. Multiple reports of the same study were linked together. We used a software program when abstracts and/or articles required translation into English.
Two reviewers independently extracted data using a standardized data extraction form created and piloted specifically for this review. A third reviewer read all records in detail to check the collected data for accuracy and ensure that no relevant information was missed. This author also resolved all errors and inconsistencies, contacted the study authors and mediated consensus on disagreements. When necessary, we consulted a fourth reviewer. We collected data on the following: record details (authors, year, journal, funding sources, conflicts of interest, aims, conclusions), study characteristics (study design, sampling, recruitment, response rate, setting, country and location, number of study centers, study period, methods of data collection, sources of data, ethical approval, informed consent), participants (number, age, sex, inclusion/exclusion criteria, comorbidities, medication use), outcomes (measurement instrument, measurement instrument adaptation, timing of outcome measurements), and miscellaneous information (contact information, correspondence required and responses, comments from the reviewers). We presented key characteristics and findings of individual studies in a 'Characteristics of included studies' table, in which studies were grouped by setting and country.
The risk of bias was assessed using the Joanna Briggs Institute (JBI) Prevalence Critical Appraisal Tool42, which contains nine items: representativeness of the sample; appropriateness of recruitment; adequateness of the sample size; appropriateness of the description of the study subjects and setting; coverage bias; validity of the measurements; reliability of the measurements; appropriateness of statistical analysis; and adequateness of the response rate. Two authors independently applied the tool to each included study and resolved all disagreements by discussion without the need for a third reviewer. The overall risk of bias was judged as high if at least one domain was at high risk or if three domains were at unclear risk. Regarding nonreporting biases, two authors independently assessed study-level selective reporting by comparing the outcomes reported in the results to those previously specified in the aims and methods sections; protocols were not available for any of the included studies. We resolved disagreements by discussion.
The eligible outcome was the PIP prevalence measured by validated explicit or implicit tools. The PIP prevalence was defined as the proportion of persons with one or more PIMs and/or PPOs at a specified point or period in time. The PIP prevalence expressed as a proportion of prescriptions was only reported in the text of the review and excluded from the data synthesis and certainty of evidence rating. When multiple outcomes within a study were available for inclusion (the same outcome measured by different tools and/or at different time points), we reported all of them in the 'Results of individual studies' table but selected a median estimate for data synthesis. Missing prevalence estimates and confidence intervals were computed from the data collected from the studies.
We grouped all outcomes in a single analysis (not prespecified in the protocol). We did not restrict the synthesis to a subset of studies, and we did not prioritize the reporting of some study findings over other findings.
Meta-analysis was not appropriate because the measurement tools were too dissimilar across studies. Therefore, to provide a quantitative assessment, we used the statistical synthesis without meta-analysis approach—summarizing effect estimates method. In this method, each included study is represented by one outcome (in our study, in the case of multiple outcomes, the median estimate was used), and the median, interquartile range and range were calculated across studies. A limitation of this synthesis method is that equal weight is given to all studies, not accounting for differences in the sample sizes. We provide a visual display of the PIP prevalence distribution by box-and-whisker plots. We also present the results from the synthesis in the 'Summary of findings' table. Statistical synthesis was performed using IBM SPSS Statistics 27. In addition, to assess the medications that are most frequently involved in PIP, we extracted the three most frequent criteria of PIP from each study and provided a brief narrative summary.
We investigated heterogeneity visually using box-and-whisker plots. We explored the following potential sources of heterogeneity using study-level variables: study quality (studies at low risk of bias and with some concerns, and studies at high risk of bias; post hoc), study setting (acute, community, LTC, and outpatient (which includes both community-dwelling and LTC residents)), and study period (before 2010 and from 2010 onward; post hoc). We could not assess the influence of several prespecified potential modifiers, including age due to differences in reporting (mean, median, missing information), country due to a small number of studies, and measurement tools due to substantial diversity.
We decided post hoc to assess the quality of evidence related to the studies included in the data synthesis using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach43–45 and created a 'Summary of findings' table. Two review authors independently judged the certainty of the evidence, with disagreements resolved by discussion. The GRADE approach specifies four categories of quality of evidence: high, moderate, low, and very low. We started at high quality because cross-sectional studies are the most appropriate research design to assess prevalence42. We considered downgrading the quality of evidence for each of the five GRADE domains (risk of bias, inconsistency, imprecision, indirectness, and publication bias) by one level or two levels in cases of severe problems. All our decisions are provided in the Discussion section and the footnotes of the 'Summary of findings' table.
We performed post hoc sensitivity analyses to investigate whether our decisions changed the results: 1) using the smallest and the largest outcomes instead of the median outcome for each study, and 2) excluding one study with a subset of eligible participants.
Results
Search results
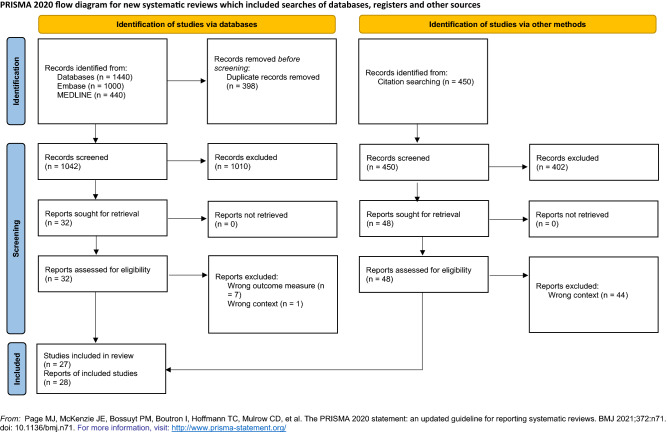
We identified 1890 records (1440 by searching electronic databases: 1000 from Embase, 440 from MEDLINE, and 450 from browsing reference lists) from our search up to 16 June 2019. After removing 398 duplicates, we screened 1492 records for eligibility and excluded 1412 records based on the title or abstract. We assessed the full text of the remaining 80 records and listed the excluded studies at this stage in the 'Characteristics of excluded studies' table (see Supplementary Table S8). Although we successfully contacted the authors of two studies, they could not provide the data on PIP prevalence (study eligibility criterion); thus, we excluded them46,47. Ultimately, 27 studies (28 records) met the inclusion criteria. One article reported two studies48. On the other hand, three papers described one study49–51. See the PRISMA flow diagram (shown in Fig. 1).
Figure 1.
PRISMA flow diagram.
Our 'top-up' search yielded 637 records, of which 477 remained after duplicates were removed. Eight of the 477 screened records were potentially eligible and are listed in the 'Studies awaiting classification' table (see Supplementary Table S9).
Characteristics of included studies
Study characteristics are summarized in the 'Characteristics of included studies' table (see Table 1). All studies were cross-sectional, except that of Stuhec et al.52, which was an uncontrolled before-after study. Different care settings were equally represented across studies—acute49–51,53–58, community48,59–64 and outpatient52,65–70 settings in seven studies, and LTC setting in six studies48,71–75. Only three studies were conducted in upper-middle-income countries: Serbia (2)62,75 and Albania (1)54. The rest of the studies were conducted in high-income countries: Croatia (5)56–58,68,70, Slovenia (5)52,60,66,67,74, Czechia (4)53,59,69,72, Poland (3)61,63,64, Slovakia (3)49–51,55,73, Romania (2)48, Hungary (1)71 and Lithuania (1)65. There were no studies from Bosnia and Herzegovina, Bulgaria, Estonia, Latvia, Montenegro, North Macedonia or the territory of Kosovo. Two studies were conducted internationally53,59, but only the data from a country in CEE were included in the review. Twelve studies were conducted up to 201049–51,53,55,58,59,61,63,64,67,69,70,74, and fifteen were conducted from 2010 onward48,52,54,56,57,60,62,65,66,68,71–73,75.
Table 1.
Characteristics of included studies.
| Study | Country | Study design | Inclusion/exclusion criteria | Data collection | Study period (year) | Medicine category | Sample size | Female (%) | Age (years) | Number of medications | Prevalence of polypharmacy (%) (cut-off) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute care setting | |||||||||||
| Hudhra54 |
Albania Non-EU, UMIC |
Cross-sectional | 60 + years & discharged from internal medicine and cardiology department | Medical charts | 2013 | Rx | 319 | 43.6 | M 69.7 SD 6.1 Ra 60–89 | M 7.8 SD 2.2 | 73.0 (7 +) |
| Matanovic56 |
Croatia EU, HIC |
Cross-sectional | 65 + years & emergency admission to the internal medicine department | Medical charts & interview (patient, GP)a | 2009–2010 | Rx, OTCb | 454 | 57.7 | M 74.8 SD 4.2 Ra 65–94a | M 5.3 SD 2.9 | 57.5 (5 +) |
| Mucalo57 |
Croatia EU, HIC |
Cross-sectional | 65 + years & 1 + medication & hospitalised at the internal medicine department & patient/proxy capable of giving consent and communicating well | Medical charts & interview (patient/caregiver, GP); standardized data collection form | 2014–2016 | Rx, OTCb | 276 | 49.3 | M 73.9 SD 6.2 Ra 65–92a | M 7.8 SD 3.2a | 91.7 (5 +) |
| Radosevic58 |
Croatia EU, HIC |
Cross-sectional | 65 + years & 1 + medication & hospitalised at the internal medicine department | Medical charts | 2007a | Rx | 142 | 52.1a | M 75.0 SD 6.3 Ra 65–97a | M 6.3 SD 2.7a | 73.2 (5 +)a |
| Gallagher53 |
Czechia EU, HIC |
Cross-sectional | 65 + years & emergency admission to the geriatric department | Medical charts & patient assessmenta | 2008 | Rx | 150 | 65.3c | Mdn 82 IQR 77–86 | Mdn 6 IQR 4–8 | 52.7 (6 +)c |
| Kostkova55 |
Slovakia EU, HIC |
Cross-sectional | 65 + years & hospitalised at the geriatric department & complete medical documentation; excluded who died | Medical charts | 2008–2009 | Rxa | 566 | 62.2 | M 77.4 SD 6.8 | NR | 68.7 admission 87.8 discharge (6 +) |
|
Wawruch51* Wawruch49 Wawruch50 |
Slovakia EU, HIC |
Cross-sectional | 65 + years & hospitalised at the internal medicine department & complete medical documentation; excluded who died | Medical charts | 2003–2005 | Rx, OTCa | 600 | 58.5 | M 76.6 SD 6.5 | NR | 60.3 admission 62.3 discharge (6 +) |
| Community setting | |||||||||||
| Fialova59 |
Czechia EU, HIC |
Cross-sectional | 65 + years & home care recipient | Patient assessment & interview (patient, caregiver) & medical charts; standardized data collection formd | 2001–2002 | Rx, OTC | 428 | 79.0 | M 81.6 SD 7.0 Ra 65–98a | M 6.7 SD 2.5a | 68.5 (6 +) |
| Kosinska61 |
Poland EU, HIC |
Cross-sectional | 65 + years & 1 + medication | Prescriptions | 2004 | Rx | 5086 (prescriptions) | 64.8 | M 74.5 Ra 65–100 | NR | NR |
| Rajska-Neumann63 |
Poland EU, HIC |
Cross-sectional | 65 + years | Questionnaire | 2002–2003a | Rx, OTC | 1000; two cities I: 680 II: 320 | 65.4c |
I: M 72.6 SD 6.5; II: M 72.5 SD 6.0 |
I: M 6.9 SD 3.2; II: M 6.6 SD 3.1 | 50.4 (7 +) |
| Rajska-Neumann64 |
Poland EU, HIC |
Cross-sectional | 100 + years | Questionnaire | 1999–2000e | Rx, OTC | 92 | 83.7c | M 101.7 SD 1.2 Ra 100–111 | M 2.5 SD 2.5 | 32.6 (5 +) |
| Primejdie48° |
Romania EU, HIC |
Cross-sectional | 65 + years | Prescriptions | 2013 | Rx | 345 | 61.2 | M 74.8 SD 6.2 Ra 65–92 | Mdn 3 | NR |
| Kovacevic62 |
Serbia Non-EU, UMIC |
Cross-sectional | 65 + years & 1 + medication; excluded who did not claim prescriptions personally | Interview (patient) & medical charts; standardized data collection form | 2012 | Rx | 509 | 57.4 | M 74.8 SD 6.5 Ra 65–95 | M 5.1 SD 2.2 | 37.0 (6 +) |
| Gorup60 |
Slovenia EU, HIC |
Cross-sectional | 65 + years & 1 + medication & capable of giving consent and communicating well & life expectancy > 1 year | Questionnaire (patient) & patient assessment | 2014–2015 | Rx | 503 | 56.7 | M 74.9 SD 6.0 Ra 65–99 | M 5.6 SD 2.9 | 62.2 (5 +) |
| LTC setting | |||||||||||
| Kalafutova72 |
Czechia EU, HIC |
Cross-sectional | 65 + years & 2 + medications & capable of giving consent and communicating well | Medical charts | 2012 | Rx, OTCb | 58 | 74.1c | M 82.4 SD 8.3 | Rx: M 8.9 OTC: M 1.2 | Rx:82.8 (6 +)c |
| Bor71 |
Hungary EU, HIC |
Cross-sectional | Residing 12 + months in the LTC; excluded who diedf | Medical charts | 2010–2015 | Rx, OTCa | 184a | 78.8a | M 82.6 SD 7.2 Ra 65–104a | M 8.5 SD 3.8a | 91.3 (4 +) |
| Primejdie48 |
Romania EU, HIC |
Cross-sectional | 65 + years | Medical charts | 2013 | Rx, OTC | 91 | 58.2 | M 80.8 SD 6.8 Ra 65–98 | Mdn 8 | NR |
| Stojanovic75 |
Serbia Non-EU, UMIC |
Cross-sectional | 65 + years & 1 + medication | Medical charts | 2018 | Rx | 400 | 69.0c | Mdn 83 IQR 11 Ra 65–99 | Mdn 8 IQR 5 | NR |
| Kolar73 |
Slovakia EU, HIC |
Cross-sectional | 65 + years | Medical charts | 2014 | Rx | 70 | 58.6 | M 79.9 SD 5.6 Ra 70–94a | M 8.1 SD 9.8a | 90.0 (5 +)a |
| Ster74 |
Slovenia EU, HIC |
Cross-sectional | 65 + years & patient/proxy capable of giving consent & complete medical documentation | Medical charts; standardized data collection form | 2006 | Rx, OTC | 2040 | 78.3 | M 82.0 SD 7.7 | M 5.8 SD 3.0 | 50.6 (6 +) |
| Outpatient setting | |||||||||||
| Popovic68 |
Croatia EU, HIC |
Cross-sectional | 65 + years & 5 + medications | Claims database | 2010 | Rx | 29,418 | 63.2 | M 77 SD 5.9 Ra 65–103a | M 7.6 SD 1.8a | NA (5 + inclusion criteria) |
| Vlahovic-Palcevski70 |
Croatia EU, HIC |
Cross-sectional | 70 + years & 1 + medication | Pharmacy database | 2002 | Rx | 10,426a | NR | NR | M 7.5 | NR |
| Vinsova69 |
Czechia EU, HIC |
Cross-sectional | 65 + years & 1 + medication | Claims database | 1997–2001 | Rx | 15,516 | NR | NR | NR | NR |
| Grina65 |
Lithuania EU, HIC |
Cross-sectional | 65 + years & 1 + medication | Claims database | 2015 | Rx | 431,625 | 68.1 | M 75.8 SD 0.0 | M 4.7 SD 0.0 | 57.5 (4 +)g |
| Jazbar66 |
Slovenia EU, HIC |
Cross-sectional | 65 + years & 1 + medication | Claims database | 2013 | Rx | 345,400 | 60.0 | M 75.4 SD 7.3 Ra 65–108a | M 7.8 SD 4.9a | 72.1 (5 +)a |
| Nerat67 |
Slovenia EU, HIC |
Cross-sectional | 65 + years & 1 + medication | Claims database | 2006 | Rx | 65 + : 298,990; 75 + : 136,076 | NR | NR | 65 + : M 7.7 SD 4.9; 75 + : M 8.3 SD 5.0 | NR |
| Stuhec52 |
Slovenia EU, HIC |
Uncontrolled before-after | 65 + years & 10 + medications & medication review & complete medical documentation | Medical charts & medication review documentation | 2012–2014 | Rx | 91 | 61.5 | M 77.5 Mdn 78 Ra 65–91 | M 13.8 Mdn 13 | NA (10 + inclusion criteria) |
EU European Union, GP General practitioner, HIC High-income country, IQR Interquartile range, LTC Long-term care, M Mean, Mdn Median, NR Not reported, OTC Over-the-counter medication, Ra Range, Rx Prescription medication, Sd Standard deviation, UMIC Upper-middle-income country.
*Indicates the major publication for the study (the study was described in three reports).
°One report described two studies.
aData were obtained and/or confirmed from study authors.
bDietary supplements were also included.
cCalculated.
dInterRAI Minimum Data Set for Home Care instrument, MDS-HC76.
eData were obtained from publication by Sikora et al.77.
fData for persons aged 65 + years were obtained by correspondence.
gDiscrepancies in publication resolved in correspondence.
The 26 studies included 1,139,693 participants, ranging from 58 to 431,625 participants. One study provided results only on prescriptions (5086)61, and one study applied a part of the tool to patients and the other part to prescriptions (1,315,624)68. In studies that reported sex, the majority of participants were female (range 52.1–83.7%)48–53,55,56,58–66,68,71–75 except in two studies (43.6 and 49.3%)54,57. One study each included participants aged over 6054, 7070, and 100 years64, and the remaining studies included participants aged over 65 years. One study included participants aged over 50 years, but the authors provided separate data for participants aged 65 years and older71, which enabled us to include this study in the review.
Data were collected from different sources/combinations of sources—medical records, claim databases, pharmacy databases, prescriptions, medication review documentation, interviews, questionnaires, and patient assessments. The use of a standardized data collection form was reported in only four studies57,59,62,74. In most studies, only prescription medications were considered48,52–55,58,60–62,65–70,73,75.
Polypharmacy, or the use of multiple medications, was defined differently across the studies: in most studies, it was defined as more than four or five medicines49–51,53,55–60,62,64,66,72–74 and in a few studies, it was defined as more than three 65,71 or six medicines 54,63. The polypharmacy prevalence ranged from 32.6 to 91.7%49–51,53–60,62–66,71–74. It was not reported in seven studies48,61,67,69,70,75, and only adults with polypharmacy were included in two studies52,68.
Overall, the prevalence of PIP was reported 52 times in 27 studies, with between one and four outcomes per study. There were differences in the concepts measured (PIMs, PPOs, and both), the measurement tools used (different domains; different versions; different adaptations; combinations), and the measurement time points (admission, discharge, admission/discharge). The predominantly measured concept was PIM use; PPOs were assessed only seven times—four times separately and three times together with PIMs. Only explicit tools were used to detect PIP, namely, the Austrian consensus panel list78, 1997 Beers criteria10, 2003 Beers criteria11, American Geriatrics Society (AGS) 2012 Beers criteria12, AGS 2015 Beers criteria13, Comprehensive protocol79, 2012 CZ expert consensus criteria80, EU(7)-PIM list81, French consensus panel list82, Ghent Older People's Prescriptions community Pharmacy Screening (GheOP3S) tool83, McLeod criteria84, PRISCUS list85, Screening Tool to Alert doctors to Right Treatment (START)15, START criteria version 216, STOPP15, and STOPP criteria version 216. Additionally, composite tools, i.e., combinations of two or more criteria, were used in the included studies (five times). The tools used most often were different versions of the Beers criteria (21 times; and four times as a part of the composite criteria; version 2003 was used most often, 14 times and two times as a part of the composite criteria) and different versions of the STOPP criteria (seven times and two times as a part of the composite criteria). Thirteen studies used only one tool49–52,58,60,61,63,64,68–70,72–74, three used only a composite tool48,71, and the remaining eleven used more than one tool and, in some cases, more than one version of the same tool53–57,59,62,65–67,75. The full versions of the tools were used in only six studies52,53,60,62,73,75. In the remaining studies, the tools were adapted. Certain sections of the tools or individual items were excluded, most often medications that were not available on the pharmaceutical market and criteria requiring some clinical or therapeutic information (such as diagnosis, dose, dosage, and duration of treatment).
Risk of bias in included studies
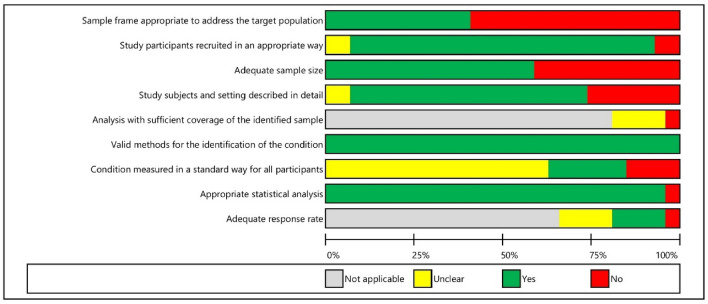
Only six studies were at low risk of bias or with some concerns59,62,65,66,68,74 (shown in Fig. 2, Supplementary Fig. S1 and Table 2). In over half of the studies48–58,61,63,71–73,75, the sample frame was not appropriate to address the target population (the country's older population), as it included only persons from one or several organizations. In contrast, in most studies48–51,53–59,61,62,65–75, participants were recruited appropriately by including everyone from the sampling frame or using random probabilistic sampling; convenience sampling was used in only a small number of studies52,63. The sample size was inadequate in almost half of the studies48,52–54,57,58,64,71–73 (determined by following the JBI Prevalence Critical Appraisal Tool recommendations). Approximately two-thirds of the studies48–57,59,62,65,66,68,71–74 described the study sample and setting in sufficient detail. All studies48–75 used valid methods (i.e., validated instruments) to assess the outcomes because this was part of the inclusion criteria. In most studies49–51,55,56,58,63–75, it was not clear if the condition was measured in the same, standard, reliable way for all participants. The statistical analysis, i.e., prevalence reporting, was appropriate in almost all studies48–51,53–75. Most studies48–51,53–56,58,61,65–71,73,75 used claims databases and medical records of all patients, and therefore the response rate and coverage bias assessment were not applicable to them. Furthermore, a small number of studies59,62,72,74 with an adequate response rate (80% or higher) had an unclear risk of coverage bias.
Figure 2.
Risk of bias: reviewers' judgements about each risk of bias item across all included studies. Presented as percentages.
Table 2.
Results of individual studies.
| Study | Country | Risk of bias | Tool | Tool adaptation | Number of patients with 1 + PIP | Sample size | Prevalence of patients with 1 + PIP (95% CI) | Timing of outcome measurement |
|---|---|---|---|---|---|---|---|---|
| Acute care setting | ||||||||
| Hudhra54 | Albania | H | Beers 201212 | Independent and considering diagnosis & registered & excluding criteria requiring follow-up dataa | 110 | 319 | 0.34 (0.29–0.40)b | Discharge |
| STOPP15 | 110 | 319 | 0.34 (0.29–0.40)b | Discharge | ||||
| STOPP version 216 | 201 | 319 | 0.63 (0.58–0.68)b | Discharge | ||||
| Matanovic56 | Croatia | H | Beers 201212 | Independent and considering diagnosis | 263 | 454 | 0.58 (0.53–0.62)b | Admissiona |
| Comprehensive protocol79 | 200 | 454 | 0.44 (0.40–0.49)b | Admissiona | ||||
| Mucalo57 | Croatia | H | Comprehensive protocol79 | Independent and considering diagnosis & registered | 102 | 276 | 0.37 (0.31–0.43)b | Discharge |
| EU(7)-PIM list81 | 184 | 276 | 0.67 (0.61–0.72)b | Discharge | ||||
| STOPP version 2 16 | 190 | 276 | 0.69 (0.63–0.74)b | Discharge | ||||
| Radosevic58 | Croatia | H | Beers 200311 | Independent of diagnosis & registered | 35 | 142 | 0.25 (0.18–0.32)b | During hospitalisation |
| Gallagher53 | Czechia | H | Beers 200311 | All criteria | 34 | 150 | 0.23 (0.17–0.30)b | Admission |
| START15 | 81 | 150 | 0.54 (0.46–0.62)b | Admission | ||||
| STOPP15 | 52 | 150 | 0.35 (0.27–0.43)b | Admission | ||||
| Kostkova55 | Slovakia | H | Beers 200311 | Independent of diagnosis | 128 | 566 | 0.23 (0.19–0.26)b | Admission |
| 157 | 566 | 0.28 (0.24–0.32)b | Discharge | |||||
| French list82 | 145 | 566 | 0.26 (0.22–0.29)b | Admission | ||||
| 172 | 566 | 0.30 (0.27–0.34)b | Discharge | |||||
| Wawruch51 * | Slovakia | H | Beers 200311 | Independent of diagnosisa | 121 | 600 | 0.20 (0.17–0.24)b | Admission |
| 120 | 600 | 0.20 (0.17–0.23)b | Discharge | |||||
| 126 | 600 | 0.21 (0.18–0.24)b | Admission & discharge | |||||
| Community setting | ||||||||
| Fialova59 | Czechia | L | Beers 199710 | Independent of diagnosis & registered & excluding criteria concerning DDIs and requiring duration of use | 67 | 428 | 0.16 (0.13–0.19)b | N/A |
| Beers 200311 | 108 | 428 | 0.25 (0.21–0.30)b | N/A | ||||
| McLeod84 | 136 | 428 | 0.32 (0.28–0.36)b | N/A | ||||
| Composite: Beers 199710& Beers 200311 & McLeod84 | 176 | 428 | 0.41 (0.37–0.46)b | N/A | ||||
| Rajska-Neumann63 | Poland | H | Beers 199710 | Independent and considering diagnosis & excluding criteria requiring dose, dosage, duration of usea | 285 | 1000 | 0.28 (0.26–0.31)b,c | N/A |
| Rajska-Neumann64 | Poland | H | Beers 200311 | Independent of diagnosis | 6 | 92 | 0.07 (0.03–0.14)b | N/A |
| Primejdie48° | Romania | H | Composite: PRISCUS list85 & START15 & STOPP15 | Registered & excluding criteria requiring clinical information and concerning OTCs | 119 | 345 | 0.34 (0.30–0.40)b | N/A |
| Kovacevic62 | Serbia | L | START15 | All criteria | 257 | 509 | 0.50 (0.46–0.55)b | N/A |
| STOPP 15 | 139 | 509 | 0.27 (0.24–0.31)b | N/A | ||||
| Gorup60 | Slovenia | H | START15 | All criteria | 216 | 503 | 0.43 (0.39–0.47)b | N/A |
| LTC setting | ||||||||
| Kalafutova72 | Czechia | H | STOPP15 | NR | 38 | 58 | 0.66 (0.53–0.77)b | N/A |
| Bor71 | Hungary | H | Composite: Austrian list78 & Beers 201513 & French list82 & PRISCUS list85 | Independent of diagnosis & registereda | 141 | 184 | 0.77 (0.70–0.82)b,d | N/A |
| Primejdie48° | Romania | H | Composite: PRISCUS list85 & START15 & STOPP15 | Registered & excluding criteria requiring clinical information | 75 | 91 | 0.82 (0.73–0.89)b | N/A |
| Stojanovic75 | Serbia | H | GheOP3S tool83 | All criteria | 383 | 400 | 0.96 (0.93–0.97)b | N/A |
| START version 216 | 399 | 400 | 1.00 (0.98–1.00)b | N/A | ||||
| STOPP version 216 | 344 | 400 | 0.86 (0.82–0.89)b | N/A | ||||
| Kolar73 | Slovakia | H | 2012 CZ criteria80 | All criteriaa | 24 | 70 | 0.34 (0.24–0.46)b | N/A |
| Ster74 | Slovenia | SC | Beers 200311 | Independent and considering diagnosis & high severity rating & registered | 355 | 2040 | 0.17 (0.16–0.19)b | N/A |
| Outpatient setting | ||||||||
| Popovic68 | Croatia | SC | Comprehensive protocol79 | Independent of diagnosis | 18,358 | 29,418 | 0.62 (0.62–0.63)b,e | N/A |
| Vlahovic-Palcevski70 | Croatia | H | Beers 199710 | Independent of diagnosis & registered & excluding criteria requiring dosage, duration of use | 864 | 10,426 | 0.08 (0.08–0.09)a,b | N/A |
| Vinsova69 | Czechia | H | Beers 200311 | Independent of diagnosis & registered & excluding criteria requiring dose, dosage, duration of use | 8351 | 15,516 | 0.54 (0.53–0.55)b | N/A |
| Grina65 | Lithuania | SC | Beers 200311 | Independent of diagnosis & registered, reimbursed & excluding criteria concerning DDIs and requiring clinical information | 111,859 | 431,625 | 0.26 (0.26–0.26)b | N/A |
| Beers 201513 | 104,126 | 431,625 | 0.24 (0.24–0.24)b | N/A | ||||
| EU(7)-PIM list81 | 246,724 | 431,625 | 0.57 (0.57–0.57)b,c | N/A | ||||
| Jazbar66 | Slovenia | SC | Austrian list78 | Independent of diagnosis & excluding criteria requiring dose | 187,186 | 345,400 | 0.54 (0.54–0.54)b | N/A |
| Beers 201212 | 192,588 | 345,400 | 0.56 (0.56–0.56)b | N/A | ||||
| EU(7)-PIM list81 | 208,085 | 345,400 | 0.60 (0.60–0.60)b | N/A | ||||
| PRISCUS list85 | 122,255 | 345,400 | 0.35 (0.35–0.36)b | N/A | ||||
| Nerat67 | Slovenia | H | Beers 200311 | Independent of diagnosis | 66,994 | 298,990 | 0.22 (0.22–0.23)b | N/A |
| French list82 | 34,999 | 136,076 | 0.26 (0.25–0.26)b | N/A | ||||
| Composite: Beers 200311 & French list82 | 48,917 | 136,076 | 0.36 (0.36–0.36)b | N/A | ||||
| Stuhec52 | Slovenia | H | PRISCUS list85 | All criteriaa | 69 | 91 | 0.76 (0.66–0.84)b, f | N/A |
DDI Drug-drug interaction, GheOP3S Ghent Older People's Prescriptions community Pharmacy Screening, H High, L Low, LTC Long-term care, N/A Not applicable, SC Some concerns, START Screening Tool to Alert doctors to Right Treatment, STOPP Screening Tool of Older Person's Prescriptions.
*Indicates the major publication for the study (the study was described in three reports).
°One report described two studies.
aData were obtained and/or confirmed from study authors.
bCalculated.
cDiscrepancies in publication resolved in correspondence.
dData for persons aged 65 + years were obtained by correspondence.
eA part of the criteria considering diagnosis was presented separately as a percentage of a total number of prescriptions (1,315,624) that was 2.0%.
fData were obtained from the master's thesis by Gorenc86.
Nonreporting bias
Reported outcomes were consistent with the stated aims and methods in all studies, except for the study of Kosinska et al.61. In this study, an additional measurement tool10 was mentioned in the abstract, but the outcome value was not clearly reported. We attempted to contact the study authors to clarify this, without success.
Prevalence findings
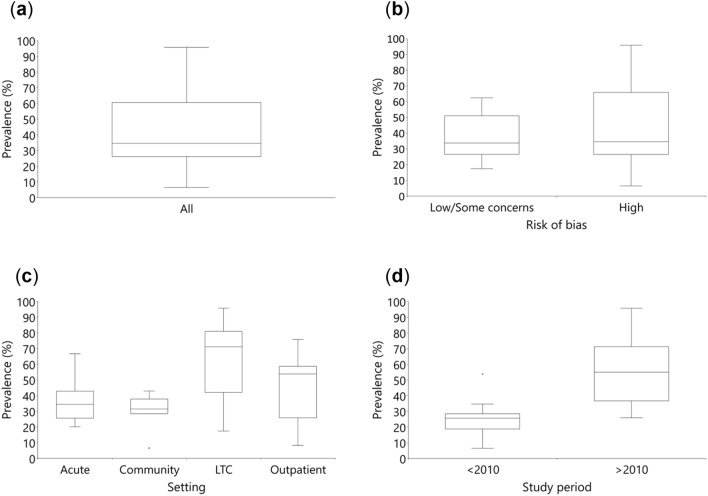
The results of individual studies that measured the PIP prevalence in patients are presented in Table 2. The two studies that measured the PIP prevalence for prescriptions instead of patients (whose results were not used in data synthesis) reported prevalence rates of 7.4%61 and 2.0%68. The results of the data synthesis, in which 26 studies were included, showed that the median PIP prevalence in older adults residing in the CEE region was 34.6% (minimum 6.5%, maximum 95.8%, interquartile range 25.9–63.2%; 1,139,693 participants; very low certainty of evidence)48–60,62–75 (see Table 3 and Fig. 3a).
Table 3.
Summary of findings.
| Outcomes | Median (range) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
|
The proportion of patients with one or more potentially inappropriate medications (PIMs) and/or potential prescribing omissions (PPOs) Assessed with: explicit validated tools |
34.6% (6.5–95.8)a | 1,139,693 (26) |
⊕ ⊝ ⊝ ⊝ VERY LOWb |
Austrian consensus panel list78, 1997 Beers criteria10, 2003 Beers criteria11, American Geriatrics Society (AGS) 2012 Beers criteria12, AGS 2015 Beers criteria13, Comprehensive protocol79, 2012 CZ expert consensus criteria80, EU(7)-PIM list81, French consensus panel list82, Ghent Older People's Prescriptions community Pharmacy Screening (GheOP3S) tool83, McLeod criteria84, PRISCUS list85, Screening Tool to Alert doctors to Right Treatment (START)15, START criteria version 216, Screening Tool of Older Person's Prescriptions (STOPP)15, STOPP criteria version 216 and composite tools (combinations of two or more tools) |
GRADE Working Group grades of evidence: High quality – we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality – we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; Low quality – our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; Very low quality – we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
aOne study by Kosinska et al.61 and part of the results from one study by Popovic et al.68 were excluded from the analysis because a unit of analysis was prescription, not a patient.
bWe downgraded the evidence three levels from high to very low due to the risk of bias (most studies at high or unclear risk of bias), imprecision (number of studies with small sample sizes), and inconsistency (considerable heterogeneity).
Figure 3.
Box-and-whisker plots of prevalence of potentially inappropriate prescribing (a) for all outcomes, (b) separately by the overall risk of bias, (c) separately by the setting, (d) separately by the study period. LTC Long-term care.
Benzodiazepines were among the top three most frequently used PIMs among almost all studies and all tools. The omission of statins for primary prevention in diabetes mellitus was among the top three PPOs in all studies48,53,60,62 using the START criteria version 115. However, this item was removed from the revised START criteria version 216 due to the lack of evidence. Only one study (Stojanovic et al.75) used other tools to assess PPOs, the START criteria version 216 and the GheOP3S tool83, which both detected a lack of vaccination as the biggest issue.
Heterogeneity assessment
An informal visual examination of heterogeneity suggested that the PIP prevalence is similar between studies at high risk of bias (20 studies)48–58,60,63,64,67,69–73,75 and those at low risk of bias or with some concerns (six studies)59,62,65,66,68,74. Furthermore, visual examination of the box-and-whisker plots showed that the PIP prevalence may be higher in LTC (six studies)48,71–75 and outpatient settings (seven studies)52,65–70 than in acute (seven studies)49–51,53–58 and community care settings (six studies)48,59,60,62–64. Finally, when informally exploring heterogeneity, we found that the prevalence might be higher in studies from 2010 onward (15 studies)48,52,54,56,57,60,62,65,66,68,71–73,75 than before 2010 (11 studies)49–51,53,55,58,59,63,64,67,69,70,74 (see Fig. 3).
Sensitivity analyses
The PIP prevalence remained consistent with the primary analysis when we reanalyzed the data using the smallest outcome from each study. However, when we used the largest outcome from each study, the PIP prevalence increased. Furthermore, the PIP prevalence remained almost unchanged when we excluded the study with a subset of relevant participants71 (see Supplementary Table S10).
Discussion
This systematic review is the first to estimate the PIP prevalence in older adults across all settings and medications in one region, countries in CEE. We identified that the issue of PIP in older adults was not comprehensively studied in the CEE region, particularly in upper-middle-income countries. Among twenty-six studies48–60,62–75, the median prevalence of PIP in older adults in the CEE region was 34.6% (interquartile range 25.9–63.2%, 26 studies, 139,693 participants, very low certainty of evidence), determined by data synthesis using the summarizing effect estimates method. Thus, our findings suggest that PIP in older adults is a highly prevalent problem and our informal visual examination of heterogeneity showed that the prevalence of PIP was higher in LTC48,71–75 and outpatient settings52,65–70 than in acute49–51,53–58 and community care settings48,59,60,62–64.
Our results are in agreement with those obtained in reviews that used similar inclusion/exclusion criteria and showed PIP prevalences of 22.6% in community-dwelling older persons from Europe31, 33.3% in older persons in primary care settings worldwide32, and 43.2% in older persons residing in LTC settings worldwide33. The results of the review by Morin et al. showed that the prevalence of PIP in LTC residents varied across regions: 49.0% in Europe, 26.8% in North America and 29.8% in other countries33. The variance of the PIP prevalence across countries was also described in the review by Liew et al.: the United Kingdom, Belgium, Australia, and New Zealand had higher PIP prevalences (35.9–59.2%) than the United States, Canada, the Netherlands, and middle-income countries (23.2–29.9%)32. Furthermore, we observed a large variation in the prevalence of PIP across studies (from 6.5 to 95.8%), which is consistent with previous reviews; in the review by Morin et al., the PIP prevalence ranged from 5.4 to 95%33, and in the review by Tommelein et al., it ranged from 0.0 to 98.8%31. We found that benzodiazepines were the most frequently prescribed PIMs, which is in agreement with the findings of Morin et al.33 and Tommelein et al.31. Only the systematic review by Tommelein et al.31 discussed the most prevalent PPOs and found, as we did, that the omission of statins for primary prevention in diabetes mellitus was the most prevalent. The systematic review by Liew et al.32 did not report which medications were most frequently involved in PIP.
Our systematic review is more comprehensive than the above-stated reviews31–33 regarding the CEE region because we included 22 studies from CEE that were not reported in these reviews. However, we excluded a study by Primejdie et al.46 reported in the review by Tommelein et al.31 because the author could not provide complete outcome data. Furthermore, our review differs from these reviews in several important methodological aspects: (1) the multiplicity of outcomes: when multiple outcomes per study were available, Tommelein et al.31 and Morin et al.33 did not select one outcome or use a statistical method that accounted for the dependency; on the other hand, Liew et al.32 used multilevel modeling to address the dependency among multiple prevalence estimates from each study; (2) data synthesis: they pooled data using a random-effect method, which we considered inappropriate in our review; (3) risk of bias: they assessed risk of bias with an adapted version of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies87 (Morin et al.33), 'a slightly adapted quality assessment scale from the Cochrane Collaboration group' (Tommelein et al.31) and the Newcastle‒Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 88 (Liew et al.32); and (4) certainty of the evidence: they did not rate the certainty of evidence (although Liew et al.32 stated in their protocol89 that they would use the GRADE approach43–45). However, despite the differences between our review and these reviews, we agree with their conclusions that the PIP prevalence in older adults is high. Additionally, our systematic review showed an increasing trend of PIP over the years, which is in line with the findings obtained by Liew et al.32 and Morin et al.33. We agree with these authors that the increasing prevalence of PIP over time might be due to the increased comprehensiveness of measurement tools, which are able to identify more prescribing problems.
Our review supports findings from the other two systematic reviews31,33 that the long-term use of benzodiazepines and the use of long-acting benzodiazepines are still highly prevalent among older adults. Benzodiazepine use in older adults is associated with cognitive impairment, sedation, delirium, dependence, withdrawal syndrome, and psychomotor impairment that increases the risk of motor vehicle accidents and falls90,91. Two especially important negative outcomes of benzodiazepine use in older adults are falls and fall-related fractures because they are common and important causes of morbidity, mortality, hospitalization, and admission to LTC facilities. Therefore, greater and continued efforts are needed to rationalize benzodiazepine prescribing.
PIP prevalence estimates vary widely across studies for several reasons. The included studies were heterogeneous regarding the inclusion criteria, participants and contexts. Regarding health status, participants varied across studies due to the different inclusion and exclusion criteria that were applied: some studies included higher-risk individuals (e.g., persons with polypharmacy), and some included healthier individuals (e.g., without cognitive impairment). Additionally, countries in CEE differ in the following aspects, which might have changed over time: health and social care systems; legislation and regulations; pharmaceutical pricing and reimbursement models; prescribing practices; the availability of medications considered PIMs in pharmaceutical markets; the availability of medication safety policies, strategies and practices; the availability of medication review and deprescribing services; the availability of interdisciplinary care models; and the availability of health care professionals who are educated and trained in various aspects of geriatrics and geriatric pharmacotherapy. We also noted variation in the types of medication regimens to which the instruments were applied, with most studies using only prescription medicines, which may also impact the PIP prevalence. However, the most important difference between the studies was the considerable variation in outcome measurements, which precluded meta-analysis. Thus, we suggest using validated measurement tools with all their items, which would enable more meaningful comparisons between studies and meta-analyses.
Studies on PIP in older patients residing in the CEE region were conducted across care settings, increasing the generalizability of our findings. However, our findings may be more applicable to high-income countries in CEE because we identified only three studies from upper-middle-income countries. None of the included studies used implicit tools to measure the PIP prevalence. Thus, the results of this review are not applicable to this type of outcome measurement.
We downgraded the certainty of evidence from high to very low for several reasons. First, most studies were at high or unclear risk of bias in one or more risk of bias domains; thus, we downgraded the quality of evidence by one level. Second, although the appropriateness of the sample size was part of the risk of bias assessment, we decided to downgrade the quality of evidence by one level for imprecision. Third, variation in the prevalence estimates across studies was considerable, and consequently, we downgraded the quality of evidence for inconsistency by one level. Finally, we did not downgrade the quality of evidence for the following: minor issues with indirectness (most studies were from high-income countries, and only explicit tools were used) and the possibility of publication bias.
The strength of this review is that we followed the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020)92. Furthermore, when potential conflicts of interest existed because the review authors were involved in the studies we considered for inclusion, we excluded these authors from screening, data extraction and the risk of bias assessment.
Although we tried to limit bias at every stage of the review, some limitations remain. First, the risk of publication bias may be considerable due to our decision to include only studies published as full papers in peer-reviewed journals. We thought this would be the most reproducible and transparent approach due to a large volume of gray literature with unverified quality in this area and the absence of study registers and protocols. Second, two authors could not provide the necessary outcome data, and we excluded these studies46,47. Finally, another potential limitation is that we did not fully incorporate the studies from our 'top-up' search into the review.
Conclusions
These results suggest that PIP in older adults is a prevalent problem throughout the CEE region. However, our findings must be interpreted with caution due to the very low certainty of the evidence.
Our review's findings could be used to raise awareness among policymakers, health care professionals, and the general public about the prevalent issue of PIP in older adults, which should be addressed in the near future at the national and international levels. Public health authorities should bring together all stakeholders to tackle this problem, primarily by raising awareness and educating health care professionals and the public about the problem of PIP in older adults and about the validated tools that should be used to minimize this issue and its negative consequences.
More research is needed to strengthen the existing evidence and increase the generalizability of the findings. Further studies should be of high-level quality, i.e., where applicable, the sample size should be calculated, probabilistic sampling should be used, a representative sample should be obtained, the response rate should be calculated, and the differences between responders and non-responders should be examined. Additionally, studies should be conducted in different care settings and countries, particularly in upper-middle-income countries where the evidence is scarce. Finally, studies should be clearly reported using appropriate guidelines.
Supplementary Information
Author contributions
J.B. and D.F. designed the study, J.B. and A.C. searched the literature, J.B. and I.K. selected studies, J.B., I.K., S.S., K.T. and V.B. extracted data, J.B. analysed the results, J.B. and B.O. assessed the risk of bias, J.B. and B.O. assessed the certainty of evidence, J.B., M.O.H. and D.F. drafted the manuscript, and all authors critically reviewed the manuscript.
Funding
This main author was solely funded by European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement, EuroAgeism project, grant number 764632. The research group and co-authors were supported by: European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement, EuroAgeism project, grant number 764632; Ministry of Education, Youth and Sports of the Czech Republic, Czech Operational Programme Research, Development and Education, Pre-application research into innovative medicines and medical technologies (InoMed) project, grant number CZ.02.1.01/0.0/0.0/18_069/0010046; Charles University project, grant number: SVV 260 551; Charles University project, Research program Cooperatio, research unit KSKF-I Ageing, Polypharmacy and Changes in the Therapeutic value of Drugs in the AgeD (Chair: Assoc. Prof. Daniela Fialova); Ministry of Education, Youth and Sports of the Czech Republic, Czech Operational Programme Research, Development and Education, START Programme, grant number: CZ.02.2.69/0.0/0.0/19_073/0016935; European Union's Horizon 2020 research and innovation programme I-CARE4OLD project, grant number 965341. Financial sponsors played no role in the design, execution, analysis and interpretation of data or study writing.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The Supplementary Information 1 file published with this Article contained an error in the order of references in the Supplementary Information.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/9/2022
A Correction to this paper has been published: 10.1038/s41598-022-25155-9
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19860-8.
References
- 1.Mekonnen AB, Redley B, de Courten B, Manias E. Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2021;87:4150–4172. doi: 10.1111/bcp.14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liew TM, Lee CS, Goh Shawn KL, Chang ZY. Potentially inappropriate prescribing among older persons: A meta-analysis of observational studies. Ann. Fam. Med. 2019;17:257–266. doi: 10.1370/afm.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malakouti SK, et al. A systematic review of potentially inappropriate medications use and related costs among the elderly. Value Health Reg. Issues. 2021;25:172–179. doi: 10.1016/j.vhri.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Cullinan S, O’Mahony D, Fleming A, Byrne S. A meta-synthesis of potentially inappropriate prescribing in older patients. Drugs Aging. 2014;31:631–638. doi: 10.1007/s40266-014-0190-4. [DOI] [PubMed] [Google Scholar]
- 5.Hill-Taylor B, et al. Effectiveness of the STOPP/START (Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: Systematic review and meta-analysis of randomized controlled studies. J. Clin. Pharm. Ther. 2016;41:158–169. doi: 10.1111/jcpt.12372. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor MN, Gallagher P, Omahony D. Inappropriate prescribing: Criteria, detection and prevention. Drugs Aging. 2012;29:437–452. doi: 10.2165/11632610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Beers MH, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch. Intern. Med. 1991;151:1825–1832. doi: 10.1001/archinte.1991.00400090107019. [DOI] [PubMed] [Google Scholar]
- 8.Spinewine A, et al. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet. 2007;370:173–184. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrow MS, Airaksinen MSA, Kivelä S-L, Lyles A, Leikola SNS. Comparison of prescribing criteria to evaluate the appropriateness of drug treatment in individuals aged 65 and older: A systematic review. J. Am. Geriatr. Soc. 2011;59:1521–1530. doi: 10.1111/j.1532-5415.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 10.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly an update. Arch. Intern. Med. 1997;157:1531–1536. doi: 10.1001/archinte.1997.00440350031003. [DOI] [PubMed] [Google Scholar]
- 11.Fick DM, et al. Updating the beers criteria for potentially inappropriate medication use in older adults: Results of a US consensus panel of experts. Arch. Intern. Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 12.American Geriatrics Society Beers Criteria Update Expert Panel American geriatrics society updated beers criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Geriatrics Society Beers Criteria Update Expert Panel American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2015;63:2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 14.2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc.67, 674–694 (2019). [DOI] [PubMed]
- 15.Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int. J. Clin. Pharmacol. Ther. 2008;46:72–83. doi: 10.5414/CPP46072. [DOI] [PubMed] [Google Scholar]
- 16.O’Mahony D, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing. 2015;44:213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanlon JT, et al. A method for assessing drug therapy appropriateness. J. Clin. Epidemiol. 1992;45:1045–1051. doi: 10.1016/0895-4356(92)90144-C. [DOI] [PubMed] [Google Scholar]
- 18.Clyne B, et al. Interventions to address potentially inappropriate prescribing in community-dwelling older adults: A systematic review of randomized controlled trials. J. Am. Geriatr. Soc. 2016;64:1210–1222. doi: 10.1111/jgs.14133. [DOI] [PubMed] [Google Scholar]
- 19.Motter FR, Fritzen JS, Hilmer SN, Paniz ÉV, Paniz VMV. Potentially inappropriate medication in the elderly: A systematic review of validated explicit criteria. Eur. J. Clin. Pharmacol. 2018;74:679–700. doi: 10.1007/s00228-018-2446-0. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Global Health Observatory data repository. Healthy life expectancy (HALE) Data by country. https://apps.who.int/gho/data/view.main.HALEXv?lang=en.
- 21.World Bank. GDP per capita, PPP (current international $). https://data.worldbank.org/indicator/NY.GDP.PCAP.PP.CD.
- 22.World Bank. World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 23.Bhagavathula AS, et al. Prevalence of polypharmacy, hyperpolypharmacy and potentially inappropriate medication use in older adults in India: A systematic review and meta-analysis. Front. Pharmacol. 2021;12:685518. doi: 10.3389/fphar.2021.685518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhagavathula AS, Gebreyohannes EA, Fialova D. Prevalence of polypharmacy and risks of potentially inappropriate medication use in the older population in a developing country: A systematic review and meta-analysis. Gerontology. 2022;68:136–145. doi: 10.1159/000516075. [DOI] [PubMed] [Google Scholar]
- 25.Hill-Taylor B, et al. Application of the STOPP/START criteria: A systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J. Clin. Pharm. Ther. 2013;38:360–372. doi: 10.1111/jcpt.12059. [DOI] [PubMed] [Google Scholar]
- 26.Opondo D, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: A systematic review. PLoS ONE. 2012;7:e43617. doi: 10.1371/journal.pone.0043617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Praxedes, M. F. D. S., Pereira, G. C. D. S., Lima, C. F. D. M., Santos, D. B. D. & Berhends, J. S. Prescribing potentially inappropriate medications for the elderly according to Beers Criteria: Systematic review. Cien. Saude Colet.26, 3209–3219 (2021). [DOI] [PubMed]
- 28.Thomas RE, Thomas BC. A systematic review of studies of the STOPP/START 2015 and American Geriatric Society Beers 2015 criteria in patients≥ 65 years. Curr. Aging Sci. 2019;12:121–154. doi: 10.2174/1874609812666190516093742. [DOI] [PubMed] [Google Scholar]
- 29.Storms H, Marquet K, Aertgeerts B, Claes N. Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: A systematic review. Eur. J. Gen. Pract. 2017;23:69–77. doi: 10.1080/13814788.2017.1288211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guaraldo L, Cano FG, Damasceno GS, Rozenfeld S. Inappropriate medication use among the elderly: A systematic review of administrative databases. BMC Geriatr. 2011;11:79. doi: 10.1186/1471-2318-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tommelein E, et al. Potentially inappropriate prescribing in community-dwelling older people across Europe: A systematic literature review. Eur. J. Clin. Pharmacol. 2015;71:1415–1427. doi: 10.1007/s00228-015-1954-4. [DOI] [PubMed] [Google Scholar]
- 32.Liew TM, Lee CS, Goh SKL, Chang ZY. The prevalence and impact of potentially inappropriate prescribing among older persons in primary care settings: Multilevel meta-analysis. Age Ageing. 2020;49:570–579. doi: 10.1093/ageing/afaa057. [DOI] [PubMed] [Google Scholar]
- 33.Morin L, Laroche ML, Texier G, Johnell K. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: A systematic review. J. Am. Med. Dir. Assoc. 2016;17(862):e1–9. doi: 10.1016/j.jamda.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Brkic, J. et al. Prevalence of potentially inappropriate medication use in older adults in Central and Eastern Europe: a systematic review. PROSPERO 2020 CRD42020152713. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020152713 (2020).
- 35.Campbell M, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ. 2020;368:16890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page MJ, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rethlefsen ML, et al. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 2021;10:39. doi: 10.1186/s13643-020-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alldred DP, Kennedy MC, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst. Rev. 2016;2:CD009095. doi: 10.1002/14651858.CD009095.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rankin A, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. 2018;9:CD008165. doi: 10.1002/14651858.CD008165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization. Active Ageing: A Policy Framework. (World Health Organization, 2002). [PubMed]
- 42.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015;13:147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 43.Guyatt GH, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guyatt G, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 45.GRADE Working Group Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Primejdie D, Bojiţǎ M, Popa A. Potential inappropriate medication use in community - dwelling elderly patients. A qualitative study. Farmacia. 2012;60:366–378. [Google Scholar]
- 47.Ilić D, Bukumirić Z, Janković S. Impact of educational intervention on prescribing inappropriate medication to elderly nursing homes residents. Srp. Arh. Celok. Lek. 2015;143:174–179. doi: 10.2298/SARH1504174I. [DOI] [PubMed] [Google Scholar]
- 48.Primejdie DP, Bojita MT, Popa A. Potentially inappropriate medications in elderly ambulatory and institutionalized patients: An observational study. BMC Pharmacol. Toxicol. 2016;17:38. doi: 10.1186/s40360-016-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wawruch M, et al. Quality indicators of pharmacotherapy in geriatrics. Klin Farmakol Farm. 2006;20:135–139. [Google Scholar]
- 50.Wawruch M, et al. Factors influencing the use of potentially inappropriate medication in older patients in Slovakia. J. Clin. Pharm. Ther. 2008;33:381–392. doi: 10.1111/j.1365-2710.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- 51.Wawruch M, et al. Perception of potentially inappropriate medication in elderly patients by Slovak physicians. Pharmacoepidemiol. Drug Saf. 2006;15:829–834. doi: 10.1002/pds.1290. [DOI] [PubMed] [Google Scholar]
- 52.Stuhec M, Gorenc K, Zelko E. Evaluation of a collaborative care approach between general practitioners and clinical pharmacists in primary care community settings in elderly patients on polypharmacy in Slovenia: A cohort retrospective study reveals positive evidence for implementation. BMC Health Serv. Res. 2019;19:118. doi: 10.1186/s12913-019-3942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallagher P, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur. J. Clin. Pharmacol. 2011;67:1175–1188. doi: 10.1007/s00228-011-1061-0. [DOI] [PubMed] [Google Scholar]
- 54.Hudhra K, et al. Prevalence and factors associated with potentially inappropriate prescriptions among older patients at hospital discharge. J. Eval. Clin. Pract. 2016;22:707–713. doi: 10.1111/jep.12521. [DOI] [PubMed] [Google Scholar]
- 55.Kostková L, Mačugová A, Drobná V, Dukát A, Wawruch M. Potentially inappropriate prescription in elderly patients: Comparison of selected quality indicators. Klin. Farmakol. Farm. 2011;25:167–171. [Google Scholar]
- 56.Matanović SM, Vlahović-Palčevski V. Potentially inappropriate prescribing to the elderly: Comparison of new protocol to Beers criteria with relation to hospitalizations for ADRs. Eur. J. Clin. Pharmacol. 2014;70:483–490. doi: 10.1007/s00228-014-1648-3. [DOI] [PubMed] [Google Scholar]
- 57.Mucalo I, et al. Potentially inappropriate medicines in elderly hospitalised patients according to the EU(7)-PIM list, STOPP version 2 criteria and comprehensive protocol. Eur. J. Clin. Pharmacol. 2017;73:991–999. doi: 10.1007/s00228-017-2246-y. [DOI] [PubMed] [Google Scholar]
- 58.Radošević N, Gantumur M, Vlahović-Palčevski V. Potentially inappropriate prescribing to hospitalised patients. Pharmacoepidemiol. Drug Saf. 2008;17:733–737. doi: 10.1002/pds.1531. [DOI] [PubMed] [Google Scholar]
- 59.Fialová D, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293:1348–1358. doi: 10.1001/jama.293.11.1348. [DOI] [PubMed] [Google Scholar]
- 60.Gorup EC, Šter MP. Number of medications or number of diseases: What influences underprescribing? Eur. J. Clin. Pharmacol. 2017;73:1673–1679. doi: 10.1007/s00228-017-2336-x. [DOI] [PubMed] [Google Scholar]
- 61.Kosińska K, Brandys J. Potentially inappropriate drugs for geriatric patients. Przegla̧d Lek. 2007;64:19–23. [PubMed] [Google Scholar]
- 62.Kovačević SV, et al. Potentially inappropriate prescribing in older primary care patients. PLoS ONE. 2014;9:e95536. doi: 10.1371/journal.pone.0095536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajska-Neumann A, Wieczorowska-Tobis K. Polypharmacy and potential inappropriateness of pharmaco-logical treatment among commuinity-dwellling elderly patients. Arch. Gerontol. Geriatr. 2007;44:303–309. doi: 10.1016/j.archger.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 64.Rajska-Neumann A, et al. Drug consumption among Polish centenarians. Arch. Gerontol. Geriatr. 2011;53:e29–e32. doi: 10.1016/j.archger.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Grina D, Briedis V. The use of potentially inappropriate medications among the Lithuanian elderly according to Beers and EU(7)-PIM list – a nationwide cross-sectional study on reimbursement claims data. J. Clin. Pharm. Ther. 2017;42:195–200. doi: 10.1111/jcpt.12494. [DOI] [PubMed] [Google Scholar]
- 66.Jazbar J, Locatelli I, Kos M. Extent and nature of inappropriate medication prescribing among elderly in Slovenia. Farm. Vestn. 2017;68:145–151. [Google Scholar]
- 67.Nerat T, Kos M. Analysis of inappropriate medication prescribing in Slovenian elderly patients based on the Beers and Laroche criteria. Zdr. Varst. 2011;50:34–44. [Google Scholar]
- 68.Popović B, et al. Potentially inappropriate prescribing in elderly outpatients in Croatia. Eur. J. Clin. Pharmacol. 2014;70:737–744. doi: 10.1007/s00228-014-1667-0. [DOI] [PubMed] [Google Scholar]
- 69.Vinšová J, et al. Prevalence and longitudinal trends in prescription of potentially inappropriate medications for the elderly in the Czech Republic. Prakt. Lek. 2006;86:722–728. [Google Scholar]
- 70.Vlahović-Palčevski V, Bergman U. Quality of prescribing for the elderly in Croatia - Computerized pharmacy data can be used to screen for potentially inappropriate prescribing. Eur. J. Clin. Pharmacol. 2004;60:217–220. doi: 10.1007/s00228-004-0747-y. [DOI] [PubMed] [Google Scholar]
- 71.Bor A, et al. Medication use and risk of falls among nursing home residents: A retrospective cohort study. Int. J. Clin. Pharm. 2017;39:408–415. doi: 10.1007/s11096-017-0426-6. [DOI] [PubMed] [Google Scholar]
- 72.Kalafutová S, Šulcová H, Jurašková B, Vlček J. A pharmacotherapy of nursing home residents. Geriatr. Gerontol. 2014;3:65–70. [Google Scholar]
- 73.Kolar J, Tinkova B, Ambrus T, Tinkova V. Analysis of pharmacotherapy in senior homes residents. Acta Pol. Pharm. 2018;75:223–228. [Google Scholar]
- 74.Šter MP, Gorup EC, Klančič D. Polypharmacy and inappropriate drug prescribing in elderly nursing home residents. Zdr. Vestn. 2009;78:231–240. [Google Scholar]
- 75.Stojanović M, Vuković M, Jovanović M, Dimitrijević S, Radenković M. GheOP3S tool and START/STOPP criteria version 2 for screening of potentially inappropriate medications and omissions in nursing home residents. J. Eval. Clin. Pract. 2020;26:158–164. doi: 10.1111/jep.13107. [DOI] [PubMed] [Google Scholar]
- 76.Morris JN, Fries BE, Steel K, Ikegami N, Bernabei R, Carpenter GI, Gilgen R, Hirdes JP, Topinková E. Comprehensive Clinical Assessment in Community Setting: Applicability of the MDS-HC. J. Am. Geriatr. Soc. 1997;45(8):1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- 77.Sikora, E. Studies on successful aging and longevity: Polish Centenarian Program. Acta Biochim. Pol.47, 487–489 (2000). [PubMed]
- 78.Mann E, et al. Potentially inappropriate medication in geriatric patients: The Austrian consensus panel list. Wien. Klin. Wochenschr. 2012;124:160–169. doi: 10.1007/s00508-011-0061-5. [DOI] [PubMed] [Google Scholar]
- 79.Matanović SM, Vlahovic-Palcevski V. Potentially inappropriate medications in the elderly: A comprehensive protocol. Eur. J. Clin. Pharmacol. 2012;68:1123–1138. doi: 10.1007/s00228-012-1238-1. [DOI] [PubMed] [Google Scholar]
- 80.Fialová D, Topinková E, Ballóková A, Matejovska-Kubesova H. 2012 CZ expert consensus for potentially inappropriate medication use in old age: Appropriate choice of drugs and drug dosing in geriatric patients (Section I.), drug-disease interactions in the old age (Section II.) Klin. Farmakol. Farm. 2013;27:18–28. [Google Scholar]
- 81.Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM list: A list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur. J. Clin. Pharmacol. 2015;71:861–875. doi: 10.1007/s00228-015-1860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: A French consensus panel list. Eur. J. Clin. Pharmacol. 2007;63:725–731. doi: 10.1007/s00228-007-0324-2. [DOI] [PubMed] [Google Scholar]
- 83.Tommelein E, et al. Older patients’ prescriptions screening in the community pharmacy: Development of the Ghent Older People’s Prescriptions community Pharmacy Screening (GheOP3S) tool. J. Public Health (Oxf) 2016;38:e158–e170. doi: 10.1093/pubmed/fdv090. [DOI] [PubMed] [Google Scholar]
- 84.McLeod PJ, Huang AR, Tamblyn RM, Gayton DC. Defining inappropriate practices in prescribing for elderly people: A national consensus panel. CMAJ. 1997;156:385–391. [PMC free article] [PubMed] [Google Scholar]
- 85.Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: The PRISCUS list. Dtsch. Arztebl. Int. 2010;107:543–551. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gorenc, K. Clinical evaluation of pharmacist consultant interventions in community health centre Ljutomer in elderly patients treated with polypharmacy (Master’s thesis). (University of Ljubljana, 2017).
- 87.von Elm E, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 88.Wells, GA. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2014).
- 89.Lee CS, Liew TM. Inappropriate prescribing among older persons in primary care: Protocol for systematic review and meta-analysis of observational studies. BMJ Open. 2017;7:e015395. doi: 10.1136/bmjopen-2016-015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: Impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493–521. doi: 10.1007/s40266-018-0544-4. [DOI] [PubMed] [Google Scholar]
- 91.Madhusoodanan S, Bogunovic OJ. Safety of benzodiazepines in the geriatric population. Expert Opin. Drug Saf. 2004;3:485–493. doi: 10.1517/14740338.3.5.485. [DOI] [PubMed] [Google Scholar]
- 92.Higgins, J. P. T. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). (Cochrane, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).