Abstract
The extracellular matrix (ECM) is a protein-and-carbohydrate meshwork that supports a variety of biological structures and processes, from tissue development and elasticity to the preservation of organ structures. ECM composition is different in each organ. It is a remarkably dynamic 3-dimensional structure that's constantly changing to maintain tissue homeostasis. This review aims to describe the involvement of ECM components in the remodeling process of spinal cord injury (SCI) and intervertebral disc degeneration (IVDD). Here, we have also described the current ECM-based therapeutic targets, which can be explored for ECM remodeling SCI is a neurological condition with intense influences resulting from a trauma inflicted on the spinal cord. SCI leads to damage to the intact ECM that leads to regeneration failure. IVDD mainly occurs due to aging and trauma. Various ECM components enable fragmentation of the disc and are thereby involved in disc degeneration. ECM manipulation can be used as an adjunct treatment in SCI and IVDD. Current treatment approaches for SCI and IVDD are conservative and unsatisfactory. Targeting ECM remodeling as an adjunct therapy may result in better disease outcomes.
Keywords: Extracellular matrix, Spinal cord injury, Intervertebral disc degeneration, Extracellular matrix remodeling
INTRODUCTION
The extracellular matrix (ECM) is a scaffold for cells and tissues composed of proteins, proteoglycans (PGs), and glycosaminoglycans (GAGs). ECM influences cell adhesion, morphology, migration, proliferation, and differentiation. Consequently, ECM material contains specific cell surface receptor-interacting domains [1]. It is an essential component of all multicellular organisms, consisting of a network of collagens, glycoproteins (GPs), and PGs that are spatially arranged [2]. Each type of tissue develops a unique composition and topology of ECM; even though each tissue type contains the unique type of ECM, some molecules are constant with the all-tissue type; these molecules are collagens, hyaluronan, elastin, fibronectins, and laminins [3]. This noncellular component is the critical modulator of cellular function and tissue behavior [4]. The viscoelastic characteristics of the microenvironment, as well as imposed mechanical stress on cells, are mechanical properties sensed by cells. Other microenvironmental parameters, such as matrix porosity and cell density, can influence cell shape, which can govern even the most fundamental of cell actions. ECM is thought to have numerous physical traits that influence cell behavior, some of which vary depending on the strain applied to the matrix. Matrix stiffness is a fundamentally intrinsic feature of the matrix that cells feel by applying cell-generated tension. Multiple properties of 3-dimensional ECM, including pore size structural organization, cross-link density, and stiffness, have been identified as a modulator of cell motility [5]. By modulating signaling pathways, the ECM helps to maintain tissue structural integrity and transduce cellular communication. Integrins, cadherins, selectins, syndecans, and other cell surface receptors interact with ECM components, influencing essential activities like proliferation, migration, and differentiation (Fig. 1) [6].
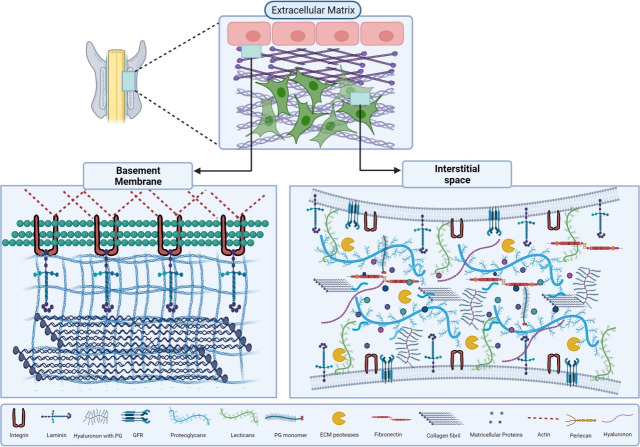
Fig. 1.
A graphical overview of extra cellular matrix (ECM) and its major components. ECM is classified into 2 major types that are basement membrane (BM) and interstitial space (IS), BM is found between epithelial cells and connective tissues. BM is composed of collagen IV meshwork that attaches with ECM components like laminins, perlecan, and minor collagens. IS is made up of collagen fibrils, secreted proteoglycans (PGs), matricellular proteins, and hyaluronan. This unique connection produces a dynamic framework for cells to adhere to ECM using surface proteins, such as cell surface PGs, integrins, glypicans and, syndecan. The signal transduction takes place by these interactions which control the various functions. GFR, growth factor receptor.
ECM components demonstrate both regenerative and degenerative potential in the central nervous system. Spinal cord injury (SCI) is a catastrophic neurological illness that occurs as a result of damage to the spinal cord due to mechanical force or pathological conditions, which results in motor and sensory functioning below the injury site [7]. Injury inflicted on the spinal cord initiates inflammation and subsequent degeneration of the ECM. In traumatic SCI, the severity of injury occurs due to cell death. It triggers a complicated secondary damage cascade that culminates in the death of neuronal cells and glial cells, in addition to ischemia and inflammation. This cascade results in the production of a glial scar and cystic cavities, as well as anomalies of spinal cord architecture and structural design. The spinal cord has a low intrinsic recuperation capability as a result of the glial scar and cystic cavities, as well as insufficient endogenous remyelination and axonal regeneration, resulting in lifelong neurological deficits after SCI. Intervertebral discs (IVDs) are fibrocartilaginous, avascular tissue of the body that resides between neighbouring vertebrae in the vertebral column. They serve as shock absorbers. They aid in the protection of the nerves which travel through the spine and vertebrae.
IVDD is a significant degenerative process and a precursor for disc herniation and low back pain [8]. Disc degeneration is caused by cellular, metabolic, and morphological changes that lead to a decrease in the density of cells and ECM components that result in compromised structure and function of IVD [9]. Early treatment appears to promote neurological recovery in preclinical research, case reports, and limited clinical trials. No conventional therapy approach has been shown to improve neurological outcomes to date [10]. The current ECM-based therapeutic approaches include neutralization of inhibitory ECM factors, stimulation of axonal regeneration via modulating ECM components. Here, in this review, we have majorly focused on the structure and composition of ECM components, ECM remodeling, major approaches to modulate ECM in SCI and IVDD. This review aims to provide the insights about ECM targets that can be explored for the therapeutic approaches.
EXTRA CELLULAR MATRIX COMPOSITION OF SPINAL CORD
In the CNS, ECM is different from the other systematic tissues. The ECM plays various functions like the migration of cells, axonal guidance, and synaptogenesis and shapes the CNS. In the mature, healthy CNS, ECM acts as a transporter and storage for growth hormones and chemicals, directly via cell membrane receptors, or as a transporter and storage for chemicals and growth factors. The ECM regulates CNS function as it preserves synapses and prevents aberrant remodeling [11]. The mature, healthy CNS includes the interstitial space (IS), basement membrane (BM), and perineuronal nets (PNNs). Collagen, laminin, fibronectin, syndecans, dystroglycan, and perlecan, which make up the majority of the BM, operate as a barrier between endothelial and parenchymal cells [12]. PNNs have an ECM that is comparable to that of the IS. However, it is more compact, resulting in a more significant amount of Chondroitin sulfate proteoglycans (CSPGs) in the body and growth-inhibitory elements like the tenascin receptor and link proteins [12]. PNNs surround the specific, but not all, neurons, as well as presynaptic terminals, nodes of Ranvier, and synaptic boutons [13]. These components are diffused and arranged in IS or in the more complex and condensed structures that constitute the small axonal coats and surround presynaptic terminal fibers, and assemblies of clustered matrix encapsulating nodes of Ranvier and PNNs around the soma, initial axon segments, and proximal dendrites [14,15].
In a normal CNS, the interstitial ECM mostly consists of hyaluronic acid (HA), sulfated PGs, and tenascin. The most common GAG in the ECM is called HA. Following injury, high-molecular-weight HA (HMW-HA) gets fragmented and creates low-molecular-weight HA (LMW-HA) fragments which can modulate inflammatory responses [16]. While HA is nonsulfated GAGs, there are various kind of sulfated GAGs found throughout the body, but the CNS is significantly abundant in them [17]. Tenascin-C is a damage-associated molecular pattern that produces innate immune cell activation by interacting with tolllike receptor [18]. Tenascins expression is essential for wound healing [19], long-term expression can be harmful, and its termination lowers neurogenic pathology and inflammation [20].
EXTRA CELLULAR MATRIX COMPOSITION OF INTERVERTEBRAL DISC
IVD mainly consists of 3 parts, i.e., central region nucleus pulposus (NP), a highly hydrated structure containing 70%– 80% water. This region contains collagen II majorly, along with other components. The second part is the annulus fibrosus (AF), which is the stiff circular outer of the IVD which surrounds the inner jelly-like NP, which mostly contains collagen I and other ECM components like collagen, PGs, HA, fibronectin, and laminin. Finally, the third part is the endplate which is made of hyaline cartilage. To maintain the homeostatic environment, the disc’s cells require nutrients like glucose and oxygen, and the endplate provides this via diffusion [21,22].
Disc degeneration is the breakdown of 2 or more discs leading to pain. IVDD results from an imbalance in catabolic and anabolic factors, which increases degradative enzymes like matrix metalloproteinase (MMPs), A disintegrin, and metalloproteinase with thrombospondin motifs (ADAMTs) and decreases the synthesis of ECM components. It is also caused as people become older due to calcification of the endplate, which can disrupt the delivery of nutrients and other metabolic components, resulting in a hypoxic environment and an acidic pH. Improper supply of nutrients causes hypertrophy, apoptosis, and reduced density of IVD cells [23]. With increasing age, the disc’s ECM undergoes significant modifications. Reduced hydration is caused by lowering the aggrecan component in the NP, leading to mechanical dysfunction. Less hydrated, more fibrous NP does not uniformly distribute compressive stresses between vertebral bodies. In addition, inflammatory cytokines like interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are upregulated, as the increase in disc degeneration leads to ECM remodeling [24].
ECM of IVD is composed of PGs, water, GAGs, collagen, and other components in lesser amounts and plays an essential role in structural integrity, stiffness, and tensile strength [25]. ECM of the disc appears to have more collagen isoforms than any other connective tissue, namely collagens V, VI, IX, XI, XII, and XIV, contributing to the matrix. Collagen serves as the primary load-bearing component in a wide range of soft tissue and is crucial to human physiology [26,27]. HA is a critical ECM component of IVD that interacts with various protein molecules essential for the signaling and cell-cell interaction. Since it is negatively charged, HA can absorb water at a rate of 10–104 times its mass, making it an osmotically active molecule [28]. It can occupy huge gaps due to its high volume when hydrated and serves as a shock absorber and lubricant [29]. Aggrecan and versican create huge aggregates with HA that offer water-binding affinity to the disc due to their sulfated GAG chains in higher concentrations [30]. Fibronectin is a huge, abundant GPs that participates in the correct construction of ECM. Collagen I, III, gelatin, thrombospondin, decorin, and latent transforming growth factor-protein-1 are among ECM molecules it may bind [31,32]. Integrins, the family of cell adhesion molecules that govern cell ECM communication and have many unique integrin receptors, have been discovered, including the fibronectin-binding integrin receptor and collagen-binding integrin receptor [33]. The broad family of unique, multiple domain trimeric BM proteins is laminins that regulate related cells behavior, including adherence, proliferation, migration, phenotypic stability, and resistance to anoikis [34].
THE EXTRA CELLULAR MATRIX REMODELING FOLLOWING SPINAL CORD INJURY
The breakdown of ECM after SCI adds to the damage of nerve tissues. Both native and permeating inflammatory cells begin secreting ECM components and MMPs, which destroy the ECM [35]. ECM controls the health and activity of neural tissue. The ECM participates in several crucial activities, namely inflammation, survival of cells, axon development, gliosis, revascularization, and adaptability after an injury. As a result, manipulating the ECM after an injury may help heal nerve tissue. After SCI, there are various endogenous repair systems, such as axon development and sprouting [36], revascularization, and neural stem cell proliferation and differentiation [37]. On the other hand, these processes may fail or succeed only partially. Following spinal cord damage, the functional impairment and tissue loss are irreversible [38]. The spinal cord is protected, regenerated, and repaired in a variety of ways. The first approach involves increasing intracellular survival systems, lowering inflammation and bleeding, or activating antioxidative pathways to reduce oxidative stress. The second approach uses regenerative processes to promote axon development and sprouting. This is accomplished by increasing intracellular axon development or decreasing growth inhibition in the damaged environment. The third method improves neural plasticity by combining electrical stimulation and rehabilitative motor training [39].
After CNS injury, the ECM’s composition alters dramatically. The nature of the injury controls this, and influences which cells are then targeted to the lesion location—for example, following blunt trauma that disrupts the blood-brain barrier but leaves the dura mater intact, such as contusive-type SCI and blunt traumatic brain injuries. Glia is generally the main source of scar matrix deposition, whereas penetrating spinal laceration, transection, or cortical stab injuries also confer more remarkable fibroblast invasion through disrupted meninges [40]. The key players in ECM breakdown are MMPs, and thus the modulation of MMP expression and activity is critical for tissue homeostasis. Changes influence ECM biology in MMP pattern expression or the equilibrium between MMPs and their tissue-specific inhibitor [41]. In addition, changes affect the modulation of cell responses facilitating tissue repair in ECM composition. Degradation of ECM proteins is used to remove dead cells and damaged tissue during the inflammatory phase of tissue restoration. This procedure produces bio-active ECM components known as matricryptins that regulate inflammatory, angiogenic, fibrogenic, and reparative pathways by interacting with cell membrane receptors [42].
THE EXTRA CELLULAR MATRIX REMODELING FOLLOWING DISC DEGENERATION
One of the essential regulators of the body’s cellular and tissue functions is ECM, which constantly responds to various central stimuli. ECM equilibrium must be strictly regulated for wound healing, proper organ homeostasis, and development [30]. Homeostasis maintenance in IVD requires tight regulation of matrix quality and turnover, and proteases and their activity enhancers mainly regulate this. This homeostasis is vulnerable to change in the expression of the proteases, which may result in ECM remodeling if altered for a long time [30,43,44]. ECM turnover increases when remodeling occurs, leading to changes in histology and architecture of tissue [45]. Excessive ECM remodeling can be a life-threatening condition, including disrupted tissue growth, repair, and degradation, finally causing degeneration [45-47]. This remodeling is governed by synthesis, secretion, alteration, deposition, and proteolytic degradation of the matrix components [47,48]. Eliminating one or more of the ECM’s constituents is an effective way to modify it. MMPs proteolytic enzymes, ADAMTs, tissue inhibitor of metalloproteinase (TIMPs), heparanase, cathepsins, hyaluronidases, and stripteases are powerful enzymes responsible for ECM remodeling and degradation of structural components of ECM involved in maintaining integrity [48-50]. Various investigations over the last few decades demonstrate that an altered mechanical environment of the disc can result in remodeling, breakdown, and rearrangement of the ECM, leading to symptoms of accelerated disc degeneration in some situations [51-54].
IVD can maintain the structural integrity and adjust to compressive loading, which regulates the anabolic and catabolic gene expression by ECM remodeling suggested in in vivo studies [55,56]. In vivo study in rabbit punctured IVD remodeling in endplate occurs naturally with degeneration [57]. Various molecules are involved in remodeling, but MMPs are supposed to cause degradation, and these MMPs are involved in angiogenesis, differentiation, apoptosis, proliferation, and migration [58-60]. Out of 23 members of MMPs, MMP-1, MMP-3, and MMP-13 are more expressed in the degenerated disc suggesting their direct role in ECM remodeling [61]. The matrix evolves throughout the remodeling phase, causing collagen bundles to expand in size and strength to substitute fibronectin, HA and PGs are also accumulated, contributing to tissue toughness [62]. Though MMP-1, 3, and 13 are increased with disc degeneration, this is associated with the rise in their inhibitors (TIMPs 1 and 2). With progressive degeneration, higher expression of ADAMTS-4 was found in immuno-positive cells, which was not accompanied by a rise in its inhibitor TIMP-3 [63]. Heparanase is a type of endo-beta-glucuronidase that targets PGs upregulation of heparanase isoforms in degenerative IVD and herniated discs, suggesting that heparan sulfate PGs play an essential role in inflammatory responses and ECM remodeling [64].
Cathepsin K is a newly found cysteine protease that breaks type I to II collagen’s triple helical domains. Factor capable of promoting cathepsin K synthesis is the receptor activator of nuclear factor-B ligand (RANKL), which is well known for its function in generating ECM remodeling enzymes. It was found that the degenerative disc has a higher expression of RANKL than in healthy disc and cathepsin K gene expression levels were found to have a positive, strong relation with RANKL expression. Based on these observations, cathepsin K is found to play an essential role in the remodeling of the disc’s ECM and degradation in the degenerative disc’s proinflammatory cytokine-rich microenvironment [65].
EXTRA CELLULAR MATRIX ASSOCIATED WITH SPINAL CORD INJURY AND INTERVERTEBRAL DISC DEGENERATION, THEIR CORRELATION
IVDD is one of the most prevalent neurological illnesses in dogs and is defined by a spontaneous explosive extrusion of the degenerative IVD into the vertebral canal, resulting in mixed contusive-compressive damage to the spinal cord [66]. Although the spinal cord is protected within the spinal canal, dislocation or shattering of the vertebrae, disruption of the IVD, or contusion within a stenotic canal can cause injury to the cord [67]. Acute thoracolumbar IVDD can result in rapid spinal cord functional impairment, ascending myelomalacia, and, eventually, decreased life expectancy and quality of life [68]. Significant and early axonal swellings characterize axonopathy in the white matter, which is amplified in the ventral portions of the lesion epicenter during naturally occurring IVDD-related SCI in dogs. Significant data suggests that lower back pain and spinal cord compression nerve pain are common clinical and public health issues caused by IVDD [69]. Degenerated discs are prone to out-pouching (herniation); the protruding disc can press against one of the spinal nerves that run from the spinal cord to the rest of the body. This pressure causes pain, weakness, and numbness in the back and legs. When the bone spurs compress the spinal cord, affected individuals can develop problems with walking and bladder and bowel control.
MMP-9 has been reported to have a severe injury in dog IVDD. Its activity in the cerebral fluid corresponds with injury severity in dogs with IVDD, suggesting that this may play a detrimental role in acute SCI [70,71]. In spinal cord-damaged mice, inhibiting MMP-9 expression improves locomotion. These MMPs are more expressed in degenerated disc cells and tissues. Human IVDD has been linked to an increase in ADAMTS-4 expression at both early and late stages [72], whereas the level of expression did not indicate any meaningful difference in SCI; this suggests that ADAMTS-4 is present in the spinal cord at all times, whether it is normal or diseased condition [73]. These findings imply that ADAMTSs and CSPGs play an important role in SCI and IVD.
Various other collagen is present like types II, VI, IX, and XI also present in the NP and types I, II, V, VI, IX, and XI from the AF type I collagen was highly expressed in the spinal cord during the scar-forming phase and induced astrocytic scar formation via the integrin-N-cadherin pathway [74]. Collagen type-III is present in both pathological and healthy IVD [75]. In SCI collagen IV meshwork that acts as a binding matrix for a variety of ECM components and inhibitory compounds such as PGs and semaphorins, as well as growth-promoting proteins [76]. After SCI, the HMW form of GAGs- HA is degraded in rat spinal cord [77]. Degradation of natural HMW-HA, has been demonstrated to activate and proliferate astrocytes and contributes to glial scar [78].
EXTRA CELLULAR MATRIX REMODELING-BASED THERAPEUTIC APPROACHES FOR SPINAL CORD INJURY
ECM protein called Periostin is linked to scar formation through fibrosis, propagation, and inflammatory signaling [79,80]. Preventing ischemia, minimizing inflammation-related secondary harm, controlling the cytotoxic and immunological response, and supporting cellular regeneration are some therapies for SCI [81]. From day 4 to day 14 post-injury, daily i.p. injections of a monoclonal antibody of mouse against Periostin were demonstrated to minimize scarring and improve sensorimotor tasks in mice [80]. In a recent study, it was found that severity of SCI could be attenuated within the 14 days after the SCI in mice when treated with N-cadherin neutralizing antibody, which disrupts the interaction between type I collagen and astrocytes, which is integrin and N-cadherin dependent which showed the reduced astroglial scar formation [74]. Pharmacologically, the scar’s fibrotic components can also be addressed. Systemic injection of the antimitotic microtubule stabilizers taxol or epothilone B reduces scar-forming fibroblast migration and suppresses substantial scar development, allowing axon regeneration and functional recovery [82,83]. In one of the studies, iron chelators 2,2-bipyridine-5,5-dicarboxylic acid inhibits prolyl 4-hydroxylase (which inhibits, a crucial enzyme in collagen IV synthesis) and cyclic adenosine monophosphate to stop collagen formation [84,85], increases neuroprotection and long-distance axon regrowth while reducing fibrotic scarring [84].
The termination of chondroitin sulfate glycosaminoglycan (CS-CAGs) through the chondroitinase ABC (ChABC) enzyme was found to improve axonal regeneration and neuroplasticity, as well as promote functional improvement, after experimental spinal cord damage [86-90]. The thoracic and cervical region of the spinal cord when undergoing contusion injury, a gene therapy approach of enzyme administration in which host cells are transduced to express the ChABC gene leads to extensive CS-GAG breakdown, resulting in decreased pathology and increased functional improvement [91-93]. Furthermore, viral administration of ChABC leads to broad CSPG regulation, which promotes macrophage conversion to a pro-resolving M2 polarisation state [91] and anti-inflammatory IL-10 modulated response [94]. The enzyme Arylsulfatase B (ARSB, N-acetylgalatosamine-4-sulfatase), which terminates the C4S portion, particularly from CS-GAGs, is the other enzymatic method that is being used to reduce CSPG inhibition. A study conducted in mice of the spinal cord damaged by compression method when treated with ARSB treatment has recently been proven to induce increased axonal outgrowth and functional locomotor recovery [95].
Alteration of the CSPG receptor through modification of the receptor protein tyrosine phosphatase σ (PTPσ) is a potential therapeutic option. PTPσ’s intracellular phosphatase domains activity is controlled by a “wedge” structure that can obstruct the catalytic site, limiting phosphorylating action and signaling downregulation. The use of a membrane-permeable peptide mimicking this wedge inhibits PTPσ signaling when ligands like CSPGs activate it. In rats with spinal contusions, systemic infusion of these peptides was demonstrated to help them regain bladder and locomotor function [96]. Wang et al. [97] reported a novel hydrogel-liposome-hydrogel delivery system, SLIP@SF, in which DTX (docetaxel) and basic fibroblast growth factor (bFGF) are encapsulated. By mending the blood-spinal cord barrier, modulating the levels of inflammatory factors, decreasing the inhibitory CSPGs level, and affecting the bipolar architecture of the glia, bFGF decreases neuronal loss and cavity region by providing a favorable environment for axonal regeneration. By enhancing microtubule stabilization, DTX promoted intrinsic axonal development. Furthermore, bFGF reduced the amount of ECM that was removed too quickly.
In a recent report, a designed nanofiber hydrogel was combined with a prolonged release of growth factor cocktail to rebuild the ECM near the lesion after severe SCI. As established by immunochemistry, such an engineered milieu might modify local inflammatory reactions, eliciting substantial axon regrowth beyond the lesion site in a coordinated manner. As a result, locomotion and electrophysiological characteristics improved significantly [98]. In a study, gene transfection and implants were used in vitro and in vivo, where they showed that lipoplexes could fix on ECM-coated poly lactic-co-glycolic acid (PLGA), with fibronectin allowing for the most efficient gene transfer. Treatment of lipoplexes with fibronectin-coated PLGA resulted in significant expression levels in vitro, allowing lipoplex attachment to complicated geometry pre-fabricated scaffolds. Several channel bridges stabilized with lipoplexes were inserted in the spinal cord following injury in vivo, leading to higher transgenic expression levels than a bare plasmid. Transport of lipoplexes through spinal cord bridges after injury was shown to be an effective mechanism for DNA carrier, as a modest amount of DNA was enough to promote transgenic expression for 3 weeks (Fig. 2) [99].
Fig. 2.
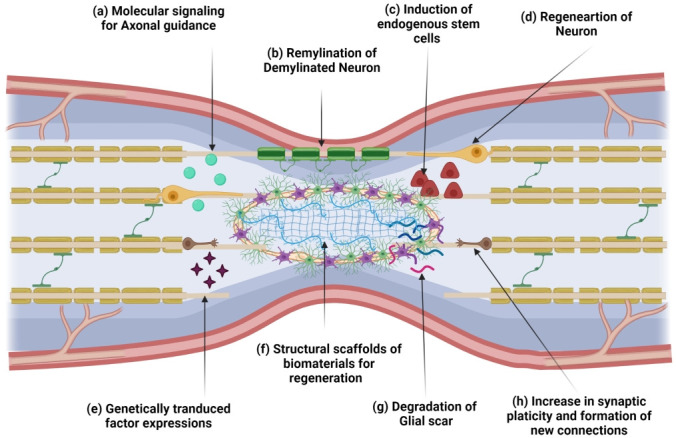
Schematic representation of different extra cellular matrix remodeling targets and applied approaches for therapeutics. (a) Axonal guidance, which stimulates axonal sprouting, requires molecular signaling. (b) The intrinsic remyelination could be promoted. (c) Stem cells from the central canal could be mobilized or induced for regeneration. (d) Regeneration of neurons with the help of different growth factors. (e) The genetically modified cells factor expression is one of the approaches to increase neuronal survival and migration. (f) Different biomaterials are used as a scaffold that maintains structural integrity and promotes regeneration. (g) Glial scar limits axonal regeneration which can be degraded by chondroitinase ABC. (h) The regeneration and plasticity of new neuronal connections could be an approach.
EXTRA CELLULAR MATRIX REMODELING-BASED THERAPEUTIC APPROACHES FOR DISC DEGENERATION
The disintegration of matrix proteins predominantly causes IVDD so potential therapy options should focus on ECM regeneration as well as cellular components [100]. Biomolecular remedies, cell-based treatments, and tissue engineering of a replacement disc have all been investigated as ways to repair and regenerate the IVD [101]. Growth factors stimulate matrix production and change the matrix balancing toward a pro-anabolic phase, which regulates disc cell metabolism. Increased mRNA expression of aggrecans, collagen I, II was detected in rabbit NP cells grown in atelocollagen and subjected to tissue growth factor-beta (TGF-β) and bone morphogenetic protein-2 (BMP2) [102]. Platelet-derived growth factor, bFGF, and insulin-like growth factor-1 are 3 effective mitogens that have been proven to amplify the proliferation of bovine NP cells [103]. Therefore, the supply of these growth factors at the proper diseased stage will be a potential therapy for IVDD.
Studies with direct biomolecule injection into the degenerative disc in vivo have yielded promising outcomes. Takegami et al. [104] found that rabbit IVD cells grown on alginate gel were treated with BMP-7 (also known as OP-1), a member of the TGF-growth factor superfamily, that showed an increase in PGs production. Synthetic peptides used to stimulate regeneration could be a highly cost-effective and safer biomolecular potential treatment. In vivo studies, Link N, a synthetic peptide with growth factor-like characteristics, has shown increasing aggrecan gene expression and downregulating protease expression [105]. The idea behind gene therapy for IVDD is that by specifically delivering genetic materials to recognised and understood components of the pathways causing IVD degeneration, it may be possible to enhance the anabolic and catabolic balance [106,107]. In recent work, Leckie et al. [108] found that injecting adeno-associated virus to disc cells induced them to release BMP-2 or TIMP-1 proteins, which slowed the progression of degenerative changes in a rabbit disc degeneration model. siRNA molecules could be used to quiet, unwanted protein molecules associated with IVD degeneration. A study by Seki et al. [109] mentioned that a single injection of ADAMTS-5 siRNA prevented degeneration and restored histological grades of NP cells in a rabbit disc degeneration model in an elegant in vivo study. Matrix degradation has been linked to TNF-α [58]. However, etanercept, a TNF-α antagonist, was discovered to be a promising therapy, resulting in considerable improvement in individuals with chronic pain related to disc [110].
Mesenchymal stem cells (MSCs) are considered to be the best option for disc repair because they can develop along a chondrogenic lineage and synthesize the PGs and collagens found in the ECM of the disc [111]. MSCs transplantation resulted in decreased height loss of disc and increased GAG content at 16 weeks in a rabbit degeneration model relative to controls, with no differences detected between MSCs and NP cell injection [112]. When MSCs and degenerated human NP cells were cocultured, the NP cells produced more PGs and collagen [113]. Hydrogel scaffolds have been employed extensively in the NP to preserve deposited PGs and facilitate the formation of osmotic pressure. Because of accessibility of manipulation and propensity to entrap released PGs, alginate [114,115] and agarose-based [116] hydrogels have been widely employed for NP cultivation. Hydrogels containing HA, a local NP ECM element, have also been employed to retain NP phenotype and improve disc biomechanics in vitro [117,118] and in vivo [119,120].
Current research is focused on improving the mechanical properties of scaffolds by introducing collagen molecules, which has resulted in enhanced compressive and tensile mechanical capabilities [121]. Scaffolds of collagen peptide nanofiber can inhibit the development of IVDD and improve tissue function by increasing GAGs and collagen accumulation [122]. Scaffold, such as polyglycolic acid and poly-DL-caprolactone and hydrogelbased natural polymers, such as collagen, fibronectin, HA, and synthetic polymers like poly-ethylene-glycol, the poly-ethylene oxide is being used [123]. LM111-hydrogels may help boost or sustain the expression of particular markers associated with phenotypic immature NP cells [124]. A potential biological therapy for initial stage IVDD involves encapsulating NP cells forming hydrogels in situ, and boosting the expression of numerous key ECM-related genes in NP cells, such as type I collagen, aggrecan, Sry-type high mobility group box transcription factor-9, and hypoxia-inducible factor-1 (Fig. 3) [125].
Fig. 3.
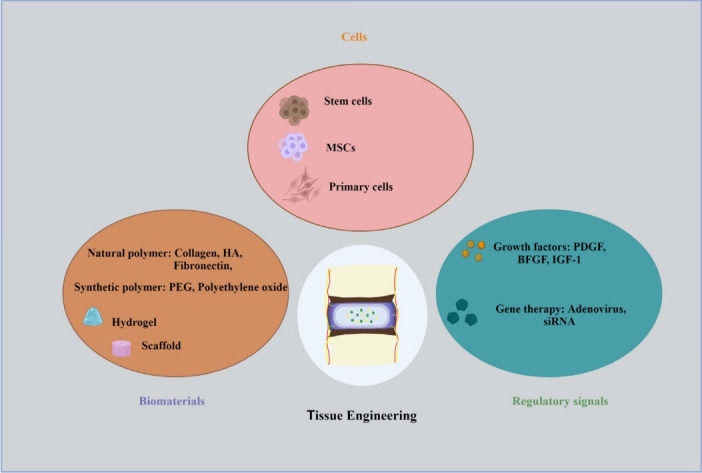
Therapeutic approaches for disc degeneration. Cells: Intervertebral disc (IVD) supplementation with reparative cells is essential due major role of cell loss in the degenerative changes. Prominently, Mesenchymal stem cells, stem cells and primary cells are considered as important source of regenerative approaches. Regulatory signals: The degenerative process can be stopped or slowed down by administering therapeutic molecules or proteins directly into the IVD and rebuilding its natural structure. Growth factors like, platelet-derived growth factor (PDGF), Insulin-like growth factor 1 (IGF-1), and basic fibroblast growth factor (BFGF) is widely used. The therapeutic approaches for regeneration of disc involve the gene transfer to localised cells inside the IVD. Biomaterials: It has been investigated to implant biomaterials to stimulate native disc cells, repair the degenerating disc structure, or even replace the complete disc. Hydrogels mimic the native extra cellular matrix and can be used as carriers for the delivery of drugs, proteins, and stem cells. Numerous natural and synthetic biomaterials have been thoroughly investigated for IVD regeneration. MSC, mesenchymal stem cell; HA, hyaluronic acid; PEG, polyethylene glycol.
CONCLUSION
The ECM is important throughout development and after a disease or injury. The ECM is involved directly in fundamental processes such as cell signaling, axon guidance, and synaptic plasticity, rather than simply providing a supportive environment. Manipulation of the ECM is a promising therapeutic method for recapitulating favorable developmental processes and/or minimizing negative remodeling following injury, either by targeting single ECM components or entire families of ECM molecules. The composition of the ECM can be deleterious to axonal regeneration, plasticity, and repair after CNS injury. Although the direct relationship between IVDD and SCI has not been established yet in humans, looking from the perspective of ECM remodeling, many ECM components share the same function in both diseases. In some of the studies, canine models of IVDD-induced SCI have shown that there is a structural and molecular interplay between IVD and SC. Correlative techniques for ECM remodeling in IVDD and SCI have not been investigated based on existing research data. Different approaches for treatment have been in use for the past few decades, but these therapeutic approaches do not provide the native microenvironment to IVD required for regeneration. We have discussed some of the regenerative methods as treatment and regeneration of the IVD using scaffolds, hydrogels, a combination of polymers. So, using ECM components as the therapeutic target would be a promising approach for regeneration.
Acknowledgments
NK and AK received the MS scholarship National Institute of Pharmaceutical Education and Research (NIPER) Ahmedabad and the Ministry of Chemicals and Fertilizers, Govt. of India. The paid version of Biorender.com was utilized to prepare the illustrations.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: HK; Data curation: ZP; Project administration: ZP, HK; Writing - original draft: NK, AK, ZP; Writing - review & editing: HK.
REFERENCES
- 1.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–10. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuzhalin AE, Lim SY, Kutikhin AG, et al. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim Biophys Acta Rev Cancer. 2018;1870:207–28. doi: 10.1016/j.bbcan.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Theocharis AD, Skandalis SS, Gialeli C, et al. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Carson DD. Extracellular matrix: forum introduction. Reprod Biol Endocrinol. 2004;2:1. doi: 10.1186/1477-7827-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraley SI, Wu PH, He L, et al. Three-dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Sci Rep. 2015;5:14580. doi: 10.1038/srep14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnaswamy VR, Benbenishty A, Blinder P, et al. Demystifying the extracellular matrix and its proteolytic remodeling in the brain: structural and functional insights. Cell Mol Life Sci. 2019;76:3229–48. doi: 10.1007/s00018-019-03182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumont RJ, Okonkwo DO, Verma S, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–64. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Loreto C, Musumeci G, Castorina A, et al. Degenerative disc disease of herniated intervertebral discs is associated with extracellular matrix remodeling, vimentin-positive cells and cell death. Ann Anat. 2011;193:156–62. doi: 10.1016/j.aanat.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira CP, Rodrigues LM, Fregni MV, et al. Extracellular matrix remodeling in experimental intervertebral disc degeneration. Acta Ortop Bras. 2013;21:144–9. doi: 10.1590/S1413-78522013000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varma AK, Das A, Wallace G, 4th, et al. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res. 2013;38:895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haggerty AE, Marlow MM, Oudega M. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci Lett. 2017;652:50–5. doi: 10.1016/j.neulet.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Lau LW, Cua R, Keough MB, et al. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci. 2013;14:722–9. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 13.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–7. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Brückner G, Brauer K, Härtig W, et al. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 15.Celio MR, Spreafico R, De Biasi S, et al. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–5. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- 16.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 17.Haddock G, Cross AK, Allan S, et al. Brevican and phosphacan expression and localization following transient middle cerebral artery occlusion in the rat. Biochem Soc Trans. 2007;35:692–4. doi: 10.1042/BST0350692. [DOI] [PubMed] [Google Scholar]
- 18.Goh FG, Piccinini AM, Krausgruber T, et al. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol. 2010;184:2655–62. doi: 10.4049/jimmunol.0903359. [DOI] [PubMed] [Google Scholar]
- 19.Sumioka T, Kitano A, Flanders KC, et al. Impaired cornea wound healing in a tenascin C-deficient mouse model. Lab Invest. 2013;93:207–17. doi: 10.1038/labinvest.2012.157. [DOI] [PubMed] [Google Scholar]
- 20.Xie K, Liu Y, Hao W, et al. Tenascin-C deficiency ameliorates Alzheimer’s disease-related pathology in mice. Neurobiol Aging. 2013;34:2389–98. doi: 10.1016/j.neurobiolaging.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Daly C, Ghosh P, Jenkin G, et al. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016;2016:5952165. doi: 10.1155/2016/5952165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 23.Kamali A, Ziadlou R, Lang G, et al. Small molecule-based treatment approaches for intervertebral disc degeneration: current options and future directions. Theranostics. 2021;11:27–47. doi: 10.7150/thno.48987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyu FJ, Cui H, Pan H, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9:7. doi: 10.1038/s41413-020-00125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461–8. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyre DR, Matsui Y, Wu JJ. Collagen polymorphisms of the intervertebral disc. Biochem Soc Trans. 2002;30:844–8. doi: 10.1042/bst0300844. [DOI] [PubMed] [Google Scholar]
- 27.Sharabi M, Wade K, Haj-Ali R. In: Biomechanics of the spine: basic concepts, spinal disorders and treatments. Galbusera F, Wilke HJ, editors. London: Academic Press; 2018. The mechanical role of collagen fibers in the intervertebral disc; pp. 105–23. [Google Scholar]
- 28.Preston M, Sherman LS. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 2011;3:1165–79. doi: 10.2741/218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 30.Ohnishi T, Novais EJ, Risbud MV. Alterations in ECM signature underscore multiple sub-phenotypes of intervertebral disc degeneration. Matrix Biol Plus. 2020;6-7:100036. doi: 10.1016/j.mbplus.2020.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt G, Hausser H, Kresse H. Interaction of the small proteoglycan decorin with fibronectin. Involvement of the sequence NKISK of the core protein. Biochem J. 1991;280:411–4. doi: 10.1042/bj2800411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velling T, Risteli J, Wennerberg K, et al. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–81. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 33.Xia M, Zhu Y. Expression of integrin subunits in the herniated intervertebral disc. Connect Tissue Res. 2008;49:464–9. doi: 10.1080/03008200802325425. [DOI] [PubMed] [Google Scholar]
- 34.Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–53. doi: 10.1146/annurev-cellbio-101011-155750. [DOI] [PubMed] [Google Scholar]
- 35.Gaudet AD, Popovich PG. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp Neurol. 2014;258:24–34. doi: 10.1016/j.expneurol.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–70. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Guth L. Experimental spinal cord injury: Wallerian degeneration in the dorsal column is followed by revascularization, glial proliferation, and nerve regeneration. Exp Neurol. 1997;147:159–71. doi: 10.1006/exnr.1997.6590. [DOI] [PubMed] [Google Scholar]
- 38.Ahuja CS, Fehlings M. Concise Review: Bridging the Gap: Novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl Med. 2016;5:914–24. doi: 10.5966/sctm.2015-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCaughey EJ, Borotkanics RJ, Gollee H, et al. Abdominal functional electrical stimulation to improve respiratory function after spinal cord injury: a systematic review and metaanalysis. Spinal Cord. 2016;54:754. doi: 10.1038/sc.2016.70. [DOI] [PubMed] [Google Scholar]
- 40.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 41.Berg G, Barchuk M, Miksztowicz V. Behavior of metalloproteinases in adipose tissue, liver and arterial wall: an update of extracellular matrix remodeling. Cells. 2019;8:158. doi: 10.3390/cells8020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frangogiannis NG. The extracellular matrix in ischemic and nonischemic heart failure. Circ Res. 2019;125:117–46. doi: 10.1161/CIRCRESAHA.119.311148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iatridis JC, MacLean JJ, Roughley PJ, et al. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg Am. 2006;88 Suppl 2:41–6. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–78. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 48.Roycik MD, Fang X, Sang QX. A fresh prospect of extracellular matrix hydrolytic enzymes and their substrates. Curr Pharm Des. 2009;15:1295–308. doi: 10.2174/138161209787846676. [DOI] [PubMed] [Google Scholar]
- 49.Vo NV, Hartman RA, Yurube T, et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331–41. doi: 10.1016/j.spinee.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Handa T, Ishihara H, Ohshima H, et al. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine (Phila Pa 1976) 1997;22:1085–91. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 52.Hutton WC, Elmer WA, Boden SD, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine (Phila Pa 1976) 1999;24:1507–15. doi: 10.1097/00007632-199908010-00002. [DOI] [PubMed] [Google Scholar]
- 53.Hsieh AH, Lotz JC. Prolonged spinal loading induces matrix metalloproteinase-2 activation in intervertebral discs. Spine (Phila Pa 1976) 2003;28:1781–8. doi: 10.1097/01.BRS.0000083282.82244.F3. [DOI] [PubMed] [Google Scholar]
- 54.Iatridis J, Risinger R, Monsey R, et al. Effects of static compression on biochemical composition of caudal discs in vivo. Vancouver (Canada): Trans International Soc Lumbar Spine (ISSLS); 2003. p. 101. [Google Scholar]
- 55.Maclean JJ, Lee CR, Alini M, et al. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 56.MacLean JJ, Lee CR, Alini M, et al. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–7. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Ashinsky BG, Bonnevie ED, Mandalapu SA, et al. Intervertebral disc degeneration is associated with aberrant endplate remodeling and reduced small molecule transport. J Bone Miner Res. 2020;35:1572–81. doi: 10.1002/jbmr.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Séguin CA, Pilliar RM, Madri JA, et al. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 2008;33:356–65. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- 59.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 60.Salo J, Mackiewicz Z, Indahl A, et al. Plasmin-matrix metalloproteinase cascades in spinal response to an experimental disc lesion in pig. Spine (Phila Pa 1976) 2008;33:839–44. doi: 10.1097/BRS.0b013e31816b1f1d. [DOI] [PubMed] [Google Scholar]
- 61.Crean JK, Roberts S, Jaffray DC, et al. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine (Phila Pa 1976) 1997;22:2877–84. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 62.Mayer SA. Intracerebral hemorrhage: natural history and rationale of ultra-early hemostatic therapy. Intensive Care Med. 2002;28 Suppl 2:S235–40. doi: 10.1007/s00134-002-1470-8. [DOI] [PubMed] [Google Scholar]
- 63.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues LM, Theodoro TR, Matos LL, et al. Heparanase isoform expression and extracellular matrix remodeling in intervertebral disc degenerative disease. Clinics (Sao Paulo) 2011;66:903–9. doi: 10.1590/S1807-59322011000500030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruber HE, Ingram JA, Hoelscher GL, et al. Constitutive expression of cathepsin K in the human intervertebral disc: new insight into disc extracellular matrix remodeling via cathepsin K and receptor activator of nuclear factor-κB ligand. Arthritis Res Ther. 2011;13:R140. doi: 10.1186/ar3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fluehmann G, Doherr MG, Jaggy A. Canine neurological diseases in a referral hospital population between 1989 and 2000 in Switzerland. J Small Anim Pract. 2006;47:582–7. doi: 10.1111/j.1748-5827.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 67.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 68.Kaki AM, El-Yaski AZ, Youseif E. Identifying neuropathic pain among patients with chronic low-back pain: use of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale. Reg Anesth Pain Med. 2005;30:422–8. doi: 10.1016/j.rapm.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 69.Luoma K, Vehmas T, Kerttula L, et al. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur Spine J. 2016;25:2873–81. doi: 10.1007/s00586-016-4715-x. [DOI] [PubMed] [Google Scholar]
- 70.Noble LJ, Donovan F, Igarashi T, et al. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–35. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagano S, Kim SH, Tokunaga S, et al. Matrix metalloprotease-9 activity in the cerebrospinal fluid and spinal injury severity in dogs with intervertebral disc herniation. Res Vet Sci. 2011;91:482–5. doi: 10.1016/j.rvsc.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Patel KP, Sandy JD, Akeda K, et al. Aggrecanases and aggrecanase-generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine (Phila Pa 1976) 2007;32:2596–603. doi: 10.1097/BRS.0b013e318158cb85. [DOI] [PubMed] [Google Scholar]
- 73.Tauchi R, Imagama S, Natori T, et al. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation. 2012;9:53. doi: 10.1186/1742-2094-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hara M, Kobayakawa K, Ohkawa Y, et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med. 2017;23:818–28. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 75.Wu JJ, Eyre DR, Slayter HS. Type VI collagen of the intervertebral disc. Biochemical and electron-microscopic characterization of the native protein. Biochem J. 1987;248:373–81. doi: 10.1042/bj2480373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klapka N, Müller HW. Collagen matrix in spinal cord injury. J Neurotrauma. 2006;23:422–35. doi: 10.1089/neu.2006.23.422. [DOI] [PubMed] [Google Scholar]
- 77.Struve J, Maher PC, Li YQ, et al. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia. 2005;52:16–24. doi: 10.1002/glia.20215. [DOI] [PubMed] [Google Scholar]
- 78.Khaing ZZ, Milman BD, Vanscoy JE, et al. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng. 2011;8:046033. doi: 10.1088/1741-2560/8/4/046033. [DOI] [PubMed] [Google Scholar]
- 79.Didangelos A, Puglia M, Iberl M, et al. High-throughput proteomics reveal alarmins as amplifiers of tissue pathology and inflammation after spinal cord injury. Sci Rep. 2016;6:21607. doi: 10.1038/srep21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yokota K, Kobayakawa K, Saito T, et al. Periostin promotes scar formation through the interaction between pericytes and infiltrating monocytes/macrophages after spinal cord injury. Am J Pathol. 2017;187:639–53. doi: 10.1016/j.ajpath.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 81.Gadot R, Smith DN, Prablek M, et al. Established and emerging therapies in acute spinal cord injury. Neurospine. 2022;19:283–96. doi: 10.14245/ns.2244176.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hellal F, Hurtado A, Ruschel J, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–31. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruschel J, Hellal F, Flynn KC, et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–52. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klapka N, Hermanns S, Straten G, et al. Suppression of fibrous scarring in spinal cord injury of rat promotes longdistance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci. 2005;22:3047–58. doi: 10.1111/j.1460-9568.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- 85.Brazda N, Müller HW. Pharmacological modification of the extracellular matrix to promote regeneration of the injured brain and spinal cord. Prog Brain Res. 2009;175:269–81. doi: 10.1016/S0079-6123(09)17518-0. [DOI] [PubMed] [Google Scholar]
- 86.Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–40. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 87.García-Alías G, Barkhuysen S, Buckle M, et al. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–51. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 88.Alilain WJ, Horn KP, Hu H, et al. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cafferty WB, Yang SH, Duffy PJ, et al. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–85. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bowes C, Massey JM, Burish M, et al. Chondroitinase ABC promotes selective reactivation of somatosensory cortex in squirrel monkeys after a cervical dorsal column lesion. Proc Natl Acad Sci U S A. 2012;109:2595–600. doi: 10.1073/pnas.1121604109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bartus K, James ND, Didangelos A, et al. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34:4822–36. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.James ND, Shea J, Muir EM, et al. Chondroitinase gene therapy improves upper limb function following cervical contusion injury. Exp Neurol. 2015;271:131–5. doi: 10.1016/j.expneurol.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burnside ER, De Winter F, Didangelos A, et al. Immuneevasive gene switch enables regulated delivery of chondroitinase after spinal cord injury. Brain. 2018;141:2362–81. doi: 10.1093/brain/awy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Didangelos A, Iberl M, Vinsland E, et al. Regulation of IL10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J Neurosci. 2014;34:16424–32. doi: 10.1523/JNEUROSCI.2927-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoo M, Khaled M, Gibbs KM, et al. Arylsulfatase B improves locomotor function after mouse spinal cord injury. PLoS One. 2013;8:e57415. doi: 10.1371/journal.pone.0057415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lang BT, Cregg JM, DePaul MA, et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015;518:404–8. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Q, Dong X, Zhang H, et al. A novel hydrogel-based combination therapy for effective neuroregeneration after spinal cord injury. Chem Eng J. 2021;415:128964. [Google Scholar]
- 98.Liu H, Xu X, Tu Y, et al. Engineering microenvironment for endogenous neural regeneration after spinal cord injury by reassembling extracellular matrix. ACS Appl Mater Interfaces. 2020;12:17207–19. doi: 10.1021/acsami.9b19638. [DOI] [PubMed] [Google Scholar]
- 99.De Laporte L, Yan AL, Shea LD. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009;30:2361–8. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54–67. doi: 10.1016/j.biomaterials.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maidhof R, Alipui DO, Rafiuddin A, et al. Emerging trends in biological therapy for intervertebral disc degeneration. Discov Med. 2012;14:401–11. [PubMed] [Google Scholar]
- 102.Lee KI, Moon SH, Kim H, et al. Tissue engineering of the intervertebral disc with cultured nucleus pulposus cells using atelocollagen scaffold and growth factors. Spine (Phila Pa 1976) 2012;37:452–8. doi: 10.1097/BRS.0b013e31823c8603. [DOI] [PubMed] [Google Scholar]
- 103.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007;16:1858–66. doi: 10.1007/s00586-007-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takegami K, An HS, Kumano F, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–8. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Mwale F, Masuda K, Pichika R, et al. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R120. doi: 10.1186/ar3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han IB. Moving forward: gene therapy for intervertebral disc degeneration. Neurospine. 2020;17:17–8. doi: 10.14245/ns.2040108.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takeoka Y, Yurube T, Nishida K. Gene therapy approach for intervertebral disc degeneration: an update. Neurospine. 2020;17:3–14. doi: 10.14245/ns.2040042.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leckie SK, Bechara BP, Hartman RA, et al. Injection of AAV2- BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12:7–20. doi: 10.1016/j.spinee.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seki S, Asanuma-Abe Y, Masuda K, et al. Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Res Ther. 2009;11:R166. doi: 10.1186/ar2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tobinick E, Davoodifar S. Efficacy of etanercept delivered by perispinal administration for chronic back and/or neck disc-related pain: a study of clinical observations in 143 patients. Curr Med Res Opin. 2004;20:1075–85. doi: 10.1185/030079903125004286. [DOI] [PubMed] [Google Scholar]
- 111.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 112.Feng G, Zhao X, Liu H, et al. Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: a comparison of 2 cell types as potential candidates for disc regeneration. J Neurosurg Spine. 2011;14:322–9. doi: 10.3171/2010.11.SPINE10285. [DOI] [PubMed] [Google Scholar]
- 113.Svanvik T, Henriksson HB, Karlsson C, et al. Human disk cells from degenerated disks and mesenchymal stem cells in co-culture result in increased matrix production. Cells Tissues Organs. 2010;191:2–11. doi: 10.1159/000223236. [DOI] [PubMed] [Google Scholar]
- 114.Abbott RD, Purmessur D, Monsey RD, et al. Regenerative potential of TGFβ3+Dex and notochordal cell conditioned media on degenerated human intervertebral disc cells. J Orthop Res. 2012;30:482–8. doi: 10.1002/jor.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gantenbein-Ritter B, Chan SC. The evolutionary importance of cell ratio between notochordal and nucleus pulposus cells: an experimental 3-D co-culture study. Eur Spine J. 2012;21 Suppl 6:S819–25. doi: 10.1007/s00586-011-2026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith LJ, Chiaro JA, Nerurkar NL, et al. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following longterm agarose culture. Eur Cell Mater. 2011;22:291–301. doi: 10.22203/ecm.v022a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park SH, Cho H, Gil ES, et al. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng Part A. 2011;17:2999–3009. doi: 10.1089/ten.tea.2010.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peroglio M, Grad S, Mortisen D, et al. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J. 2012;21 Suppl 6:S839–49. doi: 10.1007/s00586-011-1976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Collin EC, Grad S, Zeugolis DI, et al. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862–70. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 120.Malhotra NR, Han WM, Beckstein J, et al. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine (Phila Pa 1976) 2012;37:E1099–105. doi: 10.1097/BRS.0b013e31825cdfb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Calderon L, Collin E, Velasco-Bayon D, et al. Type II collagen-hyaluronan hydrogel--a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134–48. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 122.Uysal O, Arslan E, Gulseren G, et al. Collagen peptide presenting nanofibrous scaffold for intervertebral disc regeneration. ACS Appl Bio Mater. 2019;2:1686–95. doi: 10.1021/acsabm.9b00062. [DOI] [PubMed] [Google Scholar]
- 123.Siebert JR, Eade AM, Osterhout DJ. Biomaterial approaches to enhancing neurorestoration after spinal cord injury: strategies for overcoming inherent biological obstacles. Biomed Res Int. 2015;2015:752572. doi: 10.1155/2015/752572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Francisco AT, Hwang PY, Jeong CG, et al. Photocrosslinkable laminin-functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta Biomater. 2014;10:1102–11. doi: 10.1016/j.actbio.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen YC, Su WY, Yang SH, et al. In situ forming hydrogels composed of oxidized high molecular weight hyaluronic acid and gelatin for nucleus pulposus regeneration. Acta Biomater. 2013;9:5181–93. doi: 10.1016/j.actbio.2012.09.039. [DOI] [PubMed] [Google Scholar]