Abstract
Objective
To study the relationship between coronavirus disease 2019 (COVID‐19) mortality and headache among patients evaluated for COVID‐19 in Emergency Departments and hospitals.
Background
COVID‐19 has disparate impacts on those who contract it. Headache, a COVID‐19 symptom, has been associated with positive disease prognosis. We sought to determine whether headache is associated with relative risk of COVID‐19 survival.
Methods
A systematic search in PubMed was performed independently by three reviewers to identify all COVID‐19 clinical inpatient series in accordance with the PRISMA guideline. Studies were included if the study design, COVID‐19 confirmation method, disease survival ratio, and presence of headache symptom were accessible. We included 48 cohort studies with a total of 43,169 inpatients with COVID‐19: 81.4% survived (35,132/43,169) versus 18.6% non‐survived (8037/43,169). A meta‐analysis of the included studies was then performed. The study was registered on PROSPERO (ID: CRD42021260151).
Results
When considering headache as a symptom of COVID‐19, we observed a significantly higher survival rate (risk ratio: 1.90 [1.46, 2.47], p < 0.0001) among COVID‐19 inpatients with headache compared to those without headache.
Conclusion
Headache among patients with COVID‐19 presenting to hospitals may be a marker of host processes which enhance COVID‐19 survival. Future studies should further confirm these findings, in order to better understand this relation and to try to address possible limitations related to the inclusion of more severe patients who would be unable to report symptoms (e.g., patients who were intubated).
Keywords: coronavirus disease 2019, headache, meta‐analysis, mortality
Abbreviations
- CGRP
calcitonin gene‐related peptide
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- IL‐6
interleukin 6
- NOS
Newcastle–Ottawa Scale
- RR
risk ratio
- SARS‐CoV‐2
severe‐acute‐respiratory syndrome coronavirus‐2
INTRODUCTION
Some patient clinical traits have been identified as associated with increased coronavirus disease 2019 (COVID‐19) mortality, including advanced age, male sex, deprivation, and chronic diseases. 1 Moreover, multiple human genomic variants have been linked to COVID‐19 outcomes. 2 , 3 , 4 COVID‐19 traits associated with a positive disease prognosis have received less attention from investigators. One such trait may be headache.
We previously reported a prospective, consecutive cohort study of 130 patients admitted for COVID‐19 to a Spanish hospital, and found that inpatients reporting headache on admission had COVID‐19 symptoms for one less week than inpatients not reporting headache. 5
We hypothesized that headache, as a COVID‐19 symptom, is a putative marker of favorable COVID‐19 clinical outcomes, specifically mortality. We now report a meta‐analysis of 48 inpatient studies which included the presence or absence of headache as a COVID‐19 symptom and reported COVID‐19 mortality rates.
METHODS
This study was registered on PROSPERO 6 (ID: CRD42021260151) and followed the PRISMA reporting guidelines. 7 The Population, Intervention/exposure, Comparator, Outcome, and Study design (PICOS) questions were then chosen as follows: inpatients with COVID‐19 as Population; presence of headache as a COVID‐19 accompanying symptom as Intervention/exposure; absence of headache as a COVID‐19 accompanying symptom as Comparator; COVID‐19 survival as Outcome; and cohort studies as Study design.
Meta‐analysis search strategy
We conducted a systematic literature search of PubMed (April 1, 2020 to December 22, 2020) to identify all COVID‐19 clinical inpatient studies. We also included six studies published between December 2019 and March 2020 from a previous meta‐analysis. 8 There were no restrictions on study design, language, or laboratory confirmation of COVID‐19 diagnosis. Studies were included if they clearly presented in their results or in their supplementary material: (1) study design; (2) COVID‐19 confirmation method; (3) patients' demographics; (4) ratio of COVID‐19 survivors and non‐survivors; and (5) the presence of headache as a symptom of the infection in both cohorts (See Table S1 in the Supporting Information). We excluded review articles, opinion articles, case reports, preprint server articles, and studies performed either on populations <18 years old or animal models. The full references for the 48 studies included in the meta‐analysis are listed as “Meta‐Analysis References” in the Supporting Information.
Study selection and data extraction
Three investigators (V.J.G., E.C., and P.P.‐R.) examined all titles and abstracts and obtained full texts of potentially relevant papers. Data extraction was done by V.J.G. For all eligible studies, we extracted information on study country, study size, COVID‐19 confirmation (positive PCR), patients' characteristics including demographics, presence of headache among COVID‐19 accompanying symptoms (which included mainly fever, cough, dyspnea, myalgia, diarrhea, nausea or vomiting, and anosmia, along with headache), and comorbidities (cardiovascular diseases, chronic kidney diseases, chronic liver diseases, chronic respiratory diseases, and diabetes). Both COVID‐19 accompanying symptoms and comorbidities were determined through medical examination during each patient's admission.
Risk of bias assessment
Two authors (V.J.G. and E.C.) independently assessed risk of bias using the Newcastle–Ottawa Scale (NOS) to evaluate the quality of studies included in the meta‐analysis. 9 Three factors were considered for the final score: (1) selection (representativeness of the exposed cohort, selection of the non‐exposed cohort, ascertainment of exposure, and demonstration that at the beginning of the study the outcome of interest was not present); (2) comparability (study design, management of missing data, analyses performed, presence of confounding variables adjusted for age or gender); and (3) outcome (how adequately the outcome had been assessed and followed up). In the final score, we also evaluated whether all patients had a confirmed diagnosis by a COVID‐19 PCR test. We rated the quality of the studies in each domain following the guidelines of the NOS (global rating ranging from 0 to 9 stars).
Data analysis
Random‐effects pooling models were computed in order to estimate the effect size of the following binary outcome data: presence of headache in survived versus non‐survived COVID‐19 cohorts. 10 Pooled headache prevalence and 95% confidence intervals (CIs) were presented from selected publications. Risk ratio (RR) with 95% CIs were used to estimate the risk of experiencing headache in both COVID‐19 cohorts: surviving versus non‐surviving inpatients. 11 RR was computed using the Mantel–Haenszel method. 12 Headache prevalence and RR from each publication were reported using forest plots. Headache RR was also analyzed in different COVID‐19 subgroups using moderator analysis in order to study if some covariables (gender and age) had a significant effect on the observed effect size 13 and adjusted RR was computed through meta‐regression random‐effects models. Between‐study heterogeneity was assessed using the I 2 statistic and Cochran's Q‐test for statistical significance. 14 Outlier publications were discarded in the sensitivity analysis in order to check the robustness of our results. We repeated the same analysis for data that were collected on the other COVID‐19 symptoms and patients' comorbidities, although not all publications recorded the same COVID‐19 symptomatology or patients' comorbidities. Hence, we analyzed their pooled prevalence and RR in publications where headache was reported.
In case of higher heterogeneity (I 2 > 75%) in the RR analysis, if the publication's CI did not overlap with the CI of the pooled effect, we considered these studies as outliers. Influence analyses of effect size between publications were also computed in order to assess whether the influence of a particular publication distorted the overall pooled effect. Other strategies considered in the sensitivity analysis were excluding small studies (n < 250), excluding studies lacking validated COVID‐19 confirmation methods, and considering only prospective studies. Finally, publication bias was assessed through visual inspection (funnel plot) and significance test (Egger's test). All of the statistical analysis and plots were generated using metaprop (version 2.4‐0), and the meta (version 4.15‐1) and dmetar (version 0.0.9) packages of R (version 4.0.3) software.
RESULTS
The meta‐analysis included a total of 48 full‐text peer‐reviewed publications of COVID‐19 inpatient mortality studies that also reported headache as a COVID‐19 symptom (Figure 1 and Table S2). Table S3 provides quality scores for the studies, assessing risk of bias (NOS).
FIGURE 1.
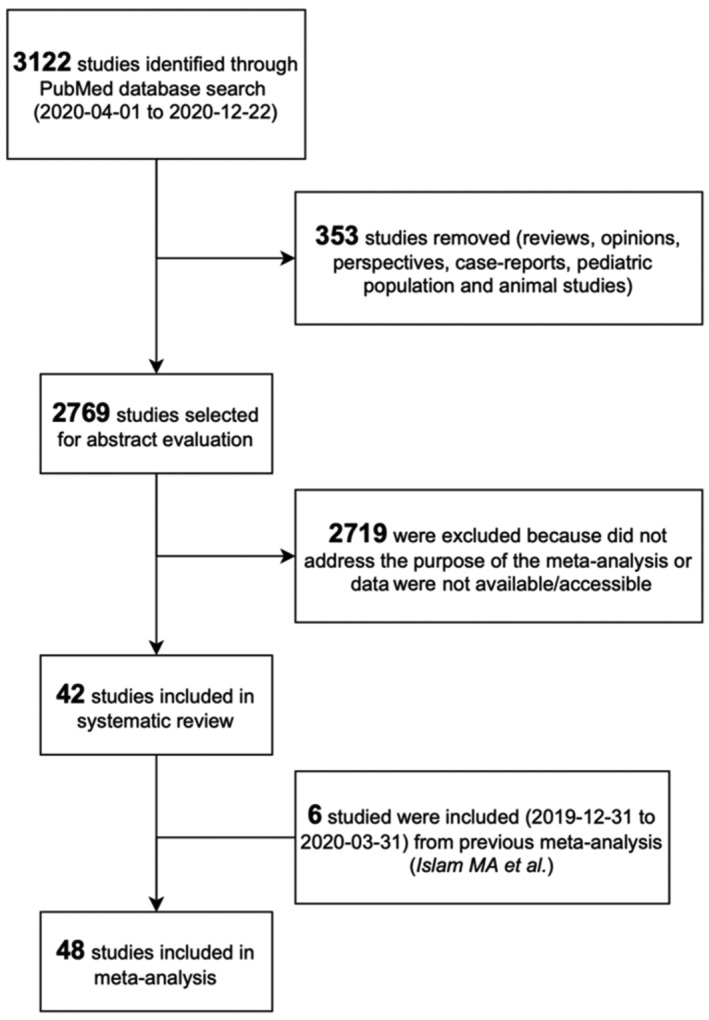
The PRISMA flow diagram of the meta‐analysis. We excluded 2719 studies after abstract evaluation for any of the following reasons: (1) original article was not accessible; (2) main outcome of the study was disease severity but not mortality; (3) total proportions of survivors/non‐survivors among hospitalized patients were not available; or (4) the number of patients with headache symptom was not reported adequately in any group (survivors or non‐survivors).
Although there was statistically significant heterogeneity between studies, the overall pooled prevalence of headache as a symptom among inpatients with COVID‐19 was 10.4% [8.3%, 12.9%] (Figure 2A). Removing outlier studies for a sensitivity analysis, the estimated pooled prevalence of headache was 9.7% [7.8%, 12.0%] (Table S4).
FIGURE 2.
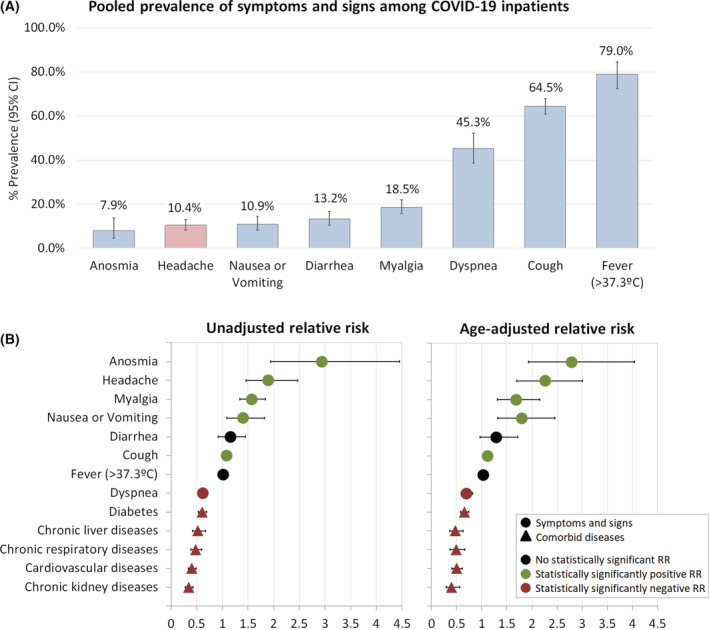
(A) Pooled prevalence of symptoms and signs among inpatients with COVID‐19. (B) Relative risk of survival for inpatients with COVID‐19 relative to their symptoms, signs and comorbid diseases. CI, confidence interval; RR, relative risk factors.
Regarding the risk of headache relative to mortality, we observed that the presence of headache symptoms was statistically significantly associated with inpatients with COVID‐19 who survived, compared to those who did not (unadjusted RR: 1.90 [1.46, 2.47], I 2 = 80.0%, p < 0.0001; age‐adjusted RR: 2.28 [1.78, 2.92], I 2 = 52.8%, p < 0.0001) (Figure 3). Moreover, risk of headache did not exhibit statistically significant publication bias following visual inspection and Egger's test (Figure S1). Notably, some other COVID‐19 symptoms were also associated with higher survival rates, including anosmia (RR: 2.94 [1.94, 4.45], I 2 = 84.5%), myalgia (RR: 1.57 [1.34, 1.83], I 2 = 48.8%), and nausea or vomiting (RR: 1.41 [1.08, 1.82], I 2 = 57.2%), whereas dyspnea and all comorbid diseases studied were associated with COVID‐19 non‐survival (Figure 2B, Tables S5 and S6). Further, we performed sensitivity analyses of headache RR, and consistently observed higher RR of headache among inpatients with COVID‐19 who survived. Excluding studies with lower quality (NOS score <7), headache RR increased without a statistically significant heterogeneity between studies (RR: 2.60 [2.03, 3.32], I 2 = 23.6%, p < 0.0001). In addition, we collected for each study, if available, the median days from the appearance of the first COVID‐19 symptom to hospital admission and we stratified patients into two categories: <1 week and ≥1 week. We observed that earlier hospital admission in patients with headache was statistically significantly associated with an even higher probability of better survival (RR: 2.98 [2.35, 3.78], I 2 = 0.0%, p = 0.010) (Table 1). Finally, we also collected for each study the timing of the medical evaluation, the setting of the medical evaluation, and data missingness in order to control for potential bias from the original studies (Table S2). Nevertheless, the presence of headache symptoms remained statistically significantly associated with patients’ survival rate (Table 1).
FIGURE 3.
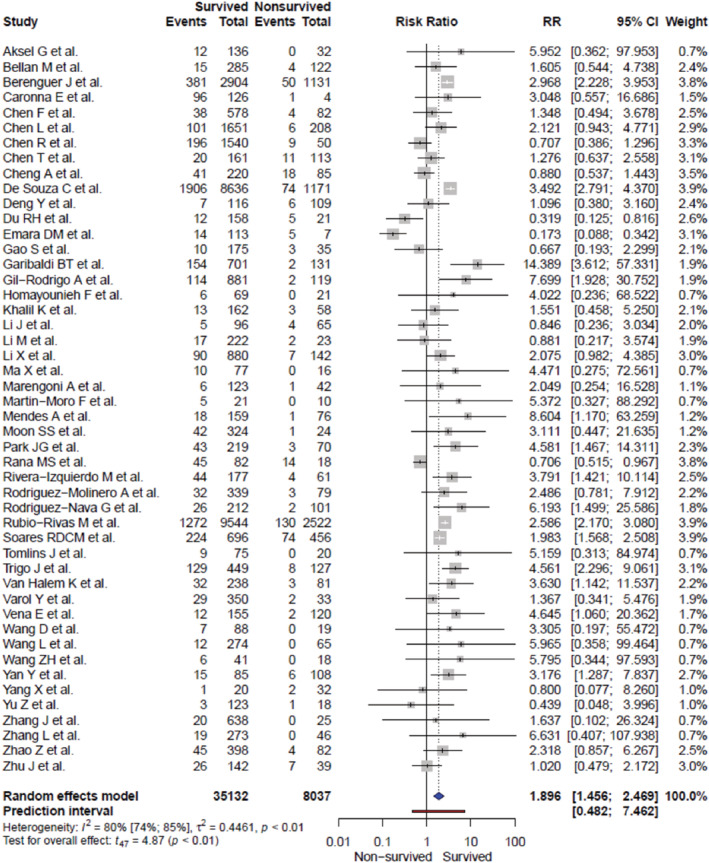
Risk of COVID‐19 inpatient survival associated with the presence of headache symptom. CI, confidence interval; RR, relative risk factors.
TABLE 1.
Sensitivity analysis of headache relative risk (RR) among inpatients with COVID‐19
| Strategy | Test for overall effect (random‐model) | Headache‐COVID19 RR [95% CI] | Between‐study heterogeneity | Number of studies included | Publication bias, Egger's test (p value) | |
|---|---|---|---|---|---|---|
| I 2 (%) | p value | |||||
| Including all studies | t = 5.18; p < 0.001 | 1.90 [1.46, 2.47] | 80.3 | <0.001 | 48/48 | 0.605 |
| Excluding outliers and over‐influencer studies a | t = 10.75; p < 0.001 | 2.18 [1.88, 2.52] | 16.8 | 0.175 | 41/48 | 0.733 |
| Excluding retrospective studies | t = 3.55; p = 0.012 | 3.38 [1.46, 7.83] | 53.5 | 0.050 | 7/48 | 0.280 |
| Excluding studies without COVID‐19 confirmation (RT‐PCR) | t = 5.18; p < 0.001 | 1.92 [1.49, 2.47] | 76.0 | <0.001 | 38/48 | 0.457 |
|
Excluding small studies (n < 250 patients) |
t = 7.26; p < 0.001 | 2.25 [1.79, 2.83] | 65.1 | <0.001 | 26/48 | 0.974 |
|
Excluding studies with lower quality (NOS score ≥7) |
t = 8.24; p < 0.001 | 2.60 [2.03, 3.32] | 23.6 | 0.180 | 21/48 | 0.829 |
| Excluding studies without information about data collection timing (only during admission) | t = 3.38; p = 0.002 | 1.80 [1.26, 2.56] | 82.6 | <0.001 | 30/48 | 0.739 |
| Excluding studies with missing cases | t = 4.53; p < 0.001 | 1.98 [1.46, 2.68] | 81.1 | <0.001 | 40/48 | 0.716 |
| Sex predominance | ||||||
| Female |
Q (1) = 0.43; p = 0.513 |
2.18 [1.32, 3.59] | 86.2 | <0.001 | 15/48 | |
| Male | 1.81 [1.30, 2.52] | 75.2 | 33/48 | |||
| Median age ≥60 years old | ||||||
| N |
Q (1) = 3.63; p = 0.047 |
1.31 [0.74, 2.30] | 91.3 | <0.001 | 16/48 | |
| Y | 2.28 [1.78, 2.92] | 52.8 | 32/48 | |||
| Medical evaluation setting | ||||||
| ER |
Q (1) = 6.49; p = 0.010 |
2.26 [1.64, 3.12] | 54.4 | <0.001 | 21/48 | |
| ICU | 1.87 [1.40, 1.92] | 74.6 | 9/48 | |||
| Median days from onset to admission | ||||||
| <1 week |
Q (1) = 6.49; p = 0.010 |
2.98 [2.35, 3.78] | 0.0 | 0.019 | 13/48 | |
| ≥1 week | 1.72 [1.09, 2.69] | 61.7 | 15/48 | |||
Note: In bold, p values <0.05.
Abbreviations: CI, confidence interval; NOS, Newcastle–Ottawa Scale.
Studies with extreme effect sizes (outliers) and studies with higher influence on overall effect (Leave‐One‐Out influence analysis) were discarded in order to obtain a homogenized between‐studies effect.
DISCUSSION
In light of our prior observations that the symptom of headache was associated with reduced length of COVID‐19 disease, 5 we performed a meta‐analysis of 48 published COVID‐19 inpatient mortality studies which captured headache as a symptom. This analysis indicates that inpatients who experience headache in the setting of the severe‐acute‐respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection are approximately twice as likely to survive, compared to those without headache. Further, headache during COVID‐19 is associated with anosmia and gastrointestinal symptoms, 15 and our meta‐analysis found that anosmia and nausea or vomiting also confer increased relative risk of COVID‐19 survival. Headache in the setting of COVID‐19 may be a marker of host defensive responses that enhance survival.
Headache is a common concomitant symptom of systemic infections, particularly viral infections. 16 COVID‐19 is no exception, as recognized by the US Centers for Disease Control and Prevention at the outset of the pandemic. 17 Multiple pathophysiological mechanisms might mediate these effects. The COVID‐19 cytokine storm is associated with pulmonary inflammation and/or vasoconstriction mediated, in part, by interleukin 6 (IL‐6). 18 We had previously observed that IL‐6 levels were lower initially, and then elevated but were significantly more stable over the course of disease, among inpatients with COVID‐19 who reported headache. 5 Significantly elevated IL‐6 levels have been reported in COVID‐19 inpatients with severe headache, and in those who had moderate COVID‐19 disease including pulmonary involvement but no serious co‐morbid medical conditions; however IL‐6 levels were not reported longitudinally over the course of disease. 19 , 20 Circulating levels of calcitonin gene‐related peptide (CGRP) are reportedly reduced during COVID‐19, 21 though it is unclear whether this is pathological or compensatory. By contrast, CGRP is elevated during migraine attacks, 22 and headache during COVID‐19 might reflect a host response that includes raising CGRP levels. Anti‐CGRP medications are FDA‐approved for migraine treatment, 23 and an anti‐CGRP drug is under trial as a potential COVID‐19 therapy; 24 however, preliminary evidence suggests that anti‐CGRP drugs do not influence COVID‐19 outcomes for patients with migraine. 25 The SARS‐CoV‐2 virus binds to the angiotensin converting enzyme 2 (ACE2) receptor on cell surfaces to gain cellular entry. 26 The renin‐angiotensin system is reported to be altered in people with migraine, and drugs that inhibit ACE1 or block angiotensin receptors are prescribed to prevent migraine attacks. 27 Finally, vitamin D deficiency may be associated with COVID‐19 mortality, 28 and vitamin D has been proposed as a migraine therapeutic. 29 Collectively, these data suggest that headaches arising during SARS‐CoV‐2 infection may share certain pathophysiological mechanisms with primary headache disorders, including migraine.
It is notable that headache was reported as a symptom in only 10.4% of inpatients with COVID‐19 included in the meta‐analysis. If headache is indeed a marker for reduced relative risk of mortality for inpatients with COVID‐19, then it appears that this may affect a small minority of COVID‐19 patients. However, this may be a misleading conclusion; COVID‐19 outpatients with headache may be less likely to visit Emergency Departments or become hospitalized. Headache can be an early 5 or isolated 30 outpatient symptom of COVID‐19, particularly for patients with prior history of migraine. 31 , 32 COVID‐19 patients presenting to Emergency Departments with headache are nearly three times more likely to have a prior history of a primary headache disorder, 5 including migraine. 33 We analyzed data from a US population‐based survey fielded in early 2020, which found that respondents with a prior diagnosis of migraine, compared to those with no history of migraine, had a 58% higher relative risk of COVID‐19 symptoms, 61% higher relative risk of testing positive for COVID‐19, but a 47% lower relative risk of visiting an Emergency Department, and a 39% lower relative risk of being hospitalized. 33 Collectively, these findings indicate that people with primary headache disorders, including migraine, may be more likely to report symptoms of COVID‐19, but they also may be relatively less likely to experience a life‐threatening COVID‐19 disease course.
We hypothesize that migraine or other primary headache disorders might encompass a phenotype of heightened sensitivity for detecting the presence of viruses, which might lead to earlier symptoms of viral infections and more effective overall defenses against them, including reduced mortality. If true, then this might provide insights into other observations linking headaches with viral infections. For example, headaches are highly prevalent with encephalitis and other viral infections (e.g., influenza), 16 , 34 or following exposure to viral antigens in the absence of virus (e.g., COVID‐19 or influenza vaccinations). 35 , 36 A prior history of migraine is particularly associated with headaches post‐COVID‐19 vaccination. 37 Persistent symptoms following acute COVID‐19 (“long COVID”) are more prevalent among patients who have symptoms of headache during acute COVID‐19. 31 Headaches themselves are prevalent symptoms of long COVID, 37 but notably are not relatively more prevalent among long COVID patients who have a prior history of migraine. 38 Finally, a prior history of migraine is associated with other presumptive post‐viral syndromes (e.g., myalgic encephalomyelitis/chronic fatigue syndrome). 39 , 40 Future studies are warranted to replicate these findings and unravel these complex relationships.
This meta‐analysis has significant strengths and limitations. The study included 48 studies from 14 nations including 43,169 patients. We replicated our analyses in studies of higher quality, and missingness was considered in the NOS. To avoid introduction of potentially confounding categorical variables, we excluded studies published following either the introduction of COVID‐19 vaccines, or the appearance of the more virulent SARS‐CoV‐2 variant strains (e.g., Delta). For this latter reason, our results may not generalize to all SARS‐CoV‐2 variants. We also included studies that did not test patients for COVID‐19; as a practical matter, COVID‐19 testing was neither widely available, nor widely reliable, during the first year of the pandemic. Further, in specifically analyzing studies of COVID‐19 patients who reported either the presence or the absence of headache symptoms, we may have introduced bias against inclusion of patients who would be unable to report these symptoms (e.g., patients who were intubated at the time of presentation to hospital). This may bias our results towards overestimating the association between headache at presentation and COVID‐19 survival. In addition, data about previous headache history in these patients are scarce and may lead to bias. However, we have to consider that the prevalence of headache in these patients was similar to the presence of anosmia, a widely recognized COVID‐19 symptom. Moreover, in the sensitivity analysis, we controlled for this possible bias by including studies only where medical examination was evaluated during the patient's admission and we compared the possible link between RR and the medical evaluation setting as a probable measure of COVID‐19 severity (ER vs. ICU). Another limitation is that data extraction was only conducted by one researcher although the current guidelines of data extraction in systematic reviews recommends that this process should be performed independently by two researchers and consensus over discrepancies reached through discussion in order to ensure the accuracy and reliability of the data extraction process. 41 However, the researcher who performed the data extraction process was aware of the importance of this stage. Moreover, it has been previously shown that there were no substantial differences between methods (single vs. double data extraction) in effect estimates for most outcomes. 42
CONCLUSIONS
Our meta‐analysis points towards a novel possibility: headache arising secondary to an infection is not a “non‐specific” symptom, but rather it may be a marker of enhanced likelihood of survival. That is, we find that patients reporting headache in the setting of COVID‐19 are at reduced risk of death. Further studies are needed to clarify the roles of headaches and headache disorders in the context of viral infections or post‐viral syndromes (e.g., long COVID). Defining specific headache mechanisms that could enhance survival from viral infections represents an opportunity for the potential discovery of improved viral therapeutics, as well as for understanding whether, and how, primary headache disorders may be adaptive.
AUTHOR CONTRIBUTIONS
Study concept and design: Robert E. Shapiro, Víctor J. Gallardo, Patricia Pozo‐Rosich. Acquisition of data: Robert E. Shapiro, Víctor J. Gallardo, Edoardo Caronna, Patricia Pozo‐Rosich. Analysis and interpretation of data: Robert E. Shapiro, Víctor J. Gallardo, Edoardo Caronna, Patricia Pozo‐Rosich. Drafting of the manuscript: Robert E. Shapiro, Víctor J. Gallardo, Edoardo Caronna, Patricia Pozo‐Rosich. Revising it for intellectual content: Robert E. Shapiro, Víctor J. Gallardo, Edoardo Caronna, Patricia Pozo‐Rosich. Final approval of the completed manuscript: Robert E. Shapiro, Víctor J. Gallardo, Edoardo Caronna, Patricia Pozo‐Rosich.
CONFLICT OF INTEREST
Dr. Shapiro has received, in the past 12 months, financial or editorial compensation as a research consultant for Eli Lilly and Lundbeck. Mr. Gallardo reports no disclosures. Dr. Caronna has received honoraria from Novartis, Chiesi, Lundbeck, and Medscape. Dr. Pozo‐Rosich has received honoraria as a consultant and speaker for Allergan‐AbbVie, Biohaven, Chiesi, Eli Lilly, Lundbeck, Medscape, Novartis, and Teva. Her research group has received research grants from Novartis; has received funding for clinical trials from Alder, Amgen, electroCore, Eli Lilly, Lundbeck, Novartis, and Teva. She is the Honorary Secretary of the International Headache Society. She is on the editorial board of Revista de Neurologia. She is an editor for Cephalalgia, Headache, Neurologia, and Frontiers of Neurology, and an advisor for The Journal of Headache and Pain. She is a member of the Clinical Trials Guidelines Committee of the International Headache Society. She has edited the Guidelines for the Diagnosis and Treatment of Headache of the Spanish Neurological Society. She is the founder of www.midolordecabeza.org. Dr. Pozo‐Rosich does not own stocks from any pharmaceutical company. There are no conflicts of interest in regard to this manuscript.
STANDARD PROTOCOL APPROVALS, REGISTRATIONS, AND PATIENT CONSENTS
The study was conducted on available studies published in the literature. No individual patients were included in the study.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We acknowledge the collaboration to this work of Dr. Rubio‐Rivas (Department of Internal Medicine, Bellvitge University Hospital, Bellvitge Biomedical Research Institute‐IDIBELL, University of Barcelona, Barcelona, Spain). We are also grateful to the other investigators who compiled the multiple datasets that we relied upon in our analyses. We also especially remember all who have died due to COVID‐19, and those who suffered or are still suffering from it. We dedicate our work to all people, especially healthcare professionals, in the fight against COVID‐19.
Gallardo VJ, Shapiro RE, Caronna E, Pozo‐Rosich P. The relationship of headache as a symptom to COVID‐19 survival: A systematic review and meta‐analysis of survival of 43,169 inpatients with COVID‐19. Headache. 2022;62:1019‐1028. doi: 10.1111/head.14376
Víctor J. Gallardo and Robert E. Shapiro contributed equally to this work.
DATA AVAILABILITY STATEMENT
All data are available and will be shared by request from any qualified investigator.
REFERENCES
- 1. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pairo‐Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in Covid‐19. Nature. 2021;591(7848):92‐98. doi: 10.1038/s41586-020-03065-y [DOI] [PubMed] [Google Scholar]
- 3. Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid‐19 with respiratory failure. N Engl J Med. 2020;383(16):1522‐1534. doi: 10.1056/nejmoa2020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeberg H, Pääbo S. A genomic region associated with protection against severe COVID‐19 is inherited from Neandertals. Proc Natl Acad Sci U S A. 2021;118(9):e2026309118. doi: 10.1073/pnas.2026309118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caronna E, Ballvé A, Llauradó A, et al. Headache: a striking prodromal and persistent symptom, predictive of COVID‐19 clinical evolution. Cephalalgia. 2020;40(13):1410‐1421. doi: 10.1177/0333102420965157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prospero International Prospective Register of Systematic Reviews . Available online: https://www.crd.york.ac.uk/prospero/ Accessed July 17, 2021.
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Islam MA, Alam SS, Kundu S, Hossan T, Kamal MA, Cavestro C. Prevalence of headache in patients with coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis of 14,275 patients. Front Neurol. 2020;11:562634. doi: 10.3389/fneur.2020.562634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells G, Shea B, O'connell D, et al. The Newcastle‐Ottawa Scale (Nos) for Assessing the Quality of Nonrandomised Studies in Meta‐analyses. Ottawa Hospital Research Institute; 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 10. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. J R Stat Soc Ser A Stat Soc. 2009;72(1):137‐159. doi: 10.1111/j.1467-985X.2008.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newcombe RG. Two‐sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med. 1998;7(8):857‐872. doi: [DOI] [PubMed] [Google Scholar]
- 12. Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel Haenszel odds ratio. Am J Epidemiol. 1986;124(5):719‐723. doi: 10.1093/oxfordjournals.aje.a114447 [DOI] [PubMed] [Google Scholar]
- 13. Borenstein M, Higgins J. Meta‐analysis and subgroups. Prev Sci. 2013;14(2):134‐143. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 15. Uygun Ö, Ertaş M, Ekizoğlu E, et al. Headache characteristics in COVID‐19 pandemic‐a survey study. J Headache Pain. 2020;21(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Marinis M, Welch KM. Headache associated with non‐cephalic infections: classification and mechanisms. Cephalalgia. 1992;12:197‐201. doi: 10.1046/j.1468-2982.1992.1204197.x [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Symptoms of Coronavirus . https://www.cdc.gov/coronavirus/2019‐ncov/symptoms‐testing/symptoms.html. Accessed January 15, 2021.
- 18. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID‐19. Nature. 2020;583(7816):437‐440. doi: 10.1038/s41586-020-2355-0 [DOI] [PubMed] [Google Scholar]
- 19. Bolay H, Karadas Ö, Oztürk B, et al. HMGB1, NLRP3, IL‐6 and ACE2 levels are elevated in COVID‐19 with headache: a window to the infection‐related headache mechanism. J Headache Pain. 2021;22(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karadaş Ö, Öztürk B, Sonkaya AR, et. al. Latent class cluster analysis identified hidden headache phenotypes in COVID‐19: impact of pulmonary infiltration and IL‐6. Neurol Sci 2021;42(5):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ochoa‐Callejero L, García‐Sanmartín J, Villoslada‐Blanco P, et al. Circulating levels of calcitonin gene‐related peptide (CGRP) are lower in COVID‐19 patients. J Endocr Soc. 2021;5(3):bvaa199. doi: 10.1210/jendso/bvaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183‐187. doi: 10.1002/ana.410280213 [DOI] [PubMed] [Google Scholar]
- 23. Charles A, Pozo‐Rosich P. Targeting calcitonin gene‐related peptide: a new era in migraine therapy. Lancet. 2019;394(10210):1765‐1774. doi: 10.1016/S0140-6736(19)32504-8 [DOI] [PubMed] [Google Scholar]
- 24. Safety and Efficacy Trial of Zavegepant* Intranasal for Hospitalized Patients With COVID‐19 Requiring Supplemental Oxygen . Accessed September 16, 2020. https://clinicaltrials.gov/ct2/show/NCT04346615
- 25. Caronna E, José Gallardo V, Alpuente A, et al. Safety of anti‐CGRP monoclonal antibodies in patients with migraine during the COVID‐19 pandemic: present and future implications. Neurologia. 2021;36(8):6111‐6617. doi: 10.1016/j.nrl.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;81(2):271‐280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halker RB, Starling AJ, Vargas BB, Schwedt TJ. ACE and ARB agents in the prophylactic therapy of migraine—how effective are they? Curr Treat Options Neurol. 2016;18(4):15. doi: 10.1007/s11940-016-0397-2 [DOI] [PubMed] [Google Scholar]
- 28. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and covid‐19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nowaczewska M, Wiciński M, Osiński S, Kázmierczak H. The role of vitamin D in primary headache–from potential mechanism to treatment. Nutrients. 2020;12(1):243. doi: 10.3390/nu12010243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toptan T, Aktan Ç, Başarı A, Bolay H. Case series of headache characteristics in COVID‐19: headache can be an isolated symptom. Headache. 2020;60(8):1788‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernandez‐de‐las‐Penas C, Gomez‐Mayordomo V, Cuadrado ML, et al. The presence of headache at onset in SARS‐CoV‐2 infection is associated with long‐term post‐COVID headache and fatigue: A case‐control study. Cephalalgia. 2021;41(13):1332‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Membrilla JA, de Lorenzo I, Sastre M, et al. Headache as a cardinal symptom of coronavirus disease 2019: a cross‐sectional study. Headache. 2020;60:2176‐2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shapiro RE, Costantino H, Pozo‐Rosich P, et al. Migraine is associated with higher incidence of COVID‐19, and higher frequency of COVID‐19 symptoms, but lower COVID‐19 – Associated health care resource utilization rates. Headache. 2021;61(S1):169. [Google Scholar]
- 34. Khandaker G, Dierig A, Rashid H, King C, Heron L, Booy R. Systematic review of clinical and epidemiological features of the pandemic influenza A (H1N1) 2009. Influenza Other Respi Viruses. 2011;5(3):148‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID‐19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663‐1669. [DOI] [PubMed] [Google Scholar]
- 36. Ekizoglu E, Gezegen H, Yalınay Dikmen P, Orhan EK, Ertaş M, Baykan B. The characteristics of COVID‐19 vaccine‐related headache: clues gathered from the healthcare personnel in the pandemic. Cephalalgia. 2021;42:366‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. Sci Rep. 2021;11(1):16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernandez‐de‐las‐Penas C, Gomez‐Mayordomo V, Garcia‐Azorin D, et al. Previous history of migraine is associated with fatigue, but not headache, as long‐term post‐COVID symptom after severe acute respiratory SARS‐CoV‐2 infection: a case‐control study. Front Hum Neurosci. 2021;15:678472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ravindran MK, Zheng Y, Timbol C, Merck SJ, Baraniuk JN. Migraine headaches in chronic fatigue syndrome (CFS): comparison of two prospective cross‐sectional studies. BMC Neurol. 2011;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chi‐Ieong L, Che‐Chen L, Wei‐Hung C, et al. Increased risk of chronic fatigue syndrome in patients with migraine: a retrospective cohort study. J Psychosom Res. 2015;79:514‐518. [DOI] [PubMed] [Google Scholar]
- 41. Alderson P. Cochrane Reviewers' Handbook 4.2. 2 (Updated December 2003). Cochrane Library; 2004. [Google Scholar]
- 42. Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59(7):697‐703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
All data are available and will be shared by request from any qualified investigator.


