Abstract
The chromosome of the filamentous bacterium Streptomyces coelicolor is linear, but the genetic map is circular. We present cytological evidence based on the use of fluorescence in situ hybridization showing that the ends of the chromosome frequently colocalize, in agreement with the idea that the ends are held together, effectively forming a circular chromosome. These observations provide a possible explanation for how a linear bacterial chromosome can exhibit a circular genetic map.
With over 500 species recognized to date, Streptomyces forms a large genus in the high-GC-content group of gram-positive bacteria. Members of this group of spore-forming, filamentous soil bacteria undergo a complex life cycle characterized by various morphologic stages (5, 6). When grown on solid media, spores germinate and develop into substrate mycelia consisting of multinucleated, long branching filaments with infrequent septa. As the colony matures, filaments termed aerial mycelia project above the colony surface. Spores, each containing a single chromosome, are then formed by synchronized septation in the multinucleated aerial filaments. Most species of Streptomyces do not sporulate when they are grown in liquid cultures.
Streptomyces coelicolor A(3)2 is the best-characterized species from a genetic point of view (17). The earliest evidence that the genetic map of S. coelicolor is circular came from conjugative mating experiments involving differentially marked strains (13, 14). All later studies, including protoplast fusion experiments (18), supported the circularity of the genetic map unequivocally. Thus, it came as a surprise when the chromosome of the related species Streptomyces lividans 66 was suggested to be linear by physical mapping data (23). Later, through the application of pulsed-field gel electrophoresis, both the S. coelicolor and S. lividans chromosomes were shown to be linear (24). Whereas most experimentally studied bacteria possess a circular genome, a few, including the Lyme disease-causing spirochete Borrelia burgdorferi (3, 9), the obligate intracellular bacterium Coxiella burnetii (36), and the erythromycin-producing actinomycete Saccharopolyspora erythraea (28), have been found to possess linear genomes. Agrobacterium tumefaciens possess two chromosomes, one that is linear and one that is circular (1). More prokaryotes are likely to be found to possess linear genomes as the physical maps of more bacterial species become available. Of the prokaryotes known to have linear genomes, only Streptomyces has a well-developed genetic system. The discovery of a linear genome exhibiting circular genetic behavior in Streptomyces leads to an interesting question: how is a circular genetic map obtained from a linear genome?
The only other known examples of a linear genome exhibiting circular genetic behavior are certain phage genomes such as that of T4 (33), where map circularity results from their circularly permutated and terminally redundant chromosomes. However, circular permutation does not apply to Streptomyces since the chromosomes were shown to have constant ends (24). In their study of phage genetics, Stahl and Steinberg (32) noted that a circular genetic map may arise from a linear genome if recombination is restricted to even numbers of crossovers. Wang et al. (34) tested this model by examining the inheritance of telomeres in plasmid-mediated interspecies crosses between S. coelicolor and S. lividans. The authors demonstrated a strong bias towards even numbers of crossovers during such matings, supporting the applicability of the Stahl and Steinberg model to the Streptomyces linear genome.
It is well known that eukaryotic chromosomes can exhibit higher-order organization, but little evidence has been presented as to whether prokaryotic chromosomes exhibit similar properties. Essentially nothing is known about how chromosomes are organized in Streptomyces. A simple explanation for the observed circular genetic behavior is that the two ends of the Streptomyces linear chromosome are physically held together to form a circle (16, 34). In such a case, even numbers of crossovers, as observed by Wang et al. (34), would be necessary if complete haploid genomes were to emerge from the mating process (32). Here, we use fluorescent in situ hybridization (FISH) to examine the localization of distinct chromosomal regions within the cell. Our results provide the first cytological evidence that the ends of a linear bacterial chromosome colocalize, suggesting that the chromosome adopts a circular configuration.
MATERIALS AND METHODS
FISH probe preparation.
FISH probe preparation was performed using a modified procedure based on the protocol described in the work of Jensen and Shapiro (22). Due to the high GC content of the S. coelicolor genome, reactions producing long PCR products are difficult to perform. Therefore, two or three separate PCRs were carried out to achieve the desired length for each probe. To prepare the 9.8-kb oriC probe, two PCRs were performed using primers MYO173 and -174 and MYO175 and -176 (primer sequences and PCR product sizes are shown in Table 1) using PfuTurbo DNA polymerase (Stratagene). The PCR fragments obtained were then cloned into the pCR2.1-TOPO vector using a TOPO TA cloning kit (Invitrogen) according to the manufacturer's instructions, producing pMYB236 and pMYB238. Five micrograms of each plasmid DNA was combined and digested with 10 U of three frequently cutting restriction enzymes (AluI, BanI, and Sau3AI) overnight to obtain fragment sizes ranging from 75 to 150 bp. The DNA was purified by phenol-chloroform extraction, concentrated by ethanol precipitation, and resuspended in 20 μl of a solution containing 10 mM Tris-HCl (pH 8.0) and 0.1 mM EDTA. DNA was denatured by incubation at 94°C for 5 min, immediately placed on ice, and then labeled with modified nucleotide FluoroLink Cy3-dCTP (Amersham) by an oligonucleotide tailing method. A reaction using a mixture containing 135 μM dCTP, 67.5 μM Cy3-dCTP, and 60 U of terminal deoxynucleotide transferase (TdT) in 1× TdT buffer (Promega) was carried out for 2 h at 37°C. The same reaction was carried out for probes labeled with digoxigenin-11-2′-dUTP (Roche Molecular Biochemicals) except that 180 μM dCTP and 20 μM digoxigenin-11-2′-dUTP were used. Proteins and free nucleotides were then removed using a QIAquick nucleotide removal kit (Qiagen) according to the manufacturer's instructions, followed by concentration by ethanol precipitation. The DNA pellet was then resuspended in a suitable volume of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) to give a probe concentration of 200 ng/μl. Labeled probes can be stored at −20°C for 2 to 4 weeks without detectable loss in labeling efficiency. The same method was used for the preparation of the TIR, LAdj, Ctrl1, and Ctrl2 probes except that different primers were used (Table 1).
TABLE 1.
PCR primers, PCR product sizes, and plasmids constructed for the preparation of FISH probes
| Probe | Forward or reverse primera | Sequence | Product size (bp) | Plasmid |
|---|---|---|---|---|
| oriC 1 | MYO173 | CGA ACG GAA GTC GGC CAG CGA CAG ATG C | 5,072 | pMYB236 |
| MYO174 | CGC CGT ACC GAA CGA ATT TGC GAA GGG C | |||
| oriC 2 | MYO175 | CGC CGT ACC GAA CGA ATT TGC GAA GGG C | 4,746 | pMYB238 |
| MYO176 | CTC CGA GAA GTT GCT CCA ATA CGC GTG G | |||
| TIR 1 | MYO258 | GCG ACC CTC GTT GAC TCC GGA GTT GTA TG | 3,252 | pMYB288 |
| MYO259 | CCC TTC GAC CAC ATC ACC GAC TCC TGA C | |||
| TIR 2 | MYO260 | GCT CAG AAG CGG CGT CAG CTC TTG AGC AC | 3,486 | pMYB290 |
| MYO261 | TGT GCA CTG GAC GAC GGG GAT GCG CTC AC | |||
| TIR 3 | MYO262 | GCC TTC CGT CCT TGA CAA AGT GGC GCT G | 3,212 | pMYB292 |
| MYO263 | CAA CCA AAC CGA ACA CGC CGC CAG CCA C | |||
| LAdj 1 | MYO264 | GCG GGA CAG AGC ATC TCG GTT GAG ATC G | 3,430 | pMYB286 |
| MYO265 | GCC AGG GTC CGC AAC GCA CCT TGG AAG | |||
| LAdj 2 | MYO254 | GCT TCC ATG CCC AGG CCC TTC GTC CTT C | 3,416 | pMYB282 |
| MYO255 | CGG ATT GGC AGC TGC TCT GAC ACA GCC TC | |||
| LAdj 3 | MYO256 | TGG AGG CTG TGT CAG AGC AGC TGC CAA TC | 3,269 | pMYB284 |
| MYO257 | GGT ACC GGT GGT CAT GTC CGG ACG GAA TC | |||
| Ctrl1 1 | MYO189 | GGT CTT GAG GTT CGT GAG GTG GTC GGT GC | 3,141 | pMYB242 |
| MYO190 | TCC CAC TTG TCG TGG ATG TCG TCG TCT | |||
| Ctrl1 2 | MYO191 | GGG AAG ACG ACG ACA TCC ACG ACA AGT GG | 3,048 | pMYB244 |
| MYO192 | CAA CAC AGG ACG GGA TTC ATG CCC ACA GC | |||
| Ctrl1 3 | MYO193 | CTG TGG GCA TGA ATC CCG TCC TGT GTT GG | 3,320 | pMYB246 |
| MYO194 | GAT CGC CGT CGG CGT TGA ACC TTG TCA GG | |||
| Ctrl2 1 | MYO240 | GCC GTT GTA CGT CAG TAG ACG AGT TCT TC | 3,350 | pMYB270 |
| MYO271 | CGG ACA GTA CTC TTC GAC CCG TAC ATC AC | |||
| Ctrl2 2 | MYO242 | GGG TGA TGT ACG GGT CGA AGA GTA CTG TC | 3,589 | pMYB272 |
| MYO243 | GAA CGC TTC CCT GCG TTC GTG GTC TGA G | |||
| Ctrl2 3 | MYO244 | CCA GAA GCC CTA CAC CTC AGA CCA CGA AC | 3,142 | pMYB274 |
| MYO245 | CCC AAG GCG ACC ACA TGT CCT CAC TGC TG |
The forward primer is listed first.
FISH procedure.
S. coelicolor strain J1508 (hisA1 uraA1 strA1 pglNF, SCP2 negative [21]) was used in all FISH experiments because of the low level of fluorescence emitted by this strain (31). Liquid yeast extract-malt extract (YEME) medium (25 ml) supplemented with 50 μg of histidine per ml and 7.5 μg of uracil per ml (19) was inoculated with 50 μl of an S. coelicolor J1508 spore suspension and incubated at 30°C with vigorous shaking for 40 to 45 h (optical density at 600 nm, ∼0.7). Formaldehyde was added directly to the culture to a final concentration of 3.7% (vol/vol), and cells were fixed at room temperature for 45 min. Cells were harvested by centrifugation at 3,000 × g for 10 min at room temperature and washed three times in an equal volume of phosphate-buffered saline (PBS; 140 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.4]). Cells were resuspended in 2.5 ml of PBS and used immediately or stored at 4°C overnight for use the next day.
One milliliter of cells was spun down, resuspended in 1 ml of GTE (50 mM glucose, 20 mM Tris-HCl [pH 7.5], 10 mM EDTA) and divided into five tubes. To each tube, lysozyme stock solution and GTE buffer were added to give a final concentration of 0.625, 1.25, 2.5, 5, or 10 mg of lysozyme per ml, and the tubes were incubated at room temperature for 10 min. Multiple lysozyme concentrations were used due to the observed variability in the optimal lysozyme concentration needed from experiment to experiment. Cells were then washed three times with 1 ml of GTE, spun down in a microcentrifuge at 2,000 × g for 2 min each time to collect the cells, and resuspended in 1 to 5 ml of GTE to give an optimal cell density. A 15-well multitest slide (ICN) was coated with poly-l-lysine by adding a drop of a 0.1% (wt/vol) poly-l-lysine solution (Sigma) into each well, left untouched for 2 min, washed with water, and then air dried completely. Ten microliters of cells from each lysozyme treatment was then pipetted into a well and incubated for 10 min at room temperature. Excess cells were removed by aspiration, and a drop of GTE was added to each well, followed by aspiration to remove loose cells. Dehydration, prehybridization, hybridization, and subsequent wash steps were performed according to the method described in the work of Jensen and Shapiro (22). For slides containing only fluorescent-nucleotide-labeled probes, the slides were mounted in ProLong Antifade (Molecular Probes) according to the manufacturer's instructions. For slides containing digoxigenin-labeled probes, blocking buffer (2% bovine serum albumin in PBS) was added to each well and the slides were incubated for 15 min at room temperature. The blocking buffer was then replaced with 2 μg of anti-digoxigenin-fluorescein Fab fragments (Roche Biochemicals) per ml in blocking buffer and incubated for 2 h at room temperature in a humidifying chamber. After antibody binding, the slide was washed twice in PBS for 5 min each time at room temperature. The slide was then mounted in ProLong Antifade as described above.
For slide observation and fluorescent-micrograph preparation, we used an Olympus BX60 microscope equipped with a MicroMax cooled-charge-coupled-device camera (Princeton Instruments) connected to an IBM personal computer running MetaMorph software version 3.0 (Universal Imaging). A UPlan Fluorite phase-contrast 100× objective was used for both slide observation and photography. A 2-s exposure was used for the collection of fluorescent images, except when DAPI (4′,6′-diamidino-2-phenylindole) was used, in which case a 0.05-s exposure was used. For the bright-field images, a 0.05-s exposure was used. Adobe Photoshop 5.0 was used for all image manipulation.
Coincident-frequency determination.
The coincident frequencies for the two types of signals in double-label FISH experiments were determined as follows. To avoid bias, the fluorescent micrographs obtained for the different probes were analyzed independently. Using Adobe Photoshop 5.0, a circle with a 0.46-μm diameter was generated and manually placed on top of each fluorescent focus centered around the central position. The images of the painted circles thus generated, representing the locations of the fluorescent foci from the different channels, were then overlaid. If the two types of painted circles overlapped, they were considered coincident, and if they did not, they were considered separate. The coincident frequency is defined as the fraction of one type of circle generated from one channel overlapping that from the other channel.
Expected coincident frequency for randomly distributed foci.
To calculate the expected coincident frequency if oriC and LAdj signals were to randomly distribute in cells, the total cellular area and the total coincident area for each type of foci from eight different fields of cells from a dual-label FISH experiment involving the oriC and the LAdj probes were determined using the program MetaMorph 3.0. The coincident area for a single focus was defined as the area (in pixels) occupied by a circle with a 0.92-μm diameter. The total coincident area for the oriC signals then equals the total number of oriC foci observed in all eight fields of cells multiplied by the coincident area. The probability that a randomly distributed LAdj focus would be found coincident with a randomly distributed oriC focus would then be the total coincident area (oriC) divided by the total cellular area. Similarly, the probability that a randomly distributed oriC focus would be found coincident with a randomly distributed LAdj focus would then be the total coincident area (LAdj) divided by the total cellular area.
RESULTS
Strategy.
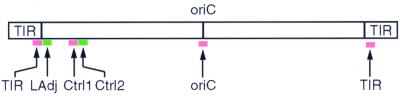
To investigate whether the ends of the S. coelicolor chromosome are held together, we performed FISH experiments to look directly at the relative locations of various chromosomal regions within the cell. The S. coelicolor chromosome is a linear molecule 8 Mb in length with a centrally located origin of replication (25). The ends of the chromosome consist of long terminal inverted repeats (TIRs) 27.5 kb in length (http://www.sanger.ac.uk/Projects/S_coelicolor/). DNA sequence available from the S. coelicolor genome project revealed the precise junctions between the TIRs and the adjacent nonrepeated regions. Accordingly, we designed three hybridization probes corresponding to (i) the origin of replication region, (ii) an internal portion of the TIR located 10 bp from the TIR junction, and (iii) a nonrepeated region close to, but outside of, the left TIR located 163 bp from the TIR junction. We refer to these probes as the oriC, TIR, and LAdj probes, respectively (Fig. 1). All probes were approximately 10 kb in length.
FIG. 1.
The positions of FISH probes on the S. coelicolor chromosome. The replication origin (oriC) is located at the center of the 8-Mb linear chromosome. Identical sequences (27.5 kb in length) named TIRs are found at both ends of the chromosome. The locations of the oriC, TIR, LAdj, Ctrl1, and Ctrl2 probes are marked with arrows below the chromosome. The physical distance between LAdj and TIR is 173 bp, and that between Ctrl1 and Ctrl2 is 40 bp. Distances shown are not to scale.
Dual-label FISH experiments involving the TIR and the LAdj probes make distinct predictions depending upon whether the ends of the S. coelicolor chromosome are held together. If the two ends of the chromosome are held together, there should be equal numbers of TIR and LAdj signals. Also, a high proportion of TIR signals should be located at or near an LAdj signal. On the other hand, if the two ends of the chromosome are not held together, there should be twice as many TIR signals as LAdj signals. In addition, half of the TIR signals should have a random location within the cell relative to the location of LAdj signals. As a control, a dual-label FISH experiment visualizing two distantly located regions of the chromosome (oriC and LAdj) was expected to show no significant association of the two signals.
Separately visualizing oriC, TIR, and LAdj.
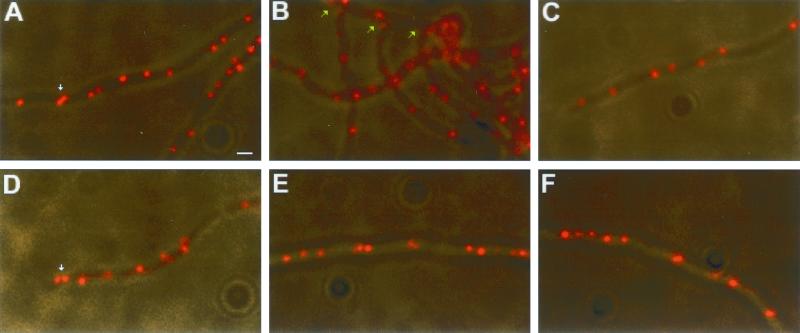
We first determined the subcellular localizations of the oriC, TIR, and LAdj regions individually in single-label FISH experiments. Vegetatively growing cells from a liquid culture of S. coelicolor were fixed and separately hybridized with the three probes. All three regions of the chromosome appeared as distinct fluorescent foci distributed along the length of the multinucleoid filaments (Fig. 2). In unicellular bacteria the replication origin region preferentially localizes near the cell poles and the terminus near the midcell (10, 26, 35). In contrast, we observed no readily recognizable pattern of focus distribution along the length of the S. coelicolor filaments with any of the probes. In S. coelicolor, cell wall growth occurs mostly in the mycelial tips (11, 31), raising the possibility that the tip region represents the major site of DNA replication. If so, the mycelial tip regions might be expected to exhibit a different chromosome organization than the nontip regions. However, we observed no difference among the distributions of oriC foci at the tips (Fig. 2C and D), the branches (Fig. 2B), and the rest of the mycelium (Fig. 2A). Twin oriC spots (bilobed oriC foci or two oriC foci closely juxtaposed) (Fig. 2A and D) were found in both the tip and the nontip regions and exhibited no preferential distribution to the mycelial tips.
FIG. 2.
Single-label FISH experiments showing the subcellular localizations of three chromosomal loci. Cy3-labeled oriC and TIR probes and the digoxigenin-labeled LAdj probe were used to detect the subcellular localizations of the replication origin region (A to D), the chromosome ends (E), and a nonrepeated region adjacent to the left TIR (F) in S. coelicolor substrate mycelia. (A) Internal portion of the mycelium. (B) Branch region of the mycelium. Green arrows point to branch regions. (C and D) Mycelial tips. White arrows point to twin oriC spots. Scale bar = 1 μm.
Detection efficiency of the FISH procedure.
To determine the staining efficiencies of our FISH probes, we constructed two probes, Ctrl1 and Ctrl2, shown in Fig. 1. Because these two probes hybridize to two regions of the chromosome immediately adjacent to each other, the two signals were expected to colocalize 100% of the time in a dual-label FISH experiment. The staining efficiencies of the probes were then determined by looking at the observed coincidence frequencies (data not shown, determined by the method illustrated in Fig. 4 and described below). The Cy3-labeled probe and digoxigenin-labeled probe were found to have 90 and 94% staining efficiencies, respectively.
FIG. 4.
Method for quantitative analysis of the relative positioning of various chromosomal sites. (A and B) Fluorescent foci representing the localizations of the TIR and the LAdj regions, respectively. (C and D) A computer-generated colored circle 0.46 μm in diameter was painted on top of each fluorescent focus centered around the central position of the focus to delineate an area considered the coincident range. Painting of the colored circles was performed entirely independently for each channel. (E) Overlay of the circles in panels C and D and bright-field image. If two different colored circles overlap, then they were considered coincident (arrows). Otherwise, they were considered separate. Scale bar = 1 μm.
Dual-label FISH experiments.
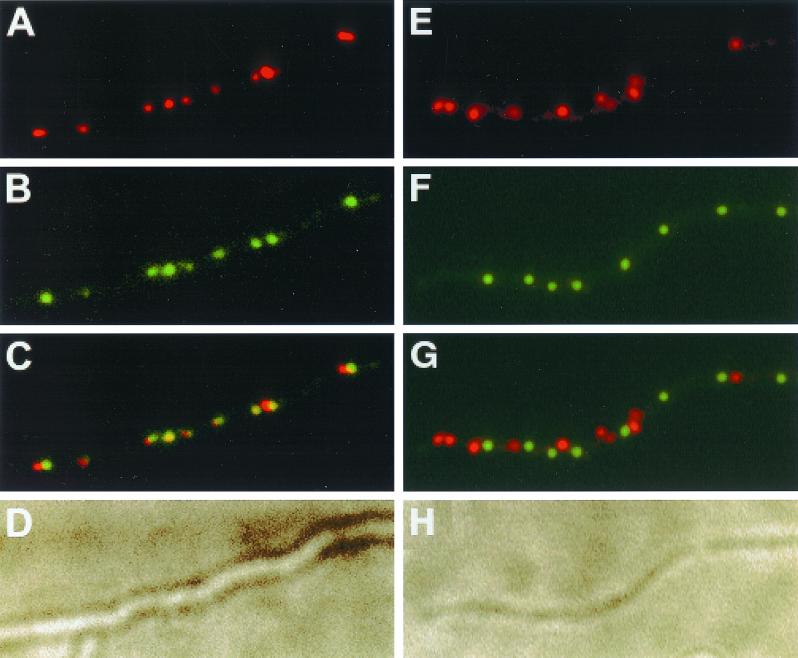
To determine whether the ends of the chromosomes were located in close proximity to each other, fixed cells from a liquid culture of S. coelicolor were hybridized with both a TIR probe and an LAdj probe. As shown in Fig. 3A to D, approximately equal numbers of TIR and LAdj signals were observed and most of the TIR signals were close to or coincident with an LAdj signal. For comparison, a dual-label FISH experiment involving the oriC probe and the LAdj probe showed little colocalization of the two fluorescent signals (Fig. 3E to H). Taken together, our data demonstrate that the terminal regions of the chromosome, which are 8 Mb apart, colocalize but that regions of the chromosome (oriC and LAdj) that are separated by 4 Mb do not. These conclusions were confirmed quantitatively as follows. One complication in our analysis was variability in the size of FISH signals, which ranged from 0.26 to 0.59 μm in diameter. To circumvent this problem, we used the Adobe Photoshop 5.0 program to assign to each focus a circle of 0.46 μm in diameter, representing the average diameter of fluorescent foci (n = 60). Thus, in Fig. 4, each focus was replaced with a computer-generated painted circle of constant diameter, centered around the central position of each fluorescent focus manually. Using this method, if the centers of two foci were closer than 0.46 μm, as demonstrated by the overlapping of the painted circles, the foci were considered to be coincident. If not, they were considered to be separate.
FIG. 3.
(A to D) Dual-label FISH experiment showing the subcellular localizations of the TIRs and the LAdj region in S. coelicolor substrate mycelia. A Cy3-labeled TIR probe corresponding to both ends of the chromosome and a digoxigenin-labeled LAdj probe corresponding to a nonrepeated region adjacent to the left TIR were used to detect the localizations of the TIR and the LAdj regions in the same cells. (A) Fluorescent foci representing the localization of the TIR regions in cells; (B) fluorescent foci representing the localization of the LAdj region in cells; (C) overlay of panels A and B; (D) bright-field image showing the mycelium. (E to H) Dual-label FISH experiment showing the subcellular localizations of the oriC region and the LAdj region. A Cy3-labeled oriC probe corresponding to the replication origin regions and a digoxigenin-labeled LAdj probe corresponding to a nonrepeated region adjacent to the left TIR were used to detect the localizations of the oriC and the LAdj regions in the same cells. (E) Fluorescent foci representing the localization of the oriC region in cells; (F) fluorescent foci representing the localization of the LAdj region in cells; (G) overlay of panels A and B; (H) bright-field image showing the mycelium. Scale bar = 1 μm.
Table 2 shows the results of this analysis starting with the control (oriC versus LAdj). We observed 47% more oriC signals than LAdj signals (305 versus 207, respectively). This excess of oriC signals was probably a simple consequence of the close proximity of the oriC probe to the site of replication initiation. In any event, 26% of the oriC signals were coincident with an LAdj signal and 35% of the LAdj signals were coincident with an oriC signal. Because the painted foci occupy a significant fraction of the area of the cell, we estimated the frequency that any two foci would be found coincident by chance. By considering the fraction of cellular area occupied by each type of painted circle (see Materials and Methods), we estimated that 25% (compare to 26% observed) of the time an oriC signal would be found to coincide with an LAdj signal by chance and that 36% (compare to 35% observed) of the time an LAdj signal would be found to coincide with an oriC signal by chance. The similarity between the expected colocalization frequencies of randomly placed foci and experimentally determined colocalization frequencies is consistent with our interpretation that the relative positioning of the oriC and the LAdj regions is random.
TABLE 2.
Coincident frequecies of the various chromosomal regions in S. coelicolor cells
| Probes used | Region | % Separatea | % Coincidenta | Totalb |
|---|---|---|---|---|
| oriC and LAdj | oriC | 74 | 26 | 305 |
| LAdj | 65 | 35 | 207 | |
| TIR and LAdj | TIR | 14 | 86 | 184 |
| LAdj | 11 | 89 | 177 |
Percentage of foci observed belonging to each class.
Total number of foci observed from eight different fields of cells.
Next, we analyzed the relative positioning of the TIR and the LAdj regions. We observed approximately equal numbers of TIR and LAdj signals (n = 184 and 177, respectively), which was expected for a chromosome configuration in which the two termini are held together. Most of the TIR and LAdj signals were found to colocalize: 86% of the TIR signals coincided with an LAdj signal, and 89% of the LAdj signals coincided with a TIR signal. The <100% colocalization was not surprising because the FISH staining frequency was not 100% as discussed previously. Thus, 86 and 89% colocalization frequencies observed are out of maximum possible observational values of 90 and 94%, respectively. Therefore, our data demonstrated that 95% of the time the two ends of the chromosomes were found to colocalize under the conditions used.
DISCUSSION
The genetic circularity of the Streptomyces chromosome was first demonstrated by conjugative mating experiments (13) and later confirmed by heteroclone and protoplast fusion experiments (14, 18). We now know, however, that the chromosome is a linear molecule with constant ends (24). How do we explain the apparent paradox between the genetic circularity and the physical linearity of the Streptomyces chromosome? One possible explanation proposed by Stahl and Steinberg (32) is that a linear genome gives rise to a circular genetic map if the genome is restricted to even numbers of crossovers. Wang et al. (34) tested the applicability of this explanation to the Streptomyces chromosome by carrying out plasmid-mediated interspecies matings and demonstrated a strong bias towards even numbers of crossovers. To explain the observed bias towards even numbers of crossovers, they proposed several hypotheses: the merozygotic nature of the conjugative mating process, the association of the chromosomal termini, the selective advantage conferred by the inheritance of telomeres from the same parent, and the homogenization of telomeres in the progeny. Wang et al. (34) discounted the last two hypotheses on the grounds that, in the matings performed, two of the progeny recovered were found to have inherited one telomere from each parent. These two progeny exhibited no growth disadvantage compared to that of their wild-type parents, and homogenization of the telomeres was not observed even after extensive subculturing.
Two possible explanations remained for the observed bias towards even numbers of crossovers: (i) the chromosome is linear and Streptomyces mating is merozygotic in nature and (ii) the chromosome adopts a circular configuration through the association of the termini. Conjugative matings in Streptomyces are merozygotic in nature; chromosome transfer from the donor to the recipient is incomplete. For complete haploid genomes to emerge, even numbers of crossovers would be required in recombination involving one partial and one complete chromosome, except when an end fragment is involved. If we assume that recombination involving end fragments is extremely rare, merozygosity may be able to explain the observed bias towards even numbers of crossovers even if the chromosome adopts a linear configuration in cells. Even numbers of crossovers would also be necessary for complete haploids to emerge if the chromosome ends associate and the chromosome adopts a circular configuration.
An additional observation, arising from the analysis of heteroclones, is the apparent transfer from the donor to the recipient of a linked segment of the genome spanning both ends of the chromosome. Heteroclones are merodiploid colonies that arise from a cross. The diploid region of a heteroclone behaves as a continuous segment of the genome. When the diploid regions from different classes of heteroclones were analyzed, they were found to overlap, covering the entire chromosome. In more than 10% of the heteroclones analyzed, the diploid regions were found to include both ends of the chromosome. Therefore, the hypothesis that Streptomyces chromosomes are linear molecules involved in merozygotic matings is not readily compatible with the results from the heteroclone experiments. A circular chromosome configuration, on the other hand, is compatible with the results of heteroclone analysis and with the observation of Wang et al. (34), regardless of whether the matings are merozygotic in nature. On balance, then, the simplest explanation for the genetic circularity of the chromosome is that the chromosome adopts a circular configuration through the association of the termini.
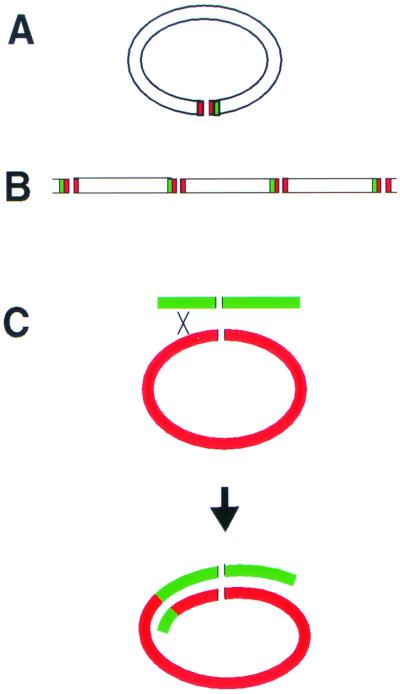
The goal of the present work was to test this hypothesis cytologically. To determine whether the Streptomyces chromosome assumes a linear or circular configuration in cells, we performed FISH experiments determining the locations of the origin of replication region (oriC), the ends of the chromosome (TIRs), and a region of the chromosome adjacent to but outside of the left TIR (LAdj). Our principal findings are that the ends of the chromosomes (TIRs) and the LAdj region colocalize in an approximately 1:1 ratio but that the oriC region and the LAdj regions of the chromosome do not to colocalize, hereby demonstrating that the ends of the Streptomyces chromosome associate in cells. The association of the chromosomal termini is consistent with a circular chromosome configuration (Fig. 5A). A circular chromosome configuration is compatible with the heteroclone chromosome structure previously proposed by Hopwood (15) (Fig. 5C). It should be noted, however, that our FISH data, considered alone, are also consistent with a model in which linear chromosomes are linked to each other in an end-to-end configuration (Fig. 5B). However, it is difficult to imagine how this chromosome configuration would be compatible with the existence of heteroclones. We therefore favor the hypothesis that the linear Streptomyces chromosome adopts a circular configuration in cells through the association of the chromosomal termini.
FIG. 5.
Models for chromosome configuration. A circular configuration (A) and an end-to-end-linked chromosome configuration (B) are shown. Green represents the LAdj region, and red represents the TIR regions. (C) Model for the generation of a heteroclone chromosome as a result of recombination with a circular chromosome as proposed by Hopwood (15).
What is the mechanism responsible for holding the ends of the Streptomyces chromosome together? To date, two types of chromosome ends have been described for linear prokaryotic replicons: those consisting of covalently closed hairpin loops without bound proteins as found in Borrelia chromosomes and linear plasmids (2, 4) and those consisting of inverted repeats with covalently attached 5′-terminal proteins as found in all characterized Streptomyces chromosomes and linear plasmids (7, 20, 24). Other linear replicons sharing the same characteristics as those of the linear Streptomyces replicons include the genomes of certain bacteriophages (e.g., Bacillus subtilis φ29, Escherichia coli PRD1, and Streptococcus pneumoniae HB-3), the mammalian adenoviruses, and the linear plasmids of plant and fungal mitochondria (12). The identity of the 5′-terminal protein of Streptomyces chromosomes is still unknown. In bacteriophages and adenoviruses, the genes for the 5′-terminal proteins have been cloned and the proteins have been shown to play an essential role in viral DNA replication.
Intriguingly, the circular form of viral DNA molecules had been found previously in DNA preparations from both φ29 and adenoviruses (27, 29). It has been suggested that a protein factor is responsible for the formation and the maintenance of the circular form (27, 29). The circular configuration observed for the Streptomyces linear chromosomes is most likely achieved by the presence of a protein(s) that is capable of holding the ends of the chromosome together, as previously suggested in a number of reports (8, 16, 30). The identities of the 5′-terminal proteins found on Streptomyces linear plasmids and chromosomes are currently unknown. Whether the 5′-terminal protein plays a role in the association of the two chromosomal ends in Streptomyces remains to be determined.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Science Foundation (MCB-9727234).
We thank K. Chater and Amy Gehring for helpful advice.
ADDENDUM IN PROOF
A recent report describes the cloning of the gene for the 5′ terminal protein of the Streptomyces chromosome (K. Bao and S. N. Cohen, Genes Dev. 15:1518–1527, 2001).
REFERENCES
- 1.Allardet-Servent A, Michaux-Charachon S, Jumas-Bilak E, Karayan L, Ramuz M. Presence of one linear and one circular chromosome in the Agrobacterium tumefaciens C58 genome. J Bacteriol. 1993;175:7869–7874. doi: 10.1128/jb.175.24.7869-7874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G, Garon C F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987;237:409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- 3.Casjens S, Huang W M. Linear chromosomal physical and genetic map of Borrelia burgdorferi, the Lyme disease agent. Mol Microbiol. 1993;8:967–980. doi: 10.1111/j.1365-2958.1993.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 4.Casjens S, Murphy M, DeLange M, Sampson L, van Vugt R, Huang W M. Telomeres of the linear chromosomes of Lyme disease spirochaetes: nucleotide sequence and possible exchange with linear plasmid telomeres. Mol Microbiol. 1997;26:581–596. doi: 10.1046/j.1365-2958.1997.6051963.x. [DOI] [PubMed] [Google Scholar]
- 5.Chater K F. Developmental decisions during sporulation in the aerial mycelium in Streptomyces. In: Brun Y V, Shimkets L J, editors. Prokaryotic development. Washington, D.C.: American Society for Microbiology; 2000. pp. 33–48. [Google Scholar]
- 6.Chater K F, Hopwood D A. Differentiation in actinomycetes. In: Ashworth J M, Smith J E, editors. Microbial differentiation. Society for General Microbiology Symposium 23. Cambridge, United Kingdom: Cambridge University Press; 1973. pp. 143–160. [Google Scholar]
- 7.Chen C W. Complications and implications of linear bacterial chromosomes. Trends Genet. 1996;12:192–196. doi: 10.1016/0168-9525(96)30014-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen C W, Lin Y-S, Yang Y-L, Tsu M-F, Chang H-M, Keiser H M, Hopwood D A. The linear chromosome of Streptomyces: structure and dynamics. Actinomycetologica. 1994;8:103–112. [Google Scholar]
- 9.Ferdows M S, Barbour A G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci USA. 1989;86:5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon G S, Sitnikov D, Webb C D, Teleman A, Straight A, Losick R, Murray A W, Wright A. Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell. 1997;90:1113–1121. doi: 10.1016/s0092-8674(00)80377-3. [DOI] [PubMed] [Google Scholar]
- 11.Gray D I, Gooday G W, Prosser J I. Apical hyphal extension in Streptomyces coelicolor A3(2) J Gen Microbiol. 1990;136:1077–1084. doi: 10.1099/00221287-136-6-1077. [DOI] [PubMed] [Google Scholar]
- 12.Hinnebusch J, Tilly K. Linear plasmids and chromosomes in bacteria. Mol Microbiol. 1993;10:917–922. doi: 10.1111/j.1365-2958.1993.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 13.Hopwood D A. A circular linkage map in the actinomycete Streptomyces coelicolor. J Mol Biol. 1965;12:514–516. doi: 10.1016/s0022-2836(65)80274-1. [DOI] [PubMed] [Google Scholar]
- 14.Hopwood D A. Lack of constant genome ends in Streptomyces coelicolor. Genetics. 1966;54:1177–1184. doi: 10.1093/genetics/54.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopwood D A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967;31:373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopwood D A. Delving into the Streptomyces chromosome. Dev Ind Microbiol. 1997;34:1–6. [Google Scholar]
- 17.Hopwood D A. Forty years of genetics with Streptomyces: from in vivo through in vitro to in silico. Microbiology. 1999;145:2183–2202. doi: 10.1099/00221287-145-9-2183. [DOI] [PubMed] [Google Scholar]
- 18.Hopwood D A, Wright H M. Bacterial protoplast fusion: recombination in fused protoplasts of Streptomyces coelicolor. Mol Gen Genet. 1978;162:307–317. doi: 10.1007/BF00268856. [DOI] [PubMed] [Google Scholar]
- 19.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: The John Innes Foundation; 1985. [Google Scholar]
- 20.Huang C H, Lin Y S, Yang Y L, Huang S W, Chen C W. The telomeres of Streptomyces chromosomes contain conserved palindromic sequences with potential to form complex secondary structures. Mol Microbiol. 1998;28:905–916. doi: 10.1046/j.1365-2958.1998.00856.x. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda H, Seno E T, Bruton C J, Chater K T. Genetic mapping, cloning and physiological aspects of the glucose kinase gene of Streptomyces coelicolor. Mol Gen Genet. 1984;196:501–507. doi: 10.1007/BF00436199. [DOI] [PubMed] [Google Scholar]
- 22.Jensen R B, Shapiro L. The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation. Proc Natl Acad Sci USA. 1999;96:10661–10666. doi: 10.1073/pnas.96.19.10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBlond P, Redenback M, Cullum J. Physical map of the Streptomyces lividans 66 genome and comparison with that of the related strain Streptomyces coelicolor A3(2) J Bacteriol. 1993;175:3422–3429. doi: 10.1128/jb.175.11.3422-3429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y S, Kieser H M, Hopwood D A, Chen C W. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol Microbiol. 1993;10:923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 25.Musialowski M S, Flett F, Scott G B, Hobbs G, Smith C P, Oliver S G. Functional evidence that the principal DNA replication origin of the Streptomyces coelicolor chromosome is close to the dnaA-gyrB region. J Bacteriol. 1994;176:5123–5125. doi: 10.1128/jb.176.16.5123-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niki H, Hiraga S. Polar localization of the replication origin and terminus in Escherichia coli nucleoids during chromosome partitioning. Genes Dev. 1998;12:1036–1045. doi: 10.1101/gad.12.7.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortin J, Vinuela E, Salas M, Vasquez C. DNA-protein complex in circular DNA from phage phi-29. Nat New Biol. 1971;234:275–277. doi: 10.1038/newbio234275a0. [DOI] [PubMed] [Google Scholar]
- 28.Reeves A R, Post D A, Vanden Boom T J. Physical-genetic map of the erythromycin-producing organism Saccharopolyspora erythraea. Microbiology. 1998;144:2151–2159. doi: 10.1099/00221287-144-8-2151. [DOI] [PubMed] [Google Scholar]
- 29.Robinson A J, Younghusband H B, Bellett A J D. A circular DNA-protein complex from adenoviruses. Virology. 1973;56:54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi K. Invertrons, a class of structurally and functionally related genetic elements that includes linear DNA plasmids, transposable elements, and genomes of adeno-type viruses. Microbiol Rev. 1990;54:66–74. doi: 10.1128/mr.54.1.66-74.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwedock J, McCormick J R, Angert E R, Nodwell J R, Losick R. Assembly of the cell division protein FtsZ into ladder-like structures in the aerial hyphae of Streptomyces coelicolor. Mol Microbiol. 1997;25:847–858. doi: 10.1111/j.1365-2958.1997.mmi507.x. [DOI] [PubMed] [Google Scholar]
- 32.Stahl F W, Steinberg C M. The theory of formal phage genetics for circular maps. Genetics. 1964;50:531–538. doi: 10.1093/genetics/50.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streisinger G, Edgar R S, Denhardt G H. Chromosome structure in phage T4, I. Circularity of the linkage map. Proc Natl Acad Sci USA. 1964;51:775–779. doi: 10.1073/pnas.51.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S J, Chang H M, Lin Y S, Huang C H, Chen C W. Streptomyces genomes: circular genetic maps from the linear chromosomes. Microbiology. 1999;145:2209–2220. doi: 10.1099/00221287-145-9-2209. [DOI] [PubMed] [Google Scholar]
- 35.Webb C D, Teleman A, Gordon S, Straight A, Belmont A, Lin D C, Grossman A D, Wright A, Losick R. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell. 1997;88:667–674. doi: 10.1016/s0092-8674(00)81909-1. [DOI] [PubMed] [Google Scholar]
- 36.Willems H, Jager C, Baljer G. Physical and genetic map of the obligate intracellular bacterium Coxiella burnetii. J Bacteriol. 1998;180:3816–3822. doi: 10.1128/jb.180.15.3816-3822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]