Abstract
Proton pump inhibitors (PPIs) reliably suppress gastric acid secretion and are therefore the first‐line treatment for gastric acid‐related disorders. Hypomagnesemia (serum magnesium [Mg2+] <0.7 mmol/L) is a commonly reported side effect of PPIs. Clinical reports demonstrate that urinary Mg2+ excretion is low in PPI users with hypomagnesemia, suggesting a compensatory mechanism by the kidney for malabsorption of Mg2+ in the intestines. However, the exact mechanism by which PPIs cause impaired Mg2+ absorption is still unknown. In this review, we show that current experimental evidence points toward reduced Mg2+ solubility in the intestinal lumen. Moreover, the absorption pathways in both the small intestine and the colon may be reduced by changes in the expression and activity of key transporter proteins. Additionally, the gut microbiome may contribute to the development of PPI‐induced hypomagnesemia, as PPI use affects the composition of the gut microbiome. In this review, we argue that the increase of the luminal pH during PPI treatment may contribute to several of these mechanisms. Considering the fact that bacterial fermentation of dietary fibers results in luminal acidification, we propose that targeting the gut microbiome using dietary intervention might be a promising treatment strategy to restore hypomagnesemia in PPI users.
Keywords: gut microbiome, magnesium, omeprazole, PPI, proton pump inhibitor
1. INTRODUCTION
Proton pump inhibitors (PPIs) are the first‐line treatment for gastric acid‐related disorders such as peptic ulcer disease (PUD), gastroesophageal reflux disease (GERD), and non‐steroid anti‐inflammatory drug (NSAID)‐induced mucosal damage. 1 In the United States alone, PPIs are used by 14.9 million patients receiving almost 160 million prescriptions annually. 2 PPI use is underestimated because PPIs are also available by over‐the‐counter sale. In general, PPIs have an excellent safety profile. 3 , 4 However, there is increasing concern in recent years about high prescription numbers and prolonged usage of PPIs. 3 , 5 , 6 , 7
In the last decade, PPI intake has been associated with an increasing number of serious side effects, including increased risk of infections, micronutrient deficiencies, fractures, diabetes mellitus, and kidney and cardiovascular disease. 8 , 9 , 10 , 11 , 12 PPI use has been demonstrated to cause Mg2+ deficiency (hypomagnesemia). 13 , 14 , 15 , 16 , 17 PPI‐induced hypomagnesemia is associated with clinical complaints including fatigue, muscle cramps, and arrhythmias. 18 , 19 In general, PPI‐induced hypomagnesemia occurs during long‐term PPI treatment (>1 year). Upon PPI withdrawal, serum Mg2+ levels rapidly restore within several days to the normal concentration range ([Mg2+] 0.7–1.0 mmol/L) but decrease again after re‐challenge with PPIs. 20 These effects are independent of the type of PPI. 21
In this review, an overview will be provided of the clinical studies that describe the prevalence and risk factors for the development of hypomagnesemia during PPI therapy. Moreover, we aim to describe the molecular mechanisms underlying the disease, as significant progress has been made toward our understanding of PPI‐induced hypomagnesemia. Based on recent advances, we will propose novel therapeutic approaches toward the treatment of PPI‐induced hypomagnesemia.
2. PROTON PUMP INHIBITORS
PPIs prevent gastric acid secretion by direct inhibition of the gastric proton‐potassium ATPase (H+, K+‐ATPase) of the epithelial cell lining in the mucosa of the stomach. 22 Orally administered PPIs are taken up in the small intestine and released to the circulation. Consequently, PPIs accumulate in the acidic secretory canaliculus of the parietal cell. The acidic environment allows the conversion of PPI‐prodrugs into active metabolites that block the gastric H+, K+‐ATPase. This mechanism contributes to the specificity of PPIs for the gastric H+, K+‐ATPase and reduces inhibition of non‐gastric H+, K+‐ATPases. 23 Clinical studies have shown that PPIs reliably suppress acid secretion up to 24 h (pH >4). 22 PPIs are more potent inhibitors of gastric acid secretion than alternative drugs, including histamine‐2‐receptor‐blockers (H2RAs) or anticholinergics. 22 , 23 Consequently, patients are often dependent on the use of PPIs since they do not respond sufficiently to H2RAs. 24
2.1. PPI ‐induced hypomagnesemia
PPI‐induced hypomagnesemia was first reported in 2006. 25 Since then, numerous clinical studies have confirmed that PPI use leads to hypomagnesemia. 11 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 As PPI users with mild hypomagnesemia (± 0.6 mmol/L) are often asymptomatic, PPI‐induced hypomagnesemia is easily missed because routine serum Mg2+ measurements during PPI therapy are often not performed. 11 By systematic analysis of cohort studies on the prevalence of PPI‐induced hypomagnesemia, we demonstrate that hypomagnesemia is a common side effect of PPI therapy (Table 1). The reported prevalence is approximately 19% (range: 2%–36%). 14 Indeed, PPI treatment increases the risk for the development of hypomagnesemia with an odds ratio (OR) of 1.83 (individual studies report ORs between 1.0 and 5.4, Table 1). 14
TABLE 1.
The association between PPI use and the development of hypomagnesemia a
| Author | Study design | Population | Cut‐off value hypomagnesemia (mmol/L) | No. of PPI users | No. of non‐users | Cases of PPI‐induced hypomagnesemia (%) | Risk assessment b (95% CI) | Adjustment variables |
|---|---|---|---|---|---|---|---|---|
|
Danziger et al. 26 2013 |
Cross‐sectional | Inpatients | <0.80 | 2632 | 8858 | 405 (15.3%) | OR: 1.10 (0.96–1.25) | Age, sex, ethnicity, comorbidities, diuretics, renal function, systolic blood pressure, heart rate, temperature, serum calcium, serum phosphorus, serum glucose, hematocrit |
|
Douwes et al. 27 2019 |
Cross‐sectional | Inpatients | <0.70 | 389 | 300 | 102 (26.0%) | OR: 2.00 (1.21–3.31) | Age, sex, BMI, eGFR, proteinuria, time since kidney transplantation, alcohol, diabetes mellitus, cardiovascular disease, diuretics, tacrolimus, cyclosporine, immunosuppressants, dietary magnesium intake |
| Gau et al. 28 2012 | Cross‐sectional | Inpatients | <0.70 | 207 | 280 | 48 (2.3%) | OR: 2.50 (1.43–4.36) | Age, sex, diabetes mellitus, heart failure, diuretics, magnesium, and potassium supplementation, acute GI illness, serum albumin, serum potassium, serum creatinine |
| Kieboom et al. 29 2015 | Cross‐sectional | Outpatients | <0.80 | 724 | 9094 | 36 (5.0%) | OR: 2.00 (1.36–2.93) | Age, sex, BMI, eGFR, diabetes mellitus, stroke, coronary heart disease, hypertension, alcohol use, diuretics |
|
Kim et al. 11 2015 |
Case–control | Inpatients | <0.70 | 105 | 210 | 32 (35.8%) | OR: 5.39 (1.06–27.49) | Age, sex, comorbidities, drugs, electrolyte levels (sodium, potassium, calcium, urea, creatinine, albumin) |
|
Lindner et al . 30 2014 |
Cross‐sectional | Inpatients | <0.75 | 423 | 4695 | 155 (3.6%) | OR: 2.10 (1.54–2.85) | CCL score, eGFR |
|
Markovits et al. 31 2014 |
Cross‐sectional | Outpatients | <0.70 | 22 458 | 69 714 | 2532 (11.0%) | OR: 1.66 (1.55–1.78) | Age, sex, diabetes mellitus, hypertension, heart failure, eGFR, diuretics, immunosuppressants, lithium, dioxin, recent hospitalization |
|
Pasina et al. 32 2015 |
Cross‐sectional | Inpatients | <0.80 | 299 | 305 | 63 (21.0%) | OR: 4.31 (2.49–7.86) | Age, sex, diabetes mellitus, chronic diarrhea, malabsorption, alcohol |
|
Sutton et al. 33 2019 |
Cross‐sectional | Inpatients | <0.80 | 329 | 5718 | 31 (9.0%) | HR: 3.16 (2.56–3.90) | Age, sex, ethnicity, CCL score, alcohol, viral suppression, index year |
Abbreviations: BMI, body mass index; CCL score, Charlson Comorbidity Index score; CI, confidence interval; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; HR, hazard ratio; OR, odds ratio.
Articles were obtained after PubMed search using the following search terms in April 2020: “proton pump inhibitor” OR “omeprazole” OR “esomeprazole” OR “lansoprazole” OR “dexlansoprazole” OR “pantoprazole” OR “rabeprazole” AND “hypomagnesemia”. Articles were only included if the study reported on the type of PPI, specified the cut‐off value for hypomagnesemia, patient population, number of PPI users, data on serum Mg2+ status, confounding factors, and included a risk assessment on PPI use and hypomagnesemia.
Risk assessment describes the risk to develop hypomagnesemia during PPI therapy.
Heterogeneity in design, population, hypomagnesemia cut‐off value, and adjustment variables may explain the variation in the prevalence of hypomagnesemia among studies. A recent meta‐analysis showed that the incidence of PPI‐induced hypomagnesemia is similar in outpatients and hospitalized patients. 15 Similarly, there were no changes in the incidence of hypomagnesemia with different cut‐off values. 15 Other factors may therefore explain the variability in the development of PPI‐induced hypomagnesemia between populations. We and others demonstrated that diuretics‐use and genetic variants (SNPs) in Mg2+ channel TRPM6 increase the risk for PPI‐induced hypomagnesemia. 29 , 34 , 35 , 36 A meta‐analysis of 12 studies showed that the PPI dose is associated with the development of hypomagnesemia (high dose OR 2.13; 95% CI 1.26–3.59). 14 Additionally, the prolonged duration of PPI treatment may be an additional factor. A PPI use of more than 6 months was associated with a higher risk (OR 2.99; 95% CI 1.73–5.15) to develop hypomagnesemia. 29 Altogether, the treatment duration and PPI dosage are demonstrated to be important factors for the development of PPI‐induced hypomagnesemia.
Urinary Mg2+ excretion is generally reduced in patients with PPI‐induced hypomagnesemia. 25 , 37 , 38 , 39 , 40 , 41 , 42 This observation suggests that the kidney is compensating for reduced intestinal Mg2+ absorption, excluding renal loss as cause for Mg2+ deficiency. Importantly, PPI use did not affect dietary Mg2+ intake or renal function that could cause the urinary Mg2+ loss. Therefore, it is postulated that PPI‐induced hypomagnesemia is caused by impaired intestinal Mg2+ absorption.
2.2. Intestinal Mg2+ absorption
Approximately 30%–50% of the daily Mg2+ intake is absorbed in the gastrointestinal (GI) tract (± 100 mg, resulting a recommended daily intake of 300–350 mg). 17 However, the absorption rate may be higher (up to 80%), when the dietary Mg2+ intake is low. 43
Two independent absorption pathways facilitate intestinal Mg2+ absorption (Figure 1). First, passive transport via tight junction complexes of two neighboring epithelial cells allows mass Mg2+ absorption. 44 This paracellular route consists of occludins, claudins, and E‐cadherin, that maintain the intestinal barrier integrity and facilitate the transport of ions, nutrients, and water. 45 Claudin‐1, −3, −4, −5, and − 8 are known for their tightening properties of intestinal epithelium. 46 They show high expression levels in the colon but are hardly expressed in the small intestine, making this segment very permeable to ions. 47 Claudin‐2, −7, and − 12 are selective for cations and enhance the paracellular permeability in duodenum and ileum. 47 In particular, the lumen‐negative transepithelial electrical potential (± 5 mV) across the tight junction determines the permeability. 48 In the small intestine, Mg2+ absorption is facilitated mainly via the paracellular absorption route. Indeed, luminal Mg2+ concentrations, and Mg2+ absorption rates are linearly correlated. 49
FIGURE 1.
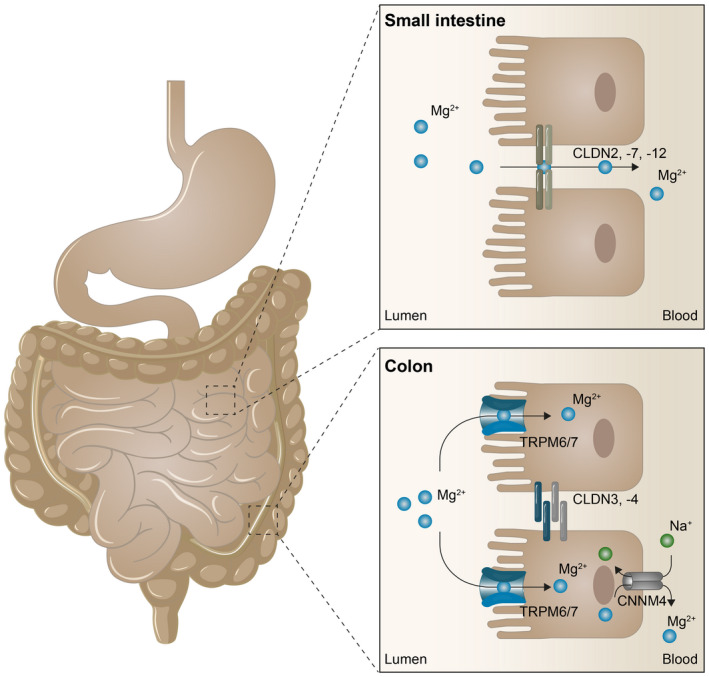
Intestinal Mg2+ absorption pathways Mg2+ absorption is mediated by two separate absorption pathways. In the small intestine, Mg2+ absorption is mainly of paracellular nature through tight junction complexes between adjacent epithelial cells. Here, CLDN2, −7, and − 12 enhance paracellular permeability. In the large intestine, Mg2+ is absorbed via active, transcellular transport facilitated by TRPM6/7 channels. Extrusion of Mg2+ to the blood compartment is mediated by CNNM4 on the basolateral side of the colonocytes.
In the colon and distal segments of the ileum, fine‐tuning of Mg2+ absorption is mediated via channels of the transient receptor potential melastatin (TRPM) family. This absorption pathway is transcellular and secondary active. Transcellular transport of Mg2+ accounts for ~30% of total Mg2+ absorption in normal physiological conditions. 17 TRPM6 and TRPM7 channels are expressed on the luminal side and cyclin M4 (CNMM4) Na+‐Mg2+ exchangers on the basolateral side of the intestinal epithelial cell. 50 TRPM6/7 channels form heterotetramers and facilitate Mg2+ uptake. 51 TRPM6 reduces the inhibition of TRPM7 by Mg‐ATP sensitivity and thereby increases the permeability for Mg2+ . 52 Mutations in the TRPM6 gene are causative for hypomagnesemia with secondary hypocalcemia (HSH), an autosomal recessive genetic disorder characterized by extremely low serum Mg2+ levels (0.1–0.3 mmol/L). 17 This hereditary disease highlights the importance of TRPM6 for intestinal Mg2+ absorption. Moreover, it was shown that TRPM6 in the intestine, but not in the kidney, is essential to maintain systemic Mg2+ balance in mice. 52 Indeed, intestine‐specific disruption of Trpm6 in mice caused severe hypomagnesemia due to a defect in intestinal Mg2+ absorption. 52
3. PPI ‐INDUCED HYPOMAGNESEMIA IS CAUSED BY INTESTINAL MALABSORPTION OF MG2+
Over the last years, several experimental studies have addressed the putative molecular mechanisms by which PPIs affect Mg2+ absorption along the intestinal tract. Here, we will critically describe the evidence for these mechanisms as potential underlying cause for the development of PPI‐induced hypomagnesemia.
3.1. PPIs affect paracellular transport of Mg2+ in the small intestine
Mg2+ absorption in the small intestine depends on passive paracellular diffusion. Consequently, two factors are essential to consider: Mg2+ availability and tight junction permeability. Both factors are potentially compromised by PPI treatment.
3.1.1. Luminal pH of the small intestine
PPIs have a direct effect on the luminal pH of the small intestine. In patients with pancreatitis, the gastric pH was correlated with the small intestinal pH during PPI treatment. 53 In general, an increased gastric pH by 2 pH units translates into a 1 pH unit increase in the small intestine. 53 Moreover, the luminal pH of the stomach, duodenum, and jejunum, but not of cecum and colon, are increased by a single omeprazole dose in Sprague–Dawley rats. 54 Consequently, paracellular Mg2+ transport in duodenum, jejunum, and ileum was reduced by 81%, 71%, and 69%, respectively. 54 Similarly, long‐term omeprazole treatment in Sprague–Dawley rats showed reduced duodenal Mg2+ absorption as result of a higher luminal pH. 55 The diminished Mg2+ absorption can be explained by the decreased solubility of Mg2+ at a higher pH. 56 Moreover, Mg2+ in the GI tract is partially bound to proteins and negatively charged ions such as Cl− and PO4 3−. This is also the reason why Mg2+‐salts are effective oral phosphate binders and are used for the treatment of chronic kidney disease (CKD). 57 However, the effectiveness is dependent on the luminal pH as Mg2+ salts bind more phosphate in an alkaline environment. 58 Together, PPIs increase the luminal pH of the segments of the small intestine and thereby might reduce the Mg2+ solubility and consequently absorption (Figure 2).
FIGURE 2.
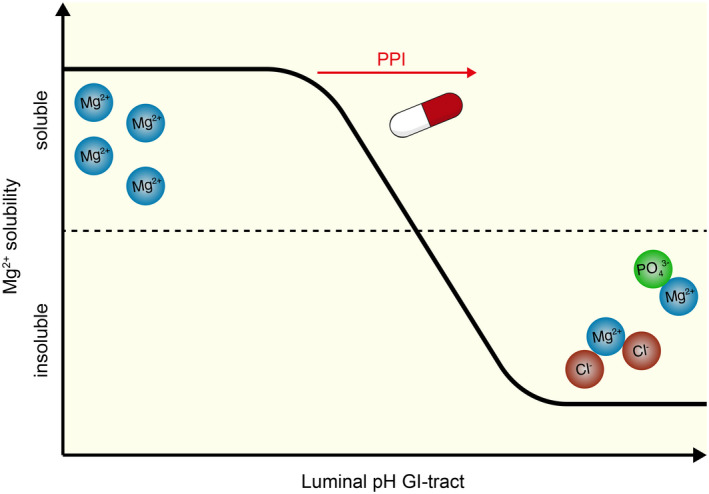
Hypothesis of the effects of PPIs on the Mg2+ solubility in the gastrointestinal tract. Schematic representation in which PPIs increase the luminal pH of the gastrointestinal (GI) tract and thereby affect Mg2+ solubility. At higher luminal pH, Mg2+ binds negatively charged molecules, such as Cl− and PO4 3−, resulting in reduced Mg2+ availability for absorption.
3.1.2. Modulation of paracellular permeability
PPIs affect paracellular permeability by direct and indirect mechanisms. Caco‐2 cells treated with omeprazole (200–600 ng/mL) for 14 and 21 days showed reduced paracellular transport as measured by Mg2+ fluxes over Caco‐2 cell monolayers. 59 Omeprazole‐treated Caco‐2 cells demonstrate reduced protein expression of permeability‐enhancing claudins, including claudin‐7 and ‐12, but not claudin‐2. 60 Consequently, the transepithelial electrical resistance (TEER) was increased, suggesting that omeprazole reduced the paracellular permeability 59 (Figure 3). Moreover, PPIs also indirectly influence paracellular permeability by increasing the luminal pH. Lowering the apical pH from 7.4 to 5.5 increased the protein expression of CLDN‐7 and ‐12 in Caco‐2 cells. 60 Under these conditions, the inhibitory effect of omeprazole was abolished and paracellular transport of Mg2+ was enhanced. 60 This study suggests that luminal pH and intestinal permeability are closely linked.
FIGURE 3.
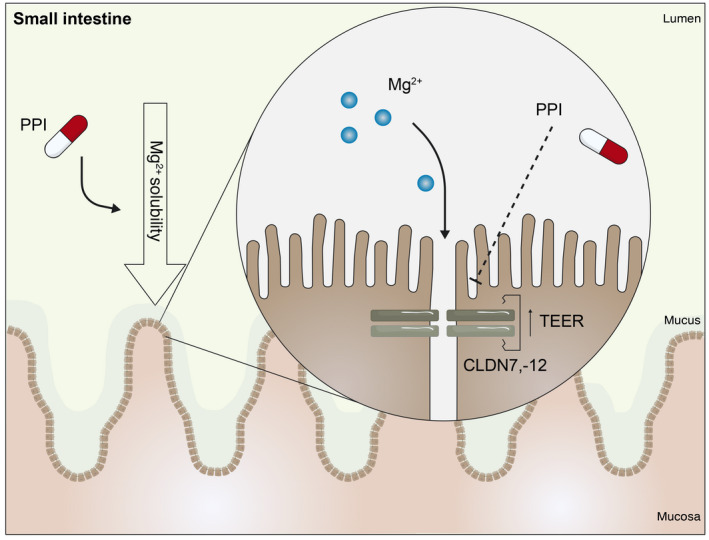
PPIs impair Mg2+ absorption in the small intestine During PPI therapy, the luminal pH of the small intestine increases. Consequently, Mg2+ solubility and absorption are reduced. Moreover, PPIs lower the expression of CLDN7, −12 and increase the transepithelial electrical resistance (TEER). Consequently, Mg2+ absorption in the small intestine is decreased.
3.2. PPIs affect transcellular transport of Mg2+ in the colon
The colon has been the main focus of studies toward the mechanisms of PPI‐induced hypomagnesemia. In this segment, a significant amount of Mg2+ transport is absorbed in healthy subjects. Moreover, absorption in the colon can compensate for reduced Mg2+ absorption in the small intestine. Although the colon is spatially separated from the stomach where PPIs predominantly act, a growing body of evidence demonstrates that colon is also affected by PPI therapy.
3.2.1. Luminal pH of the colon
The colonic H+, K+‐ATPase (ATP12A) is a close homolog of the gastric H+, K+‐ATPase (ATP4A). 61 Consequently, it has been hypothesized that omeprazole can inhibit colonic H+, K+‐ATPases, resulting in a less acidic local pH. Indeed, omeprazole treatment significantly increased the mRNA expression of colonic H+, K+‐ATPases in PPI‐treated mice. 62 An increased intraluminal pH in the colon would directly affect the solubility of Mg2+, as discussed in previous sections. Moreover, pH may also directly affect the activity of the Mg2+ channels TRPM6 and TRPM7 in the colon. However, whether PPIs have direct effects on the colonic H+, K+‐ATPase is still heavily debatable, as PPIs will not accumulate in these cells, in contrast to the parietal cells of the stomach.
3.2.2. TRPM6 function
The pH in the colon may also determine the activity TRPM6/7, which represents the luminal Mg2+ channel in the colon. 63 Given that TRPM6 activity is higher at lower pH, 63 the omeprazole‐induced increase in colonic pH might reduce TRPM6‐mediated Mg2+ absorption (Figure 4). Omeprazole treatment was shown to diminish colonic Mg2+ absorption by 39% in Sprague–Dawley rats. 54 Despite an increased protein expression of TRPM6 in the colon of omeprazole‐treated rats, which may be a compensatory response for reduced absorption. 54 Increased colonic Trpm6 expression has been demonstrated previously in omeprazole‐treated mice. 62 Genetic studies also confirm the essential role of TRPM6 in PPI‐induced hypomagnesemia. People with two single nucleotide polymorphisms (SNPs) in the TRPM6 gene (rs3750425 and rs2274924) have an increased risk for the development of hypomagnesemia in response to PPI treatment. 36 Nevertheless, the mechanisms by which PPIs affect TRPM6 are largely unresolved.
FIGURE 4.
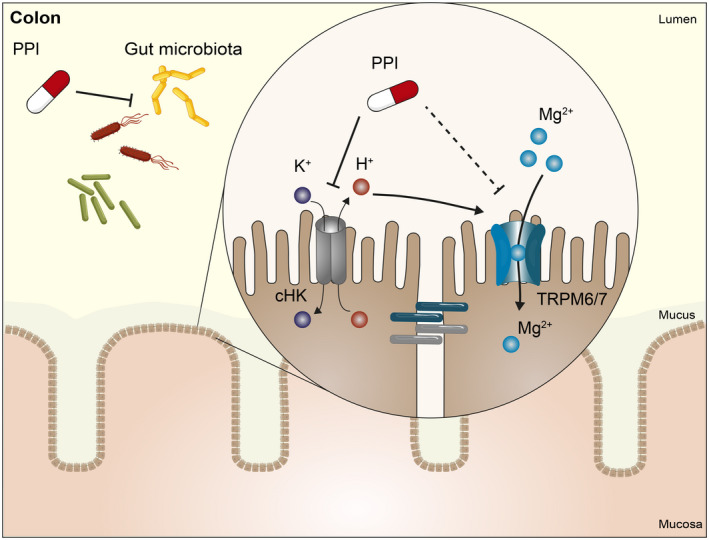
PPIs affect Mg2+ absorption in the colon. PPIs affect the composition and diversity of the gut microbiome. Additionally, PPIs inhibit the colonic H+, K+‐ATPases (cHK, ATP12A) making the pH of the colon less acidic. These factors might reduce the activity of TRPM6 channels.
3.2.3. Gut microbiome
Importantly, bacterial fermentation results in acidification of the colon. This has been shown beneficial for the solubility and absorption of Mg2+ 43. Therefore, it is interesting that PPIs have consistently been shown to change the composition of the gut microbiome (Table 2). 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 In particular, PPI users have generally a lower gut microbial diversity 64 , 66 , 67 , 69 , 70 , 71 , 72 , 73 (Figure 4). The bacterial richness and evenness (alpha diversity) of the gut microbiota is lower in PPI users compared to non‐users. 66 , 67 Additionally, the majority of studies also report significant differences in overall bacterial composition (beta diversity). 64 , 66 , 67 , 72 , 73 All studies reporting microbial changes in response to PPI treatment have been summarized in Table 2. In general, differences in overall bacterial composition are more common than differences in species richness. At the taxonomic level, PPI use is associated with an increase in the abundance of Firmicutes, including bacteria from the order Lactobacillales (with families Enterococcaceae, Lactobacillaceae, and Streptococcaceae). 64 , 66 , 68 , 69 , 70 , 72 , 73 The PPI‐induced disturbances in the gut microbiota may be the result of changes in the luminal pH. It is hypothesized that more (pathogenic) bacteria will survive the stomach with subsequent changes in the gut microbiome. 74 Indeed, several studies have shown that species in the oral microbiota are significantly enriched in the fecal microbiota of PPI users. 66 , 67 Loss of this barrier might increase the risk for enteric infections. Indeed, PPI use has been previously shown to increase the risk for the development of Clostridium difficile (CDI) infection (OR 1.26; 95% CI 1.12–1.39) or small intestinal bacterial overgrowth (SIBO) (OR 2.28; 95% CI 1.24–4.21). 75 , 76
TABLE 2.
PPI use is associated with changes in the gut microbiome a
| Author a | Study design (population) | PPI users vs non‐users | Duration of PPI treatment | Sample | Sequencing technique | Changes at the taxonomic level b | Changes in diversity | |
|---|---|---|---|---|---|---|---|---|
| Alpha (metric) | Beta (metric) | |||||||
|
Clooney et al. 64 2016 |
Cross‐sectional cohort study from population‐based database (Canada) | 32 vs. 29 | >5 years | Feces | 16S |
↑ f_Lachnospiraceae ↑ f_Streptococcaceae |
No difference (Shannon index) (Chao1 richness) | Significant shift (Bray‐Curtis) (UniFrac PCA) |
|
Freedberg et al. 65 2015 |
Prospective open‐label trial (healthy adults) | 12 | 4–8 weeks | Feces | 16S |
↑ f_Streptococcaceae ↑ f_Enterococcaceae |
No difference (Shannon index) | No difference (weighted UniFrac PCA) |
|
Imhann et al. 66 2016 |
Cross‐sectional cohort study from population‐based cohorts (Lifelines‐DEEP, IBN UMCG, IBS MUMC) | 211 vs. 1604 | Not reported | Feces | 16S |
↑ f_Lactobacillaceae ↑ f_Enterococcaceae ↑ f_Streptococcaceae ↑ f_Micrococcaceae ↓ f_Bifidobacteriacaea |
Decreased (Shannon index) (Chao1 richness ) | Significant shift (PCoA) |
|
Jackson et al. 67 2016 |
Cross‐sectional cohort study (healthy twins: TwinsUK) | 229 vs. 1598 | >3 years | Feces | 16S |
↑ f_Streptococcaceae ↑ f_Lactobacillaceae ↑ f_Micrococcaceae ↓ f_Ruminococcaceae |
Decreased (Shannon index) (OTU counts) (Chao1 richness) | Not determined |
|
Mishiro et al. 68 2018 |
Prospective open label trial (healthy adults) | 10 | 4 weeks | Feces | 16S | ↑ g_Streptococcus | No difference (Shannon index) (Chao1 richness) | No difference (Bray‐Curtis) (unweighted UniFrac) |
|
Otsuka et al. 69 2017 |
Prospective open label trial (healthy adults) | 11 | 4 weeks | Feces | 16S |
↑ g_Streptococcus ↑ g_Bacteroides |
No difference (Shannon index) (Chao1 richness) | Significant shift (UniFrac PCA) |
|
Reveles et al. 70 2018 |
Prospective open‐label trial (healthy elderly adults) | 24 | 2 weeks | Feces | 16S |
↑ f_Streptococcaceae ↓ f_Lachnospiraceae ↓ f_Bifidobacteriacaea |
No difference (Shannon index) | Significant shift (Bray‐Curtis) |
|
Seto et al. 71 2014 |
Prospective open‐label trial (healthy adults) | 9 | 4 weeks | Feces | 16S | No differences | Decreased (OTU counts) (Chao1 richness) | No difference (unweighted UniFrac) |
|
Takagi et al. 72 2018 |
Cross‐sectional cohort study (outpatients) | 36 vs. 36 | >1 year | Feces | 16S |
↑ g_Streptococcus ↑ g_Ruminococcus ↓ g_Faecalibacterium |
No difference (Shannon index) (Chao1 richness) | Significant shift (UniFrac PCA) |
|
Tsuda et al. 73 2015 |
Cross‐sectional cohort study (outpatients) | 18 vs. 27 | >2 year | Feces | 16S |
↑ g_Streptococcus ↓ g_Faecalibacterium |
No difference (Shannon index) | Significant shift (UniFrac PCA) |
Abbreviations: 16S, 16S rRNA sequencing; OTU, operational taxonomic unit; PCA, principal component analysis; PCoA, principal coordinates analysis; UniFrac, unique fraction.
Articles were obtained after PubMed search using the following search terms in April 2020: “proton pump inhibitor” AND “microbiome” OR “microbiota”.
Taxonomic composition was described as k_, kingdom; p_, phylum; c_, class; o_, order; f_, family; g_, genus; s_, species. ↑↓ describe the direction of change.
3.2.4. Short‐chain fatty acids
Bacterial fermentation has been associated with increased production of important end‐metabolites, including short‐chain fatty acids (SCFAs; acetate, propionate, butyrate). However, in a recent animal study, omeprazole did not affect the concentrations of colonic SCFAs in mice with hypomagnesemia, despite the profound effects of PPIs on the gut microbiome composition. 77 In twelve patients with reflux esophagitis, the concentrations of SCFAs were also not altered after 8 weeks of PPI treatment. 78 This finding suggests that the malabsorption of Mg2+ is not caused by direct effects of SCFAs, but likely by changes in the luminal environment of the colon. Targeting the gut microbiota with prebiotic inulin fibers (20 g/day) improved serum Mg2+ levels in patients with PPI‐induced hypomagnesemia. 79 Similar results were reported by Coudray et al. showing that inulin lowered the cecal pH and increased Mg2+ solubility from 13% to 75%–95% compared to fructose treatment. These changes significantly increased the Mg2+ absorption compared to a fiber‐free diet in rats. 80 Altogether, these studies further highlight the importance of an acidic luminal environment for the absorption of Mg2+ in the colon.
3.3. Treatment of PPI ‐induced hypomagnesemia
Currently, there are no adequate treatment strategies to restore hypomagnesemia in PPI users. PPI withdrawal still remains the gold standard. 20 Moreover, oral Mg2+ supplementation is often insufficient and causes diarrhea, nausea, and abdominal cramping at high concentrations. 17 In this section, we discuss the aforementioned treatment options, as well as future research strategies aiming to acidify the intestinal lumen in PPI users with hypomagnesemia.
3.3.1. Withdrawal
Clinical case reports show that serum Mg2+ levels restore to physiological concentrations upon PPI withdrawal, 20 but reappear after re‐challenge with a PPI. Both observations occur within days to weeks and were independent of type of PPI. 20 However, discontinuation of PPI therapy might not always be possible and, therefore, switching to different acid suppressants, such as H2RAs, could be considered. However, it is still debatable whether H2RAs also cause hypomagnesemia. Kieboom et al. demonstrated a positive correlation between H2RA use and hypomagnesemia (OR: 2.19; 95% CI 1.21–3.98), 29 while this was not observed in a different patient cohort (OR 1.06; 95% CI 0.54–2.06). 35 In previous sections, we have pointed out that treatment duration and daily dose are important contributing factors for the development of PPI‐induced hypomagnesemia. Therefore, long‐term use and high doses of PPI treatment should be prevented by healthcare professionals.
3.3.2. Mg2+ supplementation
Oral Mg2+ supplementation does not fully restore serum Mg2+ levels in patients with PPI‐induced hypomagnesemia. Indeed, high dose (30–40 mmol/day) oral Mg2+ supplements only partly restored serum Mg2+ levels in two PPI users with severe hypomagnesemia. 37 Only PPI withdrawal completely resolved the hypomagnesemia in these patients. 37 Additionally, intravenous Mg2+ infusions did not correct the Mg2+ deficiency in PPI users as shown by the consistently low levels of Mg2+ in the urine. 41 In the same study, oral Mg2+ supplementation only shortly maintained serum Mg2+ levels within the normal range. 41 Considering the fact that high oral Mg2+ supplementation often causes diarrhea, nausea, and abdominal cramping, this treatment option is both ineffective and poorly tolerated. 17
3.3.3. Prebiotics
PPIs greatly affect the luminal pH of the GI tract. Dietary fibers might therefore be a promising treatment strategy to target the microbiome and acidify the lumen of the colon. Recent intervention studies demonstrate that pre‐ and probiotic approaches can reduce PPI‐induced side effects, such as enteric infections and mineral deficiencies. Supplementation with Lactobacillus reuteri reduced the prevalence of enteric infections after 12 weeks of treatment in children with GERD. 81 Similar results were observed in reflux esophagitis patients that were treated for 8 weeks with a probiotic cocktail of Bacillus subtilis and Enterococcus faecium. 82 A recent meta‐analysis demonstrated that probiotics improve GERD‐related symptoms, including the frequency and duration of reflux episodes. 83
Moreover, prebiotic fibers have been previously shown to improve mineral absorption. Inulin supplementation successfully increased Ca2+ and Mg2+ in postmenopausal women. 84 Dietary intake of fructose oligosaccharides improved Mg2+ absorption by 18% after 36 days in adolescent girls using a stable isotope technique. 85 A recent study in PPI users with hypomagnesemia demonstrated that inulin fibers for 14 days significantly increased serum Mg2+ levels. 79 Importantly, this microbiome‐targeting therapy with prebiotics improved hypomagnesemia‐related symptoms, including generalized weakness, tetany of hands, and muscle cramps. 79
4. PPI ‐INDUCED HYPOMAGNESEMIA: DIGESTING CURRENT HYPOTHESES
Hypomagnesemia is a well‐known side effect of PPIs. Treatment duration (>1 year) and daily dose are important contributing factors for the development of PPI‐induced hypomagnesemia. Current clinical and experimental studies point to malabsorption of Mg2+ in the GI tract as underlying cause for the development of hypomagnesemia. This observation sets PPI‐induced hypomagnesemia apart from all other forms of drug‐induced hypomagnesemia that are characterized by renal Mg2+ wasting, as seen in users of gentamycin, calcineurin inhibitors, diuretics, and anti‐diabetic drugs. 86
In this review, we set out numerous mechanisms by which PPIs affect Mg2+ absorption in both small intestine and colon. Although the exact molecular mechanism remains to be elucidated, most evidence points toward reduced Mg2+ solubility in the small intestine or changes in the composition and function of the gut microbiome in the colon. This is likely caused by the increase of the luminal pH during PPI treatment. Future studies using wireless pH monitoring capsules are required to better understand the physiological pH range of different intestinal segments as well as the direct effects of PPIs on the luminal pH. This would allow to determine the optimal pH range for Mg2+ solubility. Considering the fact that high oral Mg2+ supplementation does not recover serum Mg2+ levels in PPI users with hypomagnesemia it is very likely that PPIs mainly impair active Mg2+ absorption in the colon rather than passive absorption in the small intestine.
To date, the cornerstone of hypomagnesemia treatment is still PPI withdrawal. However, this is not possible for patients who are dependent on PPIs. Alternative treatment options, such as oral Mg2+ supplementation or the use of different acid suppressants, are less effective. The gut microbiome might be a novel target to ameliorate PPI‐induced side effects using prebiotic strategies. This might rely on common mechanisms: (i) diets rich in fibers have been associated with increased bacterial diversity 87 , 88 ; (ii) fermentation of dietary fibers increases the production of SCFAs and acidification of the intraluminal pH. 89 , 90 Clinical trials studies are required to examine which dietary fibers optimally enhance intestinal Mg2+ absorption in PPI users with hypomagnesemia.
CONFLICT OF INTEREST
No conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the Netherlands Organization for Scientific Research (J.G.J. Hoenderop, NWO VICI 016.130.668 and J.H.F. de Baaij, NWO VENI 016.186.012).
Gommers LMM, Hoenderop JGJ, de Baaij JHF. Mechanisms of proton pump inhibitor‐induced hypomagnesemia. Acta Physiol. 2022;235:e13846. doi: 10.1111/apha.13846
REFERENCES
- 1. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thong BKS, Ima‐Nirwana S, Chin KY. Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int J Environ Res Public Health. 2019;16(9):1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton pump inhibitors: review of emerging concerns. Mayo Clin Proc. 2018;93(2):240‐246. [DOI] [PubMed] [Google Scholar]
- 4. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long‐term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;4:706‐715. [DOI] [PubMed] [Google Scholar]
- 5. Higuchi T. Long‐term use of proton pump inhibitors: many a little makes a mickle? Pol Arch Intern Med. 2020;130(3):174‐175. [DOI] [PubMed] [Google Scholar]
- 6. Yadlapati R, Kahrilas PJ. When is proton pump inhibitor use appropriate? BMC Med. 2017;15(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yadlapati R, Kahrilas PJ. The "dangers" of chronic proton pump inhibitor use. J Allergy Clin Immunol. 2018;141(1):79‐81. [DOI] [PubMed] [Google Scholar]
- 8. Chrysant SG. Proton pump inhibitor‐induced hypomagnesemia complicated with serious cardiac arrhythmias. Expert Rev Cardiovasc Ther. 2019;17(5):345‐351. [DOI] [PubMed] [Google Scholar]
- 9. Corsonello A, Lattanzio F. Cardiovascular and non‐cardiovascular concerns with proton pump inhibitors: are they safe? Trends Cardiovasc Med. 2019;29(6):353‐360. [DOI] [PubMed] [Google Scholar]
- 10. Kamal F, Khan MA, Molnar MZ, Howden CW. The association between proton pump inhibitor use with acute kidney injury and chronic kidney disease. J Clin Gastroenterol. 2018;52(6):468‐476. [DOI] [PubMed] [Google Scholar]
- 11. Kim S, Lee H, Park CH, et al. Clinical predictors associated with proton pump inhibitor‐induced hypomagnesemia. Am J Ther. 2015;22(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 12. Wilhelm SM, Rjater RG, Kale‐Pradhan PB. Perils and pitfalls of long‐term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6(4):443‐451. [DOI] [PubMed] [Google Scholar]
- 13. Park CH, Kim EH, Roh YH, Kim HY, Lee SK. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta‐analysis. PLoS One. 2014;9(11):e112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srinutta T, Chewcharat A, Takkavatakarn K, et al. Proton pump inhibitors and hypomagnesemia: a meta‐analysis of observational studies. Medicine. 2019;98(44):e17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liao S, Gan L, Mei Z. Does the use of proton pump inhibitors increase the risk of hypomagnesemia: an updated systematic review and meta‐analysis. Medicine. 2019;98(13):e15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. FDA . Low magnesium levels can be associated with long‐term use of proton pump inhibitor drugs (PPIs). FDA Drug Safety Communication; 2011. http://www.fda.gov/drugs/drugsafety/ucm245011.htm [Google Scholar]
- 17. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1‐46. [DOI] [PubMed] [Google Scholar]
- 18. Florentin M, Elisaf MS. Proton pump inhibitor‐induced hypomagnesemia: a new challenge. World J Nephrol. 2012;1(6):151‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perazella MA. Proton pump inhibitors and hypomagnesemia: a rare but serious complication. Kidney Int. 2013;83(4):553‐556. [DOI] [PubMed] [Google Scholar]
- 20. Hess MW, RJM HJGB, JPH D. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405‐413. [DOI] [PubMed] [Google Scholar]
- 21. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor‐associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773‐780. [DOI] [PubMed] [Google Scholar]
- 22. Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther. 2006;23(Suppl 2):2‐8. [DOI] [PubMed] [Google Scholar]
- 23. Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19(1):25‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kinoshita Y, Ishimura N, Ishihara S. Advantages and disadvantages of long‐term proton pump inhibitor use. J Neurogastroenterol Motil. 2018;24(2):182‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Epstein M, McGrath S, Law F. Proton‐pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med. 2006;355(17):1834‐1836. [DOI] [PubMed] [Google Scholar]
- 26. Danziger J, JH W, Scott DJ, et al. Proton‐pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Douwes RM, Gomes‐Neto AW, Schutten JC, et al. Proton‐pump inhibitors and hypomagnesaemia in kidney transplant recipients. J Clin Med. 2019;12:2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gau JT, Yang YX, Chen R, Kao TC. Uses of proton pump inhibitors and hypomagnesemia. Pharmacoepidemiol Drug Saf. 2012;21(5):553‐559. [DOI] [PubMed] [Google Scholar]
- 29. Kieboom BC, Kiefte‐de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population‐based cohort study. Am J Kidney Dis. 2015;66(5):775‐782. [DOI] [PubMed] [Google Scholar]
- 30. Lindner G, Funk GC, Leichtle AB, et al. Impact of proton pump inhibitor use on magnesium homoeostasis: a cross‐sectional study in a tertiary emergency department. Int J Clin Pract. 2014;68(11):1352‐1357. [DOI] [PubMed] [Google Scholar]
- 31. Markovits N, Loebstein R, Halkin H, et al. The association of proton pump inhibitors and hypomagnesemia in the community setting. J Clin Pharmacol. 2014;54(8):889‐895. [DOI] [PubMed] [Google Scholar]
- 32. Pasina L, Zanotta D, Puricelli S, Djignefa DC, Bonoldi G. Proton pump inhibitors and risk of hypomagnesemia. Eur J Intern Med. 2015;26(7):e25‐e26. [DOI] [PubMed] [Google Scholar]
- 33. Sutton SS, Magagnoli J, Cummings T, Hardin JW. The association between the use of proton pump inhibitors and the risk of hypomagnesemia in a national cohort of veteran patients with HIV. J Int Assoc Provid AIDS Care. 2019;18:2325958218821652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uehara A, Kita Y, Sumi H, Shibagaki Y. Proton‐pump inhibitor‐induced severe hypomagnesemia and hypocalcemia are clinically masked by thiazide diuretic. Intern Med. 2019;58(15):2201‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population‐based case‐control study. PLoS Med. 2014;11(9):e1001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hess MW, de Baaij JH, Broekman MM, et al. Common single nucleotide polymorphisms in transient receptor potential melastatin type 6 increase the risk for proton pump inhibitor‐induced hypomagnesemia: a case‐control study. Pharmacogenet Genomics. 2017;27(3):83‐88. [DOI] [PubMed] [Google Scholar]
- 37. Cundy T, Dissanayake A. Severe hypomagnesaemia in long‐term users of proton‐pump inhibitors. Clin Endocrinol (Oxf). 2008;69(2):338‐341. [DOI] [PubMed] [Google Scholar]
- 38. Furlanetto TW, Faulhaber GA. Hypomagnesemia and proton pump inhibitors: below the tip of the iceberg. Arch Intern Med. 2011;171(15):1391‐1392. [DOI] [PubMed] [Google Scholar]
- 39. Hoorn EJ, van der Hoek J, de Man RA, Kuipers EJ, Bolwerk C, Zietse R. A case series of proton pump inhibitor‐induced hypomagnesemia. Am J Kidney Dis. 2010;56(1):112‐116. [DOI] [PubMed] [Google Scholar]
- 40. Kuipers MT, Thang HD, Arntzenius AB. Hypomagnesaemia due to use of proton pump inhibitors‐‐a review. Neth J Med. 2009;67(5):169‐172. [PubMed] [Google Scholar]
- 41. Mackay JD, Bladon PT. Hypomagnesaemia due to proton‐pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387‐395. [DOI] [PubMed] [Google Scholar]
- 42. William JH, Nelson R, Hayman N, Mukamal KJ, Danziger J. Proton‐pump inhibitor use is associated with lower urinary magnesium excretion. Nephrol Ther. 2014;19(12):781‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schuchardt JP, Hahn A. Intestinal absorption and factors influencing bioavailability of magnesium‐an update. Curr Nutr Food Sci. 2017;13(4):260‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Baaij JH, Hoenderop JG, Bindels RJ. Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J. 2012;5(Suppl 1):i15‐i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13(1):11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amasheh S, Fromm M, Gunzel D. Claudins of intestine and nephron ‐ a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf). 2011;201(1):133‐140. [DOI] [PubMed] [Google Scholar]
- 47. Lameris AL, Huybers S, Kaukinen K, et al. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48(1):58‐69. [DOI] [PubMed] [Google Scholar]
- 48. Fordtran JS, Rector FC Jr, Carter NW. The mechanisms of sodium absorption in the human small intestine. J Clin Invest. 1968;47(4):884‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lameris AL, Nevalainen PI, Reijnen D, et al. Segmental transport of ca(2)(+) and mg(2)(+) along the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2015;308(3):G206‐G216. [DOI] [PubMed] [Google Scholar]
- 50. Yamazaki D, Funato Y, Miura J, et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet. 2013;9(12):e1003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferioli S, Zierler S, Zaißerer J, Schredelseker J, Gudermann T, Chubanov V. TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic mg(2+) and mg·ATP. Sci Rep. 2017;7(1):8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chubanov V, Ferioli S, Wisnowsky A, et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. Elife. 2016;5:e20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakamura T, Arai Y, Tando Y, et al. Effect of omeprazole on changes in gastric and upper small intestine pH levels in patients with chronic pancreatitis. Clin Ther. 1995;17(3):448‐459. [DOI] [PubMed] [Google Scholar]
- 54. Suksridechacin N, Kulwong P, Chamniansawat S, Thongon N. Effect of prolonged omeprazole administration on segmental intestinal mg(2+) absorption in male Sprague‐Dawley rats. World J Gastroenterol. 2020;26(11):1142‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thongon N, Penguy J, Kulwong S, Khongmueang K, Thongma M. Omeprazole suppressed plasma magnesium level and duodenal magnesium absorption in male Sprague‐Dawley rats. Pflugers Arch. 2016;468(11‐12):1809‐1821. [DOI] [PubMed] [Google Scholar]
- 56. Dalley DE, Isherwood P, Sykes AR, Robson AB. Effect of in vitro manipulation of pH on magnesium solubility in ruminal and caecal digesta in sheep. J Agric Sci. 1997;129(1):107‐111. [Google Scholar]
- 57. Hutchison AJ. Oral phosphate binders. Kidney Int. 2009;75(9):906‐914. [DOI] [PubMed] [Google Scholar]
- 58. Schumacher SP, Schurgers LJ, Vervloet MG, Neradova A. Influence of pH and phosphate concentration on the phosphate binding capacity of five contemporary binders. An in vitro study. Nephrology (Carlton). 2019;24(2):221‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thongon N, Krishnamra N. Omeprazole decreases magnesium transport across Caco‐2 monolayers. World J Gastroenterol. 2011;17(12):1574‐1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thongon N, Krishnamra N. Apical acidity decreases inhibitory effect of omeprazole on mg(2+) absorption and claudin‐7 and ‐12 expression in Caco‐2 monolayers. Exp Mol Med. 2012;44(11):684‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crowson MS, Shull GE. Isolation and characterization of a cDNA encoding the putative distal colon H+,K(+)‐ATPase. Similarity of deduced amino acid sequence to gastric H+,K(+)‐ATPase and Na+,K(+)‐ATPase and mRNA expression in distal colon, kidney, and uterus. J Biol Chem. 1992;267(19):13740‐13748. [PubMed] [Google Scholar]
- 62. Lameris AL, Hess MW, van Kruijsbergen I, Hoenderop JG, Bindels RJ. Omeprazole enhances the colonic expression of the mg(2+) transporter TRPM6. Pflugers Arch. 2013;465(11):1613‐1620. [DOI] [PubMed] [Google Scholar]
- 63. Li M, Jiang J, Yue L. Functional characterization of homo‐ and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127(5):525‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Clooney AG, Bernstein CN, Leslie WD, et al. A comparison of the gut microbiome between long‐term users and non‐users of proton pump inhibitors. Aliment Pharmacol Ther. 2016;43(9):974‐984. [DOI] [PubMed] [Google Scholar]
- 65. Freedberg DE, Toussaint NC, Chen SP, et al. Proton pump inhibitors Alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology. 2015;149(4):883‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mishiro T, Oka K, Kuroki Y, et al. Oral microbiome alterations of healthy volunteers with proton pump inhibitor. J Gastroenterol Hepatol. 2018;33(5):1059‐1066. [DOI] [PubMed] [Google Scholar]
- 69. Otsuka T, Sugimoto M, Inoue R, et al. Influence of potassium‐competitive acid blocker on the gut microbiome of helicobacter pylori‐negative healthy individuals. Gut. 2017;66(9):1723‐1725. [DOI] [PubMed] [Google Scholar]
- 70. Reveles KR, Ryan CN, Chan L, Cosimi RA, Haynes WL. Proton pump inhibitor use associated with changes in gut microbiota composition. Gut. 2018;67(7):1369‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2(42):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Takagi T, Naito Y, Inoue R, et al. The influence of long‐term use of proton pump inhibitors on the gut microbiota: an age‐sex‐matched case‐control study. J Clin Biochem Nutr. 2018;62(1):100‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tsuda A, Suda W, Morita H, et al. Influence of proton‐pump inhibitors on the luminal microbiota in the gastrointestinal tract. Clin Transl Gastroenterol. 2015;6(6):e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bruno G, Zaccari P, Rocco G, et al. Proton pump inhibitors and dysbiosis: current knowledge and aspects to be clarified. World J Gastroenterol. 2019;25(22):2706‐2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Macke L, Schulz C, Koletzko L, Malfertheiner P. Systematic review: the effects of proton pump inhibitors on the microbiome of the digestive tract‐evidence from next‐generation sequencing studies. Aliment Pharmacol Ther. 2020;51(5):505‐526. [DOI] [PubMed] [Google Scholar]
- 76. Singh A, Cresci GA, Kirby DF. Proton pump inhibitors: risks and rewards and emerging consequences to the gut microbiome. Nutr Clin Pract. 2018;33(5):614‐624. [DOI] [PubMed] [Google Scholar]
- 77. Gommers LMM, Ederveen THA, van der Wijst J, et al. Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI‐induced hypomagnesemia. FASEB J. 2019;33(10):11235‐11246. [DOI] [PubMed] [Google Scholar]
- 78. Hojo M, Asahara T, Nagahara A, et al. Gut microbiota composition before and after use of proton pump inhibitors. Dig Dis Sci. 2018;63(11):2940‐2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hess MW, de Baaij JH, Broekman M, et al. Inulin significantly improves serum magnesium levels in proton pump inhibitor‐induced hypomagnesaemia. Aliment Pharmacol Ther. 2016;43(11):1178‐1185. [DOI] [PubMed] [Google Scholar]
- 80. Coudray C, Tressol JC, Gueux E, Rayssiguier Y. Effects of inulin‐type fructans of different chain length and type of branching on intestinal absorption and balance of calcium and magnesium in rats. Eur J Nutr. 2003;42(2):91‐98. [DOI] [PubMed] [Google Scholar]
- 81. Belei O, Olariu L, Dobrescu A, Marcovici T, Marginean O. Is it useful to administer probiotics together with proton pump inhibitors in children with gastroesophageal reflux? J Neurogastroenterol Motil. 2018;24(1):51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sun QH, Wang HY, Sun SD, Zhang X, Zhang H. Beneficial effect of probiotics supplements in reflux esophagitis treated with esomeprazole: a randomized controlled trial. World J Gastroenterol. 2019;25(17):2110‐2121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83. Cheng J, Ouwehand AC. Gastroesophageal reflux disease and probiotics: a systematic review. Nutrients. 2020;12(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Holloway L, Moynihan S, Abrams SA, Kent K, Hsu AR, Friedlander AL. Effects of oligofructose‐enriched inulin on intestinal absorption of calcium and magnesium and bone turnover markers in postmenopausal women. Br J Nutr. 2007;97(2):365‐372. [DOI] [PubMed] [Google Scholar]
- 85. van den Heuvel EG, Muijs T, Brouns F, Hendriks HF. Short‐chain fructo‐oligosaccharides improve magnesium absorption in adolescent girls with a low calcium intake. Nutr Res. 2009;29(4):229‐237. [DOI] [PubMed] [Google Scholar]
- 86. Mohn ES, Kern HJ, Saltzman E, Mitmesser SH, McKay DL. Evidence of drug‐nutrient interactions with chronic use of commonly prescribed medications: an update. Pharmaceutics. 2018;10(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tap J, Furet JP, Bensaada M, et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol. 2015;17(12):4954‐4964. [DOI] [PubMed] [Google Scholar]
- 88. Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary Fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705‐715. [DOI] [PubMed] [Google Scholar]
- 89. Chu S, Montrose MH. Extracellular pH regulation in microdomains of colonic crypts: effects of short‐chain fatty acids. Proc Natl Acad Sci U S A. 1995;92(8):3303‐3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]


