Abstract
There is a growing interest in the psychiatric properties of the dissociative anaesthetic ketamine, as single doses have been shown to have fast‐acting mood‐enhancing and anxiolytic effects, which persist for up to a week after the main psychoactive symptoms have diminished. Therefore, ketamine poses potential beneficial effects in patients with refractory anxiety disorders, where other conventional anxiolytics have been ineffective. Ketamine is a noncompetitive antagonist of the N‐methyl‐d‐aspartate (NMDA) glutamate receptor, which underlies its induction of pain relief and anaesthesia. However, the role of NMDA receptors in anxiety reduction is still relatively unknown. To fill this paucity in the literature, this systematic review assesses the evidence that ketamine significantly reduces refractory anxiety and discusses to what extent this may be mediated by NMDA receptor antagonism and other receptors. We highlight the temporary nature of the anxiolytic effects and discuss the high discrepancy among the study designs regarding many fundamental factors such as administration routes, complementary treatments and other treatments.
Keywords: anxiety, anxiolytic, depression, ketamine, ketamine infusion, refractory anxiety, treatment‐resistant depression
1. INTRODUCTION
In the 1960s ketamine was introduced as a safer anaesthetic alternative to its structural analog phencyclidine (PCP), which may cause severe emergence delirium in humans.1 Ketamine is a water‐ and lipid‐soluble arylcycloalkylamine which exists as a racemic mixture of its two enantiomers, (S)‐ketamine and (R)‐ketamine , and acts as an antagonist of the ionotropic glutamate N‐methyl‐d‐aspartate (NMDA) receptor . 2 The G protein‐coupled NMDA receptors are widely expressed throughout the central nervous system (CNS) and require a coincident binding of glutamate and glycine (or D‐serine) and a membrane depolarization sufficient to expel magnesium (Mg2+) blocking the channel pore, before allowing the influx of calcium (Ca2+) and the propagation of excitatory neurotransmission. 3 , 4 The mechanisms whereby ketamine inhibits NMDA receptors involve reducing the mean channel opening time by occluding the channel pore, and decreasing the channel opening frequency by allosteric binding. 5 At anaesthetic intravenous (IV) doses of 1.0‐2 mg kg−1, this mechanism of action is suggested to achieve a dissociative state accompanied by analgesia, sedation and amnesia. 6
As the downstream effects of ketamine become increasingly characterised, its clinical applications have expanded to a promising therapeutic option for treatment‐resistant mood disorders. 7 The antidepressant effects of ketamine were first reported in 2000, where subanesthetic IV doses of 0.5 mg kg−1 alleviated symptoms in patients suffering from major depressive disorder within 3 days. 8 Since then, there have been increasing reports of how even a single dose of ketamine demonstrates a rapid decrease in depressive and suicidal ideation (SI) 40 minutes post‐infusion, which can last up to 14 days. 9 This is in stark contrast to the therapeutic delay of 2‐4 weeks displayed by selective serotonin reuptake inhibitors (SSRIs) and other pharmaceutical therapies used to treat depression. 10 However, the rapid improvements in mood and depressive symptoms tend to diminish after a matter of days, reflecting the transient nature of ketamine. 11 , 12
Moreover, there is increasing evidence that ketamine infusion might also be effective in treating refractory anxiety and anxiety with treatment‐resistant depression (TRD), even though clinical data is limited in comparison with the literature on depression. 13 “Refractory anxiety is defined as the persistence of anxiety symptoms in the absence of response, recovery or remission of the disorder after some form of active treatment” (p. 802). 14 Patients with comorbid anxiety and TRD are extremely difficult to treat as they often have more severe symptoms and are more susceptible to relapse. 15 , 16 Thus, it is of particular importance to understand the efficacy of ketamine in these patient populations as recently highlighted by Banov et al. 13
Refractory anxiety may cause glutamate receptor damage in the hippocampus and neuronal atrophy in the prefrontal cortex (PFC), which can result in mood disturbances and cognitive deficits. 17 NMDA receptor inhibitors have been shown to restore hippocampal function in the stressed brain by reducing NMDA receptor activity in animal models. 18 In addition, the antagonism exerted by ketamine on NMDA receptors expressed on inhibitory γ‐aminobutyric acid (GABA) interneurons causes a subsequent increase in extracellular glutamate, which in turn activates α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors. 19 By potentiating the signalling of brain‐derived neurotrophic factor (BDNF) and the mammalian target of rapamycin complex‐1 (mTORC1), AMPA receptors are critical regulators of synaptic plasticity. 20 Recent research attention has also been directed to the role of ketamine's metabolites in determining its antidepressant effectiveness (see reviews by Highland et al and Schwenk et al 21 , 22 ).
The use of ketamine to treat mood disorders remains controversial, given its popular recreational use in Europe and the United States for its sedating and hallucinogenic effects. The political nature of drug policy has also hindered therapeutic exploration of the novel mechanisms of this substance. 23 As NMDA receptors are intricately involved in the neuronal stress response, altering their function could be a key intervention for acute and refractory anxiety disorders. 24 , 25 However, studies focusing on the efficacy of ketamine treatment on refractory anxiety and anxiety with TRD are limited. The number of people suffering from anxiety is increasing worldwide, with 14% of the adult European population and 18.1% of the adults in the United States reported to be affected. 26 Consequently, refractory anxiety is emerging as a serious mental health condition on a scale with depression, and the need for effective treatment solutions is self‐evident. While recent reviews have been published on the use of ketamine in anxiety spectrum disorders, none have focused specifically on treatment‐resistant anxiety. Thus, the purpose of this current review is to systematically examine the restricted number of clinical trials and a case study which show early signs that ketamine is an effective treatment solution for refractory anxiety.
2. METHOD
This review was conducted according to recent guidelines outlined by Martinic et al. 27 for standardising the systematic review process (consult the original article for full details about this method).
2.1. Search strategy
Two reviewers (J.T. and C.J.H.) searched for relevant articles via PubMed, Web of Science, Cochrane Library and the first 200 hits on Google Scholar on September 3, 2021 and again on January 10, 2022. Databases were searched using several keywords appearing in article titles and abstracts: ((((Ketamine) AND (Anxiety)) OR (Refractory anxiety)) OR (Treatment resistant anxiety)). Manuscrips with matching titles and abstracts were removed as duplicates and the remaining articles were then screened for subject irrelevant content. An additional search was performed in the reference lists of the final selected articles, but no further relevant content was identified.
2.2. Inclusion criteria
Inclusion criteria for selected studies were (a) the article was written in English, (b) it was an original study and not a review or meta‐analysis, (c) the study was not only describing pilot data or a protocol, (d) the research was conducted on human participants and (e) the study included a diagnosis of refractory anxiety with or without TRD. As the Diagnostic and Statistical Manual (DSM‐5) no longer considers obsessive compulsive disorder (OCD) and post‐traumatic stress disorder (PTSD) anxiety spectrum disorders, articles assessing these conditions were excluded from analysis. This is because these conditions share similarities with anxiety and depressive disorders, and it was deemed necessary to look at anxiety as a prominent feature alone or with TRD, rather than as a secondary outcome.
2.3. Data extraction and analysis
Data was extracted independently by two reviewers (J.T. and C.J.H) and where there were conflicts of opinion, a third reviewer (S.B.) moderated and resolved disputes. As part of the descriptive statistical analysis, information was collected on study design, number of patients per trial, type of refractory anxiety and depression diagnosis, assessment measurements, ketamine and placebo (psychoactive or nonpsychoactive) dosing type and regimen, follow‐up period, and findings and conclusions.
2.4. Risk of bias
A risk of bias assessment was completed to establish the reliability and transparency of the findings presented in the selected studies. Using the ROBINS‐I tool, 28 bias was characterised over six domains: selection of participants, confounding variables, measurement exposure, blinding of outcome assessments, incomplete outcome data and selective outcome reporting. The risk of bias task was completed by two authors (J.T and C.J.H) independently. Results of perceived bias can be found in Figure 1. A study was judged across each category as to whether bias was clear, unclear or not present in the selected studies.
FIGURE 1.
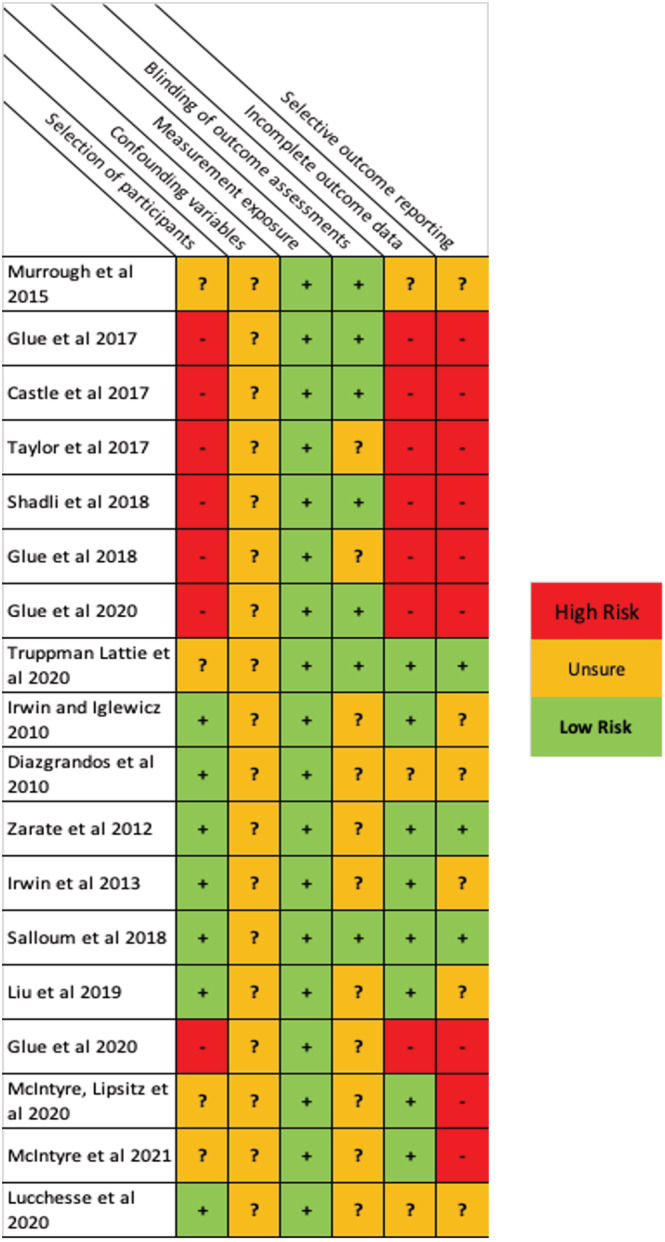
Risk of bias tables for all studies presented in this review (N = 18). A conservative vIew of bias was taken in regards to the outcome measures, sharing of participants and blinding potential of placebo control trials
3. RESULTS
Results for the systematic review are disseminated into a flow diagram in Figure 2. From 1003 articles chosen for matching keywords in the title and/or abstract, 18 studies were identified that were clinical trials, RCTs or a case study using ketamine to treat refractory anxiety with (n = 10) and without (n = 8) TRD. The total number of participants was n = 513. Studies occurred between 2010 and August 2021, with anxiety and TRD research generally taking place earlier than refractory anxiety‐only articles. Nine different measures of refractory anxiety and four measures of depression were implemented across the reviewed studies (see Table 1 for the complete list). Subcutaneous injection (n = 8) and intravenous injection (n = 8) of ketamine were the most common methods of administration, followed by oral dose (n = 2). The subsequent sections will elaborate on ketamine treatment in patients with (1) refractory anxiety, (2) refractory anxiety with TRD and (3) the abuse potential and side effects detected throughout the reviewed studies.
FIGURE 2.
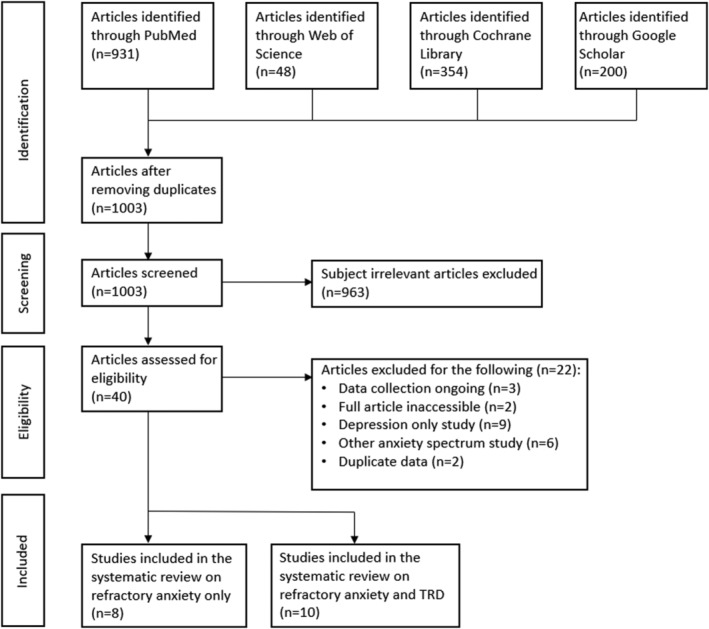
Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) flow diagram
TABLE 1.
List of studies with ketamine for refractory anxiety disorders and anxiety with depression
| Anxiety type | Authors | Sample | Study type | Dose and administration route | Follow‐up period | Findings |
|---|---|---|---|---|---|---|
| Refractory anxiety | Murrough et al. (2015) 30 | Patients (n = 24) receiving treatment for SI and some for GAD/SAD (n = 12) | Randomised, double‐blind psychoactive‐controlled study | Single 0.5 mg kg−1 of intravenous ketamine or 0.045 mg kg−1 midazolam | Pre‐dose, 1, 2, 3 and 7 days post‐treatment | Acute ketamine showed lower score on the MADRS‐SI at 24 h and BSI at 48 h compared with midazolam |
| Glue et al. (2017) 32 | Patients (n = 12) receiving treatment for GAD/SAD | Open‐label, ascending‐dose and double‐blind study | Once weekly subcutaneous ascending ketamine doses (0.25, 0.5 and 1 mg kg−1) | Pre‐dose, 1, 2, 24, 72 and 168 h post‐dose |
After 1 h ketamine showed reduced anxiety on the FA and HAM‐A lasting up to a week 10 of 12 responded to 0.5 ‐ 1 mg kg−1 doses |
|
| Castle et al. (2017) 33 | Patients (n = 18) receiving treatment for GAD/SAD | Mixed open‐label and double‐blind psychoactive‐controlled study | Once weekly open‐label or ascending subcutaneous ketamine doses (0.25, 0.5 and 1 mg kg−1) with a single 0.02 mg kg−1 midazolam dose given in the ascending dose trial | Pre‐dose, 0.5, 1, 2 up to 168 h post‐dose |
Dose‐dependent dissociation increases observed on the CADSS with ketamine while single‐dose midazolam showed no effect Anxiety scores on the FA and HAM‐A also showed a weak negative relationship with greater dissociation |
|
| Taylor et al. (2018) 31 | Patients (n = 18) receiving treatment for SAD | Randomised, double‐blind placebo‐controlled crossover study |
Single 0.5 mg kg−1 of intravenous ketamine or nonpsychoactive saline Infusions administered between 28‐day washout period |
Pre‐dose, 3 h and 1, 2, 3, 5, 7, 10, 14 and 28 days |
Acute ketamine showed reduced anxiety on the LSAS compared with placebo, but not the VAS‐anxiety Treatment responses were more likely in first 14 days |
|
| Shadli et al. (2018) 35 | Patients (n = 12) receiving treatment for GAD/SAD | Exploratory double‐blind psychoactive‐controlled study with EEG | Once weekly subcutaneous ascending ketamine doses (0.25, 0.5 and 1 mg kg−1) with 0.01 mg kg−1 midazolam counterbalanced dose as the psychoactive control | Pre‐dose, 1, 2, 24, 72 and 168 h post‐dose |
Dose‐dependent improvements on FQ but not HAM‐A reported with ketamine An increase in medium‐low theta frequency at RFL predicted ketamine effect on FQ |
|
| Glue et al. (2018) 34 | Patients (n = 20) receiving treatment for GAD/SAD | Uncontrolled, open‐label maintenance study | Once or twice weekly subcutaneous (depending on tolerability) 1 mg kg−1 ketamine dose for 3 months | Pre‐dose, 30, 60 and 120 min and then once a week for 14 weeks |
After 1 h ketamine reduced anxiety <50% on the FA and HAM‐A Post‐dose dissociation observed on the CADSS declined from 20 points at week 1 to 8.8 points at week 14 |
|
| Glue et al. (2020) 36 | Patients (n = 12) receiving treatment for GAD/SAD | Exploratory double‐blind psychoactive‐controlled replication study | Once weekly ascending subcutaneous ketamine doses (0.25, 0.5 and 1 mg kg−1) with 0.01 mg kg−1 midazolam counterbalanced dose as the psychoactive control | Pre‐dose, 0.5, 1, 2, 24, 72 and 168 h post‐dose |
Dose‐dependent anxiolytic and dissociative effects and improved anxiety ratings on HAM‐A, FQ and CADSS (within 1 h, up to a week) with ketamine Midazolam minor effect |
|
| Truppman Lattie et al. (2021) 37 | Patients (n = 24) receiving treatment for GAD/SAD | Mixed open‐label and double‐blind psychoactive‐controlled study | Once weekly ascending subcutaneous ketamine doses (0.25, 0.5 and 1 mg kg−1) | Pre‐dose, 0.5, 1, 2, 24, 72 and 168 h post‐dose |
Acute ketamine demonstrated dose‐dependent reduction in all FQ subscales and the SSAI Reductions compared to pre‐dose scores also evident during follow‐up |
|
| Anxiety with depression | Irwin & Iglewicz (2010) 38 | Hospice patients (n = 2) receiving treatment for depression and anxiety | Two case studies | In both cases, single‐dose (0.5 mg kg−1) oral ketamine was administered | Pre‐dose, 60, 120 min and 2, 4, 8, 15 days |
In both cases, acute ketamine demonstrated sharply reduced depression and anxiety on the HRSD and HADS at days 8‐15 In case 1, suicidal thoughts were also reduced on the ASC and BPRS 40 minutes post‐infusion |
| Diazgranados et al. (2010) 42 | Patients (n = 18) receiving treatment for anxiety and bipolar disorder | Randomised, double blind, placebo‐controlled, crossover, add‐on study | Two intravenous ketamine (0.5 mg kg−1) or placebo doses over two test days 2 weeks apart | Pre‐dose, 40, 80, 220, 230 min and 1, 2, 3, 7, 10, 14 days post‐dose |
Acute ketamine showed improved depressive and anxiety symptoms compared to placebo on the MADRS and HAM‐A for up to 3 days 71% of the sample responded to ketamine infusion |
|
| Zarate et al. (2012) 43 | Patients (n = 15) receiving treatment for anxiety and bipolar disorder | Randomised, double‐blind, placebo‐controlled, crossover, add‐on study | Two intravenous ketamine (0.5 mg kg−1) or placebo doses over two test days 2 weeks apart | Pre‐dose, 40, 80, 220, 230 min and 1, 2, 3, 7, 10, 14 days post‐dose |
Ketamine showed antidepressant and antisuicidal effects compared with placebo on the MADRS for 3 days but only anxiolytic effects at the first day 79% of the sample responded to ketamine |
|
| Irwin et al. (2013) 39 | Hospice patients (n = 14) receiving treatment for depression and depression with anxiety | Open‐label, proof‐of‐concept study | 28‐day, nightly oral ketamine doses (0.5 mg kg−1) | Pre‐dose, 3, 7, 14, 21 and 28 days | Nightly ketamine demonstrated improvements to anxiety on day 3 and depression on day 14 on the HADS which persisted for the remaining 28 days | |
| Salloum et al. (2018) 41 | Patients (n = 99) receiving treatment for resistant depression with (n = 45) and without (n = 54) anxiety | Multisite, randomized, double‐blind, psychoactive‐controlled study | Single intravenous dose of ketamine (0.1, 0.2, 0.5, 1.0 mg kg−1) or midazolam (0.045 mg kg−1) randomized into five arms | Pre‐dose, then 15‐20 min a time for 120 min and 1, 3 days | Acute ketamine showed no significant interaction effect between treatment arms and midazolam on the HAMD6, suggesting a similar response in anxious and nonanxious depression | |
| Liu et al. (2019) 46 | Patients (n = 50) receiving treatment for anxious (n = 30) and nonanxious (n = 20) depression | Open‐label, clinical trial | Six intravenous doses of ketamine (0.5 mg kg−1) over 12 days | Pre‐dose, 1, 3, 5, 8, 10, 12, 13 and 26 days |
Repeated ketamine showed improvements in anxiety on the HAM‐A in patients with anxious depression and depression on the MADRS with nonanxious depression Several neurocognitive improvements were also observed |
|
| Glue et al. (2020) 40 | Patients (n = 7) receiving treatment for resistant anxiety and depression | Multidose, open‐label, flexible dose, uncontrolled study | Multiple subcutaneous doses of extended release ketamine (60‐240 mg) | Pre‐dose, then every 12 h until 96 h |
Long‐release ketamine showed improvements on anxiety (HAM‐A) and depression (MADRS) over 96 h More than half of patients reported increased mood and the drug was well tolerated |
|
| McIntyre et al. (2020) 44 | Patients (n = 113) receiving treatment for TRD (n = 88) and TRD with AIA (n = 113) | Multidose, uncontrolled study | Four intravenous doses of ketamine (0.5 mg kg−1) over 6 days | Pre‐dose, then 2, 4, 6 days and 7‐day follow‐up | Ketamine showed reduced symptoms of depression on QIDS‐SR16 and AIA on GAD‐7 from baseline regardless of number of infusions received | |
| McIntyre et al (2021) 45 | Patients (n = 209) receiving treatment for resistant major depression (n = 177) or bipolar disorder (n = 26) with prominent anxiety | Multidose, uncontrolled study | Four intravenous doses of ketamine (0.5 mg kg−1) over 6 days | Pre‐dose, then 2, 4, 6 days and 7‐day follow‐up |
Ketamine exhibited reductions in depressive symptoms and SI on QIDS‐SR16 after four infusions There was also a reduction in anxiety symptoms reported on GAD‐7 after three infusions |
|
| Lucchese et al. (2021) 47 | Patients (n = 70) receiving treatment for resistant unipolar/bipolar and anxiety | Retrospective analysis of a clinical trial | Six once‐weekly subcutaneous esketamine doses between 0.5 mg kg−1 and 1 mg kg−1 based on tolerance | Pre‐dose, 2 h then 1, 2, 3, 4, 5 and 6 weeks | Acute esketamine demonstrated poor treatment outcomes for 50% of the sample assessed on the MSM, although better outcomes were observed with mild to moderate symptoms on the MADRS and with comorbid anxiety |
Abbreviations: ASC, Adverse Symptoms Checklist; AIA, anxiety, irritability and agitation; BPRS, Brief Psychiatric Rating Scale; BSI, Beck Scale for Suicidal Ideation; CADSS, Clinician‐Administered Dissociative States Scale; FQ, Fear Questionnaire; GAD7, Generalized Anxiety Disorder 7‐item; HADS, Hospital Anxiety and Depression Scale; HAM‐A, Hamilton Anxiety Scale; HAMD6, Six‐item Hamilton Rating Scale for Depression; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery‐Asberg Depression Rating Scale; MADRS‐SI, Montgomery‐Asberg Depression Rating Scale – Suicidal Ideation; MSM, Maudsley Staging Method; QIDS‐SR, Quick Inventory of Depressive Symptomatology‐Self Report 16‐item; RFL, right frontal lobe; SI, suicidal ideation; SSAI, Spielberger State Anxiety Rating Scale.
3.1. Refractory anxiety
Refractory anxiety is that which persists following successful administration of standard treatment that was ineffective (no response) or minimally effective (response but no remission). Current standard treatments include the first‐line pharmacotherapies SSRIs and selective noradrenalin reuptake inhibitors (SNRIs). Second‐line pharmacotherapies include high potency benzodiazepines (e.g. clonazepam , lorazepam , alprazolam ). First‐line psychotherapy typically involves cognitive behavioural therapy (CBT). Pseudo‐resistance is one explanation for refractory anxiety due to incorrect treatment, dose, manner in which treatment was delivered or patient nonadherence. 29 True resistance occurs when successful treatment delivery is ascertained, suggesting instead that an incorrect primary diagnosis was given, or the presence of comorbitities or other physical conditions that may hinder treatment. Studies examining single and multidose ketamine treatment for refractory anxiety show acute but transient anxiolytic effects can be found in Table 1 and are reviewed below, although findings show less statistical power than those studies assessing anxiety and depression.
3.1.1. Single dose of intravenous ketamine
In a randomised, double‐blind psychoactive‐controlled study of patients with SI (n = 24) and some with comorbid anxiety spectrum disorders (n = 12), Murrough et al 30 found that a single infusion of intravenous ketamine (0.5 mg kg−1) administered over 40 minutes showed positive treatment outcomes for patients with generalised anxiety disorder (GAD) (n = 6/24) and social anxiety disorder (SAD) (n = 6/24), among various other mood disorders. 27 Findings reached significance at 48 hours post‐infusion compared with midazolam (0.045 mg kg−1) on the Beck Scale for Suicidal Ideation (BSI) and Montgomery‐Asberg Depression Rating Scale – Suicidal Ideation (MADRS‐SI), but were nonsignificant at the end of the 7‐day follow‐up. Secondary measures showed reduced irritability and panic at 24 hours post‐infusion, although generalised anxiety remained the same. Findings demonstrated promising treatment outcomes for patients with anxiety spectrum disorders experiencing SI.
Later, a randomised, double‐blind placebo‐controlled crossover study by Taylor et al showed mixed results in patients (n = 18) being treated for SAD. 31 Single‐dose intravenous ketamine (0.5 mg kg−1) and saline placebo were given in random order with a 28‐day washout. Treatment responses were evident 3 hours post‐ketamine infusion and persisted for up to 14 days in some cases. Patients consistently showed reduced anxiety on the Liebowitz Social Anxiety Scale (LSAS), but not on an anxiety visual analogue scale (VAS‐Anxiety). Based on past research, a significant response was considered a 35% LSAS and 50% VAS‐Anxiety reduction in anxiety from baseline. Nevertheless, ketamine was associated with modest improvements on the VAS‐Anxiety relative to placebo during this period, indicating an anxiety‐reducing effect.
3.1.2. Ascending dose of subcutaneous ketamine with maintenance period
An open‐label, double‐blind study with GAD and SAD patients (n = 12) showed strong anxiety‐reducing outcomes using three ascending subcutaneous ketamine doses (0.25, 0.5 and 1 mg kg−1) administered once weekly. 32 Dose‐dependent effects were demonstrated, with greater change most notable with higher doses. Rapid onset effects were visible 1 hour post‐infusion and lasted up to a week, although 0.25 mg kg−1 showed only modest improvements. Patients self‐reported a reduction in general and social anxiety on the Fear Questionnaire (FQ) and the Hamilton Anxiety Scale (HAM‐A), which persisted for a week post‐dose. Ten participants (83%) responded to 0.5‐1 mg kg−1 doses, but two reported no anxiety reduction throughout the trial. Modest dissociative and anxiolytic effects, and heart rate and blood pressure changes were recorded with all dosing regimens. As part of the same group, Castle et al conducted a multidose, double‐blind, psychoactive‐controlled trial assessing the effects of ketamine on dissociation in patients (n = 18) with GAD and SAD. 33 The same ascending ketamine doses were used as above, and self‐reported dissociation increased on the Clinician‐Administered Dissociative States Scale (CADSS) in a dose‐dependent way compared with midazolam, which exhibited no effects. Moreover, there was a weak negative but significant correlation between increased dissociation and self‐reported anxiety reduction on the HAM‐A (r = −0.15, P = .29) and FQ (r = −0.14, P = .13). Later, the same responders (n = 20) completed an uncontrolled, open‐label maintenance study with once or twice weekly (depending on tolerability) 1 mg kg−1 ketamine for 3 months. 34 One hour post‐dose, anxiety ratings on the FQ and HAM‐A demonstrated a >50% reduction and dissociation reported on the CADSS also decreased over time from 20 points during week 1 to 8.8 points at week 14. Average systolic and diastolic blood pressure increased by 10 mmHg at 30 minutes but returned to baseline shortly after and showed no evidence of long‐term change at 14 weeks. Additionally, patients self‐reported improved social and workplace functioning on the Work and Social Adjustments Scale (WSAS) from 15 points during the first week to 11.4 points at the end of the maintenance period.
A later electroencephalogram (EEG) study by Shadli et al 35 assessed the neural basis of the therapeutic action of ketamine in patients (n = 12) with GAD and SAD who also received relaxation therapy. 31 Participants received 10 minutes of relaxation with EEG before and then 2 hours post‐dose of subcutaneously injected ketamine or midazolam . Ascending ketamine doses (0.25, 0.5 and 1 mg kg−1) randomly interspersed with single‐dose midazolam (0.01 mg kg−1) were distributed with a 1‐week washout period between doses. Anxiety measurements showed mixed results and demonstrated a ketamine dose‐dependent improvement on the FQ but not the HAM‐A relative to placebo. Nevertheless, eight of 12 patients showed a >50% anxiety reduction on the HAM‐A with 0.5 and 1 mg kg−1 ketamine. EEG also showed reduced delta and increased gamma frequency with ketamine, but only a decrease in medium‐low frequency at the right frontal theta successfully predicted reduced anxiety on the FQ. Imaging data suggested ketamine reduces refractory anxiety through related mechanisms to classic anxiolytic drugs by reducing activity in the same regions. The same authors conducted a follow‐up study with the same sample and dosing regimen as above, but with the inclusion of ratings of dissociation, blood samples, brain‐derived neurotrophic factor (BDNF) concentrations and ketamine pharmacokinetics. Ketamine showed rapid onset and significantly reduced anxiety from baseline within an hour, which continued for up to a week. 36 Effects were dose‐dependent on the FQ and HAM‐A, and midazolam showed a similar anxiety‐reducing effect with 0.05 mg kg−1 ketamine on FQ score. Ketamine significantly induced dissociation reported on the CADSS in a dose‐dependent manner when compared with placebo, which was positively associated with ketamine pharmacokinetics. The dosing regimen was well tolerated and contributed to research demonstrating ketamine's anxiolytic effects.
A recent open‐label, double‐blind, psychoactive‐controlled study by Truppman Lattie et al evaluated patients (n = 24) with refractory anxiety who received acute and maintenance ketamine therapy (the same cohort described in the studies above). 37 Ascending subcutaneous ketamine (0.25, 0.5, 1 mg kg−1) and midazolam (0.01 mg kg−1) randomly inserted into the regimen with 1 week washout between doses demonstrated a dose‐dependent reduction in anxiety with ketamine reported on the FQ and Spielberger State Anxiety Rating Scale (SSAI). Differences were most apparent between midazolam and ketamine 0.5 mg kg−1 at 2, 24 and 72 hours follow‐up. During the maintenance period, patients received 1 mg kg−1 once or twice weekly for 14 weeks and anxiety‐reducing effects were apparent 1 hour post‐dose but showed no further improvements at 2 hours. This period saw a progressive decrease in pre‐dose anxiety levels reported on the above scales, suggesting long‐term benefits of ketamine treatment. Nonetheless, the majority of patients self‐reported reoccurring anxiety once ketamine therapy finished, indicating that anxiolytic effects are temporary.
3.2. Refractory anxiety with TRD
As with refractory anxiety, comorbid TRD is minimally or unresponsive to standard treatments. The SSRIs and SNRIs are usually successful at treating both anxiety and depression, but high‐potency benzodiazepenes have no efficacy for comorbid depression and withdrawal symptoms often mimic refractory anxiety. 29 Detailed in Table 1 and following below is a review of single and multidose ketamine studies treating refractory anxiety and TRD which show some rapid anxiolitic and antidepressant effects. These studies generally test larger cohorts, although there is an over‐reliance on open label data and a lack of consistent methods between studies.
3.2.1. Single dose of oral ketamine
Irwin and Iglewicz reported two cases of patients in palliative care receiving treatment for refractory anxiety and TRD. 38 In the first case, a woman suffering respiratory failure and chronic obstructive pulmonary disease who had developed TRD and severe anxiety received a single oral ketamine infusion (0.5 mg kg−1) at home. Consequently, there was a substantial reduction (45% drop since baseline) in depressive symptoms on the Hamilton Rating Scale for Depression (HAM‐D) at 60 minutes post‐dose, which increased by day 15 (50% drop). Anxiety was also drastically reduced at 60 minutes (83% drop) on the Hospital Anxiety and Depression Scale (HADS) and remained significant at day 15 (50% drop). At 120 minutes, SI also vanished, and the patient expressed relaxation and hope for the future. At 1 month follow‐up, symptoms of depression and anxiety returned but with reduced severity. The second case was of a man in a hospice being treated for prostate cancer who had been bed‐bound for 8 months and had developed anxiety with panic attacks and TRD. He received the same ketamine dose as above and at 60 minutes post‐infusion depression significantly decreased (37% drop) on the HAM‐D, which became more pronounced at day 8 (57% drop). On the HADS there was a striking reduction to anxiety at 60 minutes (69% drop), which also increased at day 8 (85% drop). He became too sick to complete the treatment, but his wife reported that he was happier, had become more social and had accepted death prior to passing away.
3.2.2. Repeated dose of oral ketamine
In an open‐label, proof‐of‐concept trial, Irwin et al treated hospice patients (n = 14) suffering from anxiety and depression with daily oral ketamine (0.5 mg kg−1) for 28 days alongside psychiatric medications. 39 During the trial, participants showed significant improvement for symptoms of anxiety and depression on the HADS from baseline. Anxiety was significantly reduced by day 3, and for depression the same was evident at day 14. In both cases, once symptoms were improved, they remained this way through to day 28. For health reasons, only eight patients were able to complete the trial, but they all responded positively to treatment. Nevertheless, oral ketamine showed protracted effects compared to intraveous ketamine, with the therapeutic delay most evident with depressive symptoms.
A recent open‐label, multidose pilot study by Glue et al assessed long‐release ketamine for treatment of patients (n = 7) with anxiety and TRD. 40 Oral ketamine (60‐240 mg actual dose based on patient tolerability) was given on the first day at 8 am and 8 pm, and on days 2‐4 patients received doses 12‐hourly until the final dose was given at 72 hours. Anxiety was significantly reduced in all subjects on the HAM‐A and depression on the MADRS at 12 hours, with findings becoming more gradual until 96 hours. On the FQ, six out of seven patients reported 50% fear reduction from baseline, which also showed a gradual reduction at 96 hours. Additionally, only one patient reported a brief period of dissociation, demonstrating good tolerability in the sample. Consequently, oral, extended‐release ketamine was shown to have a good safety profile and strong anxiolytic and antidepressant effects.
3.2.3. Single dose of subcutaneous ketamine
Salloum et al conducted a large, multisite, psychoactive‐controlled study with patients (n = 99) suffering from TRD with (n = 45) and without (n = 54) refractory anxiety. 41 Participants were randomised to one of five conditions and received subcutaneous ketamine (01, 02, 05 or 1.0 mg kg−1) or midazolam (0.045 mg kg−1). A mixed linear effect model was used to determine anxious depression status and reaction to ketamine or midazolam during 1‐ and 3‐day follow‐up. There was a significant interaction which revealed an improvement in depressive symptoms on the HAM‐A and MADRS at day 3 with the 0.1 mg kg−1 ketamine group in patients without anxious TRD vs patients with anxious TRD relative to midazolam. No further significant findings were identified with other ketamine doses, although small group sizes might have inhibited results. Despite a single statistical difference between depression groups, other doses of ketamine showed similar nonsignificant outcomes, which suggests that ketamine treated anxious and nonanxious TRD with similar efficacy.
3.2.4. Repeated dose of subcutaneous ketamine
An early randomised, double‐blind, placebo‐controlled proof‐of‐concept study by Diazgranados et al examined ketamine treatment in patients (n = 18) with treatment‐resistant bipolar depression and anxiety who had not responded to lithium or valproate treatment. 42 Subjects received intravenous ketamine (0.5 mg kg−1) or saline solution on two test days with a 2‐week washout between doses. Depression and anxiety were rated 60 minutes prior to infusion and then at regular 40‐minute intervals post‐dose until 230 minutes with the Montgomery‐Asberg Depression Rating Scale (MADRS), the HAM‐A and other secondary outcome measures. At 40 minutes, depression and anxiety significantly improved in the ketamine group relative to placebo, which lasted for 3 days. Seventy‐one per cent of patients responded positively to ketamine therapy, which demonstrated a robust and rapid antidepressant and anxiolytic effect. The same group conducted a replication study with bipolar patients (n = 15) who received an identical ketamine dosing regimen with placebo as above and completed the same outcome measures. 43 Here, depression and SI significantly improved in the ketamine condition compared with placebo after 40 minutes infusion and remained this way until the end of day 3. Anxiety was also significantly reduced at 40 minutes follow‐up, but no further reductions were reported after the first day. Nevertheless, 79% of patients responded positively to ketamine treatment.
In another recent multidose, uncontrolled study, McIntyre et al treated patients (n = 201) receiving therapy for major depression with (n = 113) and without (n = 88) anxiety, irritability and agitation (AIA) with intravenous ketamine. 44 Four doses (0.5 mg kg−1 or 0.75 mg kg−1 for high tolerability) were delivered over 1‐2 weeks. Participants received psychometrics every 2 days post‐dose and after the fourth infusion there was a 14‐day follow‐up. Patients with AIA showed a significant reduction in anxiety, irritability and agitation measured by the Generalized Anxiety Disorder‐7 (GAD‐7) scale relative to baseline when compared with the depression without AIA group, regardless of follow‐up period. Furthermore, all participants reported reduced depressive feelings and SI on the Quick Inventory for Depressive Symptomatology Self‐Report‐16 (QIDS‐SR16) compared to baseline. A later study by this group used the same dosing regimen, psychometrics and follow‐up period to measure TRD (n = 177) or bipolar disorder (n = 26) presenting with prominent anxiety (n = 209). 45 Following all infusions, depression and anxiety were significantly reduced relative to baseline on the QID‐SR16 and GAD‐7 in patients with refractory anxiety compared to with those without. Finally, SI was also significantly reduced in both groups after the fourth infusion. In this trial, patients with TRD and anxiety responded most positively to ketamine treatment.
3.2.5. Repeated dose of intravenous ketamine
Lui et al examined repeated ketamine treatment in patients (n = 50) with anxious (n = 30) and nonanxious (n = 20) TRD and also looked at neurocognitive benefits. 46 Six intravenous ketamine infusions (0.5 mg kg−1) were given over 12 days and follow‐up occurred at day 26. In the anxiety with TRD group, anxiety significantly decreased from baseline below clinical levels on the HAM‐A by days 13 and 26, which was not apparent in the TRD‐only group. For both groups, depressive symptoms drastically improved on the MADRS on days 13 and 26. Neurocognition also showed several improvements, including faster speed of processing (SOP) in the anxious TRD group at days 13 and 26, and better verbal learning and memory (VLM) on day 13. Moreover, faster baseline SOP was correlated with better improvements in anxiety in the anxious TRD group, while better baseline VLM was linked to greater improvements in depression in the nonanxious TRD group. The link between improvements in anxiety and depression and certain neurocognitive enhancements was unique in this study.
Finally, in a new clinical trial, Lucchese et al assessed patients (n = 70) receiving esketamine treatment for resistant unipolar (n = 39) or bipolar (n = 31) disorder with refractory anxiety (n = 33). 47 Participants received once‐weekly intravenous esketamine doses (0.5 mg kg−1 or 1.0 mg kg−1 for high tolerability) for 6 weeks and the primary outcome measure was reduced feelings of depression. At baseline, patients demonstrated strong treatment resistance (65.7%) and comorbid refractory anxiety (47.1%) on the Maudsley Staging Method (MSM). At the end of the study, only 50% of patients showed improved depressive symptoms on the MADRS (below 50% of baseline score), and higher levels of treatment resistance and comorbid anxiety were associated with a poor response to treatment. Consequently, intravenous esketamine showed modest success in treating TRD and anxiety.
3.3. Abuse potential and side effects
With any pharmacotherapeutic intervention it is important to consider the relative benefits in line with the side effects the drugs have on the individual. Furthermore, potential for abuse of a substance is a key consideration for trials of new therapeutic compounds. Across these studies the side effects from ketamine administration show similar patterns of symptoms and the relative latency of these effects. Several participants report headaches, 30 , 36 , 43 nausea or other gastrointestinal complaints, 34 , 39 , 40 , 42 , 43 dizziness 30 , 34 , 36 , 40 , 42 , 43 and tachycardia, 31 , 32 , 44 with one describing unusual bodily sensations. 43 It is important to note that somatic, cognitive and gustatory side effects all appeared to normalise over the course of the experiment after an initial post‐infusion peak. Furthermore, the severity of symptoms was directly linked to the dose of the active substance with lower doses resulting in lower levels of negative side effects. The most effective dosage for avoiding negative side effects whilst subsequently improving clinical symptoms appears to be 0.5 mg kg−1. On average, these negative effects peaked around 30 minutes post‐infusion and were no longer present by 60 minutes. Interestingly, the method of infusion (whether subcutaneous, intravenous or oral) did not seem to affect the number and frequency of side effects.
Most frequently described is the symptomology of dissociation. For most of these studies these are specifically explored using the Clinician‐Administered Dissociative State Scale, 48 as this was an expected effect of ketamine infusion. It is important to consider, however, that a number of the specific side effects that participants mention, such as “feeling strange”, “blurred vision” and “dizziness”, may well be related to this feeling of dissociation. One further practical issue for clinical trials of ketamine is to consider this psychoactive effect. A number of trials fail to account for these effects in a double‐blind trial, subsequently making the blind void. Several studies discussed here used the active placebo midazolam to counteract this affect. Dissociative symptoms in all cases peak at 30 minutes post‐infusion and return to normal 60 minutes post‐infusion. No data suggest that these dissociative sensations last longer than 60 minutes.
Just one of the selected studies 36 considers the role of abuse potential in ketamine as a treatment option, but this is something that should be considered in any clinical trials. Here the authors consider the method of delivery as important in the abuse potential of such medications. Specifically, delivery as an oral substrate, such as being dissolved in a syrup or injection, reduces the potential for abuse. Furthermore, the density of the oral tablet form of ketamine may inhibit the ability to use it as an intranasal substance. The relative abuse potential for ketamine appears to be low (see review49), but this has been considered most frequently in terms of drug‐taking behaviour rather than in longitudinal clinical trials, this may for example be dose or even situation dependent.
4. DISCUSSION
To our knowledge this is the first systematic review of the role of ketamine NMDA receptor antagonism in treating refractory anxiety disorders with and without depression. It was revealed that the anxiolytic and antidepressant effects of ketamine were transient and some negative, but minor side effects increased with stronger doses of ketamine. Given these observations, the following sections will discuss in more detail the fundamental factors that need to be addressed in the currently understudied area of ketamine treatment in refractory anxiety and TRD.
In the current review, single‐dose infusions of ketamine showed significant positive treatment outcomes for patients with refractory anxiety disorders, including GAD and SAD, with reduced irritability and panic, but without significant changes in GAD. 30 Higher doses of ketamine (eg, 1 mg kg−1 weekly) have greater effects on anxiety reduction across studies, 32 , 33 , 37 , 40 , 41 , 47 with some anxiety‐reducing effects lasting for a week post‐dose, although whether an inverse U‐relationship exists between the amount of ketamine and symptom reduction is unclear.
Various signalling pathways beyond NMDA receptor antagonism have been considered when investigating its behavioral and physiologic effects. 47 Ketamine is metabolised by cytochrome P450 liver enzymes into norketamines, hydroxynorketamines (HNKs) and dehydronorketamines. 49 In addition, the metabolites (2R,6R)‐ and (2S6)‐HNK have been implicated in the antidepressant effects of ketamine in preclinical models. 50 These actions are largely attributed to AMPA receptor‐dependent synaptic transmission and the release of neurotransmitters such as serotonin and norepinephrine. 51 While initial reports claim that the antidepressant‐effects of (2R,6R)‐HNK are independent of the NMDA receptor, 50 , 52 others show that higher concentrations (50 μM) of the metabolite can inhibit NMDA receptors. 53 , 54 , 55 This may explain the longer‐lasting antidepressant effects of ketamine.
Intriguingly, the chirality of ketamine may also influence its potency, as (R)‐ketamine has been shown to have both longer‐lasting antidepressant effects and fewer side effects than (S)‐ketamine in rodent models. 56 As (R)‐ketamine is a weaker NMDA receptor inhibitor than its enantiomer, it is likely that other signal transduction pathways play a part in its psychiatric effects, including increased dopamine and opioid signaling, which have already been observed with the drug (Figure 3). 49 , 57
FIGURE 3.
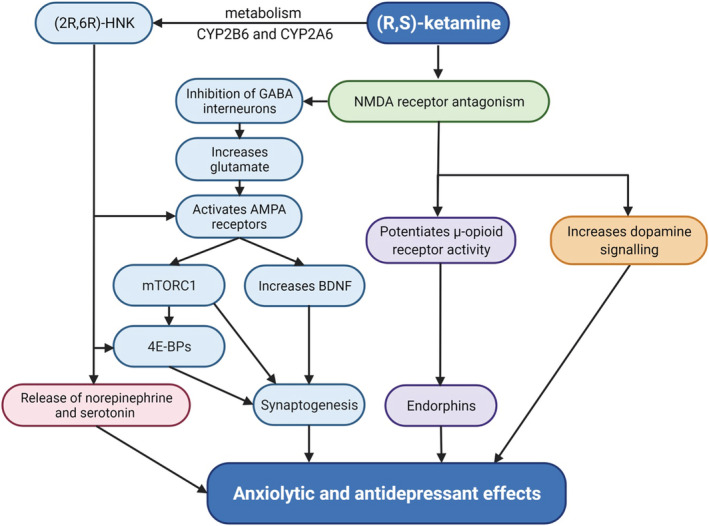
Potential mechanisms involved in ketamine's anxiolytic and antidepressant effects. AMPA, α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid; BDNF, brain‐derived neurotropic factor; CYP2B6, cytochrome P450 2B6; CYP2A6, cytochrome P450 2A6; 4E‐BPs, eukaryotic initiation factor 4E‐binding proteins; GABA, γ‐aminobutyric acid; (2R,6R)‐HNK, (2R,6R)‐hydroxynorketamine; mTORC1, mammalian target of rapamycin complex‐1; NMDA, N‐methyl‐d‐aspartate. The figure was created with BioRender.com
Anxiety is often comorbid with depression and clinical therapies typically treat both conditions concurrently, 58 therefore examining the utility of ketamine treatment in patient groups with both refractory anxiety and TRD would enhance clinical intervention. The reviewed case studies of palliative care patients show that depression comorbid with severe, life‐threatening physical ailments is significantly reduced with ketamine, alongside persistent relaxation and the patient's greater hope for the future, 38 , 39 and while these patients experienced a relapse in anxiety and depression symptoms, their subsequent reports were less severe than pre‐ketamine intake. 42 Interestingly, larger oral doses over a shorter period of hours do not appear to be any more effective than lower doses over a longer time period of days, and treatment effects may peak at 2 weeks. 32 In addition, small doses twice weekly for 3 months effectively treat other types of anxiety, including refractory social and/or generalised anxiety, with only minor adverse effects, including mild raises in blood pressure, nausea, dizziness and blurred vision. 34 These studies demonstrate the clinical, safe and efficacious utility of ketamine administration, especially for those with treatment‐resistant anxiety and depression.
Moreover, the neural basis of ketamine effects on those with refractory anxiety is related to a decrease in medium‐low frequency at the right frontal theta, in line with a >50% reduction in self‐reported anxiety. 35 However, a paucity of brain‐imaging studies of the effects of ketamine on neural processes in those with anxiety disorders prevents extensive comment on the neural mechanisms here. That said, the few imaging studies implicate hypofunction in the posterior cingulate in schizophrenia with anxiety 59 and the subgenal anterior cingulate cortex in major depressive disorder. 60
Ketamine has been shown to promote excitatory signaling of cortical pyramidal cells, via mTORC1 and eukaryotic initiation factor 4E‐binding protein (4E‐BP)‐driven synaptogenesis, which could contribute to its antidepressant effects. 61 , 62 To date, many of the ketamine refractory anxiety studies have limited statistical power when compared with those examining anxiety and depression. As such, more randomised placebo‐control, double‐blind studies with larger samples would be useful. Brain‐imaging studies utilising modalities other than EEG could determine – with enhanced spatio‐temporal resolution – neural correlates of improvements to anxiety and depression symptoms and related neurocognitive deficits (eg, functional magnetic resonance imaging and functional near‐infrared spectroscopy).
Current drug therapies for anxiety disorders demonstrate a need for alternative options: they tend to modulate monoaminergic neurons, including serotonin and noradrenaline or GABA, and vary in their anxiolytic effects. 63 SSRIs are commonly prescribed for both anxiety and depression, which block the re‐uptake of serotonin and potentiate mood‐enhancing action within the synaptic cleft. However, side effects such as sleep disturbances, increased anxiety and therapeutic lags up to 4 weeks are commonly reported. 10 SNRIs, tricyclics and monoamine oxidase inhibitors (MOIs) have similar modes of action to SSRIs, but show less efficacy, at least for anxiety. 64 Alternatively, benzodiazepines, which modulate GABA networks, or the anticonvulsant pregabalin , which reduces the release of excitatory neurotransmitters by binding presynaptic voltage‐gated Ca2+ channels, are prescribed to treat anxiety. 65 , 66 Unfortunately, these have a high abuse potential as tolerance is quickly developed. 65 , 66 Treatment‐refractory anxiety is usually diagnosed when these conventional drug therapies have been exhausted and deemed moderately or completely ineffective, which emphasises the need for additional alternatives. 67
Although the reviewed studies demonstrate heterogeneity, eg, in frequency and dosage of ketamine, wash‐out periods and variance in patient comorbidities, the effects of ketamine appear to be more potent at reducing scores on anxiety self‐report measures than midazolam, lithium or valproate 30 , 33 , 35 , 37 , 42 and psychiatric treatment alone. 39 , 67 A combination of these treatments may better enhance efficacy for reducing symptoms in the longer term. In addition, patients with anxiety disorders report modest dissociative, anxiolytic effects, and heart rate and blood pressure changes following various ketamine doses, 32 , 33 suggesting wider‐reaching clinical benefits (note: modest dissociation in patients with anxiety may correlate with the anxiolytic effects observed 33 ).
Moreover, cognitive benefits of ketamine infusion are sometimes reported. For example, one study reported better speed of processing (SOP) and verbal learning memory (VLM), implicating the efficacy of ketamine to influence NMDA receptor function within the prefrontal circuits associated with these executive functions. 46 In addition, anxiety, irritability and agitation were shown to be reduced by one study of TRD with anxiety, 45 which may be associated with improved cognitive control of affect, a neural function underpinned by fronto‐striatal circuitry. Implications for the costs/benefits of ketamine in anxiety therapy are such that severe negative reactions were not found in this review and abuse potential appeared limited, although more longitudinal data is required. 68 Thus, further exploration of the novel NMDA receptor function of ketamine, which shows signs for treating refractory anxiety, should be pursued.
5. CONCLUSIONS
The evidence suggests that anxiolytic effects of ketamine are temporary, with anxiety symptoms returning to baseline after approximately 2 weeks. However, dissociative effects and novel mechanisms underpinning psychoactive properties may contribute to clinical utility in ways yet to be explored. This review highlights different ketamine dose‐responses, administration, self‐report measures, comorbidities and other physical conditions of patients with refractory anxiety and TRD and how these influence study outcomes. To elucidate the role of NMDA receptor antagonism, receptor densities in specific brain regions and metabolism of ketamine might further explain the potentially beneficial effect of ketamine for refractory anxiety disorders.
5.1. Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 69
COMPETING INTERESTS
There are no competing interests for the authors to declare.
AUTHOR CONTRIBUTIONS
J.L.T.: conceptualization, investigation, formal analysis, data curation, writing – original draft. A.D.D.: visualization, writing – review and editing. C.J.H.: writing – review and editing. H.B.S.: supervision and editing. S.B.: conceptualization, writing – review and editing, supervision. All authors reviewed and edited the manuscript.
ACKNOWLEDGEMENT
H.B.S. is supported by the Swedish Research Council and the Swedish Brain Foundation.
Tully JL, Dahlén AD, Haggarty CJ, Schiöth HB, Brooks S. Ketamine treatment for refractory anxiety: A systematic review. Br J Clin Pharmacol. 2022;88(10):4412‐4426. doi: 10.1111/bcp.15374
Contributor Information
Jamie L. Tully, Email: j.tully@exeter.ac.uk.
Samantha Brooks, Email: s.j.brooks@ljmu.ac.uk.
DATA AVAILABILITY STATEMENT
Data from the systematic review is available upon request by emailing the corresponding authors.
REFERENCES
- 1. Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016;29(10):612‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non‐competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333(1):99‐104. [DOI] [PubMed] [Google Scholar]
- 3. Hansen KB, Yi F, Perszyk RE, et al. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol. 2018;150(8):1081‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hauser AS, Attwood MM, Rask‐Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16(s):829‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orser BA, Pennefather PS, MacDonald JF. Multiple mechanisms of ketamine blockade of N‐methyl‐d‐aspartate receptors. Anesthesiology. 1997;86(4):903‐917. [DOI] [PubMed] [Google Scholar]
- 6. Marland S, Ellerton J, Andolfatto G, et al. Ketamine: use in anesthesia. CNS Neurosci Ther. 2013;19(6):381‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gould TD, Zarate CA Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid‐acting antidepressants. Annu Rev Pharmacol Toxicol. 2019;59:213‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351‐354. doi: 10.1016/s0006-3223(99)00230-9 PMID: 10686270 [DOI] [PubMed] [Google Scholar]
- 9. Singh I, Morgan C, Curran V, Nutt D, Schlag A, McShane R. Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight. Lancet Psychiatry. 2017;4(5):419‐426. [DOI] [PubMed] [Google Scholar]
- 10. Machado‐Vieira R, Baumann J, Wheeler‐Castillo C, et al. The timing of antidepressant effects: A comparison of diverse pharmacological and somatic treatments. Pharmaceuticals (Basel). 2010;3(1):19‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. BJPsych Adv. 2016;22(4):214‐215. [DOI] [PubMed] [Google Scholar]
- 12. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950‐966. [DOI] [PubMed] [Google Scholar]
- 13. Banov MD, Young JR, Dunn T, Szabo ST. Efficacy and safety of ketamine in the management of anxiety and anxiety spectrum disorders: a review of the literature. CNS Spectr. 2020;25(3):331‐342. [DOI] [PubMed] [Google Scholar]
- 14. Bokman WA, Wetzer GAAM, Gehrels JB, Penninx BWJH, Batelaan NM, van Balkom ALJM. Aligning the many definitions of treatment resistance in anxiety disorders: A systematic review. Depress Anxiety. 2018;36:801‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR* D report. Am J Psychiatry. 2008;165(3):342‐351. [DOI] [PubMed] [Google Scholar]
- 16. Wiethoff K, Bauer M, Baghai TC, et al. Prevalence and treatment outcome in anxious versus nonanxious depression: results from the German Algorithm Project. J Clin Psychiatry. 2010;71(8). [DOI] [PubMed] [Google Scholar]
- 17. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185(1):1‐10. doi: 10.1016/0014-2999(90)90204-J [DOI] [PubMed] [Google Scholar]
- 19. Matveychuk D, Thomas RK, Swainson J, et al. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther Adv Psychopharmacol. 2020;10:2045125320916657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chater TE, Goda Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci. 2014;8:401‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Highland JN, Zanos P, Riggs LM, et al. Hydroxynorketamines: pharmacology and potential therapeutic applications. Pharmacol Rev. 2021;73(2):763‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwenk ES, Pradhan B, Nalamasu R, et al. Ketamine in the past, present, and future: Mechanisms, metabolites, and toxicity. Curr Pain Headache Rep. 2021;25(9):57‐58. [DOI] [PubMed] [Google Scholar]
- 23. Nutt D. Science and non‐science in UK drug policy. Addiction. 2010. Jul;105(7):1154. [DOI] [PubMed] [Google Scholar]
- 24. Carboni E, Carta AR, Carboni E, Novelli A. Repurposing ketamine in depression and related disorders: Can this enigmatic drug achieve success? Front Neurosci. 2021;15:15‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626(1):49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anxiety and Depression Association of America [internet] : ADAA; [2020]. Available from http://www.Adaa.Org
- 27. Martinic MK, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta‐epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roy‐Byrne P. Treatment‐refractory anxiety; definition, risk factors, and treatment challenges. Dialogues Clin Neurosci. 2015;17(2):191‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murrough JW, Soleimani L, DeWilde KE, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015;45(16):3571‐3580. [DOI] [PubMed] [Google Scholar]
- 31. Taylor JH, Landeros‐Weisenberger A, Coughlin C, et al. Ketamine for social anxiety disorder: a randomized, placebo‐controlled crossover trial. Neuropsychopharmacology. 2018;43(2):325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glue P, Medlicott NJ, Harland S, et al. Ketamine's dose‐related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J Psychopharmacol. 2017;31(10):1302‐1305. [DOI] [PubMed] [Google Scholar]
- 33. Castle C, Gray A, Neehoff S, Glue P. Effect of ketamine dose on self‐rated dissociation in patients with treatment refractory anxiety disorders. J Psychopharmacol. 2017;31(10):1306‐1311. [DOI] [PubMed] [Google Scholar]
- 34. Glue P, Neehoff SM, Medlicott NJ, Gray A, Kibby G, McNaughton N. Safety and efficacy of maintenance ketamine treatment in patients with treatment‐refractory generalised anxiety and social anxiety disorders. J Psychopharmacol. 2018;32(6):663‐667. [DOI] [PubMed] [Google Scholar]
- 35. Shadli SM, Kawe T, Martin D, McNaughton N, Neehoff S, Glue P. Ketamine effects on EEG during therapy of treatment‐resistant generalized anxiety and social anxiety. Int J Neuropsychopharmacol. 2018;21(8):717‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glue P, Neehoff S, Sabadel A, et al. Effects of ketamine in patients with treatment‐refractory generalized anxiety and social anxiety disorders: exploratory double‐blind psychoactive‐controlled replication study. J Psychopharmacol. 2020;34(3):267‐272. [DOI] [PubMed] [Google Scholar]
- 37. Truppman Lattie D, Nehoff H, Neehoff S, Gray A, Glue P. Anxiolytic effects of acute and maintenance ketamine, as assessed by the Fear Questionnaire subscales and the Spielberger State Anxiety Rating Scale. J Psychopharmacol. 2021;35(2):137‐141. [DOI] [PubMed] [Google Scholar]
- 38. Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Irwin SA, Iglewicz A, Nelesen RA, et al. Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28‐day open‐label proof‐of‐concept trial. J Palliat Med. 2013;16(8):958‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glue P, Medlicott NJ, Neehoff S, et al. CT. Safety and efficacy of extended release ketamine tablets in patients with treatment‐resistant depression and anxiety: open label pilot study. Therap Adv Psychopharmacol. 2020;10:2045125320922474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salloum NC, Fava M, Freeman MP, et al. Efficacy of intravenous ketamine treatment in anxious versus nonanxious unipolar treatment‐resistant depression. Depress Anxiety. 2018;36(3):235‐243. [DOI] [PubMed] [Google Scholar]
- 42. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add‐on trial of an N‐methyl‐d‐aspartate antagonist in treatment‐resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zarate CA Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add‐on trial. Biol Psychiatry. 2012;71(11):939‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McIntyre RS, Lipsitz O, Rodrigues NB, et al. The effectiveness of ketamine on anxiety, irritability, and agitation: implications for treating mixed features in adults with major depressive or bipolar disorder. Bipolar Disord. 2020;22(8):831‐840. [DOI] [PubMed] [Google Scholar]
- 45. McIntyre RS, Rodrigues NB, Lipsitz O, et al. The effectiveness of intravenous ketamine in adults with treatment‐resistant major depressive disorder and bipolar disorder presenting with prominent anxiety: Results from the Canadian Rapid Treatment Center of Excellence. J Psychopharmacol. 2021;35(2):128‐136. [DOI] [PubMed] [Google Scholar]
- 46. Liu W, Zhou Y, Zheng W, et al. Repeated intravenous infusions of ketamine: neurocognition in patients with anxious and nonanxious treatment‐resistant depression. J Affect Disord. 2019;259:1‐6. [DOI] [PubMed] [Google Scholar]
- 47. Lucchese AC, Sarin LM, Magalhães EJ, et al. Repeated subcutaneous esketamine for treatment‐resistant depression: impact of the degree of treatment resistance and anxiety comorbidity. J Psychopharmacol. 2021;35(2):142‐149. [DOI] [PubMed] [Google Scholar]
- 48. Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the clinician‐administered dissociative states scale (CADSS). J Traum Stress: Official Publication of the International Society for Traumatic Stress Studies. 1998;11(1):125‐136. [DOI] [PubMed] [Google Scholar]
- 49. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621‐660. doi: 10.1124/pr.117.015198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition‐independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481‐486. doi: 10.1038/nature17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ago Y, Tanabe W, Higuchi M, et al. (R)‐ketamine induces a greater increase in prefrontal 5‐HT release than (S)‐ketamine and ketamine metabolites via an AMPA receptor‐independent mechanism. Int J Neuropsychopharmacol. 2019;22(10):665‐674. doi: 10.1093/ijnp/pyz041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morris PJ, Moaddel R, Zanos P, et al. Synthesis and N‐methyl‐d‐aspartate (NMDA) receptor activity of ketamine metabolites. Org Lett. 2017;19(17):4572‐4575. doi: 10.1021/acs.orglett.7b02177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Suzuki K, Nosyreva E, Hunt K, et al. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546(7659):E1‐E3. doi: 10.1038/nature22084 [DOI] [PubMed] [Google Scholar]
- 54. Kavalali ET, Monteggia LM. The ketamine metabolite 2R,6R‐Hydroxynorketamine blocks NMDA receptors and impacts downstream signaling linked to antidepressant effects. Neuropsychopharmacology. 2018;43(1):221‐222. doi: 10.1038/npp.2017.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abbott JA, Popescu GK. Hydroxynorketamine blocks N‐methyl‐d‐aspartate receptor currents by binding to closed receptors. Mol Pharmacol. 2020;98(3):203‐210. doi: 10.1124/mol.120.119784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang JC, Li SX, Hashimoto K. R (−)‐ketamine shows greater potency and longer lasting antidepressant effects than S (+)‐ketamine. Pharmacol Biochem Behav. 2014;116:137‐141. doi: 10.1016/j.pbb.2013.11.033 [DOI] [PubMed] [Google Scholar]
- 57. Zanos P, Gould TD. Intracellular signaling pathways involved in (S)‐ and (R)‐ketamine antidepressant actions. Biol Psychiatry. 2018;83(1):2‐4. doi: 10.1016/j.biopsych.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Coplan JD, Aaronson CJ, Panthangi V, Kim Y. Treating comorbid anxiety and depression: Psychosocial and pharmacological approaches. World J Psychiat. 2015;5(4):366‐378. doi: 10.5498/wjp.v5.i4.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Northoff G, Richter A, Bermpohl F, et al. NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophr Res. 2005;72(2‐3):235‐248. doi: 10.1016/j.schres.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 60. Ionescu DF, Felicione JM, Gosai A, et al. Ketamine‐associated brain changes: a review of the neuroimaging literature. Harv Rev Psychiatry. 2018;26(6):320‐339. doi: 10.1097/HRP.0000000000000179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016;100:17‐26. doi: 10.1016/j.neuropharm.2015.07.028 [DOI] [PubMed] [Google Scholar]
- 62. Aguilar‐Valles A, De Gregorio D, Matta‐Camacho E, et al. Antidepressant actions of ketamine engage cell‐specific translation via eIF4E. Nature. 2021;590(7845):315‐319. doi: 10.1038/s41586-020-03047-0 [DOI] [PubMed] [Google Scholar]
- 63. Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7(2):193‐218. doi: 10.1017/S1461145704004080 [DOI] [PubMed] [Google Scholar]
- 64. Anxiety and panic attacks [internet] . Mind; [2020]. Available from https://www.mind.org.uk/information-support/types-of-mental-health-problems/anxiety-and-panic-attacks/treatment/
- 65. Costa E. Benzodiazepine/GABA interactions: a model to investigate the neurobiology of anxiety. In Anxiety and the anxiety disorders. Routledge; 2019:27‐52. doi: 10.4324/9780203728215-4. [DOI] [Google Scholar]
- 66. Generoso MB, Trevizol AP, Kasper S, Cho HJ, Cordeiro Q, Shiozawa P. Pregabalin for generalized anxiety disorder: an updated systematic review and meta‐analysis. Int Clin Psychopharmacol. 2017;32(1):49‐55. doi: 10.1097/YIC.0000000000000147 [DOI] [PubMed] [Google Scholar]
- 67. Dooley TP. Treating anxiety with either beta blockers or antiemetic antimuscarinic drugs: A review. Ment Health Fam Med. 2015;11(02):89‐99. doi: 10.25149/1756-8358.1102013 [DOI] [Google Scholar]
- 68. Lindqvist D, Dhabhar FS, Mellon SH, et al. Increased pro‐inflammatory milieu in combat related PTSD‐a new cohort replication study. Brain Behav Immun. 2017. Jan 1;(59):260‐264. [DOI] [PubMed] [Google Scholar]
- 69. Alexander SPH, Christopoulos A, Davenport AP, et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019. Dec;176Suppl 1(Suppl 1):S21‐S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the systematic review is available upon request by emailing the corresponding authors.


