Summary
In parallel with an increased focus on climate changes and carbon footprint, the interest in plant‐based diets and its potential health effects have increased over the past decade. The objective of this systematic review and meta‐analysis was to examine the effect of vegan diets (≥12 weeks) on cardiometabolic risk factors in people with overweight or type 2 diabetes. We identified 11 trials (796 participants). In comparison with control diets, vegan diets reduced body weight (−4.1 kg, 95% confidence interval (CI) −5.9 to −2.4, p < 0.001), body mass index (BMI) (−1.38 kg/m2, 95% CI −1.96 to −0.80, p < 0.001), glycated hemoglobin (HbA1c) (−0.18% points, 95% CI −0.29 to −0.07, p = 0.002), total cholesterol (−0.30 mmol/L, 95% CI −0.52 to −0.08, p = 0.007), and low‐density lipoprotein cholesterol (−0.24 mmol/L, 95% CI −0.40 to −0.07, p = 0.005). We identified no effect on blood pressure, high‐density lipoprotein cholesterol, and triglycerides. We found that adhering to vegan diets for at least 12 weeks may be effective in individuals with overweight or type 2 diabetes to induce a meaningful decrease in body weight and improve glycemia. Some of this effect may be contributed to differences in the macronutrient composition and energy intake in the vegan versus control diets. Therefore, more research is needed regarding vegan diets and cardiometabolic health.
Keywords: cardiometabolic health, overweight, type 2 diabetes, vegan diet
Abbreviations
- BMI
body mass index
- CIs
confidence intervals
- HbA1c
glycated hemoglobin
- HDL‐C
high‐density lipoprotein cholesterol
- LDL‐C
low‐density lipoprotein cholesterol
- RCTs
randomized controlled trials
- SD
standard deviation
1. INTRODUCTION
Plant‐based diets have existed since ancient times and the interest in plant‐based diets has increased dramatically over the past decade. 1 With climate change as a major public health crisis, 2 a plant‐based, and especially a vegan diet, may also lower the carbon footprint and thus be beneficial for the climate, 3 , 4 because a vegan diet excludes all foods of animal sources. 5 , 6 Additionally, several health benefits of vegan diets have been suggested. A meta‐analysis of observational studies concluded that vegan diets are associated with a more favorable cardiometabolic profile compared with an omnivorous diet in western countries. 7 Another systematic review including prospective cohort studies found that intake of plant protein was significantly associated with a lower risk of all‐cause mortality and cardiovascular disease mortality. 8 Systematic reviews and meta‐analyses of randomized controlled trials (RCTs) have found that various plant‐based diets (not all excluding meat products, eggs, and dairy) can lead to reductions in body weight, 9 , 10 , 11 glycated hemoglobin (HbA1c), 11 , 12 blood pressure, 13 and total cholesterol, low‐density lipoprotein cholesterol (LDL‐C), and high‐density lipoprotein cholesterol (HDL‐C). 14 , 15
Improvements in cardiometabolic risk following plant‐based diets may be explained by numerous factors associated with changes in diet quality and quantity. First, people who exclude meat products, eggs, and dairy in their diet will have a lower energy intake due to the diet's lower content of fat and higher content of dietary fiber. 6 , 16 Also, two studies have found an increase in the thermic effect of foods with this type of diet, 17 , 18 which may result in a negative energy balance and consequently a decrease in body weight. Second, weight loss is known to improve glycemic control, 19 , 20 but weight loss may not be the only responsible factor for the improvement in glycemic control. Excessive intramyocellular lipid storage following fat‐rich diets has been shown to increase production of reactive oxygen species and metabolic stress, resulting in increased insulin resistance. 21 A recent study demonstrated that following a low‐fat vegan diet for 14 weeks reduced intramyocellular lipid concentrations compared with control. 17 Third, a transition from a typical western diet to a plant‐based or vegan diet typically reduce total cholesterol, LDL‐C, and HDL‐C by a decreased dietary intake of saturated fat and cholesterol, 6 and furthermore, the high intake of dietary fibers slows down the absorption of cholesterol and the reabsorption of bile acids from the intestine. 22 , 23 On the other hand, results regarding triglyceride levels following plant‐based diets are conflicting. 14 , 15 , 24 Fourth, weight reductions following a plant‐based diet do not fully explain the observed reductions in blood pressure, 25 suggesting that other dietary components may contribute to the blood pressure‐lowering effect, for example, high potassium intake. 26
Because the term “vegetarian” reflects a wide range of plant‐based diets practiced with varying degrees of food restriction, the interpretation of previous meta‐analyses and reviews is challenging and cannot be used to guide specific dietary recommendations. The objective of this systematic review and meta‐analysis was to examine the effects of vegan diets (excluding all foods from animal sources) on cardiometabolic risk factors in people with overweight or type 2 diabetes (including prediabetic state) compared with control groups either (1) continuing habitual diet or (2) receiving other dietary interventions.
2. METHODS
2.1. Protocol registration
The protocol for this systematic review and meta‐analysis was prospectively registered at PROSPERO (CRD42021233938). The study was conducted and reported in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Version 6.2) 27 and The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. 28
2.2. Search strategy
For this systematic review and meta‐analysis, we searched the following electronic databases from inception to March 14, 2022, to identify studies: MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL). We applied no language restrictions on the search. The search strategy was designed to contain concepts of vegan diet in combination with overweight, prediabetes, or type 2 diabetes. In addition, Cochrane's recommended filters for identification of RCTs in MEDLINE, Embase, and CINAHL were applied. 29 Search strings were manually translated between databases. The search strategy is reported in Supporting Information file 1. Further, we screened reference lists of the included studies, and references cited in systematic reviews on the topic.
2.3. Eligibility criteria
We included RCTs conducted in individuals (≥18 years) with overweight (body mass index [BMI] ≥ 25 kg/m2) or type 2 diabetes (or prediabetic state). Individuals with type 2 diabetes fulfilled at least one of the following criteria stated in the original studies: being treated for type 2 diabetes at endocrinology outpatient clinics, 30 , 31 , 32 , 33 having elevated fasting plasma glucose concentrations on at least two occasions (>6.9 mmol/L 20 or >126 mg/dL 30 ) or elevated HbA1c levels (6.0%–11.0% 31 and 6.5%–10.5% 30 ), or using hypoglycemic medication for at least 6 months. 20 , 30 , 31 We excluded studies conducted in children (<18 years), pregnant or breastfeeding women, and individuals with type 1 diabetes or gestational diabetes. We included studies with a vegan diet intervention with a minimum duration of 12 weeks. The rationale for only including studies with an intervention of at least 12 weeks was that we were interested in studying the outcome HbA1c, which requires a period of 2–3 months to change. 34 To meet inclusion criteria, the vegan diet was to be compared with a passive control group (continuing habitual diet with no dietary changes prescribed) or an active control group (receiving other dietary interventions different from the vegan diet). Studies were eligible for inclusion if they provided outcome data on one or more of the following cardiometabolic outcomes: body weight, BMI, HbA1c, blood pressure, and blood lipids (total cholesterol, LDL‐C, HDL‐C, and/or triglycerides). Conference abstracts, unpublished manuscripts, and reports not published in scientific journals were not eligible for inclusion.
2.4. Study selection
After identification, we transferred the records to EPPI‐Reviewer Web, 35 and duplicates were omitted. Two authors (ADT and KKBC) screened the records by title and abstract and afterwards did a full‐text screening. The screening was performed according to the selection criteria stated above, which were prospectively registered at PROSPERO. Each author worked independently and was blinded to each other's decisions during each step of the screening process but was unblinded to information about authors, journals, and countries of origin. All discrepancies were resolved by consensus, and involvement of a third part was not needed. Multiple publications of the same study were linked together. The publication that best fitted the research question was chosen as the primary one. Information from multiple publications was collated and reconciled. In cases of unclear information in the publications, authors of the studies were contacted to clarify potential duplicate publication, to avoid biases when the same study is included more than once in a meta‐analysis. 36
2.5. Data extraction and data synthesis
One author (ADT) extracted data from the studies and entered the data into a standardized spread sheet, and another author (JSQ) reviewed the data for accuracy. Disagreements were resolved by consensus. Data sought for extraction were (1) study characteristics (first author, year of publication, country, study design [parallel group or cross‐over trial], sample size [number of participants randomized, completed, and included in analysis], and study duration [calculated as the period from randomization to the last follow‐up measurement in parallel group studies and as the duration of each period of intervention, excluding run‐in and washout, in cross‐over studies], and adherence), (2) participants' characteristics (mean age and BMI, sex, and study population [overweight, prediabetic state, or type 2 diabetes]), (3) intervention conditions (type of vegan diet, macronutrient composition, energy intake, and amount of support), (4) control conditions (type of control diet, macronutrient composition, energy intake, and amount of support), and (5) outcomes (changes in body weight, BMI, HbA1c, blood pressure, and blood lipids from baseline to end of intervention).
Studies were included in the meta‐analyses if they for the outcome of interest reported (1) number of included participants in the analysis, mean change from baseline to post intervention with corresponding standard deviations (SDs) for both the intervention group and the control group, or (2) number of included participants in the analysis, mean difference between the intervention group and the control group and corresponding 95% confidence intervals (CIs). When a study did not report SD, we used the estimation methods recommended by the Cochrane Handbook. 37 Cochrane RevMan calculator was used to estimate SD. 38
If studies reported unclear information on data sought for extraction, corresponding authors and/or last authors were contacted to clarify the information stated in the papers. If authors did not reply on our request, a reminder was sent after 2 and 4 weeks. Data provided by authors were incorporated in the manuscript up until submission. If studies only reported baseline and post intervention data, mean change for the intervention and control groups was manually calculated by subtracting baseline values from post intervention values. 37 Because this manual calculation cannot be done for SD, missing SD was imputed from other studies as suggested by the Cochrane Handbook and Furuwaka et al. 37 , 39 In case of missing SD, the study with the highest SD for the same outcome measure was used. We chose a conservative approach to avoid an overestimation of the intervention effect. This imputation of data was only done for one small pilot study (n = 11), where the authors were not in possession of the original data anymore. 32 The remaining studies provided sufficient data in the original reports, or provided data on request. 40 If blood lipid levels and HbA1c were not reported in mmol/mol or percent, data were converted to align the units of the outcome measures. When trials had multiple treatment groups, we extracted data from the vegan group and the control group stated in the study. Only one study had multiple treatment groups. 40
2.6. Assessment of study quality
We assessed the risk of bias by the Revised Cochrane risk‐of bias tool for randomized trials (RoB 2) using the Excel file provided by the RoB 2.0 development team. 41 We assigned the individual studies with “high risk,” “low risk,” or “some concerns” for each of the following domains: (1) randomization process, (2) deviation from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported result.
Overall risk of bias judgement was determined as having “some concerns” if one of the domains was rated as having “some concerns.” Likewise, studies were considered to have an overall “high risk” of bias if one of the domains was rated “high risk of bias.” Because 9 out of 11 studies were having “some concerns,” we presented all studies in the meta‐analyses and provided a narrative discussion of the risk of bias according to Cochrane Handbook. 41
We assessed the overall certainty of evidence for each of our outcomes using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework. 42 Evidence from RCTs starts at high quality, but confidence is decreased by one or two levels on the basis of four domains of limitations: (1) risk of bias, 43 (2) publication bias, 44 (3) imprecision, 45 and (4) inconsistency. 46 The fifth domain, indirectness, 47 was not assessed because systematic reviews are considered as direct evidence and will therefore only include eligible research regarding population, intervention, comparator, and outcome(s). See Supporting Information file 2 for description of the GRADE framework used. The certainty of evidence was then classified as high, moderate, low, or very low. 42 To assess the possibility of publication bias, we visually inspected the relationship between precision and effect size in funnel plots if ≥10 trials were included. When a funnel plot was not available, we downgraded the certainty of evidence for Domain 2 if more than 25% of the participants were from small studies (<20 participants in each arm). Two authors (ADT and KKBC) independently assessed risk of bias and certainty of evidence with discrepancies resolved by consensus.
2.7. Statistical analysis
We conducted meta‐analyses investigating the intervention effect on cardiometabolic outcomes with random‐effects generic inverse variance modeling using Cochrane Review Manager (RevMan) Version 5.4. 38 The DerSimmonian and Laird method was used to estimate between‐study variance. 48 We analyzed the continuous data calculating pooled mean difference and corresponding 95% CI, because all studies reported the outcomes using same scales or convertible measurements. A p‐value < 0.05 was considered statistically significant. Results are displayed as forest plots for each of the outcomes.
The random‐effect meta‐analysis was chosen because it estimates the mean of a distribution of effects rather than a single effect assumed to be common to all studies. 48 This approach fitted with the considerable heterogeneity expected from including studies with differences in population, study design, and intervention and control groups.
Statistical heterogeneity in each meta‐analysis was assessed and reported by using the I 2 statistic. 48 Cochrane Handbook roughly suggests that heterogeneity between 0% and 40% might not be important, between 30% and 60% may represent moderate heterogeneity, between 50% and 90% may represent substantial heterogeneity, and between 75% and 100% may represent considerable heterogeneity. When a study had an I 2 that placed the study in two intervals, we chose the highest and thus most conservative interval. Sources of heterogeneity were explored using sensitivity and subgroup analyses. 48
We performed subgroup analysis using study‐level variables to investigate heterogeneity and the possibility of effect modification for body weight, BMI, and HbA1c. To examine differences among subgroups, I 2 statistics were used. For the outcomes body weight and BMI, we divided the studies into subgroups based on their control groups being passive or active. We hypothesized that the effect of a vegan diet would be greater when compared with a control group making no dietary interventions (passive control), than when compared with a control group assigned to another dietary intervention (including prescribed energy restriction) (active control). In addition, we performed an analysis where the studies were divided into subgroups according to being conducted in “people with overweight” or “people with type 2 diabetes” for the outcome HbA1c. This subgroup analysis was a post hoc decision, due to a potential difference in the effect of a vegan diet in participants diagnosed with type 2 diabetes (with potentially elevated HbA1c levels and/or taking glucose‐lowering medication) than in participants with overweight, but without type 2 diabetes.
Sensitivity analyses were conducted to assess the robustness of the results. We performed analyses in which each individual trial comparison was removed from the meta‐analysis and the effect size was recalculated to determine whether a single trial comparison changed the results significantly. The sensitivity analyses were also performed in which studies with missing SD or pilot studies were removed from the meta‐analyses.
3. RESULTS
3.1. Identification and study selection
The search identified 1180 records. Of these, 919 were screened after removal of duplicates, and 862 were excluded based on titles and abstracts. Of 57 potentially relevant records screened in full text for eligibility, 11 trials 17 , 18 , 20 , 30 , 31 , 32 , 33 , 40 , 49 , 50 , 51 (n = 796) met our inclusion criteria (Figure 1). The studies by Mishra et al. 52 and Ferdowsian et al. 53 were excluded due to cluster randomization and non‐randomization, respectively.
FIGURE 1.
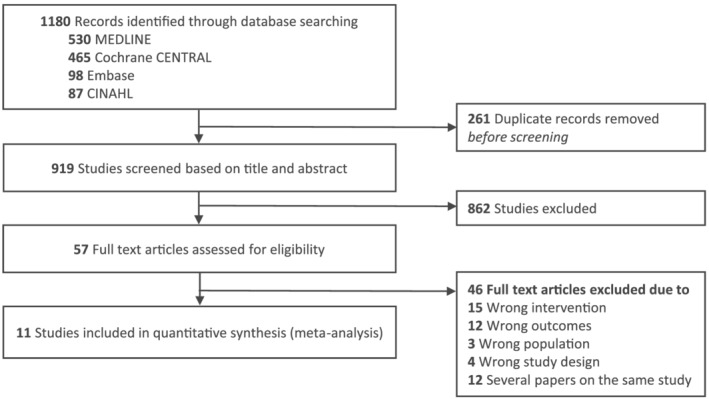
PRISMA study flow
3.2. Cohort characteristics
Table 1 shows characteristics of the included trials. In short, five studies 17 , 18 , 40 , 50 , 51 included participants with overweight, and five studies 20 , 30 , 31 , 32 , 33 participants with type 2 diabetes. One study 49 included individuals with overweight or obesity and reported that 14% of the study population had type 2 diabetes. We identified no trials investigating vegan diets in people being in the prediabetic state. Trial duration ranged from 12 to 26 weeks, with a mean duration of 19 weeks. Trial size ranged from 13 to 244 participants with a mean age ranging from 48 to 61 years. Ten studies 17 , 18 , 20 , 30 , 31 , 32 , 33 , 40 , 49 , 51 used a parallel group design, and one study a cross‐over design (Table 1). 50
TABLE 1.
Characteristics of included trials
| Authors, year, country | Study design | Sample size | Study duration (weeks) | Age (years) | BMI (kg/m2) | Sex (% female) | Population | Intervention diet | Control diet | Outcomes | Support | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Randomized Completed |
BW | BMI | HbA1c | Blood pressure | Blood lipids | ||||||||||
|
Barnard et al., 2005 18 USA |
RCT Parallel |
64 (IG: 32, CG: 32) 59 (IG: 29, CG: 30) |
14 |
IG: 57.4 [47–71] CG: 55.6 [44–73] |
IG: 33.6 ± 5.2 CG: 32.6 ± 3.3 |
100% | Overweight |
Low‐fat vegan diet Ad libitum |
Diet based on the National Cholesterol Education Program Ad libitum |
✓ | ✓ |
Both groups: No meals provided. Weekly 1‐h meetings (nutrition and cooking instruction + group discussions) |
|||
|
Barnard et al., 2006 20 USA |
RCT Parallel |
99 (IG: 49, CG: 50) 99 (IG: 49, CG: 50) |
22 |
IG: 56.7 [35–82] CG: 54.6 [27–80] |
IG: 33.9 ± 7.8 CG: 34.4 ± 7.3 |
IG: 55% CG: 66% |
T2D |
Low‐fat vegan diet + B12 Ad libitum |
Diet based on the American Diabetes Association guidelines + B12 Energy deficit: 500–1000 kcal/day |
✓ | ✓ | ✓ | ✓ | ✓ |
Both groups: No meals provided. Weekly 1‐h meetings (nutrition and cooking instruction). Continuous dietary counseling |
|
Barnard et al., 2018 30 USA |
RCT Parallel |
45 (IG: 21, CG: 24) 40 (IG: 19, CG: 19) |
20 |
IG: 61 [41–79] CG: 61 [30–75] |
IG: 34.9 ± 1.5 a CG: 33.0 ± 1.3 a |
IG: 62% CG: 46% |
T2D |
Low‐fat vegan diet + B12 Ad libitum |
Portion‐controlled diet + B12 Energy deficit: 500 kcal/day |
✓ | ✓ | ✓ | ✓ | ✓ |
Both groups: No meals provided. Weekly 1‐h meetings (nutrition and cooking instruction + support) |
|
Barnard et al., 2021 50 USA |
RCT Cross‐over |
62 52 |
2 × 26 |
IG first: 58.3 ± 8.4 a CG first: 56.6 ± 10.9 a |
IG first: 33.7 ± 3.4 a CG first: 34.3 ± 2.7 a |
77% | Overweight |
Low‐fat vegan diet + B12 Ad libitum |
Mediterranean diet Ad libitum |
✓ | ✓ | ✓ | ✓ | ✓ |
Both groups: No meals provided. Weekly meetings (nutrition and cooking instruction + support) |
|
Bunner et al., 2015 33 USA |
RCT Parallel Pilot study |
35 (IG: 17, CG: 18) 34 (IG: 17, CG: 17) |
20 |
IG: 57 ± 6 CG: 58 ± 6 |
IG: 36 ± 6 CG: 36 ± 7 |
IG: 65% CG: 47% |
T2D |
Low‐fat vegan diet + B12 Ad libitum |
No dietary changes + B12 Ad libitum |
✓ | ✓ | ✓ | ✓ | ✓ |
Intervention group: No meals provided. Weekly meetings (education + social support) |
|
Jenkins et al., 2014 51 Canada |
RCT Parallel |
39 (IG: 20, CG: 19) 23 (IG: 10, CG: 13) |
26 |
IG: 57.6 ± 1.4 a CG: 55.3 ± 1.8 a |
IG: 31.1 (29.8–32.4) CG: 31.1 (29.9–32.4) |
IG: 55% CG: 68% |
Overweight |
Low‐carbohydrate vegan diet 40% energy reduction |
High‐carbohydrate lacto‐ovo vegetarian diet 40% energy reduction |
✓ | ✓ | ✓ | ✓ | ✓ |
Both groups: No meals provided. Continuous dietary counseling. Menu plans provided |
|
Kahleova et al., 2020 17 USA |
RCT Parallel |
244 (IG: 122, CG: 122) 223 (IG: 117, CG: 106) |
16 |
IG: 53 ± 10 CG: 57 ± 13 |
IG: 33.3 ± 3.8 CG: 33.6 ± 3.7 |
IG: 86% CG: 87% |
Overweight |
Low‐fat vegan diet + B12 Ad libitum |
No dietary changes + B12 Ad libitum |
✓ | ✓ | ✓ | ✓ |
Intervention group: No meals provided. Weekly meetings (nutrition and cooking instruction) |
|
|
Lee et al., 2016 31 Korea |
RCT Parallel |
106 (IG: 53, CG: 53) 93 (IG: 46, CG: 47) |
12 |
IG: 57.5 ± 7.7 CG: 58.3 ± 7.0 |
IG: 23.9 ± 3.4 CG: 23.1 ± 2.4 |
IG: 87% CG: 75% |
T2D |
Vegan diet Ad libitum |
Korean Diabetes Association Diet Energy restricted (not specified) |
✓ | ✓ | ✓ | ✓ |
Both groups: No meals provided. Weeks 0 and 4 (nutrition education + instruction). Weekly telephone call |
|
|
Nicholson et al., 1999 32 USA |
RCT Parallel Pilot study |
13 (IG: 7, CG: 6) 11 (IG: 7, CG: 4) |
12 |
IG: 51 [34–62] CG: 60 [51–74] |
NA |
IG: 42% CG: 50% |
T2D |
Low‐fat vegan diet Ad libitum |
Low‐fat diet Ad libitum |
✓ | ✓ | ✓ | ✓ |
Both groups: Lunch and dinner provided. Twice‐weekly meetings (cooking instruction + education + support) |
|
|
Turner‐McGrievy et al., 2015 40 USA |
RCT 5‐arm b |
24 (IG: 12, CG: 12) 21 (IG: 12, CG: 9) |
26 |
IG: 48.2 ± 7.4 CG: 51.0 ± 8.6 |
IG: 32.5 ± 5.2 CG: 36.3 ± 5.5 |
IG: 67% CG: 75% |
Overweight |
Low‐fat vegan diet + B12 Ad libitum |
Healthy omnivores diet + B12 Ad libitum |
✓ | ✓ |
Intervention group: No meals provided. Weekly 1‐h meetings for 8 weeks Control group: No meals provided. Meetings at Weeks 0, 4, and 8. Weekly email (dietary information) |
|||
|
Wright et al., 2017 49 New Zealand |
RCT Parallel |
65 (IG: 33, CG: 32) 49 (IG: 25, CG: 24) |
26 |
IG: 56 ± 9.9 CG: 56 ± 9.5 |
IG: 34.5 (32.9–36.1) CG: 34.2 (31.9–36.5) |
IG: 67% CG: 53% |
Overweight (14% with T2D) |
Low‐fat vegan diet + B12 Ad libitum |
No dietary changes Ad libitum |
✓ | ✓ | ✓ | ✓ | ✓ |
Intervention group: Twice‐weekly 2‐h meetings for 12 weeks (cooking instruction + education + discussion) |
Note: Data depicted as mean ± standard deviation or [range] or (95% confidence interval).
Abbreviations: BMI, body mass index; BW, body weight; CG, control group; HbA1c, glycated hemoglobin; IG, intervention group; NA, not applicable; RCT, randomized controlled trial; T2D, type 2 diabetes.
Indicates mean ± standard error.
Only two groups of the five‐arm study are presented in this review.
Nine studies 17 , 18 , 20 , 30 , 32 , 33 , 40 , 49 , 50 prescribed a low‐fat vegan diet, one study 51 prescribed a low‐carbohydrate vegan diet, and one study 31 prescribed a vegan diet without further description (Table 1). The vegan diets varied substantially with regard to carbohydrate, protein, and fat content (Table S1). Further, none of the studies prescribed a control diet that exactly matched the intervention diet to all other aspects except veganism. All vegan diets except one 51 were ad libitum without any restriction in energy intake. Seven studies prescribed B12 supplementation to the vegan diet intervention ranging from 50 to 1000 μg/day (Table 1). 17 , 20 , 30 , 33 , 40 , 49 , 50
Three studies 17 , 33 , 49 had passive control groups, where the participants continued their habitual diet without dietary changes, all of them being non‐energy restricted. Eight studies 18 , 20 , 30 , 31 , 32 , 40 , 50 , 51 had active control groups, where participants followed other dietary interventions, for example, Mediterranean diet, different diabetes diets, and low‐fat diets (Table 1). Four of these active control diets were energy restricted, 20 , 30 , 31 , 51 and four were ad libitum diets. 18 , 32 , 40 , 50 Five studies prescribed B12 supplementation to the active control diet group that matched the B12 supplementation in the intervention groups. 17 , 20 , 30 , 33 , 40
Four studies reported that the vegan diet groups had a significantly lower energy intake than control groups, 31 , 40 , 50 , 51 and four studies identified no differences in energy intake between groups. 17 , 18 , 20 , 30 Three studies did not test for differences in energy intake between groups. 32 , 33 , 49
In general, dropout rates were low, with only two studies 49 , 51 reporting ˃20% missing outcome data.
3.3. Body weight and BMI
Ten studies evaluated the effects of vegan diets on body weight 17 , 18 , 20 , 30 , 32 , 33 , 40 , 49 , 50 , 51 and BMI. 17 , 18 , 20 , 30 , 31 , 33 , 40 , 49 , 50 , 51 The pooled analysis showed that participants following vegan diets achieved greater weight loss compared with control diets (mean difference −4.1 kg, 95% CI −5.9 to −2.4, n = 697, p < 0.001, I 2 = 91%, GRADE = moderate) (Figure 2; Table 2). BMI fell 1.38 kg/m2 in the vegan intervention groups compared with the control groups (95% CI −1.96 to −0.80, n = 780, p < 0.001, I 2 = 89%, GRADE = moderate) (Figure 3; Table 2).
FIGURE 2.
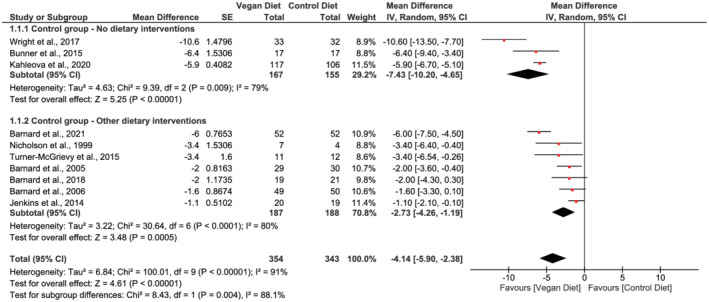
Forest plot depicting the effect of vegan diets on body weight (kg). Plots depict the effect size (mean difference) and 95% confidence interval for the individual studies and the pooled estimate overall and by subgroups. Studies are ordered by effect size. CI, confidence interval; IV, inverse variance; SE, standard error
TABLE 2.
Summary of findings and certainty of evidence
| Outcome | Summary of findings | Certainty of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of participants (no. of trials) | Mean difference (95% CI) | Risk of bias a | Publication bias b | Imprecision c | Inconsistency d | Certainty of evidence (GRADE score) | ||
| Body weight (kg) | 697 (10) | −4.1 kg (−5.9 to −2.4) | □ | □ | □ | ■ | ⊕ ⊕ ⊕ ◯ | Moderate |
| BMI (kg/m2) | 780 (10) | −1.38 kg/m2 (−1.96 to −0.80) | □ | □ | □ | ■ | ⊕ ⊕ ⊕ ◯ | Moderate |
| HbA1c (%) | 687 (9) | −0.18 percentage points (−0.29 to −0.07) | □ | □ | ■ | □ | ⊕ ⊕ ⊕ ◯ | Moderate |
| Systolic blood pressure (mmHg) | 466 (8) | 1.28 mmHg (−1.54 to 4.11) | □ | □ | ■ | □ | ⊕ ⊕ ⊕ ◯ | Moderate |
| Diastolic blood pressure (mmHg) | 466 (8) | 0.54 mmHg (−1.21 to 2.29) | □ | □ | ■ | □ | ⊕ ⊕ ⊕ ◯ | Moderate |
| Total cholesterol (mmol/L) | 605 (8) | −0.30 mmol/mol (−0.52 to −0.08) | □ | □ | ■ | ■ | ⊕ ⊕ ◯ ◯ | Low |
| LDL‐C (mmol/L) | 684 (8) | −0.24 mmol/mol (−0.40 to −0.07) | □ | □ | ■ | ■ | ⊕ ⊕ ◯ ◯ | Low |
| HDL‐C (mmol/L) | 698 (9) | −0.06 mmol/mol (−0.12 to 0.01) | □ | □ | ■ | ■ | ⊕ ⊕ ◯ ◯ | Low |
| Triglycerides (mmol/L) | 698 (9) | 0.11 mmol/mol (−0.08 to 0.29) | □ | □ | ■ | ■ | ⊕ ⊕ ◯ ◯ | Low |
Note: See further explanation in Supporting Information file 2.
Abbreviations: BMI, body mass index; CI, confidence interval; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Downgraded by one level if >25% of participants were from studies at high risk of bias.
Downgraded by one level if a funnel plot suggested the presence of publication bias, or if more than 25% of participants were from small studies.
Downgraded by one level if very wide confidence intervals or if small effect, no effect, or small worsening were observed.
Downgraded by one level if dissimilarity in point estimates and confidence intervals were observed and heterogeneity (I 2) > 50%.
FIGURE 3.
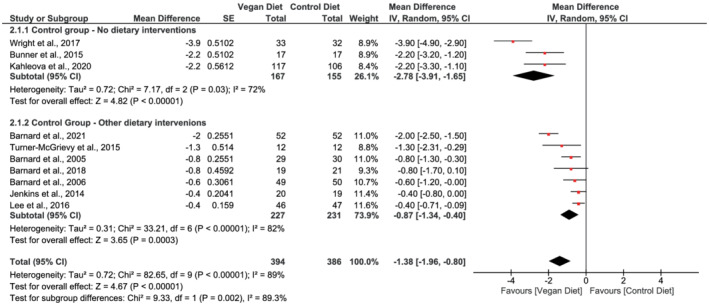
Forest plot depicting the effect of vegan diets on body mass index (kg/m2). Plots depict the effect size (mean difference) and 95% confidence interval for the individual studies and the pooled estimate overall and by subgroups. Studies are ordered by effect size. CI, confidence interval; IV, inverse variance; SE, standard error
Subgroup analysis based on comparator diet identified a greater weight loss when vegan diets were compared with habitual diet (mean difference in body weight −7.4 kg, 95% CI −10.2 to −4.7, n = 322, p < 0.001, I 2 = 79%) than compared with other dietary interventions (mean difference −2.7 kg, 95% CI −4.3 to −1.2, n = 375, p < 0.001, I 2 = 80%) (Figure 2). Test for subgroup difference was significant (χ 2 = 8.43, p = 0.004). Similarly, BMI fell by 2.78 kg/m2 in the vegan groups (95% CI −3.91 to −1.65, n = 322, p < 0.001, I 2 = 72%) as when compared with habitual diet and by 0.87 kg/m2 when compared with other dietary interventions (95% CI −1.34 to −0.40, n = 458, p < 0.001, I 2 = 82%) (Figure 3). Test for subgroup difference was significant (χ 2 = 9.33, p = 0.002).
3.4. HbA1c
Nine studies 17 , 20 , 30 , 31 , 32 , 33 , 49 , 50 , 51 reported data for HbA1c. The pooled analysis showed that vegan diets led to a reduction of 0.18 percentage points compared with control diets (95% CI −0.29 to −0.07, n = 687, p = 0.002, I 2 = 66%, GRADE = moderate) (Figure 4; Table 2).
FIGURE 4.
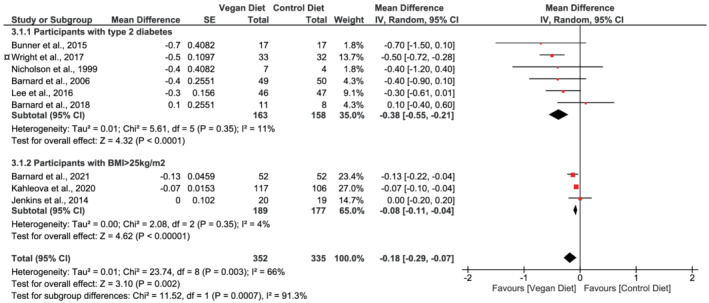
Forest plot depicting the effect of vegan diets on glycated hemoglobin (%) (HbA1c). Plots depict the effect size (mean difference) and 95% confidence interval for the individual studies and the pooled estimate overall and by subgroups. Studies are ordered by effect size. BMI, body mass index; CI, confidence interval; IV, inverse variance; SE, standard error. ¤The study by Wright and colleagues was placed in the subgroup “Participants with type 2 diabetes.” Participants were either obese (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 25 kg/m2) with either a diagnosis of type 2 diabetes or ischemic heart disease or had hypertension or hypercholesterolemia
The analysis stratified by sub‐populations showed that studies conducted in participants with type 2 diabetes had a mean reduction in HbA1c of 0.38 percentage points (95% CI −0.55 to −0.21, n = 321, p < 0.001, I 2 = 11%), and in participants with overweight, a reduction of 0.08 percentage points (95% CI −0.11 to −0.04, n = 366, p < 0.001, I 2 = 4%) when comparing vegan diets with control diets (Figure 4). Test for subgroup difference was significant (χ 2 = 11.52, p < 0.001).
3.5. Blood pressure
Eight studies 20 , 30 , 31 , 32 , 33 , 49 , 50 , 51 reported on blood pressure, with no difference observed in either systolic blood pressure (mean difference 1.28 mmHg, 95% CI −1.54 to 4.11, n = 466, p = 0.37, I = 34%, GRADE = moderate) or diastolic blood pressure (mean difference 0.54 mmHg, 95% CI −1.21 to 2.29, n = 466, p = 0.55, I 2 = 37%, GRADE = moderate), when comparing vegan diets with control diets (Figure 5; Table 2).
FIGURE 5.
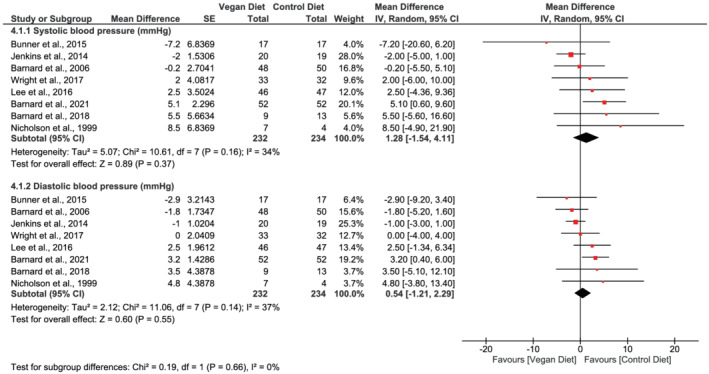
Forest plot depicting the effect of vegan diets on systolic and diastolic blood pressure (mmHg). Plots depict the effect size (mean difference) and 95% confidence interval for the individual studies and the pooled estimate overall and by subgroups. Studies are ordered by effect size. CI, confidence interval; IV, inverse variance; SE, standard error
3.6. Blood lipids
Eight studies 17 , 20 , 30 , 32 , 33 , 49 , 50 , 51 reported on total cholesterol, and the pooled analysis showed that vegan diets led to a mean 0.30 mmol/L reduction in total cholesterol compared with control diets (95% CI −0.52 to −0.08, n = 605, p = 0.007, I 2 = 65%, GRADE = low) (Figure 6; Table 2). The pooled estimate of the eight studies 17 , 20 , 30 , 31 , 33 , 49 , 50 , 51 reporting data on LDL‐C showed that vegan diets reduced LDL‐C compared with control diets (mean difference −0.24 mmol/L, 95% CI −0.40 to −0.07, n = 684, p = 0.005, I 2 = 58%, GRADE = low) (Figure 6; Table 2). The meta‐analysis of nine studies 17 , 20 , 30 , 31 , 32 , 33 , 49 , 50 , 51 reporting data on HDL‐C levels identified no difference between vegan diets and control diets (mean difference −0.06 mmol/L, 95% CI −0.12 to 0.01, n = 698, p = 0.08, I 2 = 67%, GRADE = low) (Figure 6; Table 2). In addition, pooled analysis of the same nine studies did not identify differences between vegan diets and control diets on triglyceride levels in the pooled analysis (mean difference 0.11 mmol/L, 95% CI −0.08 to 0.29, n = 698, p = 0.26, I 2 = 65%, GRADE = low) (Figure 6; Table 2).
FIGURE 6.
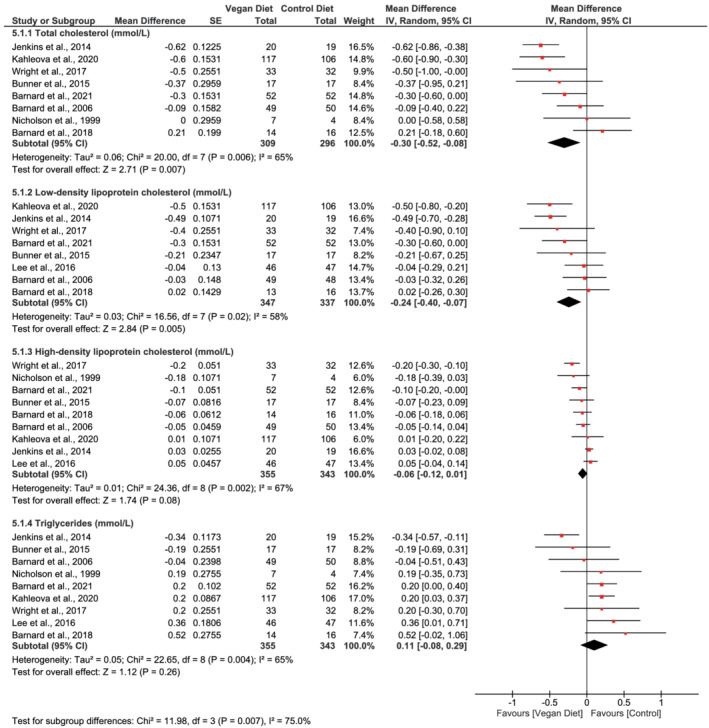
Forest plot depicting the effect of vegan diets on blood lipids (total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglycerides) (mmol/L). Plots depict the effect size (mean difference) and 95% confidence interval for the individual studies and the pooled estimate overall and by subgroups. Studies are ordered by effect size. CI, confidence interval; IV, inverse variance; SE, standard error
3.7. Adherence and support
Nine studies 18 , 20 , 30 , 31 , 33 , 40 , 49 , 50 , 51 reported on adherence to the prescribed diets through 24‐h food recalls, 20 , 30 , 40 self‐reported food diaries, 18 , 31 , 33 , 50 , 51 or average attendance at intervention sessions. 20 , 49 Four studies 18 , 30 , 40 , 50 reported similar adherence in intervention and control groups, with two studies 18 , 50 reporting high adherence, and two studies 30 , 40 did not specify adherence further. In addition, two studies only monitored adherence in their intervention groups, where one study 49 reported that the vegan diet group was highly adherent, while another study 33 reported that adherence to the plant‐based aspect of the diet was high, while adherence to the low‐fat aspect of the diet was less so. Two studies 20 , 51 followed adherence closely throughout the study period, and further dietary counseling was provided if adherence was challenged. One of the studies reported that adherence was significantly higher among participants following a vegan diet compared with the American Diabetes Association diet with same amount of support. 20 In contrast, the other study assessed adherence based on intake of three cholesterol‐lowering components from 7‐day food records and reported that the participants consumed 33% of the prescribed dietary components. 51 Lastly, one study reported that the control group (Korean Diabetes Association Diet) had significantly higher adherence compared with the vegan intervention group. 31 The latter two studies 31 , 51 provided limited amount of support compared with the other studies in this review. The remaining nine 17 , 18 , 20 , 30 , 32 , 33 , 40 , 49 , 50 studies had a high level of support with weekly or twice‐weekly sessions of cooking and nutrition instruction, group discussions, and support during the whole intervention period (see Table 1 for amount of support provided in the individual studies).
3.8. Long‐term follow‐up
Intervention duration ranged from 12 to 26 weeks, and few of the studies 49 , 54 , 55 have investigated if a vegan diet is effective in producing weight loss as well as maintaining weight loss in the long term. The 22‐week study by Barnard et al. 20 conducted in people with type 2 diabetes included a follow‐up 1 year after the end of intervention. 54 The mean weight loss at 22 weeks (−5.8 kg [vegan] and −4.3 kg [diet based on American Diabetes Association guidelines]) was partly maintained at 1‐year follow‐up (−4.4 and −3.0 kg compared with baseline). 54 Biweekly sessions of support were provided during the whole follow‐up period. Another study by Barnard et al. 18 investigated the effect of a vegan diet compared with National Cholesterol Education Program diet during a 14‐week intervention in postmenopausal women 18 and did follow‐up at 1 and 2 years post intervention. 55 At 14 weeks, and at 1‐ and 2‐year follow‐up, the vegan diet group on average lost −5.8, −4.9, and −3.1 kg compared with baseline, whereas the control group lost −3.8, −1.8, and −0.8 kg. Changes in body weight at all timepoints were significantly lower in the vegan diet group compared with the control group. 55 This study further investigated the importance of support during the follow‐up period by dividing the participants in each diet group into a supported and an unsupported group. The supported group was offered 1‐h meetings biweekly and lost more weight than their unsupported counterparts. 55 Lastly, the 26‐week study by Wright et al. 49 offered their participants to continue the vegan diet for additional 26 weeks, and 70% were followed up at 1 year (amount of support during follow‐up period is not described). The study found that the vegan group lost −12.1 and −11.5 kg at 26 weeks and at 1 year compared with baseline.
3.9. Acceptability of a vegan diet
Two of the included studies have investigated the acceptability of the vegan diets along with the efficiency in reducing body weight and improving type 2 diabetes risk. 18 , 20 The study by Barnard and colleagues 18 found that the acceptability of a low‐fat vegan diet among 64 postmenopausal women was high and not distinguishable from a more permissive National Cholesterol Education Program diet. 56 Further, 99 individuals with type 2 diabetes in the study by Barnard et al. 20 rated both the low‐fat vegan diet and the diet based on the American Diabetic Association guidelines as acceptable with no differences in acceptability between diets at both 22 and 74 weeks. 57 In addition, the study found the vegan diet to be marginally more requiring with regard to initial effort, but the difference was no longer apparent at 74 weeks. Acceptability was assessed based on rates of retention, diet adherence, and food acceptability questionnaires. 56 , 57
3.10. Changes in medication
Due to studies being conducted in participants with overweight or type 2 diabetes, several of the studies 17 , 20 , 30 , 31 , 32 , 33 , 50 , 51 reported on medication intake as medication changes may have obscured some of the observed changes in glucose control, blood pressure, and blood lipids. Most studies asked the participants not to change medication during the study duration 17 , 20 , 30 , 31 , 33 , 50 ; yet, most studies reported that medication was changed for some of the participants. 20 , 30 , 32 , 33 , 50 Only the study by Lee and colleagues reported no changes in medication throughout the study period. 31 Three studies presented results for all participants combined, 17 , 32 , 33 and two studies reported data for all participants as well as for the subgroup of participants that had consistent medication use during the intervention period. 20 , 50 However, one study 30 chose to report data only for the subgroup that did not change medication. For the remaining studies, we extracted data for all participants despite changes in medication. Jenkins et al. 51 chose in their 26‐week study to discontinue the participants' lipid‐lowering medication 2 weeks prior to starting the intervention and during the whole study duration. However, this decision caused the study physician to withdraw four participants due to failure in attaining LDL‐C targets.
3.11. Adverse events
Three studies reported on adverse events following vegan diets. The studies by Barnard et al. 20 and Bunner et al. 33 reported that there were no significant treatment‐related serious events, and Jenkins et al. 51 reported that no serious adverse events or events that involved hospitalization occurred during the study. The remaining eight studies did not report adverse events. 17 , 18 , 30 , 31 , 32 , 40 , 49 , 50
3.12. Study quality
Overall, two studies 20 , 33 were assessed to have “low risk” of bias and the remaining nine studies as having “some concerns” (Figure 7). Five trials 31 , 32 , 40 , 50 , 51 reported that the participants were randomized but did not provide further information on whether they used random sequence generation or appropriate allocation concealment and were therefore classified as having “some concerns.” Two studies 49 , 51 reported deviations from intended interventions and were rated as having “some concerns.” All studies were assessed “low risk” of bias regarding the domains “Missing outcome data” and “Measurement of the outcome.” The domain “selection of reported result” had the poorest reporting, with 54% (6/11) of trials having “some concerns.” This was mainly due to unavailability of protocols or clinical trial registrations, 18 , 32 , 40 , 49 as well as selective reporting of results, 30 and multiple outcome measurements. 17 The overall evidence should be considered fairly high quality because only RCTs were included and only a small proportion had missing data to a large extend.
FIGURE 7.
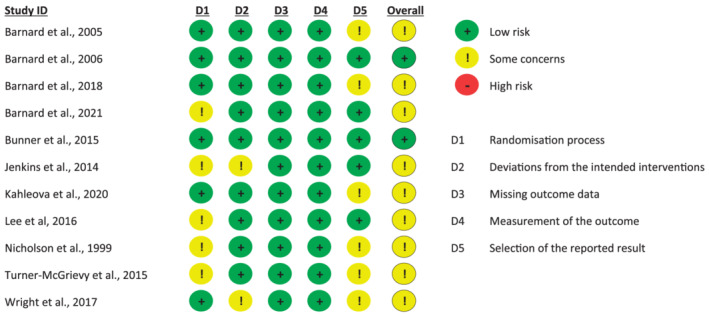
Risk of bias assessment
We only visually inspected funnel plots for the outcomes body weight and BMI, because they were the only two meta‐analyses including 10 trials. The funnel plots (Figures S1 and S2) did not show evidence of asymmetry between the studies than expected by change. Due to the small number of studies, we did not find it appropriate to test for funnel plot asymmetry, because the test power would be too low to decide whether the result was due to true asymmetry or chance.
The overall certainty of evidence for the effect of vegan diets on cardiometabolic outcomes is presented in Table 2. The evidence was graded as moderate for the effect of vegan diets on body weight, BMI, HbA1c, and systolic and diastolic blood pressure, owing to a downgrade for inconsistency for body weight and BMI, and for imprecision for HbA1c, and systolic and diastolic blood pressure. All blood lipid outcomes were downgraded for imprecision and inconsistency, and the evidence was graded low for all four outcomes.
3.13. Heterogeneity
Heterogeneity varied between the outcomes. Analyses suggested considerable heterogeneity for the outcomes body weight (I 2 = 91%, Figure 2) and BMI (I 2 = 89%, Figure 3), but the heterogeneity was reduced in the subgroup analyses when dividing the studies according to the control groups being active or passive. For the outcomes HbA1c, total cholesterol, LDL‐C, HDL‐C, and triglycerides, analyses suggested substantial heterogeneity (I 2 [58%–67%], Figure 4; Figure 6). The subgroup analyses for HbA1c eliminated the heterogeneity to 11% in the subgroup of participants with type 2 diabetes and to 4% in the subgroups with participants with BMI ≥ 25 kg/m2 (Figure 4). For systolic and diastolic blood pressure, moderate heterogeneity was suggested in the analyses (I 2 = 36% and 37%, Figure 5).
3.14. Sensitivity analysis
Sensitivity analyses excluding (1) each individual trial, (2) the studies with the missing SD, and (3) pilot studies did not substantially change the pooled estimates for the following outcomes: body weight, BMI, systolic and diastolic blood pressure, total cholesterol, LDL‐C, HDL‐C, and triglycerides. Only the removal of the study by Kahleova et al. 17 from the HbA1c meta‐analysis (n = 223) led to insignificant results in the subgroup of studies conducted in participants with overweight (mean difference −0.10 kg/m2, −0.21 to 0.01, n = 143, p = 0.09, I 2 = 26%) but did not change the overall pooled estimate for HbA1c (Figure S3).
4. DISCUSSION
4.1. Main findings
This systematic review and meta‐analysis demonstrate that consumption of vegan diets is associated with improvements in body weight, BMI, HbA1c, total cholesterol, and LDL‐C in people with overweight or type 2 diabetes. We showed that consuming vegan diets led to greater weight loss than other dietary interventions, but there was an even greater weight loss when vegan diets were compared with control groups continuing their habitual diet. We found no effects of vegan diets on levels of HDL‐C, triglycerides, and blood pressure compared with control diets.
4.2. Comparison with other studies
Our findings are in line with previous research investigating the effects of a wide range of different vegetarian diets. A recent systematic review investigating the effect of vegan diets in prevention and treatment of type 2 diabetes found similar results for all outcomes 58 ; however, a direct comparison of effect estimates is not possible due to lack of meta‐analyses. The review included eight studies of which five were included in our study. The remaining three studies were not eligible in our study due to short duration 59 (6 weeks), cluster randomization, 52 and non‐randomization. 53 Two systematic reviews and meta‐analyses 9 , 10 including 12 and 15 studies of which 8 and 11 were vegan diets, respectively, found reductions in body weight following vegetarian diets of −2.0 and −3.4 kg. The pooled estimate in our study is higher, which may be explained by the fact that only vegan diets were included in our analysis, and vegan diets likely lead to higher energy deficits due to its low energy density. 16 The reduction in HbA1c observed in our study is in agreement with a previous meta‐analysis of six vegetarian studies (including two vegan studies). 12 The decreases in total cholesterol and LDL‐C levels, as well as the finding of no changes in triglyceride levels found in our review are also in agreement with two previous meta‐analyses 14 , 15 including 11 and 19 vegetarian studies of which seven and nine were vegan studies, respectively. Both reviews reported reductions in HDL‐C levels, which we could not confirm in our meta‐analysis, although we found a tendency in the same direction. With regard to blood pressure, our analysis showed no effect of vegan diet on systolic and diastolic blood pressure, whereas another meta‐analysis 13 including seven studies found significant decreases in both diastolic and systolic blood pressure in response to a vegetarian diet. Two of the studies investigated vegan diets, and only the study by Nicholson et al. 32 was included in our analysis, because the study by Ferdowsian et al. 53 was a non‐randomized study. The inclusion of some animal products, for example, milk‐derived products and eggs, and the focus on n‐3 fatty acids could explain the reduction in blood pressure in the meta‐analysis by Yokoyama et al. 13
4.3. Adherence, support, and acceptability
Adherence and acceptability to vegan diets are important issues to address, because it may be difficult for individuals with a habitual omnivorous diet to implement and maintain a vegan diet for several months. Most of the studies reported high adherence to the vegan diets, but the same studies also provided a high amount of support during the study periods. These results suggest that providing consistent face‐to‐face contact and support may partly explain the observed adherence, which questions long‐term feasibility. Only two studies investigated the acceptability of vegan diets, and both found vegan diets to be well accepted by the participants based on rates of retention, diet adherence, and food acceptability questionnaires. 56 , 57 The participants in the two studies were highly supported by the project workers, and as with adherence, this contact may have affected the acceptability of the vegan diet as well. Unfortunately, the studies with the lowest adherence to vegan diets did not investigate diet acceptability, which would have been relevant in terms of shedding light on the difficulties and disadvantages associated with following a vegan diet.
4.4. Clinical relevance
Our results demonstrate that a vegan diet is associated with a meaningful weight loss in interventions lasting 12–26 weeks. Also, we found indications of long‐term maintenance of weight loss (up to 2 years) with vegan diets even in the absence of energy restriction. Of interest, a vegan diet reduced body weight significantly more than other diets focusing on cardiometabolic health and weight loss, for example, different diabetes diets, portion‐controlled diet, low‐fat diets, and Mediterranean diets, which often were energy restricted. Some of the studies that included data on changes in body composition 17 , 18 , 50 , 51 found that loss of lean mass accounted for 12%–40% of total weight loss in response to vegan diet interventions, which is expected during a diet‐induced weight loss. 60 As such, recommendations of eating vegan diets should optimally be combined with recommendations of being physically active to prevent loss of lean mass. 61
The American Dietetic Association states that appropriately planned vegetarian diets, including vegan diets, are nutritionally adequate and that vegan diets are considered to be a safe and effective option to obtain weight loss. 5 Furthermore, many dietary guidelines recommend to substitute red meat with plant‐based alternatives in the diet. 62 , 63 The practical advantages of a vegan diet for obtaining or maintaining a healthy weight include the absence of any restriction on caloric intake and lack of necessity for calculating food portion sizes, which may help long‐term feasibility compared with more restrictive diets. However, practical disadvantages of following a vegan diet may include less social acceptability and feasibility. In addition, one should continuously keep a focus on potential adverse events associated with consumption of a vegan diet. Ensuring a sufficient intake of essential amino acids and certain micronutrients (e.g., vitamin B12, vitamin D, iodine, iron, zink, calcium, and long‐chain n‐3 fatty acids [especially EPA and DHA]) is an important consideration. 5 , 6 , 64 A study by Kristensen et al. found that the intake of protein, vitamin D, iodine, and selenium (including supplements) among Danish vegans did not reach the 2012 Nordic Nutrition Recommendations. 64 However, with reference to the Nordic Nutrition Recommendations, the dietary content of sodium, added sugar, and fatty acids was more favorable among Danish vegans compared with age‐range‐matched omnivorous individuals from the general Danish population. 64
The high level of support during the interventions in the majority of the studies included in the meta‐analysis may limit the direct applicability of these types of vegan diet interventions in routine care.
4.5. Strengths and limitations of the study
Our systematic review has several important strengths. Firstly, it was conducted according to well‐established systematic review guidelines. The study was prospectively registered, included a thorough literature search, and we performed all screenings and assessments independently and in duplicate. It further strengthens our conclusions that we have only included RCTs. Secondly, we have only included studies that investigated vegan diets, and not a broad range of different vegetarian diets, making the interpretation across studies more comparable.
Our study is not without limitations. Firstly, only 11 studies were eligible to be included in the systematic review and meta‐analysis. Secondly, the nature of a diet intervention makes it impossible to blind the intervention for participants. Knowledge of group assignment may have affected their behavior in the trials. 65 Research staff delivering the intervention was also unblinded, but several studies reported that outcome assessors, 17 , 18 , 20 , 30 statisticians, 17 , 49 , 50 analytical technicians, 51 and endocrinologists 20 responsible for changing medications were blinded to varying degrees.
Thirdly, as expected, there was substantial heterogeneity across the included studies, which were due to differences in study population (e.g., sex, ethnicity, and morbidity) and study duration. In the stratified analysis, we observed larger effects on HbA1c in participants with type 2 diabetes compared with those without diabetes. This finding was expected because of the larger potential to improve HbA1c in the participants with diabetes. Further, the prescribed vegan diets varied in macronutrient composition, and the control groups were either continuing habitual diet or were prescribed other dietary interventions with different prescriptions in energy restriction. Therefore, the observed effects of vegan interventions on weight loss may partly be caused by differences in macronutrient composition and energy intake between the groups. Also, most studies did not adjust for changes in body weight when reporting changes in HbA1c and blood lipids. As such, the effects of vegan diet on blood markers may to some extent be mediated by weight loss. Due to the limited number of studies, we only performed three subgroup analyses, which showed that heterogeneity could be reduced (BMI and body weight) or eliminated (HbA1c) when the studies were grouped and analyzed according to control group or population, respectively. Especially for HbA1c, a considerable proportion of the variance came from the between‐grouped‐studies component and to a lesser degree from the within‐grouped‐studies component.
Fourthly, seven of the included studies 17 , 18 , 20 , 30 , 32 , 33 , 50 have been performed by the same research group; therefore, studies from different research groups would strengthen the results.
Lastly, we overall assessed the study quality as good; however, better descriptions of the randomization processes as well as transparency through protocols and clinical trial registrations would have decreased the risk of bias across studies. The certainty of evidence was graded as moderate for the effect of vegan diets on body weight, BMI, HbA1c, and systolic and diastolic blood pressure, reflecting that we believe that the true effect is probably close to the estimated effect in this review. Further, for all blood lipid outcomes, the GRADE assessment showed that the true effect might be markedly different from the estimated effect.
4.6. Unanswered questions and future research
The included studies in this review all had very high levels of individual support during the interventions, which may not be feasible in the routine clinical setting. Therefore, future studies should explore the importance of individual support for adherence to vegan diets, and further if other less costly support tools (e.g., virtual education programs and peer support) could be applied, thereby making vegan diets easier to implement in real‐life conditions. Furthermore, some studies have demonstrated that switching to a more heart‐healthy diet characterized by lower intakes of fat and sodium and higher intakes of whole grain, fruit, and vegetables reduces not only LDL‐C but also HDL‐C, 14 , 15 the so‐called vegetarian paradox. Future studies should investigate how to prevent or minimize reductions in HDL‐C when following vegan diets.
5. CONCLUSION
Moderate evidence suggests that adhering to vegan diets for at least 12 weeks may be effective in individuals with overweight or type 2 diabetes to induce a meaningful decrease in body weight and improve glycemia. Some of this effect may be contributed to differences in the macronutrient composition and energy intake in the vegan diets versus control diets. Therefore, more research is needed regarding vegan diets and cardiometabolic health.
CONFLICT OF INTEREST
No authors have received support from any organization for the submitted work. ADT, ON, LJD, JSQ, and KF are employed at Steno Diabetes Center Copenhagen, a public hospital and research institution under the Capital Region of Denmark, which is partly funded by a grant from the Novo Nordisk Foundation. KKBC is currently employed by Novo Nordisk A/S. SST has received grants from Novo Nordisk Foundation, Novo Nordisk A/S, Lundbeck Foundation, and Cambridge Weight Plan, and JSQ received grants from Danish Diabetes Academy, Danish Diabetes Association, and Novo Nordisk A/S. JMT owns stocks in Oatly group AB, KKBC, KF, and JMT own stocks in Novo Nordisk A/S, and KF owns stocks in ChemoMetec A/S and Genmab A/S. KF is a professional board member at ChemoMetec A/S. SST has received equipment/materials from Cambridge Weight Plan and Novo Nordisk. The authors have no other relationships or activities that could appear to have influenced the submitted work.
Supporting information
Table S1 Macronutrient composition in vegan intervention diets and control diets
Figure S1 Funnel plot depicting the effect of vegan diet intervention on body weight
Figure S2 Funnel plot depicting the effect of vegan diet intervention on BMI
Figure S3 Forest plot depicting the effect of vegan diets on glycated hemoglobin (HbA1c) after removal of the study by Kahleova and colleagues.
ACKNOWLEDGMENT
Open access funding enabled and organized by Projekt DEAL.
Termannsen A‐D, Clemmensen KKB, Thomsen JM, et al. Effects of vegan diets on cardiometabolic health: A systematic review and meta‐analysis of randomized controlled trials. Obesity Reviews. 2022;23(9):e13462. doi: 10.1111/obr.13462
REFERENCES
- 1. Ruby MB. Vegetarianism. A blossoming field of study. Appetite. 2012;58(1):141‐150. doi: 10.1016/j.appet.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 2. Atwoli L, Baqui AH, Benfield T, et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health. BMJ. 2021;374:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner‐McGrievy GM, Leach AM, Wilcox S, Frongillo EA. Differences in environmental impact and food expenditures of four different plant‐based diets and an omnivorous diet: results of a randomized, controlled intervention. J Hunger Environ Nutr. 2016;11(3):382‐395. doi: 10.1080/19320248.2015.1066734 [DOI] [Google Scholar]
- 4. Springmann M, Wiebe K, Mason‐D'Croz D, Sulser TB, Rayner M, Scarborough P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: a global modelling analysis with country‐level detail. Lancet Planet Heal. 2018;2(10):e451‐e461. doi: 10.1016/S2542-5196(18)30206-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Craig WJ, Mangels AR. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc. 2009;109(7):1266‐1282. doi: 10.1016/j.jada.2009.05.027 [DOI] [PubMed] [Google Scholar]
- 6. Craig WJ. Health effects of vegan diets. Am J Clin Nutr. 2009;89(5):1627S‐1633S. doi: 10.3945/ajcn.2009.26736N [DOI] [PubMed] [Google Scholar]
- 7. Benatar JR, Stewart RAH. Cardiometabolic risk factors in vegans; a meta‐analysis of observational studies. PLoS ONE. 2018;13(12):1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naghshi S, Sadeghi O, Willett WC, Esmaillzadeh A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose‐response meta‐analysis of prospective cohort studies. BMJ. 2020;370:1‐17. doi: 10.1136/bmj.m2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang RY, Huang CC, Hu FB, Chavarro JE. Vegetarian diets and weight reduction: a meta‐analysis of randomized controlled trials. J Gen Intern Med. 2016;31(1):109‐116. doi: 10.1007/s11606-015-3390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta‐analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet. 2015;115(6):954‐969. doi: 10.1016/j.jand.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 11. Toumpanakis A, Turnbull T, Alba‐Barba I. Effectiveness of plant‐based diets in promoting well‐being in the management of type 2 diabetes: a systematic review. BMJ Open Diabetes Res Care. 2018;6(1):1‐10. doi: 10.1136/bmjdrc-2018-000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta‐analysis. Cardiovasc Diagn Ther. 2014;4(5):373‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yokoyama Y, Nishimura K, Barnard ND, et al. Vegetarian diets and blood pressure: a meta‐analysis. JAMA Intern Med. 2014;174(4):577‐587. doi: 10.1001/jamainternmed.2013.14547 [DOI] [PubMed] [Google Scholar]
- 14. Wang F, Zheng J, Yang B, Jiang J, Fu Y, Li D. Effects of vegetarian diets on blood lipids: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(10):1‐14. doi: 10.1161/JAHA.115.002408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yokoyama Y, Levin SM, Barnard ND. Association between plant‐based diets and plasma lipids: a systematic review and meta‐analysis. Nutr Rev. 2017;75(9):683‐698. doi: 10.1093/nutrit/nux030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bell EA, Rolls BJ. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr. 2001;73(6):1010‐1018. doi: 10.1093/ajcn/73.6.1010 [DOI] [PubMed] [Google Scholar]
- 17. Kahleova H, Petersen KF, Shulman GI, et al. Effect of a low‐fat vegan diet on body weight, insulin sensitivity, postprandial metabolism, and intramyocellular and hepatocellular lipid levels in overweight adults: a randomized clinical trial. JAMA Netw Open. 2020;3(11):1‐14. doi: 10.1001/jamanetworkopen.2020.25454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnard ND, Scialli AR, Turner‐McGrievy G, Lanou AJ, Glass J. The effects of a low‐fat, plant‐based dietary intervention on body weight, metabolism, and insulin sensitivity. Am J Med. 2005;118(9):991‐997. doi: 10.1016/j.amjmed.2005.03.039 [DOI] [PubMed] [Google Scholar]
- 19. Rock CL, Flatt SW, Pakiz B, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(6):1573‐1580. doi: 10.2337/dc13-2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barnard ND, Cohen J, Jenkins DJA, et al. A low‐fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777‐1783. doi: 10.2337/dc06-0606 [DOI] [PubMed] [Google Scholar]
- 21. Meex RCR, Blaak EE, van Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev. 2019;20(9):1205‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jesch ED, Carr TP. Food ingredients that inhibit cholesterol absorption. Prev Nutr Food Sci. 2017;22(2):67‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naumann S, Haller D, Eisner P, Schweiggert‐Weisz U. Mechanisms of interactions between bile acids and plant compounds—a review. Int J Mol Sci. 2020;21(18):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnard ND, Scialli AR, Bertron P, Hurlock D, Edmonds K, Talev L. Effectiveness of a low‐fat vegetarian diet in altering serum lipids in healthy premenopausal women. Am J Cardiol. 2000;85(8):969‐972. doi: 10.1016/S0002-9149(99)00911-X [DOI] [PubMed] [Google Scholar]
- 25. Rouse IL, Armstrong BK, Beilin LJ, Vandongen R. Blood‐pressure‐lowering effect of a vegetarian diet: controlled trial in normotensive subjects. Lancet. 1983;1(8314–8315):5‐10. doi: 10.1016/S0140-6736(83)91557-X [DOI] [PubMed] [Google Scholar]
- 26. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ. 2013;346:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021. https://www.training.cochrane.org/handbook [Google Scholar]
- 28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lefebvre C, Glanville J, Briscoe S, et al. Technical supplement to Chapter 4: searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021. https://www.training.cochrane.org/handbook [Google Scholar]
- 30. Barnard ND, Levin SM, Gloede L, Flores R. Turning the waiting room into a classroom: weekly classes using a vegan or a portion‐controlled eating plan improve diabetes control in a randomized translational study. J Acad Nutr Diet. 2018;118(6):1072‐1079. doi: 10.1016/j.jand.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 31. Lee YM, Kim SA, Lee IK, et al. Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: a 12‐week randomized clinical trial. PLoS ONE. 2016;11(6):1‐14. doi: 10.1371/journal.pone.0155918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicholson AS, Sklar M, Barnard ND, Gore S, Sullivan R, Browning S. Toward improved management of NIDDM: a randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med (Baltim). 1999;29(2):87‐91. doi: 10.1006/pmed.1999.0529 [DOI] [PubMed] [Google Scholar]
- 33. Bunner AE, Wells CL, Gonzales J, Agarwal U, Bayat E, Barnard ND. A dietary intervention for chronic diabetic neuropathy pain: a randomized controlled pilot study. Nutr Diabetes. 2015;5(5):1‐6. doi: 10.1038/nutd.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanas R, John G. 2010 Consensus statement on the worldwide standardization of the hemoglobin A1C measurement. Diabetes Care. 2010;33(8):1903‐1904. doi: 10.2337/dc10-0953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas J, Graziosi S, Brunton J, Ghouze Z, O'Driscoll P, Bond M, Koryakina A. EPPI‐Reviewer: advanced software for systematic reviews, maps and evidence synthesis. EPPI‐Centre, UCL Social Research Institute, University College London; 2022.
- 36. Lefebvre C, Glanville J, Briscoe S, et al. Chapter 4: searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021. https://www.training.cochrane.org/handbook [Google Scholar]
- 37. Higgins JPT, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021. https://www.training.cochrane.org/handbook [Google Scholar]
- 38. The Cochrane Collaboration 2020 . Review Manager (RevMan) [Computer program]. Version 5.4.
- 39. Furuwaka TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. J Clin Epidemiol. 2006;59(1):7‐10. doi: 10.1016/j.jclinepi.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 40. Turner‐McGrievy GM, Davidson CR, Wingard EE, Wilcox S, Frongillo EA. Comparative effectiveness of plant‐based diets for weight loss: a randomized controlled trial of five different diets. Nutrition. 2015;31(2):350‐358. [DOI] [PubMed] [Google Scholar]
- 41. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021. https://www.training.cochrane.org/handbook [Google Scholar]
- 42. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401‐406. [DOI] [PubMed] [Google Scholar]
- 43. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407‐415. doi: 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 44. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64(12):1277‐1282. doi: 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 45. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64(12):1283‐1293. doi: 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 46. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294‐1302. doi: 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 47. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303‐1310. doi: 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 48. Deeks J, Higgins J, Altman D. Chapter 10: analysing data and undertaking meta‐analyses. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021. https://www.training.cochrane.org/handbook [Google Scholar]
- 49. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant‐based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):1‐10. doi: 10.1038/nutd.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barnard ND, Alwarith J, Rembert E, et al. A Mediterranean diet and low‐fat vegan diet to improve body weight and cardiometabolic risk factors: a randomized, cross‐over trial. J Am Coll Nutr. 2021;41:1‐13. doi: 10.1080/07315724.2020.1869625 [DOI] [PubMed] [Google Scholar]
- 51. Jenkins DJA, Wong JMW, Kendall CWC, et al. Effect of a 6‐month vegan low‐carbohydrate (‘Eco‐Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: a randomised controlled trial. BMJ Open. 2014;4(2):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mishra S, Xu J, Agarwal U, Gonzales J, Levin S, Barnard ND. A multicenter randomized controlled trial of a plant‐based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study. Eur J Clin Nutr. 2013;67(7):718‐724. doi: 10.1038/ejcn.2013.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferdowsian HR, Barnard ND, Hoover VJ, et al. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am J Health Promot. 2010;24(6):384‐387. doi: 10.4278/ajhp.081027-QUAN-255 [DOI] [PubMed] [Google Scholar]
- 54. Barnard ND, Cohen J, Jenkins DJA, et al. A low‐fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74‐wk clinical trial. Am J Clin Nutr. 2009;89(5):1588S‐1596S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turner‐McGrievy GM, Barnard ND, Scialli AR. A two‐year randomized weight loss trial comparing a vegan diet to a more moderate low‐fat diet. Obesity. 2007;15(9):2276‐2281. doi: 10.1038/oby.2007.270 [DOI] [PubMed] [Google Scholar]
- 56. Barnard ND, Scialli AR, Turner‐McGrievy G, Lanou AJ. Acceptability of a low‐fat vegan diet compares favorably to a step II diet in a randomized, controlled trial. J Cardiopulm Rehabil. 2004;24(4):229‐235. doi: 10.1097/00008483-200407000-00004 [DOI] [PubMed] [Google Scholar]
- 57. Barnard ND, Gloede L, Cohen J, et al. A low‐fat vegan diet elicits greater macronutrient changes, but is comparable in adherence and acceptability, compared with a more conventional diabetes diet among individuals with type 2 diabetes. J Am Diet Assoc. 2009;109(2):263‐272. doi: 10.1016/j.jada.2008.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pollakova D, Andreadi A, Pacifici F, Della‐Morte D, Lauro D, Tubili C. The impact of vegan diet in the prevention and treatment of type 2 diabetes: a systematic review. Nutrients. 2021;13(6):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wheeler ML, Fineberg SE, Fineberg NS, Gibson RG, Hackward LL. Animal versus plant protein meals in individuals with type 2 diabetes and microalbuminuria. Effects on renal, glycemic, and lipid parameters. Diabetes Care. 2002;25(8):1277‐1282. doi: 10.2337/diacare.25.8.1277 [DOI] [PubMed] [Google Scholar]
- 60. Astrup A. Energy metabolism and obesity. In: Mela D, ed. Food, Diet and Obesity. Woodhead Publishing Ltd; 2005:58‐75. doi: 10.1533/9781845690540.1.58 [DOI] [Google Scholar]
- 61. Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat‐free mass and resting metabolic rate during weight loss. Sports Med. 2006;36(3):239‐262. doi: 10.2165/00007256-200636030-00005 [DOI] [PubMed] [Google Scholar]
- 62. U.S. Department of Agriculture and U.S. Department of Health and Human . Dietary Guidelines for Americans. https://www.dietaryguidelines.gov/
- 63. Bechthold A, Boeing H, Tetens I, Schwingshackl L, Nöthlings U. Perspective: food‐based dietary guidelines in Europe‐scientific concepts, current status, and perspectives. Adv Nutr. 2018;9(5):544‐560. doi: 10.1093/advances/nmy033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kristensen NB, Madsen ML, Hansen TH, et al. Intake of macro‐ and micronutrients in Danish vegans. Nutr J. 2015;14:1‐10. doi: 10.1186/s12937-015-0103-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karanicolas PJ, Farrokhyar F, Bhandari M. Blinding: who, what, when, why, how? Can J Surg. 2010;53(5):345‐348. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Macronutrient composition in vegan intervention diets and control diets
Figure S1 Funnel plot depicting the effect of vegan diet intervention on body weight
Figure S2 Funnel plot depicting the effect of vegan diet intervention on BMI
Figure S3 Forest plot depicting the effect of vegan diets on glycated hemoglobin (HbA1c) after removal of the study by Kahleova and colleagues.


