Abstract
Background and objective
The clinical significance of sleep‐disordered breathing (SDB) in older age is uncertain. This study determined the prevalence and associations of SDB with mood, daytime sleepiness, quality of life (QOL) and cognition in a relatively healthy older Australian cohort.
Methods
A cross‐sectional analysis was conducted from the Study of Neurocognitive Outcomes, Radiological and retinal Effects of Aspirin in Sleep Apnoea. Participants completed an unattended limited channel sleep study to measure the oxygen desaturation index (ODI) to define mild (ODI 5–15) and moderate/severe (ODI ≥ 15) SDB, the Centre for Epidemiological Studies Scale, the Epworth Sleepiness Scale, the 12‐item Short‐Form for QOL and neuropsychological tests.
Results
Of the 1399 participants (mean age 74.0 years), 36% (273 of 753) of men and 25% (164 of 646) of women had moderate/severe SDB. SDB was associated with lower physical health‐related QOL (mild SDB: beta coefficient [β] −2.5, 95% CI −3.6 to −1.3, p < 0.001; moderate/severe SDB: β −1.8, 95% CI −3.0 to −0.6, p = 0.005) and with lower global composite cognition (mild SDB: β −0.1, 95% CI −0.2 to 0.0, p = 0.022; moderate/severe SDB: β −0.1, 95% CI −0.2 to 0.0, p = 0.032) compared to no SDB. SDB was not associated with daytime sleepiness nor depression.
Conclusion
SDB was associated with lower physical health‐related quality of life and cognitive function. Given the high prevalence of SDB in older age, assessing QOL and cognition may better delineate subgroups requiring further management, and provide useful treatment target measures for this age group.
Keywords: ageing, cognition, dementia, quality of life, sleep‐disordered breathing
Short abstract
The clinical implications for sleep‐disordered breathing (SDB) in older age remain uncertain. This study of healthy community‐dwelling older Australians reports significant associations between SDB and a lower physical health‐related quality of life, in contrast to other studies of SDB in older age, and between SDB and lower cognitive function.
See related editorial
INTRODUCTION
Sleep‐disordered breathing (SDB) refers to temporary reductions (hypopnoeas) and cessations (apnoeas) in respiration that occur during sleep, leading to intermittent de‐oxygenation, intra‐thoracic pressure swings, sympathetic activation and imperceptible arousals from sleep. 1 In middle‐age, these mechanisms underpin the commonly observed associations of SDB with cardiovascular 2 and cerebrovascular diseases, 3 daytime sleepiness, 4 depression 5 and reduced quality of life (QOL). 6
SDB prevalence increases with age 7 and is frequently detected in at least 50% of individuals aged 70 years and above. 8 , 9 , 10 The clinical significance remains uncertain, possibly without the same impacts on daytime sleepiness, 11 QOL 6 or mortality risk 12 as observed in the middle‐aged. Dementia, however, is a significant health issue for older people, and there are several mechanisms by which SDB may potentiate the neurodegenerative processes that lead to dementia due to Alzheimer's disease (AD) and/or vascular pathologies. 13 , 14 , 15 , 16 , 17 SDB has been linked to an increased risk of both impaired cognitive function, which usually precedes dementia, and of dementia itself, albeit inconsistently. 18 , 19 , 20 , 21 , 22
Resolving the clinical implications of SDB in older age is thus imperative. The Study of Neurocognitive Outcomes, Radiological and retinal Effects of Aspirin in Sleep Apnoea (SNORE‐ASA) is investigating the association of SDB with cognitive decline, and with neuroimaging and retinal biomarkers of vascular pathology. The SNORE‐ASA will also determine to what extent aspirin, with anti‐platelet and anti‐inflammatory properties, attenuates cognitive and imaging biomarker changes over 3 years in a cohort without major cognitive impairments. 23 This study outlines the gender‐specific prevalence of SDB in this large older community‐based cohort and investigates the associations of SDB with QOL, mood, daytime sleepiness and cognition.
METHODS
Study design
A cross‐sectional analysis of baseline data from the ASPirin in Reducing Events in the Elderly (ASPREE) and SNORE‐ASA studies was conducted. The ASPREE study was a multicentre, randomized, placebo‐controlled, double‐blind clinical trial conducted in Australia and the United States determining the effects of daily aspirin 100 mg on dementia‐free and disability‐free survival. 24 , 25 The SNORE‐ASA sub‐study was offered in Australia only.
Study participants
The ASPREE participants, all aged 70+ years, were recruited, from March 2010 to November 2014, through general practitioners. Exclusion criteria were a history of occlusive cardiovascular disease (CVD), atrial fibrillation (AF), heart failure, uncontrolled hypertension, current antithrombotic therapy, anaemia, dementia, a score of <78/100 on the Modified Mini‐Mental State Examination (3MS) or physical disability. 24
The SNORE‐ASA participation was offered during ASPREE clinical trial randomization visits in Victoria, South Australia and the Australian Capital Territory from March 2012 to November 2014. Additional exclusion criteria were a known diagnosis of obstructive sleep apnoea (OSA) and/or current treatment with continuous positive airway pressure (CPAP) therapy. 23
Sleep measures
A limited channel, unattended home sleep study was obtained using the ApneaLink Plus device (Resmed Inc, Sydney, Australia), containing a nasal cannula to detect hypopnoeas and apnoeas, a pulse oximeter and thoracic belt with pneumatic strain gauge to detect respiratory effort. 26 The output was reviewed by one of the three sleep medicine physicians (MTN, GH and FJO). A minimum trace duration of 4 hours, free of significant artefact, was required.
SDB was defined using the oxygen desaturation index 3% (ODI)—a count of how many times per hour the baseline oxygen saturation level drops by a minimum of 3%. SDB severity categories were based on standard cut‐offs: mild (ODI 5 to <15), moderate (ODI 15 to <30) and severe (ODI ≥ 30). 10 For multivariable analyses, moderate and severe SDB (based on an ODI ≥ 15) were combined. 9 , 27
The second sleep exposure of interest was the OSA syndrome, defined as an ODI ≥ 5 and daytime sleepiness, as defined by an Epworth Sleepiness Scale 28 score of ≥10 (range 0–24). The lowest and average oxygen saturation measures were also presented as descriptors of the study cohort.
Study outcomes
The 10‐question/30‐point Center for Epidemiological Studies‐Depression Scale, 29 with a cut‐off of ≥8, indicated depression. 30 The Medical Outcomes Study Short Form 12 assessed QOL with two summary scores: the physical health component score (PCS) and mental health component score (MCS) (scores 0–100, median 50, SD 10, with higher scores indicating better QOL). 31
Cognition was assessed by the 3MS (global cognition, maximal score 100) 32 ; the Hopkins Verbal Learning Test‐Revised delayed recall (HVLT‐R) (episodic memory, maximal score 12) 33 ; the single letter Controlled Oral Word Association Test (COWAT‐F) (executive function, higher score indicating better performance) 34 ; and the Symbol Digit Modalities Test (SDMT) (for psychomotor speed, maximal score 110). 35 A composite of these tests using standardized z‐scores was derived.
Statistical analysis
Participant characteristics were described separately for men and women given known differences in gender‐specific SDB prevalence. 10 For all continuous variables, probability plots indicated no significant departure from normality, so were presented as means and SD. Categorical variables were presented as counts and percentages. The SNORE‐ASA participant characteristics were compared with the ASPREE participants recruited through the same sites but who, for any reason, were not included in the final SNORE‐ASA cohort, using chi‐square tests and Student's t‐test.
The associations of SDB or the OSA syndrome, with the outcome of depression, and the association of the former with daytime sleepiness were explored using multivariable binary logistic regression models, reporting results as odds ratios (OR). Associations of SDB and the OSA syndrome with the outcomes of the PCS and MCS, individual cognitive tests and the composite cognitive score were explored using multivariate linear regression models, reporting all these results as beta coefficients (β). All models were adjusted for age, gender, education, alcohol use, smoking status and body mass index (BMI). Diabetes and hypertension are included in Table 1 for a description of the cohort; however, as both may be causally related to SDB, they were not included in models to avoid over‐adjustment. Interaction effects between measures of SDB and gender were tested and found non‐significant in all except two models (MCS and HVLT‐R); results were stratified by gender only for these two outcomes. Model outputs were each explored for multicollinearity and in all models the variance inflation factors were less than 2, indicating that there was no multicollinearity among model estimates. All analyses were performed with Stata (StataCorp. 2019. Stata Statistical Software: Release 16. StataCorp LLC, College Station, TX). Statistical significance was considered at p < 0.05.
TABLE 1.
Characteristics and SDB measures for 1399 SNORE‐ASA participants by gender
| All n = 1399 | Men n = 753 | Women n = 646 | |
|---|---|---|---|
| Age, mean (SD) | 74.0 (3.7) | 74.0 (3.7) | 74.0 (3.7) |
| Education, n (%) | |||
| <12 years | 587 (42.0) | 295 (39.2) | 292 (45.2) |
| ≥12 years | 812 (58.0) | 458 (60.8) | 354 (54.8) |
| BMI, mean (SD) | 28.2 (4.3) | 28.1 (3.6) | 28.5 (5.0) |
| Alcohol, n (%) | |||
| Current heavier use | 189 (13.5) | 155 (20.6) | 34 (5.3) |
| Current moderate use | 971 (69.4) | 497 (66.0) | 474 (73.4) |
| Former/never | 239 (17.1) | 101 (13.4) | 138 (21.4) |
| Smoking current/former, n (%) | 653 (46.7) | 416 (55.3) | 237 (36.7) |
| Hypertension, n (%) | 103 (73.8) | 568 (75.4) | 465 (72.0) |
| Diabetes, n (%) | 136 (9.7) | 88 (11.7) | 48 (7.4) |
| Depression, n (%) | 127 (9.1) | 44 (5.8) | 83 (12.9) |
| Quality of life, mean (SD) | |||
| SF‐12 PCS | 49.3 (8.3) | 50.3 (7.3) | 48.1 (9.2) |
| SF‐12 MCS | 55.9 (6.9) | 56.5 (6.3) | 55.2 (7.4) |
| Cognitive tests, mean (SD) | |||
| 3MS | 93.8 (4.2) | 93.2 (4.3) | 94.5 (4.0) |
| HVLT‐R | 7.9 (2.8) | 7.5 (2.8) | 8.3 (2.7) |
| SDMT | 38.9 (9.4) | 37.8 (9.5) | 40.3 (9.1) |
| COWAT | 12.3 (4.6) | 11.8 (4.4) | 12.9 (4.7) |
| Sleep measures | |||
| ODI, mean (SD) | 12.5 (10.1) | 13.6 (11.1) | 11.2 (8.7) |
| SDB (ODI ≥ 5), n (%) | 1129 (80.7) | 607 (80.6) | 522 (80.8) |
| Severity of SDB (ODI), n (%) | |||
| Normal <5 | 270 (19.3) | 146 (19.4) | 124 (19.2) |
| Mild (5 to <15) | 692 (49.5) | 334 (44.4) | 358 (55.4) |
| Moderate (15 to <30) | 340 (24.3) | 202 (26.8) | 138 (21.4) |
| Severe (≥30) | 97 (6.9) | 71 (9.4) | 26 (4.0) |
| Average saturation, mean (SD) | 93.1 (1.9) | 93.2 (1.7) | 93.1 (2.0) |
| Lowest desaturation, mean (SD) | 82.9 (6.8) | 83.4 (6.1) | 82.2 (7.5) |
| ESS, mean (SD) | 4.9 (3.3) | 5.2 (3.3) | 4.5 (3.3) |
| ESS ≥ 10, n (%) | 120 (9.0) | 78 (10.8) | 42 (6.9) |
| OSA syndrome, n (%) | 96 (6.8) | 58 (7.7) | 38 (5.9) |
Abbreviations: 3MS, Modified Mini‐Mental State Examination; alcohol, current heavy use: ≥125 g/week, current moderate use: <125 g/week, former/never: 0 g/week; BMI, Body mass index, weight in kilogrammes divided by height in metres squared; COWAT, Controlled Oral Word Association Test; Diabetes Mellitus, self‐report, or fasting glucose of >7 mmol/L or on treatment for diabetes mellitus; ESS, Epworth Sleepiness Scale; HVLT‐R, Hopkins Verbal Learning Test‐Revised Delayed Recall; Hypertension, average blood pressure reading of >140 mm Hg systolic blood pressure and/or >90 mm Hg diastolic blood pressure and/or on antihypertensive therapy; MCS, mental health component score; ODI, oxygen desaturation index; OSA, obstructive sleep apnoea; PCS, physical health component score; SDB, sleep‐disordered breathing; SDMT, Symbol Digit Modalities Test; SF‐12, Medical Outcomes Study Short Form 12; SNORE‐ASA, the Study of Neurocognitive Outcomes, Radiological and retinal Effects of Aspirin in Sleep Apnoea.
RESULTS
A total of 7830 participants were recruited to ASPREE from Australian sites that were also offering SNORE‐ASA participation. Of these, 347 were ineligible due to a known diagnosis of OSA and many were uninterested in the sub‐study (Figure 1). One thousand eight hundred and thirty‐four completed additional cognitive tests for SNORE‐ASA; however, only 1592 chose to complete the home sleep study, of whom 1399 had at least 4 h of ApneaLink recording free of significant artefact. This group formed the SNORE‐ASA cohort (Figure 1).
FIGURE 1.
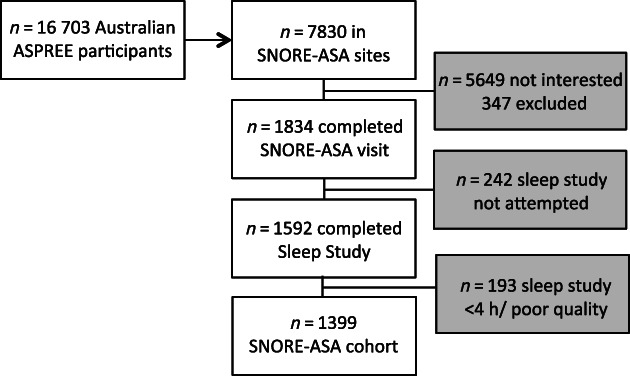
Recruitment flow chart of participants in the final study cohort. ASPREE, ASPirin in Reducing Events in the Elderly; h, hour; n, number; SNORE‐ASA, Study of Neurocognitive Outcomes, Radiological and retinal Effects of Aspirin in Sleep Apnoea
Compared to ASPREE participants recruited at the same study sites, but who were not part of the final cohort (n = 6431), SNORE‐ASA participants were younger on average (mean age 74.0 vs. 75.3 years), and a higher proportion were men (53.8% vs. 44.4%) and had greater than 11 years of formal education (58.0% vs. 52.0%) (Table S1 in the Supporting Information).
The SNORE‐ASA cohort characteristics are presented in Table 1. SDB was present in 80.7% of the cohort, similar for both men and women; 9% overall had daytime sleepiness. The proportion of participants with moderate/severe SDB was higher for men (36%) (mean ODI 13.6 [11.1]) than for women (25%) (mean ODI 11.2 [8.7]). The OSA syndrome was present in 7.7% and 5.9% of men and women, respectively.
SDB was not associated with daytime sleepiness nor depression (Table 2). The interaction term with gender and moderate/severe SDB was statistically significant for the QOL MCS score (p = 0.049). In the gender‐stratified analyses, there was no association with mild (β = 0.2, 95% CI −1.0 to 1.4, p = 0.754) or moderate/severe SDB (β = −0.8, 95% CI −2.2 to 0.5, p = 0.204) in men, nor for women with mild (β = 0.8, 95% CI −0.8 to 2.4, p = 0.294) or moderate/severe SDB (β = 1.6, 95% CI −0.3 to 3.4, p = 0.096). The QOL PCS scores were on average lower among individuals with mild SDB (β = −2.5, 95% CI −3.6 to −1.3, p < 0.001) and with moderate/severe SDB (β = −1.8, 95% CI −3.0 to −0.6, p = 0.005), compared to individuals without SDB, and were also lower in those with the OSA syndrome (β = −2.9, 95% CI −4.5 to −1.3, p = <0.001).
TABLE 2.
The association of SDB and OSA syndrome with daytime sleepiness, mood and QOL in 1399 older adults: ORs and regression beta coefficients from multivariable logistic and linear regression models, respectively
| Sleep exposures | Daytime sleepiness (ESS ≥ 10) OR (95% CI) | Depression (CES‐D ≥ 8) OR (95% CI) | QOL PCS β (95% CI) | QOL MCS β (95% CI) |
|---|---|---|---|---|
| SDB | ||||
| Normal (ODI <5) | Ref | Ref | Ref | Ref |
| Mild (5 to <15) |
1.0 (0.6 to 1.7) p = 0.915 |
0.8 (0.5 to 1.4) p = 0.536 |
−2.5 (−3.6 to −1.3) p < 0.001 |
0.4 (−0.5 to 1.4) p = 0.370 |
| Moderate/severe (≥15) |
1.0 (0.5 to 1.7) p = 0.900 |
1.1 (0.7 to 2.0) p = 0.631 |
−1.8 (−3.0 to −0.6) p = 0.005 |
0.1 (−0.9 to 1.2) p = 0.803 a |
| OSA syndrome | ||||
| Normal | N/A b | Ref | Ref | Ref |
| Present | N/A b |
1.7 (0.9 to 3.2) p = 0.130 |
−2.9 (−4.5 to −1.3) p = <0.001 |
−1.4 (−2.8 to 0.0) p = 0.050 |
Note: All models were adjusted for age, gender, education, smoking, alcohol use and BMI.
Abbreviations: β, beta coefficient; CES‐D, Center for Epidemiological Studies Depression Scale; ESS, Epworth Sleepiness Scale; MCS, Short Form 12 mental health component score; ODI, oxygen desaturation index; OSA, obstructive sleep apnoea; PCS, Short Form 12 physical health component score; QOL, quality of life; Ref, reference; SDB, sleep‐disordered breathing.
Significant interaction term for SDB and gender, see text for results of analyses stratified by gender.
Outcomes of daytime sleepiness are not relevant for the exposure of the OSA syndrome as the presence of daytime sleepiness is one component of this syndrome.
The associations of SDB and OSA syndrome with cognition are displayed in Table 3. Compared to individuals with no SDB, the adjusted mean COWAT score was lower for individuals with mild SDB (β = −0.7, 95% CI −1.4 to −0.1, p = 0.023), while the adjusted mean SDMT score was lower for individuals with moderate/severe SDB (β = −1.8, 95% CI −3.2 to −0.4, p = 0.013). The interaction term for gender and SDB was significant in the HVLT‐R model (p = 0.037 for moderate/severe SDB). In analyses stratified by gender, the mean HVLT‐R was not different for men with mild SDB (β = −0.4, 95% CI −0.9 to 0.2, p = 0.185), but was significantly lower for men with moderate/severe SDB (β = −0.6, 95% CI −1.1 to 0.0, p = 0.043). In women, these associations were not significant (mild SDB: β = 0.2, 95% CI −0.4 to 0.7, p = 0.490; moderate/severe SDB: β = 0.4, 95% CI −0.3 to 1.0, p = 0.263). SDB was associated with a lower mean composite cognitive measure for individuals with both mild SDB (β = −0.1, 95% CI −0.2 to 0.0, p = 0.022) and moderate/severe SDB (β = −0.1, 95% CI −0.2 to 0.0, p = 0.032).
TABLE 3.
The associations of SDB and OSA syndrome with cognitive function tests in 1399 men and women aged 70 plus years: Regression beta coefficients from multivariable linear regression models
| Sleep exposures | Global cognition 3MS β (95% CI) | Delayed recall HVLT‐R β (95% CI) | Psychomotor speed SDMT β (95% CI) | Executive function COWAT β (95% CI) | Composite z‐score β (95% CI) |
|---|---|---|---|---|---|
| SDB | |||||
|
Normal (ODI <5) |
Ref |
Ref | Ref | Ref | Ref |
| Mild (ODI 5 to <15) |
−0.5 (−1.1 to 0.1) p = 0.093 |
−0.1 (−0.5 to 0.3) p = 0.573 |
−1.0 (−2.3 to 0.2) p = 0.108 |
−0.7 (−1.4 to −0.1) p = 0.023 |
−0.1 (−0.2 to 0.0) p = 0.022 |
| Moderate/severe (ODI ≥ 15) |
−0.4 (−1.0 to 0.3) p = 0.286 |
−0.2 (−0.6 to 0.2) p = 0.384 a |
−1.8 (−3.2 to −0.4) p = 0.013 |
−0.5 (−1.2 to 0.2) p = 0.184 |
−0.1 (−0.2 to 0.0) p = 0.032 |
| OSA syndrome | |||||
| Normal | Ref | Ref | Ref | Ref | Ref |
| Present |
0.0 (−0.9 to 0.8) p = 0.930 |
0.1 (−0.5 to 0.6) p = 0.825 |
−0.8 (−2.6 to 1.0) p = 0.390 |
0.8 (−0.1 to 1.8) p = 0.081 |
0.0 (−0.1 to 0.2) p = 0.740 |
Note: All models were adjusted for age, gender, education, smoking, alcohol use and BMI.
Abbreviations: β, beta coefficient; 3MS, Modified Mini‐Mental State Examination; COWAT, Controlled Oral Word Association Test; HVLT‐R, Hopkins Verbal Learning Test‐Revised Delayed Recall; ODI, oxygen desaturation index; OSA, obstructive sleep apnoea; Ref, reference; SDB, sleep‐disordered breathing; SDMT, Symbol Digit Modalities Test.
Interaction was statistically significant, and the model was stratified by sex (see text for results).
DISCUSSION
In this large cohort of older Australians, SDB prevalence was 81%, and was associated with lower physical health‐related QOL and overall cognition, but not with daytime sleepiness, depression nor mental health QOL. Despite known differences in the gender‐specific prevalence of SDB, 10 these associations did not differ significantly between men and women.
The prevalence of SDB of at least moderate severity (in 36% and 25% of men and women, respectively) was lower than that observed in other older community‐based cohorts. The HypnoLaus study of 902 adults aged ≥60 years reported that 66% of men and 35% of women had an ODI of ≥15. 10 A community‐based study of 827 men and women aged 68 years reported that 53% had an apnoea–hypopnoea index (AHI) of >15. 8 The SNORE‐ASA participants, however, had lower rates of smoking and diabetes compared to the age‐matched Australian population, 36 and the comorbidities of SDB of AF, CVD and dementia were ASPREE exclusion criteria. 24 Few studies have reported the prevalence of objectively measured SDB in Australian older adults, 6 with adults aged ≥70 years not included in the largest such study. 37 Given that the ‘normal’ cut‐off of an ODI of ≥5 was exceeded by over 80% of this relatively healthy cohort, the results suggest that SDB may even be expected as part of normal ageing.
The lack of an association between SDB and daytime sleepiness is in line with several other studies in older age, 11 , 38 suggesting that daytime sleepiness may not be useful as either a measure for SDB screening or as a target for treatment in this age group. Furthermore, no association was found between SDB and depression nor with the QOL MCS. Few studies have examined the association of depression with SDB in older people as opposed to middle‐aged. 5
The association between both SDB and the OSA syndrome, with a lower QOL PCS, is novel. The MAILES study of community‐based men reported associations between SDB and lower QOL for those younger, but not older, than 70 years. 6 A further study demonstrated associations between SDB and QOL in those aged <65 years, but not those >65 years. 39 Both had smaller sample sizes, and moreover, assessed SDB based on the AHI. The ODI is a more robust measure of impact of SDB on oxygenation, and for this age group may be the more relevant measure. Indeed, a randomized controlled trial of CPAP in older adults with SDB, as defined by the ODI, demonstrated improvements in QOL in those treated with CPAP. 40 Given this, assessing QOL in older adults with SDB may be particularly useful in delineating subgroups for treatment.
There is also current interest in whether SDB is a dementia risk factor. 41 Sleep, which is disrupted in SDB, facilitates operation of the glymphatic system, which is integral for neurotoxin clearance, 13 including the clearance of beta‐amyloid which drives AD pathology. 14 , 15 The intermittent hypoxia of SDB, and resultant sympathetic activation, blood pressure variability and endothelial dysfunction may further potentiate neurodegenerative change, including via an effect on cerebral vasculature. 16 , 17 Several studies have now linked SDB to dementia risk 18 , 19 and to biomarkers of AD and vascular dementia. 41 This association, however, is not conclusive. 20 , 21 , 42 Some studies have relied upon a clinical diagnosis of SDB 43 which is more likely to be symptomatic with daytime sleepiness, a symptom itself associated with dementia risk. 44 Several large community‐based studies employing detailed neuropsychological batteries have not reported associations between SDB and significantly impaired cognition, including in longitudinal analyses and when SDB was measured in later mid‐life 45 , 46 —a time when exposure to SDB arguably would be the most critical in dementia pathogenesis.
If SDB increases dementia risk, an association between SDB and lower scores on cognitive tests should be detectable even in this SNORE‐ASA cohort that was free of major cognitive impairment, especially as subtle cognitive impairments usually precede dementia. 47 Indeed, small, but significant, associations were found for SDB (of any severity) with lower scores on a test of psychomotor speed, with a composite cognitive score, between moderate/severe SDB and lower scores on delayed recall for men only, and for mild, but not moderate/severe SDB, with lower scores on executive function. Impairments in psychomotor speed and executive function can reflect vascular pathology, 48 whereas impaired delayed recall may predict incident dementia due to AD. 49
Some caution in interpretation is required. The strongest signals were for psychomotor speed and executive function, which may have driven the overall association with reduced composite cognition. The association of mild, but not moderate/severe SDB, with reduced executive function is counterintuitive. A smaller number of participants in the moderate/severe group may have impacted the statistical significance of this result; however, and of note, there was still a trend towards lower scores for the moderate/severe group, as there was for SDB of any severity and all other cognitive tests not reaching statistical significance. While the magnitude of the lower scores associated with SDB for each test was small, collectively they may signify an increased risk of future cognitive decline and dementia. Longitudinal data are needed to definitely determine the direction of this association.
Study limitations are that several SDB co‐morbidities were exclusion criteria, the limited channel sleep study may lack sensitivity for milder SDB and was self‐administered, potentially deterring participation from individuals with poorer cognition. Thus, it has likely underestimated the true prevalence of SDB and the magnitude of the association of SDB with cognition and QOL. However, the study utilized robust and comprehensive outcome assessments, and is the largest study of SDB in an older Australian cohort to date.
In conclusion, this study has confirmed the ubiquitous nature of SDB in older age, demonstrated an association with reduced cognitive function and has shown that SDB as measured by the ODI is associated with reduced physical health QOL. For this age group, in which SDB is so common, QOL and cognitive assessments may best guide treatment decisions and targets for SDB, especially as treatment of SDB has been shown to improve QOL in older age. Whether treatment of SDB is a reversible factor towards the development of dementia remains to be seen.
AUTHOR CONTRIBUTION
Stephanie A. Ward: Conceptualization (lead); data curation (equal); formal analysis (lead); funding acquisition (equal); investigation (lead); methodology (lead); project administration (equal); writing – original draft (lead). Elsdon Storey: Conceptualization (equal); formal analysis (equal); funding acquisition (lead); methodology (equal); project administration (equal); writing – review and editing (equal). Danijela Gasevic: Formal analysis (equal); writing – review and editing (lead). Matthew T. Naughton: Conceptualization (equal); data curation (equal); funding acquisition (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Garun S. Hamilton: Conceptualization (equal); data curation (equal); funding acquisition (equal); methodology (equal); writing – review and editing (equal). Ruth E. Trevaks: Project administration (lead); writing – review and editing (equal). Rory Wolfe: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Fergal J. O'Donoghue: Conceptualization (equal); funding acquisition (equal); methodology (equal); writing – review and editing (equal). Nigel Stocks: Project administration (equal); writing – review and editing (equal). Walter P. Abhayaratna: Data curation (equal); project administration (equal); writing – review and editing (equal). Sharyn Fitzgerald: Project administration (equal); writing – review and editing (equal). Suzanne G. Orchard: Project administration (equal); writing – review and editing (equal). Joanne Ryan: Formal analysis (equal); writing – review and editing (equal). John J. McNeil: Funding acquisition (equal); writing – review and editing (equal). Christopher Reid: Methodology (equal); project administration (supporting); writing – review and editing (equal). Robyn L. Woods: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); project administration (equal); writing – review and editing (lead).
CONFLICTS OF INTEREST
Resmed leased some of the ApneaLink Plus devices used in the study and provided the nasal cannula for the devices free of cost. Garun S. Hamilton and Matthew T. Naughton have both received equipment free of charge for use in research from Resmed, and Garun S. Hamilton from Phillips Respironics and Air Liquide Healthcare for the same purpose. Fergal J. O'Donoghue has received a grant from Resmed for research purposes and also received equipment free of charge to conduct research from Resmed and Phillips Respironics.
HUMAN ETHICS APPROVAL DECLARATION
Ethics approvals for ASPREE and SNORE‐ASA were received from the Monash Human Research Ethics Committee (MUHREC CF07/3730‐2006000745) and the Alfred Hospital Ethics Committee (452/11), respectively. All participants provided informed consent.
Clinical trial registration: ASPREE Trial—ISRCTN83772183 at www.isrctn.com and NCT01038583 at www.clinicaltrials.gov. SNORE‐ASA ASPREE sub‐study—ACTRN12612000891820 at Australian New Zealand Clinical Trials Registry at www.anzctr.org.au
Supporting information
Table S1. Characteristics of SNORE‐ASA study sample compared to ASPREE participants recruited through SNORE‐ASA sites but not participating.
ACKNOWLEDGEMENTS
The authors acknowledge Resmed Pty Ltd for provision of nasal cannulas and lease of some of the ApneaLink Plus devices. The authors are most grateful to the ASPREE participants, who so willingly volunteered for this sub‐study, the general practitioners who supported the participants in the ASPREE study and the study staff at the sites where participants were recruited to SNORE‐ASA.
Research funding: ASPREE was funded by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (grant numbers U01AG029824 and U19AG062682); the National Health and Medical Research Council of Australia (grant numbers 334047 and 1127060); Monash University; and the Victorian Cancer Agency. SNORE‐ASA was funded by the National Health and Medical Research Council of Australia (grant number 1028368). Christopher Reid is supported through an NHMRC Principal Research Fellowship (GNT1136372).Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Ward SA, Storey E, Gasevic D, Naughton MT, Hamilton GS, Trevaks RE, et al. Sleep‐disordered breathing was associated with lower health‐related quality of life and cognitive function in a cross‐sectional study of older adults. Respirology. 2022;27(9):767–775. 10.1111/resp.14279
Associate Editor: M. Safwan Badr; Senior Editor: Fanny W. S. Ko
Funding information National Health and Medical Research Council, Grant/Award Numbers: 1028368, 1127060, 334047; National Institutes of Health, Grant/Award Numbers: U01AG029824, U19AG062682; NHMRC Principal Research Fellowship, Grant/Award Number: GNT1136372; Victorian Cancer Agency; Monash University
See related editorial
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Abbasi A, Gupta SS, Sabharwal N, Meghrajani V, Sharma S, Kamholz S, et al. A comprehensive review of obstructive sleep apnea. Sleep Sci. 2021;14:142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molnar MZ, Mucsi I, Novak M, Szabo Z, Freire AX, Huch KM, et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax. 2015;70:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. [DOI] [PubMed] [Google Scholar]
- 4. Lal C, Weaver TE, Bae CJ, Strohl KP. Excessive daytime sleepiness in obstructive sleep apnea. Mechanisms and clinical management. Ann Am Thorac Soc. 2021;18:757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edwards C, Almeida OP, Ford AH. Obstructive sleep apnea and depression: a systematic review and meta‐analysis. Maturitas. 2020;142:45–54. [DOI] [PubMed] [Google Scholar]
- 6. Appleton SL, Vakulin A, McEvoy RD, Vincent A, Martin SA, Grant JF, et al. Undiagnosed obstructive sleep apnea is independently associated with reductions in quality of life in middle‐aged, but not elderly men of a population cohort. Sleep Breath. 2015;19:1309–16. [DOI] [PubMed] [Google Scholar]
- 7. McMillan A, Morrell MJ. Sleep disordered breathing at the extremes of age: the elderly. Breathe (Sheff). 2016;12:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sforza E, Roche F, Thomas‐Anterion C, Kerleroux J, Beauchet O, Celle S, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli‐Israel S, Ensrud KE, et al. Associations between sleep‐disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community‐dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heinzer R, Vat S, Marques‐Vidal P, Marti‐Soler H, Andries D, Tobback N, et al. Prevalence of sleep‐disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrell MJ, Finn L, McMillan A, Peppard PE. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. Eur Respir J. 2012;40:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18:397–403. [DOI] [PubMed] [Google Scholar]
- 13. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, et al. Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann Neurol. 2018;83:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shokri‐Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, et al. Beta‐amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115:4483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chami HA, Fontes JD, Vasan RS, Keaney JF Jr, O'Connor GT, Larson MG, et al. Vascular inflammation and sleep disordered breathing in a community‐based cohort. Sleep. 2013;36:763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baril AA, Gagnon K, Arbour C, Soucy JP, Montplaisir J, Gagnon JF, et al. Regional cerebral blood flow during wakeful rest in older subjects with mild to severe obstructive sleep apnea. Sleep. 2015;38:1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta‐analysis. Sleep Med Rev. 2018;40:4–16. [DOI] [PubMed] [Google Scholar]
- 19. Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep‐disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta‐analysis. JAMA Neurol. 2017;74:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bubu OM, Andrade AG, Umasabor‐Bubu OQ, Hogan MM, Turner AD, de Leon MJ, et al. Obstructive sleep apnea, cognition and Alzheimer's disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Legault J, Thompson C, Martineau‐Dussault M, André C, Baril AA, Martinez Villar G, et al. Obstructive sleep apnea and cognitive decline: a review of potential vulnerability and protective factors. Brain Sci. 2021;11:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terpening Z, Lewis SJ, Yee BJ, Grunstein RR, Hickie IB, Naismith SL. Association between sleep‐disordered breathing and neuropsychological performance in older adults with mild cognitive impairment. J Alzheimers Dis. 2015;46:157–65. [DOI] [PubMed] [Google Scholar]
- 23. Ward SA, Storey E, Woods RL, Hamilton GS, Kawasaki R, Janke AL, et al., The Study of Neurocognitive Outcomes, Radiological and Retinal Effects of Aspirin in Sleep Apnoea‐ rationale and methodology of the SNORE‐ASA study. Contemporary Clinical Trials. 2018;64:101–111. 10.1016/j.cct.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ASPREE Investigator Group . Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of aspirin on disability‐free survival in the healthy elderly. N Engl J Med. 2018;379:1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single‐channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 27. Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep‐disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- 29. Radloff L. The CES‐D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 30. Berk M, Woods RL, Nelson MR, Shah RC, Reid CM, Storey E, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. JAMA Psychiat. 2020;77:1012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stocks NP, González‐Chica DA, Woods RL, Lockery JE, RSJ W, Murray AM, et al. Quality of life for 19,114 participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study and their association with sociodemographic and modifiable lifestyle risk factors. Qual Life Res. 2019;28:935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teng EL, Chui HC. The Modified Mini‐Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 33. Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test‐revised. Clin Neuropsychol. 1999;13:348–58. [DOI] [PubMed] [Google Scholar]
- 34. Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–38. [PubMed] [Google Scholar]
- 35. Smith A. Symbol digit modalities test (SDMT). Manual (revised). Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 36. McNeil JJ, Woods RL, Nelson MR, Murray AM, Reid CM, Kirpach B, et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J Gerontol A Biol Sci Med Sci. 2017;72:1586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cunningham J, Hunter M, Budgeon C, Murray K, Knuiman M, Hui J, et al. The prevalence and comorbidities of obstructive sleep apnea in middle‐aged men and women: the Busselton Healthy Ageing Study. J Clin Sleep Med. 2021;17:2029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fietze I, Laharnar N, Obst A, Ewert R, Felix SB, Garcia C, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences – results of SHIP‐Trend. J Sleep Res. 2019;28:e12770. [DOI] [PubMed] [Google Scholar]
- 39. Martínez‐García MA, Soler‐Cataluña JJ, Román‐Sánchez P, González V, Amorós C, Montserrat JM. Obstructive sleep apnea has little impact on quality of life in the elderly. Sleep Med. 2009;10:104–11. [DOI] [PubMed] [Google Scholar]
- 40. McMillan A, Bratton DJ, Faria R, Laskawiec‐Szkonter M, Griffin S, Davies RJ, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12‐month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804–12. [DOI] [PubMed] [Google Scholar]
- 41. Ward SA, Pase MP. Advances in pathophysiology and neuroimaging: implications for sleep and dementia. Respirology. 2020;25:580–92. [DOI] [PubMed] [Google Scholar]
- 42. Cross N, Lampit A, Pye J, Grunstein RR, Marshall N, Naismith SL. Is obstructive sleep apnoea related to neuropsychological function in healthy older adults? A systematic review and meta‐analysis. Neuropsychol Rev. 2017. Dec;27(4):389–402. [DOI] [PubMed] [Google Scholar]
- 43. Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, et al. Sleep apnea and the risk of dementia: a population‐based 5‐year follow‐up study in Taiwan. PLoS One. 2013;8:e78655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spira AP, An Y, Wu MN, Owusu JT, Simonsick EM, Bilgel M, et al. Excessive daytime sleepiness and napping in cognitively normal adults: associations with subsequent amyloid deposition measured by PiB PET. Sleep. 2018;41:zsy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lutsey PL, Bengtson LG, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, et al. Obstructive sleep apnea and 15‐year cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Sleep. 2015;39:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin MS, Sforza E, Roche F, Barthelemy JC, Thomas‐Anterion C. Sleep breathing disorders and cognitive function in the elderly: an 8‐year follow‐up study. The Proof‐Synapse Cohort. Sleep. 2014;38:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zanon Zotin MC, Sveikata L, Viswanathan A, Yilmaz P. Cerebral small vessel disease and vascular cognitive impairment: from diagnosis to management. Curr Opin Neurol. 2021. Apr 1;34(2):246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pozueta A, Rodríguez‐Rodrígue E, Vazquez‐Higuera J, Mateo I, Sánchez‐Juan P, González‐Perez S, et al. Detection of Alzheimer's disease in MCI patients by combination of MMSE and an episodic memory test. BMC Neurol. 2011;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of SNORE‐ASA study sample compared to ASPREE participants recruited through SNORE‐ASA sites but not participating.
Data Availability Statement
Data are available on request from the authors.


