Abstract
Background
Parenteral nutrition administered via central venous catheter is an established treatment option for people with intestinal failure. A serious complication of central venous catheters is the high risk of catheter‐related bloodstream infections (CRBSIs). Catheter‐locking solutions are one strategy for CRBSI prevention, with the solution taurolidine showing beneficial effects. The aim of this meta‐analysis was to identify and synthesize evidence to assess taurolidine efficacy against comparators for the prevention of CRBSI for people with intestinal failure receiving parenteral nutrition.
Methods
Six health literature databases were searched for efficacy data of rate of CRBSI for taurolidine vs control among our study population; no study design limits were applied. Individual study data were presented for the number of CRBSIs and catheter days, and rate ratio. Overall data were synthesized as a pooled risk ratio, with subgroup analyses by study design, control type, and taurolidine solution.
Results
Thirty‐four studies were included in the final analysis. At the individual level, all studies showed superior efficacy of taurolidine vs control for prevention of CRBSIs. When the data were synthesized, the pooled risk ratio was 0.49 (95% CI, 0.46–0.53; P ≤ 0.0001), indicating a 51% decreased risk of CRBSI through the use of taurolidine. Subgroup analysis showed no difference depending on study design (P = 0.23) or control type (P = 0.37) and a significant difference for taurolidine type (P = 0.0005).
Conclusion
Taurolidine showed superior efficacy over controls regardless of study design or comparator group. The results show that taurolidine provides effective CRBSI reduction for people with intestinal failure receiving parenteral nutrition.
Keywords: catheter‐related bloodstream infection, central venous catheter, venous access, parenteral nutrition, taurolidine
CLINICAL RELEVANCY STATEMENT
Prevention of catheter‐related bloodstream infections (CRBSIs) for people with intestinal failure receiving parenteral nutrition is imperative as they are at high risk of associated morbidity and mortality. The use of the catheter locking solution taurolidine has been shown to be beneficial at preventing CRBSI in a number of populations using central venous catheters, but a comprehensive data synthesis has not been carried out specifically for those with intestinal failure receiving parenteral nutrition. This meta‐analysis has identified and synthesized data from all study types to assess overall efficacy of taurolidine use for prevention of CRBSIs in this population. All individual studies showed superior efficacy of taurolidine against all comparator types. The overall data synthesis provides compelling evidence that taurolidine provides effective prevention of CRBSI for those with intestinal failure receiving parenteral nutrition, with subgroup analysis confirming the results are consistent across study types, and comparator groups. This research significantly adds to the previous literature and provides evidence for clinical decision making.
INTRODUCTION
Long term parenteral nutrition (PN) is an established treatment option for adults and children with intestinal failure (IF). 1 , 2 The principal access method for the delivery of PN and essential medications is a central venous catheter (CVC). The most serious and common complication of CVCs is catheter‐related bloodstream infections (CRBSIs), which may be life‐threatening, but may also lead to significant morbidity, requiring hospitalization, antibiotic therapy, possible line removal and replacement, and incur substantial healthcare costs. 3 , 4 , 5 , 6 , 7 For individuals requiring prolonged PN, the consequences of multiple CRBSIs may include the development of PN‐related liver failure or loss of venous access, both of which may increase the possibility of needing intestinal transplantation. 6 , 8
For people with IF receiving PN, strict catheter management protocols regarding line‐handling hygiene are essential but may be insufficient to prevent CRBSIs and additional measures may be required. 7 , 9 , 10 Catheter‐locking agents such as antibiotics, heparin, alcohol, and taurolidine are frequently used to prevent infection and clotting, and to maintain catheter patency. 11 , 12 Taurolidine locks for those receiving PN were first used in the early 1990s, and many studies have subsequently reported beneficial effects of taurolidine use, including when compared with other catheter locks. Taurolidine has broad antimicrobial and antifungal activity, inhibits biofilm development, has no reported bacterial resistance, and in combination with citrate provides additional anticoagulant benefits as a catheter lock. 13 , 14
Two previous meta‐analyses of randomized controlled trials (RCTs) have been carried out to assess the efficacy of taurolidine for combined CVC uses (PN, hemodialysis, chemotherapeutic agents). 13 , 15 These meta‐analyses identified minimal evidence available that fitted their inclusion criteria, with seven RCTs identified between both papers. Both papers stated that their findings required corroboration with further trials. An additional meta‐analysis confirmed efficacy of taurolidine specifically for those receiving PN, but minimal evidence was found fitting their inclusion criteria based on study design with the inclusion of just three RCTs. 16 The format of meta‐analysis often precludes the use of nonrandomized clinical trials, but much has been published on the benefits of taurolidine use in the form of observational studies. One systematic review that included observational studies reported on taurolidine as being beneficial but included studies where CVCs were used for (PN, hemodialysis and delivery of chemotherapeutic agents, and reported in vivo and in vitro studies. 12
The rationale for this meta‐analysis was to include evidence from observational studies as a means to enhance available data from more rigorous RCTs, and thus be able to present a broader overview of taurolidine efficacy data for people with IF receiving PN. In addition, with the use of PN itself recognized as a risk factor for CRBSIs, it is pertinent to identify literature reporting on this use, only with the exclusion of other indications for CVC use. 10 The objective of the study was to identify all literature presenting data on efficacy of taurolidine vs control to minimize the risk of CRBSIs among children and adults receiving PN administered via CVC.
METHODS
Eligibility criteria
The following inclusion criteria were required to be met to satisfy eligibility for the meta‐analysis; reporting data on patients receiving PN specifically (with exclusion of data relating to other CVC uses), and the inclusion of overall efficacy data of taurolidine vs a control group in the form of a rate of CRBSIs per 1000 days or contain data to make this calculable.
Information sources
The search strategy and implementation were performed using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 17 The following databases were searched in December 2020: Medline, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane database, Scopus, and ProQuest.
Search strategy
The individual search strategies are included (Appendix S1), but the main terms included were related to taurolidine and PN. Additional search limits were not applied.
Selection and data collection process
All identified papers were synthesized into a database, the duplicates removed, and the remaining titles and abstracts examined by two reviewers (A.V‐R. and R.N.L.) to identify those relevant for a full text review. Disputes were resolved by discussion between three reviewers (A.V‐R., R.N.L., and A.S.D.). All relevant articles were read in full text by two reviewers (A.V‐R. and R.N.L.), and those not considered as satisfying eligibility criteria were categorized with a reason for exclusion. Data from included studies were extracted and entered in to a spreadsheet by two reviewers (A.V‐R. and R.N.L.) to record study, cohort, and outcome data. If papers presented data on their cohort using different study designs (pretest‐posttest or independent cohorts), the data for each comparator group were presented and assessed separately according to the design. If papers included data on cohorts also using PN for reasons other than IF, then only data for patients with IF were extracted and assessed.
Data items
Data were collected relating to details of the study location, study design, and cohort descriptives. Outcome data were collected for taurolidine efficacy vs a control group; number of CRBSIs experienced, the number of catheter days for the cohort, and the rate of CRBSIs per 1000 catheter days where available. Additional data were collected relating to the type of taurolidine used, the control type, and secondary outcomes relating to frequency of side effects, cost, and further reports of efficacy between taurolidine and control groups.
Study risk of bias assessment
The risk of bias assessment for included studies was carried out using the Critical Appraisal Checklist for Cohort Studies developed by the Joanna Briggs Institute. 18 This checklist includes 11 items of bias assessment relating to participant selection, intervention factors, confounding, and analysis, as below:
-
1.
Were the two groups similar and recruited from the same population?
-
2.
Were the exposures measured similarly to assign people to both exposed and unexposed groups?
-
3.
Was the exposure measured in a valid and reliable way?
-
4.
Were confounding factors identified?
-
5.
Were strategies to deal with confounding factors stated?
-
6.
Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)?
-
7.
Were the outcomes measured in a valid and reliable way?
-
8.
Was the follow‐up time reported and sufficient to be long enough for outcomes to occur?
-
9.
Was follow‐up complete, and if not, were the reasons to loss to follow‐up described and explored?
-
10.
Were strategies to address incomplete follow‐up utilized?
-
11.
Was appropriate statistical analysis used?
Studies were rated for each of the 11 items according to whether they had addressed each possible source of bias appropriately with response options of yes, no, unclear, or not applicable, and these results were tabulated.
Effect measures
For the assessment of taurolidine efficacy against controls in individual studies the rate ratio was calculated, or the reported rate ratio used, for all papers. An overall pooled risk ratio (RR) was calculated using all studies reporting sufficient data on the number of CRBSIs as well as the number of catheter days for each study group.
Synthesis methods
Data presentation
Data on study characteristics and cohorts were presented in a descriptive table, as are outcome data for each study. Additional information is also reported relating to supplementary data on taurolidine/control efficacy, and secondary outcomes. Where missing data were identified for any study or patient descriptives, or results, the first or senior author of the relevant paper was contacted with a request to provide this data—this represented 24 studies, with responses received from 9.
Individual study results
To ensure consistency of data, the CRBSI rates and rate ratios were calculated using raw data on the number of CRBSIs and catheter days where available. If raw data were not available, the stated CRBSI rate and rate ratios were used. The CRBSI rate was calculated using the formula . The rate ratio was calculated using the formula: , with results <1 indicating greater efficacy of taurolidine, and results >1 indicating greater efficacy of the control solution. The rates for the taurolidine and control groups were entered in to SPSS 19 and a clustered bar graph produced to include data from each study depicting the control and taurolidine CRBSI rates.
Data synthesis
For the meta‐analysis to calculate an overall RR, and associated forest plot of results, the number of CRBSIs and the number of catheter days for each cohort were required. If one of these variables was missing, but a rate per 1000 catheter days was included, the missing data were calculated from the other two results using the CRBSI rate formula stated above. For papers not reporting the total number of catheter days for each group the reported mean or median number of days was multiplied by the cohort size to provide an estimate of the total.
The 95% confidence intervals for the RRs were extracted from the publications or calculated directly from the CRBSI number and the number of catheter days. The log RR, and standard error, were entered in to the meta‐analytical program Review Manager 5.4 20 using a random‐effects model to produce a pooled RR, with 95% confidence interval. Those studies with a rate ratio of zero, due to there being no infections in either the taurolidine or control group, were excluded from the meta‐analysis and forest plot. As heterogeneity in the rate ratios was anticipated between studies, this was specifically explored in relation to the study design, taurolidine solution, the form of the control group, and age of the study cohort, with summary measures generated and compared between these subgroups.
Certainty assessment
A certainty assessment will be discussed in relation to the assessment of bias, the populations included in this analysis, feasibility of treatment adoption, and whether potential benefits outweigh potential harms.
RESULTS
Study selection
Four hundred and forty‐one publications were identified from searches (Appendix S1 ), and 34 met the inclusion criteria of reporting the efficacy of taurolidine vs control for prevention of CRBSIs among children and adults with IF receiving PN (Figure 1).
Figure 1.
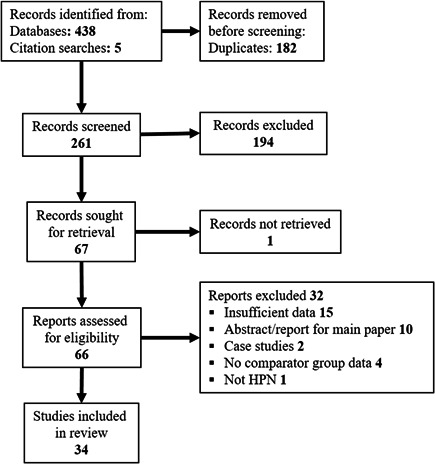
PRISMA flowchart of search strategy and identified articles. HPN, home parenteral nutrition.
Study characteristics
Study descriptives
Details of study design and cohort descriptives were extracted from the literature (Table 1). There were 26 (76%) studies carried out in European countries, 5 (15%) from Asian‐Pacific countries, and 3 (9%) from countries in the Americas. Overall study designs (three papers included data from two study designs, therefore results >100%) included 13 (38%) prospective studies, of which 5 (15% overall) were RCTs, 1 (3%) cohort control study, and 8 (24%) pretest‐posttest design. Of the 21 (62%) retrospective studies 7 (24% overall) were cohort control, and 16 (47%) pretest‐posttest design. Nineteen (56%) of the identified articles were full articles and 15 (44%) were peer reviewed conference abstracts.
Table 1.
Study and cohort characteristics of papers satisfying eligibility criteria
| First author | Year | Country | Study design | Cohort size | Cohort age | Cohort sex | Indications for PN | CVC type | CRBSI risk |
|---|---|---|---|---|---|---|---|---|---|
| Al‐Amin 3 | 2013 | UK | Retrospective pretest‐posttest | 9 |
Median 51y Range 43 to 82y |
78% F | SBS, DYS, MAL | T‐CVC | ≥2 CRBSI in 6 months |
| Barnova 21 | 2015 | UK | Retrospective pretest‐posttest | 28 | ‐ | 68% F | SBS, DYS | Single/double lumen T‐CVC | “Selected high risk” |
| Bisseling 22 | 2010 | the Netherlands | Prospective RCT | 30 | Mean 55.3y (SD 13.2) | 75% F | SBS, DYS, high‐output stoma | T‐CVC, PAC | 1 previous CRBSI |
| Buang 23 | 2017 | Singapore | Retrospective pretest‐posttest | 13 | Median 1.7y | 69% F | SBS, IBD, OBS | ‐ | ‐ |
| Chong 24 | 2020 | Singapore | Prospective pretest‐posttest | 13 | Mean 3.5y (SD 4.97) | 64% F | SBS, IBD, OBS, CD, aganglionosis, Hirschprung's | T‐CVC, PAC, PICC | ≥1 previous CRBSI |
| Chu 25 | 2012 | UK | Retrospective pretest‐posttest | 19 | Mean 69 m Range 8 to 238m | ‐ | SBS, DYS SBMD | Single‐lumen T‐CVC | 15 recurrent CRBSI, 4 prophylactic treatment |
| Clark 26 | 2019 | Australia | Prospective pretest‐posttest | 19 | Mean 6.2y (SD 5.5y) Range 0.3 to 17y | 53% F | IF malignancy | T‐CVC, PAC | Recurrent CRBSI |
| Cullis 27 | 2010 | Scotland | Retrospective cohort control + Pretest‐posttest | 49 | Median 51y Range16 to 78 | 55% F | DYS, IBD, ischemic gut, GI malignancy, enteritis | Single‐lumen T‐CVC | 7 recurrent CRBSI; control, low risk |
| German‐Diaz 28 | 2018 | Spain | Retrospectiveccohort control | 13 | Children | 38% F | SBS, DYS, FIS, CD | T‐CVC, PICC, PAC | Prior CRBSI |
| Hulshof 29 | 2017 | Netherlands | Retrospective cohort control + Pretest‐posttest | 23 | Median 85 d Range 0 to 63 m | ‐ | IF | T‐CVC | ‐ |
| Jonkers 30 | 2012 | Netherlands | Retrospective pretest‐posttest | 40 | Children and adults | ‐ | ‐ | ‐ | ‐ |
| Jurewitsch 31 | 2005 | Canada | Prospective pretest‐posttest | 7 | Range 33 to 75 | 72% F | SBS, DYS, OBS | Single‐lumen T‐CVC | Recurrent CRBSI |
| Klemesrud 32 | 2017 | Australia | Retrospectivepretest‐posttest | 7 | Children | ‐ | SBS, DYS, ENT | 3/7 previous CRBSI | |
| Lambe 33 | 2018 | France | Prospective cohort control + Pretest‐posttest | 162 | Median 1.7y (IQR 0.7–7.3) | 44% F | SBS, DYS, ENT immunodeficiency | Single‐lumen T‐CVC | Taurolidine, 2 CRBSI in 12 months; control, low risk |
| Lau 34 | ‐ | Australia | Retrospectivepretest‐posttest | 17 | Median 48y | 76% F | SBS, DYS, IBD, scleroderma | ‐ | ‐ |
| Leiberman 35 | 2020 | UK | Retrospectivepretest‐posttest | Subset from 169 | Median 56y (16–79) | 60% F | SBS, MAL, OBS, FIS | T‐CVC, PAC | Taurolidine >3 CRBSI per year |
| Lyszkowska 36 | 2019 | Poland | Prospective RCT | 86** | Range 1 to 586d | ‐ | Abdominal wall defects, necrotizing enterocolitis, stenosis/atresia of small bowel | PICC, nontunnelled CVC | ‐ |
| Merlo 37 | 2017 | Italy | Prospective pretest‐posttest | 23 | ‐ | 43% F | IF | ‐ | ≥1 CRBSI in previous 24 months |
| Merras‐Salmio 38 | 2018 | Finland | Retrospective cohort control | 100 | Median 2.4 m (IQR 0.4–12) | 38% F | SBS, DYS, ENT | Single‐lumen T‐CVC | ‐ |
| Nader 39 | 2016 | France | Retrospectivepretest‐posttest | Subset 25 from 251 | Median 0.7y (SD 0.3y) | 45% F | Overall SBS, OBS, ENT, IBD, other | T‐CVC | Subset: ≥2 CRBSIs over a 12‐month period |
| Nascimento 40 | 2019 | Brazil | Prospective pretest‐posttest | 11 | Mean 35.4 m (SD 12.7m) | ‐ | IF | ‐ | ‐ |
| Novak 41 | 2016 | Czech Republic | Retrospective cohort control | 135 | ‐ | ‐ | IF | T‐CVC, PICC, PAC | ‐ |
| Olthof 42 | 2014 | Netherlands | Retrospective cohort control | 212 | Mean 49y Range 22 to 77y | 31% F | SBS (+/− stoma), DYS, MAL | T‐CVC, PAC | ‐ |
| Parmar 43 | 2018 | UK | Prospective pretest‐posttest | 10 | Median 4.3y Range 19 m to 11y) | ‐ | IF | ‐ | 2 CRBSI within 6 months |
| Rafferty 44 | 2010 | UK | Retrospectivepretest‐posttest | 16 | Mean 44.8y Range 23 to 73y | 44% F | IF | T‐CVC | ≥3 CRBSI in 12 months |
| Rodriguez 45 | 2017 | Spain | Retrospective pretest‐posttest | 13 | Mean 61.08y (SD 14.18) | 54% F | SBS, DYS, OBS, FIS, SBMD | Single‐lumen T‐CVC | >2 CRBSI/1000 catheter days |
| Saunders 46 | 2015 | UK | Retrospective pretest‐posttest | 22 | Median 50y | ‐ | ‐ | ‐ | ≥2 in 12 months |
| Taniguchi 47 | 2009 | UK | Prospective pretest‐posttest | 6 | Mean 43y Range 36 to 46y | 33% F | DYS, IBD, mesenteric vascular disease | ‐ | ≥4 previous CRBSI |
| Toure 48 | 2012 | France | Retrospective pretest‐posttest | 15 | Mean 47y Range 18.5 to 79.6y | 47% F | SBS, OBS, CD, villous atrophy | T‐CVC, PAC | ≥1 CRBSI in 12 months |
| Tribler 49 | 2017 | Denmark | Prospective RCT | 41 | Mean 56.4y (SD 13.4) | 51% F | SBS, DYS, OBS, FIS, SBMD | Single‐lumen T‐CVC | ≥1 CRBSI in 4 years |
| Waldenvik 50 | 2016 | Sweden | Retrospective cohort control | 16 | Median 1 m | ‐ | ‐ | Single‐lumen T‐CVC | ‐ |
| Witkowski 51 | 2017 | Brazil | Prospective RCT | 28 | Median 30 m Range 1 to 165 m | 21% F | IF | T‐CVC | ‐ |
| Wouters 52 | 2018 | Europe* | Prospective RCT | 102 | Median range 47 to 59y | 62% F | SBS, DYS, OBS, FIS, SBMD | T‐CVC, PAC, PICC | ≥0.82 CRBSI/1000 catheter days |
| Zamvar 53 | 2013 | UK | Retrospective pretest‐posttest | 6 | ‐ | ‐ | ‐ | ‐ | 5/6 recurrent sepsis |
Note: Hyphens in place of cell values denote information that was not reported.
Abbreviations: CD, chronic diarrhea; CRBSI, catheter‐related bloodstream infection; CVC type: PAC, PortaCath (implanted device); DYS, dysmotility; ENT, enteropathies; F, female; FIS, fistulae; GI, gastrointestinal; IBD, inflammatory bowel disease; IF, intestinal failure; IQR, inter‐quartile range; m, months; MAL, malabsorption; OBS, obstruction (pseudo or mechanical); PAC, Port‐a‐cath (implanted device); PICC, peripherally inserted central catheter; PN, parenteral nutrition; RCT, randomized controlled trial; Sd, standard deviation; SBS, short bowel syndrome; SBMD, small bowel mucosal disease; SBS, short‐bowel syndrome; T‐CVC, tunnelled CVC; UK, United Kingdom; y, years.
*Multicenter study.
**Study Randomized by CVC.
Cohort descriptives
The combined cohort size was 1485 participants from the 34 studies, with a range of 6–212 participants per study. The combined cohort included participants with an age range from birth to 82 years, and for those studies that reported cohort gender approximately 50% were female. The most frequent indications for PN were reported as short‐bowel syndrome, dysmotility, and obstruction. The most common type of CVCs used were tunneled CVCs (Hickman or Broviac) in 23 (68%) of studies, Port‐a‐cath implanted devices in nine (26%) of studies, and peripheral CVCs in five (15%), with more than one type used in many cohorts. The CVC type was not reported in nine (26%) studies. There were 23 (68%) of studies that reported all or part of their cohort as being at high risk of CRBSIs; however, due to the wide variation in how “high risk” was classified it was not possible to do a summary of this data. In the 11 (32%) studies that did not state that their cohort was high risk for CRBSIs, the assumption was made that the cohort represented all PN patients regardless of their previous CRBSI status.
Results of individual studies
Data from each of the 34 studies were examined for overall trends (Table 2). Four studies reported data from different subgroups, therefore, data are presented for 38 comparisons. There were 12 (32%) comparisons between heparin and taurolidine, 16 (42%) comparisons were not stated but assumed as “standard care,” 3 (8%) with antibiotic locks, and 6 (16%) with saline solution, and 1 (2%) study compared both heparin and ethanol locks. “Standard care” practices were not assumed to be homogenous and may have included comparators stated in the other control groups.
Table 2.
Outcome data relating to taurolidine vs controls
| Cohort number | Number of CRBSI | Number of catheter days | Rate of CRBSI per 1000 catheter days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First author | Control type | Taurolidine type | Control | Taurolidine | Control | Taurolidine | Control | Taurolidine | Control | Taurolidine | Rate ratio |
| Al‐Amin 3 | 2 | TC (2% T, 4% C) | 9 | 9* | ‐ | ‐ | ‐ | ‐ | 6.39 | 0 | 0 |
| Barnova 21 | 2 | TC | 28 | 28* | 142 | 18 | 20,881 | 19,642 | 6.8 | 0.92 | 0.14 |
| Bisseling 22 | 1 | T | 14 | 16 | 10 | 1 | 4939 | 5370 | 2.02 | 0.19 | 0.09 |
| Buang 23 | 2 | TC | 13 | 13* | ‐ | ‐ | ‐ | ‐ | 5.3 | 0.09 | 0.17 |
| Chong 24 | 1 | TC (2% T, 4% C) | 13 | 13* | 33 | 9 | 2946 | 4737 | 11.2 | 1.9 | 0.17 |
| Chu 25 | 1 | TC (2% T, 4% C) | 19 | 19* | 57 | 10 | 6630 | 9520 | 8.6 | 1.1 | 0.13 |
| Clark 26 | 1 | TC (1.35% T, 4% C) | 19 | 19* | 39 | 5 | 7077 | 10,359 | 5.5 | 0.5 | 0.09 |
| Cullis 27 | 2 | TC (2% T, 4% C) | 42 | 7 | 88 | 6 | 36,148 | 5480 | 2.43 | 1.09 | 0.44 |
| Cullis 27 | 2 | TC (2% T, 4% C) | 7 | 7* | 60 | 6 | 6737 | 5480 | 8.9 | 1.09 | 0.12 |
| German‐Diaz 28 | 2 | T | 8 | 5 | 17 | 5 | 3288 | 2055 | 2.39 | 0.93 | 0.09 |
| Hulshof 29 | 1 | T (2% T) | 7 | 7* | 59 | 14 | 4614 | 3262 | 12.7 | 4.3 | 0.34 |
| Hulshof 29 | 3 | T (2% T) | 16 | 16* | 41 | 8 | 2747 | 2598 | 14.9 | 3.1 | 0.21 |
| Jonkers 30 | 1 | T (2% T) | 32 | 32* | 17 | 4 | 11,680 | 11,680 | 1.46 | 0.34 | 0.24 |
| Jonkers 30 | 1 | T (2% T) | 8 | 8* | 4 | 1 | 2920 | 2920 | 1.37 | 0.34 | 0.25 |
| Jurewitsch 31 | 2 | T (2% T) | 7 | 7* | 35 | 8 | 3930 | 5500 | 8.9 | 1.47 | 0.17 |
| Klemesrud 32 | 1 + 5 | T (1.35% T) | 7 | 7* | 14 | 1 | 8383 | 4000 | 1.67 | 0.25 | 0.15 |
| Lambe 33 | 4 | TC (1.35% T, 4% C) | 86 | 40 | 89 | 5 | 99,774 | 20,403 | 0.89 | 0.25 | 0.28 |
| Lambe 33 | 4 | TC (1.35% T, 4% C) | 36 | 36* | 415 | 5 | 99,774 | 20,403 | 4.16 | 0.25 | 0.06 |
| Lau 34 | 3 | TC (2% T, 4% C) | 17 | 17* | 7 | 1 | 5955 | 1753 | 1.18 | 0.57 | 0.48 |
| Leiberman 35 | 1 | TCH (2% T, 100 IU/ml H, 4% C) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 2.36 | 0.3 | 0.13 |
| Lyszkowska 36 | 2 | TC (2% T, 4% C) | 49 CVCs | 48 CVCs | 14 | 1 | 976 | 942 | 14.3 | 1.06 | 0.07 |
| Merlo 37 | 2 | TC (4% C) | 23 | 23* | 28 | 0 | 16,790 | 8395 | 1.65 | 0 | 0 |
| Merras‐Salmio 38 | 3 | TCH | 100 | 100* | 56 | 24 | 38,888 | 38,095 | 1.44 | 0.63 | 0.44 |
| Nader 39 | 2 | TC (2% T, 4% C) | 25 | 25* | 66 | 3 | 12,775 | 12,775 | 5.2 | 0.2 | 0.04 |
| Nascimento 40 | 2 | TC (2% T, 4% C) | 11 | 11* | 13 | 1 | 2281 | 3333 | 5.7 | 0.3 | 0.05 |
| Novak 41 | 1 | T | 135 | ‐ | ‐ | ‐ | ‐ | ‐ | 1.9 | 1.09 | 0.57 |
| Olthof 42 | 1 | T (2% T) | 545 CVCs | 200 CVCs | 464 | 43 | 147,842 | 71,112 | 3.1 | 0.6 | 0.19 |
| Parmar 43 | 2 | TCH (2% T, 100 IU/ml H, 4% C) | ‐ | 10 | 26 | 11 | 5274 | 5473 | 4.93 | 2.01 | 0.41 |
| Rafferty 44 | 2 | TC (2% T, 4% C) | 16 | 16* | 94 | 29 | 9632 | 8912 | 9.8 | 3.3 | 0.34 |
| Rodriguez 45 | 4 | T (2% T) | 13 | 13* | 38 | 4 | 12,186 | 5293 | 3.12 | 0.76 | 0.24 |
| Saunders 46 | 4 | T | 22 | 22* | 42 | 12 | 7351 | 12,121 | 5.71 | 0.99 | 0.17 |
| Taniguchi 47 | 2 | TC (2% T, 4% C) | 6 | 6* | 28 | 4 | 3294 | 2500 | 8.5 | 1.6 | 0.19 |
| Toure 48 | 4 | TC (1.35% T, 4% C) | 15 | 15* | 36 | 6 | 5475 | 5475 | 6.58 | 1.09 | 0.17 |
| Tribler 49 | 1 | TCH (2% T, 100 IU/ml H, 4% C) | 20 | 21 | 7 | 0 | 6956 | 9622 | 1 | 0 | 0 |
| Waldenvik 50 | 2 | TC (2% T, 4% C) | 16 | ‐ | 17 | 0 | 2920 | 2920 | 5.93 | 0 | 0 |
| Witkowski 51 | 1 | TC (2% T, 4% C) | 14 | 14 | 10 | 4 | 3889 | 4371 | 2.6 | 0.9 | 0.35 |
| Wouters 52 | 4 | T (2% T) | 50 | 52 | 18 | 5 | 12,493 | 15,318 | 1.44 | 0.33 | 0.23 |
| Zamvar 53 | 2 | TC | 16 | 16* | 42 | 0 | 8371 | 4657 | 5.02 | 0 | 0 |
Note: Control types are as follows: 1 = heparin, 2 = standard care, 3 = antibiotic, 4 = saline, 5 = ethanol. Taurolidine types are as follows: C, H, and T. Hyphens in place of cell values denote insufficient data.
Abbreviations: C, citrate; CRBSI, catheter‐related bloodstream infection; CVC, central venous catheter; H, heparin; T, taurolidine.
*Pretest/posttest.
Three different taurolidine solutions were used, with 13 (34%) stating they used taurolidine lock solution, 21 (55%) a taurolidine citrate solution, and 4 (11%) taurolidine citrate and heparin solution. There were 30 (70%) comparisons that reported either the concentration of each solution used, or the brand name of solution with manufacturer information providing specific concentration data.
Due to the variation in cohort sizes there was a wide range of reported catheter days for the control groups (976–147,842 days) and for the taurolidine group (942–71,112 days). The number of CRBSIs experienced in the control groups ranged from 4 to 464, and in the taurolidine group from 0 to 43. The rate of CRBSIs in the control groups ranged from 0.89 to 14.9 per 1000 catheter days, and the taurolidine group from 0 to 4.3 per 1000 catheter days. The calculated rate ratio ranged from 0 to 0.57, with 37 out of 38 (97%) having a rate ratio below 0.5 and in favor of taurolidine efficacy.
The calculated or reported CRBSI rate for control and taurolidine groups for each study were compared (Figure 2 ), with all studies reporting a lower CRBSI rate in the taurolidine group than control group.
Figure 2.
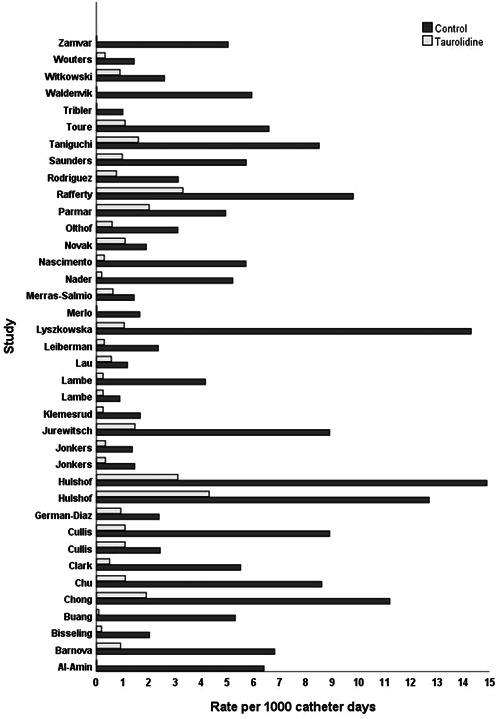
Results of individual studies: rates of catheter‐related bloodstream infections per 1000 catheter days for control and taurolidine comparisons.
Results of syntheses
Of the 34 studies identified in the searches, three were excluded from the meta‐analytical data synthesis due to insufficient raw data. 3 , 34 , 40 Of the 31 studies with sufficient data for inclusion four had a rate ratio of zero due to there being no CRBSIs in the taurolidine group, and were therefore excluded from the forest plot data synthesis. 36 , 48 , 49 , 52 Twenty‐seven studies had sufficient data for inclusion in the data synthesis and of these four studies reported data for two different cohort comparisons, therefore providing data for 31 comparisons.
Data from qualifying studies were synthesized as a forest plot according to study design (Figure 3). The pooled RR for all studies included in the synthesis was 0.49 (95% CI, 0.46–0.53; P ≤ 0.0001). This indicates a 51% decrease in risk of CRBSIs through the use of taurolidine compared with controls. This result should also be interpreted in the context of four studies being excluded from the pooled RR due to the taurolidine group having zero CRBSIs, therefore, the result favoring taurolidine efficacy is likely to be underestimated. Pooled data for each study design type all showed significant differences between taurolidine and control (P ≤ 0.0001). Tests for subgroup differences between study designs showed that there was no difference between pooled RRs depending on methodology (P = 0.23), with pooled RRs varying from 0.44 to 0.57 between the study designs. Within‐group heterogeneity was not significant for the prospective RCT's (P = 0.10), although significant for all other study design groups (P ≤ 0.0001).
Figure 3.
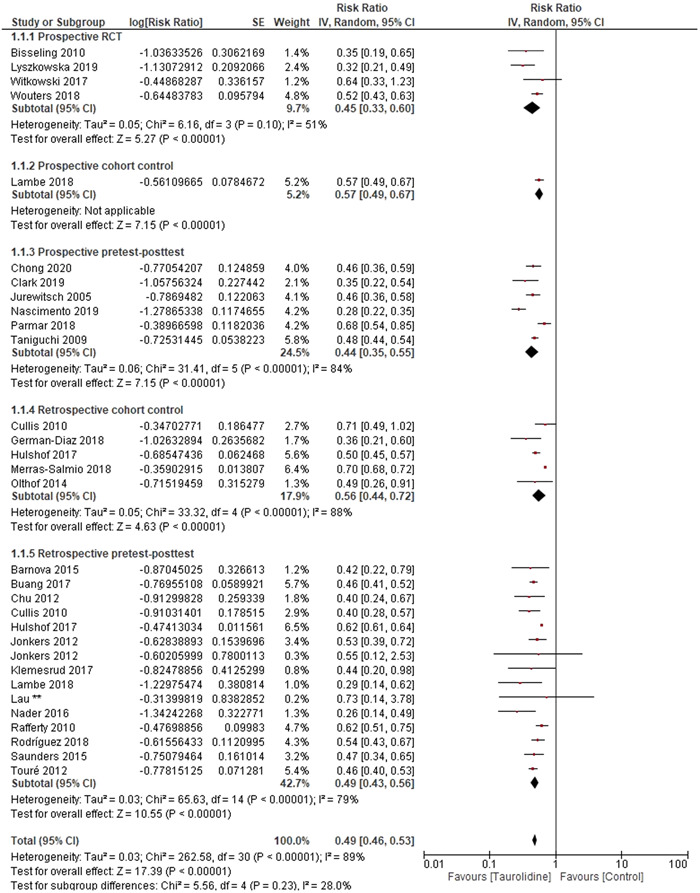
Forest plot of risk ratio for included studies for the number of catheter‐related bloodstream infections experienced in the stated number of catheter days, with subgroups for study design. SE, standard error; CI, confidence interval.
Further subgroup analysis was carried out according to the control used, the type of taurolidine solution, as well as age group (children vs adults) from studies where age data were reported. Subgroup analysis by control type showed that pooled RRs were similar among the following comparisons between taurolidine and “standard care” (RR, 0.45; 95% CI, 0.39–0.53), heparin (RR, 0.49; 95% CI, 41–0.59), saline (RR, 0.51; 95% CI, 0.46–0.56), and antibiotics (RR, 0.60; 95% CI, 0.44–0.82), all of which favored taurolidine (Figure 4). Tests for subgroup differences between control type showed that there was no difference between pooled RRs (P = 0.37). Between study heterogeneity was not significant for the saline comparator studies (P = 0.23), but significant for heparin (P = 0.01), standard care, and antibiotics (P ≤ 0.0001).
Figure 4.
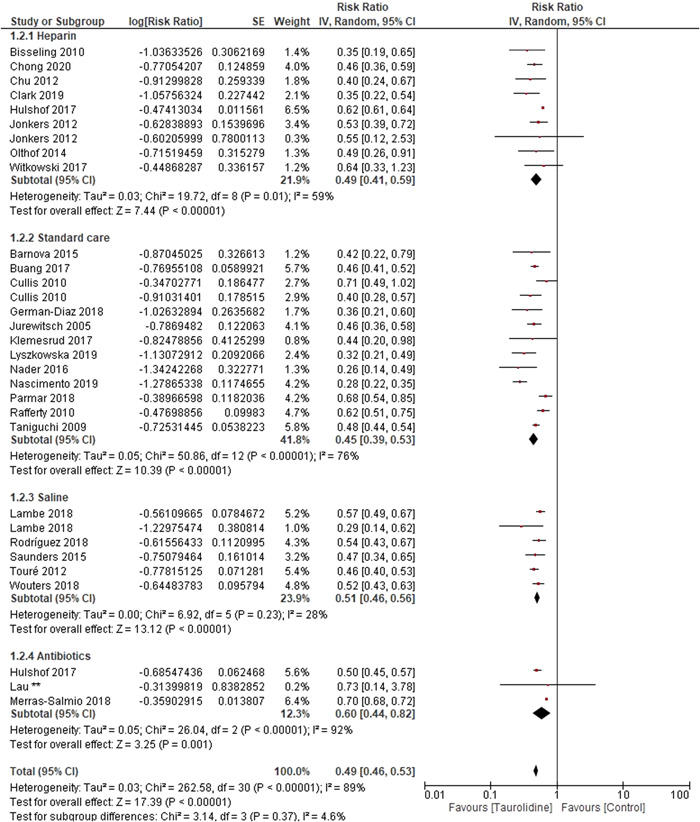
Forest plot of risk ratio for included studies for the number of catheter‐related bloodstream infections (CRBSI) experienced in the stated number of catheter days, with subgroups for control type.
Subgroup analysis by taurolidine solution type showed similar pooled RRs for taurolidine (RR, 0.51; 95% CI, 0.45–0.57) and taurolidine citrate (RR, 0.45; 95% CI, 0.4–0.51), although higher for taurolidine citrate heparin (RR, 0.7; 95% CI, 0.68–0.72) (Figure S1). The overall test for differences between subgroups was significant (P ≤ 0.0001), although when the two taurolidine citrate heparin studies were removed from the analysis this difference became nonsignificant (P = 0.13). Heterogeneity between studies were significant (P = 0.0005) except for the taurolidine citrate heparin studies (P = 0.8), although this should be interpreted with caution due to the small study numbers in this group.
The subgroup analysis by age group included 17 sets of data from 15 studies in the children's group, and 13 sets of data from 12 studies for the adults (Figure S2 ). Pooled RRs were similar for children (RR, 0.49; 95% CI, 0.44–0.54) and adults (RR, 0.50; CI, 0.47–0.54) with subgroup analysis showing that the difference between groups was not significant (P = 0.66). The data presented by studies involving children showed significant heterogeneity (P ≤ 0.0001), but not for studies involving adults (P = 0.27).
Secondary outcomes
A number of secondary outcomes were reported in the studies relating to CRBSI‐free days, CVCs, adverse events or side effects, satisfaction, and the cost difference of taurolidine vs control treatment.
CRBSI‐free days
The comparison of CRBSI‐free days was made in five papers, with all reporting superior outcomes for patients in the taurolidine group. Wouters et al 51 reported that the cumulative proportion of CRBSI‐free patients after 1 year was significantly higher in the taurolidine group (88%) than in the control group (49%, P = 0.002). The report by Bisseling et al 22 stated that the control group experienced 10 reinfections during 4939 catheter days and in the taurolidine group one reinfection during 5370 catheter days, a highly significant result. Jurewitsch et al 31 reported that CRBSI‐free days were significantly higher in the taurolidine group, and Chu et al 25 state that 74% of their patients had no infections for up to 32 months after changing to taurolidine. The mean time to the first CRBSI episode after taurolidine implementation increased from 87 to 296 days (P = 0.012) in a further study. 26
Catheter‐related outcomes
Outcomes relating to the use of taurolidine compared with control on the CVC itself were reported in seven papers. 22 , 27 , 33 , 42 , 46 , 49 , 52 The number of catheter removals due to CRBSI was significantly reduced in the taurolidine group compared with control in two studies by Wouters et al 52 (control group, 8 removals; taurolidine, 2 removals; P = 0.049) and Chong et al 24 (control group, 11 removals; taurolidine group, 1 removal). Wouters et al 52 also reported a prolonged time to CVC removal due to CRBSI in the taurolidine group, and a lower proportion requiring CVC removal due to CRBSI in the taurolidine (both results significant, P ≤ 0.05). Tribler et al 49 reported that CVC survival time was greater in the taurolidine group compared with control (control group, 159 days; taurolidine, 194 days; P = 0.06). The number of CVC changes was reported as being lower in the taurolidine group (mean, 0.71 per 1000 catheter days) than in the control group (mean, 4.71 per 1000 catheter days) in another study. 27
The number of CVC occlusions were reported as being lower in the taurolidine group by Olthof et al 42 (control group, 137; taurolidine, 34), and not being experienced by either the control or taurolidine group in a study by Bisseling et al. 22 The number of CVCs requiring salvage due to breakage was reported by Lambe et al, 33 showing no significant difference between groups (control group, 25 repairs in 99,774 days; taurolidine group, 2 repairs in 20,403 days; P = 0.18). Similarly, Saunders et al 46 reported on successful CVC salvage, with no significant difference between groups (control group, 19 [45%] salvaged; taurolidine group, 4 [33%]; P = 0.46).
Adverse events or side effects
Fourteen papers reported on whether their study cohort experienced side effects or adverse events relating to the use of taurolidine. 22 , 25 , 29 , 31 , 33 , 34 , 36 , 42 , 45 , 47 , 49 , 52 , 53 Ten papers reported that no side effects or adverse events were experienced, and 4 papers (all among the adult population), reported effects from among a total of 77 patients (5.2% of pooled study cohort). 42 , 47 , 49 , 52 Side effects or adverse reactions were reported as dysgeusia, paresthesia, palpitations, anaphylactic like reaction (N = 1), burning sensation, CVC occlusion, dizziness, nausea, vomiting, or pain.
Satisfaction
Only one study by Tribler et al 49 reported on patient satisfaction with their assigned treatment group, and no significant difference between the two groups was observed (P = 0.48).
Cost
Seven studies reported on costs associated with treatment for CRBSIs for both control and taurolidine groups 36 , 43 , 45 , 49 , 52 , 53 (Table 3). All studies showed reduced costs associated with taurolidine treatment as relating to the cost of hospital admissions for CRBSIs, drug costs, or CVC removal.
Table 3.
Comparative costs associated with taurolidine use
| Study | Associated costs | Control | Taurolidine |
|---|---|---|---|
| Lyszkowska 36 | Treatment | CRBSI treatment cost €3304/patient ($3621) | Prophylactic taurolidine cost €113/patient |
| Parmar 43 | Hospital admission bed days | 26 CRBSIs, 260 hospital admission bed days | 11 CRBSIs, 110 hospital admission bed days |
| Total: £98,800 (£380/day) ($128,893 total, $496/day) | Total: £41,800 | ||
| Rafferty 44 | Hospital days and treatment | CRBSI cost £367,000 (hospital days, antibiotics) ($478,753) | CRBSI cost £228,240 ($297,746) (£164,000 hospital days ($213,943), £64,240 taurolidine cost ($82,805) |
| Total cost savings/year: £138,760 ($181,023) | |||
| Rodriguez 45 | Hospital admission and catheter removals | €11,635.70/patient, €12.4/day ($12,754/patient, $13.6/day) | €1871.63/patient, €4.6/day ($2052/patient, $5/day) |
| Total: €151,264.14 ($165,787) | Total: €24,331.19 ($26,677) | ||
| Tribler 49 | Treatment | €6743.9/treatment year, €18.4 per day ($7392/year, $20.2/day) | €2347.7/treatment year, €6.4 per day ($2574/year, $7/day) |
| Total: €128,134 ($140,452) | Total: €61,744 total ($67,702) | ||
| Wouters 52 | CRBSI treatment | $4454 per patient | $1865 per patient (P = 0.03) |
| Zamvar 53 | Hospital days and treatment | CRBSIs led to 816 hospital days (£489,108) ($637,994), antibiotics (£14,088) ($18,376) | CRBSIs led to 136 hospital days (£68,000) ($88,696), antibiotics (£2146) ($2799) |
| Total: £503,196 ($656,394) | Total: £94,236 (including taurolidine) ($122,915) | ||
| Total cost saving: £408,960 ($533,419) |
Notes: Where costs presented in euros (€) or Great British pounds (£) these costs are also reported in the equivalent USD ($).
Abbreviation: CRBSI, catheter‐related bloodstream infection.
Risk and reporting of bias in studies
An assessment of the included studies identified a number of potential sources of bias, predominantly due to minimal consideration of confounders and missing information (Table S1). With 20 of the studies being of a pretest/posttest design, the chance of participant selection bias was minimized, although 7 further studies had insufficient information to make assumptions relating to this aspect. All studies were found to measure exposure and outcomes in a standardized way, and as such allowed for their inclusion in the meta‐analysis. Visual inspection of results tables and graphs showed no pattern of skewed data for those studies missing data for a full bias assessment. The studies that identified possible confounding factors highlighted a number of variables that may affect the risk of CRBSIs related to line type, underlying condition and comorbidities, PN frequency, PN administrator, PN composition, presence of stoma or fistula, and immune deficiency. Review of individual study characteristics (Table 1) and full text for each paper revealed that although many studies presented data on these confounders, and a number included them in their between group comparisons, few identified them as possible sources of bias and adjusted for them using multivariate regression analysis.
Certainty of evidence
The studies included in this research universally favor taurolidine regardless of demographic or clinical variables, study design, comparator type, or taurolidine type. The bias assessment identified a number of possible sources but this has not visually skewed results in favor of taurolidine or controls. The results of the overall and comparator specific meta‐analysis provide consistent evidence that taurolidine has superior efficacy over controls when viewed as pooled results, despite the expected heterogeneity between studies. The results show compelling evidence that taurolidine efficacy has been proven regardless of the rigour of study design or comparator group.
DISCUSSION
This systematic review and meta‐analysis aimed to synthesize evidence from a number of study types to address the evidence gap regarding taurolidine efficacy for the reduction of CRBSIs for those receiving PN. The overall analysis showed a universal reduction of CRBSIs for all patients using taurolidine, regardless of study design, population differences, control type, or taurolidine solution. Additional benefits were reported for catheter‐related outcomes and treatment cost.
Previous meta‐analyses have shown the superior efficacy of taurolidine compared with other catheter lock solutions for those undergoing treatment for oncology conditions, surgery, or hemodialysis. 13 , 15 Although these meta‐analysis limited their study designs to include only RCT's, with minimal evidence available for synthesis, their results showed more favorable pooled RRs than in the current synthesis that reports RR, 0.49 (95% CI, 0.45–0.53), with Sun et al's 15 paper reporting an RR of 0.23 (95% CI, 0.13–0.40) and Liu et al 13 an RR of 0.47 (95% CI, 0.25–0.89). This difference may be explained by the greater number of studies included in this meta‐analysis thereby analyzing additional representative data producing a higher RR but narrower confidence intervals. In addition, the higher RR may be due to the exclusion of all other uses of CVCs other than for PN. The formulations used in PN are susceptible to increased microbial growth due to their individual components, with dextrose and amino acids supporting fungal growth, and fat emulsions sustaining fungal and bacterial growth. 54 , 55 A further explanation may be that a high proportion of papers included in this synthesis included participants selected as having a high CRBSI base rate and, therefore, at greater risk of experiencing further infections. This factor may introduce selection bias in favor of studies with patients at low risk of CRBSIs, with neither the Liu et al 13 or Sun et al 15 paper reporting their cohorts as being patients at high risk of CRBSIs.
The studies included in this review used a number of different taurolidine lock solutions, varying in concentration as well as presence, and type, of an additive. Our synthesis showed a similar pooled rate ratio for taurolidine (RR, 0.51; CI 0.45–0.57) and taurolidine citrate (RR, 0.45; CI, 0.4–0.51) with no significant difference between these two solutions (P = 0.13), but a significant difference (P ≤ 0.0001) when studies using taurolidine citrate heparin were included (RR, 0.70; CI, 0.68–0.72). Metabolized into taurine, water, and carbon dioxide, taurolidine's mechanism of action consists of direct inhibition of pathogenicity against a broad range of microorganisms in addition to blocking their adhesion to inert surfaces. An elegant in vitro study compared the microbiocidal effects of various taurolidine containing lock solutions. 14 They concluded that 2% taurolidine and 1.34% taurolidine, with or without citrate and heparin, had potent microbiocidal effect on fungal, Gram‐positive and Gram‐negative pathogens. The more concentrated taurolidine solution did exhibit greater effect on growth inhibition, a difference thought minor and of uncertain clinical significance. Furthermore, the authors found that the addition of citrate and/or heparin did not have a bearing on the microbiocidal effect of taurolidine, despite the use of antimicrobial solution with the addition of citrate solutions previously being shown as superior to heparin alone. 56 These suggest that while the reviewed studies lacked uniformity insofar as lock solution used, the overwhelmingly positive impact of taurolidine locks on CRBSI in the home PN population is likely independent of the specific type of taurolidine lock. The pooled and individual meta‐analysis completed in this study show superior efficacy of all taurolidine formulations compared with controls, within the recognized limitations of bias.
This review included studies using a number of different control comparisons, stated as heparin, “standard care,” antibiotics, and saline. Although this factor may be considered a confounder in the assessment of bias, no clear benefit of any one of these comparisons has been shown in the available literature, and our synthesis confirmed this finding with subgroup analysis showing no significant difference (P = 0.37) between pooled rate ratios for heparin (RR, 0.49; CI, 0.41–0.59), standard care (RR, 0.45; CI, 0.39–0.53), saline (RR, 0.51; CI, 0.4–0.56), and antibiotics (RR, 0.6; CI, 0.44–0.82). A meta‐analysis carried out by Wouters et al 16 to assess different lock solutions for patients receiving PN showed the rate ratio of CRBSI to favor taurolidine over saline and heparin, and saline as being superior to heparin, although this analysis only included three datasets. Meta‐analyses by Zhang et al 57 reported superior efficacy of ethanol locks compared with heparin and saline, and Yahav et al 58 reported superiority of antibiotic and antimicrobial locks compared with heparin. In addition to the use of lock solutions, the use of standardized CVC care protocols has been shown to be beneficial in reducing CRBSI for patients with multitude uses for CVCs. 59 , 60 , 61 However, it is unclear in the included studies how “standard care” was defined and may have been just an alternative lock solution. The overall and individual meta‐analysis of taurolidine against all other controls in the current paper highlights that taurolidine has superior efficacy within the recognized limitations of bias.
The subgroup analysis performed to compare the CRBSI rate for children and adults showed that there was no difference between the two groups, despite significant heterogeneity in the studies carried out among children. While it has previously been shown that children may be at higher risk than adults of CRBSI due to hygiene factors, 62 and parents performing CVC care, 63 this meta‐analysis provides evidence that there is no difference in efficacy for taurolidine between the two groups. While it must be acknowledged that the age of participants may be a confounding factor in studies relating to CRBSI risk, this meta‐analysis shows that there is no apparent disadvantage in response to taurolidine as a way to reduce or minimize this risk.
Seven of the studies included in the present review reported, in some way, the cost implications of taurolidine lock solution use (Table 3). Across the board, the reported evidence suggests that prophylactic use of taurolidine lock solution is cost‐effective when compared against the treatment cost for CRBSI. It is worth noting that only one study reported their findings, in this regard, with statistical significance. 51 Although rates of CRBSI have decreased, the economic cost of this problem remains substantial. 64 Systematic implementation of evidence‐based intervention has proven beneficial in reducing the rates of CRBSI significantly among hospital‐based patients receiving PN in a sustained fashion. 59 Other high‐quality evidence has shown that the cost of home PN favors comparably with hospital PN. 65 Therefore, although the present review primarily sought to examine the impact of taurolidine lock solution on CRBSI rates, secondary outcome data suggests benefit to its use from a cost–benefit perspective.
Strengths
The search strategy implemented in this review adequately identified peer‐reviewed literature from a number of sources to provide data for comparison. By limiting studies to those reporting specifically on CRBSI during treatment with PN the confounding factor relating to the components of the PN solution could be mitigated. The inclusion of studies with different methodological designs in this review has provided an overview of a substantially greater amount of literature than has been presented previously. Although this methodology increases the chance of bias, the evidence reports superior efficacy of taurolidine with no clear exaggeration of effect size compared with previous meta‐analytical literature.
Limitations
The obvious limitation of this study is the inclusion of a range of study designs and control comparisons. The assessment of bias highlighted a number of shortcomings in the identified papers; however, when compared with the available literature and other meta‐analyses the results do not seem overstated as a consequence of including observational nonrandomized studies. Although some studies were missing data that would allow a comprehensive review of all results, every effort was made to retrieve this data from authors and the number of studies with insufficient data for inclusion in the meta‐analysis was low.
Conclusion
The use of CVC locking is one method among a number of CRBSI prevention techniques. However, this analysis highlights the importance of using the locking solution with superior efficacy for reducing CRBSIs in patients receiving PN. The inclusion of observational studies in this synthesis adds to the evidence base elucidated in previous meta‐analyses, while having recognized limitations relating to study methodologies. This study adds to the growing evidence base that taurolidine provides effective CRBSI reduction for people with IF receiving PN.
AUTHOR CONTRIBUTIONS
Angharad Vernon‐Roberts, Robert N. Lopez, and Andrew S. Day equally contributed to the conception and design of the research; Angharad Vernon‐Roberts and Robert N. Lopez contributed to the acquisition of the data; Angharad Vernon‐Roberts, Robert N. Lopez, Christopher M. A. Frampton, and Andrew S. Day contributed to the analysis and interpretation of the data; Angharad Vernon‐Roberts and Robert N. Lopez drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENT
Open access publishing facilitated by University of Otago, as part of the Wiley–University of Otago agreement via the Council of Australian University Librarians.
Vernon‐Roberts A, Lopez RN, Frampton CM, Day AS. Meta‐analysis of the efficacy of taurolidine in reducing catheter‐related bloodstream infections for patients receiving parenteral nutrition. J Parenter Enteral Nutr. 2022;46:1535‐1552. 10.1002/jpen.2363
REFERENCES
- 1. Staun M, Pironi L, Bozzetti F, et al. ESPEN Guidelines on Parenteral Nutrition: Home Parenteral Nutrition (HPN) in adult patients. Clin Nutr. 2009;28(4):467‐479. [DOI] [PubMed] [Google Scholar]
- 2. Cernat E, Puntis J. Paediatric parenteral nutrition: current issues. Frontline Gastroenterol. 2020;11(2):148‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Amin AH, Sarveswaran J, Wood JM, Burke DA, Donnellan CF. Efficacy of taurolidine on the prevention of catheter‐related bloodstream infections in patients on home parenteral nutrition. J Vasc Access. 2013;14(4):379‐382. [DOI] [PubMed] [Google Scholar]
- 4. Raphael BP, Hazekamp C, Samnaliev M, Ozonoff A. Analysis of healthcare institutional costs of pediatric home parenteral nutrition central line infections. J Pediatr Gastroenterol Nutr. 2018;67(4):e77‐e81. [DOI] [PubMed] [Google Scholar]
- 5. Xue Z, Coughlin R, Amorosa V, et al. Factors associated with central line‐associated bloodstream infections in a cohort of adult home parenteral nutrition patients. JPEN J Parenter Enteral Nutr. 2020;44(8):1388‐1396. [DOI] [PubMed] [Google Scholar]
- 6. Tribler S, Brandt CF, Fuglsang KA, et al. Catheter‐related bloodstream infections in patients with intestinal failure receiving home parenteral support: risks related to a catheter‐salvage strategy. Am J Clin Nutr. 2018;107(5):743‐753. [DOI] [PubMed] [Google Scholar]
- 7. Balazh JR, Franck AJ. Strategies to reduce the risk of central line‐associated bloodstream infections in parenteral nutrition. Top Clin Nutr. 2018;33(2):156‐163. [Google Scholar]
- 8. Drews BB, Sanghavi R, Siegel JD, Metcalf P, Mittal NK. Characteristics of catheter‐related bloodstream infections in children with intestinal failure: implications for clinical management. Gastroenterol Nurs. 2009;32(6):385‐390. [DOI] [PubMed] [Google Scholar]
- 9. O'Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2002;35(11):1281‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beghetto MG, Victorino J, Teixeira L, de Azevedo MJ. Parenteral nutrition as a risk factor for central venous catheter‐related infection. JPEN J Parenter Enteral Nutr. 2005;29(5):367‐373. [DOI] [PubMed] [Google Scholar]
- 11. Kolaček S, Puntis JWL, Hojsak I, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: venous access. Clin Nutr. 2018;37(6):2379‐2391. [DOI] [PubMed] [Google Scholar]
- 12. Bradshaw JH, Puntis JWL. Taurolidine and catheter‐related bloodstream infection: a systematic review of the literature. J Pediatr Gastroenterol Nutr. 2008;47(2):179‐186. [DOI] [PubMed] [Google Scholar]
- 13. Liu H, Liu H, Deng J, Chen L, Yuan L, Wu Y. Preventing catheter‐related bacteremia with taurolidine‐citrate catheter locks: a systematic review and meta‐analysis. Blood Purif. 2014;37(3):179‐187. [DOI] [PubMed] [Google Scholar]
- 14. Olthof ED, Nijland R, Gülich AF, Wanten GJA. Microbiocidal effects of various taurolidine containing catheter lock solutions. Clin Nutr. 2014;34(2):309‐314. [DOI] [PubMed] [Google Scholar]
- 15. Sun Y, Wan G, Liang L. Taurolidine lock solution for catheter‐related bloodstream infections in pediatric patients: a meta‐analysis. PLoS One. 2020;15(4):e0231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wouters Y, Causevic E, Klek S, Groenewoud H, Wanten GJA. Use of catheter lock solutions in patients receiving home parenteral nutrition: a systematic review and individual‐patient data meta‐analysis. JPEN J Parenter Enteral Nutr. 2020;44(7):1198‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moola S, Munn Z, Tufanaru C AE, et al. Chapter 7: Systematic reviews of etiology and risk. In: JBI Manual for Evidence Synthesis [Internet]. 2020. Joanna Briggs Institute. https://synthesismanual.jbi.global
- 19. IBM SPSS Statistics for Windows, Version 27.0. IBM Corp; 2020. [Google Scholar]
- 20. Review Manager. Version 5.4. The Cochrane Collaboration; 2020.
- 21. Barnova I, Fragkos K, Morgan S, et al. Significant reduction in catheter‐related bloodstream infections in high risk adult patients on home PN or IV fluids after introduction of secondary prophylaxis with taurolidine‐citrate line lock. Gut. 2015;64(suppl 1):A496. [Google Scholar]
- 22. Bisseling TM, Willems MC, Versleijen MW, Hendriks JC, Vissers RK, Wanten GJ. Taurolidine lock is highly effective in preventing catheter‐related bloodstream infections in patients on home parenteral nutrition: a heparin‐controlled prospective trial. Clin Nutr. 2010;29(4):464‐468. [DOI] [PubMed] [Google Scholar]
- 23. Buang SNH, Logarajah V, Lan NS, Liwanag MJ, Bixia A, Ong C. Home parenteral nutritional support in paediatric chronic intestinal failure – a single asian centre experience. Arch Dis Child. 2017;102(suppl 2):A119. [Google Scholar]
- 24. Chong CY, Ong RYL, Seah VXF, et al. Taurolidine–citrate lock solution for the prevention of central line‐associated bloodstream infection in paediatric haematology–oncology and gastrointestinal failure patients with high baseline central‐line associated bloodstream infection rates. J Paediatr Child Health. 2020;56(1):123‐129. [DOI] [PubMed] [Google Scholar]
- 25. Chu H‐P, Brind J, Tomar R, Hill S. Significant reduction in central venous catheter‐related bloodstream infections in children on HPN after starting treatment with taurolidine line lock. J Pediatr Gastroenterol Nutr. 2012;55(4):403‐407. [DOI] [PubMed] [Google Scholar]
- 26. Clark JE, Graham N, Kleidon T, Ullman A. Taurolidine–citrate line locks prevent recurrent central line‐associated bloodstream infection in pediatric patients. Pediatr Infect Dis J. 2019;38(1):e16‐e18. [DOI] [PubMed] [Google Scholar]
- 27. Cullis PS, McKee RF. Taurolidine lock–experience from the West of Scotland. Clin Nutr. 2010;30(3):399‐400. [DOI] [PubMed] [Google Scholar]
- 28. German‐Diaz M, Maíz‐Jimenez M, Moreno‐Villares JM. Effectiveness of taurolidine in the prevention of catheter‐related bacteremia in pediatric patients on home parenteral nutrition. Clin Nutr. 2018;37(suppl 1):S272. [Google Scholar]
- 29. Hulshof EC, Hanff LM, Olieman J, et al. Taurolidine in pediatric home parenteral nutrition patients. Pediatr Infect Dis J. 2017;36(2):233‐235. [DOI] [PubMed] [Google Scholar]
- 30. Jonkers C, Looman KI, Tabbers MM, Tas TA, Serlie MJ. Incidence of cebtral venous catheter related bloodstream infections in adults and children on home parenteral nutrition: heparin versus taurolidine catheter lock. Clin Nutr Supp. 2012;7(1):203‐204. [Google Scholar]
- 31. Jurewitsch B, Jeejeebhoy KN. Taurolidine lock: the key to prevention of recurrent catheter‐related bloodstream infections. Clin Nutr. 2005;24(3):462‐465. [DOI] [PubMed] [Google Scholar]
- 32. Klemesrud S, O'loughlin E, Stormon M, et al. Use of taurolidine catheter locks for prophylaxis against catheter‐related bloodstream infections in children receiving home parenteral nutrition. J Pediatr Gastroenterol Nutr. 2017;32(suppl 2):181. [Google Scholar]
- 33. Lambe C, Poisson C, Talbotec C, Goulet O. Strategies to reduce catheter‐related bloodstream infections in pediatric patients receiving home parenteral nutrition: the efficacy of taurolidine‐citrate prophylactic‐locking. JPEN J Parenter Enteral Nutr. 2018;42(6):1017‐1025. [DOI] [PubMed] [Google Scholar]
- 34. Lau J & Jovanovic M Comparison of Taurolidine‐Citrate Versus Gentamicin‐Citrate in Preventing Catheter Associated Infections for Home Total Parenteral Nutrition Patients. St Vincent's Hospital.
- 35. Leiberman D, Stevenson RP, Banu FW, Gerasimidis K, McKee RF. The incidence and management of complications of venous access in home parenteral nutrition (HPN): a 19 year longitudinal cohort series. Clin Nutr. 2020;37(1):34‐43. [DOI] [PubMed] [Google Scholar]
- 36. Łyszkowska M, Kowalewski G, Szymczak M, Polnik D, Mikołajczyk A, Kaliciński P. Effects of prophylactic use of taurolidine‐citrate lock on the number of catheter‐related infections in children under 2 years of age undergoing surgery. J Hosp Infect. 2019;103(2):223‐226. [DOI] [PubMed] [Google Scholar]
- 37. Merlo F, Ivaldi C, Aimasso U, De Francesco A. Taurolidine‐citrate CVC‐lock solution reduces CRBSI rate in patients with chronic intestinal failure in HPN. Clin Nutr. 2017;36(S1):S301. [Google Scholar]
- 38. Merras‐Salmio L, Mutanen A, Ylinen E, Rintala R, Koivusalo A, Pakarinen MP. Pediatric intestinal failure: the key outcomes for the first 100 patients treated in a national tertiary referral center during 1984‐2017: original communication. JPEN J Parenter Enteral Nutr. 2018;42(8):1304‐1313. [DOI] [PubMed] [Google Scholar]
- 39. Abi Nader E, Lambe C, Talbotec C, et al. Outcome of home parenteral nutrition in 251 children over a 14‐y period: report of a single center. Am J Clin Nutr. 2016;103(5):1327‐1336. [DOI] [PubMed] [Google Scholar]
- 40. Nascimento JR, Leite HP, Dos Santos LR, Uchoa KM, David AI, de Camargo MFC. Citrate‐taurolidine lock solution: impact on the incidence of catheter related bloodstream infections in children with intestinal failure receiving home parenteral nutrition. Transplantation. 2019;103(7 suppl 2):S161. [Google Scholar]
- 41. Novak F, Kralova P, Meisnerova E. Comparison of catheter‐related complications with hickmanTM catheters, subcutaneous ports and peripherally inserted central catheters in patients with intestinal failure receiving home parenteral nutrition. Clin Nutr. 2016;35(suppl 1):S119. [Google Scholar]
- 42. Olthof ED, Versleijen MWJ. Huisman‐de Waal GJ. Feuth T, Kievit W, Wanten GJA. Taurolidine lock is superior to heparin lock in the prevention of catheter related bloodstream infections and occlusions. PLoS One. 2014;9(11):e111216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parmar R, Irving S, Wong S, Tzivinikos C. Cost effectiveness of Taurolock‐Hep line lock by reducing catheter related blood stream infections in children dependent on parenteral nutrition. J Pediatr Gastroenterol Nutr. 2018;66(suppl 2):1024. [Google Scholar]
- 44. Rafferty GP, Nightingale J, Small M, Eastwood J, UgarteCano C, Gabe S. The targeted use of Taurolock® to reduce central venous catheter sepsis in a home parenteral nutrition cohort. Gut. 2010;59(suppl 1):A35. [Google Scholar]
- 45. Rodríguez M, Cordeu M, Álvarez M, et al. Clinical and economic impact of the taurolidine lock on home parenteral nutrition. Nutr Hosp. 2018;35(4):761‐766. [DOI] [PubMed] [Google Scholar]
- 46. Saunders J, Naghibi M, Leach Z, et al. Taurolidine locks significantly reduce the incidence of catheter‐related blood stream infections in high‐risk patients on home parenteral nutrition. Eur J Clin Nutr. 2015;69(2):282‐284. [DOI] [PubMed] [Google Scholar]
- 47. Taniguchi A, Eastwood J, Davidson A, Nightingale J, Gabe SM. Effectiveness of Taurolock™ in preventing recurrent catheter‐related bloodstream infections in patients on home parenteral nutrition. Proc Nutr Soc. 2009;68(OCE1):462. [Google Scholar]
- 48. Touré A, Lauverjat M, Peraldi C, et al. Taurolidine lock solution in the secondary prevention of central venous catheter‐associated bloodstream infection in home parenteral nutrition patients. Clin Nutr. 2012;31(4):567‐570. [DOI] [PubMed] [Google Scholar]
- 49. Tribler S, Brandt CF, Petersen AH, et al. Taurolidine‐citrate‐heparin lock reduces catheter‐related bloodstream infections in intestinal failure patients dependent on home parenteral support: a randomized, placebo‐controlled trial. Am J Clin Nutr. 2017;106(3):839‐848. [DOI] [PubMed] [Google Scholar]
- 50. Waldenvik K, Orden H, Engstrand H, et al. TauroLock is effective in preventing catheter related bloodstream infection in post‐surgical infants on HPN. J Pediatr Gastroenterol Nutr. 2016;62(suppl 1):884. [Google Scholar]
- 51. Witkowski M, Silveira RS, Telles AC, et al. Taurolidine lock vs heparin lock in children and adolescents on chronic parenteral nutrition: results from a randomized trial. Transplantation. 2017;101(6S2):S132. [Google Scholar]
- 52. Wouters Y, Theilla M, Singer P, et al. Randomised clinical trial: 2% taurolidine versus 0.9% saline locking in patients on home parenteral nutrition. Aliment Pharmacol Ther. 2018;48(4):410‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zamvar V, Kriel D, Sandoe J, Puntis J. Financial impact of Taurolock® during long term parenteral nutrition. J Pediatr Gastroenterol Nutr. 2013;56(suppl 2):94. [Google Scholar]
- 54. Opilla M. Epidemiology of bloodstream infection associated with parenteral nutrition. Am J Infect Control. 2008;36(10):S173e5‐8. [DOI] [PubMed] [Google Scholar]
- 55. Swindell K, Lattif AA, Chandra J, Mukherjee PK, Ghannoum MA. Parenteral lipid emulsion induces germination of Candida albicans and increases biofilm formation on medical catheter surfaces. J Infect Dis. 2009;200(3):473‐480. [DOI] [PubMed] [Google Scholar]
- 56. Mai H, Zhao Y, Salerno S, et al. Citrate versus heparin lock for prevention of hemodialysis catheter‐related complications: updated systematic review and meta‐analysis of randomized controlled trials. Int J Nephrol Urol. 2019;51(6):1019‐1033. [DOI] [PubMed] [Google Scholar]
- 57. Zhang J, Wang B, Wang J, Yang Q. Ethanol locks for the prevention of catheter‐related infection in patients with central venous catheter: a systematic review and meta‐analysis of randomized controlled trials. PLoS One. 2019;14(9):e0222408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yahav D, Rozen‐Zvi B, Gafter‐Gvili A, Leibovici L, Gafter U, Paul M. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta‐analysis of randomized, controlled trials. Clin Infect Dis. 2008;47(1):83‐93. [DOI] [PubMed] [Google Scholar]
- 59. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725‐2732. [DOI] [PubMed] [Google Scholar]
- 60. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter‐associated bloodstream infections: a systems‐based intervention. Am J Infect Control. 2006;34(8):503‐506. [DOI] [PubMed] [Google Scholar]
- 61. van der Kooi T, Sax H, Pittet D, et al. Prevention of hospital infections by intervention and training (PROHIBIT): results of a pan‐European cluster‐randomized multicentre study to reduce central venous catheter‐related bloodstream infections. Intensive Care Med. 2018;44(1):48‐60. [DOI] [PubMed] [Google Scholar]
- 62. Hodge D, Puntis JWL. Diagnosis, prevention, and management of catheter related bloodstream infection during long term parenteral nutrition. Arch Dis Child ‐ Fetal and Neonat Ed. 2002;87(1):F21‐F24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Buchman AL, Opilla M, Kwasny M, Diamantidis TG, Okamoto R. Risk factors for the development of catheter‐related bloodstream infections in patients receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2014;38(6):744‐749. [DOI] [PubMed] [Google Scholar]
- 64. Goudie A, Dynan L, Brady PW, Rettiganti M. Attributable cost and length of stay for central line‐associated bloodstream infections. Pediatrics (Evanston). 2014;133(6):e1525‐e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Arhip L, Serrano‐Moreno C, Romero I, Camblor M, Cuerda C. The economic costs of home parenteral nutrition: systematic review of partial and full economic evaluations. Clin Nutr. 2021;40(2):339‐49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.


