Summary
Restless legs syndrome (RLS) is a sensorimotor neurological disorder characterised by an urge to move the limbs with a circadian pattern (occurring in the evening/at night), more prominent at rest, and relieved with movements. RLS is one of the most prevalent sleep disorders, occurring in 5%–10% of the European population. Thomas Willis first described RLS clinical cases already in the 17th century, and Karl‐Axel Ekbom described the disease as a modern clinical entity in the 20th century. Despite variable severity, RLS can markedly affect sleep (partly through the presence of periodic leg movements) and quality of life, with a relevant socio‐economic impact. Thus, its recognition and treatment are essential. However, screening methods present limitations and should be improved. Moreover, available RLS treatment options albeit providing sustained relief to many patients are limited in number. Additionally, the development of augmentation with dopamine agonists represents a major treatment problem. A better understanding of RLS pathomechanisms can bring to light novel treatment possibilities. With emerging new avenues of research in pharmacology, imaging, genetics, and animal models of RLS, this is an interesting and constantly growing field of research. This review will update the reader on the current state of RLS clinical practice and research, with a special focus on the contribution of European researchers.
Keywords: assessment, augmentation, diagnosis, pathogenesis, RLS, treatment
1. INTRODUCTION
Restless legs syndrome (RLS) is a sensorimotor disorder characterised by a spectrum of wake and sleep symptoms, i.e., unpleasant sensations usually in the legs, urge to move the limbs, occurrence at rest and in the evening and/or at night, and improvement with movement (Allen, Picchietti, et al., 2014b). RLS leads to delayed sleep onset, poor sleep quality, psychological distress and impaired health‐related quality of life (Happe et al., 2009; Manconi et al., 2021). With its high prevalence (up to 10%) especially in European and North‐American populations (Garcia‐Borreguero, Egatz, Winkelmann, & Berger, 2006), a considerable misdiagnosis rate and the availability of only symptomatic treatments, adding to significant personal and social burden, RLS has a substantial socioeconomic impact (Trenkwalder et al., 2021). This article encompasses the key aspects of RLS, underlying relevant contributions by European researchers.
2. HISTORICAL ASPECTS OF RLS
It is worth remembering here some fundamental historical steps in the establishment of RLS as a definite nosological condition. It is widely recognised that RLS was first described by the famous English anatomist of the brain, but also neurologist and psychiatrist, Thomas Willis in the 17th century (Isler, 2010; Willis, 1672). After having been considered as a functional/psychiatric disorder for a long period (Wittmaack, 1861), from the end of the 19th century some authors began to consider it as an organic and biologically determined condition. In 1880, Beard (Beard, 1880) hypothesised the origin of the motor restlessness to be at the level of the spinal cord and in 1943 Allison (Allison, 1943) emphasised the involuntary nature of the motor component of RLS, hypothesising a vascular origin. Finally, RLS has been recognised as a well‐defined and frequent neurological disorder since its classic and detailed description by Ekbom in 1945 (Ekbom, 1945), when he reported his first eight patients, and called it “Asthenia Crurum Paraesthetica (Irritable legs)”. An additional support to the organic nature of RLS was provided in 1953 by Symonds (Symonds, 1953) with his clinical recognition of periodic leg movements in sleep (PLMS), which were subsequently demonstrated and finely described polysomnographically by Lugaresi et al. in 1965 (Lugaresi, Coccagna, Tassinari, & Ambrosetto, 1965).
3. PATHOPHYSIOLOGY OF RLS
Restless legs syndrome is a complex multifactorial condition in which a genetic and an environmental background predispose to the disease (Trenkwalder, Allen, Högl, Paulus, & Winkelmann, 2016). Several mechanisms have been proposed to play a a major role in RLS pathophysiology (Figure 1).
FIGURE 1.
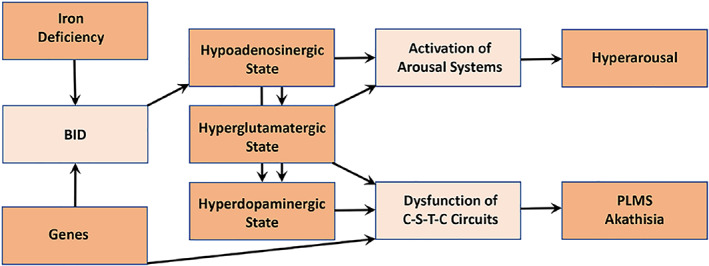
Proposed pathophysiological model of RLS. BID, brain iron deficiency; C‐S‐T‐C circuits, cortico‐striatal‐thalamic‐cortical circuits; PLMS, periodic leg movements in sleep (Ferré, García‐Borreguero, Allen, & Earley, The Neuroscientist, New Insights into the neurobiology of restless legs syndrome. Vol 25(2) 113‐125, copyright © 2019 by The Author(s). Reprinted by permission of SAGE Publications)
3.1. Brain iron deficiency
Conditions associated with serum iron deficiency show a high prevalence of RLS symptoms (Allen, Auerbach, Bahrain, Auerbach, & Earley, 2013a; Allen & Earley, 2007). However, most people with RLS do not show systemic iron deficiency but rather a brain iron deficiency (BID). This can result from altered iron acquisition by the brain (Connor et al., 2011), and is reflected by reduced cerebrospinal fluid (CSF) ferritin in the context of normal serum levels in people with RLS (Earley et al., 2000). In line with this, transcranial ultrasound of the substantia nigra showed hypoechogenicity in people with RLS (Garcia‐Malo, Miranda, et al., 2020a; Garcia‐Malo, Wanner, et al., 2020b; Godau, Schweitzer, Liepelt, Gerloff, & Berg, 2007; Godau et al., 2008; Schmidauer et al., 2005).
3.2. Dopamine dysregulation
The involvement of the dopaminergic system in the pathophysiology of RLS is supported by the therapeutic benefit of dopaminergic drugs on symptoms, suggestive of a dopamine deficit in RLS. In contrast, subsequent research has provided evidence compatible with a presynaptic hyperdopaminergic state, with increased synthesis and release of dopamine (Earley, Uhl, Clemens, & Ferré, 2017). It is likely that a post‐synaptic receptor down‐regulation secondary to increased dopaminergic stimulation is responsible for a relative evening and night‐time dopamine deficit, leading to RLS symptoms. Of note (Earley et al., 2017), brain iron deficiency in rodents confers a similar dopaminergic profile to that observed in people with RLS (Earley et al., 2014).
Besides central dopaminergic mechanisms, the descending spinal dopaminergic system originating in the dorsal‐posterior hypothalamus (A11 region), might also be involved in the pathophysiology of RLS, losing its inhibitory influence on spinal sensory inputs and thus causing symptoms (Clemens, Rye, & Hochman, 2006).
The therapeutic effects of opiates in RLS are probably related to a dopaminergic effect through the μ‐opioid receptors, as agonists of μ‐opioid receptors ultimately lead to dopaminergic activation (Altarifi et al., 2017). The μ‐opioid receptors, located in the dopaminergic ventral tegmental area and in the nucleus accumbens, mediate both the analgesic and addictive effects of opioids.
3.3. Hyperglutamatergic state
Evidence for the implication of altered glutamatergic neurotransmission in RLS comes from magnetic resonance spectroscopy showing an increase in basal glutamate levels in the thalamus (Allen, Barker, Horska, & Earley, 2013b) but also from the therapeutic effect of α2δ‐ligands (Dohin et al., 2013; Dooley, Taylor, Donevan, & Feltner, 2007; Garcia‐Borreguero et al., 2014). Furthermore, drugs that inhibit N‐methyl‐D‐aspartate (NMDA) (Inturrisi, 2005; Kapur & Friedman, 2002; Inturrisi, 2005; Silver, Allen, Senerth, & Earley, 2011), or α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA)‐glutamate receptors seem to exert a therapeutic effect on RLS symptoms (Garcia‐Borreguero, Cano, & Granizo, 2017).
However, how do dopaminergic and glutamatergic dysfunction relate to each other? It should be noted that dopamine receptor agonists are able to block the glutamate release in both brain iron deficient rats and controls (Yepes et al., 2017), suggesting that dopamine receptors localised in striatal glutamatergic terminals might be one of the targets for dopamine receptor agonists in RLS (Yepes et al., 2017).
3.4. Adenosine
Brain iron deficiency in rodents has shown to produce a significant downregulation of adenosine A1 receptors, leading to increased sensitivity of glutamatergic cortico‐striatal terminals (Ferré et al., 2018; Quiroz et al., 2016). Both A1R and A2AR receptors are localised in cortico‐striatal terminals where they form A1R‐A2AR heteromers that operate as an adenosine concentration‐dependent switch, by which high adenosine facilitates glutamate release (Ciruela et al., 2006). Thus, BID mediated changes in the adenosine receptor density or function (A1R downregulation and A2AR upregulation) lead to increased sensitivity of glutamatergic terminals, resulting in hyperdopaminergic and hyperglutamatergic states in corticostriatal pathways (Ferré et al., 2018). A recent placebo‐controlled crossover study has shown that dipyridamole, a blocker of the adenosine reuptake transporters, had a therapeutic effect on sensory symptoms, PLMS and sleep disturbances in people with RLS (Garcia‐Borreguero, Garcia‐Malo, Granizo, & Ferré, 2021).
4. NEUROPHYSIOLOGY AND PERIODIC LEG MOVEMENTS
The neurophysiological picture of RLS is dominated by the presence, in up to 85% of patients, of repetitive and somewhat stereotyped movements occurring mainly in sleep, called periodic leg movements (PLM) (Ferri, 2012); they can also occur during wakefulness (PLM during wakefulness, PLMW). PLM during sleep (PLMS) typically involve the lower limbs and are characterised by an extension of the big toe, partial flexion of the ankle, knee and, sometimes, the hip; the upper limbs can also be involved, even if more rarely (Provini et al., 2001). PLMS may be unilateral or bilateral and may be associated with cortical arousal and autonomic activation or an awakening (Ferri et al., 2007). They are defined as movements lasting from 0.5 to 10 s (15 s for bilateral movements), with an increase in the electromyographic (EMG) signal to ≥8 μV above baseline and an intermovement interval between two consecutive movements (onset‐to‐onset) of 10–90 s (Ferri et al., 2016a, 2016b); PLMS sequences are then identified by at least four consecutive limb movements with intermovement intervals within this range. Figure 2 shows an example of PLMS occurring during sleep stage 2 in a patient with RLS.
FIGURE 2.
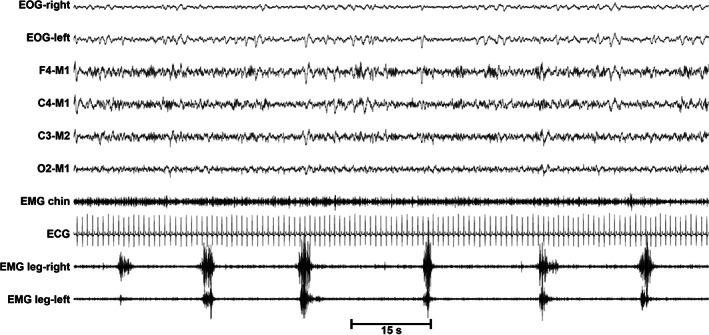
Polysomnographic aspect of a sequence of PLMS during sleep stage 2 in a patient with RLS
Although PLMS are found in the great majority of people with RLS, they may occur in other sleep disorders, sometimes with differential features, such as narcolepsy (Dauvilliers et al., 2007; Ferri et al., 2006), REM sleep behaviour disorder (RBD) (Fantini, Michaud, Gosselin, Lavigne, & Montplaisir, 2002; Manconi, Ferri, Zucconi, Fantini, et al., 2007a), and obstructive sleep apnea syndrome (Baran, Richert, Douglass, May, & Ansarin, 2003; Chervin, 2001; Manconi et al., 2014). Moreover, PLMS can be observed also in several medical conditions, such as cardiovascular disease, end‐stage renal disease, depression (Allen, Picchietti, et al., 2014b), Parkinson's disease and multiple system atrophy (Puligheddu et al., 2014; Wetter, Collado‐Seidel, Pollmächer, Yassouridis, & Trenkwalder, 2000). When they occur in the absence of RLS, narcolepsy, untreated obstructive sleep apnea or RBD, and cause clinically significant nocturnal or daytime disturbance, PLMS constitute an identifiable sleep disorder called periodic limb movement disorder (PLMD) (American Academy of Sleep Medicine, 2014). However, PLMS can also be found in the general healthy population, especially after the age of 40 years (Pennestri et al., 2006).
PLMS are the main objective parameter (Fulda & Wetter, 2008) that can be used for the assessment of treatment strategies for RLS and respond early and dramatically to even low doses of dopaminergic agents (Manconi et al., 2012; Manconi, Ferri, Zucconi, Clemens, et al., 2011a; Manconi, Ferri, Zucconi, Oldani, et al., 2007b, 2011b), thus supporting the dopaminergic hypothesis of RLS (Clemens et al., 2006).
PLMS have been reported to be increased in both adults (Yang, White, & Winkelman, 2005) and children (Ferri et al., 2022; Vendrame, Zarowski, Loddenkemper, Steinborn, & Kothare, 2011) taking selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs) antidepressants, but not the dopamine reuptake inhibitor bupropion (Kolla, Mansukhani, & Bostwick, 2018). SSRIs and SNRIs increase serotonin levels, and serotonergic projections from the raphe nuclei influence dopaminergic neurons in the substantia nigra, ventral tegmental area, nigro‐striatal pathway and mesolimbic dopaminergic system (Digiovanni, Dimatteo, Pierucci, & Esposito, 2008). These findings further support the dopaminergic theory for the generation of PLMS (Clemens et al., 2006).
Besides PLMS, neurophysiological studies of RLS have assessed central and peripheral nervous system excitability, especially by means of the transcranial magnetic stimulation (TMS) technique. The findings of these studies seem to indicate that RLS is a sensorimotor disorder with the involvement of cortical, subcortical, spinal, and peripheral nerve structures constituting a complex dysfunctional network characterised by increased excitability, decreased inhibition, and disordered synaptic plasticity (Lanza, Lanuzza, et al., 2018b). These findings also support the idea that not only dopamine is involved in the pathogenesis of RLS, but a significant contribution of different pathways, such as those involving glutamate, gamma aminobutyric acid, and opioids, is likely (Lanza et al., 2017, 2015; Lanza, Cantone, et al., 2018a; Lanza & Ferri, 2019).
Finally, a mechanism of hyperarousal, similar to that of insomnia, has been suggested to exist in RLS (Lanza & Ferri, 2019), at least in the early night hours. This is supported by studies showing increased alpha and beta EEG bands during both wakefulness preceding sleep and the sleep onset period (Ferri et al., 2014) or sympathetic activation during relaxed wakefulness preceding sleep and during sleep (DelRosso et al., 2020; Manconi, Ferri, Zucconi, Clemens, et al., 2011a).
5. NEUROIMAGING
Several imaging studies using different techniques (including magnetic resonance imaging (MRI), positron emission tomography (PET), single positron emission computed tomography (SPECT), transcranial sonography of the substantia nigra) investigated brain structures in people with RLS (Rizzo, Li, Galantucci, Filippi, & Cho, 2017).
PET and SPECT studies demonstrated a dysfunction of nigrostriatal and mesolimbic dopaminergic pathways. The most replicated finding is a reduction in D2 receptors and dopamine transporters in the striatum, compatible with an increase in synaptic dopamine (Rizzo et al., 2017). In line with the pathogenetic hypothesis of a dopaminergic dysfunction, these findings may express a post‐synaptic receptor downregulation secondary to increased dopaminergic stimulation, with subsequent relative evening and nighttime dopamine deficit (Allen, 2015).
The concept of BID in RLS is supported by most of the MRI studies using iron sensitive sequences, although an inconstant regional variability in iron content has been reported, mainly involving the substantia nigra and the thalamus (Rizzo et al., 2017). Beyond the use of neuroimaging for clarifying RLS pathophysiology, brain iron assessment through transcranial sonography of the substantia nigra has been used recently as a predictor of therapeutic response to intravenous iron therapy (Garcia‐Malo, Wanner, et al., 2020b).
Regarding MRI structural changes, there is a lack of consistency in grey matter findings (Mogavero et al., 2021; Park, Kim, Oh, Seong, & Joo, 2021; Rizzo et al., 2017; Sheng et al., 2021) whereas more consistent white matter changes in the sensory‐motor and limbic/nociceptive networks have been reported (de Paiva et al., 2020; Lee et al., 2018; Park et al., 2021; Rizzo et al., 2017; Stefani et al., 2019). As myelin synthesis depends on iron, myelin deficit in RLS could be reflected in white matter changes. Of note, a recent study reported a correlation between fractional anisotropy (FA) values in the right anterior thalamic radiation and anxiety levels, as well as between FA values in the left corticospinal tract and the number of PLM and the movement arousal index, thus linking structural changes to clinical parameters (Park et al., 2021).
A meta‐analysis on fMRI studies in people with RLS compared with healthy controls demonstrated decreased functional connectivity within the dopaminergic network in RLS including the nigrostriatal, mesolimbic, and mesocortical pathways, likely reflecting sensorimotor dysfunction (Kocar, Müller, & Kassubek, 2020). Changes in sensorimotor networks have also been found in more recent studies not included in the meta‐analysis (Ku, Lee, Kim, Chang, & Cho, 2020; Lee, Baron, Soca, & Attarian, 2016; Rizzo et al., 2017; Tuovinen et al., 2021; Zhuo et al., 2017).
Moreover, increased functional connectivity was observed bilaterally in the thalamus. In particular, diurnal changes in the thalamic circuits of the default mode network may lead to changes in arousal cortical activation thresholds and relate with the circadian expression of RLS symptoms (Ku et al., 2018). Of note, functional changes in thalamic connectivity were altered by dopamine agonist treatment (Lee et al., 2020; Tuovinen et al., 2021).
Taken together, neuroimaging data support the concept of RLS as a complex network disorder linked to brain iron deficiency and dopaminergic dysfunction, with the thalamus representing a crucial node of different networks, including the sensorimotor and limbic circuits.
6. GENETICS
Several genetic risk factors for RLS have been identified, mainly through genome‐wide association studies (GWAS) and, more recently, through mutational load analysis (Didriksen et al., 2020; Schormair et al., 2017; Tilch et al., 2020; Winkelmann et al., 2007). The identified genes are linked to neurogenesis, changes in neuronal circuit formation, synaptogenesis, and axonal guidance (Schormair et al., 2017).
The strongest genetic risk factor for RLS is an intronic regulatory element within MEIS1, a transcription factor involved in the development of the central and peripheral nervous systems. This element plays a role in the ganglionic eminences of the developing forebrain (Salminen, Lam, & Winkelmann, 2019). An increased sympathovagal variability during N2 and N3 has been reported in people with RLS with PLMS compared with control subjects. Thus, the MEIS1 variant might identify people with RLS at risk for cardiovascular disorders (Thireau et al., 2017). As MEIS1 regulation is organised in a modular pattern (i.e., disease‐associated intronic regulatory elements control MEIS1 expression with cell type and maturation stage specificity, particularly in the inhibitory neuron lineage) the precise spatiotemporal activity of these elements likely contributes to RLS pathogenesis.
Polymorphisms in BTBD9 also confer a higher risk of RLS. An MRI of the BTBD9 knockout mice revealed decreased neural activities in the cerebellum (Lyu, Xing, DeAndrade, Perez, Yokoi, et al., 2020b), and changes in the primary somatosensory cortex and in the corticostriatal pathway (Lyu, Xing, DeAndrade, Perez, Zhang, et al., 2020a), similar to the MRI alterations reported in RLS (see Neuroimaging section).
Another link between genetic findings and clinical phenotype comes from a small exploratory study suggesting that the SNP rs3104767 (related to the TOX3 gene locus) is linked to painful RLS (Karroum, Saini, Trotti, & Rye, 2020).
Two GWASs suggest a common genetic basis for RLS and insomnia (Hammerschlag et al., 2017; Lane et al., 2017), one showing also that the observed association could not be explained only by the presence of an RLS subgroup within the insomnia cases (Hammerschlag et al., 2017). In contrast, a further study in chronic insomnia disorder patients with and without RLS suggested that MEIS1 is only associated with RLS (El Gewely et al., 2018), although a lack of power precluded from refuting small pleiotropic effects.
7. ANIMAL MODELS
The limited knowledge of the mechanism behind RLS, together with the urge to identify new druggable targets and novel medications, justify the development of animal models of RLS. In the past two decades, several experiments in this direction have been attempted, however, a validated model is still not unanimously recognised.
The first pioneering attempt to replicate a RLS phenotype in animals was performed by producing a selective bilateral hypothalamic lesion of the A11 nuclei, which increased the motor activity in rodents (Ondo, 2000). Later, in the same model, a rescue treatment with dopamine agonists was proved to reduce hyperactivity (Qu et al., 2007).
Rodents subjected to an iron deficient diet showed increased wheel running activity, pain response (Dowling et al., 2011), and periodic limb movements (Lai et al., 2018). However, a milder iron deficiency did not replicate the same (Lo Martire et al., 2018).
The identification of genetic allelic variants associated with RLS by genome‐wide association studies suggested the utilisation of knockout (KO) mice for BTBD9 and MEIS 1. Mice knocked out for BTBD9 showed motor restlessness, sensory alterations, decreased sleep, and altered serum iron levels (Deandrade et al., 2012). Similar results were obtained by knocking out BTBD9 specifically in the striatum (Lyu et al., 2019). In mutant mice for MEIS1 a hyperactivity was observed with a RLS‐like circadian trend, associated with a sensory alteration of functions modulated by dopaminergic networks (Salminen et al., 2017).
However, there are no animal models reproducing all the clinical features of the complex human RLS phenotype. The main issue in achieving a trustable model concerns the face‐to‐face validity, that consists in how closely the animal reproduces RLS clinical phenotype. To foster the preclinical research in RLS, consensus guidelines on the best outcome measures to assess RLS‐like behaviour in rodents have been established recently. Motor activity and video‐PSG were recommended to assess sleep disturbances and PLMS, in combination with pharmacological interventions to rescue the RLS‐like behaviour (Salminen et al., 2021).
8. DIAGNOSIS OF RLS
8.1. Diagnostic criteria
The current diagnostic criteria for RLS have been developed by the International Restless Legs Study Group (IRLSSG) and published in 2014 (Allen, Picchietti, et al., 2014b). All five essential criteria must be met (see Table 1). As RLS diagnosis is clinical, a thorough patient history is needed.
TABLE 1.
Essential diagnostic criteria for RLS (all must be met) (Allen, Picchietti, et al., 2014b)
| 1. An urge to move the legs usually, but not always, accompanied by, or felt to be caused by, uncomfortable and unpleasant sensations in the legs |
| 2. The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity such as lying down or sitting |
| 3. The urge to move the legs and any accompanying unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues |
| 4. The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are worse in the evening or night than during the day |
| 5. The occurrence of the above features is not solely accounted for as symptoms primary to another medical or a behavioural condition (e.g., myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping) |
8.2. Differential diagnosis
In many cases of comorbidities, it is difficult to discern if RLS‐like symptoms are due to comorbidities or represent true RLS. Potential RLS mimics are listed in Table 2 (adapted from Garcia‐Borreguero et al., 2011 on behalf of the European RLS Study Group, EURLSSG) and briefly explained below.
TABLE 2.
RLS mimics (adapted from (Garcia‐Borreguero et al., 2011))
| Peripheral neuropathies |
| Radiculopathies |
| Positional discomfort |
| Painful legs and moving toes |
| Nocturnal legs cramps |
| Vascular claudication, spinal claudication |
| Peripheral vascular disease |
| Varicose veins |
| Propriospinal myoclonus |
| Sleep myoclonus |
| Fasciculations |
| Akathisia |
| Attention deficit hyperactivity disorder |
| Volitional movements, foot tapping, leg rocking |
Polyneuropathy (PN) can mimic RLS symptoms but can also represent a comorbidity in people with RLS. Typical PN symptoms are pain, numbness and tingling beginning mostly in the periphery (feet), whereas in RLS the symptoms tend to begin in the calves. Also, the classical RLS diurnal pattern of symptoms’ worsening in the evening is lacking in people with PN. Considering PN of different aetiologies, the prevalence of RLS varies from 5.2% (Rutkove, 1996) to 53.7% (Ondo & Jankovic, 1996).
Akathisia represents an internal urge to move, mostly without circadian pattern. It can be induced by medications, such as selective serotonin reuptake inhibitors (Lane, 1998), neuroleptics, and dopamine‐antagonists.
Nocturnal leg cramps are sudden painful sensations in the legs, usually unilateral, occurring during the night and mostly associated with a palpable contraction of the muscles.
Sleep myoclonus manifests with involuntary jerks when falling asleep, not associated with an urge to move.
Propriospinal myoclonus is characterised by jerky repetitive movements of the whole body.
Vascular and spinal claudication present with pain in the legs, mostly when standing or after periods of walking, without a circadian pattern.
Radiculopathies present typically with unilateral painful sensations and/or sensory/motor deficits with radicular distribution and do not improve with dopaminergic medication.
Painful legs and moving toes is a disorder characterised by irregular movements of toes, without an urge to move the legs. These movements cannot be voluntarily suppressed or only temporarily (Dressler, Thompson, Gledhill, & Marsden, 1994). In contrast to RLS, the symptoms are not relieved by movements, do not present a circadian pattern and there is no urge to move.
Attention deficit hyperactivity disorder (ADHD) can present with an urge to move associated with an internal restlessness, with increased difficulties in falling asleep. Comorbid RLS is frequent in ADHD (Cortese et al., 2005).
9. ASSESSMENT METHODS IN RLS
Methods to assess RLS have been developed for various scenarios including epidemiological studies, treatment trials, and in a diagnostic or clinical context for different aims including the assessment of the presence/absence of RLS, of RLS severity, of RLS treatment response, and of RLS augmentation. Assessment methods include scales and questionnaires (both self‐applied and in interview form), interviews, and instrumental methods. Table 3 lists the most important assessment tools for RLS.
TABLE 3.
Common instruments for the assessment of RLS
| Scope | RLS severity | Treatment response | RLS augmentation | Reference | ||
|---|---|---|---|---|---|---|
| Instrument | Epidemiological studies | Clinical setting, high pre‐test RLS probability | ||||
| Self‐completed scales and questionnaires | ||||||
| IRLS self‐administered | No | No | Yes | Possibly | Possibly | Sharon et al., 2019 |
| RLS‐6 scales | No | No | Yes | Yes | Possibly | Kohnen et al., 2016 |
| 3|4 questions [Berger] | (Yes) | Yes | No | No | No | Allen et al., 2003; Rothdach et al., 2000 |
| Cambridge Hopkins RLS questionnaire | (Yes) | Yes | No | No | No | Allen et al., 2009 |
| Interview based instruments | ||||||
| IRLS interview version | No | No | Yes | Yes | Possibly | Walters et al., 2003 |
| Hening telephone diagnostic interview (HTDI) | (Yes) | Yes | No | No | No | Hening et al., 2008; Mitterling et al., 2013 |
| Augmentation severity rating scale (ASRS) | No | No | No | No | Yes | García‐Borreguero et al., 2007 |
| Instrumental examinations | ||||||
| Suggested immobilisation test (SIT) | ‐ | Yes | Possibly | Possibly | Possibly | Michaud et al., 2002; Montplaisir et al., 1998 |
| Multiple suggested immobilisation test | ‐ | Possibly | Yes | Yes | Possibly | Garcia‐Borreguero et al., 2013 |
IRLS: International Restless Legs Syndrome Study Group Rating Scale.
For the diagnostic assessment of RLS in an epidemiological context currently no screening instruments can be recommended as stand‐alone solutions (Fulda et al., 2021). This recommendation is based on a systematic review and meta‐analysis showing that RLS screening instruments have insufficient specificity, thus identifying as RLS a large number of false‐positives (Fulda et al., 2021). Such instruments can, however, be used to screen RLS candidates, whose RLS status should then be verified by experts familiar with both RLS diagnostic criteria as well as RLS mimics. They might also be used in a clinical context with a high pretest probability of RLS, when an expert evaluation is part of the diagnostic process. Among the diagnostic instruments that have been used in multiple studies are the 3|4 questions (Rothdach, Trenkwalder, Haberstock, Keil, & Berger, 2000) and variants, the Wayne Hening Telephone Diagnostic Interview (Hening, Allen, Washburn, Lesage, & Earley, 2008), and the Cambridge Hopkins RLS questionnaire (Allen, Burchell, MacDonald, Hening, & Earley, 2009).
For the assessment of RLS severity the International Restless Legs Syndrome Study Group Scale (IRLS), a 10‐item scale with a score ranging from 0 to 40 points, is by far the most used scale and has been used in all recent RLS trials (Walters et al., 2003). The original IRLS is an interview‐based scale, but a self‐administered version has been recently validated (Sharon et al., 2019). Besides the IRLS, also the RLS‐6 scale, an instrument created by members of the EURLSSG to quantify RLS severity with six items (each ranging from 0 to 10), has been used in multiple studies, including medication trials (Kohnen et al., 2016).
For the assessment of augmentation currently only one validated instrument is available, the Augmentation Severity Rating Scale, developed by the EURLSSG (García‐Borreguero et al., 2007).
A key instrumental assessment in RLS is the suggested immobilisation test (SIT), where the subject is asked not to move for an extended period of time (typically 1 hour), during which the urge to move as well as PLMs are quantified, with both measures increasing over time and being significantly more pronounced in subjects with RLS compared with healthy controls (Montplaisir et al., 1998). Recently, an extended protocol with multiple SITs (mSIT) over the course of the day has also been validated (Garcia‐Borreguero et al., 2013). The SIT is suitable for diagnostic purposes, for the quantification of treatment response and possibly also for assessment of augmentation, especially in the case of the mSIT.
Among the assessments that have been initially validated but subsequently not independently evaluated are the RLS Diagnostic Index, a protocol combining information on the RLS diagnostic criteria, response to dopaminergic treatment, and polysomnographically demonstrated PLMS (Benes & Kohnen, 2009) and the L‐DOPA test (Stiasny‐Kolster, Kohnen, Möller, Trenkwalder, & Oertel, 2006), a standardised protocol to evaluate the acute response to a dopaminergic medication.
To the best of our knowledge there are no validated instruments for the assessment of RLS in children, although several instruments including a Pediatric RLS Severity Scale have been developed (Arbuckle et al., 2010; Riar et al., 2013).
Finally, although the presence of PLMS is a supportive criterion for RLS, the observation of PLMS has a very low diagnostic value for the presence of RLS, i.e., most subjects with PLMS do not have RLS. Since actigraphy is no longer recommended for the assessment of PLMS (Ferri et al., 2016a, 2016b; Smith et al., 2018) owing to concerns on diagnostic accuracy (Plante, 2014), polysomnography is currently the only recommended option for PLMS assessment, and is not part of the standard diagnostic process for RLS.
10. EPIDEMIOLOGY OF RLS
10.1. RLS in general population
Restless legs syndrome is a common disorder according to most of the epidemiological studies performed in both general and specific disease populations (Ferini‐Strambi, Walters, & Sica, 2014; Khachatryan, Ghahramanyan, Tavadyan, Yeghiazaryan, & Attarian, 2020; Lebrato Hernández et al., 2022; Manconi et al., 2021; Trenkwalder et al., 2016). Overall, the prevalence of RLS in the general population ranges from 2.5% to around 10% (Garcia‐Borreguero et al., 2006). The prevalence of RLS differs depending on the geographical region of Earth and altitude (Gupta, Ulfberg, Allen, & Goel, 2017; Manconi et al., 2021; Stefani, Heidbreder, Hackner, Burtscher, & Högl, 2017).
Ekbom initially reported a RLS prevalence of 5% (Ekbom, 1945), which was later confirmed in the general Swedish adult population (Ulfberg et al., 2007). The most common impression from available prevalence studies is that Western countries have a higher prevalence of RLS than Asian and South American populations. Moreover, prevalence is almost universally higher in females and increases with age (Berger, Luedemann, Trenkwalder, John, & Kessler, 2004; Cakmak et al., 2015; Castillo, Kaplan, Lin, Fredrickson, & Mahowald, 2006; Cho et al., 2008; Garcia‐Borreguero et al., 2012). Several studies confirm a high prevalence of RLS in European countries. In a large study (n = 701) from Austria, RLS was observed in 10.6% of participants with a female preponderance (Högl et al., 2005). A large telephone survey in five European countries (UK, Germany, Italy, Portugal, and Spain) found a 5.5% average prevalence of RLS (Ohayon & Roth, 2002). Prevalence studies from North America are in line with the above‐mentioned findings (Hening et al., 2004; Innes, Selfe, & Agarwal, 2011).
Of note, a study from Germany found a high incidence rate of RLS of 9.1% and 7% in two cohorts (n = 1312 and 4308 respectively) with a follow‐up duration 2.2 and 5.2 years, with a low persistence of RLS over time of 41.5% and 47.4%, respectively (Szentkiralyi, Fendrich, Hoffmann, Happe, & Berger, 2011).
The observed differences may refer to the natural course of the disease, depend on the RLS severity, pregnancy status, and various comorbidities. Also, utilisation of different methodologies may play a role, such as single practice prevalence as in the first report by Ekbom (Ekbom, 1945), versus conducting door‐to‐door interviews, sending mails, and validating telephone survey protocols (Erer, Karli, Zarifoglu, Ozcakir, & Yildiz, 2009; Garcia‐Borreguero et al., 2006; Hening et al., 2008).
10.2. RLS during pregnancy
RLS occurs in up to one third of pregnant women (21%–31%) and is more frequent in the third trimester of pregnancy. In many cases it disappears after delivery. The primary occurrence of RLS during pregnancy represents a risk factor for developing RLS later in life (4‐fold increase) or in the following pregnancies. European Research Groups in Switzerland, Italy, Slovakia, Germany published in the past years new data regarding the prevalence and management of RLS during pregnancy (Cesnik et al., 2010; Garbazza & Manconi, 2018; Goecke, Schnakenberg, Frensch, & Chechko, 2020; Manconi et al., 2004; Minar, Habanova, Rusnak, Planck, & Valkovic, 2013; Silvestri & Aricò, 2019).
10.3. RLS in children and adolescents
The prevalence of RLS of 1.9%–3.6% in children and adolescents was reported in several studies (Yilmaz, Kilincaslan, Aydin, & Kor, 2011). RLS is likely underdiagnosed in these groups, and the diagnosis can be improved with symptom description in age‐adapted terms. RLS in this special patient group is often associated with psychiatric conditions and certain medications, such as antidepressants and antipsychotics (DelRosso, Mogavero, Baroni, Bruni, & Ferri, 2021). A genetic susceptibility in RLS occurring at young age is also assumed, since children who develop RLS have at least one parent with RLS (Muhle et al., 2008).
11. TREATMENT OF RLS
11.1. European guidelines
Treatment for RLS should be initiated when symptoms impair the patient's quality of life, daytime functioning, social functioning, or sleep. Furthermore, consideration should be given to fully replenish iron stores and to maximise non‐pharmacological treatments before initiating drug treatment. It is recommended that all measures (including oral iron supplements and, in some cases, i.v. iron administration) are taken to ensure that ferritin levels are raised above 75 ng/mL (Allen et al., 2018). The use of medications that are known to exacerbate RLS symptoms should also be reconsidered; these include antihistamines, dopamine antagonists, serotonergic reuptake inhibitors, beta‐blockers, some anticonvulsants, and lithium (Winkelman, 2006).
Current European guidelines for the management of RLS were first established by the European Federation of Neurological Societies in 2012 (Garcia‐Borreguero et al., 2012) and are currently being updated by a dedicated taskforce of the European Academy of Neurology. Current guidelines recommend the use of pharmacological strategies for moderate to severe RLS and the use of dopamine agonists and α2δ‐ligands for both the short‐ and long‐term treatment of RLS. Pramipexol, ropinirole, and rotigotine were considered to be effective for up to 6 months in the treatment of RLS. However, caution is recommended during long‐term treatment with dopamine agonists due to the risk of augmentation. In 2016, the EURLSSG published guidelines on the prevention and treatment of augmentation (Garcia‐Borreguero et al., 2016) which included a change in the recommended first line treatment of RLS (to α2δ‐ligands), following the results of a long‐term comparison between a dopamine agonist and an α2δ‐ligand (Allen, Chen, et al., 2014a). Of note, the α2δ‐ligands gabapentin and pregabalin, although available, are not approved for RLS in Europe. On the other hand, prolonged‐release oxycodone‐naloxone is considered effective and is approved in Europe for treatment‐resistant RLS (Trenkwalder et al., 2013).
12. AUGMENTATION
The first operational definition of augmentation was described at a conference in Munich in 2006. Augmentation was identified as an iatrogenic worsening of symptom severity, manifested mainly by an earlier onset of symptoms in the afternoon compared with before treatment was initiated (Garcia‐Borreguero et al., 2007). Augmentation is a class‐specific complication of any dopaminergic treatment (Garcia‐Borreguero & Williams, 2014).
The actual prevalence of augmentation is controversial, as it varies according to the drug, its dose, the duration and type of study, and the criteria used to evaluate augmentation. Nevertheless, the augmentation rates increase with the duration of the studies: in short‐term studies augmentation rates of < 10% have been reported (Allen, Chen, et al., 2014a; García‐Borreguero et al., 2012; Högl et al., 2011; Oertel et al., 2008) while in studies lasting 2–3 years augmentation rates are at 30% (Allen et al., 2011; Ondo, Romanyshyn, Vuong, & Lai, 2004; Silber, Girish, & Izurieta, 2003), and in two very long‐term studies (approx. 10 years) augmentation has been reported to be 42%–68% (Garcia‐Borreguero et al., 2019; Silver et al., 2011).
Augmentation manifests as a frequently fluctuating but slowly progressive increase in symptom severity and as such, it is difficult to differentiate from RLS symptoms per se. Due to its progressive nature, it does not become evident immediately after treatment initiation.
Furthermore, the most recent guidelines (Garcia‐Borreguero et al., 2016) seek to facilitate the identification of augmentation in clinical practice by recommending physicians to ask four screening questions on any patient undergoing treatment with dopaminergic agents:
Do RLS symptoms appear earlier than when the drug was first started?
Are higher doses of the drug now needed, or do you need to take the medicine earlier, to control the RLS symptoms compared with the original effective dose?
Has the intensity of symptoms worsened since starting the medication?
Have symptoms spread to other parts of the body (e.g., arms) since starting the medication?
The deterioration of RLS severity taking place during augmentation reduces the response rate not only to dopaminergic medications, but also to future treatment with α2δ‐ligands (Garcia‐Borreguero et al., 2019). Due to the lack of response to most treatments, the EURLSSG was pivotal in the establishment of new treatment guidelines at a 2016 conference in Munich by recommending the initiation of RLS treatment with non‐dopaminergic agents (Garcia‐Borreguero et al., 2016).
13. OTHER COMPLICATIONS OF RLS TREATMENT WITH DOPAMINE AGONISTS
Reports of impulse control disorders (ICDs, mainly gambling, punding, and hypersexuality) in people with RLS under dopamine agonist therapy started around 15 years ago (D'Orsi, Demaio, & Specchio, 2011; Driver‐Dunckley et al., 2007; Evans & Stegeman, 2009; Jones & George, 2011; Quickfall & Suchowersky, 2007; Tippmann‐Peikert, Park, Boeve, Shepard, & Silber, 2007). After these first case reports, case‐control studies were conducted reporting a frequency of 7%–21% of any ICD in people with RLS treated with dopamine agonists.
Despite the well‐known association between the use of dopamine agonists and ICDs, a large study conducted by the Montpellier group comparing people with RLS treated with low‐dose dopamine agonists for a median of 11 months with drug‐free people with RLS found no differences in impulsivity scores and ICDs between the two groups (Bayard, Langenier, & Dauvilliers, 2013). A more recent smaller study reported a 39.7% frequency of ICD symptoms, and a 20.7% frequency of definitive ICDs in a cohort of people with RLS with and without augmentation. However, patients with augmentation had an almost 6‐fold increased risk of exhibiting ICD symptoms (Heim et al., 2016). A further study by the same group suggested that augmentation is associated with poorer decision making, even in the absence of ICD symptoms (Heim et al., 2021).
Adding to that, abnormalities in emotional processing (Ellmerer, Heim, et al., 2020a) and impaired executive function (Ellmerer, Stefani, et al., 2020b) have been described in people with RLS with current or past augmentation compared with RLS patients who never had augmentation and healthy controls.
A 2‐week randomised, double‐blind, crossover, and placebo‐controlled study in a single, referral centre in dopamine‐treatment‐naive patients and non‐augmented patients continuously treated with dopaminergic agents for the past five consecutive years assessed the treatment response to gabapentin enacarbil versus placebo after washout of any previous CNS‐active drug. Of note, improvement during treatment (assessed with IRLS scale, clinical global impression (CGI) scale, and mSIT) was greater in the dopamine‐treatment‐naïve group compared with the long treatment with dopamine agonists group (Garcia‐Borreguero et al., 2019).
All these findings have strong implications for the initial choice of RLS treatment, supporting current recommendations to start treatment only when symptoms are disturbing and not to start with dopaminergic agents (Garcia‐Borreguero et al., 2016), to prevent not only augmentation but also other complications of dopaminergic treatment for RLS.
14. FUTURE DIRECTIONS
Although RLS had been described centuries ago, awareness of the disease should be enhanced including the development of specific diagnostic tools in special populations such as children, and an improvement of screening methods.
Despite a considerable amount of research on RLS and improved knowledge of its pathophysiology, this is a complex multifactorial disorder and many aspects still need to be clarified. A better understanding of RLS pathophysiology is crucial for improving the management of the disease and developing new treatments. These are much needed, considering that limited options are available now. Also, awareness of augmentation and guidelines for its prevention should be improved among general practitioners and general neurologists, to avoid a misuse of dopamine agonists in people with RLS.
A better understanding of the disease can be achieved through large multicentre studies, which would allow improving clinical, neurophysiological, laboratory, imaging and genetic characterisation of RLS. This might lead to the identification of different phenotypes enabling personalised approaches with potential treatment implications.
Supporting information
APPENDIX S1 Supporting information
Khachatryan, S. G. , Ferri, R. , Fulda, S. , Garcia‐Borreguero, D. , Manconi, M. , Muntean, M.‐L. , & Stefani, A. (2022). Restless legs syndrome: Over 50 years of European contribution. Journal of Sleep Research, 31(4), e13632. 10.1111/jsr.13632
DATA AVAILABILITY STATEMENT
Data sharing is not applicable ‐ no new data generated.
REFERENCES
- Allen, R. P. (2015). Restless leg syndrome/Willis‐Ekbom disease pathophysiology. Sleep Medicine Clinics, 10(3), 207–214. 10.1016/J.JSMC.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, R. P. , Auerbach, S. , Bahrain, H. , Auerbach, M. , & Earley, C. J. (2013a). The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. American Journal of Hematology, 88(4), 261–264. 10.1002/ajh.23397 [DOI] [PubMed] [Google Scholar]
- Allen, R. P. , Barker, P. B. , Horska, A. , & Earley, C. J. (2013b). Thalamic glutamate/glutamine in restless legs syndrome increased and related to disturbed sleep. Neurology, 80(22), 2028–2034. 10.1212/WNL.0b013e318294b3f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, R. P. , Burchell, B. J. , MacDonald, B. , Hening, W. A. , & Earley, C. J. (2009). Validation of the self‐completed Cambridge‐Hopkins questionnaire (CH‐RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Medicine, 10(10), 1097–1100. 10.1016/j.sleep.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Allen, R. P. , Chen, C. , Garcia‐Borreguero, D. , Polo, O. , DuBrava, S. , Miceli, J. , … Winkelman, J. W. (2014a). Comparison of pregabalin with pramipexole for restless legs syndrome. The New England Journal of Medicine, 370(7), 621–631. 10.1056/NEJMOA1303646 [DOI] [PubMed] [Google Scholar]
- Allen, R. P. , & Earley, C. J. (2007). The role of iron in restless legs syndrome. Movement Disorders, 22(Suppl), 18). 10.1002/mds.21607 [DOI] [PubMed] [Google Scholar]
- Allen, R. P. , Ondo, W. G. , Ball, E. , Calloway, M. O. , Manjunath, R. , Higbie, R. L. , … Nisbet, P. A. (2011). Restless legs syndrome (RLS) augmentation associated with dopamine agonist and levodopa usage in a community sample. Sleep Medicine, 12(5), 431–439. 10.1016/J.SLEEP.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Allen, R. P. , Picchietti, D. , Hening, W. A. , Trenkwalder, C. , Walters, A. S. , & Montplaisi, J. (2003). Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine, 4(2), 101–119. 10.1016/s1389-9457(03)00010-8 [DOI] [PubMed] [Google Scholar]
- Allen, R. P. , Picchietti, D. L. , Auerbach, M. , Cho, Y. W. , Connor, J. R. , Earley, C. J. , … Winkelman, J. W. (2018). Evidence‐based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis‐Ekbom disease in adults and children: An IRLSSG task force report. Sleep Medicine, 41, 27–44. 10.1016/J.SLEEP.2017.11.1126 [DOI] [PubMed] [Google Scholar]
- Allen, R. P. , Picchietti, D. L. , Garcia‐Borreguero, D. , Ondo, W. G. , Walters, A. S. , Winkelman, J. W. , … Lee, H. B. (2014b). Restless legs syndrome/Willis‐Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria – History, rationale, description, and significance. Sleep Medicine, 15(8), 860–873. 10.1016/J.SLEEP.2014.03.025 [DOI] [PubMed] [Google Scholar]
- Allison, F. G. (1943). Obscure pains in the chest, back or limbs. Canadian Medical Association Journal, 48(1), 36–38. [PMC free article] [PubMed] [Google Scholar]
- Altarifi, A. A. , David, B. , Muchhala, K. H. , Blough, B. E. , Akbarali, H. , & Negus, S. S. (2017). Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. Journal of Psychopharmacology (Oxford, England), 31(6), 730–739. 10.1177/0269881116689257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine . (2014). The international classification of sleep disorders ({ICSD}‐3). American Academy of Sleep Medicine [Google Scholar]
- Arbuckle, R. , Abetz, L. , Durmer, J. S. , Ivanenko, A. , Owens, J. A. , Croenlein, J. , … Picchietti, D. L. (2010). Development of the Pediatric Restless Legs Syndrome Severity Scale (P‐RLS‐SS): A patient‐reported outcome measure of pediatric RLS symptoms and impact. Sleep Medicine, 11(9), 897–906. 10.1016/j.sleep.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Baran, A. S. , Richert, A. C. , Douglass, A. B. , May, W. , & Ansarin, K. (2003). Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep, 26(6), 717–720. 10.1093/sleep/26.6.717 [DOI] [PubMed] [Google Scholar]
- Bayard, S. , Langenier, M. C. , & Dauvilliers, Y. (2013). Decision‐making, reward‐seeking behaviors and dopamine agonist therapy in restless legs syndrome. Sleep, 36(10), 1501–1507. 10.5665/SLEEP.3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard, G. M. (1880). A practical treatise on nervous exhaustion (neurasthenia). E.B. Treat. [Google Scholar]
- Benes, H. , & Kohnen, R. (2009). Validation of an algorithm for the diagnosis of restless legs syndrome: The Restless Legs Syndrome‐Diagnostic Index (RLS‐DI). Sleep Medicine, 10(5), 515–523. 10.1016/j.sleep.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Berger, K. , Luedemann, J. , Trenkwalder, C. , John, U. , & Kessler, C. (2004). Sex and the risk of restless legs syndrome in the general population. Archives of Internal Medicine, 164(2), 196–202. 10.1001/ARCHINTE.164.2.196 [DOI] [PubMed] [Google Scholar]
- Cakmak, V. A. , Koc, B. , Nuhoglu, I. , Topbas, M. , Ucuncu, S. Y. , Deger, O. , … Velioglu, S. (2015). Prevalence of restless legs syndrome in Trabzon in the northeast Black Sea Region of Turkey: Co‐morbidities, socioeconomic factors and biochemical parameters. Neurological Research, 37(9), 763–773. 10.1179/1743132815Y.0000000058 [DOI] [PubMed] [Google Scholar]
- Castillo, P. R. , Kaplan, J. , Lin, S. C. , Fredrickson, P. A. , & Mahowald, M. W. (2006). Prevalence of restless legs syndrome among native South Americans residing in coastal and mountainous areas. Mayo Clinic Proceedings, 81(10), 1345–1347. 10.4065/81.10.1345 [DOI] [PubMed] [Google Scholar]
- Cesnik, E. , Casetta, I. , Turri, M. , Govoni, V. , Granieri, E. , Ferini Strambi, L. , & Manconi, M. (2010). Transient RLS during pregnancy is a risk factor for the chronic idiopathic form. Neurology, 75(23), 2117–2120. 10.1212/WNL.0B013E318200D779 [DOI] [PubMed] [Google Scholar]
- Chervin, R. D. (2001). Periodic leg movements and sleepiness in patients evaluated for sleep‐disordered breathing. American Journal of Respiratory and Critical Care Medicine, 164(8 I), 1454–1458. 10.1164/ajrccm.164.8.2011062 [DOI] [PubMed] [Google Scholar]
- Cho, Y. W. , Shin, W. C. , Yun, C. H. , Hong, S. B. , Kim, J. H. , Allen, R. P. , & Earley, C. J. (2008). Epidemiology of restless legs syndrome in Korean adults. Sleep, 31(2), 219–223. 10.1093/SLEEP/31.2.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela, F. , Ferré, S. , Casadó, V. , Cortés, A. , Cunha, R. A. , Lluis, C. , & Franco, R. (2006). Heterodimeric adenosine receptors: A device to regulate neurotransmitter release. Cellular and Molecular Life Sciences: CMLS, 63(21), 2427–2431. 10.1007/S00018-006-6216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, S. , Rye, D. , & Hochman, S. (2006). Restless legs syndrome: Revisiting the dopamine hypothesis from the spinal cord perspective. Neurology, 67(1), 125–130. 10.1212/01.wnl.0000223316.53428.c9 [DOI] [PubMed] [Google Scholar]
- Connor, J. R. , Ponnuru, P. , Wang, X. S. , Patton, S. M. , Allen, R. P. , & Earley, C. J. (2011). Profile of altered brain iron acquisition in restless legs syndrome. Brain, 134(4), 959–968. 10.1093/brain/awr012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese, S. , Konofal, E. , Lecendreux, M. , Arnulf, I. , Mouren, M. C. , Darra, F. , & Dalla Bernardina, B. (2005). Restless legs syndrome and attention‐deficit/hyperactivity disorder: A review of the literature. Sleep, 28(8), 1007–1013. 10.1093/SLEEP/28.8.1007 [DOI] [PubMed] [Google Scholar]
- Dauvilliers, Y. , Pennestri, M. H. , Petit, D. , Dang‐Vu, T. , Lavigne, G. , & Montplaisir, J. (2007). Periodic leg movements during sleep and wakefulness in narcolepsy. Journal of Sleep Research, 16(3), 333–339. 10.1111/j.1365-2869.2007.00601.x [DOI] [PubMed] [Google Scholar]
- de Paiva, J. P. Q. , Magalhães, S. C. , Moura, L. M. , Sato, J. R. , Amaro, E. , Sterr, A. , … Conforto, A. B. (2020). Sensorimotor white matter projections and disease severity in primary Restless Legs Syndrome/Willis‐Ekbom disease: A multimodal DTI analysis. Sleep Medicine, 73, 106–116. 10.1016/J.SLEEP.2020.05.040 [DOI] [PubMed] [Google Scholar]
- Deandrade, M. P. , Johnson, R. L. , Unger, E. L. , Zhang, L. , Van groen, T. , Gamble, K. L. , & Li, Y. (2012). Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in Btbd9 mutant mice. Human Molecular Genetics, 21(18), 3984–3992. 10.1093/HMG/DDS221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelRosso, L. M. , Hartmann, S. , Baumert, M. , Bruni, O. , Ruth, C. , & Ferri, R. (2020). Non‐REM sleep instability in children with restless sleep disorder. Sleep Medicine, 75, 276–281. 10.1016/J.SLEEP.2020.07.033 [DOI] [PubMed] [Google Scholar]
- DelRosso, L. M. , Mogavero, M. P. , Baroni, A. , Bruni, O. , & Ferri, R. (2021). Restless legs syndrome in children and adolescents. Child and Adolescent Psychiatric Clinics of North America, 30(1), 143–157. 10.1016/j.chc.2020.08.010 [DOI] [PubMed] [Google Scholar]
- Didriksen, M. , Nawaz, M. S. , Dowsett, J. , Bell, S. , Erikstrup, C. , Pedersen, O. B. , … Stefansson, K. (2020). Large genome‐wide association study identifies three novel risk variants for restless legs syndrome. Communications Biology, 3(703), 1–9. 10.1038/s42003-020-01430-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digiovanni, G. , Dimatteo, V. , Pierucci, M. , & Esposito, E. (2008). Serotonin–dopamine interaction: Electrophysiological evidence. In Progress in Brain Research (Vol. 172, pp. 45–71). Elsevier. 10.1016/S0079-6123(08)00903-5 [DOI] [PubMed] [Google Scholar]
- Dohin, E. , Högl, B. , Ferini‐Strambi, L. , Schollmayer, E. , Fichtner, A. , Bauer, L. , & García‐Borreguero, D. (2013). Safety and efficacy of rotigotine transdermal patch in patients with restless legs syndrome: A \textit{post‐hoc} analysis of patients taking 1–3 mg/24 h for up to 5 years. Expert Opinion on Pharmacotherapy, 14(1), 15–25. 10.1517/14656566.2013.758251 [DOI] [PubMed] [Google Scholar]
- Dooley, D. J. , Taylor, C. P. , Donevan, S. , & Feltner, D. (2007). Ca2+ channel α2δ ligands: Novel modulators of neurotransmission. Trends in Pharmacological Sciences, 28(2), 75–82. 10.1016/j.tips.2006.12.006 [DOI] [PubMed] [Google Scholar]
- D'Orsi, G. , Demaio, V. , & Specchio, L. M. (2011). Pathological gambling plus hypersexuality in restless legs syndrome: A new case. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 32(4), 707–709. 10.1007/S10072-011-0605-5 [DOI] [PubMed] [Google Scholar]
- Dowling, P. , Klinker, F. , Stadelmann, C. , Hasan, K. , Paulus, W. , & Liebetanz, D. (2011). Dopamine D3 receptor specifically modulates motor and sensory symptoms in iron‐deficient mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(1), 70–77. 10.1523/JNEUROSCI.0959-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler, D. , Thompson, P. D. , Gledhill, R. F. , & Marsden, C. D. (1994). The syndrome of painful legs and moving toes. Movement Disorders: Official Journal of the Movement Disorder Society, 9(1), 13–21. 10.1002/MDS.870090104 [DOI] [PubMed] [Google Scholar]
- Driver‐Dunckley, E. D. , Noble, B. N. , Hentz, J. G. , Evidente, V. G. H. , Caviness, J. N. , Parish, J. , … Adler, C. H. (2007). Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clinical Neuropharmacology, 30(5), 249–255. 10.1097/WNF.0B013E31804C780E [DOI] [PubMed] [Google Scholar]
- Earley, C. J. , Connor, J. , Garcia‐Borreguero, D. , Jenner, P. , Winkelman, J. , Zee, P. C. , & Allen, R. (2014). Altered brain iron homeostasis and dopaminergic function in restless legs syndrome (Willis‐Ekbom disease). Sleep Medicine, 15(11), 1288–1301. 10.1016/j.sleep.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Earley, C. J. , Connor, J. R. , Beard, J. L. , Malecki, E. A. , Epstein, D. K. , & Allen, R. P. (2000). Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology, 54(8), 1698–1700. 10.1212/WNL.54.8.1698 [DOI] [PubMed] [Google Scholar]
- Earley, C. J. , Uhl, G. R. , Clemens, S. , & Ferré, S. (2017). Connectome and molecular pharmacological differences in the dopaminergic system in restless legs syndrome (RLS): Plastic changes and neuroadaptations that may contribute to augmentation. Sleep Medicine, 31, 71–77. 10.1016/j.sleep.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom, K. A. (1945). Restless legs syndrome. Acta Medica Scandinavica, 158, 4–122. [DOI] [PubMed] [Google Scholar]
- El Gewely, M. , Welman, M. D. S. , Xiong, L. , Yin, S. , Catoire, H. D. S. , Rouleau, G. , … Warby, S. C. (2018). Reassessing GWAS findings for the shared genetic basis of insomnia and restless legs syndrome. Sleep, 41(11), 1–6. 10.1093/SLEEP/ZSY164 [DOI] [PubMed] [Google Scholar]
- Ellmerer, P. , Heim, B. , Stefani, A. , Peball, M. , Werkmann, M. , Holzknecht, E. , … Djamshidian, A. (2020a). Augmentation in restless legs syndrome: An eye tracking study on emotion processing. Annals of Clinical and Translational Neurology, 7(9), 1620–1627. 10.1002/ACN3.51144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmerer, P. , Stefani, A. , Heim, B. , Bergmann, M. , Seppi, K. , Poewe, W. , … Djamshidian, A. (2020b). The frontal assessment battery in RLS patients with and without augmentation. Sleep Medicine, 75, 456–458. 10.1016/J.SLEEP.2020.09.010 [DOI] [PubMed] [Google Scholar]
- Erer, S. , Karli, N. , Zarifoglu, M. , Ozcakir, A. , & Yildiz, D. (2009). The prevalence and clinical features of restless legs syndrome: A door to door population study in Orhangazi. Bursa in Turkey. Neurology India, 57(6), 729–733. 10.4103/0028-3886.59467 [DOI] [PubMed] [Google Scholar]
- Evans, A. H. , & Stegeman, J. R. (2009). Punding in patients on dopamine agonists for restless leg syndrome. Movement Disorders: Official Journal of the Movement Disorder Society, 24(1), 140–141. 10.1002/MDS.22309 [DOI] [PubMed] [Google Scholar]
- Fantini, M. L. , Michaud, M. , Gosselin, N. , Lavigne, G. , & Montplaisir, J. (2002). Periodic leg movements in {REM} sleep behavior disorder and related autonomic and {EEG} activation. Neurology, 59(12), 1889–1894. 10.1212/01.WNL.0000038348.94399.F6 [DOI] [PubMed] [Google Scholar]
- Ferini‐Strambi, L. , Walters, A. S. , & Sica, D. (2014). The relationship among restless legs syndrome (Willis‐Ekbom disease), hypertension, cardiovascular disease, and cerebrovascular disease. Journal of Neurology, 261(6), 1051–1068. 10.1007/S00415-013-7065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré, S. , García‐Borreguero, D. , Allen, R. P. , & Earley, C. J. (2019). New insights into the neurobiology of restless legs syndrome. Neuroscientist, 25(2), 113–125. 10.1177/1073858418791763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré, S. , Quiroz, C. , Guitart, X. , Rea, W. , Seyedian, A. , Moreno, E. , … García‐Borreguero, D. (2018). Pivotal role of adenosine neurotransmission in restless legs syndrome. Frontiers in Neuroscience, 11(JAN), 1–14. 10.3389/FNINS.2017.00722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri, R. , Fulda, S. , Manconi, M. , Allen, R. P. , Zucconi, M. , Ferini‐Strambi, L. , … Zak, R. S. (2016a). World {Association} of {Sleep} {Medicine} ({WASM}) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the {International} and the {European} {Restless} {Legs} {Syndrome} {Study} {Groups} ({IRLSSG}). Sleep Medicine, 86–95. 10.1016/j.sleep.2016.10.010 [DOI] [PubMed] [Google Scholar]
- Ferri, R. , Fulda, S. , Manconi, M. , Allen, R. P. , Zucconi, M. , Ferini‐Strambi, L. , … Zak, R. S. (2016b). World Association of Sleep Medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). Sleep Medicine, 26, 86–95. 10.1016/J.SLEEP.2016.10.010 [DOI] [PubMed] [Google Scholar]
- Ferri, R. (2012). The time structure of leg movement activity during sleep: The theory behind the practice. Sleep Medicine, 13(4), 433–441. 10.1016/J.SLEEP.2011.10.027 [DOI] [PubMed] [Google Scholar]
- Ferri, R. , Cosentino, F. I. I. , Manconi, M. , Rundo, F. , Bruni, O. , & Zucconi, M. (2014). Increased electroencephalographic high frequencies during the sleep onset period in patients with restless legs syndrome. Sleep, 37(8), 1375–1381. 10.5665/sleep.3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri, R. , Mogavero, M. P. , Bruni, O. , Picchietti, D. L. , Kapoor, V. , & DelRosso, L. M. (2022). Leg movements during sleep in children treated with serotonergic antidepressants. Sleep, 45(3), zsab236. 10.1093/sleep/zsab236 [DOI] [PubMed] [Google Scholar]
- Ferri, R. , Zucconi, M. , Manconi, M. , Bruni, O. , Ferini‐Strambi, L. , Vandi, S. , … Plazzi, G. (2006). Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep, 29(12), 1587–1594. 10.1093/sleep/29.12.1587 [DOI] [PubMed] [Google Scholar]
- Ferri, R. , Zucconi, M. , Rundo, F. , Spruyt, K. , Manconi, M. , & Ferini‐Strambi, L. (2007). Heart rate and spectral EEG changes accompanying periodic and non‐periodic leg movements during sleep. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118(2), 438–448. 10.1016/J.CLINPH.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Fulda, S. , Allen, R. P. , Earley, C. J. , Högl, B. , Garcia‐Borreguero, D. , Inoue, Y. , … Winkelman, J. W. (2021). We need to do better: A systematic review and meta‐analysis of diagnostic test accuracy of restless legs syndrome screening instruments. Sleep Medicine Reviews, 58, 101461. 10.1016/j.smrv.2021.101461 [DOI] [PubMed] [Google Scholar]
- Fulda, S. , & Wetter, T. C. (2008). Where dopamine meets opioids: A meta‐analysis of the placebo effect in restless legs syndrome treatment studies. Brain, 131(4), 902–917. 10.1093/brain/awm244 [DOI] [PubMed] [Google Scholar]
- Garbazza, C. , & Manconi, M. (2018). Management strategies for restless legs syndrome/Willis‐Ekbom disease during pregnancy. Sleep Medicine Clinics, 13(3), 335–348. 10.1016/J.JSMC.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Allen, R. P. , Kohnen, R. , Hogl, B. , Trenkwalder, C. , Oertel, W. , … Winkelmann, J. (2007). Diagnostic {Standards} for {Dopaminergic} {Augmentation} of {Restless} {Legs} {Syndrome}: {Report} from a {World} {Association} of {Sleep} {Medicine} – {International} {Restless} {Legs} {Syndrome} {Study} {Group} {Consensus} {Conference} at the {Max} Pl. Sleep Medicine, 8, 520–530. 10.1016/j.sleep.2007.03.022 [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Cano, I. , & Granizo, J. J. (2017). Treatment of restless legs syndrome with the selective AMPA receptor antagonist perampanel. Sleep Medicine, 34, 105–108. 10.1016/j.sleep.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Cano‐Pumarega, I. , Malo, C. G. , Cruz Velarde, J. A. , Granizo, J. J. , & Wanner, V. (2019). Reduced response to gabapentin enacarbil in restless legs syndrome following long‐term dopaminergic treatment. Sleep Medicine, 55, 74–80. 10.1016/J.SLEEP.2018.11.025 [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Egatz, R. , Winkelmann, J. , & Berger, K. (2006). Epidemiology of restless legs syndrome: The current status. Sleep Medicine Reviews, 10(3), 153–167. 10.1016/J.SMRV.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Ferini‐Strambi, L. , Kohnen, R. , O'Keeffe, S. , Trenkwalder, C. , Högl, B. , … Williams, A. M. (2012). European guidelines on management of restless legs syndrome: Report of a joint task force by the European Federation of Neurological Societies, the European Neurological Society and the European Sleep Research Society. European Journal of Neurology, 19(11), 1385–1396. 10.1111/J.1468-1331.2012.03853.X [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Garcia‐Malo, C. , Granizo, J. J. , & Ferré, S. (2021). A randomized, placebo‐controlled crossover study with dipyridamole for restless legs syndrome. Movement Disorders: Official Journal of the Movement Disorder Society, 36(10), 2387–2392. 10.1002/MDS.28668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Borreguero, D. , Högl, B. , Ferini‐Strambi, L. , Winkelman, J. , Hill‐Zabala, C. , Asgharian, A. , & Allen, R. (2012). Systematic evaluation of augmentation during treatment with ropinirole in restless legs syndrome ({Willis}‐{Ekbom} {Disease}): {Results} from a prospective, multicenter study over 66 weeks. Movement Disorders, 27(2), 277–283. 10.1002/mds.24889 [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Kohnen, R. , Boothby, L. , Tzonova, D. , Larrosa, O. , & Dunkl, E. (2013). Validation of the multiple suggested immobilization test: a test for the assessment of severity of restless legs syndrome (Willis‐Ekbom disease). Sleep, 36(7), 1101–1109. 10.5665/sleep.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Borreguero, D. , Kohnen, R. , Högl, B. , Ferini‐Strambi, L. , Hadjigeorgiou, G. M. , Hornyak, M. , … Allen, R. P. (2007). Validation of the {Augmentation} {Severity} {Rating} {Scale} ({ASRS}): {A} multicentric, prospective study with levodopa on restless legs syndrome. Sleep Medicine, 8(5), 455–463. 10.1016/j.sleep.2007.03.023 [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Patrick, J. , DuBrava, S. , Becker, P. M. , Lankford, A. , Chen, C. , … Allen, R. P. (2014). Pregabalin versus pramipexole: Effects on sleep disturbance in restless legs syndrome. Sleep, 37(4), 635–643. 10.5665/sleep.3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Silber, M. H. , Winkelman, J. W. , Högl, B. , Bainbridge, J. , Buchfuhrer, M. , … Allen, R. P. (2016). Guidelines for the first‐line treatment of restless legs syndrome/{Willis}‐{Ekbom} disease, prevention and treatment of dopaminergic augmentation: A combined task force of the {IRLSSG}, {EURLSSG}, and the {RLS}‐foundation. Sleep Medicine, 21, 1–11. 10.1016/j.sleep.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , Stillman, P. , Benes, H. , Buschmann, H. , Chaudhuri, K. R. , Rodríguez, V. M. G. , … Zucconi, M. (2011). Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurology, 28(1), 28. 10.1186/1471-2377-11-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Borreguero, D. , & Williams, A.‐M. (2014). An update on restless legs syndrome (Willis‐Ekbom disease): Clinical features, pathogenesis and treatment. Current Opinion in Neurology, 27(4), 493–501. 10.1097/WCO.0000000000000117 [DOI] [PubMed] [Google Scholar]
- Garcia‐Malo, C. , Miranda, C. , Novo Ponte, S. , Romero Peralta, S. , Cano‐Pumarega, I. , Boi, S. , … Garcia‐Borreguero, D. (2020a). Low risk of iron overload or anaphylaxis during treatment of restless legs syndrome with intravenous iron: A consecutive case series in a regular clinical setting. Sleep Medicine, 74, 48–55. 10.1016/J.SLEEP.2020.06.002 [DOI] [PubMed] [Google Scholar]
- Garcia‐Malo, C. , Wanner, V. , Miranda, C. , Romero Peralta, S. , Agudelo, L. , Cano‐Pumarega, I. , … Garcia‐Borreguero, D. (2020b). Quantitative transcranial sonography of the substantia nigra as a predictor of therapeutic response to intravenous iron therapy in restless legs syndrome. Sleep Medicine, 66, 123–129. 10.1016/j.sleep.2019.09.020 [DOI] [PubMed] [Google Scholar]
- Godau, J. , Schweitzer, K. J. , Liepelt, I. , Gerloff, C. , & Berg, D. (2007). Substantia nigra hypoechogenicity: Definition and findings in restless legs syndrome. Movement Disorders, 22(2), 187–192. 10.1002/mds.21230 [DOI] [PubMed] [Google Scholar]
- Godau, J. , Wevers, A. K. , Gaenslen, A. , Di Santo, A. , Liepelt, I. , Gasser, T. , & Berg, D. (2008). Sonographic abnormalities of brainstem structures in restless legs syndrome. Sleep Medicine, 9(7), 782–789. 10.1016/j.sleep.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Goecke, T. W. , Schnakenberg, P. , Frensch, M. , & Chechko, N. (2020). Restless legs syndrome during pregnancy and 12 weeks postpartum and its links to cardiovascular diseases, stressful life events, and psychiatric history. Journal of Clinical Medicine, 9(9), 1–16. 10.3390/JCM9093046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, R. , Ulfberg, J. , Allen, R. P. , & Goel, D. (2017). High prevalence of restless legs syndrome/Willis Ekbom Disease (RLS/WED) among people living at high altitude in the Indian Himalaya. Sleep Medicine, 35, 7–11. 10.1016/J.SLEEP.2017.02.031 [DOI] [PubMed] [Google Scholar]
- Hammerschlag, A. R. , Stringer, S. , De Leeuw, C. A. , Sniekers, S. , Taskesen, E. , Watanabe, K. , … Posthuma, D. (2017). Genome‐wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nature Genetics, 49(11), 1584–1592. 10.1038/NG.3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe, S. , Reese, J. P. , Stiasny‐Kolster, K. , Peglau, I. , Mayer, G. , Klotsche, J. , … Dodel, R. (2009). Assessing health‐related quality of life in patients with restless legs syndrome. Sleep Medicine, 10(3), 295–305. 10.1016/j.sleep.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Heim, B. , Djamshidian, A. , Heidbreder, A. , Stefani, A. , Zamarian, L. , Pertl, M. T. , … Högl, B. (2016). Augmentation and impulsive behaviors in restless legs syndrome. Neurology, 87(1), 36–40. 10.1212/WNL.0000000000002803 [DOI] [PubMed] [Google Scholar]
- Heim, B. , Ellmerer, P. , Stefani, A. , Heidbreder, A. , Brandauer, E. , Högl, B. , … Djamshidian, A. (2021). Birds of a feather flock together: disadvantageous decision making in augmented restless legs syndrome patients with and without impulse control disorders. Brain Sciences, 11(3), 383. 10.3390/BRAINSCI11030383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hening, W. A. , Allen, R. P. , Washburn, M. , Lesage, S. , & Earley, C. J. (2008). Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Medicine, 9(3), 283–289. 10.1016/J.SLEEP.2007.04.021 [DOI] [PubMed] [Google Scholar]
- Hening, W. , Walters, A. S. , Allen, R. P. , Montplaisir, J. , Myers, A. , & Ferini‐Strambi, L. (2004). Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: The REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Medicine, 5(3), 237–246. 10.1016/J.SLEEP.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Högl, B. , Kiechl, S. , Willeit, J. , Saletu, M. , Frauscher, B. , Seppi, K. , … Poewe, W. (2005). Restless legs syndrome {A} community‐based study of prevalence, severity, and risk factors. Neurology, 64(11), 1920–1924. 10.1212/01.WNL.0000163996.64461.A3 [DOI] [PubMed] [Google Scholar]
- Högl, B. , Garcia‐Borreguero, D. , Trenkwalder, C. , Ferini‐Strambi, L. , Hening, W. , Poewe, W. , … Allen, R. P. (2011). Efficacy and augmentation during 6months of double‐blind pramipexole for restless legs syndrome. Sleep Medicine, 12(4), 351–360. 10.1016/j.sleep.2010.12.007 [DOI] [PubMed] [Google Scholar]
- Innes, K. E. , Selfe, T. K. , & Agarwal, P. (2011). Prevalence of restless legs syndrome in North American and Western European populations: A systematic review. Sleep Medicine, 12(7), 623–634. 10.1016/J.SLEEP.2010.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi, C. E. (2005). The role of N‐methyl‐D‐aspartate (NMDA) receptors in pain and morphine tolerance. Minerva Anestesiologica, 71(7–8), 401–403. [PubMed] [Google Scholar]
- Isler, H. (2010). Chapter 8: The development of neurology and the neurological sciences in the 17th century. Handbook of Clinical Neurology, 95(C), 91–106 10.1016/S0072-9752(08)02108‐8 [DOI] [PubMed] [Google Scholar]
- Jones, H. B. , & George, S. (2011). “You never told me I would turn into a gambler”: A first person account of dopamine agonist‐induced gambling addiction in a patient with restless legs syndrome. BMJ Case Reports, 2011. 10.1136/BCR.07.2011.4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur, N. , & Friedman, R. (2002). Oral ketamine: A promising treatment for restless legs syndrome. Anesthesia and Analgesia, 94(6), 1558–1559. 10.1097/00000539-200206000-00034 [DOI] [PubMed] [Google Scholar]
- Karroum, E. G. , Saini, P. S. , Trotti, L. M. , & Rye, D. B. (2020). TOX3 gene variant could be associated with painful restless legs. Sleep Medicine, 65, 4–7. 10.1016/J.SLEEP.2019.07.003 [DOI] [PubMed] [Google Scholar]
- Khachatryan, S. G. , Ghahramanyan, L. , Tavadyan, Z. , Yeghiazaryan, N. , & Attarian, H. P. (2020). Sleep‐related movement disorders in a population of patients with epilepsy: Prevalence and impact of restless legs syndrome and sleep bruxism. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 16(3), 409–414. 10.5664/JCSM.8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocar, T. D. , Müller, H. P. , & Kassubek, J. (2020). Differential functional connectivity in thalamic and dopaminergic pathways in restless legs syndrome: A meta‐analysis. Therapeutic Advances in Neurological Disorders, 13, 1–10. 10.1177/1756286420941670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnen, R. , Martinez‐Martin, P. , Benes, H. , Trenkwalder, C. , Högl, B. , Dunkl, E. , & Walters, A. S. (2016). Rating of daytime and nighttime symptoms in {RLS}: {Validation} of the {RLS}‐6 scale of restless legs syndrome/{Willis}‐{Ekbom} disease. Sleep Medicine, 20, 116–122. 10.1016/j.sleep.2015.10.014 [DOI] [PubMed] [Google Scholar]
- Kolla, B. P. , Mansukhani, M. P. , & Bostwick, J. M. (2018). The influence of antidepressants on restless legs syndrome and periodic limb movements: A systematic review. Sleep Medicine Reviews, 38, 131–140. 10.1016/j.smrv.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Ku, J. , Lee, Y. S. , Chang, H. W. , Earley, C. J. , Allen, R. P. , & Cho, Y. W. (2018). Diurnal variation of default mode network in patients with restless legs syndrome. Sleep Medicine, 41, 1–8. 10.1016/J.SLEEP.2017.09.031 [DOI] [PubMed] [Google Scholar]
- Ku, J. , Lee, Y. S. , Kim, K. T. , Chang, H. W. , & Cho, Y. W. (2020). Alterations in salience network functional connectivity in individuals with restless legs syndrome. Scientific Reports, 10(1), 7643. 10.1038/S41598-020-64641-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, Y.‐Y. , Cheng, Y.‐H. , Hsieh, K.‐C. , Nguyen, D. , Chew, K.‐T. , Ramanathan, L. , & Siegel, J. M. (2018, January). Reply: The iron‐deficient rat as a model of restless legs syndrome: Was anything lost in translation? Movement Disorders: Official Journal of the Movement Disorder Society, 33, 182–183. 10.1002/mds.27263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, J. M. , Liang, J. , Vlasac, I. , Anderson, S. G. , Bechtold, D. A. , Bowden, J. , … Saxena, R. (2017). Genome‐wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nature Genetics, 49(2), 274–281. 10.1038/NG.3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, R. M. (1998). SSRI‐induced extrapyramidal side‐effects and akathisia: Implications for treatment. Journal of Psychopharmacology (Oxford, England), 12(2), 192–214. 10.1177/026988119801200212 [DOI] [PubMed] [Google Scholar]
- Lanza, G. , Bachmann, C. G. , Ghorayeb, I. , Wang, Y. , Ferri, R. , & Paulus, W. (2017). Central and peripheral nervous system excitability in restless legs syndrome. Sleep Medicine, 31, 49–60. 10.1016/j.sleep.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Lanza, G. , Cantone, M. , Aricò, D. , Lanuzza, B. , Cosentino, F. I. I. , Paci, D. , … Ferri, R. (2018a). Clinical and electrophysiological impact of repetitive low‐frequency transcranial magnetic stimulation on the sensory‐motor network in patients with restless legs syndrome. Therapeutic Advances in Neurological Disorders, 11, 1–12. 10.1177/1756286418759973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, G. , & Ferri, R. (2019). The neurophysiology of hyperarousal in restless legs syndrome: Hints for a role of glutamate/GABA. Advances in Pharmacology (San Diego, Calif.), 84, 101–119. 10.1016/BS.APHA.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Lanza, G. , Lanuzza, B. , Aricò, D. , Cantone, M. , Cosentino, F. I. I. , Bella, R. , … Pennisi, M. (2018b). Impaired short‐term plasticity in restless legs syndrome: A pilot rTMS study. Sleep Medicine, 46, 1–4. 10.1016/j.sleep.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Lanza, G. , Lanuzza, B. , Aricò, D. , Cantone, M. , Cosentino, F. I. I. , Pennisi, M. , … Ferri, R. (2015). Direct comparison of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome and restless legs syndrome. Sleep Medicine, 16(1), 138–142. 10.1016/j.sleep.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Lebrato Hernández, L. , Prieto León, M. , Cerdá Fuentes, N. A. , Uclés Sánchez, A. J. , Casado Chocán, J. L. , & Díaz Sánchez, M. (2022). Restless legs syndrome in patients with multiple sclerosis: Evaluation of risk factors and clinical impact. Neurologia (Barcelona, Spain), 37(2), 83–90. 10.1016/J.NRLENG.2018.12.018 [DOI] [PubMed] [Google Scholar]
- Lee, B. Y. , Kim, J. , Connor, J. R. , Podskalny, G. D. , Ryu, Y. , & Yang, Q. X. (2018). Involvement of the central somatosensory system in restless legs syndrome: A neuroimaging study. Neurology, 90(21), e1834–e1841. 10.1212/WNL.0000000000005562 [DOI] [PubMed] [Google Scholar]
- Lee, K. , Baron, K. , Soca, R. , & Attarian, H. (2016). The prevalence and characteristics of {REM} sleep without atonia ({RSWA}) in patients taking antidepressants. Journal of Clinical Sleep Medicine, 12(3), 351–355. 10.5664/jcsm.5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. S. , Ku, J. , Kim, K. T. , Chang, H. W. , Earley, C. J. , Allen, R. P. , & Cho, Y. W. (2020). Resting‐state connectivity and the effects of treatment in restless legs syndrome. Sleep Medicine, 67, 33–38. 10.1016/J.SLEEP.2019.10.014 [DOI] [PubMed] [Google Scholar]
- Lo Martire, V. , Alvente, S. , Bastianini, S. , Berteotti, C. , Valli, A. , Manconi, M. , … Silvani, A. (2018). Sleep and tibialis anterior muscle activity in mice with mild hypoxia and iron deficiency: implications for the restless legs syndrome. Frontiers in Physiology, 9, 1–12. 10.3389/FPHYS.2018.01818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugaresi, E. , Coccagna, G. , Tassinari, C. A. , & Ambrosetto, C. (1965). Polygraphic data on motor phenomena in the restless legs syndrome. Rivista Di Neurologia, 35(6), 550–561. [PubMed] [Google Scholar]
- Lyu, S. , Xing, H. , Deandrade, M. P. , Liu, Y. , Perez, P. D. , Yokoi, F. , … Li, Y. (2019). The role of BTBD9 in striatum and restless legs syndrome. ENeuro, 6(5), 0277‐19. 10.1523/ENEURO.0277-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, S. , Xing, H. , DeAndrade, M. P. , Perez, P. D. , Yokoi, F. , Febo, M. , … Li, Y. (2020a). The role of BTBD9 in the cerebellum, sleep‐like behaviors and the restless legs syndrome. Neuroscience, 440, 85–96. 10.1016/J.NEUROSCIENCE.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, S. , Xing, H. , DeAndrade, M. P. , Perez, P. D. , Zhang, K. , Liu, Y. , … Li, Y. (2020b). The role of BTBD9 in the cerebral cortex and the pathogenesis of restless legs syndrome. Experimental Neurology, 323, 113111. 10.1016/J.EXPNEUROL.2019.113111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manconi, M. , Ferri, R. , Zucconi, M. , Bassetti, C. L. , Fulda, S. , Aricò, D. , & Ferini‐Strambi, L. (2012). Dissociation of periodic leg movements from arousals in restless legs syndrome. Annals of Neurology, 71(6), 834–844. 10.1002/ana.23565 [DOI] [PubMed] [Google Scholar]
- Manconi, M. , Ferri, R. , Zucconi, M. , Clemens, S. , Rundo, F. , Oldani, A. , & Ferini‐Strambi, L. (2011a). Effects of acute dopamine‐agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Medicine, 12(1), 47–55. 10.1016/J.SLEEP.2010.03.019 [DOI] [PubMed] [Google Scholar]
- Manconi, M. , Ferri, R. , Zucconi, M. , Fantini, M. L. , Plazzi, G. , & Ferini‐Strambi, L. (2007a). Time structure analysis of leg movements during sleep in REM sleep behavior disorder. Sleep, 30(12), 1779–1785. 10.1093/sleep/30.12.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manconi, M. , Ferri, R. , Zucconi, M. , Oldani, A. , Fantini, M. L. , Castronovo, V. , & Ferini‐Strambi, L. (2007b). First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Medicine, 8(5), 491–497. 10.1016/j.sleep.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Manconi, M. , Ferri, R. , Zucconi, M. , Oldani, A. , Giarolli, L. , Bottasini, V. , & Ferini‐Strambi, L. (2011b). Pramipexole versus ropinirole: Polysomnographic acute effects in restless legs syndrome. Movement Disorders, 26(5), 892–895. 10.1002/mds.23543 [DOI] [PubMed] [Google Scholar]
- Manconi, M. , Garcia‐Borreguero, D. , Schormair, B. , Videnovic, A. , Berger, K. , Ferri, R. , & Dauvilliers, Y. (2021). Restless legs syndrome. Nature Reviews Disease Primers, 7(1), 1–18. 10.1038/s41572-021-00311-z [DOI] [PubMed] [Google Scholar]
- Manconi, M. , Govoni, V. , De Vito, A. , Economou, N. T. , Cesnik, E. , Casetta, I. , … Granieri, E. (2004). Restless legs syndrome and pregnancy. Neurology, 63(6), 1065–1069. 10.1212/01.WNL.0000138427.83574.A6 [DOI] [PubMed] [Google Scholar]
- Manconi, M. , Zavalko, I. , Bassetti, C. L. , Colamartino, E. , Pons, M. , & Ferri, R. (2014). Respiratory‐related leg movements and their relationship with periodic leg movements during sleep. Sleep, 37(3), 497–504. 10.5665/sleep.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud, M. , Paquet, J. , Lavigne, G. , Desautels, A. , & Montplaisir, J. (2002). Sleep laboratory diagnosis of restless legs syndrome. European Neurology, 48(2), 108–113. 10.1159/000062996 [DOI] [PubMed] [Google Scholar]
- Minar, M. , Habanova, H. , Rusnak, I. , Planck, K. , & Valkovic, P. (2013). Prevalence and impact of restless legs syndrome in pregnancy. Neuro Endocrinology Letters, 34(5), 366–371. [PubMed] [Google Scholar]
- Mitterling, T. , Frauscher, B. , Ehrmann, L. , Gschliesser, V. , Brandauer, E. , & Högl, B. (2013). Is a diagnosis of ancillary restless legs syndrome reproducible over time? {Experience} with the {Wayne} {Hening} telephone diagnostic interview. Sleep Medicine, 2012–2014, 572–574. 10.1016/j.sleep.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Mogavero, M. P. , Mezzapesa, D. M. , Savarese, M. , DelRosso, L. M. , Lanza, G. , & Ferri, R. (2021). Morphological analysis of the brain subcortical gray structures in restless legs syndrome. Sleep Medicine, 88, 74–80. 10.1016/J.SLEEP.2021.10.025 [DOI] [PubMed] [Google Scholar]
- Montplaisir, J. , Boucher, S. , Nicolas, A. , Lesperance, P. , Gosselin, A. , Rompré, P. , & Lavigne, G. (1998). Immobilization tests and periodic leg movements in sleep for the diagnosis of restless leg syndrome. Movement Disorders: Official Journal of the Movement Disorder Society, 13(2), 324–329. 10.1002/mds.870130220 [DOI] [PubMed] [Google Scholar]
- Muhle, H. , Neumann, A. , Lohmann‐Hedrich, K. , Lohnau, T. , Lu, Y. , Winkler, S. , … Stephani, U. (2008). Childhood‐onset restless legs syndrome: Clinical and genetic features of 22 families. Movement Disorders: Official Journal of the Movement Disorder Society, 23(8), 1113–1121; quiz 1203. 10.1002/mds.22016 [DOI] [PubMed] [Google Scholar]
- Oertel, W. H. , Benes, H. , Garcia‐Borreguero, D. , Geisler, P. , Högl, B. , Trenkwalder, C. , … Stiasny‐Kolster, K. (2008). One year open‐label safety and efficacy trial with rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome. Sleep Medicine, 9(8), 865–873. 10.1016/j.sleep.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Ohayon, M. M. , & Roth, T. (2002). Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. Journal of Psychosomatic Research, 53(1), 547–554. 10.1016/S0022-3999(02)00443-9 [DOI] [PubMed] [Google Scholar]
- Ondo . (2000). Clinical correlates of 6‐hydroxydopamine injections into A11 dopaminergic neurons in rats: A possible model for restless legs syndrome – PubMed. Retrieved March 29, 2022, from https://pubmed.ncbi.nlm.nih.gov/10634257/ [DOI] [PubMed]
- Ondo, W. , & Jankovic, J. (1996). Restless legs syndrome: Clinicoetiologic correlates. Neurology, 47(6), 1435–1441. 10.1212/WNL.47.6.1435 [DOI] [PubMed] [Google Scholar]
- Ondo, W. , Romanyshyn, J. , Vuong, K. D. , & Lai, D. (2004). Long‐term treatment of restless legs syndrome with dopamine agonists. Archives of Neurology, 61(9), 1393–1397. 10.1001/ARCHNEUR.61.9.1393 [DOI] [PubMed] [Google Scholar]
- Park, H. R. , Kim, H. R. , Oh, S. , Seong, J. K. , & Joo, E. Y. (2021). White matter tract‐specific alterations in patients with primary restless legs syndrome. Scientific Reports, 11(1), 16116. 10.1038/S41598-021-95238-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennestri, M. H. , Whittom, S. , Adam, B. , Petit, D. , Carrier, J. , & Montplaisir, J. (2006). PLMS and PLMW in healthy subjects as a function of age: Prevalence and interval distribution. Sleep, 29(9), 1183–1187. 10.1093/sleep/29.9.1183 [DOI] [PubMed] [Google Scholar]
- Plante, D. T. (2014). Leg actigraphy to quantify periodic limb movements of sleep: A systematic review and meta‐analysis. Sleep Medicine Reviews, 18(5), 425–434. 10.1016/j.smrv.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provini, F. , Vetrugno, R. , Meletti, S. , Plazzi, G. , Solieri, L. , Lugaresi, E. , … Montagna, P. (2001). Motor pattern of periodic limb movements during sleep. Neurology, 57(2), 300–304. 10.1212/WNL.57.2.300 [DOI] [PubMed] [Google Scholar]
- Puligheddu, M. , Figorilli, M. , Aricò, D. , Raggi, A. , Marrosu, F. , & Ferri, R. (2014). Time structure of leg movement activity during sleep in untreated Parkinson disease and effects of dopaminergic treatment. Sleep Medicine, 15(7), 816–824. 10.1016/J.SLEEP.2014.03.011 [DOI] [PubMed] [Google Scholar]
- Qu, S. , Le, W. , Zhang, X. , Xie, W. , Zhang, A. , & Ondo, W. G. (2007). Locomotion is increased in a11‐lesioned mice with iron deprivation: A possible animal model for restless legs syndrome. Journal of Neuropathology and Experimental Neurology, 66(5), 383–388. 10.1097/NEN.0B013E3180517B5F [DOI] [PubMed] [Google Scholar]
- Quickfall, J. , & Suchowersky, O. (2007). Pathological gambling associated with dopamine agonist use in restless legs syndrome. Parkinsonism & Related Disorders, 13(8), 535–536. 10.1016/J.PARKRELDIS.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Quiroz, C. , Gulyani, S. , Ruiqian, W. , Bonaventura, J. , Cutler, R. , Pearson, V. , … Ferré, S. (2016). Adenosine receptors as markers of brain iron deficiency: {Implications} for {Restless} {Legs} {Syndrome}. Neuropharmacology, 111, 160–168. 10.1016/j.neuropharm.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riar, S. K. , Leu, R. M. , Turner‐Green, T. C. , Rye, D. B. , Kendrick‐Allwood, S. R. , McCracken, C. , … Greenbaum, L. A. (2013). Restless legs syndrome in children with chronic kidney disease. Pediatric Nephrology (Berlin, Germany), 28(5), 773–795. 10.1007/s00467-013-2408-9 [DOI] [PubMed] [Google Scholar]
- Rizzo, G. , Li, X. , Galantucci, S. , Filippi, M. , & Cho, Y. W. (2017). Brain imaging and networks in restless legs syndrome. Sleep Medicine, 31, 39–48. 10.1016/J.SLEEP.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothdach, A. J. , Trenkwalder, C. , Haberstock, J. , Keil, U. , & Berger, K. (2000). Prevalence and risk factors of RLS in an elderly population: The MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology, 54(5), 1064–1068. 10.1212/wnl.54.5.1064 [DOI] [PubMed] [Google Scholar]
- Rutkove, S. B. (1996). Restless legs syndrome in patients with polyneuropathy – PubMed. Muscle Nerve, 19, 670–672. [DOI] [PubMed] [Google Scholar]
- Salminen, A. V. , Lam, D. D. , & Winkelmann, J. (2019). Role of MEIS1 in restless legs syndrome: From GWAS to functional studies in mice. In Advances in Pharmacology, 84, 175–184. 10.1016/bs.apha.2019.03.003 [DOI] [PubMed] [Google Scholar]
- Salminen, A. V. , Garrett, L. , Schormair, B. , Rozman, J. , Giesert, F. , Niedermeier, K. M. , … Winkelmann, J. (2017). Meis1: Effects on motor phenotypes and the sensorimotor system in mice. Disease Models & Mechanisms, 10(8), 981–991. 10.1242/DMM.030080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, A. V. , Silvani, A. , Allen, R. P. , Clemens, S. , Garcia‐Borreguero, D. , Ghorayeb, I. , … Manconi, M. (2021). Consensus guidelines on rodent models of restless legs syndrome. Movement Disorders: Official Journal of the Movement Disorder Society, 36(3), 558–569. 10.1002/MDS.28401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidauer, C. , Sojer, M. , Seppi, K. , Stockner, H. , Högl, B. , Biedermann, B. , … Poewe, W. (2005). Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Annals of Neurology, 58(4), 630–634. 10.1002/ANA.20572 [DOI] [PubMed] [Google Scholar]
- Schormair, B. , Zhao, C. , Bell, S. , Tilch, E. , Salminen, A. V. , Pütz, B. , … Wilson, C. H. (2017). Identification of novel risk loci for restless legs syndrome in genome‐wide association studies in individuals of {European} ancestry: A meta‐analysis. The Lancet Neurology, 16(11), 898–907. 10.1016/S1474-4422(17)30327-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon, D. , Allen, R. P. , Martinez‐Martin, P. , Walters, A. S. , Ferini Strambi, L. , Högl, B. , … Becker, P. M. (2019). Validation of the self‐administered version of the international Restless Legs Syndrome study group severity rating scale – The sIRLS. Sleep Medicine, 54, 94–100. 10.1016/j.sleep.2018.10.014 [DOI] [PubMed] [Google Scholar]
- Sheng, L. Q. , Zhao, P. W. , Ma, H. R. , Qi, L. , Yi, Z. Q. , Shi, Y. Y. , … Pan, P. L. (2021). Grey matter alterations in restless legs syndrome: A coordinate‐based meta‐analysis. Journal of Sleep Research, 30(5), e13298. 10.1111/JSR.13298 [DOI] [PubMed] [Google Scholar]
- Silber, M. H. , Girish, M. , & Izurieta, R. (2003). Pramipexole in the management of restless legs syndrome: An extended study. Sleep, 26(7), 819–821. 10.1093/sleep/26.7.819 [DOI] [PubMed] [Google Scholar]
- Silver, N. , Allen, R. P. , Senerth, J. , & Earley, C. J. (2011). A 10‐year, longitudinal assessment of dopamine agonists and methadone in the treatment of restless legs syndrome. Sleep Medicine, 12(5), 440–444. 10.1016/J.SLEEP.2010.11.002 [DOI] [PubMed] [Google Scholar]
- Silvestri, R. , & Aricò, I. (2019). Sleep disorders in pregnancy. Sleep Science (Sao Paulo, Brazil), 12(3), 232–239. 10.5935/1984-0063.20190098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. T. , McCrae, C. S. , Cheung, J. , Martin, J. L. , Harrod, C. G. , Heald, J. L. , & Carden, K. A. (2018). Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep‐wake disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 14(7), 1231–1237. 10.5664/jcsm.7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani, A. , Heidbreder, A. , Hackner, H. , Burtscher, M. , & Högl, B. (2017). Influence of high altitude on periodic leg movements during sleep in individuals with restless legs syndrome and healthy controls: {A} pilot study. Sleep Medicine, 88–89. 10.1016/j.sleep.2016.06.037 [DOI] [PubMed] [Google Scholar]
- Stefani, A. , Mitterling, T. , Heidbreder, A. , Steiger, R. , Kremser, C. , Frauscher, B. , … Scherfler, C. (2019). Multimodal magnetic resonance imaging reveals alterations of sensorimotor circuits in restless legs syndrome. Sleep, 42(12). 10.1093/SLEEP/ZSZ171 [DOI] [PubMed] [Google Scholar]
- Stiasny‐Kolster, K. , Kohnen, R. , Möller, J. C. , Trenkwalder, C. , & Oertel, W. H. (2006). Validation of the “L‐DOPA test” for diagnosis of restless legs syndrome. Movement Disorders: Official Journal of the Movement Disorder Society, 21(9), 1333–1339. 10.1002/mds.20969 [DOI] [PubMed] [Google Scholar]
- Symonds, C. P. (1953). Nocturnal myoclonus. Journal of Neurology, Neurosurgery, and Psychiatry, 16(3), 166. 10.1136/JNNP.16.3.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentkiralyi, A. , Fendrich, K. , Hoffmann, W. , Happe, S. , & Berger, K. (2011). Incidence of restless legs syndrome in two population‐based cohort studies in Germany. Sleep Medicine, 12(9), 815–820. 10.1016/J.SLEEP.2011.06.016 [DOI] [PubMed] [Google Scholar]
- Thireau, J. , Farah, C. , Molinari, N. , Bouilloux, F. , Torreilles, L. , Winkelmann, J. , … Marmigère, F. (2017). MEIS1 variant as a determinant of autonomic imbalance in restless legs syndrome. Scientific Reports, 7, 46620. 10.1038/SREP46620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilch, E. , Schormair, B. , Zhao, C. , Salminen, A. V. , Antic Nikolic, A. , Holzknecht, E. , … Oexle, K. (2020). Identification of restless legs syndrome genes by mutational load analysis. Annals of Neurology, 87(2), 184–193. 10.1002/ANA.25658 [DOI] [PubMed] [Google Scholar]
- Tippmann‐Peikert, M. , Park, J. G. , Boeve, B. F. , Shepard, J. W. , & Silber, M. H. (2007). Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology, 68(4), 301–303. 10.1212/01.WNL.0000252368.25106.B6 [DOI] [PubMed] [Google Scholar]
- Trenkwalder, C. , Tinelli, M. , Sakkas, G. K. , Dauvilliers, Y. , Ferri, R. , Rijsman, R. , … Jaarsma, J. (2021). Socioeconomic impact of restless legs syndrome and inadequate restless legs syndrome management across European settings. European Journal of Neurology, 28(2), 691–706. 10.1111/ene.14582 [DOI] [PubMed] [Google Scholar]
- Trenkwalder, C. , Allen, R. , Högl, B. , Paulus, W. , & Winkelmann, J. (2016). Restless legs syndrome associated with major diseases: {A} systematic review and new concept. Neurology, 86(14), 1336–1343 10.1212/WNL.0000000000002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkwalder, C. , Beneš, H. , Grote, L. , García‐Borreguero, D. , Högl, B. , Hopp, M. , … Kohnen, R. (2013). Prolonged release oxycodone‐naloxone for treatment of severe restless legs syndrome after failure of previous treatment: A double‐blind, randomised, placebo‐controlled trial with an open‐label extension. The Lancet. Neurology, 12(12), 1141–1150. 10.1016/S1474-4422(13)70239-4 [DOI] [PubMed] [Google Scholar]
- Tuovinen, N. , Stefani, A. , Mitterling, T. , Heidbreder, A. , Frauscher, B. , Gizewski, E. R. , … Scherfler, C. (2021). Functional connectivity and topology in patients with restless legs syndrome: A case‐control resting‐state functional magnetic resonance imaging study. European Journal of Neurology, 28(2), 448–458. 10.1111/ENE.14577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfberg, J. , Bjorvatn, B. , Leissner, L. , Gyring, J. , Karlsborg, M. , Regeur, L. , … Partinen, M. (2007). Comorbidity in restless legs syndrome among a sample of Swedish adults. Sleep Medicine, 8(7–8), 768–772. 10.1016/J.SLEEP.2006.11.015 [DOI] [PubMed] [Google Scholar]
- Vendrame, M. , Zarowski, M. , Loddenkemper, T. , Steinborn, B. , & Kothare, S. V. (2011). selective serotonin reuptake inhibitors and periodic limb movements of sleep. Pediatric Neurology, 45(3), 175–177. 10.1016/j.pediatrneurol.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Walters, A. S. , LeBrocq, C. , Dhar, A. , Hening, W. , Rosen, R. , Allen, R. P. , & Trenkwalder, C. (2003). Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Medicine, 4(2), 121–132. 10.1016/s1389-9457(02)00258-7 [DOI] [PubMed] [Google Scholar]
- Wetter, T. C. , Collado‐Seidel, V. , Pollmächer, T. , Yassouridis, A. , & Trenkwalder, C. (2000). Sleep and periodic leg movement patterns in drug‐free patients with Parkinson's disease and multiple system atrophy. Sleep, 23(3), 361–367. 10.1093/sleep/23.3.1c [DOI] [PubMed] [Google Scholar]
- Willis, T. (1672). De anima brutorum quae hominis vitalis ac sentitiva est: Exercitationes duae. Typis E.F. Impensis Ric. Davis, Oxon. [Google Scholar]
- Winkelman, J. W. (2006). Considering the causes of RLS. European Journal of Neurology, 13(Suppl 3(SUPPL. 2)), 8–14. 10.1111/J.1468-1331.2006.01586.X [DOI] [PubMed] [Google Scholar]
- Winkelmann, J. , Schormair, B. , Lichtner, P. , Ripke, S. , Xiong, L. , Jalilzadeh, S. , … Meitinger, T. (2007). Genome‐wide association study of restless legs syndrome identifies common variants in three genomic regions. Nature Genetics, 39(8), 1000–1006. 10.1038/ng2099 [DOI] [PubMed] [Google Scholar]
- Wittmaack, T. (1861). Pathologie und therapie der sensibilitat‐neurosen. E. Schafer. [Google Scholar]
- Yang, C. , White, D. P. , & Winkelman, J. W. (2005). Antidepressants and periodic leg movements of sleep. Biological Psychiatry, 58(6), 510–514. 10.1016/j.biopsych.2005.04.022 [DOI] [PubMed] [Google Scholar]
- Yepes, G. , Guitart, X. , Rea, W. , Newman, A. H. , Allen, R. P. , Earley, C. J. , … Ferré, S. (2017). Targeting hypersensitive corticostriatal terminals in restless legs syndrome. Annals of Neurology, 82(6), 951–960. 10.1002/ana.25104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz, K. , Kilincaslan, A. , Aydin, N. , & Kor, D. (2011). Prevalence and correlates of restless legs syndrome in adolescents. Developmental Medicine and Child Neurology, 53(1), 40–47. 10.1111/j.1469-8749.2010.03796.x [DOI] [PubMed] [Google Scholar]
- Zhuo, Y. , Wu, Y. , Xu, Y. , Lu, L. , Li, T. , Wang, X. , & Li, K. (2017). Combined resting state functional magnetic resonance imaging and diffusion tensor imaging study in patients with idiopathic restless legs syndrome. Sleep Medicine, 38, 96–103. 10.1016/J.SLEEP.2017.06.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supporting information
Data Availability Statement
Data sharing is not applicable ‐ no new data generated.


