AUTHOR CONTRIBUTIONS
P.H., M.T., and A.H. performed experiments. M.T. performed formal data analysis and visualization. S.H., P.A., M.R., J.T., M.K., and H.N. were involved in conceptualization. M.T. and H.N. wrote the manuscript. All authors reviewed and approved the manuscript.
CONFLICT OF INTEREST
M.T., P.A., M.R., and H.N. are shareholders and/or employees of LEO Pharma A/S. P.H. has received funding from LEO Pharma A/S. A.H. has received funding and acted as speaker for Laboratories Théa. S.H. has attended advisory boards for Sanofi‐Genzyme and received speaker honoraria from LEO Pharma, Sanofi‐Genzyme, Santen and Théa Pharmaceuticals. J.T. is an advisor for AbbVie, Almirall, Arena Pharmaceuticals, Coloplast, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly & Co, LEO Pharma, Pfizer, Regeneron, and Sanofi‐Genzyme, a speaker for AbbVie, Almirall, Eli Lilly & Co, LEO Pharma, Pfizer, Regeneron, and Sanofi‐Genzyme, and received research grants from Pfizer, Regeneron, and Sanofi‐Genzyme. M.K. is an advisor for AbbVie, Thea Pharmaceuticals and Santen, and received research grants from Thea Pharmaceuticals.
To the Editor,
Interleukin (IL)‐13 and IL‐4 are key cytokines in atopic dermatitis (AD) pathogenesis. Monoclonal antibodies inhibiting signaling of these type 2 cytokines have demonstrated clinical efficacy in moderate‐to‐severe AD patients. 1 Examples include dupilumab, which targets IL‐4Rα, inhibiting IL‐13 and IL‐4 signaling, and tralokinumab and lebrikizumab, which specifically neutralize IL‐13. These treatments have been associated with increased conjunctivitis and blepharitis in AD patients; manifestations that are regarded to be part of the AD syndrome. 2
Conjunctival goblet cell (CGC) scarcity, mucin deficiency, and immune cell infiltrates with increased numbers of Th1 cells secreting interferon‐gamma (IFN‐γ), have been reported in AD patients that developed conjunctivitis upon dupilumab treatment. 3 , 4 Inhibition of IL‐4 signaling by dupilumab may induce Th1 polarization with increased IFN‐γ production, leading to secretory dysfunction of mucins and triggering CGC apoptosis. 4 , 5
Mouse and rat CGC cultures are highly sensitive to immunomodulatory mediators, including IL‐13, IL‐4, and IFN‐γ, which directly impact cell proliferation and mucin secretion. 5 The effects of these cytokines on human CGCs are not well understood. We isolated primary CGCs from cultured conjunctiva from human donors, after ethical approval and informed consent (detailed in Data S1), and assessed cell proliferation by image‐based cell counting and mucin expression by qPCR in response to the aforementioned cytokines (Figure 1A). We confirmed that human CGCs expressed the relevant receptors for IL‐13, IL‐4, and IFN‐γ signaling (Figure 1B). Next, we assessed the cytokines’ effect on CGC proliferation using cells from three human donors. IL‐13 and IL‐4 promoted CGC cell proliferation comparably, whereas IFN‐γ had a strong negative impact on cell proliferation and viability (Figure 1C and Figure S1A). Dose‐ and time‐dependent trends were observed (Figure 1D). In murine models, IFN‐γ triggers the unfolded protein response (UPR) in CGCs. This pathway is associated with secretory dysfunction and CGC death, which may contribute to development of dry eye disease. 5 To investigate if this response was also activated by IFN‐γ in human CGCs, we assessed the expression of several UPR markers. We detected upregulation of UPR markers (DDIT3/CHOP, spliced XBP1, and HSPA5/GRP78) in human CGCs in response to IFN‐γ, but not IL‐13 or IL‐4 (Figure 2A and Figure S2A–E), indicating that IFN‐γ triggers the same cellular stress response in human CGCs as in murine models. Finally, our analysis revealed that IL‐13 and IL‐4 both lead to increased mRNA expression of two key ocular mucins, MUC2 and MUC5AC in human CGCs. MUC5AC deficiency in tear fluid has been observed in AD patients with dupilumab‐induced conjunctivitis. 6 Comparable to murine models, IFN‐γ also led to increased expression of MUC5AC. It has, however, been demonstrated that while IFN‐γ increases MUC5AC transcripts, IFN‐γ‐induced UPR signaling causes secretory dysfunction and inhibits MUC5AC secretion from CGCs. 5
FIGURE 1.
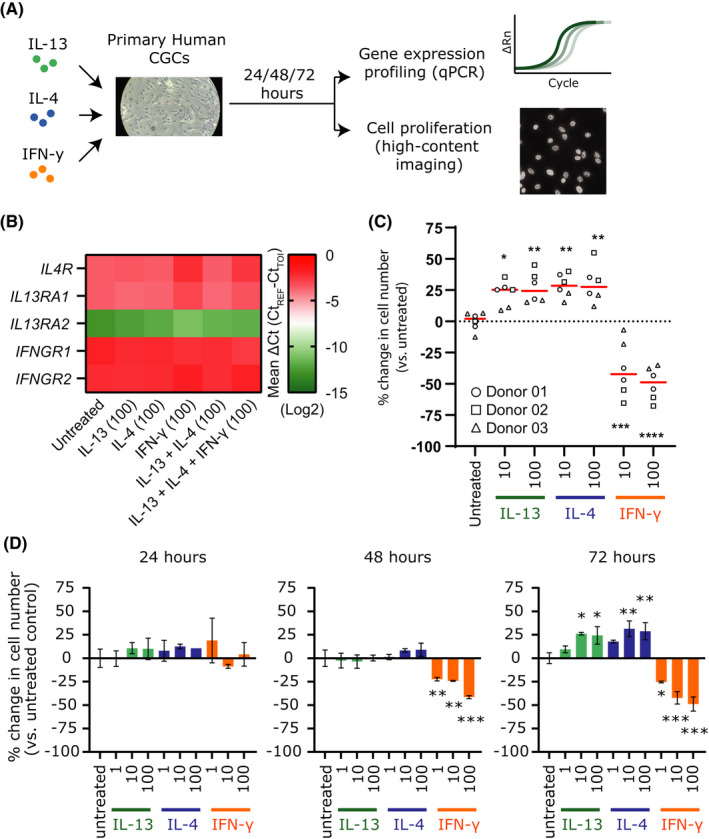
IL‐13 and IL‐4 promote conjunctival goblet cell proliferation. (A) Experimental design. (B) mRNA expression of interleukin (IL)‐13, IL‐4, and interferon‐gamma (IFN‐γ) signaling receptors in primary human conjunctival goblet cells (CGCs) measured by qPCR. (C) Change in number of CGCs from three donors after 72 h of incubation with indicated cytokines. Red bars indicate mean value per condition. (D) Proliferation of CGCs from donor #1 after 24, 48, and 72 h of incubation with indicated cytokines (mean ± SD). All concentrations shown are in ng/ml. *p < 0.05, **p < 0.01, ***p < 0.005, and ****p < 0.0001
FIGURE 2.
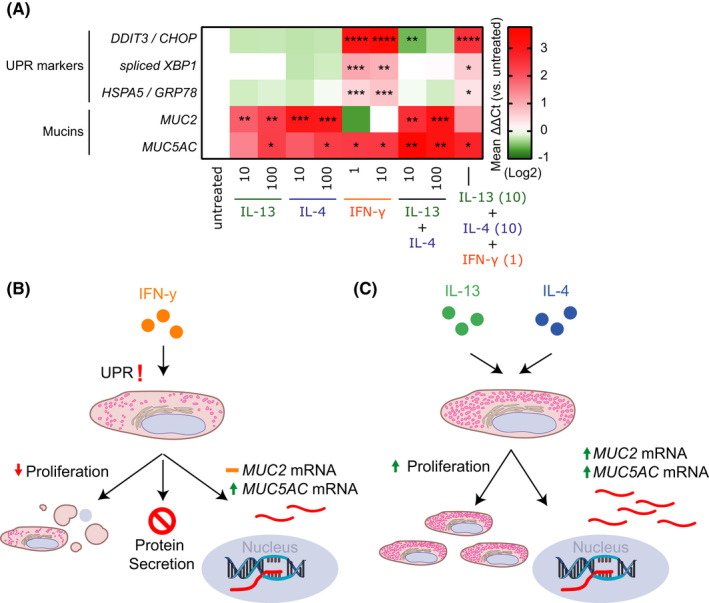
Expression of unfolded protein response and mucin genes in primary human conjunctival goblet cells. (A) mRNA expression of unfolded protein response (UPR) markers and mucins in primary human conjunctival goblet cells (CGC) upon incubation with the indicated cytokines. Change in gene expression is visualized as ΔΔCt value compared with untreated control. (B) Effects of interferon‐gamma (IFN‐γ) on primary human CGCs. (C) Functional redundant effects of IL‐13 and IL‐4 on primary human CGC proliferation and mucin mRNA expression. All concentrations shown are in ng/ml. *p < 0.05, **p < 0.01, and ***p < 0.005
While in vitro CGC cultures are appropriate to investigate molecular mechanisms, the model system in this study lacks the cellular complexity of the conjunctival tissue and associated immune cells. Also, cultures of isolated CGCs showed considerable variation in baseline expression of mucins and proliferative potential between donors. Nevertheless, our results show that IFN‐γ both reduces proliferation and viability of human CGCs and activates UPR signaling, which suggests a limitation in their capacity to produce and/or secrete mucins (Figure 2B). IL‐13 and IL‐4 on the other hand showed functional redundancy by both being able to stimulate proliferation and expression of MUC2 and MUC5AC mRNA in primary human CGCs (Figure 2C). As CGCs are essential for maintaining homeostasis of the conjunctival mucosal surface, our findings may in part provide a mechanistic explanation behind the ocular adverse events observed after treatment with biologics inhibiting IL‐4 and IL‐13 signaling. Due to the functional redundancy of IL‐13 and IL‐4, the data presented here might explain why targeted treatment neutralizing only IL‐13 is associated with a lower incidence and severity of conjunctivitis compared with inhibiting both IL‐13 and IL‐4 signaling. 7
Supporting information
Fig S1
Fig S2
Data S1
ACKNOWLEDGEMENTS
This study was sponsored by LEO Pharma A/S.
Pernille M. Hansen and Maxim A. X. Tollenaere contributed equally.
REFERENCES
- 1. Tubau C, Puig L. Therapeutic targeting of the IL‐13 pathway in skin inflammation. Expert Rev Clin Immunol. 2021;17(1):15‐25. [DOI] [PubMed] [Google Scholar]
- 2. Ravn NH, Ahmadzay ZF, Christensen TA, et al. Bidirectional association between atopic dermatitis, conjunctivitis, and other ocular surface diseases: a systematic review and meta‐analysis. J Am Acad Dermatol. 2021;85(2):453‐461. [DOI] [PubMed] [Google Scholar]
- 3. Bakker DS, Ariens L, Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180(5):1248‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakker DS, ter Linde JJM, Amini MM, et al. Conjunctival inflammation in dupilumab‐treated atopic dermatitis comprises a multicellular infiltrate with elevated T1/T17 cytokines: a case series study. Allergy. 2021;76(12):3814‐3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alam J, de Paiva CS, Pflugfelder SC. Immune ‐ goblet cell interaction in the conjunctiva. Ocul Surf. 2020;18(2):326‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnett BP, Afshari NA. Dupilumab‐associated mucin deficiency (DAMD). Transl Vis Sci Technol. 2020;9(3):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wollenberg A, Beck LA, de Bruin WM, et al. Conjunctivitis in adult patients with moderate‐to‐severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. 2021;186(3):453‐465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Data S1


