Summary
The intestinal microbiota is an important modulator of graft-versus-host disease (GVHD), which often complicates allogeneic hematopoietic stem cell transplantation (allo-HSCT). Broadspectrum antibiotics such as carbapenems increase the risk for intestinal GVHD, but mechanisms are not well-understood. In this study, we found that treatment with meropenem, a commonly used carbapenem, aggravates colonic GVHD in mice via expansion of Bacteroides thetaiotaomicron (BT). BT has a broad ability to degrade dietary polysaccharides and host mucin glycans. BT in meropenem-treated allogeneic mice demonstrated upregulated expression of enzymes involved in degradation of mucin glycans. These mice also had thinning of the colonic mucus layer and decreased levels of xylose in colon luminal contents. Interestingly, oral xylose supplementation significantly prevented thinning of the colonic mucus layer in meropenem-treated mice. Specific nutritional supplementation strategies including xylose supplementation may combat antibioticmediated microbiome injury to reduce the risk for intestinal GVHD in allo-HSCT patients.
Keywords: Mucus-degrading bacteria, Bacteroides, Bacteroides thetaiotaomicron, allogeneic hematopoietic stem cell transplantation, graft-versus-host disease, broad-spectrum antibiotics, carbapenem, xylose, mucus layer, intestinal microbiome
Graphical Abstract
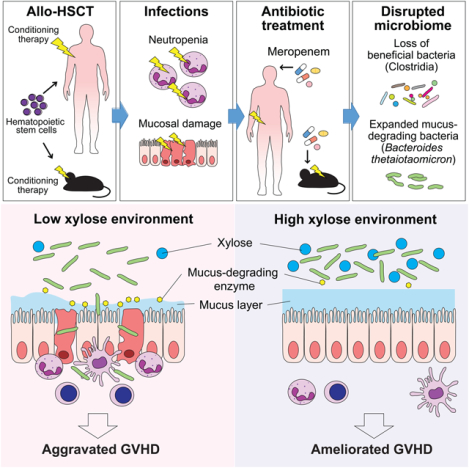
Antibiotic usage increases the risk for graft-versus-host disease (GVHD), a complication of hematopoietic stem cell transplantation, through the expansion of the colonic mucus-degrading gut microbe Bacteroides thetaiotaomicron. Supplementation oral xylose reduces thinning of the colonic mucus layer in mice and may be an option to reduce GVHD.
Introduction
The intestinal microbiota can interact with the host immune system (Hooper et al., 2012), including in the setting of allogeneic hematopoietic stem cell transplantation (allo-HSCT). Patients undergoing allo-HSCT are at risk for graft-versus-host disease (GVHD), a life-threatening inflammatory process where allogeneic donor T cells recognize recipient tissues as foreign. The composition of the intestinal microbiome has been an important modulator of GVHD (Jenq et al., 2012). In the lower intestinal tract, commensal microbes are dependent on diet- and host-derived metabolic substrates (Ley et al., 2008). They then participate in the digestion and also modulate both local and systemic immunity. Broad-spectrum antibiotics are often used in this patient population to treat infections. They have been found, however, to increase the risk for intestinal GVHD, possibly via bystander depletion of beneficial commensal bacteria. The gastrointestinal tract has been identified as a primary target of allogeneic donor T cells in allo-HSCT, and intestinal GVHD often serves to amplify systemic inflammation (Hill and Ferrara, 2000). Indeed, intestinal microbiome injury following allo-HSCT is consistently and reproducibly associated with GVHDrelated mortality and reduced overall survival (Jenq et al., 2012; Taur et al., 2014).
In allo-HSCT, major microbiome shifts are seen in association with antibiotic administration (Holler et al., 2014). Certain classes of antibiotics including carbapenems are particularly associated with increased intestinal GVHD (Elgarten et al., 2021; Hidaka et al., 2018; Lee et al., 2019; Shono et al., 2016). The intestinal microbiota, especially Clostridia, serve an important function in maintaining intestinal homeostasis (Atarashi et al., 2011; Mathewson et al., 2016). Whether loss of Clostridia during carbapenem therapy is mechanistically sufficient to aggravate intestinal GVHD is not known. In this study, we aimed to examine the effects of meropenem, a commonly used carbapenem in allo-HSCT patients, on mice undergoing allo-HSCT. We found that meropenem treatment aggravated GVHD primarily in the colon, and that a prominent effect on the colonic microbiota was expansion of Bacteroides thetaiotaomicron (BT). BT is a gram-negative obligate anaerobe with a versatile range of metabolic substrates, including dietary fiber polysaccharides and host-derived mucus-O-glycans. Meropenem-treated mice developed a thinned colonic mucus layer and bacterial translocation into mesenteric lymph nodes (MLNs). We found that expression of mucolytic enzymes by BT was increased in meropenemtreated mice, and that the colonic lumen in these mice had altered levels of carbohydrates, including xylose. Supplementation of meropenem-treated mice with xylose resulted in a significantly thicker mucus layer without changing BT abundance. Use of broad-spectrum antibiotics are important for treating infections in allo-HSCT patients but can lead to expansion and altered behavior of commensal bacteria, which in turn can contribute to increased severity of GVHD.
Results
Meropenem treatment is associated with increased intestinal GVHD
At our center, institutional guidelines recommend first-line therapy for neutropenic fever with cefepime, an antibiotic that is relatively sparing of commensal anaerobes, while second-line therapy is with meropenem, which is highly active against commensal anaerobes. We asked whether the use of meropenem was associated with a difference in incidence of intestinal GVHD. We retrospectively examined 295 patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) who underwent allo-HSCT with tacrolimus and methotrexate as GVHD prophylaxis following conditioning therapy with fludarabine and busulfan at our institution from 2011 to 2016. We evaluated the incidence of acute intestinal GVHD until day 100 after transplant and compared patients who received neither cefepime nor meropenem, those who received cefepime alone, those who received meropenem alone, and those who received both cefepime and meropenem during the period from days -10 to 30 relative to allo-HSCT. A summary of clinical characteristics is provided in Table S1; there were no significant differences between each group. Interestingly, the incidence of acute intestinal GVHD was significantly higher in patients who received either meropenem alone or both cefepime and meropenem, while the acute intestinal GVHD incidence in patients receiving only cefepime was similar to that of patients who received neither antibiotic (Figure 1A). These results indicated that meropenem exposure in allo-HSCT patients is associated with an increased incidence of acute intestinal GVHD.
Figure 1. Meropenem increased the incidence of intestinal GVHD in allo-HSCT patients and mice.
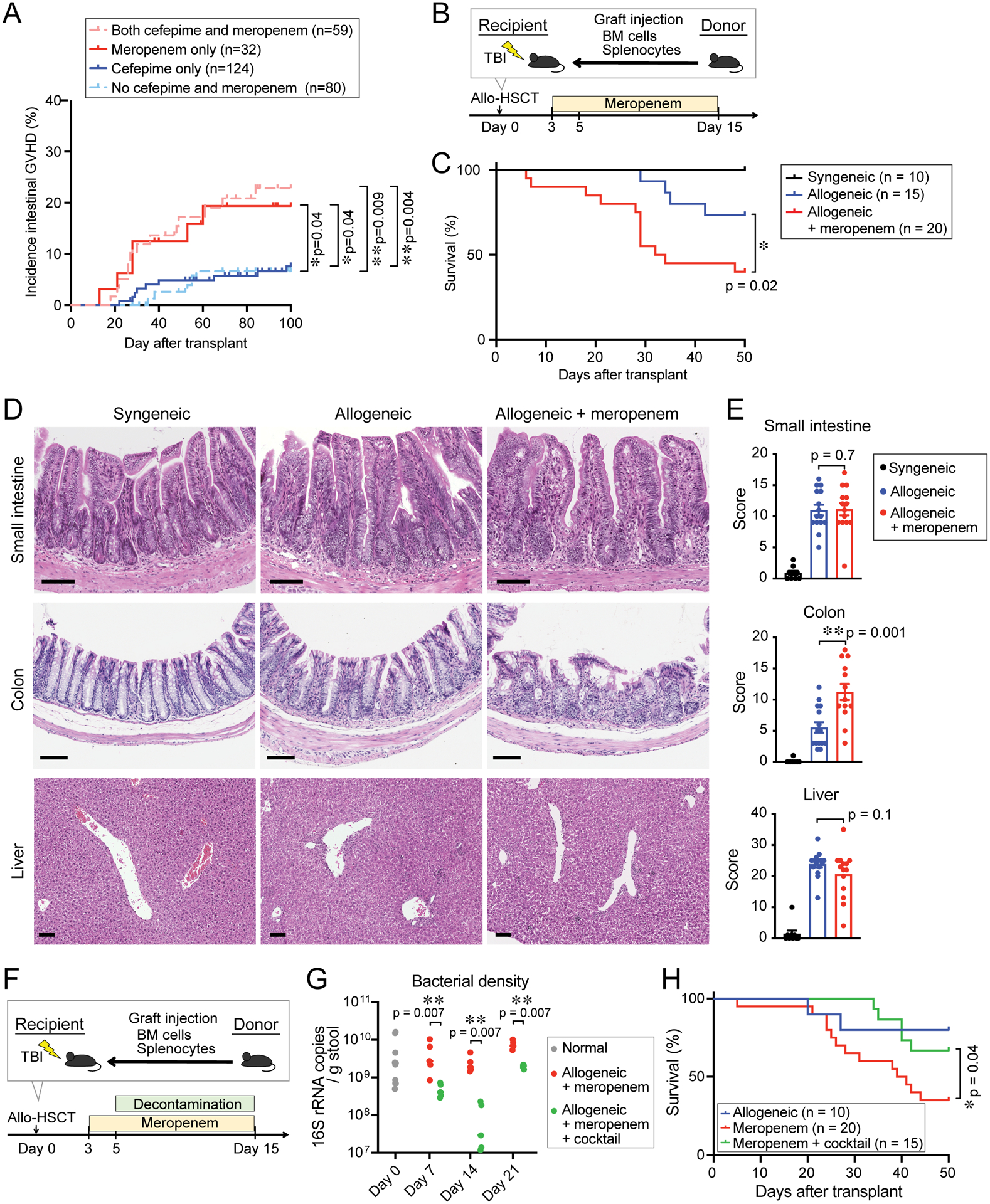
(A) Incidence of intestinal GVHD in 295 allo-HSCT patients. Patients were stratified by antibiotic treatment from days -10 to 30 relative to allo-HSCT. (B) Experimental schema of murine GVHD model. Mice were treated with meropenem from days 3 to 15. TBI, total body irradiation. (C) Overall survival. Data are combined from three independent experiments. (D) H&E staining of histological sections of GVHD target organs collected on day 18. Bar, 100 μm. (E) GVHD histology scores of GVHD target organs harvested on day 18. All GVHD histology scores were quantified by a blinded pathologist. Combined data from two independent experiments are shown as means ± SEM. (F) Experimental schema of murine GVHD model. Mice were decontaminated with piperacillin/tazobactam plus nystatin from days 5 to 15. (G) Bacterial density of fecal samples collected from mice treated as indicated. Data are shown from one representative experiment. (H) Overall survival. Data are combined from three independent experiments.
To further explore a potential relationship between meropenem and intestinal GVHD, we examined the effects of meropenem on GVHD in a mouse model of allo-HSCT. We began with developing a feasible method of administering meropenem to mice that would better mimic the effects of meropenem on the intestinal microbiome of patients. We quantified meropenem concentrations in the cecal lumen of mice 4, 8, 24, 48 and 96 hours after subcutaneous injection. Subcutaneous injection produced an increase in meropenem concentrations in the cecum at 4 hours after injection, which rapidly declined afterwards (Figure S1A), indicating that daily or even twice daily dosing in mice was unlikely to produce stable intestinal luminal concentrations of meropenem in mice. We then evaluated drinking water as a means of continuously administering meropenem to mice, using bacterial density quantified by 16S ribosomal RNA (16S rRNA) gene quantitative PCR (qPCR) to gauge reductions of the intestinal microbiota. We selected a concentration of 0.625 g/L of meropenem, which produced a detectable meropenem concentration in fecal samples (median ± SEM, 0.11 ± 0.07 μg/g stool) and a substantial one-log reduction in fecal bacterial density, as an experimental drinking water dose (Figure S1B).
Lethally irradiated B6D2F1 (H-2b/d) mice were intravenously injected with 5×106 bone marrow (BM) cells and 5×106 splenocytes from major histocompatibility complex (MHC)mismatched B6 (H-2b) or syngeneic donors on day 0. Throughout this manuscript, we refer to mice at high-risk for GVHD after receiving allogeneic donor-derived cells as “allogeneic mice,” whereas mice at no risk for GVHD after receiving syngeneic donor-derived cells are referred to as “syngeneic mice.” Meropenem was administered to allogeneic mice in the drinking water from days 3 to 15 relative to allo-HSCT infusion (Figure 1B). We found that mice treated with meropenem after allo-HSCT had significantly worsened survival (Figure 1C), with severe epithelial damage in the colon (Figure 1D) and significantly higher GVHD histological scores in the colon compared to control allogeneic mice (Figure 1E). In contrast, GVHD histology in the small intestine and liver were not substantially different in allogeneic mice treated with meropenem (Figure 1D and 1E). This indicated that meropenem primarily aggravated colonic GVHD.
Studies in the pediatric allo-HSCT population and in mice have found that intestinal Clostridia are associated with protection from GVHD (Simms-Waldrip et al., 2017). Corroborating this, Clostridia are known to be producers of short-chain fatty acids (SCFAs), including butyrate, which plays a role in maintaining epithelial integrity in murine GVHD (Mathewson et al., 2016). We asked if meropenem-treated allogeneic mice showed a loss of Clostridia abundance and a reduction in SCFA levels. We found that on day 7, or 4 days following the start of meropenem treatment, Clostridia were indeed depleted and remained so at 6 days after stopping meropenem (Figure S1C). These mice also had reduced fecal levels of SCFAs, with particularly dramatic reductions in butyrate and valerate (Figure S1D).
We also evaluated the effects of other antibiotics on colonic GVHD. Levofloxacin, cefepime, or meropenem was administered to allogeneic mice in the drinking water from days 3 to 15 relative to allo-HSCT infusion (Figure S2A). We found that allogeneic mice treated with levofloxacin or cefepime experienced colonic GVHD that was similar in severity to control allogeneic mice (Figure S2B and S2C). Consistent with this, we found that allogeneic mice treated with levofloxacin or cefepime did not show substantially reduced fecal levels of SCFAs, including in particular butyrate and valerate (Figure S2D).
To determine whether loss of Clostridia during meropenem treatment is mechanistically sufficient to aggravate intestinal GVHD, we examined the effects of intestinal microbiome decontamination in meropenem-treated allogeneic mice. To do so, we administered, in addition to meropenem, an oral cocktail of piperacillin/tazobactam and nystatin in the drinking water from days 5 to 15 after transplantation (Figure 1F), similar to a regimen given to pediatric allo-HSCT patients (Bekker et al., 2019). Bacterial density was significantly reduced by this cocktail (Figure 1G). Interestingly, we observed that intestinal decontamination improved survival in meropenemtreated allogeneic mice (Figure 1H), suggesting that meropenem treatment led to worsened GVHD not only due to depletion of beneficial bacteria, but also via expansion of pro-inflammatory bacteria.
Meropenem treatment results in loss of Clostridia and expansion of Bacteroides
We then sought to characterize the effects of meropenem on the composition of the colonic microbiota of allo-HSCT patients and mice. We collected fecal specimens from 44 patients who were treated with allo-HSCT following conditioning with fludarabine and busulfan at our center from 2014 to 2019. Of these patients, 26 did not receive and 18 did receive meropenem treatment between days -10 to 14. Among meropenem-untreated patients, all patients were treated with cefepime, except for one treated with ceftazidime and two patients treated with levofloxacin alone. A comparison of clinical characteristics demonstrated no significant differences between these groups (Table S2). We examined fecal samples collected at baseline following hospital admission around day -7 as well as on day 14 following allo-HSCT (Figure 2A). Using PERMANOVA testing of weighted UniFrac beta diversity measures, we found that at baseline, meropenemuntreated and treated patients were not significantly different, nor were they different on day 14 (Figure S3A and S3B), likely reflecting a high degree of individual patient intestinal microbiota heterogeneity which has been seen previously in this population and has been attributed to prior antibiotic treatments as well as variable nutrition (Peled et al., 2020). To better characterize microbiome effects of antibiotics in our relatively small and heterogeneous cohort, we performed a paired differential abundance analysis, asking which bacterial genera were the most changed from baseline in the two subgroups of patients. This approach demonstrated that patients treated with meropenem showed significant expansion of bacteria from the genus Bacteroides (Figure 2AC and S3C). In contrast, patients not treated with meropenem showed expansion of the genus Enterococcus, a genus belonging to Erysipelotrichaceae, and the genus UBA1819, but not Bacteroides (Figure 2A, 2C, 2E and S3C).
Figure 2. The effects of meropenem treatment on the composition of the intestinal microbiome in both patients and mice are characterized by expansion of Bacteroides.
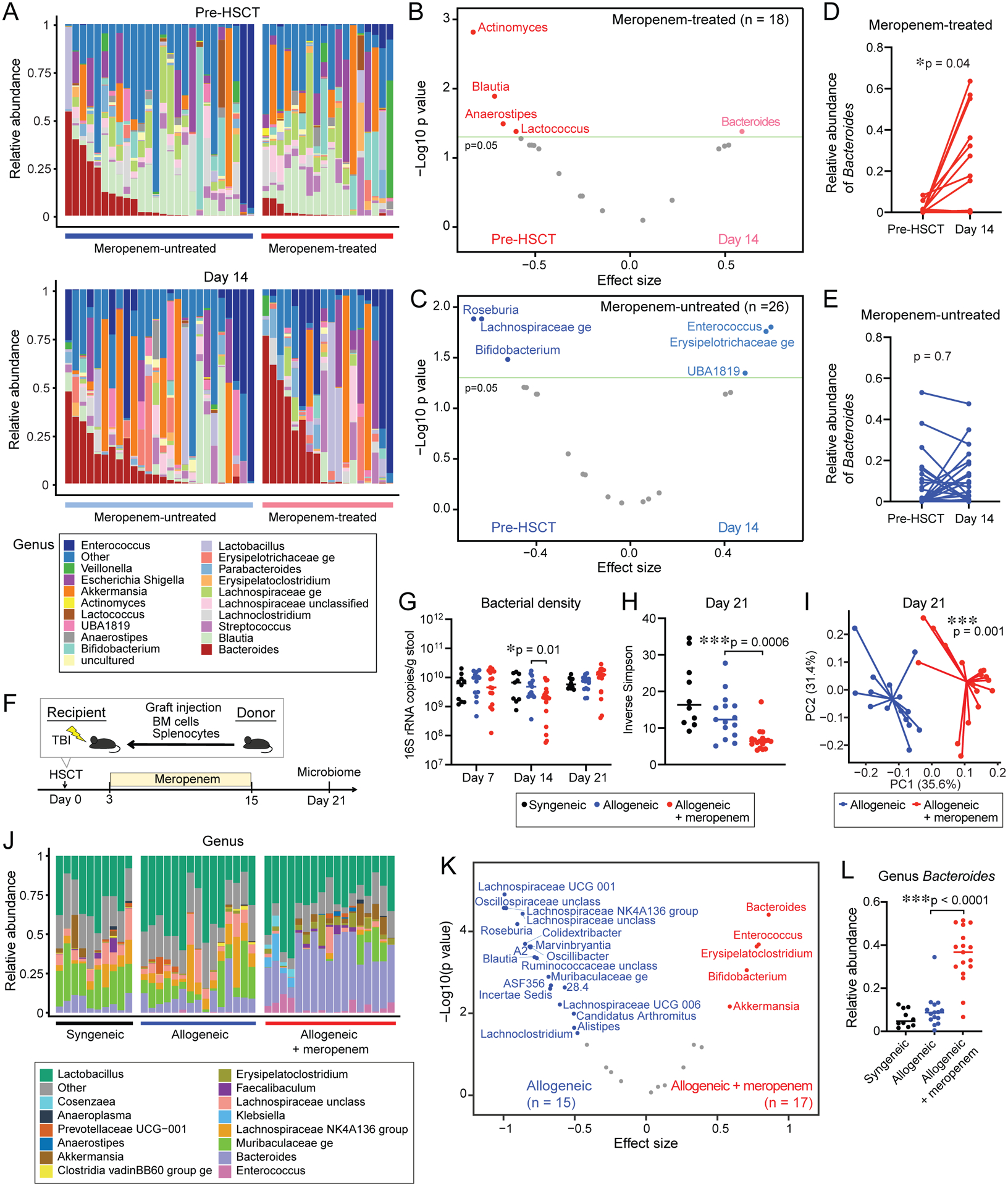
(A - C) Composition of fecal samples from 44 allo-HSCT patients collected pre-HSCT and on day 14. (A) Bacterial genera composition of fecal samples collected pre-HSCT (top) and on day 14 (bottom). (B - C) Differentially abundant bacterial genera comparing pre-HSCT and day 14 samples collected from (B) meropenem-treated or (C) meropenem-untreated patients, analyzed by the paired-Wilcoxon test and adjusted for false discovery. (D - E) Paired-Wilcoxon test of the genus Bacteroides between at pre-HSCT and on day 14 in (D) meropenem-treated or (E) meropenem-untreated patients. (F) Experimental schema of murine GVHD model. The composition of the intestinal microbiome of fecal samples collected from day 21 was evaluated. (G) Bacterial density was measured using qPCR of 16S rRNA. (H) Alpha diversity, measured by the inverse Simpson index, was quantified in fecal samples. (I) Principle coordinate analysis (PCoA) of fecal samples. (J) Bacterial genera composition of fecal samples. (K) Differentially abundant bacterial genera comparing fecal samples. (L) Relative abundance of Bacteroides in fecal samples. (G - L) Data are combined from three independent experiments.
We next asked if meropenem treatment had similar effects on the composition of the intestinal microbiota of allogeneic mice (Figure 2F). Using 16S rRNA gene qPCR, we quantified fecal bacterial densities and found that meropenem-treated allogeneic mice showed significantly decreased bacterial density on day 14 during meropenem treatment, but by day 21, or 6 days after stopping meropenem, fecal bacterial densities had recovered to a level similar to that of untreated syngeneic and allogeneic mice (Figure 2G). Most allogeneic mice treated with meropenem began to succumb to aggravated GVHD approximately 3 weeks after allo-HSCT (Figure 1C), and so we characterized the fecal microbiome on day 21 and found that alpha diversity, quantified using the inverse Simpson index, was significantly reduced in meropenem-treated mice (Figure 2H). PERMANOVA testing demonstrated significant compositional differences between meropenemuntreated and treated allogeneic mice (Figure 2I). We found that meropenem treatment led to significantly higher abundances of bacteria from several genera, including most substantially Bacteroides, as well as Enterococcus, Erysipelatoclostridium, Bifidobacterium and Akkermansia (Figure 2J, 2K and 2L). Simultaneously, many genera were depleted, including Blautia, Lachnoclostridium, and other members of Lachnospiraceae, which belong to the class Clostridia (Figure 2J and 2K).
Bacteroides thetaiotaomicron contributes to meropenem-exacerbated colonic GVHD
We then examined effects of meropenem treatment on Bacteroides subsets, which is achievable to a degree when sequencing the V4 region of the 16S rRNA gene (Jovel et al., 2016). We identified a single Bacteroides sequence variant that was significantly expanded in meropenem-treated mice and found it had 100% identity with the 16S sequences of BT, Bacteroides faecis, and Bacteroides faecichinchillae while other Bacteroides strains had 98.8% identity or less (Figure 3A). We isolated the predominant murine Bacteroides isolate and confirmed by whole genome sequencing that it was a strain of BT, with 97.4% genomic identity to the ATCC type strain of BT (ATCC 29148), and only 89.2% and 80.8% genomic identity to Bacteroides faecis and Bacteroides faecichinchillae, respectively. We have named this isolate MDA-JAX BT001, or murine BT. Interestingly, murine BT was not eliminated on days 7 and 14 by meropenem-supplemented drinking water and quickly expanded after cessation of meropenem therapy in most meropenem-treated mice, in contrast to Clostridia which consistently remained depleted (Figure 3B and S1C). These findings suggested that murine BT was less sensitive to meropenem than Clostridia.
Figure 3. Murine BT associated with meropenem-induced colonic GVHD.
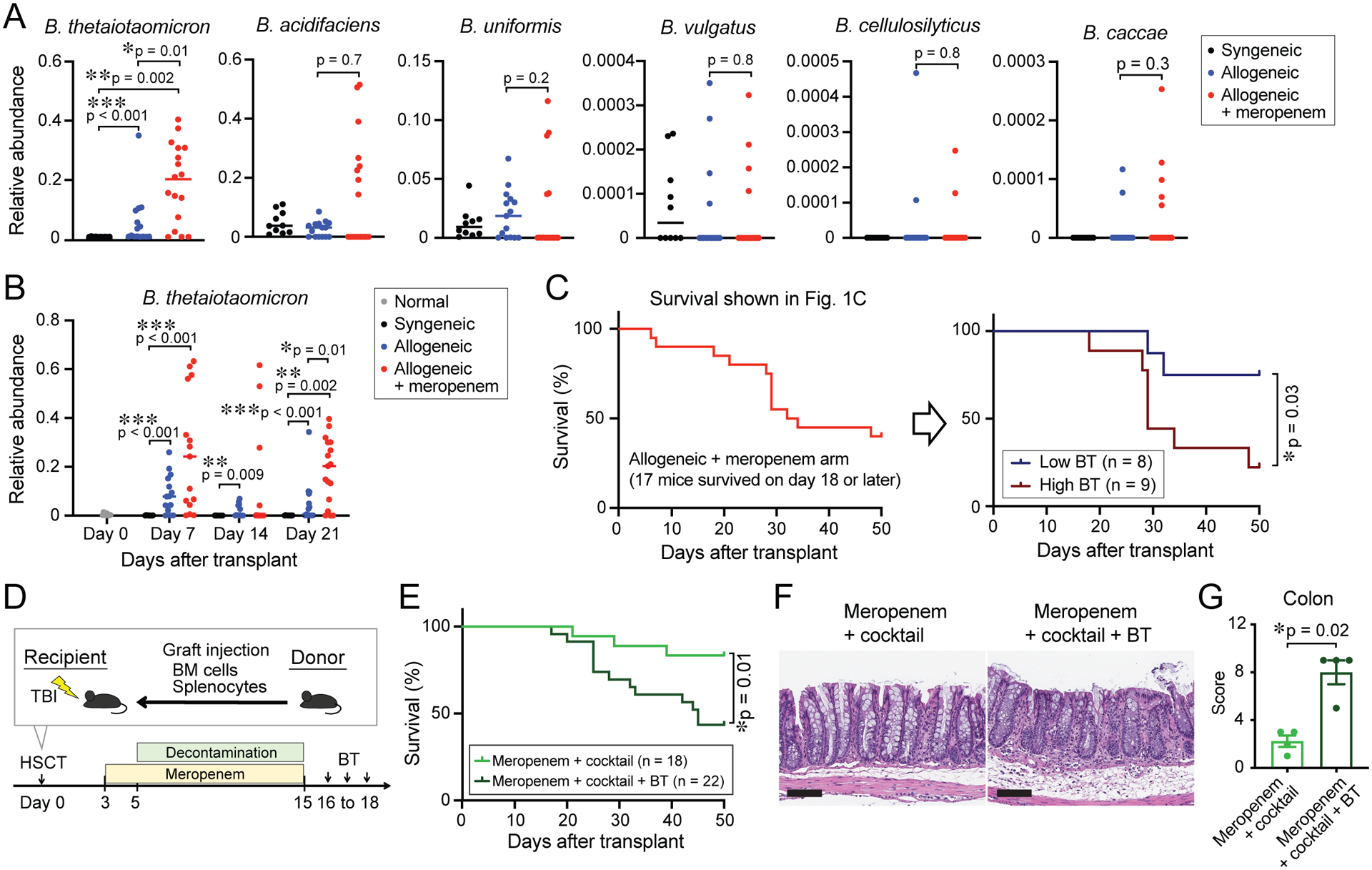
(A) Relative abundance of distinguishable Bacteroides sequence variants on day 21. (B) Longitudinal relative abundance of BT. (C) Meropenem-treated mice (Figure 1C) were stratified by median abundance of BT into a high abundant BT arm (relative abundance > 0.2) and low abundant BT arm (relative abundance < 0.2). (D) Experimental schema of murine GVHD model using decontamination therapy followed by oral introduction of BT. Mice were decontaminated as in Figure 1F followed by oral gavage of 2×107 CFUs of BT (MDA-JAX BT001) daily for 3 days. (E) Overall survival. (A - C, and E) Data are combined from three independent experiments. (F) H&E staining of histological sections of the colon collected on day 28. Bar, 100 μm. (G) GVHD histology scores of the colon collected on day 28. Data from one representative experiment are shown.
We evaluated this hypothesis by quantifying the minimum inhibitory concentration (MIC) of meropenem against several bacterial isolates (Table S3). Both mouse- and human-derived (ATCC 29148) BT strains showed only moderate sensitivity to meropenem with MICs of 4 μg/mL and 6 μg/mL, respectively. Mouse-derived Enterococcus faecalis (MDA-JAX EF001) was more resistant with an MIC of 12 μg/ml, while MICs of mouse-derived Clostridium disporicum (MDAJAX CD001), Clostridium saudiense (MDA-JAX CS001), and Lachnospiraceae unclassified (MDA-JAX LS001), which belong to the class Clostridia, had MICs of 0.094 μg/mL, 0.38 μg/mL, and 0.38 μg/mL, respectively, showing high sensitivity. Stool collected 48 hours after starting meropenem treatment in normal specific-pathogen-free (SPF) mice had a meropenem concentration of 0.11 ± 0.07 μg/g. These data suggested that BT can survive during meropenem treatment and then can have a selective advantage after discontinuation of meropenem.
We then asked if loss of Clostridia and increased abundance of BT could be seen following treatment with other antibiotics. We administered levofloxacin, cefepime or meropenem to mice with GVHD as in Figure S2A. Levofloxacin-treated mice had significantly higher abundances of Clostridia compared to meropenem-treated mice (Figure S4A and S4B) and this difference was consistent with fecal butyrate levels (Figure S2D). Additionally, both levofloxacin- and cefepimetreated mice had significantly lower abundances of BT compared to meropenem-treated mice (Figure S4A and S4C). These results indicated that meropenem is more disruptive of the microbiota, specifically with respect to loss of Clostridia and increased abundance of BT.
To further evaluate for an association between murine BT and aggravated GVHD in meropenem-treated allogeneic mice, we retrospectively stratified mice from 3 experiments shown in Figure 1C by their median relative abundance of BT. A comparison of these two cohorts showed that mice with higher abundances of BT had worsened overall survival (Figure 3C). Next, we experimentally studied the effects of murine BT on GVHD severity. In meropenem-treated allogeneic mice that had completed treatment with a decontamination cocktail, we orally inoculated 2×107 colony-forming units (CFUs) of murine BT and monitored GVHD severity and survival (Figure 3D). Strikingly, we found that allogeneic mice administered murine BT showed worsened survival (Figure 3E), with severe epithelial damage and significantly higher GVHD histological scores in the colon compared to those without inoculation of murine BT (Figure 3F and 3G), indicating that murine BT was sufficient to aggravate GVHD in allogeneic mice that had been previously decontaminated.
Meropenem treatment results in increased localization of BT to the intestinal mucosa
The composition of the intestinal microbiota is known to vary spatially, including longitudinally from proximal to distal, as well as radially from the luminal center to the mucosal surface. The mucosal surface under normal conditions is enriched with bacteria from the phylum Firmicutes including Clostridia, and other intestinal bacteria are relatively excluded, including those belonging to Bacteroidaceae, Enterococcaceae and Lactobacillaceae, which are enriched in the luminal contents (Nava and Stappenbeck, 2011). We asked how meropenem treatment can impact on the radial composition of the microbiota. We collected paired mucosal and fecal pellet samples and evaluated the bacterial composition as previously reported (Donaldson et al., 2020) (Figure 4A). In normal mice, beta diversity analysis identified significant compositional differences between luminal and mucosal samples, and the Bacteroidia-to-Clostridia ratio was significantly higher in luminal samples than mucosal samples (Figure 4B and 4C). This indicated that Clostridia are enriched in the mucosal surface while Bacteroidia including BT are enriched luminal contents at the steady state. Interestingly, we found significant compositional differences between luminal and mucosal samples in allogeneic mice (PERMANOVA testing, p = 0.02; coefficient in the linear model, R2 = 0.30), whereas meropenem-treated allogeneic mice showed loss of this distinction (PERMANOVA testing, p = 0.4; coefficient in the linear model, R2 = 0.13) (Figure 4D). We also evaluated beta diversity distances between paired luminal and mucosal samples collected from individual mice and found that these were reduced in allogeneic mice treated with meropenem, corroborating our other analyses (Figure 4E). Finally, samples from allogeneic mice showed a significant difference in the Bacteroidia-to-Clostridia ratio when comparing luminal samples with mucosal samples, whereas meropenem-treated allogeneic mice had lost this difference (Figure 4F). We also evaluated effects on radial compositional differences with other antibiotics. Cefepime-treated allogeneic mice showed loss of compositional differences between luminal and mucosal samples (PERMANOVA testing, p = 0.7; coefficient in the linear model, R2 =0.05) similar to that seen in meropenem-treated allogeneic mice, whereas levofloxacintreated mice showed less disruption (PERMANOVA testing, p = 0.1; coefficient in the linear model, R2 =0.17) (Figure S4D and S4E). Together, these data indicated that in allogeneic mice, the normal enrichment of Clostridia at the mucosal surface was preserved. Meropenem treatment, however, led to disruption of this enrichment, with high relative abundances of Bacteroides seen in mucosal samples. These results are consistent with the possibility that Clostridia function to exclude Bacteroidia from the mucosal surface, and that in the setting of reduced Clostridia secondary to eradication by meropenem treatment, Bacteroidia are then able to opportunistically colonize the mucosal surface.
Figure 4. Meropenem treatment results in increased localization of BT to the colonic mucosa in allogeneic mice.
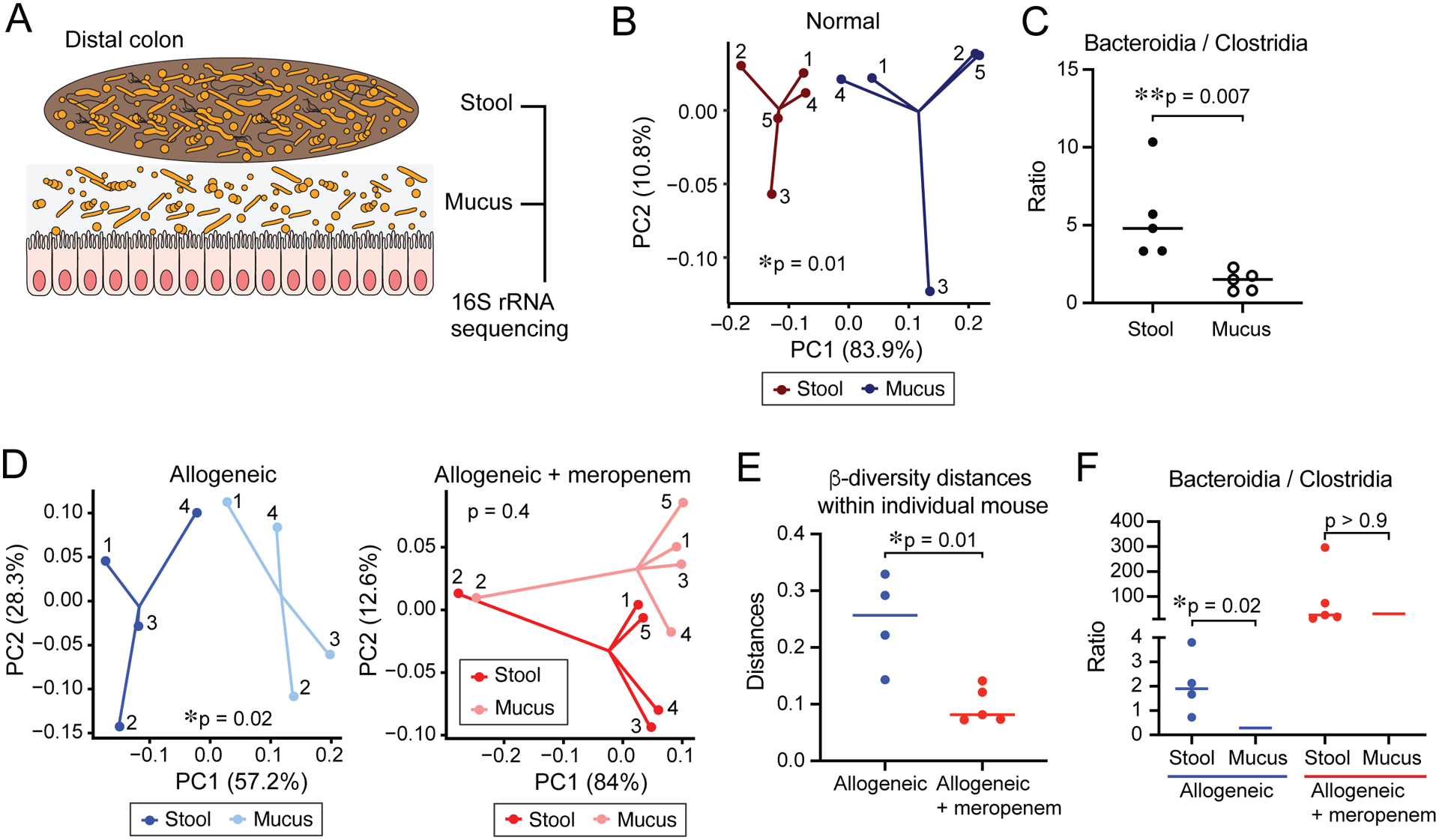
(A) Experimental schema to analyzed paired fecal and mucosal microbiome by 16S rRNA sequencing. Paired stool and mucus were collected in distal colon. (B) PCoA of stool and mucus in distal colon from normal B6D2F1 mice. (C) Ratio of relative abundance of Bacteroidia/Clostridia in stool and mucus from normal B6D2F1 mice. (D) PCoA of stool and mucus in distal colon from allogeneic mice (left) and meropenem-treated allogeneic mice (right). Numbers shown in each PCoA depict samples collected from the same individual mouse. (E) Beta diversity distances within individual mouse. (F) Ratio of relative abundance of Bacteroidia/Clostridia in stool and mucus from allogeneic mice treated or untreated with meropenem. (B - F) Data from one representative experiment are shown.
Meropenem treatment induces thinning of the colonic mucus layer and impairment of epithelial barrier integrity
BT is a gram-negative obligate anaerobe with a broad ability to degrade dietary polysaccharides as well as host-derived glycans, including mucins (Bergstrom and Xia, 2013; Tailford et al., 2015). We hypothesized that meropenem treatment during GVHD could be compromising the colonic mucus layer. We first evaluated the ability of our murine BT isolate (MDA-JAX BT001) to utilize mucin as a carbohydrate source, as well as a human-derived BT strain (ATCC 29148) and, as a comparison, non-mucolytic mouse-derived Enterococcus faecalis (MDA-JAX EF001). We found that supplementation of carbohydrate-poor media with porcine gastric mucin resulted in augmented growth of both BT strains, in contrast to no growth benefit for murine E. faecalis (Figure S5A), indicating that mucin-utilization enzymes were expressed by both BT strains. We also quantified degradation of mucin-derived carbohydrates using a PASbased colorimetric assay and confirmed that both BT strains displayed degradation of mucinderived carbohydrates (Figure S5B). We then asked if meropenem treatment impacted on the dense colonic mucus layer of allogeneic mice. PAS staining of histological sections demonstrated a significantly thinned colonic mucus layer in meropenem-treated allogeneic mice compared to untreated allogeneic mice and syngeneic mice (Figure 5A and 5B). Decontamination, in contrast, led to preservation of this mucus layer in meropenem-treated allogeneic mice, suggesting that meropenem was leading to increased mucus degradation by colonic bacteria (Figure 5A and 5B). The mucus layer provides a critical contribution to the intestinal barrier by excluding bacteria from the intestinal lumen adjacent to the intestinal epithelium. We stained our histological sections with 16S fluorescence in situ hybridization (FISH) probes and visualized dissemination of bacteria into the mucus layer and lamina propria of the colon in meropenem-treated allogeneic mice (Figure 5C). To better quantify effects of meropenem on bacterial translocation, we cultivated MLNs microbiologically and found higher bacterial loads in meropenem-treated allogeneic mice. We found that translocating bacteria included BT (Figure 5D and 5E). Bacterial translocation has previously been found to aggravate GVHD via at least two mechanisms, including recruitment of neutrophils, which can compound tissue damage, as well as by enhancing antigen presentation by dendritic cells through activation of pathogen-associated molecular pattern signaling pathways (Koyama et al., 2015; Schwab et al., 2014). We thus asked if this barrier compromise led to an inflammatory response in meropenem-treated mice and indeed observed marked colonic tissue infiltration by neutrophils and dendritic cells (Figure 5F and 5G). Altogether, these findings indicated that meropenem treatment led to compromise of the colonic mucus layer, increased bacterial translocation and an aggravated inflammatory response.
Figure 5. Meropenem-induced compromise of the colonic mucus layer in allogeneic mice.
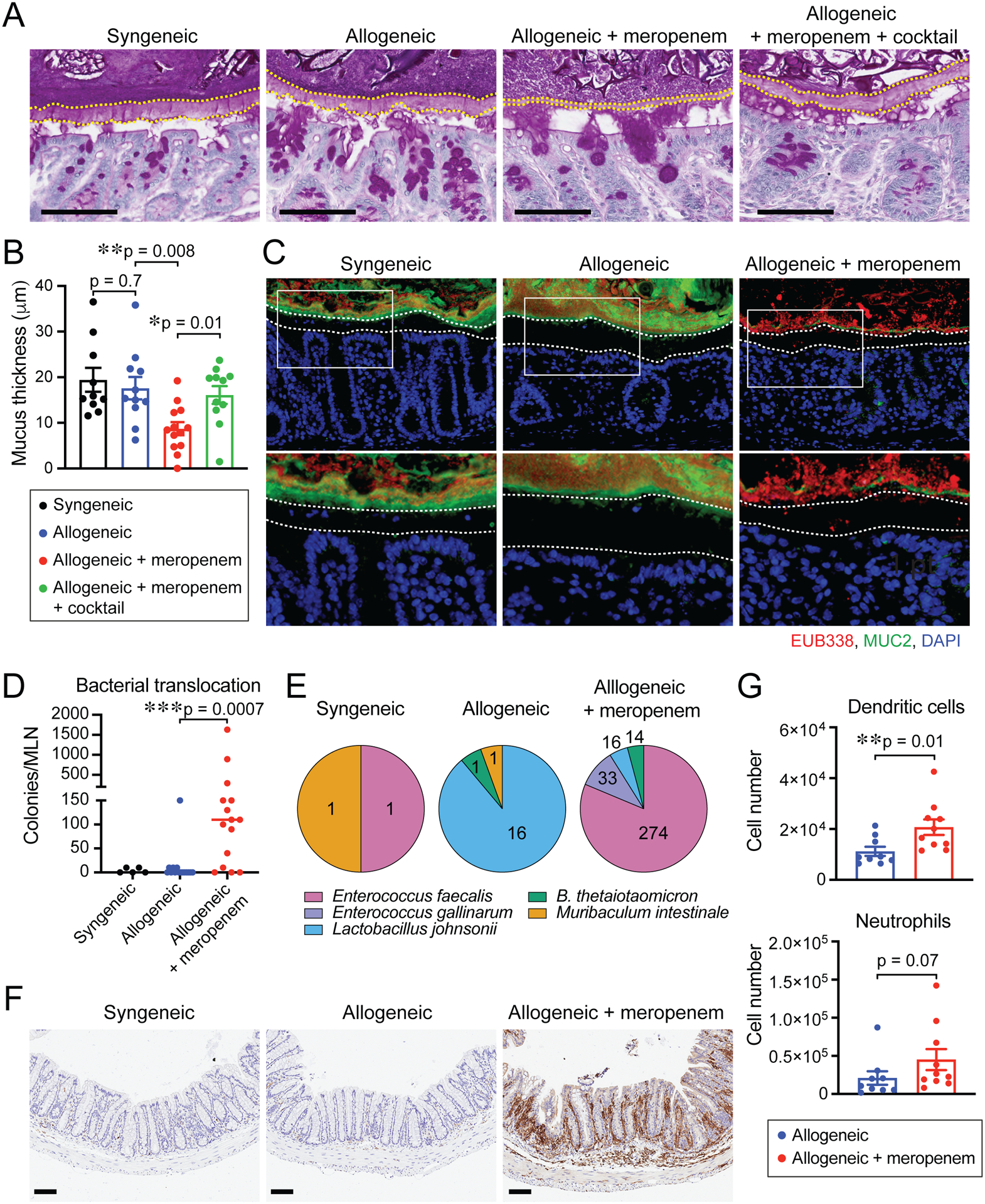
(A) PAS staining of histological colon sections collected on day 18. Bar, 100 μm. The areas inside dotted lines indicate the inner dense colonic mucus layer. (B) Mucus thickness on day 18. Combined data from two independent experiments are shown as means ± SEM. (C) Immunofluorescent staining of colon sections for MUC2 (green) with universal bacterial 16S rRNA gene in situ hybridization probe EUB338 (red) counterstained with DAPI. Bar, 100 μm. Arrowheads indicate infiltrating bacteria. Areas in white squares are magnified and shown below original images. Areas inside dotted lines indicate the inner dense colonic mucus layer. (D) Numbers of bacterial CFUs cultivated from MLNs on day 18. Combined data from three independent experiments are shown. (E) Identified translocated bacteria in MLNs by MALDI Biotyper. Number indicates bacterial CFUs. (F) Immunohistochemistry staining of CD11b in histological colon sections. Bar, 100 μm. (G) Numbers of dendritic cells and neutrophils in the colon were determined by flow cytometric analysis. Combined data from two independent experiments are shown as means ± SEM.
Meropenem treatment upregulates in vivo expression of mucus-degrading enzymes by BT
While meropenem treatment led to higher abundances of BT in allogeneic mice, we also found that GVHD itself, without meropenem treatment, also resulted in moderate increases in abundances of BT compared to syngeneic mice (Figure 3B). Allogeneic mice, however, did not display a notable thinning of the mucus layer or barrier compromise, despite increased intestinal abundances of BT. This led us to ask if meropenem treatment during GVHD may lead to alterations in the behavior of BT. To evaluate this further, we performed RNA sequencing of stool samples from allo-HSCT mice without and with meropenem treatment. We also examined the genome of our mouse-derived BT isolate (MDA-JAX BT001) (Figure S5C) and identified open-reading frames (ORF), which were then assigned to polysaccharide utilization loci (PUL) using the Polysaccharide-Utilization Loci DataBase (PULDB) (Terrapon et al., 2018) (Data S1). We examined RNA reads that aligned to the BT genome and found that meropenem treatment led to upregulated expression in murine BT of GH2 β-galactosidase, GH33 sialidase and GH29 α-Lfucosidase, all of which likely participate in the degradation of host mucin glycans (Figure 6A).
Figure 6. Mucolytic activity of BT is suppressed by ambient xylose.
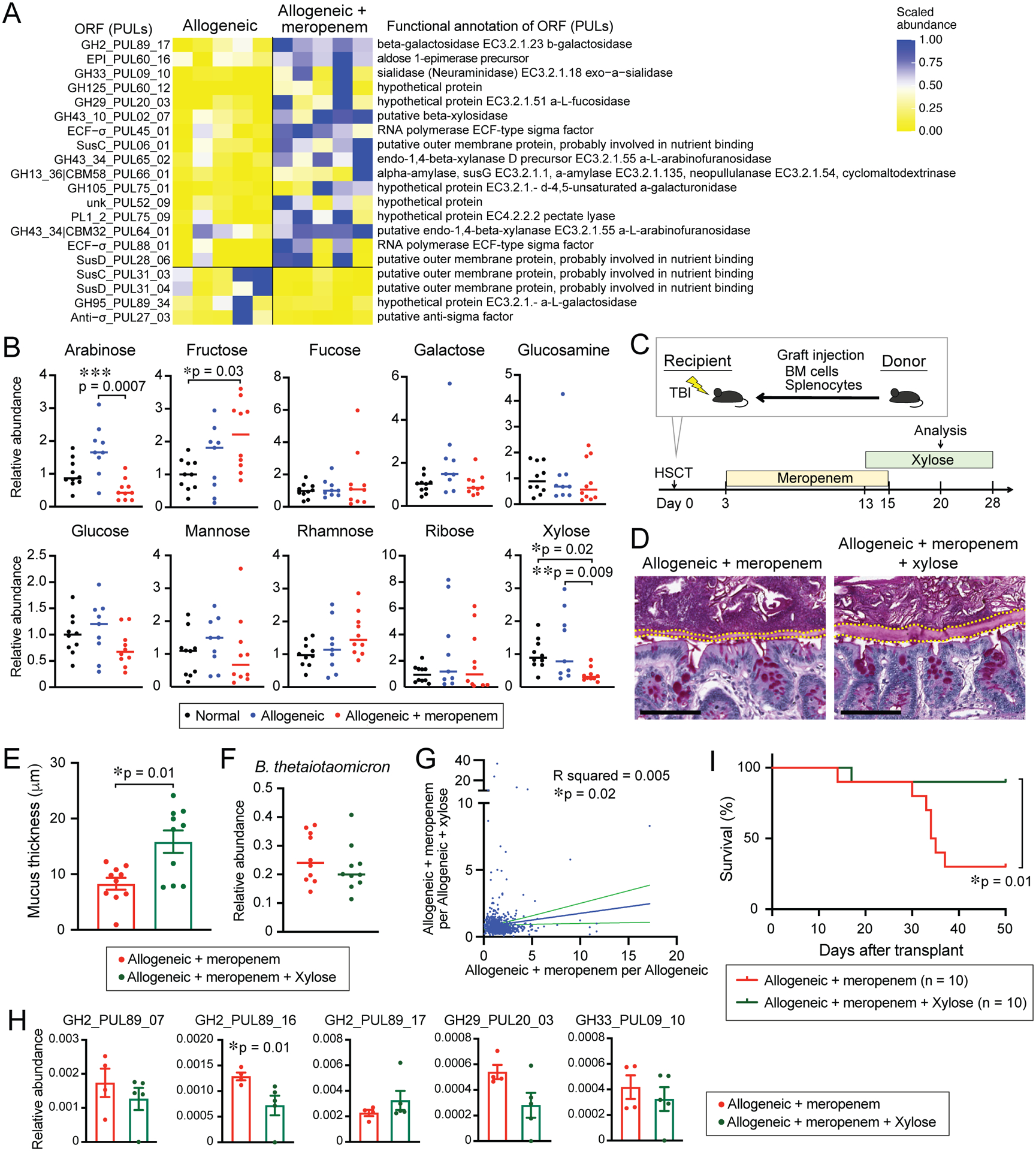
(A) Relative expression levels of PULs in BT RNA transcripts sequenced from stool collected from allogeneic mice treated or untreated with meropenem on day 18. Left: PUL identification numbers. Right: enzymatic functional annotations. (B) Relative abundances of monosaccharides of supernatants from stool collected from normal mice and allogeneic mice treated or untreated with meropenem on day 18 measured by IC-MS. (C) Experimental schema of murine GVHD model. (D) PAS staining of histological colon sections collected on day 20. Bar, 100 μm. (E) Mucus thickness on day 20. Data is shown as means ± SEM. (F) Relative abundance of BT on day 20. (G) Correlation analysis of the relative expression levels of PULs in BT RNA transcripts sequenced from stool collected from meropenem-treated allogeneic mice or those with xylose supplementation on day 21. X axis indicates calculated expression levels of PULs in BT RNA transcripts as mean expression levels of meropenem-treated allogeneic mice to allogeneic mice and Y axis indicates calculated expression levels of PULs in BT RNA transcripts as mean expression levels of meropenem-treated allogeneic mice to those with xylose supplementation. (H) Relative expression levels of PULs in BT RNA transcripts. (I) Overall survival. (E-F, I) Data are combined from two independent experiments.
To characterize the contribution of its mucus-degrading capabilities when BT exacerbates GVHD, we evaluated effects of a mucin-deficient BT strain in a murine GVHD model. This BT strain was engineered to be deficient in 11 PULs that contribute to mucin glycan degradation. This strain grew poorly in culture media containing porcine gastric mucin as the primary carbohydrate source and showed significantly decreased degradation of mucin-derived carbohydrates quantified by a PAS-based colorimetric assay compared to wild-type (WT, ATCC 29148) BT (Figure S5D and S5E). We administered a 5-day antibiotic regimen composed of piperacillin/tazobactam in the drinking water and oral gavage of both vancomycin and metronidazole to eliminate endogenous Bacteroides from the intestinal microbiome in recipient mice. We then inoculated Bacteroidesfree mice with either WT or mucin-deficient BT orally once daily for 3 days, followed 4 days later by allo-HSCT (Figure S5F). On day 21 after allo-HSCT, BT was abundant in fecal pellets from recipients that received WT BT or mucin-deficient BT but was absent in control mice (Figure S5G). Interestingly, allogeneic mice colonized by mucin-deficient BT showed significantly improved survival compared to those colonized by WT BT (Figure S5H), suggesting that mucus-degrading capabilities of BT contribute to exacerbation of GVHD.
Interestingly, in the presence of multiple suitable carbohydrate substrates, BT has been found to preferentially consume certain carbohydrates first, and only after depleting these will it then upregulate utilization genes targeting other available polysaccharides (Rogers et al., 2013). Host mucin glycans are particularly low on the metabolic hierarchy and are typically targeted only after other dietary polysaccharides have been depleted (TerAvest et al., 2014). Both ambient carbohydrates as well as metabolic byproducts have been found to be modulators of the BT transcriptional profile (Schofield et al., 2018). We asked if levels of soluble carbohydrates in the colonic lumen are perturbed by meropenem treatment, given that we had found that meropenem depletes the abundance of commensal Clostridia, which function to metabolize dietary fibers and starches (Chinda et al., 2004). Using ion chromatography-mass spectrometry (IC-MS), we characterized levels of luminal monosaccharides in the colon of mice. We found that meropenemtreatment led to significantly lower concentrations of arabinose and xylose in allogeneic mice (Figure 6B). To examine the effects of the presence of monosaccharides on mucin utilization by BT, we cultivated BT in bacterial media containing porcine gastric mucin, then subsequently added a panel of monosaccharides and finally quantified levels of remaining mucin using a colorimetric assay (Figure S6A). In the absence of additional monosaccharides, BT readily metabolized porcine gastric mucin. Mucin utilization by BT in the presence of certain monosaccharides, however, was significantly suppressed, including in particular mannose, glucose or xylose (Figure S6B). We quantified by real-time PCR expression levels of GH2 β-galactosidase, GH33 sialidase and GH29 α-L-fucosidase, and found that expression of GH33 sialidase and GH29 α-L-fucosidase by murine BT were suppressed by these monosaccharides, but expression of GH2 β-galactosidase was not significantly impacted (Figure S6C). Altogether, these data indicate that following meropenem treatment, reductions in colonic monosaccharides, particularly xylose, may lead to increased mucus-degrading behavior by BT in the colon of allogeneic mice.
This understanding led us to hypothesize that oral supplementation with xylose could mediate a benefit in allogeneic mice with GVHD aggravated by meropenem. We treated allogeneic mice with meropenem as in Figure 2F and with xylose from days 13 to 28 (Figure 6C). Interestingly, xylose supplementation prevented thinning of the colonic mucus thickness compared to allogeneic mice receiving meropenem alone, while a different monosaccharide, glucose, did not alter colonic mucus thickness (Figure 6D and 6E and S6D and S6E), suggesting that xylose could prevent thinning of the colonic mucus more than glucose. We found that the relative abundances of BT were not significantly different in meropenem-treated mice with or without oral xylose supplementation (Figure 6F). In addition, in vitro, growth of mouse- and human-derived BT in carbohydrate-poor media was augmented by xylose (Figure S6F), suggesting that xylose supplementation did not prevent thinning of the colonic mucus thickness via suppressing expansion of BT.
To profile effects of xylose on intestinal BT in the setting of GVHD, we performed RNA sequencing of stool samples from meropenem-treated allogeneic mice without and with xylose supplementation. We determined the mean relative abundances of expression of individual genes, comparing meropenem-treated allogeneic mice with and without xylose, and evaluated for a potential correlation with the mean relative abundances observed comparing allogeneic mice with and without meropenem, from Figure 6A. We found that a small amount of the changes in gene expression produced by meropenem could be attributed to changes in xylose (R = 0.08), but the slope of the linear regression was significant (p = 0.02, Figure 6G). An examination of differentially abundant genes that could contribute to mucin glycan degradation revealed that one ORF, GH2_PUL89_16, was significantly downregulated by xylose supplementation in meropenem-treated allogeneic mice. Interestingly, meropenem-treated mice receiving glucose supplementation as a control monosaccharide did not show a similar effect (Figure 6H and S6G). This ORF was not significantly upregulated by meropenem in allogeneic mice, however, and other enzymes that may contribute to mucin glycan degradation that were significantly upregulated by meropenem were not significantly downregulated by xylose. Overall, our examination of the impact of meropenem and xylose on BT gene expression indicates that xylose supplementation does not substantially restore the spectrum of effects on BT seen with meropenem treatment. Nevertheless, we asked if oral supplementation with xylose could impact on GVHD severity in meropenem-treated allogeneic mice. We found that xylose supplementation significantly improved the survival of these mice (Figure 6I). Together, these results suggest that xylose supplementation can inhibit expression of enzymes by BT that may contribute to mucus degradation, leading to better preservation of the mucus barrier and improved survival. This strategy could represent a novel approach to ameliorate GVHD in the setting of an injured commensal microbiota following antibiotic treatment.
Discussion
The use of prophylactic as well as empiric antibiotic treatment for neutropenic fever in patients undergoing allo-HSCT is supported by guidelines and effectively reduces infectious complications (Freifeld et al., 2011; Taplitz et al., 2018). In contrast, studies of effects of antibiotics on other clinical outcomes, including GVHD, have shown mixed results over time. More recent studies have demonstrated that broad-spectrum antibiotics, especially carbapenems, are associated with an increased incidence of acute intestinal GVHD (Elgarten et al., 2021; Hidaka et al., 2018; Lee et al., 2019; Shono et al., 2016), but mechanisms underlying these associations have not yet been fully identified. Here, we report establishing a mouse model of meropenemaggravated GVHD with microbiome profiles that largely recapitulate those seen in allo-HSCT patients.
Our results in patients and mice indicate that meropenem treatment led to loss of intestinal Clostridia. Previous studies have shown that Clostridia play an important role in producing SCFAs, including butyrate which plays a role in regulating intestinal immunity (Arpaia et al., 2013; Smith et al., 2013). Loss of Clostridia during GVHD results in increased epithelial injury (Mathewson et al., 2016). In this study, we confirmed that loss of Clostridia by meropenem treatment led to both decreased levels of SCFAs and compromise of the exclusion of Bacteroidia including BT from the mucosal surface. Interestingly, meropenem-treated mice that received decontamination therapy had improved survival, suggesting that meropenem aggravated intestinal GVHD not only via suppression of beneficial bacteria but also through expansion of harmful bacteria. Meropenem treatment led to increased bacteria with mucolytic functional activity, including BT, Akkermansia, and Bifidobacterium. Akkermansia muciniphila expansion and compromised intestinal barrier function following imipenem-cilastatin treatment was previously reported to be associated with exacerbated intestinal GVHD in mice (Shono et al., 2016). In this study, increases in BT following meropenem treatment were most apparent, along with thinning of the colonic mucus layer and increased bacterial translocation. Supporting the hypothesis that BT could be a key contributor to exacerbated intestinal GVHD, we found that re-introduction of BT to decontaminated mice resulted in an aggravated GVHD phenotype. BT can play a role in maintaining intestinal homeostasis by producing SCFAs, especially acetate and propionate (van der Hee and Wells, 2021; Wrzosek et al., 2013). In genetically predisposed mice, however, BT can induce colitis in a manner dependent on mucus-degrading function (Bloom et al., 2011; Hickey et al., 2015). Allo-HSCT can lead to a state that is comparably prone to inflammation due to the presence of alloreactive immune cells. Our results suggest that compromise of the colonic mucus layer by BT with increased mucolytic functional activity results in translocation of bacteria, which then results in worsened GVHD.
Allogeneic mice, even without meropenem treatment, had higher abundances of BT compared to syngeneic mice but did not show the same degree of compromise of the colonic mucus layer seen after receiving meropenem. Bacterial gene expression profiling demonstrated that meropenem treatment resulted in changes in the behavior of BT in mice, with upregulation of several enzymes predicted to be involved in degradation of mucin glycans. A mutated strain of BT deficient in multiple mucin-utilizing enzymes did not aggravate GVHD-related mortality as much as a wild-type BT strain, indicating that increased degradation of mucin glycans in allogeneic mice contributes to GVHD severity. It has been reported that ambient levels of nutrients and metabolites play a major role in determining transcriptional profiles in BT (Schofield et al., 2018). Xylose was significantly decreased by meropenem treatment in allogeneic mice, and its presence acted to downregulate mucin-degrading enzymes and suppress mucin utilization by BT both in vitro and in vivo. Because BT can utilize xylose as a carbon source (Dodd et al., 2011), our results are consistent with xylose functioning to suppress mucus-degrading behavior by BT, rather than acting as a global inhibitor.
In summary, we have found that expansion of BT in meropenem-treated mice contributes to GVHD-related mortality. Broad-spectrum antibiotics are useful for treating infections in alloHSCT. However, an altered intestinal environment due to loss of bacterial subsets can perturb ambient levels of monosaccharides, which can in turn impact on the behavior of commensal mucolytic bacteria, resulting in increased severity of GVHD. This improved understanding of how antibiotic-mediated microbiome injury leads to increased severity of intestinal GVHD should help facilitate the development of strategies to better prevent and treat this important limitation of allo-HSCT.
Limitations of the Study
The effects of xylose on intestinal bacteria are not fully characterized in this study. Our study has not examined other potential effects of xylose, including on mucin secretion by the host, or a possible impact on other intestinal bacterial subsets. We have also not investigated why xylose levels are reduced following meropenem treatment, but this could be due to the loss of many subsets of intestinal bacteria. Clostridia, in particular, may function to improve the production of xylose from dietary xylose-containing polysaccharides via enzymatic degradation. Finally, confirmation that a similar phenomenon occurs in the intestinal microbiome of allo-HSCT patients is also important for future studies.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Robert R Jenq (rrjenq@meanderson.org).
Materials availability
This study did not generate new unique reagents. All bacterial strains can be obtained from ATCC or as described in the key resources table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
| Antibodies | ||
| Rabbit anti-CD11b | Abcam | Cat# ab75476 |
| Rabbit anti-MUC2 [C3] | GeneTex | Cat# GTX100664 |
| Anti-mouse CD45, 30-F11 | BioLegend | Cat# 103113 |
| Anti-mouse CD11b, M1/70 | BioLegend | Cat# 101206 |
| Anti-mouse CD11c, N418 | BioLegend | Cat# 117310 |
| Anti-mouse CD103, 2E7 | BioLegend | Cat# 121406 |
| Anti-mouse Ly6G, 1A8 | BioLegend | Cat# 127645 |
| Anti-mouse I-A/I-E, M5/114.15.2 | BioLegend | Cat# 107628 |
| Anti-mouse F4/80, BM8 | BioLegend | Cat# 123126 |
| Secondary Antibody, Alexa Fluor 594 | Thermo Fisher | Cat# R37119 |
| Secondary Antibody, Alexa Fluor 488 | Thermo Fisher | Cat# A21206 |
| Bacterial and virus strains | ||
| Bacteroides thetaiotaomicron (MDA-JAX BT001) | This study | N/A |
| Enterococcus faecalis (MDA-JAX EF001) | This study | N/A |
| Clostridium disporicum (MDA-JAX CD001) | This study | N/A |
| Clostridium saudiense (MDA-JAX CS001) | This study | N/A |
| Lachnospiraceae unclassified (MDA-JAX LS001) | This study | N/A |
| Bacteroides thetaiotaomicron | ATCC | Cat# ATCC 29148 |
| Bacteroides thetaiotaomicron mucin-deficient mutant | This study | N/A |
| Biological samples | ||
| Human stool | This study | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Food chow (LabDiet PicoLab Rodent Diet 20) | Lab Supply | Cat# 5053 |
| D-(+)-xylose | Sigma-Aldrich | Cat# X3877 |
| D-(+)-glucose | Sigma-Aldrich | Cat# G8270 |
| D-(+)-mannose | Sigma-Aldrich | Cat# M8574 |
| Type IV collagenase | Sigma-Aldrich | Cat# C5138 |
| Porcine gastric mucin | Sigma-Aldrich | Cat# M1778 |
| DL-dithiothreitol | Bioworld | Cat# 40400120 |
| Sterilized Rumen fluid | Fisher Scientific | Cat# NC1530570 |
| Schiff’s reagent for aldehydes | Sigma-Aldrich | Cat# 84655 |
| RNase-Free DNase Set | Qiagen | Cat# 79254 |
| Critical commercial assays | ||
| QIAamp DNA mini kit | Qiagen | Cat# 51306 |
| QIAquick gel extraction kit | Qiagen | Cat# 28706X4 |
| QIAGEN Genomic-tip 20/G | Qiagen | Cat# 10223 |
| Zombie Aqua Fixable viability kit | BioLegend | Cat# 423101 |
| LiofilchemTM MTSTM Meropenem [MRP] 0.016–256 μg/mL | Fisher Scientific | Cat# 22-777-863 |
| KAPA SYBR FAST Master Mix | Roche | Cat# 07959389001 |
| SYTO™ BC Green Fluorescent Nucleic Acid Stain | Thermo Fisher | Cat# S34855 |
| Propidium Iodide Solution | BioLegend | Cat# 421301 |
| Nextera DNA Flex Library Prep Kit | Illumina | Cat# 20018704 |
| Universal Prokaryotic RNA-Seq | Tecan | Cat# 9367–32 |
| UDI 96-Plex Adaptor Plate | Tecan | Cat# S02480-FG |
| MiSeq Reagent Kit v2 (300-cycles) | Illumina | Cat# MS-102–2002 |
| NovaSeq 6000 SP Reagent Kit v1.5 | Illumina | Cat# 20028400 |
| Ovation® Complete Prokaryotic RNA-Seq DR Multiplex System | NuGEN Technologies | Cat# 0326–32/0327–32 |
| RNeasy mini kit | Qiagen | Cat# 74104 |
| Rapid Sequencing Kit | Oxford Nanopore | Cat# SQO-RAD004 |
| High-Capacity cDNA Reverse Transcription Kit | Thermo Fisher | Cat# 4368814 |
| Deposited data | ||
| 16S rRNA sequencing data of mouse fecal samples | This study | SRA PRJNA858490 |
| 16S rRNA sequencing data of patient fecal samples | This study | SRA PRJNA858489 |
| Microbiol RNA sequencing data | This study | SRA PRJNA858490 |
| Complete genome of MDA-JAX BT001 | This study | SRA PRJNA858495 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664 |
| Mouse: B6D2F1/J | The Jackson Laboratory | JAX: 100006 |
| Oligonucleotides | ||
| Alexa Fluor 594-conjugated EUB338 (5′- GCTGCCTCCCGTAGGAGT-3′) | Okumura et al., 2016 | N/A |
| 515 forward and 806 reverse primer pairs | Caporaso et al., 2012 | N/A |
| 926F (5′-AAACTCAAAKGAATTGACGG-3′) | Yang et al., 2015 | N/A |
| 1062R (5′-CTCACRRCACGAGCTGAC-3′) | Yang et al., 2015 | N/A |
| GH2 (5′-CGCACTCTTCTTGCATCTGC-3′ for the forward primer, 5′-TACCAACGGCTCACATTGGG-3′ for the reverse primer) | This study | N/A |
| GH29 (5′-GATGCTGGAAAAGGCAACGG-3′ for the forward primer, 5′-AGCGTGCCTTTTCCTTCTGA-3’ for the reverse primer) | This study | N/A |
| GH33 (5′-GGTCACCGAAAGACATTATTCATCG-3′ for the forward primer, 5′-GCCG 1 IIGATACAGATCCATTCC-3’ for the reverse primer) | This study | N/A |
| BT specific probes (5′-CACAACAGCCATAGCGTTCCA-3’ for the forward primer, 5′-ATCGCAAAAATAAGATGGGCAAA-3’ for the reverse primer) | Benjdia et al., 2011 | N/A |
| Recombinant DNA | ||
| Software and algorithms | ||
| Flow Jo | FlowJo LLC | RRID:SCR_008520 |
| GraphPad Prism | GraphPad Software | RRID:SCR_002798 |
| Flye version 2.8.2 | Kolmogorov et al., 2019 | https://github.com/fenderglass/Flye.git |
| Rebaler version 0.2.0 | Wick et al., 2019 | https://github.com/rrwick/Rebaler.git |
| BWA version 0.7.17 | Li et al., 2009 | |
| QIIME2 | Caporaso et al., 2019 | |
| VSEARCH version 2.17.1 | Rognes et al., 2014 | https://github.com/torognes/vsearch.git |
| DIAMOND version 0.9.24 | Buchfink et al., 2015 | https://github.com/bbuchfink/diamond.git |
| Other | ||
Data and code availability
16S rRNA sequencing data, RNA sequencing data and complete genome data have been deposited at Sequence Read Archive (SRA). All data are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Retrospective study design
295 patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) with fludarabine plus busulfan as conditioning therapy and tacrolimus and methotrexate as GVHD prophylaxis from 2011 and 2016 at MD Anderson Cancer Center and were analyzed retrospectively. We classified patients by antibiotic exposures, including those who received neither cefepime nor meropenem, those who received cefepime alone, those who received meropenem alone and those who received both cefepime and meropenem from day -10 to day 30 after allo-HSCT. Acute GVHD was diagnosed by clinical and/or pathological findings, and graded according to standard criteria (Przepiorka et al., 1995). For patient microbiome analyses, we identified 26 meropenem-unexposed patients and 18 meropenem-exposed patients who underwent allo-HSCT with fludarabine plus busulfan as conditioning therapy from 2014 to 2019 and provided stool samples for our biorepository on day 14 after allo-HSCT. Signed informed consent was provided by all study participants, and this study was approved by the University of Texas MD Anderson’s Institutional Review Board (PA170035).
Human samples
Samples were collected from patients undergoing stem cell transplantation and stored at 4°C for 24–48 hours until aliquoted for long-term storage at −80°C.
Mice
Female C57BL/6J (B6: H-2b) and B6D2F1 (H-2b/d, CD45.2+) were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal experiments were performed under the Guide for the Care and Use of Laboratory Animals Published by the US National Institutes of Health and was approved by the Institutional Animal Care and Use Committee. Experiments in this manuscript were performed in a non-blinded fashion.
Antibiotics administration
For subcutaneous administration, meropenem was dissolved with PBS and given at a concentration of 10 mg/day. For oral administration, meropenem was dissolved with phosphate buffer pH 8.0 and given at a concentration of 0.625 g/L in the drinking water from day 3 to day 15 after transplant.
Levofloxacin and cefepime were supplemented to autoclaved drinking water at a concentration of 0.25 g/L (Schroeder et al., 2001) and 0.625 g/L, respectively, from day 3 to day 15 after transplant. Piperacillin/tazobactam and nystatin were given at a concentration of 3.2 g/L and 320,000 IU/L respectively in combination with meropenem in the drinking water from days 5 to 15 after transplant. In a murine model where we introduced a mucin-deficient mutate strain of BT, piperacillin/tazobactam was given at a concentration of 3.2 g/L in combination with vancomycin and metronidazole dosed 1.5 mg daily by oral gavage from days −16 to −12 relative to transplant.
Xylose and glucose administration
D-(+)-xylose (X3877, Sigma-Aldrich) or D-(+)-glucose (G8270, Sigma-Aldrich) was dissolved in phosphate buffer pH 8.0 with meropenem or in water without meropenem and given at a concentration of 0.5% from days 13 to 28 after allo-HSCT.
HSCT
Mice were transplanted as previously described (Hayase et al., 2017). In brief, after receiving myeloablative total body irradiation (11 Gray) delivered in 2 doses at 4 hour intervals, (H-2b/d) mice were i.v. injected with 5 × 106 bone marrow (BM) cells and 5 × 106 splenocytesB6D2F1 from allogeneic B6 (H-2b) or syngeneic B6D2F1 donors. Female mice that were 8 to 12-weeks-old were allocated randomly to each experimental group, ensuring the mean body weight in each group was similar. Total body radiotherapy was performed using a Shepherd Mark I, Model 30, 137Cs irradiator. Mice were maintained in specific pathogen-free (SPF) condition and received normal chow (LabDiet PicoLab Rodent Diet 20 5053, Lab Supply) after HSCT. Survival after HSCT was monitored daily and the degree of clinical GVHD was assessed weekly by using an established scoring system (Cooke et al., 1996).
METHOD DETAILS
Histological and immunohistochemistry analysis
For pathological analysis, samples of the small intestine, colon and liver were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Pathology scores were quantified by a blinded pathologist. For evaluation of mucus thickness, colonic sections containing stool pellets were fixed in methanol-Carnoy’s fixative composed of methanol 60%, chloroform 30% and glacial acetic acid 10% and 5 μm sections were made and stained with Periodic acid-Schiff (PAS). Sections were imaged using an Aperio AT2. Mucus thickness of the colonic sections were measured using eSlide Manager Version 12.4.3.5008. Eight measurements per image were taken and averaged over the entire usable colon surface. Immunohistochemistry was performed using primary antibodies of rabbit anti-CD11b (ab75476, Abcam), visualized using 3,3’-diaminobenzidine (DAB) and counterstained using hematoxylin.
Immunostaining and fluorescence in situ hybridization
Colon containing stool pellets were fixed in methanol-Carnoy’s fixative and the 5 μm thin sections were made as described above. Paraffin-embedded sections were dewaxed and hydrated. Sections were incubated with 1 μg Alexa Fluor 594-conjugated EUB338 (5′-GCTGCCTCCCGTAGGAGT-3′) for detection of all bacteria in 200 μL of hybridization buffer (750 mM NaCl, 100 mM Tris-HCl (pH 7.4), 5 mM EDTA, 0.01% BSA, 10% dextran sulfate) at 40°C for 16 hours (Okumura et al., 2016). Sections were rinsed in wash buffer (50 mM NaCl, 4 mM Tris-HCl (pH 7.4), 0.02 mM EDTA), washed at 45°C for 20 min, stained with anti-Muc2 antibody [C3] (GTX100664, GeneTex) and counterstained with DAPI (Vector Laboratories).
Photographs of sections were obtained using a fluorescent microscope (Nikon NIS Elements, Advanced Research version 4.20).
Sequencing of 16S rRNA gene amplicons
Fecal samples which were collected from patients and mice and colonic mucosal samples which were collected from mice were weighed before DNA isolation. In brief, genomic DNA was isolated using the QIAamp DNA mini kit (51306, Qiagen) according to the manufacturer’s protocol that was modified to include an intensive bead-beating lysis step. The V4 region of 16S rRNA gene was amplified by PCR from 100 ng of each of extracted and purified genomic DNA using 515 forward and 806 reverse primer pairs (Caporaso et al., 2012). The quality and quantity of the barcoded amplicons were assessed on an Agilent 4200 TapeStation system (Agilent) and Qubit Fluorometer (Thermo Fisher Scientific), and libraries were prepared after pooling at equimolar ratios. The final libraries were purified using QIAquick gel extraction kit (28706X4, Qiagen) and sequenced with a 2 × 250 base pair paired-end protocol on the Illumina MiSeq platform.
Microbiome data analysis
Sequencing data from paired-end reads were de-multiplexed using QIIME 2 (Caporaso et al., 2010). Merging of paired-end reads, dereplicating, and length filtering was performed using VSEARCH 2.17.1 (Rognes et al., 2016). Following de-noising and chimera calling using the unoise3 command (Edgar, 2016), unique sequences were taxonomically classified with mothur (Schloss et al., 2009) using the Silva database (Quast et al., 2013) version 138. Weighted UniFrac distances (Lozupone et al., 2011) were determined using QIIME 2, visualized using principal coordinate analysis, and evaluated for statistical significance using permutational multivariate analysis of variance (PERMANOVA) testing. For differential abundance analysis, abundances of sequences belonging to taxonomical groups were included for analysis using the Mann-Whitney U test and adjusted for multiple comparisons using the method of Benjamini-Hochberg. Paired samples were analyzed using the Wilcoxon signed rank test with adjustment for multiple comparisons.
Quantification of fecal bacterial density
Genomic DNA was isolated from stool as described above. qPCR was performed as previously described (Yang et al., 2015). In brief, 16S rRNA gene sequences were amplified from total fecal DNA using the primers 926F (5′-AAACTCAAAKGAATTGACGG-3′) and 1062R (5′CTCACRRCACGAGCTGAC-3′). Real-time PCR were carried out in 96-well optical plates on QuantStudio Flex 6 RT PCR (Thermo Fisher) and KAPA SYBR FAST Master Mix (Roche). The PCR conditions included one initial denaturing step of 10 min at 95°C and 40 cycles of 95°C for 20 sec and 60°C for 1 min. Melting-curve analysis was performed after amplification. To determined bacterial density, a plasmid with a 16S rRNA gene of a murine Blautia isolate was generated in the pCR4 backbone and used as a standard.
Lamina propria hematopoietic cell dissociation
Murine colons were isolated, dissected longitudinally and then on a shaker in 2% fetal bovine serum in PBS with 1 mM DL-dithiothreitol (Bioworld) at 37°C for 20 min and subsequently incubated with 1.3 mM EDTA at 37°C for 40 min. They were rinsed twice and digested with 0.3 mg/ml of type IV collagenase (C5138, Sigma-Aldrich) at 37°C for 45 min, homogenized, filtered, and washed.
Flow cytometric analysis
Monoclonal antibodies conjugated with fluorescein isothiocynate, phycoerythrin, phycoerythrinCy7, peridinin-chlorophyll protein complexes, allophycocyanin, or allophycocyanin-Cy7 were purchased from BioLegend (San Diego, CA,). Lamina propria cells in colon were stained with the antibodies against murine CD45 (30-F11, BioLegend), CD11b (M1/70, BioLegend), CD11c (N418, BioLegend), CD103 (2E7, BioLegend), Ly6G (1A8, BioLegend), MHC-II (M5/114.15.2, BioLegend) and F4/80 (BM8, BioLegend) and Zombie Aqua Fixable viability kit (423101, BioLegend). In flow cytometric analysis, at least 100,000 live samples were analyzed using BD LSRFortessa™ X-20 (BD Biosciences) and FlowJo software (Tree Star, OR). The CD45+ cells were classified into neutrophils (CD11b+Ly6G+) and dendritic cells (CD11c+MHC-II+CD103+).
Construction of a BT mucin O-glycan deficient mutant
We constructed a B. thetaiotaomicron mutant with reduced ability to utilize mucin as a nutrient source by deleting 11 different PULs that were previously associated with mucin utilization either through direct growth on mucin O-glycans in vitro (Martens et al., 2008) or expression during growth in vivo in the ceca of mice fed a low fiber diet (Bjursell et al., 2006; Sonnenburg et al., 2005). The latter in vivo condition promotes expression of mucin utilization functions. A total of 93 genes encoding 37 annotated enzymes (17 sulfatases, 19 glycoside hydrolases and 1 M60-like protease) were eliminated. PUL deletions were made by allelic exchange using the counter-selectable vector, pExchange-tdk, in a thymidine kinase (tdk) deletion strain to allow counter selection using 5-fluoro-2-deoxy-uridine (FUdR) as previously described (Koropatkin et al., 2008). Briefly, 750 bp flanks were amplified adjacent to each PUL using primers listed in Table S5 and the separate products joined into a single ~1.5 kbp fragment via overlapping ends. Each fragment was cloned into pExchange-tdk, validated by sequencing and conjugated into either the B. thetaiotaomicron △tdk parent strain or a previously constructed PUL mutant in order to create the compounded mutant lines after appropriate positive selection using erythromycin and counter-selection using (FUdR) (Koropatkin et al., 2008). The order of gene deletions was as follows, with the numbers being inclusive (i.e., in the first mutant, genes BT3172 and BT3180 were part of the deletion): BT3172–80, BT1617–36 (two tandem PULs removed together), BT3796–3800, BT3092–3109, BT0752–57, BT2912–23, BT4681–84, BT4634–31, BT0865–67, BT4250–40.
Culturing of bacteria
Mouse-derived BT (MDA-JAX BT001), Enterococcus faecalis (MDA-JAX EF001) and Clostridium disporicum (MDA-JAX CD001), Clostridium saudiense (MDA-JAX CS001), and Lachnospiraceae unclassified (MDA-JAX LS001) were isolated and cultured from mouse stool samples suspended in 1 ml of chilled 20% anaerobic glycerol in a Whitley anaerobic chamber (10% H2, 5% CO2 and 85% N2). Human-derived BT (ATCC 29148) was purchased from ATCC. Bacterial number was quantified using a Nexcelom Cellometer cell counter with SYTO™ BC dye and propidium iodide. For measuring MICs against meropenem, bacteria were cultured on BYE plates including 5% sterilized rumen fluid (Fisher Scientific) with MIC test strips (Liofilchem™ MTS™ Meropenem [MRP] 0.016–256 μg/mL, Fisher Scientific). Bacterial growth experiments were performed in a novel bacterial liquid media, BYEM10, composed of a hybrid of BHI and M10 supplemented with yeast extract (Table S4). Bacteria were cultured up to 48 hours at a starting concentration of 1 × 106 bacteria/ml in BYEM10 broth (pH 7.2) with and without 5 mg/ml of porcine gastric mucin (M1778, Sigma-Aldrich) with or without 0.5 mg/ml of D-(+)xylose (X3877), D-(+)-mannose (M8574, Sigma-Aldrich) or D-(+)-glucose (G8270). Optical densities (OD600 nm) of bacterial cultures were measured with a BioTek EPOCH 2 plate reader.
Microbiologic analysis of bacterial translocation
Mesenteric lymph nodes (MLNs) were harvested from mice and homogenized in PBS and cultured anaerobically on BHI plates containing yeast extract and 5% sterilized rumen fluid (Fisher Scientific) and Columbia blood agar plates (BD) for 4 days at 37°C. Colony-forming units (CFUs) were counted and adjusted per organ. Bacteria were identified by MALDI Biotyper.
Mucin degradation assay
Levels of mucin glycans in culture supernatants were determined by a PAS-based colorimetric assay as previously described (Kilcoyne et al., 2011) with minor modifications. Briefly, culture supernatants were centrifuged at 20,000 g, 4°C for 10 minutes and collected. To perform mucin precipitation, 500 μl of culture supernatants were mixed with 1 mL of molecular grade ethanol and incubated at −30°C for overnight. Culture supernatants were centrifuged at 20,000 g, 4°C for 10 minutes. Mucin-containing pellets were washed with 1 mL of molecular grade ethanol twice and resuspended in 500 μl of PBS. 10 μl of washed culture supernatants were transferred into round bottom 96-well plate (Falcon) containing 15 μl of PBS. Serially diluted porcine gastric mucin (Sigma) standards were prepared. Freshly prepared 0.06% periodic acid in 7% acetic acid was added, and incubated at 37°C for 90 min, followed by 100 μl of Schiff’s reagent (84655, Sigma) and incubation at room temperature for 40 min. Absorbance was measured at 550 nm using a BioTek Synergy HTX plate reader.
Short Chain Fatty Acids profiling by ion chromatography-mass spectrometry (IC-MS)
To determine the relative abundance of short chain fatty acids in mouse feces samples, extracts were prepared and analyzed by ultra-high resolution mass spectrometry (HRMS). Fecal pellets were homogenized with a Precellys Tissue Homogenizer. Metabolites were extracted using 1 mL ice-cold 0.1% Ammonium hydroxide in 80/20 (v/v) methanol/water. Extracts were centrifuged at 17,000 g for 5 min at 4°C, and supernatants were transferred to clean tubes, followed by evaporation to dryness under nitrogen. Dried extracts were reconstituted in deionized water, and 5 μL was injected for analysis by IC-MS. IC mobile phase A (MPA; weak) was water, and mobile phase B (MPB; strong) was water containing 100 mM KOH. A Thermo Scientific Dionex ICS5000+ system included a Thermo IonPac AS11 column (4 μm particle size, 250 × 2 mm) with column compartment kept at 30°C. The autosampler tray was chilled to 4°C. The mobile phase flow rate was 360 μL/min, and the gradient elution program was: 0–5 min, 1% MPB; 5–25 min, 135% MPB; 25–39 min, 35–99% MPB; 39–49 min, 99% MPB; 49–50, 99–1% MPB. The total run time was 50 min. To assist the desolvation for better sensitivity, methanol was delivered by an external pump and combined with the eluent via a low dead volume mixing tee. Data were acquired using a Thermo Orbitrap Fusion Tribrid Mass Spectrometer under ESI negative ionization mode at a resolution of 240,000. Raw data files were imported to Thermo Trace Finder and Compound Discoverer software for spectrum database analysis. The relative abundance of each metabolite was normalized by sample weight.
Pharmacokinetics of meropenem by Triple Quadruple liquid chromatography-mass spectrometry (LC-MS)
C57BL/6 mice were treated with 10 mg of meropenem by subcutaneously injection. Cecal contents were collected prior to, 4, 8, 24, 48, and 96 hours after meropenem injection. To determine the relative abundance of meropenem in mouse cecum samples, extracts were prepared and analyzed with a Thermo Scientific TSQ Quantiva triple quadruple mass spectrometer coupled with a Dionex UltiMate 3000 HPLC system. Approximately 600 mgs of mouse cecal contents were homogenized with a Precellys Tissue Homogenizer. Metabolites were extracted using 100% acetonitrile. The tissue lysates were vortexed, centrifuged at 17,000 g for 5 min at 4°C, and organic layers were transferred to clean tubes, followed by evaporation to dryness under nitrogen. Dried extracts were reconstituted in 50/50 (v/v) water/Acetonitrile, and 5 μL was injected for analysis by LC-MS. The mobile phase A is 100% water and mobile phase B is 0.1% Formic Acid in acetonitrile. Separation of meropenem was achieved on an Agilent SB-C18, 1.8 μm, 100 × 3 mm column. The flow rate was 250 μL/min at 35 °C, and the gradient elution program was: 0–1 min, 5% MPB; 1–5 min, 550% MPB; 5–6 min, 50–95% MPB; 6–10 min, 95% MPB; 10–10.1 min, 95–5% MPB. The total run time was 15 min. The mass spectrometer was operated in the MRM positive ion electrospray mode with the transition m/z 384.1 -> 68.0. Raw data files were imported to Thermo Trace Finder software for final analysis. The relative abundance of meropenem was normalized by sample weight.
Carbohydrates analysis by IC-MS
To determine the relative abundance of carbohydrates in mouse feces samples, extracts were prepared and analyzed by ultra-HRMS. Fecal pellets were homogenized with a Precellys Tissue Homogenizer. Metabolites were extracted using 1 mL ice-cold 80/20 (v/v) methanol/water. Extracts were centrifuged at 17,000 g for 5 min at 4°C, and supernatants were transferred to clean tubes, followed by evaporation to dryness under nitrogen. Dried extracts were reconstituted in deionized water, and 5 μL was injected for analysis by IC-MS. IC mobile phase A (MPA; weak) was water, and mobile phase B (MPB; strong) was water containing 100 mM KOH. A Thermo Scientific Dionex ICS-5000+ system included a Thermo CarboPac PA-20-Fast column (4 μm particle size, 100 × 2 mm) with column compartment kept at 30°C. The autosampler tray was chilled to 4°C. The mobile phase flow rate was 200 μL/min, and the gradient elution program was: 0–0.5 min, 1% MPB; 0.5–10 min, 1–5% MPB; 10–15 min, 5–95% MPB; 15–20 min, 95% MPB; 20.5–25, 95–1% MPB. The total run time was 25 min. To assist the desolvation for better sensitivity, methanol was delivered by an external pump and combined with the eluent via a low dead volume mixing tee. Data were acquired using a Thermo Orbitrap Fusion Tribrid Mass Spectrometer under ESI negative ionization mode at a resolution of 240,000. Raw data files were imported to Thermo Trace Finder and Compound Discoverer software for spectrum database analysis. The relative abundance of each metabolite was normalized by sample weight.
Whole genome sequencing of BT (MDA-JAX BT001)
BT (MDA-JAX BT001) genomic DNA was isolated and purified using a Qiagen Genomic-tip 20/G column, according to the manufacturer’s instructions. For short-read Illumina sequencing, libraries were constructed with a Nextera DNA Flex Library Prep Kit (Illumina, San Diego, CA, USA), according to the manufacturer’s protocol. All libraries were quantified with a TapeStation and pooled in equal molar ratios. The final libraries were sequenced with the NovaSeq 6000 platform (Illumina) to produce 2×150 bp paired-end reads, resulting in ~5 Gb per sample. For long-read Nanopore sequencing, 500 ng of genomic DNA was used for library preparation using the Rapid Sequencing Kit (SQK-RAD004, Oxford Nanopore Technologies). Libraries were loaded into a FLO-MIN106 flow-cell for 24h sequencing run on a MinION sequencer platform (Oxford Nanopore Technologies, Oxford, UK). Data acquisition and real-time base calling were carried out by the MinKNOW software version 3.6.5. The fastq files were generated from basecalled sequencing fast5 reads.
Hybrid assembly and genome annotation of BT (MDA-JAX BT001)
To assemble the complete genome of BT, Flye version 2.8.2. (Kolmogorov et al., 2019) was used with long (Nanopore) reads and short (NovaSeq) reads combined using default settings. The genome was compared to a reference genome (BT VPI-5482) using Rebaler version 0.2.0. (https://github.com/rrwick/Rebaler) (Wick et al., 2019). The similarities of the genome of MDAJAX BT001 to other reference genomes was calculated using blastn for Bacteroides faecichinchillae (GCF_004801645.1_ASM480164v1), Bacteroides faecis (GCF_000226135.1_ASM22613v2), and BT (VPI-5482), respectively (Altschul et al., 1990). Open reading frames (ORFs) of BT (MDA-JAX BT001) were identified using the Sequence Manipulation Suite (Stothard, 2000) and annotated with polysaccharide utilization loci (PUL) (Terrapon et al., 2018) using BWA version 0.7.17 (Li and Durbin, 2009). The genome of BT and ORFs were depicted using DNA plotter software (Carver et al., 2009).
RNA sequencing and analysis
Approximately 30 mg of stool was freshly collected in 700 μL of ice cold Qiazol containing 200 μL of 0.1 mm diameter Zirconia Silica beads (11079101z, BioSpec). Samples were bead beaten twice for 2 min with a 30 second interval recovery. Samples were then centrifuged at 12,000 g for 1 min and the supernatant was collected for RNA isolation using the RNeasy mini kit (74104, Qiagen). RNA was treated on column with DNase I (79254, Qiagen) to eliminate contaminating genomic DNA. RNA quantity and quality was determined using an Agilent 4200 TapeStation system (Agilent). 250 ng of total RNA from mouse stools was used to construct libraries using the Nugen Ovation Complete Prokaryotic RNA-Seq Systems (NuGen) or the Universal Prokaryotic RNA-Seq Library Preparation Kit (9367–32, Tecan) with Unique Dual Indexes (S02480-FG, Tecan), following the manufacturer’s protocol. The cDNA libraries were sequenced on the Illumina MiSeq or NovaSeq 6000 system to produce either 1×300 bp single-end reads or 2×150 bp paired-end reads. Sequence data were demultiplexed using QIIME 2 (Caporaso et al., 2010) and their qualities were checked using VSEARCH 2.17.1 (Rognes et al., 2016). Data were filtered and truncated by quality with VSEARCH default settings. The total reads of mouse stool samples were 950923 ± 113406 (mean ± standard deviation) in Figure 6A and 137623366 ± 38865363 in Figure 6G–H and S6G. Sequences of ribosomal RNA were removed using BWA software against prokaryotic ribosomal RNA sequences from prokaryotic RefSeq genomes (Tatusova et al., 2016). Sequences of interest were further identified using DIAMOND software version 0.9.24 (Buchfink et al., 2015) to align against PULs. Features with percent identity less than 80% were excluded. The total counts of bacterial isolated samples were 104172 ± 101292 (mean ± standard deviation) in Figure 6A and 5761717 ± 2518881 in Figure 6G–H and S6G. Aligned mRNA expression changes were calculated using the Mann-Whitney U test in R software version 3.6.0 via RStudio version 1.2.1335. P values < 0.05 were considered statistically significant.
Quantitative real-time PCR analysis of BT loci
Total RNA was isolated from in vitro cultured BT as described above. The cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (4368814, Thermo Fisher Scientific). The mRNA levels of selected targets were quantified by qPCR using KAPA SYBR FAST Master Mix (Roche) and specific probes (GH2, 5′-CGCACTCTTCTTGCATCTGC-3′ for the forward primer, 5′-TACCAACGGCTCACATTGGG-3′ for the reverse primer; GH29, 5′-GATGCTGGAAAAGGCAACGG-3′ for the forward primer, 5′AGCGTGCCTTTTCCTTCTGA-3′ for the reverse primer; GH33, 5′-GGTCACCGAAAGACATTATTCATCG-3′ for the forward primer, 5′GCCGTTTGATACAGATCCATTCC-3′ for the reverse primer) and were normalized to BT specific probes (5′-CACAACAGCCATAGCGTTCCA-3′ for the forward primer, 5′ATCGCAAAAATAAGATGGGCAAA-3′ for the reverse primer) (Benjdia et al., 2011).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis
Data were checked for normality and similar variances between groups and Student’s t-tests were used when appropriate. Mann-Whitney U tests were used to compare data between two groups when the data did not follow a normal distribution. The Kaplan-Meier curves were used to depict survival probabilities and the log-rank test was applied to compare survival curves. For clinical data analysis, non-repeated ANOVA was used to compare continuous variables, while chi-square or Fisher’s exact tests were used to analyze the frequency distribution between categorical variables. Analyses were performed using R software version 3.6.0 and Prism version 7.0 (GraphPad Software, San Diego, CA). P values < 0.05 were considered statistically significant.
Supplementary Material
Figure S1. The relative abundances of Clostridia and SCFAs were significantly decreased by meropenem treatment, related to Figure 1. (A) Meropenem concentrations in the cecal contents of mice were measured 4, 8, 24, 48 and 96 hours after subcutaneously injection of meropenem using LC-MS. (B) Bacterial densities of mouse stool samples collected 7 days after administering with meropenem by drinking water. Bacterial densities were measured by qPCR of 16S rRNA. (C) Relative abundance of the class Clostridia on days 0, 7, 14 and 21. (D) Relative abundances of SCFAs in stool samples from normal mice, alloHSCT mice and meropenem-treated allo-HSCT mice on day 18 measured by IC-MS.
Figure S2. Levofloxacin and cefepime do not increase the severity of colonic GVHD in allogeneic mice, related to Figure 1. (A) Experimental schema of murine GVHD model using levofloxacin, cefepime or meropenem treatment. Allo-HSCT was performed as in Figure 1. Mice were treated with levofloxacin, cefepime or meropenem from days 3 to 15. (B) H&E staining of histological sections of the colon collected on day 20 after allo-HSCT. Bar, 100 μm. (C) GVHD histology scores of the colon collected on day 20 after allo-HSCT, quantified by a blinded pathologist. Data are combined from two independent experiments and are shown as means ± SEM. (D) Relative abundances of SCFAs in stool samples from allo-HSCT mice, levofloxacin-, cefepime-, and meropenem-treated alloHSCT mice on day 20 measured by IC-MS. Data are shown from one representative experiment as means ± SEM.
Figure S3. The intestinal microbiome in allo-HSCT patients treated or untreated with meropenem at pre-HSCT and on day 14, related to Figure 2. Additional analyses of intestinal microbiome profiling results of allo-HSCT patient samples presented in Figure 2, A–E. (A) Alpha diversity shown using the inverse Simpson index at preHSCT and on day 14. (B) PCoA between meropenem-untreated and treated patients at pre-HSCT (left) and on day 14 (right). (C) Paired-Wilcoxon test of bacteria between at pre-HSCT and on day 14 in meropenem-treated patients (shown in red) or meropenem-untreated patients (shown in blue).
Figure S4. Loss of Clostridia and expansion of BT are seen in meropenem-treated allogeneic mice and not levofloxacin- or cefepime-treated mice, related to Figure 2–4.(A) Stacked bar graphs of bacterial genera composition of fecal samples collected on day 20 after allo-HSCT. (B) Relative abundance of the class Clostridia on day 20. (C) Relative abundance of BT on day 20. (D) PCoA of stool and mucus in distal colon from allogeneic mice (left), allogeneic mice treated with levofloxacin (middle), and allogeneic mice treated with cefepime (right). Numbers shown in each PCoA depict samples collected from the same individual mouse. (E) Beta diversity distances within individual mice. (B-E) Data are shown from one representative experiment.
Figure S5. Mucin-deficient BT did not aggravate the severity of GVHD, related to Figure 5–6. (A) Bacterial culture with or without porcine gastric mucin. OD600nm was measured after 48 hours of anaerobic culture. (B) Levels of porcine gastric mucin in the culture supernatant was determined by using a PAS-based colorimetric assay. Mouse-derived Enterococcus faecalis were used as nonmucolytic bacterial control. (C) Circular plot of open reading frames (ORFs) derived from the complete genome (MDA-JAX BT001). Blue and green bars represent ORFs on the plus strand and the minus strand, respectively. Inner purple-olive ring depicts degree of GC skewing. (D) Bacterial culture of WT BT and mucin-deficient BT in carbohydrate-poor media supplemented with porcine gastric mucin. OD600nm was measured at 20 hrs. (E) Levels of porcine gastric mucin in the culture supernatant was determined by using a PAS-based colorimetric assay. (F) Experimental schema of murine GVHD model using decontamination therapy followed by oral introduction of WT BT (ATCC 29148) or mucin-deficient BT. Mice were decontaminated with piperacillin/tazobactam in the drinking water and vancomycin and metronidazole by oral gavage from days −16 to −12 followed by oral gavage of 2×107 CFUs of WT BT or mucin-deficient BT given daily from days 6 to −4 relative to allo-HSCT. (G) Relative abundance of BT in fecal samples collected on day 21. (A-B, D-E, G) Data are shown from one representative experiment as means ± SEM. (F) Overall survival. Data are combined from two independent experiments.
Figure S6. Mucus-degrading function of BT was not suppressed by glucose in allogeneic mice treated with meropenem, related to Figure 6. Experimental schema of in vitro bacterial culture assay of BT in media with porcine gastric mucin-containing medium with or without monosaccharides. (B) Relative concentrations of porcine gastric mucin in medium following culture with BT (MDA-JAX BT001). BT was first introduced to porcine gastric mucin-containing medium. At 15 hours of culture, monosaccharides were added into the culture broth and incubated for an additional 3 hours. Levels of mucin glycans in the culture supernatant were determined using a colorimetric assay. Mucin concentrations were normalized to levels of mucin glycans from non-sugar-exposed BT conditions in two independent experiments. (C) mRNA was extracted from BT pellets cultured with or without monosaccharides and subjected to qPCR analysis of GH2, GH29 and GH33 BT loci. Relative expressions of mRNA were shown by the comparative ΔCt method. (D) PAS staining of histological colon sections collected on day 28. Bar, 100 μm. The areas inside dotted lines indicate the inner dense colonic mucus layer. (E) Mucus thickness on day 28. (F) Bacterial culture with or without the indicated monosaccharide. Monosaccharides were added at 0 hr. OD600nm was measured every 2 hours up to 30 hours. (G) Relative expression levels of PULs in BT RNA transcripts. (B-C, E, G) Data are shown from one representative experiment as means ± SEM.
Highlights.
The use of meropenem aggravates colonic GVHD at least in part via expansion of BT.
BT expanded by meropenem treatment showed increased mucus-degrading behavior.
Mucus-degrading behavior by BT was associated with decreased luminal xylose.
Oral xylose supplementation improved GVHD survival in meropenem-treated mice.
Acknowledgments:
Funding:
National Institutes of Health grant 2R01HL124112-06 (RRJ)
National Institutes of Health grant R01 DK118024 (ECM)
Cancer Prevention & Research Institute of Texas RR160089 (RRJ)
Cancer Center Support (CORE) Grant 5P30CA016672-42 (PLL, RRJ)
ASTCT New Investigator Award FP00012028 (EH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
R.R.J has served as a consultant or advisory board member for Merck, Microbiome DX, Karius, MaaT Pharma, LisCure, Seres, Kaleido and Prolacta and has received patent license fee or stock options from Seres and Kaleido. E.H., M.A.J., J.L.K., and R.R.J are inventors on a patent application by University of Texas MD Anderson Cancer Center supported by results of the current study entitled, “Methods and Compositions for Treating Cancer therapy-induced Neutropenic Fever and/or GVHD.” All other authors declare no conflict of interest.
References
- Altschul SF, Gish W, Miller W, Myers EW, and Lipman DJ (1990). Basic local alignment search tool. J Mol Biol 215, 403–410. 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, and Rudensky AY (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker V, Zwittink RD, Knetsch CW, Sanders I, Berghuis D, Heidt PJ, Vossen J, de Vos WM, Belzer C, Bredius RGM, et al. (2019). Dynamics of the Gut Microbiota in Children Receiving Selective or Total Gut Decontamination Treatment during Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 25, 1164–1171. 10.1016/j.bbmt.2019.01.037. [DOI] [PubMed] [Google Scholar]
- Benjdia A, Martens EC, Gordon JI, and Berteau O (2011). Sulfatases and a radical Sadenosyl-L-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron. J Biol Chem 286, 25973–25982. 10.1074/jbc.M111.228841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom KS, and Xia L (2013). Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 23, 1026–1037. 10.1093/glycob/cwt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell MK, Martens EC, and Gordon JI (2006). Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J Biol Chem 281, 36269–36279. 10.1074/jbc.M606509200. [DOI] [PubMed] [Google Scholar]
- Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM Jr., Allen PM, and Stappenbeck TS (2011). Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe 9, 390–403. 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchfink B, Xie C, and Huson DH (2015). Fast and sensitive protein alignment using DIAMOND. Nat Methods 12, 59–60. 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. (2010). QIIME allows analysis of highthroughput community sequencing data. Nat Methods 7, 335–336. 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6, 1621–1624. 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver T, Thomson N, Bleasby A, Berriman M, and Parkhill J (2009). DNAPlotter: circular and linear interactive genome visualization. Bioinformatics 25, 119–120. 10.1093/bioinformatics/btn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinda D, Nakaji S, Fukuda S, Sakamoto J, Shimoyama T, Nakamura T, Fujisawa T, Terada A, and Sugawara K (2004). The fermentation of different dietary fibers is associated with fecal clostridia levels in men. J Nutr 134, 1881–1886. 10.1093/jn/134.8.1881. [DOI] [PubMed] [Google Scholar]
- Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J Jr., Crawford JM, and Ferrara JL (1996). An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 88, 3230–3239. [PubMed] [Google Scholar]
- Dodd D, Mackie RI, and Cann IK (2011). Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes. Mol Microbiol 79, 292–304. 10.1111/j.13652958.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GP, Chou WC, Manson AL, Rogov P, Abeel T, Bochicchio J, Ciulla D, Melnikov A, Ernst PB, Chu H, et al. (2020). Spatially distinct physiology of Bacteroides fragilis within the proximal colon of gnotobiotic mice. Nat Microbiol 5, 746–756. 10.1038/s41564020-0683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC (2016). UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv, 081257. 10.1101/081257. [DOI] [Google Scholar]
- Elgarten CW, Li Y, Getz KD, Hemmer M, Huang YV, Hall M, Wang T, Kitko CL, Jagasia MH, Nishihori T, et al. (2021). Broad spectrum antibiotics and risk of graft-versus-host disease in pediatric patients transplanted for acute leukemia: association of carbapenem use with risk of acute GVHD. Transplant Cell Ther 27, 177 e171–177 e178. 10.1016/j.jtct.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, and Infectious Diseases Society of, A. (2011). Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 52, 427–431. 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- Hayase E, Hashimoto D, Nakamura K, Noizat C, Ogasawara R, Takahashi S, Ohigashi H, Yokoi Y, Sugimoto R, Matsuoka S, et al. (2017). R-Spondin1 expands Paneth cells and prevents dysbiosis induced by graft-versus-host disease. J Exp Med 214, 3507–3518. 10.1084/jem.20170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey CA, Kuhn KA, Donermeyer DL, Porter NT, Jin C, Cameron EA, Jung H, Kaiko GE, Wegorzewska M, Malvin NP, et al. (2015). Colitogenic Bacteroides thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles. Cell Host Microbe 17, 672–680. 10.1016/j.chom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka D, Hayase E, Shiratori S, Hasegawa Y, Ishio T, Tateno T, Okada K, Goto H, Sugita J, Onozawa M, et al. (2018). The association between the incidence of intestinal graftvs-host disease and antibiotic use after allogeneic hematopoietic stem cell transplantation. Clin Transplant 32, e13361. 10.1111/ctr.13361. [DOI] [PubMed] [Google Scholar]
- Hill GR, and Ferrara JL (2000). The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 95, 2754–2759. [PubMed] [Google Scholar]
- Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, Zhu W, Sporrer D, Hehlgans T, Kreutz M, et al. (2014). Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 20, 640–645. 10.1016/j.bbmt.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, and Macpherson AJ (2012). Interactions between the microbiota and the immune system. Science 336, 1268–1273. 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, Liu C, West ML, Singer NV, Equinda MJ, et al. (2012). Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med 209, 903–911. 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovel J, Patterson J, Wang W, Hotte N, O’Keefe S, Mitchel T, Perry T, Kao D, Mason AL, Madsen KL, and Wong GK (2016). Characterization of the Gut Microbiome Using 16S or Shotgun Metagenomics. Front Microbiol 7, 459. 10.3389/fmicb.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilcoyne M, Gerlach JQ, Farrell MP, Bhavanandan VP, and Joshi L (2011). Periodic acidSchiff’s reagent assay for carbohydrates in a microtiter plate format. Anal Biochem 416, 18–26. 10.1016/j.ab.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kolmogorov M, Yuan J, Lin Y, and Pevzner PA (2019). Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 37, 540–546. 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- Koropatkin NM, Martens EC, Gordon JI, and Smith TJ (2008). Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16, 1105–1115. 10.1016/j.str.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M, Cheong M, Markey KA, Gartlan KH, Kuns RD, Locke KR, Lineburg KE, Teal BE, Leveque-El Mouttie L, Bunting MD, et al. (2015). Donor colonic CD103+ dendritic cells determine the severity of acute graft-versus-host disease. J Exp Med 212, 1303–1321. 10.1084/jem.20150329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Lim JY, Ryu DB, Kim TW, Park SS, Jeon YW, Yoon JH, Cho BS, Eom KS, Kim YJ, et al. (2019). Alteration of the Intestinal Microbiota by Broad-Spectrum Antibiotic Use Correlates with the Occurrence of Intestinal Graft-versus-Host Disease. Biol Blood Marrow Transplant 25, 1933–1943. 10.1016/j.bbmt.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Ley RE, Lozupone CA, Hamady M, Knight R, and Gordon JI (2008). Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol 6, 776–788. 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, and Durbin R (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Lladser ME, Knights D, Stombaugh J, and Knight R (2011). UniFrac: an effective distance metric for microbial community comparison. ISME J 5, 169–172. 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Chiang HC, and Gordon JI (2008). Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4, 447–457. 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, OraveczWilson K, Wu SR, Sun Y, Rossi C, et al. (2016). Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol 17, 505–513. 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava GM, and Stappenbeck TS (2011). Diversity of the autochthonous colonic microbiota. Gut Microbes 2, 99–104. 10.4161/gmic.2.2.15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura R, Kurakawa T, Nakano T, Kayama H, Kinoshita M, Motooka D, Gotoh K, Kimura T, Kamiyama N, Kusu T, et al. (2016). Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature 532, 117–121. 10.1038/nature17406. [DOI] [PubMed] [Google Scholar]
- Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, Weber D, Hashimoto D, Slingerland AE, Slingerland JB, et al. (2020). Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N Engl J Med 382, 822–834. 10.1056/NEJMoa1900623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, and Thomas ED (1995). 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15, 825–828. [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, and Glockner FO (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41, D590–596. 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TE, Pudlo NA, Koropatkin NM, Bell JS, Moya Balasch M, Jasker K, and Martens EC (2013). Dynamic responses of Bacteroides thetaiotaomicron during growth on glycan mixtures. Mol Microbiol 88, 876–890. 10.1111/mmi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognes T, Flouri T, Nichols B, Quince C, and Mahe F (2016). VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584. 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75, 7537–7541. 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield WB, Zimmermann-Kogadeeva M, Zimmermann M, Barry NA, and Goodman AL (2018). The Stringent Response Determines the Ability of a Commensal Bacterium to Survive Starvation and to Persist in the Gut. Cell Host Microbe 24, 120–132 e126. 10.1016/j.chom.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder TH, Reiniger N, Meluleni G, Grout M, Coleman FT, and Pier GB (2001). Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J Immunol 166, 7410–7418. 10.4049/jimmunol.166.12.7410. [DOI] [PubMed] [Google Scholar]
- Schwab L, Goroncy L, Palaniyandi S, Gautam S, Triantafyllopoulou A, Mocsai A, Reichardt W, Karlsson FJ, Radhakrishnan SV, Hanke K, et al. (2014). Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med 20, 648–654. 10.1038/nm.3517. [DOI] [PubMed] [Google Scholar]
- Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, Slingerland AE, Smith OM, Young LF, Gupta J, et al. (2016). Increased GVHD-related mortality with broadspectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 8, 339ra371. 10.1126/scitranslmed.aaf2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms-Waldrip TR, Sunkersett G, Coughlin LA, Savani MR, Arana C, Kim J, Kim M, Zhan X, Greenberg DE, Xie Y, et al. (2017). Antibiotic-Induced Depletion of Antiinflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol Blood Marrow Transplant 23, 820–829. 10.1016/j.bbmt.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, and Garrett WS (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, and Gordon JI (2005). Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307, 1955–1959. 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- Stothard P (2000). The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 28, 1102, 1104. 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- Tailford LE, Crost EH, Kavanaugh D, and Juge N (2015). Mucin glycan foraging in the human gut microbiome. Front Genet 6, 81. 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, Langston AA, Nastoupil LJ, Rajotte M, Rolston KV, et al. (2018). Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol 36, 3043–3054. 10.1200/JCO.18.00374. [DOI] [PubMed] [Google Scholar]
- Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, and Ostell J (2016). NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44, 6614–6624. 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, et al. (2014). The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124, 1174–1182. 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerAvest MA, He Z, Rosenbaum MA, Martens EC, Cotta MA, Gordon JI, and Angenent LT (2014). Regulated expression of polysaccharide utilization and capsular biosynthesis loci in biofilm and planktonic Bacteroides thetaiotaomicron during growth in chemostats. Biotechnol Bioeng 111, 165–173. 10.1002/bit.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrapon N, Lombard V, Drula E, Lapebie P, Al-Masaudi S, Gilbert HJ, and Henrissat B (2018). PULDB: the expanded database of Polysaccharide Utilization Loci. Nucleic Acids Res 46, D677–D683. 10.1093/nar/gkx1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hee B, and Wells JM (2021). Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol 29, 700–712. 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Wick RR, Judd LM, and Holt KE (2019). Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol 20, 129. 10.1186/s13059-019-1727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, et al. (2013). Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11, 61. 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YW, Chen MK, Yang BY, Huang XJ, Zhang XR, He LQ, Zhang J, and Hua ZC (2015). Use of 16S rRNA Gene-Targeted Group-Specific Primers for Real-Time PCR Analysis of Predominant Bacteria in Mouse Feces. Appl Environ Microbiol 81, 6749–6756. 10.1128/AEM.01906-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The relative abundances of Clostridia and SCFAs were significantly decreased by meropenem treatment, related to Figure 1. (A) Meropenem concentrations in the cecal contents of mice were measured 4, 8, 24, 48 and 96 hours after subcutaneously injection of meropenem using LC-MS. (B) Bacterial densities of mouse stool samples collected 7 days after administering with meropenem by drinking water. Bacterial densities were measured by qPCR of 16S rRNA. (C) Relative abundance of the class Clostridia on days 0, 7, 14 and 21. (D) Relative abundances of SCFAs in stool samples from normal mice, alloHSCT mice and meropenem-treated allo-HSCT mice on day 18 measured by IC-MS.
Figure S2. Levofloxacin and cefepime do not increase the severity of colonic GVHD in allogeneic mice, related to Figure 1. (A) Experimental schema of murine GVHD model using levofloxacin, cefepime or meropenem treatment. Allo-HSCT was performed as in Figure 1. Mice were treated with levofloxacin, cefepime or meropenem from days 3 to 15. (B) H&E staining of histological sections of the colon collected on day 20 after allo-HSCT. Bar, 100 μm. (C) GVHD histology scores of the colon collected on day 20 after allo-HSCT, quantified by a blinded pathologist. Data are combined from two independent experiments and are shown as means ± SEM. (D) Relative abundances of SCFAs in stool samples from allo-HSCT mice, levofloxacin-, cefepime-, and meropenem-treated alloHSCT mice on day 20 measured by IC-MS. Data are shown from one representative experiment as means ± SEM.
Figure S3. The intestinal microbiome in allo-HSCT patients treated or untreated with meropenem at pre-HSCT and on day 14, related to Figure 2. Additional analyses of intestinal microbiome profiling results of allo-HSCT patient samples presented in Figure 2, A–E. (A) Alpha diversity shown using the inverse Simpson index at preHSCT and on day 14. (B) PCoA between meropenem-untreated and treated patients at pre-HSCT (left) and on day 14 (right). (C) Paired-Wilcoxon test of bacteria between at pre-HSCT and on day 14 in meropenem-treated patients (shown in red) or meropenem-untreated patients (shown in blue).
Figure S4. Loss of Clostridia and expansion of BT are seen in meropenem-treated allogeneic mice and not levofloxacin- or cefepime-treated mice, related to Figure 2–4.(A) Stacked bar graphs of bacterial genera composition of fecal samples collected on day 20 after allo-HSCT. (B) Relative abundance of the class Clostridia on day 20. (C) Relative abundance of BT on day 20. (D) PCoA of stool and mucus in distal colon from allogeneic mice (left), allogeneic mice treated with levofloxacin (middle), and allogeneic mice treated with cefepime (right). Numbers shown in each PCoA depict samples collected from the same individual mouse. (E) Beta diversity distances within individual mice. (B-E) Data are shown from one representative experiment.
Figure S5. Mucin-deficient BT did not aggravate the severity of GVHD, related to Figure 5–6. (A) Bacterial culture with or without porcine gastric mucin. OD600nm was measured after 48 hours of anaerobic culture. (B) Levels of porcine gastric mucin in the culture supernatant was determined by using a PAS-based colorimetric assay. Mouse-derived Enterococcus faecalis were used as nonmucolytic bacterial control. (C) Circular plot of open reading frames (ORFs) derived from the complete genome (MDA-JAX BT001). Blue and green bars represent ORFs on the plus strand and the minus strand, respectively. Inner purple-olive ring depicts degree of GC skewing. (D) Bacterial culture of WT BT and mucin-deficient BT in carbohydrate-poor media supplemented with porcine gastric mucin. OD600nm was measured at 20 hrs. (E) Levels of porcine gastric mucin in the culture supernatant was determined by using a PAS-based colorimetric assay. (F) Experimental schema of murine GVHD model using decontamination therapy followed by oral introduction of WT BT (ATCC 29148) or mucin-deficient BT. Mice were decontaminated with piperacillin/tazobactam in the drinking water and vancomycin and metronidazole by oral gavage from days −16 to −12 followed by oral gavage of 2×107 CFUs of WT BT or mucin-deficient BT given daily from days 6 to −4 relative to allo-HSCT. (G) Relative abundance of BT in fecal samples collected on day 21. (A-B, D-E, G) Data are shown from one representative experiment as means ± SEM. (F) Overall survival. Data are combined from two independent experiments.
Figure S6. Mucus-degrading function of BT was not suppressed by glucose in allogeneic mice treated with meropenem, related to Figure 6. Experimental schema of in vitro bacterial culture assay of BT in media with porcine gastric mucin-containing medium with or without monosaccharides. (B) Relative concentrations of porcine gastric mucin in medium following culture with BT (MDA-JAX BT001). BT was first introduced to porcine gastric mucin-containing medium. At 15 hours of culture, monosaccharides were added into the culture broth and incubated for an additional 3 hours. Levels of mucin glycans in the culture supernatant were determined using a colorimetric assay. Mucin concentrations were normalized to levels of mucin glycans from non-sugar-exposed BT conditions in two independent experiments. (C) mRNA was extracted from BT pellets cultured with or without monosaccharides and subjected to qPCR analysis of GH2, GH29 and GH33 BT loci. Relative expressions of mRNA were shown by the comparative ΔCt method. (D) PAS staining of histological colon sections collected on day 28. Bar, 100 μm. The areas inside dotted lines indicate the inner dense colonic mucus layer. (E) Mucus thickness on day 28. (F) Bacterial culture with or without the indicated monosaccharide. Monosaccharides were added at 0 hr. OD600nm was measured every 2 hours up to 30 hours. (G) Relative expression levels of PULs in BT RNA transcripts. (B-C, E, G) Data are shown from one representative experiment as means ± SEM.
Data Availability Statement
16S rRNA sequencing data, RNA sequencing data and complete genome data have been deposited at Sequence Read Archive (SRA). All data are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


