ABSTRACT
The use of probiotics has been one of the effective strategies to restructure perturbed human gut microbiota following a disease or metabolic disorder. One of the biggest challenges associated with the use of probiotic-based gut modulation strategies is to keep the probiotic cells viable and stable during the gastrointestinal transit. Biofilm-based probiotics delivery approaches have emerged as fascinating modes of probiotic delivery in which probiotics show significantly greater tolerance and biotherapeutic potential, and interestingly probiotic biofilms can be developed on food-grade surfaces too, which is ideal for the growth and proliferation of bacterial cells for incorporation into food matrices. In addition, biofilms can be further encapsulated with food-grade materials or with bacterial self-produced biofilms. This review presents a newly emerging and unprecedently discussed techniques for the safe delivery of probiotics based on biofilms and further discusses newly emerging prebiotic materials which target specific gut microbiota groups for growth and proliferation.
KEYWORDS: Probiotic biofilms, next generation probiotics, gut microbiota, prebiotics, Bio-therapeutic potential
Introduction
Human body harbors trillions of diverse microorganisms (bacteria, protozoa, archaea, eukaryotes, and viruses), which reside on and within different parts of the body including the gut, skin and vagina. The human gastrointestinal tract contains the largest number and diversity of the known species repertoire of the human gut microbiota (> 70% of the human microbiota: 1013 to 1014), termed “gut microbiome” 1. A stable gut microbial community of the host plays a key role in host’s innate and adaptive immune system,2 metabolism, and health.3 Disruptions in the gut microbiota may occur owing to antibiotic use, changes in lifestyle, dietary habits, infection, or ageing, which lead to variegated pathogenic, metabolic, and inflammatory conditions. Today, there is almost no disease which has no relevance with the human gut microbiota. From intestinal bowel diseases (Crohn’s disease and ulcerative colitis),4 cancer,5 hypertension,6 and diabetes 7 to mental health including anxiety and depression 8 and metabolic syndrome and atopy.9
In appreciation of microbial signatures of health and disease, microbiome-modulating interventions, especially the use of probiotics, prebiotics, postbiotics, synbiotics, live biotherapeutic products, and faecal microbiota transplants (FMT) (see definitions in Box 1), have gained escalating interest in microbiome research and clinical and healthcare settings.10 Out of these approaches, FMT is considered as the most effective therapy which greatly supports microbiota colonization; however, its complicated procedure, high cost, and risk of overt immune reaction and transmission of potential pathogens are critical hurdles.11 Probiotic therapy (mainly orally) is also considered as a great intervention strategy for gut microbiota modulation and indeed a promising strategy for clinical translation because of being less costly, safer, and controllable.
Probiotics are not only used to aim restructuring of microbial dysbiosis but also they impart many useful functions to the gut including modulation of innate immune system (mainly by gut microbiota modulation), prevention of pathogen colonization, enhancement of gut barrier function, mucin production, and increased expression of tight junction proteins. Benefits of probiotics on several human health outcomes are unanimously acknowledged and reported. Probiotic enrichment and delivery in the human gut is crucial to achieve health benefits, but these techniques have met with substantial challenges for therapeutic applications and thus it is imperative to consider the principles that govern the successful establishment and persistence of probiotics in the gut environment. Many health benefits of probiotics are attenuated mainly due to a substantial loss in the viability during the gastrointestinal transit.12 The key to improving colonization of a bacterial species in the gut is to deliver it in a material resistant to many environmental stressors (for example, pH and bile salts) or to convert it into its biologically resilient form (e.g., biofilms). A biofilm is an assemblage of surface-adhered microbial cells embedded in self-produced extracellular polymeric substances (EPS) containing proteins, extracellular DNA (eDNA), polysaccharides, and lipids.13 Any exogenously introduced gut bacterium which does not form biofilm into a new environment is likely to be eliminated quickly, even when delivered in abundance; this is the reason behind the low efficiency of many probiotic strains.14
Several reviews have been published on the encapsulation of probiotics with a focus on several techniques (e.g., extrusion, emulsion, electrospinning, freeze drying, spray chilling, fluidized bed, spray drying, spray-freeze drying, coacervation) and materials, such as alginate, microbial polysaccharides, prebiotics, gums, mucilages,15 and bacterial spore coat nanomaterial.16 The focus of this review is on novel biofilm-based encapsulation systems and biofilm-based strategies to enhance oral bioavailability and gastrointestinal delivery of probiotics. We have also emphasized on the need of developing specific prebiotics which proliferate and enrich specific bacterial groups in the human gut.
Box 1. Definition of the terms probiotics, prebiotics, postbiotics, synbiotics, biotherapeutic products, and fecal microbiota transplant.
| Probiotics | “Live microorganisms which when administered in adequate amounts confer a health benefit on the host” | 17 |
| Postbiotics | “Preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” | 18 |
| Prebiotics | “A substrate that is selectively utilized by host microorganisms conferring a health benefit” | 19 |
| Synbiotic | “A mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” | 20 |
| Biotherapeutic products | “Live organisms designed and developed to treat, cure, or prevent a disease or condition in humans” | 21 |
| Fecal microbiota transplant | “A treatment that involves administration of minimally manipulated microbial community from stool of a healthy donor into the patient’s intestinal tract” | 22 |
Probiotics and the human gut microbiota
Human gut microbiota contains a large diversity of bacteria, viruses (mostly bacteriophages), and fungi which live together in an intricate balance and dynamic equilibrium. The symbiotic relationship between gut bacteria and host is very old as these symbiotic microorganisms and animals have evolved together as a result of the co-evolution that dates million years back.23,24 Bacterial colonization of humans starts from the intrauterine period when the fetus is exposed to the microbiota from mother through transplacental passage into amniotic fluid.25 Birth method (full term vaginal delivery or caesarean) also affects the acquisition and development of the gut microbiota of new born babies.26 Microbiota is further affected by introduction of oral liquid feedings (breast milk or formula milk) and food intake, and its structure keeps changing until approximately 3 years when gastrointestinal tract represents mature microbiome.25 Once established, the composition of the gastrointestinal microbiota remains relatively stable throughout adult life, but factors like disease conditions, use of antibiotics, surgical treatments, lifestyle, and long-term changes in diet cause a shift in microbiota.
The microbiota-host synergy guards the balance of the gut microbiota against invasion of pathogens. Thus, the balanced microbial community of the human gut has implications for health, immunity, and diet and nutrition. Perturbations of the gut microbiota, termed gut dysbiosis, are clearly associated with many diseases including colitis, liver diseases, diabetes mellitus (including type 2 diabetes), obesity, cardiovascular diseases, irritable bowel syndrome, allergy, asthma, protein conformational diseases, many neurodegenerative disorders, food allergies 3,27–31 as well as viral infections.32 Disruption of the gut microbiota may foster dominance of harmful pathogens over beneficial bacterial groups, for example, short chain fatty acid (SCFA)-producing or butyrogenic bacteria. Enterobacterial blooms have been widely reported in gut dysbiosis in various conditions related to inflammation in the gut.33 A recent study showed that colonization of the Caenorhabditis elegans gut with Gram-negative enteric bacterial pathogens led to aggregation and proteotoxicity of polyglutamine across several tissues of the body; whereas replacement of the microbiota with butyrogenic bacteria reversed the adverse effects by increasing host proteostasis and suppressing proteotoxicity pathogen-mediated aggregation of polyglutamine.28
Administration of bacteriocin-producing Pediococcus acidilactici strains to mice led to an increase in Ruminococcus and Lactobacillus and a decrease in Blautia sp, which shows probiotics may also affect gut microbiota dynamics, possibly due to their metabolites, among many other factors.34
Probiotics are used to restore the original gut microbiota structure which has been shown to have ameliorative effects on several metabolic disorders and diseases. Intake of probiotics does not necessarily mean enrichment of a specific bacterial group in the human gut, but microbial synergistic interactions foster growth of other bacterial groups too or in many cases trigger targeted bacterial growth, which would not have been possible otherwise. Sadiq et al. demonstrated that co-occurrence of four bacterial species of the human gut was complimentary for enhanced colonization on mucin and better growth.35 Another recent study showed that treatment with multiple probiotics (three strains of Lactiplantibacillus plantarum) diversified the gut microbiome of the subjects and resulted in an average increase of the beta diversity by 37.2% between-individual as a result of synergistic and modulatory effects of the specific strains in combination with the gut microbiota.36 Similarly, a recent study showed that feeding with a mixture of four Bifidobacterium species to mice resulted in a more favourable gut ecosystem with a significant increase in the abundance of Lactobacillus, leading to mitochondrial fitness and the IL-10–mediated suppression of Treg cells.37
Malnutrition in children has been related to perturbations in the normal development of the gut microbiota. Specific probiotic strains of the gut origin could potentially be exploited to resolve undernutrition syndromes through modulating the gut microbiota.38 Table 1 shows evidence from different studies where probiotic strains ameliorated different diseases and metabolic conditions through modulation of the human gut microbiota. In addition to their role in gut microbiota modulation, many probiotics produce complimentary enzymes required for normal metabolism as without certain bacterial taxa in the human gut, many food components may remain indigestible owing to the paucity of alimentary enzymes.61 For instance, enzymes to digest xyloglucans are only encoded by a single, complex gene locus present in Bacteroides ovatus.62
Table 1.
Role of probiotics in ameliorating various diseases and metabolic conditions through modulation of the gut microbiota.
| Name of the probiotic | Targeted disease/metabolic condition | Targeted gut microbiota | Type of the model | Reference |
|---|---|---|---|---|
| Lactobacillus acidophilus EG004 | Cognitive conditions | An increase in the Firmicutes and Proteobacteria phyla | A mouse model | 39 |
| Pediococcus pentosaceus | Colorectal cancer | Increased the abundance of Akkermansia-Verrucomicrobia clade | A mouse model | 40 |
| EcologicBarrier consisting of Lactobacilli, Lactococci, and Bifidobacteria | Stress | Increased the abundance of Butyricimonas, Parabacteroides, Alistipes, Christensenellaceae_R-7_group | Human subjects | 41 |
| Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 | Anxiety-like behavior | An increase in the population of Prevotella and Bifidobacterium species | Hamster model | 42 |
| Lactiplantibacillus plantarum TWK10 | Aging-related disorders | Increased population of SCFA-producing bacteria | A mouse model | 43 |
| L. plantarum CCFM8610 | Irritable bowel syndrome | Increased population of butyrate-producing bacteria (e.g., Anaerostipes, Anaerotruncus, Bifidobacterium, and Butyricimonas), and decreased the abundance of bacterial genus causing bloating (Methanobrevibacter) | A mouse model | 44 |
| L. Plantarum PL-02 | Fatigue and tiredness | Increased the abundance of Akkermansia muciniphila and reduced the population of Blautia coccoides and Pedobacter kwangyangensis | A mouse model | 45 |
| L. Plantarum P8 | Stress | Increased the abundance of Bifidobacterium adolescentis, Faecalibacterium prausnitzii, and B. longum | Human fecal samples | 46 |
| Lactobacillus paracasei | Type-2 diabetes | A significqnt increase in Bacteroidales_S24-7_group and the families Ruminococcaceae and Lachnospiraceae | Type 2 diabetic (T2D) rat model | 47 |
| Lactobacillus rhamnosus LRa05 | Hyperglycemia | An increase in short-chain fatty acid producing bacteria (Bacteroides and Alloprevotella) and a decrease in proinflammatory bacteria (Mucispirillum and Odoribacter) | Type 2 diabetic mice | 48 |
| Lactobacillus reuteri FGSZY33L6 and L. rhamnosus FJSYC4-1 | Metabolic syndrome | Increased the relative abundance of Desulfovibrio whilst decreasing the abundance of Romboutsia | Mice with a high-fat diet | 49 |
| Bifidobacterium breve,Bifidobacterium bifidum, B. longum, and Bifidobacterium lactis | Colitis | Increased the abundance of Lactobacillus, Bifidobacterium, Kosakonia, and Cronobacter | A mouse model | 37 |
| L. plantarum DR7 | Stress and anxiety | Increased alpha diversity and an increase in bacteria associated with dopamine and serotonin (Bacteroides and Firmicutes) | - | 50 |
| B. breve, B. bifidum and B. longum | Dextran sodium sulfate induced colitis | Decreased the abundance of pathobionts such as Klebsiella, Haemophilus and Lachnospira | A mouse model | 51 |
| L. plantarum HT121 | Obesity | Restored Firmicutes/Bacteroidetes ratio | A mouse model | 52 |
| ProBiotic-4 (B. lactis, B. bifidum, Lactobacillus casei, and L. acidophilus | Memory deficit | Decreased the abundance of Proteobacteria, Pseudomonas and Lachnospiraceae NK4A136 group | A mouse model | 53 |
| L. reuteri | - | - | - | – |
| B. bifidum CCFM16 and L. plantarum CCFM8610 | Atopic dermatitis | Increased the proportion of Bacteroidetes | Randomized controlled human trial | 54 |
| Bifidobacteria adolescentis | Atopic dermatitis | Increased the abundance of Lactobacillus species and decreased the abundance of Dorea and Pediococcus | A mouse model | 55 |
| L. reuteri | Asthma | Lactobacillus and Enterococcus abundance | A mouse model | 56 |
| L. Plantarum ZDY2013 | Colitis | Increased the abundance of Lactobacillus animalis | A mouse model | 57 |
| - | - | Stablized the abundance of Alistipes, Bacteroidetes and Alloprevotella | - | - |
| L. Plantarum LIP-1 | Hyperlipidaemia | Reduced lipopolysaccharide-producing bacteria (e.g., Sutterella, Oscillibacter, and Bilophila and increased the abundance of SCFA-producing bacteria such as Lactobacillus, Ruminococcus, Eubacterium, Alloprevotella and Coprococcus | Rats | 58 |
| L. plantarum | Chronic undernutrition | The gut microbiota which interacts with the somatotropic hormone axis | A mouse model | 59 |
| Lactobacillus curvatus HY7601 and L. plantarum KY1032 | Obesity | Increased the abundance of 11 species of phylum firmicutes | A mouse model | 60 |
Most of the probiotics that are used in either commercial probiotic development or probiotic research mostly belong to limited genera, such as Bifidobacterium and Lactobacillus and less commonly to Saccharomyces, Bacillus, Escherichia, and Weissella. With advancements in gut microbiome science, we are on the cusp of a new era of research on probiotics and synthetic microbial communities to abolish intestinal imbalance as a result of diseases and to meet consumer’s demands. An astounding range of candidates for probiotics have emerged many of which have yet to adapt the gut environment. Disease-specific next-generation probiotics have rapidly attracted much more attention than traditional probiotics with non-specific effects.63 In 2008, a clinal study on humans demonstrated the role of Faecalibacterium prausnitzii in inflammatory bowel disease (IBD) pathogenesis, where the pathological conditions of colitis were significantly related to low count of F. prausnitzii 64 and this was further supported by subsequent studies.65,66 The therapeutic potential of this commensal bacterium (as probiotic) and its supernatant (as postbiotic) has been demonstrated in chronic colitis and colitis reactivation in a TNBS (2,4,6-Trinitrobenzene sulfonic acid)-induced acute colitis mouse model.67,68 Ameliorative effects of F. prausnitzii through gut microbiota modulation have now been reported in many metabolic diseases such as, obesity 69 and liver diseases.70 In addition, many other next-generation probiotics have shown beneficial roles in the prevention of carcinogenesis by maintaining intestinal barrier integrity, improving immunotherapy efficacy, producing beneficial metabolites, and acting against pathogens.71
Controlled delivery of probiotics
Transplanting probiotics to the gut microbiome can positively modulate bacterial population dynamics and structure, and thus probiotic intervention strategies remain of great interest for disease prevention and treatment. The biggest challenge in the development of probiotic-based dietary and therapeutic supplements and in achieving their clinical efficacy is related to their targeted delivery to specific sites in the human body. Orally administered probiotics encounter several challenges during the gastrointestinal transit, and these include host and microbial enzymes (e.g., saliva in the mouth), digestive enzymes (e.g., acidic gastric fluids in the stomach, pancreatic juice and bile in the small intestine), and colonization resistance (e.g, in the colon where bacterial density is 1011 to 1012 CFU/ml) from commensal bacteria.12
Therefore, effective probiotics delivery systems and bioinspired strategies are needed to effectively use the potential of probiotics through improved tolerance during the gastrointestinal transit and rapid colonization.
Immunological and anti-inflammatory aspects probiotic biofilms
Bacterial biofilms are communities of bacteria derived from either a single or multiple bacterial species, which grow on biotic or abiotic surfaces, or remain free floating as an aggregate in liquid or at interfaces. Biofilm is a bacterial life phase which is remarkably different from the same bacteria in planktonic form in terms of physiology, genetics, and sometimes morphology and whereby specific metabolic activities are juxtaposed. Biofilm-dwelling cells remain protected from various environmental stressors (for example, antimicrobials) and bacteriophages because of complex self-produced or host-derived polysaccharide sheets, which comprise extracellular DNA (e-DNA), proteins, and lipids.72 Due to multifaceted material properties of probiotic bacterial biofilms (for example, chemically active, resilient, hydrophobic, sticky, slimy, conductive, self-healing, and safe), they can be used in a broad range of applications. In addition, properties of biofilms can be modified and improved further. For instance, porosity of biofilms can be changed which improves the biofilm permeability toward nutrients and electrons and it ultimately improves the viability of bacterial cells enclosed within the matrix. Various biologically important compounds can be integrated into the biofilm matrix such as minerals, nanoparticles, enzymes, and metal ions which make the biofilm functionally diverse and induce crosslinking effects. Biofilms can be further encapsulated with polymers and minerals to make them further tolerant towards environmental stresses.73
Probiotics delivered in the form of biofilm confer several advantages on the host (Figure 1), compared to their conventional delivery in the planktonic form. Probiotic bacteria can be effectively delivered in their biofilms where they remain viable during the gastrointestinal transit and show more adherence characteristics. Probiotics in the form of biofilms remain unaffected or at least less affected by saliva, gastric fluid, pancreatic juice, bile acids, antimicrobial agents, laminar/turbulent fluid forces, effectors of the host immune defence system, as well commensals, pathogenic bacteria and bacteriophages. In an in vivo swine study, clinical Bacillus subtilis probiotics which were coated with self-produced biofilms exhibited a 17-times higher intestinal colonization and 125-fold greater oral bioavailability compared to uncoated bacteria.74 Similarly, Lactobacillus reuteri when delivered in a biofilm formulation showed increased survival during the gastrointestinal transit and proved to be more effective in reducing NEC in a rodent model.75 Biofilm-dispersed cells may show more adherence potential compared to their counterpart planktonic cells. This has been evidenced from in vitro and in vivo study of Klebsiella pneumoniae cells spontaneously dispersed from biofilm. Biofilm-dispersed cells showed greater adhesion ability on biotic and abiotic surfaces.76
Figure 1.
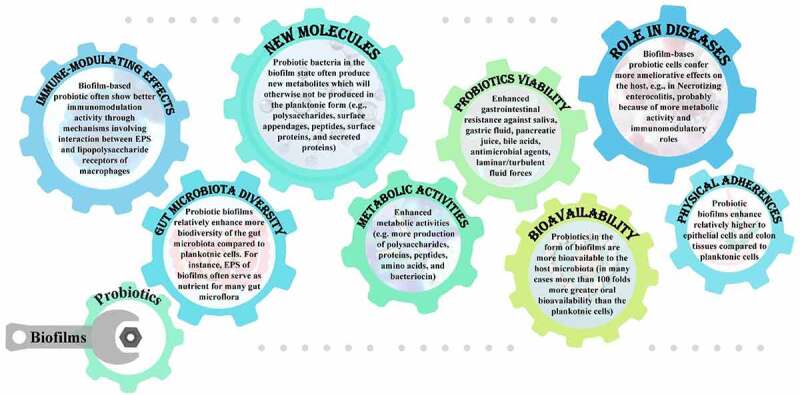
Major advantages of administrating probiotics in the form of biofilms.
Bacterial delivery in the form of biofilms is not only ideal because of enhanced physical adhesion and chemical barrier functions, but several reports indicate many beneficial effects of probiotic biofilms in terms of bacterial metabolic activities. Biofilms may exacerbate the beneficial effects of probiotics to host cells and in many cases probiotic bacteria in the form of biofilms may confer functions potentially more advantageous to the host. Bacterial cells in biofilms can also produce new molecules or larger quantities of certain molecules (e.g., polysaccharides, surface appendages, peptides, surface proteins, and secreted proteins) than counterpart planktonic cells.77 EPS provide additional functional advantages to the host which could not be achieved in the planktonic probiotic deliveries. Using untargeted metabolomics, it was recently reported that amino acids and carbohydrate metabolisms were greatly enhanced when the probiotic strain Lactobacillus paraplantarum L-ZS9 was delivered in the form of biofilms compared to its planktonic dose. Further, biofilm-based probiotic doses showed better immunomodulation activity and enhanced intestinal diversity of Lactobacillus microbiome in the dogs.78 Biofilm exopolysaccharides are produced abundantly in probiotic biofilms, like other bacterial biofilms, and thus protect bacterial species and confer many other metabolic advantages to the host. A fourfold increase in exopolysaccharide production by B. bifidum was reported in its biofilm compared to planktonic cells in a study involving untargeted metabolomics.79
There are several ways in which probiotic bacteria in the form of biofilms have better immunomodulatory and ameliorative effects compared to planktonic cells, and these are mainly related to better survival in biofilms and more EPS production (Figure 2). EPS from probiotic biofilms may interact with several carbohydrate recognition receptors present on the intestinal epithelial cells and such interactions are involved in modulating immune response. A plethora of immune-modulating effects of EPS produced by Lactobacillus and Bifidobacterium strains has been described.83
Figure 2.
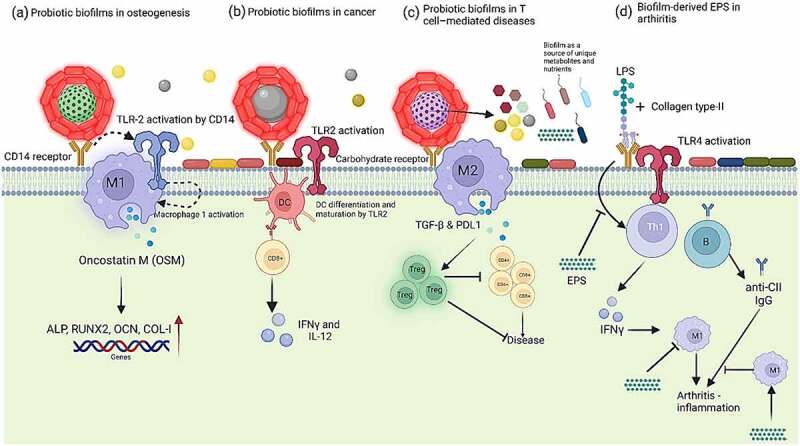
Probiotics in the form of biofilms are more effective than planktonic probiotic doses in several ways, especially in ameliorating diseases, as shown above in five different sections. (a) Polysaccharides on the surface of probiotic biofilms attach on CD14 of macrophages which leads to the activation of toll-like receptor cells (TLRs) signalling pathway. CD14 (cluster of differentiation 14) activates TLR2 to boost M1 macrophages phenotype which results in enhanced production of osteoinductive cytokines such as oncostatin M (OSM) to improve osteogenesis and it is evidenced by upregulation of osteogenic-related genes: runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and type I collagen (COL-I) 80 (b) Smectite laden with lactic acid bacteria biofilms inhibit tumor growth by activating dendritic cells (DCs) via Toll-like receptor 2 (TLR2) signalling (c) Exopolysaccharides from probiotic biofilms can induce M2 macrophages that inhibit CD4+ and CD8 + T cells by producing TGF-β and PD-L1 and possibly by induction of Tregs to prevent T-cell mediated diseases 81 (d) EPS from some LAB strain may inhibit T-cell proliferation and production of IFN-γ which leads to the polarization of M2 macrophage (an anti-inflammatory effect) and facilitates suppression of arthritogenic CII-specific IgG (T cell-dependent humoral response) – adapted from Nowak and colleagues.82 In addition, it has also been shown EPS from biofilms serve as a source of nutrients for commensals and probiotic biofilms also produce biofilm-specific metabolites which are not produced by planktonic doses.
Polysaccharides of bacterial biofilms interact with toll-like receptors (TLRs: TLR2 and TLR4 and C-type lectins) and induce the immune response in macrophages.81,84 It has been shown that EPS of bacterial biofilms can induce T cell-dependent immune responses in case of several inflammatory diseases. For instance, EPS produced by probiotic Lactobacillus rhamnosus KL37 82 and B. subtilis 81 inhibited T-cell proliferation and stimulated the production of IFN-γ. Lipopolysaccharides, on the outer surface of Gram-negative bacteria, are the main ligand for TLR4. Binding of LPS to TLR-4 leads to the activation of adaptor proteins like MyD88 and molecules like TIR-domain-containing adaptor-inducing β-interferon (TRIF) which are involved in intracellular signalling TLRs – especially TLR4. Adapter proteins phosphorylate and translocate NF-κB to the nucleus.85 NF-κB induces the expression of various genes involved in pro-inflammatory processes (e.g., cytokines and COX2), for examples those encoding cytokines and chemokines, and they also regulate inflammasome.86 EPS compete with LPS to bind to the surface of TLR-4 in the cell membrane (Figure 3. Biofilm-derived EPS from various probiotics has shown to downregulate TLR and inhibit the LPS-induced inflammatory responses, for examples EPS from L. plantarum,87 B. subtilis,81 L. rhamnosus,82 and Lactobacillus casei 80 can inhibit the LPS-induced inflammatory responses. EPS from bacterial biofilms also repress the expression of iNOS (inducible nitric oxide synthase) which causes oxidative stress by upregulating NRF2/HO-1 87 (Figure 3).
Figure 3.
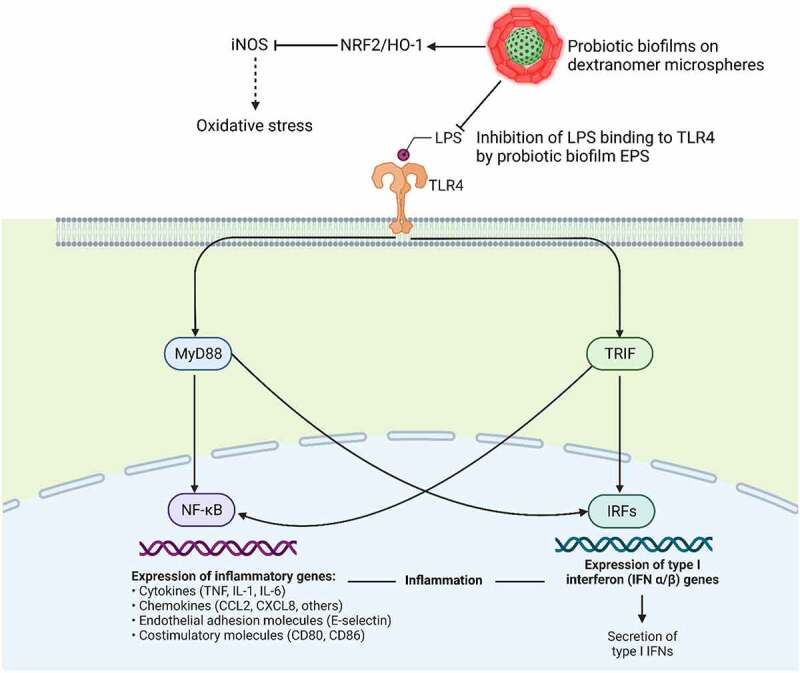
Probiotic biofilms (usuallu carbohydrate moites) compete with lipopolysaccharides on the surface of Gram-negative bacteria to bind to toll-Like receptor 4 (TLR4) cells, activation of which through MyD88 and TRIF pathways leads to the production of inflammatory factors. EPS from bacterial biofilms also repress the expression of iNOS (inducible nitric oxide synthase) which causes oxidative stress by upregulating NRF2/HO-1.81
Osteointegration ability of probiotic biofilms on medical implants is associated with their immunoregulatory and anti-infection properties. In case of osteogenesis, exopolysaccharides from inactivated food-grade L. casei biofilms interact with LPS receptors of macrophages, resulting in the activation of the TLRs pathways which stimulate the production of osteogenic cytokines (e.g., oncostatin M) by macrophages. EPS produced by L. casei biofilms on heat-treated titanium has shown to stimulate macrophages to produce larger quantities of osteogenic cytokines (e.g., oncostatin M), which helps to improve osteointegration of the titanium implant.80 High histamine production by probiotics in the biofilm state 79 may be a possible reason behind these observed phenotypes as histamine has been shown to be involved in upregulating oncostatin M in human macrophages in M1 macrophages.88 Apart from the role of EPS, another role of inactivated L. casei biofilm on the surface of titanium implants is their effective antibacterial potential against methylene resistant Staphylococcus aureus.80
Similarly, EPS produced by L. rhamnosus KL37 showed immunomodulatory properties in case of collagen-induced arthritis because of its potential to inhibit either one or both possible inflammatory signalling pathways: Th1 → IFN-γ → M1 inflammatory macrophages → arthritis and/or Th1 → IFN-γ → B cells → arthritogenic antibodies → arthritis.82
Biofilms on the surface of smectite have been reported to reduce tumor growth. Biofilm-laden smectite particles activate dendritic cells by the classical TLR2 signalling pathway and suppress tumor growth and increase the efficacy of chemotherapy, compared to planktonic doses of the same probiotic. In an in vitro trial, smectite with LAB showed to exhibit anti-tumor characteristics and enhanced the efficacy of chemotherapy and immunotherapy, when used in combination with these therapies.89
Necrotizing enterocolitis (NEC) is an inflammatory necrosis of the gut of premature infants which leads to the appearance of bloated and sensitive abdomen and results in feeding intolerance, and bloody diarrhoea and thus it results in a significant surgical emergency in neonates. A large number of meta-analyses have evaluated the effect of probiotics (e.g., Bifidobacterium and Lactobacillus) in NEC, concluding that probiotic intervention decreases the risk of NEC and reduces mortality in premature infants.90,91 A recent study using planktonic and biofilm forms of L. reuteri, on maltose loaded dextranomer microspheres (DM), to treat NEC in a rat model reported no significant effect of the planktonic doses of L. reuteri on NEC, compared to its biofilm formulation which reduced NEC in rats and this effect was attributed to more pronounced anti-bacterial and anti-inflammatory effects of L. reuteri in its biofilm form, mainly due to more production of reuterin and histamine.92 It is known that L. reuteri produces more abundant quantities of anti-bacterial and anti-inflammatory factors, especially reuterin, in its biofilm state, compared to its planktonic state.77
Another earlier study also reported the ameliorative effects of L. reuteri ATCC 23272 biofilm formulation developed on DM supplemented with biofilm-promoting substances (maltose and sucrose) against NEC. The biofilm-loaded microspheres improved mucosal barrier and inactivated the production of proinflammatory cytokines in a single prophylactic dose because the better survival of the bacterium during the gastrointestinal transit and enhanced adherence to gastrointestinal epithelial cells, compared to the delivery of the same bacterium in its planktonic form.93 In many other studies, L. reuteri biofilms developed on DM loaded with maltose have proved to be more effective, compared to planktonic cells, in ameliorating harmful effects of NEC in a rat model, which shows that the biofilm form of probiotics has more clinical potential for the deleterious effects of NEC.94,95
Human rotavirus (HRV) is one of the major causes of diarrhoea in children, causing a large-scale morbidity and mortality. Escherichia coli Nissle 1917 has been widely used in the treatment of many enteric diseases in humans; however, its clinical efficiency faces constraints due to limited stability and persistence of the strain. E. coli Nissle 1917 biofilm developed on DM reportedly enhanced innate and B cell immune responses after HRV infection and reduced diarrhoea as a result of HRV infection in malnourished pigs.96
In addition to the above-mentioned advantages of biofilm-based delivery of probiotics in different diseases/pathological conditions, EPS of biofilms also serve as a source of nutrients for some gut commensals as well as antagonists of pathogens in the gut.97
Another advantage of biofilm-based delivery system of probiotics is the encapsulation of many probiotic species/strains in the form of mixed biofilms that remain in close proximity because of bacterial interspecific interactions. Some gut species have been known to co-exist as a result of co-evolutionary processes. For example, L. reuteri has been reported to coexists in the form of mixed species biofilms with species which are part of the Lactobacillus johnsonii cluster (L. johnsonii, Lactobacillus gasseri, and Lactobacillus taiwanensis).98 This implies the idea of developing mixed species biofilm-based probiotic doses to target the human gut microbiota.
Effective biofilm-based probiotic delivery models
One of the most effective approaches for the safe delivery and complete dissemination of probiotics in the human gut is the exploitation of safe, food-grade, non-allergic, biocompatible materials as carriers for the delivery of biofilms. Polysaccharide-based materials (e.g., DM and calcium alginate beads), minerals (e.g., smectite), and food fibres (e.g., grape seeds and starch fiber of chickpea milk) have been successfully used for the development of probiotic biofilms and the use of many of these materials have been reported in successful biofilm-based probiotic delivery systems in several trials as shown in Figure 4. Probiotics delivered in the form of biofilms have been reported to exhibit immmunomodulatory and ameliorative effects evidenced from different research trials (Table 2).
Figure 4.
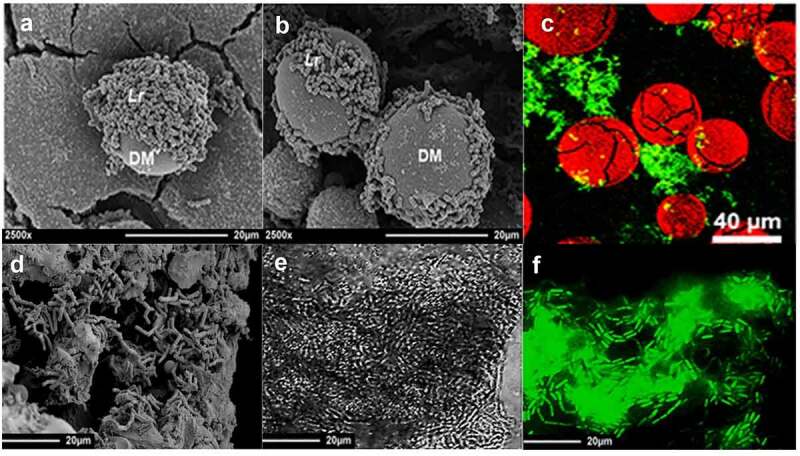
(a) Scanning electron microscopy image of Lactobacillus reuteri (Lr) biofilms on the surface of dextranomer microspheres (DM), (b) shows extracellualr polymeric substances joining the two microspheres (Olson, Navarro et al. 2018), (c) shows sucrose-filled DM (stained red with Congo Red) with biofilms of L. reuteri (stained green with SYTO9) (Navarro, Mashburn-Warren et al. 2017). (d) shows Bifidobacterium bifidum biofilms on the surface of grape seed flour, whereas (e and f) show biofilms of Bacillus subtilis on starch fiber of chickpea milk. B. subtilis is stained intense green with SYTO9, whereas starch fiber of chickpea milk is shown as faint green.99
Table 2.
Immunomodulatory and ameliorative effects of probiotics in the form of biofilms developed on different surfaces and trailed in different models.
| Name of the probiotic | Biofilm type | Any known effect | Model | Reference |
|---|---|---|---|---|
| Lactiplantibacillus plantarum | Biofilm in microtiter plates | Enhanced antibacterial activity of probiotic biofilms against Pseudomonas aeruginosa and Listeria monocytogenes | In vitro trials | 100 |
| Lactobacillus and Bifidobacterium | Biofilms on smectite (clay minerals) | Anti-cancer chemo/immunotherapy | Mouse model | 89 |
| Lactobacillus rhamnosus and L. plantarum | Biofilms developed on polystyrene surfaces | Biological activity against pathogens | In vitro trials | 100 |
| Lacticaseibacillus paracasei ATCC334 | Biofilms on calcium pectinate beads | Attenuated the effects of the dextran sulfate sodium (DSS)-induces colitis | Mouse model | 101 |
| Lactobacillus casei | Biofilm on the surface of titanium | Antibacterial activity against multidrug resistant Staphylococcus aureus and osteointegration ability | In vitro trials | 102 |
|
Biofilms on sucrose- or maltose-loaded dextranomer microspheres | Attenuated the effects of necrotizing enterocolitis | Mouse model | 93 |
| L. plantarum and Lactobacillus fermentum | Biofilm supernatant | Enhanced immunomodulatory effects | Zebrafish embryos | 103 |
| Lactobacillus casei ATCC334 | Biofilm supernatant | Enhanced immunomodulatory effects | Zebrafish larvae | 104 |
| L. reuteri | Biofilm in microtiter plates | Modulation of cytokine output and production of the antimicrobial reuterin | - | 77 |
The use of hollow semi-permeable, biodegradable and biocompatible DM has shown to be very effective in biofilm-based probiotic delivery systems. The interactions between probiotic biofilms and cross-linked dextran are mediated by glucosyltransferase-dependent adherence. Probiotic biofilms on the surface of DM have proven to be highly tolerant towards several harsh gastrointestinal conditions and resulted in successful delivery of probiotics to targeted sites with greater abilities to adhere to epithelial cells and mucosal surfaces.
DM is a porous material and thus many nutritious prebiotic substances can be loaded within the microsphere lumen, which may remain metabolically available only to probiotics and can successfully pass through the gastrointestinal tract. For instance, disaccharide (sucrose and maltose) may be metabolized and absorbed during the gastrointestinal transit if they are not protected by a suitable material. Lumen of DM cab be load with growth promoting disaccharides that are discriminatively available for the probiotics adhered to the surface of DM and the presence of these growth-promoting factors reportedly enhances adherence of probiotics on the surface of DM (Figure 5A).106 Probiotic strains on the surface of DM produce EPS which can help to stick two different DM particles together (Figure 5A and 5B). Interestingly, DM containing biofilms of L. reuteri can be loaded with L. histidine which serves as a unique method for the delivery of L-histidine to L. reuteri in the form of biofilms.106 Probiotic-biofilm containing DM particles assure a high density of probiotics on the surface of epithelial cells in a single dose – the density which could only be achieved by frequent repetition of planktonic doses.93 DM-based probiotic biofilm delivery system has also been reported to increase the probiotic potential of bacterial strains and maximize associated health benefits.
Figure 5.
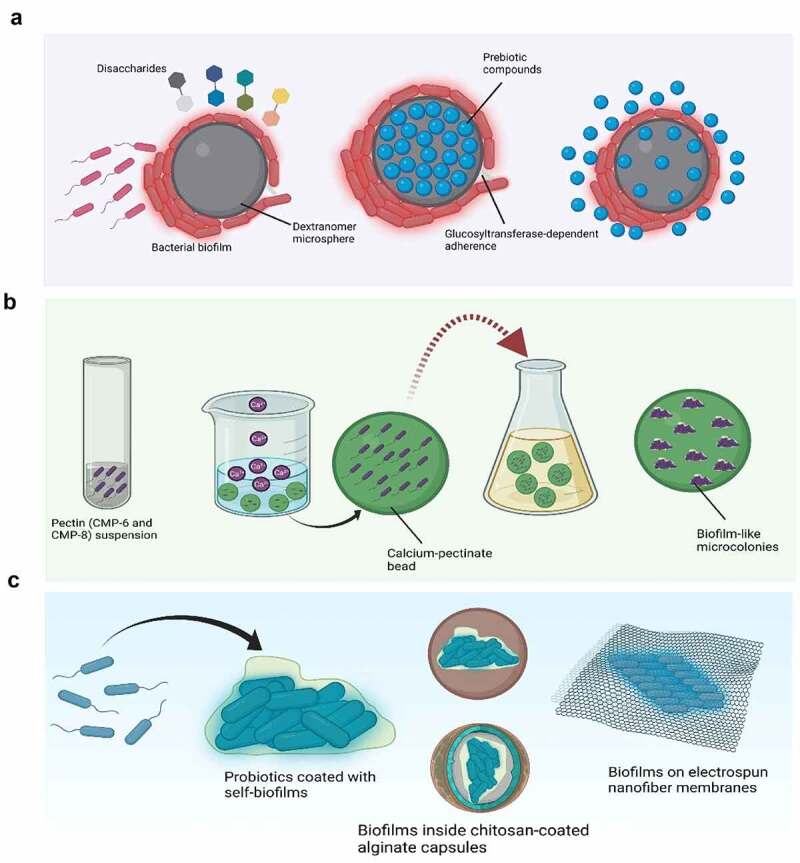
Biofilm-based probiotic delivery systems. Part A shows delivery of probiotic biofilm formed on the surface of a dextranomer (DM) where the addition of disaccharides, as growth-promoting factors, to the lumen of DM results in more probiotic growth and enhanced biofilm formation. The bright red colour outside the strains adhering to DM shows the produced extracellular polysaccharides (EPS) which help them to adhere to the surface of DM. The lumen of DM is shown to be loaded with nutritious prebiotic substances which remain available to the adhered probiotics and can survive the gastrointestinal transit. Part B shows encapsulation of probiotic cells into calcium pectinate capsules which promoted its growth into biofilm-like microcolonies and provided extra protection against environmental stressors. Part C shows coating of probiotic cells with their own biofilms containing EPS as a bioinspired strategy for oral doses of gut microbiota. Probiotic biofilm can be further encased in specific material as shown (chitosan-coated alginate capsules). Biofilms are further shown to be entrapped in electrospun cellulose acetate nanofiber membranes which has been reported to have great gastrointestinal resistance and it is ideal for the growth and biofilm formation of probiotics such as Lactoplantibacillus plantarum 105 as shown in part C.
Smectite (a type of silicate clay) has a large reactive surface area and it been reported to induce biofilm formation in many bacterial species.107 It remained ignored as a great bioactive material for the development of probiotic biofilms for a long time. The ion-exchangeable microstructure of smectite clay promotes biofilm formation, both in vitro and in vivo, by Lactobacillus and Bifidobacterium species on its surface. Organic acids produced by LAB supposedly give positive charge to the surface of smectite, which facilitates bacterial absorption because of their negatively charged cell wall as a result of teichoic acids.89 It is known that smectite can adhere to tissue surfaces such as mucus and helps to maximize bacterial contact with gastrointestinal organs. Smectite-laden biofilms have been shown to have ameliorative effects on tumors as discussed in the above section. Given the limited survival of probiotics in the gastrointestinal tract, the smectite clay, being a safe material, has potential as an ideal carrier for the delivery of dense probiotic biofilms to the gut, where it may also enhance the growth of other LAB. Role of smectite laden probiotic biofilms have clinical implications in case of chemotherapy as discussed in the above section.
Several food matrices serve as natural scaffolds for the adherence and biofilm formation of many probiotics which could be added in food or delivered directly. Different probiotic bacteria have been tested for their ability to develop biofilm on dietary fibres. Liu and others reported the biofilm-forming potential of six Bifidobacterium species on grape seed flour (insoluble dietary fibre with porous structure), where Bifidobacterium pseudo, Bifidobacterium longum, and Bifibacterium breve formed strong biofilms (more biofilm mass) with high tolerance under low pH limited nutrients.108 Wheat bran is considered as a beneficial dietary fibre with a well-known prebiotic potential. Wheat bran fraction reportedly induce microbiota changes in the human gut, specifically targeting colon butyrate producers.109 Development of probiotic biofilms by L. plantarum 8-RA-3 on wheat bran has been reported which opens further avenues of utilising wheat bran as a probiotic carrier. Survival and tolerance of B. subtilis can be enhanced by developing its biofilms on starch fibres of chickpea milk with significantly more production of pulcherrimin (an antimicrobial pigment for some pathogens).99,110 The development of B. subtilis biofilms on starch fibres confers effective adaptation strategy to the bacterium on food matrices for enduring harsh gastrointestinal conditions as well as during food processing and storage, as a part of functional food ingredients. The fibre can ultimately be used by the colonized probiotic strains for their proliferation.
Another approach for the delivery of probiotics in the form of biofilms is to encapsulate them in a material that may stimulate their biofilm formation and keep bacteria entrapped in the form of biofilms. When Lactobacillus acidophilus LMG9433T, L. rhamnosus LMG25859, and L. casei LMG6904T were encapsulated in microcapsules produced using low-methoxyl pectins (CMP-6 and CMP-8) and calcium as the wall material and allowed to grow further in a suitable growth medium, they changed into biofilms and better survived the gastrointestinal transit and heat and cold shock, compared to their planktonic cells,111 as shown in Figure 5B.
Self-coating probiotics with biofilms has also been reported as an important bioinspired strategy for oral doses of gut microbiota (Figure 5C). B. subtilis cells coated with self-produced biofilms endowed the transplanted gut microbiota with superior tolerance (a 125-fold higher oral bioavailability) and enhanced mucoadhesion capacity (17 times higher) in mice and swine models.74 Apart from developing biofilms on different surfaces as discussed above, bacterial biofilms can be encapsulated in suitable materials, usually polysaccharide-based capsules, which can successfully release the entrapped probiotics in the colonic environment. In one of the earlier investigations on probiotic biofilm delivery systems, chitosan-coated alginate capsules were proved to confer L. rhamnosus biofilms more protection against several environmental stressors (e.g., low pH and temperature).112 Heumann and others showed the successful release of biofilm-like microcolonies of Lacticaseibacillus paracasei (10 log (CFU/g) encased in calcium-pectinate beads in the gastrointestinal tract. The resulting interaction between three-dimensional network of calcium pectinate and bacterial exopolysaccharides kept bacteria adhered to the surface.101 Electrospun nanofiber membranes have three-dimensional porous structure with high bio-reactive surface area which make them ideal for the growth and proliferation of bacterial cells. Biofilms formed by a probiotic strain of L. plantarum was entrapped in electrospun cellulose acetate nanofiber membranes, which showed excellent gastrointestinal tolerance compared to bacterial cells in the planktonic form 105 (Figure 5C).
A recent study reported a new method for the development of probiotics capsules based on cellulose produced by self-aggregated biofilms of Gluconacetobacter xylinus 113 as demonstrated in Figure 6. This principle of self-produced EPS can be used for many biofilm-producing probiotic strains. Probiotic cellulose does not only protect enclosed probiotic cells, but it also serves as an antibacterial agent against many pathogens.114
Figure 6.
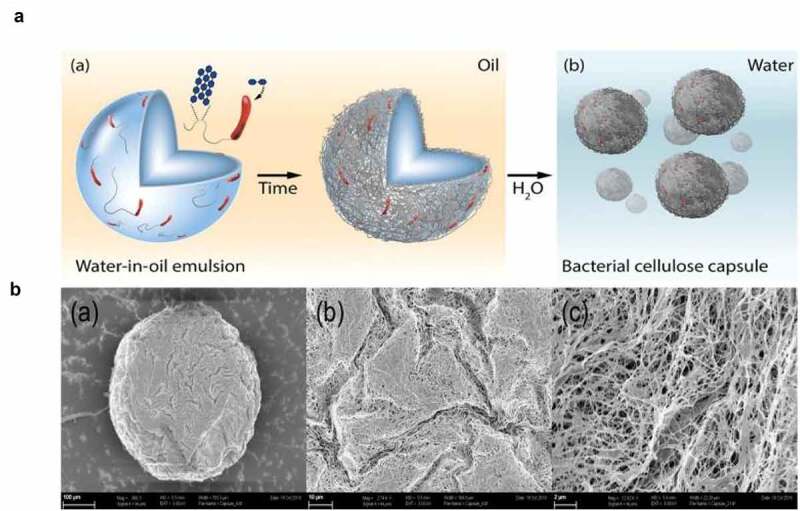
Probiotic self-grown capsules based on cellulose derived from self-assembled biofilms in an oil-water emulsion. Part A shows water-in-oil emulsion loaded with nutrients and the bacterium Gluconacetobacter xylinus, enclosed in the water phase. Over time the bacteria use the sugars and secrete cellulose which forms a uniform layer over the cells enclosed in the water phase (a) and his ultimately leads to the formation of bacterial self-produced cellulose capsules (b). Part B (a, b, and c) shows scanning electron microscopy images of the biofilm-based cellulose capsule with visible cellulose fiber network under three different magnifications (100, 10, and 2 µm). This figure is reproduced with permission.113
Mixed biofilms for probiotic delivery
Most of the probiotic bacterial species or the gut commensals have been reported to co-exist in mixed biofilms where their growth, biofilm mass and metabolic characteristics are enhanced because of inter-species interactions. Bacterial interaction in co-cultures may also lead to enhanced production of several beneficial metabolites. Increased production of bacteriocin was reported in a co-culture of Lactobacillus and Pediococcus species.115 There are many probiotic strains which do not form biofilms and thus it presents a challenge to increase their viability and probiotic potential based on the model discussed hereby. One way to deal with this challenge is to mix non-biofilm forming probiotics with some biofilm-forming probiotic strains so that they can get protection from the EPS of other strains. For instance, a few strains of B. subtilis have emerged as potential probiotics and they produce abundant EPS which can protect other probiotic bacteria and keep them entrapped within the matrix.116 Some species trigger biofilm formation in other non-biofilm forming species in combinations and thus mixed-species biofilms can be used as probiotics and can further can encapsulated based on the reported techniques discussed above. Sadiq, Wenwei, Heyndrickx, Flint, Wei, Jianxin and Zhang 35 demonstrated the existence of four different gut species together in the form of mixed-species biofilms where individual species were not able to form biofilms but existed together because of probably synergistic interactions. Species from different kingdoms (bacteria and yeasts) may also co-exist in the form of biofilms and thus this delivery system may help to deliver doses of mixed probiotics from different kingdoms. Interactions between Lactobacillus kefiri and Saccharomyces cerevisiae is probably the best examples of the existence of trans-kingdom probiotics in the form of biofilms because of interactions between bacterial surface layer proteins (SLPs) and yeast mannans.117
Novel prebiotics to enrich specific bacterial groups in the gut
Responses of the human gut microbiota to dietary components are mechanistically not well understood because of complexities involved in the interactions of the gut microbiota affecting metabolic capabilities of species. The last one decade has witnessed a surge in the development of novel prebiotics and effective delivery and enrichment strategies of probiotics. According to the consensus statement of the International Scientific Association for Probiotics and Prebiotics, a prebiotic substance must be selectively utilized and have adequate evidence of health benefit for the target host. Apart from an astounding range of established carbohydrate-based prebiotics (such as, inulin, fructooligosaccharides, galactooligosaccharides, and lactulose), several novel prebiotics have emerged which target specific microbial groups in the human gut, for instance, xylooligosaccharides, chitooligosaccharides, isomaltooligosaccharides, lactosucrose,118 protein-based prebiotics,119,120 some dietary fibres,121 and prebiotics derived from fungal 122 and algal sources, such as seaweed polysaccharides.123 Table 3 mentions many studies which reported the effectivity of selected prebiotic in promoting the growth of targeted bacterial groups in the gut.
Table 3.
Selective prebiotics targeting the growth of targeted bacteria in the gut.
| Name of the prebiotic | Source | Targeted bacteria | Reference |
|---|---|---|---|
| Inulin | - | Bifidobacterium and Lachnospiraceae | 124 |
| Omega-3 fatty acids | - | Coprococcus and Bacteroides species | 124 |
| Berberine and Curcumin | - | Bifidobacterium and Akkermansia species | 125 |
| Sulfoquinovose | Green vegetables | Faecalibacterium prausnitzii | 126 |
| Galactosyl-β1,4-l-rhamnose | - | Bifidobacterium longum subsp. Longum | 127 |
| Polyphenols | Fu brick tea | Akkermansia muciniphila, Alloprevotella, Bacteroides, and Faecalibaculum | 128 |
| Levan-type fructan | Erwinia species | Bifidobacterium and Eubacterium rectale | 129 |
| Polyphenols | Chilean currants | F. prausnitzii | 130 |
| Polyphenols | Cranberry and blueberry fruit powders | A. muciniphila | 131 |
| Protein-oligosaccharide conjugates | The conjugates were formed by mild Maillard-reaction-based covalent conjugation of galacto-oligosaccharides to lactoferrin hydrolysate | Lactobacillus casei | 119 |
| Chitin-glucan | - | Bifidobacterial communities, especially Bifidobacterium breve | 132 |
| Epigallocatechin gallate, caffeine, and theanine | Green tea | Bifidobacteria and Lactobacillus species | 133 |
| Lycium barbarum polysaccharides | Goji berries | Bifidobacterium and Lactobacillus species | 134 |
| Proanthocyanidin | Grapes | Akkermansia species | 135 |
| Anthocyanins | Black rice | Bifidobacterium and Lactobacillus species | 136 |
| Triterpenoid saponins | Gynostemma pentaphyllum (a medicinal plant) | Bacteroides, Lactobacillus and Bifidobacterium | 137 |
| Yeast α-mannan | Saccharomyces cerevisiae | Bacteroides thetaiotaomicron | 138 |
| Partially hydrolyzed guar gum | - | Bifidobacterium and butyrate producing bacteria | 139 |
| Polyfructan levan | Produced from sucrose using Lsc3 from Pseudomonas syringae | Bacteroides, Faecalibacterium, Escherichia and Streptococcus | 140 |
| Galactooligosaccharide mixture | Produced by enzymatic activity of Bifidobacterium bifidum NCIMB 41171 | Bifidobacterium bifidum | 141 |
| Lactulose | - | Bifidobacterium species | 142 |
| Xylooligosaccharide (XOS | - | Bifidobacterium longum subsp. longum CR15 | 143 |
| Pectic oligosaccharides | Sugarbeet pulp and lemon peel waste | Faecalibacterium and Roseburia | 144 |
Researchers focusing on prebiotic development are more interested in developing prebiotics targeting specific bacterial groups in the human gut at the species or strain level to increase the abundance of beneficial bacteria while reducing detrimental members in the gut microbiota, and to shift the host colonic microbiota toward a healthier composition.145
Bifidobacteria are important bacterial groups in the human gut which mainly rely on dietary carbohydrates for their growth and physiological activities. It is well established that some dietary fibers such as fructans and galacto-oligosaccharides enhance the fecal abundance of Bifidobacterium and Lactobacillus species.134,146
Prebiotics containing levan can be specifically utilized by Bacteroides thetaiotaomicron.147 Similarly, yeast mannan (indigestible water-soluble polysaccharide) has been reported to specifically promote the growth of B. thetaiotaomicron and B. ovatus in trials based on human colonic microbiota model.148 Soluble dietary fiber (10% inulin) reportedly enhances growth and abundance of Bacteroides fragilis with a concomitant increase in IgA.149
Some probiotics in the human gut particularly require some proteins for their growth and proliferation. Probiotics often contain sugar-binding proteins on their cell surface, and these mediate the uptake of many complex oligosaccharides. A protein delivery system in combination with sugars (galactooligosaccharide-lactoferrin hydrolysate micelles.) was successfully used targeting the specific growth of L. casei.120
Some dietary components have potential to be used as prebiotics because of their associated growth impacts on targeted microbiota groups. Sulfoquinovose (SQ), a sulfonated monosaccharide highly dominant in green vegetables and can be effectively used by Eubacterium rectale and F. prausnitzii leading to the production of H2S through acetate production in the human gut.126 Similarly, quinones is another food component which specifically targets Faecalibacterium species in the human gut.150 Omega-3 supplementation has been reported to result in a significant increases in Coprococcus and Bacteroides species.124 Dietary polyphenols can be utilized by certain (poly)phenol-transforming bacteria of the human gut such as Akkermansia muciniphila (a bacterial species well known for its anti-obesity properties) leading to its abundance with substantial ameliorating effects on obesity.151,152
Concluding remarks
Human gut harbors trillions of diverse microorganisms which live in an intricate balance, and dynamics of which are affected by host and environmental factors, including the diet and lifestyle. A perturbed gut leads to several pathological conditions and aberrant metabolism. The use of probiotics, among many other microbiome-modulating interventions, has been well acknowledged as a strategy to overcome the conditions associated with gut dysbiosis, because of their role in replenishing missing microbial groups. Development of specific prebiotics for targeted growth of bacteria at the species or strain level, in the human gut is also very important to modify the host colonic microbiota toward a healthier composition. Unique gut bacterial profiles with dominance of specific genera and species are linked with health and disease status.
One of the challenges in probiotic research is their effective delivery and maintenance of viability across divergent product formats. Biofilms of probiotics have been argued as the most resistant form that can better survive the gastrointestinal transit, compared to planktonic probiotic forms. Probiotic biofilms can entail a mixture of probiotic species/strains with network interactions and synergistic effects that can serve as highly resistant therapeutic microbial consortia. Probiotic biofilms can be easily subjected to many processing regimes, for instance, encapsulation, coatings, structural changes, and compound enrichment which widen their use in several applications and diverse systems as functional ingredients.
Probiotic biofilms have been proven to be more effective than their counterpart planktonic probiotics in many immunological and pathological conditions mainly due to their better survival, improved gut barrier function, more adherence, EPS production, and production of biofilm-specific metabolites such as vitamins and certain enzymes.
Probiotics in a biofilm formulation increase the efficiency of chemo- or immunotherapy by activating anti-cancer immune responses carried out by dendritic cells, which is not a property of planktonic cells. Their advantages over the conventional delivery of probiotics can be evidenced by their immunomodulatory roles in osseointegration of medical implants, necrotizing enterocolitis, and collagen-induced arthritis. Probiotics in their biofilm state produce many new metabolites and many metabolites in higher concentration which promote the growth of gut microbiota and inhibit the growth of many pathogens, apart from their metabolic and anti-inflammatory effects.
Whilst considering biofilms as the appropriate form of probiotic intake, one should consider the stage of biofilm development with the view of the production of metabolites, structural stability, and viability of cells. Many studies have provided evidence of more therapeutic potential of probiotics in the form of biofilms, mainly because of enhanced resistance of the biofilm-enclosed cells, and thus probiotic-based delivery approaches should be further investigated for drug delivery systems and for the incorporation of probiotics in foods and supplements.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant number 32101911].
Disclosure statement
The authors declare no competing financial interest.
References
- 1.Giuffrè M, Campigotto M, Campisciano G, Comar M, Crocè LS.. A story of liver and gut microbes: how does the intestinal flora affect liver disease? A review of the literature. Am J Physiol Gastrointest Liver Physiol. 2020;318(5):G889–22. doi: 10.1152/ajpgi.00161.2019. [DOI] [PubMed] [Google Scholar]
- 2.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. [DOI] [PubMed] [Google Scholar]
- 4.Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng WY, Wu C-Y, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69:1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.J-i O, Node K. Gut microbiota and hypertension. Hypertens Res. 2019;42:741–743. [DOI] [PubMed] [Google Scholar]
- 7.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster JA, Neufeld K-A M. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. [DOI] [PubMed] [Google Scholar]
- 9.Reddel S, Del Chierico F, Quagliariello A, Giancristoforo S, Vernocchi P, Russo A, et al. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci Rep. 2019;9:4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham M, Azcarate-Peril MA, Barnard A, Benoit V, Grimaldi R, Guyonnet D, Holscher HD, Hunter K, Manurung S, Obis D, et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021;29(8):667–685. doi: 10.1016/j.tim.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Giles EM, D’Adamo GL, Forster SC. The future of faecal transplants. Nat Rev Microbiol. 2019;17(12):719. doi: 10.1038/s41579-019-0271-9. [DOI] [PubMed] [Google Scholar]
- 12.Han S, Lu Y, Xie J, Fei Y, Zheng G, Wang Z, et al. Probiotic gastrointestinal transit and colonization after oral administration: a long journey. Front Cell Infect Microbiol. 2021;11:609722–609722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey ME, O’toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64(4):847–867. doi: 10.1128/MMBR.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry D. Making it stick: a compelling case for precision microbiome reconstitution. Cell Host Microbe. 2016;20(4):415–417. doi: 10.1016/j.chom.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues FJ, Cedran MF, Bicas JL, Sato HH. Encapsulated probiotic cells: relevant techniques, natural sources as encapsulating materials and food applications – a narrative review. Food Res Int. 2020;137:109682. doi: 10.1016/j.foodres.2020.109682. [DOI] [PubMed] [Google Scholar]
- 16.Song Q, Zhao H, Zheng C, Wang K, Gao H, Feng Q, Zhang H, Zhang Z, Zhang Y, Wang L, et al. A bioinspired versatile spore coat nanomaterial for oral probiotics delivery. Adv Funct Mater. 2021;31(41):2104994. doi: 10.1002/adfm.202104994. [DOI] [Google Scholar]
- 17.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 18.Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska H, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 20.Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charbonneau MR, Isabella VM, Li N, Kurtz CB. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat Commun. 2020;11(1):1738. doi: 10.1038/s41467-020-15508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13(9):508–516. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6(10):776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker WA. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr Res. 2017;82:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker AC, Bhargava R, Vaziriyan-Sani AS, Pourciau C, Donahue ET, Dove AS, et al. Colonization of the Caenorhabditis elegans gut with human enteric bacterial pathogens leads to proteostasis disruption that is rescued by butyrate. PLoS Pathog. 2021;17:e1009510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau HCH, Sung JJ-Y, Yu J. Gut microbiota: impacts on gastrointestinal cancer immunotherapy. Gut Microbes. 2021;13:1869504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Yu X, Xu X, Ming J, Wang Z, Gao B, et al. The fecal microbiota Is already altered in normoglycemic individuals who go on to have type 2 diabetes. Front Cell Infect Microbiol. 2021;11:PP598672–598672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Chang Y, Zhang K, Chen H, Tao S, Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10:5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadiq FA. Is it time for microbiome-based therapies in viral infections? Virus Res. 2021;291:198203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao Y, Qiu Z, Tian F, Yu L, Zhao J, Zhang H, et al. Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice. Food Sci Hum Wellness. 2022;11:238–246. [Google Scholar]
- 35.Sadiq FA, Wenwei L, Heyndrickx M, Flint S, Wei C, Jianxin Z, et al. Synergistic interactions prevail in multispecies biofilms formed by the human gut microbiota on mucin. FEMS Microbiol Ecol. 2021;97(8):fiab096. [DOI] [PubMed] [Google Scholar]
- 36.He Q, Huang J, Zheng T, Lin D, Zhang H, Li J, et al. Treatment with mixed probiotics induced enhanced and diversified modulation of the gut microbiome of healthy rats. FEMS Microbiol Ecol 2021;97(12):fiab151. [DOI] [PubMed] [Google Scholar]
- 37.Sun S, Luo L, Liang W, Yin Q, Guo J, Rush AM, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proceedings of the National Academy of Sciences. 2020;117(44):27509–27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351:aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon S, Kim H, Kim J, Seol D, Jo J, Choi Y, et al. Positive effect of Lactobacillus acidophilus EG004 on cognitive ability of healthy mice by fecal microbiome analysis using full-length 16S-23S rRNA metagenome sequencing. Microbiol Spectr. 2022; e01815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung Y, Ryu Y, An BC, Yoon Y-S, Choi O, Kim TY, Yoon J, Ahn JY, Park HJ, Kwon S-K, et al. A synthetic probiotic engineered for colorectal cancer therapy modulates gut microbiota. Microbiome. 2021;9(1):122. doi: 10.1186/s40168-021-01071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloemendaal M, Szopinska-Tokov J, Belzer C, Boverhoff D, Papalini S, Michels F, van Hemert S, Arias Vasquez A, Aarts E. Probiotics-induced changes in gut microbial composition and its effects on cognitive performance after stress: exploratory analyses. Transl Psychiatry. 2021;11(1):300. doi: 10.1038/s41398-021-01404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partrick KA, Rosenhauer AM, Auger J, Arnold AR, Ronczkowski NM, Jackson LM, Lord MN, Abdulla SM, Chassaing B, Huhman KL, et al. Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci Rep. 2021;11(1):3763. doi: 10.1038/s41598-021-83284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee -C-C, Liao Y-C, Lee M-C, Lin K-J, Hsu H-Y, Chiou S-Y, et al. Lactobacillus plantarum TWK10 attenuates aging-associated muscle weakness, Bone Loss, and cognitive impairment by modulating the gut microbiome in mice. Front Nutr. 2021;8:PP708096–708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Yu X, Yu L, Tian F, Zhao J, Zhang H, Qian L, Wang Q, Xue Z, Zhai Q, et al. Lactobacillus plantarum CCFM8610 alleviates irritable bowel syndrome and prevents gut microbiota dysbiosis: a randomized, double-blind, placebo-controlled, pilot clinical trial. Engineering. 2021;7(3):376–385. doi: 10.1016/j.eng.2020.06.026. [DOI] [Google Scholar]
- 45.Lee M-C, Hsu Y-J, Ho -H-H, Kuo Y-W, Lin W-Y, Tsai S-Y, Chen W-L, Lin C-L, Huang -C-C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci Rep. 2021;11(1):19469. doi: 10.1038/s41598-021-98958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma T, Jin H, Kwok L-Y, Sun Z, Liong M-T, Zhang H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol Stress. 2021;14:100294. doi: 10.1016/j.ynstr.2021.100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Z, Guo X, Zhang J, Yuan Q, Chen S. Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 2021;12(15):6809–6820. doi: 10.1039/D1FO00515D. [DOI] [PubMed] [Google Scholar]
- 48.Wu T, Zhang Y, Li W, Zhao Y, Long H, Muhindo EM, Liu R, Sui W, Li Q, Zhang M, et al. Lactobacillus rhamnosus LRa05 ameliorate hyperglycemia through a regulating glucagon-mediated signaling pathway and gut microbiota in Type 2 diabetic mice. J Agric Food Chem. 2021;69(31):8797–8806. doi: 10.1021/acs.jafc.1c02925. [DOI] [PubMed] [Google Scholar]
- 49.Zheng F, Wang Z, Stanton C, Ross RP, Zhao J, Zhang H, Yang B, Chen W. Lactobacillus rhamnosus FJSYC4-1 and Lactobacillus reuteri FGSZY33L6 alleviate metabolic syndrome via gut microbiota regulation. Food Funct. 2021;12(9):3919–3930. doi: 10.1039/D0FO02879G. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Chong HX, Chung FY, Li Y, Liong MT. Lactobacillus plantarum DR7 modulated bowel movement and gut microbiota associated with dopamine and serotonin pathways in stressed adults. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh S, Bhatia R, Khare P, Sharma S, Rajarammohan S, Bishnoi M, Bhadada SK, Sharma SS, Kaur J, Kondepudi KK, et al. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci Rep. 2020;10(1):18597. doi: 10.1038/s41598-020-75702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Huang Y, Song L, Xiao Y, Lu S, Xu J, Li J, Ren Z. Lactobacillus plantarum prevents obesity via modulation of gut microbiota and metabolites in high-fat feeding mice. J Funct Foods. 2020;73:104103. doi: 10.1016/j.jff.2020.104103. [DOI] [Google Scholar]
- 53.Yang X, Yu D, Xue L, Li H, Du J. Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta pharmaceutica Sinica B. 2020;10(3):475–487. doi: 10.1016/j.apsb.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang Z, Lu W, Zhao J, Zhang H, Qian L, Wang Q, Chen W. Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: a pilot study. Eur J Nutr. 2020;59(5):2119–2130. doi: 10.1007/s00394-019-02061-x. [DOI] [PubMed] [Google Scholar]
- 55.Fang Z, Li L, Zhao J, Zhang H, Lee Y-K, Lu W, Chen W. Bifidobacteria adolescentis regulated immune responses and gut microbial composition to alleviate DNFB-induced atopic dermatitis in mice. Eur J Nutr. 2020;59(7):3069–3081. doi: 10.1007/s00394-019-02145-8. [DOI] [PubMed] [Google Scholar]
- 56.Li L, Fang Z, Liu X, Hu W, Lu W, Lee Y-K, Zhao J, Zhang H, Chen W, et al. Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes. PLoS One. 2020;15(4):e0231865. doi: 10.1371/journal.pone.0231865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Guo Y, Chen H, Wei H, Wan C. Potential of Lactobacillus plantarum ZDY2013 and Bifidobacterium bifidum WBIN03 in relieving colitis by gut microbiota, immune, and anti-oxidative stress. Can J Microbiol. 2018;64(5):327–337. doi: 10.1139/cjm-2017-0716. [DOI] [PubMed] [Google Scholar]
- 58.Song JJ, Tian WJ, Kwok L-Y, Wang YL, Shang YN, Menghe B, Wang JG. Effects of microencapsulated Lactobacillus plantarum LIP-1 on the gut microbiota of hyperlipidaemic rats. Br J Nutr. 2017;118(7):481–492. doi: 10.1017/S0007114517002380. [DOI] [PubMed] [Google Scholar]
- 59.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 60.Park D-Y, Ahn Y-T, Park S-H, Huh C-S, Yoo S-R, Yu R, Sung M-K, McGregor RA, Choi M-S, et al. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8(3):e59470–e. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaoutari AE, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11:497–504. [DOI] [PubMed] [Google Scholar]
- 62.Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, et al. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature. 2014;506:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang C-J, Lin T-L, Tsai Y-L, T-R W, Lai W-F, C-C L, et al. Next generation probiotics in disease amelioration. J Food Drug Anal. 2019;27:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. [DOI] [PubMed] [Google Scholar]
- 65.Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Gastroenterol Res Pract. 2014;2014:872725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Siles M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M. Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. ISME J. 2017;11:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martín R, Chain F, Miquel S, Lu J, Gratadoux JJ, Sokol H, et al. The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm Bowel Dis. 2014;20:417–430. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Y, Xu H, Xu J, Guo X, Zhao H, Chen Y, et al. F. prausnitzii and its supernatant increase SCFAs-producing bacteria to restore gut dysbiosis in TNBS-induced colitis. AMB Express. 2021;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maioli TU, Borras-Nogues E, Torres L, Barbosa SC, Martins VD, Langella P, et al. Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front Pharmacol. 2021;12:PP740636–740636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017;11:1667–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Hupp T, Duchnowska R, Marek-Trzonkowska N, Połom K. Next-generation probiotics - do they open new therapeutic strategies for cancer patients? Gut Microbes. 2022;14:2035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadiq FA, Burmølle M, Heyndrickx M, Flint S, Lu W, Chen W, et al. Community-wide changes reflecting bacterial interspecific interactions in multispecies biofilms. Crit Rev Microbiol. 2021;47:338–358. [DOI] [PubMed] [Google Scholar]
- 73.Hayta EN, Ertelt MJ, Kretschmer M, Lieleg O. Bacterial materials: applications of natural and modified biofilms. Adv Mater Interfaces. 2021;8:2101024. [Google Scholar]
- 74.Wang X, Cao Z, Zhang M, Meng L, Ming Z, Liu J. Bioinspired oral delivery of gut microbiota by self-coating with biofilms. Sci Adv. 2020;6:eabb1952–eabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Hadidi A, Navarro J, Goodman SD, Bailey MT, Besner GE. Lactobacillus reuteri in its biofilm state improves protection from experimental necrotizing enterocolitis. Nutrients. 2021;13(3):918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guilhen C, Miquel S, Charbonnel N, Joseph L, Carrier G, Forestier C, et al. Colonization and immune modulation properties of Klebsiella pneumoniae biofilm-dispersed cells. NPJ Biofilms Microbiomes. 2019;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu L, Guo S, Chen X, Yang S, Deng X, Tu M, et al. Metabolic profiles of Lactobacillus paraplantarum in biofilm and planktonic states and investigation of its intestinal modulation and immunoregulation in dogs. Food Funct. 2021;12:5317–5332. [DOI] [PubMed] [Google Scholar]
- 79.Sadiq FA, Yan B, Zhao J, Zhang H, Chen W. Untargeted metabolomics reveals metabolic state of Bifidobacterium bifidum in the biofilm and planktonic states. LWT. 2020;118:108772. [Google Scholar]
- 80.Tan L, Fu J, Feng F, Liu X, Cui Z, Li B, et al. Engineered probiotics biofilm enhances osseointegration via immunoregulation and anti-infection. Sci Adv. 2020;6(46):eaba5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paynich ML, Jones-Burrage SE, Knight KL. Exopolysaccharide from Bacillus subtilis induces Anti-inflammatory M2 macrophages that prevent T cell-mediated disease. J Immunol. 2017;198:2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nowak B, Śróttek M, Ciszek-Lenda M, Skałkowska A, Gamian A, Górska S, et al. Exopolysaccharide from Lactobacillus rhamnosus KL37 inhibits t cell-dependent immune response in mice. Arch Immunol Ther Exp (Warsz). 2020;68:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laiño J, Villena J, Kanmani P, Kitazawa H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: new insights into molecular interactions with host cells. Microorganisms. 2016;4(3):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin M-H, Yang Y-L, Chen Y-P, Hua K-F, C-P L, Sheu F, et al. A novel exopolysaccharide from the Biofilm of Thermus aquaticus YT-1 induces the immune response through Toll-like receptor 2. J Biol Chem. 2011;286:17736–17745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. [DOI] [PubMed] [Google Scholar]
- 86.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduction Targeted Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwon M, Lee J, Park S, Kwon OH, Seo J, Roh S. Exopolysaccharide isolated from Lactobacillus plantarum L-14 has anti-inflammatory effects via the toll-like receptor 4 pathway in LPS-induced RAW 264.7 cells. Int J Mol Sci. 2020;21(23):9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mommert S, Hüer M, Schaper-Gerhardt K, Gutzmer R, Werfel T. Histamine up-regulates oncostatin M expression in human M1 macrophages. Br J Pharmacol. 2020;177:600–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han C, Song J, Hu J, Fu H, Feng Y, Mu R, et al. Smectite promotes probiotic biofilm formation in the gut for cancer immunotherapy. Cell Rep. 2021;34:108706. [DOI] [PubMed] [Google Scholar]
- 90.Kleerebezem M, Binda S, Bron PA, Gross G, Hill C, van Hylckama Vlieg JET, et al. Understanding mode of action can drive the translational pipeline towards more reliable health benefits for probiotics. Curr Opin Biotechnol. 2019;56:55–60. [DOI] [PubMed] [Google Scholar]
- 91.Ragan MV, Wala SJ, Goodman SD, Bailey MT, Besner GE. Next-generation probiotic therapy to protect the intestines from injury. Front Cell Infect Microbiol. 2022;12:863949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shelby RD, Mar P, Janzow GE, Mashburn-Warren L, Tengberg N, Navarro JB, et al. Antibacterial and anti-inflammatory effects of Lactobacillus reuteri in its biofilm state contribute to its beneficial effects in a rat model of experimental necrotizing enterocolitis. J Pediatr Surg. 2022;57:1382–1390. [DOI] [PubMed] [Google Scholar]
- 93.Olson JK, Navarro JB, Allen JM, McCulloh CJ, Mashburn-Warren L, Wang Y, et al. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G408–g19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Jaggers RM, Mar P, Galley JD, Shaffer T, Rajab A, et al. Lactobacillus reuteri in its biofilm state promotes neurodevelopment after experimental necrotizing enterocolitis in rats. Brain Behav Immun Health. 2021;14:100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shelby RD, Mar P, Janzow GE, Mashburn-Warren L, Tengberg N, Navarro JB, et al. Antibacterial and anti-inflammatory effects of Lactobacillus reuteri in its biofilm state contribute to its beneficial effects in a rat model of experimental necrotizing enterocolitis. J Pediatr Surg. 2021;57;7:1382–1390. [DOI] [PubMed] [Google Scholar]
- 96.Michael H, Paim FC, Miyazaki A, Langel SN, Fischer DD, Chepngeno J, et al. Escherichia coli Nissle 1917 administered as a dextranomar microsphere biofilm enhances immune responses against human rotavirus in a neonatal malnourished pig model colonized with human infant fecal microbiota. PLoS One. 2021;16:e0246193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Castro-Bravo N, Wells JM, Margolles A, Ruas-Madiedo P. Interactions of surface exopolysaccharides from bifidobacterium and lactobacillus within the intestinal environment. Front Microbiol. 2018;9:2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin XB, Wang T, Stothard P, Corander J, Wang J, Baines JF, et al. The evolution of ecological facilitation within mixed-species biofilms in the mouse gastrointestinal tract. ISME J. 2018;12:2770–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajasekharan SK, Paz-Aviram T, Galili S, Berkovich Z, Reifen R, Shemesh M. Biofilm formation onto starch fibres by Bacillus subtilis governs its successful adaptation to chickpea milk. Microb Biotechnol. 2021;14:1839–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rezaei Z, Khanzadi S, Salari A. Biofilm formation and antagonistic activity of Lacticaseibacillus rhamnosus (PTCC1712) and Lactiplantibacillus plantarum (PTCC1745). AMB Express. 2021;11(1):156. doi: 10.1186/s13568-021-01320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heumann A, Assifaoui A, Da Silva Barreira D, Thomas C, Briandet R, Laurent J, et al. Intestinal release of biofilm-like microcolonies encased in calcium-pectinate beads increases probiotic properties of Lacticaseibacillus paracasei. npj Biofilms Microbiomes. 2020;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan L, Fu J, Feng F, Liu X, Cui Z, Li B, Han Y, Zheng Y, Yeung KWK, Li Z, et al. Engineered probiotics biofilm enhances osseointegration via immunoregulation and anti-infection. Sci Adv. 2020;6(46):eaba5723. doi: 10.1126/sciadv.aba5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aoudia N, Rieu A, Briandet R, Deschamps J, Chluba J, Jego G, Garrido C, Guzzo J. Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol. 2016;53:51–59. doi: 10.1016/j.fm.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Rieu A, Aoudia N, Jego G, Chluba J, Yousfi N, Briandet R, Deschamps J, Gasquet B, Monedero V, Garrido C, et al. The biofilm mode of life boosts the anti-inflammatory properties of L actobacillus. Cell Microbiol. 2014;16(12):1836–1853. doi: 10.1111/cmi.12331. [DOI] [PubMed] [Google Scholar]
- 105.Hu MX, Li JN, Guo Q, Zhu YQ, Niu HM. Probiotics biofilm-integrated electrospun nanofiber membranes: a new starter culture for fermented milk production. J Agric Food Chem. 2019;67:3198–3208. [DOI] [PubMed] [Google Scholar]
- 106.Navarro JB, Mashburn-Warren L, Bakaletz LO, Bailey MT, Goodman SD. Enhanced probiotic potential of lactobacillus reuteri when delivered as a biofilm on dextranomer microspheres that contain beneficial cargo. Front Microbiol. 2017;8:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alimova A, Roberts M, Katz A, Rudolph E, Steiner JC, Alfano RR, et al. Effects of smectite clay on biofilm formation by microorganisms. Biofilms. 2006;3:47–54. [Google Scholar]
- 108.Liu Z, Li L, Fang Z, Lee Y, Zhao J, Zhang H, et al. The biofilm-forming ability of six Bifidobacterium strains on grape seed flour. LWT. 2021;144:111205. [Google Scholar]
- 109.D’Hoe K, Conterno L, Fava F, Falony G, Vieira-Silva S, Vermeiren J, et al. Prebiotic wheat bran fractions induce specific microbiota changes. Front Microbiol. 2018;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amoah YS, Rajasekharan SK, Reifen R, Shemesh M. Chickpea-derived prebiotic substances trigger biofilm formation by Bacillus subtilis. Nutrients. 2021;13:4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.He C, Sampers I, Van de Walle D, Dewettinck K, Raes K. Encapsulation of Lactobacillus in low-methoxyl pectin-based microcapsules stimulates biofilm formation: enhanced resistances to heat shock and simulated gastrointestinal digestion. J Agric Food Chem. 2021;69:6281–6290. [DOI] [PubMed] [Google Scholar]
- 112.Cheow WS, Hadinoto K. Biofilm-like Lactobacillus rhamnosus probiotics encapsulated in alginate and carrageenan microcapsules exhibiting enhanced thermotolerance and freeze-drying resistance. Biomacromolecules. 2013;14:3214–3222. [DOI] [PubMed] [Google Scholar]
- 113.Pepicelli M, Binelli MR, Studart AR, Rühs PA, Fischer P. Self-grown bacterial cellulose capsules made through emulsion templating. ACS Biomater Sci Eng. 2021;7:3221–3228. [DOI] [PubMed] [Google Scholar]
- 114.Sabio L, González A, Ramírez-Rodríguez GB, Gutiérrez-Fernández J, Bañuelo O, Olivares M, et al. Probiotic cellulose: antibiotic-free biomaterials with enhanced antibacterial activity. Acta Biomater. 2021;124:244–253. [DOI] [PubMed] [Google Scholar]
- 115.Gutiérrez-Cortés C, Suarez H, Buitrago G, Nero LA, Todorov SD. Enhanced bacteriocin production by Pediococcus pentosaceus 147 in co-culture with Lactobacillus plantarum LE27 on cheese whey broth. Front Microbiol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yahav S, Berkovich Z, Ostrov I, Reifen R, Shemesh M. Encapsulation of beneficial probiotic bacteria in extracellular matrix from biofilm-forming Bacillus subtilis. Artif Cells, Nanomed Biotechnol. 2018;46:974–982. [DOI] [PubMed] [Google Scholar]
- 117.Fu M, Mao K, Gao J, Wang X, Sadiq FA, Li J, et al. Characteristics of surface layer protein from Lactobacillus kefiri HBA20 and the role in mediating interactions with Saccharomyces cerevisiae Y8. Int J Biol Macromol. 2021;201:254–261. [DOI] [PubMed] [Google Scholar]
- 118.Cardoso BB, Amorim C, Silvério SC, Rodrigues LR. Novel and emerging prebiotics: advances and opportunities. Adv Food Nutr Res. 2021;95:41–95. [DOI] [PubMed] [Google Scholar]
- 119.Seifert A, Freilich S, Kashi Y, Livney YD. Protein-oligosaccharide conjugates as novel prebiotics. Polym Adv Technol. 2019;30:2577–2585. [Google Scholar]
- 120.Peled S, Livney YD. Oligosaccharide-lactoferrin shell-crosslinked particles for selective targeting of proteins to probiotic bacteria in the colon. Food Hydrocoll. 2021;120:106973. [Google Scholar]
- 121.Kang JW, Tang X, Zivkovic A. A novel prebiotic supplement increases bifidobacteria abundance in participants consuming low-fiber diets. Curr Dev Nutr. 2021;5:1163. [Google Scholar]
- 122.Oba S, Sunagawa T, Tanihiro R, Awashima K, Sugiyama H, Odani T, et al. Prebiotic effects of yeast mannan, which selectively promotes Bacteroides thetaiotaomicron and Bacteroides ovatus in a human colonic microbiota model. Sci Rep. 2020;10:17351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patel AK, Singhania RR, Awasthi MK, Varjani S, Bhatia SK, Tsai M-L, et al. Emerging prospects of macro- and microalgae as prebiotic. Microb Cell Fact. 2021;20:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vijay A, Astbury S, Le Roy C, Spector TD, Valdes AM. The prebiotic effects of omega-3 fatty acid supplementation: a six-week randomised intervention trial. Gut Microbes. 2021;13(1):1–11. doi: 10.1080/19490976.2020.1863133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Neyrinck AM, Sánchez CR, Rodriguez J, Cani PD, Bindels LB, Delzenne NM. Prebiotic effect of berberine and curcumin is associated with the improvement of obesity in mice. Nutrients. 2021;13(5):1436. doi: 10.3390/nu13051436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanson BT, Dimitri Kits K, Löffler J, Burrichter AG, Fiedler A, Denger K, Frommeyer B, Herbold CW, Rattei T, Karcher N, et al. Sulfoquinovose is a select nutrient of prominent bacteria and a source of hydrogen sulfide in the human gut. ISME J. 2021;15(9):2779–2791. doi: 10.1038/s41396-021-00968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hirano R, Sakanaka M, Yoshimi K, Sugimoto N, Eguchi S, Yamauchi Y, Nara M, Maeda S, Ami Y, Gotoh A, et al. Next-generation prebiotic promotes selective growth of bifidobacteria, suppressing Clostridioides difficile. Gut Microbes. 2021;13(1):1973835. doi: 10.1080/19490976.2021.1973835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou F, Li Y-L, Zhang X, Wang K-B, Huang J-A, Liu Z-H, Zhu M-Z. Polyphenols from fu brick tea reduce obesity via modulation of gut microbiota and gut microbiota-related intestinal oxidative stress and barrier function. J Agric Food Chem. 2021;69(48):14530–14543. doi: 10.1021/acs.jafc.1c04553. [DOI] [PubMed] [Google Scholar]
- 129.Liu C, Kolida S, Charalampopoulos D, Rastall RA. An evaluation of the prebiotic potential of microbial levans from Erwinia sp. 10119. J Funct Foods. 2020;64:103668. doi: 10.1016/j.jff.2019.103668. [DOI] [Google Scholar]
- 130.Burgos-Edwards A, Fernández-Romero A, Carmona M, Thuissard-Vasallo I, Schmeda-Hirschmann G, Larrosa M. Effects of gastrointestinal digested polyphenolic enriched extracts of Chilean currants (Ribes magellanicum and Ribes punctatum) on in vitro fecal microbiota. Food Res Int. 2020;129:108848. doi: 10.1016/j.foodres.2019.108848. [DOI] [PubMed] [Google Scholar]
- 131.Rodríguez-Daza M-C, Roquim M, Dudonné S, Pilon G, Levy E, Marette A, et al. Berry polyphenols and fibers modulate distinct microbial metabolic functions and gut microbiota enterotype-Like clustering in obese mice. Front Microbiol. 2020;11:2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alessandri G, Milani C, Duranti S, Mancabelli L, Ranjanoro T, Modica S, Carnevali L, Statello R, Bottacini F, Turroni F, et al. Ability of bifidobacteria to metabolize chitin-glucan and its impact on the gut microbiota. Sci Rep. 2019;9(1):5755. doi: 10.1038/s41598-019-42257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jung ES, Park J, Park H, Holzapfel W, Hwang JS, Lee CH. Seven-day green Tea supplementation revamps gut microbiome and caecum/skin metabolome in mice from stress. Sci Rep. 2019;9(1):18418. doi: 10.1038/s41598-019-54808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou F, Jiang X, Wang T, Zhang B, Zhao H. Lyciumbarbarum polysaccharide (LBP): a novel prebiotics candidate for Bifidobacterium and Lactobacillus. Front Microbiol. 2018;9. doi: 10.3389/fmicb.2018.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang L, Carmody RN, Kalariya HM, Duran RM, Moskal K, Poulev A, Kuhn P, Tveter KM, Turnbaugh PJ, Raskin I, et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J Nutr Biochem. 2018;56:142–151. doi: 10.1016/j.jnutbio.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu Y, Sun H, He S, Lou Q, Yu M, Tang M, Tu L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS One. 2018;13(4):e0195754–e. doi: 10.1371/journal.pone.0195754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen L, Tai WCS, Hsiao WLW. Dietary saponins from four popular herbal tea exert prebiotic-like effects on gut microbiota in C57BL/6 mice. J Funct Foods. 2015;17:892–902. doi: 10.1016/j.jff.2015.06.050. [DOI] [Google Scholar]
- 138.Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron E, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 2015;517(7533):165–169. doi: 10.1038/nature13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ohashi Y, Sumitani K, Tokunaga M, Ishihara N, Okubo T, Fujisawa T. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria in the human large intestine. Benef Microbes. 2015;6(4):451–455. doi: 10.3920/BM2014.0118. [DOI] [PubMed] [Google Scholar]
- 140.Adamberg K, Tomson K, Talve T, Pudova K, Puurand M, Visnapuu T, Alamäe T, Adamberg S. Levan enhances associated growth of Bacteroides, Escherichia, Streptococcus and Faecalibacterium in fecal microbiota. PLoS One. 2015;10(12):e0144042. doi: 10.1371/journal.pone.0144042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Depeint F, Tzortzis G, Vulevic J, I’Anson K, Gibson GR. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am J Clin Nutr. 2008;87(3):785–791. doi: 10.1093/ajcn/87.3.785. [DOI] [PubMed] [Google Scholar]
- 142.Tuohy KM, Ziemer CJ, Klinder A, Knöbel Y, Pool-Zobel BL, Gibson GR. A human volunteer study to determine the prebiotic effects of lactulose powder on human colonic microbiota. Microb Ecol Health Dis. 2002;14:165–173. [Google Scholar]
- 143.Kok CR, Quintero DFG, Niyirora C, Rose D, Li A, Hutkins R, Dudley EG. An in vitro Enrichment Strategy for Formulating Synergistic Synbiotics. Appl Environ Microbiol. 2019;85(16):e01073–19. doi: 10.1128/AEM.01073-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gómez B, Gullón B, Yáñez R, Schols H, Alonso JL. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: a comparative evaluation. J Funct Foods. 2016;20:108–121. doi: 10.1016/j.jff.2015.10.029. [DOI] [Google Scholar]
- 145.Wang S, Xiao Y, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Rational use of prebiotics for gut microbiota alterations: specific bacterial phylotypes and related mechanisms. J Funct Foods. 2020;66:103838. doi: 10.1016/j.jff.2020.103838. [DOI] [Google Scholar]
- 146.So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6):965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 147.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141(7):1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oba S, Sunagawa T, Tanihiro R, Awashima K, Sugiyama H, Odani T, Nakamura Y, Kondo A, Sasaki D, Sasaki K, et al. Prebiotic effects of yeast mannan, which selectively promotes Bacteroides thetaiotaomicron and Bacteroides ovatus in a human colonic microbiota model. Sci Rep. 2020;10(1):17351. doi: 10.1038/s41598-020-74379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nakajima A, Sasaki T, Itoh K, Kitahara T, Takema Y, Hiramatsu K, Ishikawa D, Shibuya T, Kobayashi O, Osada T, et al. A soluble fiber diet increases bacteroides fragilis group abundance and immunoglobulin a production in the gut. Appl Environ Microbiol. 2020;86(13):e00405–20. doi: 10.1128/AEM.00405-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fenn K, Strandwitz P, Stewart EJ, Dimise E, Rubin S, Gurubacharya S, Clardy J, Lewis K. Quinones are growth factors for the human gut microbiota. Microbiome. 2017;5(1):161. doi: 10.1186/s40168-017-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anonye BO. Commentary: dietary polyphenols promote growth of the gut Bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Front Immunol. 2017;8:850. doi: 10.3389/fimmu.2017.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. 2017;33:194–201. doi: 10.1016/j.jff.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


