ABSTRACT
Verticillium dahliae is a notorious soil‐borne pathogen that enters hosts through the roots and proliferates in the plant water‐conducting elements to cause Verticillium wilt. Historically, Verticillium wilt symptoms have been explained by vascular occlusion, due to the accumulation of mycelia and plant biomacromolecule aggregation, and also by phytotoxicity caused by pathogen‐secreted toxins. Beyond the direct cytotoxicity of some members of the secretome, this review systematically discusses the roles of the V. dahliae secretome in vascular occlusion, including the deposition of polysaccharides as an outcome of plant cell wall destruction, the accumulation of fungal mycelia, and modulation of plant defence responses. By modulating plant defences and hormone levels, the secretome manipulates the vascular environment to induce Verticillium wilt. Thus, the secretome of V. dahliae colludes with plant defence responses to modulate Verticillium wilt symptoms, and thereby bridges the historical concepts of both toxin production by the pathogen and vascular occlusion as the cause of wilting symptoms.
Keywords: vascular pathogen, Verticillium dahliae , secretome, toxins, vascular occlusion, Verticillium wilt.
I. INTRODUCTION
Verticillium dahliae is a well‐known hemi‐biotrophic soil‐borne pathogen that infects over 200 hosts and causes billions of dollars in losses annually (Inderbitzin & Subbarao, 2014). The pathogen attacks plants through the roots and colonizes the unique ecological niche of the plant xylem, resulting in Verticillium wilt, the symptoms of which include foliar wilting, chlorosis, stunting, necrosis and vein clearing (Fradin & Thomma, 2006; Chen et al., 2021). Over the past 60 years, two main mechanisms that may explain the induced wilt symptoms, i.e. vascular occlusion and through the involvement of toxins have been advanced in the literature (Fradin & Thomma, 2006; Báidez et al., 2007). Following successful infection of the plant, V. dahliae reaches the xylem and increases its biomass concurrent with plant defence responses that aim to restrict the pathogen, including the production of tyloses and accumulation of a colloidal matrix (Robb, Powell & Street, 1989; Báidez et al., 2007; Yadeta & Thomma, 2013). This eventually leads to vascular occlusion and plant wilting. An alternative view argues that the pathogen delivers toxins, which in this case refers broadly to a wide‐array of molecules and complex compounds such as large polysaccharides, protein‐lipopolysaccharides, glycoproteins, and enzymes including some that act as effectors that induce plant wilting (Fradin & Thomma, 2006; de Sain & Rep, 2015). Both hypotheses are equally credible (Pegg & Brady, 2002; Fradin & Thomma, 2006; Báidez et al., 2007; de Sain & Rep, 2015), and in fact, the two are tightly interlinked as the secretome, including these toxins, colludes with plant defence responses to cause Verticillium wilt symptoms. Here, we review recent findings and the mechanistic actions of the secretome of V. dahliae, which causes Verticillium wilt symptoms via both a contribution to vascular occlusion and via cytotoxicity.
(1). The role of vascular occlusion in Verticillium wilt
Following successful breaching of the endodermis, V. dahliae generally is confined to the fluid environment of the xylem cells and exerts its effects on host physiology indirectly (Yadeta & Thomma, 2013). Unlike prokaryotic vascular pathogens that break out of the vascular system and spread indiscriminately in the root and shoot parenchyma, V. dahliae seldom leaves the confines of the xylem until the death of the surrounding tissue or the host (Pegg & Brady, 2002). Key steps in the pathogenesis of V. dahliae include germination of microsclerotia, hyphal penetration of the root epidermis, and growth in the cortex that crosses the endodermis finally breaching the highly structured and rigid secondary xylem walls to enter vessels of the root (Zhao et al., 2014). Once in the xylem, the pathogen produces conidia, which germinate, ramify and penetrate adjacent vascular cells to continue colonization (Fradin & Thomma, 2006; Yadeta & Thomma, 2013).
Since V. dahliae enters the xylem tissue, it can be trapped in the pit cavities at the xylem perforation plates. Because the nutritional value of the xylem fluid is limited, the pathogen draws its nutrition by degrading the vascular cell wall using an abundance of diverse extracellular enzymes encoded within its genome (Klosterman et al., 2011; Chen et al., 2016). The insufficient degradation of the xylem walls and pit membranes causes the deposition of polysaccharides (Fradin & Thomma, 2006; Kubicek, Starr & Glass, 2014). In addition, this triggers plant defences to produce tyloses and other biomacromolecules (resins, gums, gels, etc.) from neighbouring parenchyma cells, which act as a defensive barricade to restrict pathogen spread (Benhamou, 1995; Yadeta & Thomma, 2013). Analyses of the plant vascular system using electron microscopy during Verticillium wilt of tomato (Lycopersicon esculentum L.) has indicated that terminal occlusion leading to sealing of entire veins is a limiting factor preceding the irreversible appearance of vascular wilt (Robb, Powell & Street, 1989). Thus, the pathogen rapidly proliferates, accompanied by continuous deposition of polysaccharides and plant defence‐driven structures and biomacromolecules (tyloses, suberin, etc.) that interfere with the flow of water and nutrition, ultimately leading to plant wilting (Pegg & Brady, 2002; Báidez et al., 2007; Yadeta & Thomma, 2013).
(2). The role of toxins in Verticillium wilt
The concept that toxins could underlie Verticillium‐induced foliar wilting was first proposed from results obtained using components from culture filtrates that induced wilt symptoms (Stoddart & Carr, 1966; Meyer & Dubery, 1993). Since then, efforts have been made to elucidate the role of toxins in the mechanisms of foliar wilting (Pegg & Brady, 2002). While some evidence linking toxins to foliar wilting is undeniable, other investigations have yielded ambiguous results (Fradin & Thomma, 2006). For instance, a low molecular weight phytotoxic polypeptide that exhibited toxicity to potato (Solanum tuberosum L.) leaves was detected using immunofluorescent antibodies in the xylem of infected potato stems (Nachmias, Buchner & Burstein, 1985). A similar toxin induced the typical foliar wilting when injected into Arabidopsis thaliana seedlings (Jing, Fan & Wu, 2005). Incremental progress in understanding the toxins involved has occurred with advances in genomics and metabolomics technologies, and some toxin components have been verified, including a range of proteins with enzymatic or effector activity (Klimes et al., 2015), metabolites (Zhang et al., 2016; Zhang et al., 2019a), and volatile compounds (Li & Kang, 2018). Proteins secreted from V. dahliae that serve as toxins have been shown to play critical roles in its pathogenesis resulting in foliar wilting through plant cell wall destruction, manipulation of host responses, or cytotoxicity (de Sain & Rep, 2015; Klimes et al., 2015; Chen et al., 2016). The secreted proteins have been suggested to target various sites, including the cell wall, plasma membrane, and other intracellular components (Meyer & Dubery, 1993), and also to induce changes in the microfilaments and microtubules in plant cells (Zhao et al., 2020), leading to rapid cell lesions.
II. FUNCTIONS OF THE VERTICILLIUM DAHLIAE SECRETOME
Fungal pathogens secrete numerous proteins (i.e. their secretome) to modulate plant defences by altering host cellular structure and physiology, and to facilitate colonization (Hogenhout et al., 2009). Since V. dahliae mainly resides in the vascular tissue during its disease cycle, understanding the role of the secretome in contributing to vascular occlusion or producing toxins could offer a better understanding of the mechanisms that underlie foliar wilting. Genomics‐driven research has shown that V. dahliae encodes more than 700 potential secreted proteins (Klosterman et al., 2011; Chen et al., 2018), and hundreds are delivered into the extracellular space with generalist functions (Chen et al., 2016). Currently, many secreted proteins have been shown to contribute to pathogenesis, resulting in Verticillium wilt via diverse modes of action (Table 1). Thus, V. dahliae employs its secretome to degrade plant cell walls, manipulate host immunity, exert cytotoxicity, neutralize host oxidative stress, compete with its host for nutrients, and even to affect the host microbiome to facilitate Verticillium wilt development (Fig. 1).
Table 1.
Description of some secreted proteins involved in various biological processes of Verticillium dahliae.
| Secreted protein | Function description | Cell wall degradation | Host immunity manipulation | Hormone homeostasis Interference | Cytotoxicity | Oxidative stress neutralization | Fungal nutrition | Morphological development | Microbiome manipulation | Others | Host molecular target | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VD18.5 | Phytotoxic protein | √ | Palmer, Saleeba & Lyon (2005) | |||||||||
| VDH1 | Class II hydrophobin gene | √ | Klimes & Dobinson (2006) | |||||||||
| VdEg‐1 | Endoglucanase 1 | √ | √ | Maruthachalam et al. (2011) | ||||||||
| VdAve1 | Avirulence gene of race 1 | √ | √ | √ | Ve1 | de Jonge et al. (2012); Snelders et al. (2020) | ||||||
| VdNLP1 | Necrosis‐ and ethylene‐inducing‐like protein | √ | √ | GIPC sphingolipids | Zhou et al. (2012); Santhanam et al. (2013) | |||||||
| VdNLP2 | Necrosis‐ and ethylene‐inducing‐like protein | √ | GIPC sphingolipids | Zhou et al. (2012); Santhanam et al. (2013) | ||||||||
| VdSSP1 | Cell wall degradation related protein | √ | √ | Liu et al. (2013) | ||||||||
| VdISC1 | Isochorismatase | √ | √ | Isochorismate | Liu et al. (2014) | |||||||
| VdPL3.1 | Pectin lyase | √ | Chen et al. (2016) | |||||||||
| VdPL3.3 | Pectin lyase | √ | Chen et al. (2016) | |||||||||
| Vd2LysM | Chitin‐binding lysin motif | √ | √ | Plant chitinases | Kombrink et al. (2017) | |||||||
| VdASP F2 | Allergen Asp F2‐like protein | √ | Xie, Li & Yang (2017) | |||||||||
| VdCBM1 | Cellulose binding module 1 | √ | √ | √ | Gui et al. (2017, 2018); Wang et al. (2020a) | |||||||
| VdCP1 | Cerato‐platanin protein 1 | √ | √ | Plant chitinases | Zhang et al. (2017a) | |||||||
| VdEG1 | GH12 domain‐containing protein | √ | √ | √ | Gui et al. (2017) | |||||||
| VdEG3 | GH12 domain‐containing protein | √ | √ | √ | Gui et al. (2017) | |||||||
| VdSCP7 | Small cysteine‐rich protein | √ | √ | Zhang et al. (2017b) | ||||||||
| VdPEL1 | Pectate lyase | √ | √ | √ | Yang et al. (2018) | |||||||
| VdPL1 | Polysaccharide lyase | √ | Zhang et al. (2018) | |||||||||
| VdCUT11 | Cutinase | √ | √ | √ | Gui et al. (2018) | |||||||
| PevD1 | Alt a 1 family protein | √ | √ | GhPR5, ORE1 | Zhang et al. (2021); Liang et al. (2018) | |||||||
| VdSCP41 | Small cysteine‐rich protein | √ | √ | CBP60g and SARD1 | Qin et al. (2018) | |||||||
| VdOCH1 | Alpha‐1, 6‐mannosyltransferase | √ | √ | Zhang et al. (2019b) | ||||||||
| VdPDA1 | Polysaccharide deacetylase | √ | √ | Chitin oligomer | Gao et al. (2019) | |||||||
| VdSSEP1 | Secretory Ser protease 1 | √ | √ | √ | Plant chitinases | Han et al. (2019) | ||||||
| VdSCP27 | Small cysteine‐rich protein | √ | √ | Wang et al. (2020a) | ||||||||
| VdSCP113 | Small cysteine‐rich protein | √ | √ | Wang et al. (2020a) | ||||||||
| VdSCP126 | Small cysteine‐rich protein | √ | √ | Wang et al. (2020a) | ||||||||
| VdSOD3 | Superoxide dismutase | √ | Tian et al. (2020) | |||||||||
| VdAMP2 | Antimicrobial effector | √ | Snelders et al. (2020) | |||||||||
| VdSOD5 | Superoxide dismutase | √ | Tian et al. (2021a) | |||||||||
| VdSOD1 | Cu/Zn superoxide dismutase | √ | Tian et al. (2021b) | |||||||||
| Av2 | Avirulence gene of race 2 | √ | V2 | Chavarro‐Carrero et al. (2021) | ||||||||
| VdEIX3 | Ethylene‐inducing xylanase | √ | √ | √ | EIX2 | Yin et al. (2021) | ||||||
| VdXyn4 | Xylanase | √ | √ | √ | √ | √ | Wang et al. (2021a) | |||||
| VDAL | Asp f2‐like protein | √ | PUB25/26 | Ma et al. (2021) | ||||||||
| VdAMP3 | Antimicrobial effector | √ | Snelders et al. (2021) | |||||||||
| VdR3e | Avirulence gene of race 3 | √ | Wang et al. (2021b) | |||||||||
| VdRTX1 | Ribonuclease | √ | √ | Yin et al. (2022) |
CBP60g, calmodulin binding protein 60 family member g; EIX2, leucine‐rich repeat receptor‐like protein; GIPC, glycosylinositol phosphorylceramide; ORE1, A senescence‐associated NAC transcription factor; PR5, pathogenesis‐related protein 5 (PR5)‐like protein; PUB25/26, plant U‐box 25/26; SARD1, systemic acquired resistance deficient 1; Ve1, leucine‐rich repeat receptor‐like protein.
Fig. 1.
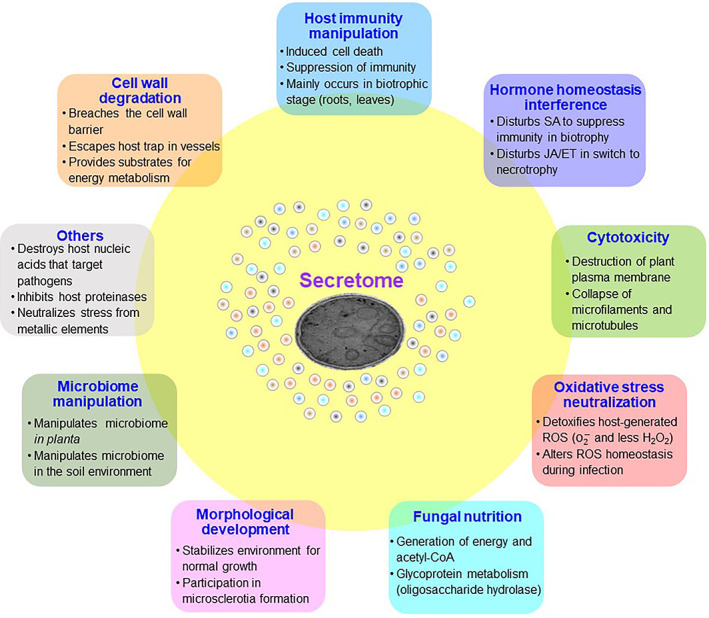
Versatile biological functions of the secretome in Verticillium dahliae. Acetyl‐coA, acetyl co‐enzyme A; ET, ethylene; JA, jasmonic acid; ROS, reactive oxygen species; SA, salicylic acid.
Verticillium dahliae employs its secretome to proliferate in the low‐nutrient confines of the xylem. Its secretome encodes a large arsenal of enzymes that allow the complete degradation of many plant polysaccharides (Klosterman et al., 2011). These processes inevitably induce xylem collapse and the deposition of polysaccharides. The host plants respond by producing biomacromolecules (gums, gels, and other resins) and tyloses as defensive structures which protrude into the vascular tissue to block progress of the pathogen. Together, the massive propagation of hyphae, the deposition of polysaccharides and the production of biomacromolecules by the plant cause xylem occlusion and plant wilting (Fig. 2).
Fig. 2.
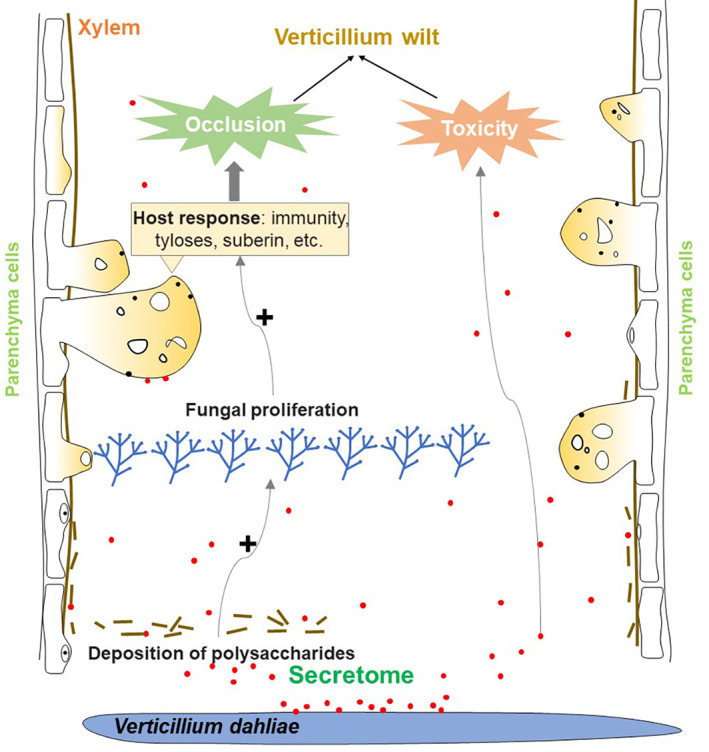
The secretome of Verticillium dahliae causes vascular occlusion which results in Verticillium wilt. Its secretome contributes to vascular occlusion by promoting fungal proliferation and polysaccharide deposition, and by inducing host responses including tyloses formation and suberin production. The secretome also contributes to Verticillium wilt through cytotoxicity.
(1). Proliferation in the plant xylem
(a). Cell wall degradation
During the infection process, V. dahliae must break through plant root cell wall barriers to reach the xylem. During this process, V. dahliae produces an array of cell wall degrading enzymes (CWDEs) (Kubicek, Starr & Glass, 2014). Some of these CWDEs destroy the highly structured and rigid secondary xylem walls, and also enable breaches of the pectin‐enriched pit cavities or the vascular end walls (Zhao et al., 2014). Additional secreted enzymes include two members of the glycoside hydrolase 12 family, the endoglucanases VdEG1 and VdEG3, which participate in cellulose/hemicellulose degradation (Gui et al., 2017) similar to the CWDEs involved in degradation of the plant cuticle and xylan (Gui et al., 2018; Yin et al., 2021; Wang et al., 2021a). Furthermore, transcription regulators of CWDEs such as the sucrose non‐fermenting protein kinase VdSNF1 (Tzima et al., 2021) and fungal‐specific transcription factor VdFTF1 (Zhang et al., 2018) regulate CWDEs expression or secretion, and significantly contribute to pathogenesis. The superabundance of secreted carbohydrate‐active enzymes suggests an extraordinary capacity of V. dahliae to degrade plant cell wall barriers during early infection stages and to degrade plant xylem walls during proliferation in these vessels.
(b). Scavenging host reactive oxygen species
Plants respond to pathogen attack with a transient burst of reactive oxygen species (ROS) that play a central role in plant immune responses (Qi et al., 2017). Pathogens can counteract this by secreting ROS‐scavenging proteins to neutralize or degrade ROS, such as superoxide dismutases (SODs), catalases, and peroxidases (Broxton & Culotta, 2016; Khademian & Imlay, 2021). Comparative analyses of the V. dahliae secretome have revealed that many secreted proteins with ROS‐scavenging and oxidative stress response functions are activated when the pathogen is cultured on host tissue or under nutrient‐starvation conditions (El‐Bebany, Rampitsch & Daayf, 2010; Chu et al., 2015; Chen et al., 2016). V. dahliae secretes SODs to detoxify host‐generated extracellular ROS, contributing significantly to virulence during plant infection (Tian et al., 2020, 2021a,b). V. dahliae ROS production facilitates penetration peg formation during the initial colonization of cotton (Gossypium hirsutum L.) roots (Zhao, Zhou & Guo, 2016). Thus, the deployment of ROS during infection combined with counter‐provisions for neutralization of host‐generated oxidative stress is key to successful infection and proliferation in the vascular tissue of host plants.
(c). Suppressing host immunity
The ability to secrete effector proteins that can enter plant cells and manipulate host immunity is a key determinant of a successful plant pathogen (He et al., 2020). In V. dahliae, the role of effectors in manipulating host immunity has been studied in detail (Fig. 3). To enable successful infection and proliferation in host plants, V. dahliae has evolved a range of effector proteins that inhibit host immune responses (Ding & Redkar, 2018), including inactivation of the salicylic acid (SA) pathway by isochorismate synthase VdIsc1 (Liu et al., 2014), cellulose‐binding module family 1‐member (VdCBM1) (Gui et al., 2017, 2018; Wang et al., 2021b), small cysteine‐rich protein VdSCP41 (Qin et al., 2018), lysin motif (LysM) protein Vd2LysM (Kombrink et al., 2017), secretory serine protease 1 (VdSSEP1) (Han et al., 2019) and polysaccharide deacetylase (VdPDA1) (Gao et al., 2019) (Fig. 3; Table 1). For instance, VdPDA1 deacetylates chitin oligomers; since the N‐acetyl group contributes to host LysM‐containing receptor recognition for ligand‐triggered immunity, the action of VdPDA1 renders the host susceptible to V. dahliae by evading host surveillance through inactivation of a chitin‐triggered host immune response (Gao et al., 2019). Together, these effectors mainly function in the early stages of infection and employ a variety of strategies to overcome immunity to facilitate pathogen colonization in roots.
Fig. 3.
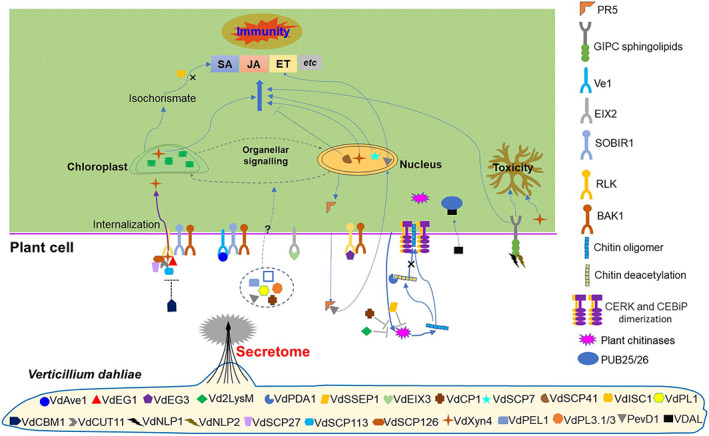
Verticillium dahliae employs its secretome to manipulate host immunity and interfere with hormone homeostasis. See Table 1 for V. dahliae protein names. VdAve1 is recognized as an avirulence determinant by tomato plants that carry the corresponding Ve1 immune receptor. VdEG1 and VdEG3 are glycoside hydrolase 12 proteins that trigger immunity dependent on the LRR‐RLPs/SOBIR1/BAK1 and LRR‐RLKs/BAK1 complexes, respectively. Vd2LysM binds long‐ or short‐chain chitin oligomers and prevents degradation of chitin by plant chitinase. VdPDA1 directs deacetylation of chitin oligomers and inhibits perception by host LysM‐containing receptors, thus avoiding ligand‐triggered immunity. VdSSEP1 hydrolyses cotton Chi28 directly, inhibiting the production of chitin oligomers. VdEIX3 exhibits immunity‐inducing activity in Nicotiana benthamiana, recognized by the leucine‐rich repeat receptor‐like protein NbEIX2. VdCP1 protects the V. dahliae cell wall from chitinase degradation. VdSCP7 targets the host nucleus to modulate plant immunity. VdSCP41 targets the plant‐specific transcription factors CBP60g and SARD1 to modulate immunity. VdISC1 disrupts the plant salicylate metabolism pathway by suppressing the transformation from isochorismate to salicylic acid. VdPL1 plays a virulence function during infection of cotton. VdCBM1 suppresses VdEG1‐, VdEG3‐, VdSCP27‐, VdSCP113‐, VdSCP126‐, VdCUT11‐ and VdXyn4‐induced cell death and some PAMPs‐triggered immunity in N. benthamiana. VdCUT11 induces plant defence responses in N. benthamania in a BAK1‐ and SOBIR1‐dependent manner. VdNLP1 and VdNLP2 are GIPC sphingolipids that act as NLP toxin receptors; NLPs form complexes with terminal monomeric hexose moieties of GIPCs and insert into the plant plasma membrane, causing cell lysis. VdSCP27, VdSCP113 and VdSCP126 induce defence responses in N. benthamania in a BAK1‐ and SOBIR1‐dependent manner. VdXyn4 plays a cytotoxic function and induces a necrosis phenotype in N. benthamania, depending on simultaneous localization to the nuclei and chloroplasts in a BAK1‐ and SOBIR1‐dependent manner. VdPEL1 exhibits pectin hydrolytic activity and induces cell death in plants. VdPL3.1/3 have virulence functions during infection of cotton. PevD1 induces ethylene biosynthesis by directly binding to ORE1. VDAL protects transcription factor MYB6 from degradation by interacting with the E3 ligases PUB25 and PUB26 to enhance Verticillium wilt resistance. BAK1, LRR‐RLK BRI1‐associated kinase‐1; CBP60g, calmodulin binding protein 60 family member g; CEBiP, chitin‐elicitor binding protein; CERK, receptor chitin elicitor receptor kinase; Chi28, chitinase 28; EIX2, leucine‐rich repeat receptor‐like protein; ET, ethylene; GIPC, glycosylinositol phosphorylceramide; JA, jasmonic acid; LRR, leucine‐rich repeat; LysM, lysin motif; MYB6, MYB domain protein 6; NLP, necrosis‐ and ethylene‐inducing‐like protein; ORE1, A senescence‐associated NAC transcription factor; PAMP, pathogen‐associated molecular pattern; PR5, pathogenesis‐related protein 5 (PR5)‐like protein; PUB25/26, plant U‐box 25/26; RLK, receptor‐like kinase; RLP, receptor‐like protein; SA, salicylic acid; SARD1, systemic acquired resistance deficient 1; SOBIR1, LRR‐RLK suppressor of BIR1‐1; Ve1, leucine‐rich repeat receptor‐like protein.
(d). Fungal nutrition
Fungi secrete many plant CWDEs such as cellulase, hemicellulase, and pectin‐degrading enzymes that act on plant tissues to produce monomeric and/or small polymeric sugars that are subsequently transported into the fungal cell via membrane‐bound transporters (Glass et al., 2013). In this way, V. dahliae obtains nutrition for proliferation in the vascular tissue. Many secreted carbohydrate‐active enzymes from V. dahliae catalyse the release of nutrients as the degradation products of cell walls (Klosterman et al., 2011; Xiong, Wang & Tian, 2015; Chen et al., 2016) (Table 1, Fig. 3), some of which function in the generation of energy and acetyl‐CoA during nutrient starvation (Chu et al., 2015; Xiong, Wang & Tian, 2015). The V. dahliae alpha‐1,6‐mannosyltransferase (VdOCH1) plays a crucial role in N‐linked oligosaccharide glycoprotein metabolism (Zhang et al., 2019b). The abundant pectate lyases secreted by V. dahliae are thought to have key roles in its adaptation to its vascular niche (Klosterman et al., 2011).
(e). Microbiome manipulation
Although pathogen effectors are typically considered to act exclusively through direct host immunity manipulation, plant pathogens also utilize effectors to target the host microbiota in order to facilitate niche colonization (Rovenich, Boshoven & Thomma, 2014; Snelders et al., 2018). Recent work (Snelders et al., 2020, 2021) suggests that V. dahliae exploits secreted proteins to manipulate the host microbiome through its antibacterial or antifungal properties, to promote infection. The V. dahliae avirulence effector (VdAve1) displays antimicrobial activity and facilitates colonization of tomato and cotton through the selective manipulation of the host microbiota in the roots, as well as in the xylem by suppressing antagonistic bacteria. The antimicrobial protein VdAMP2, is exclusively expressed by V. dahliae when in the soil and exerts antibacterial activity that contributes to niche establishment (Snelders et al., 2020). Thus, VdAMP2 and VdAve1 likely have complementary functions for optimal soil and host colonization. Another ancient antimicrobial protein (VdAMP3) is specifically expressed to ward off fungal niche competitors during resting structure formation in senescing host mesophyll tissues (Snelders et al., 2021), allowing V. dahliae to tailor the expression of microbiome‐manipulating effectors based on the microbiota that it encounters during different stages of the disease cycle. This promotes V. dahliae colonization and proliferation in host plants to accelerate symptom development.
(2). Xylem wall collapse and deposition of polysaccharides
Compared to many fungi, the genome of V. dahliae encodes a significant expansion of gene families involved in plant cell wall degradation, and specific members of this repertoire may be essential for adaptation to its vascular niche (Klosterman et al., 2011). For example, among plant cell wall polysaccharides, pectin has the highest structural and functional complexity (Mohnen, 2008; Harholt, 2010), with four major structural classes: homogalacturonan (HG), rhamnogalacturonan I (RG‐I), xylogalacturonan (XG), and rhamnogalacturonan II (RG‐II). Enzymes required for efficient degradation of pectin (Martens‐Uzunova & Schaap, 2009) include polygalacturonases and rhamnogalacturonases (glycoside hydrolases family 28, GH28), polysaccharide lyases (pectin and pectate lyases, PL1, PL2, and PL3), and rhamnogalacturonan lyases (PL4), as well as pectin methylesterases (carbohydrate esterase family 8, CE8), pectin acetylesterases (CE12 and CE13), and rhamnogalacturonan acetylesterases (CE12). Among all sequenced fungal genomes, V. dahliae has one of the most complete repertoires of polysaccharide lyases and associated glycoside hydrolases for the breakdown of complex pectin (Klosterman et al., 2011). Experimental evidence indicates that the V. dahliae polysaccharide lyase VdPL1 (Zhang et al., 2018), pectate lyase VdPEL1 (Yang et al., 2018), pectin lyases VdPL3.1 and VdPL3.3 (Chen et al., 2016), and the pathotype‐specific secretory protein VdSSP1 (Liu et al., 2013) are all involved in virulence and pectin degradation. Since pectin is enmeshed with other polysaccharides to form plant cell walls, the result is that incompletely degraded and intermediate polysaccharides contribute directly to vascular occlusion (Kubicek, Starr & Glass, 2014). In addition, in response to infections, pectin gels are usually released into the xylem (Rioux et al., 1998; Clérivet et al., 2000). Similarly, starch hydrolysis also contributes to vessel occlusion related to wilt symptoms in olive (Olea europaea L.) stems of susceptible cultivars infected by V. dahliae (Trapero et al., 2018). Thus, while degradation of the xylem walls and pit membranes by V. dahliae CWDEs supplies nutrients for pathogen proliferation in the low‐nutrient confines of the xylem, it also causes xylem collapse and blockage by deposition of insufficiently degraded polysaccharides.
(3). Induction of host response that aggravate vascular occlusion
While secretion of an array of CWDEs is required for breaching cell wall barriers and nutrient acquisition, V. dahliae also must overcome host defence responses. It achieves this with a combination of secreted effectors and interference with host hormone signalling, both of which contribute to vascular occlusion and wilt symptoms. Among plant hormones, SA, jasmonic acid (JA) and ethylene (ET) are all significant in the hormonal regulation of plant immune responses (Shigenaga & Argueso, 2016). SA has been implicated in the protection of plant tissues during the initial biotrophic phase of V. dahliae infection, while JA is more prominent following the establishment of V. dahliae in the xylem and its switch to a necrotrophic lifestyle (Dhar et al., 2020). ET activates defence responses to limit spread of the pathogen during the early stages of V. dahliae infection; but during subsequent colonization and the switch to the necrotrophic phase, ET can facilitate establishment of the pathogen and accelerate the progression of Verticillium wilt (Dhar et al., 2020). The V. dahliae secretome interferes with the intricate and delicate balance of phytohormones during V. dahliae–plant interactions, regulating the immune responses in a synergistic or antagonistic manner that contributes to Verticillium wilt progression (Dhar et al., 2020). Manipulation of SA–JA signalling has been demonstrated for several known effectors in V dahliae, including four VdSCPs (Wang et al., 2012; Wang et al., 2020a), the cutinase VdCUT11 (Gui et al., 2018), the necrosis‐ and ethylene‐inducing‐like protein VdNLP (Wang et al., 2004), the endoglucanases VdEG1 and VdEG3 (Gui et al., 2017), VdSCP7 (Zhang et al., 2017a,b) and the xylanase VdXyn4 (Wang et al., 2021a,b) (Fig. 3). Additionally, VdNLP involved in ET signalling aids in the establishment of the pathogen and accelerates the progression of Verticillium wilt and death of the host (Wang et al., 2004; Dhar et al., 2020), and PevD1 induces ET biosynthesis by directly binding to the senescence‐associated NAC transcription factor ORE1 (Zhang et al., 2021).
In response to V. dahliae infection and colonization, the plant may mount a rapid defence including deposition of coating materials such as suberin on vascular cell walls, forming a barrier to fungal penetration and horizontal spread (Robb, Powell & Street, 1989). At this stage, infection can result in vessel occlusion by gums, gels, tyloses and other deposited resins secreted by the neighbouring parenchyma cells (Robb et al., 1979; Benhamou, 1995). In addition, plant hormone levels can be manipulated by effectors secreted by pathogens, influencing plant responses to the pathogen, including vascular occlusion. During plant–pathogen interactions, many phytohormones regulate the formation of coating materials on vascular cell walls, including ET, which is involved in lignin and suberin biosynthesis, and tyloses formation, while abscisic acid coordinates suberin deposition, JA contributes to lignin biosynthesis and tyloses formation, and SA can abolish the induction of tyloses by JA (Sun et al., 2007; Leśniewska et al., 2017; Hu et al., 2018; Liu et al., 2020; Wang et al., 2020b). Some of these regulatory mechanisms have been inferred in V. dahliae–plant interactions (Hu et al., 2019; Dhar et al., 2020). In V. longisporum infections, Arabidopsis thaliana plants can generate xylem de novo by transdifferentiation of the chloroplast‐containing bundle sheath cells into functional xylem elements, enhancing water transport, storage capacity, and drought tolerance (Reusche et al., 2012). Whether a similar mechanism exists during V. dahliae–plant interactions with a role in relieving plant wilt symptoms requires further investigation. Together, the secreted effectors from V. dahliae cause host immune responses and disturb the phytohormone balance contributing to vascular occlusion and the induction of Verticillium wilt symptoms (Fig. 2).
(4). Toxicity
A cytotoxic function for all identified secreted proteins is difficult to demonstrate explicitly because a similar plant necrosis phenotype accompanies immunity‐ and cytotoxin‐induced necrosis. While some studies have shown that crude Verticillium culture extracts cause microfilaments and microtubules to collapse in plant cells (Zhao et al., 2020), it remains unclear whether it is toxic activity that directly causes necrosis. A prominent example of a fungal protein with cytotoxic activity is NLP purified from the culture filtrates of Fusarium oxysporum (Bailey, James & James, 1997). A NLP member identified from the mycelia of a V. dahliae strain pathogenic to cotton displayed the ability to cause foliar necrosis in various plant species (Wang et al., 2004). With the advent of genomics, seven additional members of the NLP family have been identified from the V. dahliae genome, two of which cause cellular necrosis (Zhou et al., 2012; Santhanam et al., 2013). The proposed mechanism of NLP‐mediated cell lysis is that glycosylinositol phosphorylceramide (GIPC) sphingolipids act as NLP receptors to form complexes with terminal monomeric hexose moieties of GIPCs that insert into the plant plasma membrane (Ottmann et al., 2009; Lenarčič et al., 2017) (Fig. 3). Additionally, a member of the xylanase family, VdXyn4 induces vein necrosis and plant wilting during advanced stages of infection, but not immunity‐triggered cell death, suggesting that it is a candidate toxicity factor (Wang et al., 2021a) (Fig. 3). Thus, constituents of the V. dahliae secretome may induce foliar wilting directly via toxicity, even if the definition of toxicity is narrow in this context.
III. HOW THE SECRETOME COMPROMISES THE HOST TO CAUSE VASCULAR OCCLUSION AND VERTICILLIUM WILT
The pathogenic status of V. dahliae is dynamic during the different stages of host infection (Fradin & Thomma, 2006), and the secretome plays a critical role in Verticillium wilt disease progression by both supplying toxins and contributing to vascular occlusion to cause irreversible and lethal plant wilting (Fig. 4).
Fig. 4.
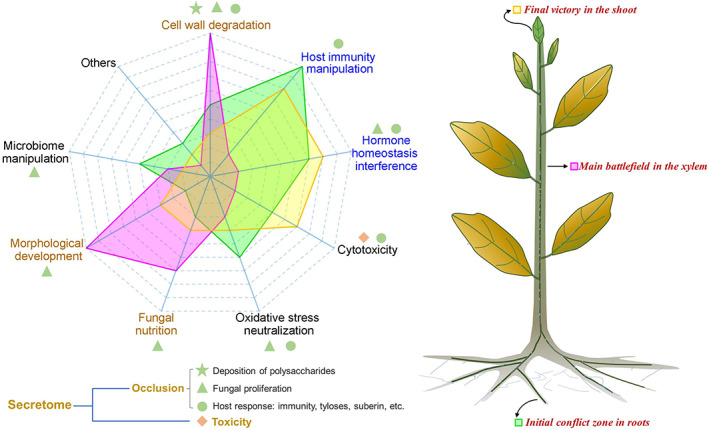
Intensity model of biological functions of the secretome at different stages of Verticillium wilt infection. Left: the main contributions of different functions of the secretome in polysaccharide deposition, fungal proliferation, host response and toxicity. Cell wall degradation, morphological development and fungal nutrition predation (shown in brown font) represent the main effects of the secretome in vessels; and host immunity manipulation and hormone interference (shown in blue font) represent the main effects of the secretome in roots and leaves. The green region shows the biological functions of the secretome that operate during the initial stages of the colonization, the pink region shows those operating when the fungus is present in the xylem, and the yellow region is for the final stage of infection when the pathogen has reached the leaves (see plant image on right).
(1). Initial conflict zone in roots
Verticillium dahliae initially attacks plants through the root tip or through lateral roots by developing an infectious structure known as hyphopodium (Zhao, Zhou & Guo, 2016), which assists in establishing biotrophy (Fradin & Thomma, 2006). The hyphopodium develops a septin ring while piercing the root epidermis and cortical cells, that acts as a functional fungus–host penetration interface for the delivery of secreted fungal proteins (Zhou, Zhao & Guo, 2017). Subsequently, the secretome overcomes host defence responses by manipulating host immunity, interfering in hormone homeostasis, neutralization of oxidative stress, and cytotoxicity. The secreted CWDEs breach the plant cell walls to allow further penetration and proliferation (Fradin & Thomma, 2006). Even with these specialized tools, only a few invading hyphae successfully traverse into the vessel cells (Zhao et al., 2014). In addition, several CWDEs are involved in manipulating host immunity and altering hormone homeostasis (Table 1). Prior to penetration of plant roots, the rhizosphere environment is also crucial for V. dahliae survival and its eventual invasion. Here, effector proteins can suppress the growth of antagonistic bacteria (Snelders et al., 2020, 2021). Thus, ‘toxic’ activities of the V. dahliae secretome are critical for successful penetration, infection, and xylem colonization (Fig. 4).
(2). The vascular battlefield
Once V. dahliae crosses the endodermis and reaches the xylem (generally 2–4 days), the hyphae begin to branch, develop terminal conidia and proliferate in the xylem (Fradin & Thomma, 2006). At this stage, the pathogen channels energy into vegetative growth resulting in hyphal extension and conidia formation in the xylem (Zhao et al., 2014). During this stage, V. dahliae uses its extensive range of CWDEs to survive in the barren environment of the xylem (Klosterman et al., 2011), and also secretes effectors with toxicity functions, for example VdXyn4 acts as a toxin during the colonization of vessels by destroying the host vascular system (Wang et al., 2021a). Concurrently, the host plants respond to the extensive sporulation and proliferation in the xylem by initiating rapid defence responses (Benhamou, 1995; Heinz et al., 1998; Pegg & Brady, 2002). In resistant hosts, colonization and proliferation of the pathogen are hindered by its compartmentalization in the vascular tissue. In susceptible hosts, the pathogen escapes these host traps by counteracting host defences via secreted effectors and hyphal growth that progresses through the occluded xylem (Fradin & Thomma, 2006). In this process, V. dahliae effectors also disturb the xylem microbiome (Snelders et al., 2020, 2021), further accelerating fungal proliferation and initiating a resistance response, that in turn leads to further xylem occlusion. Therefore, in the battle over control of the plant xylem, V. dahliae deploys its secretome to aid mycelial proliferation, resulting in release of incompletely degraded polysaccharides from the plant cell walls and induction of biomacromolecules from a host response, all of which contribute to vascular occlusion and the appearance of wilt symptoms (Fig. 4).
(3). Final victory in the shoot
The successful proliferation of V. dahliae in the xylem results in two damaging effects: vascular occlusion blocking water and nutrient transport; and rapid pathogen transmission to the leaves via the vascular plumbing (Pegg & Brady, 2002; Fradin & Thomma, 2006). The pathogen can easily degrade the cell walls of leaf tissues since these are mainly coated with aliphatic biopolymers composed of cutin rather than suberin (Schreiber, 2010). To infect foliar cells, secreted proteins are released that manipulate host immunity and break hormone homeostasis (Liu et al., 2014; Gui et al., 2017; Gao et al., 2019). For example, V. dahliae induces leaf senescence and wilting by secreting the alt a1 family protein PevD1 to regulate the ORE1–ACS6 (1‐aminocyclopropane‐1‐carboxylate synthase) cascade and enhance ET production (Zhang et al., 2021). In addition, cytotoxicity also plays a critical role in inducing cell necrosis, as VdNLPs are constitutively expressed during later stages of infection (Santhanam et al., 2013), accelerating disease symptoms and the production of microsclerotia to begin a new disease cycle. V. dahliae effectors contribute to microsclerotia formation in senescing mesophyll tissue by warding off other fungal niche competitors (Snelders et al., 2020, 2021).
IV. CONCLUSIONS
(1) Verticillium wilt caused by V. dahliae is a notorious vascular wilt disease that leads to chlorosis, stunting and wilting. Two notable hypotheses exist regarding the mechanisms underlying Verticillium wilt symptom development: vessel occlusion and direct toxicity through secreted toxins (Fradin & Thomma, 2006; Báidez et al., 2007). Vascular occlusion occurs as a result of the biomass of the pathogen present in the plant xylem, in addition to defensive tylose or colloid production by the plant (Báidez et al., 2007). The toxin hypothesis suggests that the pathogen delivers complex compounds into its host, including large polymers of polysaccharides, protein‐lipopolysaccharides, glycoproteins, enzymes, etc., that induce foliar wilting via cytotoxicity (Fradin & Thomma, 2006; de Sain & Rep, 2015).
(2) Similar to other plant pathogens, the secretome of V. dahliae has evolved to alter host structure and physiology, facilitating colonization. Specifically, components of the secretome of V. dahliae degrade plant cell walls, manipulate host immunity, exert cytotoxicity, neutralize host oxidative stresses, compete with its host for nutrients, and alter host endophytic associations and the rhizosphere microbiome, finally contributing to Verticillium wilt.
(3) Except for some V. dahliae secretome members with direct cytotoxicity, V. dahliae mainly employs its secretome to support proliferation in the plant xylem. These activities contribute to xylem wall collapse and the deposition of polysaccharides, and induction of host plant responses that promote vascular occlusion, finally compromising the host by causing total vascular occlusion. Thus, the V. dahliae secretome manipulates plant defences to contribute to vascular occlusion simultaneously with its toxicity effects to cause wilt symptoms.
(4) There are several key phases in the V. dahliae–plant interaction in which the weapons available in the secretome play critical roles in the induction of Verticillium wilt symptoms. In the initial infection zone in the roots, these effector activities specifically counter plant defences, neutralize oxidative stress, manipulate host immunity, and interfere with hormone homeostasis. Subsequently, in the vascular battlefield, the secretome aids mycelial proliferation, the release of incompletely degraded polysaccharides from plant cell walls and the induction of biomacromolecules from an active host defence response, all of which contribute to vascular occlusion. Final victory in shoot occurs as the toxic functions of the secretome manipulate immunity and hormone homeostasis to aggravate wilt symptoms further.
(5) Description of the functions of the secretory proteins in detail will allow their exact roles in vascular occlusion and toxicity to be clarified. Unravelling the bridge that links the V. dahliae secretome to the historical vascular occlusion and toxin hypotheses will shed new light into novel means to control this and related plant vascular pathogens.
V. ACKNOWLEDGEMENTS
We apologize in advance to all investigators whose research could not be cited due to space limitations. We extend special thanks to Drs Nikhil Dhar and Peter Henry for thoughtful pre‐submission comments. This work was supported by grants from the National Key Research and Development Program of China (2018YFE0112500), the Fundamental Research Funds for Central Non‐profit Scientific Institution in CAAS (Y2021XK22), and the Elite Youth Program CAAS to J.‐Y.C.
Contributor Information
Krishna V. Subbarao, Email: kvsubbarao@ucdavis.edu.
Jie‐Yin Chen, Email: chenjieyin@caas.cn.
REFERENCES
- Báidez, A. G. , Gómez, P. , Río, J. A. D. & Ortuño, A. (2007). Dysfunctionality of the xylem in Olea europaea L. plants associated with the infection process by Verticillium dahliae Kleb. Role of phenolic compounds in plant defense mechanism. Journal of Agricultural and Food Chemistry 55, 3373–3377. [DOI] [PubMed] [Google Scholar]
- Bailey, B. A. , James, C. J. & James, D. A. (1997). The 24‐kDa protein from Fusarium oxysporum f.sp. erythroxyli: occurrence in related fungi and the effect of growth medium on its production. Canadian Journal of Microbiology 43, 45–55. [DOI] [PubMed] [Google Scholar]
- Benhamou, N. (1995). Ultrastructural and cytochemical aspects of the response of eggplant parenchyma cells in direct contact with Verticillium‐infected xylem vessels. Physiological and Molecular Plant Pathology 46, 321–338. [Google Scholar]
- Broxton, C. N. & Culotta, V. C. (2016). SOD enzymes and microbial pathogens: surviving the oxidative storm of infection. PLoS Pathogens 12, e1005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro‐Carrero, E. A. , Vermeulen, J. P. , Torres, D. E. , Usami, T. , Schouten, H. J. , Bai, Y. , Seidl, M. F. & Thomma, B. P. H. J. (2021). Comparative genomics reveals the in planta‐secreted Verticillium dahliae Av2 effector protein recognized in tomato plants that carry the V2 resistance locus. Environmental Microbiology 23, 1941–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. Y. , Klosterman, S. J. , Hu, X. P. , Dai, X. F. & Subbarao, K. V. (2021). Key insights and research prospects at the dawn of the population genomics era for Verticillium dahliae . Annual Review of Phytopathology 59, 31–51. [DOI] [PubMed] [Google Scholar]
- Chen, J. Y. , Liu, C. , Gui, Y. J. , Si, K. W. , Zhang, D. D. , Wang, J. , Short, D. P. G. , Huang, J. Q. , Li, N. Y. , Liang, Y. , Zhang, W. Q. , Yang, L. , Ma, X. F. , Li, T. G. , Zhou, L. , et al. (2018). Comparative genomics reveals cotton‐specific virulence factors in flexible genomic regions in Verticillium dahliae and evidence of horizontal gene transfer from Fusarium . New Phytologist 217, 756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. Y. , Xiao, H. L. , Gui, Y. J. , Zhang, D. D. , Li, L. , Bao, Y. M. & Dai, X. F. (2016). Characterization of the Verticillium dahliae exoproteome involves in pathogenicity from cotton‐containing medium. Frontiers in Microbiology 7, 1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, X. Q. , Chen, W. , Chen, X. B. , Lu, W. J. , Han, L. , Wang, X. L. , Hao, L. L. & Guo, X. Q. (2015). The cotton WRKY gene GhWRKY41 positively regulates salt and drought stress tolerance in transgenic Nicotiana benthamiana . PLoS One 10, e0143022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clérivet, A. , Déon, V. , Alami, I. , Lopez, F. , Geiger, J. P. & Nicole, M. (2000). Tyloses and gels associated with cellulose accumulation in vessels are responses of plane tree seedlings (Platanus × acerifolia) to the vascular fungus Ceratocystis fimbriata f. sp platani . Trees‐Structure and Function 15, 25–31. [Google Scholar]
- de Jonge, R. , van Esse, H. P. , Maruthachalam, K. , Bolton, M. D. , Santhanam, P. , Saber, M. K. , Zhang, Z. , Usami, T. , Lievens, B. , Subbarao, K. V. & Thomma, B. P. H. J. (2012). Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proceedings of the National Academy of Sciences of the United States of America 109, 5110–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sain, M. & Rep, M. (2015). The role of pathogen‐secreted proteins in fungal vascular wilt diseases. International Journal of Molecular Sciences 16, 23970–23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar, N. , Chen, J. Y. , Subbarao, K. V. & Klosterman, S. J. (2020). Hormone signaling and its interplay with development and defense responses in Verticillium‐plant interactions. Frontiers in Plant Science 11, 584997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, P. T. & Redkar, A. (2018). Pathogens suppress host transcription factors for rampant proliferation. Trends in Plant Science 23, 950–953. [DOI] [PubMed] [Google Scholar]
- El‐Bebany, A. F. , Rampitsch, C. & Daayf, F. (2010). Proteomic analysis of the phytopathogenic soilborne fungus Verticillium dahliae reveals differential protein expression in isolates that differ in aggressiveness. Proteomics 10, 289–303. [DOI] [PubMed] [Google Scholar]
- Fradin, E. F. & Thomma, B. P. H. J. (2006). Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo‐atrum . Molecular Plant Pathology 7, 71–86. [DOI] [PubMed] [Google Scholar]
- Gao, F. , Zhang, B. S. , Zhao, J. H. , Huang, J. F. , Jia, P. S. , Wang, S. , Zhang, J. , Zhou, J. M. & Guo, H. S. (2019). Deacetylation of chitin oligomers increases virulence in soil‐borne fungal pathogens. Nature Plants 5, 1167–1176. [DOI] [PubMed] [Google Scholar]
- Glass, N. L. , Schmoll, M. , Cate, J. & Coradetti, S. (2013). Plant cell wall deconstruction by ascomycete fungi. Annual Review of Microbiology 67, 477–498. [DOI] [PubMed] [Google Scholar]
- Gui, Y. J. , Chen, J. Y. , Zhang, D. D. , Li, N. Y. , Li, T. G. , Zhang, W. Q. , Wang, X. Y. , Short, D. P. G. , Li, L. , Guo, W. , Kong, Z. Q. , Bao, Y. M. , Subbarao, K. V. & Dai, X. F. (2017). Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate‐binding module 1. Environmental Microbiology 19, 1914–1932. [DOI] [PubMed] [Google Scholar]
- Gui, Y. J. , Zhang, W. Q. , Zhang, D. D. , Zhou, L. , Short, D. P. G. , Wang, J. , Ma, X. F. , Li, T. G. , Kong, Z. Q. , Wang, B. L. , Wang, D. L. , Li, N.‐Y. , Subbarao, K. V. , Chen, J. Y. & Dai, X. F. (2018). A Verticillium dahliae extracellular cutinase modulates plant immune responses. Molecular Plant‐Microbe Interactions 31, 260–273. [DOI] [PubMed] [Google Scholar]
- Han, L. B. , Li, Y. B. , Wang, F. X. , Wang, W. Y. & Xia, G. X. (2019). The cotton apoplastic protein CRR1 stabilizes chitinase 28 to facilitate defense against the fungal pathogen Verticillium dahliae . Plant Cell 31, 520–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt, J. (2010). Biosynthesis of pectin. Plant Physiology 153, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Q. , Mclellan, H. , Boevink, P. C. & Birch, P. R. J. (2020). All roads lead to susceptibility: the many modes of action of fungal and oomycete intracellular effectors. Plant Communications 1, 100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz, R. , Lee, S. W. , Saparno, A. , Nazar, R. N. & Robb, J. (1998). Cyclical systemic colonization in Verticillium‐infected tomato. Physiological and Molecular Plant Pathology 52, 385–396. [Google Scholar]
- Hogenhout, S. A. , van der Hoorn, R. A. , Terauchi, R. & Kamoun, S. (2009). Emerging concepts in effector biology of plant‐associated organisms. Molecular Plant‐Microbe Interactions 22, 115–222. [DOI] [PubMed] [Google Scholar]
- Hu, Q. , Min, L. , Yang, X. Y. , Jin, S. X. , Zhang, L. , Li, Y. Y. , Ma, Y. Z. , Qi, X. W. , Li, D. Q. , Liu, H. B. , Lindsey, K. , Zhu, L. F. & Zhang, X. L. (2018). Laccase GhLac1 modulates broad‐spectrum biotic stress tolerance via manipulating phenylpropanoid pathway and jasmonic acid synthesis. Plant Physiology 176, 1808–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. P. , Puri, K. D. , Gurung, S. , Klosterman, S. J. & Subbarao, K. V. (2019). Proteome and metabolome analyses reveal differential responses in tomato–Verticillium dahliae‐interactions. Journal of Proteomics 207, 103449. [DOI] [PubMed] [Google Scholar]
- Inderbitzin, P. & Subbarao, K. V. (2014). Verticillium systematics and evolution: how confusion impedes Verticillium wilt management and how to resolve it. Phytopathology 104, 564–574. [DOI] [PubMed] [Google Scholar]
- Jing, J. , Fan, L. W. & Wu, W. H. (2005). Evidences for involvement of endogenous cAMP in Arabidopsis defense responses to Verticillium toxins. Cell Research 15, 585–592. [DOI] [PubMed] [Google Scholar]
- Khademian, M. & Imlay, J. A. (2021). How microbes evolved to tolerate oxygen. Trends in Microbiology 29, 428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes, A. & Dobinson, K. F. (2006). A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae . Fungal Genetics and Biology 43, 283–294. [DOI] [PubMed] [Google Scholar]
- Klimes, A. , Dobinson, K. F. , Thomma, B. P. H. J. & Klosterman, S. J. (2015). Genomics spurs rapid advances in our understanding of the biology of vascular wilt pathogens in the genus Verticillium . Annual Review of Phytopathology 53, 181–198. [DOI] [PubMed] [Google Scholar]
- Klosterman, S. J. , Subbarao, K. V. , Kang, S. , Veronese, P. , Gold, S. E. , Thomma, B. P. H. J. , Chen, Z. , Henrissat, B. , Lee, Y. H. , Park, J. , et al. (2011). Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathogens 7, e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink, A. , Rovenich, H. , Shi‐Kunne, X. Q. , Rojas‐Padilla, E. , van den Berg, G. C. M. , Domazakis, E. , de Jonge, R. , Valkenburg, D. J. , Sánchez‐Vallet, A. , Seidl, M. F. & Thomma, B. P. H. J. (2017). Verticillium dahliae LysM effectors differentially contribute to virulence on plant hosts. Molecular Plant Pathology 18, 596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek, C. P. , Starr, T. L. & Glass, N. L. (2014). Plant cell wall‐degrading enzymes and their secretion in plant‐pathogenic fungi. Annual Review of Phytopathology 52, 427–451. [DOI] [PubMed] [Google Scholar]
- Lenarčič, T. , Albert, I. , Böhm, H. , Hodnik, V. , Pirc, K. , Zavec, A. B. , Podobnik, M. , Pahovnik, D. , Žagar, E. , Pruitt, R. , Greimel, P. , Yamaji‐Hasegawa, A. , Kobayashi, T. , Zienkiewicz, A. , Gömann, J. , et al. (2017). Eudicot plant‐specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 358, 1431–1143. [DOI] [PubMed] [Google Scholar]
- Leśniewska, J. , Öhman, D. , Krzesłowska, M. , Kushwah, S. , Barciszewska‐Pacak, M. , Kleczkowski, L. A. , Sundberg, B. , Moritz, T. & Mellerowicz, E. J. (2017). Defense responses in aspen with altered pectin methylesterase activity reveal the hormonal inducers of Tyloses. Plant Physiology 173, 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. X. & Kang, S. C. (2018). Do volatile compounds produced by Fusarium oxysporum and Verticillium dahliae affect stress tolerance in plants? Mycology 9, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y. B. , Cui, S. C. , Tang, X. L. , Zhang, Y. , Qiu, D. W. , Zeng, H. M. , Guo, L. H. , Yuan, J. J. & Yang, X. F. (2018). An asparagine‐rich protein Nbnrp1 modulate Verticillium dahliae protein PevD1‐induced cell death and disease resistance in Nicotiana benthamiana . Frontiers in Plant Science 9, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. Y. , Chen, J. Y. , Wang, J. L. , Li, L. , Xiao, H. L. , Adam, S. M. & Dai, X. F. (2013). Molecular characterization and functional analysis of a specific secreted protein from highly virulent defoliating Verticillium dahliae . Gene 529, 307–316. [DOI] [PubMed] [Google Scholar]
- Liu, T. L. , Song, T. Q. , Zhang, X. , Yuan, H. B. , Su, L. M. , Li, W. L. , Xu, J. , Liu, S. H. , Chen, L. L. , Chen, T. Z. , Zhang, M. X. , Gu, L. C. , Zhang, B. L. & Dou, D. L. (2014). Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nature Communications 5, 4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. K. , Tao, Q. , Li, J. X. , Guo, X. Y. , Luo, J. P. , Jupa, R. , Liang, Y. C. & Li, T. Q. (2020). Ethylene‐mediated apoplastic barriers development involved in cadmium accumulation in root of hyperaccumulator Sedum alfredii . Journal of Hazardous Materials 403, 123729. [DOI] [PubMed] [Google Scholar]
- Ma, A. F. , Zhang, D. P. , Wang, G. X. , Wang, K. , Li, Z. , Gao, Y. H. , Li, H. C. , Bian, C. , Cheng, J. K. , Han, Y. N. , Yang, S. H. , Gong, Z. Z. & Qi, J. S. (2021). Verticillium dahliae effector VDAL protects MYB6 from degradation by interacting with PUB25 and PUB26 E3 ligases to enhance Verticillium wilt resistance. The Plant Cell 33, 3675–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens‐Uzunova, E. S. & Schaap, P. J. (2009). Assessment of the pectin degrading enzyme network of Aspergillus niger by functional genomics. Fungal Genetics and Biology 46, S170–S179. [DOI] [PubMed] [Google Scholar]
- Maruthachalam, K. , Klosterman, S. J. , Kang, S. , Hayes, R. J. & Subbarao, K. V. (2011). Identification of pathogenicity‐related genes in the vascular wilt fungus Verticillium dahliae by Agrobacterium tumefaciens‐mediated T‐DNA insertional mutagenesis. Molecular Biotechnology 49, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, R. & Dubery, I. A. (1993). High‐affinity binding of a protein‐lipopolysaccharide phytotoxin from Verticillium dahliae to cotton membranes. FEBS Letters 335, 203–206. [DOI] [PubMed] [Google Scholar]
- Mohnen, D. (2008). Pectin structure and biosynthesis. Current Opinion in Plant Biology 11, 266–277. [DOI] [PubMed] [Google Scholar]
- Nachmias, A. , Buchner, V. & Burstein, Y. (1985). Biological and immunochemical characterization of a low molecular weight phytotoxin isolated from a protein–lipopolysaccharide complex produced by a potato isolate of Verticillium dahliae Kleb. Physiological Plant Pathology 26, 43–55. [Google Scholar]
- Ottmann, C. , Luberacki, B. , Küfner, I. , Koch, W. , Brunner, F. , Weyand, M. , Mattinen, L. , Pirhonen, M. , Anderluh, G. , Seitz, H. U. , Nürnberger, T. & Oecking, C. (2009). A common toxin fold mediates microbial attack and plant defense. Proceedings of the National Academy of Sciences of the United States of America 106, 10359–10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, C. S. , Saleeba, J. A. & Lyon, B. R. (2005). Phytotoxicity on cotton ex‐plants of an 18.5 kDa protein from culture filtrates of Verticillium dahliae . Physiological and Molecular Plant Pathology 67, 308–318. [Google Scholar]
- Pegg, G. F. & Brady, B. L. (2002). Verticillium Wilts. CABI Publ, New York. [Google Scholar]
- Qi, J. , Wang, J. , Gong, Z. & Zhou, J. M. (2017). Apoplastic ROS signaling in plant immunity. Current Opinion in Plant Biology 38, 92–100. [DOI] [PubMed] [Google Scholar]
- Qin, J. , Wang, K. L. , Sun, L. F. , Xing, H. Y. , Wang, S. , Li, L. , Chen, S. , Guo, H. S. & Zhang, J. (2018). The plant‐specific transcription factors CBP60g and SARD1 are targeted by a Verticillium secretory protein VdSCP41 to modulate immunity. Elife 7, e34902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusche, M. , Thole, K. , Janz, D. , Truskina, J. , Rindfleisch, S. , Drübert, C. , Polle, A. , Lipka, V. & Teichmann, T. (2012). Verticillium infection triggers VASCULAR‐RELATED NAC DOMAIN7‐dependent dependent de novo xylem formation and enhances drought tolerance in Arabidopsis . The Plant Cell 24, 3823–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux, D. , Nicole, M. , Simard, M. & Ouellette, G. B. (1998). Immunocytochemical evidence that secretion of pectin occurs during gel (Gum) and tylosis formation in trees. Phytopathology 88, 494–505. [DOI] [PubMed] [Google Scholar]
- Robb, J. , Brisson, J. D. , Busch, L. & Lu, B. C. (1979). Ultrastructure of wilt syndrome caused by Verticillium dahliae. VII. Correlated light and transmission electron microscope identification of vessel coatings and tyloses. Canadian Journal of Botany 57, 822–834. [Google Scholar]
- Robb, J. , Powell, D. A. & Street, P. (1989). Vascular coating: a barrier to colonization by the pathogen in Verticillium wilt of tomato. Canadian Journal of Botany 67, 600–607. [Google Scholar]
- Rovenich, H. , Boshoven, J. C. & Thomma, B. P. H. J. (2014). Filamentous pathogen effector functions: of pathogens, hosts and microbiomes. Current Opinion in Plant Biology 20, 96–103. [DOI] [PubMed] [Google Scholar]
- Santhanam, P. , van Esse, H. P. , Albert, I. , Faino, L. , Nurnberger, T. & Thomma, B. P. H. J. (2013). Evidence for functional diversification within a fungal NEP1‐like protein family. Molecular Plant‐Microbe Interactions 26, 278–286. [DOI] [PubMed] [Google Scholar]
- Schreiber, L. (2010). Transport barriers made of cutin, suberin and associated waxes. Trends in Plant Science 15, 546–553. [DOI] [PubMed] [Google Scholar]
- Shigenaga, A. M. & Argueso, C. T. (2016). No hormone to rule them all: interactions of plant hormones during the responses of plants to pathogens. Seminars in Cell & Developmental Biology 56, 174–189. [DOI] [PubMed] [Google Scholar]
- Snelders, N. C. , Kettles, G. J. , Rudd, J. J. & Thomma, B. P. H. J. (2018). Plant pathogen effector proteins as manipulators of host microbiomes? Molecular Plant Pathology 19, 257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelders, N. C. , Petti, G. C. , van den Berg, G. C. M. , Seidl, M. F. & Thomma, B. P. H. J. (2021). An ancient antimicrobial protein co‐opted by a fungal plant pathogen for in planta mycobiome manipulation. Proceedings of the National Academy of Sciences of the United States of America 118, e2110968118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelders, N. C. , Rovenich, H. , Petti, G. C. , Rocafort, M. , van den Berg, G. C. M. , Vorholt, J. A. , Mesters, J. R. , Seidl, M. F. , Nijland, R. & Thomma, B. P. H. J. (2020). Microbiome manipulation by a soil‐borne fungal plant pathogen using effector proteins. Nature Plants 6, 1365–1374. [DOI] [PubMed] [Google Scholar]
- Stoddart, J. L. & Carr, A. J. H. (1966). Properties of wilt‐toxins produced by Verticillium albo‐atrum R & B. Annals of Applied Biology 58, 81–92. [Google Scholar]
- Sun, Q. , Rost, T. L. , Reid, M. S. & Matthews, M. A. (2007). Ethylene and not embolism is required for wound‐induced tylose development in stems of grapevines. Plant Physiology 145, 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, L. , Huang, C. M. , Zhang, D. D. , Li, R. , Chen, J. Y. , Sun, W. X. , Qiu, N. W. & Dai, X. F. (2021a). Extracellular superoxide dismutase VdSOD5 is required for virulence in Verticillium dahliae . Journal of Integrative Agriculture 20, 1858–1870. [Google Scholar]
- Tian, L. , Li, J. J. , Huang, C. M. , Zhang, D. D. , Xu, Y. , Yang, X. Y. , Song, J. , Wang, D. , Qiu, N. W. , Short, D. P. G. , Inderbitzin, P. , Subbarao, K. V. , Chen, J. Y. & Dai, X. F. (2021b). Cu/Zn superoxide dismutase (VdSOD1) mediates reactive oxygen species detoxification and modulates virulence in Verticillium dahliae . Molecular Plant Pathology 22, 1092–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, L. , Sun, W. X. , Li, J. J. , Chen, J. Y. , Dai, X. F. , Qiu, N. W. & Zhang, D. D. (2020). Unconventionally secreted manganese superoxide dismutase VdSOD3 is required for the virulence of Verticillium dahliae . Agronomy 11, 13. [Google Scholar]
- Trapero, C. , Alcántara, E. , Jiménez, J. , Amaro‐Ventura, M. C. , Romero, J. , Koopmann, B. , Karlovsky, P. , Tiedemann, A. V. , Pérez‐Rodríguez, M. & López‐Escudero, F. J. (2018). Starch hydrolysis and vessel occlusion related to wilt symptoms in olive stems of susceptible sultivars infected by Verticillium dahliae . Frontiers in Plant Science 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima, A. K. , Paplomatas, E. J. , Rauyaree, P. , Ospinagiraldo, M. D. & Kang, S. (2021). VdSNF1, the sucrose nonfermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell‐wall degradation. Molecular Plant‐Microbe Interactions 24, 129–142. [DOI] [PubMed] [Google Scholar]
- Wang, B. N. , Yang, X. F. , Zeng, H. M. , Liu, H. , Zhou, T. T. , Tan, B. B. , Yuan, J. J. , Guo, L. H. & Qiu, D. W. (2012). The purification and characterization of a novel hypersensitive‐like response‐inducing elicitor from Verticillium dahliae that induces resistance responses in tobacco. Applied Microbiology and Biotechnology 93, 191–201. [DOI] [PubMed] [Google Scholar]
- Wang, C. H. , Wang, H. , Li, P. X. , Li, H. Y. , Xu, C. M. , Cohen, H. , Aharoni, A. & Wu, S. (2020b). Developmental programs interact with abscisic acid to coordinate root suberization in Arabidopsis . Plant Journal 104, 241–251. [DOI] [PubMed] [Google Scholar]
- Wang, D. , Chen, J. Y. , Song, J. , Li, J. J. , Klosterman, S. J. , Li, R. , Kong, Z. Q. , Subbarao, K. V. , Dai, X. F. & Zhang, D. D. (2021a). Novel cytotoxic function of xylanase VdXyn4 in the plant vascular wilt pathogen Verticillium dahliae . Plant Physiology 187, 409–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Tian, L. , Zhang, D. D. , Song, J. , Song, S. S. , Yin, C. M. , Zhou, L. , Liu, Y. , Wang, B. L. , Kong, Z. Q. , Klosterman, S. , Li, J. J. , Wang, J. , Li, T. G. , Adamu, S. , et al. (2020a). Functional analyses of small secreted cysteine‐rich proteins identified candidate effectors in Verticillium dahliae . Molecular Plant Pathology 21, 667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Zhang, D. D. , Usami, T. , Liu, L. , Yang, L. , Huang, J. Q. , Song, J. , Li, R. , Kong, Z. Q. , Li, J. J. , Wang, J. , Klosterman, S. J. , Subbarao, K. V. , Dai, X. F. & Chen, J. Y. (2021b). Functional genomics and comparative lineage‐specific region analyses reveal novel insights into race divergence in Verticillium dahliae . Microbiology Spectrum 9, e0111821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. Y. , Cai, Y. , Gou, J. Y. , Mao, Y. B. , Xu, Y. H. , Jiang, W. H. & Chen, X. Y. (2004). VdNEP, an elicitor from Verticillium dahliae, induces cotton plant wilting. Applied Microbiology and Biotechnology 70, 4989–4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, C. J. , Li, Q. L. & Yang, X. Y. (2017). Characterization of VdASP F2 secretory factor from Verticillium dahliae by a fast and easy gene knockout system. Molecular Plant‐Microbe Interactions 30, 444–454. [DOI] [PubMed] [Google Scholar]
- Xiong, D. G. , Wang, Y. L. & Tian, C. M. (2015). Transcriptomic profiles of the smoke tree wilt fungus Verticillium dahliae under nutrient starvation stresses. Molecular Genetics and Genomics 290, 1963–1977. [DOI] [PubMed] [Google Scholar]
- Yadeta, K. A. & Thomma, B. P. H. J. (2013). The xylem as battleground for plant hosts and vascular wilt pathogens. Frontiers in Plant Science 4, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. K. , Zhang, Y. , Li, B. B. , Yang, X. F. , Dong, Y. J. & Qiu, D. W. (2018). A Verticillium dahliae pectate lyase induces plant immune responses and contributes to virulence. Frontiers in Plant Science 9, 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, C. M. , Li, J. J. , Wang, D. , Zhang, D. D. , Song, J. , Kong, Z. Q. , Wang, B. L. , Hu, X. P. , Klosterman, S. J. , Subbarao, K. V. , Chen, J. Y. & Dai, X. F. (2022). A secreted ribonuclease effector from Verticillium dahliae localizes in the plant nucleus to modulate host immunity. Molecular Plant Pathology. 10.1111/mpp.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Z. , Wang, N. , Pi, L. , Li, L. , Duan, W. , Wang, X. & Dou, D. L. (2021). Nicotiana benthamiana LRR‐RLP NbEIX2 mediates the perception of an EIX‐like protein from Verticillium dahliae . Journal of Integrative Plant Biology 63, 949–960. [DOI] [PubMed] [Google Scholar]
- Zhang, D. D. , Wang, J. , Wang, D. , Kong, Z. Q. , Zhou, L. , Zhang, G. Y. , Gui, Y. J. , Li, J. J. , Huang, J. Q. , Wang, B. L. , Liu, C. , Yin, C. M. , Li, R. X. , Li, T. G. , Wang, J. L. , et al. (2019a). Population genomics demystifies the defoliation phenotype in the plant pathogen Verticillium dahliae . New Phytologist 22, 1012–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. D. , Wang, X. Y. , Chen, J. Y. , Kong, Z. Q. & Dai, X. F. (2016). Identification and characterization of a pathogenicity‐related gene VdCYP1 from Verticillium dahliae . Scientific Reports 6, 27979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Zhang, Y. , Yang, J. , Kang, L. , Elorm, A. M. , Zhou, H. Y. & Zhao, J. (2019b). The α‐1,6‐mannosyltransferase VdOCH1 plays a major role in microsclerotium formation and virulence in the soil‐borne pathogen Verticillium dahliae . Fungal Biology 123, 539–546. [DOI] [PubMed] [Google Scholar]
- Zhang, L. S. , Ni, H. , Du, X. , Wang, S. , Ma, X. W. , Nürnberger, T. , Guo, H. S. & Hua, C. L. (2017b). The Verticillium‐specific protein VdSCP7 localizes to the plant nucleus and modulates immunity to fungal infections. New Phytologist 215, 368–381. [DOI] [PubMed] [Google Scholar]
- Zhang, W. Q. , Gui, Y. J. , Short, D. P. G. , Li, T. G. , Zhang, D. D. , Zhou, L. , Liu, C. , Bao, Y. M. , Subbarao, K. V. , Chen, J. Y. & Dai, X. F. (2018). Verticillium dahliae transcription factor VdFTF1 regulates the expression of multiple secreted virulence factors and is required for full virulence in cotton. Molecular Plant Pathology 19, 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Gao, Y. H. , Liang, Y. B. , Dong, Y. J. , Yang, X. F. , Yuan, J. J. & Qiu, D. W. (2017a). The Verticillium dahliae SnodProt1‐Like protein VdCP1 contributes to virulence and triggers the plant immune system. Frontiers in Plant Science 8, 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Gao, Y. H. , Wang, H. L. , Kan, C. C. , Li, Z. , Yang, X. F. , Yin, W. L. , Xia, X. L. , Nam, H. G. , Li, Z. H. & Guo, H. W. (2021). Verticillium dahliae secretory effector PevD1 induces leaf senescence by promoting ORE1‐mediated ethylene biosynthesis. Molecular Plant 14, 1901–1917. [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Chen, Q. H. , Zhou, S. , Sun, Y. H. & Li, Y. Z. (2020). H2Bub1 regulates RbohD‐dependent hydrogen peroxide signal pathway in the defense responses to Verticillium dahliae toxins. Plant Physiology 182, 640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, P. , Zhao, Y. L. , Jin, Y. , Zhang, T. & Guo, H. S. (2014). Colonization process of Arabidopsis thaliana roots by a green fluorescent protein‐tagged isolate of Verticillium dahliae . Protein & Cell 5, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. L. , Zhou, T. T. & Guo, H. S. (2016). Hyphopodium‐specific VdNoxB/VdPls1‐dependent ROS‐Ca2+ signaling is required for plant infection by Verticillium dahliae . PLoS Pathogens 12, e1005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B. J. , Jia, P. S. , Gao, F. & Guo, H. S. (2012). Molecular characterization and functional analysis of a necrosis‐ and ethylene‐inducing, protein‐encoding gene family from Verticillium dahliae . Molecular Plant‐Microbe Interactions 25, 964–975. [DOI] [PubMed] [Google Scholar]
- Zhou, T. T. , Zhao, Y. L. & Guo, H. S. (2017). Secretory proteins are delivered to the septin‐organized penetration interface during root infection by Verticillium dahliae . PLoS Pathogens 13, e1006275. [DOI] [PMC free article] [PubMed] [Google Scholar]


