Abstract
Asthma is a respiratory disorder marked by bronchial irritation and hyperresponsive airway smooth muscle. According to new research, magnesium's dual activity as an anti-inflammatory and bronchodilator may be important in asthma therapy. The goal of this study was to see how effective intravenous magnesium sulfate is in treating severe acute asthma. In addition to checking Clinicaltrials.gov, we ran a database search in Scopus, Google Scholar, PubMed, and Embase. Studies were chosen based on predetermined inclusion and exclusion criteria to prevent the chance of bias. Most researchers believed that intravenous magnesium sulfate improved symptoms and lung function significantly. Mortality and morbidity data were not available.
Keywords: allergy, literature review, acute asthma managment, intravenous magnesium sulfate, acute asthma
Introduction and background
Asthma is a chronic respiratory illness that affects a significant portion of the world’s population [1]. Asthma symptoms include coughing, wheezing, shortness of breath, and an increased respiratory rate. These symptoms are usually mild and may be controlled with medicine and avoidance of known allergens, but they can potentially result in life-threatening exacerbations. Asthma is responsible for an estimated 300,000 fatalities globally each year. The morbidity and mortality from asthma are costly and strain the healthcare system [2].
A potentially life-threatening complication of asthma is status asthmaticus, which results in adverse outcomes for the patient in the short and long term, such as respiratory failure, ICU admission, and sometimes death [3]. Inhaled oxygen, beta-agonists, corticosteroids, and bronchodilators are the mainstays of therapy. Magnesium, a calcium channel blocker, should also be considered since magnesium can aid in the relaxation of constricted bronchioles during an asthma exacerbation. Several studies have associated the onset of asthma with decreased blood levels of magnesium and inadequate nutrient intake [4,5].
Previous research has shown that administering magnesium via intravenous or inhaled routes can help manage acute exacerbations of asthma. Both forms are fast-acting and effective. The intravenous route has greater bioavailability but is associated with systemic side effects [6]. This literature review aims to examine the literature behind magnesium's role in managing asthma.
Review
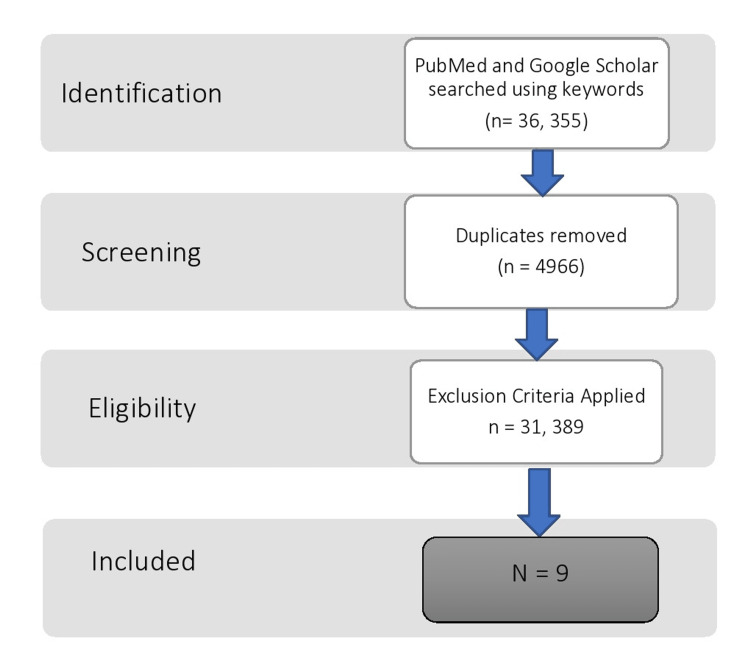
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to screen literature (Figure 1). We reviewed the existing literature using Pubmed and Google Scholar. The keywords utilized in the search were asthma, magnesium, and intravenous. This yielded 255 results on PubMed, which were reduced to 84 when the filter for free full text was applied and only 17 when review articles, systematic reviews, and meta-analyses, were removed. Google Scholar yielded 36,100 hits, of which roughly 7000 were review articles. To further narrow the search, we expanded the keywords to include severe acute asthma, which showed 1240 results, of which 285 were review articles. After applying a custom range from 2012 and narrowing the net to include only clinical trials, 122 studies were shown.
Figure 1. PRISMA framework.
PRISMA: preferred reporting items for systematic reviews and meta-analyses.
We only included studies that fit the keyword criteria with an adult population published in English. We excluded clinical trials with a crossover design, those in the recruitment process, or those without published results. We also excluded research without full-text options, research on other routes of administration of magnesium such as oral or nebulized, case reports, pre-clinical studies, veterinary studies, non-clinical reports, systematic reviews, and meta-analyses.
A clinical trial by Noppen et al. in 1990 studied the bronchodilating impact of intravenous magnesium sulfate (IVMS) in individuals with severe acute asthma. They concluded that IVMS caused a considerable increase in forced expiratory volume (FEV1) (0.94-0.39 L to 1.03-0.44 L) in ten out of twelve patients and reduced clinical signs and symptoms [7]. An earlier clinical trial by Okayama et al. [8] noted that after IVMS, the bronchodilating response was immediate, and the maximum effect of the IVMS was equivalent to that of an extra albuterol inhalation. Although it resulted in bronchodilation in moderate bronchial asthma patients, the oxygen partial pressure fell in three severe asthma patients. These findings were in contrast to a later clinical trial by Silverman et al. [9], who concluded in their trial that IVMS not only improved FEV1, but the improvement was more significant in patients who presented with poorer FEV1 on admission. However, IVMS did not impact the duration of the in-hospital stay. Another landmark trial to study, the effects of nebulized versus IVMS versus placebo, named the 3Mg trial, concurred with the clinical trial findings conducted by Silverman et al., who concluded that hospitalization rates did not differ between individuals treated with either type of magnesium (nebulized or IV). A scale called the visual analog scale (VAS) was used to quantify the severity of symptoms reported by the patient. The higher the score, the more the severity. The 3Mg study found that while there was no difference in the visual analog scale (VAS) for dyspnea between patients given magnesium and those given placebos, the difference was statistically significant for patients in the IVMS to those in the nebulized group. This meant that while magnesium itself was not superior to placebo, the intravenous form was better than the nebulized form [10]. Tiffany et al. did not find a beneficial effect of magnesium in acute asthma in a randomized, placebo-controlled trial. The participants were segmented into three groups: the first got a 2 g IVMS bolus followed by a continuous magnesium infusion of 2 g/h (16 mEq) over four hours, the second received a 2 g IVMS bolus followed by a placebo infusion, and the third received a placebo bolus followed by a placebo infusion. In this trial, neither the FEV1 nor the peak expiratory volume (PEF) increased after magnesium medication [11].
In 1938, Haury [12] presented important experimental data showing magnesium dilated bronchoconstriction in guinea pigs produced by pilocarpine, histamine, and barium chloride. A second investigation by Haury [13] in 1940 found that half of the patients with severe asthma exacerbations had low blood magnesium levels. IVMS treatment helped two of them improve their clinical conditions, including dyspnea and stridor. The study did not have an ample sample size to extrapolate the findings to the general audience. However, it was one of the first of its kind to test the relationship between magnesium and asthma. In a clinical trial by Bloch et al. [14] in 1995, all patients received IVMS and standard of care, including nebulized albuterol. Additionally, patients with a forced expiratory volume of less than 40% or those with a history of oral corticosteroids within the last six months of the trial were also administered 125 mg of methylprednisolone. After half an hour, patients were randomized to receive either 2 gm of IVMS or 50 ml of saline IV (placebo) in addition to the standard of care for acute asthma. They found that IVMS decreased hospitalization rates and improved FEV in patients with severe acute asthma but had no effect on those with moderate asthma. Green et al. [15] found similar results in their clinical study. Skobeloff et al. [16] conducted a placebo-controlled trial. Participants received either 1.2 g of IVMS in 50 mL of saline for 20 minutes or saline placebo in 50 mL over 20 minutes. Individuals who received IVMS had considerably higher peak flow and lower admission rates than placebo patients (Table 1).
Table 1. Study characteristics.
FEV1: forced expiratory volume, CHF: congestive heart failure.
| Study | Study type | Participants | Inclusion | Exclusion | Primary outcome | Secondary outcome |
| Noppen et al. [7] | Multiphase clinical trial | Six patients underwent an infusion of MgSO4 for 20 minutes | Diagnosed case of bronchial asthma, confirmed via spirometry, aged 45 to 60 years | Patients with heart failure, pneumonia, life-threatening conditions or neoplastic disorders, acidemia or hypercapnia, and patients who require mechanical ventilation | Evolution of FEV1, after MgSO4, infusion and after beta-agonist inhalation on two consecutive days | Decrease in wheezing after Mg infusion, subjective improvement in dyspnea, and adverse effects |
| Okayama et al. [8] | Clinical trial | Two groups: (1) IV saline as the control followed by IV MgSO4 (n=5). (2) IV saline as the control followed by an increased dose of IV MgSO4 followed by inhaled albuterol (n=5) | Ten randomly selected patients presented with acute asthma | None | Change in FEV1 from baseline and maximum effect compared with that of a beta-agonist | Changes in Mg and albuterol concentrations, improvement in dyspnea, and changes in oxygen partial pressure |
| Goodacre et al. [10] | Double-blind, placebo-controlled trial | IV Mg (n=396) vs nebulized Mg (n=333) vs placebo (n=358) | Adults (aged ≥16 years) attending an emergency department with severe acute asthma | Patients who had life-threatening features, a contraindication to either nebulized or intravenous MgSO4, and individuals who were unable to provide written or verbal consent | Proportion of patients admitted to hospital, either after emergency department treatment or at any time in the subsequent seven days, and patient's visual analog scale (VAS) for breathlessness in the two hours after the start of treatment | Mortality, adverse events, use of ventilation or respiratory support, length of hospital stay, admission to a high-dependency unit, change in peak expiratory flow rate and physiological variables over two hours, change in the quality of life between baseline and one month, and satisfaction with care |
| Tiffany et al. [11] | Randomized, double-blind, placebo-controlled trial | IV Mg and then maintenance Mg (n=12) vs IV Mg and then placebo (n=15) vs placebo loading dose and placebo infusion (n=21) | Forty-eight asthmatic patients aged 18 to 60 years with an initial peak expiratory flow rate (PEFR) of <200 L/min who failed to double their initial PEFR after two standardized albuterol treatments | Patients with a history of chronic bronchitis or emphysema, oral temperature >38.2°C, history of renal failure, history of CHF, or requiring tracheal intubation should have an initial of PEFR more than 200 L/min | Improvement in FEV1 or PEFR after Mg infusion | None |
| Haury [12] | Randomized, double-blinded, placebo-controlled trial | IV MgSO4 (n=18) vs placebo (n=24) | Forty-two adult patients between the ages of 18-55 with a history of asthma, peak expiratory flow of <100 l/min or 25% of predicted flow, and the ability to give informed consent | Clinical signs and symptoms are consistent with alternate causes of wheezing. Patients who were highly likely to be intubated | Change in peak expiratory flow at 60 minutes | Change in subjective symptoms of dyspnea as measured by the Borg dyspnea scale at 60 minutes and need for hospital admission |
| Haury [13] | Randomized double-blind placebo-controlled study | Two grams of MgSO4 vs placebo in 50 mL of normal saline solution IV. One hundred thirty-five patients total were randomized into two groups | Patients aged 18 to 65 years presenting with acute asthma to the ED | History of congestive heart failure, diabetes mellitus, angina, chronic renal insufficiency, temperature >380°C, pneumonia, or if pregnant | FEV1 at two hours after treatment and hospital admission rates | Follow-up after two hours and once every week for hospital visits for asthma |
| Bloch et al. [14] | Single-blind, randomized clinical trial | Two-gram IV magnesium sulfate (n=58) vs placebo (n=62) | Patients aged 18 to 65 years with acute asthma are unresponsive to a single albuterol treatment | Patients with, angina, chest pain, uncontrolled hypertension, CHF, metastatic cancer, renal disease, temperature above 38.3°C, systolic blood pressure less than 120 mm Hg, or pregnancy | Change in peak expiratory flow after Mg infusion | Number of patients requiring hospitalization, return to the ED within 72 hours, reactions to Mg infusion |
| Green et al. [15] | Randomized controlled trials | 1.2 gram of IV MgSO4 in saline (n=19) vs placebo in saline (n=19) | All patients 18 to 70 years of age presenting to the emergency department at The Medical College of Pennsylvania (Philadelphia) with an acute exacerbation of asthma | Temperature of greater than 38°C, systolic blood pressure less than 120 mm Hg, a history of kidney disease, purulent sputum, infiltrate on a chest roentgenogram, and pregnancy | Hospital admissions and PEFR after intervention | ED treatment duration, ICU admission, hospital length of stay, and vital signs |
Discussion
Previous recommendations advocated intravenous magnesium sulfate (IVMS) as a safe and effective therapy choice for adult patients with severe acute asthma who did not respond to first-line medications [17,18]. Magnesium sulfate is a physiological blood coagulation mediator that aids in releasing histamine and acetylcholine, resulting in bronchodilation. Interfering with calcium influx can also cause bronchial smooth muscle relaxation [19]. Another potential mechanism by which magnesium is thought to impact asthma is by dampening the neutrophilic burst associated with asthma leading to an improvement in the signs and symptoms of the disease [20]. According to Mohammed et al. [21], IVMS should be used as a first-line treatment for children with severe acute asthma who have not responded to other treatments. In contrast, the role of nebulized magnesium sulfate in children and the roles of both nebulized and intravenous magnesium sulfate in adults should be further investigated. Due to the low risk of severe side effects from magnesium sulfate, it is permissible to use intravenous magnesium sulfate in patients with life-threatening symptoms when any potential benefit outweighs the risks of treatment [22]. In a study done by Hashimoto et al. in 2000 [23], magnesium concentrations in serum, erythrocytes, and lymphocytes were evaluated in 25 patients with stable bronchial asthma and nine age-matched healthy participants to determine the role of magnesium in bronchial hyper-reactivity. They found that 40% of asthmatic patients had magnesium insufficiency and that the low magnesium concentration in erythrocytes represents lower magnesium storage in bronchial asthma patients. This study supports the usage of magnesium in patients with acute asthma exacerbation. While there is a wealth of information on the hazards and advantages of IVMS, more study is needed to find the best dosage and duration of magnesium administration. Our review had a few limitations: we did not include unpublished material or research that was not published in English, which might have resulted in selection bias.
Conclusions
The usefulness of magnesium in the treatment of acute asthmatic episodes is unclear. Research and local standards of care prefer to endorse the use of magnesium in severe asthma attacks. Some research supports the use of magnesium in severe asthma exacerbations since IVMS appears to lower inpatient admission rates or hospitalization rates. Also, there are additional research trials where there is an improvement in lung function, which may be the result of IVMS. A randomized, placebo-controlled trial is required to determine the actual benefit of IVMS therapy in patients with severe asthma.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.The role of oral magnesium supplements for the management of stable bronchial asthma: a systematic review and meta-analysis. Abuabat F, AlAlwan A, Masuadi E, Murad MH, Jahdali HA, Ferwana MS. NPJ Prim Care Respir Med. 2019;29:4. doi: 10.1038/s41533-019-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lozano R, Naghavi M, Foreman K, et al. Lancet. 2019;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The critically ill asthmatic--from ICU to discharge. Louie S, Morrissey BM, Kenyon NJ, et al. Clin Rev Allergy Immunol. 2012;43:30–44. doi: 10.1007/s12016-011-8274-y. [DOI] [PubMed] [Google Scholar]
- 4.Magnesium in man: implications for health and disease. de Baaij JH, Hoenderop JG, Bindels RJ, et al. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 5.Effect of magnesium chloride on rabbit bronchial smooth muscle. Spivey WH, Skobeloff EM, Levin RM. Ann Emerg Med. 1990;19:1107–1112. doi: 10.1016/s0196-0644(05)81513-6. [DOI] [PubMed] [Google Scholar]
- 6.Effect of intravenous magnesium sulfate on mortality in patients with severe acute asthma. Hirashima J, Yamana H, Matsui H, Fushimi K, Yasunaga H. Respirology. 2016;21:668–673. doi: 10.1111/resp.12733. [DOI] [PubMed] [Google Scholar]
- 7.Bronchodilating effect of intravenous magnesium sulfate in acute severe bronchial asthma. Noppen M, Vanmaele L, Impens N, Schandevyl W. https://doi.org/10.1378/chest.97.2.373. Chest. 1990;97:373–376. doi: 10.1378/chest.97.2.373. [DOI] [PubMed] [Google Scholar]
- 8.Bronchodilating effect of intravenous magnesium sulfate in bronchial asthma. Okayama H, Aikawa T, Okayama M, et al. Obstet Anesth Digest. 1987;7:53. [PubMed] [Google Scholar]
- 9.IV Magnesium sulfate in the treatment of acute severe asthma . Silverman RA, Osborn H, Runge J, et al. Chest J. 2002;122:489–497. doi: 10.1378/chest.122.2.489. [DOI] [PubMed] [Google Scholar]
- 10.Intravenous or nebulised magnesium sulphate versus standard therapy for severe acute asthma (3MG trial): a double-blind, randomised controlled trial. Goodacre S, Cohen J, Bradburn M, Gray A, Benger J, Coats T. Lancet Respir Med. 2013;1:293–300. doi: 10.1016/S2213-2600(13)70070-5. [DOI] [PubMed] [Google Scholar]
- 11.Magnesium bolus or infusion fails to improve expiratory flow in acute asthma exacerbations. Tiffany BR, Berk WA, Todd IK, White SR. Chest. 1993;104:831–834. doi: 10.1378/chest.104.3.831. [DOI] [PubMed] [Google Scholar]
- 12.The bronchodilatator action of magnesium and its antagonistic action (dilator action) against pilocarpine, histamine and barium chloride. Haury V. https://jpet.aspetjournals.org/content/64/1/58 J Pharmacol Exp Ther. 1938;64:58–64. [Google Scholar]
- 13.Blood serum magnesium in bronchial asthma and its treatment by the administration of magnesium sulphate. Haury V. https://www.cabdirect.org/cabdirect/abstract/19401403516 J Lab Clin Med. 1940;26:340–341. [Google Scholar]
- 14.Intravenous magnesium sulfate as an adjunct in the treatment of acute asthma. Bloch H, Silverman R, Mancherje N, et al. Chest. 1995;107:1576–1581. doi: 10.1378/chest.107.6.1576. [DOI] [PubMed] [Google Scholar]
- 15.Intravenous magnesium for acute asthma: failure to decrease emergency treatment duration or need for hospitalization. Green SM, Rothrock SG. Ann Emerg Med. 1992;21:260–265. doi: 10.1016/s0196-0644(05)80885-6. [DOI] [PubMed] [Google Scholar]
- 16.Intravenous magnesium sulfate for the treatment of acute asthma in the emergency department. Skobeloff EM, Spivey WH, McNamara RM, et al. JAMA. 1989;262:1210–1213. [PubMed] [Google Scholar]
- 17.British guideline on the management of asthma. British Thoracic Society Scottish Intercollegiate Guidelines Network. https://thorax.bmj.com/content/69/Suppl_1/i1. Thorax. 2014;69:1–192. [PubMed] [Google Scholar]
- 18.The effects of nebulized ketamine and intravenous magnesium sulfate on corticosteroid resistant asthma exacerbation; a randomized clinical trial. Farshadfar K, Sohooli M, Shekouhi R, Taherinya A, Qorbani M, Rezaei-Kojani M. http://10.1186/s40733-021-00081-1. Asthma Res Pract. 2021;7:15. doi: 10.1186/s40733-021-00081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnesium sulfate for acute asthma in adults: a systematic literature review. Song WJ, Chang YS. Asia Pac Allergy. 2012;2:76–85. doi: 10.5415/apallergy.2012.2.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnesium attenuates the neutrophil respiratory burst in adult asthmatic patients. Cairns CB, Kraft M. Acad Emerg Med. 1996;3:1093–1097. doi: 10.1111/j.1553-2712.1996.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 21.Intravenous and nebulised magnesium sulphate for acute asthma: systematic review and meta-analysis. Mohammed S, Goodacre S. Emerg Med J. 2007;24:823–830. doi: 10.1136/emj.2007.052050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Cochrane Database Syst Rev. 2000:0. doi: 10.1002/14651858.CD001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assessment of magnesium status in patients with bronchial asthma. Hashimoto Y, Nishimura Y, Maeda H, Yokoyama M. J Asthma. 2000;37:489–496. doi: 10.3109/02770900009055475. [DOI] [PubMed] [Google Scholar]