Abstract
Background
Controversy exists regarding the effects of a high versus a low intraoperative fraction of inspired oxygen (FiO2) in adults undergoing general anesthesia. This systematic review and meta‐analysis investigated the effect of a high versus a low FiO2 on postoperative outcomes.
Methods
PubMed and Embase were searched on March 22, 2022 for randomized clinical trials investigating the effect of different FiO2 levels in adults undergoing general anesthesia for non‐cardiac surgery. Two investigators independently reviewed studies for relevance, extracted data, and assessed risk of bias. Meta‐analyses were performed for relevant outcomes, and potential effect measure modification was assessed in subgroup analyses and meta‐regression. The evidence certainty was evaluated using GRADE.
Results
This review included 25 original trials investigating the effect of a high (mostly 80%) versus a low (mostly 30%) FiO2. Risk of bias was intermediate for all trials. A high FiO2 did not result in a significant reduction in surgical site infections (OR: 0.91, 95% CI 0.81–1.02 [p = .10]). No effect was found for all other included outcomes, including mortality (OR = 1.27, 95% CI: 0.90–1.79 [p = .18]) and hospital length of stay (mean difference = 0.03 days, 95% CI −0.25 to 0.30 [p = .84). Results from subgroup analyses and meta‐regression did not identify any clear effect modifiers across outcomes. The certainty of evidence (GRADE) was rated as low for most outcomes.
Conclusions
In adults undergoing general anesthesia for non‐cardiac surgery, a high FiO2 did not improve outcomes including surgical site infections, length of stay, or mortality. However, the certainty of the evidence was assessed as low.
Keywords: complications, fraction of inspired oxygen, general anesthesia, meta‐analysis, outcomes, systematic review
Editorial Comment
Oxygen levels and oxygenation targets in hospitalized patients have received a lot of attention in recent years. In this systematic review, the desirable and undesirable effects of the inspired fraction of oxygen in patients undergoing general anesthesia for non‐cardiac surgery were assessed. Based on low certainty evidence from 25 RCTs, a high fraction of inspired oxygen did not seem to improve outcome, which is in accordance with other published systematic reviews.
1. INTRODUCTION
Each year, millions of patients undergo general anesthesia for therapeutic and diagnostic procedures. 1 During such procedures, anesthetic staff may optimize several ventilator settings with the purpose of reducing post‐operative morbidity and mortality. One key setting is the fraction of inspired oxygen (FiO2).
Previous studies have investigated the effects of different levels of FiO2 in the acute and intensive care population, however, the effect of different levels of FiO2 on post‐operative clinical outcomes in patients undergoing general anesthesia remains unclear. Currently, the World Health Organization recommends the use of an intraoperative FiO2 of 80% with the aim of reducing the risk of surgical site infections. 2 However, the review that constitutes the basis of this recommendation has been criticized for not thoroughly considering the potential adverse effects of a high intraoperative FiO2. 3 , 4 A high FiO2 is not recommended in many other settings such as the emergency department or intensive care unit. 5 , 6 Thus, some uncertainty remains regarding this balance of beneficial versus deleterious effects, and it might depend on certain patient and surgical characteristics. Although there are recent systematic reviews on this topic, 7 , 8 there are new trials to consider, 9 , 10 and these previous reviews have not extensively explored the potential effect heterogeneity between trials.
The goal of this systematic review was to (1) perform a comprehensive review of randomized trials assessing the effect of different levels of intraoperative FiO2 during general anesthesia for non‐cardiac surgery on patient‐centered outcomes and (2) explore whether heterogeneity exists according to trial and patient characteristics.
2. METHODS
2.1. Protocol and registration
This review is part of a series of reviews of clinical trials assessing various respiratory and hemodynamic targets or strategies for patients undergoing general anesthesia for non‐cardiac surgery. This part of the review focuses on FiO2 settings or oxygenation targets (PaO2 or oxygen saturation), and findings for other targets (i.e., goal‐directed hemodynamic therapy, 11 blood pressure, and ventilation) are reported separately. As no trials on oxygenation targets were identified, we focus on FiO2 in the remainder of the manuscript. The protocol was uploaded to figshare.com on June 11, 2020, and updated on August 19, 2020 and is provided in the Appendix S1. The reporting of this review followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 12 The PRISMA checklist is provided in the Appendix S1.
2.2. Eligibility criteria and outcomes
The research question was framed according to the PICO format: (1) In adults undergoing general anesthesia with invasive mechanical ventilation for non‐cardiac surgery (P), does a specific intraoperative FiO2 (I), as compared to a different intraoperative FiO2 (C) result in better clinical outcomes (O); (2) Trials including very short duration of anesthesia (e.g., for electroconvulsive therapy), cesarean sections, interventional radiology, and surgery requiring one‐lung ventilation were excluded.
Only randomized clinical trials were included, including quasi‐randomized (e.g., intervention assignment by day or week) trials as well as cluster‐randomized trials. Randomized cross‐over trials, where the cross‐over occurred within individual patients, were not included. Only trials published as full‐text articles and in English language were included. There was no limitation regarding the year of publication.
The main clinically relevant outcomes reported were mortality, hospital length of stay, and surgical site infection. Additional reported outcomes included other post‐operative complications (e.g., pulmonary complications and cardiac complications), as well as other patient‐centered outcomes (e.g., intensive care unit admission and quality of life). Additional details about the categories and definitions used for post‐operative complications are provided in the Supplement. Trials focusing only on physiological or surrogate outcomes were not included. Outcomes related to post‐operative pain, nausea, and vomiting will be reported in a separate manuscript.
2.3. Information sources and search strategy
We searched PubMed and Embase on May 28, 2020, July 24, 2020, and March 8, 2021. The search was updated on March 22, 2022. The search strategy reflects that the current review on FiO2 is part of a series of reviews, with a combined search strategy evaluating multiple respiratory and hemodynamic targets during general anesthesia. The search included a combination of various text and indexing search terms for general anesthesia or surgery and the various targets. To identify randomized trials, the Cochrane sensitivity‐maximizing search strategy was used. 13 The full search strategy for both databases is provided in the protocol. The updated search strategy is provided in the Supplement.
To identify registered ongoing trials, the International Clinical Trials Registry Platform was searched on April 5, 2021 and again on June 28, 2021. Additional details are provided in the Supplement.
2.4. Study selection
Pairs of two reviewers independently screened all titles and abstracts retrieved from the systematic searches. Any disagreements regarding inclusion or exclusion were resolved via discussion between the reviewers and with a third reviewer as needed. Two reviewers then independently reviewed the full texts of all potentially relevant publications passing the first level of screening. Any disagreement regarding eligibility was resolved via discussion. The Kappa values for inter‐observer variance were calculated. In case of poor inter‐reviewer agreement (i.e., κ < 0.4), a third reviewer reviewed all excluded titles and abstracts to ensure optimized sensitivity. The bibliographies of included articles as well as recent reviews 14 were reviewed for potential additional relevant manuscripts.
2.5. Data collection
Two reviewers, using a pre‐defined standardized data extraction form, extracted data from individual manuscripts. Any discrepancies in the extracted data were resolved via discussion.
2.6. Risk of bias in individual studies
Two investigators independently assessed the risk of bias for the included trials using the revised Cochrane risk‐of‐bias tool for randomized trials. 15 Disagreements were resolved via discussion. Risk of bias was assessed for each outcome within a trial but is reported at the trial level as the highest risk of bias score across all outcomes. If the bias varied according to the outcomes, this was noted.
2.7. Data synthesis and confidence in cumulative evidence
Included trials were assessed for clinical (i.e., participants, interventions, comparators), methodological (ie study design or risk of bias), and statistical heterogeneity. If no major clinical or methodological heterogeneity was identified, meta‐analyses were performed using Review Manager 5.4.1 (Cochrane Collaboration, Nordic Cochrane Centre). For dichotomous variables, Peto's odds ratio (OR) method was used for all meta‐analyses, including meta‐regression. This method was used as many of the outcomes were infrequent or occurred in zero patients in one of the treatment arms. 16 , 17 Results for the dichotomous variables are reported as ORs with 95% confidence intervals. For continuous variables (ie hospital length of stay), the inverse variance method with random effects was used for meta‐analyses. Results from these analyses are reported as the mean difference with 95% confidence intervals. Several manuscripts reported hospital length of stay using medians and quartiles. In order to use these results in meta‐analyses, we estimated means and standard deviations, assuming normality of the data. 18
Based on the available data, we conducted several post hoc subgroup analyses according to surgical characteristics. These included ≥50% versus <50% of the included patients requiring acute surgery and ≥50% versus <50% of the included patients undergoing abdominal surgery. Subgroup analyses according to other patient‐ and interventional characteristics, for example laparoscopic versus non‐laparoscopic surgery, were not feasible in this context due to insufficient trials reporting relevant data.
Meta‐regression was performed to evaluate the relationship between selected potential continuous moderators and the outcomes of mortality, hospital length of stay, and surgical site infection. Only comparisons with at least 10 trials were considered. Moderators included median year of patient inclusion, duration of surgery (in minutes), and sample size, as well as mortality and hospital length of stay (in days) in the control group as a reflection of the illness severity in the underlying trial population. The latter two analyses should be interpreted with caution due to the potential for regression to the mean. 19 , 20 Results are presented using bubble plots with the size of each bubble corresponding to the inverse variance of the effect size in each trial. Meta‐regression was performed using STATA version 16 (StataCorp LP).
We performed sensitivity analyses excluding trials with a FiO2 level different from 80% and/or 30%. To assess for potential publication bias for the primary outcomes, funnel plots were created and visually interpreted.
2.8. Cumulative evidence (GRADE)
The certainty of the overall evidence for a given comparison and outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology and classified within one of four categories: very low, low, moderate, or high certainty of evidence. 21 Additional details are provided in the Supplement. GRADEpro (McMaster University, 2020) was used for drafting of the GRADE table.
3. RESULTS
3.1. Overview
The systematic search yielded 23,936 unique titles/abstracts, of which 23,393 were excluded during the initial screening (κ = 0.61, Figure S1). Five hundred and forty‐three full manuscripts were screened, of which 34 manuscripts investigated different levels of intraoperative FiO2 with no trials investigating other oxygen related targets (eg oxygen saturation). Four of these trials 22 , 23 , 24 , 25 were excluded due to data irregularities. 26 Three additional manuscripts were identified by reviewing bibliographies. A total of 33 manuscripts published between 2000 and 2022 were therefore included. The manuscripts represented 24 separate randomized trials, 22 , 23 , 24 , 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 one alternating intervention trial, 48 and eight post hoc or subgroup analyses of these trials. 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Data on a total of 15,032 patients were included. We additionally identified 25 ongoing or unpublished trials with details provided in the (Appendix S1).
All but six trials compared an intraoperative FiO2 of 80% to an intraoperative FiO2 of 30%. Two trials investigated an intraoperative FiO2 of 100% 45 or 50% 32 in the intervention arm, one trial an FiO2 of 80% in the intervention arm and 40% in the control arm, 9 one trial an FiO2 of 65% in the intervention arm and 35% in the control arm, 57 and finally two trials an FiO2 of 33% 37 or 35% 29 FiO2 in the control arm. Most of the trials were small (median sample size = 252) with only 32% (n = 8) including 500 patients or more. A total of 64% of the trials (n = 16) primarily included patients undergoing abdominal surgery.
An overview of the included manuscripts is provided in Table 1, and patient and surgical characteristics are provided in the Table S2.
TABLE 1.
Characteristics of included manuscripts
| Trial | Country | Years of patient inclusion | Main inclusion criteria | No. of patients | Intervention FiO2 | Control FiO2 |
|---|---|---|---|---|---|---|
| Kotani (2000) 45 | Japan | NR | Scheduled to undergo orthopedic surgery >6 h | 60 | 100% | 30% |
| Greif (2000) 36 | Austria, Germany | 1996–1998 | Age 18–80, elective open colorectal resection | 500 | 80% | 30% |
| Purhonen (2002) 71 | Finland | NR | Female, ASA I‐II, ambulatory gynecologic laparoscopy | 100 | 80% | 30% |
| Pryor (2004) 29 | USA | 2001–2003 | Major open abdominal surgery | 165 | 80% | 35% |
| Mayzler (2005) 38 | Israel | 2001–2002 | Elective colorectal surgery for malignant disease | 38 | 80% | 30% |
| Belda (2005) 32 | Spain | 2003–2004 | Elective colorectal resection | 300 | 50% | 30% |
| Myles (2007) 40 | Multiple | 2003–2004 | Expected duration >2 h, anticipated length of hospital stay ≥3 days | 2050 | 80% | 30% a |
| Meyhoff (2009) 39 | Denmark | 2006–2008 | Abdominal laparotomy | 1400 | 80% | 30% |
| McKeen (2009) 28 | Canada | 2003–2005 | Ambulatory laparoscopic tubal ligation, ASA I‐II | 304 | 80% | 30% |
| Bickel (2011) 33 | Israel | 2006–2009 | ASA I‐IV, open appendectomy for acute appendicitis | 210 | 80% | 30% |
| Thibon (2012) 43 | France | 2003–2007 | Elective abdominal, gynecological, or breast surgery | 434 | 80% | 30% |
| Staehr (2012) 56 | Denmark | 2008 | Scheduled for laparotomy for ovarian cancer | 35 | 80% | 30% |
| Meyhoff b (2012) 49 | Denmark | 2006–2008 | Abdominal laparotomy | 1400 | 80% | 30% |
| Stall (2013) 42 | USA | 2007–2010 | High‐energy lower extremity fracture | 228 | 80% | 30% |
| Chen (2013) 46 | China | 2009–2011 | Elective open colorectal surgery | 60 | 80% | 30% |
| Meyhoff b (2014) 50 | Denmark | 2006–2008 | Abdominal laparotomy | 1400 | 80% | 30% |
| Kurz (2015) 27 | Ireland, Switzerland, Austria, USA | 2002–2007 | Age ≤80, elective colorectal resection expected to last 2–6 h | 586 | 80% | 30% |
| Wasnik (2015) 47 | India | NR | Open appendectomy for acute appendicitis | 64 | 80% | 30% |
| Fonnes (2016) 51 | Denmark | 2006–2008 | Abdominal laparotomy | 1377 | 80% | 30% |
| Chiang (2017) 34 | New Zealand | 2009–2011 | Infrainguinal bypass surgery | 80 | 80% | 30% |
| Kurz (2017) 48 | USA | 2013–2016 | Colorectal surgery | 5749 | 80% | 30% |
| Mayank (2018) 37 | India | NR | Elective colorectal surgery, expected duration >1 h | 94 | 80% | 33% |
| Kongebro c (2018) 52 | Denmark | 2006–2008 | Abdominal laparotomy | 1386 | 80% | 30% |
| Alvandipour (2018) 31 | Iran | NR | Colorectal surgery | 85 | 80% | 30% |
| Ruetzler d (2019) 53 | USA | 2013–2016 | Colorectal surgery | 4481 | 80% | 30% |
| Cohen e (2019) 54 | USA | 2013–2016 | Colorectal surgery | 5056 | 80% | 30% |
| Ferrando (2019) 35 | Spain | 2017–2018 | BMI < 35, major abdominal surgery, expected duration >2 h | 717 | 80% | 30% |
| Li (2020) 44 | China | 2018 | Age ≥18, ASA I‐III, elective abdominal surgery expected to last >2 h | 252 | 80% | 30% |
| Jiang e (2021) 55 | USA | 2013–2016 | Colorectal surgery | 3471 | 80% | 30% |
| Lin (2021) 9 | China | 2018–2020 | Laparoscopic surgery for gastric and colorectal malignancies, ASA I‐III, NYHA I‐II, age 65–85 years | 630 | 80% | 40% |
| Park (2021) 57 | South Korea | 2020 | Age ≥50 years, ASA I‐III, elective abdominal surgery, expected duration >1 h | 190 | 60% | 35% |
| Reiterer (2021) 72 | Austria | 2017–2019 | Elective moderate to high‐risk abdominal surgery, expected duration >2 h, age >45 years, cardiovascular risk | 260 | 80% | 30% |
| Holse (2022) 58 | Denmark | 2018–2020 | Cardiovascular risk factors, age ≥45 years | 576 | 80% | 30% |
Abbreviations: FiO2, fraction of inspired oxygen; NR, not reported; GA, general anesthesia; PONV, postoperative nausea and vomiting; NYHA, New York Heart Association Functional Classification.
± N2O.
Follow‐up study of Meyhoff (2009). 39
Post‐hoc analysis of Meyhoff (2009). 39
Subanalysis of Kurz (2017). 48
Post‐hoc analysis of Kurz, (2017). 48
The included trials reported various outcomes (Table S3), of which we performed meta‐analyses on a total of nine outcomes (Figure 1). The remaining outcomes were not eligible for meta‐analyses as a limited number of trials reported these outcomes or due to very heterogeneous definitions. The outcomes included in the meta‐analysis were short‐ and long‐term mortality, hospital length of stay, surgical site infection, anastomotic leakage, wound dehiscence, need for reoperation, atelectasis, pneumonia, and myocardial injury/infarction.
FIGURE 1.
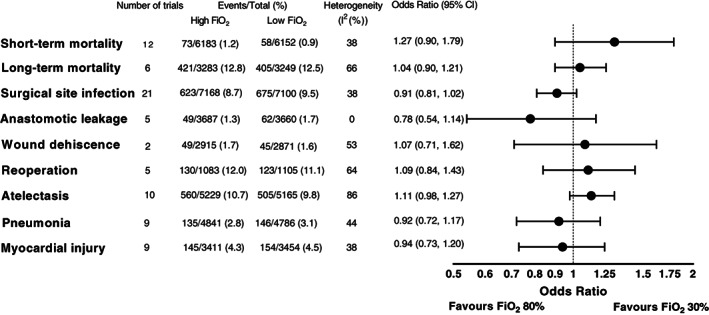
Overview of results from meta‐analyses of binary outcomes. FiO2, fraction of inspired oxygen; CI, confidence interval.
All trials were assessed as having an overall intermediate risk of bias (Table S4).
3.2. Mortality
We identified 16 trials that addressed the effect of high versus low intraoperative FiO2 on mortality (Table S3). Of these, 12 reported short‐term mortality, which included in‐hospital mortality, mortality during the trial period, and 7‐, 15‐, and 30‐day mortality, and six trials reported long‐term mortality with median follow‐up ranging from 180 days to 4 years across trials. A total of 12,335 and 6532 patients were included in the meta‐analysis on short‐ and long‐term mortality, respectively.
For both short‐ and long‐term mortality, we found no difference in survival between high and low FiO2 (OR = 1.27, 95% CI 0.90–1.79 [p = .18] and OR = 1.04, 95% CI 0.90–1.21 [p = .60], respectively (Figure 1 and Figure 2, Figure S2]). The results from the subgroup analyses for long‐term mortality were similar but was limited by the low number of trials (Figure S3). In an analysis of overall mortality including 13,293 patients, there was no difference between high and low FiO2 (OR = 1.03, 95% CI 0.75–1.40 [p = .87], Figure S4).
FIGURE 2.
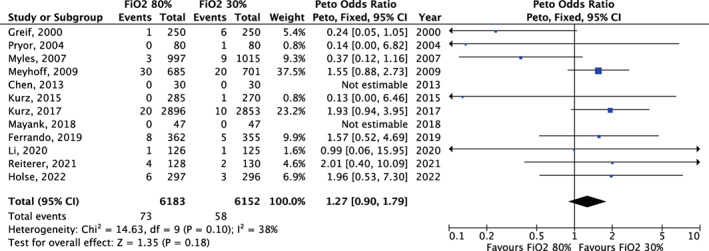
Meta‐analysis for short‐term mortality. FiO2, Fraction of inspired oxygen; CI, confidence interval.
Results from meta‐regressions are provided in Figures S5–S7 and Table S5. There was no clear effect measure modification according to short‐term mortality in the control group or sample size. More recent trials, as compared to older trials, favored a lower FiO2. Specifically, each subsequent median year of patient inclusion increased the effect size by 1.08 (95% CI 1.02–1.13 [p = .005]), indicating a more harmful effect of high oxygen compared to low oxygen.
In the funnel plot, we found no clear evidence of publication bias for short‐term mortality, although the interpretation was limited by the low number of trials (Figure S8).
The results were similar in the sensitivity analysis (Table S6).
3.3. Hospital length of stay
Seventeen trials reported data on hospital length of stay for a total of 9064 patients (Table S3). Two trials included patients scheduled for ambulatory surgery where hospital length of stay was only a few hours, and another trial only reported the outcome's variation as a range. These trials were not included in the meta‐analysis. We found no evidence of a difference in hospital length of stay between the high FiO2 group and the low FiO2 group (mean difference = 0.03 days, 95% CI ‐0.25 to 0.30, [p = .84], Figure S9). No differences between groups were found in subgroup analyses (Figures S10 and S11).
In the meta‐regression, there were no clear effect measure modification according to median year of patient inclusion, short‐term mortality in the control group, length of stay in the control group, and duration of surgery (all p > .05 [Figures S12–S16 and Table S5]). Larger as compared to smaller trials favored a higher FiO2. Specifically, each additional 100 subjects changed the mean difference in the effect size by 0.03 (95% CI, −0.06 to −0.00 [p = .03]) in favor of a higher FiO2.
In the funnel plot, we found no clear evidence of publication bias, although the interpretation was limited by the low number of trials (Figure S17).
The result was similar in the sensitivity analysis (Table S6).
3.4. Surgical site infection
Twenty‐one trials reported data on postoperative surgical site infection for a total of 14,268 patients (Table S3). In the meta‐analysis, high FiO2 versus low FiO2 did not result in a significant decrease in the risk of surgical site infection (OR = 0.91, 95% CI 0.81–1.02 [p = .10], Figure 3).
FIGURE 3.
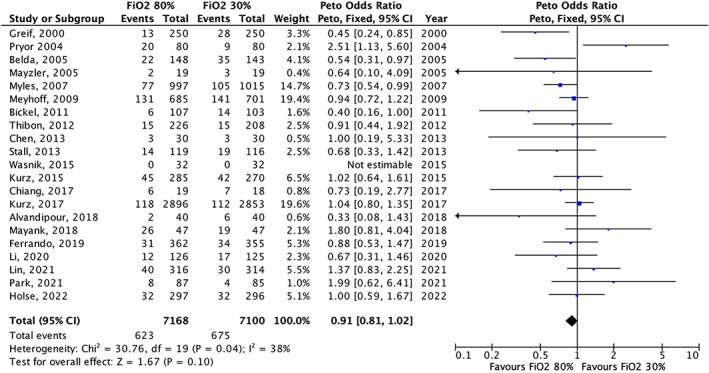
Meta‐analysis for surgical site infection. FiO2, Fraction of inspired oxygen; CI, confidence interval.
In the subgroup on ≥50% versus <50% acute surgery, we found the most prominent effect of high FiO2 in the subgroup with ≥50% acute surgery (OR = 0.55, 95% CI 0.31–0.98 [p = .04]) versus <50% acute surgery (OR = 0.92, 95% CI 0.82–1.04 [p = .20], Figure S18), although the test for subgroup differences was not significant (p = .09). There was no subgroup difference according to ≥50% versus <50% abdominal surgery (Figure S19).
In the meta‐regression, there were no clear effect measure modifiers (all p > .05 Figures S20–S24 and Table S5]).
In the funnel plot, we found no clear evidence of publication bias (Figure S25).
The result was similar in the sensitivity analysis (Table S6).
3.5. Postoperative complications
In addition to surgical site infection, some of the included trials reported data on other postoperative surgical complications, including five on anastomotic leakage for 7347 patients, two on wound dehiscence for 5786 patients, and five on reoperation for 2188 patients. We found no evidence of differences in the incidence of anastomotic leakage (OR = 0.78, 95% CI 0.54–1.14 [p = .21], Figure S26), wound dehiscence (OR = 1.07, 95% CI 0.71–1.62 [p = .74], Figure S27), or reoperation (OR = 1.09, 95% CI 0.84–1.43 [p = .51], Figure S28) between the trial groups. Results were similar in the subgroup analyses for reoperation, although this analysis was limited by the low number of trials (Figure S29).
Ten trials reported data on atelectasis for 10,394 patients and nine trials on pneumonia for 9627 patients. The pooled estimates did not indicate a difference between groups (OR = 1.11, 95% CI 0.98–1.27 [p = .11], Figure S30 and OR = 0.92, 95% CI 0.72–1.17 [p = .50], Figure S31). The same was evident in the subgroup analyses, although these analyses were limited by the low number of trials and patients Figures S32 and S33).
Nine trials reported data on myocardial injury/infarction for 6865 patients. In the meta‐analysis, we found no difference between groups (OR = 0.94, 95% CI 0.73–1.20, Figure S34 [p = .61]). Similar results were found in the subgroup analysis (Figure S35) although this analysis was limited by the low number of trials.
Sensitivity analyses on these postoperative complications generally showed similar results to the primary analyses, although they were limited by the low number of trials (Table S6).
3.6. Cumulative evidence (GRADE)
Using GRADE, the overall certainty for most of the included outcomes was assessed as low. For anastomotic leakage and hospital length of stay the overall certainty was assessed as moderate. The GRADE assessment is found in Table S7.
4. DISCUSSION
This systematic review included 33 manuscripts describing results from 25 separate trials with almost 15,000 patients. The trials all investigated the effect of high (mostly 80%) versus low (mostly 30%) FiO2 on various clinical and postoperative outcomes. In the meta‐analyses, there was no significant difference between a high and a low FiO2 for all outcomes including surgical site infection, length of stay, and mortality. The overall certainty in the evidence was considered low for most of the outcomes.
The included trials were generally small with only eight trials including 500 patients or more 9 , 27 , 35 , 36 , 39 , 40 , 48 , 58 and only three trials including more than 1000 patients. 39 , 40 , 48 A noticeable proportion of the trials did not report patient and surgical characteristics deemed crucial for determining potential clinical heterogeneity (e.g., ASA score, length of surgery). Moreover, in some cases, the outcomes were poorly and heterogeneously defined, leading to difficulties in including the outcomes in meta‐analyses. However, there was only little between‐study heterogeneity in the interventions and comparators used, as most of the trials compared an FiO2 of 80% to 30%.
No trials were assessed as having a low risk of bias. All the included trials had an intermediate risk of bias. This was largely because no trials had intraoperative blinding of the clinical team performing the intervention, which cannot rule out a risk of bias due to the possibility of deviations from the intended interventions.
The trade‐off between the beneficial and potential detrimental effects of hyperoxemia has been subject to vigorous debate and research. The potential beneficial effects of hyperoxemia on surgical site infection are twofold. First, the risk of surgical site infection is inversely related to tissue oxygenation in observational settings. 59 , 60 It is therefore plausible that ensuring a high tissue oxygenation will decrease the risk of surgical site infection. Second, it has been shown in the laboratory that the intrinsic ability of the immune system to eliminate pathogens is highly oxygen dependent. 61 As such, tissue hyperoxygenation might promote phagocytosis and consequently prevent infection. In the current review, there was no significant improvement in surgical site infection with a high FiO2. This is in contrast with findings in previous reviews, 14 , 62 and likely reflects the inclusion of newer trials. However, the point estimate in the current meta‐analysis suggested fewer surgical site infections with a high FiO2 but there was some heterogeneity in the results from the included trials and wide confidence intervals (Figure 2), and the overall certainty in the evidence was therefore rated as low. Although there was some indication that the results suggesting benefit were primarily driven by older trials, we did not find a significant association between the year of patient inclusion and the effect size in meta‐regression (Table S5). Based on this, we did not downgrade the evidence for indirectness. Lastly, the effect size on surgical site infection was relatively small. The importance of this finding, given the uncertainty and especially in the context of the remaining outcomes, is therefore unclear.
On the other hand, a high FiO2 might potentially promote atelectasis, 63 , 64 which in turn might promote respiratory infection. Furthermore, exposure to a high oxygen concentration might cause cellular damage and lung injury through the formation of reactive oxygen species, 65 an effect that is evident in animal models after only short exposure to high levels of oxygen. 66 There is some evidence of deleterious effects of high levels of oxygen in the acute and intensive care population, where it has been associated with worse clinical outcomes in some studies, 67 although the pooled evidence is inconclusive. 68 Recent evidence suggests that in patients with acute hypoxemic respiratory failure, there is no effect on mortality with higher versus lower oxygenation targets. 69 In the present review, as well as in previously published reviews, 14 , 62 , 70 no adverse effects of high levels of oxygen on pulmonary (including atelectasis), cardiovascular, and clinical complications (ie mortality, length of hospital stay) were evident. However, for most of these outcomes, the certainty of evidence was low (Table S7).
While some trials have suggested that a high FiO2 could result in increased mortality, 39 , 48 we found no difference in short‐ or long‐term mortality in the meta‐analyses. Given the relatively low mortality in the included population, it is difficult to exclude a clinically important difference in mortality between the groups. For example, if a trial was designed to detect a difference in short‐term mortality of 1.0% versus 1.5%, approximately 15,000 to 20,000 patients would have to be included.
This systematic review provides an update on intraoperative FiO2 and was performed using rigorous methodology. The review differs from previous reviews by including newly published trials and by considering the effect of the intervention in subgroups that have not previously been investigated. Future trials should focus on reporting and consistently defining clinically relevant and patient‐centered outcomes, and in this context aim at including cohorts of considerable size to allow for detection of meaningful differences in these outcomes. Furthermore, future trials should aim at blinding all personnel involved in assessment of the outcomes as this would minimize the risk of bias.
This review has some limitations. First, specific outcomes and their definitions were not prespecified in the protocol. This was done to capture all the clinically relevant outcomes that were reported in the included trials. However, this approach might have introduced some subjectivity into which outcomes were included in the manuscript. Second, the subgroup analyses performed were not prespecified and were based on the available data provided in the included trials. For many of the subgroups, very few trials were available. Third, there was no limit on trial publication year leading to pooling of trials spanning more than 20 years. We did, however, perform meta‐regression to assess the effect of publication year. Fourth, patient and surgical characteristics as well as outcomes were poorly, or in some cases not, defined leading to some degree of subjectivity and unreliability in classifying trials and in meta‐analysis inclusion. Fifth, while the intervention was similar across trials, there was some heterogeneity in the included patient populations and outcomes. While we explored this heterogeneity in subgroup analyses and meta‐regression, the results from the meta‐analyses should be carefully interpreted. Sixth, we did not contact trial authors for additional information about outcomes that were not reported. Lastly, we did not include unpublished trials, including trials only published as abstracts. As such, we might have missed relevant trials.
In adults undergoing general anesthesia for non‐cardiac surgery, a high FiO2 does not improve clinically relevant postoperative outcomes. For most outcomes, the certainty in the evidence was assessed as low and it therefore remains unclear whether applying a high FiO2 is beneficial or harmful. Our findings do not support current WHO guidelines to use a FiO2 of 80%. 2
AUTHOR CONTRIBUTIONS
LWA, AG, and MJH were involved in study conception and design. All authors were involved in data acquisition, data interpretation, and critical revision of the manuscript for important intellectual content. MH, PCL, MH, and LWA were involved in data analysis. MH, PCL, and LWA were involved in drafting the manuscript. All authors reviewed the results and approved the final version of the manuscript.
CONFLICT OF INTEREST
None of the authors have any conflict of interest.
Supporting information
Appendix S1 Supporting Information
Høybye M, Lind PC, Holmberg MJ, et al. Fraction of inspired oxygen during general anesthesia for non‐cardiac surgery: Systematic review and meta‐analysis . Acta Anaesthesiol Scand. 2022;66(8):923‐933. doi: 10.1111/aas.14102
Maria Høybye and Peter C. Lind contributed equally.
REFERENCES
- 1. Weiser TG, Haynes AB, Molina G, et al. Size and distribution of the global volume of surgery in 2012. Bull World Health Organ. 2016;94:201‐209F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Guidelines for the Prevention of Surgical Site Infection. 2nd ed. World Health Organization; 2018. [PubMed] [Google Scholar]
- 3. Hedenstierna G, Perchiazzi G, Meyhoff CS, Larsson A. WHO can make sense of the WHO guidelines to prevent surgical site infection? Anesthesiology. 2017;126:771‐773. [DOI] [PubMed] [Google Scholar]
- 4. Volk T, Peters J, Sessler DI. The WHO recommendation for 80% perioperative oxygen is poorly justified. Anaesthesist. 2017;66:227‐229. [DOI] [PubMed] [Google Scholar]
- 5. O'Driscoll BR, Howard LS, Earis J, Mak V. British Thoracic Society guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Respir Res. 2017;4:e000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbateskovic M, Schjorring OL, Russo Krauss S, et al. Higher versus lower fraction of inspired oxygen or targets of arterial oxygenation for adults admitted to the intensive care unit. Cochrane Database Syst Rev. 2019;2019:CD012631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim CH, Han JY, Cha SH, Kim YH, Yoo KY, Kim HJ. Effects of high versus low inspiratory oxygen fraction on postoperative clinical outcomes in patients undergoing surgery under general anesthesia: a systematic review and meta‐analysis of randomized controlled trials. J Clin Anesth. 2021;75:110461. [DOI] [PubMed] [Google Scholar]
- 8. Fasquel C, Huet O, Ozier Y, Quesnel C, Garnier M. Effects of intraoperative high versus low inspiratory oxygen fraction (FiO2) on patient's outcome: a systematic review of evidence from the last 20 years. Anaesth Crit Care Pain Med. 2020;39:847‐858. [DOI] [PubMed] [Google Scholar]
- 9. Lin X, Wang P, Liu DW, et al. Intraoperative oxygen concentration and postoperative delirium after laparoscopic gastric and colorectal malignancies surgery: a randomized, double‐blind, controlled trial. Clin Interv Aging. 2021;16:1085‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holse C, Aasvang EK, Vester‐Andersen M, et al. Hyperoxia and antioxidants for myocardial injury in noncardiac surgery: a 2 × 2 factorial, blinded, randomized clinical trial. Anesthesiology. 2022;136:408‐419. [DOI] [PubMed] [Google Scholar]
- 11. Jessen MK, Vallentin MF, Holmberg MJ, et al. Goal‐directed haemodynamic therapy during general anaesthesia for noncardiac surgery: a systematic review and meta‐analysis. Br J Anaesth. 2022;128:416‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006‐1012. [DOI] [PubMed] [Google Scholar]
- 13. JPT Higgins, Green S. (2021) Cochrane Handbook for Systematic Reviews of Interventions. [www document]. Accessed May 9, 2021. https://training.cochrane.org/handbook/current
- 14. Mattishent K, Thavarajah M, Sinha A, et al. Safety of 80% vs 30‐35% fraction of inspired oxygen in patients undergoing surgery: a systematic review and meta‐analysis. Br J Anaesth. 2019;122:311‐324. [DOI] [PubMed] [Google Scholar]
- 15. Julian Higgins JT. Revised cochrane risk‐of‐bias tool for randomized trials (RoB 2). In Julian PT, Higgins JS, Page MJ, JAC Sterne, on behalf of the RoB2 Development Group , eds. 2019.
- 16. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med. 2004;23:1351‐1375. [DOI] [PubMed] [Google Scholar]
- 17. Bradburn MJ, Deeks JJ, Berlin JA, Russell LA. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Stat Med. 2007;26:53‐77. [DOI] [PubMed] [Google Scholar]
- 18. Shi J, Luo D, Weng H, et al. Optimally estimating the sample standard deviation from the five‐number summary. Res Synth Methods. 2020;11:641‐654. [DOI] [PubMed] [Google Scholar]
- 19. Sharp SJ, Thompson SG, Altman DG. The relation between treatment benefit and underlying risk in meta‐analysis. BMJ. 1996;313:735‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reade MC, Delaney A, Bailey MJ, Angus DC. Bench‐to‐bedside review: avoiding pitfalls in critical care meta‐analysis – funnel plots, risk estimates, types of heterogeneity, baseline risk and the ecologic fallacy. Crit Care. 2008;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schietroma M, Cecilia EM, Carlei F, et al. Prevention of anastomotic leakage after total gastrectomy with perioperative supplemental oxygen administration: a prospective randomized, double‐blind, controlled, single‐center trial. Ann Surg Oncol. 2013;20:1584‐1590. [DOI] [PubMed] [Google Scholar]
- 23. Schietroma M, Pessia B, Colozzi S, Carlei F, Shehaj I, Amicucci G. Effect of high perioperative oxygen fraction on surgical site infection following surgery for acute sigmoid diverticulitis. A prospective, randomized, double blind, controlled, monocentric trial. Chirurgia (Bucur). 2016;111:242‐250. [PubMed] [Google Scholar]
- 24. Schietroma M, Cecilia EM, De Santis G, Carlei F, Pessia B, Amicucci G. Supplemental Peri‐operative oxygen and incision site infection after surgery for perforated peptic ulcer: a randomized double‐blind monocentric trial. Surg Infect (Larchmt). 2016;17:106‐113. [DOI] [PubMed] [Google Scholar]
- 25. Schietroma M, Colozzi S, Pessia B, Carlei F, Amicucci G. The effects of high‐concentration oxygen on inflammatory markers in laparoscopic cholecystectomy: a randomized controlled trial. Surg Laparosc Endosc Percutan Tech. 2017;27:83‐89. [DOI] [PubMed] [Google Scholar]
- 26. Myles PS, Carlisle JB, Scarr B. Evidence for compromised data integrity in studies of liberal peri‐operative inspired oxygen. Anaesthesia. 2019;74:573‐584. [DOI] [PubMed] [Google Scholar]
- 27. Kurz A, Fleischmann E, Sessler DI, Buggy DJ, Apfel C, Akça O. Effects of supplemental oxygen and dexamethasone on surgical site infection: a factorial randomized trial‡. Br J Anaesth. 2015;115:434‐443. [DOI] [PubMed] [Google Scholar]
- 28. McKeen DM, Arellano R, O'Connell C. Supplemental oxygen does not prevent postoperative nausea and vomiting after gynecological laparoscopy. Can J Anaesth. 2009;56:651‐657. [DOI] [PubMed] [Google Scholar]
- 29. Pryor KO, Fahey TJ 3rd, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population: a randomized controlled trial. JAMA. 2004;291:79‐87. [DOI] [PubMed] [Google Scholar]
- 30. Purhonen S, Niskanen M, Wüstefeld M, Mustonen P, Hynynen M. Supplemental oxygen for prevention of nausea and vomiting after breast surgery. Br J Anaesth. 2003;91:284‐287. [DOI] [PubMed] [Google Scholar]
- 31. Alvandipour M, Mokhtari‐Esbuie F, Baradari AG, Firouzian A, Rezaie M. Effect of Hyperoxygenation during surgery on surgical site infection in colorectal surgery. Ann Coloproctol. 2019;35:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belda FJ, Aguilera L, García de la Asunción J, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA. 2005;294:2035‐2042. [DOI] [PubMed] [Google Scholar]
- 33. Bickel A, Gurevits M, Vamos R, Ivry S, Eitan A. Perioperative hyperoxygenation and wound site infection following surgery for acute appendicitis: a randomized, prospective, controlled trial. Arch Surg. 2011;146:464‐470. [DOI] [PubMed] [Google Scholar]
- 34. Chiang N, Rodda OA, Sleigh J, Vasudevan T. Perioperative warming, oxygen, and Ilomedin on oxygenation and healing in infrainguinal bypass surgery. J Surg Res. 2017;220:197‐205. [DOI] [PubMed] [Google Scholar]
- 35. Ferrando C, Aldecoa C, Unzueta C, et al. Effects of oxygen on post‐surgical infections during an individualised perioperative open‐lung ventilatory strategy: a randomised controlled trial. Br J Anaesth. 2020;124:110‐120. [DOI] [PubMed] [Google Scholar]
- 36. Greif R, Akça O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical‐wound infection. N Engl J Med. 2000;342:161‐167. [DOI] [PubMed] [Google Scholar]
- 37. Mayank M, Mohsina S, Sureshkumar S, Kundra P, Kate V. Effect of perioperative high oxygen concentration on postoperative SSI in elective colorectal surgery‐a randomized controlled trial. J Gastrointest Surg. 2019;23:145‐152. [DOI] [PubMed] [Google Scholar]
- 38. Mayzler O, Weksler N, Domchik S, Klein M, Mizrahi S, Gurman GM. Does supplemental perioperative oxygen administration reduce the incidence of wound infection in elective colorectal surgery? Minerva Anestesiol. 2005;71:21‐25. [PubMed] [Google Scholar]
- 39. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543‐1550. [DOI] [PubMed] [Google Scholar]
- 40. Myles PS, Leslie K, Chan MT, et al. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology. 2007;107:221‐231. [DOI] [PubMed] [Google Scholar]
- 41. Purhonen S, Niskanen M, Wüstefeld M, Hirvonen E, Hynynen M. Supplemental 80% oxygen does not attenuate post‐operative nausea and vomiting after breast surgery. Acta Anaesthesiol Scand. 2006;50:26‐31. [DOI] [PubMed] [Google Scholar]
- 42. Stall A, Paryavi E, Gupta R, Zadnik M, Hui E, O'Toole RV. Perioperative supplemental oxygen to reduce surgical site infection after open fixation of high‐risk fractures: a randomized controlled pilot trial. J Trauma Acute Care Surg. 2013;75:657‐663. [DOI] [PubMed] [Google Scholar]
- 43. Thibon P, Borgey F, Boutreux S, Hanouz JL, Le Coutour X, Parienti JJ. Effect of perioperative oxygen supplementation on 30‐day surgical site infection rate in abdominal, gynecologic, and breast surgery: the ISO2 randomized controlled trial. Anesthesiology. 2012;117:504‐511. [DOI] [PubMed] [Google Scholar]
- 44. Li XF, Jiang D, Jiang YL, et al. Comparison of low and high inspiratory oxygen fraction added to lung‐protective ventilation on postoperative pulmonary complications after abdominal surgery: a randomized controlled trial. J Clin Anesth. 2020;67:110009. [DOI] [PubMed] [Google Scholar]
- 45. Kotani N, Hashimoto H, Sessler DI, et al. Supplemental intraoperative oxygen augments antimicrobial and proinflammatory responses of alveolar macrophages. Anesthesiology. 2000;93:15‐25. [DOI] [PubMed] [Google Scholar]
- 46. Chen Y, Liu X, Cheng CH, et al. Leukocyte DNA damage and wound infection after nitrous oxide administration: a randomized controlled trial. Anesthesiology. 2013;118:1322‐1331. [DOI] [PubMed] [Google Scholar]
- 47. Wasnik N. Role of supplemental oxygen in reducing surgical site infection in acute appendicities: our experience of sixty four cases. Int J Biomed Adv Res. 2015;6(02):124‐127. [Google Scholar]
- 48. Kurz A, Kopyeva T, Suliman I, et al. Supplemental oxygen and surgical‐site infections: an alternating intervention controlled trial. Br J Anaesth. 2018;120:117‐126. [DOI] [PubMed] [Google Scholar]
- 49. Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS. Increased long‐term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow‐up of a randomized clinical trial. Anesth Analg. 2012;115:849‐854. [DOI] [PubMed] [Google Scholar]
- 50. Meyhoff CS, Jorgensen LN, Wetterslev J, Siersma VD, Rasmussen LS. Risk of new or recurrent cancer after a high perioperative inspiratory oxygen fraction during abdominal surgery. Br J Anaesth. 2014;113(Suppl 1):i74‐i81. [DOI] [PubMed] [Google Scholar]
- 51. Fonnes S, Gögenur I, Søndergaard ES, et al. Perioperative hyperoxia ‐ long‐term impact on cardiovascular complications after abdominal surgery, a post hoc analysis of the PROXI trial. Int J Cardiol. 2016;215:238‐243. [DOI] [PubMed] [Google Scholar]
- 52. Kongebro EK, Jorgensen LN, Siersma VD, Meyhoff CS. Association between perioperative hyperoxia and cerebrovascular complications after laparotomy‐a post‐hoc follow‐up study. Acta Anaesthesiol Scand. 2019;63:164‐170. [DOI] [PubMed] [Google Scholar]
- 53. Ruetzler K, Cohen B, Leung S, et al. Supplemental intraoperative oxygen does not promote acute kidney injury or cardiovascular complications after noncardiac surgery: subanalysis of an alternating intervention trial. Anesth Analg. 2020;130:933‐940. [DOI] [PubMed] [Google Scholar]
- 54. Cohen B, Ruetzler K, Kurz A, et al. Intra‐operative high inspired oxygen fraction does not increase the risk of postoperative respiratory complications: alternating intervention clinical trial. Eur J Anaesthesiol. 2019;36:320‐326. [DOI] [PubMed] [Google Scholar]
- 55. Jiang Q, Kurz A, Zhang X, Liu L, Yang D, Sessler DI. Supplemental intraoperative oxygen and long‐term mortality: subanalysis of a multiple crossover cluster trial. Anesthesiology. 2021;134:709‐721. [DOI] [PubMed] [Google Scholar]
- 56. Staehr AK, Meyhoff CS, Henneberg SW, Christensen PL, Rasmussen LS. Influence of perioperative oxygen fraction on pulmonary function after abdominal surgery: a randomized controlled trial. BMC Res Notes. 2012;5:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park M, Jung K, Sim WS, et al. Perioperative high inspired oxygen fraction induces atelectasis in patients undergoing abdominal surgery: a randomized controlled trial. J Clin Anesth. 2021;72:110285. [DOI] [PubMed] [Google Scholar]
- 58. Holse C, Aasvang EK, Vester‐Andersen M, et al. Hyperoxia and antioxidants for myocardial injury in noncardiac surgery: a 2 x 2 factorial, blinded, randomized clinical trial. Anesthesiology. 2022;136:408‐419. [DOI] [PubMed] [Google Scholar]
- 59. Govinda R, Kasuya Y, Bala E, et al. Early postoperative subcutaneous tissue oxygen predicts surgical site infection. Anesth Analg. 2010;111:946‐952. [DOI] [PubMed] [Google Scholar]
- 60. Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997‐1004. discussion 05. [DOI] [PubMed] [Google Scholar]
- 61. Qadan M, Battista C, Gardner SA, Anderson G, Akca O, Polk HC Jr. Oxygen and surgical site infection: a study of underlying immunologic mechanisms. Anesthesiology. 2010;113:369‐377. [DOI] [PubMed] [Google Scholar]
- 62. Hovaguimian F, Lysakowski C, Elia N, Tramèr MR. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta‐analysis of randomized controlled trials. Anesthesiology. 2013;119:303‐316. [DOI] [PubMed] [Google Scholar]
- 63. Koo CH, Park EY, Lee SY, Ryu JH. The effects of intraoperative inspired oxygen fraction on postoperative pulmonary parameters in patients with general anesthesia: a systemic review and meta‐analysis. J Clin Med. 2019;8:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hedenstierna G, Rothen HU. Respiratory function during anesthesia: effects on gas exchange. Compr Physiol. 2012;2:69‐96. [DOI] [PubMed] [Google Scholar]
- 65. Pagano A, Barazzone‐Argiroffo C. Alveolar cell death in hyperoxia‐induced lung injury. Ann N Y Acad Sci. 2003;1010:405‐416. [DOI] [PubMed] [Google Scholar]
- 66. Matute‐Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379‐L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta‐analysis. Lancet. 2018;391:1693‐1705. [DOI] [PubMed] [Google Scholar]
- 68. Barbateskovic M, Schjorring OL, Krauss SR, et al. Higher vs lower oxygenation strategies in acutely ill adults: a systematic review with meta‐analysis and trial sequential analysis. Chest. 2021;159:154‐173. [DOI] [PubMed] [Google Scholar]
- 69. Schjørring OL, Klitgaard TL, Perner A, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384:1301‐1311. [DOI] [PubMed] [Google Scholar]
- 70. Wetterslev J, Meyhoff CS, Jørgensen LN, Gluud C, Lindschou J, Rasmussen LS. The effects of high perioperative inspiratory oxygen fraction for adult surgical patients. Cochrane Database Syst Rev. 2015;2015:CD008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Purhonen S, Turunen M, Ruohoaho UM, Niskanen M, Hynynen M. Supplemental oxygen does not reduce the incidence of postoperative nausea and vomiting after ambulatory gynecologic laparoscopy. Anesth Analg. 2003;96:91‐96. table of contents. [DOI] [PubMed] [Google Scholar]
- 72. Reiterer C, Kabon B, Taschner A, et al. Perioperative supplemental oxygen and NT‐proBNP concentrations after major abdominal surgery ‐ a prospective randomized clinical trial. J Clin Anesth. 2021;73:110379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


