Abstract
Objective
Emerging evidence has shown that ambient air pollution affects brain health, but little is known about its effect on epileptic seizures. This work aimed to assess the association between daily exposure to ambient air pollution and the risk of epileptic seizures.
Methods
This study used epileptic seizure data from two independent data sources (NeuroVista and Seer App seizure diary). In the NeuroVista data set, 3273 seizures were recorded using intracranial electroencephalography (iEEG) from 15 participants with refractory focal epilepsy in Australia in 2010–2012. In the seizure diary data set, 3419 self‐reported seizures were collected through a mobile application from 34 participants with epilepsy in Australia in 2018–2021. Daily average concentrations of carbon monoxide (CO), nitrogen dioxide (NO2), ozone (O3), particulate matter ≤10 μm in diameter (PM10), and sulfur dioxide (SO2) were retrieved from the Environment Protection Authority (EPA) based on participants’ postcodes. A patient‐time‐stratified case‐crossover design with the conditional Poisson regression model was used to determine the associations between air pollutants and epileptic seizures.
Results
A significant association between CO concentrations and epileptic seizure risks was observed, with an increased seizure risk of 4% (relative risk [RR]: 1.04, 95% confidence interval [CI]: 1.01–1.07) for an interquartile range (IQR) increase of CO concentrations (0.13 parts per million), whereas no significant associations were found for the other four air pollutants in the whole study population. Female participants had a significantly increased risk of seizures when exposed to elevated CO and NO2, with RRs of 1.05 (95% CI: 1.01–1.08) and 1.09 (95% CI: 1.01–1.16), respectively. In addition, a significant association was observed between CO and the risk of subclinical seizures (RR: 1.20, 95% CI: 1.12–1.28).
Significance
Daily exposure to elevated CO concentrations may be associated with an increased risk of epileptic seizures, especially for subclinical seizures.
Keywords: air pollution, Australia, case‐crossover design, epilepsy, seizures
Key points.
A total number of 6692 seizure counts were obtained from 49 participants with epilepsy during a total of 23 349 follow‐up days using long‐term intracranial electroencephalography (iEEG) and a seizure diary mobile application.
A patient‐time‐stratified case‐crossover design with the conditional Poisson regression model was used to determine the associations between ambient air pollutants and epileptic seizures.
Daily exposure to carbon monoxide (CO) concentrations showed a positive association with the risk of epileptic seizures, especially for subclinical seizures.
1. INTRODUCTION
Epilepsy is a common, serious, chronic neurological disorder that affects ~65 million people worldwide. 1 It is characterized by recurring seizures that can severely impact the quality of life and increase the risk of injury and mortality. As defined by the International League Against Epilepsy (ILAE), an epileptic seizure is “the transient occurrence of signs or symptoms resulting from abnormal excessive or synchronous neuronal activity in the brain.” 2 Some seizures are visible in electroencephalography (EEG) recordings and have evident clinical symptoms (termed clinical seizures), whereas others are present in EEG recordings but are asymptomatic (termed subclinical seizures). 3
It has been demonstrated consistently that air pollution can threaten human health, 4 , 5 , 6 especially for cardiovascular and respiratory systems. 7 , 8 , 9 Recently emerging evidence shows that air pollution could also have adverse effects on brain health. 10 Several studies have shown that air pollution is linked to the elevated risk of neurological disorders including stroke, 11 , 12 migraine, 13 cognitive deficit, 10 , 14 neurodegenerative diseases, 15 and psychiatric disorders. 16 Epidemiological and animal toxicological studies have indicated that air pollution could induce oxidative stress and neuroinflammation and alter the immune response of the brain. 17 Neural activity can be affected, leading to changes in neurobehavioral functions. 10 , 18 Furthermore, changes in chemicals and compositions of environment exposure have been shown to affect brain metabolism and increase neuronal excitability, and they may induce seizures. 17 , 19 , 20 , 21 , 22 Thus air pollution may be a risk factor for epileptic seizures.
Recent studies have reported that air pollution is associated with the increased risk of hospitalization or outpatient visits for people with epilepsy. 23 , 24 , 25 However, these hospital‐based studies were limited to analyses of the numbers of hospital visits by patients with epilepsy, and the actual times of seizures were not considered. To date, no investigation has reported whether air pollution is associated with the risk of epileptic seizures. Uncovering an association between air pollution and epileptic seizures may improve seizure prevention and prediction, and thereby improve the safety and health of people living with epilepsy. In this panel study, we investigated the association between daily exposure to air pollutants and the risks of both clinical and subclinical seizures in participants with epilepsy based on long‐term seizure records.
2. METHODS
2.1. Study population
This study used epileptic seizure data from two independent data sets. The first data set came from the NeuroVista (NV) study, 26 a clinical trial of a seizure advisory system and so far the longest continuous intracranial EEG (iEEG) recording in humans. Specifically, iEEG were recorded from 15 refractory patients with focal epilepsy in Australia, who were each implanted with a personal seizure advisory device for a median follow‐up period of 557 days (interquartile range [IQR]: 384–725 days per participant) between June 2010 and August 2012. The device included 16 intracranial electrodes implanted over the cortical surface near the epileptogenic zone. The continuous iEEG recordings were transmitted wirelessly and collected on an external device. Both the clinical seizures and subclinical seizures were included in this study. In total, 3273 seizures (median: 151, IQR: 27–429 seizures per participant) were recorded. All 15 participants had both clinical and subclinical seizures (total clinical seizures: 1539; total subclinical seizures: 1734). For further information about the data collection procedure and patient demographics, readers can refer to the initial clinical trial. 26 The initial clinical trial and this study were approved by the human research ethics committee of St Vincent's Hospital, Melbourne, Australia. All patients signed informed consent before participation for the research analysis of their data.
The other data set was seizure diary (SD) data collected using the Seer mobile application, a freely available mobile application to self‐report seizures, and track seizure cycles and medication adherence. In total, 3419 self‐reported seizures (clinical seizures) were collected from 34 participants with a clinical diagnosis of epilepsy in Australia from January 2018 to February 2021. All 34 participants with a minimum of three recorded seizures and at least 1.5 months of recording duration were included. The recording duration of each participant spanned between the time of their first and last reported seizures on the Seer mobile application. This study was approved by the human research ethics committee of St Vincent's Hospital, Melbourne, Australia. All patients provided written informed consent for the research analysis of their data.
2.2. Exposure assessment
Hourly concentrations of ambient carbon monoxide (CO), nitrogen dioxide (NO2), particulate matter ≤10 μm in diameter (PM10), ozone (O3), and sulfur dioxide (SO2) were measured by the Australian Environment Protection Authority's (EPA’s) air quality monitoring stations around the states of Victoria, New South Wales, Queensland, and Tasmania. The air pollutant data used in this study have been validated and quality assured by the EPA. The measurement and reporting methods of air pollutants are accredited by the National Association of Testing Authorities (NATA). We retrieved the hourly ambient temperature (at 2 m above the land surface) and ambient dew point temperature at 0.1°×0.1° spatial resolution from the ERA5 data set (https://cds.climate.copernicus.eu/cdsapp#!/home). All hourly observations were aggregated into daily mean data. In addition, we calculated daily mean relative humidity from the collected daily mean temperature and daily mean dew point temperature using an algorithm provided by the “humidity” R package. 27 We linked the center of every participant's postcode with their nearest monitoring stations to obtain the participant's environmental exposure data. The distribution of the distances to the nearest monitoring stations is shown in Figure S1. When data from the nearest station were missing, values from the second nearest monitoring station were used.
2.3. Statistical analyses
A patient‐time‐stratified case‐crossover design was used to examine the associations between air pollutants and epileptic seizures. This design has been applied widely to investigate the effects of short‐term environmental exposures on the risk of acute events. 28 , 29 The design compares exposure levels between the event periods (“cases”) and the self‐matched normal periods (“controls”). Because the comparisons are conducted within individuals and within the same time stratum, this design is able to control for time trends and interpatient variations. 29
In this study, the calendar month was selected as a fixed time window. 30 Days with seizures were treated as “case” periods and control periods were selected by matching the same day of the week in the same calendar month and the same year for the same patients. We applied a conditional quasi‐Poisson regression model that has been shown to be an alternative to the conditional logistic model but with the advantage of allowing for overdispersion and autocorrelation 31 to fit the time‐stratified case‐crossover design,
where Yt,s is the observed daily seizure counts on day t and stratum s, assumed to arise from a quasi‐Poisson distribution. Stratum s was a stratum variable combining the year, calendar month, day of the week, and participant number to adjust for the long‐term trend, inter‐month, day of week, and interpatient variations. E(Yt,s ) is the expected value of Yt,s , αs is the intercept, β is the coefficient, and Xt,s is the daily average concentration of a certain air pollutant. To control for potential time‐variant confounding factors, which are likely to have nonlinear effects, 28 we included the temperature (Tt,s ), relative humidity (Ht,s ), sun radiation (Rt,s ) and precipitation (Pt,s ) using natural cubic spline functions S(), each with three degrees of freedom. We also explored the associations between air pollutant concentrations and epileptic seizure risks by using natural cubic splines with three degrees of freedom for pollutant variables in the conditional quasi‐Poisson regression model described above.
Furthermore, we investigated the potential delayed (i.e., lagged) association between pollutant levels and epileptic seizure risks, as it is possible that the pollutant level of the previous days (e.g., yesterday's exposure level) might affect the present day's seizure risk. 32 The potential single‐lag day association (i.e., l‐day lagged association) was examined by shifting the pollutant level l‐day forward and fitted in the model described above. We also investigated the accumulative effect of multiple‐lag days by calculating the moving average pollutant level of the previous 0 to l‐day and fitted in the model described above. The temperature, relative humidity, sun radiation, and precipitation were controlled with the same lag as pollutants.
Subgroup analyses were performed by stratifying the participants’ sex, data set, and seizure type. The association results were measured by the relative risk (RR) of epileptic seizures for an interquartile range (IQR) increase in the pollutant concentration.
2.4. Sensitivity analyses
Sensitivity analyses were performed to examine the robustness of the results. First, we added any one of the other pollutants in the single‐pollutant models (i.e., two‐pollutant models) to examine the combined effects of two different pollutants. In addition, we used different degrees of freedom values (e.g., 4 and 5) in the S() function for meteorological variables to test the variation of the exposure‐response association. Furthermore, we changed the time‐stratified window length from a calendar month to 21 days and 14 days to control for the potential temporal trends other than the possible yearly, monthly, and weekly cycles. Finally, we incorporated the seizure counts of the previous month or week in the single‐pollutant model to control for the potential impact of previous seizure counts on current seizures (reflecting autocorrelation).
All analyses were performed with R software (version 3.5.2). The “gnm” package was used to conduct the conditional Poisson regression. A two‐sided p‐value less than .05 was considered statistically significant.
3. RESULTS
This study included 49 participants (55% female) with a clinical diagnosis of epilepsy collected from the NeuroVista (NV) and seizure dairy (SD) data sets in Australia (15 and 34 participants, respectively). Table 1 shows participant information and seizure characteristics in the two data sets. Overall, a total of 6692 epileptic seizures (median: 71; IQR: 19–201 seizures per participant) in 3639 seizure days (median: 50; IQR: 10–135 days per participant) were recorded during a total of 23 349 follow‐up days (median: 465; IQR: 272–671 days per participant) between 2010–2012 and 2018–2021.
TABLE 1.
Participant information and seizure characteristics
| NeuroVista data set | Seizure diary data set | Overall | |
|---|---|---|---|
| No. of participants | 15 | 34 | 49 |
| Male | 9 (60%) | 13 (38%) | 22 (45%) |
| Female | 6 (40%) | 21 (62%) | 27 (55%) |
| Study period | June 2010‐Aug 2012 | Jan 2018‐Feb 2021 | June 2010‐Feb 2021 |
| Seizure types | Clinical and subclinical | Self‐report (clinical) | Clinical and subclinical |
| Follow‐up days | 557 (384–725) | 432 (235–652) | 465 (272–671) |
| Seizure days | 80 (24–167) | 27 (10–96) | 50 (10–135) |
| Seizure counts | |||
| Clinical | 1539 (47%) | 3419 (100%) | 4958 (74%) |
| Subclinical | 1734 (53%) | NA | 1734 (26%) |
Numerical data are presented as n (%) or median (interquartile range).
NA, not applicable.
The daily average concentrations of air pollutants and meteorological variables are shown in Table 2. The median daily average concentrations of air pollutants during the follow‐up periods were 0.15 parts per million (ppm) for CO, 6.78 parts per billion (ppb) for NO2, 14.74 ppb for O3, 15.02 µg/m3 for PM10, and 0.30 ppb for SO2. All daily concentrations of CO and SO2, and at least 95% of daily concentrations of NO2, O3, and PM10 were within Australian air quality standards (Table S1). The correlations between air pollutants and meteorological variables are shown in Table S2. The pollutant concentrations are slightly and positively correlated with each other (R = 0.01–0.43), except for the negative correlations between O3 and CO, and between O3 and NO2 (R = –0.30 and –0.06, respectively).
TABLE 2.
Descriptive statistics for air pollutants and meteorological variables
| NeuroVista data set | Seizure diary data set | Overall | |
|---|---|---|---|
| CO (ppm) | 0.19 (0.11, 0.30) | 0.14 (0.10, 0.21) | 0.15 (0.10, 0.23) |
| NO2 (ppb) | 8.04 (5.30, 11.74) | 6.22 (3.15, 9.78) | 6.78 (3.91, 10.46) |
| O3 (ppb) | 14.78 (11.26, 19.29) | 14.74 (8.65, 19.86) | 14.74 (9.80, 19.70) |
| PM10 (µg/m3) | 14.13 (10.21, 19.50) | 15.54 (11.42, 21.24) | 15.02 (11.00, 20.60) |
| SO2 (ppb) | 0.52 (0.14, 1.17) | 0.27 (0.09, 0.57) | 0.30 (0.09, 0.69) |
| T (℃) | 12.49 (9.26, 16.63) | 14.41 (10.86, 18.61) | 13.81 (10.27, 17.99) |
| R (%) | 74.83 (67.80, 82.18) | 71.55 (62.79, 78.84) | 72.67 (64.45, 80.00) |
| P (mm) | 0.25 (0.02, 1.26) | 0.16 (0.01, 1.01) | 0.19 (0.01, 1.09) |
| S (J/m2) | 10 027 716 (6 168 135, 14 796 773) | 9 220 435 (6 092 152, 13 232 344) | 9 419 401 (6 120 211, 13 728 569) |
Data are presented as median (interquartile range).
Abbreviations: CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; P, precipitation; PM10, particulate matter ≤10 µm in diameter; ppb, parts per billion; ppm, parts per million; R, relative humidity; S, sun radiation; SO2, sulfur dioxide; T, temperature; μg/m3, micrograms per cubic meter.
Table 3 shows the overall associations between epileptic seizures and air pollutants, and the results of the stratified analyses. Overall, we observed a significant positive association between CO concentrations and epileptic seizure risks, with an increased seizure risk of 4% (relative risk [RR]: 1.04, 95% confidence interval [CI]: 1.01–1.07) for an IQR increase of CO (0.13 ppm), whereas no significant relationships were found in the other four air pollutants in the whole study population.
TABLE 3.
Association between epileptic seizures and an interquartile range (IQR) increase in pollutant concentrations in the single‐pollutant model
| CO | NO2 | O3 | PM10 | SO2 | |
|---|---|---|---|---|---|
| Overall | 1.04 (1.01, 1.07)** | 1.04 (0.98, 1.10) | 0.99 (0.94, 1.05) | 1.00 (0.97, 1.03) | 0.98 (0.95, 1.00) |
| Sex | |||||
| Female | 1.05 (1.01, 1.08)* | 1.09 (1.01, 1.16)* | 0.97 (0.90, 1.04) | 1.02 (0.99, 1.06) | 0.98 (0.95, 1.02) |
| Male | 1.02 (0.97, 1.07) | 0.92 (0.83, 1.02) | 1.10 (0.95, 1.28) | 0.96 (0.92, 1.01) | 0.99 (0.96, 1.01) |
| Dataset | |||||
| NV | 1.10 (1.03, 1.17)** | 1.05 (0.96, 1.14) | 0.98 (0.90, 1.06) | 0.94 (0.88, 1.00) | 0.93 (0.87, 1.00) |
| SD | 1.00 (0.96, 1.03) | 1.04 (0.97, 1.12) | 1.01 (0.93, 1.10) | 1.03 (1.00, 1.06) | 1.03 (0.99, 1.06) |
| Seizure type | |||||
| Clinical (NV) | 0.99 (0.92, 1.06) | 1.01 (0.91, 1.12) | 0.93 (0.85, 1.02) | 1.02 (0.95, 1.10) | 0.99 (0.90, 1.08) |
| Subclinical (NV) | 1.20 (1.12, 1.28)*** | 1.09 (0.99, 1.20) | 1.02 (0.93, 1.12) | 0.87 (0.82, 0.93)*** | 0.91 (0.85, 0.98)* |
The association was measured by the relative risk (RR) with 95% confidence interval (CI).
Abbreviations: Clinical (NV), clinical seizures from NeuroVista dataset; CO, Carbon monoxide; NO2, Nitrogen dioxide; NV, NeuroVista dataset; O3, Ozone; PM10, Particulate matter ≤10 µm in diameter; SD, Seizure dairy dataset; SO2, Sulfur dioxide; Subclinical (NV), subclinical seizures from NeuroVista dataset.
***p < .001; **p < .01; *p < .05.
In terms of the associations in different sexes, female participants had a significantly increased risk of epileptic seizures when exposed to elevated CO (RR: 1.05, 95% CI: 1.01–1.08) and NO2 (RR: 1.09, 95% CI: 1.01–1.16) concentrations, whereas no significant associations were found in male participants for any air pollutants.
The association results in the two data sets showed that there was a significant association between CO concentration and epileptic seizure risk in the NV data set (RR: 1.10, 95% CI: 1.03–1.17), whereas no significant associations were found in the SD data set for any air pollutants. Further analysis of different seizure types in the NV data set revealed that the epileptic seizure risk significantly increased by 20% when considering only subclinical seizures (RR: 1.20, 95% CI: 1.12–1.28), for an IQR increase of CO concentration, and decreased by 13% (RR: 0.87, 95% CI: 0.82–0.93) and 9% (RR: 0.91, 95% CI: 0.85–0.98) for subclinical seizures, for an IQR increase of PM10 and SO2 concentrations, respectively, whereas no significant associations were observed when considering clinical seizures for any air pollutants.
The associations between air pollutant concentrations and epileptic seizure are shown in Figure 1. The associations between pollutant concentrations and epileptic seizure risks tended to be linear, except for NO2 and O3. However, only CO concentration demonstrated a significant positive association with the risk of epileptic seizures. We found a close to “J” shaped association between the NO2 concentration and seizure risks. However, a significant association was observed only when NO2 concentration was above 16.83 ppb (95th percentile of NO2 concentrations, RR: 1.11, 95% CI: 1.01–1.22).
FIGURE 1.
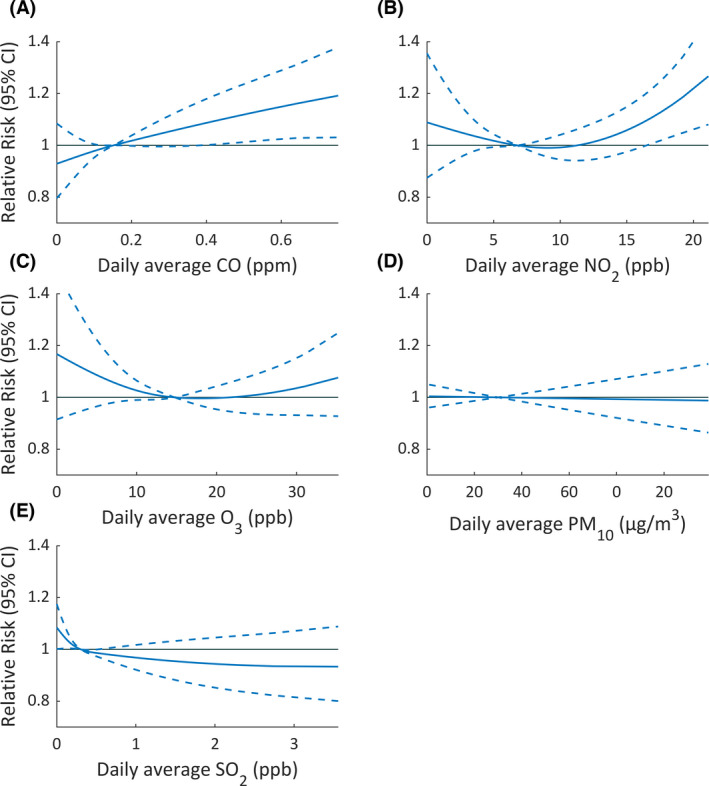
Association between daily average concentrations of air pollutants and the relative risk (95% confidence interval) of epileptic seizures in study participants with epilepsy. (A) Carbon monoxide (CO). (B) Nitrogen dioxide (NO2). (C) Ozone (O3). (D) Particulate matter ≤10 µm in diameter (PM10). (E) Sulfur dioxide (SO2). Associations were examined using cubic splines with 3 degrees of freedom for pollutant variables in the conditional quasi‐Poisson regression model. The median concentration of each pollutant was set as the reference value
Associations between air pollutants and epileptic seizures in two‐pollutant models are shown in Figure 2. The deleterious effect of CO remained statistically significant after adjustment for any one of the other pollutants. SO2 after adjustment for CO or NO2 showed a slightly negative association with seizures.
FIGURE 2.
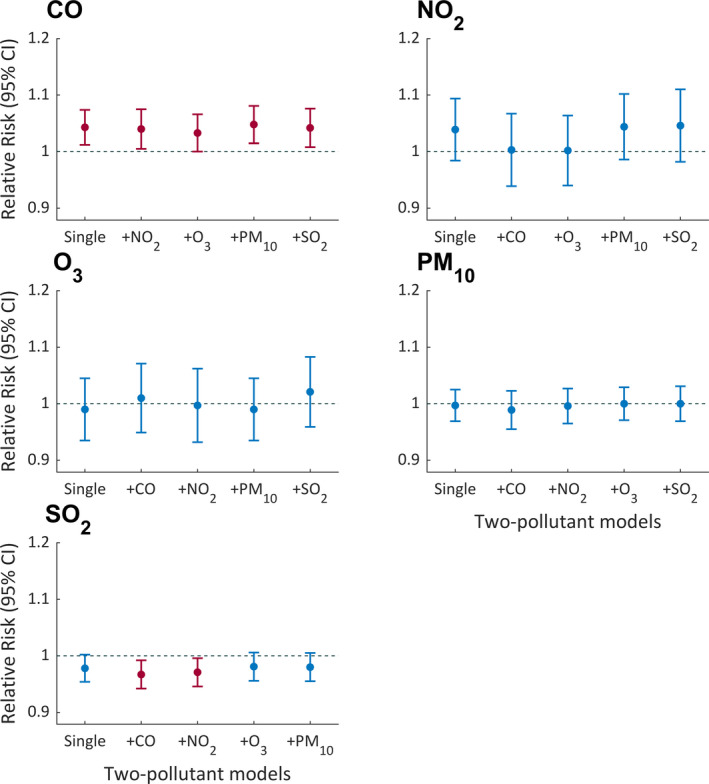
Association between epileptic seizures and air pollutants in two‐pollutant models. The point and the bar lines show relative risk (RR) and 95% confidence interval (CI) for an interquartile range (IQR) increase in a certain air pollutant by adjusting for another kind of air pollutant. The red bar indicates significant relative risk where the 95% CI does not include 1.00
Figure 3 shows the lagged pattern of the associations between air pollutants and seizures. As for the single‐lag day effect, CO at lag0‐1 and O3 at lag1‐3 showed significant positive associations with the risk of seizures, although NO2 at lag2 and PM10 at lag3 showed slightly negative associations with seizures, respectively. As for the effect of moving average of multiple lag days, except for CO at mv01‐03, other air pollutants did not show significant associations with seizures.
FIGURE 3.
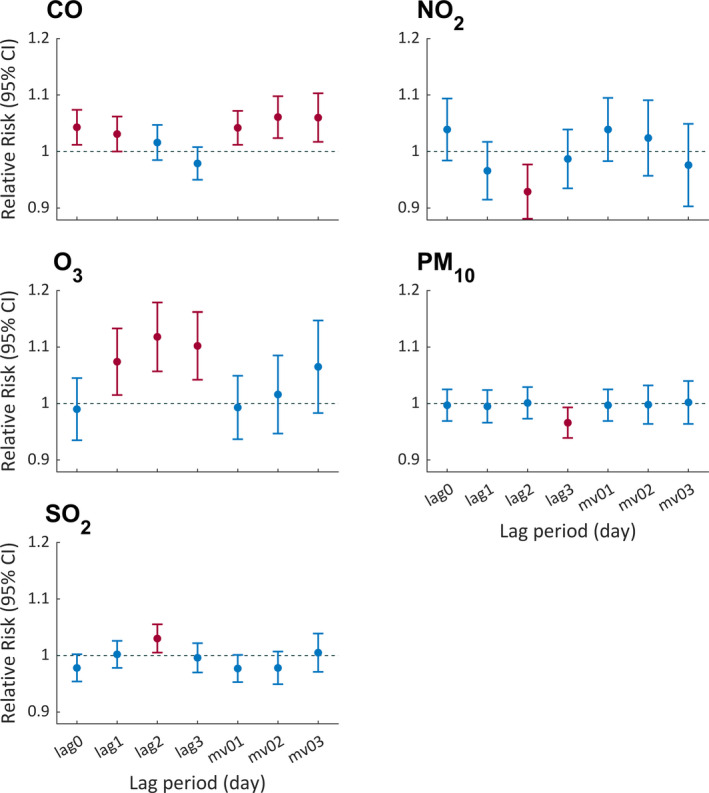
The relative risk (95% confidence interval) of epileptic seizures associated with an interquartile range (IQR) increase of air pollutants using 0–3 lag days in the single‐pollutant model. Lag0, lag1, lag2, and lag3 represent the corresponding single lag day. Mv01, mv02, and mv03 represent the moving average of the previous 0–1 days, 0–2 days, and 0–3 days, respectively. The red bars represent significant relative risk where the 95% confidence interval (CI) does not include 1.00
Sensitivity analyses suggested that our main results were robust when changing the degrees of freedom (df) from 3 to 5 for meteorological variables (Table S3), modifying the different time strata (Table S4), and adding the seizure counts of the previous month or week in the model (Table S5).
4. DISCUSSION
There are no studies so far, to the best of our knowledge, that have examined the impact of air pollution on the risks of epileptic seizures based on iEEG measures. This panel study quantified the association between ambient air pollution and the risk of epileptic seizures based on two independent long‐term seizure data sets recorded for 6 years (2010–2012 and 2018–2021) in Australia. Our findings indicate that elevated CO concentrations, although within Australian air quality standards, could significantly increase the risk of epileptic seizures. This positive association was observed in subclinical seizures but not in clinical seizures, which suggests that low‐level CO exposure may be more likely to associate with subclinical seizures, whereas it may not be strong enough to associate directly with clinical seizures.
Although mounting evidence has demonstrated adverse neurological effects of exposure to air pollutants, 10 , 33 few studies have focused on associations between air pollution and epilepsy. According to a recent systematic review, six epidemiological studies in the literature have explored the impact of air pollution on epilepsy. 19 Most of the studies were based on hospital databases or registers for epilepsy and delineated that air pollution has an adverse influence on hospitalization or outpatient visits for epilepsy. For instance, a recent study based on 47 hospitals from 10 Chinese cities indicated that an IQR increase of CO and NO2 was correlated with an increased admission of 2.0% and 1.1% for epilepsy, respectively. 23 Likewise, a study conducted in seven Chilean cities showed that the increase of air pollutant levels (CO, NO2, O3, SO2, PM10, and PM2.5) may be risk factors for epilepsy hospitalization. 24 Using hospital epilepsy hospitalization as a surrogate of epileptic seizures cannot capture many seizures not leading to hospital admission for various reasons (e.g., not severe enough, limited health care resources), and cannot record the time of seizures accurately. Our home‐based epileptic seizure data obtained from long‐term iEEG and a mobile application could overcome these limitations, which can give a more accurate estimation of the association between air pollution and epileptic seizures. In this study, we found that CO concentration had a significant positive association with the risk of epileptic seizures (RR: 1.04, 95% CI: 1.01–1.07), but no significant associations were found for other air pollutants.
Apart from the difference in home‐based data sources, the large difference in the air pollution concentration exposure is another reason that our findings cannot be directly compared with previous studies. Compared with previous studies conducted in high air pollution countries, such as China and Chile, 23 , 24 , 25 the present study was conducted in Australia, and most of the daily air pollutant concentrations were within Australian air quality standards (see Table S1). Consistent evidence has shown that exposure to high levels of air pollution is associated with markers of neuroinflammation and neuropathology that may be linked to neurodegenerative conditions 34 as well as epilepsy. 35 , 36 In this study, apart from CO, the other four studied air pollutants (NO2, O3, SO2, and PM10) did not show significant associations with the risk of epileptic seizures, which is possibly due to their low concertation levels.
In addition, apart from epileptic seizures with clinical symptoms (clinical seizures), we have taken advantage of the iEEG recordings in the NV data set to investigate the relationships between ambient air pollution and subclinical seizures (electrographic seizures without obvious clinical symptoms). Studies have suggested that subclinical seizures have an adverse effect on cognition 37 and may also associate with some psychiatric and compulsive disorders. 38 Therefore, it is important to explore the effects of air pollution on subclinical seizures. Our results in the NV data set demonstrated that, unlike clinical seizures, significant associations were observed between air pollutants (CO, PM10, and SO2) and subclinical seizures, even though PM10 and SO2 showed a negative relationship. Furthermore, consistent with the results for the clinical seizures in the NV data set, no significant associations were found in any pollutants in the SD data set. It should be noted that all seizures recorded in the SD data set were essentially clinical seizures, as only seizures with clinical symptoms can be noticed and reported by participants. Therefore, one possible interpretation of our finding is that low‐level air pollution may be more likely to affect subclinical seizures but may not be strong enough to directly associate with clinical seizures.
The results of the discrepancy effects stratified by sex revealed that females had a higher risk of seizures for the increase in CO and NO2 concentration than males. One possible explanation is the sex differences in outdoor activities and behavior patterns (e.g., smoking, exercise, etc.), which may cause a difference in the environmental exposure. 39 However, further analysis is not possible in this study due to the lack of individual‐specific environmental exposure and activity information. Future work is warranted to study the potential reasons for sex differences in the relationship between ambient air pollutants and epileptic seizures.
Evidence has shown that short‐term exposure to low‐level CO could impair cognitive functions 40 and even increase the risk of mortality. 41 Acute exposure to CO can cause neurotoxic symptoms like headaches, dizziness, and disorientation by reducing the amount of oxygen reaching the brain and tissues. 42 At high concentrations, CO poisoning might induce seizures. 43 , 44 A study in rats indicated that CO could regulate cerebral blood flow in epileptic seizures. 45 Even though the underlying causes and pathways involved are unclear, our findings provide important clues for further exploration of the effect of CO exposure on epileptic seizures.
Air pollution may impact brain metabolism, thereby increasing susceptibility to seizures. 19 Air pollution components can indirectly enter the bloodstream and the brain through the lungs and the gastrointestinal tract by changing the permeability of the blood‐brain barrier. 17 , 19 , 46 , 47 In addition, pollutants can directly influence the brain through the olfactory nerve from the nose. 19 Inflammation and oxidative stress have been identified as the main mechanisms for the damage to the central nervous system induced by air pollution. 17 Inflammatory reactions in the brain can enhance neuronal excitability, impair cell survival, and alter the innate immune response. 47 Experimental findings have indicated that brain inflammation can contribute to the occurrence of seizures. 48 Moreover, chronic mitochondrial oxidative stress and resultant dysfunction have been implicated as contributing factors to render the brain more susceptible to epileptic seizures. 49 Inflammation and oxidative stress have also been identified as the main factors associated with epileptogenesis. 19 , 49 Other potential pathology mechanisms include cerebrovascular damage, neuron damage through activating reactive microgliosis, and astroglia and microglia activation. 17 , 19 Given the complex nature of air pollution, the neurological effects of air pollutants are probably a result of the synergistic interaction of the multiple pathways. 17 Further research into the association between air pollutant exposures and epileptic seizure risks is of crucial importance to better protect health, especially given the current trend of climate change.
There are some unique strengths of this study. This is the first panel study so far to investigate the association between air pollutants and epileptic seizures. The data of epileptic seizures obtained from long‐term iEEG recordings have higher accuracy than the previous hospital‐based studies performed using medical records. In addition, this is the first study to investigate the impact of pollutants at low concentration levels on subclinical seizures, and the results showed that CO exposure, although within the Australian air quality standard, could contribute to the increased risks of subclinical seizures. Finally, we used the time‐ and patient‐stratified case‐crossover design to investigate the association between air pollution and epileptic seizures. The designed self‐matching approach controlled for inter‐patient variations and the temporal trends or cycles of epileptic seizures and air pollution.
We should also acknowledge several limitations. The potential inaccuracy of self‐reported seizures by patients in the SD data set might underestimate the influence of air pollution on seizures. In addition, we used the ambient air pollution levels in participants’ postcodes as proxies for individual exposures, which inevitably introduces some measurement errors and could underestimate the associations. Moreover, because of the difficulty in obtaining long‐term epileptic seizure data from individuals, only 49 participants with epilepsy were included in this study, which may have limited the statistical power to detect associations. Additional larger studies are warranted to further evaluate these effects.
Knowledge gained from our study could have important clinical and public health implications. Our findings suggest that CO exposure could be explored as a potential new feature for seizure‐risk forecasting, which may be used to reduce the uncertainty of seizures and guide epilepsy management. Furthermore, our study could also drive new potential approaches to reduce seizure risks by managing behavior when pollutant levels are high or using air filtration systems to reduce exposure to high CO.
In conclusion, our study found that daily exposure to elevated CO, even though within the Australian air quality standard, is significantly associated with the increased risk of epileptic seizures, especially for subclinical seizures. These findings indicate that CO exposure could be a potential seizure risk factor, and further larger‐scale studies are warranted to explore the effect of CO exposure on epileptic seizures.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This research is supported by the Melbourne Monash Consciousness Research Seed Funding and the Australian National Health and Medical Research Council Ideas Grant (APP1183119). ZC and RX are supported by the China Scholarship Council (grant numbers: 201709120011 and 201806010405, respectively). WY is supported by Monash Graduate Scholarship, Monash International Tuition Scholarship, and PhD Top Up Funding from the Centre for Air Pollution, Energy and Health Research. YG is supported by a Career Development Fellowship of the Australian National Health and Medical Research Council (APP1163693). We thank Environment Protection Authority in Victoria, Tasmania, New South Wales, and Queensland for sharing the air quality data. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians. [Correction added on 16 May, 2022, after first online publication: CAUL funding statement has been added.]
Chen Z, Yu W, Xu R, Karoly PJ, Maturana MI, Payne DE, et al. Ambient air pollution and epileptic seizures: A panel study in Australia. Epilepsia. 2022;63:1682–1692. 10.1111/epi.17253
Yuming Guo and David B. Grayden are joint senior authors.
Contributor Information
Zhuying Chen, Email: zhuyingc@student.unimelb.edu.au.
Wenhua Yu, Email: wenhua.yu@monash.edu.
REFERENCES
- 1. Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. 2015;385:884–98. [DOI] [PubMed] [Google Scholar]
- 2. Fisher RS, Boas WVE, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46:470–2. [DOI] [PubMed] [Google Scholar]
- 3. Sperling MR, O'Connor MJ. Auras and subclinical seizures: characteristics and prognostic significance. Ann Neurol. 1990;28:320–8. [DOI] [PubMed] [Google Scholar]
- 4. Liang D, Shi L, Zhao J, Liu P, Sarnat JA, Gao S, et al. Urban air pollution may enhance COVID‐19 case‐fatality and mortality rates in the United States. Innovation. 2020;1:100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu C, Chen R, Sera F, Vicedo‐Cabrera AM, Guo Y, Tong S, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. 2019;381:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu W, Guo Y, Shi L, Li S. The association between long‐term exposure to low‐level PM2.5 and mortality in the state of Queensland, Australia: a modelling study with the difference‐in‐differences approach. PLoS Med. 2020;17(6):e1003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamra GB, Guha N, Cohen A, Laden F, Raaschou‐Nielsen O, Samet JM, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta‐analysis. Environ Health Perspect. 2014;122:906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117:1697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Y, Qi J, Ruan Z, Yin P, Zhang S, Liu J, et al. Changes in life expectancy of respiratory diseases from attaining daily PM2.5 standard in China: A Nationwide Observational Study. Innovation. 2020;1:100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X, Ha SU, Basnet R. A review of epidemiological research on adverse neurological effects of exposure to ambient air pollution. Front Public Health. 2016;4:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen G, Wang A, Li S, Zhao X, Wang Y, Li H, et al. Long‐term exposure to air pollution and survival after ischemic stroke: The china national stroke registry cohort. Stroke. 2019;50:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. Short term exposure to air pollution and stroke: systematic review and meta‐analysis. The BMJ. 2015;350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szyszkowicz M, Rowe B, Kaplan G. Ambient sulphur dioxide exposure and emergency department visits for migraine in Vancouver, Canada. Int J Occup Med Environ Health. 2009;22:7–12. [DOI] [PubMed] [Google Scholar]
- 14. Kulick ER, Wellenius GA, Boehme AK, Joyce NR, Schupf N, Kaufman JD, et al. Long‐term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology. 2020;94:e1782–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kioumourtzoglou M‐A, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, et al. Long‐term PM2. 5 exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect. 2016;124:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu P, Zhang Y, Xia G, Zhang W, Xu R, Wang C, et al. Attributable risks associated with hospital outpatient visits for mental disorders due to air pollution: a multi‐city study in China. Environ Int. 2020;143:105906. [DOI] [PubMed] [Google Scholar]
- 17. Block ML, Calderón‐Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crüts B, van Etten L, Törnqvist H, Blomberg A, Sandström T, Mills NL, et al. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part Fibre Toxicol. 2008;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandes M, Carletti C, de Araújo LS, Santos R, Reis J. Respiratory gases, air pollution and epilepsy. Rev Neurol (Paris). 2019;175:604–13. [DOI] [PubMed] [Google Scholar]
- 20. Lee WG. Carbon monoxide poisoning presenting as non‐convulsive status epilepticus treated with hyperbaric oxygen therapy. J Epilepsy Res. 2018;8:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woodbury DM, Rollins LT, Gardner MD, Hirschi WL, Hogan JR, Rallison ML, et al. Effects of carbon dioxide on brain excitability and electrolytes. Am J Physiol‐Legacy Content. 1957;192:79–90. [DOI] [PubMed] [Google Scholar]
- 22. Requena M, Parrón T, Navarro A, García J, Ventura MI, Hernández AF, et al. Association between environmental exposure to pesticides and epilepsy. Neurotoxicology. 2018;68:13–8. [DOI] [PubMed] [Google Scholar]
- 23. Bao X, Tian X, Yang C, Li Y, Hu Y. Association between ambient air pollution and hospital admission for epilepsy in Eastern China. Epilepsy Res. 2019;152:52–8. [DOI] [PubMed] [Google Scholar]
- 24. Cakmak S, Dales RE, Vidal CB. Air pollution and hospitalization for epilepsy in Chile. Environ Int. 2010;36:501–5. [DOI] [PubMed] [Google Scholar]
- 25. Xu C, Fan Y‐N, Kan H‐D, Chen R‐J, Liu J‐H, Li Y‐F, et al. The novel relationship between urban air pollution and epilepsy: a time series study. PLoS One. 2016;11:e0161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cook MJ, O'Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, et al. Prediction of seizure likelihood with a long‐term, implanted seizure advisory system in patients with drug‐resistant epilepsy: a first‐in‐man study. Lancet Neurol. 2013;12:563–71. [DOI] [PubMed] [Google Scholar]
- 27. Cai J. Humidity: Calculate Water Vapor Measures from Temperature and Dew Point; 2019. [cited 2020 Sep 4] Available from: https://cran.r‐project.org/web/packages/humidity/index.html
- 28. Rakers F, Walther M, Schiffner R, Rupprecht S, Rasche M, Kockler M, et al. Weather as a risk factor for epileptic seizures: a case‐crossover study. Epilepsia. 2017;58:1287–95. [DOI] [PubMed] [Google Scholar]
- 29. Janes H, Sheppard L, Lumley T. Case‐crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 2005;16:717–26. [DOI] [PubMed] [Google Scholar]
- 30. Mittleman MA. Optimal referent selection strategies in case‐crossover studies: a settled issue. Epidemiology. 2005;16:715–6. [DOI] [PubMed] [Google Scholar]
- 31. Armstrong BG, Gasparrini A, Tobias A. Conditional Poisson models: a flexible alternative to conditional logistic case cross‐over analysis. BMC Med Res Methodol. 2014;14:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol. 2013;42:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim H, Kim WH, Kim YY, Park HY. Air pollution and central nervous system disease: a review of the impact of fine particulate matter on neurological disorders. Front Public Health. 2020;8:575330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cipriani G, Danti S, Carlesi C, Borin G. Danger in the air: air pollution and cognitive dysfunction. Am J Alzheimer's Dis Other Dement. 2018;33:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459–72. [DOI] [PubMed] [Google Scholar]
- 37. Ung H, Cazares C, Nanivadekar A, Kini L, Wagenaar J, Becker D, et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain. 2017;140:2157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yeager CL, Guerrant JS. Subclinical epileptic seizures; impairment of motor performance and derivative difficulties. Calif Med. 1957;86:242–7. [PMC free article] [PubMed] [Google Scholar]
- 39. Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amitai Y, Zlotogorski Z, Golan‐Katzav V, Wexler A, Gross D. Neuropsychological impairment from acute low‐level exposure to carbon monoxide. Arch Neurol. 1998;55:845–8. [DOI] [PubMed] [Google Scholar]
- 41. Chen K, Breitner S, Wolf K, Stafoggia M, Sera F, Vicedo‐Cabrera AM, et al. Ambient carbon monoxide and daily mortality: a global time‐series study in 337 cities. Lancet Planet Health. 2021;5:e191–e9. [DOI] [PubMed] [Google Scholar]
- 42. Varrassi M, Di Sibio A, Gianneramo C, Perri M, Saltelli G, Splendiani A, et al. Advanced neuroimaging of carbon monoxide poisoning. Neuroradiol J. 2017;30:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herman LY. Carbon monoxide poisoning presenting as an isolated seizure. J Emerg Med. 1998;16:429–32. [DOI] [PubMed] [Google Scholar]
- 44. Mori T, Nagai K. Carbon‐monoxide poisoning presenting as an afebrile seizure. Pediatr Neurol. 2000;22:330–1. [DOI] [PubMed] [Google Scholar]
- 45. Montécot C, Seylaz J, Pinard E. Carbon monoxide regulates cerebral blood flow in epileptic seizures but not in hypercapnia. NeuroReport. 1998;9:2341–6. [DOI] [PubMed] [Google Scholar]
- 46. Calderón‐Garcidueñas L, Solt AC, Henríquez‐Roldán C, Torres‐Jardón R, Nuse B, Herritt L, et al. Long‐term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood‐brain barrier, ultrafine particulate deposition, and accumulation of amyloid β‐42 and α‐synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310. [DOI] [PubMed] [Google Scholar]
- 47. Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–43. [DOI] [PubMed] [Google Scholar]
- 48. Marchi N, Granata T, Ghosh C, Janigro D. Blood–brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia. 2012;53:1877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. 2004;37:1951–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


