Summary
Sunflecks are transient patches of direct radiation that provide a substantial proportion of the daily irradiance to leaves in the lower canopy. In this position, faster photosynthetic induction would allow for higher sunfleck‐use efficiency, as is commonly reported in the literature. Yet, when sunflecks are too few and far between, it may be more beneficial for shade leaves to prioritize efficient photosynthesis under shade.
We investigated the temporal dynamics of photosynthetic induction, recovery under shade, and stomatal movement during a sunfleck, in sun and shade leaves of Fagus sylvatica from three provenances of contrasting origin.
We found that shade leaves complete full induction in a shorter time than sun leaves, but that sun leaves respond faster than shade leaves due to their much larger amplitude of induction. The core‐range provenance achieved faster stomatal opening in shade leaves, which may allow for better sunfleck‐use efficiency in denser canopies and lower canopy positions.
Our findings represent a paradigm shift for future research into light fluctuations in canopies, drawing attention to the ubiquitous importance of sunflecks for photosynthesis, not only in lower‐canopy leaves where shade is prevalent, but particularly in the upper canopy where longer sunflecks are more common due to canopy openness.
Keywords: canopy vertical gradients, Fagus sylvatica, photosynthetic induction, provenance trial, stomatal dynamics, sun and shade leaves, sunfleck
Introduction
The distribution of foliage and branches within a forest canopy, along with the density and relative arrangement of the trees themselves, produce a highly heterogeneous spatial environment. As light penetrates this medium, elements of the canopy will act as a filter, scattering and preferentially absorbing certain regions of the spectrum. This creates a vertical gradient in the distribution of light and its composition, arranged in different proportions of shade, partial shade, and full sunlight (Smith et al., 1989). Although patterns of irradiance and spectral quality are highly localized, average light availability will exponentially decline with canopy depth (Monsi & Saeki, 1953); a decline which will be steeper in denser canopies. Additionally, daytime temperatures usually decline while air humidity rises with depth in the canopy (Stiegel et al., 2017; Miller et al., 2021). It is often assumed that the photosynthetic machinery is optimized towards carbon gain for a given environment (Pons, 2012; Retkute et al., 2015), therefore this vertical variation in the microclimate means that leaves need to adopt distinct responses, depending on their location within the canopy, if they are to attain optimal carbon uptake for their position.
A distinction is often made between leaves that developed under high irradiance, usually at the top of the canopy (henceforth called ‘sun leaves’) and those that developed under low irradiance, mostly found lower in the canopy (henceforth called ‘shade leaves’). Sun leaves tend to have a higher leaf mass per area (LMA), nitrogen, chlorophyll and carotenoid content on an area basis, as well as a higher ratio of chlorophyll a/b, compared with shade leaves (Lichtenthaler et al., 2007; Lichtenthaler et al., 2013). This means that sun leaves can usually reach higher rates of electron transfer and carboxylation than shade leaves, at the expense of higher respiration rates and a higher photosynthetic light compensation point (Scartazza et al., 2016; Liu et al., 2019; Zhuang et al., 2021). When accounting for the decline in irradiance through the canopy, these differences in leaf traits result in a corresponding vertical gradient of leaf nitrogen, photosynthetic rate and capacity (Campany et al., 2016; Hikosaka, 2016; Liu et al., 2019). Yet, at the canopy level, shaded leaves often contribute more than half of the gross primary production, because of the large proportion of the canopy they occupy (He et al., 2018).
In the canopy, leaves also experience patches of substantially higher irradiance than that of the canopy shade, commonly called sunflecks (McLean, 1919). These can be very brief (less than a second, Durand et al., 2021), or last for several minutes (sometimes called sun patches, Smith & Berry, 2013), depending on whether they originate from the movement of the sun, clouds, or are due to the wind causing mechanical deformations in the canopy (Kaiser et al., 2018; Burgess et al., 2021). Sunflecks can be responsible for a large (up to 90%) but variable proportion of the total incident irradiance available in the lower forest canopy (Chazdon, 1988; Pfitsch & Pearcy, 1989). This will impact carbon uptake, but exactly how photosynthesis is affected by these fluctuations in irradiance depends both on their timescale, and on the physiological state of the leaf when they occur. Usually, sunflecks are thought to be more important for the lower canopy, where shade leaves have been reported to take less time to complete full photosynthesis induction (Way & Pearcy, 2012).
We can generally delineate three physiological limitations on the carbon gained from longer sunflecks, that mainly originate from the movement of the sun and clouds: (1) the speed of photosynthetic induction, (2) photoprotection in the form of nonphotochemical quenching (NPQ) and (3) the rate of stomatal movements. All three factors influence the use of fluctuating light by the photosynthetic machinery and are described in more detail later.
Leaves previously acclimated to a long period of shade (typically > 5 min) will be limited by induction requirements of the photosynthetic machinery (Kobza & Edwards, 1987; Sassenrath‐Cole & Pearcy, 1992). When an uninduced leaf is exposed to light, a small initial rise in CO2 assimilation is produced, supported by the leaf's residual metabolite pools and enzyme activation state. This is followed by the first phase of the induction response, which lasts 1–2 min, consisting of rapid light‐activation of enzymes involved in the ribulose‐1,5‐biphosphate (RuBP) regeneration pathway (Kirschbaum & Pearcy, 1988; Sassenrath‐Cole & Pearcy, 1992). For leaves experiencing longer periods of shade, activation of Rubisco is also needed, involving Rubisco activase (Taylor et al., 2022), itself requiring electron transport through photosystem I (PSI) and adenosine 5′‐triphosphate (ATP) production (Campbell & Ogren, 1992). This process can take > 10 min (Woodrow & Mott, 1989). Even after full induction, photosynthesis does not respond instantaneously to a sunfleck, which is something that can hinder carbon uptake in short (< 1 min) sunflecks when integrated over long periods (Way & Pearcy, 2012). Other concurrent processes, such as chloroplast relocation and components of mesophyll conductance may also substantially affect light‐use efficiency during a sunfleck (Flexas et al., 2008; Sztatelman et al., 2016). For example, mesophyll conductance can limit photosynthetic induction by up to 35% (Liu et al., 2022). Genotypic variability behind these processes has been found to affect photosynthetic efficiency under fluctuating light (Sun et al., 2016; Acevedo‐Siaca et al., 2020; Cowling et al., 2021).
Under high irradiance, leaves may receive more energy than they can use, over‐exciting the photosynthetic machinery. To dissipate this excess energy without damaging and inactivating the photosystems, several protective mechanisms exist such as dissipation of energy as heat during NPQ. NPQ is principally associated with the activity of the photosystem II (PSII) subunit S protein (PsbS) and the xanthophyll cycle (Demmig‐Adams & Adams, 1992; Li et al., 2000), but also with the reversible phosphorylation of light‐harvesting complexes, known as state transitions (Allen et al., 1981). Whilst the induction of NPQ is rapid, its relaxation can take several minutes, thus lagging behind fluctuations in irradiance in the canopy (Murchie & Ruban, 2020), which can limit photosynthesis in shade leaves under low light (Kromdijk et al., 2016; Y. Wang et al., 2020). We will hereafter refer to photosynthetic recovery as the slow (> 1 min) increase in photosynthesis which follows its initial sudden decline during the transition from sunfleck to shade.
Finally, for longer sunflecks (> 1 min), slow stomatal opening in leaves previously under a prolonged period in the shade can impose a further limitation on the induction of photosynthesis by restricting the diffusion of CO2 into the leaf (Allen & Pearcy, 2000; Way & Pearcy, 2012). The temporal dynamics of stomatal movements are dependent on growing conditions (glasshouse and field‐grown poplars: Durand et al., 2020), and modulated by specific environmental factors (drought: Durand et al., 2019; ozone: Dusart et al., 2019). The light environment during growth can have an especially large impact on stomatal dynamics (sun vs shade: Gérardin et al., 2018; fluctuating light: Matthews et al., 2018), suggesting that depth in the canopy may present a limitation to stomatal movement. Stomatal response speed can also vary greatly both within (Durand et al., 2019) and between (McAusland et al., 2016) species. Thus, adaptations to the local environment, such as differences in canopy openness, and to the latitudinal gradient in day length could create further environmental pressures to display a specific syndrome with regard to stomatal speed and response.
The dynamic regulation of photosynthesis and stomatal conductance in sun and shade leaves of field‐grown trees are yet to be studied. Here, we report on the temporal dynamics of photosynthetic induction, recovery under low light, and stomatal movements in sun and shade leaves of 12‐yr old European beech (Fagus sylvatica) trees from Swedish, German, and Spanish provenances growing outside their natural range in Helsinki, Finland. We investigate whether beech trees can be considered shade‐tolerant because of better sunfleck use, or higher photosynthetic rates under shade. We hypothesize that: (1) leaves growing deeper in the canopy would achieve faster photosynthesis induction, recovery, and/or stomatal movements, allowing them to use sunflecks more efficiently than leaves at the top of the canopy; (2) we expect trees from different origins to exhibit different temporal responses for the three processes because their adaptation was driven by different environmental selection pressures; (3) when considering time‐integrated photosynthetic rates, we predict that shade leaves would benefit most from more‐frequent, longer and more‐intense sunflecks compared with sun leaves.
Materials and Methods
Study site and plant material
We performed the experiment in a Fagus sylvatica, L. provenance trial, at the University of Helsinki, Finland (60.227°N, 25.018°E, 10 m above sea level). The most extreme range‐edge provenances were selected, including from Blaviksliarna southern Sweden (SE) (leading edge) and Montejo de la Sierra, Madrid (ES), in Spain (trailing edge) (Supporting Information Table S1; F. Wang et al., 2020), plus one provenance from the heart of the distribution in Eichelberg (DE) central Germany (Table S1). These provenances were chosen for their potentially different behaviour with regards to sunflecks, driven by adaptations to strongly contrasting climates of origin. Single trees from each provenance were arranged randomly within five blocks over 22 rows (separated by 120 cm) of 12 trees 100 cm apart, planted in 2010. The outermost trees, composing the border, were excluded from the analyses. In May 2020, the plot was thinned by removing half of the trees (see Fig. S1 for a map of the plot), and in the summer of 2021 the average height was 6 m.
Light measurements
To assess the light environment through the canopy, we recorded spectral photon irradiance (μmol m−2 s−1) with a CCD array spectroradiometer Maya 2000 Pro (Ocean Optics, Dunedin, FL, USA) to which a cosine diffuser (D7‐H‐SMA; Bentham Instruments Ltd, Reading, UK) was attached with a fibre‐optic cable (FC‐UV400‐2 400 μm; Avantes, Leatherhead, UK). The device was calibrated by the Finnish Radiation and Nuclear Safety Authority (STUK; Ylianttila et al., 2007) in April 2021. Measurements were taken at four locations in the plot, and at depths of 2, 3 and 4.5 m from the top of the canopy (representing the top, middle and bottom of the canopy, respectively) once between 11 and 21 June 2021. The four measuring locations were typical of the light environment within the plot and were encircled approximately equidistant from a representative tree of each focal beech provenance. We recorded sets of 500 scans at an integration time of 100 ms at each location and height (n = 4). The diffuser was levelled in a horizontal position, and each recorded spectrum comprised a nonequispaced set of wavelengths: 250.14, 250.62, 251.09, 251.57, …, 899.77 nm. A consecutive sequence of spectra was measured to apply corrections for instrument noise in the dark, and for stray light in ultraviolet (UV) following the protocol in Hartikainen et al. (2018) the using ooacquire R package (Aphalo & Ylianttila, 2021). All data were recorded within 3 h of solar noon (c. 13:30) from 10:30 to 15:30, local time. For each measuring point and depth, we extracted the scans showing the 5% (shade), 50% (median light environment) and 95% (sunfleck) quantile in photosynthetically‐active radiation (PAR).
Sunfleck duration distribution was estimated using hemispherical photographs, taken in RAW format with a Sigma 4.5 mm f2.8 EX DC HSM circular fisheye lens (Sigma Corp.) combined with a Nikon D7100 (Nikon Corp., Tokyo, Japan) camera body with a CMOS 24 MP image sensor. Pictures were taken using a tripod, under overcast sky, at 2, 3 and 4 m depth, representing the top to bottom of the canopy in 2020. ISO was fixed at 100 and aperture at F22, varying only exposure time. To reduce variation due to exposure time, we produced a gamma‐corrected and contrast‐stretched blue channel 8‐bit jpeg from the RAW files following Macfarlane et al. (2014). Sunfleck duration distribution was then calculated during the growing season (1 May–30 November) for each photograph with Gap Light Analyser (Simon Fraser University, Burnaby, BC, Canada) using automatic thresholding according to Nobis & Hunziker (2005). The software defined a sunfleck as the period for which the sensor experienced direct sunlight.
Light induction by sunflecks
Six leaves, each sampled from a different tree, were measured for each of the three provenances (Swedish, Spanish, and German) at two depths (2 and 4 m canopy depth, hereafter referred as sun and shade leaves), summing to 36 leaves. We chose leaves at the top of the canopy (2 m depth) that nonetheless experienced shade part of the day due to surrounding foliage, because we considered them more representative of the upper canopy than the very few leaves at the very tip of the main stem that very rarely experience shading. Since we could not reach the very highest canopy to measure photosynthesis, the sampling was performed by randomly choosing a leaf from a cut branch holding at least 10 leaves. The cut end of each branch was immediately submerged under water and kept in the shade, to prevent dehydration during the measurements (completed within c. 90 min from cutting). Measurements were recorded every 5 s using an infrared gas analyser (IRGA, LI‐6400 or LI‐6800; Li‐Cor, Lincoln, NE, USA) from 09:00 to 18:00 between 9 and 28 June 2021. Time of day did not significantly affect the speed of photosynthesis and stomatal movement (P > 0.64). Leaves were left to acclimate to the low light conditions inside the gas‐exchange chamber for 30 min (PAR: 20 μmol m−2 s−1; CO2 concentration: 400 ppm; block temperature: 25°C; relative humidity: 60%). This acclimation time allowed us to minimize potential effects of having cut branches. At the end of the acclimation period (SS0), PAR was raised to 1200 μmol m−2 s−1 (SS1), then lowered back to 20 μmol m−2 s−1 (SS2). At each step, leaves were left for 30 min, or until stomatal conductance (g s) reached a steady‐state. PAR levels represented typical irradiance in the shade and at the top of the canopy under a clear sky. These measurements also defined the blue (420–490 nm) to red (620–680 nm) ratio (B : R), following Sellaro et al. (2010) we used (B : R was 0.96 ± 0.08 in the shade and 0.78 ± 0.01 during sunflecks), in order to better reflect the spectral composition of natural sunlight. At 20 μmol m−2 s−1, B : R was set to 1 (equal proportion), and at 1200 μmol m−2 s−1, B : R was set to 0.71 (500 and 700 μmol m−2 s−1 of blue and red light, respectively). At high irradiance, the B : R ratio was also decided by instrumental limitations (maximum blue light of 500 μmol m−2 s−1).
Dynamics of photosynthesis and stomatal conductance
To describe the response of CO2 assimilation (A n) and stomatal conductance to a change of irradiance, we fitted a sigmoid of the form:
| (Eqn 1) |
with the fitted A n or g s , y 0 and Y the starting and ending steady‐state values, τ and λ fitted parameters representing the response time (in seconds) and the lag time (in seconds), and t the time. More information on this model and its parameters can be found in Durand et al. (2019), and Vialet‐Chabrand et al. (2013). From this model an estimator of the rate of change, or speed, the maximum slope (SLmax), can be calculated as:
| (Eqn 2) |
This model was used to fit both stomatal opening and closure, as well as the increase of A n during photosynthetic induction (after PAR was raised from 20 to 1200 μmol m−2 s−1), and the increase of A n after its initial decrease when PAR was lowered back to 20 μmol m−2 s−1 (Fig. S2).
Assessing photosynthesis under various sunfleck patterns (optimization routine)
In order to examine how leaves at different canopy depths are physiologically disposed to use different patterns of light fluctuations, we modelled photosynthesis integrated over time at different canopy depths and under diverse sunfleck patterns. Measurements of gas‐exchange on leaves 2 and 4 m deep in the canopy were performed on 9 and 18 June 2021 using the LI‐6400 IRGA. We used a chamber with a transparent top made from cellulose diacetate film, with conditions inside the chamber similar to those outside to avoid the need for acclimation to the chamber's conditions (PAR: variable; CO2 concentration: 400 ppm; block temperature: 23°C, relative humidity: 45–65%, unregulated). The leaf was left inside the chamber until the conditions were equalized (typically 90 s), then the measurement was recorded. From the measured A n and PAR irradiance (I), a nonrectangular hyperbola was fitted (Xu et al., 2019), separately for the two canopy depths (Fig. S3), of the form:
| (Eqn 3) |
with α the quantum yield of assimilation, A sat the light‐saturated net photosynthetic rate, C the convexity of the curve and R d the dark respiration rate. Due to light being highly heterogeneous in the canopy, measured A n can be lower than expected among those leaves that have been uninduced by prolonged shade and then inadvertently transferred to higher irradiance during the measurements, or because of discrepancies between the irradiance experienced by the leaf in the chamber and that recorded by the PAR sensor a few centimetres away. To account for these scenarios we used only the data showing maximum A n at each PAR.
Adding the calculated dynamic parameters of photosynthetic induction and recovery allowed us to calculate the time‐integrated A n for a sunfleck of any duration, frequency and amplitude against a background shade of 20 μmol m−2 s−1. For a change in PAR different from the one we measured (20–1200 μmol m−2 s−1), we first need to calculate steady‐state A n for a given PAR using Eqn 3. Then, the time‐related parameters τ (response time) and λ (lag time) have to be recalculated because they are dependent on the amplitude of the response (i.e. for a change from 20 to 600 μmol m−2 s−1, τ would be about half as long). We re‐calculated τ using steady‐state A n and SLmax by re‐arranging Eqn 2, and λ by assuming a constant τ : λ ratio. Time‐integrated A n was then calculated for a sunfleck‐shade cycle (as in Fig. 1), according to different given sunfleck durations and frequencies, and assuming the same degree of photosynthetic recovery regardless of sunfleck properties. This exercise also presumes leaf photosynthesis is uninduced, in order to fit the conditions under which the dynamic parameters were estimated. Finally, we ran an optimization routine (‘optim’ in R) to find sets of sunfleck properties (amplitude, duration and frequency) that would result in similar time‐integrated A n between sun and shade leaves, giving us insight into the behaviour of sun and shade leaves under fluctuating light.
Fig. 1.
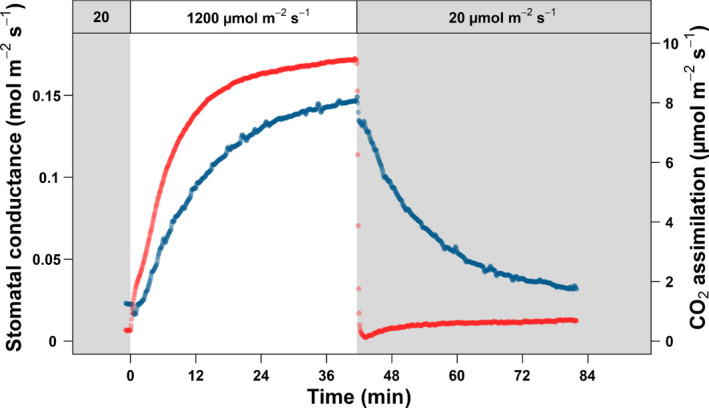
Example of a time course of light induction by a sunfleck. The example curve shown here was recorded on a sun leaf at the top of the canopy, from the Fagus sylvatica Swedish provenance. The CO2 assimilation and stomatal conductance are shown in red and blue, respectively. The grey and white area represent periods of low (20 μmol m−2 s−1) and high (1200 μmol m−2 s−1) irradiance, respectively.
Statistical analysis
Statistical analyses were made using R v.4.0.2 (R Core Team, 2021) with the packages car (Fox & Weisberg, 2019), emmeans (Searle et al., 1980) and multcomp (Hothorn et al., 2008). Differences between canopy depth, category of light condition (i.e. sunfleck or shade), sun and shade leaf, and provenances were assessed using Type II ANOVAs (Table S2). Normality and homoscedasticity were checked graphically. Post hoc contrast analyses were performed to test for differences between modalities of each factor, and P values were adjusted to control the false discovery rate. Significant differences were considered at P < 0.05 for all tests.
Results
Gradients in the light environment and leaf morpho‐physiological traits with canopy depth
Gradients in the amount and properties of light in the beech provenance trial were highly variable, but nevertheless there were clear trends in the strength, composition, and heterogeneity of irradiance according to canopy depth, and sunfleck/shade distinction. This produced strong physiological differences between leaves at the top and bottom of the canopy (Tables 1, S3). Median PAR irradiance at the canopy top was 6.7 and 10 times higher than in the middle and bottom of the canopy, respectively (Fig. 2a, P < 0.01). Similarly, the typical irradiance during a sunfleck at the top of the canopy was approximatively double that of sunflecks in the middle or bottom layers. Finally, while the pairwise comparison did not detect significant differences between canopy depths in the shade, there was a significant correlation (P < 0.001, R 2 = 0.8) of decreasing irradiance in shade from top to the bottom canopy layer.
Table 1.
Summary of steady‐state values for photosynthetic induction by a sunfleck in leaves from the bottom or top of the canopy, in three provenances of Fagus sylvatica trees grown in Helsinki.
| Provenance | Canopy depth | State | Net CO2 assimilation, A n (μmol m−2 s−1) | Stomatal conductance, g s (mol m−2 s−1) | Intrinsic water use efficiency, A n/g s (μmol mol−1) |
|---|---|---|---|---|---|
|
Blaviksliarna Sweden (SE) |
Bottom | SS0 | 1.01 ± 0.14 a | 0.013 ± 0.002 ab | 93.33 ± 25.03 e |
| SS1 | 4.76 ± 0.47 b | 0.059 ± 0.009 abc | 89.49 ± 15.89 e | ||
| SS2 | 1.11 ± 0.15 a | 0.013 ± 0.002 ab | 97.72 ± 17.33 e | ||
| Top | SS0 | 0.49 ± 0.18 a | 0.05 ± 0.018 abc | 24.35 ± 10.52 abc | |
| SS1 | 14.37 ± 1.37 c | 0.183 ± 0.029 de | 86.52 ± 14.7 de | ||
| SS2 | 0.57 ± 0.15 a | 0.047 ± 0.015 abc | 19.44 ± 7.14 a | ||
|
Eichelberg Germany (DE) |
Bottom | SS0 | 0.71 ± 0.16 a | 0.014 ± 0.002 ab | 58.57 ± 14.77 abcde |
| SS1 | 5.51 ± 0.56 b | 0.078 ± 0.013 c | 79.06 ± 12.02 bcde | ||
| SS2 | 0.73 ± 0.11 a | 0.014 ± 0.002 ab | 62.86 ± 16.28 abcde | ||
| Top | SS0 | 0.29 ± 0.15 a | 0.027 ± 0.009 ab | 22.34 ± 9.68 ab | |
| SS1 | 12.53 ± 1.63 c | 0.144 ± 0.028 d | 99.23 ± 15.05 e | ||
| SS2 | 0.34 ± 0.12 a | 0.027 ± 0.006 ab | 31.05 ± 19.1 abcd | ||
|
Montejo de la Sierra Spain (ES) |
Bottom | SS0 | 0.77 ± 0.18 a | 0.012 ± 0.003 a | 89.97 ± 34.29 e |
| SS1 | 6.43 ± 0.66 b | 0.091 ± 0.014 c | 78.9 ± 10.94 cde | ||
| SS2 | 0.79 ± 0.18 a | 0.018 ± 0.004 ab | 50.15 ± 9.39 abcde | ||
| Top | SS0 | 0.31 ± 0.19 a | 0.055 ± 0.009 abc | 6.26 ± 4.03 a | |
| SS1 | 16.41 ± 1.25 d | 0.229 ± 0.031 e | 80.79 ± 15.94 cde | ||
| SS2 | 0.31 ± 0.11 a | 0.061 ± 0.013 bc | 6.42 ± 2.61 a |
SS0, low light before the sunfleck (20 μmol m−2 s−1); SS1, high light during the sunfleck (1200 μmol m−2 s−1); SS2, low light after the sunfleck. Values are means ± standard error. Letters represent statistically significant differences between groups tested by post hoc pairwise comparisons (P < 0.05).
Fig. 2.
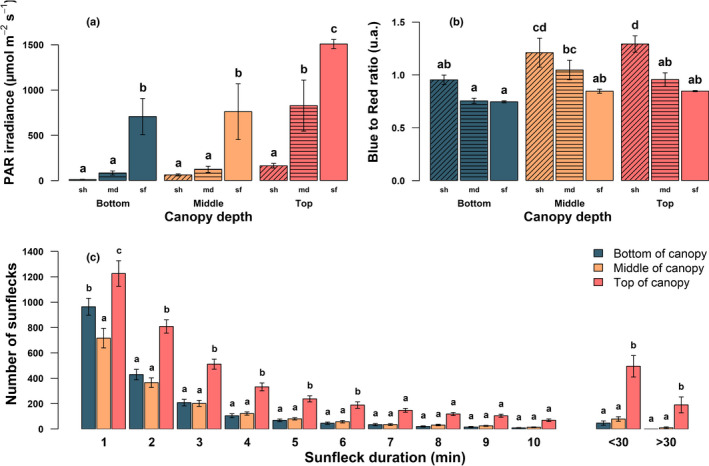
Properties of the light environment in a 12‐yr‐old Fagus sylvatica provenance trial in Helsinki, Finland. (a, b) Photosynthetically‐active radiation (PAR) irradiance and blue (420–490 nm) to red (620–680 nm) ratio at the bottom (teal), middle (yellow) and top (pink) of the canopy. Measurements were performed once between 11 and 21 June 2021. We recorded four sets of 500 spectrometer recordings at four locations in the plot (i.e. 8000 in total), and extracted for each location the recording representing the 5%, 50%, and 95% quantile of the PAR irradiance distribution. These scans are respectively referred to in the figure as ‘shade’ (sh, diagonal hatching), ‘median’ (md, horizontal hatching), and ‘sunfleck’ (sf, no hatching). The four locations within the stand were used as replicates. Thus, ‘shade’ represents the average of four scans (one at each location) for which only 100 scans (5% of the 2000 scans recorded) had a lower PAR irradiance. (c) Density distribution of sunfleck duration measured with Gap Light Analyzer using hemispherical photographs. Each day of the growing season (between 1 May and 30 November 2021) was used for the analysis. Values are means ± 1 standard deviation. Different letters represent statistically significant differences between groups tested by post hoc pairwise comparisons (P < 0.05). In (c), letters were applied separately for each class of sunfleck duration.
While the strength of irradiance did not differ between the bottom and middle of the canopy, we did find a change in its spectral composition (Fig. 2b). The B : R was 27% higher in the shade at the middle of the canopy than at the bottom, but there was no significant difference in B : R among sunflecks between canopy layers. In the shade the B : R was 28%, 43%, and 53% higher than during a sunfleck for the bottom, middle and top of the canopy, respectively.
Concerning the patterns of sunfleck duration (Fig. 2c), we found that over the whole growing season the top of the canopy experienced 1.7 and 1.3 times as many 1 min‐long sunflecks relative to the middle and bottom of the canopy, respectively. For longer sunflecks this trend is exacerbated, with 6.2 and 10.4 times as many sunflecks of 10 to 30 min‐long in the top of the canopy, compared with the middle or bottom canopy, respectively. For sunflecks longer than 30 min, there were more than 250 times as many in the top of the canopy (191.1) than at the bottom where sunflecks were almost nonexistent (0.75 on average over the whole season); with the longest recorded sunfleck in the upper canopy being 168 min‐long. Overall, irradiances at the bottom and middle of the canopy were relatively similar. The greater openness of the top layer of the canopy meant that its periods of shade and sunflecks were more equitably distributed than in the lower layers.
Dynamic response of photosynthesis and stomatal conductance to a sunfleck
When looking at the dynamic response of photosynthetic induction during a 30 min‐long simulated sunfleck (Figs 3a, 4a–d), we found large differences both according to canopy depth and provenance of the trees. Leaves at the top of the canopy exhibited a greater amplitude of induction than leaves at the bottom (3.6, 2.5 and 2.8 times higher for trees from Sweden, Germany, and Spain, respectively, Fig. 4a). Correspondingly, the response time (τ) was also 1.6, 2.6, and 1.4 times longer in leaves from the top of the canopy, for Swedish, German, and Spanish trees, respectively. The trees of German origin had the largest differences in τ with canopy depth, and uniquely had a significantly longer lag time (λ, almost doubled) in leaves at the top of the canopy. Lastly, due to the proportionally smaller increases in τ than amplitude, the speed of photosynthetic induction of leaves at the top of the canopy was about twice as fast as that of leaves at the bottom of the canopy, in all except for the German provenance (Fig. 4d).
Fig. 3.
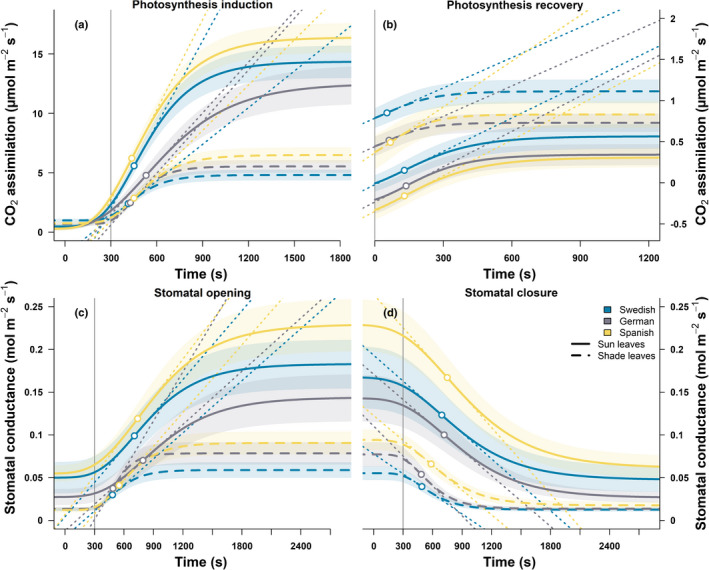
Fitted sigmoidal curves used to derive the parameters τ, λ, SLmax of photosynthesis induction (a), recovery (b), stomatal opening (c) and closure (d) for leaves at the bottom (dashed lines) and top (continuous lines) of the canopy, in three provenances of Fagus sylvatica trees (Sweden in blue, Germany in grey, Spain in yellow) grown in Helsinki. The average sigmoidal response is drawn in bold with the coloured areas showing the standard error around the mean. The open points indicate the timepoint when the rate of change is at its maximum, with the dotted lines representing the maximum speed (SLmax). The vertical grey line shows when the change in illumination happened.
Fig. 4.
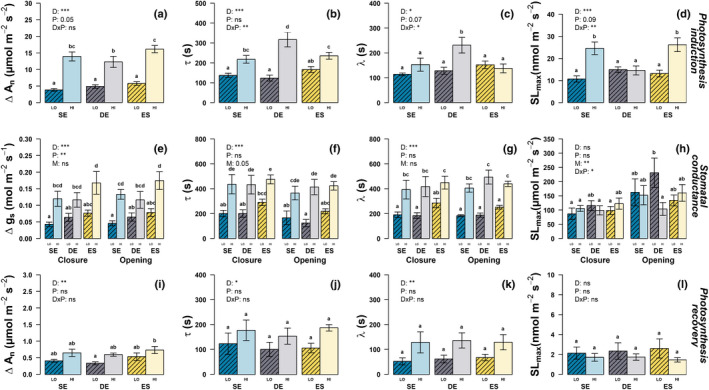
Dynamic parameters of the photosynthesis induction (a–d), stomatal conductance (e–h), and photosynthesis recovery (i–l) for leaves at the canopy top (HI, light, unshaded) and bottom (LO, dark, shaded), in three provenances of Fagus sylvatica trees (SE: Sweden in blue, DE: Germany in grey, ES: Spain in yellow) grown in Helsinki. Δ, amplitude, τ, time constant; λ, lag time; SLmax, maximum slope of the sigmoidal response. Values are means ± standard error. Letters represent statistically significant differences between groups tested by post hoc pairwise comparisons (P < 0.05). Results of two‐way ANOVA are given for main effects (D, canopy depth; P, provenance, M, stomatal opening and closing) and interaction (D × P). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
Steady‐state g s was very similar under low light before and after the 30 min‐long sunfleck (Table 1; Fig. 3c,d), giving a similar amplitude between stomatal opening and closing (Fig. 4e). Overall, g s amplitude was 35% and 43% higher in the Spanish trees than in the Swedish or German trees, respectively, and 2.8, 1.8, and 2.2 times higher in leaves at the top of the canopy compared to those at the bottom for the Swedish, German, and Spanish trees, respectively. The τ and λ for g s responded similarly, with no significant differences between provenances, being about twice as high in leaves at the top than those at the bottom of the canopy (Fig. 4f,g). Overall, τ was 20% longer during stomatal closure than during their opening (P = 0.05). Differences between opening and closure were neither dependent on provenance nor canopy depth, however stomatal opening was on average 50% faster than closure (P < 0.01, Fig. 4h), since they had a very similar amplitude. This difference was particularly striking in trees from Germany in which the speed of stomatal opening of canopy‐top leaves was less than half that of leaves at the bottom of the canopy (adjusted P = 0.03).
Under low light, A n in leaves at the top of the canopy was about half that of those at the bottom, but under high light, A n at the top was three times greater than at the bottom of the canopy (Table 1). Differences in g s between canopy depths were between two and four times higher in leaves at the top of the canopy compared to those at the bottom, depending on provenance. Under high light, A n increased by 4.5, 7.6, and 8.2 times, and g s by 4.5, 5.6 and 7.4 times for leaves at the bottom of the canopy in Swedish, German and Spanish trees. However, the A n of leaves at the top of the canopy increased under high light by 27, 40 and 53 times from their value under low light, while g s increased only by 3.7, 5.3 and 4.0 times for Swedish, German and Spanish trees, respectively. Thus, increases under high light between A n and g s were much more similar for leaves at the bottom of the canopy than for those at the top. This led to significant increases in intrinsic water‐use efficiency under high light in leaves at the top of the canopy (by 3.9, 3.7 and 12.7 times in Swedish, German and Spanish trees, respectively, Table 1), but not at the bottom.
Regarding photosynthetic recovery, overall there was a 52% larger amplitude (P < 0.01), 57% longer τ (P < 0.05), and λ more than doubled (P < 0.01) in leaves at the top of the canopy compared to those at the bottom (Figs 3b, 4i–k). Also, there was a small but nonsignificant trend towards faster photosynthetic recovery in leaves at the bottom of the canopy (Fig. 4l). However, large variations in the data, partly due to noise inherent to the measurements of small fluxes using the gas‐exchange system prevented us from detecting specific differences between provenances by pairwise comparisons.
Photosynthesis under different sunfleck properties
An optimization routine was applied to find sets of sunfleck properties giving the same time‐integrated A n for leaves at the top and at the bottom of the canopy during a sunfleck–shade cycle. An example using beech trees from the Swedish provenance is presented in Fig. 5. For a sunfleck lasting 5 min with an amplitude of 1500 μmol m−2 s−1, we found that a frequency of 0.3 h−1 (or once every 20 min) leads to the same A n between leaves at the top and bottom of the canopy (Fig. 5a). A sunfleck with the same frequency and amplitude, but lasting longer will result in higher A n for leaves at the top of the canopy, due to their CO2 assimilation under high light being three times higher (Table 1), and their photosynthetic induction faster (Fig. 4). The same is true for sunflecks of increasing frequency or amplitude, until light saturation is reached. Generally, sunfleck properties that increase the amount of time a leaf spends under high irradiance (increased amplitude, frequency or duration) will benefit leaves at the top of the canopy, and faster induction only reinforces this trend. In contrast, sunflecks properties that increase the amount of time spent under shade (reduced amplitude, frequency or duration) will result in higher A n in leaves at the bottom of the canopy that attain double the photosynthesis under shade.
Fig. 5.
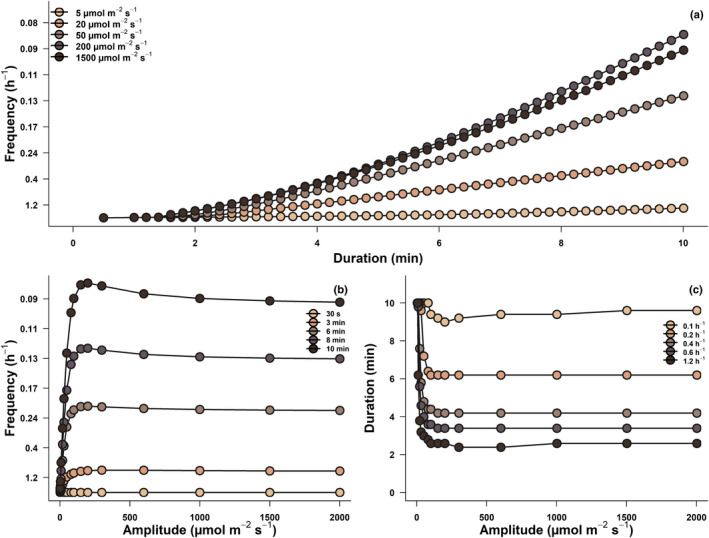
Two‐dimensional representation of simulation results showing the sets of sunfleck properties (amplitude, duration and frequency) resulting in the same time‐integrated CO2 assimilation between leaves from the top and bottom of the canopy for a sunfleck–shade cycle (as in Fig. 1), in Fagus sylvatica trees of Swedish provenance, grown in Helsinki. Colour gradients show series of increasing (a) sunfleck amplitude, (b) sunfleck duration, and (c) sunfleck frequency from light to dark brown.
Theoretically, to reach similar A n between canopy depths as sunflecks increase in amplitude, sunflecks must either decrease in frequency (Fig. 5b), or occur for a shorter duration (Fig. 5c). For example, a similar A n between canopy layers is reached with sunflecks lasting 2 min occurring every 50 min, and for 6‐min sunflecks occurring every 5 h. The peak in Fig. 5(b) and corresponding trough in Fig. 5(c) are due to differences in convexity (parameter C in Eqn 3) between the light response curves of top and bottom canopy leaves (Fig. S3), resulting in larger differences in A n between canopy depths at 300 μmol m−2 s−1 than at 1500 μmol m−2 s−1.
Discussion
Faster photosynthetic induction in leaves at the top of the canopy
Shade leaves are thought to be better adapted than sun leaves to the dynamic light environment of the understorey (Way & Pearcy, 2012). One proposed mechanism is that faster rates of photosynthetic induction under shade would improve light‐use efficiency during a sunfleck, and thus enhance carbon assimilation in the lower layers of the canopy. Support for this hypothesis has been provided by studies reporting shorter response time in shade leaves during induction (Küppers & Schneider, 1993; Chen & Klinka, 1997; Urban et al., 2007) compared to sun leaves (but see: Bai et al., 2008). In accordance with these reports, our results also show shorter response time (τ) in leaves at the bottom of the canopy, compared to those at the top (Fig. 4b). Although, the reason for this was because of much higher rates of A n after full induction in leaves at the top. In fact, when taking into account the magnitude of induction in both types of leaves, we find that leaves at the top of the canopy are capable of faster induction than leaves at the canopy bottom (Fig. 4d), which runs contrary to our expectations. This means that the larger size of the Calvin cycle metabolite pool and enzymes in leaves at the top of the canopy takes proportionally less time to achieve full activation than in leaves at the bottom of the canopy. Indeed, differences between A n before and after induction are much larger in leaves at the top of the canopy than at the bottom, due to their higher A n at saturating irradiance, as has been often reported (Lichtenthaler et al., 2007; Urban et al., 2007; Liu et al., 2019; F. Wang et al., 2020).
It should be noted that 30 min of acclimation to low irradiance simulated the Rubisco activation level we would expect under shade rather than inducing complete deactivation of Rubisco. Thus, differences of activation levels under low irradiance, and/or of deactivation speed during this 30 min period, may partly contribute to the differences in photosynthesis induction between canopy depths. The speed of Rubisco deactivation has been found to vary between species (Pearcy et al., 1996), and is increasingly considered as a potential target for productivity improvements under fluctuating light (Taylor et al., 2022).
Our findings thus show that induction times are not, by themselves, an adequate indicator of induction speed, as the time to complete photosynthetic induction is dependent on the magnitude of induction itself. Confusion may also be born from considering a process to be ‘faster’ because of its shorter duration in reaching completion, or because of differences in the speed of induction, both meanings commonly used in the scientific literature. Somewhat corollary to our findings, Naumburg & Ellsworth (2000) concluded from their review that species' shade tolerance is not generally related to photosynthetic induction speed. However, our conclusion that leaves in the upper and lower canopy differ in their photosynthetic induction, was only possible because of a novel and comprehensive examination of induction processes. We accounted for the amplitude, duration and speed of these processes, in a large dataset from a measurement campaign specifically designed for this purpose. Therefore we advocate reporting both speed and amplitude of photosynthetic induction in future studies evaluating light‐use efficiency under dynamic light conditions.
Differences in lag times (λ) of photosynthetic induction, but also λ of stomatal response when induction is limited by stomatal opening, can affect the duration, and thus the speed of the overall induction response (Wachendorf & Küppers, 2017). In our experiment, λ of the stomatal response was longer in leaves at the top of the canopy and was positively correlated with the magnitude of the stomatal response (R 2 = 0.49). This suggests that to achieve a larger change in stomatal conductance more time for perception and signalling may be required, e.g. to activate and deactivate the ion channels on guard cells that are responsible for the change in stomatal aperture; processes thought to be related to λ (Durand et al., 2019).
Faster photosynthetic recovery during a high to low irradiance transition has been related to faster NPQ relaxation in Nicotiana tabacum (Kromdijk et al., 2016). We found that recovery of photosynthesis was similar across canopy layers among beech trees, although the initial loss of A n was greater in leaves at the top of the canopy (Fig. 4i,j). If NPQ relaxation is the dominant process affecting photosynthesis recovery, the pattern we report is in agreement with the similarly longer relaxation times found in Avocado (Persea americana) leaves with increased NPQ capacity (Jia et al., 2013). The kinetics of NPQ relaxation are seldom explored, especially in situ on tree species (D'Haese et al., 2004; Murchie & Ruban, 2020), and very little is known of their environmental determinants. Still, NPQ induction and relaxation kinetics were found to be cultivar‐specific and function independently from each other in Triticum aestivum (McAusland et al., 2019), whereas we did not detect differences in photosynthesis recovery among provenances. It should be emphasized that photosynthetic recovery is not only due to NPQ relaxation, but the product of many processes including: chloroplast relocation (Sztatelman et al., 2016), state transitions (Mullineaux & Emlyn‐Jones, 2005), and respiration rates (Atkin et al., 2000). The relative contribution of each to photosynthetic recovery currently remains unexplored, yet understanding these processes may hold the key to improve productivity (Kromdijk et al., 2016; Taylor et al., 2022).
Dynamics of stomatal movements in sun and shade leaves is dependent on local adaptations at the provenance level
To our knowledge, ours is the first study comparing the dynamics of stomatal movements in sun and shade leaves of mature trees. Similar studies generally use seedlings grown in the understorey or in the open (Bai et al., 2008), or under various levels of shade in the glasshouse (Küppers & Schneider, 1993). We found evidence for intra‐specific differences in stomatal response speed, with the leaves of one German beech provenance, Eichelberg, displaying faster stomatal opening at the bottom of the canopy. A study of photosynthesis in rice canopies by Acevedo‐Siaca et al. (2021) found no difference in stomatal response speed between two canopy levels in three closely related species, and likewise between upper and lower canopy leaves in two shade‐intolerant poplar species (Roden & Pearcy, 1993b). Although, it should be noted that both studies also reported a lack of those physiological and morphological differences between leaves or canopy levels that typically allow for a distinction between sun and shade leaves. Three other studies have focused on the stomatal dynamics of plants growing under high or low irradiance. In Arabidopsis thaliana, plants grown under an average irradiance of 460 μmol m−2 s−1 in controlled conditions tended to have faster stomatal opening than those grown under half this irradiance (Matthews et al., 2018). Similarly, tobacco plants in growth chambers under 400 μmol m−2 s−1 illumination displayed faster stomatal closure, but not opening, compared to those grown under 40 μmol m−2 s−1 (Gérardin et al., 2018). This report appears contrary to our results. However, across a set of 11 rainforest tree species, Kardiman & Ræbild (2018) concluded that the speed of stomatal movement between saplings grown under high and low irradiance was strongly species‐specific, as stomatal movement in some species was faster and some slower under shade, a pattern that was not necessarily dependent on successional stage. In our study, not only was stomatal opening faster in the German beech provenance in the leaves at the bottom of the canopy, but we also found it to be the only provenance where photosynthetic induction operated at a similar speed in leaves at the top and bottom of the canopy. This consistency may be partly explained by a reduced lag time of induction in leaves in lower canopy layers (Fig. 4c). Taken together, these results suggest that this provenance from the core of the species distribution for beech may be able to benefit from a more efficient use of sunflecks in the lower canopy, compared to the other two range‐edge provenances. While we do not have information on canopy structure at the provenances' origin, it is possible that the more favourable environment in central Germany could allow for a denser canopy (Rajsnerová et al., 2015), placing stronger selection pressures on efficient sunfleck use in the lower canopy in this provenance.
Are sun leaves more efficient at using sunflecks than shade leaves?
By comparing time‐integrated A n in leaves at the top and bottom of the canopy under a common set of sunfleck properties (Fig. 5), we found that the canopy traits that produce a longer duration of shade (i.e. shorter and less frequent sunflecks) resulted in leaves at the bottom of the canopy exhibiting higher time‐integrated A n than leaves at the top. When added to our findings of faster induction in the leaves (Fig. 4), and longer and more frequent sunflecks (Fig. 2) at the top of the canopy, this paints a picture of leaves in the upper canopy being more efficient at using sunflecks. On the contrary, leaves at the bottom appear more efficient at using the lower irradiance of the shaded environment (Fig. 5; Table 1).
Typically, sunflecks are thought to be more common in, and useful for, the lower layers of the canopy. Therefore, most research on sunflecks has focused on understorey species (Chazdon, 1988; Kirschbaum et al., 1988; Pfitsch & Pearcy, 1989; Chazdon & Pearcy, 1991) and tree regeneration (Pearcy, 1983; Küppers & Schneider, 1993; Kursar & Coley, 1993). Our findings thus represent a paradigm shift for future sunfleck research, drawing attention to the importance of sunflecks for photosynthesis in the upper canopy.
That is not to say that sunflecks in the lower canopy are unimportant. The total amount of time under direct radiation due to sunflecks was 95 h and 977 h at the bottom and top of the canopy, respectively. This represented 3.4% and 34.4% of the total daylight hours between 1 May and 31 October 2021. However, this was equivalent to 70% of the total PAR irradiance received in the lower canopy, compared with 83% (only 13% more) at the top of the canopy (Fig. 2c), because sunflecks had an irradiance 67 times higher than the shade at the bottom of the canopy, but only nine times higher at the top (Fig. 2a). Moreover, shade leaves seem to maintain a high photosynthetic induction state for a longer period in low light than sun leaves (Küppers et al., 1996), which could partly compensate for the lower likelihood of sunfleck occurrence in the bottom canopy layers. The lack of an increase in water‐use efficiency under sunflecks in leaves at the bottom of the canopy, has parallels with findings from Eucalyptus tereticornis, and also supports the idea that shade leaves may ‘lie in wait’ for sunflecks (Campany et al., 2016).
It should be noted that our modelling simulations considered only a static view of the canopy light environment. In natural conditions, changes in wind speed and direction will produce movement of branches, and leaf fluttering (Roden & Pearcy, 1993a), depending on the mechanical properties of the canopy (Burgess et al., 2016). In turn, this will create a highly dynamic light environment, with often very short sunflecks (< 1 s) named ‘windflecks’ (Burgess et al., 2021; Durand et al., 2021). Because of their short duration, individual windflecks are less likely to affect induction processes than leaf processes operating at similar timescales, such as the short‐lived sustained CO2 assimilation after illumination (Pons & Pearcy, 1992). Nonetheless, sunflecks also often occur in clusters (Vierling & Wessman, 2000), for example as a result of gusts of wind, and in those cases induction speeds are likely to affect overall carbon uptake. Short frequent sunflecks also serve as an impediment to Rubisco deactivation. Our modelling assumed leaves had an appropriate Rubisco activation state for a constant 20 μmol m−2 s−1 illumination, while in practice this would likely be higher when accounting for sunflecks. Since leaves at the bottom of the canopy were slower to reach full induction, this difference may mitigate their lower capacity to use sunflecks, as compared to leaves at the canopy top. We also assumed a similar photosynthetic recovery regardless of sunfleck properties. In practice, natural conditions provide stronger and more frequent sunflecks, likely to induce stronger photoprotective mechanisms, which will depress A n to various degrees under shade especially in leaves at the top of the canopy where sunflecks last longer and occur more frequently, and where leaves have greater photoprotective potential (Demmig‐Adams, 1998).
Conclusion
The study of photosynthesis under fluctuating light is rapidly becoming a central question in plant science (Murchie et al., 2009; Kaiser et al., 2018). With the recognition that plants rarely experience stable steady‐state conditions in natural conditions, their slow response to fluctuations in light presents great potential for improvements in the efficiency of carbon assimilation (Lawson et al., 2012; Carmo‐Silva et al., 2015; Ort et al., 2015). In this article, we have shown how ubiquitous sunflecks are, being prevalent throughout all canopy depths of European beech trees of diverse origin. In particular, the relatively high openness in the upper canopy layers, creates many opportunities for light fluctuations to occur. We also found leaves in the upper layers of the canopy to be more efficient at using longer sunflecks (> 5 min), both because of their faster photosynthetic induction and higher rates of CO2 assimilation under high light. This challenges the paradigm that sunflecks are primarily valuable for carbon uptake in the lower canopy and understorey and highlights their relevance for photosynthesis throughout the canopy. Our modelling allowed us to utilize the dynamic response of photosynthesis to calculate carbon uptake under any sunfleck. Further examination of the dynamics of induction loss under shade of different depths and durations will allow us to model photosynthesis under any time series of light fluctuations. Beyond this, accounting for the dynamics of photosynthesis under fluctuations in light that are shorter than a second (see fig. 1 in Way & Pearcy, 2012) will pave the way for accurate estimation of carbon uptake in naturally fluctuating conditions integrated across timescales.
Competing interests
None declared.
Author contributions
MD, ZRS and TMR contributed to the experimental design, data collection, and data analysis and interpretation. MD, ZRS, YS, AJB, EHM and TMR contributed to the writing of the manuscript.
Supporting information
Fig. S1 Map of the provenance trial plot.
Fig. S2 Example curve fitting for photosynthesis and stomatal conductance.
Fig. S3 Photosynthetic light response curves from gas‐exchange on beech leaves at the top and bottom of the canopy.
Table S1 Information on the four beech provenance in the trial plot.
Table S2 Dataset used for statistical analysis.
Table S3 Leaf‐mass per area and chlorophyll content per leaf area with regard to canopy depth in three provenances of beech trees grown in Helsinki, Finland.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
This research was funded by Academy of Finland decisions #324555 and #323843, and partially supported by two awards from Viikki Plant Science Centre (ViPS) and HiLIFE Grand Challenges Programme at the University of Helsinki granted to YS and TMR. This experiment was located within Viikki Plant Growth and Experimental Field Facilities (ViGoR) HiLIFE infrastructure, and maintained with the assistance of Daniel Richterich and Leena Grönholm. Beechnuts were kindly donated by Ismael Aranda (Montejo), Rolf Övergaard (Blaviksliarna) and Gerhard Huber (Eichelberg). The authors thank Baiba Matule, Teemu Paljakka, Xin Zhuang, Alexandre Poutier and Pedro J. Aphalo for their technical help in the 2021 field campaign.
Data availability
The data that supports the findings of this study are available in the Supporting Information of this article. More information is available upon request.
References
- Acevedo‐Siaca LG, Coe R, Wang Y, Kromdijk J, Quick WP, Long SP. 2020. Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytologist 227: 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo‐Siaca LG, Dionora J, Laza R, Paul Quick W, Long SP. 2021. Dynamics of photosynthetic induction and relaxation within the canopy of rice and two wild relatives. Food and Energy Security 10: e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JF, Bennett J, Steinback KE, Arntzen CJ. 1981. Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291: 25–29. [Google Scholar]
- Allen MT, Pearcy RW. 2000. Stomatal versus biochemical limitations to dynamic photosynthetic performance in four tropical rainforest shrub species. Oecologia 122: 479–486. [DOI] [PubMed] [Google Scholar]
- Aphalo PJ, Ylianttila L 2021. R package: ooacquire v.0.2.3.9005: acquire data from OO spectrometers . [WWW document] URL https://docs.r4photobiology.info/ooacquire/, https://github.com/aphalo/ooacquire [accessed 1 June 2022].
- Atkin OK, Evans JR, Ball MC, Lambers H, Pons TL. 2000. Leaf respiration of snow gum in the light and dark. Interactions between temperature and irradiance. Plant Physiology 122: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K‐D, Liao D‐B, Jiang D‐B, Cao K‐F. 2008. Photosynthetic induction in leaves of co‐occurring Fagus lucida and Castanopsis lamontii saplings grown in contrasting light environments. Trees 22: 449–462. [Google Scholar]
- Burgess AJ, Durand M, Gibbs JA, Retkute R, Robson TM, Murchie EH. 2021. The effect of canopy architecture on the patterning of “windflecks” within a wheat canopy. Plant, Cell & Environment 44: 3524–3537. [DOI] [PubMed] [Google Scholar]
- Burgess AJ, Retkute R, Preston SP, Jensen OE, Pound MP, Pridmore TP, Murchie EH. 2016. The 4‐dimensional plant: effects of wind‐induced canopy movement on light fluctuations and photosynthesis. Frontiers in Plant Science 7: 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campany CE, Tjoelker MG, von Caemmerer S, Duursma RA. 2016. Coupled response of stomatal and mesophyll conductance to light enhances photosynthesis of shade leaves under sunflecks. Plant, Cell & Environment 39: 2762–2773. [DOI] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. 1992. Light activation of rubisco by rubisco activase and thylakoid membranes. Plant and Cell Physiology 33: 751–756. [Google Scholar]
- Carmo‐Silva E, Scales JC, Madgwick PJ, Parry MA. 2015. Optimizing rubisco and its regulation for greater resource use efficiency. Plant, Cell & Environment 38: 1817–1832. [DOI] [PubMed] [Google Scholar]
- Chazdon R. 1988. Sunflecks and their importance to forest understorey plants. Advances in Ecological Research 18: 1–63. [Google Scholar]
- Chazdon RL, Pearcy RW. 1991. The importance of sunflecks for forest understory plants. Bioscience 41: 760–766. [Google Scholar]
- Chen HY, Klinka K. 1997. Light availability and photosynthesis of Pseudotsuga menziesii seedlings grown in the open and in the forest understory. Tree Physiology 17: 23–29. [DOI] [PubMed] [Google Scholar]
- Cowling SB, Treeintong P, Ferguson J, Soltani H, Swarup R, Mayes S, Murchie EH. 2021. Out of Africa: characterising the natural variation in dynamic photosynthetic traits in a diverse population of African rice (Oryza glaberrima). Journal of Experimental Botany 73: 3283–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Haese D, Vandermeiren K, Caubergs RJ, Guisez Y, De Temmerman L, Horemans N. 2004. Non‐photochemical quenching kinetics during the dark to light transition in relation to the formation of antheraxanthin and zeaxanthin. Journal of Theoretical Biology 227: 175–186. [DOI] [PubMed] [Google Scholar]
- Demmig‐Adams B. 1998. Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant and Cell Physiology 39: 474–482. [Google Scholar]
- Demmig‐Adams B, Adams WW. 1992. Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology 43: 599–626. [Google Scholar]
- Durand M, Brendel O, Buré C, Le Thiec D. 2019. Altered stomatal dynamics induced by changes in irradiance and vapour‐pressure deficit under drought: impact on the whole plant transpiration efficiency of poplar genotypes. New Phytologist 222: 1789–1802. [DOI] [PubMed] [Google Scholar]
- Durand M, Brendel O, Bure C, Le Thiec D. 2020. Changes in irradiance and vapour pressure deficit under drought induce distinct stomatal dynamics between glasshouse and field‐grown poplars. New Phytologist 227: 392–406. [DOI] [PubMed] [Google Scholar]
- Durand M, Matule B, Burgess AJ, Robson TM. 2021. Sunfleck properties from time series of fluctuating light. Agricultural and Forest Meteorology 308–309: 108554. [Google Scholar]
- Dusart N, Vaultier MN, Olry JC, Bure C, Gerard J, Jolivet Y, Le Thiec D. 2019. Altered stomatal dynamics of two Euramerican poplar genotypes submitted to successive ozone exposure and water deficit. Environmental Pollution 252: 1687–1697. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas‐Carbo M, Diaz‐Espejo A, Galmes J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell & Environment 31: 602–621. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. 2019. An R companion to applied regression. Thousand Oaks, CA, USA: Sage. [Google Scholar]
- Gérardin T, Douthe C, Flexas J, Brendel O. 2018. Shade and drought growth conditions strongly impact dynamic responses of stomata to variations in irradiance in Nicotiana tabacum . Environmental and Experimental Botany 153: 188–197. [Google Scholar]
- Hartikainen SM, Jach A, Grane A, Robson TM. 2018. Assessing scale‐wise similarity of curves with a thick pen: as illustrated through comparisons of spectral irradiance. Ecology and Evolution 8: 10206–10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Chen JM, Gonsamo A, Luo X, Wang R, Liu Y, Liu R. 2018. Changes in the shadow: the shifting role of shaded leaves in global carbon and water cycles under climate change. Geophysical Research Letters 45: 5052–5061. [Google Scholar]
- Hikosaka K. 2016. Optimality of nitrogen distribution among leaves in plant canopies. Journal of Plant Research 129: 299–311. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Jia H, Forster B, Chow WS, Pogson BJ, Osmond CB. 2013. Decreased photochemical efficiency of photosystem II following sunlight exposure of shade‐grown leaves of avocado: because of, or in spite of, two kinetically distinct xanthophyll cycles? Plant Physiology 161: 836–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E, Morales A, Harbinson J. 2018. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiology 176: 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardiman R, Ræbild A. 2018. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiology 38: 696–705. [DOI] [PubMed] [Google Scholar]
- Kirschbaum MU, Pearcy RW. 1988. Gas exchange analysis of the fast phase of photosynthetic induction in Alocasia macrorrhiza. Plant Physiology 87: 818–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum MUF, Gross LJ, Pearcy RW. 1988. Observed and modelled stomatal responses to dynamic light environments in the shade plant Alocasia macrorrhiza . Plant, Cell & Environment 11: 111–121. [Google Scholar]
- Kobza J, Edwards GE. 1987. The photosynthetic induction response in wheat leaves: net CO2 uptake, enzyme activation, and leaf metabolites. Planta 171: 549–559. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Glowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354: 857–861. [DOI] [PubMed] [Google Scholar]
- Küppers M, Schneider H. 1993. Leaf gas exchange of beech (Fagus sylvatica L.) seedlings in lightflecks: effects of fleck length and leaf temperature in leaves grown in deep and partial shade. Trees 7: 160–168. [Google Scholar]
- Küppers M, Timm H, Orth F, Stegemann J, Stober R, Schneider H, Paliwal K, Karunaichamy KS, Ortiz R. 1996. Effects of light environment and successional status on lightfleck use by understory trees of temperate and tropical forests. Tree Physiology 16: 69–80. [DOI] [PubMed] [Google Scholar]
- Kursar TA, Coley PD. 1993. Photosynthetic induction times in shade‐tolerant species with long and short‐lived leaves. Oecologia 93: 165–170. [DOI] [PubMed] [Google Scholar]
- Lawson T, Kramer DM, Raines CA. 2012. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Current Opinion in Biotechnology 23: 215–220. [DOI] [PubMed] [Google Scholar]
- Li XP, Bjorkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK. 2000. A pigment‐binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Ač A, Marek MV, Kalina J, Urban O. 2007. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiology & Biochemistry 45: 577–588. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Babani F, Navrátil M, Buschmann C. 2013. Chlorophyll fluorescence kinetics, photosynthetic activity, and pigment composition of blue‐shade and half‐shade leaves as compared to sun and shade leaves of different trees. Photosynthesis Research 117: 355–366. [DOI] [PubMed] [Google Scholar]
- Liu Q, Dong L, Li F. 2019. Modification of a photosynthetic light‐response (PLR) model for modeling the vertical gradient in the response of crown PLR curves. Canadian Journal of Forest Research 49: 949–959. [Google Scholar]
- Liu T, Barbour MM, Yu D, Rao S, Song X. 2022. Mesophyll conductance exerts a significant limitation on photosynthesis during light induction. New Phytologist 233: 360–372. [DOI] [PubMed] [Google Scholar]
- Macfarlane C, Ryu Y, Ogden GN, Sonnentag O. 2014. Digital canopy photography: exposed and in the raw. Agricultural and Forest Meteorology 197: 244–253. [Google Scholar]
- Matthews JSA, Vialet‐Chabrand S, Lawson T. 2018. Acclimation to fluctuating light impacts the rapidity of response and diurnal rhythm of stomatal conductance. Plant Physiology 176: 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Atkinson JA, Lawson T, Murchie EH. 2019. High throughput procedure utilising chlorophyll fluorescence imaging to phenotype dynamic photosynthesis and photoprotection in leaves under controlled gaseous conditions. Plant Methods 15: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Vialet‐Chabrand S, Davey P, Baker NR, Brendel O, Lawson T. 2016. Effects of kinetics of light‐induced stomatal responses on photosynthesis and water‐use efficiency. New Phytologist 211: 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RC. 1919. Studies in the ecology of tropical rain‐forest: with special reference to the forests of South Brazil. Journal of Ecology 7: 121–172. [Google Scholar]
- Miller BD, Carter KR, Reed SC, Wood TE, Cavaleri MA. 2021. Only sun‐lit leaves of the uppermost canopy exceed both air temperature and photosynthetic thermal optima in a wet tropical forest. Agricultural and Forest Meteorology 301–302: 108347. [Google Scholar]
- Monsi M, Saeki T. 1953. Über den Lictfaktor in den Pflanzengesellschaften und sein Bedeutung für die Stoffproduktion. Japanese Journal of Botany 14: 22–52. [Google Scholar]
- Mullineaux CW, Emlyn‐Jones D. 2005. State transitions: an example of acclimation to low‐light stress. Journal of Experimental Botany 56: 389–393. [DOI] [PubMed] [Google Scholar]
- Murchie EH, Pinto M, Horton P. 2009. Agriculture and the new challenges for photosynthesis research. New Phytologist 181: 532–552. [DOI] [PubMed] [Google Scholar]
- Murchie EH, Ruban AV. 2020. Dynamic non‐photochemical quenching in plants: from molecular mechanism to productivity. The Plant Journal 101: 885–896. [DOI] [PubMed] [Google Scholar]
- Naumburg E, Ellsworth DS. 2000. Photosynthetic sunfleck utilization potential of understory saplings growing under elevated CO2 in FACE. Oecologia 122: 163–174. [DOI] [PubMed] [Google Scholar]
- Nobis M, Hunziker U. 2005. Automatic thresholding for hemispherical canopy‐photographs based on edge detection. Agricultural and Forest Meteorology 128: 243–250. [Google Scholar]
- Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP et al. 2015. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proceedings of the National Academy of Sciences, USA 112: 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy RW. 1983. The light environment and growth of C3 and C4 tree species in the understory of a Hawaiian forest. Oecologia 58: 19–25. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Krall JP, Sassenrath‐Cole GF. 1996. Photosynthesis in fluctuating light environments. In: Baker NR, ed. Advances in photosynthesis and respiration. Dordrecht, the Netherlands: Springer, 321–346. [Google Scholar]
- Pfitsch WA, Pearcy RW. 1989. Daily carbon gain by Adenocaulon bicolor (Asteraceae), a redwood forest understory herb, in relation to its light environment. Oecologia 80: 465–470. [DOI] [PubMed] [Google Scholar]
- Pons TL. 2012. Interaction of temperature and irradiance effects on photosynthetic acclimation in two accessions of Arabidopsis thaliana . Photosynthesis Research 113: 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TL, Pearcy RW. 1992. Photosynthesis in flashing light in soybean leaves grown in different conditions. II. Lightfleck utilization efficiency. Plant, Cell & Environment 15: 577–584. [Google Scholar]
- R Core Team . 2021. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL https://www.R‐project.org/ [accessed 1 June 2022]. [Google Scholar]
- Rajsnerová P, Klem K, Holub P, Novotná K, Večeřová K, Kozáčiková M, Rivas‐Ubach A, Sardans J, Marek MV, Peñuelas J et al. 2015. Morphological, biochemical and physiological traits of upper and lower canopy leaves of European beech tend to converge with increasing altitude. Tree Physiology 35: 47–60. [DOI] [PubMed] [Google Scholar]
- Retkute R, Smith‐Unna SE, Smith RW, Burgess AJ, Jensen OE, Johnson GN, Preston SP, Murchie EH. 2015. Exploiting heterogeneous environments: does photosynthetic acclimation optimize carbon gain in fluctuating light? Journal of Experimental Botany 66: 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden JS, Pearcy RW. 1993a. Effect of leaf flutter on the light environment of poplars. Oecologia 93: 201–207. [DOI] [PubMed] [Google Scholar]
- Roden JS, Pearcy RW. 1993b. Photosynthetic gas exchange response of poplars to steady‐state and dynamic light environments. Oecologia 93: 208–214. [DOI] [PubMed] [Google Scholar]
- Sassenrath‐Cole GF, Pearcy RW. 1992. The role of ribulose‐1,5‐bisphosphate regeneration in the induction requirement of photosynthetic CO2 exchange under transient light conditions. Plant Physiology 99: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scartazza A, Di Baccio D, Bertolotto P, Gavrichkova O, Matteucci G. 2016. Investigating the European beech (Fagus sylvatica L.) leaf characteristics along the vertical canopy profile: leaf structure, photosynthetic capacity, light energy dissipation and photoprotection mechanisms. Tree Physiology 36: 1060–1076. [DOI] [PubMed] [Google Scholar]
- Searle SR, Speed FM, Milliken GA. 1980. Population marginal means in the linear model: an alternative to least squares means. The American Statistician 34: 216–221. [Google Scholar]
- Sellaro R, Crepy M, Trupkin SA, Karayekov E, Buchovsky AS, Rossi C, Casal JJ. 2010. Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiology 154: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WK, Berry ZC. 2013. Sunflecks? Tree Physiology 33: 233–237. [DOI] [PubMed] [Google Scholar]
- Smith WK, Knapp AK, Reiners WA. 1989. Penumbral effects on sunlight penetration in plant communities. Ecology 70: 1603–1609. [Google Scholar]
- Stiegel S, Entling MH, Mantilla‐Contreras J. 2017. Reading the leaves' palm: leaf traits and herbivory along the microclimatic gradient of forest layers. PLoS ONE 12: e0169741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ye M, Peng S, Li Y. 2016. Nitrogen can improve the rapid response of photosynthesis to changing irradiance in rice (Oryza sativa L.) plants. Scientific Reports 6: 31305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztatelman O, Labuz J, Hermanowicz P, Banas AK, Bazant A, Zglobicki P, Aggarwal C, Nadzieja M, Krzeszowiec W, Strzalka W et al. 2016. Fine tuning chloroplast movements through physical interactions between phototropins. Journal of Experimental Botany 67: 4963–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SH, Gonzalez‐Escobar E, Page R, Parry MAJ, Long SP, Carmo‐Silva E. 2022. Faster than expected rubisco deactivation in shade reduces cowpea photosynthetic potential in variable light conditions. Nature Plants 8: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban O, Kosvancova M, Marek MV, Lichtenthaler HK. 2007. Induction of photosynthesis and importance of limitations during the induction phase in sun and shade leaves of five ecologically contrasting tree species from the temperate zone. Tree Physiology 27: 1207–1215. [DOI] [PubMed] [Google Scholar]
- Vialet‐Chabrand S, Dreyer E, Brendel O. 2013. Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level. Plant, Cell & Environment 36: 1529–1546. [DOI] [PubMed] [Google Scholar]
- Vierling LA, Wessman CA. 2000. Photosynthetically active radiation heterogeneity within a monodominant Congolese rain forest canopy. Agricultural and Forest Meteorology 103: 265–278. [Google Scholar]
- Wachendorf M, Küppers M. 2017. The effect of initial stomatal opening on the dynamics of biochemical and overall photosynthetic induction. Trees 31: 981–995. [Google Scholar]
- Wang F, Israel D, Ramírez‐Valiente J‐A, Sánchez‐Gómez D, Aranda I, Aphalo PJ, Robson TM. 2020. Seedlings from marginal and core populations of European beech (Fagus sylvatica L.) respond differently to imposed drought and shade. Trees 35: 53–67. [Google Scholar]
- Wang Y, Burgess SJ, de Becker EM, Long SP. 2020. Photosynthesis in the fleeting shadows: an overlooked opportunity for increasing crop productivity? The Plant Journal 101: 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way DA, Pearcy RW. 2012. Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiology 32: 1066–1081. [DOI] [PubMed] [Google Scholar]
- Woodrow IE, Mott KA. 1989. Rate limitation of non‐steady‐state photosynthesis by ribulose‐1,5‐bisphosphate carboxylase in spinach. Functional Plant Biology 16: 487–500. [Google Scholar]
- Xu J, Lv Y, Liu X, Wei Q, Qi Z, Yang S, Liao L. 2019. A general non‐rectangular hyperbola equation for photosynthetic light response curve of rice at various leaf ages. Scientific Reports 9: 9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylianttila L, Visuri R, Huurto L, Jokela K. 2007. Evaluation of a single‐monochromator diode array spectroradiometer for sunbed UV‐radiation measurements. Photochemistry and Photobiology 81: 333–341. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Zhou L, Wang Y, Chi Y. 2021. Nitrogen allocation regulates the relationship between maximum carboxylation rate and chlorophyll content along the vertical gradient of subtropical forest canopy. Agricultural and Forest Meteorology 307: 108512. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Map of the provenance trial plot.
Fig. S2 Example curve fitting for photosynthesis and stomatal conductance.
Fig. S3 Photosynthetic light response curves from gas‐exchange on beech leaves at the top and bottom of the canopy.
Table S1 Information on the four beech provenance in the trial plot.
Table S2 Dataset used for statistical analysis.
Table S3 Leaf‐mass per area and chlorophyll content per leaf area with regard to canopy depth in three provenances of beech trees grown in Helsinki, Finland.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article. More information is available upon request.


