Summary
Lumbar epidural is the gold standard for labour analgesia. Low concentrations of local anaesthetic are recommended. This network meta‐analysis investigated whether further reducing the concentration of local anaesthetic can improve maternal and neonatal outcomes without compromising analgesia. We conducted a systematic search of relevant databases for randomised controlled trials comparing high (>0.1%), low (>0.08% to ≤0.1%) or ultra‐low (≤0.08%) concentration local anaesthetic (bupivacaine or equivalent) for labour epidural. Outcomes included mode of delivery, duration of labour and maternal/neonatal outcomes. Bayesian network meta‐analysis with random‐effects modelling was used to calculate odds ratios or weighted mean differences and 95% credible intervals. A total of 32 studies met inclusion criteria (3665 women). The total dose of local anaesthetic received increased as the concentration increased; ultra‐low compared with low (weighted mean difference −14.96 mg, 95% credible interval [−28.38 to −1.00]) and low compared with high groups (weighted mean difference −14.99 [−28.79 to −2.04]), though there was no difference in the number of rescue top‐ups administered between the groups. Compared with high concentration, ultra‐low concentration local anaesthetic was associated with increased likelihood of spontaneous vaginal delivery (OR 1.46 [1.18 to 1.86]), reduced motor block (Bromage score >0; OR 0.32 [0.18 to 0.54]) and reduced duration of second stage of labour (weighted mean difference −13.02 min [−21.54 to −4.77]). Compared with low, ultra‐low concentration local anaesthetic had similar estimates for duration of second stage of labour (weighted mean difference −1.92 min [−14.35 to 10.20]); spontaneous vaginal delivery (OR 1.07 [0.75 to 1.56]; assisted vaginal delivery (OR 1.35 [0.75 to 2.26]); caesarean section (OR 0.76 [0.49 to 1.22]); pain (scale 1–100, weighted mean difference −5.44 [−16.75 to 5.93]); and maternal satisfaction. Although a lower risk of an Apgar score < 7 at 1 min (OR 0.43 [0.15 to 0.79]) was reported for ultra‐low compared with low concentration, this was not sustained at 5 min (OR 0.12 [0.00 to 2.10]). Ultra‐low concentration local anaesthetic for labour epidural achieves similar or better maternal and neonatal outcomes as low and high concentration, but with reduced local anaesthetic consumption.
Keywords: Bayesian, epidural, labour analgesia, local anaesthetic, maternal outcomes, meta‐analysis, obstetric outcomes
Introduction
Lumbar epidural has been used for labour analgesia since the 1930s and remains the gold standard. Recent estimates for 13 high‐income countries suggest that epidurals are used in 10–64% of births, with use continuing to rise [1]. Epidurals have been associated with prolonged labour and increased rates of operative/instrumental delivery; however, it is unclear if these effects are causative or related to an increased analgesic requirement for more difficult labour [2]. The James Lind Alliance identified the influence of epidural on the progress and outcome of labour and the minimisation of adverse effects as a research priority [3].
The use of high concentrations of local anaesthetic for labour epidural has fallen out of favour, and 0.1% bupivacaine or levobupivacaine are most commonly used in the UK [4]. Meta‐analysis supports the association of low concentrations (≤0.1% bupivacaine or equivalent) with reduced rates of assisted vaginal delivery compared with higher local anaesthetic concentrations [5]. Worldwide, there is no universally agreed standard technique and many randomised controlled studies use further reduced concentrations of local anaesthetic (< 0.1% bupivacaine), though any additional benefit of this remains unclear.
This network meta‐analysis combines the evidence for three different local anaesthetic concentrations, ultra‐low (≤0.08% bupivacaine or equivalent), low (>0.08% to ≤0.1%) and high (>0.1%) to explore whether further reducing local anaesthetic concentration can improve outcomes for mother and baby, while maintaining satisfactory analgesia.
Methods
A literature search was conducted using the following databases: MEDLINE Ovid; Embase Ovid; CINAHL EBSCO; Cochrane Central Register of Controlled Trials; and US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) from date of inception to 5 October 2020 with repetition on 11 October 2021. We included randomised and quasi‐randomised controlled trials (by month of delivery) comparing ultra‐low and/or low and/or high concentrations of bupivacaine, levobupivacaine or ropivacaine, and at least one obstetric, maternal, neonatal or early childhood outcome, in both primiparous and parous women. Full details of the search methodology, classification of local anaesthetic doses, outcomes assessed and statistical methodology are provided in online Supporting Information, Appendix S1, Tables S1 and S2. Excluded studies are listed in online Supporting Information, Appendix S2.
Variables were extracted as counts for binary data and as mean and standard deviations for continuous variables. Where mean and standard deviations were not available, they were estimated from median, range, IQR and CI [6, 7]. Data presented in graphical form were extracted using the metaDigitise software on R studio (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria) where possible. Results are present in accordance with Bayesian Analysis Reporting Guidelines [8].
Results
Thirty‐two studies met inclusion criteria (3665 women, Fig. 1). Of these, 1578 women received high concentration, 746 received low concentration and 1341 received ultra‐low concentration (Fig. 2). The primary outcome of mode of delivery was included in all studies. Not all papers provided data for all of the secondary outcomes. A summary of study characteristics is included in online Supporting Information, Table S3. Twenty‐four studies looked at bupivacaine, seven at ropivacaine and three at levobupivacaine. One study by Baliuliene et al. [9] had two groups comparing bupivacaine and levobupivacaine, which were counted as separate trials. The addition of opioids varied between different local anaesthetic concentrations. Opioids were included in the epidural infusion in all study groups investigating low concentration (8 out of 8 studies), in 27 out of 30 study groups investigating ultra‐low concentration and in 16 out of 30 study groups investigating high concentration. Fourteen studies included only primiparous participants, 11 included patients of mixed parity and 7 did not specify (online Supporting Information, Table S3). Risk of bias assessment [10] is presented in online Supporting Information, Fig. S1. Thirteen studies were assessed as being low risk of bias, 9 moderate risk and 10 at high or unclear risk of bias. Results for all outcomes are shown in Table 1 and Fig. 3.
Figure 1.
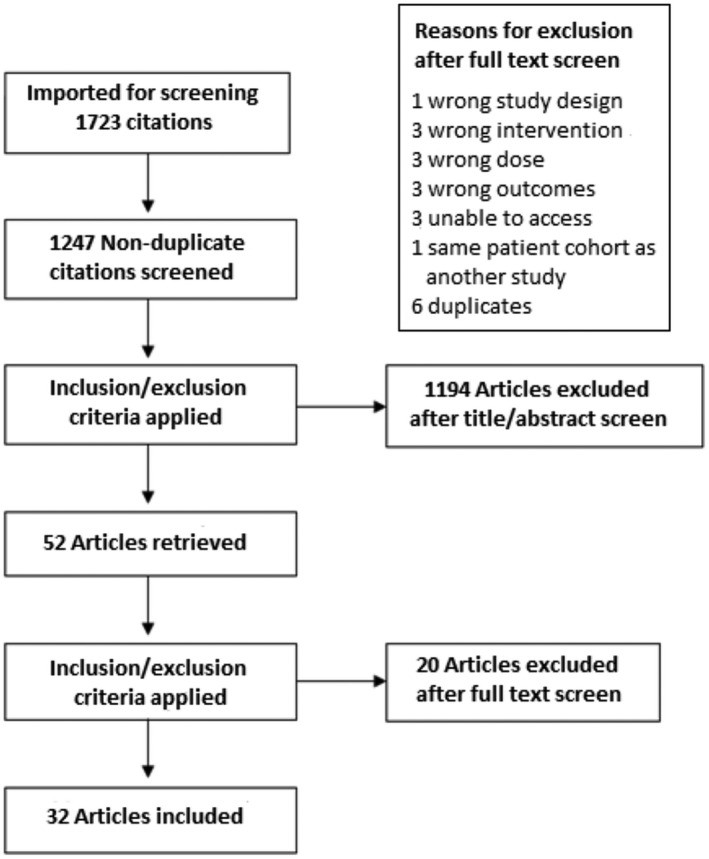
PRISMA flow chart for study selection.
Figure 2.

Network comparisons for assisted vaginal delivery. Edges are weighted according to the number of studies included in each comparison. Six compared ultra‐low concentration with low concentration, 27 compared ultra‐low concentration with high concentration and 5 compared low concentration with high concentration. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 1.
Odds ratios/weighted mean difference and 95% credible intervals for all outcomes.
| Outcome | Total studies | High: low OR [95% credible interval] | High: ultra‐low OR [95% credible interval] | Low: ultra‐low OR [95% credible interval] |
|---|---|---|---|---|
| Spontaneous vaginal delivery | 32 (n = 3665) | 1.36 [0.97 to 1.94] | 1.46 [1.18 to 1.86] | 1.07 [0.75 to 1.56] |
| Assisted vaginal delivery | 32 (n = 3665) | 0.71 [0.43 to 1.25] | 0.87 [0.64 to 1.16] | 1.23 [0.68 to 2.04] |
| Caesarean section | 32 (n = 3665) | 1.03 [0.65 to 1.57] | 0.78 [0.58 to 1.05] | 0.76 [0.49 to 1.22] |
| Top‐up dose required | 16 (n = 1494) | 1.15 [0.31 to 4.35] | 1.27 [0.75 to 2.16] | 1.10 [0.30 to 4.04] |
| Pruritus | 20 (n = 2048) | 4.13 [0.94 to 20.4] | 5.55 [2.18 to 16.3] | 1.35 [0.31 to 5.74] |
| Nausea and vomiting | 19 studies (n = 1912) | 1.09 [0.51 to 2.18] | 1.30 [0.85 to 2.08] | 1.20 [0.63 to 2.47] |
| Hypotension | 20 (n = 1584) | 0.85 [0.02 to 29.89] | 1.08 [0.36 to 2.95] | 1.28 [0.04 to 40.13] |
| Urinary retention | 10 (n = 1078) | 1.06 [0.24 to 4.59] | 0.83 [0.29 to 2.10] | 0.78 [0.18 to 3.04] |
| Bromage score >0 | 27 (n = 2529) | 0.72 [0.26 to 2.05] | 0.32 [0.18 to 0.54] | 0.44 [0.16 to 1.17] |
| Apgar score <7 at 1 min | 18 (n = 2315) | 2.00 [1.16 to 3.81] | 0.85 [0.55 to 1.27] | 0.43 [0.21 to 0.79] |
| Apgar score <7 at 5 min | 19 (n = 2428) | 3.05 [0.17 to 243.69] | 0.35 [0.02 to 4.49] | 0.11 [0 to 2.26] |
| Outcome | Total studies | High: low weighted mean difference [95% credible interval] | High: ultra‐low weighted mean difference [95% credible interval] | Low: ultra‐low weighted mean difference [95% credible interval] |
|---|---|---|---|---|
| Duration first stage of labour | 14 (n = 2319) | 39.96 [10.84 to 70.58] | 3.50 [−15.33 to 27.88] | −36.15 [−63.23 to −8.52] |
| Duration second stage of labour | 17 (n = 2559) | −11.14 [−23.45 to 0.97] | −13.02 [−21.54 to −4.77] | −1.92 [−14.35 to 10.20] |
| Total dose local anaesthetic | 23 (n = 2825) | −14.99 [−28.79 to −2.04] | −30.10 [−38.21 to −22.20] | −14.96 [−28.38 to −1.00] |
| Visual analogue score at 30 min (scale 1:100) | 8 (n = 687) | 8.68 [−2.34 to 20.03] | 3.09 [−0.10 to 7.69] | −5.44 [−16.75 to 5.93] |
| Visual analogue score at 60 min (scale 1:100) | 9 (n = 940) | 4.49 [−2.43 to 12.07] | 3.16 [−1.10 to 7.21] | −1.36 [−9.00 to 5.54] |
| Maternal satisfaction (scale 1:100) | 6 (n = 527) | 4.45 [−4.87 to 13.54] | −0.65 [−7.14 to 6.31] | −5.04 [−13.11 to 3.93] |
| Umbilical artery pH | 4 (n = 590) | 0.01 [−0.03 to 0.06] |
Figure 3.

Box and whisker plot for (a) binary and (b) continuous outcomes. Low concentration is the reference concentration to which high and ultra‐low concentrations are compared. The box represents the IQR and the whiskers represent the 95% credible intervals. The credible intervals for hypotension have been truncated to fit (see Table 1 for upper limits of credible intervals). AVD, assisted vaginal delivery; SVD, spontaneous vaginal delivery.
Spontaneous vaginal delivery
Thirty‐two studies (n = 3665) reported mode of delivery as an outcome measure. Out of these, 6 compared ultra‐low with low concentration, 27 compared ultra‐low with high concentration and 5 compared low with high concentration (Fig. 3). The rates of spontaneous vaginal delivery were greater with ultra‐low compared with high concentration (median OR 1.46, 95% credible interval [1.18 to 1.86]). While the comparisons of ultra‐low vs. low concentration and low vs. high concentration did not reach statistical significance (Table 1, Fig. 2), using the results of the Bayesian analysis, we can infer that the estimated probability of low concentration increasing the incidence of spontaneous vaginal delivery compared with high concentration is 96%. Furthermore, there is a 65.5% probability that ultra‐low concentration increases the chance of a spontaneous vaginal delivery compared with low concentration.
Assisted vaginal delivery
There was no significant difference in the incidence of assisted vaginal delivery between the three groups using the 95% credible interval threshold (Table 1, Fig. 2). The estimated probability that low concentration reduces the incidence of assisted vaginal delivery compared with high concentration is 95.5% (favouring low concentration), and that ultra‐low concentration reduces the incidence of assisted vaginal delivery compared with high concentration is 88.5%. On comparison of ultra‐low and low concentration, the estimated probability of ultra‐low concentration reducing assisted vaginal delivery is 22%.
Funnel plots were created for the incidence of assisted vaginal delivery for each of the three pairwise comparisons of local anaesthetic concentration (online Supporting Information, Fig. S2). These plots appeared symmetrical, suggesting minimal publication bias. Frequentist pairwise analyses using only direct evidence showed similar results to the main analysis (online Supporting Information, Fig. S3).
Caesarean section
No statistically significant difference was detected between ultra‐low, low and high concentration for caesarean section (Table 1; Fig. 2). From Bayesian analysis, the estimated probability that low concentration decreases the incidence of caesarean section compared with high concentration is 45%, and for ultra‐low to high concentration is 96%. The estimated probability that ultra‐low decreases the incidence of caesarean section compared with low concentration is 88.5%.
Mode of delivery limited to papers published in the last 10 years
Five studies were published between 2011 and 2021 (511 women) [9, 11, 12, 13, 14]. A total of 147 women received high concentration, 194 received low concentration and 170 received ultra‐low concentration. Results for spontaneous vaginal delivery and caesarean section were similar to those in the main analysis, but with wider credible intervals. For assisted vaginal delivery, the credible intervals were wide and no further conclusions can be drawn (online Supporting Information, Table S4).
Total local anaesthetic dose
This outcome was reported in 23 studies (2825 parturients). The total dose of local anaesthetic was significantly lower for both low and ultra‐low compared with high concentration. Ultra‐low total dose was significantly lower than low concentration (weighted mean difference −14.96 mg, 95% credible interval [−28.38, −1.00]). There were no significant differences in the number of rescue top‐ups required between low and ultra‐low concentration groups (Table 1; Fig. 2).
Maternal outcomes
Fourteen studies (n = 2319) reported results for duration of the first stage of labour. This was significantly reduced in ultra‐low compared with low concentration groups (weighted mean difference −36.15 min, 95% credible interval [−63.23 to −8.52]), though was increased in low compared with high concentration groups (weighted mean difference 39.96 min [10.84 to 70.58]) (Table 1; Fig. 2). There was no difference between ultra‐low and high concentration.
Duration of the second stage of labour (17 studies, n = 2559) was decreased in ultra‐low compared with high concentration (weighted mean difference −13.02 min [−21.54 to −4.47]) but did not reach significance between low and high, nor low and ultra‐low concentration (Table 1; Fig. 2).
The 30‐ or 60‐min VAS pain scores and maternal satisfaction scores were similar between the three groups. There were no significant differences in the rates of pruritus, nausea and vomiting, urinary retention or hypotension (Table 1; Fig. 2). Parturients receiving ultra‐low concentration were significantly less likely to have a Bromage score >0 compared with high concentration (OR 0.32, 95% credible interval [0.18, 0.54]) but there was no difference between low and ultra‐low concentration (Table 1; Fig. 2). No studies reported second or third‐degree tears or post‐partum haemorrhage.
Neonatal outcomes
Four studies (n = 823) reported need for ‘high‐level’ neonatal resuscitation (defined as one or more of bag/mask ventilation, intubation or administration of naloxone) as an outcome measure. Of these, three studies reported no requirement for neonatal resuscitation for either group and one study [15] reported ‘any requirement’ for neonatal resuscitation (no specific definition). The rates of ‘any neonatal resuscitation’ did not differ between low and high concentration. Low concentration was associated with an increased rate of ‘high‐level’ neonatal resuscitation compared with high concentration (5% vs. 1%, p = 0.02). The Comparative Obstetric Mobile Epidural Trial (COMET) group was the only trial to report on the rates of admission to neonatal intensive care, these did not differ significantly between low and high concentration (28% vs. 25%, p = 0.4) [15].
Apgar score < 7 at 1 min was reported in 18 trials (Fig. 2). Neonates in the low concentration group had a significantly higher risk of having an Apgar score <7 compared with the high concentration group (OR 2.00, 95% credible interval [1.16 to 3.81]). There was no difference between ultra‐low and high concentration (Table 1; Fig. 2). Compared with low concentration, neonates in the ultra‐low group were significantly less likely to have an Apgar score <7 at 1 min (OR 0.43 [0.21 to 0.79]). We repeated the analysis after removing results from the COMET study [15]. This was the largest study included in the analysis and its findings of a less favourable Apgar at 1 min in the low concentration group were highly influential in the overall meta‐analysis of this outcome. However, this finding persisted when the COMET study was excluded from the analysis (OR 0.34 [0.10 to 0.98]).
An Apgar score < 7 at 5 min was reported in 19 trials (Fig. 2). There were no significant differences between the three local anaesthetic concentrations for the incidence of low Apgar score at 5 min.
Umbilical artery pH was not recorded in any of the low concentration studies. Between ultra‐low and high concentration, it was recorded in four studies and there were no significant differences. Breastfeeding at 6 weeks was only recorded in the COMET trial, where it did not find significant differences between the control and low concentration epidural groups. No studies reported the rates of breastfeeding within 24 h. No studies reported outcomes related to early childhood development.
Discussion
This meta‐analysis found that ultra‐low concentration local anaesthetic is associated with reduced total local anaesthetic dose, shorter first stage of labour and reduced incidence of Apgar < 7 at 1 min compared with low concentration, without compromising maternal analgesia, side‐effect profile, satisfaction or neonatal outcomes. Both ultra‐low and low concentration appear to increase the chance of a spontaneous vaginal delivery compared with high concentration and there is some evidence that ultra‐low concentration may increase the likelihood of spontaneous vaginal delivery and reduce the incidence of caesarean section (though not assisted vaginal delivery) compared with low concentration. High concentration offered no advantages compared with ultra‐low concentration local anaesthetic.
Using Bayesian analysis, we demonstrated a 65.5% probability that the rates of spontaneous vaginal delivery were increased with ultra‐low compared with low concentration. Consistent with this finding, the estimated probability that ultra‐low compared with low concentration decreases the incidence of assisted vaginal delivery and caesarean section is 22% and 88.5%, respectively. Although these results do not meet the predetermined cut‐off level of 95% specified in our methods section, they may represent a potential benefit for further reducing local anaesthetic concentrations from low to ultra‐low concentration to reduce the rates of caesarean section and increase the rate of spontaneous vaginal delivery. The benefit of a Bayesian analysis is that we can directly present these probabilities, allowing individual clinicians to interpret them in the wider clinical context and deciding whether 65.5% or 88.5% probabilities represent a potential benefit for clinical practice.
We are not aware of any meta‐analyses which have compared low and ultra‐low concentration local anaesthetic for maternal and neonatal outcomes. Two meta‐analyses have examined epidural vs. non‐epidural analgesia for mode of delivery, finding no difference in assisted vaginal delivery (when studies after 2005 were excluded) or caesarean delivery [2, 16]. A 2013 meta‐analysis (11 studies, 1997 women) comparing high (>0.1% bupivacaine or equivalent ropivacaine) and low concentrations of local anaesthetic (≤0.1% bupivacaine) found increased incidence of assisted vaginal delivery, but not of caesarean section, when high concentrations were compared with low [5]. Collectively, these studies have led to the widespread adoption of low concentration local anaesthetic in epidurals. Our own study reaffirms the benefits of lower concentrations of local anaesthetic vs. high concentration with respect to decreased local anaesthetic consumption, reduced incidence of assisted vaginal delivery, improved chance of spontaneous vaginal delivery and generally more favourable maternal and neonatal outcomes. The current analysis was designed to provide further evidence regarding the comparison of high and low concentrations, but more importantly to specifically compare any additional benefits of ultra‐low to low concentration.
Ultra‐low concentration was associated with a significantly lower total dose of local anaesthetic than low concentration. This may be associated with potential benefits for the parturient including decreased motor block, which may be more comfortable for the patient, and a reduced risk of local anaesthetic toxicity. In addition, high doses of local anaesthetic are more likely to block the autonomic nerves involved in uterine contraction. This could explain the significant increase in duration of the first stage of labour observed in low compared with ultra‐low concentration, though may also reflect the lack of a clearly defined starting point for the first stage of labour. The first stage of labour can be divided into a latent first stage (irregular contractions with cervix long and <4 cm dilated) and the active first stage (cervix >4 cm dilated and regular contractions). None of the papers clarify a definition for the starting point of labour, so this result may reflect heterogeneity of outcome reporting. Data on duration of epidural analgesia were not available but should be considered for inclusion in the future studies.
It is reassuring that there does not appear to be any significant differences in neonatal outcomes, with the exception of 1‐min Apgar score < 7. A 5‐min Apgar score <7 is more predictive of poor neonatal outcomes than 1‐min scores [17, 18], and there were no significant differences in the rate of 5‐min Apgar score <7 nor umbilical cord pH between the different local anaesthetic concentrations. Patients in the low concentration group were around twice as likely to have a 1‐min Apgar score < 7 compared with high and ultra‐low concentration groups. The authors of the COMET study suggested this phenomenon may reflect an increased use of epidural opioids in the low concentration group; however, a meta‐analysis looking at the effects of epidural or spinal opioids for labour analgesia (21 randomised controlled trials, 2859 participants) found no difference in Apgar score at 1 or 5 min compared with those not receiving neuraxial opioids [19]. Furthermore, patients receiving ultra‐low concentration would arguably be as likely to receive epidural opioids as those in the low concentration group. When the COMET study was excluded from our analysis, ultra‐low concentration was still significantly less likely to be associated with 1‐min Apgar score <7 than low concentration. Unlike 5‐min Apgar score, a 1‐min Apgar score < 7 is not associated with poorer long‐term developmental outcomes [20]. None of the included studies reported any childhood developmental outcome data.
We performed a sub‐group analysis to evaluate more contemporary clinical practice, despite this being limited to only five studies (511 women). Results for spontaneous vaginal delivery and caesarean section were similar to those in the main analysis. Both obstetric and anaesthetic practice has changed during this time, with rates of assisted vaginal delivery falling [21] and caesarean section rates increasing 4% per year worldwide [22]. Evidence is accumulating supporting the use of intermittent bolus and patient‐controlled epidural analgesia rather than continuous epidural regimes to reduce the total dose of local anaesthetic given [23, 24]. Reducing the concentration of local anaesthetic is a straightforward intervention that would be easy to implement. A large multicentre randomised controlled trial comparing low and ultra‐low concentration local anaesthetics could potentially further clarify any benefits of lowering the concentration of local anaesthetic in labour epidurals.
Our study has several strengths, including a systematic search strategy, a robust Bayesian network meta‐analysis incorporating 32 studies and the use of Bayesian inference to calculate the probabilities of attaining a spontaneous vaginal delivery or requiring operative delivery for each local anaesthetic group. We acknowledge several weaknesses, including the heterogeneity of the underlying studies, the variable background rates of operative delivery reflecting the different geographical settings and local clinical practices. Local anaesthetic concentrations were not the sole intervention as adjuvant drugs such as adrenaline or opioids were frequently used. This reflects current practice where most institutions routinely use opioids as part of epidural solutions. In some of the included studies, the addition of opioids varied between different local anaesthetic concentrations with ultra‐low (27 of 30 studies) and low concentration (8 of 8 studies) more likely to be combined with an opioid than high concentration solutions (16 of 30 studies). However, we did not find any significant differences between these, other than Apgar score at 1 min as described above and increased local anaesthetic concentration being associated with higher Bromage score, which is more plausibly explained by the increased local anaesthetic concentration. Reducing the concentration of local anaesthetic may increase requirements for epidural opioids. The threshold local anaesthetic concentrations requiring co‐administration with epidural opioids is unknown and this may be a key area for future work. All methods of epidural maintenance were included in this meta‐analysis. This may influence our results as there is evidence that women require lower total doses of local anaesthetic when using patient‐controlled epidural analgesia, or intermittent bolus rather than continuous infusions [23, 24]. However, only two of the studies used different methods of administration of epidural maintenance for different groups of the trial meaning that the variable being assessed was the local anaesthetic concentration rather than the method of administration [14, 15]. Parity may influence the local anaesthetic requirement of labouring women. In 14 out of the 32 studies, only primiparous participants were included, 11 studies included patients of mixed parity and 7 did not specify. Since there was no difference in the inclusion criteria between the groups of differing concentration, and given that participants were randomised, this is unlikely to have significantly affected the results. Finally, the decisions on whether or not to intervene by the obstetrician were not standardised. Again, this is unlikely to significantly affect our results as this would be common to all groups of study. Differences in co‐administration of opioids, method of local anaesthetic administration and obstetric practice between studies remain a limitation of this meta‐analysis and further make the case for a randomised controlled trial of low vs. ultra‐low concentration local anaesthetic for labour epidural.
In conclusion, our network meta‐analysis has found that ultra‐low concentration local anaesthetic for labour epidural achieves similar or better maternal and neonatal outcomes as low and high concentrations of local anaesthetic, but with reduced local anaesthetic consumption. This information may be used to aid clinician decision‐making towards further optimising epidural local anaesthetic regimes in labour. A randomised controlled trial comparing low and ultra‐low concentration local anaesthetic for labour epidural is warranted.
Supporting information
Appendix S1. Search methodology.
Appendix S2. Excluded studies.
Table S1. Search strategy for Ovid MEDLINE®.
Table S2. Inclusion and exclusion criteria.
Table S3. Characteristics of included studies.
Table S4. Subgroup analysis for mode of delivery of papers published in 2011‐2021.
Figure S1. Risk of bias assessment.
Figure S2. Funnel plots to assess for publication bias.
Figure S3. Forest plots for rate of assisted vaginal delivery.
Acknowledgements
The study protocol was registered on PROSPERO (CRD42020210878). RK was supported via grant funding from the Obstetric Anaesthetists' Association and the Scottish Society of Anaesthetists and by an NHS Research Scotland Career Researcher Fellowship. The views expressed in this publication are those of the author(s) and not necessarily those of the UK National Health Service, the National Institute for Health Research, or the UK Department of Health and Social Care or any other funders mentioned here. SN has participated in Advisory Boards and received consultancy or speakers' fees from Access Fertility, Beckman Coulter, Ferring, Finox, Merck, Modern Fertility, MSD, Roche Diagnostics and The Fertility Partnership. No other competing interests declared.
Contributor Information
L. Halliday, Email: lucy.halliday@glasgow.ac.uk, @LucyHalliday7.
M. Kinsella, @mkinsella462.
S. M. Nelson, @profscottnelson.
R. J. Kearns, rjharrison79.
References
- 1. Seijmonsbergen‐Schermers AE, van den Akker T, Rydahl E, et al. Variations in use of childbirth interventions in 13 high‐income countries: a multinational cross‐sectional study. PLoS Medicine 2020; 17: e1003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anim‐Somuah MSR, Cyna AM, Cuthbert A. Epidural versus non‐epidural or no analgesia for pain management in labour. Cochrane Database of Systematic Reviews 2018; 5: CD000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boney O, Bell M, Bell N, et al. Identifying research priorities in anaesthesia and perioperative care: final report of the joint National Institute of Academic Anaesthesia/James Lind Alliance Research Priority Setting Partnership. British Medical Journal Open 2015; 5: e010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vedagiri Sai R, Rappai G, Johnstone C. Survey of obstetric epidural anaesthetic practises in Scotland. Abstract presented at the Obstetric Anaesthetists' Association Annual Meeting, Bournemouth, 2013: 43. https://www.oaa-anaes.ac.uk/assets/_managed/editor/File/Courses/2013/13.92%20non-IJOA%20posters.pdf (accessed 27/04/2022).
- 5. Sultan P, Murphy C, Halpern S, Carvalho B. The effect of low concentrations versus high concentrations of local anesthetics for labour analgesia on obstetric and anesthetic outcomes: a meta‐analysis. Canadian Journal of Anesthesia 2013; 60: 840–54. [DOI] [PubMed] [Google Scholar]
- 6. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kruschke JK. Bayesian analysis reporting guidelines. Nature Human Behaviour 2021; 5: 1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baliuliene V, Macas A, Rimaitis K. The optimal concentration of bupivacaine and levobupivacaine for labor pain management using patient‐controlled epidural analgesia: a double‐blind, randomized controlled trial. International Journal of Obstetric Anesthesia 2018; 35: 17–25. [DOI] [PubMed] [Google Scholar]
- 10. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. British Medical Journal 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 11. Çakırca M, Bektaş M, Özcan A, Doğulu F. Comparison of two different bupivacaine doses with sufentanil for epidural obstetric analgesia. Turkiye Klinikleri Tip Bilimleri Dergisi 2013; 33: 516–21. [Google Scholar]
- 12. El‐Shaarawy AM, Asfour MS, Rashwan DA, Amer MM, El‐Menshawe SF, Elkomy MH. Comparison of three different concentrations of levobupivacaine for epidural labor analgesia: clinical effect and pharmacokinetic profile. Anesthesia, Essays and Researches 2018; 12: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ayşegül K, Ari ED, Firdevs O, Ayhan C, Fatma NA. The comparison of 0.125% bupivacaine+2 mcg/ml fentanyl and 0.0625% bupivacaine+2 mcg/ml fentanyl in patient controlled epidural analgesia during labor. Journal of Clinical Anaesthesia and Management 2016; 1: 1–6. [Google Scholar]
- 14. Nunes J, Nunes S, Veiga M, Cortez M, Seifert I. A prospective, randomized, blinded‐endpoint, controlled study ‐ continuous epidural infusion versus programmed intermittent epidural bolus in labor analgesia. Revista Brasileira de Anestesiologia 2016; 66: 439–44. [DOI] [PubMed] [Google Scholar]
- 15. Comparative Obstetric Mobile Epidural Trial (COMET) Study Group UK . Effect of low‐dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial. Lancet 2001; 358: 19–23. [DOI] [PubMed] [Google Scholar]
- 16. Wang T‐T, Sun S, Huang S‐Q. Effects of epidural labor analgesia with low concentrations of local anesthetics on obstetric outcomes: a systematic review and meta‐analysis of randomized controlled trials. Anesthesia and Analgesia 2017; 124: 1571–80. [DOI] [PubMed] [Google Scholar]
- 17. Iliodromiti S, Mackay DF, Smith GC, Pell JP, Nelson SM. Apgar score and the risk of cause‐specific infant mortality: a population‐based cohort study. Lancet 2014; 384: 1749–55. [DOI] [PubMed] [Google Scholar]
- 18. Cnattingius S, Johansson S, Razaz N. Apgar score and risk of neonatal death among preterm infants. New England Journal of Medicine 2020; 383: 49–57. [DOI] [PubMed] [Google Scholar]
- 19. Wang K, Cao L, Deng Q, et al. The effects of epidural/spinal opioids in labour analgesia on neonatal outcomes: a meta‐analysis of randomized controlled trials. Canadian Journal of Anesthesia 2014; 61: 695–709. [DOI] [PubMed] [Google Scholar]
- 20. Razaz N, Cnattingius S, Persson M, Tedroff K, Lisonkova S, Joseph KS. One‐minute and five‐minute Apgar scores and child developmental health at 5 years of age: a population‐based cohort study in British Columbia, Canada. British Medical Journal Open 2019; 9: e027655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nolens B, Capelle M, van Roosmalen J, et al. Use of assisted vaginal birth to reduce unnecessary caesarean sections and improve maternal and perinatal outcomes. Lancet Global Health 2019; 7: e408–e9. [DOI] [PubMed] [Google Scholar]
- 22. The Lancet . Stemming the global caesarean section epidemic. Lancet 2018; 392: 1279. [DOI] [PubMed] [Google Scholar]
- 23. Liu XZH, Zhang H, Guo M, Gao Y, Du C. Intermittent epidural bolus versus continuous epidural infusions for labor analgesia: a meta‐analysis of randomized controlled trials. PLoS One 2020; 15: e0234353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Vyver M, Halpern S, Joseph G. Patient‐controlled epidural analgesia versus continuous infusion for labour analgesia: a meta‐analysis. British Journal of Anaesthesia 2002; 89: 459–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search methodology.
Appendix S2. Excluded studies.
Table S1. Search strategy for Ovid MEDLINE®.
Table S2. Inclusion and exclusion criteria.
Table S3. Characteristics of included studies.
Table S4. Subgroup analysis for mode of delivery of papers published in 2011‐2021.
Figure S1. Risk of bias assessment.
Figure S2. Funnel plots to assess for publication bias.
Figure S3. Forest plots for rate of assisted vaginal delivery.


