Abstract
The therapeutic potential of the tocotrienol group stems from its nutraceutical properties as a dietary supplement. It is largely considered to be safe when consumed at low doses for attenuating pathophysiology as shown by animal models, in vitro assays, and ongoing human trials. Medical researchers and the allied sciences have experimented with tocotrienols for many decades, but its therapeutic potential was limited to adjuvant or concurrent treatment regimens. Recent studies have focused on targeted drug delivery by enhancing the bioavailability through carriers, self‐sustained emulsions, nanoparticles, and ethosomes. Epigenetic modulation and computer remodeling are other means that will help increase chemosensitivity. This review will focus on the systemic intracellular anti‐cancer, antioxidant, and anti‐inflammatory mechanisms that are stimulated and/or regulated by tocotrienols while highlighting its potent therapeutic properties in a diverse group of clinical diseases.
Keywords: antioxidant behavior, cancer studies, chemotherapy, disease pathology, tocotrienol in cancer immunology, tocotrienols, vitamin E
Abbreviations
- 5‐FU
5‐fluorouracil
- ABC transporters
ATP‐cassette binding protein family
- ADCY1
adenylyl cyclase type 1.
- AKI
acute kidney injury
- Akt
protein kinase‐B
- alpha TTP
alpha‐tocopherol transfer protein
- AMPK
adenosine mono phosphate kinase
- Ang‐1
angiopoietin‐1
- ATF3
activating transcription factor‐3
- Bcl‐2
B‐cell lymphoma 2
- Bcl‐xL
B‐cell lymphoma‐extra‐large
- BMDM
bone marrow‐derived macrophages
- BSG
basigin‐encoding plasma membrane protein in spermatogenesis
- CAC
colitis‐associated colorectal cancer
- CD3‐epsilon
cluster of differentiation 3‐epsilon
- CDK 1, CDK2, CDK4, CDK6
cyclin‐dependent kinase 1, 2, 4, and 6
- cDNA
complimentary deoxyribonucleic acid
- CFLAR
apoptosis regulator
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disorder
- COX‐2
cyclooxygenase‐2
- CSC
cancer stem cells
- CVD
cardiovascular disease
- DAMP
damage‐associated molecular patterns
- DCs
dendritic cells
- DHA
docosahexaenoic acid
- DKD
diabetic kidney disease
- DR5
death receptor 5
- DSS
dextran sulfate sodium
- EGFR
epidermal growth factor receptor
- EMT
epithelial‐to‐mesenchymal transition
- ER
endoplasmic reticulum
- ERK1 and ERK2
extracellular signal‐regulated protein kinase 1 and 2
- FasL
Fas ligand
- FGF18
fibroblast growth factor 18
- FLICE
Fas‐associated death domain‐like IL‐1‐converting enzyme
- G6PD
glucose‐6‐phosphate dehydrogenase
- G‐CSF
granulocyte colony‐stimulating factor
- GnRH
gonadotropin‐releasing hormone
- GSSG
glutathione disulfide
- HCC
hepatocarcinoma cell line
- HDL
high‐density lipoprotein
- HIF‐1 α
hypoxia‐inducible 1‐alpha
- HMG‐CoA
3‐hydroxy 3‐methyl glutaryl coenzyme A
- HPLC
high‐pressure liquid chromatography
- IDL
intermediate‐low‐density lipoprotein
- IL‐6
interleukin‐6
- iNOS
inducible nitric oxide synthase
- Insigs
insulin‐induced genes
- Jak/stat‐3/6
Janus kinase/signal transducer and activator of transcription proteins‐induce cellular senescence
- JNK
c‐Jun N‐terminal kinase
- K562 cells
chronic myeloid leukemia
- LDL
low‐density lipoprotein
- LH
luteinising hormone
- LPS
lipopolysaccharide
- MAPK
mitogen‐activated protein kinase
- MCF‐7
Michigan Cancer Foundation‐7
- MDA
malondialdehyde
- MDA‐MB‐231
Anderson‐metastatic breast 231
- MDR‐1
multidrug‐resistant protein‐1
- MDRSM
mixture design response surface methodology
- miR
microribonucleic acid
- MMP‐2 and MMP‐9
matrix metalloproteinase‐2 and ‐9
- mTOR
mammalian target of rapamycin
- NF‐kB/p65
nuclear factor kappa beta/protein 65
- NKX3‐1
androgen‐regulated homeobox gene‐1
- NLRP3
nod‐like receptor protein‐3
- Nox‐2
NADPH oxidase
- Nrf2
nuclear factor‐erythroid factor 2‐related factor 2
- NSCLC
nonsmall cell lung cancer
- OPG
osteoprotegerin
- p‐eIf2α
translation initiation factor 2
- p16
protein 16
- p21
protein 21
- p27
protein 27
- p38
protein 38
- p53
protein 53
- PARP
poly‐ADP‐ribose polymerase
- PCa
prostate cancer
- PD
Parkinsonian disease
- PERK/eIF2alpha/ATF‐4
activation‐mediated protein kinase‐like ER kinase/eukaryotic translational initiation factor/activating transcription factor 4
- P‐gp
P‐glycoprotein
- PI3K/Akt
phosphoinositide‐3‐kinase/protein kinase B
- p‐JNK
jun N‐terminal kinase
- PSP
polysaccharopeptide
- PUFA
poly unsaturated fatty acids
- RA
rheumatoid arthritis
- Raf–ERK
rapidly accelerated fibrosarcoma–extracellular signal‐regulated kinases
- RANKL
receptor activator of nuclear factor‐kappa‐Β ligand
- Ras
rat sarcoma‐derived guanine nucleotide‐binding protein
- RIPI
receptor‐interacting protein serine/threonine kinase
- ROS
reactive oxygen species
- RSK
ribosomal protein S6 kinase
- SCC
squamous cell carcinoma
- SHP1 and SHP2
Src homology 2 domain‐containing protein tyrosine phosphatase 1 and 2
- siRNA
small interfering ribonucleic acid
- SIRT‐1
sirtuin 1
- SNX9
sorting nexin 9
- SOD
superoxide dismutase
- STAT3
signal transducer and activator of transcription 3
- TAK1
activated kinase 1
- TGF‐β
transforming growth factor‐β
- TGF‐β
transforming growth factor‐beta
- TMP1 and TMP2
tissue metalloproteinase 1 and 2
- TNM
tumor, nodes, and metastases
- TP53
tumor protein 53
- TRAIL
TNF‐alpha‐related apoptosis inducing‐ligand
- TRF
tocotrienol rich fraction
- UACR
urine albumin to creatinine ratio
- uPA
urokinase‐type plasminogen activator
- VEGF
vascular endothelial growth factor
- VLDL
very‐low‐density lipoproteins
- γ‐CEHC
gamma‐carboxyethyl hydroxychromanol
1. INTRODUCTION
1.1. What are tocotrienols?
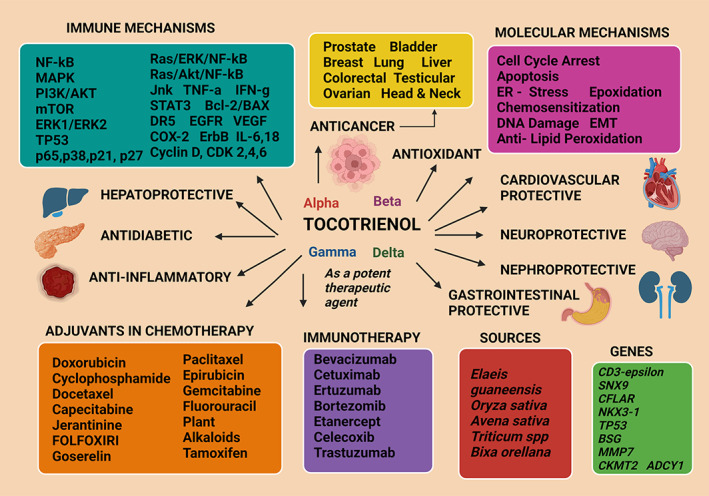
Tocotrienols and tocopherols are two groups of vitamin E 1 which possess considerable therapeutic potential owing mostly to their anticancer, 2 antioxidant, 3 anti‐diabetic, 4 anti‐inflammatory, 5 nephroprotective, 6 neuroprotective, 7 hepatoprotectives, 8 and cardiovascular‐protective properties. 9 These different forms of vitamin E can only be obtained from plants 10 and are known to be the principal fat‐soluble, antioxidants present in the human blood plasma. 11 Thus, they show promise in attenuating a spectrum of human diseases (Figure 1). 12 These are dietary components which can be transported due to lipophilic nature 13 and their nutraceutical value has bestowed a beneficial paradigm of clinical attributes in both animal models and human studies. 14 The use of vitamin E compounds dates to the late 19th century when the tocopherols were first identified as having medicinal effects. 15 Early studies were focused more on tocopherol benefits but the current trend is to evaluate the therapeutic potential of tocotrienols in clinical research, 16 with emphasis on biological functions performed by this group including cellular proliferation, cell differentiation, cellular regeneration, repair mechanisms, apoptosis, and autophagy. 17 Tocotrienols have gained increasing attention with the discovery of being a novel candidate for noncommunicable disease therapy and are evaluated in clinical trials. 18 , 19
FIGURE 1.
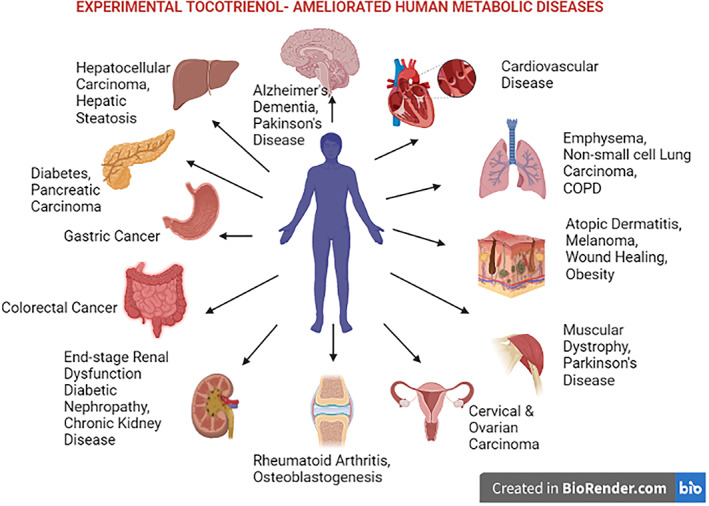
Human diseases attenuated by experimental tocotrienol treatment
1.2. Chemical structure and sources
The chemical structure of vitamin E was elucidated in 1938 by Fenholz. 20 The two main forms of vitamin E, the tocopherols and tocotrienols, comprise four sub‐forms each (named alpha, beta, gamma, and delta) and share structural similarity 21 except for a few differences that account for functional modifications. The tocotrienol sub‐forms offer superior therapeutic potential compared to the tocopherol counterparts. 22 , 23 In addition to the eight different forms of vitamin E that are established, two more variants named des‐methyl and di‐des‐methyl tocotrienols have been added to this vitamer profile. 24 This eight‐member vitamin E family shares a chromanol head having a hydrocarbon tail attached at the 2‐position of the ring. The tocopherol group consists of a saturated, phytyl side‐chain whereas tocotrienol has an unsaturated isoprenoid side chain comprising of three trans‐carbon–carbon double bonds (Figure 2). 25 The four different forms of each differ in their R1 and R2 groups on the chromanol ring substituted with either a methyl group or hydrogen atoms. 26 There is evidence that the tocotrienols have a better ability to diffuse through the phospholipid bilayer of the cell membrane because of their unsaturated isoprenoid tail compared to the phytyl tail of tocopherol. 27 Their lipid‐solubility and hydrophobicity have required membrane contact or specific protein carriers for its cellular transportation which occurs in plasma in association with plasma phospholipid transfer proteins. 28 Out of the eight different forms of vitamin E, alpha‐tocopherol was identified before tocotrienols 29 but with the progress of research, tocotrienols became recognized as superior to RRR‐alpha‐tocopherol in their ability to penetrate the blood–brain barrier and the liver because of their small size and lipophilic nature. 30 The tocotrienol therapeutic efficacy was overshadowed by the tocopherols until recent years during which the tocotrienols were recognized as being more potent in treating a wide range of diseases thus playing a prominent role in health and disease. As such, more preclinical and clinical studies are needed to improve its medicinal properties which are confirmed as being more curative than the tocopherol forms which were thought to be more therapy‐wise superior to the tocotrienols. An array of other different synthetic vitamin E forms exist, such as the tocopherol‐member family that includes the acetate and succinate forms of tocopherols, and the phosphorylated tocopherols in addition to the four principal forms of tocopherols—alpha, beta, gamma, and delta. Early research showed that treatment with alpha‐tocopherol and alpha‐tocopheryl acetate was suppressive on mitomycin‐stimulated—human Tenon's fibroblast proliferation. 31
FIGURE 2.
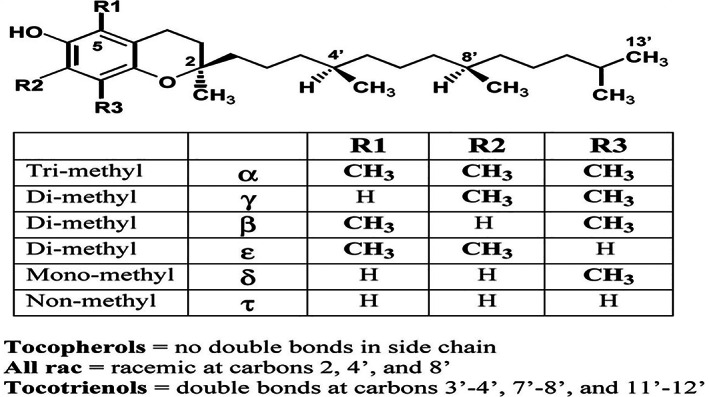
Stereochemical structure of tocotrienols and tocopherols 314
The succinate form of alpha‐tocotrienol was more effective than the aforementioned two vitamin‐E forms in suppressing restimulated fibroblasts via a decrease in glucose‐6‐phosphate dehydrogenase (G6PD) activity and upregulated necrosis. 31 Another vitamin E‐form is tocopheryl phosphate which is involved in gene regulation, inhibition of cell proliferation, and limiting lipid accumulation in cells. 32 An antagonistic effect between alpha‐tocopherol and alpha‐tocopheryl phosphate on the phosphoinositide‐3‐kinase/protein kinase B (PI3K/Akt) pathway was observed and the stimulation of the PI3Kγ/Akt pathway by alpha‐tocopheryl phosphate leads to the induction of vascular endothelial growth factor (VEGF) expression. 32 Additionally, the redox‐inactive tocotrienol (6‐O‐carboxypropyl‐α‐tocotrienol) was tested against malignant mesothelioma, which is an aggressive form of cancer that has poor prognosis, that produced enhanced anti‐cancer effects than with tocotrienol given alone. 33 Further, its oral administration in a xenografted mouse model augmented anti‐tumor effects without the loss of body weight, than the normal tocotrienols administered through the same route. 33 Vitamin E was first identified in 1922 from a green leafy vegetable extract 34 where the ill effects of ingesting rancid fat were thus overcome by consuming it and research which had ensued thereafter had designated it an essential vitamin. 35 Vitamin E is synthesized exclusively by all photosynthetic organisms. The biosynthesis of tocotrienols occurs in the seed endosperm of most monocotyledons, for example, cereal crops such as wheat, barley, and rice, and in some dicotyledons (families Apiaceae and Solanaceae, which includes Tobacco). 36 , 37 Crude palm oil extracts are known to contain up to 800 mg/kg of tocotrienol, obtained particularly from the fruits of Elaeis guineensis, commonly known as the African oil palm or Macaw‐fat, which is the principal source of palm oil. 38 Palm oil is a major source of tocotrienol which is extracted from the palm fruit mesocarp, the endosperm, and kernel oil, and contains up to 30% of tocopherol and 70% of tocotrienol comprising alpha, gamma, and delta forms as the principal constituents). 39 It also consists of 50% saturated fatty acids, 40% of unsaturated fatty acids and 10% of polyunsaturated fatty acids unlike any other edible plant or animal oil 40 and is devoid of trans fatty acids. 41 Gamma‐tocotrienol is the abundant and active natural form of tocotrienol present in rice bran oil (Oryza sativa) which consists of antioxidant activity 42 although in oats (Avena sativa) the active ingredient is alpha‐tocotrienol. 43 Hulled and dehulled wheats (Triticum spp.,) contain beta‐tocotrienol as its natural source of tocotrienol, 44 although it is doubted whether the consumption of these foods provides an adequate supply of tocotrienol that is required to maintain good health in the human populations. The biologically effective doses have been described to be between 100–200 g for palm oil or rice bran oil and 1.5–4 kg are needed from wheat‐germ, barley, or oats where notably a 1000 kg of crude palm oil is needed to derive 1 kg of a commercially produced tocotrienol and tocopherol supplementation, named Tocomin (containing 50% of tocopherols and tocotrienols) which points out that the consumption of commercial preparations are more beneficial than consuming natural sources of tocotrienols. 15 Tocotrienol is also synthesized by annatto (Bixa orellana) seeds 45 and nonfood plants such as rubber (Hevea brasiliensis) and is contained in the rubber latex. 46
1.3. Genetic basis of tocotrienol action
Genetic studies by a clinical trial using peripheral blood mononuclear cells have identified the putative genes that are associated with the tocotrienol therapeutic action. This gene profile consisted of the cluster of differentiation 3‐epsilon (CD3‐epsilon), sorting nexin 9 (SNX9), tumor protein 53 (TP53), androgen‐regulated homeobox gene‐1 (NKX3‐1), apoptosis regulator (CFLAR), basigin‐encoding plasma membrane protein in spermatogenesis (BSG), fibroblast growth factor 18 (FGF18), matrix metalloproteinase‐7 (MMP7), mitochondrial creatine kinase‐2 (CKMT2), and adenylyl cyclase type 1 (ADCY1). 47 When treatment was continued for 3 months with the tocotrienol rich fraction (TRF), it produced the upregulation of the gene expression of; cell division, transcription, G protein‐coupled receptor signaling, multicellular organismal growth, protein kinase activity, cell surface receptor signaling, and response to glucocorticoids with the downregulation of the aging‐related genes. 47 Additionally, the modulation of biological functions through gene expressions has been reported. They included downregulating G‐protein coupled receptor signaling, phosphoinositide, and integrin‐mediated signaling, apoptotic processes, cell–cell signaling, extracellular signal‐regulated protein kinase 1 and 2 (ERK1 and ERK2) signaling cascade, cell surface receptor signaling, and stimulating multicellular organismal growth, when treated with TRF for 6 months. 47
Gene expression analysis of MCF‐7 cells treated with gamma‐tocotrienol revealed alterations in the expression of multiple genes involved in cell growth and proliferation, cell death, cell cycle, cellular development, cellular movement, and gene expression. Further analysis of differentially modulated genes using Ingenuity Pathway Analysis software suggested modulation of canonical signal transduction or metabolic pathways such as nuclear factor‐erythroid factor 2‐related factor 2 (Nrf2)‐mediated oxidative stress response, transforming growth factor‐beta (TGF‐β) signaling and Endoplasmic Reticulum (ER) stress response by the different forms of tocotrienol.
1.4. Tocotrienol metabolism
The major drawback of tocotrienol therapy is that it is metabolized and excreted rapidly from the body. 48 It is either removed after solubilization in bile acids and egested in feces or excreted in urine after shortening of the side chains to make it more water‐soluble. 49 The bioavailability is of short duration and is not sustained in plasma for an adequate period of time, which is not more than 3.5–4 h at which the tocotrienol concentration in the blood reaches the peak, but it completely disappears from blood after 24 h. 50 An investigation into quantifying the tocotrienol level in nonsupplemented adult plasma has revealed a very low concentration of the common metabolite, gamma‐carboxyethyl hydroxychromanol (γ‐CEHC) to be 307 nM/128 μg L−1 although it is more than what was previously reported. 51 Tocotrienol is hydrophobic, so requires protein carriers to enter a cell and is absorbed into the enterocytes lining the upper part of the small intestine and similar to other dietary lipids, are incorporated into chylomicrons that enter the blood plasma from the lymphatics. 52 Vitamin E metabolism is controlled by the liver where its uptake, secretion, and transport occur via the alpha‐tocopherol transfer protein (alpha TTP) for which tocotrienols have a greater affinity. 53 Absorption of tocotrienols is enhanced when consumed together with fat, to increase the bile secretion and because it protects poly unsaturated fatty acids (PUFA) from lipid peroxidation, it is delivered more to the sites of PUFA that is present in the body. Tocotrienols are metabolized in the liver, which is the nexus of vitamin E metabolism, where it undergoes omega‐hydroxylation by the cytochrome P450 activity followed by beta‐oxidation, conjugation, and excretion. 53 Research has shown the involvement of the cellular senescence regulator, Sirtuin 1 (SIRT‐1) which delays senescence in cells playing a role in the cellular uptake and metabolism of tocotrienols. 50 Tocotrienols show differential bioavailability in various organs such as the skin, brain, adipose tissue, muscles, cardiac muscle fiber, and liver suggesting the existence of another intracellular transport mechanism, that is independent of the alpha TTP carrier. 54 However, among the different forms of tocotrienol, delta‐tocotrienol uptake was the highest followed by gamma‐tocotrienol and then alpha‐tocotrienol, in a study that utilized human diploid fibroblasts. 53 Recent research has revealed preferential uptake and metabolism of tocotrienol forms, raising the need to identify the mechanisms for its intracellular augmentation and delivery to sites that otherwise hinders the therapeutic benefit of tocotrienols.
2. ANTI‐CANCER ACTIVITY OF TOCOTRIENOL
The quest for discovering a potential treatment strategy for cancer is ongoing (Table 1) as existing therapies have limited benefits. The conventional anti‐cancer treatment including chemotherapy, radiotherapy, and surgery are presented with many side effects and hence, novel drugs are needed which could promote bioavailability (the half‐maximal inhibitory concentration or IC50) and systemic efficacy (reaching and alleviating the disease states in different organs, for an example metastasis), while minimizing toxicity in cancer patients. 55 In recent years, gamma and delta forms of tocotrienol have shown promising therapeutic efficacy in targeting common metabolic pathways 56 to inhibit tumor progression (invasion, proliferation, angiogenesis, and metastasis) and cell cycle activity (through DNA damage and arresting G1/S phase of the cell cycle). 57 They also promote the mechanisms of autophagy and apoptosis (via the caspase‐dependent and caspase‐independent pathways). 58 Tocotrienols are more potent than the tocopherols in their ability to precisely target the cancerous cells from the noncancerous cells by the combination of a hypomethylated chroman ring (such as the alpha form that is fully methylated and delta form being the least methylated) and the isoprenoid tail that could evoke a well‐defined response of bioavailability and transformation. 59 Data using breast and prostate cancer cells treated with gamma‐tocotrienols have revealed the destruction of the cancerous cells leaving the epithelial noncancerous cells unaffected. 60 It makes tocotrienols superior anti‐neoplastic compounds 61 as the conventional therapies induce nonspecific cellular destruction, causing side effects. 62 Furthermore, gamma and delta tocotrienols are considered to be more potent anti‐cancer therapeutics compared to the other familial forms, in mediating cell death in research studies conducted with in vitro cell lines sourced from cancerous cervical, 63 lung, 64 breast, 65 colon, 66 liver, 67 skin, 68 prostate, 56 blood, 69 pancreas, 70 gastric, 71 and brain tissue 72 (Figure 4). The anticancer pharmacokinetic action of tocotrienol is thought to increase with neoadjuvant combined therapy 73 and several clinical trials are continuing at present. 18 , 74 Gamma‐tocotrienol in combination with a single dose of chemotherapy each with docetaxel (at concentrations of 0.5, 1, and 2 nM with 50, 75, and 100 μM for 6 days in an in vitro assay), 75 cyclophosphamide (dosage not found) with a T3 mixture (Malaysian Palm Oil Board, Malaysia) that was composed of 27.45% alpha‐tocotrienol, 57.99%, gamma‐tocotrienol, and 14.56% delta‐tocotrienol, diluted in corn oil to the desired concentration 76 are expected to induce anti‐cancer effects in an ongoing clinical trial. Additionally, epirubicin [at 0.33 mg/g and tocotrienol at 750 mg/g:tocotrienol mixture containing total tocotrienols 75% (750 mg/g); delta‐tocotrienol 89%; gamma‐tocotrienol and other tocotrienols 11%; Tocopherols 1% maximum], 77 erlotinib (gamma‐tocotrienol at 0–4 μM and erlotinib at 0–5 μM), 78 or ertuzumab (with gamma‐tocotrienol, delta‐tocotrienol and the synthetic derivative alpha‐tocopheryl polyethylene glycol 1000 succinate 73 are under investigation in breast cancer patients in Denmark. Another human phase II trial is being conducted to determine the tocotrienol effect on ameliorating the side effects of the cancer treatment, FOLFOXIRI (5‐fluorouracil, oxaliplatin, and irinotecan). 79 The first anti‐cancer drug to have experimented with in tocotrienol adjuvant therapy, was the breast cancer drug, tamoxifen which was unsuccessful with the researchers concluding that estrogen receptor‐positive‐early stages of breast cancer [of tumor, nodes, and metastases (TNM) Stages I and II] remained unresponsive to the tocotrienol‐rich‐fraction (TRF) administered with tamoxifen. 80 However, the delta form of tocotrienol displayed efficacy in attenuating pancreatic ductal carcinoma (reaching adequate bioavailability levels in blood with significant neoplastic cell apoptosis) in a cohort of 25 persons given a dose of 200–1600 mg daily for 2 weeks before performing surgery. This highlights the usefulness of tocotrienol in pancreatic neoplasia, warranting further investigations. 81 The adequate bioavailability was displayed by the IC50 of tumor cell growth inhibition, which was 40, 45, and 60 μM for delta, gamma, and beta tocotrienol compounds, respectively. The IC50 of growth inhibition for gemcitabine was 20 μM. In contrast alpha‐tocotrienol, alpha‐tocopherol, and delta‐tocopherol had no growth inhibitory activity. Malignant transformation was inhibited by 70, 30, 19, and 6% with delta, gamma, beta, and alpha tocotrienol compounds, respectively in MiaPaca‐2 cells. Malignant transformation inhibition for gemcitabine was 89%. 81 In this study, delta‐tocotrienol was the most bioactive form which was remarkably nontoxic to nontransformed pancreatic cancer cells. Both gamma and delta‐tocotrienol forms inhibited the nuclear DNA binding activity of nuclear factor kappa beta/protein 65 (NF‐kB/p65) and the delta form was more apoptotic towards pancreatic cancer compared to gemcitabine treatment. 81 There are two other clinical trials which are ongoing; nonsmall cell lung carcinoma being treated with delta‐tocotrienol and standard chemotherapy 82 and ovarian carcinoma, given tocotrienol synergized with bevacizumab 74 which will elucidate the possibility of augmenting cancer treatment concomitant with tocotrienol administration.
TABLE 1.
The latest studies on tocotrienol in cancer (published in 2021)
| Model | Disease | Tocotrienol treatment regimen | Results | Outcome/Conclusion | References |
|---|---|---|---|---|---|
| Balb/c mouse | Breast cancer | Delta‐tocotrienol and its metabolite delta‐tocotrienol, 13′‐carboxychromanol in the ratio of 8/1 given mixed with diet. Duration not stated. | Reduced tumor multiplicity, reduced pro‐inflammatory cytokine release, modulation of gut microbiota | Anti‐cancer activity is promoted. | 98 |
| Human CNE1 cell line | Nasopharyngeal carcinoma | In vitro assay with delta‐tocotrienol 10–80 μm concentrations for 72 h. | Induced cell cycle arrest and apoptosis | Anti‐cancer activity is promoted. | 316 |
| MCF‐7/Adr cell line | Breast cancer | Tocotrienol 25–50 μg for 72 h with doxorubicin | NF‐kB pathway was inhibited with suppressed mdr1 promoter activity and p‐gp efflux. | Anti‐cancer activity was confirmed. | 163 |
| MBA‐MB‐231 and MBA‐MB‐453 cell lines | Triple‐negative breast cancer | Gamma tocotrienol 0–12 μM | Reduction in proliferation, migration, and EMT, inhibition of androgen receptor | Anti‐cancer efficacy determined. | 317 |
| TRF entrapped in an oil‐in‐water emulsion | Entrapment efficiency of TRF | Homogenized at 5000 rpm for 15 min with 0.75% calcium carbonate as stabilizer | Above 95% entrapment efficiency and small droplet size | Enhanced efficiency in drug delivery established. | 318 |
| Mouse +SA and TS/A mammary tumor cells | Mammary tumors/breast cancer | Gamma tocotrienol 5 and 6 μM | Attenuation of galectin‐3 distribution, blocks histamine‐induced cell migration, active binding to glycoconjugate receptors, and extracellular matrix proteins | Anticancer activity is promoted. | 319 |
| BLM human melanoma cells and A375 stem cells | Melanoma | Delta tocotrienol (data taken from abstract) | Induces paraptosis cell death, impairs mitochondria in vemurafenib‐treated A375 stem cells | Anti‐cancer activity is promoted | 120 |
| MDA‐MB‐231 cell line | Triple‐negative breast cancer | Gamma tocotrienol 8 μM with D3 (10 nM) for 4 days | Higher apoptosis, higher necrosis, lowered invasiveness | Anti‐cancer effect confirmed | 320 |
| A549, HEP G2 cell lines | Lung and liver cancer | TRF (0.05–400 μM), caffeic acid entrapped in water‐in‐oil‐in‐water multiple emulsion, and cisplatin | Enhanced cell cycle arrest at G0/G1, enhanced apoptosis, enhanced ROS, high encapsulation, and loading efficiency | Anti‐cancer efficacy determined. | 321 |
| Panc 10.05, SW 1990, AsPC‐1, BxPC‐3 cells | Pancreatic cancer | Tocotrienols with gemcitabine | Enhanced cell cytotoxicity and higher cellular uptake of gemcitabine | Anti‐cancer efficacy confirmed | 322 |
FIGURE 4.
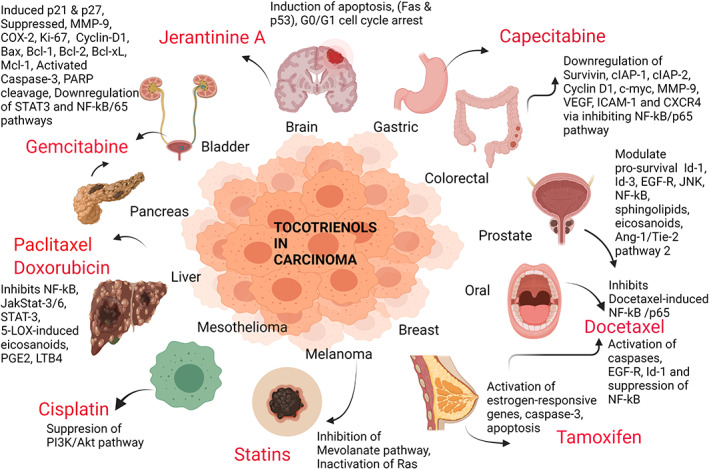
The different anti‐cancer drugs and the molecular mechanisms with which Tocotrienols have been combined successfully. p21—a cyclin‐dependent kinase inhibitor—cell cycle inhibitor in G1/S phases; p27‐another cyclin‐dependent kinase inhibitor‐regulating the cell cycle in G0 to S phases; MMP—matrix metalloproteinases—degrades matrix and non‐matrix proteins; COX—cyclooxygenase—Produces prostaglandins‐initiate inflammation; KI‐67—a nuclear protein inducing cell proliferation; cyclin D1—a cell cycle regulatory subunit of cyclin‐dependent kinases; Bax—Bcl‐2‐associated X protein—an apoptosis regulator; Bcl‐1, Bcl‐2, Bcl‐XL—B cell lymphoma family members—regulator proteins of apoptosis, cell death; Mcl‐1‐myeloid cell leukemia 1—a regulator of mitochondrial homeostasis and an apoptotic member of the Bcl—family of proteins; Caspase‐3—an apoptosis regulator in DNA fragmenting and degradation of cytoskeletal proteins; PARP—poly adenosine di phosphate (ADP) ribose polymerase—a protein which helps cells to repair by itself; STAT3—signal transducer and activator of transcription‐3—protein involved in cell growth, proliferation, migration, and apoptosis; NF‐kB/69—nuclear factor kappa beta subunit of the protein 65—induces inflammation and its progression; Jak/STAT‐3/6—janus kinase/signal transducer and activator of transcription proteins—induce cellular senescence; 5‐LOX‐induced eicosanoids—5‐lipoxygenase‐induced eicosanoids—induces prostaglandins and leukotrienes; PGE2—prostaglandin 2—inducers of inflammation; LTB4—leukotriene B4‐involved in inflammation; PI3K—phosphatidylinositol‐3‐kinase—plasma membrane associated lipid kinases involved in cell proliferation and survival; Akt—protein kinase B—involved in cell growth, proliferation, angiogenesis, vasorelaxation; Ras—a family of proteins involved in cellular functions and survival; EGF‐R—epidermal growth factor‐receptor—controls cell division and survival; Id‐1—inhibitor of differentiation‐1 associated with docetaxel—promotes longer relapse‐free survival in cancer patients; Ang‐1/Tie‐2—angiopoietin‐1 and receptor tyrosine kinase—promotes endothelial cell survival, vascular protective effects; Survivin—inhibitor of apoptosis; cIAP‐1 and 2—Cellular inhibitor of apoptosis proteins—inhibits apoptosis; c‐Myc—human oncogene overexpressed in various cancers; VEGF—vascular endothelial growth factor; ICAM‐1—intercellular adhesion molecule‐1—Facilitates leukocyte‐endothelial trans migration; CXCR4—chemokine receptor protein which spans the outer membrane of cells in white blood cells and many other cell types
There are several studies performed on cancer stem cells (CSC) of prostate 83 and pancreatic cancers 84 and human epithelial CSC from breast, 85 colon and cervical cancers, 55 where the delta and gamma forms of tocotrienol were markedly effective as adjuvants. These tocotrienol forms reportedly suppressed tumor progression, invasion of tissues, and metastasis, when treated combinedly with anti‐neoplastic medication (5‐azathioprine—in prostate CSC, 86 simvastatin—in breast CSC 85 ). It was achieved by the activation of several immune, metabolic, and cell signaling pathways (by downregulating signal transducer and activator of transcription 3 (STAT3) and inhibiting the mevalonate pathway, inducing Src homology 2 domain‐containing protein tyrosine phosphatase 1 and 2 (SHP1 and SHP2) proteins and mitogen‐activated protein kinase (MAPK) pathway, activation of de novo ceramide pathway, and under hypoxia through the hypoxia‐inducible 1‐alpha (HIF‐1α) signaling and strong induction of apoptosis of CSC (through the Docosahexaenoic Acid [DHA]‐induced apoptosis). 55 Furthermore, the anticancer efficacy of tocotrienol [gamma, delta‐tocotrienol forms, and the tocotrienol rich fraction (TRF)] was demonstrated in studies involving in vitro cell lines (prostate cancer cells—LNCaP and PC3, gastric cancer cells—SGC‐7901, melanoma cells—C32 and G361), where they displayed protection against invasion, metastasis, and tumor‐angiogenesis by stimulating the E‐cadherin and beta‐catenin pathways, downregulating mRNA expression of matrix metalloproteinase‐2 and ‐9 (MMP‐2 and MMP‐9) and stimulating the inhibition of tissue metalloproteinase 1 and 2 (TMP1 and TMP2)]. 55 , 69
The tocotrienol‐mediated anti‐cancer effects have already been established in both in vitro studies where exogenous gamma‐tocotrienol was able to suppress growth and proliferation of cells, and in vivo experiments where it had the potential to suppress tumor metastasis and angiogenesis. 87 Another study had demonstrated gamma‐tocotrienol‐driven downregulation of tumor angiogenesis in prostate cancer cells in vitro. 88 This was achieved through the activation of adenosine mono phosphate kinase (AMPK)‐innervated, angiopoietin‐1 (Ang‐1), and tyrosine‐protein kinase receptor‐2 (Tie‐2 receptor) inhibition which was projected as a potential downstream target of gamma‐tocotrienol in this tumor angiogenesis pathway. 88 The researchers had noted the inhibition of Ang‐1 gene transcription from complimentary deoxyribonucleic acid (cDNA) microarray construction as well as diminished protein secretion, with gamma‐tocotrienol treatment but not tocopherol, adding a new dimension to curtailing tumor angiogenesis in advanced prostate cancer. 88
In the continued search for new therapeutics to overcome the drug‐resistance in cancers, a group of researchers had synthesized oxazine derivatives of both gamma‐ and delta‐tocotrienol as (Figure 3) a novel treatment option in in vitro and in vivo models of breast cancer. 89 Oxazines are heterocyclic compounds that exhibit anti‐cancer effects better than the parent compounds and significantly inhibited tumor proliferation in syngeneic mouse mammary glands. 89 The mode of action of those oxazine derivatives included suppression of Protein kinase‐B (Akt) and nuclear factor‐kappa beta (NF‐kB) pathways, increased cell cycle arrest proteins, protein 21 (p21), and protein 27 (p27) as well as the inhibition of cyclin D1, cyclin‐dependent kinase 2, 4, and 6 (CDK2, CDK4, CDK6) and cyclooxygenase‐2 (COX‐2) combined immune‐metabolic processes. 89
FIGURE 3.
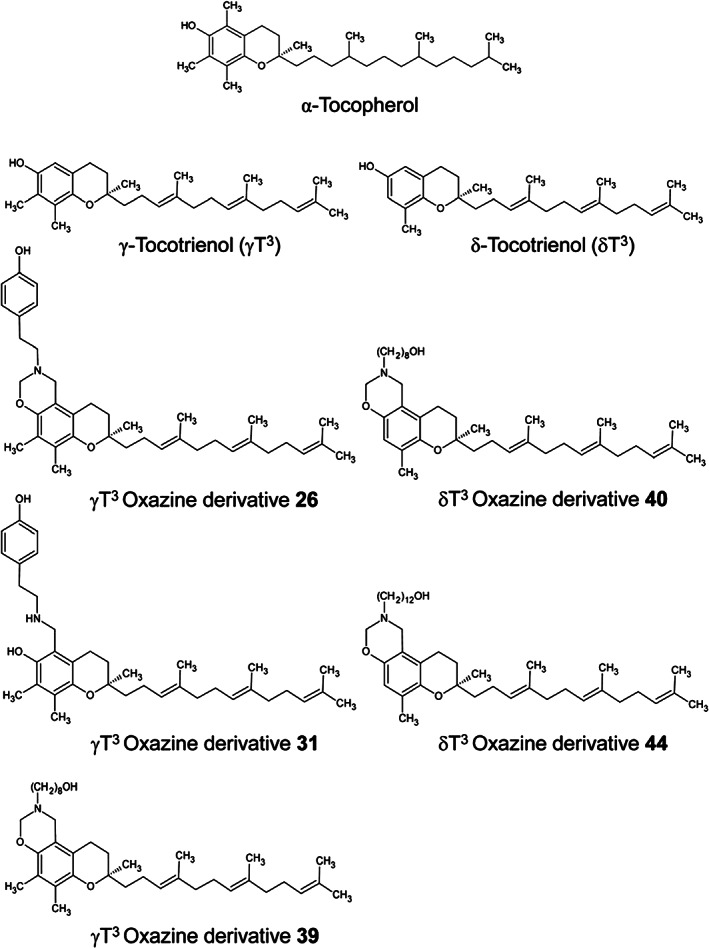
Chemical structures of gamma‐ and delta‐tocotrienol and their respective oxazine derivatives. 89 [Reprinted from ANTICANCER RESEARCH, Vol. 34, Suryatheja Ananthula, Parash Parajuli, Fathy A. Behery, Alaadin Y. Alayoubi, Khalid A. El Sayed, Sami Nazzal and Paul W. Sylvester, “Oxazine Derivatives of γ‐ and δ‐Tocotrienol Display Enhanced Anticancer Activity In Vivo”, Pages 2715–2726, Copyright (2014), with permission from ANTICANCER RESEARCH.]
The importance of gamma‐tocotrienol is further justified by its ability to influence multiple gene activation mechanisms such as cell growth, proliferation, cell cycle, cell death, cellular movement, development, apoptosis, ER stress, and gene expression itself. 90 A study performed on breast cancer tumorigenesis using Michigan cancer foundation‐7 (MCF‐7) and M.D. Anderson‐metastatic breast 231 (MDA‐MB‐231) cell lines have revealed that gamma‐tocotrienol is a multi‐faceted anti‐cancer therapy as mentioned earlier. 90
A simulation software linked to gene transcription analysis has revealed a significant increase in activating transcription factor‐3 (ATF3), synergized by gamma‐tocotrienol treatment. When ATF3 activity was inhibited by small interfering ribonucleic acid (siRNA) activity, gamma‐tocotrienol was able to restore ATF3‐induced apoptotic effects which in turn downregulated tumor proliferation. 91
Radiation therapy is one of the most common adjuvant cancer therapies initiated after surgery and gamma‐tocotrienol have demonstrated significant recovery in some mouse studies such as CD2FI mouse exposed to total body gamma irradiation‐induced cell death. 92 The radioprotective properties elicited by gamma and delta‐tocotrienol were documented to include recovery from radiation‐induced cell death via extracellular signal‐regulated kinase (ERK)/mammalian target of rapamycin (mTOR) pathways with enhanced survival and protection of hematopoietic cells, where the hematopoietic system is regarded mostly at risk from irradiation. Further, radioprotection from gamma‐ and delta‐tocotrienol forms also included cancer stem cells and progenitor cell foci in mouse bone marrow, where gamma‐tocotrienol induced and promoted hematopoiesis as well. 93 , 94 Additionally, gamma‐tocotrienol treatment yielded protection from radiation therapy‐induced damage in healthy cells as well as promoted irradiation‐induced cell death in tumors by increasing tumor cell sensitization to irradiation. 95 Gamma‐tocotrienol is known to inhibit cancer cell invasion and metastasis by reversing epithelial‐to‐mesenchymal transition (EMT—being the salient mechanism of invasion and cancer metastasis), in studies involving melanoma and prostate cancer cells. 96 Thus, gamma‐tocotrienol treatment promoted the expression of epithelial markers such as E‐cadherin and gamma‐catenin, while mesenchymal markers, alpha‐smooth muscle‐actin, and vimentin, were suppressed. 97 Furthermore, the anti‐cancer stem cell properties of tocotrienol were linked to its potential for reversing EMT, that simultaneously hedged against disease recurrence. 97
In a murine model of colitis‐associated colorectal cancer (CAC), treatment with delta‐tocotrienol and its metabolite, delta‐tocotrienol 13 carboxychromanol together, have displayed a role in modulating the gut microbiome composition but not richness. 98 CAC was induced in mice by jointly administered azoxymethane and dextran sulfate sodium (DSS) in the diet that was followed by treatment with a delta‐tocotrienol mixture that could prevent the multiplicity of large adenomas in the colon. 98 These observations suggest that tocotrienol's anti‐tumorigenic properties may also result from the modulation of the gut microbial flora because there was a conspicuous increase in the Lactococcus and the Bacteroides in the intervention group compared to the control group. 98 The therapeutic combination inhibited tumorigenesis and suppressed proinflammatory cytokines in addition to influencing the gut microbial community. The modulation of the gut microflora by tocotrienols may present a mechanism toward attenuating tumorigenesis in the colon. 98
2.1. Anti‐cancer mechanisms of tocotrienol
2.1.1. Cell cycle arrest by tocotrienol
An increasing number of research studies investigating the chemotherapeutic action of tocotrienol has risen in recent years, which have confirmed a substantial effect on cell death in cancerous tumors through cell cycle arrest. 99 Abrogation of the cell cycle is an effective gateway to controlling the uninhibited cell growth and proliferation observed in all types of cancer. 100 Therefore, the regulation of cell cycle checkpoints offers a viable strategy in cancer therapy, which can be achieved by tocotrienol treatment, either alone or combined with standard chemotherapeutics. 101 Cell cycle block has been reported with simultaneous gamma‐tocotrienol treatment in suppressing tumor growth and development in estrogen‐dependent breast cancer cells (MCF‐7) and estrogen‐independent breast cancer cells (MDA‐MB‐231), 91 delta‐tocotrienol in hepatocellular carcinoma; HepG2 cells, 102 gamma‐tocotrienol in prostate cancer; LNCaP, DU145, PC‐3, and human VCaP cells, 69 delta‐tocotrienol in melanoma cells; B16, 103 gamma‐tocotrienol in human cervical carcinoma; HeLa cells, 104 through immune‐metabolic modulation including the upregulation of p21 and p27, and the diminishing of cyclin D1, CDK‐2, 4, and 6, and phosphorylated Rb. 105 Tocotrienol activity in the modulation of cell cycle proteins have been reported in glioblastoma and leukemia (by delta‐tocotrienol), pancreatic cancer (by gamma‐tocotrienol), and gastric cancers (by gamma‐tocotrienol). 104 , 106 The suppression of brain cancer cells (U87MG) has been documented with gamma‐tocotrienol administered together with Jerantinine A, an indole alkaloid which is a potent anti‐proliferative agent that disrupted the G0/G1 interphase and microtubular polymerization, through Fas Ligand (FasL) and protein 53 (p53)‐stimulated apoptosis via the intrinsic mitochondrial pathway. 72
Gamma‐tocotrienol combined with Doxorubicin reversed multi‐drug resistance in breast cancer in the multi‐drug resistant breast cancer cell line (MCF‐7/Adr breast cancer cells) through enhanced P‐glycoprotein expression which led to G2/M arrest and apoptosis. 107 P glycoprotein is an ATP‐dependent drug efflux pump present in tumors. Gamma‐tocotrienol was able to arrest the cell cycle at G0/G1 phase in HeLa cells as well. 63
2.1.2. Apoptosis by tocotrienol
Apoptosis is an innate immune mechanism involved in cancer cell death which is targeted by most cancer drugs. There are evidence‐based studies carried out on tocotrienol effectiveness in triggering cellular apoptosis, as a means of enhancing its anti‐tumorigenic properties. 108 Gamma‐tocotrienol has shown potent apoptotic activity and growth inhibitory effects in prostate cancer (PCa) cells and in downregulating the expression of several oncogenic products through the inhibition of NF‐kB pathway and apoptosis, as well as anti‐invasive effects and chemo sensitization (in prostate cancer). 56 Furthermore, gamma‐tocotrienol has demonstrated regulatory effects on B‐cell lymphoma 2 (Bcl‐2) protein and caspase‐3 activity in a gastric cancer cell line (SCG 7901), by mediating apoptosis through the rapidly accelerated fibrosarcoma–extracellular signal‐regulated kinases (Raf–ERK) signaling cascade. 109 Additionally, studies of alpha‐tocotrienol treatment on cancerous cell lines have produced favorable results such as anti‐proliferation effects and apoptosis in human cervical cancer‐HeLa cells by the suppression of cyclin D, protein 16 (p16), CDK6, and inducing interleukin‐6 (IL‐6) expression and the mitochondrial apoptosis pathway. 100 Triple‐negative breast cancer (MDA‐MB 231) was attenuated by activating micro ribonucleic acid (miR)‐429 and estrogen‐dependent MCF‐7 cells 110 and inducing DNA damage, NF‐kB inhibition, and poly ADP‐ribose polymerase (PARP) cleavage. 111 Similarly, apoptosis was induced in a breast cancer cell line (66‐cl‐4‐GFP) by the expression of death receptor 5 (DR5) dependent on c‐Jun N‐terminal kinase (JNK), protein 38 (p38), mitogen‐activated protein kinase (MAPK) pathway, and ERK‐mediated endoplasmic reticulum (ER) stress pathway. 112 Apoptosis was induced in bladder cancer (T24, 5637, J82, and UMUC‐3) by inhibiting the signal transducer and activator of the transcription 3 (STAT3) pathway. 113 Chronic myeloid leukemia (K562 cells) was suppressed by both intrinsic and extrinsic mechanisms, 114 and nonsmall cell lung cancer (NSCLC) was inhibited by the downregulation of NF‐kB and Notch‐1 pathways. 115 Gamma‐tocotrienol combined with lovastatin had induced apoptosis in HL60 cells by suppressing rat sarcoma‐derived guanine nucleotide‐binding protein, Ras/ERK/NF‐kB, and Ras/Akt/NF‐kB mechanisms and downregulating glyoxalase 1 and 3‐hydroxy 3‐methyl glutaryl coenzyme A (HMG‐CoA) reductase activity. 116 There are more studies on which tocotrienol treatment had been fruitful, that were performed with cell lines that included human lung adenocarcinoma (A549), 117 and glioblastoma (U87MG) 117 by beta‐tocotrienol, cervical cancer (Caski cells), 118 and hepatic cancer by gamma‐tocotrienol. 119 All these studies have highlighted the potent apoptotic and anti‐neoplastic strategies possible with tocotrienol therapy that are based on its multiple effects on different aspects of immune function (Figures 4, 5, 6, 7, 8, 9, 10, 11, 12, 13). Therefore, further studies on tocotrienols could yield significant data to confirm its anti‐tumor therapeutic potential.
FIGURE 5.
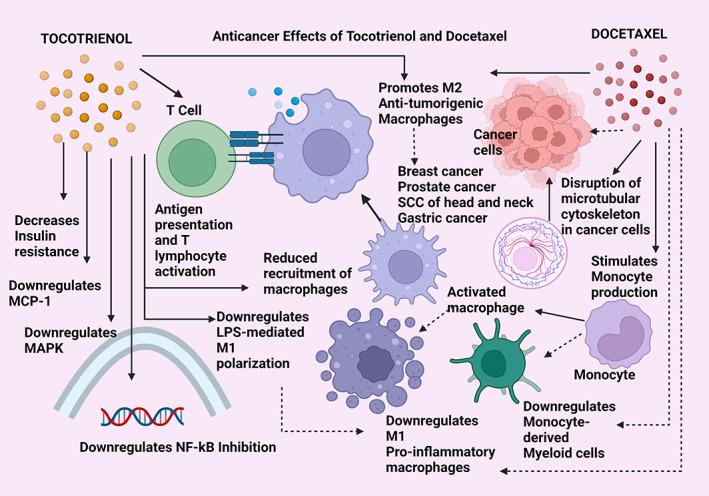
Anticancer effects of tocotrienol compared with docetaxel. MCP‐1—monocyte chemo‐attractant protein‐1; MAPK—mitogen‐activated protein kinase pathway; NF‐kB—nuclear factor kappa beta pathway; LPS—lipopolysaccharide; M1 and M2—the two broad divisions of macrophage types; SCC—squamous cell carcinoma. Straight arrows indicate stimulation/upregulation and dotted arrows represent downregulation/inhibition mechanisms.
FIGURE 6.
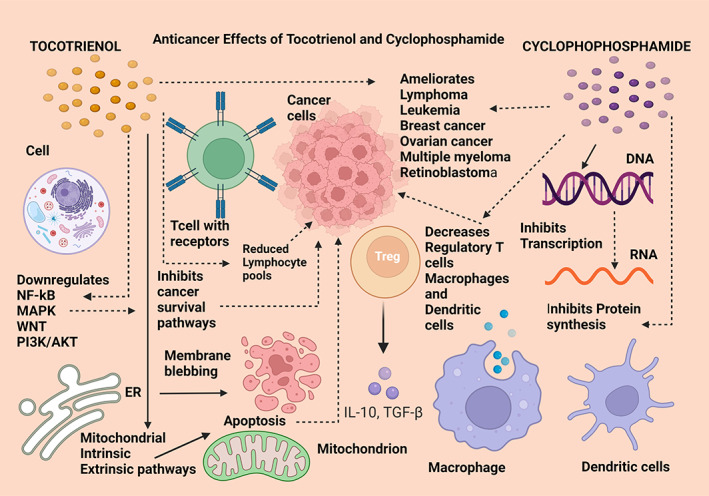
Anticancer effects of tocotrienol compared with cyclophosphamide. NF‐kB—nuclear factor kappa beta; MAPK—mitogen‐activated protein kinase; PI3K/AKT—phosphatidylinositol‐3‐kinase/protein kinase B; ER—endoplasmic reticulum; IL‐10—interleukin 10; TGF‐β—transforming growth factor‐beta; DNA—deoxyribonucleic acid; RNA—ribonucleic acid. Straight arrows indicate stimulation, dotted arrows indicate inhibition
FIGURE 7.
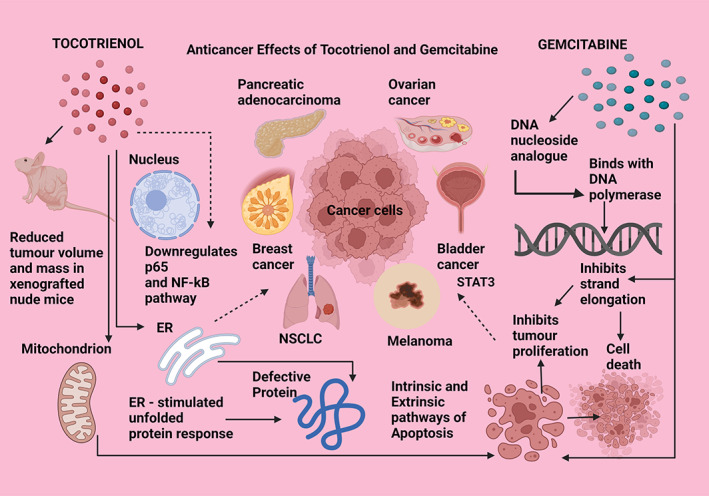
Anticancer effects of tocotrienol compared with gemcitabine. p65—protein 65; NF‐kB—nuclear factor kappa beta pathway; ER—endoplasmic reticulum; NSCLC—nonsmall cell lung cancer; DNA—deoxyribonucleic acid. Straight arrows indicate stimulation, dotted arrows indicate inhibition
FIGURE 8.
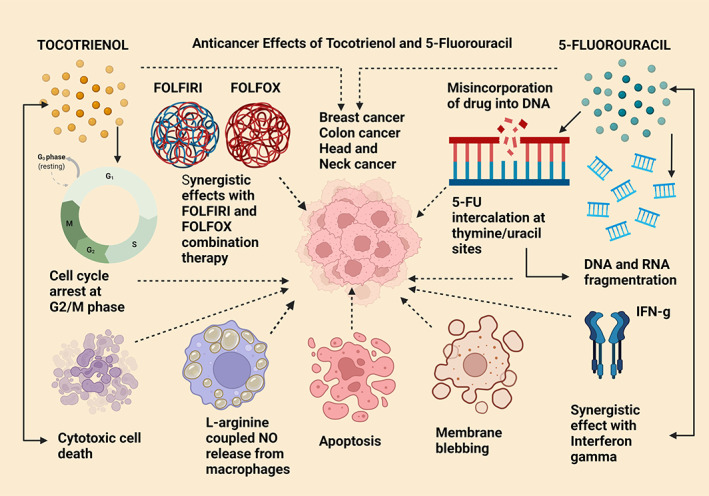
Anticancer effects of tocotrienol compared with 5‐fluorouracil. G2/M—growth phase 2/mitotic phase; NO—nitric oxide; DNA—deoxyribonucleic acid; 5‐FU—5‐fluorouracil; RNA—ribonucleic acid; IFN‐g—gamma interferon; FOLFIRI—folinic acid, leucovorin, 5‐fluorouracil, and irinotecan; FOLFOX—folinic acid, fluorouracil, and oxaliplatin. Straight arrows indicate stimulation, dotted arrows indicate inhibition
FIGURE 9.
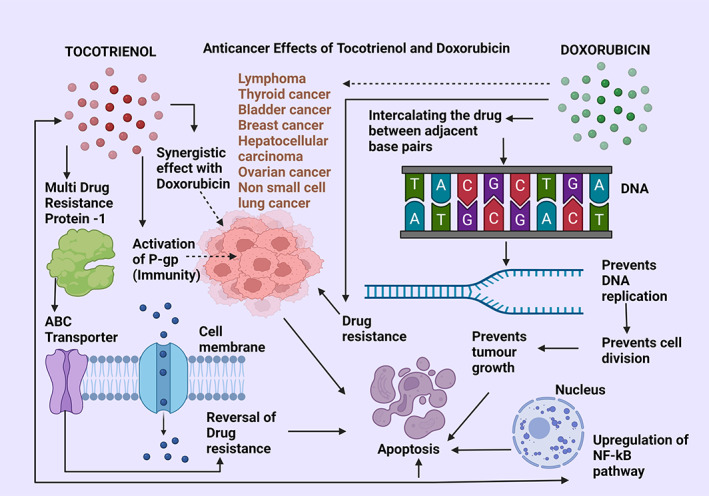
Anticancer effects of tocotrienol compared with doxorubicin. ABC transporters—ATP‐binding cassette transporters; P‐gp—protein glycoprotein; NF‐kB—nuclear factor kappa beta pathway; DNA—deoxyribonucleic acid. Straight arrows indicate stimulation, dotted arrows indicate inhibition
FIGURE 10.
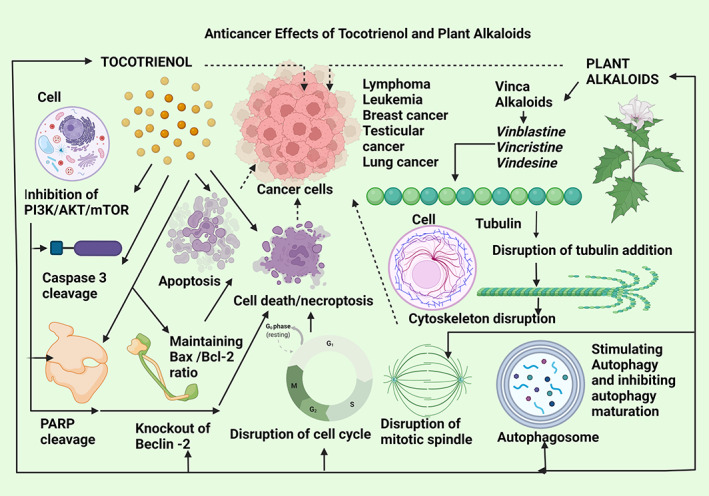
Anticancer effects of tocotrienol compared with plant alkaloids. PI3K/AKT/mTOR—phosphatidylinositol‐3‐kinase/protein kinase B/mammalian target of rapamycin, poly adenosine diphosphate‐ribose polymerase. Straight arrows indicate stimulation, dotted arrows indicate inhibition
FIGURE 11.
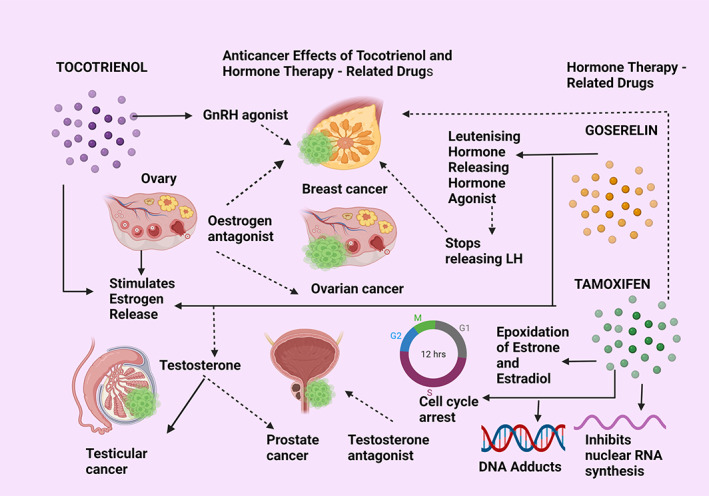
Anticancer effects of tocotrienol compared with hormone therapy. GnRH—gonadotropin‐releasing hormone; LH—luteinizing hormone; DNA—deoxyribonucleic acid; RNA—ribonucleic acid. Straight arrows indicate stimulation, dotted arrows indicate inhibition
FIGURE 12.
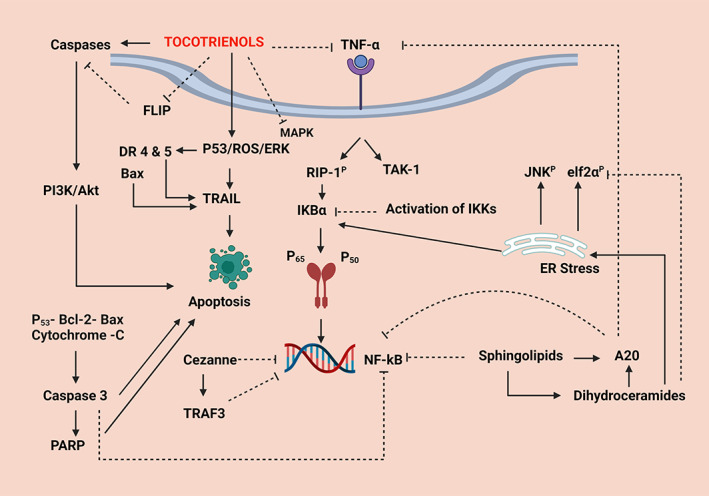
The common molecular mechanisms induced by tocotrienol therapy in experimental studies: Straight arrows indicate upregulation, and the dotted arrows show inhibition. TNF‐α—tumor necrosis factor‐alpha—a pro‐inflammatory cytokine—an inflammatory marker; FLIP—FLICE inhibitory protein—an inhibitor of apoptosis—a cytoplasmic protein complex which activates the inhibition of caspases; DR 4 & 5—death receptor 4 and 5; P‐53—A protein which acts as a tumor suppressor—inhibits uncontrolled cell proliferation; ROS—reactive oxygen species; ERK—extracellular signal‐regulated kinase; Bax—a member of the Bcl‐2 gene family which are nuclear encoded proteins which mediate apoptosis through mitochondrial activation; TRAIL—TNF‐related apoptosis‐inducing ligand—a protein ligand that induces cell death; PI3K—phosphoinositide 3—kinase, a protein which favors cellular functions in cancer; Akt—protein kinase (B)—a serine/threonine specific protein kinase—an enzyme which plays a key role in many cellular functions; NF‐kB—nuclear factor kappa beta‐pathway; PARP—poly‐ADP ribose polymerase—enables repair of DNA damage; Cezanne—deubiquitinating protein which inhibits NF‐kB translocation; TRAF3—a member of the TNF‐receptor associated factor protein family—which negatively regulates NF‐kB activity; RIP1P—receptor interacting serine/threonine‐protein kinase 1—Induces apoptosis and necroptosis by participating in NF‐kB, Akt, and JNK pathways; TAK1—transforming growth factor‐beta‐activated kinase‐1—mediates NF‐kB, p38, and JNK pathways, JNKP‐phosphorylated c‐Jun‐N—terminal kinase—controls apoptosis and cell proliferation—A member of the MAPK—mitogen‐activated protein kinase pathway; IKBα, nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor‐alpha—inhibits NF‐kB transcription; IKK—Inhibitor of upstream IKB kinase—inhibits IKB phosphorylation, degradation, subsequent NF‐kB nuclear translocation; p65—a subunit of NF‐kB nuclear transcription‐involved in inducing the NF‐kB pathway‐immunity, tumorigenesis, inflammation; p50—a component transcription factor of the NF‐kB pathway; ER—endoplasmic reticulum; elf2αP—phosphorylated eukaryotic translation initiation factor‐alpha‐subunit 1‐involved in translation in protein synthesis; A20—plays an anti‐tumorigenesis role in cancer
FIGURE 13.
The summary of tocotrienol functions that highlight its therapeutic potential
A study in 2021 has described delta‐tocotrienol therapy‐activated cellular paraptosis, which is noncanonical programmed cell death. Paraptosis is a prominent anti‐cancer strategy utilized to suppress the progression of melanoma. 120 Delta‐tocotrienol was capable of inducing membrane dysfunction in both mitochondria and ER at a concentration of 15 μg/ml kept for 12 h in A375 and BLM cell lines. This treatment resulted in an overproduction and influx of calcium ions, which led to a marked increase in reactive oxygen species (ROS). 120 Membrane dysfunction was achieved by cytoplasmic vacuolization produced by ER and mitochondrial membrane defects that caused downregulation of metabolic oxidative phosphorylation. It also induced hypoxia that diminished adenosine triphosphate (ATP) production leading to defective adenosine mono phosphate activated‐protein kinase (AMPK) phosphorylation, which are integral steps in cellular energy homeostasis. 120 Another recent cancer research study (in 2019) has confirmed the effectiveness of delta‐tocotrienol treatment as a novel strategy in suppressing pancreatic carcinoma, because of its ability to induce apoptosis via the TNF‐alpha‐related apoptosis inducing‐ligand (TRAIL). 121 TRAIL‐induced apoptosis which happens through the mediation of death receptors synergistically acting with caspase 8, was implicated in this study for mediating the downregulation of Fas‐associated death domain‐Like IL‐1‐converting enzyme (FLICE)‐inhibitory proteins, FLIP‐c in particular, that is known to suppress apoptosis through the action of caspase 3 cleaving enzymes. 121 In brief, this programmed cellular apoptosis was induced by delta, gamma, and alpha‐tocotrienol forms through promoting FLIP‐c ubiquitination leading to the degradation of the FLICE‐inhibitory proteins. 121 There are several more studies which have been published recently in 2021 (Table 1).
2.1.3. Chemo‐sensitization by tocotrienol
Tocotrienols have displayed the potential to promote chemo sensitization in cancer cells, a process which increases the sensitivity of tumors to chemotherapy while reducing its toxicity in normal cells. 122 Chemosensitivity to cytotoxic cancer treatment is recognized as a way to lower side effects arising from, present‐day anti‐cancer drugs. 122 The ability of both gamma‐ 71 , 123 and delta‐tocotrienol 113 to induce chemosensitization, when treated simultaneously with a broad range of drugs, are documented in cancer‐related studies. They include the concurrent supplementation of tocotrienols with the following drugs: gemcitabine in pancreatic 60 and bladder 113 cancer, capecitabine in colorectal 124 and gastric 71 cancer, tamoxifen 125 and docetaxel 126 in breast cancer, paclitaxel 123 and doxorubicin 127 in hepatic cancer, docetaxel 128 in prostate cancer, cisplatin 129 in mesothelioma H28 cells, statins 130 in melanoma cells, and docetaxel in human oral cancer cells 75 (Figure 4).
The advantage of combination therapy is that it can reduce drug resistance in the targeted cells, improve potency, and minimize the nonselective toxicity in noncancerous cells. 72 Stand‐alone gamma‐tocotrienol therapy in brain cancer cells had been toxic at high doses (3.17 μg/ml) and therefore, was restrictive in its therapeutic potential when used by itself. 131 This was shown in an in vitro study on brain cancer cells which had gamma‐tocotrienol (at 1.29 μg/ml) as an adjuvant with the phytochemical, Jerantinine A (at 0.16 μg/ml), in combined therapy that was successful in promoting anti‐cancer efficacy at a lower dose. 72 Compared to the gamma‐tocotrienol high dose, the percentage decrease in concentration at which the combined therapy was effective was 59% less than either gamma‐tocotrienol or Jerantinine given alone because Jerantinine was also highly toxic to healthy cells at 0.62 μg/ml. Therefore, the combined treatment had achieved anti‐cancerous benefits (decreased tumorigenic proliferation) through activating the Fas‐p53 signal transduction to induce apoptosis via the mitochondrial pathway. 72 Another study on a statistical modeling system using a software called mixture design response surface methodology (MDRSM) aimed at identifying the best combination of a curative drug design for prostate cancer, has been reported. 132 The best combination treatment was identified as gamma‐tocotrienol with alpha‐tocopherol ether acetate and docetaxel that could evade drug resistance in prostate cancer treatment than docetaxel given alone. 132 The synergistic action of gamma‐tocotrienol and polysaccharopeptide (PSP) has been investigated in which, prostate cancer cell growth, proliferation, and cytotoxicity were seen to reduce, through the mediation of AMPK. 133 The researchers of this study had observed an increase in AMPK with the simultaneous inactivation of acetyl‐co A carboxylase, an integral step in cellular energy homeostasis, evidenced by the Ser 79‐activated phosphorylation. 133 Further, gamma‐tocotrienol possessed the ability to sensitize the prostate cancer tumors to PSP, in vivo. 133
2.1.4. Anti‐metastasis mechanisms of tocotrienol
Cancer metastasis is a dynamic process that underpins the need for effective cancer medicines that can potentially prevent the motility of extracellular matrix and cells within the tumors. 104 Tocotrienols are endowed with anti‐cancer metastasis properties which could modulate the numerous extrinsic and intrinsic factors that are responsible for tumor metastasis and therapeutic resistance in most cancers. Delta‐tocotrienol exhibited anti‐metastasis properties by down‐regulating Notch‐1 and NF‐kB signaling pathways through the repression of protease activity of MMP‐9/urokinase‐type plasminogen activator (uPA) in NSCLC. 134 Delta‐tocotrienol has further produced anti‐metastasis ability in pancreatic tumors by attenuating tumor cell migration, invasion, and EMT, involving vimentin, E‐cadherin, and N‐cadherin transition. 81 Gamma‐tocotrienol was effective in repressing metastasis in in vitro breast carcinoma by the suppression of cytoskeletal re‐organization and cellular membrane protrusions. Gamma‐tocotrienol further demonstrated anti‐metastasis effects in breast tissue by inhibiting EMT through the canonical Wnt signaling mechanism. 97 Interestingly, most of the in vitro studies that were conducted were corroborated with in vivo research that has validated the anti‐metastatic effects of tocotrienols in malignant tumors. A plethora of such studies are on record and a handful is mentioned here, that include cell lines from colorectal, prostate, cervical, melanoma, neuroblastoma, and ovarian cancers. 104
2.2. Similarities between anti‐cancer mechanisms of tocotrienol and common chemotherapeutics
The existing anticancer chemotherapies belong to five broad classes: alkylating agents, anti‐metabolites, anthracyclines, plant alkaloids, and hormone‐regulated anticancer drugs. This section summarizes the similarities between these different chemotherapeutic groups and tocotrienol in cancer therapy. The aim is to draw a parallel with the chemotherapeutic molecular mechanisms that are compatible or synonymous with the tocotrienol therapeutic potential. Given the similarities which are described in the subsections below, a more detailed study to match the pharmacokinetics of tocotrienols and toxicity profile with those drugs in preclinical studies is warranted. The hypothesis is that the immune and molecular anti‐cancer mechanisms between the two groups are not dissimilar and therefore tocotrienol possesses chemotherapeutic properties that can be utilized in the treatment of cancers.
2.2.1. Tocotrienol and docetaxel
Docetaxel is one of the standard chemotherapies used particularly against metastatic breast and prostate cancer and its primary mechanism of action is promoting the disruption of the microtubular cytoskeleton in cancer cells. Its main immune effect is to promote the proinflammatory activation of the M1 phenotype of the tumor‐associated macrophages and the induction of antigen‐presenting cells to activate T cytotoxic lymphocytes. The polarization of macrophages into its pro and anti‐inflammatory responses is mostly mediated and determined by the tumor microenvironment. Docetaxel is a Taxane‐derivative that produces anti‐tumor immune activation involving the monocyte‐derived macrophage activation and the suppression of the NF‐kB pathway. Tocotrienols are being used as an adjuvant with Docetaxel in an ongoing clinical trial (NCT02909751) that is investigating its potential as a combined anti‐neoplastic therapy. The immune effect of gamma‐tocotrienol is evident by its ability to induce the suppression of the monocyte‐derived proinflammatory macrophages. The interesting connection between Docetaxel and gamma‐tocotrienol is the immune activation of both these therapeutics leading to the stimulation of the macrophage repertoires that mediate an anti‐inflammatory, anti‐tumorigenic effect (Figure 5). 135 , 136 Tocotrienols have shown increased chemo‐sensitization when administered as an adjuvant with Docetaxel, where gamma‐tocotrienol served to increase the Docetaxel‐induced apoptosis in melanoma and prostate cancer. 60 Gamma‐tocotrienol combined with Docetaxel showed enhanced tumor‐suppressive effects in mice implanted with breast cancer than when treated by the two therapeutics separately. 60 Synergistic anti‐cancer effects in prostate cancer were reported by co‐treatment of Docetaxel and gamma‐tocotrienol on apoptosis mechanisms by upregulating the pro‐apoptotic proteins (caspases 3, 7, 8, 9, and cleaved PARP) and downregulating pro‐survival proteins (Id‐1, NF‐kB, p65, ik‐B, and EGF‐R). 56
2.2.2. Tocotrienol and cyclophosphamide
Cyclophosphamide is an anticancer drug that is a potent immune‐suppressive agent, prescribed mainly for various leukemias, lymphomas, breast cancer, multiple myeloma, ovarian cancer, and retinoblastoma. It was shown to downregulate both humoral and cell‐mediated immune responses where its main pharmacokinetic action is selectively suppressing the regulatory T cells and being an alkylating agent, inhibiting transcription of DNA to RNA which disrupts protein synthesis. Cyclophosphamide has produced remarkable effects in reducing the lymphocyte proliferation with markedly diminished macrophage and dendritic cell populations in peritoneal exudates. Cyclophosphamide has been combined with tocotrienols (with no information on the exact form of vitamin E) as a synergistic adjuvant therapy in clinical trials (NCT02909751) which are ongoing at present and in drawing a parallel with cyclophosphamide mode of action, tocotrienols too, possess anticancer activity by reducing pools of lymphocytes as well as achieving anti‐tumor capabilities by inducing cell death. They modulate cancer cell survival via the mitochondria and ER ‐stimulated apoptosis mechanisms and by the downregulation of cancer cell survival pathways including NF‐kB, MAPK, Wnt, and phosphatidylinositol‐3‐kinase/protein kinase B (PI3K/AKT) immune regulators (Figure 6). 137 , 138
2.2.3. Tocotrienol and gemcitabine
Gemcitabine is an anticancer drug that belongs to the antimetabolite category of cancer drugs that is very effective against pancreatic adenocarcinoma, and solid tumors in breast, bladder, ovarian, and nonsmall cell lung cancer. It is a DNA nucleoside analog that exerts multiple mechanisms of action, of which the main action is to incorporate gemcitabine into DNA polymerase that prevents strand elongation. It also stimulates apoptosis via the intrinsic and extrinsic mitochondrial cytolytic pathways as well as the ER stress‐innervated unfolded protein response. In pancreatic tumors, gemcitabine targets the knockdown of the p65 subunit in the NF‐kB pathway, thereby causing immune suppression and abrogation of cell proliferation as the combined result of the different anticancer modalities of gemcitabine. Similar to the anti‐neoplastic action of gemcitabine, tocotrienols also display similar action by suppressing malignant tumorigenesis, evidenced by the activation of intrinsic apoptosis in human melanoma cell lines BLM and A375 in vitro while leaving the normal melanocytes unaffected. The similarity of gemcitabine and delta‐tocotrienol is that they both activate intrinsic apoptosis and ER stress‐related anticancer action. Further, the A375 xenografts in nude mice treated with delta‐tocotrienol resulted in hampered tumor progression and marked reduction in tumor volume and mass, indicating the efficacy of delta‐tocotrienol as a cytolytic agent in cancers (Figure 7). 139 , 140 Gamma‐tocotrienol showed increased sensitivity with gemcitabine in suppressing orthotopic pancreatic tumors in both in vitro and in vivo models. 60
2.2.4. Tocotrienol and 5‐fluorouracil
5‐Fluorouracil (5‐FU) is another antimetabolite type of anticancer drug which is now outdated due to the loss of chemosensitivity. Therefore, it is used in combined therapy to attenuate breast, colon, and head, and neck cancers. Its mechanism of action is the inhibition of thymidylate synthase which prevents the incorporation of thymine nucleotides thereby interfering with DNA synthesis, replication, and finally promoting cell death. Additionally, 5‐FU causes misincorporation with DNA and RNA polynucleotides in place of thymine and/or uracil, stimulation of apoptosis, cytotoxic cell death, cell cycle arrest, synergistic effects with gamma interferon, and conjugation with l‐arginine to increase endogenous nitric oxide release from macrophages. Combination therapy with FOLFIRI and FOLFOX was also shown to be effective in prolonging the lifespan of advanced metastatic cancers. In relating these mechanisms with tocotrienol cytotoxic and cytolytic action, in SW48 and Caco‐2 colonic cancer cell lines, the combination of delta‐tocotrienol with 5‐FU had enhanced antitumorigenic effects by way of DNA fragmentation, membrane blebbing, cell cycle arrest at the G2/M phase, nuclear condensation, and increased apoptosis. It is of interest that either 5‐FU and tocotrienol single therapy or in combined therapy, both 5‐FU and delta‐tocotrienols have achieved similar outcomes in promoting anticancer mechanisms of action particularly, in colorectal cancer (Figure 8). 141 , 142 This means there was no additive effect or synergism when treatment was combined or given alone.
2.2.5. Tocotrienol and doxorubicin
Doxorubicin belongs to the anthracycline category of anticancer drugs which interferes with both DNA and RNA synthesis by intercalating with the adjacent DNA base pairs, thus blocking DNA replication and therefore, cell division. Doxorubicin is a highly efficacious anticancer drug that is prescribed for many cancer types, including lymphomas, nonsmall cell lung cancer, ovarian, bladder, thyroid, and breast cancers. Its main mechanism is to slow down or impede cancer growth by inhibiting Topoisomerase II which is required for cancer growth. A study on a hepatocarcinoma cell line (HCC) and breast cancer treated with Doxorubicin has revealed drug resistance, which was thought to happen via the induction of the multidrug‐resistant protein‐1 (MDR‐1) belonging to the ATP‐cassette binding protein family (ABC transporters) which occur due to changes in the tumor microhabitat and its associated signaling cascades. It is of interest to note that gamma‐tocotrienol can reverse the MDR type 1 protein action in breast cancer as well as provide a synergistic effect with Doxorubicin on the HCC cell line. It exerts its effects through conjugated therapy as well as stimulating proapoptotic mechanisms to abrogate cancer cell proliferation while augmenting Doxorubicin accumulation inside the HCC cell line. The reversal of drug resistance in tumors was achieved by gamma‐tocotrienol treatment through inhibition of the NF‐kB pathway and P‐glycoprotein (P‐gp) action which controls Doxorubicin efflux through the plasma membrane‐bound receptors (Figure 9). 107 , 143
2.2.6. Tocotrienol and plant alkaloids
Plant alkaloids are another group of anticancer agents in use as chemotherapeutic drugs and they are a highly diverse group of compounds available for cancer therapy. Around 3000 different plant alkaloids with the US FDA approval for cancer therapy exist. Among those, indole vinca alkaloids are of importance for Vinblastine, Vincristine, and Vindesine, extracted from the plant, Catharanthus roseus in the family Apocynaceae have been tested for anticancer efficacy in combination therapy. They were used against lymphomas, leukemias, breast, testicular and lung cancers, and Kaposi's sarcoma. The chief mechanism of action is stimulating autophagy in cancer cells and inhibiting autophagy maturation in those tumors. Vinblastine combined with an autophagy stimulator (C‐ceramide), had induced autophagy and apoptosis in cell lines from hepatocarcinoma and colon cancers. The main mechanism of action is the disruption of tubulin addition and prevention of the mitotic spindles which lead to tumor cell death while arresting the cell cycle and hence cancer cell proliferation. The knockout of Beclin‐2, a protein associated with autophagy is also suppressed by vinca alkaloid combination therapy. In comparison, gamma‐tocotrienol also possesses the capability for inducing autophagy as shown in breast cancer and prostate cancer cells in which it stimulated an increased Beclin‐2 and Bax/Bcl‐2 ratio, cleaved caspase 3, cleaved PARP, and diminished signaling of PI3K/AKT/mTOR activation that adds to anticancer properties of tocotrienol in inducing apoptosis and autophagy leading to tumor cell death (Figure 10). 144 , 145
2.2.7. Tocotrienol and hormone therapy
Hormone therapy is another category of anticancer treatment which is essentially used against sex hormone‐related cancers, such as breast, ovarian, testicular, and prostate cancers. Anti‐neoplastic drugs, Tamoxifen, and Goserelin are examples of hormonal/endocrine anticancer therapy which blocks the gonadotropin‐associated hormone release to suppress cancer proliferation that occurs due to increased endogenous hormone release. Another effective treatment modality is using luteinizing hormone receptor agonists, which help to suppress tumor growth. Goserelin when given as an adjuvant with radiation therapy for advanced prostate cancer was successful in extending survival in those patients while Tamoxifen, an estrogen antagonist is used to treat breast cancers in premenopausal women. Tamoxifen inhibits the epoxidation and activation of the two steroid hormones, oestradiol, and oestrone, which allows for DNA adduct formation as well as inhibiting nuclear RNA synthesis, which prevents cell division, caused by G2/M phase cell cycle arrest. The therapeutic value of epoxides comes from enzyme inhibition, cell cycle arrest, and apoptosis. Goserelin functions as an agonist analog of the gonadotropin‐releasing hormone that is used for blocking testosterone release in advanced prostate cancer treatment. Interestingly, tocotrienols too have been able to act as estrogen hormone antagonists, where both estrogen receptor‐negative and positive breast cancer cell lines had shown improved results in suppressing cancer cell proliferation either as single tocotrienol isomers or combinedly as the tocotrienol‐rich fraction (TRF). There are studies in which tocotrienols have been combined with Tamoxifen to treat breast cancer, where both together had been more synergistic in their anticancer action. Further, tocotrienols were reported to stimulate the gonadotropin‐releasing hormone (GnRH) agonists in a study of bone loss induced by testosterone deficiency (Figure 11). 146 , 147 , 148 , 149
2.3. Tocotrienol in cancer immunology
Tocotrienol as adjuvants in immunotherapy has made promising advances in cancer treatment. A mouse model induced with a mammary tumor has revealed tocotrienol efficacy as an adjuvant in a vaccine, containing dendritic cells (DCs) important for antigen‐sampling and conveying the antigens for priming of inactive lymphocytes. Sub‐dermally injected TRF, together with dendritic cells and tumor lysate showed a better immunogenic response (with increased secretion of the pro‐inflammatory cytokines; gamma‐interferon and interleukin‐12), in attenuating tumor growth, compared to the control group given only the DCs, vehicle and the tumor lysate. The intervention group also produced more cytotoxic CD8 cells and natural killer cells in the peripheral circulation. 150 There is more evidence on the ability of tocotrienol to enhance DC‐mediated tumor immunity where tocotrienol had facilitated tumor elimination with increased lymphocyte proliferation. Adding to these observations is another reported study in which Inhibitor of differentiation/DNA binding Id‐1 (a member of the helix–loop–helix protein family expressed in actively proliferating cells), and beta‐catenin, two stem cell proteins promoted stem cell survival and their self‐renewal. Self‐renewal is the process of perpetuating the stem cell pool throughout life and division with the maintenance of the undifferentiated state. This requires cell cycle control and often maintenance of multipotency or pluripotency, depending on the stem cell type. 60 There are many more studies that have reported the induction of the NF‐kB 151 and PI3K/Akt 152 and the inhibition of MAPK 153 signaling cascade in suppressing tumor growth, proliferation, survival, and metastasis. Delta‐tocotrienol was able to significantly inhibit the release of pro‐inflammatory cytokines—tumor necrosis factor‐alpha (TNF‐α), IFN‐γ, IL‐1β, and IL‐6, in lipopolysaccharide (LPS) induced RAW 264.7 macrophages in addition to suppressing the phosphorylation of JNK and the ERK1/2 and MAPK pathways. 154 There are several different immune‐modulatory treatments which have been combined with tocotrienol as therapy for alleviating disease. They are celecoxib [which inhibits cyclooxygenase (COX)‐2], cetuximab [epidermal growth factor receptor (EGFR)], bortezomib (NF‐kB), etanercept (TNF‐alpha), trastuzumab [human epidermal growth factor receptor (HER)‐2], and bevacizumab [vascular endothelial growth factor (VEGF)] modulators. 108 The monotherapy of celecoxib, an anti‐cancer medication is limited in use by severe gastrointestinal and cardiovascular toxicity. When combined with gamma‐tocotrienol, it produced more potent inhibitory effects on cell proliferation by blocking the anti‐inflammatory COX‐2‐dependent as well as independent pathways along with decreased prostaglandin (PG‐2) levels and suppressed phosphorylated Akt and NF‐kB immune mechanisms. 155 Monotherapy of gamma‐tocotrienol is also limited by its inability to obtain and sustain effective therapeutic levels in tissues but the synergized action of celecoxib with gamma‐tocotrienol has produced enhanced anti‐cancer therapeutic effects. This effect was achieved at the concentrations of 0.25 μM gamma‐tocotrienol and 2.5 μM celecoxib in highly malignant in vitro + SA mammary epithelial cell cultures. 155 The clinical success of cetuximab or trastuzumab, which are monoclonal antibody therapy that targets ErbB receptor‐mediated anti‐neoplastic effects, was diminished as the receptors of the ErbB family together act to rescue mammary cancer cells from anti‐proliferative agents targeting single ErbB receptors. When combined with gamma‐tocotrienol, this EGF‐mediated anti‐cancer immune therapeutic drug produced an improvement by inhibiting the ErbB3 heterodimer transphosphorylation and selectively inhibiting the interactions between the ErbB3 receptor with the other ErbB receptor family members. 156 The VEGF‐targeted combined delta‐tocotrienol with bevacizumab therapy elicited a favorable outcome in refractory ovarian cancer. 74 The overall survival of patients was more in which HOXA9, a circulating tumor DNA biomarker of prognostic value was increased. Its usefulness was that the ineffective treatment regimens could be stopped during the early disease stages, suggesting that tocotrienol exerts an additive effect on bevacizumab in innervating anti‐tumor angiogenesis which the researchers of this study thought was necessary to suppress cancer progression. 74
The primary immune/metabolic mechanism associated with tocotrienol activity appears to be the inhibition of the NF‐kB pathway, 157 which is a central transcription factor that stimulates several genes involved in cell survival, proliferation, and inflammation (all of which are cancer‐promoting cellular metabolic processes). 158 In resting conditions, NF‐kB remains inactive, bound to the nuclear proteins p65 and p50 which are sequestered by the inhibitory nuclear factor of kappa light polypeptide gene enhancer in B‐cells inhibitor, alpha [IKBα—a 40 kDa protein that inhibits NF‐kB action]. 159 When pro‐inflammatory cytokines are released, such as TNF‐alpha, a signaling cascade commences with the stimulation of receptor proximal signaling complexes containing receptor‐interacting protein serine/threonine kinase (RIPI) and transforming growth factor‐β (TGF‐β)‐activated kinase 1 (TAK1), which begin the phosphorylation of RIPI leading to the activation of IKBα. 160 Activated IKBα releases the p65 and p50 dimers that translocate to the nucleus and bind to the respective gene promoters. 160 Tocotrienol exerts its effects by inactivating NF‐kB via sphingolipids, which in turn activate A20 (an immunogenic protein related to both innate and adaptive immunity), which can inhibit the NF‐kB activity during the early stages of transcription. 160 , 161 The sphingolipids activate A20, by first stimulating dihydroceramides. The biosynthesis of dihydroceramides is initiated by serine combining with palmitoyl‐coenzyme A which is catalyzed by serine palmitoyl transferase to produce 3‐ketosphinganine which is converted to sphinganine. Sphinganine produces dihydroceramide when catalyzed by dihydroceramide synthase. 162 Dihydroceramides promote ER stress by upregulating p‐IKBα, the protein of Jun N‐terminal kinase (p‐JNK), and translation initiation factor 2 (p‐eIf2α), those of which induce the action of A20 alongside Cezanne. Cezanne is a negative regulator of the NF‐kB by deubiquitinating TRAF3, an inhibitor of NF‐kB. 160 , 161 A20 with or without Cezanne can induce the TNF‐alpha receptor blockade by the TAKI, JNK, and NF‐KB pathways. 161
Gamma‐tocotrienol treatment as an adjuvant in breast cancer therapy was found to reverse the action of MDR‐1, by inhibiting the NF‐kB pathway. 163 Drug resistance is a considerable problem encountered in cancer therapy and the P‐gp, a plasma membrane protein pump, is associated with promoting drug efflux, thus inducing suboptimal or no drug accumulation within cancer cells. 164 The MDR phenotype is critically associated with the overexpression of ABC transporters of which the P‐gp plays a pivotal role. 165 Gamma‐tocotrienol action in cancer treatment attenuated the expression levels of MDR‐1 and P‐gp mRNA, in an in vitro study utilizing MCF‐7/Adriamycin breast cancer cell line, which also demonstrated its ability to reverse the drug efflux by using NF‐kB agonists/antagonists, MDR‐1 promoter activity, and P‐gp transporter activity. 163 The TRF induced apoptosis in another experiment conducted on the breast cancer cell line, MDA‐MB‐231. 111 The results indicated the induction of PARP cleavage (a hallmark of the cellular apoptosis process) with the inhibition of the NF‐kB pathway, leading to the suppression of unlimited cell proliferation ending up in anti‐tumorigenesis action. 111
In addition to the inhibitory modulation seen on the NF‐kB signal transduction by tocotrienol and specifically by gamma‐tocotrienol, the PI3K/Akt signaling mechanism inhibition also is reported by other studies. 166 PI3K/Akt pathway is a mitogen‐dependent signaling cascade which leads toward marked increases in cell proliferation, thus worsening the tumorigenesis observed in cancer patients. 166 The upside of tocotrienol is that it does not affect the normal cell viability, but suppresses PI3K/Akt signaling by activating caspases, particularly downregulating FLIP, an endogenous caspase inhibitor in mammary tumor cells. 166
Beta‐tocotrienol was reported to be a more potent anti‐cancer therapeutic tocotrienol form than the gamma‐tocotrienol in modulating a host of metabolic pathways, in an in vitro study performed on two breast cancer cell lines. 167 This study investigated the function of the apoptosis‐inducing proteins, p‐53, Bcl‐2, Bax, cytochrome C, cleaved PARP‐1 and caspase‐3, and key cell survival proteins; p‐PI3K and p‐GSK3‐α/β, which reported high sensitivity to the treatment and improved anti‐cancer efficacy, and hence was introduced as a more promising therapeutic agent given its innervation of multiple immune/metabolic pathways. 167 Beta‐tocotrienol was speculated to act via a p53‐independent, PI3K/Akt signaling pathway. 167 Additionally, the therapeutic action of beta‐tocotrienol executing a different anticancer mechanism to the previous study was published as in vitro studies on human lung and brain adenocarcinoma cells. 117 Anti‐cancer effects were produced by inducing double‐stranded breakages in DNA leading to apoptotic effects with chromatin condensation and formation of apoptotic bodies. 117 The underlying mechanism of beta‐tocotrienol was described as activating the cell death receptors and caspase‐8‐mediated mitochondrial apoptosis pathway. 117
Another mechanism of gamma‐tocotrienol was to induce TRAIL in human colon cancer tumor cells, by working through the death receptors (DR4 and 5) for which p53 and Bax upregulation are also required. 168 TRAIL is known to act as an apoptotic mechanism, which promotes anti‐cancer properties by downregulating cell survival proteins. 168 The TRAIL response was upregulated by gamma‐tocotrienol acting via ERK1, because ERK1 sequestration by siRNA subdues TRAIL, and was concluded that TRAIL upregulation by gamma‐tocotrienol proceeds through the stimulation of ROS/ERK/p53 pathway. 168
In prostate cancer cells (LNCaP) compared with a normal prostate cell line (PC‐3), the gamma‐tocotrienol therapeutic mechanism was identified to upregulate apoptosis by activating c‐JUN, caspases 3 and 9, and the Ras/Raf/ERK pathway. 169 The results indicated no therapeutic effect with alpha‐tocopherol on in vitro anti‐cancer therapy compared to gamma‐tocotrienol, irrespective of androgen sensitivity in those cells. 169 The mode of action of both gamma and delta‐tocotrienol on pancreatic cancer cell lines showed significant anti‐proliferative and apoptotic ability by suppressing; ERK/MAP kinase, phosphorylation of Akt, and ribosomal protein S6 kinase (RSK), through the downregulation of Her2/ErbB2 mechanism at the messenger level. 170 Another contradictory study on epidermal growth factor (EGF)‐induced neoplastic +SA mammary epithelial cells reported that gamma‐tocotrienol exerts its anti‐proliferative effects by the upregulation of ErbB3 tyrosine receptor phosphorylation (with heterodimerization), and not by ErbB1 and ErbB2 receptor mediation. 156 However, it was reported that optimal signal transduction with the ErbB family receptors occurs when ErbB3 is activated concurrently combined with either ErbB1 and/or ErbB2 receptors. 156 When the neoplastic mammary epithelial cells were treated with 0–8 μm, in vitro, gamma‐tocotrienol suppressed the PI3K/PI3K‐dependent kinase‐1 (PDK‐1)/Akt mitogenic signaling cascade in a dose‐dependent manner. 156
A similar in vitro study on +SA mammary neoplasm treated with combinatorial therapy consisting of a COX‐inhibitor, Celecoxib, and gamma‐tocotrienol was also reported to enhance antiproliferative effects as an improved formula with potential anti‐neoplastic properties. 155 This treatment combination together achieved the suppression of phosphorylated Akt, NF‐kB, COX‐2, and prostaglandin levels, where the researchers described it as a new therapeutic option for breast cancer because of the antiproliferation was dependent on the dose given. 155 Importantly, the suppression of COX‐inhibition by this formula also served to reduce the toxicity of Celecoxib, as monotherapy. 155
Delta‐tocotrienol treatment was reported successful in reducing ionizing radiation injury in CD2FI mice (measured against a 30‐day survival post‐Cobalt‐60‐gamma irradiation) through the induction of the granulocyte colony‐stimulating factor (G‐CSF). 171 Delta‐tocotrienol treatment had upregulated many different cytokines and proved to be exerting its radioprotective mechanism by stimulating a G‐CSF‐mediated mechanism because when the mice were injected simultaneously with delta‐tocotrienol and G‐CSF‐neutralizing antibodies, the radiation‐protectivity afforded by delta‐tocotrienol was abrogated. 171 In addition to delta‐tocotrienol, gamma‐tocotrienol is also reported to induce radioprotection by mediating a G‐CSF mechanism. 94 The tocopherols were known to exert radioprotection by inducing antioxidant action because the ionizing radiation damage was elicited by ROS and free radicals. Alpha‐tocopherol succinate, delta‐ and gamma‐tocotrienol have been introduced as more effective radiation countermeasures. However, delta‐tocotrienol seems to induce radioprotection by stimulating signal transduction of various molecular mechanisms and signaling pathways in addition to its antioxidant action. 171
In summarizing the key metabolic mechanisms through which the tocotrienols broadly exert their anticancer therapeutic actions (Figure 12), they primarily involve the downregulation of the NF‐kB, PI3K/Akt/GSK, p38 (MAPK), TGF‐β, Ras/Raf/Mek/ERK, activation of the caspases and reduction in cyclin D1, cyclin‐dependent—kinases 2,4,6 and apoptosis induction.
3. ANTIOXIDANT AND ANTI‐INFLAMMATORY ACTIVITIES OF TOCOTRIENOL
3.1. Antioxidant behavior of tocotrienol
Vitamin E leads the cell antioxidant defense system, as it is a major lipid‐soluble antioxidant bound to the cellular and organelle membranes and is one that offers protection to the membranes by preventing lipid peroxidation. 172 Antioxidant efficacy of tocotrienol is well‐documented and already established. 173 , 174 Oxidative stress arises from the disruption of redox‐signaling and molecular damage that erupts from endogenous free radicals such as ROS, hydroxyl free radicals, unstable superoxides, and nitric oxide (NO), released from intracellular metabolic activities and the breakdown of immune pathways. 175 , 176 A study conducted on evaluating the antioxidant properties of tocotrienol using primary hippocampal neurons have shown that gamma‐tocotrienol provides neuroprotection by upregulating the B‐cell lymphoma‐extra‐large (Bcl‐xL) family of proteins known to induce anti‐apoptosis and maintain neurotransmission at its optimum. 177 The neuronal excitotoxicity was shown to release free radicals such as ROS due to post‐translational cleavage of Bcl‐xL proteins and the accumulation of its products within the mitochondrial membranes leading to mitochondrial dysfunction and depletion of intracellular ATP, resulting in subsequent neuronal death. 177 The hippocampal neurons when treated with alpha‐tocotrienol and glutamate (which induces ROS), was found to offer neuroprotection through exerting its antioxidant properties which prevented the loss of mitochondrial membrane potential. 177 The endoplasmic reticulum (ER)‐associated stress is mostly induced by misfolded proteins and is linked to oxidative stress because a disruption of redox homeostasis also yields an unfolded protein response. 178 Another study investigating the anti‐ER stress mechanisms of gamma‐tocotrienol using +SA mammary tumor epithelial cells have reported that its mode of action in producing anti‐apoptotic effects is not by mitochondrial dysfunction or the death receptors but by increasing PARP cleavage and activation‐mediated protein kinase‐like ER kinase/eukaryotic translational initiation factor/activating transcription factor 4 (PERK/eIF2alpha/ATF‐4) pathway, which is a marker of ER stress response. 178
An investigation into the impact of oxidative stress on bone formation, osteoblasts were exposed to hydrogen peroxide and were treated with varying gamma‐tocotrienol concentrations ranging from 1 to 100 μM, in which the production of malondialdehyde, a final product of lipid peroxidation, free radical release, a marker of oxidative stress, and antioxidant status in cancerous patients 179 was dose‐dependent. 131 Further, in evaluating the antioxidant enzyme activity, gamma‐tocotrienol prevented the reduction in superoxide dismutase (SOD, using tetrazolium assay into measuring the superoxide radicals), catalase (needed to breakdown hydrogen peroxide), and glutathione peroxidase activity (catalyzing the reduction of hydrogen peroxide and peroxide radicals) even at lower concentrations. 131 At the higher dose, gamma‐tocotrienol itself had become toxic to the osteoblasts, suggesting that at higher concentrations, it could become pro‐oxidant or pro‐apoptotic to bone tissue. 131 A study which had investigated the TRF‐mediated antioxidant efficacy in experimental diabetic rats had reported increased SOD activity, increased vitamin C levels, reduced lipid peroxidation in the thoracic aorta homogenates measured with malondialdehyde. 180 Tocotrienol treatment had served to lessen the oxidative stress inflicted upon the blood vessels reiterating that the type 1 diabetes mellitus leads to atherosclerosis, resulting in vascular damage. 180 TRF‐treated diabetic rat thoracic aorta showed reduced vascular smooth muscle cell proliferation and degeneration, a smooth lining of the intima with fewer defects, a more regular elastic lamina, squamous characteristics in the endothelium, and increased SOD activity in aortic homogenates, 180 These results suggest that the oxidative stress and DNA damage, induced by diabetes, is attenuated by TRF treatment. 180 Additionally, the ability of the TRF to inhibit oxidative stress arising from UV radiation in murine skin and improve wound healing mechanisms in diabetic animals by a possible free radical scavenging role has been reported. 181
The development of diabetes mellitus also produces impaired skeletal muscle growth. 182 A study in which diabetes was induced by a high fat diet and streptozotocin, a C57BL/6J mouse model, had shown reduced skeletal muscle‐related protein levels (4‐hydroxynonenal, protein carbonyls, Nrf2, and HO‐1), associated with oxidative damage upon treatment with TRF. 182 These results had suggested a protective role in TRF against skeletal muscle atrophy which could arise from oxidative stress in diabetic mice, explained partly due to TRF‐induced mitochondrial biogenesis. 182 It was achieved by the upregulation of SIRT1 (Sirtuin or silent mating type information regulation 2 homolog) 1 (S. cerevisiae), SIRT3, and AMPK pathways which aid glucose metabolism via insulin signaling in type 2 diabetic mice. 182 Further studies are summarized in Table 2. The main strength of tocotrienols arise from their ability to influence the endogenous fat‐soluble lipids [chylomicrons, chylomicron remnants, very‐low‐density lipoproteins (VLDL), intermediate‐low‐density lipoprotein (IDL), low‐density lipoprotein (LDL), high‐density lipoprotein (HDL), cholesterol, triglycerides, and apoliproteins] preventing lipid peroxidation and attenuating oxidative stress. 183
TABLE 2.
The latest tocotrienol studies on oxidative stress (published in 2021)
| Model | Disease | Tocotrienol treatment regimen | Results | Outcome | Reference |
|---|---|---|---|---|---|
| Strain (N2) of Caenorhabditis elegans (L4 stage) | Oxidative stress | 100 μg/ml at 20°C for 4 h in a petri‐dish | Survival of the nematodes was considered as having antioxidant activity | Delta tocotrienol extracted from Achiote seeds possesses antioxidant properties | 323 |
| Mouse | Oxidative stress in the liver | 2000 mg/kg for 14 days | Induced maximal Nrf‐2 gene expression within 1 h. | Tocotrienols (TRF) promote antioxidant‐related hepatoprotection in mice, induced by cytoprotective Nrf‐2 gene upregulation. | 324 |
| Sprague Dawley Rats | Oxidative stress | 60 mg/kg bodyweight for 8 weeks | Increased bone strength, bone microstructure, calcium content in ovariectomized rats with antioxidant activity. | Annatto‐derived delta‐tocotrienol induces antioxidant activity and bone health in estrogen‐deficient rats. | 325 |
| Characterization of physicochemical properties of vitamin E combined with cyclodextrin (CD) | Antioxidant properties | Formation of Vitamin E and βcyclodextrin complex by solution method. | Vitamin was physically encapsulated by the β‐cyclodextrin with extended lifetime dependent on β‐CD concentration. | Antioxidant activity increased with free radical scavenging ability in the inclusion complex measured by fluorescence intensity. | 326 |
| Assessment of tocotrienol therapeutic potential in Sea Buckthorn juice. | Antioxidant, anti‐inflammatory, and neuroprotection effects | Measured by ultra‐performance liquid chromatography coupled with fluorescence detector | Anti‐enzyme and antioxidant properties of the juice correlated selectively with the composition | Valuable dietary source of vitamins E and A and which enhances antioxidant, anti‐inflammatory, and protection from neurodegeneration processes. | 327 |
| C57BL/6J WT and db/db mouse | Type 2 Diabetes | A modified form of tocotrienol‐β (deh‐T3β) given mixed with diet for 2 months | Improved kidney and liver function, reduced liver steatosis, faster wound healing, insulin efficiency in adipose tissue, and improved heart recovery after ischemia. | Antioxidant properties and mitochondrial function were improved. | 328 |
| C57BL/6J mouse | Obesity and obesity‐related metabolic disorders | High‐fat diet +800 mg/kg BW for 14 weeks | Lowered serum metabolites, inflammatory and antioxidant biomarkers, and improved carbohydrate metabolism. | A nontoxic dose of tocotrienol improved obesity‐associated hypercholesterolemia and hyperglycemia | 196 |
3.2. Anti‐inflammatory behavior of tocotrienol
Inflammation is an integral component of innate immune reactions and comprises acute and chronic inflammatory responses. 184 They are elicited as part of immune surveillance and host defense mechanisms for maintaining immune homeostasis. 184 Acute inflammation is needed for prompt resolution of a disease state that is self‐regulatory while chronic inflammation is a pathophysiological defect that progresses into impaired homeostasis. 184 Importantly, both these immune aberrant mechanisms are attenuated by tocotrienol therapy. Chronic inflammation has manifested as chronic kidney disease, rheumatoid arthritis, CVD, diabetes, cancers, neurodegeneration, metabolic syndrome, and liver disease. 185 Tocotrienols have successfully curbed inflammation by a variety of physiological mechanisms of which the downregulation of the gene expression of pro‐inflammatory cytokines and the repression of inflammatory biomarkers are more common strategies. 186 The prominent biomarkers that are suppressed with tocotrienols include IL‐6, TNF‐a, MDA, and C‐reactive protein although most results have been inconsistent. 187 The dosage and the individual tocotrienol isomer profiles had reported different outcomes and in general, tocotrienols were not very successful in attenuating inflammation as per a meta‐analysis that was published recently. 184 TRF was more effective against single isomers, of which both delta and gamma tocotrienols produced better results than alpha and beta counterparts. A dose of 400 mg/day or above had reduced MDA levels while CRP was fair downregulated. 184 For the lack of high‐quality clinical data, the anti‐inflammatory status of tocotrienols remains inconclusive. The main concern is the discrepancy between preclinical studies and clinical studies, the former being nonreproducible in humans. There was an increase in HDL cholesterol levels following tocotrienol treatment but no effect on total cholesterol level, LDL‐cholesterol, or triglycerides. 188 The oral administration was insufficient to upregulate tocotrienol bioavailability to repress inflammation due to shorter half‐life, fast elimination, and hindered transportation in plasma. A high quantity of dietary lipids were needed to stimulate bile secretion and emulsification for efficient absorption of tocotrienols and the cellular transportation of tocotrienols was not efficient although ready incorporation into cell membranes made cellular influx more favorable. Among the anti‐inflammatory mechanisms of tocotrienols are inhibitory action of NF‐kB and STAT‐3 pathways 189 and suppression of inflammatory mediators such as IL‐1, IL‐6, IL‐18, IL‐8, TNF‐alpha, and inducible nitric oxide synthase. 190 The biomarkers of oxidative stress have also shown inflammation resolution that is caused by ROS and RNS, free fatty acids, chemotherapy, and ionizing radiation. When the individual tocotrienol isomeric forms were compared, delta‐tocotrienol was more inhibitory on IL‐6 and TNF‐alpha levels in LPS‐stimulated RAW 264.6 and RAW 264.7 macrophages respectively than the gamma‐ and alpha‐tocotrienol or the TRF. 186
Obesity and insulin resistance are two disease states in which the adipose tissue develops inflammation by the Nod‐like receptor protein‐3 (NLRP3) inflammasome activation. It leads to impaired lipid metabolism by stimulating the release of eicosanoids. Saturated fatty acid—stimulation activates the NLRP3 inflammasome in innate immune cells and thus, the gamma‐tocotrienol treatment showed attenuation of lipopolysaccharide (LPS) and SFA‐stimulated NLRP‐3 inflammasome activation in bone marrow‐derived macrophages (BMDM) in vitro. 191 Gamma‐tocotrienol repressed the NLRP3 inflammasome activation by the downregulation of arachidonic acid metabolism (a precursor of eicosanoids), diacylglycerol, prostaglandin release, and COX‐2 activation, which led to the modulation of the BMDM lipid profile. It modulated the ensuing glycerolipid, glycerophospholipid, lysophospholipid, and sphingolipid production via the suppression of eicosanoid release and inhibited the de novo synthesis of ceramides of the dihydroceramide pathway. The lipid metabolism, which is altered during inflammation, impacts the energy homeostasis in macrophages and was shown partly rescued by gamma‐tocotrienol. 191 The activation of the NLRP‐3 inflammasome results in aberrant innate immunity that develops into metabolic diseases, particularly type‐2 diabetes. Another in vitro study of macrophages treated with Annatto seed‐derived delta‐tocotrienol showed a marked decrease in ROS, which is the second signal needed for the priming and activation of the NLRP‐3 inflammasome. 192 The activation of the inflammasome occurs through intracellular sensing of endogenous damage‐associated molecular patterns (DAMP), from molecules of microbial infections that produce pro‐inflammatory cytokines such as interleukin 1‐beta (IL‐1β) which is a hallmark of progression into chronic inflammatory diseases. Delta‐tocotrienol was effective in inhibiting the priming of the inflammasome by suppressing the NF‐kB pathway and the ROS production leading to the cleavage of caspase‐1 needed for IL‐1β secretion in murine macrophages, at a concentration of 1 μM that suggested its use in immune modulation (MAPK, p‐ERK, p38) and delaying of the onset of chronic inflammation. 192 Further, the NLRP3 inflammasome activity in a chronic inflammation mimetic of type 2‐diabetes was attenuated by gamma‐tocotrienol in a model of leptin receptor knockout mice. 193 This study introduced a two‐prong approach by gamma‐tocotrienol consisting of downregulating A20‐induced TNF‐alpha interacting protein 3‐activated NF‐kB signaling cascade and the suppression of caspase‐1 cleavage by adenosine mono phosphate (AMP)‐activated protein kinase autophagy, the mechanisms of which were successful in curtailing the progression of type‐2 diabetes in those mice. 193
3.3. Link between antioxidant and anti‐inflammatory properties of tocotrienol
Cellular oxidative stress can produce chronic tissue inflammation. 194 Antioxidant properties of a compound can also be anti‐inflammatory because the former protects the tissues from ROS‐activated tissue damage. 194 Thus, both these metabolic pathways synergize each other. A compound which is capable of neutralizing oxidative damage, invariably serves to enhance anti‐inflammatory reactions as well. 195 Tocotrienols are major tissue antioxidants as well as anti‐inflammatory which together produce attenuation of tissue injury that arises from oxidative agents that could continue to ignite tissue inflammation. 196 Vitamin E forms are well‐known antioxidant nutrient supplements that act to prevent unwanted inflammatory responses that are caused by exogenous and endogenous oxidants in the cellular environment. 195 Antioxidant agents comprise enzymatic and nonenzymatic compounds, of which, tocotrienol, a nonenzymatic compound, can induce the action of the enzymes; superoxide dismutase, catalase, glutathione oxidase, glutathione transferase, heme oxygenase‐1 Redox proteins, and peroxiredoxin. 197 Thus, the therapeutic value of vitamin E compounds, particularly the tocotrienols is further enhanced due to its ability to stimulate dual roles by which its anti‐cancer properties are augmented. 198 The prolonged localized cellular inflammatory reactions (generated by dysfunctional electron transport chain in mitochondria, ER and cell membrane, nicotinamide adenine dinucleotide phosphate [NADPH] oxidase, peroxidases, and nitric oxide synthase) can give way to tumorigenesis from which cancers arise, and those cancers have been attenuated by tocotrienols. 199 Among the different types of oxidative damage, DNA oxidation, protein oxidation, and lipid peroxidation are known to produce carcinogenesis, and tocotrienols were identified as having anti‐cancer properties that ameliorated the malignant tumors by apoptosis, cell cycle arrest, and disrupted DNA replication and curbed metastasis. 199 DNA oxidation impairs the DNA replication process, and transcription, and upregulates transversion mutations, protein oxidation will lead to peptide chain breakages and susceptibility to proteolysis, while lipid peroxidation results in cell membrane damage in all membrane‐bound organelles. 200 Disruption of the plasma membrane would result in the inactivation of signal transduction by denaturing receptors and enzymes. The oxidative stress and chronic inflammation form an interconnected vicious cycle where both situations impinge upon the individual's immune system. 201 The most abundant immune cell cohort that protects the organelles, tissues, and the organs from infections and injury onslaughts are the macrophages which release free radical intermediates during the process of promoting both innate and adaptive immunity. 202 As the putative therapeutic role of the tocotrienols is fortified by its multiple roles in downregulating the oxidative, inflammatory, and cancerous responses. 203 The mechanism of action in antioxidants is neutralizing ROS and ROS‐induced intermediates by donating hydrogen atoms, which make them potent hydrogen (or proton) donors. 204 The antioxidant agents prevent further oxidation of ROS‐related biomolecules by participating in many rounds of peroxyl radical quenching and reduction by ascorbate or ubiquinone. 205
4. TOCOTRIENOL ACTIVITY IN SYSTEMIC DISEASE
As shown in Figure 1, tocotrienols have been effective in ameliorating many diseases in preclinical and clinical studies. A plethora of research studies bears witness to the therapeutic potential of the tocotrienol isomers in health and disease. This section includes a review of experimental tocotrienol therapies that have induced beneficial effects in many human organ systems. The importance of tocotrienol as a putative therapeutic agent 206 has been recognized with decades of research which has reported its diverse biological functions as shown in Figure 4. 207 Tocotrienol is now well known for its antioxidant, 208 anti‐inflammatory, 190 anti‐cancer, 89 immune‐modulation, 209 damaged DNA‐repair, 210 plasma cholesterol‐reducing, 211 anti‐diabetic, 212 neuro, 213 renal 214 and skin protective 215 effects in addition to longevity, 216 bone health, 217 and improved cognition. 218 It also modulates the plasma lipid profile 219 and the white matter lesions in the brain. 220 The influence of tocotrienols as modulators of many cellular metabolic pathways has also been reported with the discovery of its involvement in producing cytokines, 94 growth factors, 221 kinases, 109 transcription factors, 222 adhesion molecules, 223 and apoptotic regulators. 111 Although tocotrienol has been widely recognized as a multi‐faceted, 224 multi‐beneficial 225 treatment option for many different human diseases, whether as a single therapy 92 or in combination with other drugs, 226 but an effective, cumulative, dose for its use has not been elucidated yet, 54 and varies widely depending on the disease type or malignancy. The prospective recommended dosages have been described as 100–200 mg/day, 227 for neuro and renal protection, anti‐inflammatory, and anti‐aging related diseases, but for cardio protection, it is 100–960 mg/day. 1 The consumption of alpha‐tocopherol above 400 IU/day or about 270 mg/day is excessive or wasteful. 228 The plasma concentration of the tocotrienol forms alpha, gamma, and delta are considerably increased in the fed‐state with a two‐fold greater absorption. 229 Double dosing or the same recommended dose taken twice daily had appeared effective since tocotrienol has a short half‐life and such a regimen serves to increase the circulating plasma level of those tocotrienol forms compared to tocopherol. 230
4.1. Renal disease
Renal disease can occur as an adverse effect of anti‐cancer treatments 231 as well as other drugs prescribed for a multiarray of human diseases. 232 Acute kidney failure could develop within a short time or because of aging or chronic disease conditions. 233 In this section, the therapeutic potential of tocotrienol is discussed relevant to renal dysfunction, which has been a promising treatment strategy to restore renal homeostasis. 234 , 235 An imbalance of the steady‐state in renal functionality could evolve due to the toxicity of different pharmacotherapy or overloading of various metabolic compounds or drugs given to alleviate ill health that is excreted by renal ultrafiltration. Renal nephropathies are a combination of several disease conditions associated with the kidneys and may include, kidney stones, analgesic abuse, multiple myeloma, untreated urinary tract infections, glomerulopathies, non‐diabetic kidney disease, and hypertension. 236 , 237
A randomized, double‐blinded, placebo‐controlled clinical trial was conducted in patients having diabetic nephropathy (usually a complication of diabetes which requires either a renal transplant or dialysis), and were supplemented with tocotrienol (as Tocovid, 200 mg twice daily for 12 weeks). 238 Tocotrienol treatment had produced a remarkable decrease in serum creatinine and a marked increase in estimated glomerular filtration rate (eGFR; which are common biomarkers of end‐stage renal complications). 238 These biomarkers had remained static for 6–9 months post‐treatment and other renal dysfunction parameters such as serum HBA1C, MDA, TNF‐alpha, VCAM‐1, and urine albumin and creatinine ratio had not been reduced. 239 This trial was limited to hyperglycemic patients having type 2 diabetes and those receiving treatment for cancer, cardiovascular, and liver disease, and those taking vitamin C and E, polyphenols, or glutathione were excluded. 239 Moreover, as the serum tocotrienol bioavailability diminishes after around 2 months from treatment, and is mostly deposited in adipocytes, any measurement relating to its tissue retention must be made by sampling the adipose tissue. 239
The Phase II trial of the previously mentioned study [that sampled individuals diagnosed with later stages of Diabetic Kidney Disease (DKD)] had Tocovid administered for 16 weeks, was extended to 12 months of twice‐daily supplementation of 200 mg of tocotrienol as another separate study. 18 The treatment‐washout period (when a participant is taken off a study drug or other medication to eliminate the effects of the treatment) remained up to only 6 months with no improvement in serum creatinine level or eGFR. 18 However, up to 2 months post‐treatment, serum creatinine levels remained reduced and the eGFR increased, without a significant improvement in the urine albumin to creatinine ratio (UACR). 18 This investigation was hampered by the Covid‐19 situation, but testing had continued every 2 months during the 12‐month trial duration. The statistics on DKD have revealed that up to 40% of diabetic patients are likely to develop DKD and most of those suffering from DKD present up to 50% of end‐stage renal disease. 18
Renal injury is induced by both hyperlipidemia and hyperglycemic conditions acting synergistically with each other, and patients with diabetes mellitus are highly prone to developing diabetic nephropathy as dyslipidemia, a high increase of fats and cholesterol accumulation in blood, which commonly occurs in those individuals. 240 A study that explored the efficacy of the TRF as a treatment offered for nephroprotection, had evaluated the expression of TGF‐β on fibronectin and collagenase IV levels in kidney tissue of type‐2 diabetic rats. 240 The outcome was the attenuation of kidney injury, after continued treatment for 16 weeks. 240 This study demonstrated that the TRF acts as a potent hypolipidemic, hypoglycemic, and an antioxidant agent which protects against the development of diabetic nephropathy in rats. 240
The chromium compounds, specifically those of chromium IV poses a high risk of developing acute kidney injury (AKI) through industrial or occupational exposure, or by inhalation, ingestion, or intravenous administration, in both humans and animals. 241 A study on male Wistar rats in which AKI was induced by potassium dichromate injection and treated with TRF (with 200 mg/kg daily for 3 weeks) to investigate the nephroprotection offered by tocotrienol, reported sustainable glomerular function, redox status, and selective reabsorption in the proximal convoluted tubule. 242 The untreated group developed AKI within 48 hours with significant oxidative stress‐induced tissue damage also validated by kidney histology. 242 Prolonged chronic renal disease is known to be induced by hyperlipidemia, which also causes atherosclerosis. An animal study using Wistar rats were treated with the TRF (extracted from palm oil) after inducing atherosclerosis by feeding an atherogenic diet consisting of high cholesterol, cholic acid, and beef tallow having high calorific value. 243 After 6 weeks of TRF treatment (given 100 mg/kg body weight), the animals displayed significant improvement in kidney function including eGFR. 243 The untreated controls had developed dyslipidemia, as well as atherosclerosis and the researchers, concluded that the TRF offers nephroprotection against an atherogenic diet‐induced progressive chronic renal dysfunction in Wistar rats. 243
A study done on the dietary intervention of vitamin E in chronic hemodialysis patients given a combination of tocotrienol (TRF, 180 mg/daily) and tocopherol (40 mg/day) reported a significant lowering of the lipid profiles after a 16‐week supplementation. 244 At this end‐stage, renal functioning showed no response to either inflammatory markers (IL‐6 and C reactive protein) or antioxidant markers (MDA and total antioxidant power) for the duration of this study. 244 The TRF intervention group displayed higher apolipoprotein A1 (the main lipid component in high‐density lipoprotein (HDL) or good cholesterol and a biomarker of lipid metabolism) and cholesteryl‐ester transfer protein activity which is a plasma protein bound to HDL, that transports cholesterol). 244
4.2. Cardiovascular disease
Cardiovascular disease (CVD) is considered as the number one global cause of mortality with around 18 million persons dying each year from it. 245 CVD risk factors are multifocal and high lipid profiles, lead to atherosclerosis and other vascular defects which arise commonly in most populations that consume high fat (lipid peroxidation, dyslipidemia) and/or high sugar diet. 246 These defects arise from mitochondrial glucose oxidation resulting in mitochondrial ROS, which causes oxidative stress damage to the vasculature. 247 The superior benefits of tocotrienol treatment in lowering CVD are established because of its pharmacotherapeutic actions such as the inhibition of vascular smooth muscle proliferation, 248 HMG‐CoA reductase activity, which is the major rate‐limiting enzyme in cholesterol biosynthesis, 249 oxidation of low‐density lipoproteins (LDL), 206 monocytes endothelial cell adhesion, 250 downstream inhibition of protein kinase C (PKC) 251 and platelet aggregation. 252 However, there are some studies which have reported not having either superior or detrimental effects upon tocotrienol supplementation in CVD, possibly due to insufficient bioavailability, 253 improper delivery (oral gavage being a reason for sub‐optimal accumulation and tissue retention) 254 and poor metabolism. 48 Also, atherosclerosis results as a response toward vascular injury caused by hyperlipidemia that progresses into hypertension. 255
The CVD risk increases with aging, particularly in the above 70‐year category. 256 The common treatment available for those aged individuals is the use of statins to lower cholesterol levels 257 and beta‐blockers to control hypertension. 258 However, these medications are prone to side effects, such as cardiomyocyte thickening 259 atrial or ventricular hypertrophy of the heart walls and wasting muscle diseases (such as rhabdomyolysis), muscular pain, and soreness. 260 Therefore, tocotrienols have been experimented with by many researchers, given its antioxidant, anti‐inflammatory, and anti‐diabetic properties. Those individuals contracting diabetes run a very high risk of presenting with CVD symptoms, particularly vascular injuries. 261 The onset of aging can create a vicious cycle of diseases such as diabetes, renal disease, and CVD which are interdependent and the therapeutic potency of tocotrienol is that it consists of nutraceutical properties which could alleviate many metabolic deficiencies (Figure 13). 262 , 263
Varying concentrations of alpha‐, gamma‐, and delta‐tocotrienol forms in combination with alpha‐tocopherol and Tocomin, a patented self‐emulsifying tocotrienol and tocopherol complex having better absorption as a commercially available dietary supplement, were tested for their efficacy in inducing antioxidant functions in a pyrogallol‐induced oxidative stress condition in an in vitro study of the rat thoracic aorta. 264 The inhibition of the superoxide generated by the treatment combinations used were measured by the hypoxanthine/xanthine oxidase assay and in the presence of NADPH oxidase, a potent generator of ROS. 264 This study reported that Tocomin had a superior antioxidant effect followed by alpha‐tocopherol than the other tocotrienol forms on vascular function and for optimal antioxidant activity in the aorta, a combination of both tocotrienol and tocopherols was needed. The same study also measured the endothelium‐mediated relaxation induced by oxidative stress in the presence of acetylcholine (known to induce endothelium‐dependent relaxation) and sodium nitroprusside (a vasodilator which induces endothelium‐independent relaxation) in which Tocomin was 100 times more effective than alpha‐tocopherol alone or when the different tocotrienol forms taken individually. 264 This research confirmed that the tocotrienols together act as a cardioprotective agent against oxidative stress‐induced vascular dysfunction. 264 Importantly, vitamin E was found to increase nitric oxide (NO) production which in turn serves to improve the endothelial‐dependent vasodilatation by relaxing the vascular smooth muscle cells. 265 NO is produced by the enzyme l‐arginine‐mediated by eNOS which is beneficial in CVD. 266 NO at low levels is known to neutralize free radicals to improve oxidative stress damage in blood vessels. 266
4.3. Nervous system disease
The beneficial role of vitamin E in neuroprotection is already recognized. 267 The importance of tocotrienols is established given its ability to maintain neuronal membrane integrity (being lipophilic in nature) by counteracting the ill effects of lipid peroxidation. 268 Orally delivered tocotrienols reach brain tissue 30 and cerebrospinal fluid 269 through systemic circulation and protect against free radical‐induced oxidative damage leading to neuronal death and defective synapses. 270 This process has been validated by its neuroprotective action against glutamate‐induced ROS, NO, and/or neurotoxic metabolites. 271 Tocotrienol supplementation was very effective in sustaining the structure and function of the nervous system. 272 Tocotrienol consists of properties which can nullify the exposure to damaging neurotoxic metabolites produced within the body and significantly attenuating subsequent neurodegeneration which impairs cognitive functions. 213 , 218 Although the mode of action of tocotrienols in neurology is still largely unknown, it is deemed beneficial to maintaining the health and prevent diseases of the nervous system. 273 Tocotrienol treatment of HT4 cells, which are mouse neurons taking the properties of differential neurons at restrictive temperatures such as 39 Celsius after glutamate challenge, was promising although it is speculated as acting along with an antioxidant‐independent mechanism. 274 These may include stimulating other signal transduction pathways leading to the modulation of cytokine production.
The cerebral small vessel disease is a hitherto unexplored neurological condition which runs a high risk for the development of several debilitating neuropathies such as ischemic stroke, dementia (up to 40% risk in older people), Alzheimer's disease, and acute physical disabilities (mostly in the over 65 ages). 275 It occurs because of perforations in the blood vessels in the brain which is concurrent with the inability to self‐regulate the cerebral blood flow. 275 These hemodynamic changes are produced by hypertension and arterial stiffness caused due to oxidative stress which arises in the cerebral vasculature and when treated with the vitamin E derivatives (alpha‐tocopherol and alpha‐tocotrienol), was significantly downregulated. 275 Vitamin E is a beneficial treatment for neuropathological conditions because of its ability to protect PUFA which offers neuroprotection to the brain. 22 Arachidonic acid is the major PUFA present in the central nervous system (CNS) and is metabolized by both enzymatic and nonenzymatic pathways, which produce neurotoxic metabolites. 276 Neurotoxicity was attenuated by nanomolar concentrations of palm oil‐derived alpha‐tocotrienol, thus preventing neurodegeneration. 277 The effectiveness of tocotrienol forms was tested in microglia that are the most abundant, (up to 80%) resident immune cells of the CNS, which respond to injury and stress by releasing NO at toxic levels to the brain tissue. 278 Mouse BV2 microglia were tested in vitro by adding different concentrations of three palm oil‐extracted tocotrienols (alpha, gamma, and delta) to examine the NO release induced by lipopolysaccharide (LPS). 278 The results revealed a significant lowering of NO production by the microglia treated with the different tocotrienol forms (at 48 h LPS posttreatment) and delta‐tocotrienol was the most effective at a 50 μM concentration. None of the isomers was found to compromise the microglial viability. 278
Chronic cerebral hypo‐perfusion (known to set in with aging) develops low cerebral blood flow which results in neuronal cell death leading to neurodegeneration of cognitive functions. 277 A Sprague–Dawley rat model of similar neurodegeneration was induced by bilateral ligation in two carotid arteries to lower the blood flowing into the brain and were named the two vessel‐occluded (2VO) group. These mice were compared with a Sham group and a vitamin E tocotrienol‐treated group in which the oxidative stress levels were ascertained by measuring the isoprostane level in brain homogenates, which is a prostaglandin‐like accurate marker of lipid peroxidation in both animal and human models of oxidative stress in the CNS. 277 Neurodegeneration status was assessed by counting the dead neurons in the hippocampus, cerebral cortex, white matter, and the visual system. There was no significant difference either in the isoprostane levels or the neuronal death count between the vitamin E tocotrienol‐treated group and the sham group. 277 It provided evidence that tocotrienol offers neuroprotection in conditions of insufficient cerebral blood flow that reduces the ATP supply to the brain. 277
Parkinsonian disease (PD) is a progressively debilitating neurodegenerative pathologic condition induced by a marked reduction in the dopaminergic neurons which causes motor and nonmotor dysfunction. 279 In a recent study (in 2021), PD was induced by a single dose of intracisternal injection of 6‐hydroxy dopamine (6‐OHDA) in Sprague–Dawley rats, and the efficacy of tocotrienol was measured utilizing behavioral parameters such as paw retraction, beam travel, and cylinder tests and immunohistochemical specimens obtained from the substantia nigra in the mid brain. The diseased animals showed depigmentation, loss of dopaminergic neurons, and striatum fiber density. 279 and alpha‐tocotrienol showed amelioration of the symptoms of PD and was able to reverse the neurodegeneration also by improving motor deficiencies. 279
The multi‐drug resistance protein‐1 (MRP‐1) is a key factor associated with the induction of ischemic stroke in mice and was found elevated in glutathione‐induced mouse brain lesions. 280 Alpha‐tocotrienol was identified as a neuroprotector which shields against stroke in vivo, by suppressing the neurotoxicity that arises from glutathione depletion and microRNA activity in the areas of brain affected by stroke. 280 Primary cortical neurons challenged with glutathione oxidase in vitro, showed reduced ischemic stroke lesions when treated with alpha tocotrienol, but its mode of action was attenuated in MRP‐1‐knockout mice. 280 Thus, MRP‐1 also appears to play a protective role in downregulating neuronal cell death by increasing glutathione efflux from neural cells and it was suggested that the therapeutic action of alpha‐tocotrienol may be mediated via a novel mechanism involving MRP‐1 which makes stroke‐related MRP‐1 and microRNA as sensitive targets of tocotrienol. 280 Further, the elevation of intracellular glutathione disulfide (GSSG, the oxidized form of glutathione) triggers neural cell death. Excessive cellular GSSG is toxic and therefore pumped out at the expense of ATP. Inefficient clearance of intracellular GSSG was shown to build‐up neurotoxicity. These observations highlight the significance of cellular GSSG clearing systems in neuroprotection, especially in the context of the stroke where oxidative stress is overt. 281
White matter lesions in the brain are related to cerebral small vessel disease. In evaluating tocotrienol treatment on white matter lesions, twice daily supplementation of an oral tocotrienol mix, was significantly effective compared to the control group. 220 It was concluded that the tocotrienol therapeutic potential in neuropathies considerably increases when supplied as a mix of the different forms than single isomer supplementation. 220 Their conclusion was further supported by studies carried out with Tocomin, which displayed superiority against treatment with individual tocotrienol forms since it consists of a mix of both tocotrienols and tocopherols. 282 The superiority of tocomin compared to those individual vitamin E forms was reported in impaired cellular antioxidant behavior. It may be attributed to the release of nitric oxide (NO), less eNOS and NADPH oxidase (Nox‐2) activity, activation of diacyl glycerol, and subsequent protein kinase C (PKC). Less NO leads to accumulation of superoxide radicals that increase oxidative stress resulting in impaired cellular functions. 264
Hyperalgesia is extreme sensitivity to pain, and results from damaged nerves and/or chemical changes in nerve pathways and also is associated with microvascular complications of diabetes mellitus. 283 This excruciating pain is related to oxidative and nitrosative stress arising from ROS, NO, cytokines, and apoptosis that is referred to as diabetic neuropathic pain and was induced in an experimental diabetic rat model to investigate the effectiveness of tocotrienol treatment in alleviating the pain. 283 Those rats were treated with tocotrienol combined with insulin, from which the hyperglycemia was improved with a marked reduction in pain by modulating the oxidative and nitrosative stress factors, caspase 3, and proinflammatory cytokine release. 283
Alpha‐tocotrienol is well known for its neuroprotective effects in ischemic stroke. 284 In a canine model of ischemic stroke treated with tocotrienol‐enriched fraction for 10 weeks, the fluoroscopy‐guided angiography images and magnetic resonance imaging of the brain in the intervention group revealed improvement in the areas of stroke attributed to sustained collateral cerebrovascular circulation in the ischemic territory. 284 Tocotrienol enrichment showed upregulation of the tissue inhibitor of metalloprotease‐1 (associated with vascular remodeling) and downregulation of matrix metalloproteinase‐2 which is a biomarker of neural inflammation, the breakdown of the blood–brain barrier, and degeneration of the myelin proteins. 284 Hence these results warrant further investigation to assess the effectiveness of tocotrienol in patients presenting transient ischemic attacks and those persons are at high risk of developing severe stroke. 276 , 284
The medicinal efficacy of tocotrienol is strengthened by its ability for inducing hypolipidemia and reducing plasma cholesterol levels. 285 A low concentration of tocotrienol (between 2 μM gamma‐tocotrienol giving 50% inhibition and 10 μM giving 80% inhibition) is deemed sufficient to suppress the activity of the hepatic enzyme, HMG‐CoA reductase (3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase/HMGCR) required for cholesterol synthesis in the liver via the mevalonate pathway. 286 Furthermore, the delta and gamma forms of tocotrienol are involved in the sterol‐regulated degradation of proteins through the ER membrane‐associated proteins such as insulin‐induced genes (Insigs) that activate the degradation of misfolded and dysfunctional proteins via ubiquitin ligases. It is also linked to the regulation of the HMG‐CoA reductase activity in a complex feedback loop. 287 Similarly, a nanomolar concentration of gamma and delta forms of tocotrienol were found to be an order of magnitude higher than what was required to protect the neurons from neurotoxic insults, as per the data from cell culture studies. 288 One drawback of tocotrienol metabolism is that it has low bioavailability when taken through the oral route. 54 Among the cohort of tocotrienol isomers, alpha‐tocotrienol can suppress neurodegeneration of nerve cells at nanomolar concentrations by regulating specific mediators which induce cell death and oral supplementation of tocotrienol is known to protect against the adverse effects of stroke. 289
4.4. Lipidomic disease
As indicated by previous studies, tocotrienol transportation within tissues and all vital organs has been described, where it had reached the skin, liver, heart, spinal cord, skeletal muscles, lung, adipose tissue, and brain even when consumed as an oral supplement. 290 , 291 Additionally, tocotrienol was detected in measurable amounts in new‐born rat tissues when pregnant rats were orally administered. 292 The absorption of tocotrienol takes place in the small intestine where it enters the systemic circulation first as chylomicrons which later breakdown and attach to circulating lipoprotein carriers for transportation into cells. 293 A common method that enhances fat‐soluble vitamin absorption is to deliver it in the form of an emulsion, and ingested tocotrienol forms an emulsion with the gastrointestinal juices as soon as it enters the intestine. 294 However, there is evidence that when both tocopherol and tocotrienol are digested together, the tocopherols outcompete tocotrienol during absorption, which suggests that it is prudent not to consume both these vitamin E forms, at the same time. 295 Tocopherol cellular uptake and transport conventionally occur through binding with tocopherol transfer protein (TTP) and it is extended to the cellular uptake of the tocotrienols as well. 296 It is also known that tocotrienol absorption is enhanced when taken together with food, such as after a meal in healthy individuals. 297 Tocotrienol absorption is deemed dependent on the intake of food, the lipid content of food, and bioavailability. 298
Obesity is considered as a pathological condition due to excessive fat deposits in the adipocytes arising from defective lipid metabolism and glucose homeostasis. 196 Obesity is also characterized as being a reservoir of signaling molecules involving cytokines, chemokines, oxidative stress, and inflammatory biomarkers and consequently is prone to persisting chronic low‐grade inflammation in fatty tissue. 196 Tocotrienols have reported efficacy in attenuating obesity and was validated by an investigation on the impact of tocotrienol supplementation in high‐fat diet‐induced obesity in mice. 196 It revealed beneficial changes in serum metabolites related to multiple metabolic pathways which modulated oxidative stress, lipid peroxidation, metabolism of fatty acids, amino acids, phospholipids, sphingolipids, and the gut‐inhabitant microbiome in obese mice, supplemented with tocotrienol. 196 A comparison between high‐fat diet‐fed obese mice with a low‐fat diet‐fed counterparts, supplemented with delta‐tocotrienol revealed attenuation of fat deposition in adipocytes (being smaller in size), elevated anti‐inflammatory adipokines, and low infiltration of macrophages into adipose tissue. 299 Delta‐tocotrienol supplementation had reduced the markers of fat oxidation and fatty acid synthesis in adipose tissue and liver. 299 The outcome of this study indicates that tocotrienol supplementation promotes metabolically healthy status in obese mice which could be extended into clinical studies. 299
4.5. Skin disease
Atopic dermatitis treated with gamma‐tocotrienol combined with alpha‐tocopherol was delivered to the skin as a nano‐emulsion entrapped in nanoparticles enhanced by microwave exposure [pretreatment by microwave (1 mW/3985 MHz). This treatment promoted therapeutics accumulation in the epidermis through enhancing nano‐emulsion penetration into the skin, which was successful in attenuating the disease. 300 The available standard therapy for dermatitis consists of steroids and other immunosuppressive drugs which produce side effects and gamma‐tocotrienol administered via the oral route is deemed ineffective due to its low bioavailability, in the epidermis and dermis. 300 This delivery mechanism presents a de novo treatment modality in skin diseases because targeted delivery of gamma‐tocotrienol to the skin had proven to be more effective in alleviating dermatitis symptoms. 300 Wound healing is yet another function of tocotrienol treatment. 301 Mice exposed to deep‐partial thickness burns were treated with TRF as a cream combined with epidermal growth factor (EGF) and macroscopic and microscopic images of the skin were analyzed along with tests on biochemical parameters. 301 The EGF and TRF formulation reduced neutrophils, lymphocytes, and myofibroblasts with no change in adipocytes. 301 Additionally, lowering of lipid peroxidation and nitrite levels were observed indicating reduced oxidative stress. TRF together with EGF presents a new therapeutic combination in the treatment of burn wounds. 301
4.6. Glycemic disease
In a clinical study of type‐2‐diabetes, delta‐tocotrienol administered concurrently with oral glycemic therapy reported a significant reduction in the glycemic condition, an increase in plasma insulin, and insulin resistance alongside the attenuation of markers related to oxidative stress and inflammation. The expression of a repertoire of micro ribonucleic acid (miRNA are short, noncoding RNAs, which repress the synthesis of certain proteins by post‐transcriptional destabilizing of mRNA) is indicative of glycemic control in which some were elevated (miRNA‐126, 132) and some were downregulated (miRNA‐375, 34a, and 21). 302 Thus, the glycemic therapy synergized by delta‐tocotrienol has proven its ability in ameliorating long‐term type‐2 diabetes in patients without causing adverse effects. 302
4.7. Lung disease
Asthmatic Norway brown rats were treated with synthetic anti‐asthmatic drugs with either one of adjunct tocotrienol, carotene, or dexamethasone post‐challenge with ovalbumin, to examine the therapeutic outcome of tocotrienol in allergic asthma. 39 Allergic asthma is a chronic airway inflammatory disease which compromises respiration and its synthetic drugs exhibit multiple adverse side effects. 39 The groups treated with either tocotrienol or carotene, which are natural phytochemical nutrients, reported favorable outcomes in alleviating asthmatic symptoms in this rat model. 39 The observations were supported by decreased; rat plasma C‐reactive protein, immunoglobulin E, and the absence of histologically validated immune cell infiltration in bronchial walls and edema, which signified healthy lungs. 39
Emphysema is a chronic obstructive pulmonary disorder (COPD) that occurs partly due to inflammation and oxidative stress. 303 The standard treatment offered are corticosteroids which do not alleviate oxidative stress and some patients become steroid‐resistant. 303 Since the tocotrienols are antioxidant and anti‐inflammatory agents, a study in which emphysema was induced by exposure to cigarette smoke in BALB/c mice, reported attenuation of disease when given gamma‐tocotrienol compared to prednisolone, a steroid. 303 Gamma‐tocotrienol was effective in ameliorating emphysema lesions of murine lungs, reduce bronchial wall thickening, pulmonary pro‐oxidant, and pro‐inflammatory gene expression, biomarkers of oxidative stress and inflammation which also restored the normal lung antioxidant capacity. 303 This study provides evidence of tocotrienol having a therapeutic role in COPD treatment in a mouse model.
4.8. Eye disease
The development of cataracts in the eyes is a leading cause of visual impairment which arises as an early‐onset complication of diabetes. 304 There are studies that have reported the cataract‐inhibiting properties of tocotrienol such as reducing lens stress and inhibiting cataract‐genesis in mice fed a galactose‐rich diet. 305 A third study in which diabetes was induced by streptozotocin injection reported curtailment of cataract progression in the delta‐tocotrienol‐treated group compared to the control group. Topical tocotrienol application was capable of suppressing; the NF‐kB pathway, inducible nitric oxide synthase (iNOS) activation, and oxidative‐nitrosative stress, while improving ATP synthesis and ATPase activity, restoring lens proteins, and calpain activity as well as reducing lens aldose reductase enzymatic activity. 304
4.9. Bone disease
Osteoblast migration is needed for bone remodeling and repairing the fractures in support of which delta‐tocotrienol has been identified as a potential source of osteoanabolic mechanisms. 306 This study delineated a role for tocotrienol therapy in human bone marrow mesenchymal stem cells exerting its influence through the mediation of Akt phosphorylation and the activation of the Wnt/beta‐catenin pathway. 306 Tocotrienols strengthen the structure and functions of the bone mass in the skeletal system. 306 Delta‐tocotrienol was shown to stimulate beta catenin‐regulated osteogenic targeted genes involved in osteoblast differentiation and migration. 306 Therefore, delta‐tocotrienol plays a vital role in maintaining bone health by reducing bone loss by inhibiting osteoclast formation and bone resorption through the modulation of the Receptor activator of nuclear factor‐kappa‐Β ligand (RANKL) and osteoprotegerin (OPG) expression. 307 Another study investigating the bone properties in obese mice treated with annatto‐oil‐extracted tocotrienol combined with green tea polyphenols displayed increased bone volume through the modulation of the gut microbiome composition and the biosynthesis of vitamin K2. 308 Thus, it presents a link between bone health maintenance and the gut‐inhabitant microbiome which could be a novel diet‐modulated therapy in promoting bone vitality in obesity models. 308
Rheumatoid arthritis (RA) is a systemic and autoimmune disease of the joints, which causes severe joint pain, bone erosion, and cartilage destruction stimulated by inflammatory mediators. 309 RA was induced in Dark Agouti rats by collagen type II intradermal injection and were treated with the TRF. 309 Compared to the group without TRF, the intervention group displayed significant attenuation of collagen‐induced arthritis in this in vivo rat model by reducing the expression of inflammatory mediators and joint destruction. 309
5. RAMIFICATIONS OF THERAPEUTIC USE
Although this review has focussed on the beneficial effects of Tocotrienols, negative outcomes have also been reported, although in‐depth analysis of these studies is beyond the scope of this review. Notwithstanding the large amount of experimental data that is published on the beneficial characteristics of the different forms of tocotrienol, negative outcomes have been reported in certain studies. Dairy animals treated with gamma‐tocotrienol had recorded a loss of endothelial barrier integrity in bovine mammary endothelial cells at lower concentrations without displaying adverse effects on cell viability in other cell types at like concentrations. The impact of antioxidant therapeutic effect of vitamin E forms is important during calving and peak lactation which otherwise produce inflammation and destruction of macromolecules (lipids, nucleic acids, and proteins) leading to the manifestation of immune dysfunction and metabolic disease. The same study also showed superior antioxidant effects of the tocopherols compared to the tocotrienol counterparts. 310 The metabolism of both tocopherol and tocotrienols undergo a similar process although the tocotrienols degrade in a more complex manner. In vitro differences have been observed in a HepG2 cell culture model although it was postulated to be different in in vivo systems. Thus, the metabolism and biodegradation of these two cohorts are correlated to their bioavailability and retention within the cells which showed less accumulation in the case of tocotrienols making the tocopherols have a more therapeutic effect. 311 Other instances of tocotrienols being inferior to its tocopherol forms is having low plasma bioavailability in cells when consumed through the oral route. It clearly is an impeding factor in the tocotrienol therapeutic spectrum. Further, tocopherol bioactivity in the cellular system is augmented by the hepatic plasma tocopherol transfer proteins and tocopherol‐associated proteins, both of which assist in the transportation of the tocopherol forms whereas the tocotrienols have less affinity towards those carrier‐mediated cellular influx mechanisms. 312 Tocopherols and tocotrienols display unique concentrations in various tissues as per a study that quantified the vitamin E forms in various mouse tissue. The study using high‐pressure liquid chromatography (HPLC), had reported the existence of a selective mechanism that is based on the antioxidant capacity of the toco family forms although it is debatable. This study utilized hairless mouse tissue to quantify these lipophilic antioxidants that recorded almost 99.8% of alpha‐tocopherol in the brain with no significant presence of tocotrienols. The overall quantification showed under 1% tissue tocotrienol whereas most other tissues had considerably high percentages of the tocopherol forms. 313 The biopotency of tocopherols is greater than that of tocotrienols and is a measure of the biological half‐life, which is longer in tocopherols. Thus, tocotrienol degradation is faster with it being metabolized quicker than the tocopherols. This selective metabolism is based on the activity of the human cytochrome P450 also known as (CYP4F2) which mediates the ω‐hydroxylation step that converts both tocopherols and tocotrienols to carboxychromanol, where CYP4F2 is considered the only enzyme that catalyzes the metabolic breakdown of intracellular vitamin E forms. This degradation step is determined by the respective molecular structures of the vitamin E forms and depends upon the stereochemistry of the sidechain and the number of methyl groups present in the chromanol ring structure. Thus, tocotrienols are metabolized and excreted in urine faster than tocopherols because of the unsaturated isoprenoid sidechain composition. 314
6. FUTURE DIRECTIONS
There are many avenues along which tocotrienol research could be guided in the future to gain clinical benefits from tocotrienol therapy. Thus, continued research is encouraged that focuses more on the clinical therapeutic potential of tocotrienol in ameliorating the inflammatory diseases for which it had exhibited experimental efficacy. One drawback in tocotrienol research is lacking clinical models possibly due to needing invasive techniques for investigations. Most of the research has been conducted with in vitro studies that do not depict the in vivo conditions. There are extensive studies performed on microRNA and small interfering RNA‐involving cytolytic mechanisms which do not represent the clinical picture. A gap in the present tocotrienol research still prevails because the means to elicit a long‐lasting therapeutic effect has not been successful. Interestingly, tocotrienol possesses strong inhibitory anticancer properties which could be the highlight of its therapeutic spectrum. A detailed assessment of the drug–drug interactions has not been conducted with tocotrienol therapy in cancer patients, because the cancer physiology varies significantly among the cancer types. The cancer studies have so far concentrated on adjuvant tocotrienol therapy in attenuating the cancers although the ability of tocotrienol to overcome the toxicity of chemotherapy is still lacking. Since tocotrienols are plant‐derived phytochemicals, there are abundant resources from which these compounds could be extracted. Because certain studies have shown dose‐dependent activity in tocotrienols, new methods are needed for obtaining extract, with an extended half‐life, and to overcome the side effects. To enhance its therapeutic efficacy, the bioavailability threshold and duration of retention should be increased. These properties could be enhanced by combining with appropriate adjuvants that can be identified by analytical computer software which matches membrane‐bound receptors and their docking compounds (such as CADD). 315
7. CONCLUSIONS
This review has summarized the salient aspects of experimental tocotrienol therapy combined with the latest findings that focused on modulating the immune‐metabolic responses in different disease states, particularly, cancer. The superiority of tocotrienols in disease treatment (Figure 13) compared to tocopherols is already established. Tocotrienols are considered superior to the tocopherols for many reasons that are associated with biological functions related to health and disease. Alpha‐, gamma‐, and delta‐tocotrienol forms are identified to be more effective than alpha‐tocopherol which was more commonly experimented with during the early days of its discovery. More recent research studies have revealed that tocotrienols surpass tocopherols by being better antioxidant, antihypertensive, anti‐cancer metastatic, anti‐inflammatory, anti‐hypercholesterolemic, anti‐atherogenic, anti‐tumor, anti‐proliferative, apoptotic, anti‐angiogenic, and neuroprotective therapy that are documented through a large number of preclinical and clinical studies. The preferential uptake of topically applied tocotrienols into ocular tissues, maintaining normal bone calcification in rats, better therapeutic effects in obesity, diabetes, and osteoporosis have been documented. The maintenance of tocotrienol levels up to 24 h after intragastric administration was documented in adipose tissue of epididymis, renal, subcutaneous, and brown fat tissue. The highest concentration of tocotrienols was reported at 8 h in serum, liver, mesenteric lymph nodes, lungs, and spleen in rats. Tocotrienols are transported within membranes and incorporated into membranes more than tocopherols in rats. There is paucity of information in identifying a biochemically, physiologically, and immunologically active, formulation to overcome its deficiencies as a therapeutic agent that have been reported by certain other studies such as having low bioavailability, low tissue penetration efficiency except in skin, short half‐life, and higher drug efflux from cells. The protective role of tocotrienol on PUFA could be employed in mediating anti‐oxidation of dietary fatty acids and regulating endocytosis and exocytosis via ER. Those mechanisms act to restrict or enhance drug influx or efflux, thus augmenting drug metabolism and increasing chemosensitivity to curative compounds. Importantly, tocotrienols can cross the blood–brain barrier in restoring cognitive functions in neurodegenerative diseases which warrants further investigations.
AUTHOR CONTRIBUTIONS
Concept, writing, and organizing by Ranmali Ranasinghe. Constructive criticism, Guidance, and Editing by Anthony Zulli and Michael Mathai.
FUNDING INFORMATION
No funding was received to assist with the preparation of this manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare that are relevant to the content of this article.
ACKNOWLEDGEMENT
Ranmali Ranasinghe is a recipient of the Research Training Programme (RTP) Scholarship from the Australian Government for PhD candidature. Open access publishing facilitated by Victoria University, as part of the Wiley ‐ Victoria University agreement via the Council of Australian University Librarians.
Ranasinghe R, Mathai M, Zulli A. Revisiting the therapeutic potential of tocotrienol. BioFactors. 2022;48(4):813–856. 10.1002/biof.1873
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Kanchi MM, Shanmugam MK, Rane G, Sethi G, Kumar AP. Tocotrienols: the unsaturated sidekick shifting new paradigms in vitamin E therapeutics. Drug Discov Today. 2017;22(12):1765–81. [DOI] [PubMed] [Google Scholar]
- 2. Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: an insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008;123(4):739–52. [DOI] [PubMed] [Google Scholar]
- 3. Sylvester PW. Vitamin E and apoptosis. Vitam Horm. 2007;76:329–56. [DOI] [PubMed] [Google Scholar]
- 4. Ahangarpour A, Sayahi M, Sayahi M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: a review study. Diabetes Metab Syndr Clin Res Rev. 2019;13(1):854–7. [DOI] [PubMed] [Google Scholar]
- 5. Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, et al. Vitamin E has a dual effect of anti‐inflammatory and antioxidant activities in acetic acid‐induced ulcerative colitis in rats. Can J Surg. 2011;54(5):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abd AH, Qasim B, Sahib H, Raheem H. Nephroprotective effect of vitamin E and Origanum vulgare extracts against vancomycin induced nephrotoxicity in rats. Int J Pharmaceut Sci Rev Res. 2016;36:89–96. [Google Scholar]
- 7. Behl . Vitamin E and other antioxidants in neuroprotection. Int J Vitam Nutr Res. 1999;69(3):213–9. [DOI] [PubMed] [Google Scholar]
- 8. Tan CY, Saw TY, Fong CW, Ho HK. Comparative hepatoprotective effects of tocotrienol analogs against drug‐induced liver injury. Redox Biol. 2015;4:308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibrahim MA, Bakhaat GA, Tammam HG, Mohamed RM, el‐Naggar SA. Cardioprotective effect of green tea extract and vitamin E on cisplatin‐induced cardiotoxicity in mice: toxicological, histological and immunohistochemical studies. Biomed Pharmacother. 2019;113:108731. [DOI] [PubMed] [Google Scholar]
- 10. Trela A, Szymańska R. Less widespread plant oils as a good source of vitamin E. Food Chem. 2019;296:160–6. [DOI] [PubMed] [Google Scholar]
- 11. Traber MG. Determinants of plasma vitamin E concentrations. Free Radic Biol Med. 1994;16(2):229–39. [DOI] [PubMed] [Google Scholar]
- 12. Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ Med J. 2014;14(2):e157–65. [PMC free article] [PubMed] [Google Scholar]
- 13. Müller L, Theile K, Böhm V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol Nutr Food Res. 2010;54(5):731–42. [DOI] [PubMed] [Google Scholar]
- 14. Bendich A, Machlin LJ. Safety of oral intake of vitamin E. Am J Clin Nutr. 1988;48(3):612–9. [DOI] [PubMed] [Google Scholar]
- 15. Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel V, Rink C, Khanna S, Sen CK. Tocotrienols: the lesser known form of natural vitamin E. Indian J Exp Biol. 2011;49(10):732–8. [PMC free article] [PubMed] [Google Scholar]
- 17. Chin K‐Y, Pang K‐L, Soelaiman I‐N. Tocotrienol and its role in chronic diseases. Anti‐inflammatory nutraceuticals and chronic diseases. 2016;928:97–130. [DOI] [PubMed] [Google Scholar]
- 18. Koay YY, Tan GCJ, Phang SCW, Ho JI, Chuar PF, Ho LS, et al. A phase IIb randomized controlled trial investigating the effects of Tocotrienol‐rich vitamin E on diabetic kidney disease. Nutrients. 2021;13(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vitamin E in Neuroprotection Study (VENUS) Investigators , Hor CP, Fung WY, Ang HA, Lim SC, Kam LY, et al. Efficacy of oral mixed tocotrienols in diabetic peripheral neuropathy: a randomized clinical trial. JAMA Neurol. 2018;75(4):444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Das S, Nesaretnam K, Das DK. Tocotrienols in cardioprotection. Vitam Horm. 2007;75:285–99. [DOI] [PubMed] [Google Scholar]
- 21. Zingg J‐M. Vitamin E: an overview of major research directions. Mol Aspects Med. 2007;28(5–6):400–22. [DOI] [PubMed] [Google Scholar]
- 22. Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab. 2014;11(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80(11):1613–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das S, Das M, Das DK. 21 vitamin E isomers, Tocotrienols, in Cardioprotection. Tocotrienols: vitamin E beyond tocopherols. 2008;285. [Google Scholar]
- 25. Aggarwal B, Nesaretnam K. Vitamin E tocotrienols: life beyond tocopherols . BioMed Central. 2012;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mishima K, Tanaka T, Pu F, Egashira N, Iwasaki K, Hidaka R, et al. Vitamin E isoforms α‐tocotrienol and γ‐tocopherol prevent cerebral infarction in mice. Neurosci Lett. 2003;337(1):56–60. [DOI] [PubMed] [Google Scholar]
- 27. Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008;44(5):739–64. [DOI] [PubMed] [Google Scholar]
- 28. Lemaire‐Ewing S, Desrumaux C, Néel D, Lagrost L. Vitamin E transport, membrane incorporation and cell metabolism: is α‐tocopherol in lipid rafts an oar in the lifeboat? Mol Nutr Food Res. 2010;54(5):631–40. [DOI] [PubMed] [Google Scholar]
- 29. Kaempf‐Rotzoll D, Igarashi K, Aoki J, Jishage K, Suzuki H, Tamai H, et al. α‐Tocopherol transfer protein is specifically localized at the implantation site of pregnant mouse uterus. Biol Reprod. 2002;67(2):599–604. [DOI] [PubMed] [Google Scholar]
- 30. Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med. 2007;28(5–6):692–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyenberg A, Goldblum D, Zingg JM, Azzi A, Nesaretnam K, Kilchenmann M, et al. Tocotrienol inhibits proliferation of human Tenon's fibroblasts in vitro: a comparative study with vitamin E forms and mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2005;243(12):1263–71. [DOI] [PubMed] [Google Scholar]
- 32. Khadangi F, Azzi A. Vitamin E–the next 100 years. IUBMB Life. 2019;71(4):411–5. [DOI] [PubMed] [Google Scholar]
- 33. Sato A, Arai T, Fusegi M, Ando A, Yano T. A redox‐inactive derivative of Tocotrienol suppresses tumor growth of mesothelioma cells in a xenograft model. Biol Pharm Bull. 2019;42(6):1034–7. [DOI] [PubMed] [Google Scholar]
- 34. Ribeiro AM, Estevinho BN, Rocha F. One century of vitamin E: the progress and application of vitamin E encapsulation—a review. Food Hydrocoll. 2021;121:106998. [Google Scholar]
- 35. Sen CK, Khanna S, Roy S. Tocotrienols: vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harinantenaina L. Tocotrienols in plants: sources and importance. In: Watson RR, Preedy VR, editors. Tocotrienols: vitamin E beyond tocopherols. Champaign, IL: The American Oil Chemist's Society (AOCS); 2008. p. 43. [Google Scholar]
- 37. Horvath G, Wessjohann L, Bigirimana J, Jansen M, Guisez Y, Caubergs R, et al. Differential distribution of tocopherols and tocotrienols in photosynthetic and non‐photosynthetic tissues. Phytochemistry. 2006;67(12):1185–95. [DOI] [PubMed] [Google Scholar]
- 38. Ng MH, Choo YM, Ma AN, Chuah CH, Hashim MA. Separation of vitamin E (tocopherol, tocotrienol, and tocomonoenol) in palm oil. Lipids. 2004;39(10):1031–5. [DOI] [PubMed] [Google Scholar]
- 39. Zainal Z, Abdul Rahim A, Khaza'ai H, Chang SK. Effects of palm oil tocotrienol‐rich fraction (TRF) and carotenes in ovalbumin (ova)‐challenged asthmatic Brown Norway rats. Int J Mol Sci. 2019;20(7):1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan C‐P, Nehdi IA. The physicochemical properties of palm oil and its components. Palm Oil. Netherlands: Elsevier; 2012. p. 377–91. [Google Scholar]
- 41. Nesaretnam K. Palm oil's impact on nutrition and health. 3rd International Symposium on Recombined Milk and Milk Products, Penang (Malaysia), 23–26 May 1999. Schaerbeek, Belgium: International Dairy Federation; 1999. [Google Scholar]
- 42. Sarmento CMP, Ferreira SRS, Hense H. Supercritical fluid extraction (SFE) of rice bran oil to obtain fractions enriched with tocopherols and tocotrienols. Brazil J Chem Eng. 2006;23:243–9. [Google Scholar]
- 43. McIntosh G, Bulman F, Russell G. Cereal grains, alpha tocotrienol and cholesterol metabolism in the rat. Asia Pac J Clin Nutr. 1992;1:89–94. [PubMed] [Google Scholar]
- 44. Hejtmánková K, Lachman J, Hejtmánková A, Pivec V, Janovská D. Tocols of selected spring wheat (Triticum aestivum L.), einkorn wheat (Triticum monococcum L.) and wild emmer (Triticum dicoccum Schuebl [Schrank]) varieties. Food Chem. 2010;123(4):1267–74. [Google Scholar]
- 45. Frega N, Mozzon M, Bocci F. Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography‐mass spectrometry. J Am Oil Chem Soc. 1998;75(12):1723–7. [Google Scholar]
- 46. Babura SR, Abdullah SNA, Khaza H. Advances in genetic improvement for tocotrienol production: a review. J Nutr Sci Vitaminol. 2017;63(4):215–21. [DOI] [PubMed] [Google Scholar]
- 47. Ghani SMA, Goon JA, Azman NHEN, Zakaria SNA, Hamid Z, Ngah WZW. Comparing the effects of vitamin E tocotrienol‐rich fraction supplementation and α‐tocopherol supplementation on gene expression in healthy older adults. Clinics. 2019;74:e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lodge JK, Ridlington J, Leonard S, Vaule H, Traber MG. α‐ and γ‐tocotrienols are metabolized to carboxyethyl‐hydroxychroman derivatives and excreted in human urine. Lipids. 2001;36(1):43–8. [DOI] [PubMed] [Google Scholar]
- 49. Liu KY, Jiang Q. Tocopherols and tocotrienols are bioavailable in rats and primarily excreted in feces as the intact forms and 13′‐carboxychromanol metabolites. J Nutr. 2020;150(2):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jaafar F, Abdullah A, Makpol S. Cellular uptake and bioavailability of tocotrienol‐rich fraction in SIRT1‐inhibited human diploid fibroblasts. Sci Rep. 2018;8(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Giusepponi D, Galarini R, Barola C, Torquato P, Bartolini D, Moretti S, et al. LC‐MS/MS assay for the simultaneous determination of tocopherols, polyunsaturated fatty acids and their metabolites in human plasma and serum. Free Radic Biol Med. 2019;144:134–43. [DOI] [PubMed] [Google Scholar]
- 52. Reboul E. Vitamin E bioavailability: mechanisms of intestinal absorption in the spotlight. Antioxidants. 2017;6(4):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Traber MG. Mechanisms for the prevention of vitamin E excess. J Lipid Res. 2013;54(9):2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fu J‐Y, Che HL, Tan DMY, Teng KT. Bioavailability of tocotrienols: evidence in human studies. Nutr Metab. 2014;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Constantinou C, Charalambous C, Kanakis D. Vitamin E and cancer: an update on the emerging role of γ and δ tocotrienols. Eur J Nutr. 2020;59(3):845–57. [DOI] [PubMed] [Google Scholar]
- 56. Yap W, Chang PN, Han HY, Lee DT, Ling MT, Wong YC, et al. γ‐Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple‐signalling pathways. Br J Cancer. 2008;99(11):1832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Samant G, Wali V, Sylvester P. Anti‐proliferative effects of γ‐tocotrienol on mammary tumour cells are associated with suppression of cell cycle progression. Cell Prolif. 2010;43(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiang Q, Rao X, Kim CY, Freiser H, Zhang Q, Jiang Z, et al. Gamma‐tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int J Cancer. 2012;130(3):685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Viola V, Pilolli F, Piroddi M, Pierpaoli E, Orlando F, Provinciali M, et al. Why tocotrienols work better: insights into the in vitro anti‐cancer mechanism of vitamin E. Genes Nutr. 2012;7(1):29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ling MT, Luk SU, al‐Ejeh F, Khanna KK. Tocotrienol as a potential anticancer agent. Carcinogenesis. 2012;33(2):233–9. [DOI] [PubMed] [Google Scholar]
- 61. Springett GM, Husain K, Neuger A, Centeno B, Chen DT, Hutchinson TZ, et al. A phase I safety, pharmacokinetic, and Pharmacodynamic Presurgical trial of vitamin E δ‐tocotrienol in patients with pancreatic ductal neoplasia. EBioMedicine. 2015;2(12):1987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reddy RS, Dathar S. Nano drug delivery in oral cancer therapy: an emerging avenue to unveil. J Med Radiol Pathol Surg. 2015;1(5):17–22. [Google Scholar]
- 63. Xu W, Mi Y, He P, He S, Niu L. γ‐Tocotrienol inhibits proliferation and induces apoptosis via the mitochondrial pathway in human cervical cancer HeLa cells. Molecules. 2017;22(8):1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zarogoulidis P, Cheva A, Zarampouka K, Huang H, Li C, Huang Y, et al. Tocopherols and tocotrienols as anticancer treatment for lung cancer: future nutrition. J Thorac Dis. 2013;5(3):349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loganathan R, Selvaduray KR, Nesaretnam K, Radhakrishnan AK. Differential and antagonistic effects of palm tocotrienols and other phytonutrients (carotenoids, squalene and coenzyme Q10) on breast cancer cells in vitro. J Oil Palm Res. 2013;25:208–15. [Google Scholar]
- 66. Wada S, Naito Y, Matsushita Y, Nouchi M, Kawai M, Minami E, et al. δ‐Tocotrienol suppresses tumorigenesis by inducing apoptosis and blocking the COX‐2/PGE2 pathway that stimulates tumor–stromal interactions in colon cancer. J Funct Foods. 2017;35:428–35. [Google Scholar]
- 67. Har CH, Keong CK. Effects of tocotrienols on cell viability and apoptosis in normal murine liver cells (BNL CL. 2) and liver cancer cells (BNL IME a. 7R. 1), in vitro. Asia Pac J Clin Nutr. 2005;14(4):374–80. [PubMed] [Google Scholar]
- 68. Chang PN, Yap WN, Wing Lee DT, Ling MT, Wong YC, Yap YL. Evidence of γ‐tocotrienol as an apoptosis‐inducing, invasion‐suppressing, and chemotherapy drug‐sensitizing agent in human melanoma cells. Nutr Cancer. 2009;61(3):357–66. [DOI] [PubMed] [Google Scholar]
- 69. Sailo BL, Banik K, Padmavathi G, Javadi M, Bordoloi D, Kunnumakkara AB. Tocotrienols: the promising analogues of vitamin E for cancer therapeutics. Pharmacol Res. 2018;130:259–72. [DOI] [PubMed] [Google Scholar]
- 70. Husain K, Francois RA, Yamauchi T, Perez M, Sebti SM, Malafa MP. Vitamin E δ‐tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF‐κB activation in pancreatic cancer. Mol Cancer Ther. 2011;10(12):2363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manu KA, Shanmugam MK, Ramachandran L, Li F, Fong CW, Kumar AP, et al. First evidence that γ‐tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF‐κB pathway. Clin Cancer Res. 2012;18(8):2220–9. [DOI] [PubMed] [Google Scholar]
- 72. Abubakar IB, Lim KH, Kam TS, Loh HS. Enhancement of apoptotic activities on brain cancer cells via the combination of γ‐tocotrienol and jerantinine a. Phytomedicine. 2017;30:74–84. [DOI] [PubMed] [Google Scholar]
- 73. Constantinou C, Charalambous C, Kanakis D, Kolokotroni O, Constantinou AI. Update on the anti‐cancer potency of tocotrienols and α‐tocopheryl polyethylene glycol 1000 succinate on leukemic cell lines. Nutr Cancer. 2020;73:1–7. [DOI] [PubMed] [Google Scholar]
- 74. Thomsen CB, Andersen RF, Steffensen KD, Adimi P, Jakobsen A. Delta tocotrienol in recurrent ovarian cancer. A phase II trial. Pharmacol Res. 2019;141:392–6. [DOI] [PubMed] [Google Scholar]
- 75. Kani K, Momota Y, Harada M, Yamamura Y, Aota K, Yamanoi T, et al. γ‐Tocotrienol enhances the chemosensitivity of human oral cancer cells to docetaxel through the downregulation of the expression of NF‐κB‐regulated anti‐apoptotic gene products. Int J Oncol. 2013;42(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Saleh H, Omar E, Froemming GRA, Said RM. Tocotrienol preserves ovarian function in cyclophosphamide therapy. Hum Exp Toxicol. 2015;34(10):946–52. [DOI] [PubMed] [Google Scholar]
- 77. Nasr M, Nafee N, Saad H, Kazem A. Improved antitumor activity and reduced cardiotoxicity of epirubicin using hepatocyte‐targeted nanoparticles combined with tocotrienols against hepatocellular carcinoma in mice. Eur J Pharm Biopharm. 2014;88(1):216–25. [DOI] [PubMed] [Google Scholar]
- 78. Bachawal S, Sylvester P, Wali V. Antiproliferative effects of combined EGFR inhibitor and γ‐tocotrienol treatment is associated with the suppression of ErbB3 and ErbB4 receptor activity in neoplastic mammary epithelial cells. Cancer Res. 2008;68:669. [Google Scholar]
- 79. Hansen TF, Qvortrup C, Pfeiffer P. Angiogenesis inhibitors for colorectal cancer. A review of the clinical data. Cancers. 2021;13(5):1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nesaretnam K, Meganathan P, Veerasenan SD, Selvaduray KR. Tocotrienols and breast cancer: the evidence to date. Genes Nutr. 2012;7(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Husain K, Centeno BA, Coppola D, Trevino J, Sebti SM, Malafa MP. Delta‐tocotrienol is the most bioactive natural tocotrienol in the prevention of pancreatic cancer transformation. Oncotarget. 2017;8(19):31554–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Durovski D, Ornella R, Godefridus JP, Elisa G. The role of developmental signaling pathways in non‐small cell lung carcinoma. J Mol Clin Med. 2019;2(2):41–53. [Google Scholar]
- 83. Kaneko S, Sato C, Shiozawa N, Sato A, Sato H, Virgona N, et al. Suppressive effect of delta‐tocotrienol on hypoxia adaptation of prostate cancer stem‐like cells. Anticancer Res. 2018;38(3):1391–9. [DOI] [PubMed] [Google Scholar]
- 84. Husain K, Centeno BA, Coppola D, Trevino J, Sebti SM, Malafa MP. δ‐Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem‐like cells and prevents pancreatic cancer metastasis. Oncotarget. 2017;8(19):31554–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gopalan A, Yu W, Sanders BG, Kline K. Eliminating drug resistant breast cancer stem‐like cells with combination of simvastatin and gamma‐tocotrienol. Cancer Lett. 2013;328(2):285–96. [DOI] [PubMed] [Google Scholar]
- 86. Lee SO, Ma Z, Yeh CR, Luo J, Lin TH, Lai KP, et al. New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non‐stem/progenitor cells. J Mol Cell Biol. 2013;5(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nesaretnam K. Multitargeted therapy of cancer by tocotrienols. Cancer Lett. 2008;269(2):388–95. [DOI] [PubMed] [Google Scholar]
- 88. Tang KD, Liu J, Russell PJ, Clements JA, Ling MT. Gamma‐tocotrienol induces apoptosis in prostate cancer cells by targeting the Ang‐1/Tie‐2 signalling pathway. Int J Mol Sci. 2019;20(5):1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ananthula S, Parajuli P, Behery FA, Alayoubi AY, el Sayed KA, Nazzal S, et al. Oxazine derivatives of γ‐and δ‐tocotrienol display enhanced anticancer activity in vivo. Anticancer Res. 2014;34(6):2715–26. [PubMed] [Google Scholar]
- 90. Patacsil D, Tran AT, Cho YS, Suy S, Saenz F, Malyukova I, et al. Gamma‐tocotrienol induced apoptosis is associated with unfolded protein response in human breast cancer cells. J Nutr Biochem. 2012;23(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sylvester PW, Akl MR, Malaviya A, Parajuli P, Ananthula S, Tiwari RV, et al. Potential role of tocotrienols in the treatment and prevention of breast cancer. Biofactors. 2014;40(1):49–58. [DOI] [PubMed] [Google Scholar]
- 92. Satyamitra MM, Kulkarni S, Ghosh SP, Mullaney CP, Condliffe D, Srinivasan V. Hematopoietic recovery and amelioration of radiation‐induced lethality by the vitamin E isoform δ‐tocotrienol. Radiat Res. 2011;175(6):736–45. [DOI] [PubMed] [Google Scholar]
- 93. Srinivasan V, Xiao M. Delta‐Tocotrienol: radiation protection and effects on signal transduction pathways. Bethesda, MD: Armed Forces Radiobiology Research Institute; 2011. [Google Scholar]
- 94. Kulkarni SS, Cary LH, Gambles K, Hauer‐Jensen M, Kumar KS, Ghosh SP. Gamma‐tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony‐stimulating factor. Int Immunopharmacol. 2012;14(4):495–503. [DOI] [PubMed] [Google Scholar]
- 95. Singh VK, Beattie LA, Seed TM. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 2013;54(6):973–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. De Silva L, Chuah LH, Meganathan P, Fu JY. Tocotrienol and cancer metastasis. Biofactors. 2016;42(2):149–62. [DOI] [PubMed] [Google Scholar]
- 97. Ahmed RA. γ‐Tocotrienol Reversal of Epithelial‐to‐Mesenchymal Transition in Human Breast Cancer Cells is Mediated through a Suppression of Canonical Wnt and Hedgehog Signaling. Vitamin E in Health and Disease. Croatia: InTech Open; 2018. [Google Scholar]
- 98. Yang C, Zhao Y, Im S, Nakatsu C, Jones‐Hall Y, Jiang Q. Vitamin E delta‐tocotrienol and metabolite 13′‐carboxychromanol inhibit colitis‐associated colon tumorigenesis and modulate gut microbiota in mice. J Nutr Biochem. 2021;89:108567. [DOI] [PubMed] [Google Scholar]
- 99. Srivastava JK, Gupta S. Tocotrienol‐rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun. 2006;346(2):447–53. [DOI] [PubMed] [Google Scholar]
- 100. Wu S‐J, Ng L‐T. Tocotrienols inhibited growth and induced apoptosis in human HeLa cells through the cell cycle signaling pathway. Integr Cancer Ther. 2010;9(1):66–72. [DOI] [PubMed] [Google Scholar]
- 101. Wali VB, Bachawal SV, Sylvester PW. Combined treatment of γ‐tocotrienol with statins induce mammary tumor cell cycle arrest in G1. Exp Biol Med. 2009;234(6):639–50. [DOI] [PubMed] [Google Scholar]
- 102. Wada S, Satomi Y, Murakoshi M, Noguchi N, Yoshikawa T, Nishino H. Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett. 2005;229(2):181–91. [DOI] [PubMed] [Google Scholar]
- 103. Fernandes NV, Katuru R, Dutta D, Mills N, Mo H. The impact of d‐δ‐Tocotrienol and Geranylgeraniol on cell cycle progression and apoptosis in human and murine melanoma cells. FASEB J. 2010;24:lb237–7. [Google Scholar]
- 104. Aggarwal V, Kashyap D, Sak K, Tuli H, Jain A, Chaudhary A, et al. Molecular mechanisms of action of tocotrienols in cancer: recent trends and advancements. Int J Mol Sci. 2019;20(3):656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Huang Y, Wu R, Su ZY, Guo Y, Zheng X, Yang CS, et al. A naturally occurring mixture of tocotrienols inhibits the growth of human prostate tumor, associated with epigenetic modifications of cyclin‐dependent kinase inhibitors p21 and p27. J Nutr Biochem. 2017;40:155–63. [DOI] [PubMed] [Google Scholar]
- 106. Kabel AM. Statins: a new hope for cancer therapy. J Cancer Res Treat. 2013;2:36–8. [Google Scholar]
- 107. Ding Y, Peng Y, Deng L, Fan J, Huang B. Gamma‐tocotrienol reverses multidrug resistance of breast cancer cells with a mechanism distinct from that of atorvastatin. J Steroid Biochem Mol Biol. 2017;167:67–77. [DOI] [PubMed] [Google Scholar]
- 108. Kannappan R, Gupta SC, Kim JH, Aggarwal BB. Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr. 2012;7(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sun W, Wang Q, Chen B, Liu J, Liu H, Xu W. γ‐Tocotrienol‐induced apoptosis in human gastric cancer SGC‐7901 cells is associated with a suppression in mitogen‐activated protein kinase signalling. Br J Nutr. 2008;99(6):1247–54. [DOI] [PubMed] [Google Scholar]
- 110. Wang C, Ju H, Shen C, Tong Z. miR‐429 mediates δ‐tocotrienol‐induced apoptosis in triple‐negative breast cancer cells by targeting XIAP. Int J Clin Exp Med. 2015;8(9):15648–56. [PMC free article] [PubMed] [Google Scholar]
- 111. Loganathan R, Selvaduray KR, Nesaretnam K, Radhakrishnan AK. Tocotrienols promote apoptosis in human breast cancer cells by inducing poly (ADP‐ribose) polymerase cleavage and inhibiting nuclear factor kappa‐B activity. Cell Prolif. 2013;46(2):203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Park SK, Sanders BG, Kline K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress‐dependent increase in extrinsic death receptor signaling. Breast Cancer Res Treat. 2010;124(2):361–75. [DOI] [PubMed] [Google Scholar]
- 113. Ye C, Zhao W, Li M, Zhuang J, Yan X, Lu Q, et al. δ‐Tocotrienol induces human bladder cancer cell growth arrest, apoptosis and chemosensitization through inhibition of STAT3 pathway. PLoS One. 2015;10(4):e0122712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ng K, Radhakrishnan A, Selvaduray K. Gamma‐tocotrienol inhibits proliferation of human chronic myeloid leukemic cells via activation of extrinsic and intrinsic apoptotic pathways. J Blood Dis Ther. 2016;1:102. [Google Scholar]
- 115. Ji X, Wang Z, Geamanu A, Sarkar FH, Gupta SV. Inhibition of cell growth and induction of apoptosis in non‐small cell lung cancer cells by delta‐tocotrienol is associated with notch‐1 down‐regulation. J Cell Biochem. 2011;112(10):2773–83. [DOI] [PubMed] [Google Scholar]
- 116. Chen C‐C, Liu TY, Huang SP, Ho CT, Huang TC. Differentiation and apoptosis induction by lovastatin and γ‐tocotrienol in HL‐60 cells via Ras/ERK/NF‐κB and Ras/Akt/NF‐κB signaling dependent down‐regulation of glyoxalase 1 and HMG‐CoA reductase. Cell Signal. 2015;27(11):2182–90. [DOI] [PubMed] [Google Scholar]
- 117. Lim S‐W, Loh HS, Ting KN, Bradshaw TD, Zeenathul NA. Antiproliferation and induction of caspase‐8‐dependent mitochondria‐mediated apoptosis by β‐tocotrienol in human lung and brain cancer cell lines. Biomed Pharmacother. 2014;68(8):1105–15. [DOI] [PubMed] [Google Scholar]
- 118. Hasani N, Yusoff PA, Bak K, Gapor M, Ngah WZW. The possible mechanism of action of palm oil γ‐tocotrienol and α‐tocopherol on the cervical carcinoma CaSki cell apoptosis. Biomed Res. 2008;19(3):194–200. [Google Scholar]
- 119. Sakai M, Okabe M, Tachibana H, Yamada K. Apoptosis induction by γ‐tocotrienol in human hepatoma Hep3B cells. J Nutr Biochem. 2006;17(10):672–6. [DOI] [PubMed] [Google Scholar]
- 120. Raimondi M, Fontana F, Marzagalli M, Audano M, Beretta G, Procacci P, et al. Ca2+ overload‐and ROS‐associated mitochondrial dysfunction contributes to δ‐tocotrienol‐mediated paraptosis in melanoma cells. Apoptosis. 2021;26:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Francois RA, Zhang A, Husain K, Wang C, Hutchinson S, Kongnyuy M, et al. Vitamin E δ‐tocotrienol sensitizes human pancreatic cancer cells to TRAIL‐induced apoptosis through proteasome‐mediated down‐regulation of c‐FLIP s. Cancer Cell Int. 2019;19(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Husain K, Malafa MP. Role of tocotrienols in chemosensitization of cancer. Role of nutraceuticals in cancer chemosensitization. Netherlands: Elsevier; 2018. p. 77–97. [Google Scholar]
- 123. Rajendran P, Li F, Manu KA, Shanmugam MK, Loo SY, Kumar AP, et al. γ‐Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: potential role as an antiproliferative, pro‐apoptotic and chemosensitizing agent. Br J Pharmacol. 2011;163(2):283–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. γ‐Tocotrienol suppresses growth and sensitises human colorectal tumours to capecitabine in a nude mouse xenograft model by down‐regulating multiple molecules. Br J Cancer. 2016;115(7):814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yap WN, Zaiden N, Tan YL, Ngoh CP, Zhang XW, Wong YC, et al. Id1, inhibitor of differentiation, is a key protein mediating anti‐tumor responses of gamma‐tocotrienol in breast cancer cells. Cancer Lett. 2010;291(2):187–99. [DOI] [PubMed] [Google Scholar]
- 126. Luk SU, Yap WN, Chiu YT, Lee DTW, Ma S, Lee TKW, et al. Gamma‐tocotrienol as an effective agent in targeting prostate cancer stem cell‐like population. Int J Cancer. 2011;128(9):2182–91. [DOI] [PubMed] [Google Scholar]
- 127. Pérez‐Hernández M, Arias A, Martínez‐García D, Pérez‐Tomás R, Quesada R, Soto‐Cerrato V. Targeting autophagy for cancer treatment and tumor chemosensitization. Cancer. 2019;11(10):1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yap W, Zaiden N, Luk SY, Lee DT, Ling MT, Wong YC, et al. In vivo evidence of γ‐tocotrienol as a chemosensitizer in the treatment of hormone‐refractory prostate cancer. Pharmacology. 2010;85(4):248–58. [DOI] [PubMed] [Google Scholar]
- 129. Nakashima K, Virgona N, Miyazawa M, Watanabe T, Yano T. The tocotrienol‐rich fraction from rice bran enhances cisplatin‐induced cytotoxicity in human mesothelioma H28 cells. Phytother Res. 2010;24(9):1317–21. [DOI] [PubMed] [Google Scholar]
- 130. McAnally JA, Gupta J, Sodhani S, Bravo L, Mo H. Tocotrienols potentiate lovastatin‐mediated growth suppression in vitro and in vivo. Exp Biol Med. 2007;232(4):523–31. [PubMed] [Google Scholar]
- 131. Abd Manan N, Mohamed N, Shuid AN. Effects of low‐dose versus high‐dose γ‐tocotrienol on the bone cells exposed to the hydrogen peroxide‐induced oxidative stress and apoptosis. Evid Based Complement Alternat Med. 2012;2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Asay S, Graham A, Hollingsworth S, Barnes B, Oblad RV, Michaelis DJ, et al. γ‐Tocotrienol and α‐tocopherol ether acetate enhance docetaxel activity in drug‐resistant prostate cancer cells. Molecules. 2020;25(2):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu J, Lau EYT, Chen J, Yong J, Tang KD, Lo J, et al. Polysaccharopeptide enhanced the anti‐cancer effect of gamma‐tocotrienol through activation of AMPK. BMC Complement Altern Med. 2014;14(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rajasinghe LD, Pindiprolu RH, Gupta SV. Delta‐tocotrienol inhibits non‐small‐cell lung cancer cell invasion via the inhibition of NF‐κB, uPA activator, and MMP‐9. Onco Targets Ther. 2018;11:4301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhao L, Kang I, Fang X, Wang W, Lee MA, Hollins RR, et al. Gamma‐tocotrienol attenuates high‐fat diet‐induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int J Obes (Lond). 2015;39(3):438–46. [DOI] [PubMed] [Google Scholar]
- 136. Millrud CR, Mehmeti M, Leandersson K. Docetaxel promotes the generation of anti‐tumorigenic human macrophages. Exp Cell Res. 2018;362(2):525–31. [DOI] [PubMed] [Google Scholar]
- 137. Winkelstein A. Mechanisms of immunosuppression: effects of cyclophosphamide on cellular immunity. Blood. 1973;41(2):273–84. [PubMed] [Google Scholar]
- 138. Tham S‐Y, Loh HS, Mai CW, Fu JY. Tocotrienols modulate a life or death decision in cancers. Int J Mol Sci. 2019;20(2):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Montagnani Marelli M, Marzagalli M, Moretti RM, Beretta G, Casati L, Comitato R, et al. Vitamin E δ‐tocotrienol triggers endoplasmic reticulum stress‐mediated apoptosis in human melanoma cells. Sci Rep. 2016;6(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014;741:8–16. [DOI] [PubMed] [Google Scholar]
- 141. Zhang N, Yin Y, Xu SJ, Chen WS. 5‐Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13(8):1551–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tham SY. Combinatorial application of tocotrienols and chemotherapeutic drugs enhances apoptosis and autophagy in colorectal cancer cells. UK: University of Nottingham; 2020. [Google Scholar]
- 143. Sevinc A, Ozyurt I, Severcan FZ. Possible therapeutic effects of gamma‐Tocotrienol conjugation therapy with doxorubicin on hepatocellular carcinoma cell line HEPG2. Biophys J. 2017;112(3):280. [Google Scholar]
- 144. Mondal A, Gandhi A, Fimognari C, Atanasov AG, Bishayee A. Alkaloids for cancer prevention and therapy: current progress and future perspectives. Eur J Pharmacol. 2019;858:172472. [DOI] [PubMed] [Google Scholar]
- 145. Tiwari RV, Parajuli P, Sylvester PW. γ‐Tocotrienol‐induced autophagy in malignant mammary cancer cells. Exp Biol Med. 2014;239(1):33–44. [DOI] [PubMed] [Google Scholar]
- 146. Yu F, Bender W. The mechanism of tamoxifen in breast cancer prevention. Breast cancer research. Volume 3. Switzerland; Springer Nature; 2001. [Google Scholar]
- 147. Gomes AR, Varela CL, Tavares‐da‐Silva EJ, Roleira FMF. Epoxide containing molecules: a good or a bad drug design approach. Eur J Med Chem. 2020;201:112327. [DOI] [PubMed] [Google Scholar]
- 148. Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337(5):295–300. [DOI] [PubMed] [Google Scholar]
- 149. Chin K‐Y, Ima‐Nirwana S. The role of tocotrienol in preventing male osteoporosis—a review of current evidence. Int J Mol Sci. 2019;20(6):1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hafid SRA, Radhakrishnan AK, Nesaretnam K. Tocotrienols are good adjuvants for developing cancer vaccines. BMC Cancer. 2010;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang Y, Park NY, Jang Y, Ma A, Jiang Q. Vitamin E γ‐tocotrienol inhibits cytokine‐stimulated NF‐κB activation by induction of anti‐inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J Immunol. 2015;195(1):126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ayoub NM, Bachawal SV, Sylvester PW. γ‐Tocotrienol inhibits HGF‐dependent mitogenesis and met activation in highly malignant mammary tumor cells. Cell Prolif. 2012;44(6):516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wali V, Sylvester P. The synergistic inhibitory effect of combined statin and γ‐tocotrienol treatment on the growth of the highly malignant+ SA mammary epithelial cells in culture is mediated through the inhibition of MAPK and Akt mitogenic signaling. Cancer Res. 2007;67:4784. [Google Scholar]
- 154. Shen J, Yang T, Xu Y, Luo Y, Zhong X, Shi L, et al. δ‐Tocotrienol, isolated from rice bran, exerts an anti‐inflammatory effect via MAPKs and PPARs signaling pathways in lipopolysaccharide‐stimulated macrophages. Int J Mol Sci. 2018;19(10):3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shirode AB, Sylvester PW. Synergistic anticancer effects of combined γ‐tocotrienol and celecoxib treatment are associated with suppression in Akt and NFκB signaling. Biomed Pharmacother. 2010;64(5):327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Samant G, Sylvester P. γ‐Tocotrienol inhibits ErbB3‐dependent PI3K/Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell Prolif. 2006;39(6):563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kaileh M, Sen R. Role of NF‐κB in the anti‐inflammatory effects of tocotrienols. J Am Coll Nutr. 2010;29(sup3):334S–9S. [DOI] [PubMed] [Google Scholar]
- 158. Ng L‐T, Ko H‐J. Comparative effects of tocotrienol‐rich fraction, α‐tocopherol and α‐tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem. 2012;134(2):920–5. [DOI] [PubMed] [Google Scholar]
- 159. Magnani M, Crinelli R, Bianchi M, Antonelli A. The ubiquitin‐dependent proteolytic system and other potential targets for the modulation of nuclear factor‐kB (NF‐kB). Curr Drug Targets. 2000;1(4):387–99. [DOI] [PubMed] [Google Scholar]
- 160. Wang Y. Mechanistic investigation of gamma‐tocotrienol‐mediated anti‐inflammatory actions. USA: Purdue University; 2012. [Google Scholar]
- 161. Yang C, Jiang Q. Vitamin E δ‐tocotrienol inhibits TNF‐α‐stimulated NF‐κB activation by up‐regulation of anti‐inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J Nutr Biochem. 2019;64:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Van IJzendoorn SC, Van Der Wouden JM, Liebisch G, Schmitz G, Hoekstra D. Polarized membrane traffic and cell polarity development is dependent on dihydroceramide synthase‐regulated sphinganine turnover. Mol Biol Cell. 2004;15(9):4115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ding Y, Fan J, Fan Z, Zhang K. γ‐Tocotrienol reverses multidrug resistance of breast cancer cells through the regulation of the γ‐Tocotrienol‐NF‐κB‐P‐gp axis. J Steroid Biochem Mol Biol. 2021;209:105835. [DOI] [PubMed] [Google Scholar]
- 164. Amin ML. P‐glycoprotein inhibition for optimal drug delivery. Drug Target Insights. 2013;7:S12519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kanagasabai R, Krishnamurthy K, Druhan LJ, Ilangovan G. Forced expression of heat shock protein 27 (Hsp27) reverses P‐glycoprotein (ABCB1)‐mediated drug efflux and MDR1 gene expression in Adriamycin‐resistant human breast cancer cells. J Biol Chem. 2011;286(38):33289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sylvester WP, Ayoub NM. Tocotrienols target PI3K/Akt signaling in anti‐breast cancer therapy. Anti‐Cancer Agents Med Chem (Formerly Curr Med Chem Anti‐Cancer Agents). 2013;13(7):1039–47. [DOI] [PubMed] [Google Scholar]
- 167. Idriss M, Hodroj MH, Fakhoury R, Rizk S. Beta‐tocotrienol exhibits more cytotoxic effects than gamma‐tocotrienol on breast cancer cells by promoting apoptosis via a P53‐independent PI3‐kinase dependent pathway. Biomolecules. 2020;10(4):577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kannappan R, Ravindran J, Prasad S, Sung B, Yadav VR, Reuter S, et al. γ‐Tocotrienol promotes TRAIL‐induced apoptosis through reactive oxygen species/extracellular signal‐regulated kinase/p53‐mediated upregulation of death receptors. Mol Cancer Ther. 2010;9(8):2196–207. [DOI] [PubMed] [Google Scholar]
- 169. Moore C, Palau VE, Mahboob R, Lightner J, Stone W, Krishnan K. Upregulation of pERK and c‐JUN by γ‐tocotrienol and not α‐tocopherol are essential to the differential effect on apoptosis in prostate cancer cells. BMC Cancer. 2020;20:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Shin‐Kang S, Ramsauer VP, Lightner J, Chakraborty K, Stone W, Campbell S, et al. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic Biol Med. 2011;51(6):1164–74. [DOI] [PubMed] [Google Scholar]
- 171. Singh VK, Wise SY, Scott JR, Romaine PLP, Newman VL, Fatanmi OO. Radioprotective efficacy of delta‐tocotrienol, a vitamin E isoform, is mediated through granulocyte colony‐stimulating factor. Life Sci. 2014;98(2):113–22. [DOI] [PubMed] [Google Scholar]
- 172. Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid‐soluble, chain‐breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983;221(1):281–90. [DOI] [PubMed] [Google Scholar]
- 173. Qureshi AA, Mo H, Packer L, Peterson DM. Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J Agric Food Chem. 2000;48(8):3130–40. [DOI] [PubMed] [Google Scholar]
- 174. Das S, Powell SR, Wang P, Divald A, Nesaretnam K, Tosaki A, et al. Cardioprotection with palm tocotrienol: antioxidant activity of tocotrienol is linked with its ability to stabilize proteasomes. Am J Physiol Heart Circ Physiol. 2005;289(1):H361–7. [DOI] [PubMed] [Google Scholar]
- 175. Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–48. [DOI] [PubMed] [Google Scholar]
- 176. Finaud J, Lac G, Filaire E. Oxidative stress. Sports Med. 2006;36(4):327–58. [DOI] [PubMed] [Google Scholar]
- 177. Park H‐A, Mnatsakanyan N, Broman K, Davis AU, May J, Licznerski P, et al. Alpha‐tocotrienol prevents oxidative stress‐mediated post‐translational cleavage of Bcl‐xL in primary hippocampal neurons. Int J Mol Sci. 2020;21(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wali VB, Bachawal SV, Sylvester PW. Endoplasmic reticulum stress mediates γ‐tocotrienol‐induced apoptosis in mammary tumor cells. Apoptosis. 2009;14(11):1366–77. [DOI] [PubMed] [Google Scholar]
- 179. Bergin P, Leggett A, Cardwell CR, Woodside JV, Thakkinstian A, Maxwell AP, et al. The effects of vitamin E supplementation on malondialdehyde as a biomarker of oxidative stress in haemodialysis patients: a systematic review and meta‐analysis. BMC Nephrol. 2021;22(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of palm oil tocotrienol‐rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin‐induced diabetic rats. Clinics. 2009;64:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sample A, He YY. Mechanisms and prevention of UV‐induced melanoma. Photodermatol Photoimmunol Photomed. 2018;34(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lee H, Lim Y. Tocotrienol‐rich fraction supplementation reduces hyperglycemia‐induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice. J Nutr Biochem. 2018;57:77–85. [DOI] [PubMed] [Google Scholar]
- 183. Feingold KR, Grunfeld C. Introduction to lipids and lipoproteins. South Dartmouth, MA: MDText.com, Inc; 2015. [Google Scholar]
- 184. Khor B‐H, Tiong HC, Tan SC, Wong SK, Chin KY, Karupaiah T, et al. Effects of tocotrienols supplementation on markers of inflammation and oxidative stress: a systematic review and meta‐analysis of randomized controlled trials. PLoS One. 2021;16(7):e0255205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Zainal Z, Khaza'ai H, Kutty Radhakrishnan A, Chang SK. Therapeutic potential of palm oil vitamin E‐derived tocotrienols in inflammation and chronic diseases: evidence from preclinical and clinical studies. Food Res Int. 2022;156:111175. [DOI] [PubMed] [Google Scholar]
- 186. Yam ML, Abdul Hafid SR, Cheng HM, Nesaretnam K. Tocotrienols suppress proinflammatory markers and cyclooxygenase‐2 expression in RAW264. 7 macrophages. Lipids. 2009;44(9):787–97. [DOI] [PubMed] [Google Scholar]
- 187. Qureshi A, Khan D, Wajeeha D, Trias AM. Impact of δ‐tocotrienol on inflammatory biomarkers and oxidative stress in hypercholesterolemic subjects. J Clin Exp Cardiol. 2015;6(367):2. [Google Scholar]
- 188. Pang K‐L, Chin K‐Y. The role of tocotrienol in protecting against metabolic diseases. Molecules. 2019;24(5):923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Malavolta M, Pierpaoli E, Giacconi R, Basso A, Cardelli M, Piacenza F, et al. Anti‐inflammatory activity of tocotrienols in age‐related pathologies: a SASPected involvement of cellular senescence. Biol Proc Online. 2018;20(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wu SJ, Liu PL, Ng LT. Tocotrienol‐rich fraction of palm oil exhibits anti‐inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol Nutr Food Res. 2008;52(8):921–9. [DOI] [PubMed] [Google Scholar]
- 191. Kim Y, Gromovsky AD, Brown JM, Chung S. Gamma‐tocotrienol attenuates the aberrant lipid mediator production in NLRP3 inflammasome‐stimulated macrophages. J Nutr Biochem. 2018;58:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Buckner T, Fan R, Kim Y, Kim J, Chung S. Annatto tocotrienol attenuates NLRP3 inflammasome activation in macrophages. Curr Dev Nutr. 2017;1(6):e000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kim Y, Wang W, Okla M, Kang I, Moreau R, Chung S. Suppression of NLRP3 inflammasome by γ‐tocotrienol ameliorates type 2 diabetes. J Lipid Res. 2016;57(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Gupta A, Kumar R, Ganguly R, Singh AK, Rana HK, Pandey AK. Antioxidant, anti‐inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol Rep. 2021;8:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ungurianu A, Zanfirescu A, Nițulescu G, Margină D. Vitamin E beyond its antioxidant label. Antioxidants. 2021;10(5):634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Shen C‐L, Ramamoorthy S, Kaur G, Dufour JM, Wang R, Mo H, et al. Dietary annatto‐extracted Tocotrienol reduces inflammation and oxidative stress, and improves macronutrient metabolism in obese mice: a metabolic profiling study. Nutrients. 2021;13(4):1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Irato P, Santovito G. Enzymatic and non‐enzymatic molecules with antioxidant function. Basel, Switzerland: Multidisciplinary Digital Publishing Institute; 2021. p. 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ji X, Yao H, Meister M, Gardenhire DS, Mo H. Tocotrienols: dietary supplements for chronic obstructive pulmonary disease. Antioxidants. 2021;10(6):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Trujillo M, Kharbanda A, Corley C, Simmons P, Allen AR. Tocotrienols as an anti‐breast cancer agent. Antioxidants. 2021;10(9):1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Cejas P, Casado E, Belda‐Iniesta C, Castro JD, Espinosa E, Redondo A, et al. Implications of oxidative stress and cell membrane lipid peroxidation in human cancer (Spain). Cancer Causes Control. 2004;15(7):707–19. [DOI] [PubMed] [Google Scholar]
- 201. Neganova M, Liu J, Aleksandrova Y, Klochkov S, Fan R. Therapeutic influence on important targets associated with chronic inflammation and oxidative stress in cancer treatment. Cancer. 2021;13(23):6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121(11):2381–6. [DOI] [PubMed] [Google Scholar]
- 203. Szewczyk K, Chojnacka A, Górnicka M. Tocopherols and tocotrienols—bioactive dietary compounds; what is certain, what is doubt? Int J Mol Sci. 2021;22(12):6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Loi M, Paciolla C. Plant antioxidants for food safety and quality: exploring new trends of research. Basel, Switzerland: Multidisciplinary Digital Publishing Institute; 2021. p. 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Diekemper D, Pölloth B, Schwarzer S. From agricultural waste to a powerful antioxidant: Hydroxytyrosol as a sustainable model substance for understanding antioxidant capacity. J Chem Educ. 2021;98(8):2610–7. [Google Scholar]
- 206. Theriault A, Chao JT, Wang Q, Gapor A, Adeli K. Tocotrienol: a review of its therapeutic potential. Clin Biochem. 1999;32(5):309–19. [DOI] [PubMed] [Google Scholar]
- 207. Meganathan P, Fu J‐Y. Biological properties of tocotrienols: evidence in human studies. Int J Mol Sci. 2016;17(11):1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Muid S, Ali AM, Yusoff K, Nawawi HM. Optimal antioxidant activity with moderate concentrations of Tocotrienol rich fraction (TRF) in in vitro assays. Int Food Res J. 2013;20(2):687–94. [Google Scholar]
- 209. Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71(4):487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Chin S‐F, Hamid NAA, Latiff AA, Zakaria Z, Mazlan M, Yusof YAM, et al. Reduction of DNA damage in older healthy adults by tri E® Tocotrienol supplementation. Nutrition. 2008;24(1):1–10. [DOI] [PubMed] [Google Scholar]
- 211. Roza JM, Xian‐Liu Z, Guthrie N. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Alternative Ther Health Med. 2007;13(6):44–8. [PubMed] [Google Scholar]
- 212. Sivamaruthi BS, Kesika P, Chaiyasut C. A comprehensive review on anti‐diabetic property of rice bran. Asian Pac J Trop Biomed. 2018;8(1):79. [Google Scholar]
- 213. Tiwari V, Kuhad A, Chopra K. Suppression of neuro‐inflammatory signaling cascade by tocotrienol can prevent chronic alcohol‐induced cognitive dysfunction in rats. Behav Brain Res. 2009;203(2):296–303. [DOI] [PubMed] [Google Scholar]
- 214. Budin SB, Han KJ, Jayusman PA, Taib IS, Ghazali AR, Mohamed J. Antioxidant activity of tocotrienol rich fraction prevents fenitrothion‐induced renal damage in rats. J Toxicol Pathol. 2013;26(2):111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Traber MG, Podda M, Weber C, Thiele J, Rallis M, Packer L. Diet‐derived and topically applied tocotrienols accumulate in skin and protect the tissue against. Asia Pac J Clin Nutr. 1997;6(1):63–7. [PubMed] [Google Scholar]
- 216. Durani L, Jaafar F, Tan JK, Tajul Arifin K, Mohd Yusof YA, Wan Ngah WZ, et al. Targeting genes in insulin‐associated signalling pathway, DNA damage, cell proliferation and cell differentiation pathways by tocotrienol‐rich fraction in preventing cellular senescence of human diploid fibroblasts. Clin Ter. 2015;166(6):e365–73. [DOI] [PubMed] [Google Scholar]
- 217. Shen CL, Klein A, Chin KY, Mo H, Tsai P, Yang RS, et al. Tocotrienols for bone health: a translational approach. Ann N Y Acad Sci. 2017;1401(1):150–65. [DOI] [PubMed] [Google Scholar]
- 218. Mangialasche F, Xu W, Kivipelto M, Costanzi E, Ercolani S, Pigliautile M, et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol Aging. 2012;33(10):2282–90. [DOI] [PubMed] [Google Scholar]
- 219. Chin S‐F, Ibahim J, Makpol S, Abdul Hamid NA, Abdul Latiff A, Zakaria Z, et al. Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: a randomized controlled study. Nutr Metab. 2011;8(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Gopalan Y, Shuaib IL, Magosso E, Ansari MA, Abu Bakar MR, Wong JW, et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke. 2014;45(5):1422–8. [DOI] [PubMed] [Google Scholar]
- 221. Azlina MFN, Qodriyah H, Chua KH, Kamisah Y. Comparison between tocotrienol and omeprazole on gastric growth factors in stress‐exposed rats. World J Gastroenterol. 2017;23(32):5887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Wilankar C, Sharma D, Checker R, Khan NM, Patwardhan R, Patil A, et al. Role of immunoregulatory transcription factors in differential immunomodulatory effects of tocotrienols. Free Radic Biol Med. 2011;51(1):129–43. [DOI] [PubMed] [Google Scholar]
- 223. Theriault A, Chao J‐T, Gapor A. Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis. 2002;160(1):21–30. [DOI] [PubMed] [Google Scholar]
- 224. Vasanthi HR, Parameswari R, Das DK. Multifaceted role of tocotrienols in cardioprotection supports their structure: function relation. Genes Nutr. 2012;7(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Peh HY, Tan WSD, Liao W, Wong WSF. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther. 2016;162:152–69. [DOI] [PubMed] [Google Scholar]
- 226. Sylvester PW, Wali VB, Bachawal SV, Shirode AB, Ayoub NM, Akl MR. Tocotrienol combination therapy results in synergistic anticancer response. Front Biosci. 2011;17:3183–95. [DOI] [PubMed] [Google Scholar]
- 227. Qureshi AA, Sami SA, Salser WA, Khan FA. Dose‐dependent suppression of serum cholesterol by tocotrienol‐rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002;161(1):199–207. [DOI] [PubMed] [Google Scholar]
- 228. Murphy BF. Hypervitaminosis E. JAMA. 1974;227(12):1381–1. [DOI] [PubMed] [Google Scholar]
- 229. Yap S, Yuen K, Wong J. Pharmacokinetics and bioavailability of α‐, γ‐and δ‐tocotrienols under different food status. J Pharm Pharmacol. 2001;53(1):67–71. [DOI] [PubMed] [Google Scholar]
- 230. Compadre CM, Singh A, Thakkar S, Zheng G, Breen PJ, Ghosh S, et al. Molecular dynamics guided design of tocoflexol: a new radioprotectant tocotrienol with enhanced bioavailability. Drug Dev Res. 2014;75(1):10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Lameire NH, Flombaum CD, Moreau D, Ronco C. Acute renal failure in cancer patients. Ann Med. 2005;37(1):13–25. [DOI] [PubMed] [Google Scholar]
- 232. Perazella MA. Drug‐induced renal failure: update on new medications and unique mechanisms of nephrotoxicity. Am J Med Sci. 2003;325(6):349–62. [DOI] [PubMed] [Google Scholar]
- 233. Murtagh FE, Murphy E, Sheerin NS. Illness trajectories: an important concept in the management of kidney failure. Oxford: Oxford University Press; 2008. [DOI] [PubMed] [Google Scholar]
- 234. Damiano S, Navas L, Lombari P, Montagnaro S, Forte IM, Giordano A, et al. Effects of δ‐tocotrienol on ochratoxin A‐induced nephrotoxicity in rats. J Cell Physiol. 2018;233(11):8731–9. [DOI] [PubMed] [Google Scholar]
- 235. Gupta A, Chopra K. Effect of tocotrienols on iron‐induced renal dysfunction and oxidative stress in rats. Drug Chem Toxicol. 2009;32(4):319–25. [DOI] [PubMed] [Google Scholar]
- 236. He JC, Chuang PY, Ma'ayan A, Iyengar R. Systems biology of kidney diseases. Kidney Int. 2012;81(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Singh A, Levy J, Pusey C. Fast facts: renal disorders. Basel, Switzerland: Karger Medical and Scientific Publishers; 2013. [Google Scholar]
- 238. Tan SMQ, Chiew Y, Ahmad B, Kadir KA. Tocotrienol‐rich vitamin E from palm oil (tocovid) and its effects in diabetes and diabetic nephropathy: a pilot phase II clinical trial. Nutrients. 2018;10(9):1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Tan GCJ, Tan S, Phang S, Ng YT, Ng EY, Ahmad B, et al. Tocotrienol‐rich vitamin E improves diabetic nephropathy and persists 6–9 months after washout: a phase IIa randomized controlled trial. Ther Adv Endocrinol Metab. 2019;10:2042018819895462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Siddiqui S, Ahsan H, Khan MR, Siddiqui WA. Protective effects of tocotrienols against lipid‐induced nephropathy in experimental type‐2 diabetic rats by modulation in TGF‐β expression. Toxicol Appl Pharmacol. 2013;273(2):314–24. [DOI] [PubMed] [Google Scholar]
- 241. Wedeen RP, Qian L. Chromium‐induced kidney disease. Environ Health Perspect. 1991;92:71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Khan MR, Siddiqui S, Parveen K, Javed S, Diwakar S, Siddiqui WA. Nephroprotective action of tocotrienol‐rich fraction (TRF) from palm oil against potassium dichromate (K2Cr2O7)‐induced acute renal injury in rats. Chem Biol Interact. 2010;186(2):228–38. [DOI] [PubMed] [Google Scholar]
- 243. Rashid Khan M, Ahsan H, Siddiqui S, Siddiqui WA. Tocotrienols have a nephroprotective action against lipid‐induced chronic renal dysfunction in rats. Ren Fail. 2015;37(1):136–43. [DOI] [PubMed] [Google Scholar]
- 244. Daud ZAM, Tubie B, Sheyman M, Osia R, Adams J, Tubie S, et al. Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vasc Health Risk Manag. 2013;9:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Redfern J, Neubeck L. E‐health in cardiovascular medicine. Basel, Switzerland: Multidisciplinary Digital Publishing Institute; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Amano Y, Kawakubo K, Lee JS, Tang AC, Sugiyama M, Mori K. Correlation between dietary glycemic index and cardiovascular disease risk factors among Japanese women. Eur J Clin Nutr. 2004;58(11):1472–8. [DOI] [PubMed] [Google Scholar]
- 247. Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of oxidative stress on the heart and vasculature: part 2 of a 3‐part series. J Am Coll Cardiol. 2017;70(2):212–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Vasanthi R, ShriShriMal N, Das DK. Phytochemicals from plants to combat cardiovascular disease. Curr Med Chem. 2012;19(14):2242–51. [DOI] [PubMed] [Google Scholar]
- 249. Ramanathan N, Tan E, Loh LJ, Soh BS, Yap WN. Tocotrienol is a cardioprotective agent against ageing‐associated cardiovascular disease and its associated morbidities. Nutr Metab. 2018;15(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Schaffer S, Müller WE, Eckert GP. Tocotrienols: constitutional effects in aging and disease. J Nutr. 2005;135(2):151–4. [DOI] [PubMed] [Google Scholar]
- 251. Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–74. [DOI] [PubMed] [Google Scholar]
- 252. Cicero AF, Derosa G. Rice bran and its main components: potential role in the management of coronary risk factors. Curr Top Nutraceut Res. 2005;3(1):29–46. [Google Scholar]
- 253. Packer L, Weber SU, Rimbach G. Molecular aspects of α‐tocotrienol antioxidant action and cell signalling. J Nutr. 2001;131(2):369S–73S. [DOI] [PubMed] [Google Scholar]
- 254. Alam Z. Nano formulations of natural compounds for enhanced delivery. J Dis. 2017;4(1):14–20. [Google Scholar]
- 255. Alexander RW. Hypertension and the pathogenesis of atherosclerosis: oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25(2):155–61. [DOI] [PubMed] [Google Scholar]
- 256. Karavidas A, Lazaros G, Tsiachris D, Pyrgakis V. Aging and the cardiovascular system. Hellenic J Cardiol. 2010;51(5):421–7. [PubMed] [Google Scholar]
- 257. Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des. 2009;15(5):467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH, Cochrane Hypertension Group . Beta‐blockers for hypertension. Cochrane Database Syst Rev. 2017;1:1–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Li C, Chen Z, Yang H, Luo F, Chen L, Cai H, et al. Selumetinib, an oral anti‐neoplastic drug, may attenuate cardiac hypertrophy via targeting the ERK pathway. PLoS One. 2016;11(7):e0159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Antons KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of statin‐induced rhabdomyolysis. Am J Med. 2006;119(5):400–9. [DOI] [PubMed] [Google Scholar]
- 261. Williamson JR, Kilo C, Tilton RG. Mechanisms of glucose‐and diabetes‐induced vascular dysfunction. Hyperglycemia, diabetes, and vascular disease. USA: Springer; 1992. p. 107–32. [Google Scholar]
- 262. Di Vincenzo A, Tana C, El Hadi H, Pagano C, Vettor R, Rossato M. Antioxidant, anti‐inflammatory, and metabolic properties of tocopherols and tocotrienols: clinical implications for vitamin E supplementation in diabetic kidney disease. Int J Mol Sci. 2019;20(20):5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Cascella M, Bimonte S, Barbieri A, del Vecchio V, Caliendo D, Schiavone V, et al. Dissecting the mechanisms and molecules underlying the potential carcinogenicity of red and processed meat in colorectal cancer (CRC): an overview on the current state of knowledge. Infect Agents Cancer. 2018;13(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Ali SF. The effect of tocotrienols on vascular function, PhD thesis. Melbourne: RMIT University; 2017. [Google Scholar]
- 265. Alauddina M, Islama J, Shirakawaa H, Kosekib T, Ardiansyahc , Komaia M. Rice bran as a functional food: an overview of the conversion of rice bran into a superfood/functional food. Superfood and Functional Food‐An Overview of Their Processing and Utilization. London: IntechOpen; 2017. [Google Scholar]
- 266. Ruschitzka FT, Wenger RH, Stallmach T, Quaschning T, de Wit C, Wagner K, et al. Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin. Proc Natl Acad Sci USA. 2000;97(21):11609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Ismail M, Alsalahi A, Imam MU, Ooi DJ, Khaza'ai H, Aljaberi MA, et al. Safety and neuroprotective efficacy of palm oil and tocotrienol‐rich fraction from palm oil: a systematic review. Nutrients. 2020;12(2):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Kamat J, Devasagayam T. Tocotrienols from palm oil as potent inhibitors of lipid peroxidation and protein oxidation in rat brain mitochondria. Neurosci Lett. 1995;195(3):179–82. [DOI] [PubMed] [Google Scholar]
- 269. Sakakibara R, Kawai T. Cerebrospinal fluid oxidative stress markers in Alzheimer's disease. Neurol Clin Neurosci. 2020;8(5):232–40. [Google Scholar]
- 270. Ambrogini P, Torquato P, Bartolini D, Albertini MC, Lattanzi D, di Palma M, et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy‐derived neurodegeneration: the role of vitamin E. Biochim Biophys Acta (BBA) Mol Basis Dis. 2019;1865(6):1098–112. [DOI] [PubMed] [Google Scholar]
- 271. Selvaraju TR, Khaza'ai H, Vidyadaran S, Sokhini Abd Mutalib M, Ramachandran V, Hamdan Y. Cytoprotective effect of tocotrienol‐rich fraction and α‐tocopherol vitamin E isoforms against glutamate‐induced cell death in neuronal cells. Int J Vitam Nutr Res. 2014;84(3–4):140–51. [DOI] [PubMed] [Google Scholar]
- 272. Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A. α‐Tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology. 2004;47(6):904–15. [DOI] [PubMed] [Google Scholar]
- 273. Biswas N, Khanna S. Tocotrienol Vitamin E and Neurodegenerative Disorders. Antioxidants and functional foods for neurodegenerative disorders. Boca Raton, FL: CRC Press; 2021. p. 229–39. [Google Scholar]
- 274. Sen CK, Khanna S, Roy S, Packer L. Molecular basis of vitamin E action: tocotrienol potently inhibits glutamate‐induced pp60c‐Src kinase activation and death of HT4 neuronal cells. J Biol Chem. 2000;275(17):13049–55. [DOI] [PubMed] [Google Scholar]
- 275. Mustapha M, Nasril CM, Hay YK, Yee FW, Hamid HA. Neuroprotective potentials of natural vitamin E for cerebral small vessel disease. Neuroprotection—New Approaches and Prospects. London: InTech Open; 2019. [Google Scholar]
- 276. Rink C, Khanna S, Sen CK. Arachidonic acid metabolism and lipid peroxidation in stroke: alpha‐tocotrienol as a unique therapeutic agent. Studies on experimental models. Berlin: Springer; 2011. p. 63–90. [Google Scholar]
- 277. Mohamed WM, Sayeed S, Saxena AK, Oothuman P. Oxidative stress status and neuroprotection of tocotrienols in chronic cerebral hypoperfusion‐induced neurodegeneration rat animal model. Int J Nutr Pharmacol Neurol Dis. 2018;8(2):47. [Google Scholar]
- 278. Tan SW, Ramasamy R, Abdullah M, Vidyadaran S. Inhibitory effects of palm α‐, γ‐and δ‐tocotrienol on lipopolysaccharide‐induced nitric oxide production in BV2 microglia. Cell Immunol. 2011;271(2):205–9. [DOI] [PubMed] [Google Scholar]
- 279. Kumari M, Ramdas P, Radhakrishnan AK, Kutty MK, Haleagrahara N. Tocotrienols ameliorate neurodegeneration and motor deficits in the 6‐OHDA‐induced rat model of parkinsonism: Behavioural and immunohistochemistry analysis. Nutrients. 2021;13(5):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Park H‐A, Kubicki N, Gnyawali S, Chan YC, Roy S, Khanna S, et al. Natural vitamin E α‐tocotrienol protects against ischemic stroke by induction of multidrug resistance‐associated protein 1. Stroke. 2011;42(8):2308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. White AR, Cappai R. Neurotoxicity from glutathione depletion is dependent on extracellular trace copper. J Neurosci Res. 2003;71(6):889–97. [DOI] [PubMed] [Google Scholar]
- 282. Ali SF, Woodman OL. Tocomin restores endothelium‐dependent relaxation in the diabetic rat aorta by increasing NO bioavailability and improving the expression of eNOS. Front Physiol. 2019;10:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Kuhad A, Chopra K. Tocotrienol attenuates oxidative–nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology. 2009;57(4):456–62. [DOI] [PubMed] [Google Scholar]
- 284. Rink C, Christoforidis G, Khanna S, Peterson L, Patel Y, Khanna S, et al. Tocotrienol vitamin E protects against preclinical canine ischemic stroke by inducing arteriogenesis. J Cereb Blood Flow Metab. 2011;31(11):2218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Choi Y‐M, Lee J‐S. Hypolipidemic and antioxidative properties of tocotrienol‐rich fraction (TRF) supplementation in high fat‐fed rats. Food Sci Biotechnol. 2009;18(6):1528–31. [Google Scholar]
- 286. Parker R, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate cholesterol production in mammalian cells by post‐transcriptional suppression of 3‐hydroxy‐3‐methylglutaryl‐coenzyme a reductase. J Biol Chem. 1993;268(15):11230–8. [PubMed] [Google Scholar]
- 287. DeBose‐Boyd RA. Feedback regulation of cholesterol synthesis: sterol‐accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008;18(6):609–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Miyazawa T, Nakagawa K, Sookwong P. Health benefits of vitamin E in grains, cereals and green vegetables. Trends Food Sci Technol. 2011;22(12):651–4. [Google Scholar]
- 289. Patel V, Rink C, Gordillo GM, Khanna S, Gnyawali U, Roy S, et al. Oral tocotrienols are transported to human tissues and delay the progression of the model for end‐stage liver disease score in patients. J Nutr. 2012;142(3):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Khanna S, Patel V, Rink C, Roy S, Sen CK. Delivery of orally supplemented α‐tocotrienol to vital organs of rats and tocopherol‐transport protein deficient mice. Free Radic Biol Med. 2005;39(10):1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Patel V, Khanna S, Roy S, Ezziddin O, Sen CK. Natural vitamin E α‐tocotrienol: retention in vital organs in response to long‐term oral supplementation and withdrawal. Free Radic Res. 2006;40(7):763–71. [DOI] [PubMed] [Google Scholar]
- 292. Nagapan G, Meng Goh Y, Shameha Abdul Razak I, Nesaretnam K, Ebrahimi M. The effects of prenatal and early postnatal tocotrienol‐rich fraction supplementation on cognitive function development in male offspring rats. BMC Neurosci. 2013;14(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Napolitano G, Fasciolo G, di Meo S, Venditti P. Vitamin E supplementation and mitochondria in experimental and functional hyperthyroidism: a mini‐review. Nutrients. 2019;11(12):2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Yap SP, Yuen KH. Influence of lipolysis and droplet size on tocotrienol absorption from self‐emulsifying formulations. Int J Pharm. 2004;281(1–2):67–78. [DOI] [PubMed] [Google Scholar]
- 295. Drotleff AM, Bohnsack C, Schneider I, Hahn A, Ternes W. Human oral bioavailability and pharmacokinetics of tocotrienols from tocotrienol‐rich (tocopherol‐low) barley oil and palm oil formulations. J Funct Foods. 2014;7:150–60. [Google Scholar]
- 296. Chiroma AA, Khaza'ai H, Abd Hamid R, Chang SK, Zakaria ZA, Zainal Z. Analysis of expression of vitamin E‐binding proteins in H2O2 induced SK‐N‐SH neuronal cells supplemented with α‐tocopherol and tocotrienol‐rich fraction. PLoS One. 2020;15(11):e0241112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Kabir M, Adnan M, Rahman M. Natural sources of tocotrienols: a note on absorption. J Silico Vitr Pharmacol. 2017;3:1–5. [Google Scholar]
- 298. Borel P, Preveraud D, Desmarchelier C. Bioavailability of vitamin E in humans: an update. Nutr Rev. 2013;71(6):319–31. [DOI] [PubMed] [Google Scholar]
- 299. Allen L, Ramalingam L, Menikdiwela K, Scoggin S, Shen CL, Tomison MD, et al. Effects of delta‐tocotrienol on obesity‐related adipocyte hypertrophy, inflammation and hepatic steatosis in high‐fat‐fed mice. J Nutr Biochem. 2017;48:128–37. [DOI] [PubMed] [Google Scholar]
- 300. Harun MS, Wong TW, Fong CW. Advancing skin delivery of α‐tocopherol and γ‐tocotrienol for dermatitis treatment via nanotechnology and microwave technology. Int J Pharm. 2021;593:120099. [DOI] [PubMed] [Google Scholar]
- 301. Guo H‐F, Abd Hamid R, Mohd Ali R, Chang SK, Rahman MH, Zainal Z, et al. Healing properties of epidermal growth factor and tocotrienol‐rich fraction in deep partial‐thickness experimental burn wounds. Antioxidants. 2020;9(2):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Mahjabeen W, Khan DA, Mirza SA, Pervez MA. Effects of delta‐tocotrienol supplementation on glycemic control, oxidative stress, inflammatory biomarkers and miRNA expression in type 2 diabetes mellitus: a randomized control trial. Phytother Res. 2021;35:3968–76. [DOI] [PubMed] [Google Scholar]
- 303. Peh HY, Tan WSD, Chan TK, Pow CW, Foster PS, Wong WSF. Vitamin E isoform γ‐tocotrienol protects against emphysema in cigarette smoke‐induced COPD. Free Radic Biol Med. 2017;110:332–44. [DOI] [PubMed] [Google Scholar]
- 304. Abdul Nasir NA, Agarwal R, Sheikh Abdul Kadir SH, Vasudevan S, Tripathy M, Iezhitsa I, et al. Reduction of oxidative‐nitrosative stress underlies anticataract effect of topically applied tocotrienol in streptozotocin‐induced diabetic rats. PLoS One. 2017;12(3):e0174542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Nasir NAA, Agarwal R, Vasudevan S, Tripathy M, Alyautdin R, Ismail NM. Effects of topically applied tocotrienol on cataractogenesis and lens redox status in galactosemic rats. Mol Vis. 2014;20:822–35. [PMC free article] [PubMed] [Google Scholar]
- 306. Pang K‐L, Chin K‐Y. Comment on: food for bone: evidence for a role for Delta‐Tocotrienol in the physiological control of osteoblast migration. Int J Mol Sci. 2020;21(18):4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Radzi NFM, Ismail NA, Alias E. Tocotrienols regulate bone loss through suppression on osteoclast differentiation and activity: a systematic review. Curr Drug Targets. 2018;19(9):1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Elmassry MM, Chung E, Cao JJ, Hamood AN, Shen CL. Osteoprotective effect of green tea polyphenols and annatto‐extracted tocotrienol in obese mice is associated with enhanced microbiome vitamin K2 biosynthetic pathways. J Nutr Biochem. 2020;86:108492. [DOI] [PubMed] [Google Scholar]
- 309. Zainal Z, Rahim AA, Radhakrishnan AK, Chang SK, Khaza'ai H. Investigation of the curative effects of palm vitamin E tocotrienols on autoimmune arthritis disease in vivo. Sci Rep. 2019;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Kuhn M, Sordillo L. Vitamin E analogs limit in vitro oxidant damage to bovine mammary endothelial cells. J Dairy Sci. 2021;104(6):7154–67. [DOI] [PubMed] [Google Scholar]
- 311. Birringer M, Pfluger P, Kluth D, Landes N, Brigelius‐Flohé R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132(10):3113–8. [DOI] [PubMed] [Google Scholar]
- 312. Zielinski H. Tocotrienols: distribution and sources cereals—role in human health. Tocotrienols: Vitamin E Beyond Tocopherols. Boca Raton: CRC Press; 2008. p. 23–42. [Google Scholar]
- 313. Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996;37(4):893–901. [PubMed] [Google Scholar]
- 314. Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their ω‐oxidation by tocopherol‐ω‐hydroxylase. J Lipid Res. 2007;48(5):1090–8. [DOI] [PubMed] [Google Scholar]
- 315. Muegge I, Bergner A, Kriegl JM. Computer‐aided drug design at Boehringer Ingelheim. J Comput Aided Mol Des. 2017;31(3):275–85. [DOI] [PubMed] [Google Scholar]
- 316. Shen J, Yang T, Tang Y, Guo T, Guo T, Hu T, et al. δ‐Tocotrienol induces apoptosis and inhibits proliferation of nasopharyngeal carcinoma cells. Food Funct. 2021;12:6374–88. [DOI] [PubMed] [Google Scholar]
- 317. Sultana TA, Aldabaan N, Sylvester PW. γ‐Tocotrienol inhibition of androgen receptor (AR) expression and activation in triple negative breast cancer (TNBC) MBA‐MB‐231 and MDA‐MB‐453 cells is associated with a reduction in proliferation, migration and epithelial‐to‐mesenchymal transition (EMT). Cancer Res. 2021;81:2572. [Google Scholar]
- 318. Tan PY, Tey BT, Chan ES, Lai OM, Chang HW, Tan TB, et al. Stabilization and release of palm Tocotrienol emulsion fabricated using pH‐sensitive calcium carbonate. Foods. 2021;10(2):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Grazier JJ, Sylvester PW. γ‐Tocotrienol inhibition of metastatic phenotypic behavior is associated with a decrease in galectin‐3 expression and distribution in the highly malignant mouse+ SA & TS/a mammary tumor cells. Cancer Res. 2021;81:2889. [Google Scholar]
- 320. Anwar MR, Hossian AKMN, Matthaiolampakis G, Sylvester PW. The anticancer effects of the vitamin E isoform, γ‐tocotrienol, and vitamin D3 act synergistically to inhibit MDA‐MB‐231 triple negative breast cancer (TNBC) cell proliferation and viability in vitro. Cancer Res. 2021;81:2573. [Google Scholar]
- 321. Raviadaran R, Ng MH, Chandran D, Ooi KK, Manickam S. Stable W/O/W multiple nanoemulsion encapsulating natural tocotrienols and caffeic acid with cisplatin synergistically treated cancer cell lines (A549 and HEP G2) and reduced toxicity on normal cell line (HEK 293). Mater Sci Eng C. 2021;121:111808. [DOI] [PubMed] [Google Scholar]
- 322. Maniam G, Mai CW, Zulkefeli M, Fu JY. Co‐encapsulation of gemcitabine and tocotrienols in nanovesicles enhanced efficacy in pancreatic cancer. Nanomedicine. 2021;16(5):373–89. [DOI] [PubMed] [Google Scholar]
- 323. Gómez‐Linton DR, Navarro‐Ocaña A, Román‐Guerrero A, Alavez S, Pinzón‐López L, Mendoza‐Espinoza JA, et al. Environmentally friendly achiote seed extracts with higher δ‐tocotrienol content have higher in vitro and in vivo antioxidant activity than the conventional extract. J Food Sci Technol. 2021;58(7):2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Atia A, Al‐Rawaiq NS, Abdullah A. Tocotrienols activate Nrf2 nuclear translocation and increase the antioxidant‐related hepatoprotective mechanism in mice liver. Curr Pharm Biotechnol. 2021;22:1085–98. [DOI] [PubMed] [Google Scholar]
- 325. Mohamad N‐V, Ima‐Nirwana S, Chin K‐Y. Therapeutic potential of annatto tocotrienol with self‐emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed Pharmacother. 2021;137:111368. [DOI] [PubMed] [Google Scholar]
- 326. Chen W, Liu D, Zhou L, Li Q, Wu D. Antioxidant activity of vitamin E enhanced by cyclodextrin inclusion complex. Br Food J. 2021;123:3988–98. [Google Scholar]
- 327. Tkacz K, Gil‐Izquierdo Á, Medina S, Turkiewicz IP, Domínguez‐Perles R, Nowicka P, et al. Phytoprostanes, phytofurans, tocopherols, tocotrienols, carotenoids and free amino acids and biological potential of sea buckthorn juices. J Sci Food Agric. 2021;102:185–97. [DOI] [PubMed] [Google Scholar]
- 328. Dallner G, Bentinger M, Hussain S, Sinha I, Yang J, Schwank‐Xu C, et al. Dehydro‐Tocotrienol‐β counteracts oxidative‐stress‐induced diabetes complications in db/db mice. Antioxidants. 2021;10(7):1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


