Abstract
Habitat loss and shifts associated with climate change threaten global biodiversity, with impacts likely to be most pronounced at high latitudes. With the disappearance of the tundra breeding habitats, migratory shorebirds that breed at these high latitudes are likely to be even more vulnerable to climate change than those in temperate regions. We examined this idea using new distributional information on two subspecies of Black‐tailed Godwits Limosa limosa in Asia: the northerly, bog‐breeding L. l. bohaii and the more southerly, steppe‐breeding L. l. melanuroides. Based on breeding locations of tagged and molecularly assayed birds, we modelled the current breeding distributions of the two subspecies with species distribution models, tested those models for robustness and then used them to predict climatically suitable breeding ranges in 2070 according to bioclimatic variables and different climate change scenarios. Our models were robust and showed that climate change is expected to push bohaii into the northern rim of the Eurasian continent. Melanuroides is also expected to shift northward, stopping in the Yablonovyy and Stanovoy Ranges, and breeding elevation is expected to increase. Climatically suitable breeding habitat ranges would shrink to 16% and 11% of the currently estimated ranges of bohaii and melanuroides, respectively. Overall, this study provides the first predictions for the future distributions of two little‐known Black‐tailed Godwit subspecies and highlights the importance of factoring in shifts in bird distribution when designing climate‐proof conservation strategies.
Keywords: climate change, East Asian‐Australasian Flyway, IPCC, Limosa limosa, Maxent, shorebirds, species distribution modelling
Due to climate change, the breeding range of the two Asian subspecies of Black‐tailed Godwit would shift northward in the future, accompanied by a significant decline in suitable breeding range size.
Abstract
气候变化导致的物种栖息地丧失和转移,威胁着全球生物多样性,其影响在高纬度地区尤为明显。我们推测,在北半球极地繁殖的鸻鹬类较之于低纬度繁殖的种类更易受到干扰。通过预测黑尾塍鹬渤海亚种 (Limosa limosa bohaii) 和东方亚种 (L. l. melanuroides) 在亚洲的繁殖区分布,我们对该假说进行了验证。使用两个亚种繁殖地的坐标,我们首先对它们当前繁殖区的分布进行了模拟,并验证了模型的准确性。然后根据未来两种碳排放场景下的气候条件,预测两个亚种50年后的气候适宜繁殖区。结果显示,在2070年渤海亚种的繁殖地将转移至欧亚大陆的北缘(北极滨海地区);同时东方亚种也会向北转移,但会止步于Yablonovyy和Stanovoy山脉南麓,且繁殖地海拔会随之升高。未来渤海和东方亚种的繁殖区面积将骤减到目前估测的16%和11%。综上所述,该研究首次对黑尾塍鹬两个亚洲种群的当前和未来分布进行了预测,同时探讨了全球暖化对该物种繁殖分布的影响。
1. INTRODUCTION
The global temperature has risen by 0.08°C per decade since the Industrial Revolution in the late 19th century (Lindsey & Dahlman, 2020; Root et al., 2003). This warming has been particularly severe in the Arctic (Collins et al., 2013; Houghton et al., 2001; Kåresdotter et al., 2021; Serreze & Barry, 2011). It has led to advanced snowmelt (Chen et al., 2021; Zhong et al., 2021) and earlier emergence of insects (Saalfeld et al., 2021), forcing animals such as shorebirds to breed and migrate earlier (Lameris et al., 2017), with one cost being lower adult survival (Rakhimberdiev et al., 2018). In general, many organisms have advanced their timing of reproduction to counter the adverse effects of the warming climate (Jenni & Kéry, 2003; Root et al., 2003; Walther et al., 2002); the taxa that have advanced the least have shown the most substantial population declines (Lameris et al., 2018; Ross et al., 2018).
To mitigate the detrimental effects of global warming, species are expected to leave their current breeding range and move to more climatically suitable breeding habitats. Indeed, birds that breed at low latitudes in Eurasia and North America tend to move to the cooler north (Hitch & Leberg, 2007; McClure et al., 2012; Thomas & Lennon, 1999; Virkkala et al., 2018; Zuckerberg et al., 2009). However, in the Arctic, northward shifts are constrained by the presence of the Arctic Ocean; this results in a compressed breeding range and smaller population sizes, in addition to longer migrations in terms of distance and time (Parmesan, 2006; Rehfisch & Crick, 2003; Wauchope et al., 2017).
Black‐tailed Godwits Limosa limosa (hereafter ‘godwits’) are migratory shorebirds that breed across the temperate and boreal zones of the Palearctic (Prater et al., 1977; Zhu et al., 2020). In Europe, L. l. limosa shows little evidence of change in the timing of homeward migration and breeding (Kleijn et al., 2010; Schroeder et al., 2012), but advancing nesting dates were detected in L. l. islandica (Gill et al., 2014). With the gradual increase in average April temperatures over the past century, observations have also suggested a northward expansion of L. l. limosa in NW Russia (Popov & Starikov, 2015). Studies on the East Asian godwits are scarce, with little knowledge on L. l. melanuroides in the East Asian‐Australasian Flyway and a recent discovery of the hitherto unknown subspecies L. l. bohaii in China (Zhu, Verkuil, et al., 2021). The previously defined breeding range of melanuroides, which included seven fragmented sites from ca. 45°N to ca. 68°N (BirdLife International, 2018), is consequently being reconsidered because some of those areas are occupied by bohaii. Recent tracking revealed two areas in the Arctic region of the Russian Far East where bohaii breeds (59°N to ca. 65°N). Breeding melanuroides are mainly found in the temperate steppe of East Asia (45°N to ca. 52°N, Zhu, Verhoeven, et al., 2021).
The latitudinal contrast between the two East Asian godwit populations provides a suitable system to investigate and compare how climate change may differentially impact the breeding range. On the basis of species distribution models (SDMs, Pacifici et al., 2015), we delineate the breeding ranges of the two subspecies bohaii and melanuroides and examine which environmental factors determine these distributions. We then use this understanding to evaluate how global warming will affect the currently climatically suitable breeding habitats of the two subspecies; to do this, we project two greenhouse emissions scenarios in the models, capturing a range of possibilities using both a moderate and a pessimistic climate change trend (IPCC, 2013).
2. MATERIALS AND METHODS
2.1. Breeding locations
We obtained breeding ground locations for genetically confirmed bohaii individuals by satellite tracking (Zhu, Verhoeven, et al., 2021), considering stationary locations recorded during June–August as breeding ground locations (Figure 1). Stationary locations were identified when a transmitter recorded a speed of ≈0 km/h and a local elevation of ≈0 m. Breeding ground locations of melanuroides were obtained from our previous genetic work (Zhu, Verkuil, et al., 2021). We used known genetic differences between the subspecies to confirm that the individuals breeding in locations identified through satellite tracking versus those breeding in locations identified through published records did indeed belong to the two subspecies (see Zhu, Verkuil, et al., 2021). This yielded 60 known breeding ground locations for bohaii (2015–2018) and 41 for melanuroides (1993–2016) to parameterize our species distribution models (SDMs).
FIGURE 1.
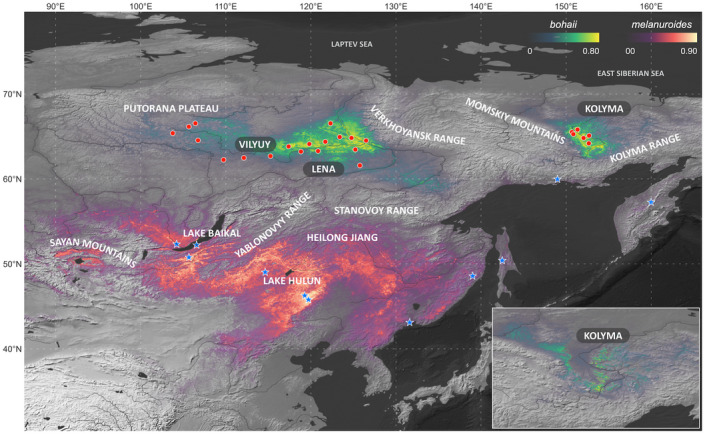
Predicted current breeding ranges of two subspecies of godwits in Asia. The dots and stars represent occurrence records of bohaii and melanuroides, respectively. The map in the bottom right: the test model returned a similar result when predicting the eastern breeding range of bohaii using only the western part of the occurrence records.
2.2. Environmental variables
To capture the current breeding habitat in our species distribution models, we obtained (a) 19 bioclimatic variables and (b) surface elevation data from WorldClim (www.worldclim.org, v 2.1, 1970–2000), along with (c) six breeding habitat features variables (e.g. vegetation type, snow cover) from Earthenv (http://www.earthenv.org//landcover), (d) the average normalized difference vegetation index (NDVI) for May–July during 2014–2018 from NOAA (https://www.ncei.noaa.gov/data/avhrr‐land‐normalized‐difference‐vegetation‐index/access/), and (e) the Global Human Footprint and Influence indexes which summarize data on human population density, land use, infrastructure and access (https://sedac.ciesin.columbia.edu/data/collection/wildareas‐v2). See Table S1 for a complete overview and detailed information on all variables. As the distributional record of the bohaii subspecies was collected more recently than the 1970–2000 bioclimatic data, we verified that the variable that contributed the most to predicting its distribution (Table S2), that is, temperature seasonality (Bio4), from 1970–2000 to 2010–2018 increased by 1% only.
To predict the breeding range for both subspecies in 2070, we obtained data from the Intergovernmental Panel on Climate Change (IPCC) AR5 from the Global Climate Model (GCM) downscaled data portal (http://www.ccafs‐climate.org/data_spatial_downscaling/). We selected two different emissions scenarios to take the uncertainty of the future into account: (1) the Representative Concentration Pathway 4.5 (RCP), a moderate scenario in which greenhouse gas emissions peak around 2040 and then decline and (2) the more pessimistic RCP 8.5, in which emissions are predicted to continue to grow throughout the 21st century (https://ar5‐syr.ipcc.ch/index.php). For each of the two emissions scenarios, we selected two different Global Climate Models (GCMs), GFDL‐CM3 and MIROC‐ESM (Wauchope et al., 2017), that predict future bioclimatic conditions, resulting in two sets of predicted bioclimatic conditions for each subspecies (Table S1).
In considering potential godwit breeding habitats, we constrained the range of all environmental variables to above 40°N on the Eurasian continent (BirdLife International, 2018). Though there may also be suitable breeding habitats in Alaska or northern Canada, ca. 3000 km to the east of our easternmost records, the likelihood that these areas will become occupied by godwits within the next five decades appears low due to the lack of suitable steppingstone habitats between the current breeding locations and North America. In Qgis 3.8, all environmental variables were resampled to 2.5 arc‐min spatial resolution (ca. 4.5 km at the equator) using the nearest neighbour interpolation. Any missing values were filled with the mean values within five pixels of the missing pixel using the maximum distance method, followed by repeating the smoothing iterations five times after each interpolation. The mean NDVI for a given pixel was calculated using the raster calculator.
2.3. Species distribution modelling
We constructed current and future SDMs for these two subspecies using maximum entropy modelling (Maxent, version 3.3.4). Maxent is a machine‐learning algorithm that uses presence‐only data to determine the predicted suitability of local conditions for a given species (Phillips et al., 2006; Phillips & Dudík, 2008). Several studies have suggested that Maxent has good predictive power for birds (Hu & Liu, 2014; Wauchope et al., 2017). We used occurrence records only when the subspecies present was genetically confirmed, and the breeding status was known because this approach to predicting subspecies distribution has proven more accurate than traditional methods that rely solely on occurrence records (Ikeda et al., 2017).
To model the current breeding habitats of the two subspecies, we first tested Pearson's correlations on all 29 environmental variables. Each highly correlated variable pair (|r| ≥ 80%) that gave higher values when testing the correlations against the remaining ones were retained. Next, we determined the final selection of the variables by their contributions and jackknife tests. Those variables that contributed less than 1% to the model were removed (Table S2). As a result, 14 variables were selected for the final analyses: annual mean temperature (°C), mean diurnal temperature range (°C), temperature seasonality (SD × 100), mean temperature of the wettest quarter (°C), mean temperature of the driest quarter (°C), mean temperature of the coldest quarter (°C), annual precipitation (mm), precipitation seasonality (variation coefficient), precipitation of the driest quarter (mm), human footprint (range: 1–100, a higher value indicates higher anthropogenic impacts), herbaceous plants coverage (%), shrub coverage (%), elevation (m) and NDVI (see correlation matrix in Table S3). To test the robustness of our models in predicting the current range, we used a model that contained only the western occurrence records of bohaii to see whether the model would correctly predict the eastern range more than 1000 km away (Figure 1). We were unable to do the same for melanuroides because the subspecies did not have such widely dispersed breeding occurrence records.
The godwit breeding habitat in 2070 was estimated by changing the current climatic conditions as predicted in two IPCC greenhouse gas emissions scenarios (RCP 4.5 & 8.5). Since there were no non‐climatic variables available in the future scenarios, for example, landcover, anthropogenic impacts or NDVI, we only used the 19 bioclimatic variables and elevation data for these models. Therefore, the predictions only estimate the future change in climatically suitable breeding habitat ranges and do not deal with other global changes. Consequently, the predictions are relatively conservative.
In all models, 70% of the occurrence records were randomly assigned to the training dataset and 30% to the testing dataset. The “Remove duplicate presence records” option was used to avoid the inclusion of duplicated records in a grid cell. The model performance was evaluated using the mean AUC score (area under the receiving operating curve, mean ± SD) and threshold‐based evaluation methods (Phillips et al., 2006). The model output was summarized into a logistic suitability value ranging from 0: unsuitable to 1: suitable. In our case, we defined three suitability levels: low: 0–0.29, medium: 0.3–0.59 and high: 0.6–1. We ran 20 bootstrap replicates for all models and selected the point‐wise mean of the 20 output grids to draw the predicted breeding habitat ranges. Results from the two Global Climate Models (GCMs) in each emissions scenario were averaged, yielding two consensus grids for each subspecies. Lastly, we overlaid the predicted current breeding habitat ranges and the predicted climatically suitable breeding habitat for both subspecies in 2070 onto maps in Qgis 3.18 (Figure 2).
FIGURE 2.
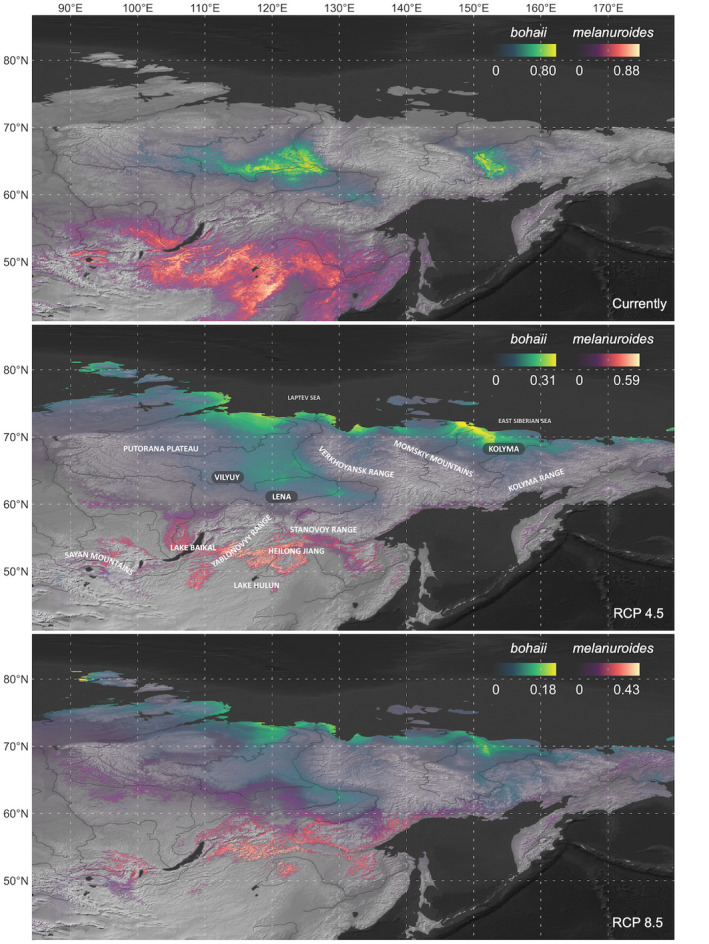
Predicted range shifts of two subspecies of godwits in 2070, based on RCP 4.5 and RCP 8.5 scenarios. Habitat suitability was presented by logistic values (from 0: unsuitable to 1: suitable).
2.4. Spatial analysis
The values of the 14 variables that best explained the current range of the breeding distribution were extracted at the occurrence locations using the function “sample raster values” in Qgis 3.18. We used Student’s t‐tests to explore differences between the subspecies for these environmental and climatic variables. To assess the predicted shift in breeding habitat range in 2070, we used Student’s t‐tests to compare mean breeding latitude and elevation in 2020 with those predicted for each subspecies in 2070. Since the highest logistic suitability in 2070 differed between subspecies (see Results), we constructed the polygons with values greater than 0.15 (for bohaii) and 0.3 (melanuroides) in Qgis 3.18. We randomly extracted 200 latitude and elevation datums from each modelled habitat range. We calculated the sizes of the breeding habitats with low, medium and high suitability in current and future scenarios using the “r.report” function. Statistics were performed in R v 3.6.0 (R Core Team, 2018) and then visualized in the package ggplot2.
3. RESULTS
3.1. Current breeding habitat ranges
Confirming the known breeding distribution of bohaii, the model identified two isolated suitable breeding areas, both located around the Arctic Circle in the Russian Far East (61–68°N; Figure 1). The western area was in the Sakha Republic, extending from 100°E westwards to the confluence of the Vilyuy and Lena Rivers and ending in the western foothills of the Verkhoyansk Range (128°E; Figure 1). The eastern area was between the Momskiy and Kolyma Ranges, in the Kolyma River basin of Magadan Oblast (150–155°E; Figure 1). For melanuroides, the range of suitable breeding habitat was further south (42–52°N). It covered a vast swathe of Asia's temperate interior, stretching from Lake Baikal to the Heilongjiang River and the Sea of Japan (90–140°E; Figure 1). Some coastal areas in the Russian Far East were also found to be suitable for melanuroides, namely Sakhalin Island and an area south of the Kolyma Range (142–160°E; Figure 1). The AUC scores and threshold statistics suggest that these models provided reasonable predictions for both bohaii (AUCtraining = 0.996 ± 0.001; AUCtest = 0.993 ± 0.001; all threshold statics p < 10−13) and melanuroides (AUCtraining = 0.991 ± 0.007; AUCtest = 0.957 ± 0.049; all threshold statics p < 10−5). We verified the robustness of our model predictions by testing whether our models would also predict the eastern breeding area of bohaii using only the occurrence records from the western part. Encouragingly, the models successfully predicted the eastern area (Figure 1). The final and test models indicated that the eastern breeding area is suitable for bohaii (the highest suitabilities were 0.86 and 0.77, respectively), with breeding range sizes of 292 × 103 km2, 304 × 103 km2, respectively.
Concerning habitat features, bohaii bred near rivers in swampy areas with low shrub coverage and low anthropogenic impact (Figure 3; Figure S1). Melanuroides bred mainly in pasture areas close to lakes and with more human activity (Figure 3; Figure S1). The annual mean temperature and precipitation in the breeding habitats for bohaii were significantly lower than for melanuroides (Figure 3). Within the modelling range where the logistic suitability values were higher than 0, 64% (8.4 × 105 km2) and 63% (4.2 × 106 km2) of grid cells were predicted to have low breeding suitability for bohaii and melanuroides, respectively. In comparison, 30% (3.9 × 105 km2) and 32% (2.1 × 106 km2) of grid cells had medium suitability, and 7% (0.9 × 104 km2) and 5% (0.4 × 106 km2) of grid cells had high suitability (Figure 4, Table S4).
FIGURE 3.
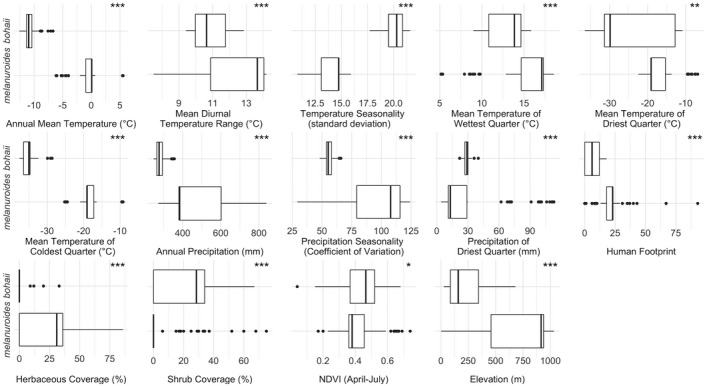
Variables were used to model current breeding habitats and compare the climatical and environmental features of two subspecies’ breeding habitats. Values were collected from the occurrence records (Significance level: *p < .05, **p < .001, ***p < .0001).
FIGURE 4.
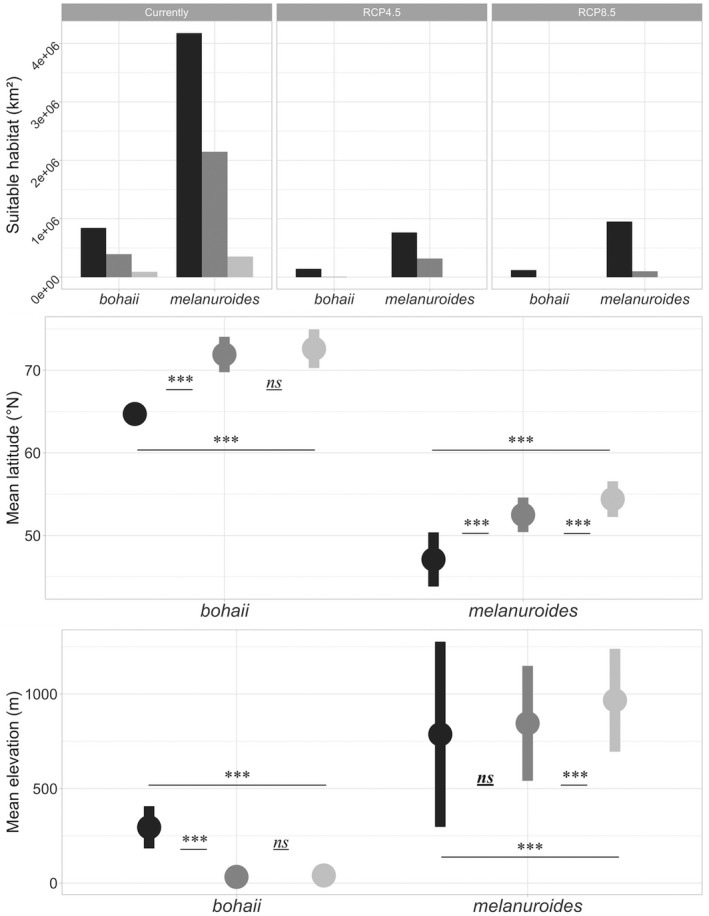
Top panel: loss of the suitable breeding habitat ranges in 2070 based on two climate change scenarios, black, grey and light grey represent low (0–0.29), medium (0.3–0.59) and high suitability (0.6–1), respectively; Middle panel: mean latitudinal (±SD) shifts of two bohaii and melanuroides’ breeding habitat ranges; Bottom panel: mean elevation (±SD) shifts of both subspecies. For middle and bottom panels, black, grey and light grey represent currently, RCP 4.5 and RCP 8.5 scenarios. Significance level: *p < .05, **p < .001, ***p < .0001.
3.2. Climatically suitable breeding habitat ranges in 2070
The models predicted a dramatic range shift and decline of climatically suitable breeding habitats by 2070 for both subspecies under RCP 4.5 and RCP 8.5 scenarios (Figure 2). The mean latitude of bohaii's breeding habitat range is predicted to shift northward from the current 64.7 ± 7.6°N to 71.9 ± 2.1°N (p < .0001) in RCP 4.5 and 72.6 ± 2.3°N (p < .0001) in RCP 8.5 (Figure 4). In other words, the only remaining climatically suitable breeding habitat would lie at the northern margin of the continent along the Arctic Ocean coast in the Russian Far East (68–70°N, 60–180°E). With this latitudinal shift, the breeding elevation of bohaii would show a significant decrease from the current 295 ± 112 m to 33 ± 31 m (p < .0001) in RCP 4.5 and 40 ± 28 m (p < .0001) in RCP 8.5 (Figure 4). In RCP 4.5, the habitat range would decline to 11% of the current extent (1.5 × 105 km2), of which 96% (1.4 × 105 km2) of grid cells were predicted to have low suitability, 5% (6742 km2) of grid cells were predicted to have medium suitability, and no high suitability habitats were expected to remain. In RCP 8.5, the habitat range declined to 9% of the current area, and all areas (1.2 × 105 km2) were predicted to have low suitability (Figure 4, Table S4).
The climatically suitable breeding habitat range of melanuroides is predicted to be split by the Yablonovy and Stanovoy Ranges, with the central part of Mongolia and the south of Lake Baikal no longer suitable for melanuroides (Figures 1, 2). The habitat range would shift to the north of the Heilongjiang River basin, towards the foothills of the Stanovoy Range. The mean latitude of the habitat range is predicted to shift significantly from the current 47.1 ± 3.3°N to 52.5 ± 2°N (p < .0001) in RCP 4.5 and 54.5 ± 2.1°N (p < .0001) in RCP 8.5 (Figure 4). The current mean elevation (787 ± 490 m) is predicted to remain the same as in RCP 4.5 (845 ± 304 m, p = 0.16), but increased significantly in RCP 8.5 (957 ± 272 m, p < .0001, Figure 4). Under RCP 4.5, the habitat range declined to 16% of the current area (1.1 × 106 km2), of which 71% (7.6 × 105 km2) of grid cells were predicted to have low suitability, 29% (3.2 × 105 km2) of grid cells were predicted to have medium suitability, and no high suitability habitats were expected to remain. In RCP 8.5, the habitat range declined to 15% of the current area (1 × 106 km2), with 90% (9.5 × 105 km2) of the habitat range predicted to have low suitability, 10% (1 × 105 km2) of grid cells predicted to have medium suitability, and no high suitability habitats remained (Figure 4, Table S4).
4. DISCUSSION
The modelling shows that, consistent with common patterns, the breeding ranges of both subspecies of Black‐tailed Godwits are projected to shift northward due to a warming climate. More crucial, however, is our finding that both subspecies are also expected to suffer a large decline in climatically suitable breeding habitats because of climate change. The breeding range and climatic suitability for the more northerly breeding subspecies bohaii would suffer the most significant decline because there is no land further to the north for the birds to use. In addition, due to the lack of climatically suitable breeding habitat in the north, the southerly subspecies melanuroides is expected to be forced onto higher ground to breed. Our results highlight the importance of factoring in future shifts in bird distribution when designing climate‐proof conservation strategies.
4.1. Current breeding ranges
Currently, bohaii godwits are known to breed in Russia along the Arctic circle in two isolated regions east and west of the Verkhoyansk Mountain Range. Our model was able to predict the eastern region based on records from the western region alone (Figure 1.), demonstrating the model's robustness and the similarity of the environmental conditions in both regions. Degtyarev et al. (2020) describe a population of ca.11,000 godwits breeding in an area stretching from 62°N to 68°N and from 100°E to 125°E. This area closely matches our prediction of the western breeding range of bohaii. The measured body dimensions of members of this breeding population also match those described for bohaii (E. Shemyakin & P. Tomkovich, pers. comm., Zhu, Verhoeven, et al., 2021), which further adds to our confidence that our model correctly predicts the current breeding range for bohaii.
The current breeding range of melanuroides is not well‐known, but the latest summary assessments (BirdLife International, 2018) assume that melanuroides breeds relatively far north (60–65°N) and thus overlaps with bohaii. However, this is not consistent with either our or previous results. Our tracking and modelling efforts do not support the idea that melanuroides breeds north of 60°N, and neither do the findings of Lappo et al. (2012), while the modelled breeding range for melanuroides is significantly further to the south (42–52°N) and therefore does not overlap with bohaii at all. Instead, the identified breeding ranges of the two subspecies are separate from one another and differ substantially in landscape, environment and climate: while bohaii breeds in swampy natural lowland with lower annual mean temperatures and precipitation, melanuroides mainly inhabits warmer and wetter semi‐natural pastures near lakes (Figure 1). Intriguingly, bohaii's current medium and highly suitable breeding habitat range is less than one‐fifth of melanuroides' (Table S4). A potential explanation for this difference is that there is less open area for bohaii to breed due to the presence of Boreal (Taiga) forest and mountain ranges (Figure 5; Figure S1). In contrast, the much larger breeding range of melanuroides is not restricted in this way, since it does not include forests and mountains (Figure S1).
FIGURE 5.
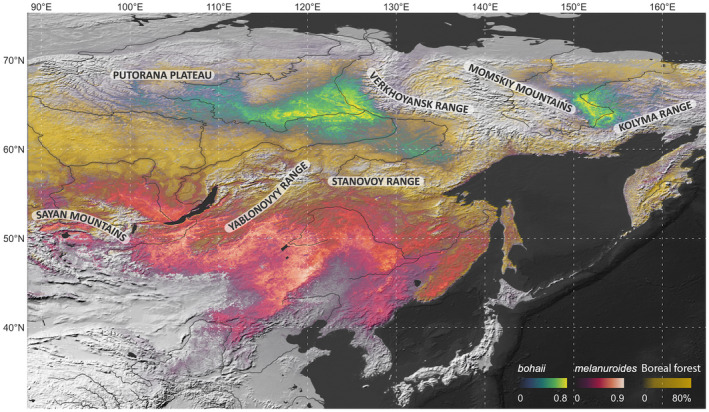
Predicted current breeding ranges of two subspecies of godwits in relation to the boreal forests. The range of the boreal forests was constructed using the tree canopy density product (source: https://glad.geog.umd.edu/projects/gfm/boreal/data.html).
4.2. Projected breeding ranges under climate change scenarios
The modelling results indicate that the climatically suitable breeding ranges of bohaii and melanuroides may shrink by 89% and 84% while also shifting northward, assuming no adaptive change in habitat preferences. Under both climate change scenarios, the modelled climatically suitable breeding habitat range of bohaii is concentrated in the estuaries along the Arctic coast. Here, further northward expansion is constrained by the absence of land (Figure 2). Meanwhile, melanuroides is projected to leave the temperate pastures due to significant loss of climatically suitable habitat in central‐eastern Mongolia and NE China (45–50°N). We observed a projected increase in their breeding elevation, with the southern foothills of the Stanovoy Range becoming suitable for melanuroides (Figure 2). However, the latitudinal shift is not as drastic as predicted for bohaii because, climatically, most areas to the north of the Stanovoy Range will remain too cold and dry to suit melanuroides. Moreover, the northward shift required to reach the next potentially suitable breeding habitat would be nearly impossible to make in only five decades; there are ca. 1900 km of continuous boreal forest separating the current breeding area from the basins of the Vilyuy and Lena rivers in the north (Figures 1, 5). It is important to reemphasize that these modelled ranges take only projected future climate into account. If other changes (e.g. climate‐driven changes in land or vegetation cover, water bodies) were also factored in, the actual future distribution of these two subspecies would probably be even smaller than we have projected.
Consistent with the predictions given here, continental godwits (L. l. limosa) in NW Russia have already been observed to shift their breeding range northward from 60°N in 1970 to 64°N in 2012 (Popov & Starikov, 2015). In addition, godwits started colonizing the basins of the Vilyuy and Lena rivers from the south in the early 1990s (Degtyarev et al., 2020). Meanwhile, godwits have been observed to vacate southerly regions like the Selenga Delta near Lake Baikal, after a period of continuous population decline, which might be due to the prolonged periods of low water since the late 2000s (I. Fefelov, pers. comm).
4.3. The bigger picture
While these two East Asian godwit subspecies do not suffer from as much direct anthropogenic impact on their breeding grounds as the Continental European subspecies (Kleijn et al., 2010; Kruk et al., 1997), they appear to be threatened by hunting (Zhu, Verhoeven, et al., 2021) and deteriorating staging sites in the Yellow Sea (Chan et al., 2019; Piersma et al., 2016; Yang et al., 2011). We now show that the two subspecies will also likely have to cope with climate change (1) heavily diminishing the suitability of their current breeding habitat and (2) shifting and considerably shrinking the climatically suitable breeding habitat range. As a result, we expect a decline in the adult survival and reproductive success of those godwits that remain in their current breeding range (Jetz et al., 2007; Saalfeld et al., 2021).
This expected reduction in suitable breeding habitat contrasts interestingly with observations made for a different subspecies, the Icelandic godwit Limosa limosa islandica. At the start of the 20th century, the breeding area for islandica was constrained to Iceland's relatively warm southwest corner, and the population numbered only a few thousand (Gill et al., 2007). As the climate warmed and many areas were converted to farmland, large parts of the island became more suitable for breeding, and the islandica population consequently grew to ca. 47,000 (Gunnarsson et al., 2005; Gunnarsson et al., 2006). Nevertheless, the impact of a changing climate has also been negative; the original primary breeding area in the southwest of Iceland now appears to have become too warm, leading to reduced reproductive success in that area (Alves et al., 2019). These islandica observations neatly illustrate that climate change can benefit a godwit population by increasing the suitable breeding habitat, and it can negatively impact a godwit population by decreasing the suitable breeding habitat. Though both effects have been detected in the case of islandica, our results show that only the negative effect is expected for bohaii and melanuroides.
In the longer term, we expect the populations of bohaii and melanuroides to become smaller than at present. We also expect the adverse effects of climate change to be greater for bohaii than for melanuroides, because bohaii has a substantially smaller range and potentially small population size, precisely the two factors identified as affecting the susceptibility and extinction risk of populations in response to climate change (Beyer & Manica, 2020; Gaston & Blackburn, 1996). In addition, bohaii would face more extreme weather events (e.g. drought and wildfires, see Kharuk et al., 2021, Yasunari et al., 2021) while experiencing both higher predation rates and increased competition for breeding space as more species and populations become concentrated in the Arctic rim (Killengreen et al., 2007; Layton‐Matthews et al., 2020; Vallejos et al., 2020). These factors might partially explain the decline of wintering waterbirds breeding in Siberia compared to temperate Asia (Sung et al., 2021). Our results highlight the importance of thinking ahead and factoring in shifts in bird distribution when designing climate‐proof conservation strategies to salvage our heritage of migratory birds and, ultimately, the ecosystems we all belong to.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Table S1
Table S2
Table S3
Table S4
Figure S1
ACKNOWLEDGEMENTS
We thank Igor Fefelov, Pavel Tomkovich, Evgeny Shemyakin, Nikolai Egorov, Nyambayar Batbayar and Gankhuyag Purev‐Ochir for providing their local knowledge of the Black‐tailed Godwit in Russia and Mongolia. Jesse Conklin, Eldar Rakhimberdiev, Yvonne Verkuil and Alice McBride gave suggestions that improved the manuscript. This research was funded by the Natural Science Foundation of China (31830089) and International Wetlands and River Beijing.
Zhu, B‐R , Verhoeven, M. A. , Velasco, N. , Sanchez‐Aguilar, L. , Zhang, Z. , & Piersma, T. (2022). Current breeding distributions and predicted range shifts under climate change in two subspecies of Black‐tailed Godwits in Asia. Global Change Biology, 28, 5416–5426. 10.1111/gcb.16308
Contributor Information
Bing‐Run Zhu, Email: drewbingrun@outlook.com.
Zhengwang Zhang, Email: zzw@bnu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Dryad at https://doi.org/10.5061/dryad.gqnk98sr3.
REFERENCES
- Alves, J. A. , Gunnarsson, T. G. , Sutherland, W. J. , Potts, P. M. , & Gill, J. A. (2019). Linking warming effects on phenology, demography, and range expansion in a migratory bird population. Ecology and Evolution, 9, 2365–2375. 10.1002/ece3.4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BirdLife International . (2018). Limosa limosa (amended version of 2016 assessment). ‐ The IUCN Red List of Threatened Species 2017: e.T22693150A111611637. 10.2305/IUCN.UK.2017-1.RLTS.T22693150A111611637.en. [DOI]
- Beyer, R. M. , & Manica, A. (2020). Historical and projected future range sizes of the world's mammals, birds, and amphibians. Nature Communications, 11, 1–8. 10.1038/s41467-020-19455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, Y. C. , Peng, H. B. , Han, Y. X. , Chung, S. S. W. , Li, J. , Zhang, L. , & Piersma, T. (2019). Conserving unprotected important coastal habitats in the Yellow Sea: Shorebird occurrence, distribution and food resources at Lianyungang. Global Ecology and Conservation, 20, e00724. 10.1016/j.gecco.2019.e00724 [DOI] [Google Scholar]
- Chen, X. , Yang, Y. , Ma, Y. , & Li, H. (2021). Distribution and attribution of terrestrial snow cover phenology changes over the Northern Hemisphere during 2001–2020. Remote Sensing, 13, 1843. 10.3390/rs13091843 [DOI] [Google Scholar]
- Collins, M. , Knutti, R. , Arblaster, J. , Dufresne, J. L. , Fichefet, T. , Friedlingstein, P. , Gao, X. , Gutowski, W. J. , Johns, T. , Krinner, G. , & Shongwe, M. (2013). Long‐term climate change: Projections, commitments and irreversibility. In Climate change 2013–The physical science basis: Contribution of Working Group I to the fifth assessment report of the intergovernmental panel on climate change (pp. 1029–1136). Cambridge University Press. [Google Scholar]
- Degtyarev, V. G. , Egorov, N. N. , & Afanasiev, М. А. (2020). The Vilyui population of the Black‐tailed Godwit (Limosa limosa melanuroides, Charadriiformes, Scolopacidae). Зоологический Журнал, 99, 430–435. 10.1134/S1062359021070104 [DOI] [Google Scholar]
- Gaston, K. J. , & Blackburn, T. M. (1996). Global scale macroecology: interactions between population size, geographic range size and body size in the Anseriformes. Journal of Animal Ecology, 65, 701–714. 10.2307/5669 [DOI] [Google Scholar]
- Gunnarsson, T. G. , Gill, J. A. , Potts, P. M. , Atkinson, P. W. , Croger, R. E. , Gélinaud, G. , Gardarsson, A. , & Sutherland, W. J. (2005). Estimating population size in black‐tailed godwits Limosa limosa islandica by colour‐marking. Bird Study, 52, 153–158. 10.1080/00063650509461385 [DOI] [Google Scholar]
- Gunnarsson, T. G. , Gill, J. A. , Appleton, G. F. , Gíslason, H. , Gardarsson, A. , Watkinson, A. R. , & Sutherland, W. J. (2006). Large‐scale habitat associations of birds in lowland Iceland: Implications for conservation. Biological Conservation, 128, 265–275. 10.1016/j.biocon.2005.09.034 [DOI] [Google Scholar]
- Gill, J. A. , Langston, R. H. W. , Alves, J. A. , Atkinson, P. W. , Bocher, P. , Vieira, N. C. , Crockford, N.J. , Gélinaud, G. , Groen, N. , Gunnarsson, T. G. , & Piersma, T. (2007). Contrasting trends in two Black‐tailed Godwit populations: A review of causes and recommendations. Wader Study Group Bulletin, 114, 43–50. https://www.waderstudygroup.org/article/2966/ [Google Scholar]
- Gill, J. A. , Alves, J. A. , Sutherland, W. J. , Appleton, G. F. , Potts, P. M. , & Gunnarsson, T. G. (2014). Why is timing of bird migration advancing when individuals are not? Proceedings of the Royal Society B: Biological Sciences, 281, 20132161. 10.1098/rspb.2013.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitch, A. T. , & Leberg, P. L. (2007). Breeding distributions of North American bird species moving north as a result of climate change. Conservation Biology, 21, 534–539. 10.1111/j.1523-1739.2006.00609.x [DOI] [PubMed] [Google Scholar]
- Houghton, J. T. , Ding, Y. D. J. G. , Griggs, D. J. , Noguer, M. , van der Linden, P. J. , Dai, X. , Maskell, K. , & Johnson, C. A. (Eds.). (2001). Climate change 2001: The scientific basis: Contribution of working group I to the third assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press. [Google Scholar]
- Hu, J. , & Liu, Y. (2014). Unveiling the conservation biogeography of a data‐deficient endangered bird species under climate change. PLoS One, 9, e84529. 10.1371/journal.pone.0084529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, D. H. , Max, T. L. , Allan, G. J. , Lau, M. K. , Shuster, S. M. , & Whitham, T. G. (2017). Genetically informed ecological niche models improve climate change predictions. Global Change Biology, 23, 164–176. 10.1111/gcb.13470 [DOI] [PubMed] [Google Scholar]
- IPCC . (2013) Annex I: Atlas of global and regional climate projections (eds van Oldenborgh GJ, Collins M, Arblaster J et al.). In: Stocker TF, Qin D, Plattner GK et al. (Eds.) Climate change 2013: The physical science basis. Contributions of Working Group I to the fifth assessment report of the intergovernmental panel on climate change (pp. 1311–1394). Cambridge University Press, . [Google Scholar]
- Jenni, L. , & Kéry, M. (2003). Timing of autumn bird migration under climate change: Advances in long‐distance migrants, delays in short‐distance migrants. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270, 1467–1471. 10.1098/rspb.2003.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz, W. , Wilcove, D. S. , & Dobson, A. P. (2007). Projected impacts of climate and land‐use change on the global diversity of birds. PLoS Biology, 5, e157. 10.1371/journal.pbio.0050157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kåresdotter, E. , Destouni, G. , Ghajarnia, N. , Hugelius, G. , & Kalantari, Z. (2021). Mapping the vulnerability of Arctic wetlands to global warming. Earth's Futures, 9, e2020EF001858. 10.1029/2020EF001858 [DOI] [Google Scholar]
- Kharuk, V. I. , Ponomarev, E. I. , Ivanova, G. A. , Dvinskaya, M. L. , Coogan, S. C. , & Flannigan, M. D. (2021). Wildfires in the Siberian taiga. Ambio, 50, 1953–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killengreen, S. T. , Ims, R. A. , Yoccoz, N. G. , Bråthen, K. A. , Henden, J. A. , & Schott, T. (2007). Structural characteristics of a low Arctic tundra ecosystem and the retreat of the Arctic fox. Biological Conservation, 135, 459–472. 10.1016/j.biocon.2006.10.039 [DOI] [Google Scholar]
- Kleijn, D. , Schekkerman, H. , Dimmers, W. J. , Van Kats, R. J. , Melman, D. , & Teunissen, W. A. (2010). Adverse effects of agricultural intensification and climate change on breeding habitat quality of Black‐tailed Godwits Limosa l. limosa in The Netherlands. Ibis, 152, 475–486. 10.1111/j.1474-919X.2010.01025.x [DOI] [Google Scholar]
- Kruk, M. , Noordervliet, M. A. W. , & Ter Keurs, W. J. (1997). Survival of black‐tailed godwit chicks Limosa limosa in intensively exploited grassland areas in The Netherlands. Biological Conservation, 80, 127–133. 10.1016/S0006-3207(96)00131-0 [DOI] [Google Scholar]
- Lameris, T. K. , Scholten, I. , Bauer, S. , Cobben, M. M. , Ens, B. J. , & Nolet, B. A. (2017). Potential for an Arctic‐breeding migratory bird to adjust spring migration phenology to Arctic amplification. Global Change Biology, 23, 4058–4067. 10.1111/gcb.13684 [DOI] [PubMed] [Google Scholar]
- Lameris, T. K. , van der Jeugd, H. P. , Eichhorn, G. , Dokter, A. M. , Bouten, W. , Boom, M. P. , Litvin, K. E. , Ens, B. J. , & Nolet, B. A. (2018). Arctic geese tune migration to a warming climate but still suffer from a phenological mismatch. Current Biology, 28, 2467–2473. 10.1016/j.cub.2018.05.077 [DOI] [PubMed] [Google Scholar]
- Lappo, Е. , Tomkovich, P. , & Syroechkovskiy, E. (2012). Atlas of breeding waders in the Russian Arctic (pp. 381–385). Cambridge University Press. [Google Scholar]
- Layton‐Matthews, K. , Hansen, B. B. , Grøtan, V. , Fuglei, E. , & Loonen, M. J. (2020). Contrasting consequences of climate change for migratory geese: Predation, density dependence and carryover effects offset benefits of high‐arctic warming. Global Change Biology, 26, 642–657. 10.1111/gcb.14773 [DOI] [PubMed] [Google Scholar]
- Lindsey, R. , & Dahlman, L. (2020). Climate change: Global temperature. Climate.gov, 16. https://www.climate.gov/news‐features/understanding‐climate/climate‐change‐global‐temperature [Google Scholar]
- McClure, C. J. , Rolek, B. W. , McDonald, K. , & Hill, G. E. (2012). Climate change and the decline of a once common bird. Ecology and Evolution, 2, 370–378. 10.1002/ece3.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici, M. , Foden, W. B. , Visconti, P. , Watson, J. E. , Butchart, S. H. , Kovacs, K. M. , Scheffers, B. R. , Hole, D. G. , Martin, T. G. , Akçakaya, H. R. , & Corlett, R. T. (2015). Assessing species vulnerability to climate change. Nature Climate Change, 5, 215–224. 10.1038/nclimate2448 [DOI] [Google Scholar]
- Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics, 37, 637–669. 10.1146/annurev.ecolsys.37.091305.110100 [DOI] [Google Scholar]
- Phillips, S. J. , Anderson, R. P. , & Schapire, R. E. (2006). Maximum entropy modelling of species geographic distributions. Ecological Modelling, 190, 231–259. 10.1016/j.ecolmodel.2005.03.026 [DOI] [Google Scholar]
- Phillips, S. J. , & Dudík, M. (2008). Modeling of species distribution with Maxent: New extensions and a comprehensive evaluation. Ecography, 31, 161–175. 10.1111/j.0906-7590.2008.5203.x [DOI] [Google Scholar]
- Piersma, T. , Lok, T. , Chen, Y. , Hassell, C. J. , Yang, H. Y. , Boyle, A. , Slaymaker, M. , Chan, Y. C. , Melville, D. S. , Zhang, Z. W. , & Ma, Z. (2016). Simultaneous declines in summer survival of three shorebird species signals a flyway at risk. Journal of Applied Ecology, 53, 479–490. 10.1111/1365-2664.12582 [DOI] [Google Scholar]
- Popov, I. , & Starikov, D. (2015). Recent northward expansion of breeding Black‐tailed Godwits Limosa limosa in NW Russia. Wader Study, 122, 173–183. https://www.waderstudygroup.org/article/7355/ [Google Scholar]
- Prater, A. J. , Prater, T. , Marchant, J. , & Vourinen, J. (1977). Guide to the identification and ageing of Holarctic waders (Vol. 17). British Trust for Ornithology. [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing. [Google Scholar]
- Rakhimberdiev, E. , Duijns, S. , Karagicheva, J. , Camphuysen, C. J. , Dekinga, A. , Dekker, R. , Gavrilov, A. , Ten Horn, J. , Jukema, J. , Saveliev, A. , & Soloviev, M. (2018). Fuelling conditions at staging sites can mitigate Arctic warming effects in a migratory bird. Nature Communications, 9, 4263. 10.1038/s41467-018-06673-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfisch, M. M. , & Crick, H. Q. (2003). Predicting the impact of climatic change on Arctic‐breeding waders. Wader Study Group Bulletin, 100, 86–95. [Google Scholar]
- Root, T. L. , Price, J. T. , Hall, K. R. , Schneider, S. H. , Rosenzweig, C. , & Pounds, J. A. (2003). Fingerprints of global warming on wild animals and plants. Nature, 421, 57–60. 10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- Ross, M. V. , Alisauskas, R. T. , Douglas, D. C. , Kellett, D. K. , & Drake, K. L. (2018). Density‐dependent and phenological mismatch effects on growth and survival in lesser snow and Ross's goslings. Journal of Avian Biology, 49, 1–12. 10.1111/jav.01748 [DOI] [Google Scholar]
- Saalfeld, S. T. , Hill, B. L. , Hunter, C. M. , Frost, C. J. , & Lanctot, R. B. (2021). Warming Arctic summers are unlikely to increase productivity of shorebirds through renesting. Scientific Reports, 11, 15277. 10.1038/s41598-021-94788-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J. , Piersma, T. , Groen, N. M. , Hooijmeijer, J. C. , Kentie, R. , Lourenço, P. M. , Schekkerman, H. , & Both, C. (2012). Reproductive timing and investment in relation to spring warming and advancing agricultural schedules. Journal of Ornithology, 153, 327–336. 10.1007/s10336-011-0747-5 [DOI] [Google Scholar]
- Serreze, M. C. , & Barry, R. G. (2011). Processes and impacts of Arctic amplification: A research synthesis. Global and Planetary Change, 77, 85–96. 10.1016/j.gloplacha.2011.03.004 [DOI] [Google Scholar]
- Sung, Y. H. , Pang, C. C. , Li, T. C. H. , Wong, P. P. Y. , & Yu, Y. T. (2021). Ecological correlates of 20‐year population trends of wintering waterbirds in Deep Bay, South China. Frontiers in Ecology and Evolution, 9, 247. 10.3389/fevo.2021.658084 [DOI] [Google Scholar]
- Thomas, C. D. , & Lennon, J. J. (1999). Birds extend their ranges northwards. Nature, 399, 213–213. 10.1038/20335 [DOI] [Google Scholar]
- Vallejos, M. A. V. , Padial, A. A. , Vitule, J. R. S. , & Monteiro‐Filho, E. L.d. A. (2020). Effects of crowding due to habitat loss on species assemblage patterns. Conservation Biology, 34, 405–415. 10.1111/cobi.13443 [DOI] [PubMed] [Google Scholar]
- Virkkala, R. , Rajasärkkä, A. , Heikkinen, R. K. , Kuusela, S. , Leikola, N. , & Pöyry, J. (2018). Birds in boreal protected areas shift northwards in the warming climate but show different rates of population decline. Biological Conservation, 226, 271–279. 10.1016/j.biocon.2018.08.015 [DOI] [Google Scholar]
- Walther, G. R. , Post, E. , Convey, P. , Menzel, A. , Parmesan, C. , Beebee, T. J. , Fromentin, J. M. , Hoegh‐Guldberg, O. , & Bairlein, F. (2002). Ecological responses to recent climate change. Nature, 416, 389–395. 10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Wauchope, H. S. , Shaw, J. D. , Varpe, Ø. , Lappo, E. G. , Boertmann, D. , Lanctot, R. B. , & Fuller, R. A. (2017). Rapid climate‐driven loss of breeding habitat for Arctic migratory birds. Global Change Biology, 23, 1085–1094. 10.1111/gcb.13404 [DOI] [PubMed] [Google Scholar]
- Yang, H. Y. , Chen, B. , Barter, M. , Piersma, T. , Zhou, C. F. , Li, F. S. , & Zhang, Z. W. (2011). Impacts of tidal land reclamation in Bohai Bay, China: Ongoing losses of critical Yellow Sea waterbird staging and wintering sites. Bird Conservation International, 21, 241–259. 10.1017/S0959270911000086 [DOI] [Google Scholar]
- Yasunari, T. J. , Nakamura, H. , Kim, K. M. , Choi, N. , Lee, M. I. , Tachibana, Y. , & da Silva, A. M. (2021). Relationship between circum‐Arctic atmospheric wave patterns and large‐scale wildfires in boreal summer. Environmental Research Letters, 16, 064009. 10.1088/1748-9326/abf7ef [DOI] [Google Scholar]
- Zhong, X. , Zhang, T. , Kang, S. , & Wang, J. (2021). Spatiotemporal variability of snow cover timing and duration over the Eurasian continent during 1966–2012. Science of the Total Environment, 750, 141670. 10.1016/j.scitotenv.2020.141670 [DOI] [PubMed] [Google Scholar]
- Zhu, B. R. , Hassell, C. J. , Verkuil, Y. I. , Gunnarson, T. G. , Hooijmeijer, J. C. , Zhang, Z. , & Piersma, T. (2020). Size, shape and sex differences in three subspecies of Black‐tailed Godwits Limosa limosa . Bird Study, 67, 45–52. 10.1080/00063657.2020.1733930 [DOI] [Google Scholar]
- Zhu, B. R. , Verkuil, Y. I. , Conklin, J. R. , Yang, A. , Lei, W. , Alves, J. A. , Hassell, C. J. , Dorofeev, D. , Zhang, Z. , & Piersma, T. (2021). Discovery of a morphologically and genetically distinct population of Black‐tailed Godwits in the East Asian‐Australasian Flyway. Ibis, 163, 448–462. 10.1111/ibi.12890 [DOI] [Google Scholar]
- Zhu, B. R. , Verhoeven, M. A. , Loonstra, A. H. , Sanchez‐Aguilar, L. , Hassell, C. J. , Leung, K. K. , Lei, W. , Zhang, Z. , & Piersma, T. (2021). Identification of breeding grounds and annual routines of the newly discovered bohaii subspecies of Black‐tailed Godwits. Emu‐Austral Ornithology, 121, 292–302. 10.1080/01584197.2021.1963287 [DOI] [Google Scholar]
- Zuckerberg, B. , Woods, A. M. , & Porter, W. F. (2009). Poleward shifts in breeding bird distributions in New York State. Global Change Biology, 15, 1866–1883. 10.1111/j.1365-2486.2009.01878.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Figure S1
Data Availability Statement
The data that support the findings of this study are available from Dryad at https://doi.org/10.5061/dryad.gqnk98sr3.


