Abstract
Hypotheses concerning the biologic embedding of early adversity via developmental neuroplasticity mechanisms have been proposed on the basis of experimental studies in animals. However, no studies have demonstrated a causal link between early adversity and neural development in humans. Here, we present evidence from a randomized controlled trial linking psychosocial deprivation in early childhood to changes in cortical development from childhood to adolescence using longitudinal data from the Bucharest Early Intervention Project. Changes in cortical structure due to randomization to foster care were most pronounced in the lateral and medial prefrontal cortex and in white matter tracts connecting the prefrontal and parietal cortex. Demonstrating the causal impact of exposure to deprivation on the development of neural structure highlights the importance of early placement into family-based care to mitigate lasting neurodevelopmental consequences associated with early-life deprivation.
An RCT demonstrates that early-life deprivation alters patterns of neural development from childhood to adolescence.
INTRODUCTION
Exposure to psychosocial deprivation in the form of institutional rearing is associated with a wide range of adverse long-term outcomes, including impaired cognitive abilities (1–3), increased psychopathology (4, 5), and poor social functioning (6, 7). We and others have proposed that these negative developmental outcomes are the result of biological embedding of adversity, whereby early psychosocial experiences alter brain development, contributing to lasting changes in cognitive, emotional, and behavioral development (8, 9). However, to date, most support for these hypotheses comes from observational studies.
Early in development, the presence of sensitive and responsive caregivers not only provides young children with safety but also provides sensory, motoric, linguistic, and social stimulation that fosters learning (8, 10, 11). The absence of an invested caregiver deprives children of the back-and-forth interactions that facilitate the regulation of arousal and distress and limits opportunities for early learning that are critical for developing typical neural architecture (12). While experimental evidence demonstrates lasting differences in cognitive (13) and socioemotional development (14) among institutionally reared children, to date, no studies have demonstrated mechanisms via developmental neuroplasticity. Current evidence concerning the impact of institutional rearing on neurobiological development largely relies on observational evidence from children adopted out of institutions, limiting the degree to which causal relationships can be determined (12, 15–22). Here, we demonstrate the impact of exposure to psychosocial deprivation in early life on the development of neural structure into adolescence using an experimental design.
Previous evidence from the Bucharest Early Intervention Project (BEIP) has demonstrated that random assignment to a supportive caregiving environment through removal from institutional rearing and placement into foster care in early childhood can normalize stress response systems, basic learning mechanisms, cognitive function, and psychopathology in early and middle childhood among institutionally reared children (2, 4, 13, 23, 24). Our previous study of brain structure in the BEIP at 9 years revealed that exposure to institutional rearing was associated with smaller gray and white matter volume and global reductions in cortical thickness. At this time point, randomization out of institutional care and into foster care was associated with higher white matter integrity in the anterior and superior corona radiata, the internal and external capsule, and the cingulum (24) but not volume or thickness of cortical or subcortical structures (12, 20). With the exception of these findings, all prior evidence linking institutionalization with neural structure comes from observational studies of previously institutionalized children that may be hampered by selection effects influencing both the placement of children into institutions and adoption out of institutions (25, 26) [but see also (27)].
Observational studies show that institutional rearing has consistently been associated with reduced cortical gray matter volume in childhood (12), adolescence (18), and adulthood (28) and reduced cortical thickness across many regions of the cortex in childhood (20). Changes in cortical development following institutional rearing may contribute to the high rates of psychopathology and reductions in cognitive ability observed in previously institutionalized children [e.g., (1)]. Decreases in volume and surface area of prefrontal cortex (PFC) regions specifically—including the superior, middle, and inferior frontal gyri (IFG) and orbitofrontal cortex—have been associated with institutionalization in both adolescent and adult samples (16, 28, 29). These associations vary across development: Institutionalization is associated with reduced thickness and volume of the anterior cingulate (ACC) and lateral temporal cortex in early adolescence (16, 20) and greater thickness and volume in these regions in adulthood (28). This pattern of findings suggests that institutionalization may shift not only early structural brain development but also the trajectory of gray matter development in adolescence. This possibility is consistent with processes of neural development observed in noninstitutionalized samples. Cortical structures—particularly in the prefrontal, temporal, and parietal cortex—undergo profound change during adolescence (30). Specifically, cortical thinning progresses relatively linearly across childhood and adolescence, whereas surface area has a parabolic association with age with steep increases followed by gradual decreases in adolescence (31). This change reflects an extended period of developmental plasticity for the association cortex (32, 33). Recent evidence from the BEIP additionally suggests that adolescence may be a second period of elevated sensitivity to environmental influences and that positive caregiving experiences during this period may ameliorate the impact of early psychosocial deprivation on cognitive and neurobiological outcomes (34, 35). To date, no study has examined the impact of institutionalization on developmental change in cortical structure.
Institutionalization has also been associated with global reductions in white matter volume and integrity, suggesting less robust structural connectivity. Previously institutionalized children demonstrate reductions in the volume and integrity of the corpus callosum (12, 18, 24) and reductions in white matter integrity in the uncinate fasciculus (36, 37), frontal-striatal tracts (24, 38, 39), and frontal-parietal tracts (37), compared to children raised in families.
Here, we examine developmental change in neural structure from childhood to adolescence in the only randomized controlled trial of foster care as an alternative to institutional care for abandoned children (40). To characterize the neural structure in our sample, we examined the total gray matter volume, subcortical volume, cortical surface area and thickness, and structural integrity of white matter tracts. We first evaluate the impact of institutionalization on developmental change from 9 to 16 years of age and then on neural structure in late adolescence (at age 16). We examine associations between exposure to institutionalization and neural development compared to family rearing from birth. Next, we examine whether randomized placement into family-based care early in life alters neural development and whether the timing of placement influences neurodevelopment among the children randomized to foster care. Children were randomized into foster care between the ages of 6 and 36 months, allowing us to evaluate the presence of a sensitive period in early childhood (see consort diagram in fig. S1). Last, we examined whether the impact of institutional rearing on cortical structure is associated with psychopathology and cognitive ability in adolescence.
The unique experimental and longitudinal design of the BEIP study allows us to draw conclusions about the causal impact of early deprivation on neural development in adolescence. We hypothesized that random assignment to foster care intervention would alter patterns of developmental change, which we could observe, between 9 and 16 years, resulting in altered neural structure at 16 years. Given the known sensitive periods for neural development in infancy, we expected that earlier placement into foster care intervention would be associated with a more normative pattern of change of neural structure at age 16. Last, we predicted that differences in neural structure observed at 16 years would be associated with psychopathology and cognitive ability in adolescence.
RESULTS
Total brain volume
First, we examined change in whole-brain volume for gray and white matter at 9 and 16 years as a function of exposure to institutionalization, randomization to foster care, and timing of placement into foster care intervention. Changes in whole-brain gray and white matter volume across age did not differ for any group comparison (all P > 0.53).
Next, we examined group differences in total gray and white matter volume at 16 years. As we observed previously in this sample at 9 years, children exposed to institutionalization had significantly lower global gray matter (F1,111 = 7.72, P = 0.006, η2 = 0.065), white matter (F1,111 = 11.25, P = 0.001, η2 = 0.092), and intracranial volume (F1,111 = 5.89, P = 0.004, η2 = 0.096) compared to never-institutionalized children. Exposure to institutionalization resulted in a 2.5 to 5.5% decrease in brain volume across these metrics, but gray matter volume for all groups still fell within about 1 SD of the mean on recently published brain charts (41) (https://brainchart.shinyapps.io/brainchart/). There were no differences as a function of randomization to the foster care intervention in gray matter (t80 = 0.10, P = 0.92, d = 0.023), white matter (t80 = 1.40, P = 0.16, d = 0.310), or total intracranial volume (t80 = 1.13, P = 0.13, d = 0.249; see table S3). Furthermore, age at placement into foster care intervention was not related to gray matter (F1,37 = 2.93, P = 0.10, η2 = 0.073), white matter (F1,37 = 1.73, P = 0.19, η2 = 0.045), or total intracranial volume (F1,37 = 1.62, P = 0.21, η2 = 0.042).
Subcortical volume
Change in subcortical volume from 9 to 16 years did not differ as a function of institutionalization, random assignment to foster care, or timing of the foster care intervention. There were no statistically significant effects of ever being exposed to institutionalization, foster care intervention, or timing of placement into foster care on subcortical gray matter volume at 16 years in any region (see the Supplementary Materials and table S3 for further information).
Cortical thickness and surface area
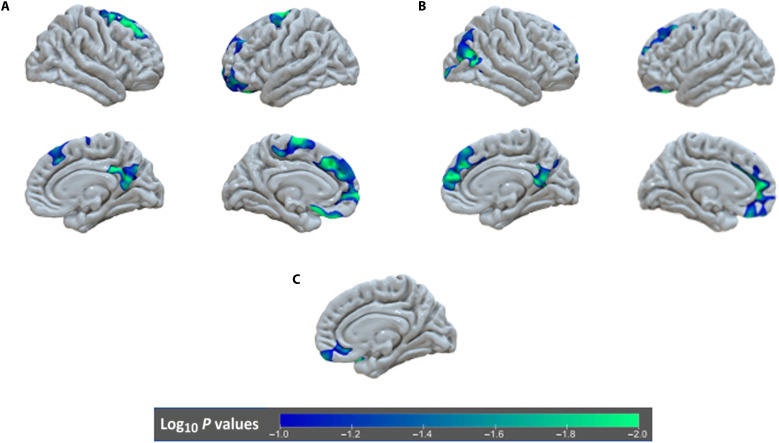
First, we examined change in cortical thickness and surface area from 9 to 16 years as a function of exposure to institutionalization, randomization to foster care, and timing of placement into foster care using a vertex-wise analysis corrected for multiple comparisons [false discovery rate (FDR) correction to P < 0.05]. There were no differences in cortical development among previously institutionalized relative to never-institutionalized children when we examined change in thickness or surface area from 9 to 16 years. However, randomization to foster care and earlier timing of placement were associated with changes in cortical thickness over time. Specifically, cortical thickness decreased more from 9 to 16 years in the lateral and medial PFC as well as the precuneus for children randomized to foster care compared to children who remained in care as usual (Fig. 1 and Table 1). Children randomized into foster care before 24 months exhibited greater decreases in thickness in the lateral and medial PFC and precuneus from 9 to 16 years (Fig. 1 and Table 1). Surface area in the medial orbital frontal cortex also decreased more markedly between 9 and 16 years for children randomized before 24 months of age.
Fig. 1. Differences in longitudinal change in gray matter structure by group.
(A) Regions with increased thinning between 9 and 16 years of age for children randomly assigned to foster care relative to those in care as usual. (B) Regions with accelerated thinning for children randomly assigned to foster care before 24 months of age, relative to those randomly assigned after 24 months of age. (C) A region in the ventral medial PFC that showed significant decreases in surface area from 8 to 16 years for children randomly assigned to foster care before 24 months of age, relative to those randomly assigned after 24 months of age.
Table 1. Differences in longitudinal change in gray matter structure by group.
Regions with significant change in cortical thickness and surface area from 9 to 16 years of age for the children who remained in care as usual relative to children randomly assigned to foster care intervention as well as differences for children within the FCG who were randomly assigned before and after 24 months of age. L, left; R, right.
| Cluster size |
z value of max vertex |
P value of cluster |
Approximate coordinates of max vertex in MNI space |
||||||
| (mm2) | z | P | x | y | z | ||||
| Prolonged institutionalization (n = 27) > foster care (n = 23; thickness) | |||||||||
| L post sup front cortex |
1419 | 3.33 | 0.04 | −9.1 | −12.1 | 69.0 | |||
| L ant sup front cortex |
3924 | 2.93 | >0.001 | −7.8 | 28.5 | 42.1 | |||
| R mid cingulate |
1494 | 2.98 | 0.03 | 8.7 | −42.0 | 31.7 | |||
| R sup front cortex |
2133 | 2.93 | 0.002 | 20.4 | 19.3 | 56.8 | |||
| After 24 months (n = 13) > before 24 months (n = 10; thickness) | |||||||||
| L med orbital front cortex |
2153 | 3.59 | >0.001 | −13.4 | 44.9 | 2.2 | |||
| L sup front cortex |
1329 | 5.77 | 0.03 | −22.2 | 4.2 | 47.9 | |||
| R sup front cortex |
1343 | 2.43 | 0.04 | 9.4 | 55.7 | 19.0 | |||
| R lat occipital cortex |
3252 | 3.80 | >0.001 | 35.2 | −81.1 | 2.0 | |||
| R precuneus | 1371 | 3.77 | 0.03 | 11.4 | −50.1 | 29.2 | |||
| After 24 months (n = 13) > before 24 months (n = 10; area) | |||||||||
| R lat orbital front cortex |
1041 | 2.24 | 0.03 | 17.0 | 17.0 | −22.8 | |||
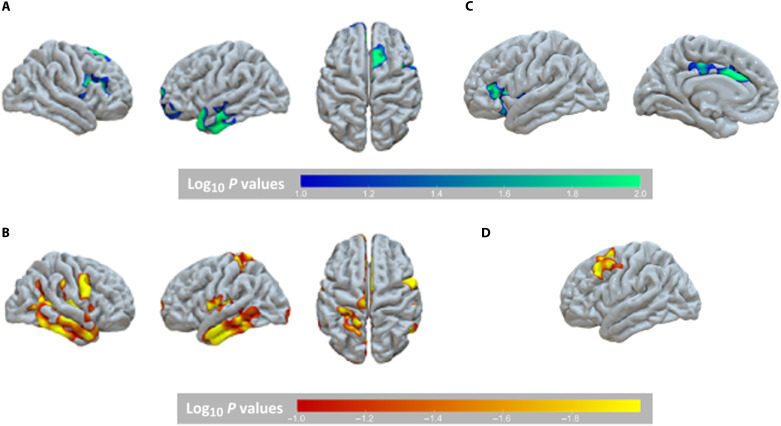
We then examined group differences in cortical thickness and surface area at 16 years using the same type of vertex-wise analysis, corrected for multiple comparisons. We observed differences in neural structure as a function of institutionalization, randomization to foster care, and timing of placement. At 16 years, children ever exposed to institutionalization had thicker cortex in the ACC, lateral PFC, and temporal pole and reduced cortical surface area relative to never-institutionalized adolescents in the bilateral ACC, bilateral medial and lateral inferior temporal cortex, bilateral insula, and posterior IFG (Fig. 2 and Table 2). Children randomized to foster care had a thinner cortex in the left ACC and IFG compared to adolescents who remained in prolonged institutional care at 16 years (Fig. 2 and Table 2). In contrast, children placed before versus after 24 months of age had thicker cortex in the caudal middle frontal gyrus (MFG) (Fig. 2 and Table 2). No effects of randomization to foster care or timing of placement were observed for surface area at 16 years.
Fig. 2. Differences in gray matter structure by group at 16 years.
At the age of 16 years, children who had been raised in families from birth had (A) a thinner cortex in the medial prefrontal, IFG, and temporal pole and (B) a larger surface area in the superior parietal, insula, and inferior temporal cortex compared to those ever exposed to institutionalization. (C) Children randomly assigned to foster care had a thinner cortex in the left IFG and ACC relative to those who remained in institutional care. (D) Children assigned to foster care before relative to after 24 months of age had thicker cortex in the left MFG.
Table 2. Differences in gray matter structure by group at 16 years.
Regions with significant differences in cortical thickness at 16 years of age among children exposed to institutionalization relative to community control subjects, for those in care as usual compared to those randomized to foster care intervention, and for those within the foster care. Surface area was not significantly different between participants in care as usual compared to those randomized to foster care intervention
| Area | Cluster size |
z value of max vertex |
P value of cluster |
Approximate coordinates of max vertex in MNI space |
||
| (mm2) | z | P | x | y | z | |
| Ever (n = 81) versus never-institutionalized (n = 33; thickness) | ||||||
| L inf temporal cortex |
2447 | 4.75 | >0.001 | −47.1 | −9.2 | −39.8 |
| L sup frontal cortex |
2961 | 3.31 | >0.001 | −7.4 | 37.3 | 28.5 |
| R sup frontal cortex |
1409 | 4.47 | 0.02 | 10.2 | 23.1 | 57.1 |
| R inf frontal cortex |
1430 | 3.50 | 0.02 | 39.6 | 11.9 | 21.7 |
| Ever (n = 81) versus never-institutionalized (n = 33; area) | ||||||
| L postcentral gyrus |
2702 | 5.09 | 0.008 | −46.6 | −12.6 | 18.1 |
| L sup frontal cortex |
6046 | 4.82 | >0.001 | −12.7 | 16.1 | 35.9 |
| L fusiform gyrus |
9667 | 4.60 | >0.001 | −36.3 | −25.5 | −21.6 |
| R precentral gyrus |
11,717 | 4.92 | >0.001 | 53.1 | 0.1 | 36.8 |
| R caudal ant cingulate |
2574 | 4.11 | 0.01 | 10.6 | 22.1 | 28.4 |
| Prolonged institutionalization (n = 40) > foster care (n = 41; thickness) | ||||||
| L caudal ant cingulate |
1404 | 3.47 | 0.02 | −12.7 | 26.2 | 25.0 |
| L inf frontal cortex |
1595 | 2.64 | 0.009 | −30.9 | 14.8 | 2.6 |
| After 24 months (n = 20) > before 24 months (n = 21; thickness) | ||||||
| L mid frontal gyrus |
1654 | −4.07 | 0.003 | −40.4 | 22.1 | 38.5 |
To identify whether differences in neural structure by randomization into foster care observed at 16 years were directly related to the impact of randomization on longitudinal change in neural structure, we performed a region of interest (ROI) analysis. We extracted thickness at 9 and 16 years in the two regions that were significantly different in the whole-brain analysis between foster care and care-as-usual participants at 16 years (i.e., ACC and IFG). In this analysis, every participant with at least one data point at 9 and/or 16 years was included [N = 90, care-as-usual group (CAUG) = 45 and foster care group (FCG) = 45]. We used latent change score analysis (42) to examine change in thickness from 9 to 16 years in the ACC and IFG (see fig. S2 for a depiction of the model). Analyses were run in Mplus version 7.0. and were performed using an estimator that is robust to non-normality when there are missing data (maximum likelihood with robust SEs). Missing data were handled using full information maximum likelihood estimation. We fit two separate models (one for ACC and one for IFG) with randomization group as a predictor of change in thickness over time. For both the ACC and IFG, there was a significant association between intervention group and change in thickness from 9 to 16 years {ACC: B [95% confidence interval (CI)] = −0.10 [−0.17, −0.02], P = 0.01; IFG: B [95% CI] = −0.08 [−0.13, −0.03], P = 0.003}, such that those assigned to foster care showed greater declines in thickness over time.
Neural structure, psychopathology, and cognition
Last, we sought to characterize the relevance of observed differences in neural structure by intervention group for psychopathology given the impact that early institutionalization has on mental health (14, 43). We examined associations between psychopathology as measured by bifactor scores [“p” factor and internalizing and externalizing factors (43); see the Supplementary Materials for methods] and cortical thickness in the two regions (ACC and IFG), which showed a significant impact of randomization to foster care at 16 years. All analyses controlled for age at scan and gender. Thickness in the ACC was positively associated with externalizing factor scores (β = 0.22, t = 2.02, P = 0.047, f2 = 0.10) but not the p factor (β = 0.03, t = 0.29, P = 0.78, f2 = 0.001) or internalizing factor (β = 0.07, t = 0.69, P = 0.54, f2 = 0.005; fig. S3). Thickness in the IFG was not related to any psychopathology factor (see the Supplementary Materials). The association was in the expected direction where thicker ACC at 16 years was associated with increased externalizing psychopathology. These associations were not robust to additional controls for group membership.
We also examined associations between cognitive ability as measured by full-scale intelligence quotient (FSIQ) at 18 years (13) and thickness in the same two regions. ACC thickness was not associated with FSIQ at 18 years (β = 0.13, t = 1.15, P = 0.25, f2 = 0.019), but IFG thickness was (β = 0.24, t = −1.97, P = 0.05, f2 = 0.055). The association was in the expected direction where thinner cortex in the IFG at 16 years was associated with higher IQ at age 18. These associations were not robust to additional controls for group membership.
White matter integrity
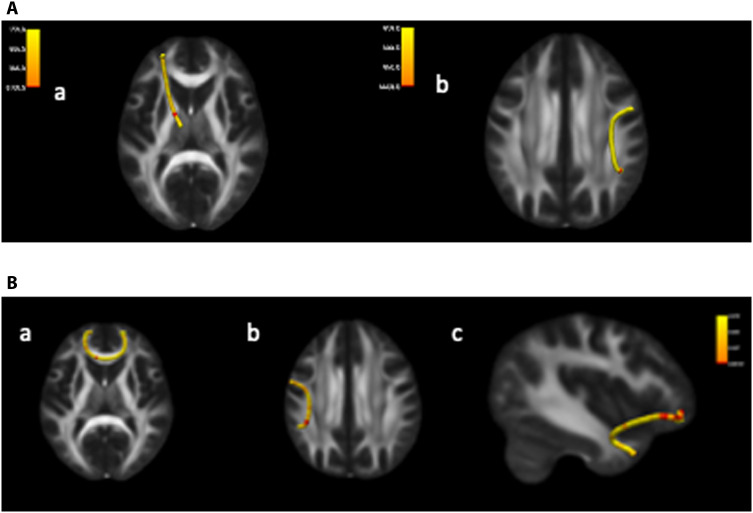
We examined white matter integrity as a function of institutionalization, randomization to foster care, and timing of placement after correcting for multiple comparisons across each of the 18 tracts, locations (n = 33 to 72 locations per tract), and diffusivity measures [fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD)]. Two locations on the left anterior thalamic radiation had significantly increased MD and RD for children exposed to institutionalization relative to those raised in families since birth, and one location on the right superior longitudinal fasciculus (parietal division) had significantly increased MD (Fig. 3 and Table 3). Ten locations on three tracts differed as a function of randomization to foster care. Seven of the 10 locations were located in the left uncinate fasciculus and 6 of these 7 locations had significantly increased AD for children who experienced prolonged institutionalization relative to those randomized to foster care (Fig. 3 and Table 3). One location in a different area had significantly increased MD for the children who remained in care as usual compared to the FCG. Moreover, two locations on the superior longitudinal fasciculus (parietal division) had significantly decreased FA, and one region on the forceps minor had significantly decreased MD for children in care as usual relative to the FCG (Fig. 3 and Table 3). No regions exhibited significantly different FA, MD, RD, or AD values between children who were randomized to foster care before and after 24 months of age after correcting for multiple comparisons.
Fig. 3. Differences in white matter structure by group at 16 years.
(A) Clusters of significant difference in white matter integrity between children ever exposed to institutionalization and those never exposed to institutionalization in the (a) left anterior thalamic radiation and (b) the right longitudinal fasciculus. (B) Clusters of significant difference in white matter integrity between children randomly assigned to foster care relative to those who remained in institutions in the (a) forceps minor, the (b) left longitudinal fasciculus, and the (c) right uncinate fasciculus.
Table 3. Differences in white matter structure by group at 16 years.
Significant differences in white matter integrity between children who had ever been exposed to institutionalization and those raised in families as well as between those randomized to foster care intervention and those assigned to remain in care as usual.
| Tract | Metric | z value | FDR-corrected |
Approximate coordinates of max vertex in MNI space |
||
| z | P | x | y | z | ||
| Ever-institutionalized (n = 81) > never-institutionalized (n = 33) | ||||||
| L ant thalamic radiation |
RD | 3.663 | 0.022 | −13.5 | −2.7 | 7.9 |
| RD | 3.452 | 0.028 | −12.7 | −4.4 | 7.5 | |
| MD | 3.541 | 0.042 | −12.7 | −4.4 | 7.5 | |
| MD | 3.16 | 0.054 | −12.7 | −4.4 | 7.5 | |
| R sup longitudinal fasciculus |
MD | 3.67 | 0.034 | 43.2 | −46.3 | 32.3 |
| Prolonged institutionalization (n = 41) > foster care (n = 41) | ||||||
| Forceps minor | MD | −3.67 | 0.023 | −9.5 | 28.8 | 4.5 |
| L sup longitudinal fasciculus |
FA | −3.29 | 0.031 | −39.0 | −40.2 | 30.3 |
| FA | −3.45 | 0.031 | −39.9 | −41.4 | 30.4 | |
| L uncinate fasciculus | AD | 4.44 | 0.002 | −19.4 | 27.3 | −6.2 |
| AD | 3.27 | 0.024 | −19.3 | 29.6 | −7.0 | |
| AD | 3.35 | 0.024 | −19.6 | 25.9 | −6.1 | |
| AD | 3.20 | 0.024 | −19.9 | 24.4 | −6.1 | |
| AD | −3.69 | 0.024 | −16.1 | 24.4 | −5.9 | |
| AD | −3.41 | 0.024 | −15.7 | 30.9 | −5.2 | |
| MD | 3.44 | 0.053 | −32.4 | 0 | −12.8 | |
DISCUSSION
Here, we provide the first evidence from a randomized controlled trial that profound psychosocial neglect early in life affects structural neurodevelopment from middle childhood to adolescence using a longitudinal randomized controlled trial design. We show that longitudinal changes in cortical thickness from middle childhood to adolescence vary as a function of foster care intervention and timing of removal from institutional care in infancy. Specifically, children randomly assigned to foster care had greater thinning of the PFC from 9 to 16 years than children who remained in institutional care. In addition, among those randomized to foster care, children removed from institutional care before 24 months showed increased thinning in these same regions relative to those removed later. Rapid cortical thinning in association cortices occurs during adolescence (44, 45), making the reduced thinning observed among children exposed to prolonged institutional rearing a deviation from the expected developmental pattern.
These altered patterns of neurodevelopment from 9 to 16 years resulted in a thinner cortex in prefrontal areas (e.g., dorsal ACC and right IFG) by 16 years for children who were randomized to family care relative to those who remained in care as usual. Previous observational studies have associated exposure to institutionalization with atypical gray matter structure in the ACC (16, 29) and IFG (28), and our findings indicate that the impact of early exposure to deprivation on developmental change of gray matter may drive this effect. Cortical thickness in these regions was associated with relevant outcomes. Thickness in the ACC at 16 years was positively associated with externalizing psychopathology from a bifactor model. This finding is consistent with a significant body of work linking the function and structure of the ACC with externalizing symptoms in populations of participants not exposed to institutionalization (46, 47) and with data demonstrating that ACC function and connectivity mediate the link between other forms of adversity and externalizing psychopathology (48, 49). Thickness in the IFG, but not ACC, was negatively associated with cognitive ability at 18 years. This is consistent with a robust body of work linking lateral PFC function and structure with IQ [e.g., (50)].
Compared to children exposed to institutional care, children raised in families from birth had a thinner cortex in the medial PFC, IFG, and temporal pole. These findings were in similar areas and in the same direction as the differences observed as a function of randomization to foster care, suggesting a long-term impact of institutional care on brain structure through adolescence.
These results are substantively different from those that we previously reported at 9 years, at which point we observed more marked thinning, particularly in the posterior regions of the brain, for previously institutionalized children (20), and no effects of the foster care intervention. However, our current results are consistent with recent findings from the English-Romanian Adoptees study acquired in postpubertal participants (28), showing that previously institutionalized young adults had increased thickness, relative to never-institutionalized peers, in the right temporal pole and IFG, a finding that we replicate when comparing similar groups in this analysis. Together, these patterns suggest meaningful differences in the impact of deprivation on neurodevelopment in childhood relative to adolescence.
While proliferation of synaptic connections in the PFC occurs in early prenatal life just as in the primary sensory cortex, pruning of these connections occurs most robustly in adolescence (45). One possible explanation for these findings is that the reduction in expected social and cognitive inputs from caregivers (8, 10) in early life among institutionally reared children results in decreased synaptic proliferation or early pruning as sensitive periods in the PFC open (51). This is consistent with the pattern of reduced gray matter volume and widespread cortical thinning for children exposed to institutionalization at 9 years in this sample. For children who do not experience these expected social and cognitive inputs before 24 months, this may lead to early closing of windows of plasticity before the developmental period—in this case, adolescence—when neural circuits in the association cortex are most likely to be influenced by experiential inputs (52). Thus, this early deprivation appears to stunt ongoing circuit refinement in adolescence, flattening the typically steep trajectory of cortical thinning during this period. Given the long trajectory of structural development in the association cortex, these findings are consistent with an early sensitive period for PFC development in the first 2 years of life that lays the foundation for subsequent developmental trajectories and windows of sensitivity to environmental influences that reopen in adolescence. These findings are consistent with many other examples from this sample where children randomized to foster care earlier versus later exhibit higher IQ (13), reduced blunting of the stress response (23), and more typical neural function (53) in middle childhood and adolescence.
In addition, when we examined the impact of timing of randomization out of the institution, we saw that children randomized into family care before 24 months of age had relatively thicker cortex in the MFG in adolescence than those randomized later. In contrast, children randomized to foster care had a thinner cortex in the ACC and IFG at 16 years. The MFG has a longer developmental trajectory than most other areas in the brain, including other regions of the PFC (44, 45). It may be that the period of marked pruning where adolescent environmental experiences refine circuit development involving the MFG remains ongoing beyond the age of 16 years and that we will observe a thinner cortex in this region in early adulthood for children randomized to foster care, particularly when placement occurs in the first 2 years of life.
We also observed decreased white matter integrity for children randomized to prolonged institutional care compared to those randomized to foster care, and for previously institutionalized children compared to never-institutionalized children, in the superior longitudinal fasciculus, a white matter tract connecting the lateral PFC to the parietal cortex that supports working memory and executive functions (54). Randomization to foster care was also associated with greater white matter integrity in the uncinate fasciculus, a tract that connects the inferior PFC with the amygdala and anterior temporal cortex, consistent with prior observational studies documenting reduced structural integrity of this tract among previously institutionalized children (36, 37).
The results of this investigation are remarkably consistent across measurements of cortical thickness, surface area, and white matter integrity, as well as when examining deprivation as a function of institutionalization relative to family rearing, as a function of random assignment to foster care, or timing of placement into foster care. In all cases, we observe a profound effect of early and prolonged exposure to deprivation on the development of the PFC. These findings document the effects of institutionalization on trajectories of frontoparietal circuit refinement during adolescence that may (55) account for the influence of early deprivation on executive function (56). In addition, we see changes in the development and structure of cortical thickness in the ventral ACC as well as in the integrity of the uncinate fasciculus as a function of exposure to institutional care. Given the role of this tract and medial prefrontal regions in emotional processing and social cognition (57), these differences may contribute to long-term risk for psychopathology in previously institutionalized children (14). Future studies are needed to clarify associations between altered trajectories of structural brain development and adverse cognitive and socioemotional outcomes among institutionally reared children.
We found that previously institutionalized adolescents had smaller overall gray and white matter volumes compared to their never-institutionalized peers in adolescence, replicating other studies (17, 28, 58), including findings from this sample at 9 years (12). Although we did not observe a significant effect of randomization into foster care on total gray, white, or intracranial volume, the effect sizes that we observed for these comparisons were small, and it is possible that we would have observed this effect with a larger sample size. Relatedly, we observed that ever-institutionalized adolescents had a smaller surface area compared to those never exposed to institutionalization across much of the cortex including the superior parietal, insula, and temporal cortex. This pattern of findings could reflect either selection effects associated with exposure to early institutionalization (e.g., children who thrive in the first few weeks of life are less likely to be placed in an institution initially) or the impact of institutionalization on brain development in infancy. Work in other samples examining the association between polygenic risk scores and indices of prenatal risk on total brain volume are consistent with the possibility that early experiences during infancy contribute to the overall reductions in total cortical volume in institutionalized children (28). In addition, the BEIP sample was carefully screened for prenatal risk (e.g., alcohol) and genetic and neurological abnormalities before enrollment (40), reducing the likelihood that selection effects explain this finding and further highlighting the importance of early experience in shaping total brain volume.
Institutionalization, randomization to foster care, and timing of placement were not related to subcortical volume for any structure, including the amygdala, replicating earlier results in this sample (12) and other studies (16, 28). This finding contrasts with two prior studies reporting institutionalization-related differences in the amygdala (22) and hippocampus (18), both examining children who were adopted internationally. It is possible that methodological differences in measurement (e.g., our use of FreeSurfer) or sample composition (e.g., the bias that might occur among children adopted out of institutions) may contribute to our observation of this null result. Given that more recent studies using larger samples and improved methods for estimating subcortical volume have not found an impact of institutionalization on subcortical structure (12, 16, 28), the preponderance of evidence suggests that this association, if it exists, is small and varies markedly across development (59).
Limitations
We note that magnetic resonance imaging (MRI) data at age 9 and 16 years were acquired using different scanners, although scanners were matched for manufacture and scanner strength, and pulse sequences used were similar between time points. We elected to perform longitudinal processing across these time points, taking steps to confirm the appropriateness of this analytic decision. Combining data across scanners has become commonplace (60). In line with previous research, we used tests of heteroskedasticity to examine whether combining samples was appropriate but, because we do not have the same age of participants acquired at both scanners, were unable to perform ComBat or a similar batch correction method. However, we were able to replicate known developmental trends in this dataset (31) (e.g., general reductions in cortical thickness across time; fig. S4 and table S2), which reassured us that our estimates of cortical structure were reliable and comparable across time points.
Recent policies, such as the incarceration and/or separation of children and families attempting to enter the United States, have reinvigorated the debate about the long-term impacts of institutionalization of children, even for short periods (61). Here, we provide evidence for causal effects of early-life institutionalization on neurodevelopmental trajectories lasting into the second decade of life. The unique longitudinal and experimental design of the BEIP provides strong evidence for effects of early-life deprivation on neurodevelopment that persist into late adolescence, with the largest effects in the association cortex, most notably the PFC and white matter tracts connecting the PFC to other regions. In addition, these results indicate that ongoing exposure to institutionalization into adolescence continues to affect further refinement of neural structure, which typically occurs in adolescence. Our findings suggest that these lasting neurodevelopmental effects of early deprivation may explain the enduring impacts of institutional rearing on multiple domains of functioning, including cognitive ability and psychopathology. These findings highlight the critical importance of identifying family-based alternatives to institutionalization for children and the importance of working through policy and practice to reduce the likelihood that children are separated from their families.
MATERIALS AND METHODS
Participants
The BEIP is a longitudinal study of children raised from early infancy in institutions in Bucharest, Romania. It is the only randomized controlled trial of foster care as an alternative to institutional rearing. A sample of 136 children (aged 6 to 30 months) was recruited from six institutions for young children in Bucharest. An age-matched sample of 72 community-reared children born at the same hospitals as the children in institutions was recruited from pediatric clinics in Bucharest and comprised the never-institutionalized group (NIG). Between 6 and 33 months of age, half of the children in the institutionalized group were randomized to a high-quality foster care intervention, resulting in two groups: the FCG and the group exposed to prolonged institutional care (CAUG). Together, this comprises the ever-institutionalized group (EIG). The BEIP was initiated at the request of the Secretary of State for Child Protection in Romania. All study procedures were approved by the local commissions on child protection in Bucharest, the Romanian ministry of health, and the institutional review boards of the home institutions of the three principal investigators (C.A.N., N.A.F., and C.H.Z.). At each round of data collection, consent and assent were obtained from the participant and their legal guardian. A more complete description of procedures used to ensure ethical integrity has been published previously and commented on by the scientific community (62, 63).
Previously, we reported on neural structure in this sample from data acquired when participants were about 9 years of age (12, 20, 24). At our earlier time point, we acquired data from N = 81 participants ranging from 8.10 to 11.66 years of age (M = 9.76, SD = 0.79; 52% female). Here, we report on longitudinal change between 9 and 16 years of age for N = 64 participants with data at both time points (N = 23 FCG; N = 27 CAUG; N = 14 NIG; 51.5% female; on average, 9.84 years at time 1 and 16.68 years at time 2). We additionally report on longitudinal change in individuals in the FCG who were randomized before 24 months (N = 10) and those randomized after 24 months (N = 13). When participants were 16 years of age, 11 years after the formal randomized controlled trial had ended, we acquired structural MRI data from 115 participants (N = 41 FCG; N = 41 CAUG; N = 33 NIG; 49% female; 15.55 to 17.97 years of age). Of the children in the FCG group, N = 21 were randomized before 24 months and N = 20 were randomized after 24 months. For the ROI analysis where we use linear mixed effects (LME) models to examine longitudinal change in regions observed to be different between groups at 16 years, we have N = 90 participants in the FCG (N = 45) and CAUG (N = 45) with at least one time point of data at 8 or 16 years. Compared to the NIG, children in the EIG were younger [t(115) = 2.31, P = 0.02], and there was a marginally longer time between their time 1 and time 2 imaging sessions [t(115) = 1.95, P = 0.06], but they did not differ in gender distribution (P = 0.39). No significant differences were found between the CAUG and FCG in age (P = 0.44), gender (P = 0.66), or duration between time 1 and time 2 (P = 0.24) (see table S1). In all analyses examining brain structure between EIG and NIG, we include age in months at imaging session and gender as covariates. One participant who had been randomized to the CAUG group did not have usable structural MRI data, and their cortical thickness and surface area data were removed from all analyses involving those outcomes (see consort diagram in fig. S1).
MRI acquisition
At time 1 and time 2, structural magnetic resonance images were acquired on Siemens 1.5 T systems. Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu). Diffusion-weighted data were analyzed using TRActs Constrained by UnderLying Anatomy [TRACULA; (64)], an automatic reconstruction tool for identifying major white matter tracts from diffusion-weighted images and measuring the diffusion within these tracts at a set number of locations. The acquisition sequences for gray matter data differed in minor ways across acquisitions. Following recommendations (60, 65), we documented that there was no evidence of heteroskedasticity before completing our longitudinal analysis. Please see the Supplementary Materials for details about data acquisition, analysis, and tests of heteroskedasticity.
Data acquisition parameters
At time 1, structural magnetic resonance images were acquired at Regina Maria Health Center (Bucharest, Romania) on a Siemens Magnetom Avanto 1.5 T Syngo system. Images were obtained using a transverse magnetization–prepared rapid gradient echo three-dimensional sequence [echo time (TE) = 2.98 ms, TI = 1000 ms, flip angle = 8, 176 slices with isometric voxels of 1 mm by 1 mm by 1 mm] with a 16-channel head coil. The repetition time (TR) for this sequence varied between 1650 and 1910. Data from five subjects were acquired in the sagittal plane; data from one subject were acquired in the coronal plane. Acquisition parameters did not differ by group membership nor were they associated with scan quality.
At time 2, structural magnetic resonance images were acquired at Santador Hospital (Bucharest, Romania) on a Siemens Magnetom 1.5 T Syngo system. Images were obtained using an MPRAGE sequence [TE = 3.5 ms, TI = 1100 ms, flip angle = 7, 192 slices with isometric voxels of 1 mm by 1 mm by 1 mm, TR = 2530, acceleration factor 2 (using GRAPPA)] with an eight-channel head coil. In addition, at this time point, multiple diffusion-weighted images were acquired in 30 directions. Scan parameters were as follows: b = 1000 s/mm2, TE/TR = 88 ms/8500 s, with a partial Fourier coverage of 7/8 and an acceleration factor of 2, 216-mm field of view, and slices of 2 mm without gap, to yield a voxel size of 2 mm by 2 mm by 2 mm.
The pulse sequences and scanner differed between time 1 and time 2. Combining data from multiple sites has become commonplace, particularly when it comes to structural neuroimaging studies (60). In studies that compare structural data from the same participant acquired on multiple scanners, scanner manufacturer, field strength, and utilization of a similar pulse sequence are the strongest predictors of differences in estimations of neural structure (65). Because we hold these factors constant from baseline to follow-up and use similar pulse sequences to acquire our data, it is likely that quantification of the neural structure from the study sites we combine here is comparable. While we were unable to acquire structural imaging data from each site on the same participant at the same age to directly test the comparability of our acquisitions, we tested for heteroskedasticity in models including scanner site before completing our longitudinal analysis. Specifically, we found—in univariate models that included age at the time of scan, gender, group membership, and site of data acquisition—that there was no evidence for heteroskedasticity using White’s test for total cortical volume (χ2 = 7.51, P = 0.82), right amygdala volume (χ2 = 8.70, P = 0.73), left amygdala volume (χ2 = 14.75, P = 0.26), mean right hemisphere cortical thickness (χ2 = 17.45, P = 0.13), or mean left hemisphere cortical thickness (χ2 = 19.38, P = 0.08). In addition, we found no evidence for heteroskedasticity of cortical thickness in two areas of the cortex that emerged as significantly different between groups, the ACC [right (χ2 = 14.93, P = 0.25) and left (χ2 = 12.19, P = 0.43)] and the IFG [right (χ2 = 17.16, P = 0.14) and left (χ2 = 14.03, P = 0.29)]. In addition, on visual inspection, none of the residual plots for these variables exhibited evidence of heteroskedasticity. We begin the longitudinal data analysis results with a description of our longitudinal results that do not consider moderation by group membership entirely to demonstrate that we replicate commonly described changes in neural structure related to age. We provide this information as a reassurance that the current longitudinal analysis is likely to be valid.
Gray matter analysis
FreeSurfer (version 5.0 for 8-year and 5.3 for 16-year and longitudinal analysis) processing includes motion correction of a volumetric T1-weighted image, removal of nonbrain tissue using a hybrid watershed/surface deformation procedure (66), automated Talairach transformation, previously validated in pediatric populations, and segmentation of the subcortical white matter and deep gray matter volumetric structures, separately validated for use with pediatric populations (67). This analysis yields estimates of cortical thickness and surface area that can be analyzed vertex by vertex across the entire cortex. FreeSurfer morphometric procedures have demonstrated good test-retest reliability across scanner manufacturers and field strengths (68). For the longitudinal analysis, images were processed with the longitudinal stream (69). An unbiased within-subject template space and image were created using robust, inverse consistent registration (70). Processing steps such as skull stripping, Talairach transforms, atlas registration, spherical surface maps, and parcellations are then initialized with common information from the within-subject template. These methods, in summary, significantly increase reliability and statistical power (69).
White matter analysis
TRACULA uses probabilistic tractography constrained by the individual subject’s anatomy derived from FreeSurfer cortical parcellation and subcortical segmentation; tracts and diffusion are identified in native space. For each participant, 18 tracts were identified (forceps major and forceps minor, right and left anterior thalamic radiation, cingulum angular bundle and cingulate gyrus, cortical spinal tract, inferior longitudinal fasciculus, superior longitudinal fasciculus parietal segment and temporal segment, and uncinate fasciculus). Each tract is composed of between 33 and 79 locations. For each location, FA, MD, RD, and AD were calculated. We examined group differences in FA, MD, RD, and AD using two-sample t tests. We report significant differences between groups after FDR correction for multiple comparisons (71, 72).
Psychopathology
General psychopathology (p factor) as well as internalizing- and externalizing-specific factors were measured as saved factor scores from a previously estimated bifactor model in which multiple domains of psychopathology were assessed from caregiver and teacher reports on the MacArthur Health and Behavior Questionnaire (73). Each of the eight domains of psychopathology was set to load simultaneously onto a general factor (p factor) that captured common variance across all psychopathology domains, as well as their respective internalizing- and externalizing-specific factors. More details on the estimation of this model can be found in a prior study by our group (43). The factor scores (p, internalizing, and externalizing) at age 16 from this model were then output and used as manifest variables in the current analysis.
Cognitive ability
At the age of 18 years, cognitive ability was assessed via the Wechsler Intelligence Scale for Children, Fourth Edition [WISC-IV (74)]. The WISC-IV includes 10 subtests, with 5 additional supplemental subtests, and assesses cognitive ability in four domains: verbal comprehension, perceptual reasoning, working memory, and processing speed. The WISC-IV was translated from English to Romanian, and specific items were altered, given cultural context (e.g., names of historical figures). We computed an FSIQ composite score using the 10 subtest scores and age 16 norms. We followed test rules and substituted tests that were “spoiled” with substitutions from supplemental tests. Because of the need to translate the WISC-IV into Romanian for this study, a policy was implemented whereby substitutions for subtests by a supplemental test were used if participants achieved a standard score of less than 5 on the original subtest. When both an original unspoiled subtest and a supplemental subtest in the same domain were administered, the subtest with the highest score was used to estimate the FSIQ score [for more details about this “benefit of the doubt” approach to scoring, see (75)]. Five participants were identified by BEIP staff as too cognitively impaired to participate in the WISC-IV; these participants’ scores were imputed to 40.
Given the complexity of administering a normed assessment in a context and language for which official norms were not available, we took several steps to address potential floor effects in this sample. We administered the WISC-IV rather than the Wechsler Adult Intelligence Scale (76) and used the oldest possible age norm to standardize scores (i.e., 16 years). Second, trained Romanian psychologists, supervised by U.S. clinical psychologists, provided the supplemental tests in relevant domains if participants obtained a scaled score of 5 or below on one of the 10 primary subtests. Scoring was then determined by taking the higher of the two subscales if a supplemental test was administered.
Group comparisons
First, we examine the volume of cortical gray matter, white matter, corpus callosum, and six subcortical structures (amygdala, hippocampus, caudate, putamen, globus pallidus, and thalamus). For diffusion-weighted imaging findings, we first compare FA, MD, RD, and AD at each location on 18 tracts. We performed all analyses of these data in SPSS 28. We first compare ever-institutionalized children (EIG) to never-institutionalized children (NIG). This comparison documents the likely impact of exposure to institutionalization on neural structure and is similar to analyses in other postinstitutionalized samples. For comparisons at 16 years, we use a general linear model (GLM) analysis controlling for covariates, and for longitudinal analysis, we use a mixed between-within repeated-measures GLM, again controlling for covariates; we correct for multiple comparisons using FDR. Next, we examine the impact of random assignment to foster care intervention within the EIG using two-sample, two-tailed t tests, and we correct for multiple comparisons using FDR. This comparison is unique to this sample and allows us to draw causal conclusions about the impact of prolonged institutionalization on neural structure. Last, within the foster care intervention group, we perform an exploratory analysis where we examine whether the age at which children were randomly assigned to foster care intervention affected cortical development, again using two-sample, two-tailed t tests; we correct for multiple comparisons using FDR. GLM analyses control for age and sex; longitudinal analyses control for time (in months) elapsed between the two scans.
Next, we use a whole-brain vertex-wise analysis to examine cortical surface area and thickness; again, using GLM in longitudinal analyses, we control for the time between scans, and when comparing EIG to NIG, we additionally control for age and gender. All analyses are two-tailed, and we report directionality of effects as well as presence of differences. These analyses are controlled for multiple comparisons using a cluster correction method implemented in FreeSurfer (vertex level −log10P = 1.3, cluster correction to P < 0.05) (77). For gray matter analyses, we first report longitudinal change from 9 to 16 years for the subset of participants for which we had two time points of data, followed by overall group differences at 16 years of age. For white matter, we focus only on group differences at age 16 (and not longitudinal change), because the diffusion acquisition and analytic strategy differed substantially between these two time points.
We examined associations between cortical thickness in regions affected by institutionalization at 16 years of age and both psychopathology at the age of 16 years and IQ at the age of 18 years using an ROI analysis. Specifically, we extracted cortical thickness in the regions that were significantly different between the CAUG and FCG (the left ACC and IFG) in the whole-brain analysis. We examined associations between cortical structure and psychopathology at 16 years of age using these ROIs controlling for age at MRI scan and gender. We additionally conducted models in which group assignment was covaried (dummy-coded as 0 = CAUG and 1 = FCG).
Acknowledgments
We thank the caregivers and children who participated in this project and the Bucharest Early Intervention Project staff for the tireless work on our behalf.
Funding: This research was supported by a grant from the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development (to C.A.N.) and the NIH R01-MH091363 (to C.A.N.), K01-MH092526 (to K.A.M.), and K01-MH092555 and RO1-MH115004 (to M.A.S.). These funders provided support for all data collection and analysis.
Author contributions: M.A.S., C.A.N., and K.A.M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization: M.A.S., K.A.M., C.A.N., C.H.Z., and N.A.F. Methodology: M.A.S., K.A.M., S.G., K.G., K.P., K.L.H., and M.W. Visualization: M.A.S., K.G., S.G., K.P., and M.W. Funding acquisition: C.A.N., N.A.F., and C.H.Z. Project administration: M.A.S., K.A.M., C.A.N., C.H.Z., N.A.F., and K.L.H. Supervision: M.A.S., K.A.M., C.A.N., C.H.Z., N.A.F., and K.L.H. Writing—original draft: M.A.S. and C.E.M. Writing—review and editing: K.A.M., C.E.M., C.A.N., C.H.Z., N.A.F., K.L.H., and M.W.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. In addition, the extracted neural metrics generated and/or analyzed during the current study are available in the OSF repository associated with the corresponding author’s name (https://osf.io/476m8/?view_only=a9178a169ad346a094043d037827f4e8).
Supplementary Materials
This PDF file includes:
Region of interest analysis
Impact of institutionalization on subcortical volume without controls for ICV
Nonsignificant results (group effects)
Associations between neural structure and psychopathology
Longitudinal change
Figs. S1 to S4
Tables S1 to S3
References
REFERENCES AND NOTES
- 1.Bos K. J., Fox N., Zeanah C. H., Nelson C. A. III, Effects of early psychosocial deprivation on the development of memory and executive function. Front. Behav. Neurosci. 3, 16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan M. A., McLaughlin K. A., Winter W., Fox N., Zeanah C., Nelson C. A., Early deprivation disruption of associative learning is a developmental pathway to depression and social problems. Nat. Commun. 9, 2216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tibu F., Sheridan M. A., McLaughlin K. A., Nelson C. A., Fox N. A., Zeanah C. H., Disruptions of working memory and inhibition mediate the association between exposure to institutionalization and symptoms of attention deficit hyperactivity disorder. Psychol. Med. 46, 529–541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphreys K. L., McGoron L., Sheridan M. A., McLaughlin K. A., Fox N. A., Nelson C. A., Zeanah C. H., High-quality foster care mitigates callous-unemotional traits following early deprivation in boys: A randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 54, 977–983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeanah C. H., Egger H. L., Smyke A. T., Nelson C. A., Fox N. A., Marshall P. J., Guthrie D., Institutional rearing and psychiatric disorders in Romanian preschool children. Am. J. Psychiatry 166, 777–785 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Almas A. N., Degnan K. A., Radulescu A., Nelson C. A. III, Zeanah C. H., Fox N. A., Effects of early intervention and the moderating effects of brain activity on institutionalized children’s social skills at age 8. Proc. Natl. Acad. Sci. U.S.A. 109, 17228–17231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin A. R., Fox N. A., Zeanah C. H. Jr., Nelson C. A., Social communication difficulties and autism in previously institutionalized children. J. Am. Acad. Child Adolesc. Psychiatry 54, 108–115.e1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin K. A., Sheridan M. A., Nelson C. A., Neglect as a violation of species-expectant experience: Neurodevelopmental consequences. Biol. Psychiatry 82, 462–471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tottenham N., The importance of early experiences for neuro-affective development. Curr. Top. Behav. Neurosci. 16, 109–129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheridan M. A., McLaughlin K. A., Neurobiological models of the impact of adversity on education. Curr. Opin. Behav. Sci. 10, 108–113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gee D. G., Cohodes E. M., Influences of caregiving on development: A sensitive period for biological embedding of predictability and safety cues. Curr. Dir. Psychol. Sci. 30, 376–383 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheridan M. A., Fox N. A., Zeanah C. H., McLaughlin K. A., Nelson C. A. III, Variation in neural development as a result of exposure to institutionalization early in childhood. Proc. Natl. Acad. Sci. U.S.A. 109, 12927–12932 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson C. A. III, Zeanah C. H., Fox N. A., Marshall P. J., Smyke A. T., Guthrie D., Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science 318, 1937–1940 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Humphreys K. L., Gleason M. M., Drury S. S., Miron D., Nelson C. A. III, Fox N. A., Zeanah C. H., Effects of institutional rearing and foster care on psychopathology at age 12 years in Romania: Follow-up of an open, randomised controlled trial. Lancet Psychiatry 2, 625–634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff B., Gee D. G., Telzer E. H., Humphreys K. L., Gabard-Durnam L., Flannery J., Tottenham N., Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience 249, 129–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodel A. S., Hunt R. H., Cowell R. A., Van Den Heuvel S. E., Gunnar M. R., Thomas K. M., Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage 105, 112–119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta M. A., Gore-Langton E., Golembo N., Colvert E., Williams S. C. R., Sonuga-Barke E., Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J. Cogn. Neurosci. 22, 2316–2325 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Mehta M. A., Golembo N. I., Nosarti C., Colvert E., Mota A., Williams S. C. R., Rutter M., Sonuga-Barke E. J. S., Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J. Child Psychol. Psychiatry 50, 943–951 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Pollak S. D., Nelson C. A., Schlaak M. F., Roeber B. J., Wewerka S. S., Wiik K. L., Frenn K. A., Loman M. M., Gunnar M. R., Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Dev. 81, 224–236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin K. A., Sheridan M. A., Winter W., Fox N. A., Zeanah C. H., Nelson C. A., Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol. Psychiatry 76, 629–638 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvers J. A., Lumian D. S., Gabard-Durnam L., Gee D. G., Goff B., Fareri D. S., Caldera C., Flannery J., Telzer E. H., Humphreys K. L., Tottenham N., Previous institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. J. Neurosci. 36, 6420–6430 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tottenham N., Hare T. A., Quinn B. T., McCarry T. W., Nurse M., Gilhooly T., Millner A., Galvan A., Davidson M. C., Eigsti I.-M., Thomas K. M., Freed P. J., Booma E. S., Gunnar M. R., Altemus M., Aronson J., Casey B. J., Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 13, 46–61 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin K. A., Sheridan M. A., Tibu F., Fox N. A., Zeanah C. H., Nelson C. A. III, Causal effects of the early caregiving environment on development of stress response systems in children. Proc. Natl. Acad. Sci. U.S.A. 112, 5637–5642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bick J., Zhu T., Stamoulis C., Fox N. A., Zeanah C., Nelson C. A., Effect of early institutionalization and foster care on long-term white matter development: A randomized clinical trial. JAMA Pediatr. 169, 211–219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bongaarts J., Guilmoto C., How many more missing women? Lancet 386, 427 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Grant M. J., Yeatman S., The relationship between orphanhood and child fostering in sub-Saharan Africa, 1990s-2000s. Popul. Stud. (Camb). 66, 279–295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutter M., Kumsta R., Schlotz W., Sonuga-Barke E., Longitudinal studies using a “natural experiment” design: The case of adoptees from Romanian institutions. J. Am. Acad. Child Adolesc. Psychiatry 51, 762–770 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Mackes N. K., Golm D., Sarkar S., Kumsta R., Rutter M., Fairchild G., Mehta M. A., Sonuga-Barke E. J. S.; on behalf of the ERA Young Adult Follow-up team , Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc. Natl. Acad. Sci. U.S.A. 117, 641–649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzberg M. P., Hodel A. S., Cowell R. A., Hunt R. H., Gunnar M. R., Thomas K. M., Risk taking, decision-making, and brain volume in youth adopted internationally from institutional care. Neuropsychologia 119, 262–270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills K. L., Goddings A.-L., Herting M. M., Meuwese R., Blakemore S.-J., Crone E. A., Dahl R. E., Güroğlu B., Raznahan A., Sowell E. R., Tamnes C. K., Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage 141, 273–281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeWinn K. Z., Sheridan M. A., Keyes K. M., Hamilton A., McLaughlin K. A., Sample composition alters associations between age and brain structure. Nat. Commun. 8, 874 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gogtay N., Thompson P. M., Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain Cogn. 72, 6–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman-Rakic P. S., Development of cortical circuitry and cognitive function. Child Dev. 58, 601–622 (1987). [PubMed] [Google Scholar]
- 34.Colich N. L., Sheridan M. A., Humphreys K. L., Wade M., Tibu F., Nelson C. A., Zeanah C. H., Fox N. A., McLaughlin K. A., Heightened sensitivity to the caregiving environment during adolescence: Implications for recovery following early-life adversity. J. Child Psychol. Psychiatry. 62, 10.1111/jcpp.13347, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunnar M., DePasquale C., Reid B., Donzella B., Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proc. Natl. Acad. Sci. U.S.A. 116, 23984–23988 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eluvathingal T. J., Chugani H. T., Behen M. E., Juhász C., Muzik O., Maqbool M., Chugani D. C., Makki M., Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics 117, 2093–2100 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Govindan R. M., Behen M. E., Helder E., Makki M. I., Chugani H. T., Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS). Cereb. Cortex 20, 561–569 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behen M. E., Muzik O., Saporta A. S. D., Wilson B. J., Pai D., Hua J., Chugani H. T., Abnormal fronto-striatal connectivity in children with histories of early deprivation: A diffusion tensor imaging study. Brain Imaging Behav. 3, 292–297 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanson J. L., Adluru N., Chung M. K., Alexander A. L., Davidson R. J., Pollak S. D., Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev. 84, 1566–1578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeanah C. H., Nelson C. A., Fox N. A., Smyke A. T., Marshall P., Parker S. W., Koga S., Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev. Psychopathol. 15, 885–907 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Bethlehem R. A. I., Seidlitz J., White S. R., Vogel J. W., Anderson K. M., Adamson C., Adler S., Alexopoulos G. S., Anagnostou E., Areces-Gonzalez A., Astle D. E., Auyeung B., Ayub M., Bae J., Ball G., Baron-Cohen S., Beare R., Bedford S. A., Benegal V., Beyer F., Blangero J., Blesa Cábez M., Boardman J. P., Borzage M., Bosch-Bayard J. F., Bourke N., Calhoun V. D., Chakravarty M. M., Chen C., Chertavian C., Chetelat G., Chong Y. S., Cole J. H., Corvin A., Costantino M., Courchesne E., Crivello F., Cropley V. L., Crosbie J., Crossley N., Delarue M., Delorme R., Desrivieres S., Devenyi G. A., Di Biase M. A., Dolan R., Donald K. A., Donohoe G., Dunlop K., Edwards A. D., Elison J. T., Ellis C. T., Elman J. A., Eyler L., Fair D. A., Feczko E., Fletcher P. C., Fonagy P., Franz C. E., Galan-Garcia L., Gholipour A., Giedd J., Gilmore J. H., Glahn D. C., Goodyer I. M., Grant P. E., Groenewold N. A., Gunning F. M., Gur R. E., Gur R. C., Hammill C. F., Hansson O., Hedden T., Heinz A., Henson R. N., Heuer K., Hoare J., Holla B., Holmes A. J., Holt R., Huang H., Im K., Ipser J., Jack C. R., Jackowski A. P., Jia T., Johnson K. A., Jones P. B., Jones D. T., Kahn R. S., Karlsson H., Karlsson L., Kawashima R., Kelley E. A., Kern S., Kim K. W., Kitzbichler M. G., Kremen W. S., Lalonde F., Landeau B., Lee S., Lerch J., Lewis J. D., Li J., Liao W., Liston C., Lombardo M. V., Lv J., Lynch C., Mallard T. T., Marcelis M., Markello R. D., Mathias S. R., Mazoyer B., McGuire P., Meaney M. J., Mechelli A., Medic N., Misic B., Morgan S. E., Mothersill D., Nigg J., Ong M. Q. W., Ortinau C., Ossenkoppele R., Ouyang M., Palaniyappan L., Paly L., Pan P. M., Pantelis C., Park M. M., Paus T., Pausova Z., Paz-Linares D., Binette A. P., Pierce K., Qian X., Qiu J., Qiu A., Raznahan A., Rittman T., Rodrigue A., Rollins C. K., Romero-Garcia R., Ronan L., Rosenberg M. D., Rowitch D. H., Salum G. A., Satterthwaite T. D., Schaare H. L., Schachar R. J., Schultz A. P., Schumann G., Schöll M., Sharp D., Shinohara R. T., Skoog I., Smyser C. D., Sperling R. A., Stein D. J., Stolicyn A., Suckling J., Sullivan G., Taki Y., Thyreau B., Toro R., Traut N., Tsvetanov K. A., Turk-Browne N. B., Tuulari J. J., Tzourio C., Vachon-Presseau É., Valdes-Sosa M. J., Valdes-Sosa P. A., Valk S. L., van Amelsvoort T., Vandekar S. N., Vasung L., Victoria L. W., Villeneuve S., Villringer A., Vértes P. E., Wagstyl K., Wang Y. S., Warfield S. K., Warrier V., Westman E., Westwater M. L., Whalley H. C., Witte A. V., Yang N., Yeo B., Yun H., Zalesky A., Zar H. J., Zettergren A., Zhou J. H., Ziauddeen H., Zugman A., Zuo X. N.; 3R-BRAIN; AIBL; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Repository Without Borders Investigators; CALM Team; Cam-CAN; CCNP; COBRE; cVEDA; ENIGMA Developmental Brain Age Working Group; Developing Human Connectome Project; FinnBrain; Harvard Aging Brain Study; IMAGEN; KNE96; The Mayo Clinic Study of Aging; NSPN; POND; The PREVENT-AD Research Group; VETSA, Bullmore E. T., Alexander-Bloch A. F., Brain charts for the human lifespan. Nature 604, 525–533 (2022).35388223 [Google Scholar]
- 42.Kievit R. A., Brandmaier A. M., Ziegler G., van Harmelen A.-L., de Mooij S. M. M., Moutoussis M., Goodyer I. M., Bullmore E., Jones P. B., Fonagy P.; NSPN Consortium, Lindenberger U., Dolan R. J., Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Dev. Cogn. Neurosci. 33, 99–117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wade M., Fox N. A., Zeanah C. H., Nelson C. A., Effect of foster care intervention on trajectories of general and specific psychopathology among children with histories of institutional rearing: A randomized clinical trial. JAMA Psychiat. 75, 1137–1145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gogtay N., Giedd J. N., Lusk L., Hayashi K. M., Greenstein D., Vaituzis A. C., Nugent T. F. III, Herman D. H., Clasen L. S., Toga A. W., Rapoport J. L., Thompson P. M., Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 101, 8174–8179 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huttenlocher P. R., Dabholkar A. S., Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 387, 167–178 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Tanzer M., Derome M., Morosan L., Salaminios G., Debbané M., Cortical thickness of the insula and prefrontal cortex relates to externalizing behavior: Cross-sectional and prospective findings. Dev. Psychopathol. 33, 1437–1447 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Shannon K. E., Sauder C., Beauchaine T. P., Gatzke-Kopp L. M., Disrupted effective connectivity between the medial frontal cortex and the caudate in adolescent boys with externalizing behavior disorders. Crim. Justice Behav. 36, 1141–1157 (2009). [Google Scholar]
- 48.Silveira S., Boney S., Tapert S. F., Mishra J., Developing functional network connectivity of the dorsal anterior cingulate cortex mediates externalizing psychopathology in adolescents with child neglect. Dev. Cogn. Neurosci. 49, 100962 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fava N. M., Trucco E. M., Martz M. E., Cope L. M., Jester J. M., Zucker R. A., Heitzeg M. M., Childhood adversity, externalizing behavior, and substance use in adolescence: Mediating effects of anterior cingulate cortex activation during inhibitory errors. Dev. Psychopathol. 31, 1439–1450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung R. E., Haier R. J., The parieto-frontal integration theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav. Brain Sci. 30, 135–154 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Tooley U. A., Bassett D. S., Mackey A. P., Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 22, 372–384 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larsen B., Luna B., Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci. Biobehav. Rev. 94, 179–195 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanderwert R. E., Marshall P. J., Nelson C. A., Zeanah C. H., Fox N. A., Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLOS ONE 5, e11415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vestergaard M., Madsen K. S., Baaré W. F. C., Skimminge A., Ejersbo L. R., Ramsøy T. Z., Gerlach C., Akeson P., Paulson O. B., Jernigan T. L., White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J. Cogn. Neurosci. 23, 2135–2146 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Sowell E. R., Thompson P. M., Tessner K. D., Toga A. W., Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J. Neurosci. 21, 8819–8829 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wade M., Fox N. A., Zeanah C. H., Nelson C. A. III, Long-term effects of institutional rearing, foster care, and brain activity on memory and executive functioning. Proc. Natl. Acad. Sci. U.S.A. 116, 1808–1813 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olson I. R., Von Der Heide R. J., Alm K. H., Vyas G., Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev. Cogn. Neurosci. 14, 50–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Ijzendoorn M. H., Bakermans-Kranenburg M. J., Juffer F., Plasticity of growth in height, weight, and head circumference: Meta-analytic evidence of massive catch-up after international adoption. J. Dev. Behav. Pediatr. 28, 334–343 (2007). [DOI] [PubMed] [Google Scholar]
- 59.VanTieghem M., Korom M., Flannery J., Choy T., Caldera C., Humphreys K. L., Gabard-Durnam L., Goff B., Gee D. G., Telzer E. H., Shapiro M., Louie J. Y., Fareri D. S., Bolger N., Tottenham N., Longitudinal changes in amygdala, hippocampus and cortisol development following early caregiving adversity. Dev. Cogn. Neurosci. 48, 100916 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Horn J. D., Toga A. W., Multi-site neuroimaging trials. Curr. Opin. Neurol. 22, 370–378 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teicher M. H., Childhood trauma and the enduring consequences of forcibly separating children from parents at the United States border. BMC Med. 16, 146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeanah C. H., Fox N. A., Nelson C. A., The Bucharest Early Intervention Project: Case study in the ethics of mental health research. J. Nerv. Ment. Dis. 200, 243–247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller F. G., The randomized controlled trial as a demonstration project: An ethical perspective. Am. J. Psychiatry 166, 743–745 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Yendiki A., Panneck P., Srinivasan P., Stevens A., Zöllei L., Augustinack J., Wang R., Salat D., Ehrlich S., Behrens T., Jbabdi S., Gollub R., Fischl B., Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform. 5, 23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reig S., Sánchez-González J., Arango C., Castro J., González-Pinto A., Ortuño F., Crespo-Facorro B., Bargalló N., Desco M., Assessment of the increase in variability when combining volumetric data from different scanners. Hum. Brain Mapp. 30, 355–368 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ségonne F., Dale A. M., Busa E., Glessner M., Salat D., Hahn H. K., Fischl B., A hybrid approach to the skull stripping problem in MRI. Neuroimage 22, 1060–1075 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Ghosh S. S., Kakunoori S., Augustinack J., Nieto-Castanon A., Kovelman I., Gaab N., Christodoulou J. A., Triantafyllou C., Gabrieli J. D. E., Fischl B., Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuroimage 53, 85–93 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jovicich J., Czanner S., Han X., Salat D., van der Kouwe A., Quinn B., Pacheco J., Albert M., Killiany R., Blacker D., Maguire P., Rosas D., Makris N., Gollub R., Dale A., Dickerson B. C., Fischl B., MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 46, 177–192 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reuter M., Schmansky N. J., Rosas H. D., Fischl B., Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reuter M., Rosas H. D., Fischl B., Highly accurate inverse consistent registration: A robust approach. Neuroimage 53, 1181–1196 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B. Methodol. 57, 289–300 (1995). [Google Scholar]
- 72.Menyhart O., Weltz B., Győrffy B., MultipleTesting.com: A tool for life science researchers for multiple hypothesis testing correction. PLOS ONE 16, e0245824 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Essex M. J., Boyce W. T., Goldstein L. H., Armstrong J. M., Kraemer H. C., Kupfer D. J., The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the Macarthur Health and Behavior Questionnaire. J. Am. Acad. Child Adolesc. Psychiatry 41, 588–603 (2002). [DOI] [PubMed] [Google Scholar]
- 74.D. Wechsler, The Wechsler Intelligence Scale for Children—Fourth Edition (The Psychological Corporation, 2004). [Google Scholar]
- 75.K. L. Humphreys, L. S. King, K. L. Guyon-Harris, M. A. Sheridan, K. A. McLaughlin, A. Radulescu, C. A. Nelson, N. A. Fox, C. H. Zeanah, Foster care leads to sustained cognitive gains following severe early deprivation, in Proceedings of the National Academy of Sciences (2022). [DOI] [PMC free article] [PubMed]
- 76.D. Wechsler, Wechsler Adult Intelligence Scale—Fourth Edition (Pearson Assessment, 2008). [Google Scholar]
- 77.Greve D. N., Fischl B., False positive rates in surface-based anatomical analysis. Neuroimage 171, 6–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desikan R. S., Ségonne F., Fischl B., Quinn B. T., Dickerson B. C., Blacker D., Buckner R. L., Dale A. M., Maguire R. P., Hyman B. T., Albert M. S., Killiany R. J., An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Region of interest analysis
Impact of institutionalization on subcortical volume without controls for ICV
Nonsignificant results (group effects)
Associations between neural structure and psychopathology
Longitudinal change
Figs. S1 to S4
Tables S1 to S3
References