Abstract
Background and Purpose
It is well established that the nucleus accumbens and glutamate play a critical role in the motivation to take drugs of abuse. We have previously demonstrated that rats with ablation of the serotonin (5‐HT) transporter (SERT−/− rats) show increased cocaine intake reminiscent of compulsivity.
Experimental Approach
By comparing SERT−/− to SERT+/+ rats, we set out to explore whether SERT deletion influences glutamate neurotransmission under control conditions as well as after short access (1 h/session) or long access (6 h/session) to cocaine self‐administration.
Key Results
Rats were killed at 24 h after the final self‐administration session for ex vivo molecular analyses of the glutamate system (vesicular and glial transporters, post‐synaptic subunits of NMDA and AMPA receptors and their related scaffolding proteins). Such analyses were undertaken in the nucleus accumbens core. In cocaine‐naïve animals, SERT deletion evoked widespread abnormalities in markers of glutamatergic neurotransmission that, overall, indicate a reduction of glutamate signalling. These results suggest that 5‐HT is pivotal for the maintenance of accumbal glutamate homeostasis. We also found that SERT deletion altered glutamate homeostasis mainly after long access, but not short access, to cocaine.
Conclusion and Implications
Our findings reveal that SERT deletion may sensitize the glutamatergic synapses of the nucleus accumbens core to the long access but not short access, intake of cocaine.
LINKED ARTICLES
This article is part of a themed issue on New discoveries and perspectives in mental and pain disorders. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v179.17/issuetoc
Keywords: cocaine self‐administration, glutamate, nucleus accumbens, serotonin transporter
Abbreviations
- 5‐HTTLPR
5‐HT (serotonin) transporter‐linked polymorphic region
- FR1
fixed ratio 1
- GLT‐1
glial glutamate transporter
- SAP102
synapse associated protein 102; NMDA receptor scaffolding protein
- SAP97
s
ynapse associated protein 97; AMPA receptor scaffolding protein
- SERT−/−
serotonin transporter knockout
- SERT+/+
wild‐type counterpart of serotonin transporter knockout rat
- vGLUT1
vesicular glutamate transporter 1
What is already known
Rats lacking the serotonin transporter (SERT) show moderate and escalated cocaine self‐administration.
What does this study adds
Escalated, but not moderate, cocaine self‐administration activates accumbal glutamate synapses in SERT−/− rats
What is the clinical significance
Inherited serotonin transporter down‐regulation may explain elevated use of cocaine in such subjects.
1. INTRODUCTION
In drug addiction, compulsivity is considered the terminal ending of the shift from the hedonic, regular use of a given drug into a non‐controlled drug use, characterized by craving and a high risk of relapse. A critical trigger of this transition is a negative emotional state that emerges as a result of drug abstinence (Koob & Le Moal, 2008). Since 5‐hydroxytryptamnie (5‐HT; serotonin) plays a critical role in emotional states, it is very likely that this neurotransmitter is crucial for the above‐mentioned transition (Nonkes et al., 2011). Indeed, reduction of the serotonin transporter (SERT/SLC6A4) contributes to intake of cocaine and the related anxiety‐like phenotype (Verheij et al., 2018), possibly by changing the extracellular levels of 5‐HT (Homberg et al., 2008; Lesch et al., 1996; Rocha et al., 1998; Verheij et al., 2014).
Previous work from our laboratory elucidated that cocaine taking was enhanced in SERT knockout (SERT−/−) rats under conditions that simulate the moderate (the so‐called short‐access condition) or heightened (the so‐called long‐access condition) use of cocaine (Homberg et al., 2008; Karel et al., 2018; Verheij et al., 2018). Further, previous data showed that rats exposed to both cocaine conditions were anxious when exposed to specific behavioural tests 24 h later (Verheij et al., 2018), allowing us to hypothesize that the higher cocaine intake observed under both experimental conditions could be triggered, at least partially, by increased anxiety, which is known to be a critical contributor of addiction (Verheij et al., 2018). In addition, we have recently investigated, under both short access or long access conditions, the role of SERT in the modulation of glutamate neurotransmission, another neurotransmitter known to play a role in the action of cocaine and its related negative emotional states (Richard & Berridge, 2011). In the habenula and medial prefrontal cortex, we found a significant dysregulation in the expression of various proteins of the glutamatergic synapse under both experimental conditions in SERT−/− rats suggesting that, indeed, the interaction between 5‐HT and glutamate represents a critical biological substrate for drug addiction (Caffino et al., 2019; Caffino, Mottarlini, et al., 2021). These findings also suggest that SERT‐induced alterations of glutamate homeostasis can span multiple brain regions implicated in reward and withdrawal.
The nucleus accumbens is a limbic structure that plays a central role in the cocaine‐induced neuroadaptive changes underlying the development of addiction, in particular with respect to drug intake, withdrawal and motivation to seek for drugs. Several lines of evidence show that cocaine affects glutamatergic signalling in the nucleus accumbens (Cornish & Kalivas, 2000; LaCrosse et al., 2016; Logan et al., 2018; Scofield, Li, et al., 2016; Stefanik et al., 2018). Further, accumbal 5‐HT has been reported to influence glutamate homeostasis not only in drug‐naïve (Muramatsu et al., 1998) but also cocaine‐exposed animals (Zayara et al., 2011). In this study, we decided to dissect the influence of SERT deletion on glutamate homeostasis in a specific subregion of the nucleus accumbens, that is, the core nucleus accumbens. This choice was based on two different lines of evidence: (1) selective excitotoxic lesions of the nucleus accumbens core profoundly impair the acquisition of cocaine self‐administration and cocaine‐seeking behaviours (Ito et al., 2004) and (2) in previous publications from our group, we had observed differences in the acquisition of cocaine self‐administration behaviour in SERT−/− rats (Homberg et al., 2008; Karel et al., 2019; Nonkes et al., 2013). SERT−/− rats and their wild‐type (SERT+/+) counterparts were exposed to both short access or long access cocaine self‐administration to mimic moderate or heightened consumption of cocaine, respectively (Ahmed & Koob, 1998). Our main goal was to study the complex machinery that regulates glutamate levels, focusing on the level of the specific proteins regulating presynaptic release) and glial reuptake (GLT‐1/EAAT2/SLC1A2) together with the expression of the main subunits of NMDA (GluN1, GluN2A and GluN2B) and AMPA (GluA1 and GluA2) receptors and their related scaffolding protein expression (SAP97 and SAP102). We therefore set out to explore these critical determinants of glutamate neurotransmission in the whole homogenate of the core nucleus accumbens taking advantage of brain material, unused so far, which we collected in a previous study (Caffino et al., 2019). Our findings reveal that SERT deletion may sensitize the glutamatergic synapses of the nucleus accumbens core to the heightened, but not moderate, intake of cocaine.
2. METHODS
2.1. Animals
Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley et al., 2020).
We employed rats in this study because the rat is the preferred species for preclinical addiction research (Homberg et al., 2017). SERT−/− rats (SLC6A41Hubr) were generated by N‐ethyl‐N‐nitrosurea (ENU) induced mutagenesis (Smits et al., 2006) outcrossed with commercially available Wistar rats (Harlan, Ter Horst, the Netherlands) for at least 10 generations (Homberg et al., 2007). Rats were housed in groups of two in enriched Macrolon type III cages (42 × 26 × 15 cm; Techniplast 1291H, Tecnilab‐BMI) with corncobs bedding (irradiated, SPPS COB12, Bio Services) under conventional conditions (no filtertops). The animals had access to food (dried pellets of standard chow food; Ssniff RM V1534‐703 diet supplied by BioServices) and water ad libitum, except during test phases. The rats were housed under a reversed day and night cycle (lights off at 08:00 AM) in temperature (21 ± 1°C) and humidity (55 ± 5%) controlled rooms. Testing (cocaine self‐administration) took place in the dark phase of the light/dark cycle. Male SERT−/− and SERT+/+ rats were subjected to short access (1‐h daily self‐administration session) and long access (6‐h daily self‐administration session) cocaine self‐administration according to the procedures described in Caffino et al. (2019). The number of animals analysed for protein expression levels was naïve:‐ SERT+/+: n = 7, SERT−/−: n = 7; short access: SERT+/+: n = 6, SERT−/−: n = 6 and long access: SERT−/−: n = 7, SERT−/−: n = 7). Behavioural testing was always done blindly by an experimenter who was unaware of the genotype of the animals. Allocation of the rats to the treatment groups (short access and long access) were random. The experimental procedures were performed under a project license from the Central Committee on Animal Experiments (Centrale Commissie Dierproeven, The Hague, The Netherlands), in full compliance with the legal requirements of Dutch legislation on the use and protection of laboratory animals (Animal Testing Act). All efforts were made to reduce the number of animals used and their suffering.
2.2. Cocaine self‐administration
Cocaine was provided by the (National Institute on Drug Abuse, Rockville, MD), and was dissolved in saline 0.9%. Briefly, 1 week after surgery, rats were trained to self‐administer cocaine (0.5 mg·kg‐1 infusion) under a fixed ratio 1 (FR1) schedule of reinforcement (for details, please see Caffino et al., 2019; Verheij et al., 2016, 2018). Two days after cocaine self‐administration training, rats were allowed to self‐administer cocaine during daily 6‐h sessions (extended or long access group of rats) or 1‐h session (limited of short access group of rats) for a total of 15 days (Ahmed & Koob, 1998). Additional groups of cocaine‐naive SERT−/− and SERT+/+ rats also underwent intravenous catheterization, were handled daily and received daily infusion of heparinized saline, but were not exposed to the self‐administration chambers (Verheij et al., 2016, 2018).
2.3. Tissue collection
Twenty‐four hours following the last cocaine self‐administration session, rats were sacrificed by decapitation without anaesthesia. This procedure was used to avoid effects of anaesthetics on protein expression and it is in accordance with the Dutch legal regulations for sacrificing rodents. Brains were quickly collected and stored at −80°C. Using the rat brain atlas of Paxinos and Watson (2005), the core nucleus accumbens (from Bregma +2.76 mm to Bregma +0.84 mm) was punched from frozen brain sections of 220 μm using a sterile 1‐mm‐diameter needle (Giannotti et al., 2016). Punches from the right and left hemisphere were pooled. core nucleus accumbens tissue was stored at −80°C until being processed for molecular analysis (see below).
2.4. Protein extraction and western blot analyses
The immune‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). Proteins were extracted as previously described with minor modifications (Caffino et al., 2017).
Briefly, bilateral punches of core nucleus accumbens were homogenized in a glass–glass potter in cold 0.32‐M sucrose buffer pH 7.4 containing 1‐mM HEPES, 0.1‐mM PMSF, in presence of commercial cocktails of protease (Roche, Monza, Italy) and phosphatase (Sigma‐Aldrich, Milan, Italy) inhibitors and then sonicated. Total proteins have been measured in the total homogenate according to the Bradford Protein Assay procedure (Bio‐Rad, Milan, Italy), using bovine serum albumin as calibration standard.
Western blots were run as previously described (Caffino et al., 2017). Briefly, 10 μg of proteins for each sample were run on a sodium dodecyl sulfate‐8% polyacrylamide gel under reducing conditions and then electrophoretically transferred onto nitrocellulose membranes (GE Healthcare, Milan, Italy). Blots were blocked 1 h at room temperature with I‐Block solution (Life Technologies Italia, Italy) in TBS + 0.1% Tween‐20 buffer and then incubated with antibodies against the total proteins of interest.
The conditions of the primary antibodies were the following: anti‐vGlut1 (1:1000, Cell Signaling Technology Inc., RRID:AB_2797887), anti‐GLT‐1 (1:5000, AbCam, RRID:AB_1566262), anti‐GluN1 (1:1000, Invitrogen, RRID:AB_2533060), anti‐GluN2B (1:1000, Santa Cruz Biotechonology, RRID:AB_670229), anti‐GluN2A (1:1000, Invitrogen, RRID:AB_2536209), anti‐SAP102 (1:1000, Cell Signaling Technology Inc., RRID:AB_2092180), anti‐GluA1 (1:2000, Cell Signaling Technology Inc, RRID:AB_641040), anti‐GluA2 (1:2000, Cell Signaling Technology Inc., RRID:AB_10622024), anti‐SAP97 (1:1000, AbCam, RRID:AB_2091910) and anti‐β‐Actin (1:10000, Sigma‐Aldrich, RRID:AB_476697). Expression levels of every single protein were normalized using its own β‐Actin loading control, which was detected by evaluating the band density at 43 kDa. Optic density (OD) of immunocomplexes was visualized by chemiluminescence using the Chemidoc MP Imaging System (Bio‐Rad Laboratories, RRID:SCR_008426). Gels were run two times each and the results represent the average from two different runs. We used a correction factor to average the different gels: correction factor gel 2 = average of (OD protein of interest/OD β‐actin for each sample loaded in gel 1)/(OD protein of interest/OD β‐actin for the same sample loaded in gel 2) (Caffino, Verheij, et al., 2020).
2.5. Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). All animals tested were treated as independent values, there were no technical replicates.
The Kolmogorov–Smirnov test was employed to determine normality of residuals: no significant variance in homogeneity was found. Thus, the molecular changes produced by genotype and cocaine exposure alone as well as by their combination were analysed using a two‐way ANOVA, with the factors genotype and type of cocaine access as independent variables. When dictated by relevant interaction terms, Tukey's multiple comparisons test was used to characterize differences among individual groups of rats. The post hoc tests were conducted only if F in ANOVA achieved P < .05 and there was no significant variance in homogeneity. Two‐way ANOVA analyses were performed using raw data (Table S1). Then, data were normalised as percentages of the cocaine‐naïve SERT+/+ control rats that were not exposed to either cocaine short access or long access to enable visual comparisons across genotypes with different degrees of expression of glutamatergic molecular determinants. Values are presented as percentage of control rats. Subjects were eliminated from the final dataset if their data deviated from the mean by 2 SDs. Prism 6.0 (GraphPad, RRID:SCR_002798) was used to analyse all the data. Data are shown as mean ± SEM and as % of baseline to control for unwanted sources of variation.
Significance for all tests was assumed at p < .05.
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org (Harding et al., 2018) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Kelly, et al., 2019; Alexander, Mathie, et al., 2019).
3. RESULTS
3.1. Cocaine intake
As we reported previously (Caffino et al., 2019), no genotype differences were observed during the acquisition of cocaine self‐administration. Under short access conditions, the daily number of cocaine infusions was higher in SERT−/− versus SERT+/+ rats, leading to a significant higher total cocaine intake in SERT−/− (172 ± 24 infusions) versus SERT+/+ (81 ± 13 infusions) rats. When the rats were allowed to self‐administer cocaine under long access, the daily number of cocaine infusions was significantly higher in SERT−/− versus SERT+/+ rats, leading to a higher total cocaine intake in SERT−/− (1209 ± 88 infusions) versus SERT+/+ (823 ± 157 infusions) rats. Under both short access and long access conditions, the number of inactive lever presses was similar in both genotypes.
3.2. Expression levels of the vesicular glutamate transporter in the homogenate of the core nucleus accumbens following short access and long access to cocaine in SERT+/+ and SERT−/− rats
We first evaluated the expression level of the vesicular glutamate transporter 1 (vGLUT1/SLC17A7), that is, the molecule responsible of packaging glutamate into presynaptic vesicles before release, in the homogenate of core nucleus accumbens of SERT−/− and SERT+/+ rats under naive conditions and following the different paradigms of cocaine self‐administration. Two‐way ANOVA (for details, see Table S1) revealed a significant cocaine access × genotype interaction (Figure 1). Examining the individual treatment effects, we found that SERT deletion significantly reduced vGLUT1 expression in naïve animals. Interestingly, the expression of vGLUT1 was reduced in SERT+/+ rats after long access conditions, whereas vGLUT1 enhanced in SERT−/− rats after the same conditions. Conversely, no effects of the short access procedure were observed.
FIGURE 1.
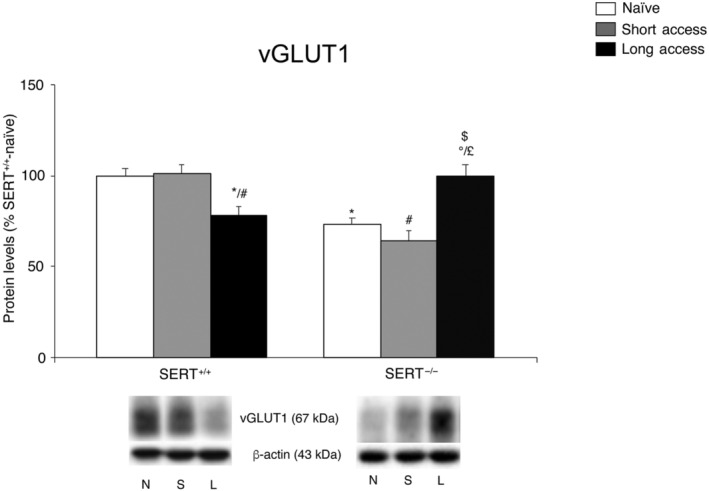
Interaction between SERT deletion and cocaine self‐administration (short or long access) on the vesicular glutamate transporter 1 (vGLUT1) in the core nucleus accumbens. Protein levels of vGlut1 in core nucleus accumbens are expressed as percentages of SERT+/+‐ naïve rats. Below the graphs, representative immunoblots are shown for vGLUT1 (60 kDa) and β‐actin (43 kDa) proteins. Histograms represent the mean ± SEM of the following number of rats: naïve (SERT+/+ n = 7; SERT−/− n = 6), short access (SERT+/+ n = 6; SERT−/− n = 5) and long access (SERT+/+ n = 6; SERT−/− n = 6). *P < .05, versus SERT+/+‐naive; # P < .05 versus SERT+/+‐short access; $ P < .05 versus SERT+/+‐long access; £ P < .05 versus SERT−/−‐naive; °P < .05 versus SERT−/−‐short access (Tukey's multiple comparisons test). N, naïve; S, Cocaine Short‐Access; L, Cocaine Long‐Access
3.3. Expression levels of the glial glutamate transporter (GLT‐1/EAA2) in the homogenate of the core nucleus accumbens following short access and long access to cocaine in SERT+/+ and SERT−/− rats
In order to have a comprehensive idea of the release/uptake system functioning under our experimental conditions, we evaluated the expression level of the main glial glutamate transporter responsible for the clearance of glutamate from the synaptic cleft, that is, GLT‐1, in the homogenate of core nucleus accumbens of SERT−/− and SERT+/+. These analyses were undertaken under naive conditions and following the different paradigms of cocaine self‐administration. Two‐way ANOVA (for details, see Table S1) revealed a main effect of cocaine access, genotype and a cocaine access × genotype interaction (Figure 2). Further intergroup subtesting revealed reduced expression of GLT‐1 in SERT−/− rats in cocaine‐naïve rats. We also found increased expression of GLT‐1 in SERT−/− rats exposed to long access, but not short access, whereas SERT+/+ rats exhibited an increase of GLT‐1 following short access, but not long access, procedure.
FIGURE 2.
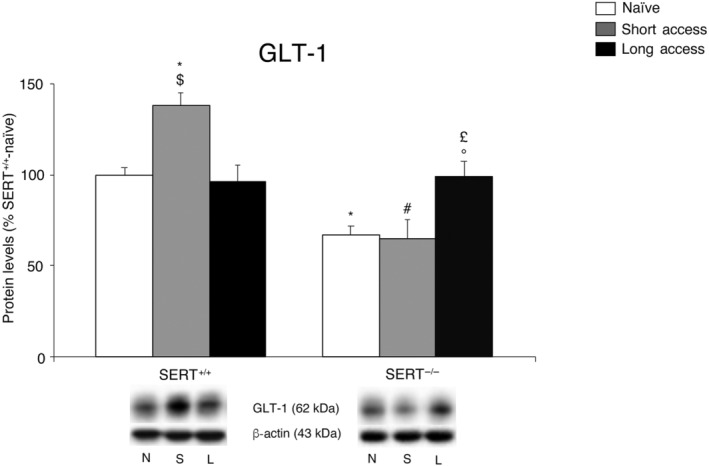
Interaction between SERT deletion and cocaine self‐administration (short or long access) on the glial glutamate transporter 1 (GLT‐1) in the core nucleus accumbens. Protein levels of GLT‐1 in core nucleus accumbens are expressed as percentages of SERT+/+‐naive rats. Below the graphs, representative immunoblots are shown for GLT‐1 (62 kDa) and β‐actin (43 kDa) proteins. Histograms represent the mean ± SEM of the following number of rats: naïve (SERT+/+ n = 7; SERT−/− n = 7), short access (SERT+/+ n = 6; SERT−/− n = 6) and long access (SERT+/+ n = 7; SERT−/− n = 7). *P < .05 versus SERT+/+‐naive; # P < .05 versus SERT+/+‐short access; $ P < .051 versus SERT+/+‐ short access; £ P < .05 versus SERT−/−‐naive; °P < .05 versus SERT−/−‐short access (Tukey's multiple comparisons test). N, naïve; S, Cocaine Short‐Access; L, Cocaine Long‐Access
3.4. Expression levels of NMDA receptor subunits in the homogenate of the core nucleus accumbens following short access and long access to cocaine in SERT+/+ and SERT−/− rats
We then analysed protein expression level of the obligatory subunit of the NMDA receptor, that is, GluN1, in the core nucleus accumbens of SERT−/− and SERT+/+ rats under naive conditions and following different paradigms of cocaine self‐administration (Figure 3a), in the whole homogenate. Two‐way ANOVA (for details, see Table S1) revealed a significant effect of cocaine access and a significant cocaine access × genotype interaction. Given the interaction of the two treatment paradigms, we made all intergroup comparisons. Under cocaine‐naïve conditions, no effects were caused by the genotype. However, in SERT−/− rats exposed to cocaine, long access, but not short access, GluN1 expression increased, an effect that was not observed in SERT+/+ rats.
FIGURE 3.
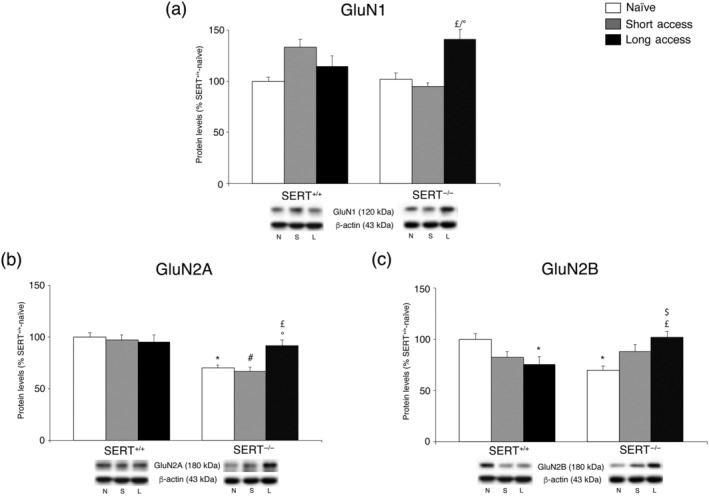
Interaction between SERT deletion and cocaine self‐administration (short or long access) on the NMDA receptor subunits in the core nucleus accumbens. Protein levels of GluN1 (a), GluN2A (b) and GluN2B (c) in core nucleus accumbens are expressed as percentages of SERT+/+‐naive rats. In panel (d), representative immunoblots are shown for GluN2A (180 kDa), GluN2B (180 kDa), GluN1 (120 kDa) and β‐actin (43 kDa) proteins. Histograms represent the mean ± SEM of the following number of rats: naïve (SERT+/+ n = 7; SERT−/− n = 7), short access (SERT+/+ n = 6; SERT−/− n = 6) and long access (SERT+/+ n = 7; SERT−/− n = 7). *P < .05 versus SERT+/+‐naive; # P < .05 versus SERT+/+‐ short access; $ P < .05 versus SERT+/+‐short access; £ P < .05, £ P < .05 versus SERT−/−‐naive; °P < .05 versus SERT−/−‐short access (Tukey's multiple comparisons test). N, naïve; S, Cocaine Short‐Access; L, Cocaine Long‐Access
Subsequently, we investigated the expression level of two accessory subunits of the NMDA receptor: GluN2A and GluN2B (Figure 3b,c, respectively), in the whole homogenate of the core nucleus accumbens. With respect to GluN2A, two‐way ANOVA (for details, see Table S1) revealed a main effect of genotype and a cocaine access × genotype interaction effect (Figure 3b). Therefore, we again subdivided the data for individual intergroup comparisons. Under cocaine‐naïve conditions, the removal of SERT evoked a significant decrease of GluN2A levels. In the core nucleus accumbens of SERT−/− rats, subsequent exposure to long access elicited a significant elevation of GluN2A levels whereas short access did not. In the core nucleus accumbens of SERT+/+ rats, there was no effect in response to both short access or long access.
With respect to GluN2B, two‐way ANOVA (for details, see Table S1) revealed a significant cocaine access × genotype interaction (Figure 3c). Examining the individual treatment effects, we found that, similarly to GluN2A, the removal of SERT evoked a significant decrease of GluN2B levels in cocaine‐naïve rats. The overall patterns of cocaine‐related effects on GluN2B expression were completely different between genotypes: in fact, whereas long access elicited a reduction in the expression of GluN2B in SERT+/+ rats, the same treatment evoked an increase in the core nucleus accumbens of SERT−/− rats. The short access procedure did not cause a significant effect in both genotypes.
3.5. Expression levels of the scaffold protein SAP102 in the homogenate of the core nucleus accumbens following short access and long access to cocaine in SERT+/+ and SERT−/− rats
To further characterize the impact of the combination of SERT deletion and cocaine self‐administration on the stability of NMDA receptor in the core nucleus accumbens, we evaluated SAP102 protein levels, a scaffolding protein that anchors and stabilizes NMDA receptors in the post‐synaptic membrane, in the core nucleus accumbens of SERT−/− and SERT+/+ rats under cocaine‐naive conditions and following different paradigms of cocaine self‐administration (Figure 5a), in the whole homogenate.
FIGURE 5.
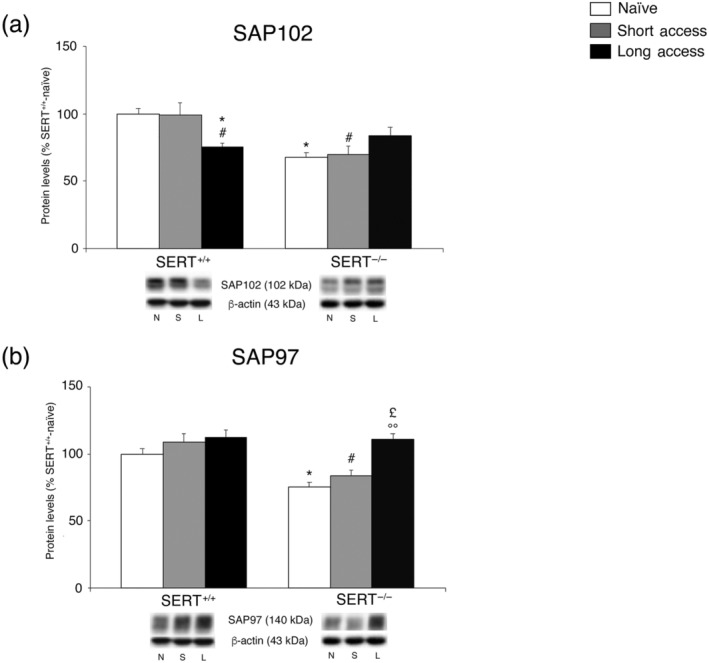
Interaction between SERT deletion and cocaine self‐administration (short or long access) on the scaffolding proteins SAP102 and SAP97 in the core nucleus accumbens. Protein levels of SAP102 (a) and SAP97 (b) are expressed as percentages of SERT+/+‐naive rats. Below the graphs, representative immunoblots are shown for SAP102 (102 kDa), SAP97 (97 kDa) and β‐actin (43 kDa) proteins. Histograms represent the mean ± SEM of the following number of rats: naïve (SERT+/+ n = 7; SERT−/− n = 7), short access (SERT+/+ n = 6; SERT−/− n = 6) and long access (SERT+/+ n = 7; SERT−/− n = 7). *P < .05 versus SERT+/+‐naive; # P < .05 versus SERT+/+‐short access; £ P < .05 versus SERT−/−‐naïve; °P < .05 versus SERT−/−‐short access (Tukey's multiple comparisons test). N, naïve; S, Cocaine Short‐Access; L, Cocaine Long‐Access
Two‐way ANOVA (for details, see Table S1) revealed a significant effect of genotype and a cocaine access × genotype interaction. Examining the individual treatment effects, we found that deletion of SERT reduced the expression of SAP102 in drug‐naïve rats without altering, though, the response to both short access or long access. Interestingly, the long access, but not short access, procedure significantly reduced SAP102 expression in SERT+/+ rats (Figure 5a).
3.6. Expression levels of AMPA receptor subunits in the homogenate of the core nucleus accumbens following short access and long access to cocaine in SERT+/+ and SERT−/− rats
Subsequently, we investigated the expression level of the two main subunits of the AMPA receptor GluA1 and GluA2, in the core nucleus accumbens of SERT−/− and SERT+/+ rats under naive conditions and following different paradigms of cocaine self‐administration (Figure 4a,b, respectively), in the whole homogenate. With respect to the AMPA subunit GluA1, two‐way ANOVA (for details, see Table S1) revealed a significant cocaine access and genotype effect and a cocaine access × genotype interaction effect (Figure 4a). Post hoc testing of the main treatment effects indicated a significant reduction in GluA1 caused by the ablation of SERT in drug‐naïve animals. The removal of SERT altered the subsequent response to the long access cocaine regimen, which led to a significant increase in GluA1 levels. Interestingly, neither short access nor long access procedures altered the expression of GluA1 in SERT+/+ rats.
FIGURE 4.
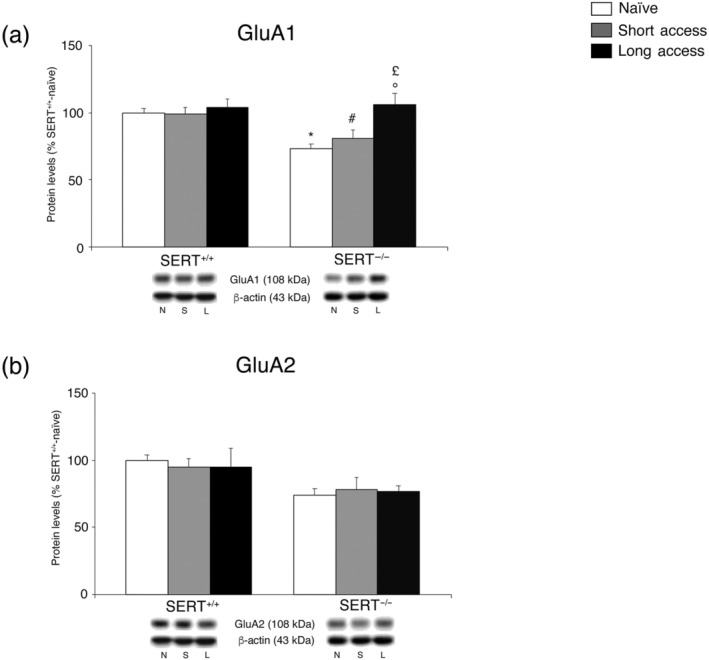
Interaction between SERT deletion and cocaine self‐administration (short or long access) on the AMPA receptor subunits in the core nucleus accumbens. Protein levels of GluA1 (a) and GluA2 (b) are expressed as percentages of SERT+/+‐naïve rats. Below the graphs, representative immunoblots are shown for GluA1 (108 kDa), GluA2 (108 kDa) and β‐actin (43 kDa) proteins. Histograms represent the mean ± SEM of the following number of rats: naïve (SERT+/+ n = 7; SERT−/− n = 7), short access (SERT+/+ n = 6; SERT−/− n = 6) and long access (SERT+/+ n = 7; SERT−/− n = 7). *P < .05 versus SERT+/+‐naive; # P < .05 versus SERT+/+‐short access; £ P < .01 versus SERT−/−‐naive; °P < .05 versus SERT−/−‐short access (Tukey's multiple comparisons test). N, naïve; S, Cocaine Short‐Access; L, Cocaine Long‐Access
Only a significant effect of genotype was observed in GluA2 expression (for details, see Table S1) and therefore, the data were not subdivided for post hoc testing (Figure 4b).
3.7. Expression levels of the scaffold protein SAP97 in the homogenate of the core nucleus accumbens following short access and long access to cocaine in SERT+/+ and SERT−/− rats
To complete the characterization of the impact of the combination of SERT deletion and cocaine self‐administration on the stability of AMPA receptor in the core nucleus accumbens, we evaluated SAP97 protein levels, a scaffolding protein that anchors and stabilizes AMPA receptors in the post‐synaptic membrane, in the core nucleus accumbens of SERT−/− and SERT+/+ rats under naive conditions and following different paradigms of cocaine self‐administration (Figure 5b), in the whole homogenate. Two‐way ANOVA (for details, see Table S1) revealed an effect of cocaine access and a cocaine access × genotype interaction effect. In light of the interactions of treatment effects, we found that SERT deletion reduced SAP97 levels in drug‐naïve animals. Long access, but not short access, enhanced SAP97 expression in SERT−/− rats whereas no effect of either treatment was observed in SERT+/+ animals (Figure 5b).
4. DISCUSSION
Our findings reveal that SERT regulates glutamate homeostasis in the core nucleus accumbens after early withdrawal from cocaine self‐administration, with low expression of SERT sensitizing the glutamatergic synapses of the nucleus accumbens core to the escalated, but not moderate, intake of cocaine.
First, our results indicate that the deletion of SERT affects the glutamate synapse as virtually all the glutamatergic determinants analysed (with the exception of the GluN1 and GluA2 subunits) show a reduced expression in the core nucleus accumbens of cocaine‐naïve SERT−/− rats. It is widely established that glutamate signalling requires a dynamic and well‐co‐ordinated interaction among neurons and glial cells (Bridges et al., 2012). Our results reveal that a proper functioning of SERT is critical for the maintenance of accumbal glutamate homeostasis. We hypothesize that the lack of SERT in drug‐naïve animals may reduce the release of glutamate into the synaptic cleft (as measured by the reduced expression of vGLUT1, the protein responsible of packaging glutamate into vesicles before synaptic release). These reduced extracellular levels of glutamate may be balanced by a reduced clearance of the transmitter as evidenced by reduced GLT‐1 expression, the main glial glutamate transporter, in an attempt to maintain a physiological concentration of glutamate in the synapse. As a result of this reduced reuptake of glutamate, the post‐synaptic glutamate response may be attenuated in the core nucleus accumbens of SERT−/− naïve rats, as shown by the reduced expression of the main NMDA and AMPA receptor subunits and their main scaffolding proteins. We suggest that the overall down‐regulation of glutamate neurotransmission in cocaine naïve SERT−/− rats represents an adaptive response of the glutamate system to buffer the long‐lasting absence of SERT.
Since GLT‐1 expression is significantly reduced in cocaine‐naïve SERT−/− rats, an effect that has been shown to promote cocaine seeking (Knackstedt et al., 2010), these findings provide the ground to explain, at least partially, the proneness shown by SERT−/− rats to self‐administer cocaine (Homberg et al., 2008; Karel et al., 2018; Nonkes et al., 2011; Verheij et al., 2018).
The second main issue brought about by our findings relies on the influence exerted by high 5‐HT extracellular levels on the response to either short access or long access procedures. At first glance, it appears quite clear that 5‐HT does not interact with short access to alter glutamate homeostasis, whereas long access affects most of the glutamate determinants examined, in a way that is different from the effects of long access in the core nucleus accumbens of SERT+/+ rats. The cocaine self‐administration results suggest that the glutamatergic synapse of the core nucleus accumbens may require a prolonged daily period of cocaine self‐administration for these alterations to become manifest, since a shorter daily exposure is ineffective. These results suggest that the baseline reduction in glutamate homeostasis caused by SERT ablation interacts with long access to reactivate the glutamate synapse bringing the levels of the main determinants of glutamate neurotransmission back to control levels. These results provide a molecular mechanism for accumbal 5‐HT as gateway transmitter amplifying the effects of cocaine. Taken together, these results suggest that 5‐HT‐induced changes in glutamate may play a prominent role for the elevated, but not moderate, intake of cocaine (Homberg et al., 2008; Karel et al., 2018; Nonkes et al., 2011; Verheij et al., 2018).
We found that, after long access to cocaine, not only vGLUT1 and GLT‐1 levels were markedly enhanced in SERT−/− rats but also the levels of the main subunits of NMDA and AMPA receptors were up‐regulated. It is interesting to note that the increased NMDA receptor expression in the core nucleus accumbens of these SERT−/− rats is not accompanied by a similar increase of the main scaffolding protein SAP102. This may lead to a less stable glutamate synapse in the core nucleus accumbens of SERT−/− rats (Ortinski et al., 2013). Notably, a discrepancy between expression of glutamate receptors and their anchoring proteins has been observed in the core nucleus accumbens of SERT−/− rats exposed to long access to amphetamine (Caffino, Verheij, et al., 2020), suggesting that NMDA receptor instability may generalize across various psychostimulants. Given the reduced expression of scaffolding proteins, NMDA receptor may move easily to extrasynaptic zones where they can trigger the activation of pathways that may lead to maladaptive consequences (Ortinski et al., 2013). Conversely, long access to cocaine in SERT−/− rats increased the expression of both GluA1 and its specific scaffolding protein SAP97, without altering GluA2 expression. This imbalance in AMPA receptor subunits suggests an enhanced formation of GluA2‐lacking AMPA receptor that are stabilized in the synapse by the increased expression of its anchoring protein. Notably, increased synaptic formation of GluA2‐lacking and Ca2+‐permeable AMPA receptor is considered an index of incubation of drug craving and a maladaptive mechanism critical for drug‐seeking behaviour (Conrad et al., 2008; McCutcheon et al., 2011; Purgianto et al., 2013), pointing to changes in these receptors as crucial for elevated cocaine use in subjects with inherited serotonin transporter down‐regulation. Further, it is interesting to point out that, after extended access to cocaine self‐administration, local alterations in the glutamate synapse have been observed following prolonged withdrawal only (Scofield, Heinsbroek, et al., 2016; Wolf, 2016). Of note, in SERT−/− rats, glutamatergic alterations were found in the core nucleus accumbens much earlier (i.e. after 24 h). These results suggest that SERT deletion may have accelerated cocaine‐induced glutamatergic dysfunctions.
A limitation of the current study relies on the fact that, due to the paucity of the brain material that can be obtained by punching the core nucleus accumbens within the entire nucleus accumbens, we limited our analyses to the whole homogenate, without having the possibility to make preparations of the postsynaptic density, for which more tissue is needed. Thus, we cannot distinguish glutamate receptor localization between synaptic versus extrasynaptic sites to get a more precise information on glutamate homeostasis. Further, as the current data were collected from male rats, we do not know whether findings generalize to female rats.
In conclusion, we have shown that early withdrawal following exposure of SERT−/− rats to long access, but not short access, of cocaine leads to widespread alterations in glutamate homeostasis at presynaptic, postsynaptic as well as glia cell level, which combined would reactivate the glutamate system that was inhibited by the deletion of SERT. Interestingly, enhanced glutamatergic neurotransmission in the nucleus accumbens has increasingly been implicated in the pathophysiology of depressive‐like behaviours following chronic stress (Abdallah et al., 2018; Vialou et al., 2010). Our data suggest that knockout of SERT at time of conception might set the brain's response to cocaine later in life and may, therefore, indicate that 5‐HT‐glutamate interactions may contribute to the negative emotional state observed in drug users after drug discontinuation (Gawin, 1991; Perrine et al., 2008).
AUTHOR CONTRIBUTIONS
L.C. conducted the western blot experiments, analysed the data and contributed to the writing of the manuscript. F.M. conducted the western blot analyses, made the graphic abstract, overviewed figures and made literature research. G.T. contributed to sample preparation and conducted the western blot analyses and contributed to overview figures and literature research. M.V. conducted the self‐administration experiment and contributed to the writing of the manuscript. J.R. conceived and planned the experiments, contributed to the interpretation of the results and contributed to the writing of the manuscript. F.F. conceived and planned the experiments, supervised the molecular analyses, contributed to the interpretation of the results, contributed to the writing of the manuscript and supervised the project. All authors discussed the results and contributed to the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for, Design and Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1. Uncropped immunoblot related to the expression levels of GluN2A (180 kDa; Figure 3), GluN2B (180 kDa; Figure 3), GluN1 (120 kDa; Figure 3), SAP97 (97 kDa, predicted 140 kDa; Figure 5), SAP102 (102 kDa; Figure 5), GluA1 (108 kDa; Figure 4), GluA2 (108 kDa; Figure 4), vGLUT1 (67 kDa; Figure 1), GLT‐1 (62 kDa; Figure 2) and β‐Actin (43 kDa) measured in the homogenate of the nucleus accumbens (cNAc) of SERT+/+ and SERT−/− rats exposed to short access (ShA) and long acces (LgA) cocaine self‐administration.
Table S1. F and p values of molecular data presented in the results section.
Table S2. Pearson's product–moment correlation analyses between cocaine intake and glutamatergic targets of interest.
ACKNOWLEDGEMENTS
This work was supported by the Dipartimento delle Politiche Antidroga (Rome, Italy) through the ERANID Grant “STANDUP” awarded to F.F. and by ZonMW through the ERANID Grant “STANDUP” awarded to J.H., as well as by grants from MIUR Progetto Eccellenza.
Caffino, L. , Mottarlini, F. , Targa, G. , Verheij, M. M. M. , Homberg, J. , & Fumagalli, F. (2022). Long access to cocaine self‐administration dysregulates the glutamate synapse in the nucleus accumbens core of serotonin transporter knockout rats. British Journal of Pharmacology, 179(17), 4254–4264. 10.1111/bph.15496
Judith R. Homberg and Fabio Fumagalli share the senior authorship.
Funding information Dipartimento delle Politiche Antidroga, Italy, Grant/Award Number: STANDUP; ZonMW
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
REFERENCES
- Abdallah, C. G. , Sanacora, G. , Duman, R. S. , & Krystal, J. H. (2018). The neurobiology of depression, ketamine and rapid‐acting antidepressants: Is it glutamate inhibition or activation? Pharmacology & Therapeutics, 190, 148–158. 10.1016/j.pharmthera.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, S. H. (1998). Transition from Moderate to Excessive Drug Intake: Change in Hedonic Set Point. Science, 282(5387), 298–300. 10.1126/science.282.5387.298 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , Anderson, C. M. H. , Bröer, S. , Dawson, P. , Hagenbuch, B. , Hammond, J. R. , Hancox, J. , Inui, K. , … Verri, T . (2019). The concise guide to PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176(S1). 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , Aldrich, R. W. , Becirovic, E. , Biel, M. , Catterall, W. A. , Conner, A. C. , Davies, P. , … Zhu, M , (2019). The concise guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176(S1). 10.1111/bph.14749 [DOI] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Mangum, J. , Wonnacott, S. , & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges, R. , Lutgen, V. , Lobner, D. , & Baker, D. A. (2012). Thinking Outside the Cleft to Understand Synaptic Activity: Contribution of the Cystine‐Glutamate Antiporter (System xc−) to Normal and Pathological Glutamatergic Signaling. Pharmacological Reviews, 64(3), 780–802. 10.1124/pr.110.003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffino, L. , Mottarlini, F. , Van Reijmersdal, B. , Telese, F. , Verheij, M. M. M. , Fumagalli, F. , & Homberg, J. R. (2021). The role of the serotonin transporter in prefrontal cortex glutamatergic signaling following short‐ and long‐access cocaine self‐administration. Addiction Biology, 26(2). 10.1111/adb.12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffino, L. , Piva, A. , Giannotti, G. , Di Chio, M. , Mottarlini, F. , Venniro, M. , Yew, D. T. , Chiamulera, C. , & Fumagalli, F. (2017). Ketamine self‐administration reduces the homeostasis of the glutamate synapse in the rat brain. Molecular Neurobiology, 54, 7186–7193. 10.1007/s12035-016-0231-6 [DOI] [PubMed] [Google Scholar]
- Caffino, L. , Verheij, M. M. M. , Que, L. , Guo, C. , Homberg, J. R. , & Fumagalli, F. (2019). Increased cocaine self‐administration in rats lacking the serotonin transporter: a role for glutamatergic signaling in the habenula. Addiction Biology, 24(6), 1167–1178. 10.1111/adb.12673 [DOI] [PubMed] [Google Scholar]
- Caffino, L. , Verheij, M. M. M. , Roversi, K. , Targa, G. , Mottarlini, F. , Popik, P. , Nikiforuk, A. , Golebiowska, J. , Fumagalli, F. , & Homberg, J. R. (2020). Hypersensitivity to amphetamine's psychomotor and reinforcing effects in serotonin transporter knockout rats: Glutamate in the nucleus accumbens. British Journal of Pharmacology, 177(19), 4532–4547. 10.1111/bph.15211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, K. L. , Tseng, K. Y. , Uejima, J. L. , Reimers, J. M. , Heng, L. J. , Shaham, Y. , Marinelli, M. , & Wolf, M. E. (2008). Formation of Accumbens GluR2‐lacking AMPA receptors mediates incubation of cocaine craving. Nature, 454, 118–121. 10.1038/nature06995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish, J. L. , & Kalivas, P. W. (2000). Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. The Journal of Neuroscience, 20(15), 1–5. 10.1523/JNEUROSCI.20-15-j0006.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Sobey, C. G. , Stanford, S. C. , Teixeira, M. M. , Wonnacott, S. , & Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin, F. (1991). Cocaine addiction: psychology and neurophysiology. Science, 251(5001), 1580–1586. 10.1126/science.2011738 [DOI] [PubMed] [Google Scholar]
- Giannotti, G. , Caffino, L. , Mottarlini, F. , Racagni, G. , & Fumagalli, F. (2016). Region‐specific effects of developmental exposure to cocaine on fibroblast growth factor‐2 expression in the rat brain. Psychopharmacology, 233, 2699–2704. 10.1007/s00213-016-4315-9 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , Gray, A. J. G. , Bruce, L. , Alexander, S. P. H. , Anderton, S. , Bryant, C. , Davenport, A. P. , Doerig, C. , Fabbro, D. , Levi‐Schaffer, F. , Spedding, M. , Davies, J. A. , & Nc I . (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg, J. R. , De Boer, S. F. , Raasø, H. S. , Olivier, J. D. A. , Verheul, M. , Ronken, E. , Cools, A. R. , Ellenbroek, B. A. , Schoffelmeer, A. N. M. , Vanderschuren, L. J. M. J. , De Vries, T. J. , & Cuppen, E. (2008). Adaptations in pre‐ and postsynaptic 5‐HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats. Psychopharmacology, 200(3), 367–380. 10.1007/s00213-008-1212-x [DOI] [PubMed] [Google Scholar]
- Homberg, J. R. , Olivier, J. D. , Smits, B. M. , Mul, J. D. , Mudde, J. , Verheul, M. , Nieuwenhuizen, O. F. M. , Cools, A. R. , Ronken, E. , Cremers, T. , Schoffelmeer, A. N. M. , Ellenbroek, B. A. , & Cuppen, E. (2007). Characterization of the serotonin transporter knockout rat: A selective change in the functioning of the serotonergic system. Neuroscience, 146, 1662–1676. 10.1016/j.neuroscience.2007.03.030 [DOI] [PubMed] [Google Scholar]
- Homberg, J. R. , Wohr, M. , & Alenina, N. (2017). Comeback of the rat in biomedical research. ACS Chemical Neuroscience, 8, 900–903. 10.1021/acschemneuro.6b00415 [DOI] [PubMed] [Google Scholar]
- Ito, R. , Robbins, T. W. , & Everitt, B. J. (2004). Differential control over cocaine‐seeking behavior by nucleus accumbens core and shell. Nature Neuroscience, 7, 389–397. 10.1038/nn1217 [DOI] [PubMed] [Google Scholar]
- Karel, P. , Almacellas‐Barbanoj, A. , Prijn, J. , Kaag, A. M. , Reneman, L. , Verheij, M. M. M. , & Homberg, J. R. (2019). Appetitive to aversive counter‐conditioning as intervention to reduce reinstatement of reward‐seeking behavior: The role of the serotonin transporter. Addiction Biology, 24, 344–354. 10.1111/adb.12596 [DOI] [PubMed] [Google Scholar]
- Karel, P. , Calabrese, F. , Riva, M. , Brivio, P. , Van der Veen, B. , Reneman, L. , Verheij, M. , & Homberg, J. (2018). d‐Cycloserine enhanced extinction of cocaine‐induced conditioned place preference is attenuated in serotonin transporter knockout rats. Addiction Biology, 23, 120–129. 10.1111/adb.12483 [DOI] [PubMed] [Google Scholar]
- Knackstedt, L. A. , Moussawi, K. , Lalumiere, R. , Schwendt, M. , Klugmann, M. , & Kalivas, P. W. (2010). Extinction training after cocaine self‐administration induces glutamatergic plasticity to inhibit cocaine seeking. The Journal of Neuroscience, 30, 7984–7992. 10.1523/JNEUROSCI.1244-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob, G. F. , & Le Moal, M. (2008). Addiction and the brain antireward system. Annual Review of Psychology, 59, 29–53. 10.1146/annurev.psych.59.103006.093548 [DOI] [PubMed] [Google Scholar]
- LaCrosse, A. L. , Hill, K. , & Knackstedt, L. A. (2016). Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. European Neuropsychopharmacology, 26, 186–194. 10.1016/j.euroneuro.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch, K. P. , Bengel, D. , Heils, A. , Sabol, S. Z. , Greenberg, B. D. , Petri, S. , Benjamin, J. , Muller, C. R. , Hamer, D. H. , & Murphy, D. L. (1996). Association of anxiety‐related traits with a polymorphism in the serotonin transporter gene regulatory region. Science, 274, 1527–1531. 10.1126/science.274.5292.1527 [DOI] [PubMed] [Google Scholar]
- Lilley, E. , Stanford, S. C. , Kendall, D. E. , Alexander, S. P. H. , Cirino, G. , Docherty, J. R. , George, C. H. , Insel, P. A. , Izzo, A. A. , Ji, Y. , Panettieri, R. A. , Sobey, C. G. , Stefanska, B. , Stephens, G. , Teixeira, M. , & Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology, 177(16), 3611–3616. 10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, C. N. , LaCrosse, A. L. , & Knackstedt, L. A. (2018). Nucleus accumbens GLT‐1a overexpression reduces glutamate efflux during reinstatement of cocaine‐seeking but is not sufficient to attenuate reinstatement. Neuropharmacology, 135, 297–307. 10.1016/j.neuropharm.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon, J. E. , Wang, X. , Tseng, K. Y. , Wolf, M. E. , & Marinelli, M. (2011). Calcium‐permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self‐administration but not experimenter‐administered cocaine. The Journal of Neuroscience, 31, 5737–5743. 10.1523/JNEUROSCI.0350-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu, M. , Lapiz, M. D. , Tanaka, E. , & Grenhoff, J. (1998). Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5‐HT1B receptors. The European Journal of Neuroscience, 10, 2371–2379. 10.1046/j.1460-9568.1998.00248.x [DOI] [PubMed] [Google Scholar]
- Nonkes, L. J. , Maes, J. H. , & Homberg, J. R. (2013). Improved cognitive flexibility in serotonin transporter knockout rats is unchanged following chronic cocaine self‐administration. Addiction Biology, 18, 434–440. 10.1111/j.1369-1600.2011.00351.x [DOI] [PubMed] [Google Scholar]
- Nonkes, L. J. , van Bussel, I. P. , Verheij, M. M. , & Homberg, J. R. (2011). The interplay between brain 5‐hydroxytryptamine levels and cocaine addiction. Behavioural Pharmacology, 22, 723–738. 10.1097/FBP.0b013e32834d6260 [DOI] [PubMed] [Google Scholar]
- Ortinski, P. I. , Turner, J. R. , & Pierce, R. C. (2013). Extrasynaptic targeting of NMDA receptors following D1 dopamine receptor activation and cocaine self‐administration. The Journal of Neuroscience, 33, 9451–9461. 10.1523/JNEUROSCI.5730-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos, G. , & Watson, C. (2005). The rat brain in stereotaxic coordinates. Elsevier Academic Press. [DOI] [PubMed] [Google Scholar]
- Percie du Sert, N. , Hurst, V. , Ahluwalia, A. , Alam, S. , Avey, M. T. , Baker, M. , Browne, W. J. , Clark, A. , Cuthill, I. C. , Dirnagl, U. , Emerson, M. , Garner, P. , Holgate, S. T. , Howells, D. W. , Karp, N. A. , Lazic, S. E. , Lidster, K. , MacCallum, C. J. , Macleod, M. , … Würbel, H. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine, S. A. , Sheikh, I. S. , Nwaneshiudu, C. A. , Schroeder, J. A. , & Unterwald, E. M. (2008). Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety‐ and depression‐like behaviors in the rat. Neuropharmacology, 54, 355–364 S0028‐3908(07)00325‐5 [pii]. 10.1016/j.neuropharm.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgianto, A. , Scheyer, A. F. , Loweth, J. A. , Ford, K. A. , Tseng, K. Y. , & Wolf, M. E. (2013). Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self‐administration regimens. Neuropsychopharmacology, 38, 1789–1797. 10.1038/npp.2013.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, J. M. , & Berridge, K. C. (2011). Metabotropic glutamate receptor blockade in nucleus accumbens shell shifts affective valence towards fear and disgust. The European Journal of Neuroscience, 33, 736–747. 10.1111/j.1460-9568.2010.07553.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, B. A. , Fumagalli, F. , Gainetdinov, R. R. , Jones, S. R. , Ator, R. , Giros, B. , Miller, G. W. , & Caron, M. G. (1998). Cocaine self‐administration in dopamine‐transporter knockout mice. Nature Neuroscience, 1, 132–137. 10.1038/381 [DOI] [PubMed] [Google Scholar]
- Scofield, M. D. , Heinsbroek, J. A. , Gipson, C. D. , Kupchik, Y. M. , Spencer, S. , Smith, A. C. , Roberts‐Wolfe, D. , & Kalivas, P. W. (2016). The nucleus accumbens: Mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacological Reviews, 68, 816–871. 10.1124/pr.116.012484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield, M. D. , Li, H. , Siemsen, B. M. , Healey, K. L. , Tran, P. K. , Woronoff, N. , Boger, H. A. , Kalivas, P. W. , & Reissner, K. J. (2016). Cocaine self‐administration and extinction leads to reduced glial fibrillary acidic protein expression and morphometric features of astrocytes in the nucleus accumbens core. Biological Psychiatry, 80, 207–215. 10.1016/j.biopsych.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits, B. M. , Mudde, J. B. , van de Belt, J. , Verheul, M. , Olivier, J. , Homberg, J. , Guryev, V. , Cools, A. R. , Ellenbroek, B. A. , Plasterk, R. H. A. , & Cuppen, E. (2006). Generation of gene knockouts and mutant models in the laboratory rat by ENU‐driven target‐selected mutagenesis. Pharmacogenetics and Genomics, 16, 159–169. 10.1097/01.fpc.0000184960.82903.8f [DOI] [PubMed] [Google Scholar]
- Stefanik, M. T. , Sakas, C. , Lee, D. , & Wolf, M. E. (2018). Ionotropic and metabotropic glutamate receptors regulate protein translation in co‐cultured nucleus accumbens and prefrontal cortex neurons. Neuropharmacology, 140, 62–75. 10.1016/j.neuropharm.2018.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij, M. M. , Karel, P. , Cools, A. R. , & Homberg, J. R. (2014). Reduced cocaine‐induced serotonin, but not dopamine and noradrenaline, release in rats with a genetic deletion of serotonin transporters. European Neuropsychopharmacology, 24, 1850–1854. 10.1016/j.euroneuro.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Verheij, M. M. , Vendruscolo, L. F. , Caffino, L. , Giannotti, G. , Cazorla, M. , Fumagalli, F. , Riva, M. A. , Homberg, J. R. , Koob, G. F. , & Contet, C. (2016). Systemic delivery of a brain‐penetrant TrkB antagonist reduces cocaine self‐administration and normalizes TrkB signaling in the nucleus Accumbens and prefrontal cortex. The Journal of Neuroscience, 36, 8149–8159. 10.1523/JNEUROSCI.2711-14.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij, M. M. M. , Contet, C. , Karel, P. , Latour, J. , van der Doelen, R. H. A. , Geenen, B. , van Hulten, J. A. , Meyer, F. , Kozicz, T. , George, O. , Koob, G. F. , & Homberg, J. R. (2018). Median and dorsal raphe serotonergic neurons control moderate versus compulsive cocaine intake. Biological Psychiatry, 83, 1024–1035. 10.1016/j.biopsych.2017.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou, V. , Maze, I. , Renthal, W. , LaPlant, Q. C. , Watts, E. L. , Mouzon, E. , Ghose, S. , Tamminga, C. A. , & Nestler, E. J. (2010). Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. The Journal of Neuroscience, 30, 14585–14592. 10.1523/JNEUROSCI.2496-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, M. E. (2016). Synaptic mechanisms underlying persistent cocaine craving. Nature Reviews. Neuroscience, 17, 351–365. 10.1038/nrn.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayara, A. E. , McIver, G. , Valdivia, P. N. , Lominac, K. D. , McCreary, A. C. , & Szumlinski, K. K. (2011). Blockade of nucleus accumbens 5‐HT2A and 5‐HT2C receptors prevents the expression of cocaine‐induced behavioral and neurochemical sensitization in rats. Psychopharmacology, 213, 321–335. 10.1007/s00213-010-1996-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Uncropped immunoblot related to the expression levels of GluN2A (180 kDa; Figure 3), GluN2B (180 kDa; Figure 3), GluN1 (120 kDa; Figure 3), SAP97 (97 kDa, predicted 140 kDa; Figure 5), SAP102 (102 kDa; Figure 5), GluA1 (108 kDa; Figure 4), GluA2 (108 kDa; Figure 4), vGLUT1 (67 kDa; Figure 1), GLT‐1 (62 kDa; Figure 2) and β‐Actin (43 kDa) measured in the homogenate of the nucleus accumbens (cNAc) of SERT+/+ and SERT−/− rats exposed to short access (ShA) and long acces (LgA) cocaine self‐administration.
Table S1. F and p values of molecular data presented in the results section.
Table S2. Pearson's product–moment correlation analyses between cocaine intake and glutamatergic targets of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


