Abstract
It is important to define the accuracy of fine‐needle aspiration cytology (FNAC) in the diagnosis of Warthin tumor (WT). This systematic review and meta‐analysis evaluated the accuracy of FNAC in the diagnosis of WT in the parotid gland and WT growth rate. For determination of FNAC accuracy, 17 studies, encompassing 1710 cases, were included. Pulled random model estimates of sensitivity, specificity, PPV, and NPV were 93.7% (95%CI: 92.1, 95.3), 97.9% (95%CI: 97, 98.9), 93.3% (95%CI: 91.5, 95.1), and 97.4% (95%CI: 96.4, 98.4), respectively. FNAC is highly reliable for the diagnosis of WT of the parotid. The high PPV value suggests that patients with a cytological diagnosis of WT of the parotid may be assigned to active surveillance.
Keywords: diagnosis, fine‐needle aspiration, parotid gland, Warthin tumor
Abbreviations
- FN
false negative
- FNAC
fine‐needle aspiration cytology
- FP
false positive
- NPV
negative predictive value
- PPV
positive predictive value
- TN
true negative
- TP
true positives
- WT
Warthin tumor
1. INTRODUCTION
Warthin tumor (WT), also known as papillary cystadenoma lymphomatosum or adenolymphoma, is a benign neoplasm that arises almost exclusively in the parotid gland, which is the origin of most salivary gland tumors. It comprises 15% of all parotid tumors and is the second most frequent neoplasm in the parotid gland, after pleomorphic adenoma. 1 , 2 , 3 WT is more common in Caucasians in the 6th and 7th decades of life, smokers, and males, although a narrowing of the gender gap has recently been observed, likely due to increased smoking among women. 3 Several etiological factors have been suggested, including Epstein Barr virus (EBV) infection, autoimmune diseases, radiation, chronic inflammation, and most importantly, cigarette smoking. 1 , 2 In the last few years, there has been an increased trend in the diagnosis of WT in comparison to other parotid tumors; in some studies, WT was found to be more common than pleomorphic adenoma. One study showed that this trend cannot be explained by changes in smoking patterns. 4 , 5 , 6 The same study suggested metabolic syndrome and obesity as two central risk factors. 6 The typical clinical manifestation of WT is a painless firm swelling in the upper neck, but some cases will be asymptomatic, and others will show symptoms of facial nerve branch irritation, ear pain, tinnitus, and hearing impairment. 2 In general, WT grows slowly, and malignant transformations are rare, occurring at a rate of less than 0.1%. 3 The malignant transformation can arise from the epithelial or lymphoid cells of WT. 7 , 8 Synchronous or metachronous tumors, some of which are malignant, in proximity to WT of the parotid, occur rarely. 9 Preoperative assessment of WT with fine‐needle aspiration (FNA) cytological analysis (FNAC) typically identifies a combination of necrotic debris, lymphocytes, and oncocytic epithelial clusters. 10 , 11 , 12
Despite the reports of slow growth, some studies reported on cases in which WT doubled in size within 1 year. 13 , 14 , 15 It is therefore of prime importance to identify the patients at risk of rapid WT growth. However, the literature is scarce and does not include important demographics and clinical data. 13 , 14 , 15 Several studies evaluated the performance of FNA for the diagnosis of WT but showed considerably conflicting results. So et al. reported on 95.8% sensitivity and 97.2% positive predictive value (PPV), and Viguer et al. reported on 90.4% and 98.1%, respectively, whereas other studies demonstrated a rate of false diagnosis of about 25%–40%. 16 , 17 , 18 , 19
This systematic review and meta‐analysis considered publications that include a comparison between preoperative FNAC (index test) and postsurgical histopathological diagnosis (reference test) of WT in the parotid gland. In addition, the mean growth rate of the tumor as measured by imaging modalities was calculated.
2. METHODS
2.1. Search strategy
This systemic review and meta‐analysis followed the referred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) extension for diagnostic test accuracy guidelines. 20 A comprehensive search of PubMed and Scopus was conducted on July 25, 2021 to identify relevant publications. The search terms used were “fine needle aspiration” OR “fine needle sampling” OR “FNA,” AND “Warthin tumor” OR “adenolymphoma” OR “parotid” AND “tumor” OR “neoplasm” OR “mass” as well as “growth rate.” No date restriction was applied. To expand the search, “similar articles” function in PubMed and “related articles” function in Google Scholar were used. In addition, the reference list of selected articles was screened.
2.2. Eligibility criteria
Articles that used FNAC for the diagnosis of a parotid gland lesion and histopathological assessment for a final postoperative diagnosis were included. Cases with a nondiagnostic FNAC result were not included for the assessment of FNAC accuracy. Case reports, letters, or comments to the authors, article not in English, and cases or articles that did not address WT were excluded. To calculate WT growth rate, we included articles that diagnosed WT using histopathology or cytology. WT size evaluations were based on articles reporting on CT‐ or MRI‐based tumor size evaluation at least twice with a minimum time interval of 3 months, a measured dimension, and which mention follow‐up duration. If more than two size evaluations were available, only the first and the last were included. In cases where several articles used the same database in overlapping years, only the most recent published article was included to avoid data duplication.
2.3. Data extraction, processing, and synthesis
The following data were extracted for each case: method of needle guidance, needle size, patient characteristics, the time (years) range of the data, FNAC diagnosis, and final histopathological diagnosis. Data regarding follow‐up duration for each lesion, imaging modality used, and initial and final size of WT were also extracted.
The following parameters were calculated: true positives (TP): FNAC and histopathological diagnosis is WT; false positive (FP): FNAC result is WT, but histopathological diagnosis is not; false negative (FN): FNAC result is a different lesion (not WT), but histopathological result is WT; true negative (TN): both FNAC and histopathology results are not WT. Our goal was to conduct meta‐analysis of individual FNAC estimates in the diagnosis of WT; for this reason, all the included studies contain cases of FP, FN, and TP and some studies also contain TN cases. Studies that lack case of FP, FN, or TP were excluded. All the false positive and false negative results were classified as malignant, benign, or normal. Cytodiagnostic or histopathologic results that were classified as “probably malignant,” “suspected malignant,” “suspicious for malignancy,” “cannot exclude malignancy” were included in the malignant group.
2.4. Quality assessment
The included studies were assessed for quality of methodology based on the Diagnostic Accuracy Research Quality Assessment‐2 (QUADAS‐2) tool. 21 The risk of bias was rated as “low,” “high,” or “unclear,” corresponding to a score of “2,” “1,” and “0,” respectively. A study awarded a cumulative score ≥6 was considered of high quality.
2.5. Statistical analysis
IBM SPSS Statistics for Windows, Version 27.0 was used for data analysis. R software and related packages were used for the meta‐analysis. The pooled sensitivity, specificity, PPV, and NPV were calculated to assess the diagnostic value of FNAC. Mean percent diameter change for the entire population and percent diameter change for subgroups were calculated to assess WT growth rate. Dependent variables were assessed for normality using the Kolmogorov–Smirnov test and by graphically comparing frequencies distribution to bell shape. The T test was used to compare the means of subgroups; p‐value <0.05 was considered statistically significant. Between‐study heterogeneity was evaluated using the Cochran Q test and the Higgins I square test, where I 2 > 50% indicates statistically significant heterogeneity. Random and fixed models were used for pooled estimates. Publication bias was evaluated by visual inspection of the symmetry of the funnel plot.
3. RESULTS
The literature search yielded 451 records, of which 17 articles met the inclusion criteria (Figure 1). Table 1 presents key study design elements of all the included articles. The reports were published between 1996 and 2019, conducted in 12 countries, and encompassed a total of 1710 cases, with study samples sizes ranging between 5 and 223 cases. Needle type and needle guidance technique were mentioned occasionally, patient sex was reported in 9 studies, and patient age was reported in 10 studies. The years range of presented data was unavailable for two articles. All the studies were retrospective and involved a medical records database search.
FIGURE 1.
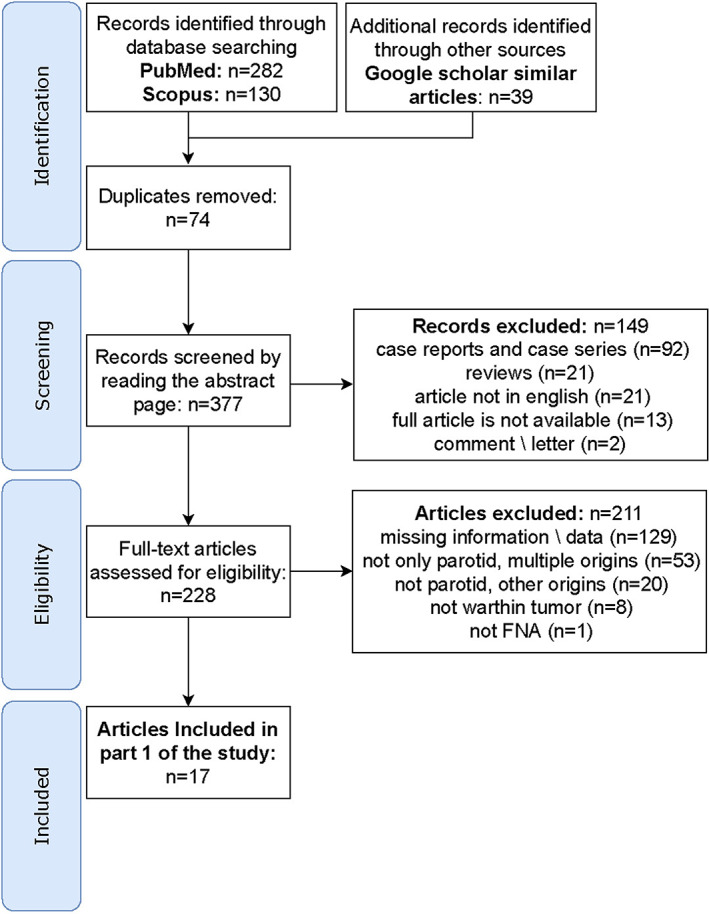
Flow diagram for identification of studies for assessment of fine‐needle aspiration cytology accuracy in diagnosing Warthin tumor in the parotid gland [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Summary of studies reviewed to determine fine‐needle aspiration cytology accuracy
| Article, year published | Years range | Location | Study design | Needle type | Needle guidance | Age (range) | Males |
|---|---|---|---|---|---|---|---|
| Al‐Khafaji et al. 27 | 1986–1996 | USA | Retrospective | N/A | N/A | Mean: 56 (5–90) | 50.6% |
| Altin et al. 16 | 2008–2017 | Turkey | Retrospective | 23 G | N/A | Mean: 47.5 (7–82) | 54.6% |
| Atula et al. 28 | 1984–1991 | Finland | Retrospective | 23 G | N/A | Unknown | Unknown |
| Edizer et al. 29 | 2005–2013 | Turkey | Retrospective | 23 or 25 G | US | Unknown | Unknown |
| Huang et al. 30 | N/A | Taiwan | Retrospective | N/A | US | Unknown | Unknown |
| Jafari et al. 31 | 2000–2006 | France | Retrospective | 27 G | US or palpation | Mean: 50.5 (17–87) | 60% |
| Jayaram et al. 32 | N/A | Malaysia | Retrospective | 22 G | N/A | Unknown | Unknown |
| Jechova et al. 33 | 2006–2016 | Czechia | Retrospective | N/A | US | Median: 57 (12–96) | 42.6% |
| Raymond et al. 34 | 1992–2000 | Canada | Retrospective | N/A | N/A | Mean: 60.2 (14–88) | 1.4:1 |
| So et al. 18 | 2006–2017 | Canada | Retrospective | N/A | N/A | Mean: 63.2 (SD 10.4) | Unknown |
| Suzuki et al. 35 | 1999–2017 | Japan | Retrospective | 21 or 22 G | US or free hand technique | Unknown | Unknown |
| Zabren et al. 19 | 1990–1998 | Switzerland | Retrospective | 22 G | N/A | Mean: 55 (16–97) | 46% |
| Akbas et al. 36 | 1994–2000 | Turkey | Retrospective | 25 G | US | Unknown | Unknown |
| Behzatoglu et al. 37 | 1997–2002 | Turkey | Retrospective | 22 G | N/A | Mean: 44 (12–80) | 54.6% |
| Ali et al. 38 | 2002–2010 | Pakistan | Retrospective | 22 G | Free hand technique | Mean: 44 (15–78) | 56.5% |
| Riley et al. 39 | 1996–2000 | New Zealand | Retrospective | 24 G | N/A | Unknown | Unknown |
| Weinberger et al. 40 | 1985–1989 | USA | Retrospective | 22 G | Free hand technique | Mean: 57 (SD 12.9) | 77.7% |
Abbreviations: N/A, not available; US, ultrasound.
In seven studies, the TN rate was not reported, as the publication focused on evaluation of the concordance between FNA and histopathology in the diagnosis of WT. Study data, individual diagnostic estimates and pooled estimates are summarized in Table 2. Individual and pooled estimates are also presented in a forest plot in Figures 2 and 3. The pooled sensitivity and PPV were calculated based on cases from 17 studies. The random effects model of the 17 studies showed a pooled sensitivity of 93.7% (95%CI: 92.1, 95.3) and pooled PPV of 93.3% (95%CI: 91.5, 95.2). Pooled specificity and NPV were calculated based on the 10 studies in which TN data were reported. The random effects model of these 10 studies showed a pooled specificity of 97.9% (95%CI: 97, 98.9) and a pooled NPV of 97.4% (95%CI: 96.4, 98.4). Heterogeneity assessments showed that PPV and specificity estimates were homogenous (Q = 20.2, p = 0.210, I 2 = 20.8 for PPV, and Q = 10.9, p = 0.282, I 2 = 17.4% for specificity). NPV and sensitivity estimates were found to be heterogenous (Q = 74.3, p < 0.0001, I 2 = 78.5% for sensitivity, and Q = 32.3, p = 0.0002, I 2 = 72.1% for NPV). Visual assessment of the funnel plots for each of the four FNAC estimates showed no asymmetrical distribution (Figure 4). All included studies were of high quality (Figure 5).
TABLE 2.
Summary of the fine‐needle aspiration accuracy analysis
| Article | NPV | PPV | Specificity | Sensitivity | TN | FN | FP | TP |
|---|---|---|---|---|---|---|---|---|
| Al‐Khafaji et al. 27 | 97.7 | 83.3 | 97.7 | 83.3 | 129 | 3 | 3 | 15 |
| Altin et al. 16 | 93.3 | 78.6 | 93.8 | 71.7 | 137 | 13 | 9 | 33 |
| Atula et al. 28 | 68.3 | 87.5 | 13 | 4 | 28 | |||
| Edizer et al. 29 | 93.0 | 95.2 | 3 | 2 | 40 | |||
| Huang et al. 30 | 78.3 | 74.2 | 90.6 | 88.5 | 29 | 8 | 3 | 23 |
| Jafari et al. 31 | 100 | 91.7 | 96.7 | 100 | 59 | 0 | 2 | 22 |
| Jayaram et al. 32 | 100.0 | 80.0 | 1 | 0 | 4 | |||
| Jechova et al. 33 | 93.1 | 96.6 | 7 | 15 | 201 | |||
| Raymond et al. 34 | 89.2 | 89.2 | 4 | 4 | 33 | |||
| So et al. 18 | 97.2 | 95.8 | 3 | 2 | 69 | |||
| Suzuki et al. 35 | 94.5 | 92.6 | 11 | 8 | 137 | |||
| Zbaren et al. 19 | 91.2 | 84.4 | 97 | 62.8 | 167 | 15 | 5 | 27 |
| Akbas et al. 36 | 100 | 94.7 | 98.4 | 100.0 | 62 | 0 | 1 | 18 |
| Behzatoglu et al. 37 | 98.4 | 100 | 100 | 75.0 | 63 | 1 | 0 | 3 |
| Ali et al. 38 | 98.2 | 90.0 | 99.1 | 81.8 | 111 | 2 | 1 | 9 |
| Riley et al. 39 | 95.7 | 66.7 | 97.8 | 50.0 | 90 | 4 | 2 | 4 |
| Weinberger et al. 40 | 90.4 | 60.0 | 95 | 42.9 | 38 | 4 | 2 | 3 |
| Total (1710 cases) | 885 | 93 | 63 | 669 | ||||
| Pooled value [95% CI] | ||||||||
| Random effects model | 97.4 [96.4, 98.4] | 93.3 [91.5, 95.2] | 97.9 [97.0, 98.9] | 93.7 [92.1, 95.3] | ||||
| Fixed effect model | 94.0 [92.1, 95.8] | 86.6 [81.8, 91.4] | 96.5 [95.0, 98.0] | 79.5 [74.4, 84.6] | ||||
Abbreviations: FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; TN, true negative, TP, true positive.
FIGURE 2.
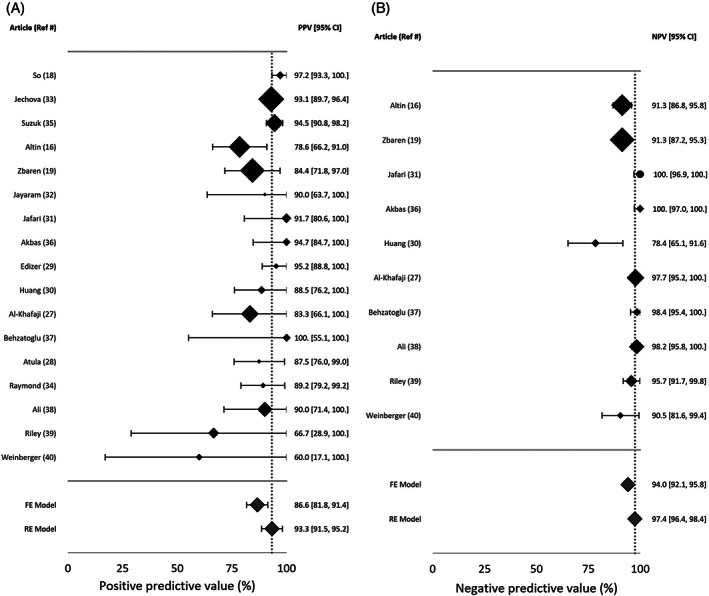
Forest plot of (A) positive predictive value (PPV) and (B) negative predictive value (NPV). Dashed line depicts value of random model estimate. FE, fixed model estimate; RE, random model estimate
FIGURE 3.
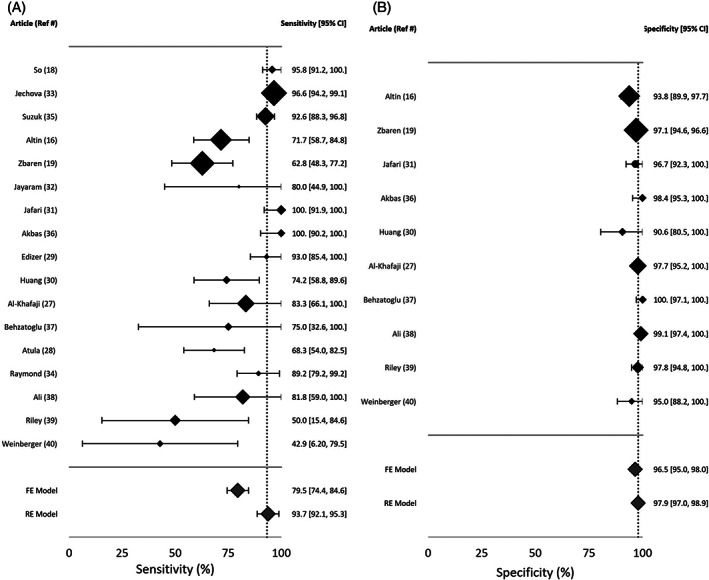
Forest plot of (A) sensitivity and (B) specificity. Dashed line depicts value of random model estimate. FE, fixed model estimate; RE, random model estimate
FIGURE 4.
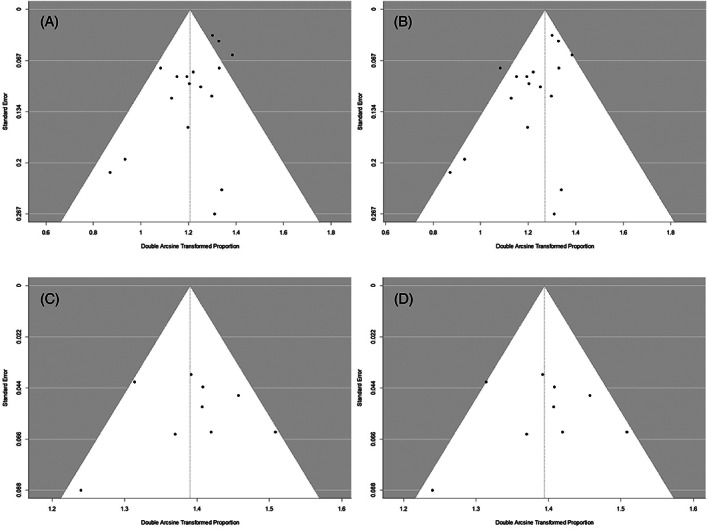
Funnel plots for assessment of publication bias. Each point represents a separate study. (A) PPV, (B) sensitivity, (C) specificity, (D) NPV. Horizontal axis represents the effect of the studies, vertical axis represents study size, vertical dashed line indicates effect summary, white triangle shape depicts the values extending 1.96 standard errors around the effect summary, this area should include 95% of studies. Studies with a larger sample size and hence, higher precision, are located at the top, studies with higher estimates are located at the right. When publication bias occurs, one expects asymmetry in the scatter around the effect summary, with more studies showing a positive as opposed to a negative result. NPV, negative predictive value
FIGURE 5.
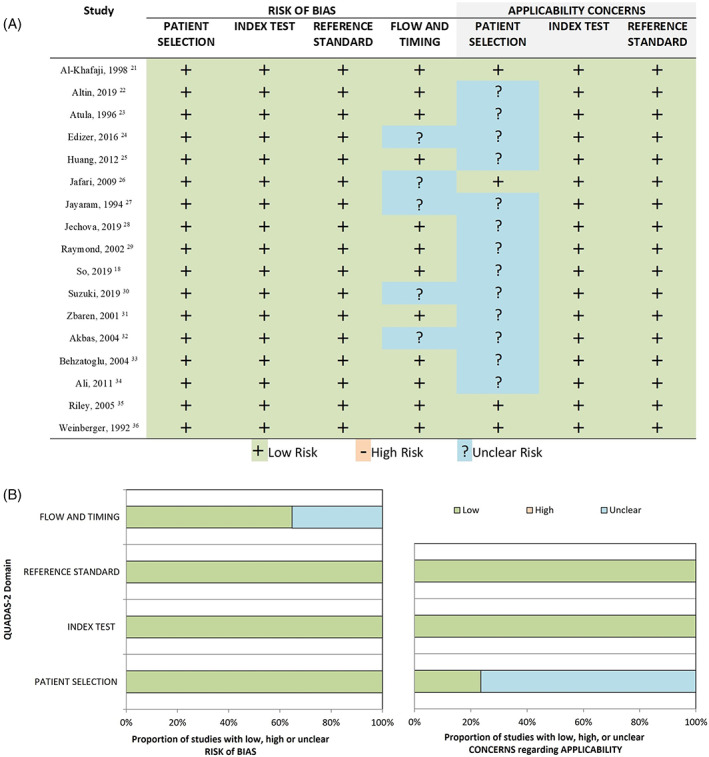
Risk‐of‐bias and applicability concerns summary for each domain of the Diagnostic Accuracy Research Quality Assessment (QUADAS‐2) for each included study. (A) Risk‐of‐bias table. (B) Risk‐of‐bias graph [Color figure can be viewed at wileyonlinelibrary.com]
A summary of all cases falsely diagnosed by FNAC is presented in Figure 6. The total FP rate was 3.6% (63 out of 1710 patients), and the FP rate of malignant tumors was 2% (35 out of 1710 patients). When considering all positive FNAC results (n = 732), the rate of malignant FP was 4.7% (35 out of 732 patients). Most of the cases in the FP category were classified as malignant (55.5%, n = 35), and the leading FP diagnosis was adenoid cystic carcinoma (n = 12), followed by mucoepidermoid carcinoma (n = 11). The total FN rate was 5.4% (93 out of 1710 patients); 21 (22.5%) were classified as malignant.
FIGURE 6.
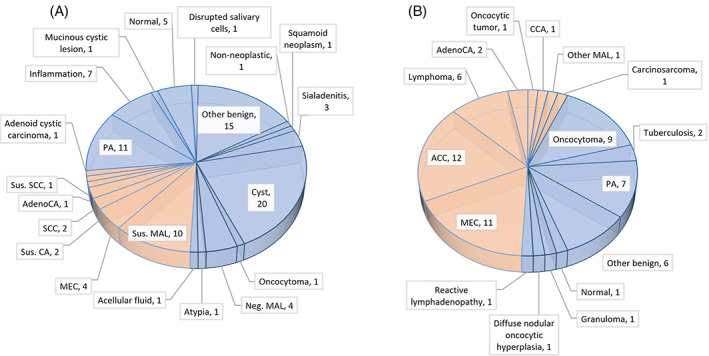
False negative (A) and false positive (B) results of fine‐needle aspiration cytology diagnosis of Warthin tumor of the parotid gland in 17 studies. Blue and orange colors represent benign + normal and malignant cases, respectively. ACC, acinic cell carcinoma; adenoCA, adenocarcinoma; CCA, cribriform cystadenocarcinoma; DBCL, diffuse B cell lymphoma; MEC, mucoepidermoid carcinoma; Neg. MAL, negative for malignancy; PA, pleomorphic adenoma; SCC, squamous cell carcinoma; Sus. CA, suspicion of carcinoma; Sus. MAL, suspicion of malignancy; Sus. SCC, suspicion of squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This systematic review and meta‐analysis evaluated the accuracy of FNAC in the diagnosis of WT and investigated WT growth rate. The study found FNAC to have a high specificity (97.9% [95%CI: 97, 98.9]), and PPV (93.3% [95%CI: 91.5, 95.1]), yet a variable sensitivity (93.7% [95%CI: 92.1, 95.3]) and NPV (97.4% [95%CI: 96.4, 98.4]). Although FNAC is highly specific in the diagnosis of WT, the review found that 35/732 (4.7%) positive results proved malignant is postoperative histopathology, hence, patients choosing an observational approach based on preoperative FNAC WT diagnosis should be followed up with caution. False FNAC results involving WT is a well‐known phenomenon. 22 The falsely diagnosed FNAC cases may be the result of sampling error, when WT cysts with acellular fluid are sampled. 12 In addition, WT oncocytes tend to undergo necrosis and to change to squamous or mucinous epithelium which may lead to diagnosis of a malignant tumor. 12 WT necrosis can also cause cyst spillage and subsequent inflammation and reactive changes, thus challenging cytodiagnosis. 10 Other sources of sampling errors are mixed tumors of WT and synchronous benign and malignant lesions. 9 Both FN and FP cases may harbor malignant tumors and should raise concern of progression of malignant disease. Yet, characteristics of these cases were not available for review; thus, future research is warranted to identify the features of falsely diagnosed cases. When considering the overall high PPV, a positive diagnosis of WT by FNAC can be a reasonable option in selected cases with close follow‐up.
The cited malignant transformation rate of WT tumors diagnosed by histopathology is 0.1%. 3 Yet, in the case of FNAC diagnosis, false results are mostly due to a sampling error. Although malignant transformation can contribute to lead to a false diagnosis, we think it has a small effect overall. Additionally, cases of malignant transformation were not included in the review, and this subject is beyond the scope of the current study.
We chose not to present data regarding WT growth rate in this review. The data obtained in appraised publications were very heterogeneous. Moreover, factors influencing growth are many and unknown, all of which may lead to imprecise results. We suggest that futures studies conduct more comprehensive, three‐dimensional size assessments of WT.
5. LIMITATION
This review had several limitations. Articles reviewed to determine FNA accuracy were retrospective in nature, and some had a small sample size. Another limitation was that sensitivity and NPV showed a high degree of heterogeneity. Cytodiagnosis terminology used when assessing the salivary glands has some variability between medical centers, which might have been even more pronounced in articles published before 2015, before the Milan system was developed. 23 Given the variability in reported FN and FP malignancy rates and established by this review, it is important that physicians be familiar with the institutional rate for malignancy when FNAC fails to diagnose correctly. This is key for advising the patient and informing them adequately for decision making. FNAC results are impacted by many factors, including collection method, physician FNA training, freehand versus ultrasound‐guided technique, and pathologist versus physician performed FNA. Most of the included studies failed to adequately report on these factors. 24 , 25 , 26
6. CONCLUSIONS
To the best of our knowledge, this is the first systematic review and meta‐analysis of diagnostic accuracy of WT of the parotid gland. The study found that FNAC has high performance in the diagnosis of WT at this site. Although FP results were not common, most turned out to be malignant. The overall high PPV value suggests that selected patients with a cytological diagnosis of WT of the parotid can be assigned to active surveillance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGMENTS
We wish to thank Mr. Basem Hijazi from the Azrieli Faculty of Medicine, Bar Ilan University, for the statistical analysis and Galilee‐CBR for editorial assistance. The article was written as part of the requirements of the Azrieli Faculty of Medicine, Bar‐Ilan University, Safed, Israel, for an MD degree of Roie Fisher.
Fisher R, Ronen O. Cytologic diagnosis of parotid gland Warthin tumor: Systematic review and meta‐analysis . Head & Neck. 2022;44(10):2277‐2287. doi: 10.1002/hed.27099
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhan KY, Khaja SF, Flack AB, Day TA. Benign parotid tumors. Otolaryngol Clin North Am. 2016;49(2):327‐342. doi: 10.1016/j.otc.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 2. Teymoortash A, Krasnewicz Y, Werner JA. Clinical features of cystadenolymphoma (Warthin's tumor) of the parotid gland: a retrospective comparative study of 96 cases. Oral Oncol. 2006;42(6):569‐573. doi: 10.1016/j.oraloncology.2005.10.017 [DOI] [PubMed] [Google Scholar]
- 3. Faur A, Lazǎr E, Cornianu M, Dema A, Vidita CG, Gǎluşcan A. Warthin tumor: a curious entity—case reports and review of literature. Rom J Morphol Embryol. 2008;50(2):269‐273. [PubMed] [Google Scholar]
- 4. Luers JC, Guntinas‐Lichius O, Klussmann JP, Küsgen C, Beutner D, Grosheva M. The incidence of Warthin tumours and pleomorphic adenomas in the parotid gland over a 25‐year period. Clin Otolaryngol. 2016;41(6):793‐797. doi: 10.1111/coa.12694 [DOI] [PubMed] [Google Scholar]
- 5. Tunç O, Gönüldaş B, Arslanhan Y, Kanlıkama M. Change in Warthin's tumor incidence: a 20‐year joinpoint trend analysis. Eur Arch Otorhinolaryngol. 2020;277(12):3431‐3434. doi: 10.1007/s00405-020-06081-w [DOI] [PubMed] [Google Scholar]
- 6. Psychogios G, Vlastos I, Thölken R, Zenk J. Warthin's tumour seems to be the most common benign neoplasm of the parotid gland in Germany. Eur Arch Otorhinolaryngol. 2020;277(7):2081‐2084. doi: 10.1007/s00405-020-05894-z [DOI] [PubMed] [Google Scholar]
- 7. Alnoor F, Gandhi JS, Stein MK, Gradowski JF. Follicular lymphoma diagnosed in Warthin tumor: a case report and review of the literature. Head Neck Pathol. 2020;14(2):386‐391. doi: 10.1007/s12105-019-01045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JE, Kim TG. Squamous cell carcinoma arising from Warthin's tumor in the parotid gland. BJR Case Rep. 2019;5(4):20190032. doi: 10.1259/bjrcr.20190032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Srivastava S, Nadelman C. Synchronous ipsilateral warthin tumor encased by a separate mucoepidermoid carcinoma of the parotid gland: a case report and review of the literature. Diagn Cytopathol. 2010;38(7):533‐537. doi: 10.1002/dc.21267 [DOI] [PubMed] [Google Scholar]
- 10. Cobb CJ, Greaves TS, Raza AS. Fine needle aspiration cytology and diagnostic pitfalls in Warthin's tumor with necrotizing granulomatous inflammation and facial nerve paralysis: a case report. Acta Cytol. 2009;53(4):431‐434. doi: 10.1159/000325346 [DOI] [PubMed] [Google Scholar]
- 11. Manucha V, Gonzalez MF, Akhtar I. Impact of the Milan system for reporting salivary gland cytology on risk assessment when used in routine practice in a real‐time setting. J Am Soc Cytopathol. 2021;10(2):208‐215. doi: 10.1016/j.jasc.2020.08.005 [DOI] [PubMed] [Google Scholar]
- 12. Parwani A, Ali SZ. Diagnostic accuracy and pitfalls in fine‐needle aspiration interpretation of Warthin tumor. Cancer. 2003;99(3):166‐171. doi: 10.1002/cncr.11207 [DOI] [PubMed] [Google Scholar]
- 13. Schwalje AT, Uzelac A, Ryan WR. Growth rate characteristics of Warthin's tumours of the parotid gland. Int J Oral Maxillofac Surg. 2015;44(12):1474‐1479. doi: 10.1016/j.ijom.2015.07.019 [DOI] [PubMed] [Google Scholar]
- 14. Mantsopoulos K, Goncalves M, Koch M, Iro H. Watchful waiting in carefully selected metachronous cystadenolymphomas of the parotid gland: a reliable option? Br J Oral Maxillofac Surg. 2019;57(5):425‐429. doi: 10.1016/j.bjoms.2018.12.018 [DOI] [PubMed] [Google Scholar]
- 15. Seok J, Jeong WJ, Ahn SH, Jung YH. The growth rate and the positive prediction of needle biopsy of clinically diagnosed Warthin's tumor. Eur Arch Otorhinolaryngol. 2019;276(7):2091‐2096. doi: 10.1007/s00405-019-05493-7 [DOI] [PubMed] [Google Scholar]
- 16. Altin F, Alimoglu Y, Acikalin RM, Yasar H. Is fine needle aspiration biopsy reliable in the diagnosis of parotid tumors? Comparison of preoperative and postoperative results and the factors affecting accuracy. Braz J Otorhinolaryngol. 2019;85:275‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viguer JM, Vicandi B, Jiménez‐Heffernan JA, López‐Ferrer P, González‐Peramato P, Castillo C. Role of fine needle aspiration cytology in the diagnosis and management of Warthin's tumour of the salivary glands. Cytopathology. 2010;21(3):164‐169. doi: 10.1111/j.1365-2303.2009.00667.x [DOI] [PubMed] [Google Scholar]
- 18. So T, Sahovaler A, Nichols A, et al. Utility of clinical features with fine needle aspiration biopsy for diagnosis of Warthin tumor. J Otolaryngol Head Neck Surg. 2019;48(1):1‐5. doi: 10.1186/s40463-019-0366-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zbaren P, Schr C, Hotz MA, Loosli H. Value of fine‐needle aspiration cytology of parotid gland masses. Laryngoscope. 2001;111:1989‐1992. [DOI] [PubMed] [Google Scholar]
- 20. McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta‐analysis of diagnostic test accuracy studies the PRISMA‐DTA statement. JAMA. 2018;319(4):388‐396. doi: 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 21. Reitsma JB, Leeflang MMG, Sterne JAC, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(4):529‐536. [DOI] [PubMed] [Google Scholar]
- 22. Sučić M, Ljubić N, Perković L, et al. Cytopathology and diagnostics of Warthin's tumour. Cytopathology. 2020;31(3):193‐207. doi: 10.1111/cyt.12830 [DOI] [PubMed] [Google Scholar]
- 23. Barbarite E, Puram SV, Derakhshan A, Rossi ED, Faquin WC, Varvares MA. A call for universal acceptance of the Milan system for reporting salivary gland cytopathology. Laryngoscope. 2020;130(1):80‐85. doi: 10.1002/lary.27905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ganguly A, Burnside G, Nixon P. A systematic review of ultrasound‐guided FNA of lesions in the head and neck‐focusing on operator, sample inadequacy and presence of on‐spot cytology service. Br J Radiol. 2014;87(1044):20130571. doi: 10.1259/bjr.20130571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine‐needle aspiration biopsy is determined by physician training in sampling technique. Cancer. 2001;93(4):263‐268. doi: 10.1002/cncr.9040 [DOI] [PubMed] [Google Scholar]
- 26. Pinki P, Alok D, Ranjan A, Chand MN. Fine needle aspiration cytology versus fine needle capillary sampling in cytological diagnosis of thyroid lesions. Iran J Pathol. 2015;10(1):47‐53. doi: 10.7508/ijp.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al‐Khafaji BM, Nestok BR, Katz RL. Fine‐needle aspiration of 154 parotid masses with histologic correlation: ten‐year experience at the University of Texas M. D. Anderson Cancer Center. Cancer. 1998;84:153‐159. [PubMed] [Google Scholar]
- 28. Atula T, Grénman R, Laippala P, Klemi P. Fine‐needle aspiration biopsy in the diagnosis of parotid gland lesions: evaluation of 438 biopsies. Diagn Cytopathol. 1996;15(3):185‐190. [DOI] [PubMed] [Google Scholar]
- 29. Edizer DT, Server EA, Yigit O, Yildiz M. Role of fine‐needle aspiration biopsy in the management of salivary gland masses. Turk Arch Otolarengol. 2016;54(3):105‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang YC, Wu CT, Lin G, Chuang WY, Yeow KM, Wan YL. Comparison of ultrasonographically guided fine‐needle aspiration and core needle biopsy in the diagnosis of parotid masses. J Clin Ultrasound. 2012;40:189‐194. [DOI] [PubMed] [Google Scholar]
- 31. Jafari A, Royer B, Lefevre M, Corlieu P, Périé S, St Guily JL. Value of the cytological diagnosis in the treatment of parotid tumors. Otolaryngol Head Neck Surg. 2009;140(3):381‐385. doi: 10.1016/j.otohns.2008.10.032 [DOI] [PubMed] [Google Scholar]
- 32. Jayaram G, Verma AK, Sood N, Khurana N. Fine needle aspiration cytology of salivary gland lesions. J Oral Pathol Med. 1994;23(6):256‐261. doi: 10.1111/j.1600-0714.1994.tb00055.x [DOI] [PubMed] [Google Scholar]
- 33. Jechova A, Kuchar M, Novak S, et al. The role of fine‐needle aspiration biopsy (FNAB) in Warthin tumour diagnosis and management. Eur Arch Otorhinolaryngol. 2019;276(10):2941‐2946. doi: 10.1007/s00405-019-05566-7 [DOI] [PubMed] [Google Scholar]
- 34. Raymond MR, Yoo JH, Heathcote JG, McLachlin CM, Lampe HB. Accuracy of fine‐needle aspiration biopsy for Warthin's tumours. J Otolaryngol. 2002;31(5):263‐270. doi: 10.2310/7070.2002.34289 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki M, Kawata R, Higashino M, et al. Values of fine‐needle aspiration cytology of parotid gland tumors: a review of 996 cases at a single institution. Head Neck. 2019;41(2):358‐365. doi: 10.1002/hed.25503 [DOI] [PubMed] [Google Scholar]
- 36. Akbaş Y, Tuna EU, Demireller A, Ozcan H, Ekinci C. Ultrasonography guided fine needle aspiration biopsy of parotid gland masses. Kulak Burun Bogaz Ihtis Derg. 2004;13:15‐18. [PubMed] [Google Scholar]
- 37. Behzatoğlu K, Bahadir B, Kaplan HH, Yücel Z, Durak H, Bozkurt ER. Fine needle aspiration biopsy of the parotid gland: diagnostic problems and 2 uncommon cases. Acta Cytol. 2004;48(2):149‐154. doi: 10.1159/000326308 [DOI] [PubMed] [Google Scholar]
- 38. Ali NS, Akhtar S, Junaid M, Awan S, Aftab K. Diagnostic accuracy of fine needle aspiration cytology in parotid lesions. ISRN Surg. 2011;2011:1‐5. doi: 10.5402/2011/721525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Riley N, Allison R, Stevenson S. Fine‐needle aspiration cytology in parotid masses: our experience in Canterbury, New Zealand. ANZ J Surg. 2005;75(3):144‐146. doi: 10.1111/j.1445-2197.2005.03331.x [DOI] [PubMed] [Google Scholar]
- 40. Weinberger MS, Rosenberg WW, Meurer WT, Robbins KT. Fine‐needle aspiration of parotid gland lesions. Head Neck. 1992;14(6):483‐487. doi: 10.1002/hed.2880140611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


