Abstract
Premise
Leaf lobing and leaf size vary considerably across and within species, including among grapevines (Vitis spp.), some of the best‐studied leaves. We examined the relationship between leaf lobing and leaf area across grapevine populations that varied in extent of leaf lobing.
Methods
We used homologous landmarking techniques to measure 2632 leaves across 2 years in 476 unique, genetically distinct grapevines from five biparental crosses that vary primarily in the extent of lobing. We determined to what extent leaf area explained variation in lobing, vein length, and vein to blade ratio.
Results
Although lobing was the primary source of variation in shape across the leaves we measured, leaf area varied only slightly as a function of lobing. Rather, leaf area increases as a function of total major vein length, total branching vein length, and vein to blade ratio. These relationships are stronger for more highly lobed leaves, with the residuals for each model differing as a function of distal lobing.
Conclusions
For leaves with different extents of lobing but the same area, the more highly lobed leaves have longer veins and higher vein to blade ratios, allowing them to maintain similar leaf areas despite increased lobing. These findings show how more highly lobed leaves may compensate for what would otherwise result in a reduced leaf area, allowing for increased photosynthetic capacity through similar leaf size.
Keywords: ampelography, grapevine, leaf morphology, leaf shape, Vitis
Each leaf on a plant is shaped by genetics, the environment, and development, which all interact to contribute to variation in form and size (Chitwood and Sinha, 2016). Across diverse species, paleobotanists have long‐recognized a relationship between leaf teeth and climate. In particular, among woody angiosperms, more‐toothed species are significantly negatively correlated with mean annual temperature (Bailey and Sinnott, 1916; Wilf, 1997). These relationships have been confirmed in extant populations (Peppe et al., 2011; Schmerler et al., 2012; Wright et al., 2017).
Why variation in leaf form exists across different climates remains is an ongoing area of study, with many possible explanations (Nicotra et al., 2011; Edwards et al., 2016). For example, toothed species examined in a colder region had higher transpiration and photosynthate production early in the growing season in comparison to the untoothed species. As a result, this mechanism could provide an advantage to the toothed species by allowing them to maximize the duration of their growing season (Royer and Wilf, 2006). Teeth may also serve as hydathodes, expelling water in early spring in temperate species and avoiding mesophyll flooding due to root pressure (Feild et al., 2005). Additional explanations include that temperate leaves are thinner and rely on structural support from veins, forming a wedge shape and leading to a toothy margin (Givnish, 1979), or that highly dissected leaves reduce feeding efficiency by herbivores (Brown and Lawton, 1991). The shape of the temperate leaf may also be due to selection on leaf primordia inside overwintering buds, with the bounded space resulting in physical pressures influencing the adult leaf form (Edwards et al., 2016). Indeed, similar shapes can arise for many reasons, and the explanations behind the variations across climates may be a combination of these reasons and others.
In addition to leaf serrations or teeth, leaf size varies considerably, with an over 100,000‐fold difference in leaf size among species worldwide (Díaz et al., 2016; Wright et al., 2017). Similar to variation in teeth, leaf size varies with climate, with larger leaves generally found in wetter, warmer areas, the same zones where less‐toothed leaves are found (Webb, 1968; Peppe et al., 2011; Chitwood and Sinha, 2016). For example, in a study of the Australian rainforest, leaves were found to be smaller with a reduction in rainfall (Webb, 1968). However, variation in leaf size has trade‐offs: larger leaves have a thicker boundary layer of still air, which slows heat loss and may increase respiration rates more than photosynthesis rates. Additional water is required to cool the leaf by transpiration. At the same time, larger leaves have more photosynthetic potential due to their larger surface area. Thus, when access to water decreases, smaller leaves are favored (Givnish, 1987; Westoby et al., 2002).
In recent efforts to estimate leaf area, accurate estimates required a specific correction factor for leaf shape in addition to leaf length and width. The correction factor differed depending on the extent of lobing of the leaf, indicating that leaves differed allometrically—differences in size were correlated with other differences in shape, in this case, lobing. More highly lobed leaves had smaller leaf areas in comparison to unlobed leaves with the same length and width (Schrader et al., 2021).
Many plant leaves have traits with allometric relationships that occur as a result of lobing and other aspects of leaf shape. For example, across leaves measured from 869 apple (Malus spp.) accessions differences in the length‐to‐width ratio were the primary source of variation, with variation in the width of the blade, not the length, being significantly correlated with variation in leaf shape (Migicovsky et al., 2018). Allometric relationships for the length‐to‐width ratios of leaves have also been described in numerous other plant species, including Passiflora (Chitwood and Otoni, 2017), Solanum (Chitwood et al., 2013), and Vitis (Klein et al., 2017). A recent study of Vitis species measured across 4 years and multiple nodes of development identified vein to blade ratio as an allometric variation of leaf size. As leaf size increased, the proportion of the leaf area composed of blade exponentially increased, while the proportion composed of vein area decreased (Chitwood et al., 2021).
Among the most well‐studied leaves are those belonging to grapevine species (Vitis), with an entire field of study, ampelography (“vine” + “writing”), dedicated to their study. Ampelography was first described by Louis Ravaz (1902) and brought to widespread attention for its use in wine grapes by Pierre Galet (1979). The use of ampelography has continued in contemporary times through the use of comprehensive morphometric techniques, in particular homologous landmarks (Chitwood et al., 2014, 2016a; Klein et al., 2017; Chitwood, 2021; Harris et al., 2021).
Homologous landmarking is based on the shared morphology across grapevine leaves: (1) five major veins—a midvein, two distal veins, and two proximal veins, each of which terminate at the tip of a lobe; (2) six major branching veins—two petiolar veins that branch off of proximal veins, two veins that branch off distal veins, and two that branch off of each side of the midvein (Figure 1A). Despite numerous studies characterizing the shape of grapevine leaves across species (Chitwood et al., 2016a), development (Chitwood et al., 2016a), and years (Chitwood et al., 2016b, 2021), questions remain. In grapevine, lobing is a major source of variation in leaf shape (Galet, 1979; Chitwood, 2021). Lobing permits sunlight to permeate the canopy of the grapevine, which provides desirable benefits to grape growers, but if more highly lobed leaves have a smaller area, then the photosynthetic capacity of the vine would be reduced.
Figure 1.
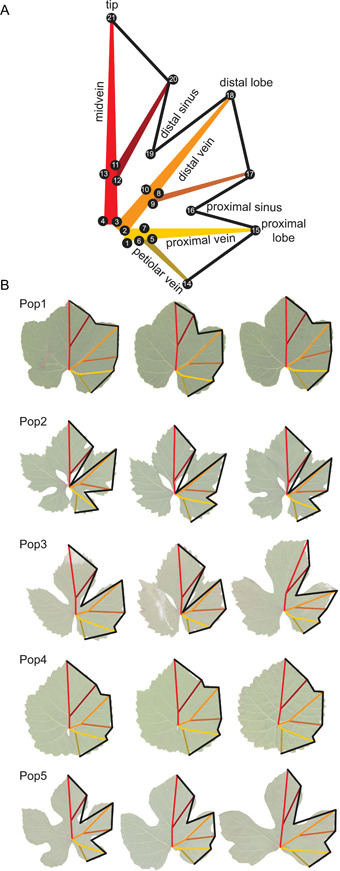
(A) Schematic diagram of 21 landmarks used in this study to measure leaf shape and size. Major veins are labeled, with branching veins indicated by darker shades. Numbers indicate the order the landmarks were placed. (B) Shape information captured by the 21 landmarks across each of the five populations examined in this study. Three leaves from a single vine are shown from each population. Given the range of lobing within an individual population (Appendix S4), the leaves shown are not representative of an individual population, but rather, the variation in lobing across all populations.
Here, we measured 2632 leaves across 2 years in 476 unique, genetically distinct grapevine accessions from five biparental crosses that vary primarily in the extent of lobing. We demonstrate that leaves of varying sizes do not differ in lobing, but rather, more highly lobed leaves with the same total leaf area as less‐lobed leaves show increases in leaf vein length and vein to blade ratio. These findings provide evidence for a mechanism in which more‐lobed leaves maintain similar leaf surface area compared to less‐lobed leaves, despite lobing.
MATERIALS AND METHODS
Sampling
Leaves were sampled from seedlings of five biparental Vitis (Vitaceae) populations located in a vineyard planted in Madera County, California. There were 500 seedlings, with 450 sharing a seed parent, DVIT 2876. The remaining 50 seedlings had DVIT 2876 as a grandparent. DVIT 2876 ‘Olmo b55‐19’ is a compound‐leafed accession from the U. S. Department of Agriculture‐Agricultural Research Service (USDA‐ARS) National Clonal Germplasm repository, suspected to include Vitis piasezkii Maximowicz, as one of its parents (or grandparents). Thus, all of the populations had one parent with compound leaves of the V. piasezkii type, and each population had a different second parent with simple (non‐compound) leaves. The populations were created to examine variation in leaf lobing, and the resulting progeny from each cross had a range of leaf shapes from very lobed to entire.
The vines were composed of 125 individuals from a DVIT 2876 × unnamed Vitis vinifera L. selection cross (Pop1), 100 individuals from a DVIT 2876 × a different unnamed V. vinifera selection cross (Pop2), 150 individuals from a DVIT 2876 × unnamed Vitis hybrid cross (Pop3), 75 individuals from a DVIT 2876 × a different unnamed Vitis hybrid cross (Pop4), and 50 individuals from a seedling (DVIT 2876 × unnamed V. vinifera selection) × DVIT 3374 (Vitis mustangensis Buckley) cross (Pop5). The selections used in these crosses are unnamed because they are the result of breeding crosses.
The vines sampled were planted in 2017. They were trained to a unilateral cordon and spur pruned. Leaf samples were collected on 22 June and 12 July 2018, then again in 2019 on 14 and 19 June and 4 July. Although 500 vines were initially planted, some vines were not large enough to sample, and so the number of vines sampled each year is smaller. Across the sampling dates within a given year, a total of three mature, representative leaves were sampled from each of the vines. Due to the young age (2–3 years old) of the vines, it was not possible to sample an entire shoot without potentially damaging the health of many vines, and so the three leaves selected were based on an overall visual assessment of the vine to capture any variation in lobing, leaf color, or other within‐vine variation. These leaves were placed into labeled plastic bags. The plastic bags were stored in a cooler during collection and scanned, vein side down, later the same day using a flatbed scanner. Files were saved as JPEGs and named using the accession ID. All original scans used in this study are available from the Dryad repository (Migicovsky et al., 2022).
Landmarking
Leaves were analyzed using 21 landmarks as previously described (Chitwood et al., 2016b, 2021; Bryson et al., 2020). A visualization of these landmarks and the shape information captured using the landmarks across leaves from each of the five populations sampled are shown in Figure 1. In the case of complete dissection, we marked the distal sinus as the intersection of the naked veins of the midvein and the distal vein at the petiolar junction. These landmarks were placed manually using ImageJ on one side of the leaf (Abràmoff et al., 2004). The resulting table was saved as a text file with the coordinates for all landmarks and then visualized using ggplot2 v.3.3.5 (Wickham, 2016) in R v.4.1.0 (R Core Team, 2021) to detect mistakes. If the resulting visualization did not look like a leaf, indicating that the landmarking had been performed incorrectly, the landmarking was done again. The resulting data excluded vines that were too small to sample as well as leaves that were damaged and could not be landmarked.
Data analyses
The resulting text files from each scan were merged in R. In addition, we determined the image size for each scan using the R package imager (version 0.42.10; Barthelme, 2021) and used ImageJ (Abràmoff et al., 2004) to calculate the number of pixels per centimeter for all subsequent area calculations. Total leaf area and blade and vein areas were calculated using the shoelace algorithm, which calculates the area of a polygon using the landmarks as vertices, as described previously (Chitwood et al., 2021). In addition, we calculated the ratio of vein to blade area.
The degree of distal and proximal lobing of each leaf was calculated by first calculating the length of the distal and proximal veins. For this calculation, we used the midpoint of the landmarks at the base of the vein and the landmark at the tip of the lobe to calculate the distance between the two points. Similarly, we calculated the distance between the distal and proximal sinus and the landmark at the base of the leaf. Distal lobing values were calculated as the ratio of the length of the distal sinus to the length of the distal lobe, with values increasing as lobing decreases and the same ratio was calculated for proximal lobing. In addition, we calculated the length of the remaining major vein (the midvein) and the three branching veins. Total major vein length was calculated as the length of the midvein + the length of the distal vein + the length of the proximal vein. Similarly, total branching vein length was the sum of all three branching veins, including the petiolar vein as well as the veins which branch off of the midvein and distal vein (Figure 1A).
Landmarks were adjusted using a generalized Procrustes analysis (GPA) in the R package shapes version 1.2.6 with the reflect=TRUE option (Dryden, 2021) before performing principal component analysis (PCA). To compare differences in size across years, we used a Mann–Whitney U‐test to contrast 2018 and 2019 leaf areas for vines that were fully sampled (3 leaves) in both years.
Subsequent analyses were performed in R, and code for analyzing the data is available at the GitHub repository https://github.com/zoemigicovsky/grape_leaf_lobing. Data were visualized using ggplot2 (Wickham, 2016). Briefly, for accessions with three leaves sampled in both 2018 and 2019, we examined variation in leaf area, blade area, vein area, and the ratio between vein area and blade area for each leaf across both years. For all leaves measured, we used Pearson's correlation to determine the relationship between distal lobing and principal component 1 (PC1) based on homologous landmark data. We compared PC1 values, distal lobing, total major vein length, and total branching vein length across years for vines that were fully sampled. Next, we examined the variation in distal lobing across all leaves sampled for each of the five crosses sampled in this study.
We modeled the natural logarithm of area (cm2) vs distal lobing and the natural logarithm of area (cm2) vs natural logarithm of total major vein length (cm). We extracted the residuals from the model describing the relationship between ln (Area) ~ ln (Total major vein length) and modeled the relationship between the residuals vs. distal lobing. Next, we calculated the correlation between total major vein length and total branching vein length, before modeling the relationship between the natural logarithm of area (cm2) and natural logarithm of total branching vein length (cm) and the residual values vs. distal lobing. Lastly, we repeated the analysis, examining the relationship between the natural logarithm of area (cm2) vs the natural logarithm of the ratio of vein area to blade area, then modeling the relationship between the residuals from that model and distal lobing.
RESULTS
For each genetically distinct accession, a total of 1–6 leaves were sampled across the 2 years of the study. These accessions were the result of five biparental crosses, each with one parent with compound leaves and a different second parent with simple leaves, resulting in progeny with a range of leaf shapes. To compare the shape and size of leaves across the 2 years, for this analysis only, only accessions with three leaves sampled for both years were included. The total number of accessions with three leaves sampled in both years was 398, for a total of 2388 leaves. Across these 398 accessions, area was significantly greater for the whole leaf as well as blade and vein regions in 2019 compared to 2018 (Appendix S1A). While the median leaf area for 2018 was 24.45 cm2, it increased in 2019 by 4.24 cm2, resulting in a median leaf area of 28.69 cm2. Since proportionally more of the leaf is composed of blade than vein, the increase in blade area contributes more to the increase in overall leaf area than the increase in vein area does. The ratio of vein to blade ratio did not differ significantly between years, although the larger leaves in 2019 had a slightly lower vein to blade ratio (0.0603) in comparison to 2018 (0.0607) (Appendix S1B). Thus, differences in leaf size across years were not correlated with differences in the vein to blade ratio, indicating that leaf expansion was proportional for both blade and veins.
To determine the primary source of variation in leaf shape, we used all 2632 leaves sampled across 476 vines in 2018 and 2019 in all downstream analyses. First, we subjected the homologous landmark data to a PCA. The primary axis of variation, PC1, explained approximately 43.7% of the variation in the data set. PC1 was also significantly correlated with distal lobing (r = 0.99, P < 1 × 10−15; Figure 2A). In contrast, proximal lobing was significantly correlated with PC1, but the correlation was weaker (r = 0.66, P < 1 × 10−15; Appendix S2). An examination of the mean shape of a leaf for each PC1 quartile (Figure 2B) showed that distal lobing is the primary source of variation in these populations with leaves ranging from nearly entire to highly lobed along PC1. We also compared the subset of 2388 leaves from vines fully sampled in 2018 and 2019 for values along PC1 (Appendix S3A), distal lobing values (Appendix S3B), total major vein length (Appendix S3C), and total branching vein length (Appendix S3D). Across years, vines did not significantly differ in values along PC1 or distal lobing, indicating that there was no significant variation in shape. However, leaves from 2019 had significantly longer total major vein length and total branching length, which supports the overall increase in leaf size in 2019 (Appendix S1A) but indicates that this increase is not correlated with differences in leaf shape. For all subsequent analyses, the complete set of 2632 leaves was also used. Among this complete set, we identified substantial variation in distal lobing in each of the five populations (Appendix S4).
Figure 2.
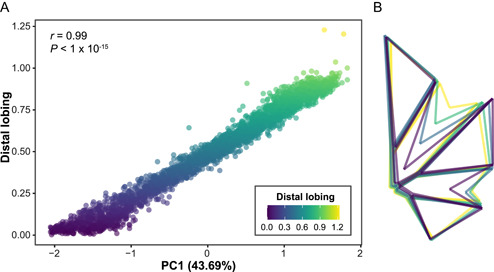
(A) Correlation between principal component 1 (PC1) and distal lobing. Each leaf (n = 2632) is plotted with the color of the point indicating the distal lobing value. Distal lobing values were calculated as the ratio of the length of the distal sinus to the length of the distal lobe terminus, with values increasing as lobing decreases. The Pearson's correlation coefficient between PC1 and distal lobing is indicated, as is the amount of variance explained by PC1. (B) For each PC1 quartile, a mean leaf is plotted and colored according to distal lobing value.
After calculating the total area of each leaf, we modeled the natural logarithm of leaf area as a function of distal lobing (Figure 3). While the natural logarithm of total leaf area varied significantly as a function of distal lobing (P = 0.026), the adjusted R 2 was 0.003, indicating that the effect of distal lobing on total leaf area is small, and the shape of the leaf is decoupled from its overall area.
Figure 3.
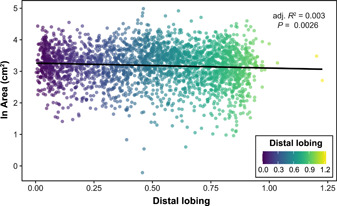
Natural logarithm of area (cm2) vs distal lobing. Each leaf (n = 2632) is plotted with the color of the point indicating the distal lobing value. The natural log of leaf area varies significantly (p = 0.026) as a function of distal lobing, with an adjusted R 2 of 0.003.
Modeling the natural logarithm of leaf area as a function of the natural logarithm of total major vein length (the sum of the midvein, distal vein, and proximal vein lengths, indicated in Figure 1A), total leaf area increases as major vein length increases (Figure 4A). This linear relationship (R 2 = 0.95, P < 1 × 10−15) indicates that leaf area increases as a function of vein length. The residuals from this model have a linear relationship with distal lobing (Figure 4B, R 2 = 0.32, P < 1 × 10−15). Thus, a highly lobed leaf (one with a lower distal lobing value) of the same area as a more entire leaf (one with a higher distal lobing value) will have longer veins. By increasing vein length, highly lobed leaves can maintain total leaf areas similar to those with less lobing. Indeed, when visualizing the mean leaf for each PC1 quartile (Figure 2B), it is apparent that the distal lobe is longer in the more highly lobed leaves.
Figure 4.
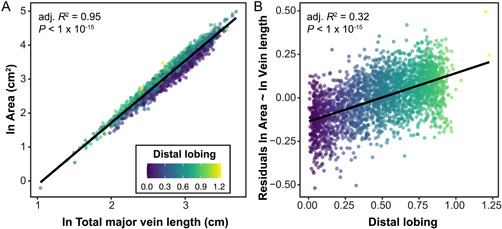
(A) Natural logarithm of area (cm2) vs. natural logarithm of total major vein length. Total major vein length (cm) was calculated by combining the length of the proximal, distal, and midvein. (B) Residuals from the model of natural logarithm of area (cm2) vs natural logarithm of total major vein length, as indicated in panel A, vs. distal lobing. In both panels, each leaf (n = 2632) is plotted with the color of the point indicating the distal lobing value. The natural log of leaf area varies significantly as a function of the natural log of total major vein length, and residuals from that model vary significantly as a function of distal lobing. The adjusted R 2 for each model is indicated on the plots.
In addition to total major vein length, we also calculated total branching vein length across other veins measured in this study and found that the two measurements were highly correlated (Appendix S5, r = 0.97, P < 1 × 10−15). Branching vein length included the length of the petiolar vein and the veins that branch off the midvein and distal vein. Leaves measured for this study with higher major vein lengths also had longer branching veins. A similar linear relationship between the natural logarithm of leaf area and the natural logarithm of branching vein length occurred (Appendix S6A, R 2 = 0.96, P < 1 × 10−15), indicating that like major vein length, leaf area increases as a function of branching vein length. However, when we model the residuals from this relationship as a function of distal lobing, the amount of variation in distal lobing explained by these residuals was lower (Appendix S6B, R 2 = 0.14, P < 1 × 10−15). Therefore, distal lobing is a better predictor of the residuals for the relationship between leaf area and major vein length, than branching vein length. For leaves with the same surface area, those which are more highly lobed will tend to have both longer major veins and branching veins, but this difference is more pronounced for the major veins, indicating that leaves compensate for area lost to lobing primarily through length of the major veins, and not the length of branching veins.
While increasing vein length is one way for a more highly lobed leaf to achieve a similar surface area to a more entire leaf, an alternative or complementary hypothesis is that the leaf has a higher vein to blade ratio. Although we did not observe a strong difference in vein to blade across years despite leaves increasing in size (Appendix S1), previous work has identified vein to blade ratio as a strong indicator of allometric variation, with larger leaves decreasing the proportion of the area composed of vein relative to blade (Chitwood et al., 2021). By modeling the relationship between the natural logarithm of leaf area and the natural logarithm of vein to blade ratio, we identified a significant linear relationship, with total leaf area decreasing as vein to blade increased (Figure 5A, R 2 = 0.24, p < 1 × 10−15). This linear, allometric relationship is similar, albeit weaker, than the one seen between area and total major vein length (Figure 4A) and total branching vein length (Appendix S6A). However, it occurs in the opposite direction, with leaves having smaller total areas as the vein to blade ratio increases and the total major and branching vein lengths decrease.
Figure 5.
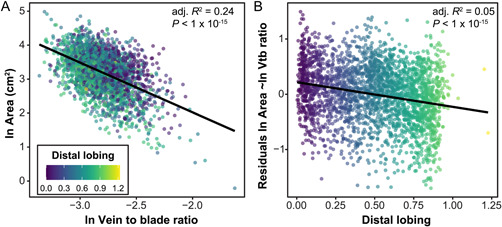
(A) Natural logarithm of area (cm2) vs. natural logarithm of vein to blade ratio. (B) Residuals from the model of natural logarithm of area (cm2) vs. natural logarithm of vein to blade ratio, as indicated in panel A, vs. distal lobing. In both panels, each leaf (n = 2632) is plotted with the color of the point indicating the distal lobing value. The natural logarithm of leaf area varies significantly as a function of the natural logarithm of vein to blade ratio, and residuals from that model vary significantly as a function of distal lobing. The adjusted R 2 for each model is indicated on the plots.
The residuals from the model between the natural logarithm of leaf area and the natural logarithm of vein to blade ratio explain a significant amount of the variance in distal lobing (Figure 5B, R 2 = 0.05, p < 1 × 10−15), but the R 2 is much smaller than for the same models applied using total major or branching vein lengths. Thus, leaf area is maintained by more highly lobed leaves primarily through increases in length, not increases in the surface area of veins relative to the blade. However, this subtle relationship still indicates that for leaves of the same size, those that are more highly lobed will have higher vein to blade ratios, which partly explains how they are able to compensate for the reduction in blade area. Ultimately, more highly lobed leaves (with lower distal lobing values) have more negative residuals for total major vein length and total branching vein length, and more positive residuals for vein to blade ratio. These relationships indicate that for a given leaf area, more highly lobed leaves have longer veins (both major and branching) and higher vein to blade ratios, allowing them to maintain similar leaf areas despite increased lobing.
DISCUSSION
By quantifying variation in shape for 2632 leaves sampled across 476 grapevines that showed immense variation in leaf lobing, we were able to evaluate the relationship between leaf size and leaf shape. Previous work performing linkage mapping in grapevine identified quantitative trait loci on chromosome 1 associated with the depth of the leaf sinus, or “lobiness” (Welter et al., 2007; Demmings et al., 2019). Although we did not perform linkage mapping with the biparental crosses examined in this work, we did identify distal lobing as the primary source of variation. This finding is supported by the historical literature (Galet, 1979) and a detailed study of 60 wine and table grape varieties, in which the distal sinus was one of the strongest indicators of variety (Chitwood, 2021).
Although lobing was the primary source of variation in shape across the leaves measured, leaf area varied only slightly as a function of lobing, with an R 2 of 0.003. Indeed, due to the presence of a handful of smaller, less‐lobed leaves, the slope of the linear relationship between the natural log of leaf area and distal lobing was negative (–0.038), indicating that less‐lobed leaves with distal lobing values closer to 1 actually had slightly smaller areas than the larger leaves had, although as noted, this relationship is very minor. Without other compensating mechanisms, increasing lobing would reduce leaf area. The lack of any substantial relationship between lobing and leaf area indicates the existence of compensating mechanisms that allow lobed leaves to maintain overall area.
We determined that leaf area increases as a function of total major vein length, and total branching vein length and leaf area decreases as a function of vein to blade ratio. These relationships are stronger for more highly lobed leaves, with the residuals for each model differing as a function of distal lobing. These findings show how more highly lobed leaves may compensate for what would otherwise be a reduced leaf area, allowing for increased photosynthetic capacity through similar leaf size.
Our analyses here are restricted to five biparental populations, all with a shared parent or grandparent responsible for lobing, DVIT 2876. Lobing in DVIT 2876 is derived from V. piasezkii, in which blade dissection can extend completely to the petiolar junction as a 3–5‐foliate leaf. The populations also contain lobing introduced from V. vinifera. It is possible that the relationships observed here are not necessarily true across Vitis more broadly, especially in species with more entire leaves. In addition, due to a single sampling time point for each vine in each year, as well as only sampling three representative leaves, we were unable to capture within‐vine variation due to non‐genetic effects that occur within the lifespan of a leaf. For example, it is well established that changes in leaf morphology occur both within the lifespan of a leaf (ontogeny) and at successive nodes along the shoot (heteroblasty) (Chitwood et al., 2016a,b; Baumgartner et al., 2020). Variation throughout the growing season and the effects of climate beyond the two years sampled (Chitwood et al., 2021) would also be missed by our study. Such effects warrant future research to determine whether the compensatory mechanisms for leaf shape we observe are maintained.
Determining how lobing influences variation in other measurements of leaf shape is not just botanically fascinating, but also important because more highly lobed leaves may have a viticultural benefit in the vineyard. For example, in cotton (Gossypium hirsutum L.), leaf shape has an impact on chemical spray penetration, with the highly lobed leaves allowing more spray to be delivered deeper into the canopy (Andres et al., 2016). However, in cotton, the switch from entire to highly lobed leaves reduces the leaf area (Andres et al., 2016). In grapevine, extensive canopy management practices such as planting distance, pruning including shoot length and architecture, training system, and trellis design are used to influence access to sunlight (Reynolds and Vanden Heuvel, 2009; Keller, 2020b). Given the central position of grape berries in the canopy, access to sunlight is particularly important, and leaf removal or thinning may be used to increase sunlight penetration around the canopy fruit zone. Leaves within the canopy may receive only 1/100 of photosynthetically active radiation that exterior leaves do (Smart et al., 1982). Light in the canopy has an important influence on grape composition. For example, in one study of ‘Merlot’ grapes, leaf removal significantly reduced titratable acidity and increased the concentrations of phenols, anthocyanins, flavonols, and flavan‐3‐ols (Anić et al., 2021). Similarly, ‘Riesling’ grapes had higher monoterpene alcohols and bound aromatic alcohols (Zoecklein et al., 1998) and ‘Sauvignon Blanc’ grapes had increased total soluble solids and reduced titratable acidity (Bledsoe et al., 1988) when leaves were removed. In all these cases, leaf removal resulted in more light in the canopy fruit zone, and the changes in grape composition were attributed to the increase in light.
In addition to grape composition, light is also known to influence fertility of latent buds. Warm, sunny conditions, water and nutrient access, and sufficient photosynthesizing leaf area, are necessary to maximize the number of primordia. Similar conditions are also required for flowering, fruit set, and berry development (Keller, 2020a). Like cotton, access to the grapes within the canopy due to highly lobed leaves or leaf thinning could improve spray penetration and improve the deposition of chemicals. Indeed, there is evidence that grapevine varieties do differ in the extent that sprays can penetrate the canopy; in work on two grape varieties, the vineyard spraying system needed to be calibrated based on variety (Palleja and Landers, 2017).
While leaf thinning may have benefits, it does reduce the leaf area per meter of canopy, by partially or entirely removing leaves. Ultimately, more highly lobed leaves with the same leaf surface area could enable grape growers to capture the benefits of leaf thinning (sunlight and spray penetration) while not removing leaves, thereby not reducing leaf area and associated photosynthetic capacity as well as saving on leaf removal costs.
Across both the paleorecord and extant populations, less‐toothed leaves and larger leaves have been identified in similar climates (Chitwood and Sinha, 2016). Within the grapevines studied here, leaf shape and size are decoupled from each other. Increases in vein length compensate for leaf area lost due to lobing, providing evidence of one mechanism that could allow leaves to maintain area while increasing lobing. This mechanism could allow sunlight to permeate the grapevine canopy without reducing the photosynthetic capacity of the vine.
CONCLUSIONS
By examining the relationship between leaf size and shape, we showed that highly lobed leaves do not differ in overall leaf area compared to less‐lobed leaves. Future work is still needed to determine whether such variation in leaf shape could be harnessed through breeding to improve grapevine management. These results may suggest use of a novel allometric mechanism in Vitaceae in which reductions in leaf area due to lobing are compensated through increases in vein length.
AUTHOR CONTRIBUTIONS
P.C. generated the seedlings and supervised the maintenance of the vineyard. Z.M., J.F.S., P.C., and D.H.C. conceived of the initial idea for this study. Z.M. coordinated the research. Z.M., J.F.S., Z.H., L.L.K., A.L., M.M., and K.W. sampled the leaves for this study. A.F., M.K., A.J.M., P.C., and D.H.C. acquired funding for this study and provided supervisory support. Z.M. analyzed the data with input from D.H.C. Z.M. wrote the first draft of the manuscript, which all authors read, commented on, and edited.
CONFLICTS OF INTEREST
P.C. is employed by E. & J. Gallo Winery. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation.
Supporting information
Appendix S1. (A) Leaf area (cm2) for all leaves sampled in 2018 and 2019. The area values are shown for the whole leaf and for the blade and vein portions of the leaves separately. Because values are small for vein area, that section of the plot is enlarged below. (B) The ratio between vein area and blade area for each leaf is shown for 2018 and 2019.
Appendix S2. Correlation between PC1 and proximal lobing. Each leaf (n = 2632) is plotted; the color of the point indicates the proximal lobing value.
Appendix S3. (A) PC1 values for all leaves sampled in 2018 and 2019. (B) Distal lobing values for all leaves sampled in 2018 and 2019. (C) Total major vein length for all leaves sampled in 2018 and 2019. (D) Total branching vein length for all leaves sampled in 2018 and 2019.
Appendix S4. Density plots indicating the distribution of distal lobing values for each of the five populations examined in this study.
Appendix S5. Correlation between the total major vein length (cm) and total branching vein length (cm).
Appendix S6. (A) Natural logarithm of area (cm2) vs natural logarithm of total branching vein length. (B) Distal lobing vs residuals from the model of natural logarithm of area (cm2) vs natural logarithm of total branching vein length, as indicated in panel A.
ACKNOWLEDGMENTS
The research conducted for this study was supported by the National Science Foundation (NSF) Plant Genome Research Program 1546869. J.F.S. was supported by an NSF Graduate Research Fellowship under Grant No. 1758713 and by Saint Louis University. This research was also funded through the USDA National Institute of Food and Agriculture and Michigan State University AgBioResearch.
We acknowledge all individuals involved in maintaining the vineyard evaluated for this study. We acknowledge Leah Brand (Missouri State University), Julie Curless (Missouri State University), Dalton Gilig (University of Missouri), and Ilona Natsch (Saint Louis University) for assistance in sampling and landmarking the leaves. We also acknowledge Laszlo Kovacs (Missouri State University) for student supervisory support and two anonymous reviewers for feedback that improved this article.
Migicovsky, Z. , Swift J. F., Helget Z., Klein L. L., Ly A., Maimaitiyiming M., Woodhouse K., Fennell A., Kwasniewski M., Miller A. J., Cousins P., and Chitwood D. H.. 2022. Increases in vein length compensate for leaf area lost to lobing in grapevine. American Journal of Botany 109(7): 1063–1073. 10.1002/ajb2.16033
Contributor Information
Zoë Migicovsky, Email: zoe.migicovsky@acadiau.ca.
Daniel H. Chitwood, Email: dhchitwood@gmail.com.
DATA AVAILABILITY STATEMENT
The original scans of leaves used for this study are available from the Dryad Digital Repository: doi:10.5061/dryad.3ffbg79m8 (Migicovsky et al., 2022). All data and code used in this study can be found on GitHub: https://github.com/zoemigicovsky/grape_leaf_lobing.
REFERENCES
- Abràmoff, M. D. , Magalhães P. J., and Ram S. J.. 2004. Image processing with ImageJ. Biophotonics International 11: 36–42. [Google Scholar]
- Andres, R. J. , Bowman D. T., Jones D. C., and Kuraparthy V.. 2016. Major leaf shapes of cotton: genetics and agronomic effects in crop production. Journal of Cotton Science 20: 330–340. [Google Scholar]
- Anić, M. , Osrečak M., Andabaka Ž., Tomaz I., Večenaj Ž., Jelić D., Kozina B., et al. 2021. The effect of leaf removal on canopy microclimate, vine performance and grape phenolic composition of Merlot (Vitis vinifera L.) grapes in the continental part of Croatia. Scientia Horticulturae 285: 110161. [Google Scholar]
- Bailey, I. W. , and Sinnott E. W.. 1916. The climatic distribution of certain types of angiosperm leaves. American Journal of Botany 3: 24–39. [Google Scholar]
- Barthelme, S. 2021. imager: Image processing library based on ‘CImg’. R package version 0.42.10. Website: https://CRAN.R-project.org/package=imager
- Baumgartner, A. , Donahoo M., Chitwood D. H., and Peppe D. J.. 2020. The influences of environmental change and development on leaf shape in Vitis. American Journal of Botany 107: 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe, A. , Kliewer W., and Marois J.. 1988. Effects of timing and severity of leaf removal on yield and fruit composition of Sauvignon Blanc grapevines. American Journal of Enology and Viticulture 39: 49–54. [Google Scholar]
- Brown, V. , and Lawton J. H.. 1991. Herbivory and the evolution of leaf size and shape. Philosophical Transactions of the Royal Society, B, Biological Sciences 333: 265–272. [Google Scholar]
- Bryson, A. E. , Brown M. W., Mullins J., Dong W., Bahmani K., Bornowski N., Chiu C., et al. 2020. Composite modeling of leaf shape along shoots discriminates Vitis species better than individual leaves. Applications in Plant Sciences 8: e11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. 2021. The shapes of wine and table grape leaves: an ampelometric study inspired by the methods of Pierre Galet. Plants, People, Planet 3: 155–170. [Google Scholar]
- Chitwood, D. H. , Klein L. L., O'Hanlon R., Chacko S., Greg M., Kitchen C., Miller A. J., and Londo J. P.. 2016a. Latent developmental and evolutionary shapes embedded within the grapevine leaf. New Phytologist 210: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , Kumar R., Headland L. R., Ranjan A., Covington M. F., Ichihashi Y., Fulop D., et al. 2013. A quantitative genetic basis for leaf morphology in a set of precisely defined tomato introgression lines. Plant Cell 25: 2465–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , Mullins J., Migicovsky Z., Frank M., VanBuren R., and Londo J. P.. 2021. Vein‐to‐blade ratio is an allometric indicator of leaf size and plasticity. American Journal of Botany 108: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , and Otoni W. C.. 2017. Morphometric analysis of Passiflora leaves: the relationship between landmarks of the vasculature and elliptical Fourier descriptors of the blade. GigaScience 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , Ranjan A., Martinez C. C., Headland L. R., Thiem T., Kumar R., Covington M. F., et al. 2014. A modern ampelography: a genetic basis for leaf shape and venation patterning in grape. Plant Physiology 164: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , Rundell S. M., Li D. Y., Woodford Q. L., Yu T. T., Lopez J. R., Greenblatt D., et al. 2016b. Climate and developmental plasticity: interannual variability in grapevine leaf morphology. Plant Physiology 170: 1480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , and Sinha N. R.. 2016. Evolutionary and environmental forces sculpting leaf development. Current Biology 26: R297–R306. [DOI] [PubMed] [Google Scholar]
- Demmings, E. M. , Williams B. R., Lee C.‐R., Barba P., Yang S., Hwang C.‐F., Reisch B. I., et al. 2019. Quantitative trait locus analysis of leaf morphology indicates conserved shape loci in grapevine. Frontiers in Plant Science 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz, S. , Kattge J., Cornelissen J. H. C., Wright I. J., Lavorel S., Dray S., Reu B., et al. 2016. The global spectrum of plant form and function. Nature 529: 167–171. [DOI] [PubMed] [Google Scholar]
- Dryden, I. L. 2021. Shapes: statistical shape analysis. R package version 1.2.6. Website: https://CRAN.R-project.org/package=shapes
- Edwards, E. J. , Spriggs E. L., Chatelet D. S., and Donoghue M. J.. 2016. Unpacking a century‐old mystery: winter buds and the latitudinal gradient in leaf form. American Journal of Botany 103: 975–978. [DOI] [PubMed] [Google Scholar]
- Feild, T. S. , Sage T. L., Czerniak C., and Iles W. J.. 2005. Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation‐induced flooding of the mesophyll. Plant, Cell & Environment 28: 1179–1190. [Google Scholar]
- Galet, P. 1979. A practical ampelography. Cornell University Press, Ithaca, NY, USA. [Google Scholar]
- Givnish, T. 1979. On the adaptive significance of leaf form. In Solbrig O. T., Jain S., Johnson G. B., and Raven P. H. [eds.], Topics in plant population biology, 375–407. Columbia University Press, NY, NY, USA. [Google Scholar]
- Givnish, T. J. 1987. Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytologist 106: 131–160. [Google Scholar]
- Harris, Z. N. , Awale M., Bhakta N., Chitwood D. H., Fennell A., Frawley E., Klein L. L., et al. 2021. Multi‐dimensional leaf phenotypes reflect root system genotype in grafted grapevine over the growing season. GigaScience 10: giab087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, M. 2020a. Phenology and growth cycle. In The science of grapevines, 3rd ed., 61–103. Academic Press, San Diego, CA, USA. [Google Scholar]
- Keller, M. 2020b. Developmental physiology. In The science of grapevines, 3rd ed., 199–277. Academic Press, San Diego, CA, USA. [Google Scholar]
- Klein, L. L. , Caito M., Chapnick C., Kitchen C., O'Hanlon R., Chitwood D. H., and Miller A. J.. 2017. Digital morphometrics of two North American grapevines (Vitis: Vitaceae) quantifies leaf variation between species, within species, and among individuals. Frontiers in Plant Science 8: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migicovsky, Z. , Li M., Chitwood D. H., and Myles S.. 2018. Morphometrics reveals complex and heritable apple leaf shapes. Frontiers in Plant Science 8: 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migicovsky, Z. , Swift J. F., Helget Z., Klein L. L., Ly A., Maimaitiyiming M., Woodhouse K., et al. 2022. Data from: increases in vein length compensate for leaf area lost to lobing in grapevine. Dryad Digital Repository. 10.5061/dryad.3ffbg79m8 [DOI] [PMC free article] [PubMed]
- Nicotra, A. B. , Leigh A., Boyce C. K., Jones C. S., Niklas K. J., Royer D. L., and Tsukaya H.. 2011. The evolution and functional significance of leaf shape in the angiosperms. Functional Plant Biology 38: 535–552. [DOI] [PubMed] [Google Scholar]
- Palleja, T. , and Landers A. J.. 2017. Real time canopy density validation using ultrasonic envelope signals and point quadrat analysis. Computers and Electronics in Agriculture 134: 43–50. [Google Scholar]
- Peppe, D. J. , Royer D. L., Cariglino B., Oliver S. Y., Newman S., Leight E., Enikolopov G., et al. 2011. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytologist 190: 724–739. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Website: https://www.r-project.org [Google Scholar]
- Ravaz, L. 1902. Les vignes américaines: porte‐greffes et producteurs‐directs: caractères, aptitudes. Coulet et Fils, Montpelier, France. [Google Scholar]
- Reynolds, A. G. , and Vanden Heuvel J. E.. 2009. Influence of grapevine training systems on vine growth and fruit composition: a review. American Journal of Enology and Viticulture 60: 251–268. [Google Scholar]
- Royer, D. L. , and Wilf P.. 2006. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. International Journal of Plant Sciences 167: 11–18. [Google Scholar]
- Schmerler, S. B. , Clement W. L., Beaulieu J. M., Chatelet D. S., Sack L., Donoghue M. J., and Edwards E. J.. 2012. Evolution of leaf form correlates with tropical–temperate transitions in Viburnum (Adoxaceae). Proceedings of the Royal Society, B, Biological Sciences 279: 3905–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader, J. , Shi P., Royer D. L., Peppe D. J., Gallagher R. V., Li Y., Wang R., and Wright I. J.. 2021. Leaf size estimation based on leaf length, width and shape. Annals of Botany 128: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart, R. E. , Shaulis N. J., and Lemon E. R.. 1982. The effect of Concord vineyard microclimate on yield. I. The effects of pruning, training, and shoot positioning on radiation microclimate. American Journal of Enology and Viticulture 33: 99–108. [Google Scholar]
- Webb, L. J. 1968. Environmental relationships of the structural types of Australian rain forest vegetation. Ecology 49: 296–311. [Google Scholar]
- Welter, L. J. , Göktürk‐Baydar N., Akkurt M., Maul E., Eibach R., Töpfer R., and Zyprian E. M.. 2007. Genetic mapping and localization of quantitative trait loci affecting fungal disease resistance and leaf morphology in grapevine (Vitis vinifera L). Molecular Breeding 20: 359–374. [Google Scholar]
- Westoby, M. , Falster D. S., Moles A. T., Vesk P. A., and Wright I. J.. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- Wickham, H. 2016. ggplot2: elegant graphics for data analysis. Springer‐Verlag, NY, NY, USA. [Google Scholar]
- Wilf, P. 1997. When are leaves good thermometers? A new case for leaf margin analysis. Paleobiology 23: 373–390. [Google Scholar]
- Wright, I. J. , Dong N., Maire V., Prentice I. C., Westoby M., Díaz S., Gallagher R. V., et al. 2017. Global climatic drivers of leaf size. Science 357: 917–921. [DOI] [PubMed] [Google Scholar]
- Zoecklein, B. W. , Wolf T. K., Marcy J. E., and Jasinski Y.. 1998. Effect of fruit zone leaf thinning on total glycosides and selected aglycone concentrations of Riesling (Vitis vinifera L.) grapes. American Journal of Enology and Viticulture 49: 35–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. (A) Leaf area (cm2) for all leaves sampled in 2018 and 2019. The area values are shown for the whole leaf and for the blade and vein portions of the leaves separately. Because values are small for vein area, that section of the plot is enlarged below. (B) The ratio between vein area and blade area for each leaf is shown for 2018 and 2019.
Appendix S2. Correlation between PC1 and proximal lobing. Each leaf (n = 2632) is plotted; the color of the point indicates the proximal lobing value.
Appendix S3. (A) PC1 values for all leaves sampled in 2018 and 2019. (B) Distal lobing values for all leaves sampled in 2018 and 2019. (C) Total major vein length for all leaves sampled in 2018 and 2019. (D) Total branching vein length for all leaves sampled in 2018 and 2019.
Appendix S4. Density plots indicating the distribution of distal lobing values for each of the five populations examined in this study.
Appendix S5. Correlation between the total major vein length (cm) and total branching vein length (cm).
Appendix S6. (A) Natural logarithm of area (cm2) vs natural logarithm of total branching vein length. (B) Distal lobing vs residuals from the model of natural logarithm of area (cm2) vs natural logarithm of total branching vein length, as indicated in panel A.
Data Availability Statement
The original scans of leaves used for this study are available from the Dryad Digital Repository: doi:10.5061/dryad.3ffbg79m8 (Migicovsky et al., 2022). All data and code used in this study can be found on GitHub: https://github.com/zoemigicovsky/grape_leaf_lobing.


