Abstract
Objective
The primary aim was assessing the cost‐effectiveness of an internet‐based self‐help program, expert‐patient support, and the combination of both compared to a care‐as‐usual condition.
Method
An economic evaluation from a societal perspective was conducted alongside a randomized controlled trial. Participants aged 16 or older with at least mild eating disorder symptoms were randomly assigned to four conditions: (1) Featback, an online unguided self‐help program, (2) chat or e‐mail support from a recovered expert patient, (3) Featback with expert‐patient support, and (4) care‐as‐usual. After a baseline assessment and intervention period of 8 weeks, five online assessments were conducted over 12 months of follow‐up. The main result constituted cost‐utility acceptability curves with quality‐of‐life adjusted life years (QALYs) and societal costs over the entire study duration.
Results
No significant differences between the conditions were found regarding QALYs, health care costs and societal costs. Nonsignificant differences in QALYs were in favor of the Featback conditions and the lowest societal costs per participant were observed in the Featback only condition (€16,741) while the highest costs were seen in the care‐as‐usual condition (€28,479). The Featback only condition had the highest probability of being efficient compared to the alternatives for all acceptable willingness‐to‐pay values.
Discussion
Featback, an internet‐based unguided self‐help intervention, was likely to be efficient compared to Featback with guidance from an expert patient, guidance alone and a care‐as‐usual condition. Results suggest that scalable interventions such as Featback may reduce health care costs and help individuals with eating disorders that are currently not reached by other forms of treatment.
Public significance statement
Internet‐based interventions for eating disorders might reach individuals in society who currently do not receive appropriate treatment at low costs. Featback, an online automated self‐help program for eating disorders, was found to improve quality of life slightly while reducing costs for society, compared to a do‐nothing approach. Consequently, implementing internet‐based interventions such as Featback likely benefits both individuals suffering from an eating disorder and society as a whole.
Keywords: cost–benefit analysis, cost‐effectiveness, eating disorders, economic evaluation, eHealth, expert patient, internet‐based intervention, quality of life, randomized controlled trial
1. INTRODUCTION
Eating disorders are burdensome in terms of disability, quality of life and mortality (Arcelus et al., 2011; Smink et al., 2013) and also from an economic perspective (Erskine et al., 2016; van Hoeken & Hoek, 2020). There exists a large treatment gap for eating disorders, meaning that many individuals with an eating disorder do not get help specifically for their eating disorder, despite having substantial symptoms (Austin et al., 2020). Nevertheless, they generally make more use of health care services compared to people without an eating disorder (Hart et al., 2011; van Son et al., 2012; Weissman & Rosselli, 2017), which is reflected in higher health care costs (Ágh et al., 2016; Samnaliev et al., 2015). Eating disorder related costs may become larger still when also considering costs outside health care, such as productivity losses and caregiver costs (Deloitte Access Economics, 2020). These substantial costs warrant well‐advised resource allocation decisions. In fact, investing in evidence‐based treatment for eating disorders might ultimately result in cost savings (Bode et al., 2017). Apart from such policy changes, helping individuals with an eating disorder while reducing costs for society requires continued effort from researchers and clinicians to make treatments more effective, accessible, and less expensive.
Cost‐effectiveness research, where outcomes and costs of two different courses of action are compared, is necessary to distinguish interventions that are more efficient than others. Internet interventions, often coined as cost‐effective alternative to other treatment options, have frequently been confirmed in their effectiveness (Linardon et al., 2020; Loucas et al., 2014; Melioli et al., 2016; Pittock et al., 2018), but cost‐effectiveness research in scarce. Across mental disorders, evidence from systematic reviews cautiously suggests internet‐based interventions might indeed be cost‐effective, at least compared to do‐nothing approaches (Ahern et al., 2018; Donker et al., 2015; Paganini et al., 2018). A few studies investigated the cost‐effectiveness of internet interventions compared to face‐to‐face eating disorder treatment (Crow et al., 2009; König et al., 2018; Watson et al., 2018) and found internet interventions to be slightly less effective in reducing eating disorder symptoms, but also less costly. Consequently, such interventions might be especially efficient as a first step in a stepped‐care treatment model, as they have the potential to reach individuals that currently do not get appropriate care for their eating disorder (Aardoom, Dingemans, & Van Furth, 2016). When researching these first step internet‐based interventions for eating disorders, care as usual may be used as a reference, since it represents the, often inappropriate, care individuals with eating disorders in society receive. Unfortunately, there is a paucity of cost‐effectiveness research comparing online interventions for eating disorders with care as usual. A simulation study on US college students with eating disorders indicated that a stepped‐care treatment model with online guided self‐help was less costly and resulted in fewer individuals in need of additional treatment than usual care (Kass et al., 2017). Recently, Akers et al. (2021) showed an online version of the cognitive‐dissonance based intervention “the Body Project” to have health benefits compared to enhanced usual care, while health utilization was similar. Additionally, Aardoom, Dingemans, van Ginkel, et al. (2016) found that Featback, an online automatic monitoring and feedback system for people with an eating disorder, was cost‐effective compared to a care‐as‐usual condition, regardless of whether the intervention was complemented with chat or e‐mail support by a psychologist. Taken together, the limited evidence available suggests that online interventions for eating disorders may be cost‐effective compared to care as usual, which is especially interesting considering that such interventions are scalable and easily accessible and can reach people at an early stage of eating disorder development. Recently, a second randomized controlled trial to replicate and extend the results on the effectiveness and cost‐effectiveness of Featback compared to care as usual was conducted. In the first RCT (Aardoom, Dingemans, Spinhoven, et al., 2016), support by a psychologist did not add to the effectiveness of Featback. Possibly, support by expert patients (i.e., recovered individuals) is more fitting and effective for those reluctant to seek help (Rohrbach et al., 2019).
2. AIMS
The primary aim of this study was to investigate the cost‐effectiveness of the three conditions, (1) the fully automated internet intervention Featback, (2) chat or email support from expert patients, and (3) the combination of both interventions compared to (4) a care‐as‐usual condition from a societal perspective. The three active online interventions were expected to be more efficient than care as usual.
3. METHOD
3.1. Design and randomization
This economic evaluation was part of a randomized controlled trial, pre‐registered at the Dutch Trial Register (NL7065) and approved by an independent medical ethics committee (METC‐LDD; NL64553.058.18). Detailed information on the interventions and methods can be found in the study protocol (Rohrbach et al., 2019). Results on the clinical effectiveness will be reported elsewhere. A two‐by‐two factorial design with the internet‐based interventions Featback and expert‐patient support was used, resulting in four conditions: (1) Featback, (2) Featback with expert‐patient support, (3) expert‐patient support, and (4) care‐as‐usual condition. All conditions had a duration of 8 weeks. Assessments on quality of life and costs were all online and completed by participants at postintervention and 3, 6, 9, and 12 month follow up. Participants were randomized and distributed across conditions in blocks of 40. For randomization, a computer‐generated random numbers list was made by an independent researcher, concealing it from the principal investigator before and during the trial. The economic analysis maintains a societal perspective, meaning that both health care costs and nonhealthcare costs were included. Data concerning costs and utility covered a period of 14 months (i.e., 8 weeks intervention or waiting plus 12 months follow up).
3.1.1. Participants
Participants were recruited mainly via Proud2Bme, a Dutch online community for people with eating‐related problems or eating disorders, from October 2018 to October 2019. After expressing interest to participate, they received a screening questionnaire. Eligible participants were 16 years or older, had internet access and reported at least mild eating disorder symptoms. Specifically, they scored 52 or higher on the Weight Concerns Scale (Killen et al., 1993) or reported a body mass index lower than or equal to 18.5, or one or more weekly binge eating episodes or compensatory behaviors in the past 4 weeks on the Short Evaluation of Eating Disorders (Bauer et al., 2005). Participants with severe eating disorder symptoms were advised to seek professional help but were not excluded as they too may benefit from the offered interventions.
3.2. Interventions
Participants in all conditions were free to undergo any other type of intervention or treatment, representing individuals in society with varying levels of treatment. Consequently, the waiting list control condition can be seen as care as usual for individuals with eating disorder symptoms in (Dutch speaking) society.
3.2.1. Featback
Participants could make weekly use of an automated monitoring and feedback system for 8 weeks. Based on the answers of a short monitoring questionnaire, participants received a supportive feedback message with a summary of self‐reported eating problems, psychoeducation, and guidance on how to counter eating disorder related symptoms. Current level of impairments as well as improvements or deteriorations in eating disorder related symptoms compared to the previous week were captured in the messages. Additionally, participants could access the Featback website with psycho‐educative material on eating disorders at their own convenience.
3.2.2. Expert‐patient support
Five expert patients (sometimes referred to as peers or mentors) were recruited, who had a lived experience of an eating disorder and were fully recovered. They received a protocol and were trained on how to use their own experience to help others overcome their eating disorder via chat and e‐mail. Monthly supervision from an experienced expert patient and clinical psychologist during the trial was included. Participants allocated to the conditions with expert‐patient support were assigned to one of the expert patients for 8 weeks and could schedule a 20‐minute chat or e‐mail session every week. Chat sessions closed automatically after 20 min. For e‐mail sessions, participants sent an e‐mail to their expert patient before the scheduled time slot and the expert patient responded during the appointment.
3.2.3. Featback with expert‐patient support
Participants in this condition were able to make use of both Featback and weekly 20‐minute chat or e‐mail support from an expert patient.
3.2.4. Care‐as‐usual condition
Participants in this condition were placed on a waiting list for 14 months. After the waiting period, participants were offered 8 weeks of Featback with weekly expert‐patient support.
3.3. Measures
3.3.1. Demographics
Assessed baseline variables were age, gender, nationality, education level, eating disorder treatment history, marital status, weight, height, eating disorder duration, internet usage, eating disorder symptoms assessed with the Eating Disorder Examination Questionnaire global scores (Fairburn & Beglin, 2008).
3.3.2. Quality of life
The primary outcome measure for the economic evaluation was quality‐of‐life adjusted life years (QALYs) as assessed with the EQ‐5D‐5L (EuroQol Group, 1990), which demonstrates adequate psychometric properties (Feng et al., 2021). The Dutch tariff (Versteegh et al., 2016) was used to translate EQ‐5D‐5L scores to utility values. Subsequently, QALYs were calculated over the 14 month follow‐up period using the area‐under‐the‐curve method.
Because generic health questionnaires like the EQ‐5D‐5L might be limited in their extent to detect changes in wellbeing for interventions aimed at mental health (Pietersma et al., 2013) the economic evaluation was also conducted using the ICECAP‐A (Al‐Janabi et al., 2012). Psychometric properties of the ICECAP‐A have been found to be adequate (Afentou & Kinghorn, 2020; Rohrbach, Dingemans, Essers, et al., 2021). A capability value anchored at 0 (no capability) and 1 (full capability) was calculated for each participant using the ICECAP‐A Dutch tariffs (Rohrbach, Dingemans, Groothuis‐Oudshoorn, et al., 2021) over the 14 month study period. Details on the used quality‐of‐life instruments and accompanying transformations can be found in supplemental material (S1).
3.3.3. Costs
Health care costs included intervention costs and use of health care services. Intervention costs for Featback included 5 min of technical support by a researcher (including setting up an account, redirecting participants to professional help in the case of severe symptom deterioration and responding to technical problems) multiplied by their hourly rate (€31.50). For expert‐patient support, costs were calculated by multiplying their hourly rate (€22.31) with the time spent on support sessions (i.e., estimated at 30 min for each session, including preparation and administration). Additionally, supervision costs were calculated by dividing the total time spent on supervision (i.e., 14 one‐hour sessions attended by six expert patients with €22.31 hourly wage, one researcher with €31.50 hourly wage, and one clinical psychologist with €106.17 hourly wage) by the number of participants in the two conditions with the possibility of expert‐patient support. All wages were determined based on the real wages during the conduct of the study.
Health care costs were measured with the TiC‐P midi (Timman et al., 2015) at each assessment (i.e., over an 8‐week period at postintervention and 3 months at all other follow‐up assessments). The midi version was chosen over the full version as it reduced the time burden for participants while maintaining a reliable estimate of health service use (Timman et al., 2015). Finally, visits to the general practitioner, dietician, psychologist based in mental health institutions, the private section or hospitals, medical specialist, the emergency department, daycare in mental health institutions, and hospitalizations either in the hospital or a mental health institution were included as health care costs. After inspecting the data for errors and possible double counts, the number of visits to each health care provider was multiplied with their cost prices as indicated by the Dutch guidelines for cost research in health care (Hakkaart‐van Roijen et al., 2015; Kanters et al., 2017). All assessed health care services with their reference price are presented in Table 1.
TABLE 1.
Price references
| Category | Reference price | CPI index 2014–2021 | CPI index 2019–2021 | Final cost price (2021) | |
|---|---|---|---|---|---|
| Intervention costs a | |||||
| Featback (5 min researcher coordination per participant; hourly wage of €30.72) | €2.56 | 1.025 | €2.62 | ||
| Expert‐patient support session (30 min per session; hourly wage of €22.31) | €11.16 | 1.025 | €11.44 | ||
| Supervision costs per participant | €21.38 | ||||
| Direct health care costs | |||||
| General practitioner | €33.00 | 1.095 | €36.15 | ||
| Dietician | €33.00 | 1.095 | €36.15 | ||
| Psychologist, psychotherapist or psychiatrist–mental health care | €98.00 | 1.095 | €107.35 | ||
| Psychologist, psychotherapist or psychiatrist ‐ independent | €94.44 | 1.095 | €103.45 | ||
| Psychologist, psychotherapist or psychiatrist ‐ hospital | €91.00 | 1.095 | €99.68 | ||
| Medical specialist | €91.00 | 1.095 | €99.68 | ||
| Emergency department | €259.00 | 1.095 | €283.70 | ||
| Day treatment ‐ mental health care | €183.05 | ||||
| Hospitalization ‐ mental health care | €302.36 | 1.095 | €331.20 | ||
| Hospitalization–hospital | €476.00 | 1.095 | €521.40 | ||
| Indirect costs | |||||
| Average gross hourly female wage | €31.60 | 1.095 | €34.61 | ||
| Average gross hourly domestic worker wage | €14.00 | 1.095 | €15.34 | ||
Note: Dutch CPI indexes for 2021, 2019 and 2014 were 108.88, 106.2, and 99.4 respectively.
Abbreviation: CPI, cost price index.
Wages of the research coordinator, expert patient, and clinical psychologist (supervision) were based on the real wages during the conduct of the study.
Nonhealth care costs were measured with the Productivity Costs Questionnaire (PCQ) (Bouwmans et al., 2015), including costs related to absence from work (absenteeism), reduced productivity at work because of health problems (presenteeism), and reduced productivity of unpaid work such as domestic chores because of health problems. Absenteeism costs were calculated by multiplying the recalled hours of missed work over the last 4 weeks extrapolated to 8 weeks (at postintervention) or 3 months (at follow‐up measurements) by the average gross hourly wage of female working individuals in the Netherlands (Hakkaart‐van Roijen et al., 2015). In cases of longer absence through illness the friction cost method was applied, meaning that no costs were incurred after being absent for 12 weeks, because initial production levels were expected to have been restored by that time. Presenteeism costs were calculated by multiplying the recalled hours with reduced productivity because of health problems over the last 4 weeks extrapolated to 8 weeks or 3 months by the average gross hourly wage of female working individuals in the Netherlands (Hakkaart‐van Roijen et al., 2015). Lastly, costs related to reduced productivity of unpaid work was calculated by multiplying the recalled hours in which others had to perform domestic chores instead of the participant in the last 4 weeks extrapolated to 8 weeks or 3 months by the average gross hourly wage of a domestic worker (Hakkaart‐van Roijen et al., 2015). Gross hourly wages are presented in Table 1.
All costs were indexed to the year 2021 using the Dutch consumer price index (OECD, 2021). No discounting was applied to QALYs and costs, given that the time horizon was slightly more than 1 year.
3.4. Missing data
Baseline values of the EQ‐5D‐5L and ICECAP‐A were not collected. As these variables appear stable over a relatively short period (i.e., 8 weeks) of time, they were estimated to be equal to those at postintervention for the main analyses. This assumption was tested using sensitivity analyses.
According to the intention‐to‐treat approach, all participants who completed baseline were included throughout the analyses. Missing data were multiply imputed (Rubin, 1987) using the software program R version 3.5.1. Details on the multiple imputation procedure can be found in the supplemental materials (S2).
3.5. Statistical analyses
Costs, both health care and societal, and effects in terms of QALYs (EQ‐5D‐5L) and capabilities (ICECAP‐A) over the 14 month period were compared between the four conditions using analyses of variance (ANOVA) pooled across imputations (Rubin, 1987; Van Ginkel & Kroonenberg, 2014). Multiple testing was corrected for using Holm's method (Holm, 1979). Cost‐utility analyses were conducted with QALYs and societal costs over the 14 month follow‐up period. Specifically, QALYs and costs were averaged over the 100 imputed datasets. Subsequently, a bootstrap procedure simulating 1000 samples drawn from the average imputation sample was conducted in Microsoft Excel to estimate the uncertainty regarding mean costs and QALYs. Mean costs and QALYs per study condition were used to calculate the incremental net benefit (INB) for each condition. To calculate the INB, first, society's willingness to pay (WTP) for one extra year lived in perfect health (i.e., 1 QALY) was multiplied with the QALY gain in a condition, which expresses the effect in monetary terms. Subtracting the costs for this condition resulted in its INB. The 1000 INBs for each condition were used to calculate the probability of a condition to be cost‐effective compared to the other conditions for a range of WTP values. In the Netherlands the willingness to pay is assumed to vary between 20,000 euro per QALY for interventions in the context of “low disease burden” to 80,000 euro per QALY in the context of severe diseases (Zwaap et al., 2015). To accommodate all relevant WTP values, the current study explored values ranging from €0 to €100,000. The results were presented in cost‐utility acceptability curves for the four conditions separately.
Four sensitivity analyses were conducted to investigate the robustness of the results. Specifically, using the average imputation sample, cost‐utility analyses were repeated with (1) capability values based on ICECAP‐A scores resulting in cost‐capability acceptability curves, (2) QALYs based on utility scores obtained from the visual analogue scale of the EQ‐5D‐5L (raw scores divided by 100), and (3) direct health care costs only instead of societal costs. Lastly, because baseline scores of the EQ‐5D‐5L were unavailable, a sensitivity analysis (4) was performed where baseline scores of the EQ‐5D‐5L were estimated using the 4‐item Patient Health Questionnaire (PHQ‐4). Equipercentile mapping was used to translate baseline PHQ‐4 scores into EQ‐5D‐5L scores. These were then used to calculate adjusted QALYs for the cost‐utility acceptability curves. Details on the mapping procedure can be found in the supplemental materials (S3).
4. RESULTS
4.1. Participants
In total, 355 participants completed informed consent and the baseline assessment and were included in the analyses. Retention of participants at baseline (T0), postintervention (8 weeks; T1) and 3, 6, 9, and 12 month follow‐up (T2–T5) was 355 (100%), 280 (78.9%), 252 (71.0%), 244 (68.7%), 233 (65.6%), and 242 (68.2%) respectively. Study drop‐out rates did not differ between conditions at postintervention, χ 2 (3) = 3.99, p = .26, or 12 month follow‐up, χ 2 (3) = 4.90, p = .18. No differences in stopping with the intervention between the three active interventions were found, χ 2 (2) = 1.24, p = .54. Baseline characteristics of participants are presented in Table 2.
TABLE 2.
Baseline characteristics of participants
| Characteristics | Featback (N = 88) | Featback + expert patient support (N = 90) | Expert patient support (N = 87) | Waiting list (N = 90) | Total sample (N = 355) | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female (%) | 82 (93.2) | 89 (98.9) | 84 (96.6) | 88 (97.8) | 343 (96.7) | |
| Male (%) | 5 (5.7) | 1 (1.1) | 1 (1.1) | 2 (2.2) | 9 (2.5) | |
| Other (%) | 1 (1.1) | 0 (0.0) | 2 (2.3) | 0 (0.0) | 3 (0.8) | |
| Nationality | ||||||
| Dutch (%) | 78 (88.6) | 80 (88.9) | 80 (92.0) | 81 (90.0) | 319 (89.9) | |
| Belgian (%) | 9 (10.2) | 9 (10.0) | 6 (6.9) | 8 (8.9) | 32 (9.0) | |
| Other (%) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 4 (1.1) | |
| Education | ||||||
| Low (%) | 5 (5.6) | 12 (13.3) | 12 (13.7) | 18 (20.5) | 47 (13.3) | |
| Middle (%) | 33 (37.5) | 31 (34.4) | 34 (39.0) | 35 (39.3) | 133 (37.6) | |
| High (%) | 50 (56.8) | 47 (52.2) | 41 (47.1) | 36 (40.4) | 174 (49.2) | |
| Treatment history for ED | ||||||
| Yes (%) | 46 (52.3) | 54 (54.0) | 53 (60.9) | 49 (54.4) | 202 (56.9) | |
| No (%) | 42 (47.7) | 36 (36.0) | 34 (39.1) | 41 (45.6) | 153 (43.1) | |
| Self‐reported diagnosis status | ||||||
| Officially diagnosed with ED | 52 (59.1) | 60 (66.7) | 52 (59.8) | 58 (64.4) | 222 (62.5) | |
| No diagnosis, but assumed to have ED | 24 (27.3) | 22 (24.4) | 23 (26.4) | 22 (24.4) | 91 (25.6) | |
| Eating problems, but likely no ED diagnosis | 12 (13.6) | 8 (8.9) | 12 (13.7) | 10 (11.1) | 42 (11.8) | |
| Marital status | ||||||
| Married/living together (%) | 20 (22.7) | 22 (24.4) | 26 (29.9) | 30 (33.3) | 98 (27.6) | |
| Living alone (%) | 68 (77.3) | 66 (73.3) | 58 (66.7) | 58 (64.4) | 250 (70.4) | |
| Divorced (%) | 0 (0.0) | 1 (1.1) | 3 (3.4) | 2 (2.2) | 6 (1.6) | |
| Widow (%) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 1 (0.2) | |
| Age (Years) | 28.0 (1.7) | 28.3 (10.4) | 26.8 (9.4) | 28.1 (12.4) | 27.8 (10.8) | |
| Weight (kg) | 64.0 (21.0) | 62.2 (18.3) | 63.6 (22.0) | 64.7 (23.4) | 63.6 (21.2) | |
| Height (cm) | 169.9 (7.2) | 168.5 (6.9) | 169.7 (7.1) | 169.5 (6.9) | 169.4 (7.0) | |
| Years with ED | 10.1 (9.1) | 10.3 (8.8) | 8.6 (8.2) | 11.4 (12.0) | 10.1 (9.7) | |
| Internet usage (hours per day) | 4.2 (2.6) | 3.7 (2.2) | 3.9 (2.3) | 3.4 (2.8) | 3.8 (2.5) | |
| Eating disorder symptoms (EDE‐Q) | 3.9 (1.1) | 4.1 (1.1) | 4.3 (1.0) | 4.3 (1.0) | 4.1 (1.0) | |
Note: Data are presented as means (standard deviation) unless indicated otherwise.
Abbreviations: ED, eating disorder; EDE‐Q, Eating Disorder Examination Questionnaire.
4.2. Quality of life
EQ‐5D‐5L utility values and ICECAP‐A index scores for all measurement points, as well as QALYs and capability values over the total study duration (12 months + 8 weeks) are presented in Table 3. Average QALYs were highest in the Featback with expert‐patient support condition and lowest for the care‐as‐usual condition. However, no significant differences in QALYs between the four conditions were found. Similarly, no differences in improvements on capabilities as derived from the ICECAP‐A between the four conditions were found.
TABLE 3.
Means (standard errors) of utilities, QALYs and capabilities
| Mean value per participant (SE) | |||||||
|---|---|---|---|---|---|---|---|
| Category | Featback (n = 88) | Featback + expert patient support (n = 90) | Expert patient support (n = 87) | Waiting list (n = 90) | Total (n = 355) | Pooled F‐statistic | |
| EQ‐5D‐5L utilities | |||||||
| Postintervention (T1; 8 weeks) | 0.68 (0.03) | 0.68 (0.03) | 0.61 (0.03) | 0.58 (0.03) | 0.64 (0.01) | F(3, 333) = 3.01, p = .03 | |
| 3‐month follow‐up (T2) | 0.62 (0.03) | 0.68 (0.03) | 0.60 (0.03) | 0.58 (0.04) | 0.62 (0.02) | F(3, 326) = 1.88, p = .13 | |
| 6‐month follow‐up (T3) | 0.69 (0.03) | 0.69 (0.03) | 0.62 (0.03) | 0.60 (0.04) | 0.65 (0.02) | F(3, 321) = 1.90, p = .13 | |
| 9‐month follow‐up (T4) | 0.61 (0.04) | 0.65 (0.04) | 0.63 (0.04) | 0.62 (0.04) | 0.63 (0.02) | F(3, 311) = 0.19, p = .91 | |
| 12‐month follow‐up (T5) | 0.66 (0.03) | 0.71 (0.03) | 0.66 (0.03) | 0.64 (0.04) | 0.67 (0.02) | F(3, 317) = 0.61, p = .61 | |
| EQ‐5D Visual Analogue Scale utilities | |||||||
| Postintervention (T1; 8 weeks) | 0.60 (0.02) | 0.59 (0.02) | 0.55 (0.02) | 0.55 (0.02) | 0.57 (0.01) | F(3, 331) = 1.05, p = .37 | |
| 3‐month follow‐up (T2) | 0.55 (0.02) | 0.56 (0.02) | 0.55 (0.02) | 0.57 (0.02) | 0.56 (0.01) | F(3, 333) = 0.22, p = .88 | |
| 6‐month follow‐up (T3) | 0.61 (0.02) | 0.57 (0.02) | 0.54 (0.02) | 0.58 (0.02) | 0.57 (0.01) | F(3, 325) = 1.77, p = .15 | |
| 9‐month follow‐up (T4) | 0.57 (0.02) | 0.59 (0.02) | 0.56 (0.02) | 0.59 (0.02) | 0.58 (0.01) | F(3, 318) = 0.43, p = .73 | |
| 12‐month follow‐up (T5) | 0.60 (0.02) | 0.61 (0.02) | 0.57 (0.02) | 0.59 (0.02) | 0.59 (0.01) | F(3, 326) = 0.52, p = .67 | |
| ICECAP‐A capability values | |||||||
| Postintervention (T1; 8 weeks) | 0.69 (0.02) | 0.68 (0.02) | 0.63 (0.02) | 0.65 (0.03) | 0.66 (0.01) | F(3, 329) = 1.40, p = .24 | |
| 3‐month follow‐up (T2) | 0.68 (0.02) | 0.69 (0.02) | 0.62 (0.02) | 0.66 (0.03) | 0.66 (0.01) | F(3, 324) = 1.48, p = .22 | |
| 6‐month follow‐up (T3) | 0.70 (0.03) | 0.69 (0.03) | 0.64 (0.03) | 0.65 (0.03) | 0.67 (0.03) | F(3, 316) = 1.01, p = .39 | |
| 9‐month follow‐up (T4) | 0.67 (0.03) | 0.68 (0.03) | 0.65 (0.03) | 0.69 (0.03) | 0.67 (0.01) | F(3, 305) = 0.44, p = .72 | |
| 12‐month follow‐up (T5) | 0.72 (0.02) | 0.72 (0.03) | 0.64 (0.03) | 0.72 (0.03) | 0.70 (0.01) | F(3, 313) = 2.09, p = .10 | |
| Total QALYs EQ‐5D‐5L a | 0.75 (0.03) | 0.78 (0.03) | 0.71 (0.03) | 0.69 (0.03) |
0.74 (0.02) |
F(3, 337) = 1.87, p = .14 | |
| Total QALYs EQ‐5D Visual Analogue Scale a | 0.67 (0.02) | 0.64 (0.02) | 0.69 (0.02) | 0.65 (0.02) | 0.66 (0.01) | F(3, 339) = 0.74, p = .53 | |
| Total capability values ICECAP‐A a | 0.79 (0.02) | 0.79 (0.02) | 0.73 (0.02) | 0.77 (0.03) | 0.77 (0.01) |
F(3, 328) = 1.31, p = .27 |
|
Calculated over the entire 14‐month study duration.
Abbreviation: SE, standard error.
4.3. Costs
Intervention costs, health care costs and nonhealth care costs are presented in Table 4. Intervention costs were significantly higher in conditions with expert‐patient support. Lowest health care costs were found in the Featback only condition, while highest costs were found in the care‐as‐usual condition. The relatively low‐health care costs in the Featback only condition could mostly be attributed to fewer participants being hospitalized in that condition. Average societal costs per participant over the study duration were again lowest in the Featback only condition and highest in the care‐as‐usual condition. Although the omnibus test was significant, after a Holm correction for multiple testing, pooled ANOVA tests revealed no significant difference between the four conditions for health care costs and societal costs.
TABLE 4.
Means (standard errors) of costs per study condition over the course of 14 months in 2021 euros with the percentage of participants that incurred the costs
| Mean costs per participant (SE) (% of participants incurring costs) | |||||||
|---|---|---|---|---|---|---|---|
| Category | Featback (n = 88) | Featback + expert patient support (n = 90) | Expert patient support (n = 87) | Waiting list (n = 90) | Total (n = 355) | Pooled F‐statistic | |
| Total intervention costs | 3 (0) | 65 (4) | 72 (4) | 0 (0) | 35 (2) | F(3, 351) = 226,45, p < .001 | |
| Health care costs | |||||||
| General practitioner | 288 (33) [93%] | 260 (39) [92%] | 215 (28) [87%] | 320 (36) [95%] | 271 (17) [92%] | F(3, 335) = 1.69, p = .17 | |
| Dietician | 122 (25) [52%] | 217 (37) [61%] | 133 (31) [53%] | 202 (38) [58%] | 169 (17) [56%] | F(3, 334) = 2.12, p = .10 | |
| Psychologist, psychotherapist or psychiatrist–mental health care | 1483 (298) [55%] | 1745 (369) [58%] | 2723 (448) [70%] | 1810 (312) [66%] | 1936 (184) [62%] | F(3, 336) = 2.23, p = .08 | |
| Psychologist, psychotherapist or psychiatrist ‐ independent | 1005 (186) [57%] | 869 (153) [59%] | 1195 (222) [64%] | 1052 (188) [63%] | 1029 (95) [61%] | F(3, 335) = 0.52, p = .67 | |
| Psychologist, psychotherapist or psychiatrist ‐ hospital | 617 (181) [40%] | 241 (75) [32%] | 714 (188) [43%] | 549 (138) [43%] | 528 (76) [39%] | F(3, 340) = 1.83, p = .14 | |
| Medical specialist | 220 (50) [46%] | 225 (47) [56%] | 204 (43) [46%] | 297 (53) [57%] | 237 (24) [52%] | F(3, 332) = 0.73, p = .53 | |
| Emergency department | 74 (68) [3%] | 28 (27) [5%] | 9 (11) [3%] | 54 (26) [7%] | 41 (20) [5%] | F(3, 346) = 0.52, p = .67 | |
| Day treatment ‐ mental health care | 827 (264) [25%] | 642 (244) [24%] | 1102 (338) [33%] | 1542 (547) [28%] | 1029 (186) [27%] | F(3, 339) = 1.14, p = .33 | |
| Hospitalization ‐ mental health care | 2557 (1105) [14%] | 4385 (1758) [19%] | 4417 (1908) [20%] | 9158 (2524) [30%] | 5150 (967) [21%] | F(3, 338) = 2.24, p = .08 | |
| Hospitalization–hospital | 528 (282) [15%] | 1537 (589) [19%] | 1923 (900) [19%] | 2836 (952) [27%] | 1711 (370) [20%] | F(3, 338) = 1.71, p = .16 | |
| Total health care costs | 7722 (1535) | 10,215 (2281) | 12,705 (2808) | 17,820 (3349) | 12,135 (1316) | F(3, 340) = 2.81, p = .04 | |
| Nonhealth care costs | |||||||
| Absenteeism | 1456 (388) [38%] | 2602 (690) [49%] | 1925 (514) [39%] | 2117 (518) [43%] | 2029 (271) [42%] | F(3, 328) = 0.78, p = .51 | |
| Presentism | 3142 (630) [59%] | 5122 (937) [77%] | 1968 (437) [62%] | 2588 (567) [68%] | 3216 (342) [66%] | F(3, 333) = 4.16, p = .01 | |
| Substitution of unpaid work | 4421 (870) [79%] | 6042 (1183) [82%] | 7022 (1489) [88%] | 5954 (1139) [79%] | 5858 (586) [82%] | F(3, 337) = 0.8, p = .49 | |
| Total societal costs | 16,741 (2023) | 23,980 (3277) | 23,620 (3365) | 28,479 (3736) | 23,238 (1612) | F(3, 340) = 2.34, p = .07 | |
Abbreviation: SE, standard error.
4.4. Cost‐effectiveness
Cost‐utility acceptability curves are presented in Figure 1. For values of the WTP for one additional QALY between €0 and €100,000, offering the Featback only condition had the highest probability of being efficient for the four alternatives (66%–86%). In other words, between the four conditions, Featback only had the highest probability of having the largest INB across the 1000 bootstrap samples, regardless of the WTP. At very high‐WTP values, the probability of the combination of Featback with expert‐patient support to be efficient compared to the alternatives increased, but still did not exceed the Featback only condition. The care‐as‐usual condition had a probability of up to 13% of being optimal compared to the alternatives for all WTP values. This probability was around 1% across all WTP values for the expert‐patient support only condition.
FIGURE 1.
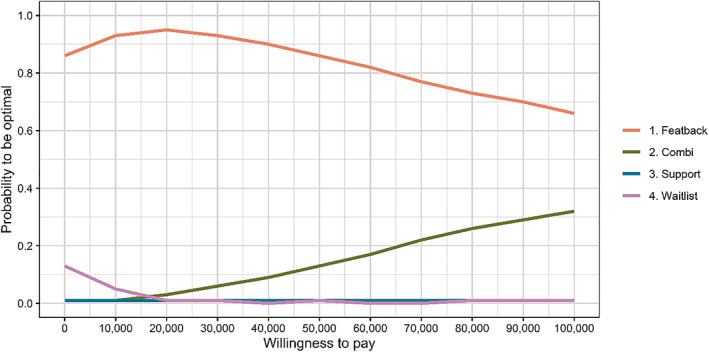
Cost‐utility acceptability curves with EQ‐5D QALYs for the four study conditions derived from 1000 bootstrap samples.
4.5. Sensitivity analyses
As can be deduced from Figure 2, results were highly similar for cost‐capability acceptability curves, where gains for a particular condition were measured as capability values as assessed with the ICECAP‐A. Specifically, the Featback only condition had the highest probability of being efficient compared to the other three conditions across all WTP values (72%–86%). A second sensitivity analysis using the visual analogue scale of the EQ‐5D‐5L to assess QALYs also showed results comparable to the main analysis. Third, cost‐utility acceptability curves of the EQ‐5D‐5L with direct health care costs only showed the Featback only condition to have the highest probability of being efficient (51%–78%) compared to the three alternatives for WTP values of €60,000 or less. For WTP values between €70,000 and €100,000, Featback with expert‐patient support had the highest probability of being efficient among the four conditions (52%–57%). Fourth, when baseline values of the EQ‐5D‐5L were estimated using equipercentile mapping with the PHQ‐4, an almost identical pattern to the main analysis emerged with the Featback only condition having the highest probability to be efficient compared to the three other conditions across all WTP values (67%–95%). Cost‐effectiveness acceptability curves of the last three sensitivity analyses can be found in the supplemental materials (S4).
FIGURE 2.
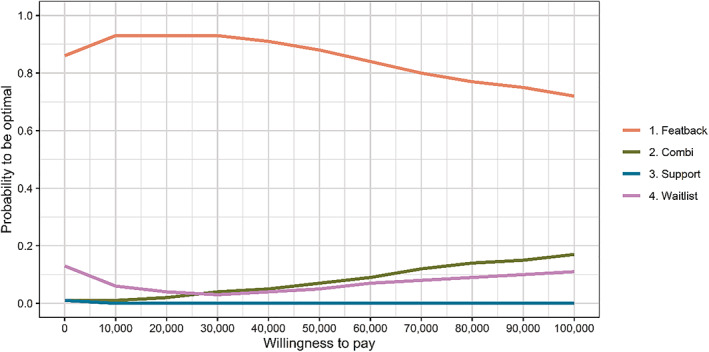
Cost‐capability acceptability curves with ICECAP‐A capability values for the four study conditions derived from 1000 bootstrap samples.
5. DISCUSSION
In the current study, an economic evaluation based on a randomized controlled trial covering a period of 14 months was conducted comparing (1) a fully automated internet‐based intervention 'Featback', (2) online support by expert patients via mail and chat, and (3) the combination of these to (4) care as usual for people with eating disorders. Primarily, from a societal perspective, the Featback intervention had the highest probability of being efficient for a wide range of WTP values compared to the three other conditions. Secondly, expert‐patient support alone and care as usual had very low probabilities of being efficient compared to the alternatives over the whole range of explored WTP values. Lastly, the combination of Featback and expert‐patient support was more efficient than a care‐as‐usual condition for WTP values over €20,000, but less than Featback alone. The results suggest that, between the four investigated conditions, Featback is the intervention of choice from a (societal) economic perspective. Despite severe and long‐lasting symptoms, 43% of participants in the sample never received treatment for their eating problems, demonstrating the potential of internet‐based interventions to reach an underserved population. Notably, as Featback and expert‐patient support are brief interventions, it might be that their impact on quality of life or health care and societal costs is more distinct for people with less severe symptoms or at the beginning stages of their eating disorder, but it proved difficult to reach this group. Furthermore, while around 97% of the Dutch population older than 12 years has internet access, some individuals with eating disorder symptoms cannot be reached through internet or find it challenging to work with and require a different approach. However, implementing the unguided Featback intervention could help to reach individuals with an eating disorder who currently do not receive appropriate care and is likely to lead to similar or slightly better quality of life while reducing costs for society, compared to not implementing it.
The findings are in line with the two studies mentioned in the introduction, cautiously indicating online interventions for eating disorders to be cost‐effective compared to care as usual (Akers et al., 2021; Kass et al., 2017). A previous trial concerning Featback indicated that Featback with or without guidance from a psychologist had higher probabilities of being efficient compared to a care‐as‐usual condition (Aardoom, Dingemans, van Ginkel, et al., 2016). In the current trial, the automed feedback messages were improved and more personalized towards users and support from a psychologist was replaced with expert‐patient support, in an attempt to increase the effectiveness of and satisfaction with the interventions. Unexpectedly, conditions with expert‐patient guidance were less efficient than Featback alone for all acceptable WTP values. The contrast with the previous trial, where Featback was equally efficient with and without therapist support, might partly be explained by the improvements to the automated monitoring system, possibly increasing its effectiveness. However, this is unlikely given that the combination condition also included the improved monitoring system, but was outperformed by Featback alone. Another explanation of the favorable probabilities of the Featback only condition in the cost‐utility acceptability curves were the (nonsignificantly) lower costs for several categories, resulting in nonsignificantly lower total costs. Mainly, a low percentage of people in the Featback condition was hospitalized compared to the other conditions. The finding suggests that brief weekly monitoring and feedback messages can prevent hospitalization. Self‐monitoring has been found to be important in preventing psychiatric hospitalization (Ådnanes et al., 2020). However, if the Featback monitoring system would have this effect, lower hospitalization rates in the combination condition would also be expected. Therefore, other explanations why the Featback condition had favorable results, such as chance, cannot be ruled out. Looking across mental disorders, adding guidance appears to increase the probability of being cost‐effective compared to unguided alternatives (Donker et al., 2015). However, given the scarcity of evidence, more research directly comparing guided and unguided internet‐based interventions is required for decisive conclusions.
More generally, an increasing number of studies is substantiating the claim that internet interventions are likely to be cost‐effective compared to care as usual for a number of mental disorders (Ahern et al., 2018; Donker et al., 2015; Hedman et al., 2012; Paganini et al., 2018), but evidence is mixed (Kolovos et al., 2018). The systematic reviews and meta‐analysis highlight the need to continue economic evaluation research, since heterogeneity in intervention content and application of guidance make it difficult to reach definitive conclusions. For eating disorder treatment, current evidence indicates that online self‐monitoring is likely an efficient alternative to usual care, while more information is needed on whether and how to add guidance. Concordantly, internet‐based interventions such as Featback have the potential to help individuals currently not reached by traditional treatment options, while being worth the investment.
5.1. Strengths and limitations
The current study had several strengths, including a 12‐month follow‐up period with a societal perspective, a large sample size, several sensitivity analyses and multiple imputation procedures to handle missing data. Some limitations can also be noted. First, baseline values of quality of life and wellbeing questionnaires were not collected. Although a sensitivity analysis with estimated baseline scores produced similar results, the missing values may have led to a slight underestimation of the QALYs and capability values in the active interventions. Relatedly, it could be worthwhile to study which generic preference‐based measures are sensitive in eating disorder populations, as both the EQ‐5D and ICECAP might be limited in this regard. A second limitation pertains to missing data due to dropout of the current sample. While missing data were handled adequately, imputing cost data was challenging since for some categories only a small percentage of people incurred relatively high costs while others incurred no costs. Thirdly, although many costs were accounted for, assessed direct and nonhealth care costs were not exhaustive. For example, medication costs, costs related to internet access and use, and costs attributable to alleviating symptoms were not captured, which may have influenced results slightly. Lastly, cost data were based on self‐reported health care visits and work productivity over a period of 4 weeks. This may have introduced recall bias.
6. CONCLUSION
A brief fully automated internet‐based self‐help program (Featback) for eating disorders was found to be efficient compared to care as usual. Results suggest that such interventions may be especially valuable as a first step in a stepped‐care model of eating disorder treatment, as it is preferable over a do‐nothing approach. Implementing highly scalable and low‐threshold interventions such as Featback would not only benefit individuals suffering from an eating disorder, but society as a whole. Mental health professionals and researchers would profit from further investigating how to widely disseminate such interventions to optimize the potential benefits, both in terms of effects and costs. Furthermore, subsidy providers, policy makers, and health insurancies should consider wider funding to make installment of evidence‐based internet interventions for eating disorders possible, as they appear to be worth the investment.
AUTHOR CONTRIBUTIONS
Pieter J. Rohrbach: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; software; validation; visualization; writing – original draft; writing – review and editing. Alexandra E. Dingemans: Conceptualization; funding acquisition; methodology; project administration; supervision; writing – original draft; writing – review and editing. Eric F. van Furth: Conceptualization; funding acquisition; methodology; project administration; supervision; writing – review and editing. Philip Spinhoven: Conceptualization; funding acquisition; methodology; project administration; supervision; writing – review and editing. Joost R. van Ginkel: Conceptualization; data curation; formal analysis; methodology; resources; software; writing – review and editing. Stephanie Bauer: Resources; software; writing – review and editing. M. Elske van den Akker‐Van Marle: Conceptualization; formal analysis; methodology; project administration; resources; software; supervision; validation; writing – original draft; writing – review and editing.
CONFLICT OF INTEREST
Pieter J. Rohrbach reports a grant from ZonMw during the conduct of the study. No other disclosures were reported.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGMENTS
The work was funded by ZonMw (636310001).
Rohrbach, P. J. , Dingemans, A. E. , van Furth, E. F. , Spinhoven, P. , van Ginkel, J. R. , Bauer, S. , van den Akker‐Van Marle, M. E. (2022). Cost‐effectiveness of three internet‐based interventions for eating disorders: A randomized controlled trial. International Journal of Eating Disorders, 55(8), 1143–1155. 10.1002/eat.23763
Action Editor: Ruth Striegel Weissman
Funding information ZonMw, Grant/Award Number: 636310001
DATA AVAILABILITY STATEMENT
Data, materials, and code are available upon reasonable request.
REFERENCES
- Aardoom, J. J. , Dingemans, A. E. , Spinhoven, P. , van Ginkel, J. R. , de Rooij, M. , & van Furth, E. F. (2016). Web‐based fully automated self‐help with different levels of therapist support for individuals with eating disorder symptoms: A randomized controlled trial. Journal of Medical Internet Research, 18(6), e159. 10.2196/jmir.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aardoom, J. J. , Dingemans, A. E. , & van Furth, E. F. (2016). E‐health interventions for eating disorders: Emerging findings, issues, and opportunities. Current Psychiatry Reports, 18(4), 1–8. 10.1007/s11920-016-0673-6 [DOI] [PubMed] [Google Scholar]
- Aardoom, J. J. , Dingemans, A. E. , van Ginkel, J. R. , Spinhoven, P. , van Furth, E. F. , & van den Akker‐van Marle, M. E. (2016). Cost‐utility of an internet‐based intervention with or without therapist support in comparison with a waiting list for individuals with eating disorder symptoms: A randomized controlled trial. International Journal of Eating Disorders, 49(12), 1068–1076. 10.1002/eat.22587 [DOI] [PubMed] [Google Scholar]
- Ådnanes, M. , Cresswell‐Smith, J. , Melby, L. , Westerlund, H. , Šprah, L. , Sfetcu, R. , Straßmayr, C. , & Donisi, V. (2020). Discharge planning, self‐management, and community support: Strategies to avoid psychiatric rehospitalisation from a service user perspective. Patient Education and Counseling, 103(5), 1033–1040. 10.1016/j.pec.2019.12.002 [DOI] [PubMed] [Google Scholar]
- Afentou, N. , & Kinghorn, P. (2020). A systematic review of the feasibility and psychometric properties of the ICEpop CAPability measure for adults and its use so far in economic evaluation. Value in Health, 23(4), 515–526. 10.1016/j.jval.2019.12.010 [DOI] [PubMed] [Google Scholar]
- Ágh, T. , Kovács, G. , Supina, D. , Pawaskar, M. , Herman, B. K. , Vokó, Z. , & Sheehan, D. V. (2016). A systematic review of the health‐related quality of life and economic burdens of anorexia nervosa, bulimia nervosa, and binge eating disorder. Eating and Weight Disorders, 21(3), 353–364. 10.1007/s40519-016-0264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern, E. , Kinsella, S. , & Semkovska, M. (2018). Clinical efficacy and economic evaluation of online cognitive behavioral therapy for major depressive disorder: A systematic review and meta‐analysis. Expert Review of Pharmacoeconomics & Outcomes Research, 18(1), 25–41. 10.1080/14737167.2018.1407245 [DOI] [PubMed] [Google Scholar]
- Akers, L. , Rohde, P. , Shaw, H. , & Stice, E. (2021). Cost‐effectiveness comparison of delivery modalities for a dissonance‐based eating disorder prevention program over 4‐year follow‐up. Prevention Science, 22, 1086–1095. 10.1007/s11121-021-01264-1 [DOI] [PubMed] [Google Scholar]
- Al‐Janabi, H. , Flynn, T. N. , & Coast, J. (2012). Development of a self‐report measure of capability wellbeing for adults: The ICECAP‐A. Quality of Life Research, 21(1), 167–176. 10.1007/s11136-011-9927-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcelus, J. , Mitchell, A. J. , Wales, J. , & Nielsen, S. (2011). Mortality rates in patients with anorexia nervosa and other eating disorders: A meta‐analysis of 36 studies. Archives of General Psychiatry, 68(7), 724–731. 10.1001/archgenpsychiatry.2011.74 [DOI] [PubMed] [Google Scholar]
- Austin, A. , Flynn, M. , Richards, K. , Hodsoll, J. , Duarte, T. A. , Robinson, P. , Kelly, J. , & Schmidt, U. (2020). Duration of untreated eating disorder and relationship to outcomes: A systematic review of the literature. European Eating Disorders Review, 1‐17, 329–345. 10.1002/erv.2745 [DOI] [PubMed] [Google Scholar]
- Bauer, S. , Winn, S. , Schmidt, U. , & Kordy, H. (2005). Construction, scoring and validation of the short evaluation of eating disorders (SEED). European Eating Disorders Review, 13, 191–200. 10.1002/erv.637 [DOI] [Google Scholar]
- Bode, K. , Götz von Olenhusen, N. M. , Wunsch, E. M. , Kliem, S. , & Kröger, C. (2017). Population‐based cost‐offset analyses for disorder‐specific treatment of anorexia nervosa and bulimia nervosa in Germany. International Journal of Eating Disorders, 50(3), 239–249. 10.1002/eat.22686 [DOI] [PubMed] [Google Scholar]
- Bouwmans, C. , Krol, M. , Severens, H. , Koopmanschap, M. , Brouwer, W. , & Hakkaart‐van Roijen, L. (2015). The IMTA productivity cost questionnaire: A standardized instrument for measuring and valuing health‐related productivity losses. Value in Health, 18, 753–758. 10.1016/j.jval.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Crow, S. J. , Mitchell, J. E. , Crosby, R. D. , Swanson, S. A. , Wonderlich, S. , & Lancanster, K. (2009). The cost effectiveness of cognitive behavioral therapy for bulimia nervosa delivered via telemedicine versus face‐to‐face. Behaviour Research and Therapy, 47(6), 451–453. 10.1016/j.brat.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloitte Access Economics . (2020). The social and economic cost of eating disorders in The United States of America: A report for the strategic training initiative for the prevention of eating disorders and the academy for eating disorders. https://www.hsph.harvard.edu/striped/report-economic-costs-of-eating-disorders/
- Donker, T. , Blankers, M. , Hedman, E. , Ljótsson, B. , Petrie, K. , & Christensen, H. (2015). Economic evaluations of internet interventions for mental health: A systematic review. Psychological Medicine, 45, 3357–3376. 10.1017/S0033291715001427 [DOI] [PubMed] [Google Scholar]
- Erskine, H. E. , Whiteford, H. A. , & Pike, K. M. (2016). The global burden of eating disorders. Current Opinion in Psychiatry, 29(6), 346–353. 10.1097/YCO.0000000000000276 [DOI] [PubMed] [Google Scholar]
- EuroQol Group . (1990). EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy, 16, 199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- Fairburn, C. G. , & Beglin, S. J. (2008). Assessment of eating disorders: Interview or self‐report questionnaire? International Journal of Eating Disorders, 16(4), 363–370. [DOI] [PubMed] [Google Scholar]
- Feng, Y. S. , Kohlmann, T. , Janssen, M. F. , & Buchholz, I. (2021). Psychometric properties of the EQ‐5D‐5L: A systematic review of the literature. Quality of Life Research, 30(3), 647–673. 10.1007/s11136-020-02688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkaart‐van Roijen, L. , Van der Linden, N. , Bouwmans, C. M. A. , Kanters, T. , & Tan, S. S. (2015). Kostenhandleiding: methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. KOS, 38–55. https://www.zorginstituutnederland.nl/binaries/zinl/documenten/publicatie/2016/02/29/richtlijn‐voor‐het‐uitvoeren‐van‐economische‐evaluaties‐in‐de‐gezondheidszorg/Richtlijn+voor+het+uitvoeren+van+economische+evaluaties+in+de+gezondheidszorg+%28verdiepingsmodules%29.pdf [Google Scholar]
- Hart, L. M. , Granillo, M. T. , Jorm, A. F. , & Paxton, S. J. (2011). Unmet need for treatment in the eating disorders: A systematic review of eating disorder specific treatment seeking among community cases. Clinical Psychology Review, 31(5), 727–735. 10.1016/j.cpr.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Hedman, E. , Ljótsson, B. , & Lindefors, N. (2012). Cognitive behavior therapy via the internet: A systematic review of applications, clinical efficacy and cost‐effectiveness. Expert Review of Pharmacoeconomics & Outcomes Research, 12(6), 645–764. 10.1586/ERP.12.67 [DOI] [PubMed] [Google Scholar]
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70. [Google Scholar]
- Kanters, T. , Bouwmans, C. M. A. , Van der Linden, N. , Tan, S. S. , & Hakkaart‐van Roijen, L. (2017). Update of the Dutch manual for costing studies in health care. PLoS One, 12(11), e0187477. 10.1371/journal.pone.0187477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass, A. E. , Balantekin, K. N. , Fitzsimmons‐Craft, E. E. , Jacobi, C. , Wilfley, D. E. , & Taylor, C. B. (2017). The economic case for digital interventions for eating disorders among United States college students. International Journal of Eating Disorders, 50(3), 250–258. 10.1002/eat.22680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen, J. D. , Taylor, C. B. , Hammer, L. D. , Litt, I. , Wilson, D. M. , Rich, T. , Hayward, C. , Simmonds, B. , Kraemer, H. , & Varady, A. (1993). An attempt to modify unhealthful eating attitudes and weight regulation practices of young adolescent girls. International Journal of Eating Disorders, 13(4), 369–384. [DOI] [PubMed] [Google Scholar]
- Kolovos, S. , van Dongen, J. M. , Riper, H. , Buntrock, C. , Cuijpers, P. , Ebert, D. D. , Geraedts, A. S. , Kenter, R. M. , Nobis, S. , Smith, A. , Warmerdam, L. , Hayden, J. A. , van Tulder, M. W. , & Bosmans, J. E. (2018). Cost effectiveness of guided internet‐based interventions for depression in comparison with control conditions: An individual‐participant data meta‐analysis. Depression and Anxiety, 35, 209–219. 10.1002/da.22714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König, H. H. , Bleibler, F. , Friederich, H. C. , Herpertz, S. , Lam, T. , Mayr, A. , Schmidt, F. , Svaldi, J. , Zipfel, S. , Brettschneider, C. , Hilbert, A. , de Zwaan, M. , & Egger, N. (2018). Economic evaluation of cognitive behavioral therapy and internet‐based guided self‐help for binge‐eating disorder. International Journal of Eating Disorders, 51(2), 155–164. 10.1002/eat.22822 [DOI] [PubMed] [Google Scholar]
- Linardon, J. , Shatte, A. , Messer, M. , Firth, J. , & Fuller‐Tyszkiewicz, M. (2020). E‐mental health interventions for the treatment and prevention of eating disorders: An updated systematic review and meta‐analysis. Journal of Consulting and Clinical Psychology, 88(11), 994–1007. 10.1037/ccp0000575 [DOI] [PubMed] [Google Scholar]
- Loucas, C. E. , Fairburn, C. G. , Whittington, C. , Pennant, M. E. , Stockton, S. , & Kendall, T. (2014). E‐therapy in the treatment and prevention of eating disorders: A systematic review and meta‐analysis. Behaviour Research and Therapy, 63, 122–131. 10.1016/j.brat.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melioli, T. , Bauer, S. , Franko, D. L. , Moessner, M. , Ozer, F. , Chabrol, H. , & Rodgers, R. F. (2016). Reducing eating disorder symptoms and risk factors using the internet: A meta‐analytic review. International Journal of Eating Disorders, 49(1), 19–31. 10.1002/eat.22477 [DOI] [PubMed] [Google Scholar]
- OECD . (2021). Consumer price indices (CPIs). https://stats.oecd.org/Index.aspx?DataSetCode=PRICES_CPI
- Paganini, S. , Teigelkoetter, W. , Buntrock, C. , & Baumeister, H. (2018). Economic evaluations of internet‐and mobile‐based interventions for the treatment and prevention of depression: A systematic review. Journal of Affective Disorders, 225, 733–755. 10.1016/j.jad.2017.07.018 [DOI] [PubMed] [Google Scholar]
- Pietersma, S. , van den Akker‐Van Marle, M. E. , & de Vries, M. (2013). Generic quality of life utility measures in health‐care research: Conceptual issues highlighted for the most commonly used utility measures. International Journal of Wellbeing, 3(2), 173–181. [Google Scholar]
- Pittock, A. , Hodges, L. , & Lawrie, S. M. (2018). The effectiveness of internet‐delivered cognitive behavioural therapy for those with bulimic symptoms: A systematic review. BMC Research Notes, 11(1), 748. 10.1186/s13104-018-3843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach, P. J. , Dingemans, A. E. , Essers, B. A. , Furth, E. F. , Spinhoven, P. , Groothuis‐Oudshoorn, C. G. M. , van Til, J. A. , & van den Akker‐Van Marle, M. E. (2021). The ICECAP‐A instrument for capabilities: Assessment of construct validity and test–retest reliability in a general Dutch population. Quality of Life Research, 31, 687–696. 10.1007/s11136-021-02980-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach, P. J. , Dingemans, A. E. , Groothuis‐Oudshoorn, C. G. M. , van Til, J. A. , Essers, B. A. , Spinhoven, P. , Furth, E. F. , & van den Akker‐Van Marle, M. E. (2021). The ICEpop capability measure for adults instrument for capabilities: Development of a tariff for the Dutch general population. Value in Health, 25(1), 125–132. 10.1016/j.jval.2021.07.011 [DOI] [PubMed] [Google Scholar]
- Rohrbach, P. J. , Dingemans, A. E. , Spinhoven, P. , van den Akker‐Van Marle, E. , van Ginkel, J. R. , Fokkema, M. , Moessner, M. , Bauer, S. , & van Furth, E. F. (2019). A randomized controlled trial of an internet‐based intervention for eating disorders and the added value of expert‐patient support: Study protocol. Trials, 20(1), 1–17. 10.1186/s13063-019-3574-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, D. B. (1987). Multiple imputation for nonresponse in surveys. Wiley. [Google Scholar]
- Samnaliev, M. , Noh, H. L. , Sonneville, K. R. , & Austin, S. B. (2015). The economic burden of eating disorders and related mental health comorbidities: An exploratory analysis using the US medical expenditures panel survey. Preventive Medicine Reports, 2, 32–34. 10.1016/j.pmedr.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smink, F. R. E. , van Hoeken, D. , & Hoek, H. W. (2013). Epidemiology, course, and outcome of eating disorders. Current Opinion in Psychiatry, 26(6), 543–548. 10.1097/YCO.0b013e328365a24f [DOI] [PubMed] [Google Scholar]
- Timman, R. , Bouwmans, C. M. A. , Busschbach, J. J. V. , & Hakkaart‐van Roijen, L. (2015). Development of the treatment inventory of costs in psychiatric patients: TiC‐P mini and Midi. Value in Health, 18(8), 994–999. 10.1016/j.jval.2015.07.006 [DOI] [PubMed] [Google Scholar]
- van Ginkel, J. R. , & Kroonenberg, P. M. (2014). Analysis of variance of multiply imputed data. Multivariate Behavioral Research, 49(1), 78–91. 10.1080/00273171.2013.855890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoeken, D. , & Hoek, H. W. (2020). Review of the burden of eating disorders: Mortality, disability, costs, quality of life, and family burden. Current Opinion in Psychiatry, 33(6), 521–527. 10.1097/YCO.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Son, G. E. , Hoek, H. W. , van Hoeken, D. , Schellevis, F. G. , & van Furth, E. F. (2012). Eating disorders in the general practice: A case–control study on the utilization of primary care. European Eating Disorders Review, 20(5), 410–413. 10.1002/erv.2185 [DOI] [PubMed] [Google Scholar]
- Versteegh, M. M. , Vermeulen, K. M. , Evers, S. M. A. A. , de Wit, G. A. , Prenger, R. , & Stolk, E. A. (2016). Dutch tariff for the five‐level version of EQ‐5D. Value in Health, 19(4), 343–352. 10.1016/j.jval.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Watson, H. J. , McLagan, N. , Zerwas, S. C. , Crosby, R. D. , Levine, M. D. , Runfola, C. D. , Peat, C. M. , Moessner, M. , Zimmer, B. , Hofmeier, S. M. , Hamer, R. M. , Marcus, M. D. , Bulik, C. M. , & Crow, S. J. (2018). Cost‐effectiveness of internet‐based cognitive‐behavioral treatment for bulimia nervosa: Results of a randomized controlled trial. Journal of Clinical Psychiatry, 79(1), 16m11314. 10.4088/JCP.16m11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman, R. S. , & Rosselli, F. (2017). Reducing the burden of suffering from eating disorders: Unmet treatment needs, cost of illness, and the quest for cost‐effectiveness. Behaviour Research and Therapy, 88, 49–64. 10.1016/j.brat.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Zwaap, J. , Knies, S. , van der Meijden, C. , Staal, P. , & van der Heiden, L. (2015). Cost‐effectiveness in practice (pp. 5–40). Diemen, Netherlands: Zorginstituut Nederland. https://english.zorginstituutnederland.nl/binaries/zinl-eng/documents/reports/2015/06/16/cost-effectiveness-in-practice/Cost-effectiveness+in+practice.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
Data, materials, and code are available upon reasonable request.


