Abstract
Objectives
The complement component 5 (C5) inhibitor ravulizumab demonstrated non‐inferiority to eculizumab following 26 weeks of treatment in complement inhibitor‐naïve and complement inhibitor‐experienced patients with paroxysmal nocturnal hemoglobinuria (PNH; studies 301 and 302, respectively). This study aims to describe the results of both studies from 27 weeks to 2 years.
Methods
Patients (N = 441) continued to receive ravulizumab throughout the extension period. Efficacy endpoints included lactate dehydrogenase (LDH) normalization, transfusion avoidance and fatigue score (FACIT‐F). Safety analyses were also performed.
Results
From 27 weeks to 2 years, improvements in LDH levels were maintained in both study populations. Transfusion avoidance was maintained in 81.9% (study 301) and 85.6% (study 302) of patients, and FACIT‐F scores remained stable. Ravulizumab was well tolerated, and the incidence of adverse events (AEs) were similar between patients of both studies. Incidence of serious AEs deemed related to ravulizumab treatment was low (<3%).
Conclusions
This study reports, to date, the longest period of follow‐up in over 400 patients with PNH treated with ravulizumab (662 patient‐years). Long‐term, ravulizumab demonstrated durable efficacy and was well tolerated, highlighting the importance of C5 inhibitors as the mainstay of PNH treatment.
Keywords: breakthrough hemolysis, complement inhibitor, lactate dehydrogenase, paroxysmal nocturnal hemoglobinuria, ravulizumab
Novelty statement.
This study reports the longest period of follow‐up in both complement inhibitor‐naïve and complement inhibitor‐experienced patients with paroxysmal nocturnal hemoglobinuria (PNH) treated with ravulizumab to date. From 27 weeks to 2 years, ravulizumab demonstrated durable efficacy, which was well tolerated, with no new safety signals; supporting the importance of immediate, complete and sustained terminal complement inhibition as the mainstay of PNH treatment.
1. INTRODUCTION
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare chronic hematologic disorder caused by uncontrolled terminal complement activation, which can lead to intravascular hemolysis, organ damage (e.g., kidney impairment and pulmonary hypertension), thrombotic events (TEs), and increased morbidity and mortality. 1 , 2 , 3 , 4
Complement component 5 (C5) inhibitors, such as eculizumab and ravulizumab, are the current standard of care in patients with PNH, where commercially available. 5 Eculizumab (Soliris®, Alexion Pharmaceuticals) is a first‐generation humanized monoclonal antibody which binds to C5 and was the first disease‐specific treatment approved for patients with PNH by both the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2007. Ravulizumab (Ultomiris®, Alexion Pharmaceuticals), is a second‐generation humanized monoclonal antibody, which binds to C5 that was developed to reduce the treatment burden associated with eculizumab through an improved dosing regimen. It provides the same benefits as eculizumab but has a substantially longer half‐life (50 days vs. 11 days), thereby permitting a longer intravenous dosing interval (every 8 weeks [q8w] vs. every 2 weeks [q2w]).
Ravulizumab was approved by the FDA in 2018 for the treatment of adult patients with PNH and approved by the EMA in 2019 for the treatment of adult patients with PNH symptoms indicative of high disease activity (HDA; defined as a lactate dehydrogenase [LDH] ratio ≥1.5 times upper limit of normal [ULN; 246 U/L] and the presence of at least one symptom of fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia [hemoglobin (Hb) <10 g/dl], major adverse vascular events [MAVEs; including thromboembolism], dysphagia, or erectile dysfunction), or those who are clinically stable after having been treated with eculizumab for at least the past 6 months. 6 , 7 In 2021, both approvals were extended to include pediatric patients one month of age and older with PNH (FDA) and pediatric patients weighing ≥10 kg with PNH (EMA).
In two, phase 3, multicenter, randomized trials of patients with PNH (studies 301 10 and 302 11 ), ravulizumab q8w was shown to be non‐inferior to eculizumab q2w for all efficacy endpoints and demonstrated a comparable safety profile with no unexpected safety signals at 26 weeks. Following the primary evaluation period (26 weeks), patients either switched from eculizumab to ravulizumab or continued ravulizumab for up to 5 years. At open‐label extension, both treatment pathways demonstrated durable efficacy and were well tolerated at 1 year for both studies. 12 , 13 Here, we present the combined long‐term efficacy and safety results from the extension periods of both studies (27 weeks to 2 years); the longest duration of follow‐up in patients with PNH receiving ravulizumab.
2. METHODS
2.1. Patient population
Adult patients (≥18 years of age) with a diagnosis of PNH, confirmed by high sensitivity flow cytometry of red and white blood cells, with PNH granulocyte/monocyte clone sizes of at least 5% were eligible for inclusion in the studies. Key exclusion criteria were body weight < 40 kg at screening, history of bone marrow transplantation, and history of meningococcal infection. All enrolled patients with PNH provided written informed consent.
2.2. Study design
Clinical trials 301 (NCT02946463) 10 and 302 (NCT03056040) 11 were phase 3, randomized, open‐label, active‐controlled, non‐inferiority, multicenter studies. Study 301 included patients with PNH who were naïve to C5 inhibitors, with LDH levels ≥1.5 × ULN and at least one sign or symptom of PNH at screening; all characteristics which are associated with HDA. Study 302 included clinically stable patients with PNH who had received eculizumab treatment for ≥6 months and had LDH levels ≤1.5 × ULN at screening.
In the initial 26‐week primary evaluation period of both studies, patients received either weight‐based dosing of ravulizumab q8w (consisting of an initial loading dose at Day 1 of 2400 mg for patients weighing ≥40 kg to <60 kg and 2700 mg for patients weighing ≥60 kg to <100 kg, followed by a maintenance dose on Day 15; and q8w thereafter of 3000 mg for patients weighing ≥40 kg to <60 kg or 3300 mg for patients weighing ≥60 kg to <100 kg), or the approved eculizumab dose (consisting of an initial loading dose of 600 mg on Days 1, 8, 15, and 22, followed by a maintenance dose of 900 mg on Day 29 and q2w thereafter). The primary evaluation period was followed by an extension period, during which eligible patients either continued receiving ravulizumab or switched from eculizumab to ravulizumab, respectively, for up to 5 years.
2.3. Efficacy endpoints
Efficacy endpoints considered in analysis comprised of proportion of patients achieving LDH normalization, proportion of patients achieving LDH ≤1.5 × ULN, percentage change in LDH levels, proportion of patients avoiding transfusion, proportion of patients with stabilized Hb, proportion of patients with breakthrough hemolysis, and quality of life (QoL) measured using Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT‐F) scale (range, 0–52; higher scores indicate less fatigue) and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (EORTC QLQ‐C30) global health status, physical functioning, and fatigue subscales (range, 0–100; higher scores for functional scales indicate better QoL; lower scores for symptom scales indicate lower symptom levels). Patients were considered to have achieved transfusion avoidance if they remained transfusion free and did not require a transfusion per protocol‐specified guidelines. LDH normalization was defined as LDH ≤1 × ULN and stabilized Hb was defined as avoidance of a ≥2 g/dl decrease in Hb level from baseline in the absence of transfusion during that period. BTH was defined as ≥1 new or worsening sign or symptom of intravascular hemolysis (fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia [Hb <10 g/dl], MAVEs [including thrombosis], dysphagia, or erectile dysfunction) in the presence of LDH ≥2 × ULN. In study 301, BTH was defined as above following prior reduction of LDH to <1.5 × ULN on treatment.
2.4. Safety endpoints
Safety outcomes assessed included the total exposure of patients with PNH to ravulizumab, incidence of treatment‐emergent adverse events (TEAEs), serious adverse events (SAEs), TEAEs considered as MAVEs and immunogenicity—reflected by the development of anti‐drug antibodies (ADAs).
2.5. Statistical analysis
The data cut‐off date for this analysis was the 2‐year study visit except for early terminations (i.e., Day 743 or Day 757 for patients initially randomized to ravulizumab or eculizumab, respectively). Efficacy and safety outcomes were compared using descriptive statistics. The rate of adverse events (AEs) adjusted by patient‐years of exposure was calculated for this analysis and was defined as (number of events)/(100 patient‐years).
3. RESULTS
3.1. Patient characteristics
For studies 301 and 302, 246 and 195 patients with PNH were included in the randomized primary evaluation periods, respectively. Baseline patient demographics (Table 1) and clinical characteristics (Table 2) were similar across both studies. Patients (N = 441, 47.4% female, mean [standard deviation; SD] age at first study drug infusion: study 301, 45.5 [15.7] years; study 302, 47.7 [14.2] years) were predominantly Asian (49.2%, including Japanese race) or White/Caucasian (46.5%). At baseline, patients of study 301 reported a mean (SD) LDH level of 1606.4 (752.7) U/L, whereas patients of study 302 reported a mean (SD) LDH level of 231.6 (49.2) U/L.
TABLE 1.
Patient demographics in the 301 and 302 studies
| Characteristic | Study 301 | Study 302 | ||||
|---|---|---|---|---|---|---|
| Ravulizumab (n = 125) | Eculizumab (n = 121) | Total (N = 246) | Ravulizumab (n = 97) | Eculizumab (n = 98) | Total (N = 195) | |
| Sex, female, n (%) | 60 (48.0) | 52 (43.0) | 112 (45.5) | 47 (48.5) | 50 (51.0) | 97 (49.7) |
| Age at first infusion of study drug, years, mean (SD) | 44.8 (15.2) | 46.2 (16.2) | 45.5 (15.7) | 46.6 (14.4) | 48.8 (14.0) | 47.7 (14.2) |
| Race, n (%) | ||||||
| Asian | 72 (57.6) | 57 (47.1) | 129 (52.4) | 23 (23.7) | 19 (19.4) | 42 (21.5) |
| Japanese | 19 (15.2) | 15 (12.4) | 34 (13.8) | 5 (5.2) | 7 (7.1) | 12 (6.2) |
| White/Caucasian | 43 (34.4) | 51 (42.1) | 94 (38.2) | 50 (51.5) | 61 (62.2) | 111 (56.9) |
| Black/African American | 2 (1.6) | 4 (3.3) | 6 (2.4) | 5 (5.2) | 3 (3.1) | 8 (4.1) |
| American Indian/Alaska Native | 1 (0.8) | 1 (0.8) | 2 (0.8) | NR | NR | NR |
| Other/multiple | 4 (3.2) | 4 (3.3) | 8 (3.3) | 3 (3.1) | 1 (1.0) | 4 (2.1) |
| Not reported/Unknown | 3 (2.4) | 4 (3.3) | 7 (2.8) | 16 (16.5) | 14 (14.3) | 30 (15.4) |
| Weight, kg, mean (SD) | 68.2 (15.6) | 69.2 (14.9) | 68.7 (15.2) | 72.4 (16.8) | 73.4 (14.6) | 72.9 (15.7) |
Abbreviations: NR, not reported; SD, standard deviation.
TABLE 2.
Baseline patient characteristics in the 301 and 302 studies
| Characteristic | Study 301 | Study 302 | ||||
|---|---|---|---|---|---|---|
| Ravulizumab (n = 125) | Eculizumab (n = 121) | Total (N = 246) | Ravulizumab (n = 97) | Eculizumab (n = 98) | Total (N = 195) | |
| History of MAVEs, n (%) | 17 (13.6) | 25 (20.7) | 42 (17.1) | 28 (28.9) | 22 (22.4) | 50 (25.6) |
| Age at PNH diagnosis, years, mean (SD) | 37.9 (14.9) a | 39.6 (16.7) b | 38.7 (15.8) c | 34.1 (14.4) | 36.8 (14.1) d | 35.5 (14.3) e |
| PNH clone size, %, mean (SD) | ||||||
| Total RBCs | 38.4 (23.7) | 38.7 (23.2) | 38.6 (23.4) | 60.6 (32.5) | 59.5 (31.4) | 60.1 (31.9) |
| Granulocytes | 84.2 (21.0) | 85.3 (19.0) | 84.7 (20.0) | 82.6 (23.6) | 84.0 (21.4) | 83.3 (22.5) |
| Monocytes | 86.9 (18.1) | 89.2 (15.2) | 88.0 (16.7) | 85.6 (20.5) | 86.1 (19.7) | 85.9 (20.0) |
| Hemoglobin, g/L, mean (SD) f | 94.1 (14.6) | 95.9 (17.1) | NA | 110.8 (18.4) | 109.1 (18.4) | NA |
| LDH level, U/L, mean (SD) g | 1633.5 (778.8) | 1578.3 (727.1) | 1606.4 (752.7) | 228.0 (48.7) | 235.2 (49.7) | 231.6 (49.2) |
| LDH level group, n (%) | ||||||
| <3 × ULN h | 8 (14.4) | 16 (13.2) | 34 (13.8) | NR | NR | NR |
| ≥3 × ULN h | 107 (85.6) | 105 (86.8) | 212 (86.2) | NR | NR | NR |
| Haptoglobin, g/L, mean (SD) i | 0.194 (0.034) | 0.197 (0.024) | NA | 0.283 (0.235) | 0.255 (0.174) | NA |
| History of aplastic anemia, n (%) | 41 (32.8) | 38 (31.4) | 79 (32.1) | 34 (35.1) | 39 (39.8) | 73 (37.4) |
| Patients with pRBC/whole blood transfusions within 1 year before first dose, n (%) | 103 (82.4) | 100 (82.6) | 203 (82.5) | 13 (13.4) | 12 (12.2) | 25 (12.8) |
| pRBC units received within 1 year prior to study entry, randomization strata, n (%) | ||||||
| 0 units | 23 (18.4) | 21 (17.4) | 44 (17.9) | NR | NR | NR |
| 1–14 units | 79 (63.2) | 78 (64.5) | 157 (63.8) | NR | NR | NR |
| >14 units | 23 (18.4) | 22 (18.2) | 45 (18.3) | NR | NR | NR |
| Time on eculizumab before first study infusion, years, mean (SD) | NA | NA | NA | 6.0 (3.5) | 5.6 (3.5) | 5.8 (3.5) |
| Time from PNH diagnosis to consent, years, mean (SD) | 6.7 (8.1) a | 6.4 (7.5) b | 6.6 (7.8) c | 12.4 (8.4) | 11.9 (9.4) d | 12.2 (8.9) e |
Abbreviations: LDH, lactate dehydrogenase; MAVE, major adverse vascular event; NA, not available; NR, not reported; PNH, paroxysmal nocturnal hemoglobinuria; pRBC, packed red blood cell; RBC, red blood cell; SD, standard deviation; ULN, upper limit of normal.
n = 123.
n = 118.
n = 241.
n = 97.
N = 194.
Normal range, 11.5–16.0 g/dl (women) and 13.0–17.5 g/dl (men).
Normal range, 120–246 U/L.
LDH ULN level 246 U/L.
Normal range, 0.4–2.4 g/dl.
For the extension period, a combined total of 434 patients with PNH from studies 301 and 302 received ravulizumab, and of these, 219 patients received eculizumab prior to switching to ravulizumab in the extension period.
3.2. Efficacy endpoints
Mean LDH levels over time are shown in Figure 1A (study 301) and Figure 1B (study 302). At Day 183, LDH normalization was achieved by 114 (47.3%; study 301) and 120 (63.2%; study 302). At 2 years, LDH normalization was achieved by 108 (48.2%; study 301) and 96 patients (56.5%; study 302). Of those patients who achieved Day 183 LDH normalization, 76.4% (study 301) and 73.9% (study 302) maintained this response at 2 years. In addition, at 2 years, LDH normalization was achieved in a further 26 (22.4%; study 301) and 14 (24.1%; study 302) patients who had not achieved LDH normalization at Day 183.
FIGURE 1.
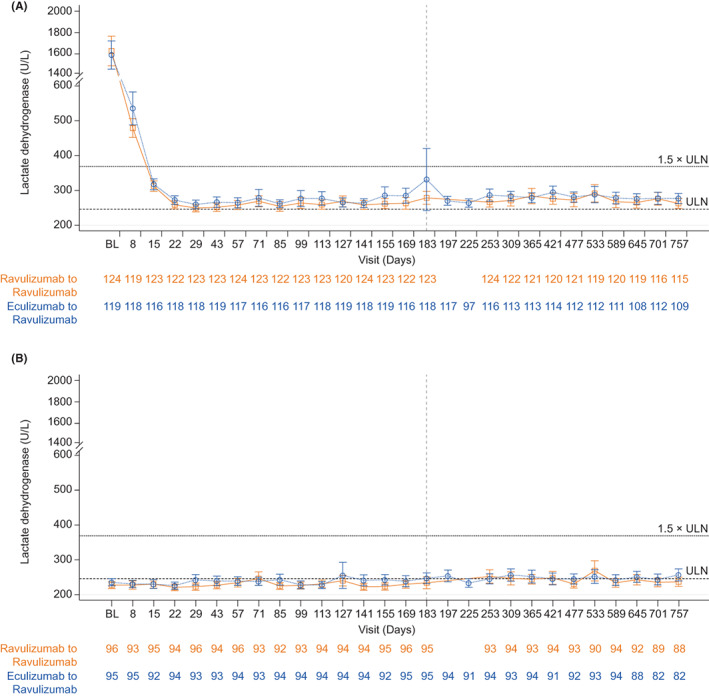
Mean (95% CI) LDH levels (U/L) over time in (A) study 301 and (B) study 302, by treatment. Abbreviations: BL, baseline; CI, confidence interval; LDH, lactate dehydrogenase; ULN, upper limit of normal. ULN = 246 U/L; 1.5 × ULN = 369 U/L
The LDH ≤1.5 × ULN response threshold was achieved by 218 patients (90.5%; study 301) and 183 patients (96.3%; study 302) at Day 183 and in 203 patients (90.6%; study 301) and 161 patients (94.7%; study 302) at 2 years. Most patients (93.0%, study 301; 94.5%, study 302) who achieved LDH ≤1.5 × ULN at Day 183 maintained this response at 2 years. In addition, a further 14 patients (66.7%; study 301) and 6 patients (100.0%; study 302) who had not achieved LDH ≤1.5 × ULN at Day 183 achieved this response at 2 years.
Patients of both studies showed durable responses for percentage change in LDH levels from Day 183, similar to those observed at the 26‐week primary analysis endpoint and 52‐week open‐label extension. At 2 years, patients in study 301 demonstrated a mean (SD) 1.9% (26.9%) increase in LDH levels from the primary analysis endpoint, whereas patients in study 302 had an 8.8% (28.9%) increase in LDH levels from primary analysis endpoint. During the randomized period, 171 patients (70.4%) and 160 patients (83.8%) avoided transfusion in studies 301 and 302, respectively. During >12–18 months of extension, in the same respective studies, transfusion was avoided in 178 patients (73.3%) and 163 patients (85.3%). Between the randomized period and the extension period, transfusion avoidance was maintained in 81.9% and 85.6% of patients in the 301 and 302 studies, respectively. In total, 162 patients (66.7%) and 142 patients (74.4%) achieved Hb stabilization during the randomized period and 69.1% and 83.8% of patients maintained this response during the extension period, in studies 301 and 302, respectively.
At 2 years, mean (SD) FACIT‐F scores were similar between both studies (study 301, 43.5 [8.10]; study 302, 41.2 [10.70]) and the mean (SD) percentage change from Day 183 baseline scores were minimal (study 301, 1.6% [36.38]; study 302, −1.2% [25.62]; Figure 2A,B). Mean (SD) EORTC QLQ‐C30 subscale scores at 2 years were also similar between studies (301 vs. 302: global health status, 70.4 [20.57] vs. 71.6 [20.07]; physical functioning, 88.4 [14.74] vs. 86.8 [17.83]; fatigue, 20.9 [20.27] vs. 28.3 [24.65]).
FIGURE 2.
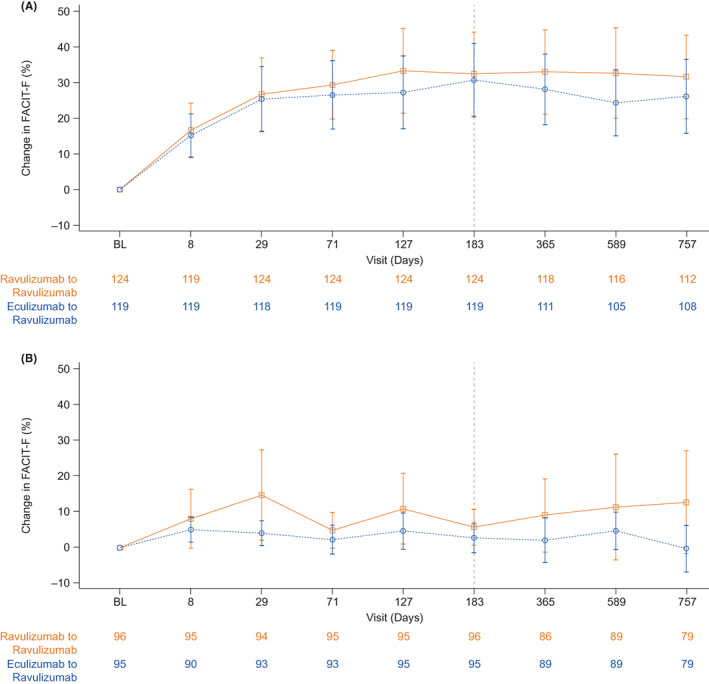
Mean (95% CI) percentage change from baseline FACIT‐F over time in (A) study 301 and (B) study 302, by treatment. Abbreviations: BL, baseline; CI, confidence interval; FACIT‐F, Functional Assessment of Chronic Illness Therapy—Fatigue
During the extension period, the proportion of patients from either study who experienced BTH events whilst receiving ravulizumab were similar: 15 C5 inhibitor‐naïve patients (6.2%; ravulizumab to ravulizumab, n = 10; eculizumab to ravulizumab, n = 5; study 301) and 11 C5 inhibitor‐experienced patients (5.8%; ravulizumab to ravulizumab, n = 7; eculizumab to ravulizumab, n = 4; study 302), respectively (Figure 3). The number of BTH events reported did differ between studies (301 vs. 302: 25 vs. 11). Of the patients who experienced BTH during the extension periods (n = 26), one BTH event per patient was reported in patients of study 302, whereas several patients in study 301 experienced multiple BTH events (0–6 months of extension, 1 patient experienced two BTH events; >6–12 months of extension, 3 patients experienced two BTH events; >12–18 months of extension, 1 patient experienced two BTH events). None of the BTH events (n = 16) with free C5 level data available at the time of the event showed suboptimal terminal complement inhibition. Of the BTH events that occurred during the extension periods, 16/25 (64.0%) and 4/11 (36.4%) in the 301 and 302 studies, respectively, did not have available data on the free C5 levels at the time of the event. For the BTH events without available data on the free C5 levels at the time of the event, all free C5 samples before or after the event showed effective terminal complement inhibition (<0.5 μg/ml).
FIGURE 3.
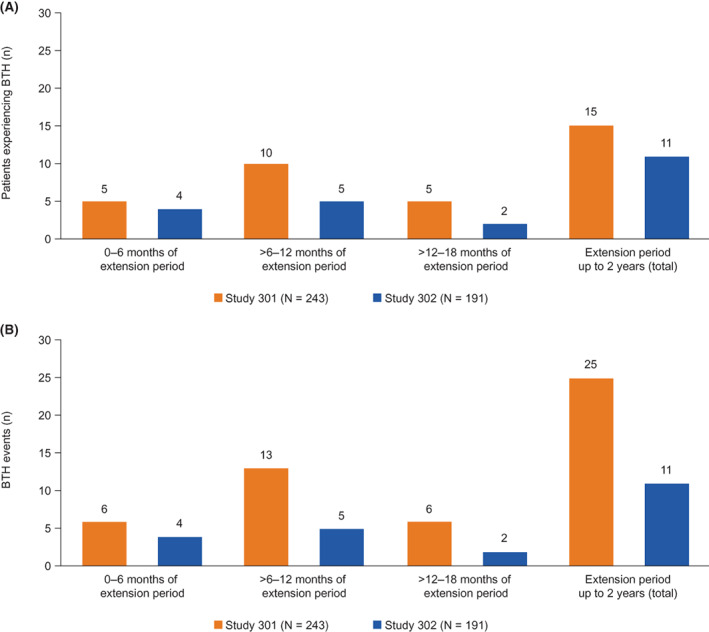
Number of patients with PNH experiencing BTHa (A) and the number of BTH events reported (B) per 6‐month interval of the extension period in study 301b and study 302. Abbreviations: BTH, breakthrough hemolysis; PNH, paroxysmal nocturnal hemoglobinuria. aIn study 301, 15 patients experienced BTH events during the extension period up to 2 years. Of these patients, 5/15 experienced BTH events across multiple periods of extension. bIn study 301, at 0–6 months of extension, one patient experienced two BTH events; at >6–12 months of extension, three patients experienced two BTH events; at >12–18 months of extension, one patient experienced two BTH events
Of the 25 BTH events reported during the extension period in study 301, 9 (36.0%) were associated with infectious events, which included flu‐like symptoms, gingival abscess, influenza A infection, and upper respiratory infection. However, a greater proportion of BTH events reported in study 302 was associated with infectious events (7/11 [63.6%]), and included events such as fever, a cold, an infection of unknown source and H1N1 influenza infection.
No patients with PNH discontinued ravulizumab treatment owing to a lack of efficacy.
3.3. Safety endpoints
The total exposure of patients with PNH to ravulizumab over the extension period (27 weeks to 2 years) was 662 patient‐years. Overall, 391 patients (90.1%) with PNH reported TEAEs in the extension period, of whom 98 patients (22.6%) experienced treatment‐related TEAEs (Table 3). There were 6 patients (1.4%) who experienced a TEAE that was considered a MAVE; none of which occurred owing to a BTH event. The five most commonly reported TEAEs (in ≥10.0% of patients) were upper respiratory tract infection (n = 80 [18.4%]), followed by nasopharyngitis (n = 70 [16.1%]), headache (n = 56 [12.9%]), pyrexia (n = 44 [10.1%]), and fatigue (n = 39 [9.0%]).
TABLE 3.
Safety outcomes from the extension periods of the combined 301 and 302 studies period (27 weeks to 2‐year data cut)
| 27 weeks to 2‐year data cut (N = 434) | ||
|---|---|---|
| Patients, n (%) | Events, n (rate) a | |
| TEAEs | ||
| Any TEAE b | 391 (90.1) | 2327 (351.5) |
| Related TEAEs | 98 (22.6) | 236 (35.6) |
| Unrelated TEAEs | 381 (87.8) | 2091 (315.8) |
| Grade 1 | 333 (76.7) | 1347 (203.5) |
| Grade 2 | 280 (64.5) | 794 (119.9) |
| Grade 3 | 100 (23.0) | 159 (24.0) |
| Grade 4 | 19 (4.4) | 25 (3.8) |
| TEAE considered a MAVE | 6 (1.4) c | 8 (1.2) |
| Most common TEAEs (in ≥ 10% of patients) | ||
| Upper respiratory tract infection | 80 (18.4) | 103 (15.6) |
| Nasopharyngitis | 70 (16.1) | 108 (16.3) |
| Headache | 56 (12.9) | 75 (11.3) |
| Pyrexia | 44 (10.1) | 52 (7.9) |
| Fatigue | 39 (9.0) | 70 (10.6) |
| SAEs | ||
| Any SAE | 86 (19.8) | 121 (18.3) |
| Related SAE | 10 (2.3) | 12 (1.8) |
| Unrelated SAE | 80 (18.4) | 109 (16.5) |
| SAE leading to study discontinuation | 3 (0.7) d | 3 (0.5) |
| Death | 4 (0.9) e , f | – |
Abbreviations: AE, adverse event; MAVE, major adverse vascular event; SAE, serious adverse event, TEAE, treatment‐emergent adverse event.
Rate of AE adjusted by patient‐years of exposure, defined as (number of events)/(100 patient‐years). The data cut‐off is the 2‐year visit except early terminations (i.e. Day 743 or Day 757 for patients randomized to ravulizumab or eculizumab, respectively).
TEAEs are AEs with a start date and start time on or after the date and time of the first infusion of study drug; grade 1 = mild, grade 2 = moderate, grade 3 = severe, grade 4 = life‐threatening.
Eight MAVEs were recorded; 1 patient had two events of pulmonary embolism, 1 patient had two events of cerebral infarction; the other MAVEs included thrombophlebitis, deep vein thrombosis, jugular vein thrombosis, and peripheral artery thrombosis. Six events were considered to be unrelated to treatment and two events were unlikely related to treatment. None of these events led to change in dose.
Three discontinuations were recorded: acute myeloid leukemia, myelodysplastic syndrome, and lung adenocarcinoma.
Four deaths unrelated to study drug were reported: pulmonary sepsis, acute myeloid leukemia, lung adenocarcinoma, and lung neoplasm malignant.
The two deaths leading to study drug discontinuation were acute myeloid leukemia and lung adenocarcinoma.
SAEs were reported in 86 patients (19.8%) with PNH during the extension period, of whom 10 patients (2.3%) experienced SAEs that were deemed treatment related. Three patients (0.7%) experienced treatment‐emergent SAEs which were considered MAVEs; peripheral artery thrombosis (n = 1 [0.2%]), jugular vein thrombosis (n = 1 [0.2%]), and pulmonary embolism (n = 1 [0.2%]). SAEs that led to study discontinuation were reported in 3 patients (0.7%), and included myelodysplastic syndrome, acute myeloid leukemia, and lung adenocarcinoma; all of which were deemed unrelated to treatment. Four deaths were reported during the extension period (acute myeloid leukemia, lung adenocarcinoma, pulmonary sepsis, and malignant lung neoplasm), two of which were of patients who discontinued the study owing to SAEs (acute myeloid leukemia and lung adenocarcinoma). All deaths reported in the extension period were deemed unrelated to study treatment (Table 3).
No meningococcal infections were reported during the extension period, and immunogenicity at 2 years was low across both studies with five patients (1.2%) testing positive for non‐neutralizing ADAs.
4. DISCUSSION
C5 inhibitors, eculizumab and ravulizumab, are the mainstay of PNH treatment. Although the safety profile of eculizumab has been well characterized, with a favorable risk–benefit profile demonstrated in a large 10‐year post‐marketing pharmacovigilance surveillance analysis, 14 safety data for ravulizumab are limited due to its more recent approvals by the FDA and EMA. However, in all phase 3 clinical trials of ravulizumab to date, it has demonstrated a safety and efficacy profile comparable to eculizumab. 10 , 11 , 12 , 13 Results from this analysis provide, to date, the longest period of follow‐up in more than 400 patients with PNH treated with ravulizumab (662 patient‐years). This study demonstrated the durable efficacy of ravulizumab, which was well tolerated in both C5 inhibitor‐naïve and C5 inhibitor‐experienced patients with PNH, with no new safety signals reported in up to 2 years of treatment.
Lack of normalization of LDH levels is associated with a significant increased risk of thrombosis 15 and mortality. 4 Throughout the extension period, LDH normalization was maintained, with many patients achieving LDH ≤1.5 × ULN. In addition, mean LDH levels increased from the primary analysis endpoint and remained stable, and both Hb stabilization and transfusion avoidance rates were comparable to those reported at 52‐week open‐label extension. 12 , 13 Similarly, the improvement in FACIT‐F scores reported at the end of the primary evaluation period were maintained through the 2‐year extension period. EORTC QLQ‐C30 scores also confirmed relatively high QoL at the start of the primary evaluation period, with similar scores reported at the end of the primary evaluation and 2‐year extension periods of both studies. 12 , 13
Approximately 90% of patients with PNH treated with ravulizumab reported TEAEs, the majority of which were deemed unrelated to study treatment. Among the most commonly reported TEAEs were infection and headache. No patients with PNH discontinued ravulizumab treatment owing to a lack of efficacy, and 3 patients discontinued owing to SAEs that were deemed unrelated to study treatment. Additionally, the incidence of SAEs deemed related to study treatment were very low (<3%).
MAVEs, particularly thromboembolic events, are a major clinical concern in patients with PNH, accounting for 40–67% of deaths for which the cause is known. 2 , 16 At present, the mechanisms of thrombosis in PNH are complex and numerous, and include complement‐mediated platelet and white cell activation, intravascular hemolysis, nitric oxide depletion, impairment of the fibrinolytic system, and increased inflammatory mediators. 17 , 18 In this analysis, only 6 patients with PNH experienced a TEAE that was considered a MAVE, none of which were precipitated by BTH, suggesting that terminal complement inhibition was not compromised.
BTH can occur in patients with PNH with suboptimal terminal complement inhibition, or when experiencing stressful events such as pregnancy, surgery, or infection. 19 , 20 For patients with PNH treated with eculizumab, incidences of BTH due to suboptimal C5 inhibition can be managed through either increasing/additional dosages or shortening the dosing interval to <14 days. 21 , 22 In this analysis of patients with PNH receiving ravulizumab, the proportion of patients who experienced BTH was low in the extension periods of both 301 and 302 studies (<10%), and more than 40% of BTH events experienced by these patients were associated with infection events (the majority of which were viral or bacterial infections). However, the number of BTH events reported in study 301 during the extension period was more than double that for study 302 (25 vs. 11, respectively). The disparity in BTH events may potentially be due to differences in the baseline disease activity of patients in the two studies, as patients with PNH in study 301 had HDA compared with those in study 302 who had clinically stable disease having been on prior eculizumab therapy; however, further investigation into these differences may be required. Overall, these findings suggest that ravulizumab provided effective terminal complement inhibition throughout the extension period, and that the reported BTH events were likely due to stressful events experienced by the patients.
4.1. Limitations
A key limitation of this study is the absence of available data on free C5 levels at the time of BTH events for all patients from both studies, although reassuringly, data up to 26 weeks reported that the number of BTH events associated with free C5 levels was low (n = 11) and occurred in patients who received eculizumab. 10 , 11 However, without this information, it is difficult to ascertain if terminal complement inhibition was compromised and was subsequently associated with the event occurrence.
5. CONCLUSION
The efficacy and safety data presented here represent the longest period of follow‐up in the largest sample of patients with PNH treated with ravulizumab to date. These findings add to the body of evidence that C5 inhibitors are effective and well tolerated in patients with PNH and that terminal complement inhibition should continue to be the mainstay of PNH treatment.
CONFLICT OF INTEREST
AGK has received consultancy fees/honoraria/speaker's bureau from Achillion, Akari, Alexion, AstraZeneca Rare Disease, Amgen, Apellis, Biocryst, Celgene, F. Hoffmann‐La Roche, Novartis and Ra Pharma and also research funding from Celgene. MG has received honoraria and advisory board from Alexion, AstraZeneca Rare Disease, and Sobi, consultancy fees and advisory board from BioCryst, consultancy fees from Regeneron Pharmaceuticals, and support for Medscape education in PNH from Apellis. KU has received research funding from Apellis, Alexion, AstraZeneca Rare Disease, Chugai and Roche, and speaker's bureau from Novartis. AK has received honoraria and research support (to Pavlov University) from Alexion, AstraZeneca Rare Disease. MO, JY and AM are employees and stockholders of Alexion, AstraZeneca Rare Disease. JN has received honoraria and provided consultancy to Alexion, AstraZeneca Rare Disease, Apellis, Biocryst, Chugai, Novartis and Roche. JWL received honoraria, consulting fees, and grants (to Seoul St. Mary's Hospital) from Alexion, AstraZeneca Rare Disease. RPL has provided consultancy, honoraria, membership on an Alexion, AstraZeneca Rare Disease. Board of Directors or advisory committees and has received research funding, Speaker's Bureau from Alexion, AstraZeneca Rare Disease.
ACKNOWLEDGMENTS
This study was sponsored by Alexion, AstraZeneca Rare Disease. Medical writing support was provided by Stephen McKenna, MSc, and Rebecca Hornby, PhD, of Oxford PharmaGenesis, Oxford, UK, with funding from Alexion, AstraZeneca Rare Disease.
Kulasekararaj AG, Griffin M, Langemeijer S, et al. Long‐term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2‐year results from two pivotal phase 3 studies. Eur J Haematol. 2022;109(3):205‐214. doi: 10.1111/ejh.13783
Funding information Alexion, AstraZeneca Rare Disease
Contributor Information
Austin G. Kulasekararaj, Email: austin.kulasekararaj@nhs.net.
301/302 Study Group:
Aigul Latypova, Wilma Barcellini, Fiorenza Barraco, Donald Beam, Peter Bettelheim, Elena Borisenkova, Andres Brodsky, Benedict Carnley, Jaroslav Cermak, Tsai‐Yun Chen, Lee Ping Chew, Teng Keat Chew, Chul Won Choi, Yunsuk Choi, Joo Seop Chung, Sophie De Guibert, Timothy Devos, Yuri Dunaev, Jadwiga Dwilewicz‐Trojaczek, Yoko Edahiro, Iliya Elykomov, Maria Ines Engelberger, Fabrizio Pomponi, Wolfgang Fuereder, Nobuharu Fujii, Shinichiro Fujiwara, Piero Galieni, Anna Gaya Valls, Stéphane Girault, David Gomez Almaguer, F. Ataulfo Gonzalez Fernandez, Sergey Gritsaev, Eren Gunduz, Chattree Hantaweepant, Hiroshi Harada, Martin Höglund, Wei‐Han Huang, Azlan Husin, Takayuki Ikezoe, Ken Ishiyama, Yoshikazu Ito, Jun Ho Jang, Deog‐Yeon Jo, Ka‐Won Kang, James Kennedy, Hyo Jung Kim, Jeong‐A Kim, Jin Seok Kim, Fumihiko Kimura, Masayoshi Kobune, Hiroshi Kosugi, Alexander Kulagin, Austin Kulasekararaj, Kuan‐Ming Lai, Loree Larratt, Gyeong‐Won Lee, Jae Hoon Lee, Je‐Hwan Lee, Jong Wook Lee, Chien‐Chin Lin, Elena Lukina, Elena Martynova, Itaru Matsumura, Galina Meike, Sandra Fátima Menosi Gualandro, Natalia Minaeva, Yasuo Mori, Kimio Morita, Fernanda Maria Morselli Ramalho, Yeung‐Chul Mun, Petra Muus, Alexander Myasnikov, Kensuke Naito, Haruhiko Ninomiya, Ayako Nogami, Rosario Notaro, Emilio Ojeda Gutierrez, Masaya Okada, Shinichiro Okamoto, Tatiana Olkhovik, Fabrizio Pane, Ronald Paquette, Joon Seong Park, Régis Peffault de la Tour, Caroline Piatek, Agnieszka Piekarska, Marcos Laercio Pontes Reis, Tatiana Pospelova, Vadim Ptushkin, Alexander Roeth, Ponlapat Rojnuckarin, Viviani de Lourdes Rosa Pessoa, Blanca Rossi, Sinari Salleh, Marco Aurelio Salvino de Araujo, Desmond Samuel, Natalya Saraeva, Hubert Schrezenmeier, Yury Shatokhin, Tatiana Shelekhova, Sang Kyun Sohn, Katerina Steinerova, Kazutaka Sunami, Sharifah Shahnaz Syed Abdul Kadir, Shinobu Tamura, Koen Theunissen, See Guan Toh, Akihiro Tomita, José Miguel Torregrosa Diaz, Yasutaka Ueda, Kensuke Usuki, Alessandro Maria Vannucchi, Pongtep Viboonjuntra, Iige Viigimaa, Svetlana Volkova, Ming‐Chung Wang, Jong‐Ho Won, Lily Lee Lee Wong, Voon Fei Wong, Eng Soo Yap, Su‐Peng Yeh, Ho‐Young Yhim, Yuji Yonemura, Sung‐Soo Yoon, and Andrey Zhuravkov
DATA AVAILABILITY STATEMENT
Alexion will consider requests for disclosure of clinical study participant‐level data provided that participant privacy is assured through methods like data de‐identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant‐level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion‐sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
REFERENCES
- 1. Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124:2804‐2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253‐1258. [DOI] [PubMed] [Google Scholar]
- 3. Holguin MH, Fredrick LR, Bernshaw NJ, Wilcox LA, Parker CJ. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989;84:7‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jang JH, Kim JS, Yoon SS, et al. Predictive factors of mortality in population of patients with paroxysmal nocturnal hemoglobinuria (PNH): results from a Korean PNH registry. J Korean Med Sci. 2016;31:214‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bektas M, Copley‐Merriman C, Khan S, Sarda SP, Shammo JM. Paroxysmal nocturnal hemoglobinuria: current treatments and unmet needs. J Manag Care Spec Pharm. 2020;26:S14‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Medicines Agency . CHMP summary of positive opinion for ultomiris. European Medicines Agency; 2019. [Accessed: 23 February 2022]. Available from: https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-ultomiris_en.pdf. [Google Scholar]
- 7. US Food and Drug Administration . FDA approves ravulizumab‐cwvz for paroxysmal nocturnal hemoglobinuria. 2018. [Accessed: 23 February 2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ravulizumab-cwvz-paroxysmal-nocturnal-hemoglobinuria. [Google Scholar]
- 8. European Medicines Agency . CHMP post‐authorisation summary of positive opinion for ultomiris (II‐10). European Medicines Agency; 2021. [Accessed: 23 February 2022]. Available from: https://www.ema.europa.eu/en/documents/smop/chmp‐post‐authorisation‐summary‐positive‐opinion‐ultomiris‐ii‐10_en.pdf. [Google Scholar]
- 9. US Food and Drug Administration. FDA approves therapy for pediatric patients with serious rare blood disease. 2021. [Accessed: 23 February 2022]. Available from: https://www.fda.gov/drugs/news‐events‐human‐drugs/fda‐approves‐therapy‐pediatric‐patients‐serious‐rare‐blood‐disease
- 10. Lee JW, Sicre de Fontbrune F, Lee LWL, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133:530‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5‐inhibitor‐experienced adult patients with PNH: the 302 study. Blood. 2019;133:540‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kulasekararaj AG, Hill A, Langemeijer S, et al. One‐year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab. Eur J Haematol. 2021;106:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schrezenmeier H, Kulasekararaj A, Mitchell L, et al. One‐year efficacy and safety of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria naïve to complement inhibitor therapy: open‐label extension of a randomized study. Ther Adv Hematol. 2020;11:2040620720966137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Socié G, Caby‐Tosi M‐P, Marantz JL, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10‐year pharmacovigilance analysis. Br J Haematol. 2019;185:297‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JW, Jang JH, Kim JS, et al. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean registry. Int J Hematol. 2013;97:749‐757. [DOI] [PubMed] [Google Scholar]
- 16. Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121:4985‐4996. [DOI] [PubMed] [Google Scholar]
- 17. Peacock‐Young B, Macrae F, Newton D, Hill A, Ariëns R. The prothrombotic state in paroxysmal nocturnal hemoglobinuria: a multifaceted source. Haematologica. 2018;103:9‐17. [DOI] [PubMed] [Google Scholar]
- 18. Krisinger MJ, Goebeler V, Lu Z, et al. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120:1717‐1725. [DOI] [PubMed] [Google Scholar]
- 19. Brodsky RA. A complementary new drug for PNH. Blood. 2020;135:884‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Risitano AM, Marotta S, Ricci P, et al. Anti‐complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hillmen P, Muus P, Röth A, et al. Long‐term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162:62‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peffault de Latour R, Fremeaux‐Bacchi V, Porcher R, et al. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2015;125:775‐783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Alexion will consider requests for disclosure of clinical study participant‐level data provided that participant privacy is assured through methods like data de‐identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant‐level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion‐sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).


