Abstract
Background
Secretory leukocyte protease inhibitor (SLPI), a ~12 kDa protein is an important regulator of innate and adaptive immunity and a component of tissue regenerative programmes. SLPI expression is markedly elevated in chronically inflamed skin, including that of individuals suffering from psoriasis. However, the role of SLPI in these diseases remains elusive.
Objectives
The poor understanding of the early stages of the development of psoriasis is a major obstacle to successful intervention in the skin pathology. We hypothesized that SLPI and peripheral nerves that might be activated early in the progression of the disease likely form a functional relationship to maintain skin barrier homeostasis and respond to a variety of threats.
Methods
We used skin biopsies of healthy donors and individuals with psoriasis to show expression pattern of SLPI. A role of SLPI in psoriasis was mechanistically assessed using SLPI‐deficient mice and an imiquimod (IMQ)‐induced experimental model of psoriasis.
Results
We show that mice lacking SLPI had exaggerated skin alterations that extended beyond the treatment site in an imiquimod‐induced psoriasis. The spatiotemporally distinct skin responses in SLPI‐deficient mice, compared to their wild‐type littermates, resulted from a compromised skin barrier function that manifested itself in heightened transepidermal water loss through the larger skin area surrounding the IMQ‐challenged skin. The increased pathogenic skin changes in the absence of SLPI were reversible through pharmacological treatment that blocks a nerve‐reflex arc.
Conclusions
Together, these data indicate that SLPI plays a protective role in psoriasis through preventing skin dryness, inherent in the pathogenesis of psoriasis and that this SLPI action depends on neuronal input operating in a reflex manner. These findings reveal a previously unrecognized mechanism that maintains cutaneous homeostasis, which involves a crosstalk between the nervous system and a protein anatomically poised to fortify the epidermal permeability barrier.
Keywords: antimicrobial peptides, chronic inflammation, psoriasis, sensory afferents
Introduction
Psoriasis is a chronic inflammatory skin disease with a prevalence of approx. 2%–3% of people across the world, and is associated with psoriatic arthritis, Crohn’s disease or cardiovascular and psychological/psychiatric comorbidities (reviewed in Refs [1, 2]). Although various treatments are currently used to clear the symptoms of psoriasis, there is no cure for the disease. 3 The most efficacious modulating therapy is based on neutralizing specific inflammatory mediators such as IL‐17 and IL‐23 with biologic agents. 3 This approach primarily targets dysregulated immune responses. Therefore, there is a need to focus on less‐described molecular and cellular skin constituents that might be activated early in the progression of the disease, including antimicrobial peptides (AMPs) and peripheral nerves. Overexpression of AMPs, including cathelicidin/LL‐37 and β‐defensins is one of the alterations that mark psoriatic skin. 4 , 5 These AMPs have been proposed to play modulatory role in skin inflammation and generally worsen psoriatic skin lesions, but also contribute to a return to homeostasis. For example, LL‐37 and/or β‐defensins can drive skin inflammation by enabling self‐DNA or self‐RNA‐mediated ‐responses in dendritic cells and/or keratinocytes. 5 , 6 On the other hand, LL‐37 was also found to block activation of the DNA‐sensing inflammasomes, suggesting its anti‐inflammatory role in psoriasis. 7
Among AMPs, highly expressed in psoriatic skin is secretory leukocyte protease inhibitor (SLPI). 4 Although SLPI has been implicated in controlling microbial growth, the protein is best known for its inhibitory role against serine proteases, such as neutrophil elastase (reviewed in Refs [8]). The role of SLPI in skin pathophysiology remains obscure. In a model of cutaneous injury, SLPI was shown to promote the repair of injured skin. The lack of SLPI was accompanied by increased leukocyte infiltration and delayed re‐epithelialization in mice subjected to full‐thickness dorsal incisional wounding. 9 , 10 SLPI can also play immune‐modulatory role in the skin by its capacity to decorate DNA and contribute to activation of TLR9 in plasmacytoid dendritic cells, 11 as well as by inhibiting a deposition of neutrophil extracellular traps (NETs). 12 These actions of SLPI suggest an important regulatory role for this molecule in psoriasis pathophysiology.
Here, we show that SLPI deficiency leads to pathologic skin alterations that extend beyond imiquimod (IMQ)‐induced skin changes in an experimental model of psoriasis, and that these extended skin changes are a consequence of a dysregulated pathway‐mediated by a neural reflex arc. Our data suggest that SLPI in dialogue with the nervous system is involved in controlling spatiotemporal psoriasis‐defining parameters and may ameliorate the breakdown of epidermal homeostasis intrinsic to the pathology of psoriasis.
Materials and methods
Human skin biopsy samples
All human studies were performed in accordance with guidelines established by the Jagiellonian University Institutional Bioethics Committee under approved protocols (#87/B/2014; 1072.6120.30.2020). After obtaining informed consent from patients, approximately 6‐mm punch biopsies were taken from lesional skin of psoriasis patients (n = 8; F:M 3:5; age 44 ± 16 years, PASI score 18.5 ± 7.5). Unchanged areas of skin surrounding moles were obtained from healthy individuals undergoing cosmetic surgery for mole removal and served as a negative control (n = 6; F:M 3:3; age 41 ± 15 years).
Experimental psoriasis and bupivacaine treatment
All animal procedures and experiments were performed in accordance with national and European legislation, after approval by the Second Local Ethical Committee on Animal Testing at the Jagiellonian University in Krakow (approval # 298/2017). Slpi‐/‐ and WT mice from C57BL/6 background and a mixed C57BL/6 × 129/SvJ background were generously donated by Dr. Sharon M. Wahl. 9 The mice used in this study were sex‐ and age‐matched 8‐ to10‐week‐old littermates. Mice were housed under pathogen‐free conditions in the animal facility at the Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University. An IMQ model of psoriasis was induced as previously described 12 , 13 with minor modifications. The mice were treated twice a day for up to 6 days with 15 mg of Aldara™ cream (5% imiquimod), (Meda AB, Solna, Sweden) or Vaseline (Unilever) on 1 cm2 shaved and depilated back skin. The disease severity was evaluated daily using a scoring system based on the clinical Psoriasis Area and Severity Index (PASI). Erythema, scaling, thickening and the affected area were scored independently on a scale from 0 to 4: 0 – none; 1 – slight; 2 – moderate; 3 – marked; 4 – very marked. The skinfold thickness was measured on the last day of experiments with a caliper. Trans‐epidermal water loss (TEWL) was measured on the central (IMQ‐treated) and adjacent, untreated area of the back skin using a Tewameter® TM300 (Courage + Khazaka Electronic). Where indicated, a single dose of BUV (Sigma‐Aldrich) (5 mmol/L in 20 µL) or 0.9% saline was injected twice a day intradermally (i. d.) in the centre of the back skin, followed by IMQ treatment 20 min later ( 14 with modifications).
Immunohistochemistry
Frozen 6–10 μm sections were prepared from the skin biopsies of psoriatic and healthy donors, fixed in acetone and stained with the monoclonal‐biotin‐mouse‐anti‐human SLPI (clone: 31, Abcam) or biotin mouse IgG1 к isotype control (clone: MOPC‐31C, BD Pharmingen, BD Biosciences), followed by PE‐streptavidin (BD Pharmingen). Frozen skin sections (10 µm) from WT mice and SLPI KO mice as a negative control were stained with biotinylated goat anti‐mouse SLPI (R&D Systems) followed by streptavidin conjugated to APC (eBioscience, San Diego, CA, USA). The sections were counterstained with Hoechst dye 33258 (Life Technologies, Carlsbad, CA, USA). Images were captured with a fluorescence microscope (NIKON, Eclipse) and analysed using NIS elements software (Nikon).
Flow cytometry
0.5‐cm2 skin samples (from the central and lateral skin) were incubated with 2.5 mg/mL of Collagenase D (Roche Diagnostics) solution. Single‐cell suspensions from the skin were obtained by mashing the samples through 40‐μm cell strainers in RPMI1640 medium (Biowest) supplemented with 2% FBS (Gibco). The cells were stained for viability assessment (Zombie Aqua Fixable Viability Kit; BioLegend) and then blocked with anti‐CD16/CD32 antibodies (Fc block; eBioscience) followed by staining with directly conjugated antibodies: CD45.2‐APC/Cy7 (clone 104, BioLegend), CD11b‐eFluor450 (clone M1/70, eBioscience), Ly6G‐APC (clone 1A8, BioLegend) and CD3‐Alexa Fluor 594 (clone 17A2, BioLegend). Data were acquired on a BD LSRII (BD Biosciences). Singlets were selected based on FCS‐A vs FCS‐H. Dead cells were routinely excluded from the analysis. Analyses were performed with FCS Express (De Novo Software).
qPCR analysis
Total RNA was extracted using Total RNA Zol‐Out kit (A&A Biotechnology) and converted to cDNA using NxGen M‐MulV reverse transcriptase (Lucigen) with random primers (Promega). Real‐time PCR was performed on the 7500 Fast (Applied Biosystems) using SYBR Green I containing universal PCR master mix (A&A Biotechnology) and primers specific for: mouse Slpi (5′‐TGAGAAGCCACAATGCCGTA‐3′, 5′‐CTCCACACTGGTTTGCGAAT‐3′), and Gapdh (5′‐ TGTGTCCGTCGTGGATCTGA‐3′, 5′‐ TTGCTGTTGAAGTCGCAGGAG‐3′). Levels of the SLPI mRNA in each sample were analysed in duplicates, normalized to the housekeeping gene Gapdh and either shown as relative expression (2−ΔCT) or fold change (2−ΔΔCT method). 15
Statistical analysis
Statistical analyses were performed using Statistica 13 software (StatSoft Inc.). Data are shown as mean ± standard deviation (SD) or mean ± standard error of the mean (SEM). The specific tests performed and number of samples per group are described in the figure legends.
Results
Psoriasis‐prone skin tends to express high levels of SLPI. 4 Using biopsies from the affected (lesional) skin of psoriasis patients, and skin biopsies from healthy individuals, we confirmed by immunostaining that SLPI is abundantly expressed, often in a patchy pattern, in the epidermis of patients with psoriasis but not in healthy donors. The upregulated SLPI levels were primarily detected in the superficial epidermal layers (Fig. 1a).
Figure 1.
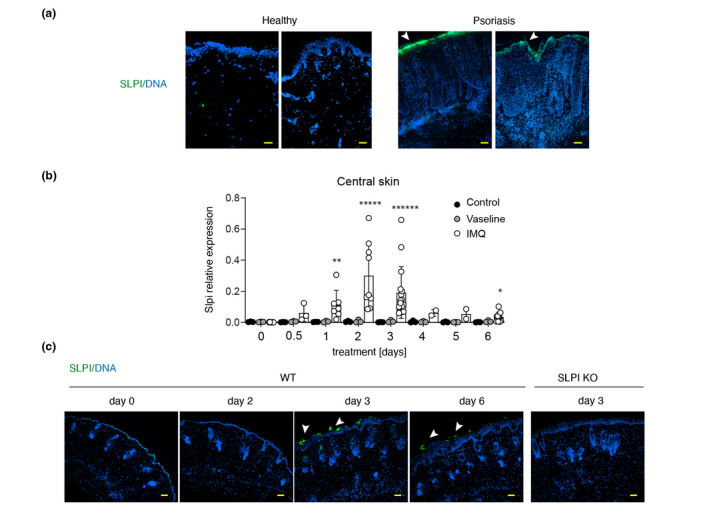
Secretory leukocyte protease inhibitor expression increases in lesional skin of psoriasis patients and in an experimental model of psoriasis. (a) Fluorescence microscopy images of human skin from the donors indicated. Overlay images from 2 healthy and 2 psoriatic donors are shown. Data are representative for at least 4 donors in each group. (b) Slpi gene expression changes during IMQ and Vaseline treatment in C57BL/6 mice. Control = non‐treated mice. The data are shown as mean ± SD from three independent experiments. Dots indicate individual mice. *, P < 0.05; **, P < 0.01; *****, P < 0.00001; ******, P < 0.000001; by Kruskal–Wallis test with multiple comparisons. (c) Representative images of SLPI protein expression in mouse skin during IMQ treatment. Skin sections were stained for SLPI (green) and DNA (blue). Data are from one experiment and are representative of at least five experiments. Image from SLPI KO mouse is shown as a negative control. Arrowheads in A & C point to SLPI‐stained epidermis. Scale bar = 50 μm.
We analysed the kinetics of SLPI expression in an experimental, IMQ‐based model of psoriasis. The topical application of IMQ, induces psoriasis‐like skin changes that recapitulate key features of the human disease. 13 In this model, signs of psoriasis are usually visible at day 2–3 such as skin thickening, redness and scaling that progressively worsen over time up to 6 days when they resemble fully developed skin lesions. 13 C57BL/6 mice were treated with IMQ on the dorsal skin twice a day for 6 days, and SLPI expression in the treated skin was monitored using qPCR and immunohistochemistry. Significant cutaneous Slpi expression approx. 12‐times above the base line (day 0) was noted as early as 12 h following IMQ administration, rising further until day 2, when Slpi levels on average exceeded control levels 84 times (Fig. 1b). Upon longer treatment Slpi expression progressively declined, but even at day 6 was approx. 10‐times as high as in untreated controls. In contrast, topical administration of Vaseline instead of IMQ, did not cause significant Slpi upregulation (Fig. 1b). The kinetics of SLPI expression at mRNA level was paralleled by immunohistochemistry data, showing the most apparent SLPI protein expression in the epidermis at day 3 and reduced albeit still upregulated SLPI protein levels at day 6 (Fig. 1c). Thus, high expression of SLPI accompanies both the early and later stages of psoriasis development. In common with human psoriasis, SLPI was mostly detected in the upper epidermis of mice undergoing psoriasis‐like changes (Fig. 1a and c).
To determine whether the cutaneous presence of SLPI is functionally relevant, we subjected SLPI‐deficient mice (SLPI KO) and their wild‐type littermates (WT) to IMQ treatment. Since different strains of mice vary in terms of the magnitude and pattern of psoriasis‐like changes following IMQ application, 13 , 15 we initially employed mice from two genetic backgrounds; mixed strain C57BL/6 × 129/SvJ as well as C57BL/6 strain. SLPI KO developed less‐demarcated plaques compared to their WT littermates, specifically in the C57BL/6 × 129/SvJ strain (Fig. 2a and b). Thus, the total SLPI ablation tended to result in more robust skin lesion size regardless of strain. A recent RNA‐seq analysis revealed that among different mouse strains, the IMQ‐induced response in C57BL/6 mice best parallels the expression patterns specific to human psoriasis. 15 Therefore, in subsequent experiments we exclusively used mice from the C57BL/6 strain.
Figure 2.
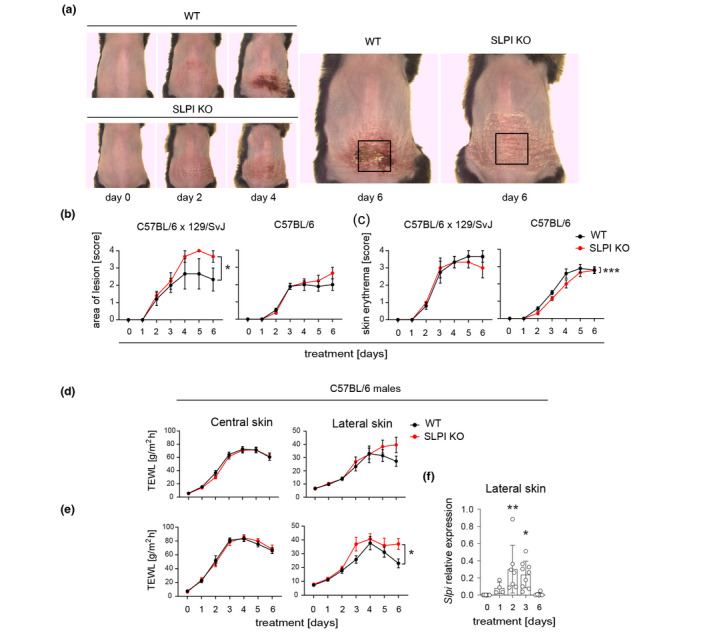
Secretory leukocyte protease inhibitor affects size of skin lesions and skin barrier function beyond the IMQ‐challenged site. The mice indicated were subjected to IMQ‐treatment followed by kinetic assessment of the lesion size and skin erythema. (a) The representative image of female WT and SLPI KO mice from mixed background C57BL/6 × 129/SvJ is shown with the indicated site of treatment. (b) Cumulative data (the mean ± SEM) showing size of lesion. n = 3–10 mice in each group. (c) Cumulative data (the mean ± SEM) showing skin erythema. (d) WT and SLPI KO C57BL6 males were subjected to IMQ‐treatment followed by TEWL measurement at the days indicated at the central and lateral skin. The mean ± SEM is shown. n = at least 10 mice in each group. (e) WT and SLPI KO C57BL6 males were injected with 0.9% NaCl i.d. at the stimulated site prior to IMQ‐treatment. TEWL was then measured at the days indicated at the central and lateral skin. The mean ± SEM is shown. n = at least 7 mice in each group. (f) The lateral skin of WT mice was harvested at the days indicated and subjected to qPCR analysis for Slpi mRNA levels. The data are shown as mean ± SD from three independent experiments. Dots indicate individual mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001; by ANCOVA followed by Bonferroni post hoc test.
Psoriatic skin is characterized by dramatically increased transepidermal water loss (TEWL) largely due to a compromised skin barrier function. 16 , 17 To determine whether SLPI deficiency affects the barrier ability in this condition, we measured TEWL in the skin from the treated dorsal site (central skin) and adjacent, untreated site within the altered region (lateral skin) of SLPIKO as well as WT littermates over the course of the IMQ treatment. Whereas the central skin showed a similar progressive increase in TEWL in both SLPI KO and WT mice, the lateral skin appeared to be more functionally compromised in SLPI‐deficient mice as evidenced by a tendency towards enhanced lateral TEWL readings (Fig. 2d). Psoriasis is associated with dry skin. Since the skin’s diminished ability to retain moisture contributes to an enhanced TEWL, we next assessed whether SLPI is required to prevent water loss while the skin is hydrated. To improve skin hydration, we administered 0.9% saline to the central skin i. d. at the centre of the IMQ‐treated site, prior to each round of IMQ administration. Under this condition, SLPI KO mice lost a significantly higher amount of water through the lateral but not central skin compared to the WT controls (Fig. 2e). The biggest fold change in TEWL induction in SLPI KO mice compared to WT mice, was observed at days 3 and 6 (Fig. 2e). Thus, the expanded skin lesion in SLPI‐deficient mice was concurrent with heightened TEWL (which was augmented by skin hydration), indicating impaired barrier function beyond the IMQ‐treated site.
Given the localized effect of SLPI on water retention that was confined to the lateral skin, we next assessed whether the lateral and central skin differed in terms of SLPI expression levels. qPCR demonstrated that Slpi transcription at the lateral skin paralleled Slpi expression levels at the central skin in the course of IMQ‐induced psoriasis (Figs 2f and 1b, respectively). Collectively, these data suggested that upregulated SLPI levels alone cannot account for the different rate of water loss at the central and lateral skin in experimental psoriasis.
Two key symptoms of psoriasis at cellular level are dysregulated keratinocyte proliferation leading to a thickened epidermis, and robust skin infiltration by leukocytes, among which neutrophils are considered a hallmark of psoriasis, whereas T cells are crucial to the manifestation of the disease. 18 IMQ‐treatment resulted in a markedly increased skin thickness in both WT and SLPI KO mice (Fig. 3). However, SLPI‐deficiency had neither a macroscopic effect on the skinfold thickness (Fig. 3a), nor were found to affect the epidermal thickness in the central and lateral sites as revealed by histology (Fig. 3b).
Figure 3.
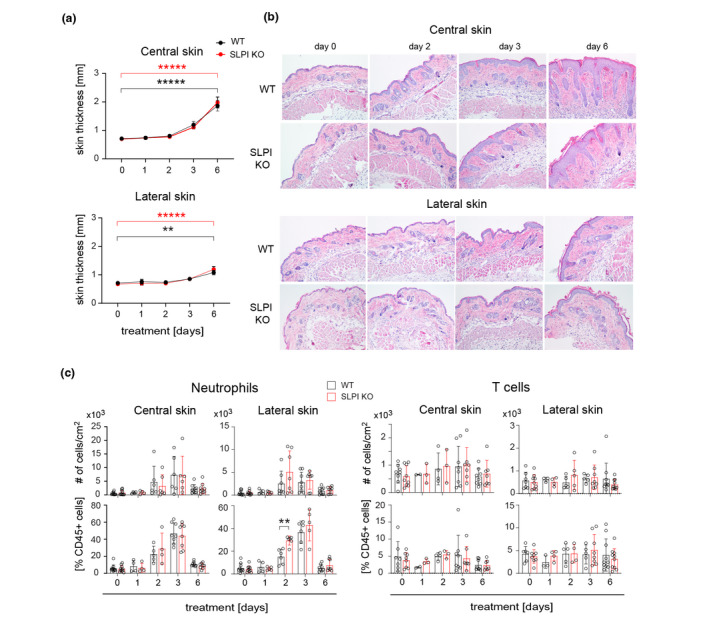
Secretory leukocyte protease inhibitor deficiency does not affect psoriatic skin thickness and has only a marginal effect on skin inflammation in experimental psoriasis. WT and SLPI KO C57BL/6 males were subjected to IMQ‐treatment. (a) Skinfold thickness was measured at the days indicated using a caliper. The mean ± SEM is shown. n = 3–10 mice in each group. *****, P < 0.00001; **, P < 0.01; by two‐way ANOVA followed by Bonferroni post hoc test. (b) Skin was harvested and analysed using histology. Results are representative of at least three independent experiments. (c) Skin was harvested and subjected to flow cytometry analysis. Total leukocytes were detected using anti CD45 mAbs, whereas neutrophils were detected using anti Ly6G and CD11b mAbs and T cells were detected using anti‐CD3 mAbs. The mean of n = 7–10 mice ± SD is shown. *P < 0.05 by t‐test.
A flow cytometry analysis showed that IMQ challenge led to similar rate of skin inflammation in SLPI KO and WT mice, which manifested itself in a comparable number and percentile of neutrophils and T cells infiltrating the treated skin (Fig. 3c). Some differences were only noted in an adjacent, untreated site at day 2, where a significantly increased percentile of neutrophils among CD45+ leukocytes were found in SLPI KO mice. Together, these data suggested that enlarged skin lesions in SLPI KO mice cannot be attributed to altered epidermal thickness or the scope of skin inflammation.
To determine the mechanism underlying the different presentation of the diseased skin in SLPI KO mice compared to WT mice, we focused on the cutaneous sensory neurons and their recently reported ability to trigger an inflammatory reflex arc. 14 The free endings of neurons that transmit sensory information from the skin can be activated by a variety of cutaneous stimuli, and the resulting action potentials are propagated to the neuronal bodies localized primarily in the dorsal root ganglia (DRG), close to the spinal cord. 19 In the axon reflex, this neuronal activation is diverted and redirected back to axonal terminals in the skin. 19 , 20 Local activation of cutaneous afferents with C. albicans was recently found to augment host immunity at adjacent, uninfected skin through pathways that operate in a reflex manner. 14 Although inflammation at adjacent sites in IMQ‐treated mice was only mildly affected by SLPI deficiency, the lack of SLPI phenocopied some aspects of nerve reflex arc‐propagated skin responses, namely skin involvement beyond the site of the original insult. Therefore, we next asked whether SLPI can give rise to a smaller altered skin area by restricting the nerve reflex arc‐mediated effects on the skin barrier function. To block voltage‐gated Na+ channels and thus activation at the nerve terminals in the skin surrounding the original stimulus, we injected SLPI KO and WT littermates with 5 mmol/L BUV, a long‐acting local anaesthetic. 14 BUV, or 0.9% saline as a vehicle, was administered i.d. at the IMQ‐treated site before each IMQ application. BUV treatment, when compared to NaCl treatment, did not significantly alter TEWL scores at the central skin of both SLPI KO and WT mice, indicating BUV‐unperturbed skin barrier function (Fig. 4a). In stark contrast, TEWL was markedly reduced at the lateral skin of SLPI KO mice, and slightly lowered in WT mice when the reflex arc was disabled by BUV treatment (Fig. 4a). Thus, in the presence of BUV, SLPI KO and WT mice demonstrated comparable TEWL over the lateral skin. BUV administration also tended to reduce the size of the skin area involved in the IMQ‐challenged SLPI KO mice but not the WT mice (Fig. 4b). Other pathological features, including skin erythema, scaling or thickness, were overall unaffected in SLPI KO mice by BUV administration (Fig. S1a). Likewise, the skinfold thickness or percentile of neutrophils and T cells that accumulated in the central and lateral skin following IMQ challenge, remained similar in BUV‐and NaCl‐ treated SLPI KO and WT mice (Fig. S1b & c, respectively). Taken together, these data suggested that specific skin barrier function such as water loss protection is mediated by SLPI in a nerve‐reflex arc‐dependent manner.
Figure 4.
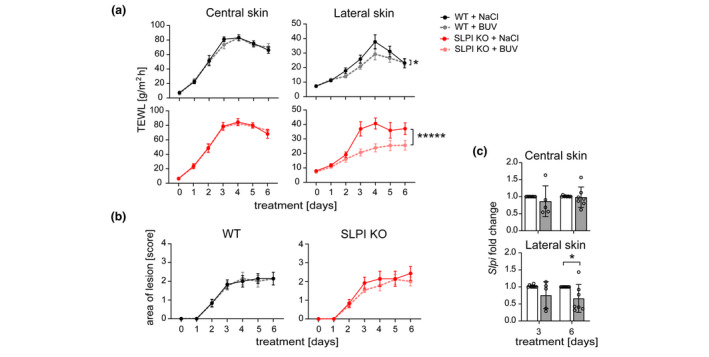
Secretory leukocyte protease inhibitor prevents water loss in cutaneous regions in a nerve‐reflex arc‐controlled manner. 5 mmol/L BUV or vehicle (0.9% NaCl) was administered i.d. to WT or SLPI KO C57BL/6 mice followed by IMQ treatment. (a) TEWL was measured at the days indicated and presented as the means ± SEM. n = 7–9 mice per group. *, P < 0.05; *****, P < 0.00001; by ANCOVA followed by Bonferroni post hoc test. (b) The area of skin lesions was evaluated using manual scoring at the days indicated and presented as the means ± SEM. n = 7–9 mice per group. (c) Slpi mRNA levels in WT mice treated with 0.9% NaCl (empty bars) or BUV (shaded bars) were analysed at the days indicated by qPCR. Data are shown as the means ± SD and are presented relative to NaCl‐treated central and later skin at days 3 and 6. n = 3 experiments with data points showing individual mice. *P < 0.05 by Mann–Whitney U test.
The upregulation of SLPI levels observed in the IMQ‐treated skin of WT mice at both central and lateral sites (Figs 1b and 2f) raised the question as to whether the nerve‐reflex arc regulates SLPI expression in surrounding unchallenged skin. A statistically significant decrease in SLPI mRNA levels within the adjacent site but not the IMQ‐treated central site was observed at day 6 but not day 3 when the reflex arc was blocked by BUV (Fig. 4c). Thus, TEWL and SLPI expression are both controlled, albeit with different kinetics, by the nerve‐reflex arc in the extended area of psoriasiform skin.
Discussion
Here, we report a previously undescribed level of functional organization of the skin barrier responses; namely interaction between the protein SLPI, with the neuronal network which transmits the information vital for permeability barrier homeostasis. Our findings support and extend the emerging notion that both the nervous system and the neural reflex mediate crucial processes associated with infection and inflammation at body barriers, including skin (reviewed in Ref. [19]). The nervous and immune systems cooperate to provide better skin protection against environmental insults. This is illustrated by nerve reflex arc‐mediated protective innate anticipatory immunity. Local activation of cutaneous sensory neurons with C. albicans augmented host immunity at adjacent, uninfected skin by triggering inflammatory type 17 immune responses, potentially to prevent or alleviate infection spread to the surrounding not yet infected areas. 14 On the other hand, direct interaction between the nervous and immune system can also promote pathological skin inflammation. Selective disruption of IL‐23 or IL‐17 signalling leads to significant improvements in psoriasis but the disease recurs following cessation of therapy. Notably, sensory nerves drive psoriasiform inflammation by affecting IL‐23 production by local dendritic cells in experimental psoriasis, 21 suggesting that neuronal activity is key to pathogenic inflammation in psoriasis.
Alterations in skin thickness, more robust skin inflammation and/or problems with barrier maintenance could potentially lead to pathogenic skin presentation in mice lacking SLPI. The weakened barrier function beyond the challenged site was found to be the main skin defect associated with SLPI deficiency. Although the extended area of altered skin in SLPI KO mice exhibited some other features characteristic of more severe psoriasis, such as neutrophil‐dominated skin infiltration, these changes were subtle, and likely ameliorated by enforced skin hydration. On the other hand, increased skin moisture by applying saline locally, brought to light epidermal permeability dysfunction at lateral cutaneous sites of mice lacking SLPI. These findings suggest that potential skin damaging immune mechanisms associated with SLPI deficiency in psoriasis would likely be secondary to a perturbed barrier function at the lateral cutaneous site.
The uppermost skin layer, the stratum corneum, together with the tight junctions that seal neighbouring keratinocytes in the underlying layer‐stratum granulosum, forms an interdependent permeability and antimicrobial barrier. 22 Our data indicate that SLPI and a nerve‐reflex arc are functionally linked in regulating skin barrier function. Skin desiccation is considered one of the preventive strategies that lower microbial mass and reduce the risk of infection. 22 However, skin dryness is also a poorly explored feature shared by multiple cutaneous chronic inflammatory disorders, including atopic dermatitis (AD) and psoriasis. 23 , 24 Notably, excessively dry skin often precedes the development of disease flares or worsens their symptoms. Therefore, while the nervous system may protect the host against infection by desiccating the skin surface, its activity can also lead to or accelerate cutaneous pathology by promoting water loss through the epidermis at sites adjacent to lesional skin.
Secretory leukocyte protease inhibitor‐deficient mice had restored the ability to retain water within the lateral site if the neural reflex was disabled by BUV treatments. The strategic positioning of SLPI in the upper epidermis and its non‐uniform, patchy distribution in the uppermost layers of psoriatic skin, supports the role of SLPI as a molecule that locally seals in skin moisture in a reflex‐arc‐regulated fashion. This scenario is also consistent with the regulation of SLPI expression at the lateral skin in response to a nerve‐reflex arc. However, it is also possible that SLPI is involved in ameliorating neuronal sensitivity to IMQ‐induced stimuli by raising the threshold for evoking action potentials or directly inhibiting sensory neurons engaged in communicating these alterations to the lateral, unchallenged cutaneous sites.
Secretory leukocyte protease inhibitor expression is upregulated not only in psoriasis but also other inflammatory skin disorders, such as AD. 4 Therefore, SLPI involvement in preventing excessive skin dryness might not be limited to psoriasis but also play a role in other dermatoses associated with a disturbed skin barrier function.
In conclusion: On the basis of our findings we propose a model, in which IMQ challenge results in the local activation of the neuronal network innervating the skin. This then triggers nerve reflex arc responses which promote loss of moisture at the neighbouring skin regions. The presence of SLPI tightens the skin barrier function in these regions at the level of the epidermis and/or inhibits neuronal circuits (Fig. 5). A better knowledge of the skin environment in which chronic inflammatory changes take place may lead to more effective and rapid intervention with skin pathology.
Figure 5.
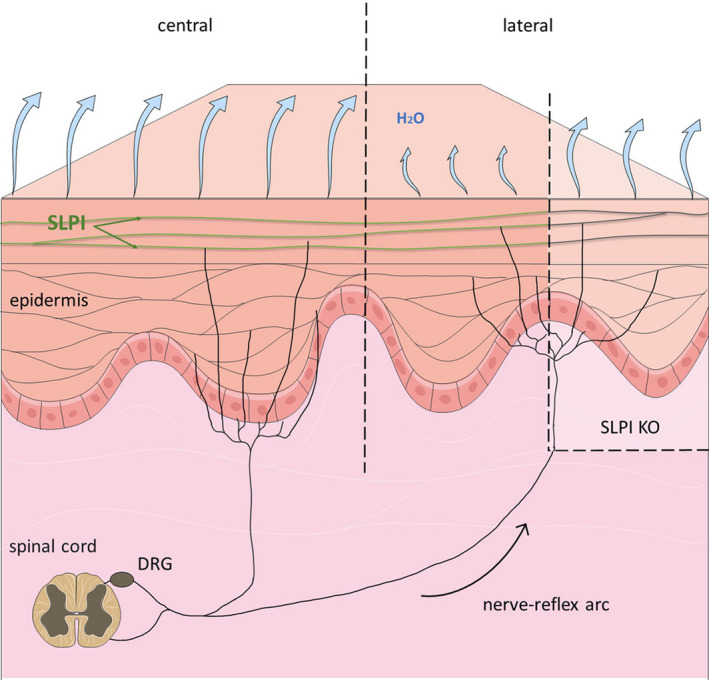
A model of SLPI involvement in preventing excessive water loss in a reflex‐arc regulated manner.
Supporting information
Figure S1 Skin redness, scaling and thickness as well cutaneous neutrophil and T cell content as are not significantly regulated in IMQ‐challenged SLPI KO and WT mice by a nerve‐reflex arc.
Funding sources
This work was supported by Polish National Science Center grants 2011/02/A/NZ5/00337 and UMO‐2017/25/B/NZ6/01003. The open‐access publication of this article was funded by the Priority Area BioS under the programme ‘Excellence Initiative‐Research University’ at the Jagiellonian University in Krakow.
Conflicts of Interest
The authors (PK, BG, AM, ASD, MMG, MK, MKM, GP, JC) declare no conflict of interest. Research funding disclosure (JC) is provided above.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Boehncke WH, Schon MP. Psoriasis. Lancet 2015; 386: 983–994. [DOI] [PubMed] [Google Scholar]
- 2. Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol 2005; 5: 699–711. [DOI] [PubMed] [Google Scholar]
- 3. Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL‐23/IL‐17 signaling pathway and the treatment of psoriasis. J Immunol 2018; 201: 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Jongh GJ, Zeeuwen PLJM, Kucharekova M et al. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol 2005; 125: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 5. Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol 2012; 39: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lande R, Gregorio J, Facchinetti V et al. Plasmacytoid dendritic cells sense self‐DNA coupled with antimicrobial peptide. Nature 2007; 449: 564–569. [DOI] [PubMed] [Google Scholar]
- 7. Dombrowski Y, Peric M, Koglin S et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 2011; 3: 82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Majchrzak‐Gorecka M, Majewski P, Grygier B, Murzyn K, Cichy J. Secretory leukocyte protease inhibitor (SLPI), a multifunctional protein in the host defense response. Cytokine Growth Factor Rev 2016; 28: 79–93. [DOI] [PubMed] [Google Scholar]
- 9. Ashcroft GS, Lei K, Jin W et al. Secretory leukocyte protease inhibitor mediates non‐redundant functions necessary for normal wound healing. Nat Med 2000; 6: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 10. Zhu J, Nathan C, Jin W et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 2002; 111: 867–878. [DOI] [PubMed] [Google Scholar]
- 11. Skrzeczynska‐Moncznik J, Wlodarczyk A, Zabieglo K et al. Secretory leukocyte proteinase inhibitor‐competent DNA deposits are potent stimulators of plasmacytoid dendritic cells: implication for psoriasis. J Immunol 2012; 189: 1611–1617. [DOI] [PubMed] [Google Scholar]
- 12. Zabieglo K, Majewski P, Majchrzak‐Gorecka M et al. The inhibitory effect of secretory leukocyte protease inhibitor (SLPI) on formation of neutrophil extracellular traps. J Leukoc Biol 2015; 98: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Fits L, Mourits S, Voerman JSA et al. Imiquimod‐induced psoriasis‐like skin inflammation in mice is mediated via the IL‐23/IL‐17 axis. J Immunol 2009; 182: 5836–5845. [DOI] [PubMed] [Google Scholar]
- 14. Cohen JA, Edwards TN, Liu AW et al. Cutaneous TRPV1(+) neurons trigger protective innate type 17 anticipatory immunity. Cell 2019; 178: 919–932 e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swindell WR, Michaels KA, Sutter AJ et al. Imiquimod has strain‐dependent effects in mice and does not uniquely model human psoriasis. Genome Med 2017; 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grice KA, Bettley FR. Skin water loss and accidental hypothermia in psoriasis, ichthyosis, and erythroderma. Br Med J 1967; 4: 195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tagami H, Yoshikuni K. Interrelationship between water‐barrier and reservoir functions of pathologic stratum corneum. Arch Dermatol 1985; 121: 642–645. [PubMed] [Google Scholar]
- 18. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496–509. [DOI] [PubMed] [Google Scholar]
- 19. Tamari M, Ver Heul AM, Kim BS. Immunosensation: Neuroimmune Cross Talk in the Skin. Annu Rev Immunol 2021; 39: 369–393. [DOI] [PubMed] [Google Scholar]
- 20. Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol 2018; 36: 783–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riol‐Blanco L, Ordovas‐Montanes J, Perro M et al. Nociceptive sensory neurons drive interleukin‐23‐mediated psoriasiform skin inflammation. Nature 2014; 510: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aberg KM, Man M‐Q, Gallo RL et al. Co‐regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol 2008; 128: 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pons‐Guiraud A. Dry skin in dermatology: a complex physiopathology. J Eur Acad Dermatol Venereol 2007; 21(Suppl 2): 1–4. [DOI] [PubMed] [Google Scholar]
- 24. Werner Y, Lindberg M. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm Venereol 1985; 65: 102–105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Skin redness, scaling and thickness as well cutaneous neutrophil and T cell content as are not significantly regulated in IMQ‐challenged SLPI KO and WT mice by a nerve‐reflex arc.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


