Abstract
Objective
Nonadherence to antiseizure drugs is a significant problem in pediatric epilepsy and is linked to increased morbidity and mortality, clinically unnecessary medication changes, and increased health care costs. Family interventions can improve adherence. However, it is challenging to determine which families will struggle with nonadherence and require intervention. This study aims to identify specific parent, family, child, and medical factors that predict which families most need family‐based adherence interventions.
Methods
Families enrolled in a randomized clinical trial of a family‐based adherence intervention completed measures assessing parent, family, child, and medical factors. Families also used an electronic adherence monitor. Adherence of ≥95% was considered high adherence (not requiring intervention), and <95% was considered suboptimal adherence (requiring intervention). We conducted a stepwise logistic regression analysis to assess demographic, medical, child, family, and parent predictors of membership to the suboptimal adherence group.
Results
Of the 200 families of children with new onset epilepsy who enrolled, 177 families completed the study. Of these families, 121 (68%) were in the high adherence group and 56 (32%) were in the suboptimal adherence group. Families with lower socioeconomic status (SES), children of color, lower general family functioning, and more parent distress were more likely to be in the suboptimal adherence group.
Significance
We identified that parent and family factors, as well as sociodemographic characteristics, predicted membership in the suboptimal adherence group. It is critical to find creative and practical solutions for assessing and intervening upon key adherence predictors. These may include streamlined screening for parental distress and family functioning, as well as recognition that families of lower SES and communities of color may be at heightened risk for suboptimal adherence.
Keywords: adherence, epilepsy, family functioning, race, socioeconomic status
Key Points.
To optimize and maximize the delivery of family‐based adherence interventions, it is important to identify who is in need of intervention
Family, parent, and sociodemographic factors appear to predict whether a family has suboptimal adherence after new onset epilepsy
Creative approaches to screening for and addressing adherence barriers are essential to maximize the health of children with epilepsy
1. INTRODUCTION
Pediatric epilepsy affects roughly 472 000 children in the United States, 1 and the first line of treatment is the use of antiseizure medication (ASM), which leads to seizure freedom in ~65% of children with epilepsy 2 ; however, approximately 60% of children and families struggle to maintain adherence to their ASM. 3 Nonadherence (i.e., behaviors that do not align with recommendations made by a medical professional or health care provider) 4 , 5 is linked to increased morbidity (e.g., continued seizures) 6 , clinically unnecessary medication changes, 5 and increased health care costs. 7 For example, children who were nonadherent in the first 6 months of ASM treatment were 3.24 times more likely to have ongoing seizures 4 years following diagnosis. 6 Thus, identifying and mitigating adherence barriers early in the disease process is imperative to optimizing quality of life.
As outlined by the Pediatric Self‐Management Model, individual, family, community, and health care system factors contribute to pediatric self‐management. 4 , 5 For younger children with epilepsy, members of the family system, such as caregivers, play a particularly large role, taking on primary responsibility for completing treatment‐related tasks, including scheduling appointments, obtaining medicines, developing dosing routines, and managing competing demands to achieve optimal adherence. 8 Thus, parent and family factors can play a large role in a child's adherence, including socioeconomic status (SES), family communication, and family problem‐solving. 4 , 5 , 9 , 10 Additionally, parents who lack knowledge about epilepsy and those experiencing psychosocial distress may struggle to optimally follow medication regimens. 4 , 5 , 9 , 10
Child‐specific factors such as behavioral problems, medical factors such as medication side effects, and other community and health care system factors can also help or hinder adherence among young children with epilepsy. 4 , 5 For young children, these child‐, medical‐, and health care system‐specific factors often indirectly contribute to adherence behaviors by leading to additional family stress and burden, which may interfere with administering ASM. Thus, these data clearly indicate a need for family‐focused adherence interventions, given the many barriers that families can face in maintaining adherence to their child's ASM.
Despite the need for family‐focused interventions to improve adherence in pediatric epilepsy, few studies exist. 11 , 12 , 13 , 14 , 15 Several pilot trials and a recent large randomized controlled clinical trial, known as Supporting Treatment Adherence Regimens (STAR 16 ), which focuses on improving parent epilepsy knowledge and family‐based problem‐solving skills around adherence barriers, have demonstrated preliminary efficacy in improving ASM adherence. However, we still lack the ability to identify which families would most benefit from a family‐focused adherence intervention.
Evidence‐based treatments can take years to integrate into clinical practice, with a 17‐year research to practice gap. 17 One first important step in the translation of research to practice is to identify families that require an intervention based on clinical information to inform practitioners. Thus, the key aim of the current paper is to identify specific parent, family, child, and medical factors to identify families that most need family‐based adherence interventions. Specifically, this study utilizes baseline data from a randomized controlled trial (NCT01851057) for a family‐focused adherence intervention (STAR) among 200 families of young children (2–12 years old) newly diagnosed with epilepsy. 16 , 18 STAR trial uses an enrichment design, which screens and identifies nonadherent participants (e.g., run‐in period to identify nonadherence) and only randomizes individuals who would most benefit from the intervention. 19 Thus, participants who had an adherence rate of ≥95% during the baseline period were considered to represent families that did not require intervention and thus ended study participation. In contrast, families that exhibited <95% were randomized to either the control (i.e., education) or treatment group (i.e., education and problem‐solving). We hypothesized that demographic (SES, child race, parent marital status), medical (presence of a seizure since last clinic visit, medication side effects), child (internalizing symptoms, externalizing symptoms, adaptive functioning, history of psychosocial functioning), family (family functioning variables), and parent (epilepsy knowledge, parent psychosocial distress) factors would predict membership in either a suboptimal adherence group needing intervention or a high adherence group not needing intervention. Importantly, we believe that these data can help to shed light on families that may be most at risk for suboptimal adherence, and therefore in need of family‐focused interventions as a part of routine clinical care.
2. MATERIALS AND METHODS
2.1. Study participants
Participants included young children with epilepsy and their caregivers recruited from the Comprehensive Epilepsy Center at Cincinnati Children's Hospital Medical Center from April 2013 to December 2018. Inclusion and exclusion criteria included (1) child between 2 and 12 years of age; (2) child diagnosed with epilepsy in the past 7 months; (3) ASM monotherapy; (4) child and parent able to read and speak English; (5) no nonepilepsy medical disorders requiring daily medications for the child, with the exception of allergies and asthma; (6) no significant child developmental delay (i.e., autism); and (7) family living within 75 miles of the hospital.
2.2. Design and procedures
A sequential randomization, enrichment design clinical trial (NCT01851057) was used to evaluate a family‐tailored adherence intervention (i.e., STAR) for children with epilepsy and their caregivers compared to an education only intervention. 16 , 18 Two hundred participants were enrolled and screened for intervention based on baseline adherence rates (nonadherence < 95%). Participants demonstrating nonadherence were randomized to either the attention control group (education only) or treatment group (education and problem‐solving). Both treatment groups received eight sessions. Participants with near‐perfect adherence (≥95%) were monitored over the course of 7 total months for nonadherence. If nonadherence was identified during this time frame, randomization occurred. Participants with near‐perfect adherence during the entire 7‐month screening period ended study participation. Randomized participants received eight intervention sessions over a 4‐month period and were followed for three additional follow‐up visits (3, 6, and 12 months following intervention).
Eligible participants were screened in outpatient epilepsy clinics for recruitment by study coordinators and, if eligible, approached to learn about the study. Once all questions were answered and consent/assent forms were signed, study participation commenced. At the initial baseline visit, parents completed questionnaires and were given an adherence electronic monitor to track their daily medication taking. Subsequent assessments included downloading of adherence monitors and questionnaire completion. Medical chart reviews also occurred at each visit. For purposes of the current study, only baseline questionnaires were used. Baseline for each participant was considered the time point at which patients first demonstrated a month of adherence of <95%, which could have been at 1, 3, or 6 months following initial enrollment in the study. See Figure 1 for a simplified version of the study timeline. Participants were compensated for their time and effort. This study was approved by the hospital institutional review board. A detailed overview of the study methods can be found in Ref. [18].
FIGURE 1.
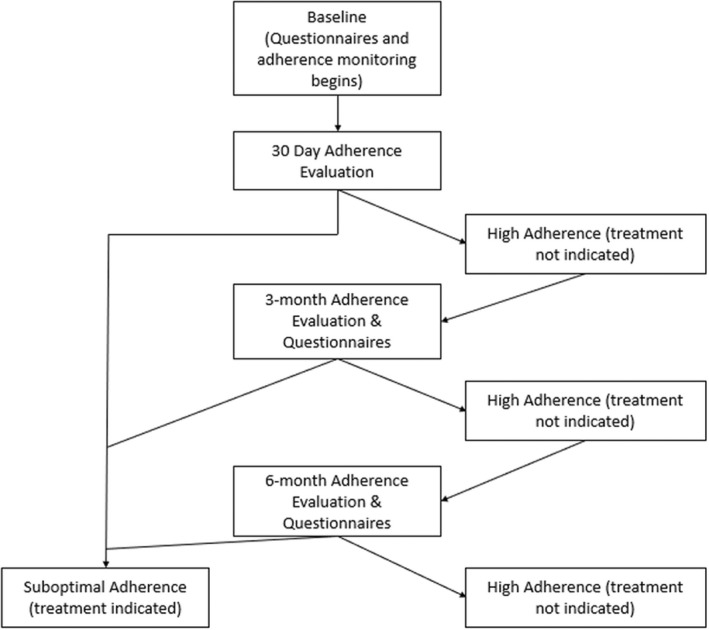
Study timeline
2.3. Measures
2.3.1. Background information form
The Background Information Form is a demographic questionnaire completed by caregivers that provides general information about the child's age, race/ethnicity, sex, and parent marital status. Race and ethnicity data were determined from self‐report. A family Duncan score was used as a proxy for SES, with scores ranging 15–97 and higher scores representing higher SES. 20
2.3.2. Medical chart review form
Medical information was extracted from the electronic medical record to document seizure type/etiology, seizure frequency, and treatment regimen.
2.3.3. Electronic monitoring
The Medication Event Monitoring Systems TrackCap (MEMS 6 TrackCap) made by AARDEX Corporation is an electronic monitoring system measuring the dosing histories of patients prescribed oral medications (i.e., standard plastic vial or microelectronic circuit in bottle that registers dates and times bottle is accessed). The SimpleMed + Pillbox is an electronic pillbox provided to families to organize and administer their seizure medication. Of note, the SimpleMed pillbox was not available for the study until July 2015.
Daily data from these two devices were used to calculate monthly adherence rates by dividing the number of doses taken by the number of doses prescribed and multiplying by 100% (e.g., 45 doses taken/60 doses prescribed * 100% = 75%). Monthly adherence rates were used for analyses.
2.3.4. Pediatric Epilepsy Side Effects Questionnaire
The Pediatric Epilepsy Side Effects Questionnaire is a caregiver‐completed questionnaire that assesses seizure medication side effects in youth aged 2–18 years with epilepsy.21 Items cover a broad range of neurological, behavioral, gastrointestinal, skin, and motor side effects and are summed to obtain a total side effects severity score. Item responses range from 1 (not present) to 6 (high severity), and total scores can range from 0 to 100. The measure has demonstrated excellent reliability and internal consistency.
2.3.5. Behavior Assessment Schedule for Children: Parent Rating Scale
The Behavior Assessment Schedule for Children: Parent Rating Scale provides a parental assessment of behavioral and emotional difficulties in children and adolescents. 22 It yields several composite and subscale scores, including Externalizing Problems (Hyperactivity, Aggression, Conduct Problems subscales), Internalizing Problems (Anxiety, Depression, Somatization subscales), and Adaptive Skills (Adaptability, Social Skills, Leadership, Activities of Daily Living, Functional Communication subscales). Individual raw scores are converted to standardized T‐scores, with elevations detected for ≥70 for all except Adaptive Skills, for which low scores reflect elevations (i.e., ≤30).
2.3.6. Brief Symptom Inventory
The Brief Symptom Inventory is a self‐report measure assessing parental symptoms of emotional and behavioral disorders across 10 dimensions, including Somatization, Obsession–Compulsion, Interpersonal Sensitivity, Depression, Anxiety, Hostility, Phobic, Anxiety, Paranoid Ideation, and Psychoticism. 23 A global index score was calculated to assess general distress: the Global Severity Index (GSI; distress level). The GSI raw scores were converted to T‐scores. Higher scores are indicative of more symptoms. Internal consistency coefficients ranged from .71 to .85. 24
2.3.7. Epilepsy Knowledge Questionnaire–Revised
The original Epilepsy Knowledge Questionnaire is a 55‐item questionnaire with statements regarding epilepsy knowledge and 21 statements on social knowledge of epilepsy. 25 Items were modified or deleted for the current study to be consistent with language and medical practice in the United States with 47 items. The final score represents the percentage of questions the parents answered correctly. Psychometric properties of the revised total knowledge score are adequate.
2.3.8. McMaster Family Assessment Device
The Family Assessment Device (FAD) is a parent‐report questionnaire designed to assess family functioning based on the McMaster model of family functioning. 26 The FAD contains six specific behavioral dimensions of family functioning (Problem‐Solving, Communication, Roles, Affective Responsiveness, Affective Involvement, Behavior Control) and one separate scale of overall functioning, which was used for this study (alpha coefficients = .72–.92). 27 Higher scores indicate worse functioning, with a range of possible scores from 1 to 4.
2.4. Statistical analysis
All analyses were conducted using IBM SPSS Statistics version 26. Descriptive data were evaluated including means, SDs, and percentages. Bivariate correlations were run to evaluate the relationships between variables of interest. The sample was dichotomized into two groups: (1) families that had adherence > 95% (i.e., high adherence group) and (2) families that had adherence ≤ 95% (i.e., suboptimal adherence group). Individuals in the high adherence group were assigned a value of 0, and individuals in the suboptimal adherence group were assigned a value of 1 for adherence group membership. Adherence group classifications align with the criteria for qualifying for adherence‐focused family treatment in the larger clinical trial (NCT01851057 18 ).
A stepwise logistic regression was conducted to assess predictors of being in the suboptimal adherence group relative to the high adherence group among the full sample of study completers (N = 177). Pairwise deletion was used to handle missing data. Demographic factors, including SES, parent marital status, and child race, were included in Step 1. Medical factors, including the presence of a seizure since the last clinic visit and reported side effects of ASM medication, were included in Step 2. Child factors, including internalizing symptoms, externalizing symptoms, adaptive functioning, and history of psychosocial concerns, were added in Step 3. Family factors, including the seven domains of the FAD, were added in Step 4. Finally, parent factors, including epilepsy knowledge and parent psychological distress, were included for Step 5. When using logistic regressions, an R 2 cannot be calculated; thus, a pseudo R 2 is most commonly used to assess model fit. We used the Nagelkerke R 2, which has an upper limit of 1, and higher numbers indicate better fit. The Nagelkerke R 2 is reflective of improvement above and beyond a null model (model with no predictors).
3. RESULTS
Two hundred parents of children with new onset epilepsy enrolled in the study, but 23 withdrew from the study or were lost to follow‐up, resulting in a total of 177 families that completed the study. Of these families, 121 (68%) were in the high adherence group and 56 (32%) were in the suboptimal adherence group. Of the 56 families in the suboptimal adherence group, 52% were randomized after the initial 1‐month of monitoring. The remaining families were randomized at later time points due to high initial adherence (4‐month randomization = 35%, 7‐month randomization = 13%). Based on self‐report, parents were predominantly White, non‐Hispanic (86.5%); female (94.5%); and married (70%; see Table 1).
TABLE 1.
Descriptive characteristics at baseline
| Characteristic | Suboptimal adherence, n (%) | High adherence, n (%) | Total, n (%) |
|---|---|---|---|
| Sample size | 56 | 121 | 200 |
| Parent ethnicity | |||
| White | 46 (82.1%) | 109 (90.1%) | 173 (86.5%) |
| Black | 8 (14.3%) | 6 (5%) | 18 (9%) |
| Asian | 0 (0%) | 3 (2.5%) | 3 (1.5%) |
| Multiracial | 2 (3.6%) | 2 (1.7%) | 4 (2%) |
| Other/unknown | 0 (0%) | 1 (.8%) | 2 (1%) |
| Parent sex | |||
| Female | 56 (100%) | 114 (94.2%) | 189 (94.5%) |
| Marital status | |||
| Married | 28 (50%) | 99% (81.8%) | 140 (70%) |
| Single, divorced, or other | 28 (50%) | 22 (18.2%) | 60 (30%) |
| Child age, mean (SD) | 7.7 (3.1) | 7.6 (2.9) | 7.5 (2.9) |
| Child sex | |||
| Female | 29 (51.8%) | 65 (53.7%) | 105 (52.5%) |
| Seizure type | |||
| Partial | 11 (20%) | 45 (37%) | 60 (30%) |
| General | 31 (55%) | 47 (39%) | 87 (43.5%) |
| Unclassified | 14 (25%) | 29 (24%) | 53 (26.5%) |
| Initially prescribed antiseizure drug | |||
| Carbamazapine | 6 (11%) | 18 (15%) | 27 (13.5%) |
| Ethosuximide | 18 (32%) | 32 (26%) | 54 (27%) |
| Levetiracetam | 12 (21%) | 39 (32%) | 60 (30%) |
| Oxcarbazepine | 3 (5%) | 11 (9%) | 15 (7.5%) |
| Lamotrigine | 0 (0%) | 3 (2%) | 3 (1.5%) |
| Topiramate | 1 (2%) | 1 (1%) | 2 (1%) |
| Valproic acid | 16 (29%) | 17 (14%) | 39 (19.5%) |
| Parent BSI global severity index T‐score, mean (SD) | 52.3 (11.4) | 47.7 (10.9) | 48.9 (11.2) |
| BASC‐PRS, mean (SD) | |||
| Internalizing T‐score | 52 (11.3) | 49.4 (10.7) | 50.1 (11.4) |
| Externalizing T‐score | 51.2 (10) | 48.8 (10) | 49.2 (9.9) |
| Adaptive functioning T‐score | 46.7 (11) | 51.8 (10) | 50.5 (10.7) |
| Family functioning, mean (SD) | |||
| General | 1.6 (.4) | 1.5 (.4) | 1.5 (.4) |
| Problem‐solving | 1.8 (.4) | 1.7 (.4) | 1.7 (.4) |
| Communication | 1.9 (.4) | 1.7 (.4) | 1.8 (.4) |
| Roles | 2.1 (.4) | 1.9 (.4) | 1.9 (.4) |
| Affective responsiveness | 1.7 (.5) | 1.6 (.4) | 1.6 (.4) |
| Affective involvement | 1.9 (.4) | 1.8 (.5) | 1.8 (.5) |
| Behavioral control | 1.5 (.4) | 1.4 (.3) | 1.4 (.4) |
Note: Two hundred families completed the baseline assessment, but 23 families withdrew or were lost to follow‐up, so these families are not represented in the 56 and 121 who respectively were categorized into the suboptimal and high adherence groups, but are captured in the descriptive statistics for the total n.
Abbreviations: BASC‐PRS, Behavior Assessment Schedule for Children: Parent Rating Scale; BSI, Brief Symptom Inventory.
A small subset of parents reported clinically significant global psychological distress (14%). A slightly larger group reported psychosocial concerns about their child, with 20% reporting clinically concerning internalizing problems, 13.5% reporting clinically concerning externalizing problems, and 18.4% reporting clinically concerning adaptive functioning difficulties. A subset of families reported low family functioning across all domains: Problem‐Solving (6.1%), Communication (14.4%), Affective Responsiveness (6.1%), Roles (13.9%), Affective Involvement (26.1%), Behavioral Control (14.4%), and General (6.7%).
3.1. Logistic regression
In Step 1 (demographics, Nagelkerke R 2 = .20), individuals with higher SES (odds ratio [OR] = .98, p < .001) were less likely to be in the suboptimal adherence group. Specifically, for each 1 unit increase in SES (range = 15–97), individuals were 3.3% less likely to be in the suboptimal adherence group. Families with children of color (i.e., Black, Asian, or multiracial; OR = 3.26, p = .009) were 226% times more likely to be in the suboptimal adherence group. There were no significant predictors of adherence group in Step 2 (medical factors, Nagelkerke R 2 change = 0) or Step 3 (child psychosocial factors, Nagelkerke R 2 change = .02). In Step 4 (family factors, Nagelkerke R 2 change = .07), those with better general family functioning (OR = .08, p = .032) were less likely to be in the suboptimal adherence group. Specifically, for each 1 unit increase in family functioning (range = 1–4), individuals were 92% less likely to be in the suboptimal adherence group. In Step 5 (parent factors, Nagelkerke R 2 change = .05), parents with higher psychosocial distress (OR = 1.05, p = .027) were 5% more likely to be in the suboptimal adherence group. Specifically, for each 1 unit increase in parent distress, individuals were 5% more likely to be in the suboptimal adherence group. Thus, an individual with higher parent distress (1 SD above the mean) was 112% more likely to be in the suboptimal adherence group than an individual with low parent distress (1 SD below the mean). In the final model with all five steps, lower SES, children of color, lower general family functioning, and more parent distress all remained unique and significant predictors of being in the suboptimal adherence group (final Nagelkerke R 2 = .34). See Table 2 for a list of the final ORs.
TABLE 2.
Final ORs (N = 177)
| Predictors | OR | SE | p |
|---|---|---|---|
| Duncan (SES) | 0.98 | 0.01 | .02 |
| Marital status | 1.04 | 0.25 | .88 |
| Race | 3.67 | 0.55 | .02 |
| Seizures since last visit | 1.30 | 0.44 | .55 |
| Side effects | 0.98 | 0.02 | .38 |
| Externalizing symptoms (CBCL) | 0.97 | 0.03 | .32 |
| Internalizing symptoms (CBCL) | 0.98 | 0.03 | .47 |
| Adaptive skills (CBCL) | 0.98 | 0.03 | .44 |
| History of psychosocial problems | 2.04 | 0.50 | .16 |
| FAD problem‐solving | 1.74 | 0.94 | .55 |
| FAD communication | 4.53 | 0.92 | .10 |
| FAD roles | 2.19 | 0.82 | .342 |
| FAD affective responsiveness | 0.86 | 0.74 | .84 |
| FAD affective involvement | 0.91 | 0.69 | .89 |
| FAD behavioral control | 3.11 | 0.97 | .24 |
| FAD general | 0.08 | 1.22 | .04 |
| Epilepsy knowledge | 0.96 | 0.03 | .22 |
| BSI GSI (parent distress) | 1.05 | 0.02 | .03 |
Abbreviations: BSI, Brief Symptom Inventory; CBCL, Child Behavior Checklist; FAD, Family Assessment Device; GSI, Global Severity Index; OR, odds ratio; SES, socioeconomic status.
3.2. Exploratory analyses
We conducted exploratory analyses to better understand the odds of being in the suboptimal adherence group by race. The dichotomization of race into White and non‐White is not ideal; however, this decision was made due to small sample sizes of children of color. Therefore, we decided to run two exploratory logistic regressions evaluating the odds of being in the suboptimal adherence group for Black children relative to White children and multiracial children relative to White children, as these two groups were large enough for comparisons. We controlled for income and SES in these analyses. These analyses indicated that families with children who are Black (OR = 2.90, p = .002) were nearly three times more likely to be in the suboptimal adherence group and that families with children who are multiracial (OR = 1.22, p = .56) were no more likely to be in the suboptimal adherence group compared to White children. However, these results should be interpreted with caution, given the relatively small sample sizes of the comparator groups (n = 20 and 15, respectively).
4. DISCUSSION
The current study is a comprehensive evaluation of demographic, medical, child, caregiver, and family factors that relate to adherence behaviors in young children with newly diagnosed epilepsy. Building from the Pediatric Self‐Management Model, we identified four key predictors of suboptimal adherence. These factors include both nonmodifiable and modifiable factors that should be assessed and addressed within clinical practice settings.
Nonmodifiable factors included SES and race/ethnicity, which are intrinsically linked. 28 Specifically, lower SES and the child being of color were associated with being in the suboptimal adherence group, and exploratory analyses found that relative risk of being in the suboptimal adherence group was even higher among children with Black racial identities. Lower SES being associated with suboptimal adherence has long been established in the pediatric epilepsy adherence literature. 3 However, findings related to the child's race are novel in the field and mirror some of the adult epilepsy adherence literature. Specifically, Bautista et al. 29 found that relative to White non‐Hispanic adults, Black adults had lower ASM adherence after controlling for key adherence predictors (e.g., education, age, epilepsy type). The reasons for these differences may be attributed to the perception that ASMs are more harmful than beneficial among groups that have faced historic mistreatment and therefore have broken trust with medical providers and systems. 30 , 31 Furthermore, the outcomes for individuals with epilepsy who are socially disadvantaged and/or Black appear to be worse. 29 , 32 This may be due to historic barriers that have resulted in a lack of resources and access to comprehensive multidisciplinary epilepsy care 33 or systemic and institutional racism that exists in our medical system. Furthermore, recent research by our team has identified that barriers to adherence are different for White families as compared to Black families. 34
This intersectionality of SES and race on adherence highlights the need to identify families seen in epilepsy care that are less resourced due to these nonmodifiable factors and speaks to the need to provide proactive interventions. Because historic systems of racism and inequality have contributed to this current disparity for Black children, the proactive identification of systemic barriers and subsequent interventions to mitigate these barriers are essential to adherence promotion among Black children with epilepsy. For instance, perhaps adherence interventions should first seek to assess and address systemic barriers (e.g., access to pharmacies), and then provide individual adherence interventions only if adherence problems persist once barriers are addressed. Social workers may be ideally positioned to identify systemic barriers together with families and connect them with appropriate resources. The Epilepsy Learning Healthcare System is currently evaluating ways to systematically assess adherence barriers, with the goal of addressing both the systems and individual/family barriers. 35 Another approach is to address adherence barriers at point of care 36 via interdisciplinary teams, which are timely and cost‐effective. 37 Valenzuela and Smith 31 encourage interdisciplinary care with psychologists to promote patient–provider interactions that are sensitive to the family and likely contribute to reductions in health disparities. In general, adherence interventions at point of care need to be culturally tailored when possible, as this typically facilitates improved adherence behaviors. 38 , 39
Modifiable factors that predicted suboptimal adherence group status included general family functioning and parental distress. These findings are consistent with the literature, 9 , 40 as caring for a child with a chronic condition can be stressful to caregivers 41 and lead to parental distress. Studies have demonstrated that caregivers of children with chronic conditions are more likely to be have symptoms of depression, anxiety, and posttraumatic stress disorder. 42 , 43 , 44 Thus, it is critical for pediatric epilepsy health care providers to recognize the critical need to address the family unit in care, as parent functioning is directly linked to child outcomes. 45 , 46 , 47 Despite a growing body of literature that delineates the role of parent psychosocial factors in medication adherence among children with epilepsy, screening for parent distress and family functioning has largely been confined to research endeavors and is virtually nonexistent during pediatric clinical health encounters. Thus, the current standards for clinical practice fail to capture dynamics critical to patient success, and thereby miss opportunities to address modifiable factors that influence medication adherence.
Overall, these data highlight several areas that warrant attention in clinical settings. Although comprehensive screening of child, parent, and family factors that lead to suboptimal adherence is recommended, the busy pace of clinic visits and inability to screen in smaller clinical practices makes this recommendation difficult to implement. Furthermore, interdisciplinary epilepsy care is vital in the treatment of both physical and mental health symptoms of youth with epilepsy. Having psychologists integrated into routine epilepsy visits would allow for clinical assessment and short‐term therapeutic solutions that are proactive and preventative in nature. For example, if a young child is exhibiting more behavioral issues, which could lead to parent distress, a psychologist may be able to provide strategies for behavior early on to mitigate larger behavioral issues. Such interventions likely would influence adherence and self‐management, given that children with oppositional behaviors are less likely to adhere to their treatment regimen. As noted above, psychologists can also serve to improve patient–provider communications within the team, which could additionally benefit family adherence and thereby health (e.g., seizure control) and patient‐reported outcomes (e.g., quality of life).
Although the study has several strengths, including a large sample size and well operationalized outcomes, there are several notable limitations. First, the larger STAR randomized clinical trial was conducted at only a single site, which limits its generalizability. Multisite adherence intervention studies, some of which are ongoing (NCT03817229, NCT03958331), should replicate these study findings. Although electronic monitors are used for research purposes, they have not been supported well for clinical use due to cost in epilepsy. Thus, identifying new biomarkers of adherence (e.g., hair samples, blood levels) or having robust and simple adherence patient‐reported outcomes 48 could prove useful in clinical settings. Families enrolled in clinical trials, as represented in the current study, may not represent all families seen in clinical practice, 49 and thus the sample may not be generalizable. This may be especially true for families of color. 50 Furthermore, the inclusion criteria for this study may limit the generalizability of these findings to those with treatment‐resistant epilepsy who may be on polytherapy or who are not newly diagnosed. Adherence predictors may change as children's medical regimens become more complicated and as they manage their epilepsy for longer. Thus, further research in this area is essential. Finally, this study was completed as a secondary data analysis, and therefore we did not conduct a priori power analyses. This may limit the interpretation of our findings and speaks to the importance of replication through ongoing prospective research on these predictors of adherence.
Despite limitations of this study, the current data suggest the importance of finding creative and practical solutions for assessment and treatment of key adherence predictors. These may include streamlined screening for parental distress and family functioning, as well as recognition that families of lower SES and communities of color may be at heightened risk for suboptimal adherence. Thus, interdisciplinary care is highly recommended to optimize outcomes for youth with epilepsy.
AUTHOR CONTRIBUTIONS
Dana M. Bakula: Conceptualization, formal analysis, methodology, validation, visualization, writing–original draft preparation, writing–review & editing. Katherine W. Junger: Conceptualization, visualization, writing–review & editing. Shanna M. Guilfoyle: Conceptualization, visualization, writing–review & editing. Constance A. Mara: Conceptualization, formal analysis, methodology, visualization, writing–review & editing. Avani C. Modi: Conceptualization, visualization, writing–review & editing, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing–original draft preparation.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICAL APPROVAL
This study received Institutional Review Board approval and followed ethical guidelines of the American Psychological Association.
PATIENT CONSENT STATEMENT
All patients enrolled in this study provided informed consent for participation.
ACKNOWLEDGMENTS
We would like to thank the research coordinators, predoctoral interns, and postdoctoral fellows, and our epilepsy medical team for helping to conduct this study. We would also like to acknowledge all the children and their families that participated in the research.
Bakula DM, Junger KW, Guilfoyle SM, Mara CA, Modi AC. Key predictors of the need for a family‐focused pediatric epilepsy adherence intervention. Epilepsia. 2022;63:2120–2129. 10.1111/epi.17302
Funding information
This work was supported by a grant from the National Institutes of Health (R01HD073115) to the senior author.
DATA AVAILABILITY STATEMENT
These data will be available upon request and we will follow journal guidelines for data sharing and availability.
REFERENCES
- 1. Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy—United States, 2015. MMWR. Morbidity and Mortality Weekly Report. 2017;66(31):821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Chadwick D, Guerreiro C, et al. ILAE treatment guidelines: evidence‐based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47(7):1094–120. [DOI] [PubMed] [Google Scholar]
- 3. Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305(16):1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Modi AC, Pai AL, Hommel KA, Hood KK, Cortina S, Hilliard ME, et al. Pediatric self‐management: a framework for research, practice, and policy. Pediatrics. 2012a;129(2):e473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Modi AC, Wu YP, Guilfoyle SM, Glauser TA. Uninformed clinical decisions resulting from lack of adherence assessment in children with new‐onset epilepsy. Epilepsy & Behavior. 2012b;25(4):481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modi AC, Rausch JR, Glauser TA. Early pediatric antiepileptic drug nonadherence is related to lower long‐term seizure freedom. Neurology. 2014;82(8):671–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia. 2008;49(3):446–54. [DOI] [PubMed] [Google Scholar]
- 8. Gutierrez‐Colina AM, Smith AW, Mara CA, Modi AC. Adherence barriers in pediatric epilepsy: from toddlers to young adults. Epilepsy & Behavior. 2018;80:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loiselle K, Rausch JR, Modi AC. Behavioral predictors of medication adherence trajectories among youth with newly diagnosed epilepsy. Epilepsy & Behavior. 2015;50:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith AW, Gutierrez‐Colina AM, Guilfoyle SM, Modi AC. Pediatric epilepsy. Adherence and self‐Management in Pediatric Populations. Academic Press; 2020. p. 207–33. [Google Scholar]
- 11. Al‐aqeel S, Al‐sabhan J. Strategies for improving adherence to antiepileptic drug treatment in patients with epilepsy. Cochrane Database of Systematic Reviews. 2011;(1). 10.1002/14651858.CD008312.pub2 [DOI] [PubMed] [Google Scholar]
- 12. Modi AC, Guilfoyle SM, Mann KA, Rausch JR. A pilot randomized controlled clinical trial to improve antiepileptic drug adherence in young children with epilepsy. Epilepsia. 2016;57(3):e69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Modi AC, Guilfoyle SM, Rausch J. Preliminary feasibility, acceptability, and efficacy of an innovative adherence intervention for children with newly diagnosed epilepsy. Journal of Pediatric Psychology. 2013;38(6):605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews. 2014;(11). 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner JL, Modi AC, Johnson EK, Shegog R, Escoffery C, Bamps Y, et al. Self‐management interventions in pediatric epilepsy: what is the level of evidence? Epilepsia. 2017;58(5):743–54. [DOI] [PubMed] [Google Scholar]
- 16. Modi AC, Guilfoyle SM, Glauser TA, Mara CA. Supporting treatment adherence regimens in children with epilepsy: a randomized clinical trial. Epilepsia. 2021;62(7):1643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. Journal of the Royal Society of Medicine. 2011;104(12):510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Modi AC, Glauser TA, Guilfoyle SM. Supporting treatment adherence regimens in young children with epilepsy and their families: trial design and baseline characteristics. Contemporary Clinical Trials. 2020;90:105959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Temple RJ. Special study designs: early escape, enrichment, studies in non‐responders. Communications in Statistics‐Theory and Methods. 1994;23(2):499–531. [Google Scholar]
- 20. Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Social Science Research. 1981;10(4):364–95. [Google Scholar]
- 21. Morita DA, Glauser TA, Modi AC. Development and validation of the pediatric epilepsy side effects questionnaire. Neurology. 2012;79(12):1252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamphaus RW. Behavior assessment system for children, (BASC‐2). The Encyclopedia of Clinical Psychology. 2014;1–6. 10.1002/9781118625392.wbecp447 [DOI] [Google Scholar]
- 23. Derogatis LR, Spencer PM. The brief symptom inventory manual. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 24. Kellett S, Beail N, Newman DW, Frankish P. Utility of the brief symptom inventory in the assessment of psychological distress. Journal of Applied Research in Intellectual Disabilities. 2003;16(2):127–34. [Google Scholar]
- 25. Jarvie S, Espie CA, Brodie MJ. The development of a questionnaire to assess knowledge of epilepsy: I. general knowledge of epilepsy. Seizure. 1993;2:187–93. [DOI] [PubMed] [Google Scholar]
- 26. Epstein NB, Baldwin LM, Bishop DS. The McMaster family assessment device. Journal of Marital and Family Therapy. 1983;9(2):171–80. [Google Scholar]
- 27. Miller IW, Epstein NB, Bishop DS, Keitner GI. The McMaster family assessment device: reliability and validity. Journal of Marital and Family Therapy. 1985;11(4):345–56. [Google Scholar]
- 28. Jackson PB, Williams DR. The intersection of race, gender, and SES: health paradoxes. In: Schulz AJ, Mullings L, editors. Gender, race, class, & health: intersectional approaches. Jossey‐Bass/Wiley; 2006. p. 131–62. [Google Scholar]
- 29. Bautista RED, Graham C, Mukardamwala S. Health disparities in medication adherence between African‐Americans and Caucasians with epilepsy. Epilepsy & Behavior. 2011;22(3):495–8. [DOI] [PubMed] [Google Scholar]
- 30. Paschal AM, Ablah E, Wetta‐Hall R, Molgaard CA, Liow K. Stigma and safe havens: a medical sociological perspective on African‐American female epilepsy patients. Epilepsy & Behavior. 2005;7(1):106–15. [DOI] [PubMed] [Google Scholar]
- 31. Valenzuela JM, Smith L. Topical review: provider–patient interactions: an important consideration for racial/ethnic health disparities in youth. Journal of Pediatric Psychology. 2016;41(4):473–80. [DOI] [PubMed] [Google Scholar]
- 32. Bautista RED, Jain D. Detecting health disparities among Caucasians and African‐Americans with epilepsy. Epilepsy & Behavior. 2011;20(1):52–6. [DOI] [PubMed] [Google Scholar]
- 33. Szaflarski M, Szaflarski JP, Privitera MD, Ficker DM, Horner RD. Racial/ethnic disparities in the treatment of epilepsy: what do we know? What do we need to know? Epilepsy & Behavior. 2006;9(2):243–64. [DOI] [PubMed] [Google Scholar]
- 34. Gutierrez‐Colina AM, Wetter SE, Mara CA, Guilfoyle SM, Modi AC. Racial disparities in medication adherence barriers: pediatric epilepsy as an exemplar. Journal of Pediatric Psychology. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donahue MA, Herman ST, Dass D, Farrell K, Kukla A, Abend NS, et al. Establishing a learning healthcare system to improve health outcomes for people with epilepsy. Epilepsy Behav. 2021;117:107805. 10.1016/j.yebeh.2021.107805 [DOI] [PubMed] [Google Scholar]
- 36. Guilfoyle SM, Follansbee‐Junger K, Modi AC. Development and preliminary implementation of a psychosocial service into standard medical care for pediatric epilepsy. Clinical Practice in Pediatric Psychology. 2013;1(3):276–88. [Google Scholar]
- 37. Ryan JL, McGrady ME, Guilfoyle SM, Junger K, Arnett AD, Modi AC. Health care charges for youth with newly diagnosed epilepsy. Neurology. 2015;85(6):490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosal MC, Ockene IS, Restrepo A, White MJ, Borg A, Olendzki B, et al. Randomized trial of a literacy‐sensitive, culturally tailored diabetes self‐management intervention for low‐income Latinos: Latinos en control. Diabetes Care. 2011;34(4):838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Utz SW, Williams IC, Jones R, Hinton I, Alexander G, Yan G, et al. Culturally tailored intervention for rural African Americans with type 2 diabetes. The Diabetes Educator. 2008;34(5):854–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Everhart RS, Fiese BH, Smyth JM, Borschuk A, Anbar RD. Family functioning and treatment adherence in children and adolescents with cystic fibrosis. Pediatric Allergy, Immunology and Pulmonology. 2014;27(2):82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cousino MK, Hazen RA. Parenting stress among caregivers of children with chronic illness: a systematic review. Journal of Pediatric Psychology. 2013;38(8):809–28. [DOI] [PubMed] [Google Scholar]
- 42. Pinquart M. Featured article: depressive symptoms in parents of children with chronic health conditions: a meta‐analysis. Journal of Pediatric Psychology. 2019a;44(2):139–49. [DOI] [PubMed] [Google Scholar]
- 43. Pinquart M. Meta‐analysis of anxiety in parents of young people with chronic health conditions. Journal of Pediatric Psychology. 2019b;44(8):959–69. [DOI] [PubMed] [Google Scholar]
- 44. Pinquart M. Posttraumatic stress symptoms and disorders in parents of children and adolescents with chronic physical illnesses: a meta‐analysis. Journal of Traumatic Stress. 2019c;32(1):88–96. [DOI] [PubMed] [Google Scholar]
- 45. Bakula DM, Sharkey CM, Perez MN, Espeleta HC, Gamwell KL, Baudino M, et al. Featured article: the relationship between parent and child distress in pediatric cancer: a meta‐analysis. Journal of Pediatric Psychology. 2019;44(10):1121–36. [DOI] [PubMed] [Google Scholar]
- 46. Bakula DM, Sharkey CM, Perez MN, Espeleta HC, Gamwell KL, Baudino M, et al. The relationship between parent distress and child quality of life in pediatric cancer: a meta‐analysis. Journal of Pediatric Nursing. 2020;50:14–9. [DOI] [PubMed] [Google Scholar]
- 47. Drotar D. Relating parent and family functioning to the psychological adjustment of children with chronic health conditions: what have we learned? What do we need to know? Journal of Pediatric Psychology. 1997;22(2):149–65. [DOI] [PubMed] [Google Scholar]
- 48. Plevinsky JM, Gutierrez‐Colina AM, Carmody JK, Hommel KA, Crosby LE, McGrady ME, et al. Patient‐reported outcomes for pediatric adherence and self‐management: a systematic review. Journal of Pediatric Psychology. 2020;45(3):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sanson‐Fisher RW, Bonevski B, Green LW, D'Este C. Limitations of the randomized controlled trial in evaluating population‐based health interventions. American Journal of Preventive Medicine. 2007;33(2):155–61. [DOI] [PubMed] [Google Scholar]
- 50. Moye LA, Powell JH. Evaluation of ethnic minorities and gender effects in clinical trials: opportunities lost and rediscovered. Journal of the National Medical Association. 2001;93(12 Suppl):29S–34S. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These data will be available upon request and we will follow journal guidelines for data sharing and availability.


